Abstract
Context
Subarachnoid hemorrhage (SAH) has a high fatality rate and many suffer from delayed neurological deficits. Biomarkers may aid in the identification of high-risk patients, guide treatment/management and improve outcome.
Objective
The aim of this review was to summarize biomarkers of SAH associated with outcome.
Methods
An electronic database query was completed, including an additional review of reference lists to include all potential human studies.
Results
A total of 298 articles were identified; 112 were reviewed; 55 studies were included.
Conclusion
This review details biomarkers of SAH that correlate with outcome. It provides the basis for research investigating their possible translation into the management of SAH patients.
Keywords: Biomarkers, critical care, markers, outcome, subarachnoid hemorrhage, vasospasm
Introduction
Subarachnoid hemorrhage (SAH) comprises about 5% of all strokes (Ostrowski et al., 2006). The mortality from this devastating condition is high, with a case fatality rate of about 50% (Hop et al., 1997), with more than 30% of patients dying from the initial hemorrhage or rehemorrhage (Ostrowski et al., 2006). Of those who survive, more than 40% have long-term delayed neurological deficits (DNDs), including stroke, cognitive and neuropsychological abnormalities that adversely affect overall function and quality of life. The multi-faceted pathophysiology of SAH, combined with poor functional outcome, underscores the need for prognostic serum biological markers that can be used to aid in the identification of patients at high-risk for vasospasm, ischemia and stroke prior to neurological deterioration.
A number of factors contribute to DNDs and poor outcome after SAH including cerebral vasospasm, ischemia, stroke, microthrombi, oxidative damage and inflammation (Kassell et al., 1985). Of these, vasospasm has been the most widely researched. Although it is a common, potentially treatable, complication of SAH, its pathogenesis is not completely understood. Results from the CONSCIOUS trials (Macdonald et al., 2008, 2010, 2011, 2013; Meyers & Connolly, 2011) demonstrated that the prevention of angiographic vasospasm has equivocal effects on outcome. This finding has led to a reevaluation of the pathogenesis of brain injury in SAH and a resurgence of interest in neuroinflammation as a primary culprit. Neuroinflammation, vasospasm, ischemia and stroke may be interdependent manifestations of a worsening clinical course.
Parameters associated with outcome in SAH assess the state of the patient on admission and include age, admission neurologic grade (i.e. Hunt and Hess grade and World Federation of Neurological Surgeons grade) and the amount of blood on admission computed tomography (CT) scan (i.e. Fisher grade or similar classification). The current management of SAH patients, during the acute phase of the disease process, is centered on identifying changes in neurological examination or imaging studies, such as CT or transcranial Doppler (TCD). Imaging studies are performed either routinely or are obtained following changes in clinical signs or symptoms. Imaging that are triggered by patient decline, and preemptive medical treatment that is often initiated before imaging, results in appropriate management for some patients, but for others, can result in unnecessary treatment and multiple unnecessary imaging studies. Medical treatment of SAH is not without risk and can increase morbidity, length of hospital stay and mortality (Suarez et al., 2006). 40% of SAH patients experience medical complications including pulmonary edema, cardiac arrhythmia and electrolyte disturbances (Solenski et al., 1995; Suarez, et al., 2006). These complications are secondary to the treatment and the nature of the disease. Irregardless, utilizing prognostic serum biomarkers to aid in predicting which patients will have neurological sequelae before symptomatic presentation may help guide treatment during the acute phase, would allow for standardized monitoring that could identify high-risk patients and initiate early therapy prior to clinical deterioration. This approach could also improve cost effectiveness, decrease unnecessary treatment, reduce the need for emergent imaging, and ultimately improving patient care and outcome after SAH.
Biomarkers are a measurable entity representing a biological or pathophysiological process. Some serum biomarkers have proven to be reliable tools for diagnosis, therapeutic decision making and prognosis in many disease states. Prominent examples of utilization of biomarkers in medicine include the use of cardiac troponin I (cTnI) for acute myocardial infarction, B-type naturetic peptide for congestive heart failure and C-reactive protein (CRP) and lactate for septic shock. Although the kinetics of each biomarker of outcome in SAH is beyond the scope of this review, this article aims to focus on the evidence-based research and potential utility of these biomarkers in the prognosis of patients after SAH. This systematic review summarizes biomarkers that have been specifically associated with clinical outcome, focusing on serum biomarkers, in hopes of providing an impetus for further research towards the development and implementation in the management of SAH patients.
Methods
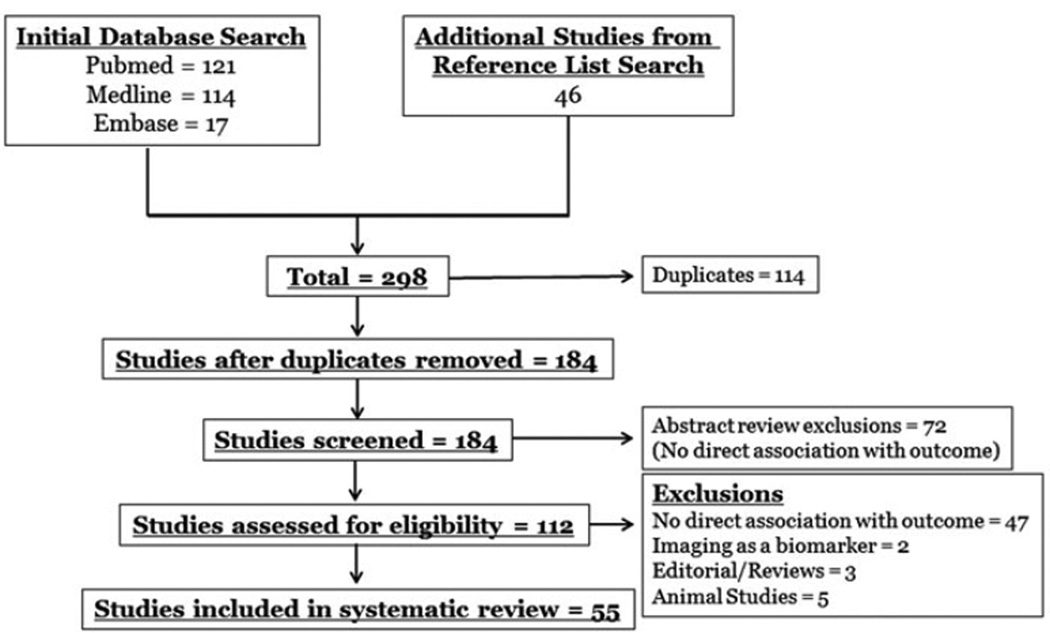
MEDLINE (1946–January 2013), PubMed (1809–January 2013) and EMBASE (2009–January 2013) electronic databases were queried. The search included the medical subject heading and keywords “subarachnoid hemorrhage” AND “biomarkers” AND “outcome”. There were no restrictions in language. The initial search identified 252 potentially usable studies. An extensive detailed review of reference lists was done to include all potential studies, as not all study results were identified as “biomarkers” of SAH. This resulted in 46 additional studies. A total of 298 studies resulted. All duplicates were eliminated. The remaining studies were reviewed by the authors for inclusion via abstract review. 72 publications were excluded by abstract review secondary to no outcome analysis. Of the remaining 112 studies, 57 studies were excluded after manuscript review by the primary author, and confirmed by all other authors. These included studies with no direct association with outcome after SAH, animal studies, editorial/reviews and imaging used as a “biomarker”. A total of 55 studies were retained and included in this systematic review (Figure 1). All 55 articles were agreed by all authors to support the goal of this review, identifying biomarkers that have been directly associated with outcome after SAH.
Figure 1.
Search results.
Results
Manual abstract screening and exclusion of articles were completed as describe above. All the studies included in this review were human and written in English. There were a significant amount of studies identified from the detailed reference list review. These studies did not specifically state their findings were “biomarkers” and therefore were not found on the initial search. A total of 46 additional studies resulted. A summary of studies included in this systematic review is given, by group, in Table 1.
Table 1.
Summary of studies reporting biomarkers associated with outcome in SAH.
| Biomarker | #of patients |
Detection substrate |
Summary of findings and outcome scale used | Author, year |
|---|---|---|---|---|
| Neuron and astrocyte-specific markers | ||||
| S100β | 32 | CSF | Univariate analysis demonstrated increased S100β associated with poor outcome (GOS) | Kaneda et al., 2010 |
| 109 | Blood | S100β is excellent predictor of 12-month outcome (GOS) | Sanchez-Pena et al., 2008 | |
| 55 | CSF/Blood | Increased CSF and blood S100β was associated with outcome prognosis (GOS) | Moritz et al., 2010 | |
| 52 | Blood | S100β indicator of scale and severity as well as prognostic indicator of 1 year outcome. (GOS) | Stranjalis et al., 2007 | |
| 74 | Blood | Level of S100β correlated with initial SAH severity and at day 8 was an independent predictive factor for outcome at 6 months (GOS) | Weiss et al., 2006 | |
| 51 | Blood | Elevated S100β associated with increased mortality and unfavorable outcome at 6 months (GOS) | Oertel et al., 2006 | |
| 67 | Blood | Elevations of S100β was associated with poor outcome (GOSE) | Vos et al., 2006 | |
| 43 | Blood | Mean values of S100β predictor of outcome (GOS) | Schick et al., 2003 | |
| 71 | Blood | Elevations of S100β correlate with early neurological deficits (7 days) and worse outcome at 6 months (GOS) | Wiesmann et al., 1997 | |
| 45 | CSF | Increased S100β associated with severity of injury, clinical course, delayed ischemic deterioration and outcome (GOS) | Hardemark et al., 1989 | |
| Neuron-specific enolase (NSE)-conflicting | 55 | CSF | Increased CSF levels of NSE associated with poor outcome at discharge from ICU (GOS) | Moritz et al., 2010 |
| 29 | Blood | Increased NSE levels associated with poor outcome at discharge (GOS) | Mabe et al., 1991 | |
| Glial fibrillary acid protein (GFAP) | 32 | CSF | Univariate analysis demonstrated increased GFAP associated with poor outcome (GOS) | Kaneda et al., 2010 |
| 116 | Blood | GFAP independent predictor of outcome after 1 year (GOSE) | Nylen et al., 2007 | |
| 67 | Blood | Elevations of GFAP associated with poor outcome (GOSE) | Vos et al., 2006 | |
| Apolipoprotein E (ApoE) | 47 | CSF | Decreases correlated with poor clinical outcome at 3 months (GOS) | Kay et al., 2003a,b |
| APOE gene | 46 | Genotyping | E4 carriers trend towards detrimental long term effect on cognitive function (10 year longitudinal study) | Louko et al., 2006 |
| 101 | Genotyping | E4 expression prognostic for clinical VSP and higher risk of definitive neurologic deficits at least 6 months (MMSE and RDI) | Lanterna et al., 2005 | |
| 108 | Genotyping | E4 gene expression prognostic factor for poor outcome with a risk of unfavorable outcome 7.1 times higher noncarriers (GOS) | Niskakangas et al., 2001 | |
| 227 | Genotyping | E4 association with unfavorable outcome at 3 months (GOS) | Tang et al., 2003 | |
| 72 | Genotyping | E4 carriers associated with poor outcome at 6 months (GOS) | Leung et al., 2002 | |
| Neurofilament | 30 | CSF | Phosphorylated neurofilament subunit (pNF-H) elevations from the second week predict poorer outcome (GOSE) | Lewis et al., 2010 |
| 148 | CSF | Neurofilament heavy chain elevations in CSF associated with bad outcome at 3 months (GOS) | Petzold et al., 2006 | |
| 48 | CSF | Neurofilament protein elevation in CSF correlates with neurological status in-hospital and 1-year outcome (GOSE) | Nylen et al., 2006 | |
| Amyloid B-protein | 47 | CSF | Decreases levels correlated with poor clinical outcome at 3 months (GOS) | Kay et al., 2003a,b |
| Ubiquitin C terminal Hydrolase 1 (UCHL1) | 30 | CSF | Elevation from the second week predict poorer outcome (GOSE) | Lewis et al., 2010 |
| Inflammatory markers | ||||
| CRP | 116 | Blood | Early elevations of CRP prognostic for poor outcome (mRS) | Jeon et al., 2012 |
| 178 | Blood | Elevated CRP predict risk for poor outcome (GOS) | Juvela, 2000 | |
| 25 | Blood | Elevated CRP associated with worse admission grade and poor GCS and NIHSS scores | Frontera et al., 2012 | |
| 82 | Blood | Inverse relationship between CRP and GOS at discharge (GOS and mRS) | Romero et al., 2012 | |
| 110 | Blood | hsCRP in patients who had delayed cerebral ischemia and poorer outcome at 3 months (mRS) | Badjatia et al., 2011 | |
| 41 | Blood | CRP levels were inversely related to GOS and mRS scores on discharge | Fountas et al., 2009 | |
| 33 | Blood | Increased levels of CRP were associated with delayed ischemic neurologic deficit at day 0 and day 7 after SAH | Kubo et al., 2008 | |
| TNF-α | 52 | Blood | Elevations of TNF-α on Day 2 to 3 is significantly associated with poor at 3-month with a similar trend at 6 months (mRS) | Chou et al., 2012 |
| 52 | CSF | Increased TNF-α associated with unfavorable outcome at 3 months (GOS) | Nakahara et al., 2009 | |
| 22 | CSF | Increased TNF-α associated with unfavorable outcome at 12 days post SAH (GOS) | Mathiesen et al., 1997 | |
| IL-1 | 22 | CSF | Increased IL-1Ra (IL-1 receptor antagonist) associated with unfavorable outcome at 12 days post SAH (GOS) | Mathiesen et al., 1997 |
| IL-6 | 52 | CSF | Increased IL-6 associated with unfavorable outcome at 3 months (GOS) | Nakahara et al., 2009 |
| 35 | CSF/Blood | Increased IL-6 in CSF significantly increased in patients with poor clinical outcome (Day 11) (GOS) | Fassbender et al., 2001 | |
| IL-8 | 52 | CSF | Increased IL-8 associated with unfavorable outcome at 3 months (GOS) | Nakahara et al., 2009 |
| High-mobility Group Box 1 protein (HMGB1) | 52 | CSF | Increased HMGB1 associated with unfavorable outcome at 3 months (GOS) | Nakahara et al., 2009 |
| Catecholamine | 102 | CSF | Elevated CSF epinephrine independent predictor of mortality and disability at 30 days | Moussouttas et al., 2012 |
| 21 | CSF/Blood | Elevated CSF epinephrine and norepinephrine associated with patients with focal ischemic deficits | Dilraj et al., 1992 | |
| Microalbuminuria | 51 | Urine | High microablumin/creatinine ration (MACR), >200 mg/g, in first 8 days from SAH predictor of unfavorable neurological outcome at 3 months (GOS) | Terao et al., 2007 |
| Molecular adhesion and extracellular matrix markers | ||||
| MMP-9 | 55 | CSF/Blood | Elevation of MMP-9 associated with poor 3-month clinical outcome (mRS) | Chou et al., 2011 |
| ICAM-1, VCAM-1 | 33 | Blood | Increased levels of ICAM-1 and VCAM-1 were associated with delayed ischemic neurologic deficit at day 0 and day 7 after SAH | Kubo et al., 2008 |
| 78 | CSF/Blood | Very high CSF ICAM-1 and VCAM-1 associated with unfavorable outcome and/or symptomatic VSP (GOS at discharge) | Kaynar et al., 2004 | |
| 158 | Blood | ICAM-1 associated with patients with SAH with poor outcome at day 14 (mRS) | Mack et al., 2002 | |
| Selectin | 21 | Blood | sL-selectin associated with poor outcome at 6 months (BI) | Wang et al., 2011 |
| Vascular and angiogenic markers | ||||
| Endothelial microparticles | 20 | Blood | MP higher at discharge but no significant differences after 6 months (mRS and GOS) | Lackner et al., 2010 |
| VEGF | 38 | Blood | Increased levels of VEGF predict onset of delayed VSP before onset or neurological deterioration | Mcgirt et al., 2002 |
| Caspase-3 | 52 | Blood | Regression analysis demonstrated early C3a strong independent predictor of functional outcome | Mack et al., 2007 |
| Coagulation factor markers | Blood | |||
| vWF | 90 | Blood | Early levels of vWF associated with delayed cerebral ischemia and poor outcome at 12–14 weeks (mTS) | Frinjs et al., 2006a,b |
| Fibrinogen | 25 | Blood | Elevated fibrinogen correlated with worse Barthel scores at 14 days and trended to worse Barthel index at 3 months (BI) | Frontera et al., 2012 |
| 72 | Blood | Elevated levels strongly correlated with mortality | Morga et al., 2007 | |
| Thrombin-antithrombin complex (TAT) | 72 | Blood | Elevated levels strongly correlated with mortality | Morga et al., 2007 |
| Cardiac markers | ||||
| Cardiac troponin I (cTnI) | 41 | Blood | Neurologic outcome during hospital stay was adversely related to increased cTnI and wall motion abnormalities, predicting poor GCS on admission and increased hospital stay (GOS) | Kumar et al., 2011 |
| 239 | Blood | Patients with elevated cTnI more severe aSAH symptoms and levels ≥0.3 ng/ml independent predictor of poor functional outcome at 2 months (GOS and mRS) | Miketic et al., 2010 | |
| 378 | Blood | In-hospital mortality significantly increased with increased TnI | Sandhu et al., 2008 | |
| 83 | Blood | Elevated cTnI predictor of in-hospital mortality | Ramappa et al., 2008 | |
| 300 | Blood | Strong association between TnI and in-patient mortality | Yarlagadda et al., 2006 | |
| 68 | Blood | cTnI elevations independent prognostic indicator of poor outcome at 3 months (mRS) | Schuiling et al., 2005 | |
| Other markers | ||||
| Malondialdehyde (MDA) | 32 | CSF | At 2 weeks, elevations of MDA predictor of poor neurological outcome at 6 months (GOS) | Kaneda et al., 2010 |
| Kallikrein-related peptidase-6 (KLK6) | 13 | Blood | Kallikrein-related peptidase 6 (KLK6) was significantly lower in patients with severe disability or death. | Martinez-Morillo et al., 2012 |
| Creatine Kinase-BB (CK-BB) | 30 | CSF | Increased levels of CK-BB > greater than 40 U/L were associated with unfavorable outcome at 1 week and 2 months (GOS) | Coplin et al., 1999 |
| Microdialysis (MD) | ||||
| Lactate | 95 | Brain tissue | Significantly higher in patients with acute focal neurological deficits and correlated with neurological worsening at 6 and 12 months (GOS) | Sarrafzadeh et al., 2003 |
| 10 | Brain tissue | Elevations significantly correlated with poor outcome at 3 months (GOS) | Staub et al., 2000 | |
| Pyruvate | 95 | Brain tissue | Decreased levels in patients with acute focal neurological deficits and correlated with neurological worsening at 6 and 12 months (GOS) | Sarrafzadeh et al., 2003 |
| Excitatory amino acids (EAA) | 95 | Brain tissue | Significantly higher in patients with acute focal neurological deficits and correlated with neurological worsening at 6 and 12 months (GOS) | Sarrafzadeh et al., 2003 |
| 10 | Brain tissue | Elevations significantly correlated with poor outcome at 3 months (GOS) | Staub et al., 2000 | |
| Nitrate | 10 | Brain tissue | Elevations significantly correlated with poor outcome at 3 months (GOS) | Staub et al., 2000 |
GOS: Glasgow Outcome Scale; GOSE: Glasgow Outcome Scale Extended; MMSE: Mini-Mental State Examination; RDI: Rankin Disability Index; mRS: Modified Rankin Score; mTS: Modified Tardieu Scale; BI: Barthel Index.
There were a number of variabilities within these studies including the study size, the timing of assessment of outcome, the outcome scoring scale used and the substrate tested. The study sizes varied from 20 to 378 subjects. There was short-term and long-term timing for assessments of outcome. This included 3–12 days after SAH, discharge from hospital and 1 month to 1 year after SAH. There was also a variety of outcome scoring scales used included Glasgow Outcome Scale (GOS), Glasgow Outcome Scale Extended (GOSE), Mini-Mental State Examination (MMSE), Rankin Disability Index (RDI), modified Rankin Score (mRS), modified Tardieu Scale (mTS) and the Barthel Index (BI). Tested substrates include blood, CSF and brain tissue (microdialysis). Direct comparisons between the studies were not done for this systematic review secondary to such variabilities, however discussed below are cited sensitivities and specificities.
A biomarkers’ ability to accurately determine negative and positive subjects will guide their utility in clinical practice. In SAH, acceptable sensitivities and specificities were demonstrated with CRP, selectin, thrombin antithrombin complex (TAT), creatine kinase-BB (CK-BB) and malondialdehyde (MDA) (83 versus 84%, 90 versus 83%, 72 versus 70%, 70 versus 100% and 88 versus 75%, respectively) (Coplin et al., 1999; Jeon et al., 2012; Kaneda et al., 2010; Morga et al., 2007; Wang et al., 2011). Although most of the sensitivities and specificities are not greater than 90%, each of these markers may be able to identify subjects that have poor outcome and those that will potentially recover well with some reasonableness.
Some biomarkers have acceptable sensitivities but poor specificities. These included GFAP, neurofilament, ubiquitin C terminal hydrolase 1 (UCHL1) and D-dimer (92 versus 40%, 40–100% versus 14–91%, 90 versus 50%, 88 versus 36%, respectively) (Lewis et al., 2010; Morga, et al., 2007; Nylen et al., 2007; Petzold et al., 2006). Microalbuimuria had a poor sensitivity but an acceptable specificity (60 versus 96%, respectively) (Terao et al., 2007). Surprisingly, although S100β had the most number of studies secondary to the logical promise of its utilization, a study demonstrated poor sensitivity and specificity with outcome, 50 and 67% respectively (Weiss et al., 2006). In this review, we discuss biomarkers of SAH and the evidence of their applicability to outcome.
Discussion
Neuron and astrocyte specific markers
Central nervous system (CNS)-specific markers have been the focus of research as potential biomarkers for outcome after SAH, specifically markers originating from neurons and astrocytes. S100β, neuron-specific enolase (NSE), glial fibrillary acid protein (GFAP), apolipoprotein E (apoE) and APOE gene, neurofilament, amyloid β-protein and ubiquitin C terminal hydrolase 1 (UCHL1) have been studied. Of these, S100β has been used as a prognostic adjunct tool, monitoring outcome following therapeutic treatment with magnesium and atorvastatin in SAH patients (Hassan et al., 2012; Schmid-Elsaesser et al., 2006).
S100β
S100β is a group of calcium-binding protein dimers found predominantly in astrocytes, glial and Schwann cells in the CNS. It is released secondary to pathological brain injury such as SAH, acute brain injury, traumatic brain injury (TBI), acute ischemic stroke and cardiac arrest reflecting neuroinflammation and injury. Increased blood levels of S100β are associated with brain injury in TBI (Ingebrigtsen et al., 1999; Kanner et al., 2003) and stroke (Kanner et al., 2003), and has demonstrated some validity in SAH. In 1998, Persson et al. (1988) were among the first to demonstrate high cerebral spinal fluid (CSF) levels of S100β after SAH. Concentrations were related to the severity of hemorrhage, the development of delayed ischemia secondary to vasospasm and outcome. Since this early study, the relationship between S100β and vasospasm has been controversial. Moritz et al. (2010) and Jung et al. (2013) demonstrated no association with increased S100β levels and vasospasm. Interestingly, Herrmann et al. (2000) found that patients with strokes that had low blood levels of S100β completely recovered, with reversible pathology. This correlated with finding by Jung et al. (2013) who demonstrated a similar association in SAH. They showed that patients with vasospasm and no elevation of S100β had no delayed cerebral ischemia.
More recently, Sanchez-Pena et al. (2008) demonstrated that the mean 15-day S100β blood level is a prognostic indicator of 12-month outcome in SAH. Few studies that have investigated S100β in CSF found a similar correlation with outcome (Hardemark et al., 1989; Kaneda et al., 2010). CSF sampling is difficult to collect and exposes the patient to additional risks. Therefore, serum sampling would be more efficacious and safe in comparison. S100β may be a prognostic biomarker of SAH, and brain injury in general, specifically with the potential to identify patients with reversible injury. Further research must focus on its efficacy in guiding the management of SAH, identifying appropriate high-risk patients and its impact as a prognostic tool in SAH.
Neuron-specific enolase
Neuron-specific enolase (NSE) is a cytoplasmic enzyme released by neurons and neuroendocrine cells after damage to the CNS. Although NSE has been shown to increase with SAH, there have been controversial results regarding its associations with outcome Kacira et al. (2007) demonstrated an increase in CSF and blood NSE levels after SAH. Kaneda et al. (2010) and Oertel et al. (2006) found no correlation with CSF or blood NSE levels with outcome, respectively. Mortiz et al. (2010) investigated both CSF and blood NSE and found a correlation with CSF only. Mabe et al. (1991) demonstrated a significant correlation with serum NSE and outcome. The studies demonstrating an association with NSE and outcome determined Glasgow outcome scale (GOS) at discharge from the intensive care unit or hospital (Mabe et al., 1991; Moritz et al., 2010). Therefore, NSE may be a biomarker of prognosis in the acute phase after SAH, as there was no correlation with 6-month outcome (Oertel et al., 2006).
Glial fibrillary acid protein
Glial fibrillary acid protein (GFAP) is a cytoskeleton protein that serves as an intermediate filament in mature astrocytes. It is increased in CSF in brain pathologies such as stroke, intracerebral hemorrhage, dementia and SAH and has been associated with predicting functional outcome after stroke and TBI (Herrmann et al., 2000; Mondello et al., 2011, 2012). Vos et al. (2004) concur that GFAP levels in blood are a prognostic indicator of functional outcome after stroke and TBI. In SAH, although there are no studies demonstrating a direct association with GFAP and vasospasm, there are three studies that demonstrate GFAP as an independent predictor of poor outcome (Kaneda et al., 2010; Vos et al., 2006) as far out as 1 year (Nylen et al., 2007). Although Vos et al. (2006) demonstrated that both S100β and GFAP in blood were associated with increased odds for poor outcome at hospital discharge, S100β was a better predictor. This differentiation may be due to the timing of release after injury, level of vasogenic edema, cytotoxicity and apoptosis after SAH. Regardless, GFAP shows promise as a marker of outcome in SAH. Further research is required for validation and applicability.
Apolipoprotein E (ApoE)
ApoE is a polymorphic protein produced in the brain that exerts neurotrophic and neuroprotective effects. It has been linked to neurological disease states such as TBI and Alzheimer’s disease (Lanterna & Biroli, 2009). The biological effects of ApoE after SAH include modulation of inflammation in the brain, free radical scavenger, membrane repair, excitotoxicity protection, protection against apoptosis and smooth muscle contraction (Lanterna & Biroli, 2009). In SAH, decreased CSF levels of ApoE correlate with poor outcome (Kay et al., 2003a). Moreover, an ApoE-mimetic peptide, derived from the receptor binding site of the protein, demonstrated therapeutic benefit in a mouse model, improving functional outcome, reducing mortality and decreasing vasospasm (Gao et al., 2006).
The APOE gene has been associated with outcome and delayed cerebral ischemia after SAH. In a more than 10-year longitudinal study, Louko et al. (2006) demonstrated that, although not statistically conclusive, carriers of the E4 isoform had a greater risk for impaired memory and color naming. However, Morris et al. (2004) demonstrated no significant association between E4 carriers and cognitive impairment. Although APOE, specifically the E4 isoform, has been shown to be associated with mortality and poor outcome (Dunn et al., 2001; Gao et al., 2006; Kay et al., 2003a,b; Lanterna et al., 2005; Leung et al., 2002; Louko et al., 2006; Niskakangas et al., 2001; Tang et al., 2003), data supporting its use as a biomarker for prognosis are weak. Furthermore, the absolute (positive or negative) value of genotyping, and the inability to identify abnormal elevations or depressions, limits the potential usefulness in monitoring the progression of disease in SAH.
Others
Other neuronal-specific biomarkers that have been associated with outcome in SAH are neurofilament (NF), amyloid β-protein and ubiquitin C terminal hydrolase 1 (UCHL1). All three markers reflect a level of brain injury or degeneration and have been associated with poor outcome (Kay et al., 2003b; Lewis et al., 2010; Nylen et al., 2006; Petzold et al., 2006). Neurofilament is a structural component of motor axons and a marker of neuronal injury in white matter. There are three studies demonstrating that elevated levels of neurofilament in CSF is associated with outcome in SAH (Lewis et al., 2010; Nylen et al., 2006; Petzold et al., 2006) as far out as 1 year (Nylen et al., 2006). However, Zanier et al. (2011) demonstrated no correlation with neurofilament CSF levels and adverse events or long-term clinical outcome. Amyloid β-protein activates enzymatic processes, protects against oxidation and is a transcriptional factor. Kay et al. (2003b) demonstrated, in a small human study, that decreased levels of CSF amyloid β-protein correlated with poor clinical outcome at 3 months. UCHL1 is an enzyme specific to neurons and neuroendocrine cells and is concentrated in dendrites in gray matter, representing 1–2% of human brain protein. It is released into the CSF following neuronal and dendritic injury. Lewis et al. (2010) demonstrated that increased levels of CSF UCHL1 for more than 10 days post-aneurysm rupture were predictive of poor outcome. The research on these three markers is in its infancy and further work must be done to determine systemic correlations and their possible utility as predictors of outcome in SAH.
Inflammatory biomarkers
Inflammation plays a key role in the pathology of SAH and contributes to functional and cognitive outcome. In SAH, the action of blood in the subarachnoid space initiates the rapid activation of inflammatory cascades that include vascular and cellular components, such as leukocyte migration and cell adhesion molecules in endothelial cells. This initial, acute, neuroinflammatory response plays an important role in the treatment and prognosis of patients with SAH.
A number of cytokines, proinflammatory markers and products of metabolism have been correlated with poor outcome after SAH. These include C-reactive protein (CRP), TNF-α, IL-1 family members (i.e. IL-1 receptor antagonist: IL-1Ra), IL-6, IL-8, high-mobility group box 1 protein (HMGB1), catecholamines and microalbuminuria. Other serum markers such as procalcitonin (PCT) and myeloperoxidase (MPO) have not been demonstrated to be associated with outcome, but have been associated with inflammation in SAH (Cengiz et al., 2007; Kofoed et al., 2007; Luzzani et al., 2003; Oconnor et al., 2004; Reinhart & Meisner, 2011). Lactate, a product of anaerobic metabolism, is a biomarker of mortality in patients with septic shock (Manikis et al., 1995; Mikkelsen et al., 2009). In SAH, lactate has been associated with poor outcome in a mouse model (Cengiz et al., 2007); however, no human studies have been reported.
C-reactive protein
C-reactive protein (CRP) is a member of the acute phase reactant protein family and has been used as a prognostic biomarker in bacterial infection and sepsis. CRP is produced by hepatocytes secondary to cytokine and inflammatory stimulation. It is one of the most widely used marker of infection in the critically ill, with varying levels of sensitivity and specificity (Vincent et al., 2011). When used in combination with other markers of infection, such as temperature, there is an increased specificity and sensitivity. CRP is a marker of inflammation after SAH and has been associated with vasospasm and poor outcome. The study by Fountas et al. (2009) was the first to investigate CRP and its association with outcome in SAH, and is the only study that assessed both blood and CSF levels of CRP. They showed that elevated CRP was associated with vasospasm and less favorable outcome at discharge, with a peak on post-admission day 3. Within the past few years, many investigators have concurred that CRP is a marker of poor outcome in SAH patients. Badjatia et al. (2011) demonstrated that elevated high sensitivity CRP (hsCRP), in the first 14 days after SAH, is an independent predictor of delayed cerebral ischemia. Kubo et al. (2008) additionally demonstrated that elevated hsCRP, at day 0 and day 7, was associated with delayed ischemic neurological deficits. Romero et al. (2012) demonstrated that elevated blood levels of CRP were associated with less favorable prognosis on discharge and that CRP blood levels were related to severity of SAH. Frontera et al. (2012) concurred showing that CRP blood levels were significantly higher in poorer grade patients over time and correlated with severity of SAH on admission. Jeon et al. (2012) demonstrated specifically in surgical SAH patients, that pre-operative and post-operative blood levels of CRP were associated with poor outcome. In this study, postoperative day 1–2 elevated CRP levels were associated with severe neurological deterioration on admission, cerebral infarction, intracerebral hemorrhage and surgical decompression.
Although the above mentioned data supporting the potential utility of CRP as a prognostic serum biomarker in SAH is mounting, it is important to recognize that CRP is not specific for neurologic injury. CRP is a marker of a systemic inflammatory response and is not produced by brain tissue. It is uncertain if it enters the brain and contributes to the pathogenesis of cerebral tissue damage or if it is a systemic marker of the severity of SAH. In addition, it is common to have a secondary process, pneumonia or another infective process, during the acute phase after SAH that may confound the sensitivity and specificity of CRP. Nevertheless, CRP has been shown to be a useful predictive indicator of outcome, making it an appealing measure in SAH.
Cytokines
TNF-α and IL-6 are acute phase reactant cytokines produced by a number of cells in response to inflammatory stimulus. IL-1β is a proinflammatory cytokine that is produced by activated macrophages. IL-1Ra is an antiinflammatory cytokine, which is also a part of the milieu of acute phase reactants produced by immune cells and hepatocytes. However, its role in the inflammatory process is unclear. IL-8 is a neutrophil chemotactic factor that is produced by a number of cells, including macrophages and endothelial cells, after a stimulus. Increased levels of these cytokines have been associated with poor outcome in SAH (Chou et al., 2012; Fassbender et al., 2001; Mathiesen et al., 1997; Nakahara et al., 2009; Sozen et al., 2009). The role of TNF-α and IL-6 in the acute phase of inflammation after SAH is well documented. However, few studies have looked at their association with outcome. Chou et al. (2012) demonstrated an association between serum TNF-α and a poor outcome at 6 months after SAH. Sozen et al. (2009) demonstrated that inhibition of IL-lβ in a mouse model of SAH reduced mortality and improved neurological function. However, there are no human studies investigating outcome and IL-1β in SAH. Mathiesen et al. (1997) demonstrated that levels of IL-1Rα and TNF-α were increased after SAH and were associated with unfavorable outcome at 12 days post-SAH. Fassbender et al. (2001) demonstrated that increased CSF IL-6 levels were significantly associated with poor outcome. Nakahara et al. (2009) showed increased levels of IL-8 in blood were associated with unfavorable outcome. These primary studies provide a basis for the potential use of these neuroinflammatory biomarkers as prognostic markers in SAH.
Importantly, the levels of cytokines in CSF and blood are not equivalent. Inflammatory markers peak initially in the CSF with a variable delayed secondary peak in the blood. This variable secondary peak of blood cytokines must be further investigated, as blood is the most practical source for sampling. As with any marker of inflammation, patients having a concurrent inflammatory or infectious process in addition to SAH may have “non-specific” elevations in cytokines.
Catecholamines and other products of metabolism
SAH initiates a profound neuroinflammatory response, including a centrally-mediated sympathetic response. Moussouttas et al. (2012) and Dilraj et al. (1992) demonstrated that plasma and CSF levels of epinephrine are independent predictors of morbidity and mortality in SAH. Although epinephrine detection assays of blood and urine are well-developed and simplistic, epinephrine should be combined with other markers of disease to increase sensitivity and specificity for SAH.
Microalbuminuria is a product of metabolism and a biomarker of infection and sepsis. It corresponds to a low rate of albumin excretion in the urine and is a non-specific marker of inflammation associated with increased vascular permeability and is measured as an albumin/creatinine ratio. Terao et al. (2007) found a microalbumin/creatinine ratio greater than 200 mg/g to be a potent independent predictor of unfavorable neurological outcome. Although non-specific, this biomarker has potential as it has successfully been utilized in other inflammatory disease states.
Molecular adhesion and extracellular matrix markers
Early brain injury after SAH involves the contraction of cerebral arteries resulting in the release of several vasoactive factors that may lead to endothelial cell damage and vasospasm. Intimal proliferation is a consequence of disturbed endothelial cells that can last up to 2 weeks after the onset of SAH. Molecular adhesion and extracellular matrix proteins are critical in this process and are associated with outcome in SAH. These proteins include matrix metalloproteinase-9 (MMP-9), intracellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and L-selectin. They are intimately involved with the vasculature and acute pathogenesis of disease, signifying the potential for use as biomarkers in SAH.
Matrix metalloproteinase-9
Matrix metalloproteinase-9 (MMP-9) is an extracellular matrix protein predominately released by neutrophils after brain injury secondary to ischemia and neurodegenerative disorders. It is one of the first factors to be prominently upregulated in smooth muscle cells and leukocytes that can account for the onset of myeloproliferative intimal lesions leading to vasospasm in SAH. MMP-9 plays a key role in the pathophysiology of acute brain injury in SAH as it (1) breaks down the blood brain barrier, (2) enhances cerebral edema and (3) facilitates neuronal and vascular apoptosis. McGirt et al. (2002) showed an increase in serum MMP-9 was associated with vasospasm. Chou et al. (2011) demonstrated an increase in blood and CSF MMP-9 levels were associated with poor clinical outcome at 3 months, but not with vasospasm. These studies demonstrate the potential of serum MMP-9 as a prognostic biomarker in the acute phase of SAH.
Intracellular adhesion molecule-1 and vascular cell adhesion molecule
Adhesion molecules have long been implicated in mediating a robust inflammatory response following an ischemic insult. Intracellular adhesion molecule-1 (ICAM-1) is expressed at low concentrations in endothelial cells and leukocytes under normal physiological conditions. However, with inflammatory stimuli, ICAM-1 activates an inflammatory cascade that may lead to vasospasm. The adherence of leukocytes to the vascular endothelium is a hallmark of inflammation and is induced by these adhesion molecules. Elevated serum ICAM-1 levels have been correlated with poor clinical outcome (Kaynar et al., 2004; Kubo et al., 2008; Mack et al., 2002). Kaynar et al. (2004) have postulated that simultaneous release of soluble ICAM-1 and vascular cell adhesion molecule-1 (VCAM-1) in CSF and blood are risk factors for unfavorable outcome and post-hemorrhagic vasospasm. These adhesion molecules can serve as serum biomarkers of outcome for SAH and may be the basis of specific antibody inhibition that may improve functional outcome.
Selectin
Selectin is another cell adhesion molecule family. There are three main types, depending on origin, endothelial (E), platelets (P) and leukocytes (L). Mean serum P- and L-selectins in SAH patients are associated with an onset of delayed ischemic neurologic deficits (Nissen et al., 2000). However, only L-selectin, was associated with poor outcome (Wang et al., 2011). This is a single study, demonstrating an opportunity for further investigation.
Biomarkers of the vasculature and clotting cascade
The search for markers of brain injury and neurological deficits in the setting of SAH has been heavily focused on vascular components and the clotting cascade. This is largely a consequence of the research emphasis on vasospasm and the associated clinical deterioration. The results from the CONSCIOUS-2 trial (Macdonald et al., 2013), which showed that prevention of angiographic vasospasm had equivocal effects on outcome, has led to a reevaluation of the pathogenesis of brain injury in SAH. However, these findings do not discount endothelin-1 (ET-1) as a potential biomarker for SAH, as it parallels vasospasm, an independently strong indicator of worsening clinical status. A detailed discussion of vasospasm, and its prognostic biomarkers, is beyond the scope of this review. However, ET-1 warrants some discussion. Several lines of evidence have implicated ET-1 in the pathogenesis of vasospasm: (1) ET-1 is a very potent vasoconstrictor (Webb et al., 1998), (2) ET-1 is found in elevated quantities in CSF and blood in SAH patients (Masaoka et al., 1989; Suzuki et al., 1990), (3) ET-1 can evoke vasospasm experimentally (Zubkov et al., 2000) and (4) specific antagonists of ET-1 attenuate angiographic vasospasm in experimental SAH (Juvela, 2000). Production of ET-1 has been attributed to endothelial cells, smooth muscle cells, neurons, astrocytes and CSF leukocytes (Fassbender et al., 2000). Seifert et al. (1995) have shown that ET-1 levels are strongly predictive of hematoma volume and development of delayed neurological deficits.
Inasmuch as vasospasm is an independent marker of worsening clinical course, serum biomarkers of vascular injury and coagulation upregulation may be of clinical use. A more thorough understanding of these biomarkers, and the signaling cascades involved, may lead to their development and implementation into the clinical management of SAH and novel therapeutic options for this devastating disease.
Endothelial cell activation and microparticles
Markers relating to endothelial cell damage or activation may be among the most specific early markers relating to SAH. Fibronectin containing extra type III domain (ED1-fn) is a sensitive marker of endothelial cell activation in SAH and is increased 72 hours after SAH (Frijns et al., 2002). ED1-fn is associated with the clinical condition on admission, and is increased in delayed cerebral ischemia. Regardless of whether endothelial cell activation is a consequence or a cause of delayed cerebral ischemia, activated endothelial cells may be involved in other aspects of SAH-related injury. A recent study by Lackner et al. (2010) looked at levels of endothelial microparticles in SAH, as a measure of endothelial cell damage. In patients with any level of disability (modified Rankin Scale ≥1), microparticles were elevated with vasospasm and associated with unfavorable outcome at discharge. These patients were compared to those that made a complete recovery prior to discharge. However, microparticle levels were not associated with outcomes at 6-months. This is the only study that has investigated microparticles and outcome in SAH.
Vascular endothelial growth factor and anti-angiogenic factors
Vascular endothelial growth factor (VEGF) stimulates endothelial cell proliferation and permeability, increases intercellular adhesion molecule expression and leukocyte infiltration, and facilitates vascular smooth muscle cell migration and intimal proliferation (Carmeliet & Collen, 1998; Grosskreutz et al., 1999; McGirt et al., 2002; Melder et al., 1996). Therefore, it may play a key role in several signaling cascades related to injury in SAH. The increase in blood VEGF levels occur several days prior to the onset of vasospasm and neurological deterioration, and are strongly associated with the severity of the initial insult, clinical status upon admission, and the volume of SAH (McGirt et al., 2002). The specificity of VEGF for a particular damaging process has yet to be determined, but it may be an important early indicator of injury progression. VEGF and other vascular mitogens are regulated by circulating antiangiogenic factors, soluble-fms-like tyrosine kinase 1 (sFlt1) (Olsson et al., 2006) and soluble endoglin (sEng) (Bernabeu et al., 2007), and changing levels of these factors may be associated with outcome in SAH. Testai et al. (2011) recently examined levels of sFlt1 and sEng in a cohort of SAH patients and found an early increase, particularly in patients who developed focal neurological signs and/or deterioration in consciousness. In the same way that VEGF is involved in a host of cellular cascades, antiangiogenic factors may be involved in the modulation of a variety pathways leading to damage in SAH. The specificity of VEGF may be an important early indicator of injury progression and monitoring levels on a regular basis may be advantageous.
Complement cascade
The complement cascade, involved in erythrocyte hemolysis and activation of inflammation, may be involved in the pathogenesis of SAH-induced injury. Studies have demonstrated that upregulation of C3a, C4a and the membrane attack complex takes place after SAH (Kasuya & Shimizu, 1989; Lindsberg et al., 1996; Mack et al., 2007; Ostergaard et al., 1987). Mack et al. (2007) found that early C3a and C5a levels were significantly increased in SAH patients and were independent of clinical status upon admission. Early C3a levels strongly correlated with outcome and may be a useful serum biomarker for early inflammatory mediated damage after SAH.
von Willebrand factor
von Willebrand factor (vWF) is a large adhesive glycoprotein that is stored in endothelial cell Weibel-Palade bodies. Its specific cell source makes it a suitable serum biomarker of endothelial cell activation in SAH. Frijns et al. (2002, 2006a,b) found that SAH patients with vWF greater than or equal to the median value had an increased risk of poor outcome, with no increased risk for ischemia. In contrast, McGirt et al. (2002) found that vWF levels could accurately predict the occurrence of vasospasm. These preliminary studies demonstrate that vWF may be a useful marker for severity of injury and prognosis of clinical course in SAH.
Fibrinogen degradation products and thrombin-antithrombin (TAT) complex
Fibrinolytic agents may be administered after SAH to aid in clot removal. Anti-fibrinolytic therapy is known to increase the risk of vasospasm (Kassell et al., 1984). Patients with vasospasm have been shown to have lower blood levels of fibrinolytic activity (including D-dimer and TAT complexes) (Morga et al., 2007; Suzuki et al., 1997), increased fibrinogen (Frontera et al., 2012) and smaller hematoma load (Morga et al., 2007; Suzuki et al., 1997), which has been associated with worsening clinical status (Frontera et al., 2012; Morga, et al., 2007. Morga et al. (2007) demonstrated that D-dimer and TAT complex formation were related to the clinical status of patients after SAH. They found that D-dimer and TAT levels were associated with hematoma volume and worsening of clinical status. Given these findings, D-dimer, fibrinogen and TAT serum levels may be useful biomarkers for worsening clinical course after SAH.
Cardiac markers
Cardiac abnormalities, including dysrhythmias and left ventricular systolic dysfunction, are a well-recognized phenomenon after SAH. The pathophysiology and contribution of cardiac dysfunction to outcome after SAH is controversial and is usually reversible. The most likely mechanism is excessive catecholamine release. Over the past 5 years, there have been a number of studies, in humans, that demonstrate a significant association between increased cardiac troponin I (cTnI) levels and morbidity and mortality in patients with SAH (Kumar et al., 2011; Miketic et al., 2010; Naidech et al., 2005; Ramappa et al., 2008; Sandhu et al., 2008; Schuiling et al., 2005; Yarlagadda et al., 2006). This neurologically mediated cardiac dysfunction is an independent predictor of mortality (Kumar et al., 2011; Miketic et al., 2010; Naidech et al., 2005; Ramappa et al., 2008; Sandhu et al., 2008; Schuiling et al., 2005; Yarlagadda et al., 2006). It is associated with severity of initial neurological injury including lower admission Glasgow coma scale, increased hospital stay, poor neurologic outcome (Ramappa et al., 2008) including functional recovery and disability (Miketic et al., 2010) and increased in-hospital morbidity and mortality (Kumar et al., 2011; Miketic et al., 2010; Naidech et al., 2005; Ramappa et al., 2008; Sandhu et al., 2008; Schuiling et al., 2005; Yarlagadda et al., 2006) after SAH. In recent years, cardiac dysfunction in patients after SAH have been associated with increased levels of blood B-type natriuretic peptide (BNP) as well (Taub et al., 2011). SAH patients with associated cardiac dysfunction present a unique challenge with regards to medical management. As mentioned in the introduction, complications of medical management can be quite devastating in these patients, in particular. Utilizing cTnI levels may assist in identifying those at risk for cardiopulmonary dysfunction as well as high-risk patients that may require modifications to therapy.
Other markers
There are other biomarkers that have been associated with outcome in SAH patients that are not categorized in any of the groups denoted above. They include malondialdehyde (MDA), kallikrein-6 (KLK6) and creatine kinase–BB (CK-BB). They each have a single study that demonstrates their association with poor outcome in SAH patients. MDA is a byproduct of lipid peroxidation and arachidonic acid metabolism and frequently used as an indicator of oxidative stress. Kaneda et al. (2010) demonstrated elevated MDA levels at Day 14 were associated with poor outcome. KLK6 is a serine protease enzyme whose physiological function is unknown. Nevertheless, Martinez-Morillo et al. (2012) demonstrated that decreased blood levels of KLK6 were associated with increased mortality. CK-BB is a creatine kinase found in the brain and is involved in energy maintenance within mitochondria. Coplin et al. (1999) demonstrated that increased CSF levels of CK-BB, greater than 40 U/L, was associated with unfavorable outcome at 1 week and 2 months after SAH.
Microdialysis
Intracerebral microdialysis is a technique used for sampling the extracellular molecular chemistry within brain tissue. Although the volume of brain tissue being analyzed is limited, it has been utilized in neurocritical care to monitor markers of ischemia and cell damage (Ungerstedt & Rostami, 2004). The most commonly utilized marker of microdialysis is the lactate/pyruvate ratio. However, other excitatory amino acids, such as glycerol and nitrate, have also been investigated. The lactate/pyruvate ratio is a marker of changes in the reduction and oxidation states associated with ischemia. Increased lactate/pyruvate ratios, excitatory amino acids and nitrate have been correlated to poor outcomes and neurological deterioration (Nilsson et al., 1999; Sarrafzadeh et al., 2003; Staub et al., 2000). Microdialysis has been used for the early detection of secondary insults after brain injury, such as SAH. Although there is great theoretical promise in monitoring molecular changes and metabolic disturbances associated with secondary insults early after brain injury, the quality of information gained from microdialysis will have to be exceptional to justify its added risk. Nevertheless, microdialysis demonstrates promise in regards to monitoring molecular changes and metabolic disturbances associated with secondary insults early after brain injury.
Conclusion
Subarachnoid hemorrhage is a devastating condition, whose progression, fatality and secondary debilitating outcome is the basis for ongoing research towards improving clinical management. Current standards of incorporating age, admission neurologic grade and admission CT scan assess the state of the patient on admission. Utilizing a prognostic serum biomarker, with continuous daily evaluation and ability to monitor trends, would allow patients to be monitored during the acute phase and assist in the management and treatment of SAH patients. These biomarkers would identify those that necessitate more aggressive management, those that may warrant closer monitoring, promote early treatment models and predict long-term outcome. The elevation in certain biomarkers may be detected prior to the presentation of neurological deterioration. Therefore, patients may be identified earlier, in the acute period, as high risk for poor outcome. These patients may require a prolonged duration of a higher level of care (i.e. intensive critical care) including frequent neurological examinations and continuous monitoring. Currently, some SAH patients are transferred to a lower level of care based on their neurological examinations and progress, only to return to the intensive care unit after neurological deterioration. Management for these “high-risk” patients, once identified, may include initiation of increased systolic blood pressure and mean arterial pressure goals as well as increased fluid resuscitation (early initiation of “triple H” therapy). This preemptive management may decrease the risk of deterioration in some of these patients and may ultimately improve their outcome. This may also decrease the number of emergent imaging studies that are initiated after acute neurological deterioration, as it may prevent some of these patients from deteriorating; therefore, enhance the feasibility of biomarker monitoring. However, due to the lack of evidence and excellent sensitivities and specificities of any single biomarker, their utilization in the standard of care in the acute phase of SAH has not been accepted. Further research to identify specific biokinetics of these biomarkers, including appropriate ranges, specific peaks and half-lives, must be done to improve their sensitivities and specificities and their translation into clinical medicine. Currently, prognostic biomarkers after SAH include neuronal-specific markers, inflammatory markers, molecular adhesion and matrix markers, vascular and angiogenic markers, coagulation function, cardiac markers and molecules of microdialysis.
This review details biomarkers that potentially, used in combination with current imaging and neurological examinations, may innately have the ability to predict and monitor high-risk patients and may improve outcome. Focusing specifically on blood biomarkers, secondary to the ease of access and sampling, in the acute period after SAH, S100β, CRP, adhesion and matrix markers, vasogenic markers and cardiac markers (TnI) demonstrate promise. Each of them have been demonstrated to correlate with outcome; however, their sensitivities and specificities must be improved before translation and utilization. Further research in the development and implementation of serum biomarkers of outcome in SAH would establish a safe, more cost effective standard monitoring tool that would allocate resources more efficiently, improve clinical management and may decrease morbidity and mortality.
Acknowledgments
This work is supported in part by a Scientist Development Grant (13SDG14370030) to Dr Caron Hong from the American Heart Association, and by grants to Dr J. Marc Simard from the National Institute of Neurological Disorders and Stroke (NS061808) and the National Heart, Lung and Blood Institute (HL082517).
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Badjatia N, Carpenter A, Fernandez L, et al. Relationship between C-reactive protein, systemic oxygen consumption, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke. 2011;42:2436–2442. doi: 10.1161/STROKEAHA.111.614685. [DOI] [PubMed] [Google Scholar]
- Bernabeu C, Conley BA, Vary CP. Novel biochemical pathways of endoglin in vascular cell physiology. J Cell Biochem. 2007;102:1375–1388. doi: 10.1002/jcb.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Collen D. Vascular development and disorders: molecular analysis and pathogenic insights. Kidney Int. 1998;53:1519–1549. doi: 10.1046/j.1523-1755.1998.00936.x. [DOI] [PubMed] [Google Scholar]
- Cengiz SL, Ak A, Ustun ME, Karakose S. Lactate contents from cerebrospinal fluid in experimental subarachnoid hemorrhage, well correlate with vasospasm: ongoing and neurologic status. J Neurosurg Anesthesiol. 2007;19:166–170. doi: 10.1097/ANA.0b013e3180461278. [DOI] [PubMed] [Google Scholar]
- Chou SH, Feske SK, Atherton J, et al. Early elevation of serum tumor necrosis factor-alpha is associated with poor outcome in subarachnoid hemorrhage. J Investig Med. 2012;60:1054–1058. doi: 10.231/JIM.0b013e3182686932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SH, Feske SK, Simmons SL, et al. Elevated peripheral neutrophils and matrix metalloproteinase 9 as biomarkers of functional outcome following subarachnoid hemorrhage. Transl Stroke Res. 2011;2:600–607. doi: 10.1007/s12975-011-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplin WM, Longstreth WT, Jr, Lam AM, et al. Cerebrospinal fluid creatine kinase-BB isoenzyme activity and outcome after subarachnoid hemorrhage. Arch Neurol. 1999;56:1348–1352. doi: 10.1001/archneur.56.11.1348. [DOI] [PubMed] [Google Scholar]
- Dilraj A, Botha JH, Rambiritch V, et al. Levels of catecholamine in plasma and cerebrospinal fluid in aneurysmal subarachnoid hemorrhage. Neurosurgery. 1992;31:42–50. doi: 10.1227/00006123-199207000-00007. [DOI] [PubMed] [Google Scholar]
- Dunn LT, Stewart E, Murray GD, et al. The influence of apolipoprotein E genotype on outcome after spontaneous subarachnoid hemorrhage: a preliminary study. Neurosurgery. 2001;48:1006–1010. doi: 10.1097/00006123-200105000-00007. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Hodapp B, Rossol S, et al. Endothelin-1 in subarachnoid hemorrhage: an acute-phase reactant produced by cerebrospinal fluid leukocytes. Stroke. 2000;31:2971–2975. doi: 10.1161/01.str.31.12.2971. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Hodapp B, Rossol S, et al. Inflammatory cytokines in subarachnoid haemorrhage: association with abnormal blood flow velocities in basal cerebral arteries. J Neurol Neurosurg Psychiatry. 2001;70:534–537. doi: 10.1136/jnnp.70.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountas KN, Tasiou A, Kapsalaki EZ, et al. Serum and cerebrospinal fluid C-reactive protein levels as predictors of vaso-spasm in aneurysmal subarachnoid hemorrhage. Clinical article. Neurosurg Focus. 2009;26:E22. doi: 10.3171/2009.2.FOCUS08311. [DOI] [PubMed] [Google Scholar]
- Frijns CJ, Fijnheer R, Algra A, et al. Early circulating levels of endothelial cell activation markers in aneurysmal subarachnoid haemorrhage: associations with cerebral ischaemic events and outcome. J Neurol Neurosurg Psychiatry. 2006a;77:77–83. doi: 10.1136/jnnp.2005.064956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijns CJ, Kasius KM, Algra A, et al. Endothelial cell activation markers and delayed cerebral ischaemia in patients with subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2006b;77:863–867. doi: 10.1136/jnnp.2005.081539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijns CJ, Rinkel GJ, Castigliego D, et al. Endothelial cell activation after subarachnoid hemorrhage. Neurosurgery. 2002;50:1223–1229. doi: 10.1097/00006123-200206000-00009. [DOI] [PubMed] [Google Scholar]
- Frontera JA, Aledort L, Gordon E, et al. Early platelet activation, inflammation and acute brain injury after a subarachnoid hemorrhage: a pilot study. J Thromb Haemost. 2012;10:711–713. doi: 10.1111/j.1538-7836.2012.04651.x. [DOI] [PubMed] [Google Scholar]
- Gao J, Wang H, Sheng H, et al. A novel apoE-derived therapeutic reduces vasospasm and improves outcome in a murine model of subarachnoid hemorrhage. Neurocrit Care. 2006;4:25–31. doi: 10.1385/NCC:4:1:025. [DOI] [PubMed] [Google Scholar]
- Grosskreutz CL, Anand-Apte B, Duplaa C, et al. Vascular endothelial growth factor-induced migration of vascular smooth muscle cells in vitro. Microvasc Res. 1999;58:128–136. doi: 10.1006/mvre.1999.2171. [DOI] [PubMed] [Google Scholar]
- Hardemark HG, Almqvist O, Johansson T, et al. S-100 protein in cerebrospinal fluid after aneurysmal subarachnoid haemorrhage: relation to functional outcome, late CT and SPECT changes, and signs of higher cortical dysfunction. Acta Neurochir (Wien) 1989;99:135–144. doi: 10.1007/BF01402322. [DOI] [PubMed] [Google Scholar]
- Hassan T, Nassar M, Elhadi SM, Radi WK. Effect of magnesium sulfate therapy on patients with aneurysmal subarachnoid hemorrhage using serum S100B protein as a prognostic marker. Neurosurg Rev. 2012;35:421–427. doi: 10.1007/s10143-011-0368-8. [DOI] [PubMed] [Google Scholar]
- Herrmann M, Vos P, Wunderlich MT, et al. Release of glial tissue-specific proteins after acute stroke: a comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke. 2000;31:2670–2677. doi: 10.1161/01.str.31.11.2670. [DOI] [PubMed] [Google Scholar]
- Hop JW, Rinkel GJ, Algra A, van GJ. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke. 1997;28:660–664. doi: 10.1161/01.str.28.3.660. [DOI] [PubMed] [Google Scholar]
- Ingebrigtsen T, Waterloo K, Jacobsen EA, et al. Traumatic brain damage in minor head injury: relation of serum S-100 protein measurements to magnetic resonance imaging and neurobehavioral outcome. Neurosurgery. 1999;45:468–475. doi: 10.1097/00006123-199909000-00010. [DOI] [PubMed] [Google Scholar]
- Jeon YT, Lee JH, Lee H, et al. The postoperative C-reactive protein level can be a useful prognostic factor for poor outcome and symptomatic vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2012;24:317–324. doi: 10.1097/ANA.0b013e31826047a2. [DOI] [PubMed] [Google Scholar]
- Jung CS, Lange B, Zimmermann M, Seifert V. CSF and serum biomarkers focusing on cerebral vasospasm and ischemia after subarachnoid hemorrhage. Stroke Res Treat. 2013;2013:560305. doi: 10.1155/2013/560305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvela S. Plasma endothelin concentrations after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2000;92:390–400. doi: 10.3171/jns.2000.92.3.0390. [DOI] [PubMed] [Google Scholar]
- Kacira T, Kemerdere R, Atukeren P, et al. Detection of caspase-3, neuron specific enolase, and high-sensitivity C-reactive protein levels in both cerebrospinal fluid and serum of patients after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2007;60:674–679. doi: 10.1227/01.NEU.0000255394.77538.BB. [DOI] [PubMed] [Google Scholar]
- Kaneda K, Fujita M, Yamashita S, et al. Prognostic value of biochemical markers of brain damage and oxidative stress in post-surgical aneurysmal subarachnoid hemorrhage patients. Brain Res Bull. 2010;81:173–177. doi: 10.1016/j.brainresbull.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Kanner AA, Marchi N, Fazio V, et al. Serum S100beta: a noninvasive marker of blood-brain barrier function and brain lesions. Cancer. 2003;97:2806–2813. doi: 10.1002/cncr.11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985;16:562–572. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- Kassell NF, Torner JC, Adams HP., Jr Antifibrinolytic therapy in the acute period following aneurysmal subarachnoid hemorrhage. Preliminary observations from the Cooperative Aneurysm Study. J Neurosurg. 1984;61:225–230. doi: 10.3171/jns.1984.61.2.0225. [DOI] [PubMed] [Google Scholar]
- Kasuya H, Shimizu T. Activated complement components C3a and C4a in cerebrospinal fluid and plasma following subarachnoid hemorrhage. J Neurosurg. 1989;71:741–746. doi: 10.3171/jns.1989.71.5.0741. [DOI] [PubMed] [Google Scholar]
- Kay A, Petzold A, Kerr M, et al. Decreased cerebrospinal fluid apolipoprotein E after subarachnoid hemorrhage: correlation with injury severity and clinical outcome. Stroke. 2003a;34:637–642. doi: 10.1161/01.STR.0000057579.25430.16. [DOI] [PubMed] [Google Scholar]
- Kay A, Petzold A, Kerr M, et al. Temporal alterations in cerebrospinal fluid amyloid beta-protein and apolipoprotein E after subarachnoid hemorrhage. Stroke. 2003b;34:e240–e243. doi: 10.1161/01.STR.0000100157.88508.2F. [DOI] [PubMed] [Google Scholar]
- Kaynar MY, Tanriverdi T, Kafadar AM, et al. Detection of soluble intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in both cerebrospinal fluid and serum of patients after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004;101:1030–1036. doi: 10.3171/jns.2004.101.6.1030. [DOI] [PubMed] [Google Scholar]
- Kofoed K, Andersen O, Kronborg G, et al. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: a prospective study. Crit Care. 2007;11:R38. doi: 10.1186/cc5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y, Ogasawara K, Kakino S, et al. Serum inflammatory adhesion molecules and high-sensitivity C-reactive protein correlates with delayed ischemic neurologic deficits after subarachnoid hemorrhage. Surg Neurol. 2008;69:592–596. doi: 10.1016/j.surneu.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Kumar PV, Vannemreddy P, Kumar D, et al. Cardiac troponin I levels are a marker of myocardial dysfunction in subarachnoid hemorrhage and predicts poor neurologic outcome. J Louisiana State Medical Soc. 2011;163:257–260. [PubMed] [Google Scholar]
- Lackner P, Dietmann A, Beer R, et al. Cellular microparticles as a marker for cerebral vasospasm in spontaneous subarachnoid hemorrhage. Stroke. 2010;41:2353–2357. doi: 10.1161/STROKEAHA.110.584995. [DOI] [PubMed] [Google Scholar]
- Lanterna LA, Biroli F. Significance of apolipoprotein E in subarachnoid hemorrhage: neuronal injury, repair, and therapeutic perspectives – a review. J Stroke Cerebrovasc Dis. 2009;18:116–123. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Lanterna LA, Rigoldi M, Tredici G, et al. APOE influences vasospasm and cognition of noncomatose patients with subarachnoid hemorrhage. Neurology. 2005;64:1238–1244. doi: 10.1212/01.WNL.0000156523.77347.B4. [DOI] [PubMed] [Google Scholar]
- Leung CH, Poon WS, Yu LM, et al. Apolipoprotein e genotype and outcome in aneurysmal subarachnoid hemorrhage. Stroke. 2002;33:548–552. doi: 10.1161/hs0202.102326. [DOI] [PubMed] [Google Scholar]
- Lewis SB, Wolper R, Chi YY, et al. Identification and preliminary characterization of ubiquitin C terminal hydrolase 1 (UCHL1) as a biomarker of neuronal loss in aneurysmal subarachnoid hemorrhage. J Neurosci Res. 2010;88:1475–1484. doi: 10.1002/jnr.22323. [DOI] [PubMed] [Google Scholar]
- Lindsberg PJ, Ohman J, Lehto T, et al. Complement activation in the central nervous system following blood-brain barrier damage in man. Ann Neurol. 1996;40:587–596. doi: 10.1002/ana.410400408. [DOI] [PubMed] [Google Scholar]
- Louko AM, Vilkki J, Niskakangas T. ApoE genotype and cognition after subarachnoid haemorrhage: a longitudinal study. Acta Neurol Scand. 2006;114:315–319. doi: 10.1111/j.1600-0404.2006.00676.x. [DOI] [PubMed] [Google Scholar]
- Luzzani A, Polati E, Dorizzi R, et al. Comparison of procalcitonin and C-reactive protein as markers of sepsis. Crit Care Med. 2003;31:1737–1741. doi: 10.1097/01.CCM.0000063440.19188.ED. [DOI] [PubMed] [Google Scholar]
- Mabe H, Suzuki S, Mase M, et al. Serum neuron-specific enolase levels after subarachnoid hemorrhage. Surg Neurol. 1991;36:170–174. doi: 10.1016/0090-3019(91)90108-l. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Higashida RT, Keller E, et al. Preventing vasospasm improves outcome after aneurysmal subarachnoid hemorrhage: rationale and design of CONSCIOUS-2 and CONSCIOUS-3 trials. Neurocrit Care. 2010;13:416–424. doi: 10.1007/s12028-010-9433-3. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Higashida RT, Keller E, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2) Lancet Neurol. 2011;10:618–625. doi: 10.1016/S1474-4422(11)70108-9. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Higashida RT, Keller E, et al. Randomised trial of clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid hemorrhage undergoing surgical clipping (CONSCIOUS-2) Acta Neurochir. 2013;(Suppl 115):27–31. doi: 10.1007/978-3-7091-1192-5_7. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Kassell NF, Mayer S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–3021. doi: 10.1161/STROKEAHA.108.519942. [DOI] [PubMed] [Google Scholar]
- Mack WJ, Ducruet AF, Hickman ZL, et al. Early plasma complement C3a levels correlate with functional outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2007;61:255–260. doi: 10.1227/01.NEU.0000255518.96837.8E. [DOI] [PubMed] [Google Scholar]
- Mack WJ, Mocco J, Hoh DJ, et al. Outcome prediction with serum intercellular adhesion molecule-1 levels after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002;96:71–75. doi: 10.3171/jns.2002.96.1.0071. [DOI] [PubMed] [Google Scholar]
- Manikis P, Jankowski S, Zhang H, et al. Correlation of serial blood lactate levels to organ failure and mortality after trauma. Am J Emerg Med. 1995;13:619–622. doi: 10.1016/0735-6757(95)90043-8. [DOI] [PubMed] [Google Scholar]
- Martinez-Morillo E, Diamandis A, Romaschin AD, Diamandis EP. Kallikrein 6 as a serum prognostic marker in patients with aneurysmal subarachnoid hemorrhage. PLoS One. 2012;7:e45676. doi: 10.1371/journal.pone.0045676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaoka H, Suzuki R, Hirata Y, et al. Raised plasma endothelin in aneurysmal subarachnoid haemorrhage. Lancet. 1989;2:1402. doi: 10.1016/s0140-6736(89)92019-9. [DOI] [PubMed] [Google Scholar]
- Mathiesen T, Edner G, Ulfarsson E, Andersson B. Cerebrospinal fluid interleukin-1 receptor antagonist and tumor necrosis factor-alpha following subarachnoid hemorrhage. J Neurosurg. 1997;87:215–220. doi: 10.3171/jns.1997.87.2.0215. [DOI] [PubMed] [Google Scholar]
- McGirt MJ, Lynch JR, Blessing R, et al. Serum von Willebrand factor, matrix metalloproteinase-9, and vascular endothelial growth factor levels predict the onset of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2002;51:1128–1134. doi: 10.1097/00006123-200211000-00005. [DOI] [PubMed] [Google Scholar]
- Melder RJ, Koenig GC, Witwer BP, et al. During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat Med. 1996;2:992–997. doi: 10.1038/nm0996-992. [DOI] [PubMed] [Google Scholar]
- Meyers PM, Connolly ES., Jr Stroke: disappointing results for clazosentan in CONSCIOUS-2. Nat Rev Neurol. 2011;7:660–661. doi: 10.1038/nrneurol.2011.168. [DOI] [PubMed] [Google Scholar]
- Miketic JK, Hravnak M, Sereika SM, Crago EA. Elevated cardiac troponin I and functional recovery and disability in patients after aneurysmal subarachnoid hemorrhage. Am J Crit Care. 2010;19:522–528. doi: 10.4037/ajcc2010156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37:1670–1677. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- Mondello S, Jeromin A, Buki A, et al. Glial neuronal ratio: a novel index for differentiating injury type in patients with severe traumatic brain injury. J Neurotrauma. 2012;29:1096–1104. doi: 10.1089/neu.2011.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondello S, Papa L, Buki A, et al. Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. Crit Care. 2011;15:R156. doi: 10.1186/cc10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morga R, Czepko R, Dembinska-Kiec A, Danilewicz B. Assessment of the haemostatic system in patients surgically treated for ruptured cerebral aneurysm. Neurol Neurochir Pol. 2007;41:296–305. [PubMed] [Google Scholar]
- Moritz S, Warnat J, Bele S, et al. The prognostic value of NSE and S100B from serum and cerebrospinal fluid in patients with spontaneous subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2010;22:21–31. doi: 10.1097/ANA.0b013e3181bdf50d. [DOI] [PubMed] [Google Scholar]
- Morris PG, Wilson JT, Dunn LT, Nicoll JA. Apolipoprotein E polymorphism and neuropsychological outcome following subarachnoid haemorrhage. Acta Neurol Scand. 2004;109:205–209. doi: 10.1034/j.1600-0404.2003.00206.x. [DOI] [PubMed] [Google Scholar]
- Moussouttas M, Huynh TT, Khoury J, et al. Cerebrospinal fluid catecholamine levels as predictors of outcome in subarachnoid hemorrhage. Cerebrovasc Dis. 2012;33:173–181. doi: 10.1159/000334660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidech AM, Kreiter KT, Janjua N, et al. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation. 2005;112:2851–2856. doi: 10.1161/CIRCULATIONAHA.105.533620. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Tsuruta R, Kaneko T, et al. High-mobility group box 1 protein in CSF of patients with subarachnoid hemorrhage. Neurocrit Care. 2009;11:362–368. doi: 10.1007/s12028-009-9276-y. [DOI] [PubMed] [Google Scholar]
- Nilsson OG, Brandt L, Ungerstedt U, Saveland H. Bedside detection of brain ischemia using intracerebral microdialysis: sub-arachnoid hemorrhage and delayed ischemic deterioration. Neurosurgery. 1999;45:1176–1184. doi: 10.1097/00006123-199911000-00032. [DOI] [PubMed] [Google Scholar]
- Niskakangas T, Ohman J, Niemela M, et al. Association of apolipoprotein E polymorphism with outcome after aneurysmal subarachnoid hemorrhage: a preliminary study. Stroke. 2001;32:1181–1184. doi: 10.1161/01.str.32.5.1181. [DOI] [PubMed] [Google Scholar]
- Nissen JJ, Mantle D, Blackburn A, et al. The selectin superfamily: the role of selectin adhesion molecules in delayed cerebral ischaemia after aneurysmal subarachnoid haemorrhage. Acta Neurochir. 2000;76:55–60. doi: 10.1007/978-3-7091-6346-7_11. [DOI] [PubMed] [Google Scholar]
- Nylen K, Csajbok LZ, Ost M, et al. CSF-neurofilament correlates with outcome after aneurysmal subarachnoid hemorrhage. Neurosci Lett. 2006;404:132–136. doi: 10.1016/j.neulet.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Nylen K, Csajbok LZ, Ost M, et al. Serum glial fibrillary acidic protein is related to focal brain injury and outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:1489–1494. doi: 10.1161/STROKEAHA.106.478362. [DOI] [PubMed] [Google Scholar]
- Oconnor E, Venkatesh B, Mashongonyika C, et al. Serum procalcitonin and C-reactive protein as markers of sepsis and outcome in patients with neurotrauma and subarachnoid haemorrhage. Anaesth Intensive Care. 2004;32:465–470. doi: 10.1177/0310057X0403200402. [DOI] [PubMed] [Google Scholar]
- Oertel M, Schumacher U, McArthur DL, et al. S-100B and NSE: markers of initial impact of subarachnoid haemorrhage and their relation to vasospasm and outcome. J Clin Neurosci. 2006;13:834–840. doi: 10.1016/j.jocn.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, et al. VEGF receptor signalling – in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- Ostergaard JR, Kristensen BO, Svehag SE, et al. Immune complexes and complement activation following rupture of intracranial saccular aneurysms. J Neurosurg. 1987;66:891–897. doi: 10.3171/jns.1987.66.6.0891. [DOI] [PubMed] [Google Scholar]
- Ostrowski RP, Colohan AR, Zhang JH. Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res. 2006;28:399–414. doi: 10.1179/016164106X115008. [DOI] [PubMed] [Google Scholar]
- Persson L, Hardemark H, Edner G, et al. S-100 protein in cerebrospinal fluid of patients with subarachnoid haemorrhage: a potential marker of brain damage. Acta Neurochir (Wien) 1988;93:116–122. doi: 10.1007/BF01402892. [DOI] [PubMed] [Google Scholar]
- Petzold A, Keir G, Kay A, et al. Axonal damage and outcome in subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2006;77:753–759. doi: 10.1136/jnnp.2005.085175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramappa P, Thatai D, Coplin W, et al. Cardiac troponin-I: a predictor of prognosis in subarachnoid hemorrhage. Neurocritical Care. 2008;8:398–403. doi: 10.1007/s12028-007-9038-7. [DOI] [PubMed] [Google Scholar]
- Reinhart K, Meisner M. Biomarkers in the critically ill patient: procalcitonin. Crit Care Clin. 2011;27:253–263. doi: 10.1016/j.ccc.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Romero FR, Bertolini EF, Figueiredo EG, Teixeira MJ. Serum C-reactive protein levels predict neurological outcome after aneurysmal subarachnoid hemorrhage. Arq Neuropsiquiatr. 2012;70:202–205. doi: 10.1590/s0004-282x2012000300009. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pena P, Pereira AR, Sourour NA, et al. S100B as an additional prognostic marker in subarachnoid aneurysmal hemorrhage. Crit Care Med. 2008;36:2267–2273. doi: 10.1097/CCM.0b013e3181809750. [DOI] [PubMed] [Google Scholar]
- Sandhu R, Aronow WS, Rajdev A, et al. Relation of cardiac troponin I levels with in-hospital mortality in patients with ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage. Am J Cardiol. 2008;102:632–644. doi: 10.1016/j.amjcard.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Sarrafzadeh A, Haux D, Sakowitz O, et al. Acute focal neurological deficits in aneurysmal subarachnoid hemorrhage: relation of clinical course CT findings, and metabolite abnormalities monitored with bedside microdialysis. Stroke. 2003;34:1382–1388. doi: 10.1161/01.STR.0000074036.97859.02. [DOI] [PubMed] [Google Scholar]
- Schick U, Dohnert J, Meyer JJ, et al. Prognostic significance of SSEP, BAEP and serum S-100b monitoring after aneurysm surgery. Acta Neurol Scand. 2003;108:161–169. doi: 10.1034/j.1600-0404.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- Schmid-Elsaesser R, Kunz M, et al. Intravenous magnesium versus nimodipine in the treatment of patients with aneurysmal subarachnoid hemorrhage: a randomized study. Neurosurgery. 2006;58:1054–1065. doi: 10.1227/01.NEU.0000215868.40441.D9. [DOI] [PubMed] [Google Scholar]
- Schuiling WJ, Dennesen PJW, Tans J, et al. Troponin I in predicting cardiac or pulmonary complications and outcome in subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2005;76:1565–1569. doi: 10.1136/jnnp.2004.060913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert V, Loffler BM, Zimmermann M, et al. Endothelin concentrations in patients with aneurysmal subarachnoid hemorrhage. Correlation with cerebral vasospasm, delayed ischemic neurological deficits, and volume of hematoma. J Neurosurg. 1995;82:55–62. doi: 10.3171/jns.1995.82.1.0055. [DOI] [PubMed] [Google Scholar]
- Solenski NJ, Haley EC, Jr, Kassell NF, et al. Medical complications of aneurysmal subarachnoid hemorrhage: a report of the multicenter, cooperative aneurysm study. Participants of the Multicenter Cooperative Aneurysm Study. Crit Care Med. 1995;23:1007–1017. doi: 10.1097/00003246-199506000-00004. [DOI] [PubMed] [Google Scholar]
- Sozen T, Tsuchiyama R, Hasegawa Y, et al. Role of interleukin-1beta in early brain injury after subarachnoid hemorrhage in mice. Stroke. 2009;40:2519–2525. doi: 10.1161/STROKEAHA.109.549592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub F, Graf R, Gabel P, et al. Multiple interstitial substances measured by microdialysis in patients with subarachnoid hemorrhage. Neurosurgery. 2000;47:1106–1115. doi: 10.1097/00006123-200011000-00016. [DOI] [PubMed] [Google Scholar]
- Stranjalis G, Korfias S, Psachoulia C, et al. The prognostic value of serum S-100b protein in spontaneous subarachnoid haemorrhage. Acta Neurochir (Wien) 2007;149:231–238. doi: 10.1007/s00701-006-1106-9. [DOI] [PubMed] [Google Scholar]
- Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354:387–396. doi: 10.1056/NEJMra052732. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Sato S, Suzuki Y, et al. Endothelin immunoreactivity in cerebrospinal fluid of patients with subarachnoid haemorrhage. Ann Med. 1990;22:233–236. doi: 10.3109/07853899009148932. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kudo A, Otawara Y, et al. Fibrinolytic activity in the CSF and blood following subarachnoid haemorrhage. Acta Neurochir (Wien) 1997;139:1152–1154. doi: 10.1007/BF01410975. [DOI] [PubMed] [Google Scholar]
- Tang J, Zhao J, Zhao Y, et al. Apolipoprotein E epsilon4 and the risk of unfavorable outcome after aneurysmal subarachnoid hemorrhage. Surg Neurol. 2003;60:391–396. doi: 10.1016/s0090-3019(03)00323-9. [DOI] [PubMed] [Google Scholar]
- Taub PR, Fields JD, Wu AH, et al. Elevated BNP is associated with vasospasm-independent cerebral infarction following aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2011;15:13–18. doi: 10.1007/s12028-011-9535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao Y, Takada M, Tanabe T, et al. Microalbuminuria is a prognostic predictor in aneurysmal subarachnoid hemorrhage. Intensive Care Med. 2007;33:1000–1006. doi: 10.1007/s00134-007-0617-z. [DOI] [PubMed] [Google Scholar]
- Testai FD, Aiyagari V, Hillmann M, et al. Proof of concept: endogenous antiangiogenic factors predict the occurrence of symptomatic vasospasm post subarachnoid hemorrhage. Neurocrit Care. 2011;15:416–420. doi: 10.1007/s12028-011-9559-y. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U, Rostami E. Microdialysis in neurointensive care. Curr Pharm Des. 2004;10:2145–2152. doi: 10.2174/1381612043384105. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Donadello K, Schmit X. Biomarkers in the critically ill patient: C-reactive protein. Crit Care Clin. 2011;27:241–251. doi: 10.1016/j.ccc.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Vos PE, Lamers KJ, Hendriks JC, et al. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology. 2004;62:1303–1310. doi: 10.1212/01.wnl.0000120550.00643.dc. [DOI] [PubMed] [Google Scholar]
- Vos PE, van GM, Beems T, et al. Increased GFAP and S100beta but not NSE serum levels after subarachnoid haemorrhage are associated with clinical severity. Eur J Neurol. 2006;13:632–638. doi: 10.1111/j.1468-1331.2006.01332.x. [DOI] [PubMed] [Google Scholar]
- Wang HC, Lin WC, Yang TM, et al. The association between symptomatic delayed cerebral infarction and serum adhesion molecules in aneurysmal subarachnoid hemorrhage. Neurosurgery. 2011;68:1611–1617. doi: 10.1227/NEU.0b013e318210c871. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Monge JC, Rabelink TJ, Yanagisawa M. Endothelin: new discoveries and rapid progress in the clinic. Trends Pharmacol Sci. 1998;19:5–8. doi: 10.1016/s0165-6147(97)01144-9. [DOI] [PubMed] [Google Scholar]
- Weiss N, Sanchez-Pena P, Roche S, et al. Prognosis value of plasma S100B protein levels after subarachnoid aneurysmal hemorrhage. Anesthesiology. 2006;104:658–666. doi: 10.1097/00000542-200604000-00008. [DOI] [PubMed] [Google Scholar]
- Wiesmann G, Missler U, Hagenström H, Gottmann D. S-100 protein plasma levels after aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien) 1997;139:1155–1160. doi: 10.1007/BF01410976. [DOI] [PubMed] [Google Scholar]
- Yarlagadda S, Rajendran P, Miss JC, et al. Cardiovascular predictors of in-patient mortality after subarachnoid hemorrhage. Neurocritical Care. 2006;5:102–107. doi: 10.1385/NCC:5:2:102. [DOI] [PubMed] [Google Scholar]
- Zanier ER, Refai D, Zipfel GJ, et al. Neurofilament light chain levels in ventricular cerebrospinal fluid after acute aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2011;82:157–159. doi: 10.1136/jnnp.2009.177667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubkov AY, Rollins KS, Parent AD, et al. Mechanism of endothelin-1-induced contraction in rabbit basilar artery. Stroke. 2000;31:526–533. doi: 10.1161/01.str.31.2.526. [DOI] [PubMed] [Google Scholar]