Abstract
Mycosis Fungoides (MF), the most common type of cutaneous T-cell lymphoma (CTCL) is characterized by a helper T cell 2 (Th2)-skewing with a mature CD4+ memory T-cell phenotype. Using skin samples from MF patients (n=21), healthy volunteers (n=17), individuals with atopic dermatitis (n=17) and psoriasis (n=9), we found interleukin (IL)-32 mRNA expression significantly higher in MF samples than in samples from benign inflammatory skin diseases, and its expression increases with disease progression. By immunohistochemistry and immunofluorescence, we confirmed IL-32 protein expression in many CD3+CD4+ T cells and some epidermotropic T cells in MF lesions. MyLa cells (a MF cell line) express IL-32, which in turn could promote cellular proliferation and viability in a dose-dependent fashion. IL-32-treated MyLa and CTCL HH cells up-regulated cell proliferation and survival genes. Of the major “polarizing” T-cell cytokines, only IFNγ mRNA increases with MF progression and positively correlates with IL-32 mRNA expression. Th2 cytokines do not positively correlate with IL-32 mRNA expression or MF progression. Furthermore, by flow cytometry, IL-32 production by circulating activated T-cells in healthy individuals was found in both IFNγ+ and IFNγ− cells but not in IL-4+ or IL-13+ cells. In conclusion, we have identified IL-32+ cells as the likely tumor cells in MF, and demonstrated that IL-32 mRNA expression increases with MF progression and is significantly higher than those in other skin diseases, and that some IL-32+ T cells are independent from the defined Th subsets. Thus IL-32 may play a unique role in MF progression as an autocrine cytokine.
Keywords: cutaneous T-cell lymphoma, interferon-γ, tumor microenvironment, disease progression, biomarker
Introduction
Cutaneous T-cell lymphoma (CTCL) is characterized by clonal expansion of malignant T cells, typically exhibiting the phenotype of mature CD4+ memory T-cells. Similar to other malignant diseases, interaction between malignant T cells and surrounding nonmalignant inflammatory cells is involved in the pathogenesis and progression of CTCL (1). The most common type of CTCL is Mycosis Fungoides (MF). MF accounts for approximately 70% of all CTCL cases (2) and shows different characteristics compared to Sézary syndrome, the second most common type of CTCL. MF initially presents as flat erythematous patches covering limited areas of the body (patch stage). In the patch stage, MF typically exhibits an indolent clinical behavior and the disease can remain stable for many years. Some of the patch lesions progress to indurated plaque lesions (plaque stage), while only limited cases develop large tumors (tumor stage). With disease progression, the malignant T cells tend to disseminate to lymph nodes, peripheral blood and internal organs, which carries an unfavorable prognosis (3).
Adhesion molecules and chemokines, attracting T cells to the lesional skin, as well as numerous cytokines, possibly relating the microenvironment to the progression of MF, have been found (1). Among them, helper T cell 2 (Th2) cytokines, particularly IL-5, are regarded as important in MF progression (4). The reason why some patients develop advanced stage disease is still unknown, but characteristics of tumor cells and the overall immune microenvironment are likely to control disease progression.
Recently, IL-32 mRNA expression was detected in MF skin as well as in MyLa cells, the cell line derived from MF lesional skin (5, 6), but the cells producing this cytokine were not identified and its function is presently unknown. IL-32 is a proinflammatory cytokine known to be involved in many inflammatory diseases such as rheumatoid arthritis and Crohn’s disease, as well as malignant diseases, including lung and pancreatic cancer (7–10). In rheumatoid arthritis, injection of IL-32 into knees induces joint swelling (7). IL-32 expression in tumor cells is associated with a poor prognosis in lung cancer, and IL-32 prompts pancreatic cancer cell proliferation (9, 10).
Here, we show that IL-32 mRNA expression levels in MF are significantly higher than those in other inflammatory skin diseases, and that IL-32 expression levels increase with MF progression. IL-32 is more consistently expressed than Th2 cytokines in MF lesions and is not correlated with the expression of Th2 cytokines. Thus IL-32 might contribute to tumor progression in MF independent of Th2 cytokines.
Materials and Methods
Skin and blood samples
MF skin samples (patch stage; n=8, plaque stage; n=8, tumor stage; n=5) were obtained at the Charité-University Medical Center Berlin under its approved protocols. Skin samples from atopic dermatitis (AD; n=17), psoriasis (Pso; n=9), and healthy volunteers (n=17), as well as whole blood from healthy volunteers were collected at The Rockefeller University under its IRB-approved protocols. Written, informed consent was obtained and the studies were performed in adherence with the Principles of the Declaration of Helsinki.
Immunohistochemistry
Standard procedures were used for immunohistochemistry and immunofluorescence as previously described (11). Frozen tissue sections were stained with mouse anti-human IL-32αβγδ (KU32–52, BioLegend). Biotin-labeled horse anti-mouse antibody (Vector Laboratories) was used to detect mouse monoclonal antibody. The staining signal was amplified with avidin-biotin complex (Vector Laboratories) and developed with chromogen 3-amino-9-ethylcarbazole (Sigma-Aldrich).
Immunofluorescence
Frozen skin sections were fixed with acetone and blocked with 10% normal goat serum (Vector Laboratories) for 30 minutes. Primary antibodies were incubated overnight at 4°C and amplified with the appropriate Alexa Fluor® 488 (A-488) or 568 (A-568) conjugated secondary antibody for 30 minutes at room temperature. Antibodies used are: IL-32αβγδ (KU32–52, BioLegend), CD3 (SK7, BD Biosciences), CD4 (SK3, BD Biosciences), CD8 (SK1, BD Biosciences), hNKp46 (195314, R&D Systems), CD20 (L27, BD Biosciences), CD14 (M5E2, BioLegend), CD11c (B-ly6, BD Pharmingen), CD303 (AC144, Miltenyi Biotec), and CD163 (5C6-FAT, Acris Antibodies).
Quantitative reverse transcription (RT)-PCR assay
mRNA was extracted from skin samples using the RNeasy Mini Kit (Qiagen). cDNA was synthesized using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Preamplification RT-PCR was performed using TaqMan® PreAmp Master Mix Kit, primers and probes (Applied Biosystems). The primers and probes used in our study are listed in Supplementary Table S1. The results were normalized to human acidic ribosomal protein (hARP) housekeeping gene and analyzed with Applied Biosystems PRISM 7700.
Cell culture
MyLa cells were generously donated by Dr. Kaltoft (Skin Cancer Center Charité, Berlin). They were tested for purity by the fragment lengths of T-cell receptor beta and gamma rearrangements (12). HH cell line was obtained from ATCC (CRL-2105™) in January 2014; no authentication assay was performed. MyLa and HH cells were maintained in RPMI1640 (Gibco) with 10% fetal calf serum (FCS) (Gibco) and 1% pen/strep (Gibco) at 37°C, 5% CO2. Cell proliferation was examined by WST-1 assay (Roche Applied Science) according to the company’s protocol. Recombinant IL-32γ was obtained from R&D Systems. For microarray analysis, MyLa and HH cells were cultured in 0.1% FCS with and without 5ng/ml IL-32γ for 40h. Both MyLa and HH cells were tested and confirmed to be mycoplasma-free.
RNA extraction, amplification, and hybridization for microarray
RNA extraction from MyLa and HH cells was performed by using RNeasy Micro Kit (Qiagen). Total RNA was subjected to two-cycles of cDNA synthesis (Affymetrix), and Human Genome U133 plus A2.0 arrays (Affymetrix) were used.
Flow Cytometry
Blood cells from healthy volunteers were activated with 25ng/ml PMA (Sigma-Aldrich) and 2ug/ml Ionomycin (Sigma-Aldrich) in RPMI1640 with 5% human AB serum (cellgro), 1% HEPES (Sigma-Aldrich), and 0.5% Gentamycin (Gibco) at 37°C, 5% CO2 for 4 hours. Thereafter the blood cells were treated with 0.2nM EDTA, and RBCs were lysed (BD FACS lysing solution, BD FACS™) and used for flow cytometry assays. LIVE/DEAD Fixable Blue (viable dye, Invitrogen), PE-Alexa Fluor® 610-CD3 (7D6, Invitrogen), Brilliant Violet 711™-Ki-67 (BioLegend), Alexa Fluor 700-IFN-γ (B27, BD Pharmingen), BV421-IL-4 (8D4–8, BD Horizon™), PerCP/Cy5.5-IL-13 (JES10-5A2, BioLegend), FITC-TNF-α (6401.1111, BD Fastimmune™), PE-Cyanine7-IL-22 (22URTI, eBioscience), APC-eFluor® 780-IL-17 (eBio64DEC17, eBioscience), PE-IL-9 (MH9A4, BioLegend), biotin-IL-32αβγδ (KU32–52, BioLegend) and APC-streptavidin (BioLegend) were used for cell-surface and intracellular staining. Fluorescence minus one (FMO) and Isotype-matched control antibodies were used to set up baselines to exclude background from the analysis. After LIVE/DEAD and surface-staining, cells were fixed and permeabilized for intracellular staining. Cell acquisition was performed using the LSR II flow cytometer supported by the FACSDiva v6.1.1 software (BD Biosciences). Data were analyzed by using FlowJo v.X (Treestar, Inc).
Enzyme-linked immunosorbent assay
After culture in various concentrations of FCS for 40 hours, MyLa cells were centrifuged, and supernatant was collected to measure IL-32 levels. Enzyme-linked immunosorbent assay (ELISA) kits were used for measuring IL-32 levels (Creative Diagnostics) in supernatant, according to the manufacturer’s protocols. The measured values from individual samples were plotted by dots. The detection range of the assay was from 15.6–1000 pg/ml.
Statistics
All values obtained from RT-PCR were transformed to log10 before analysis. Analysis between two groups was performed using the Student’s t-test. For RT-PCR experiments where identical values were obtained within a group (e.g. healthy volunteers for IL-5), a one sample t-test was used. For comparisons involving more than two groups, One-way ANOVA followed by Tukey’s multiple-comparison test was carried out. Correlation coefficients were determined by using the Spearman’s rank correlation test. P-values of < 0.05 were considered statistically significant.
To analyze microarray data, expression values were obtained using GC Robust Multi-array Average (GCRMA) algorithm. Fold-change (FCH) between each treated sample vs. its control were calculated and genes with FCH of > 2.0 were considered as differentially expressed. We used Ingenuity Pathways Analysis (IPA) to identify pathways enriched in our list of genes, which were dysregulated by treatment by FCH of > 2.0. Pathways with enrichment P-values lower than 0.05 were considered significantly dysregulated.
Results
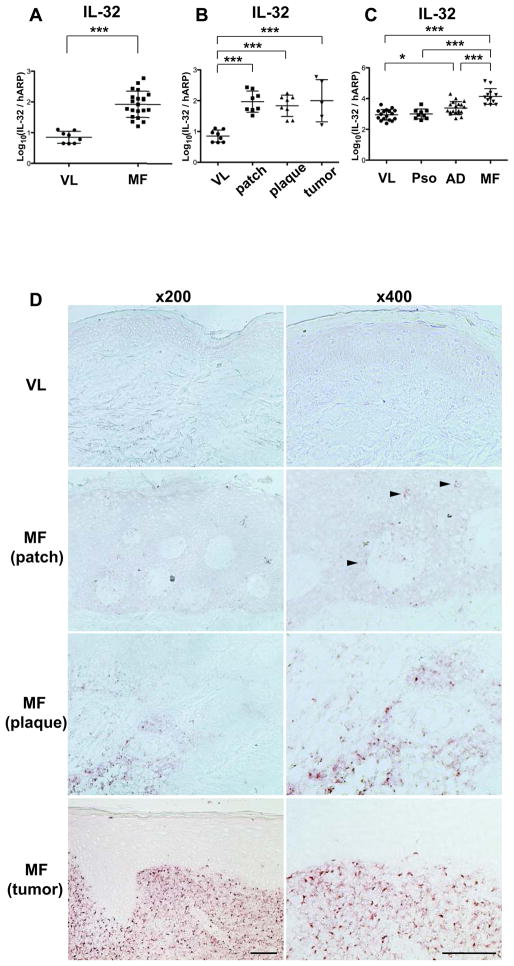
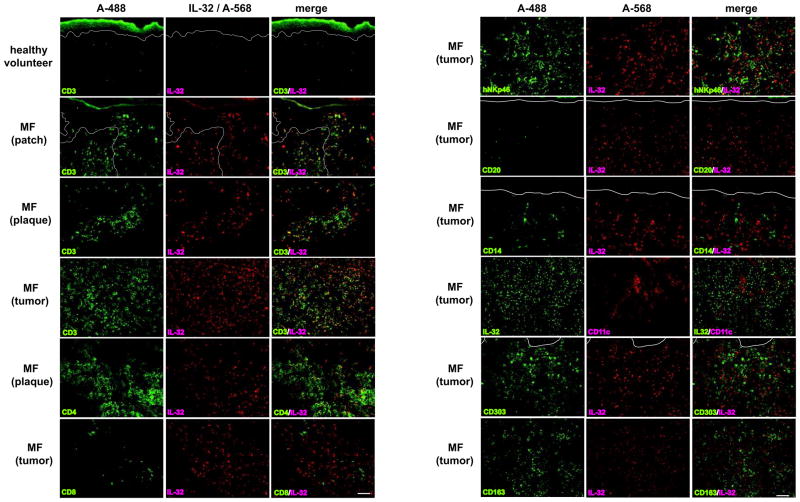
We investigated IL-32 mRNA expression in skin samples obtained from healthy volunteers vs. MF skin lesions. IL-32 mRNA is highly expressed in MF skin samples (Fig. 1A) and its expression levels increase with disease progression (Fig. 1B). Since high IL-32 expression has been recognized in inflammatory skin diseases, such as Pso (13) and AD (14), we compared their expression levels. While IL-32 mRNA expression levels in Pso were not significantly higher than those in healthy volunteers, its expression levels in AD were significantly higher than those in healthy volunteers. MF lesions showed substantially and significantly higher IL-32 mRNA expression levels compared to both Pso and AD (Fig. 1C). By immunohistochemistry, we confirmed IL-32 protein expression in MF (Fig. 1D). The number of IL-32+ cells increased with disease progression, and we detected IL-32+ cells in the epidermis in the patch stage. To identify IL-32+ cell subsets, we next did two-color immunofluorescence (IF) (Fig. 2). There were few IL-32+CD3+ cells in healthy skin, while many IL-32+CD3+ cells were found in MF lesions. Most of the IL-32+ cells were CD3+, and the number of IL-32+CD3+ cells increased with disease progression. Moreover, some CD3+ cells infiltrating the epidermis (epidermotropic T cells) expressed IL-32. We found numerous IL-32+CD4+ cells but could not find any IL-32+CD8+ cells. To explore the possibility that another cell population expresses IL-32, we stained MF skin with antibodies for natural killer cells (NK cells; hNKp46), B cells (CD20), monocytes (CD14), myeloid dendritic cells (mDCs; CD11c), plasmacytoid dendritic cells (pDCs; CD303), and macrophages (CD163). While a few IL-32+ NK cells were detected, IL-32 positivity was not found on any other cell type tested. The cell counts at ×200 magnification were as follows (mean ± SD); CD3+IL-32+: 119.8 ± 76.7, CD3+IL-32−: 22.6 ± 14.0, CD4+IL-32+: 99.4 ± 72.4, CD8+IL-32+: 2.4 ± 1.5, hNKp46+IL-32+: 21.7 ± 12.5, CD20+IL-32+: 0.0 ± 0.0, CD14+IL-32+: 0.0 ± 0.0, CD11c+IL-32+: 0.0 ± 0.0, CD303+IL-32+: 0.0 ± 0.0, CD163+IL-32+: 0.0 ± 0.0. We did find abundant mDCs, pDCs, and macrophages in MF skin in close proximity to IL-32+ cells. Overall, our IF results indicate that most of the IL-32+ cells in MF skin have helper T-cell phenotypes (CD3+CD4+), the same phenotype as MF tumor cells.
Figure 1.
IL-32 is highly expressed in MF lesional skin. A–C, IL-32 mRNA levels using skin (VL; healthy volunteers, MF / patch stage, plaque stage, tumor stage, Pso, AD). Data in Fig. 1C are from a different experiment than those in Fig. 1A–B. Horizontal bars are mean ± SD. *P<0.05, ***P<0.001. D, Immunohistochemistry for IL-32 using skin from VL and MF patients (patch, plaque, and tumor stage). Arrows show cells positive for IL-32. Magnification, ×200 (left panels) and ×400 (right panels). Scale bar represents 100μm.
Figure 2.
CD4+ T-cells are the main source for IL-32+ cells. Immunofluorescence using skin samples from healthy volunteers and MF patients. A-488 labeled CD3, CD4, CD8, hNKp46, CD20, CD14, IL-32, CD303 or CD163 (left panels) and A-568 labeled IL-32 or CD11c (center panels) are shown, with merged images in right panels. White lines indicate the epidermis-dermis junctions. Magnification at ×200. Scale bar represents 100μm.
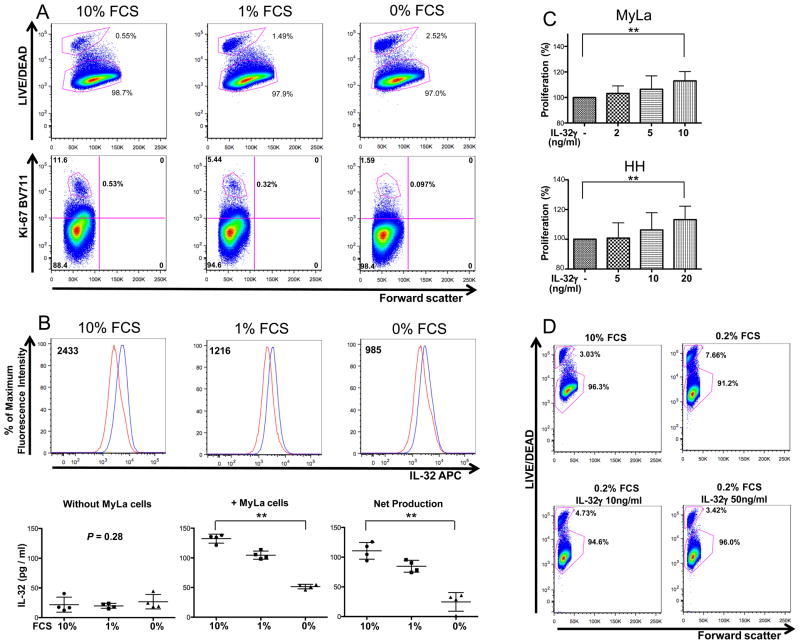
We next studied the influence of IL-32 on cell proliferation and viability using MyLa cells. In the normal culture condition (10% FCS), most MyLa cells are viable (98.7% LIVE/DEAD−), and proliferate well (high Ki-67 positivity), with consistent IL-32 expression (Fig. 3A and B). When we cultured MyLa cells in low FCS concentrations, viability and Ki-67+ cell numbers decreased (Fig. 3A). IL-32 expression and production levels in MyLa cells were reduced in parallel with these decrements (Fig. 3B). We next cultured MyLa cells in lower concentration of FCS, 0.1% or 0.2%, with and without IL-32. Compared to media alone, we observed dose-dependent increases in cell proliferation in the media with IL-32 (Fig. 3C). Likewise, IL-32 improved cell viability in a dose-dependent fashion. Cell viability in 0.2% FCS was increased by higher concentration of IL-32 to almost the same levels as in 10% FCS (Fig. 3D). Since MyLa is the only well-established MF cell line, we also used HH cells, which were derived from the peripheral blood of patients with aggressive CTCL (15), to confirm IL-32-induced cell proliferation. An increase in cell proliferation was observed in HH cells upon the addition of IL-32 in a dose-dependent manner (Fig. 3C). Thus, IL-32 levels correlate with proliferation and viability of CTCL cell lines, indicating that this cytokine can promote both processes.
Figure 3.
IL-32 facilitates cell proliferation and augments viability of CTCL cells. A, After MyLa cells were cultured in media with the indicated FCS concentrations for 40 hours, they were stained with LIVE/DEAD (top panels), Ki-67 (bottom panels). B, After MyLa cells were cultured in media with the indicated FCS concentrations for 40 hours, they were stained with IL-32 (top panels). Red and blue lines represent FMO isotype-control and APC-IL-32 antibodies, respectively. Numbers represent the median fluorescence intensity value’s difference between FMO isotype-control and APC-IL-32. In bottom panels, IL-32 production in media including indicated FCS concentrations without MyLa cells (left panel), and in media containing indicated FCS concentrations with MyLa cells (center panel) are shown. In the right panel, we show the net production of IL-32 by MyLa cells cultured in indicated FCS concentrations (values shown in the left panel were subtracted from those shown in the center panel). C, After MyLa cells and HH cells were cultured in media containing 0.1% FCS with and without IL-32γ for 40 hours, cell proliferation rates were analyzed by using WST-1 assay. n=4–7. Values are mean ± SD. **P<0.01. D, After MyLa cells were cultured in the four conditions shown in the panels for 18 hours, they were analyzed for LIVE/DEAD expression. A, B (top panels), D, Representative results. A, B, D, experiments were done three times.
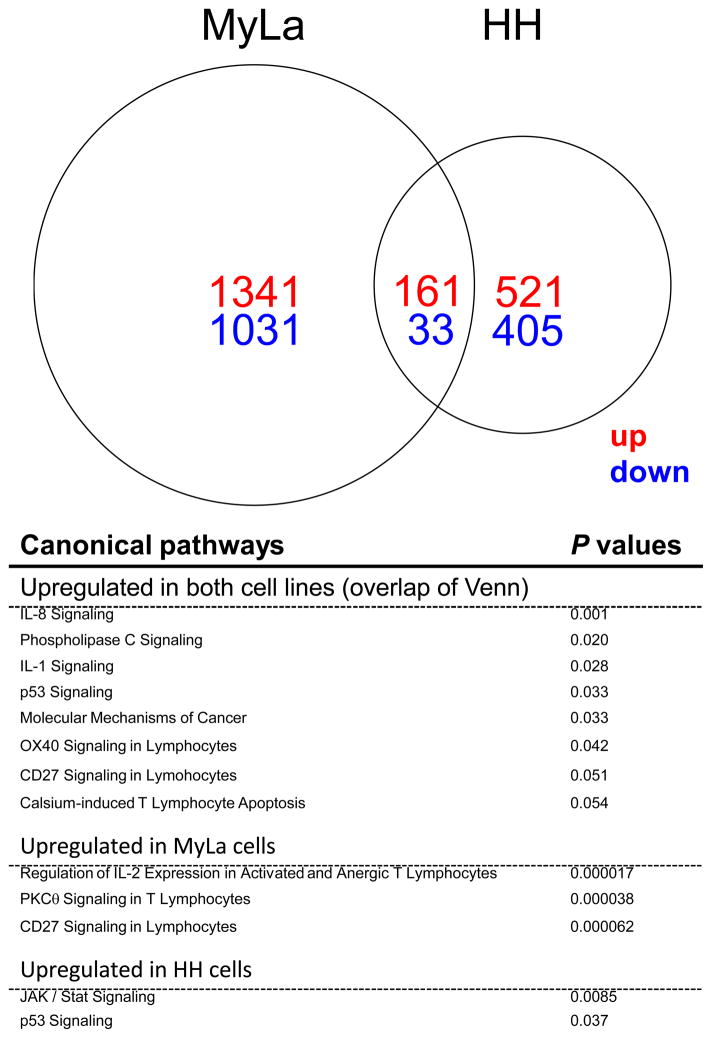
To elucidate the influence of IL-32 on MF cells, we analyzed genomic changes induced by IL-32 in MyLa and HH cells using transcriptome profiling on Affymetrix Human Genome U 133 plus A2.0 arrays. As shown in Figure 4, we identified a total of 1502 up-regulated and 1064 down-regulated probe sets in MyLa cells and a total of 682 up-regulated and 438 down-regulated probe sets in HH cells. 161 up-regulated and 33 down-regulated probe sets were detected in both MyLa and HH cells. Those genes related to cell proliferation, cell survival, cell death, cell cycle, cell invasion, and tumor immunity are listed in Supplementary Table 2. By Ingenuity canonical pathway analysis, 127 pathways, including Regulation of IL-2 Expression in Activated and Anergic T Lymphocytes (P = 0.000017), PKCθ Signaling in T Lymphocytes (P = 0.000038), and CD27 signaling in Lymphocytes (P = 0.000062) were significantly up-regulated in IL-32-treated MyLa cells compared to MyLa cells cultured without IL-32. Selected up-regulated pathways are summarized in Figure 4 and Supplementary Table 3. On the other hand, only 15 pathways, including JAK/Stat Signaling (P = 0.0085) and p53 signaling (P = 0.037), were significantly up-regulated in IL-32-treated HH cells compared to HH cells without IL-32. Pathways which were up-regulated in both IL-32-treated MyLa and HH cells compared to their controls are also shown. Thus, the effect of IL-32 on MyLa cells was much stronger than that on HH cells. Induction of BCL-2 or BCL2L1 in MF cells could be mediators that increase viability of these cells in low concentration serum (Fig. 3).
Figure 4.
IL-32 prompts cell activation and cancer related pathways. A Venn diagram reveals the numbers of up-regulated (red) and down-regulated (blue) probe sets in IL-32-treated MyLa cells and / or HH cells. Significantly up-regulated canonical pathways (p<0.05) and selected canonical pathways (with p values close to 0.05) in IL-32-treated MyLa cells and / or HH cells are shown with P values.
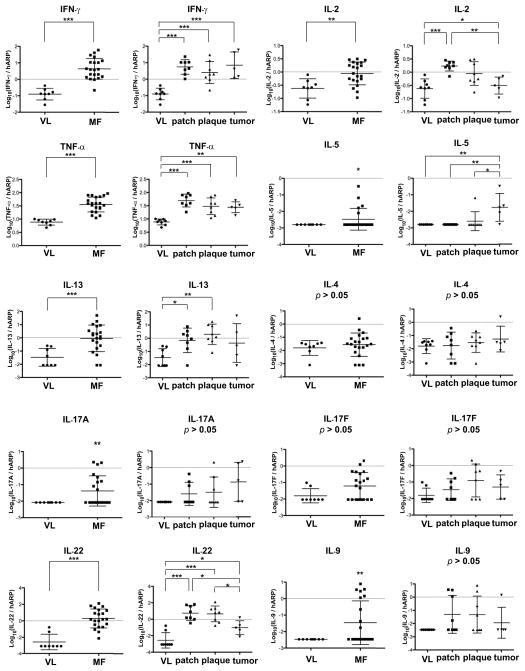
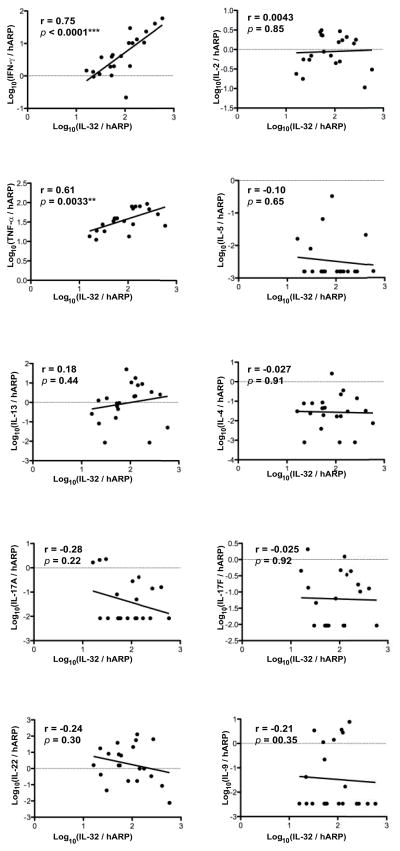
To determine the relationship between production of IL-32 and other cytokines synthesized in MF lesions, we measured mRNA expression levels (Fig. 5) and then correlated expression levels with IL-32 mRNA (Fig. 6). In Fig. 5, expression of cytokines that define Th1, Th2, Th9, Th17 and Th22 T-cell subsets is shown for 21 patients according to their stage of MF lesions. Consistent with past reports of elevated Th2 levels in MF lesions, high expression of IL-13 was seen in patch and plaque, but not tumor stage lesions and IL-5 was high in tumor stage lesions. However, IL-4 mRNA was not significantly elevated. Interestingly, high expression of IFNγ was seen in all stages of MF, while IL-2 levels progressively decreased from patch to tumor stages. IL-22 mRNA was elevated in MF lesions, with high expression in patch and plaque stages. Some patients also had elevated expression of IL-17A, IL-17F, and IL-9, but not in a pattern consistently associated with disease stage. Elevated levels of TNF-α were found in all stages of MF lesions. The relative expression of IL-32 mRNA vs. T-cell subset-defining cytokines is shown in Fig. 6. Production of IL-32 mRNA had strong and significant correlations with levels of IFNγ and TNF-α mRNAs, but not with other cytokines.
Figure 5.
Only IFNγ shows consistently increased mRNA expression in MF lesions. mRNA expression levels of various cytokines in the skin of VL and MF. Horizontal bars are mean ± SD. For IL-5, IL-17A, and IL-9 mRNA expression levels, a one sample t-test was done to compare the non-identical values to the identical values only in MF lesions. The percentages of samples showing non-identical values are 23.8% (IL-5), 42.9% (IL-17A) and 42.9% (IL-9). *P<0.05, **P<0.01, ***P<0.001.
Figure 6.
Only IFNγ and TNF-α show positive, significant correlations with IL-32 mRNA expression in MF lesions, while Th2 cytokines do not. Correlations between mRNA expression levels of IL-32 (x-axis) and other cytokines (y-axis). **P<0.01, ***P<0.001.
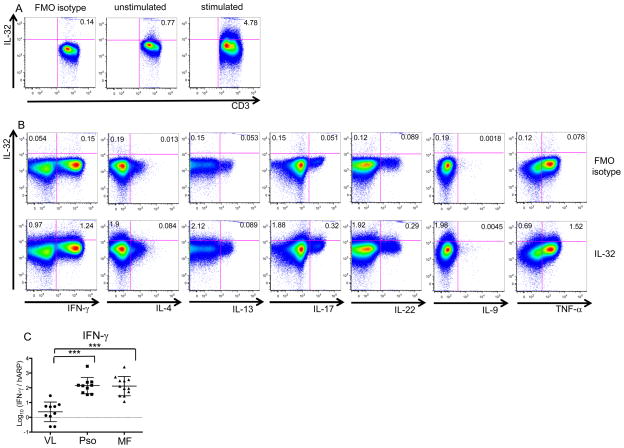
To determine whether IL-32 is produced exclusively by Th1 T cells (IFNγ–producing T cells), we activated peripheral blood T cells with PMA/Ionomycin and performed intracellular cytokine staining and flow cytometry analysis to assess their ability to present defined Th subsets (Fig. 7). By using unstimulated whole blood from healthy volunteers, we detected small IL-32+ populations (0.77%) among CD3+ cells (Fig. 7A), compatible with the few IL-32+CD3+ cells seen in healthy skin (Fig. 2). After stimulation with PMA/Ionomycin, IL-32 production among CD3+ cells increased (4.78%) (Fig. 7A). We found ~55% of IL-32 were produced by Th1 (IFNγ+) T cells, with lesser production by Th22 (IL-22+) and Th17 (IL-17+) T cells. Th2 (IL-4+ or IL-13+) and Th9 (IL-9+) T cells did not produce IL-32 as a rule. Hence, multiple T-cell subsets have the ability to produce IL-32, but it is not a Th2-axis cytokine. Additionally, roughly 30–60% of IFNγ−IL-32+ cells were negative for other cytokines including TNF-α and those defining Th1, Th2, Th17, Th22, and Th9 T cells (Sup Fig. 1), thus indicating that IL-32 is not restricted to already known T-cell subsets. IFNγ up-regulation and its correlation with disease activity are known in Pso (16), but Pso skin samples do not show significantly higher IL-32 mRNA expression compared to healthy skin samples (Fig. 1C). Furthermore, Pso and MF have similar IFNγ mRNA expression levels, both of which are significantly higher than those in healthy volunteers (Fig. 7C). Hence, it is likely that factors beyond IFNγ also control IL-32 expression.
Figure 7.
IL-32 production is detected in both IFNγ+ and IFNγ− cells in whole blood from healthy volunteers. A, B, Whole blood samples from healthy volunteers were stained with indicated antibodies. A, no stimulation (center panel) and stimulation with PMA/Ionomycin for 4 hours (right panel). B, stimulation with PMA/Ionomycin for 4 hours. A, B, Panels show a representative experiment out of three. C, IFNγ mRNA levels using skin (VL, Pso, MF). Horizontal bars are mean ± SD. ***P<0.001.
Discussion
Our study revealed that MF lesions exhibit high IL-32 expression levels unrelated to already known Th polarization, and that IL-32 could accelerate proliferation and augment viability of CTCL cell lines. T cells, NK cells, monocytes, macrophages, dendritic cells, epithelial cells, keratinocytes, and endothelial cells have all been reported to express or produce IL-32, depending on the situation (14, 17, 18). In MF, the main cellular source of IL-32 appears to be CD3+CD4+ T cells, which is the neoplastic cell type of MF. Our IF (Fig. 2), flow cytometry and ELISA (Fig. 3) results indicate that MF tumor cells could be the main source of IL-32 in MF. mDCs, pDCs, and macrophages are very close to IL-32+ cells in MF lesions, indicating possible interactions between these surrounding cells and tumor cells (Fig. 2). The number of mDCs, pDCs, and CD163+ macrophages has been reported to increase in MF (19, 20). Though their roles in MF remain to be elucidated, the involvement of mDCs, pDCs and macrophages in other malignancies is well established (21). While mDCs and macrophages can produce TNF-α, pDCs may contribute IFNγ production indirectly via type I interferon release. TNF-α and IFNγ are highly expressed in MF (Fig. 5), and are known to be able to induce IL-32 production (18, 22). Therefore, mDCs, pDCs, and macrophages in MF might induce IL-32 production from tumor cells. In addition, activated T cells can produce TNF-α and IFNγ (Fig. 7B) and are thus another candidates for inducing IL-32 production.
Detailed functions of IL-32 in MF remain to be established, but we showed that IL-32 could accelerate proliferation and/or viability of CTCL cell lines. Up-regulated probe sets and canonical pathways related to cell survival, cell proliferation, and cell growth found in IL-32-treated CTCL cell lines further support this idea.
This study presents a quantitative analysis of cytokines produced by distinctive T-cell subsets during MF disease progression. Classically, it was believed that early stage MF skews to Th1-dominant phenotype, while late stage MF shows Th2 profiles (1). While Th2 cytokines were clearly produced in many MF samples, not all tumors showed increased levels of IL-4, IL-5 and IL-13 mRNAs, and a clear progression with stage was not evident. In contrast, expression of IL-32 and IFNγ increased with disease progression and mRNAs for both cytokines were consistently expressed across samples. IL-32 levels were not significantly correlated with Th2 cytokines, such as IL-17, IL-22, or IL-9 but were positively and significantly correlated with IFNγ and TNF-α mRNA levels. Although IL-22 has been found to regulate IL-32 production in keratinocytes (23), there was no correlation between IL-22 and IL-32 mRNA expression levels in MF. Results from our immunohistochemistry and IF studies suggest that the bulk of IL-32 is associated with T cells and not epidermal keratinocytes, explaining the lack of correlation between IL-32 and IL-22 in our study. In general, hyperkeratosis is seen in early stage MF whereas the epidermis becomes thinner in tumor stage. In parallel with the thickness of the epidermis, IL-22 mRNA expression was high in patch and plaque stages, but decreased from plaque to tumor stage. Hence, high IL-22 mRNA expression in patch and plaque stages might be involved in hyperkeratosis in these stages of MF since IL-22 induces keratinocyte hyperplasia (24).
The association between IL-32 and IFNγ might be explained by the induction of IL-32 through IFNγ (22) or by co-production within a subset of Th1 T cells. However, IL-32 cannot be viewed as a Th1 marker, since both IFNγ+ and IFNγ− T cells produce this cytokine. Moreover, approximately 50% of IL-32+IFNγ− cells were negative for the other cytokines that we examined in our study. In addition, MF and Pso skin samples show comparably high IFNγ expression, while IL-32 expression levels in MF are significantly higher than those in Pso. Further study would be necessary to clarify the role of IL-32 in MF as well as IL-32-related interactions between tumor cells and surrounding cells. Nonetheless, our results improve our understanding of the microenvironment in MF, especially regarding Th cytokines and IL-32.
In conclusion, we have characterized the high IL-32 expression in MF. IL-32 expression is not linked to a specific Th paradigm, and tumor cells in MF are the likely source of IL-32. Hence, if IL-32 marks malignant T cells in MF, increases in its expression would be the most direct measure of disease progression.
Supplementary Material
Acknowledgments
Grant support; This study was partially supported by the TTCL (CTSA, RUCCTS Grant#8 UL1 TR000043) from the National Center for Advancing Translational Sciences (NCATS, NIH). NG was supported by NIH MSTP grant GM07739.
Footnotes
Conflicts of interest: The authors state no conflict of interest.
References
- 1.Wong HK, Mishra A, Hake T, Porcu P. Evolving insights in the pathogenesis and therapy of cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome) Br J Haematol. 2011;155:150–66. doi: 10.1111/j.1365-2141.2011.08852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973–2002. Arch Dermatol. 2007;143:854–9. doi: 10.1001/archderm.143.7.854. [DOI] [PubMed] [Google Scholar]
- 3.Weinstock MA, Gardstein B. Twenty-year trends in the reported incidence of mycosis fungoides and associated mortality. Am J Public Health. 1999;89:1240–4. doi: 10.2105/ajph.89.8.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benton EC, Crichton S, Talpur R, Agar NS, Fields PA, Wedgeworth E, et al. A cutaneous lymphoma international prognostic index (CLIPi) for mycosis fungoides and Sezary syndrome. Eur J Cancer. 2013;49:2859–68. doi: 10.1016/j.ejca.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Vowels BR, Lessin SR, Cassin M, Jaworsky C, Benoit B, Wolfe JT, et al. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. J Invest Dermatol. 1994;103:669–73. doi: 10.1111/1523-1747.ep12398454. [DOI] [PubMed] [Google Scholar]
- 6.van Kester MS, Borg MK, Zoutman WH, Out-Luiting JJ, Jansen PM, Dreef EJ, et al. A meta-analysis of gene expression data identifies a molecular signature characteristic for tumor-stage mycosis fungoides. J Invest Dermatol. 2012;132:2050–9. doi: 10.1038/jid.2012.117. [DOI] [PubMed] [Google Scholar]
- 7.Kaltoft K, Bisballe S, Dyrberg T, Boel E, Rasmussen PB, Thestrup-Pedersen K. Establishment of two continuous T-cell strains from a single plaque of a patient with mycosis fungoides. In Vitro Cell Dev Biol. 1992;28A:161–7. doi: 10.1007/BF02631086. [DOI] [PubMed] [Google Scholar]
- 8.Joosten LA, Netea MG, Kim SH, Yoon DY, Oppers-Walgreen B, Radstake TR, et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2006;103:3298–303. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Netea MG, Azam T, Ferwerda G, Girardin SE, Walsh M, Park JS, et al. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci U S A. 2005;102:16309–14. doi: 10.1073/pnas.0508237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorrentino C, Di Carlo E. Expression of IL-32 in human lung cancer is related to the histotype and metastatic phenotype. Am J Respir Crit Care Med. 2009;180:769–79. doi: 10.1164/rccm.200903-0400OC. [DOI] [PubMed] [Google Scholar]
- 11.Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J Clin Invest. 2007;117:2517–25. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukowsky A, Muche JM, Möbs M, Assaf C, Humme D, Hummel M, et al. Evaluation of T-cell clonality in archival skin biopsy samples of cutaneous T-cell lymphomas using the biomed-2 PCR protocol. Diagn Mol Pathol. 2010;19:70–7. doi: 10.1097/PDM.0b013e3181b2a1b7. [DOI] [PubMed] [Google Scholar]
- 13.Kempuraj D, Conti P, Vasiadi M, Alysandratos KD, Tagen M, Kalogeromitros D, et al. IL-32 is increased along with tryptase in lesional psoriatic skin and is up-regulated by substance P in human mast cells. Eur J Dermatol. 2010;20:865–7. doi: 10.1684/ejd.2010.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer N, Zimmermann M, Burgler S, Bassin C, Woehrl S, Moritz K, et al. IL-32 is expressed by human primary keratinocytes and modulates keratinocyte apoptosis in atopic dermatitis. J Allergy Clin Immunol. 2010;125:858–865. e10. doi: 10.1016/j.jaci.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Starkebaum G, Loughran TP, Jr, Waters CA, Ruscetti FW. Establishment of an IL-2 independent, human T-cell line possessing only the p70 IL-2 receptor. Int J Cancer. 1991;49:246–53. doi: 10.1002/ijc.2910490218. [DOI] [PubMed] [Google Scholar]
- 16.Lew W, Bowcock AM, Krueger JG. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and “Type 1” inflammatory gene expression. Trends Immunol. 2004;25:295–305. doi: 10.1016/j.it.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity. 2005;22:131–42. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Heinhuis B, Netea MG, van den Berg WB, Dinarello CA, Joosten LA. Interleukin-32: a predominantly intracellular proinflammatory mediator that controls cell activation and cell death. Cytokine. 2012;60:321–7. doi: 10.1016/j.cyto.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Schwingshackl P, Obermoser G, Nguyen VA, Fritsch P, Sepp N, Romani N. Distribution and maturation of skin dendritic cell subsets in two forms of cutaneous T-cell lymphoma: mycosis fungoides and Sezary syndrome. Acta Derm Venereol. 2012;92:269–75. doi: 10.2340/00015555-1220. [DOI] [PubMed] [Google Scholar]
- 20.Sugaya M, Miyagaki T, Ohmatsu H, Suga H, Kai H, Kamata M, et al. Association of the numbers of CD163(+) cells in lesional skin and serum levels of soluble CD163 with disease progression of cutaneous T cell lymphoma. J Dermatol Sci. 2012;68:45–51. doi: 10.1016/j.jdermsci.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Netea MG, Azam T, Lewis EC, Joosten LA, Wang M, Langenberg D, et al. Mycobacterium tuberculosis induces interleukin-32 production through a caspase-1/IL-18/interferon-gamma-dependent mechanism. PLoS Med. 2006;3:e277. doi: 10.1371/journal.pmed.0030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–85. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.