Abstract
Telomeres are specialized structures providing chromosome integrity during cellular division along with protection against premature senescence and apoptosis. Accelerated telomere attrition in patients with Myelodysplastic Syndrome (MDS) occurs by an undefined mechanism. Although the MDS clone originates within the myeloid compartment, T-lymphocytes display repertoire contraction and loss of naïve T-cells. The replicative lifespan of T-cells is stringently regulated by telomerase activity. In MDS cases, we show that purified CD3+ T-cells have significantly shorter telomere length and reduced proliferative capacity upon stimulation compared to controls. To understand the mechanism, telomerase enzymatic activity and telomerase reverse transcriptase (hTERT) gene expression were compared in MDS cases (n=35) and healthy controls (n=42) within different T-cell compartments. Telomerase activity is greatest in naïve T-cells illustrating the importance of telomere repair in homeostatic repertoire regulation. Compared to healthy controls, MDS cases had lower telomerase induction (p<0.0001) that correlated with significantly lower hTERT mRNA (p<0.0001), independent of age and disease stratification. hTERT mRNA deficiency affected naïve but not memory T-cells, and telomere erosion in MDS occurred without evidence of an hTERT-promoter mutation, copy number variation or deletion. Telomerase insufficiency may undermine homeostatic control within the hematopoietic compartment and promote a change in the T-cell repertoire in MDS.
Keywords: myelodysplastic syndrome, telomere, T lymphocyte, transcription, bone marrow failure
Introduction
Myelodysplastic syndromes (MDS) include a spectrum of age-related hematological neoplasms characterized by dysplasia, cytopenias and potential for Acute Myeloid Leukemia (AML) progression (1). Because complex mutations are acquired within the myeloid progenitor or stem cell pool, the biological characteristics are enormously heterogeneous (2, 3). The early manifestations of MDS, however, are relatively well conserved and include inflammation within the bone marrow microenvironment coupled with excessive proliferation and apoptosis of myeloid progenitors (4, 5). In MDS patients, telomere length is shortened by an unknown process related possibly to proliferative stress or by ineffective telomeric repair.
Telomeres are a repetitive hexanucleotide (TTAGGG) region that protect from chromosomal deterioration (6). T-cells and hematopoietic stem/progenitor cells need telomere replenishment by telomerase, which is a specialized reverse transcriptase composed of the rate-limiting telomerase reverse transcriptase (hTERT) enzyme, the Telomerase RNA Component (hTERC) template, and the shelterin stabilizing proteins. hTERT gene expression is transcriptionally regulated and its induction limits replicative senescence and protects regenerating somatic cells from apoptosis and genome instability (7). Mutations in telomere components have demonstrated the role for telomerase in hematopoietic repopulation and in immune homeostasis (8). hTERC, hTERT and mutations in shelterin proteins in patients with aplastic anemia and dyskaratosis congenital (DKC) have been informative to demonstrate the consequences of telomerase deficiency in bone marrow failure and predisposition to cancer (6, 9). In patients with mutations in telomere components, T-cells undergo TCR signal transduction but fail to display normal clone size after activation (6). In previous studies, siRNA silencing of hTERT in T-cells renders them sensitive to apoptosis and hinders their regeneration indicating a direct role for telomerase in replication potential (10). Compared to myeloid cells, there is no data regarding the length of telomeres or telomerase function within the T-cell compartment in MDS patients (2). Of all somatic cells, T-cells are dependent on telomere repair because of their high rate of turnover. This activity is particularly important for maintenance of naïve cells after age-dependent thymic involution (10). In MDS, the T-cells are characterized by skewed repertoire distribution (11) and form suppressive lymphoid aggregates within the bone marrow of some patients where they can directly suppress hematopoiesis (12, 13).
This study was conducted to examine the mechanism of MDS T-cell deregulation related to telomere function. We report that deficiency in hTERT mRNA expression is important in MDS and reveal a novel mechanistic link between aplastic anemia and other primary telomere repair disorders.
Materials and Methods
Cell isolation in patients and healthy controls
MDS patients were recruited from 2005–2010 through a collaborative study involving four centers: the Hematologic Malignancy Program at the Moffitt Cancer Center (“Moffitt”), the Cleveland Clinic Cancer Center, UCLA Medical Center and Penn State Cancer Center. All study participants signed informed consents approved by the institutional review boards (IRB) at the participating institutions. All diagnoses were histologically-confirmed after review at the participating center and classified according to standard criteria including the World Health Organization (WHO)(14) and International Prognostic Scoring System (IPSS)(1). Inclusion into this study (n=35) was based on having a sufficient volume of frozen peripheral blood mononuclear (PBMCs) available for T-cell isolation (30×106 cells). Details describing the isolation of PBMCs and purification of CD3+ T-cells is provided in supplemental material.
Measurement of telomere length
DNA was extracted from unstimulated purified CD3+ T-cells using the PureLink Genomic DNA Kits according to manufacturer’s instructions (Invitrogen, Carlsbad, California), and relative telomere lengths were measured by a modified version of the quantitative real-time (qRT) PCR–based telomere assay, as described previously (15, 16). Details of the assay are provided in supplemental methods and supplemental Fig. 1.
T-cell activation
Purified CD3+ T-cells were stimulated in RPMI 1640 medium (Gibco, Grand Island, NY) containing 10% FBS with CD3/CD28 T-cell activator beads (Dynabead®, Invitrogen, Carlsbad, CA, USA) for 3 days.
Measurement of TREC DNA in purified T-cells
Signal-joint T-cell receptor excision circles (sjTRECs) are circular extrachromosomal fragments of DNA generated during TCR gene rearrangement in the thymus (17). Since they are non-transferable to daughter cells, expression decreases with each round of cell division in culture and quantification is a reliable estimate of population doubling (18). Genomic DNA was extracted from CD3+ T-cell, as described above. Detailed methods for sjTREC quantification can be found in supplemental material.
Measurement of hTERT mRNA expression
Details of qRT-PCR methods is provided in the supplemental material.
Telomerase activity
Telomerase activity was determined using the telomerase PCR-ELISA kit (TRAP) (Roche Molecular Biochemicals, Indianapolis, IN, USA) according to the manufacturer’s instructions. Details are provided in supplemental methods. Experiments were carried out at least twice to improve the reliability of the results. The relative telomerase activity within each sample was calculated as follows: (the absorbance of the sample - the absorbance of the heat-treated sample)/the absorbance of the internal standard of the sample.
Flow cytometry to detect BrdU incorporation
To access entry into S-phase, bromodeoxyuridine (BrdU) incorporation (BrdU flow kit, BD Biosciences, San Diego, CA) was used, as described previously (19). Detailed methods are provided in supplemental information.
Naïve and memory cell purification and flow assays
T-cells subsets were isolated from purified CD3+ T-cells using CD45RO and CD45RA antibody staining and isolation with the Aria flow sorter (BD Biosciences). Subset purity was >95% in post-sort analyses. Memory (CD45RO+CD45RA-) and naïve (CD45RA+CD45RO−)(17) cells were stimulated with CD3/CD28 activator beads (Dynabeads®, Dynal Corp.) for 3 days. Telomere length was detected in sorted naïve and memory T-cells before stimulation and telomerase activity and hTERT expression was measured using the TRAP assay and qRT-PCR reaction, respectively, before and after the 3 days of stimulation. DAPI exclusion discriminated viable and dead cells during activation and CD69 staining was used to determine the TCR response. Detailed methods are provided in supplemental material.
Sequencing of hTERT promoter
The core promoter of hTERT was amplified using genomic DNA from purified CD3+ T-cells for sequence analysis. Detailed methods are provided in supplemental material.
Statistical analysis
Telomere length, proliferation (BrdU and PD), and hTERT mRNA data were compared as continuous variables between MDS cases and controls using the Wilcoxon rank-sum test. Data on telomerase activity was normally distributed and compared as a continuous variable using a T-test. The mean age of controls was 52 (range 17–84y) and MDS cases was 67 (range 28–87y) (Wilcoxon, p=0.1). For all variables, the statistical significance of the interaction with age as a continuous variable and sex in relation to case-control status was tested by including an interaction term in the logistic regression model of log-transformed data. However, hTERT mRNA data was square-root transformed to achieve normality because log-transformation failed to achieve a normal distribution. Correlations between telomere length and age and hTERT activity and mRNA expression were assessed using the Spearman correlation coefficient.
Since T-cell homeostasis is altered by thymic involution, and younger MDS patients (i.e. under the age of 65 years old) are suspected of having a different pathobiologic mechanism for impaired hematopoiesis related to an immunological attack, we stratified the analyses by age <65 or ≥ 65 years. For these subset analyses, the 5 youngest healthy controls were omitted to better approximate the age range among younger MDS cases. In these subgroups, the mean age of controls was 47y (range 26–63y, n=23) and the mean age of cases was 52y (n=9, range 28–61y), (Wilcoxon, p=0.27). The older group was balanced with regard to age with a mean of 73 (n=14, range 65–84y) in controls and a mean of 73 (n=26, range 65–87y) in cases (Wilcoxon, p=0.97). All statistical analyses were performed with the SPSS 19.0 software package (SPSS Inc., Chicago, IL, USA).
Results
Clinical characteristics of MDS patients
Characteristics of the 35 MDS patients and 42 controls are summarized in Table 1. Of the 42 controls and 35 cases, 18 (43%) and 24 (69%) were male, respectively (p=0.02). MDS patients were classified as refractory anemia with or without ringed sideroblast (RA, RARS) (n=2, 5.7%), refractory cytopenia with multilineage dysplasia (RCMD) (n=15, 42.9%), refractory anemia with excess blasts (RAEB)-1 (n=5, 14.3%) and RAEB-2 (n=9, 25.7%) and MDS-unclassified (MDS-U, n=4, 11.4%) based on WHO classification criteria (14). Based on IPSS, 12 (34.3%) were low risk, 9 (25.7%) intermediate-1, 8 (22.9%) intermediate-2, and 6 (17.1%) high risk (1). Twenty of 35 (57.1%) had identifiable cytogenetic abnormalities by metaphase karyotyping or FISH, and 15 (42.9%) had normal cytogenetics.
Table 1.
Clinical characteristics of MDS cases and controls
| Characteristics of Cases and Controls | ||
|---|---|---|
| Age | ||
| Case:Controls | Mean age (range) | p-value |
| Controls (n=42) | 52 (17–84) | |
| MDS Cases (n=35) | 67 (28–87) | 0.1 |
| Younger Case:Control group (<65y)* | ||
| Controls (n=23) | 47 (26–63) | |
| MDS Cases (n=9) | 52 (28–61) | 0.26 |
| Older Case:Control group (≥65y) | ||
| Controls (n=14) | 73 (65–84) | 0.97 |
| MDS Cases (n=26) | 73 (65–87) | |
| Sex (Male/Female) | N (M/F) | % (M/F) | |
| Controls (n=42) | 24/11 | 69/31 | |
| MDS Cases (n=35) | 18/24 | 43/57 | 0.02 |
| Clinical Characteristics of MDS Cases | n | % | |
| IPSS** classification | |||
| Low | 12 | 34.3 | |
| Intermediate-1 | 9 | 25.7 | |
| Intermediate-2 | 8 | 22.9 | |
| High | 6 | 17.1 | |
| Cytogenetics | |||
| Normal | 15 | 42.9 | |
| Abnormal | 20 | 57.1 | |
| WHO## MDS subtype | |||
| RA# with ringed sideroblasts (RARS) | 2 | 5.7 | |
| RCMD$ | 15 | 42.9 | |
| RA with excess blasts (RAEB)-1% | 5 | 14.3 | |
| RAEB-2 | 9 | 25.7 | |
| MDS-unclassified (MDS-U) | 4 | 11.4 | |
Within the younger groups, subset statistical analyses were also conducted excluding the 5 youngest controls with an age range that more closely approximated cases as described in the text.
IPSS=International Prognostic Scoring System;
WHO=World Health Organization;
RA=Refractory anemia
RCMD=Refractory cytopenia with multilineage dysplasia including patients with (n=2) or without ringed sideroblasts.
Refractory anemia with excess blasts −1 and 2.
Age-independent telomere attrition in purified T-cells in MDS
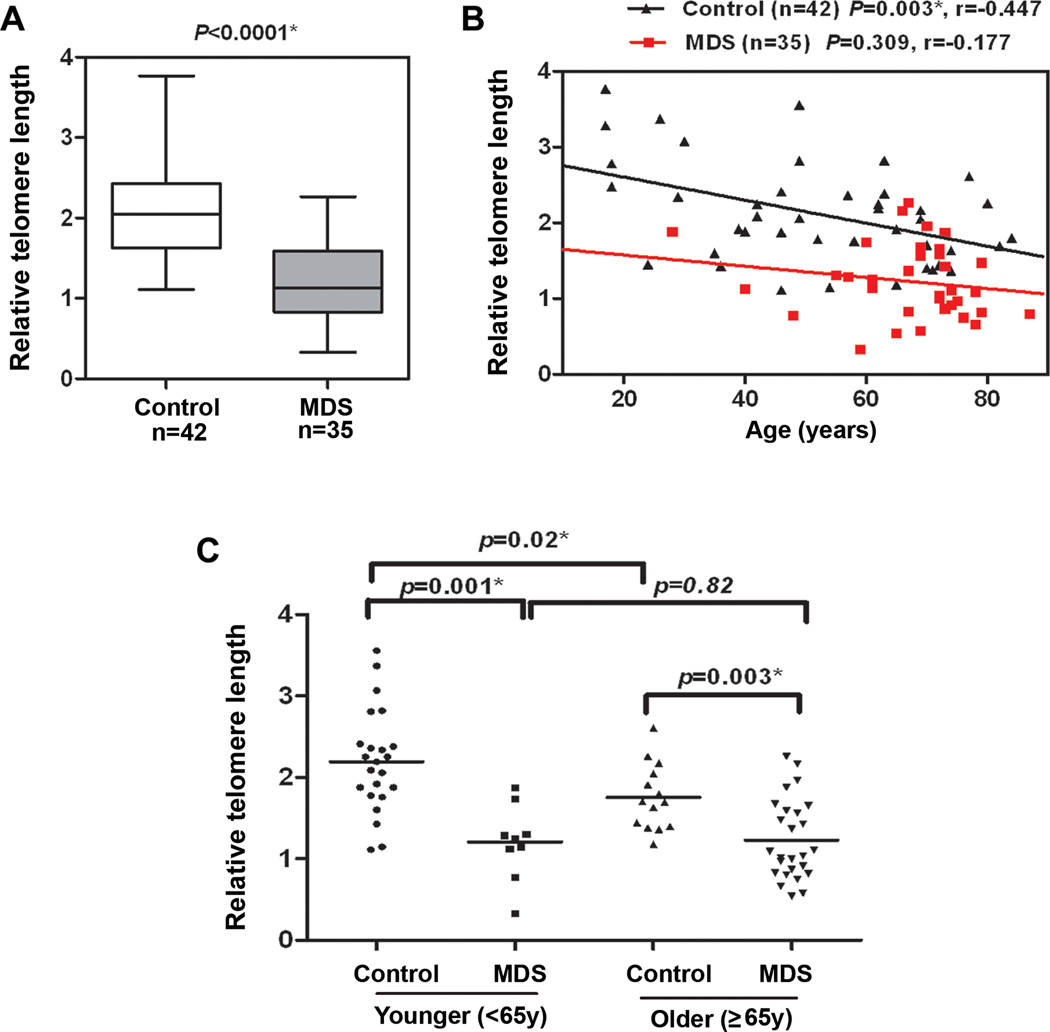
The mean relative telomere length in purified T-cells was compared relative to hemoglobin and found to be significantly shorter among MDS cases (n=35) (median=1.1, 95% CI, 1.1–1.4) compared to controls (n=42) (median=2.1, 95% CI, 1.9–2.3) after adjustment for age and sex (p<0.0001) (Fig. 1A) using log-transformed data. In healthy individuals, T-cell telomere length declined progressively with age (r=−0.447, p=0.003) (Fig. 1B) in agreement with previous reports reflecting the proliferative pressure on the T-cell compartment (20). Although not statistically significant, the age-related trend was similar in cases (r=−0.177, p=0.309) (Fig. 1B). To further evaluate the effect of age, we compared telomere length between MDS cases and controls stratified into younger (<65 yrs) and older (≥ 65 yrs) age groups (Fig. 1C). As expected, telomere lengths were significantly shorter among older controls compared to younger controls (p=0.001), although no difference in telomere lengths was observed between older and younger MDS cases. MDS cases tended to have shorter telomeres than controls in both the <65 age group (p=0.001) and the 65+ age group (p=0.003). In general, MDS patients within the younger group had telomeres that were shorter than the oldest healthy individuals attesting to the proliferative stress or loss of telomeric repair in the T-cell compartment.
Figure 1. Shortened telomere length in CD3+ T-cells in MDS patients.
CD3+T-cells were purified by negative selection from blood of MDS patients (n=35) and healthy donors (n=42). Telomere length was detected using quantitative PCR with 293 T-cells were used as a calibrator, as previously described (16). Results were analyzed using the ∆∆Ct method, as described in supplemental methods. Case-control differences for the telomere lengths in CD3+ T-cells were compared using the Wilcoxon sum rank test. P-values for the case-control differences are shown at the top of each panel. Log-transformed values for telomere length were then used in multivariate logistic regression analyses to adjust for age (as continuous variable) and sex, and the case-control difference in telomere length in CD3+ T-cells was statistically significant (p<0.0001). Correlation between telomere length and age was assessed in cases and controls using the Spearman rank correlation coefficient. p<0.05 was considered statistically significant. (A) Box and whisker-plots of telomere length in CD3+ T-cells in MDS patients compared to controls. (B) Telomere length (y-axis) relative to hgb was inversely correlated with the age in CD3+ T-cells from cases and controls. (C) Age-stratified analysis among younger <65 years and older (≥65y). All tests were two-sided and associations were considered statistically significant at a significance level of p<0.05.
Proliferative defects in MDS T-cells
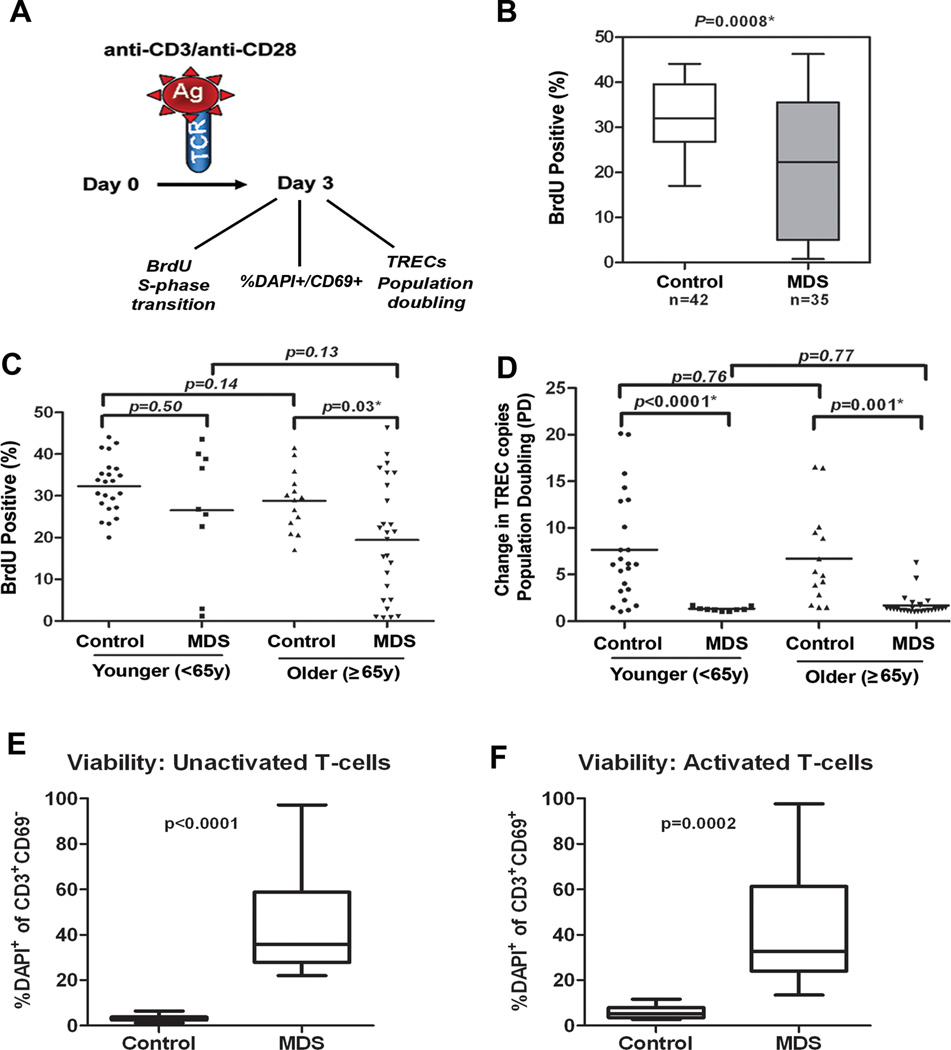
Replicative potential is closely linked to telomere length and telomerase activation(10, 21). To define the proliferative capacity of T-cells in MDS compared to healthy controls, both the percentage of cells capable of entering the cell cycle (ie, percentage of cells in S-phase as indicated by BrdU incorporation), cell death (percentage of CD69+ DAPI+ cells) and their proliferative burst potential (ie, population doubling) were assessed after anti-CD3/CD28 bead stimulation (Fig. 2A). The percentage of BrdU positive T-cells is significantly reduced in MDS cases (median=22.3, 95% CI, 16.3–26.3) compared to controls (median=32.0, 95% CI, 30.0–34.6) (p=0.0008) (Fig. 2B). However, wide variability was observed in the percentage of BrdU positive cells in MDS. The discrepancy in cell cycle regulation was more relevant in older cases compared to younger cases (case:control difference, p=0.03 in older group compared to case:control difference p=0.50 in younger group), but there were patients in both age groups with normal capacity for S-phase transition (Fig. 2C).
Figure 2. Deficiency of the proliferative capacity of CD3+ T-cells in MDS patients.
CD3+ T-cells were negatively separated from blood of MDS patients (n=35) and healthy donors (n=42) and samples were divided into two aliquots. One aliquot was used for assays on day 0 and the other stimulated with anti-CD3/CD28 beads for 3 days. (A) Shows a diagram of the analyses conducted. On day 0 cells were used for TREC baseline measurements, stained with CFSE and examined for DAPI on CD69+ cells (as defined in supplemental material). On day 3, BrdU incorporation was measured by flow cytometry to determine the percentage of T-cells capable of entering S-phase in MDS patients and healthy donors after stimulation. Samples were also collected for TREC DNA content, % DAPI within the CD69+ population and for CFSE dilution assays (as defined in supplemental material). (B) BrdU indicates the percentage of cells capable of entering S-phase. The box and whisker plots of data are shown for 42 controls and 35 MDS patients. (C) BrdU data divided into groups of younger and older patients and controls based on age <65y and ≥65y. (D) Dilution of the number of TRECs per cell indicates the replication potential. Population doubling (PD, ie, replication potential) was calculated as the ratio of the TREC DNA copy number per cell in unstimulated T-cells and stimulated T-cells on day 3 after antiCD3/CD28 stimulation. Case-control difference in PD analyzed in groups based on age<65y and ≥65y. Case-control differences in BrdU and PD in purified CD3+ T-cells were compared using the Wilcoxon signed rank. (E) percentage of DAPI+CD69+ cells in unactivated T-cells at baseline (Time 0) and the (F) percentage of these cells after activation in MDS patients and control. P-values based on Wilcoxon test for the case-control differences are shown at the top of each panel. Log-transformed values were then used in multivariate logistic regression analyses to adjust for age (as continuous variable) and sex, and the case-control. Exemplary CFSE staining in a control and case sample and summary of data in 6 donors tested is shown in supplementary Figure 2 and confirm deficiency in population doubling. All tests were two-sided and associations were considered statistically significant at a significance level of p<0.05.
Telomerase deficiency primarily impacts replication burst potential as failure to induce the telomere repair machinery leads to apoptosis and premature growth arrest (6). Estimation of replication potential was assessed using sjTREC dilution (Fig. 2A) as a measure of population doubling (PD) after in vitro activation with anti-CD3/CD28-coated beads. The change in copy number reflects the dilution of DNA through expansion after activation. Results were compared in younger and older cases and controls. In controls (n=42), the mean sjTREC DNA copy number decreased from 80 to 15 copies per cell after stimulation, indicating that an average of 6 PDs occurred in the three-day period of the assay. Fluorescent lipophilic dye 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) staining confirmed that T-cell populations in controls progress through roughly 5–7 generations within the 3-day expansion period, which is consistent with the sjTREC dilution assay (supplemental Figure 2). In the CSFE assay, ~50% of the cells are able to divide in bead-activated control T-cells. Compared to controls, PD was significantly decreased in MDS cases, both in the younger (p<0.0001) and older (p=0.001) groups (Fig. 2D). In MDS, expansion progressed past one generation of division on average in only 15% of cells using the CSFE assay (supplemental Figure 2). This indicates that there is a defect in the proliferative clonal burst in MDS T-cells after stimulation. Reduced clonal burst potential suggests that the cells undergo premature cell death or apoptosis. The % of DAPI positive cells prior to and after anti-CD3/CD28 stimulation was assessed in CD69+-activated T-cells. As shown in Fig. 2 E and F, cell death was significantly higher in activated T-cells from MDS patients compared to controls.
Short telomere length in naive T-cells in MDS patients suggests inherent loss of telomere maintenance
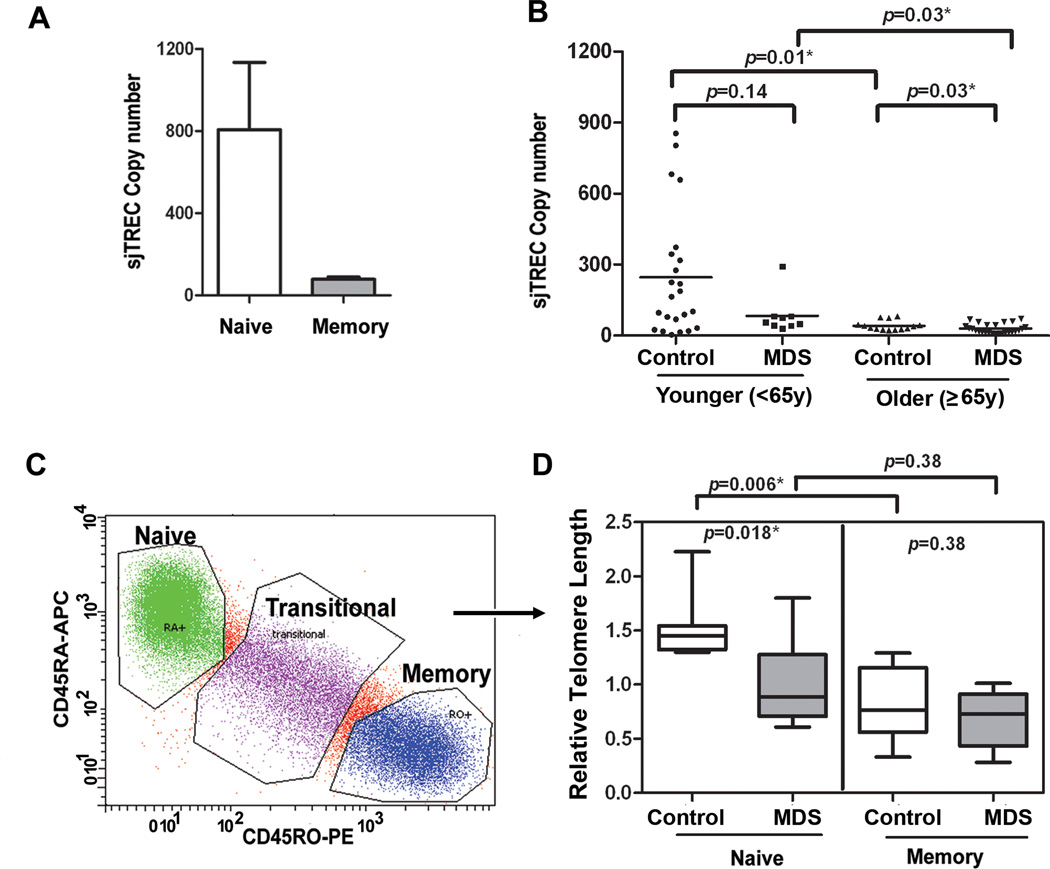
Telomeres may shorten secondary to proliferative stress, as previously hypothesized in MDS myeloid progenitors. In this case, telomere length should be shortest in memory cells that have been exposed to antigenic stimulation. To assess whether telomere dysfunction is a feature of antigen exposure, naïve and memory populations were compared in cases and controls. sjTREC expression was examined and found, as expected, to reside primarily within the naïve (CD45RA+CD45RO−) not the memory (CD45RO+CD45RA−) population (Fig. 3A)(17) in sorted populations, as shown in Fig. 3C. Using phenotyping, naïve T-cells have been shown to be reduced in MDS (19, 22, 23). Since naïve T-cells are lost through a normal aging process, sjTREC copy number was compared in unstimulated samples from younger and older groups of cases and controls (Fig. 3B). As indicated by sjTREC copies, MDS cases tended to have less naïve cells in the 65+ age group (p=0.01). Although decreased in younger MDS cases compared to younger controls, this difference was not statistically significant (p=0.14, Wilcoxon).
Figure 3. Telomere attrition within naïve T-cells.
(A) Naïve T-cells exclusively express sjTRECs and our data on sorted cell populations confirm that sjTREC DNA is present within the phenotype of naïve (CD45RA+CD45RO−) compared to memory (CD45RO+CD45RA−) T-cells. Using sjTREC expression as an indicator of the number of naïve cells in peripheral blood T-cells, bulk CD3+T-cells from blood of MDS patients were examined. Case-control difference in TRECs were analyzed in groups based on age<65y and ≥65y. The same DNA was used to determine telomere length in Fig 1 and at Day 0 for PD. sjTREC in peripheral CD3+ T-cell was detected using quantitative PCR and GAPDH was used as normalization for cellular DNA content in the sample. (C) Naïve and memory T-cells were isolated by flow sorting to 99% purity using the gating strategy shown. Flow cytometry was conducted after gating on viable cells and gating on CD3+ T-cells. CD45RA-APC and CD45RO-PE were used for the analysis. (D) From MDS patients (n=8, gray box) and age-matched healthy controls (n=8, white box), DNA was extracted for telomere length analysis from sorted (highly purified) naïve and memory populations. Telomere length was detected using qRT-PCR, with 293 T-cell as a calibrator, and the data was analyzed using ∆∆Ct, as described in supplemental methods. Case-control differences were compared using the Wilcoxon signed rank and p-values for the case-control differences are shown at the top of each panel. All tests were two-sided and associations were considered statistically significant at a significance level of p<0.05.
Flow sorting was then used to isolate naïve and memory cells, as shown in Fig. 3C. Purified (sorted) naïve and memory T-cells were compared in a subset of MDS cases (n=8) and healthy controls (n=8) with a sufficient number of cells available for sorting. This subgroup of cases and controls were individually age-matched. In healthy controls, the mean relative telomere length was significantly longer in naïve T-cells compared to memory cells (p=0.006) reflecting their history of proliferative expansion (Fig. 3D)(10). The telomere length in sorted naïve cells was significantly decreased among MDS cases compared to controls (p=0.018), whereas no case-control difference was observed for telomere length measured in memory cells. Thus, telomere length is shortest within the naïve T-cell compartment in MDS patients consistent with an inherent telomere defect. Since it is critical to confirm the naïve phenotype of the sorted MDS T-cells, additional surface markers including CD27, CD28, and CCR7 (24) were used and indeed confirmed that the isolated naïve cells have no evidence of antigen-activation-associated phenotypic changes (data not shown). These results indicate that shorter telomere length in bulk T-cell populations reflects in part a reduction in naïve cells, as well as premature telomere attrition in antigen inexperienced, naïve T-cells indicating that the defect is not dependent on past antigen exposure.
Impaired inducible telomerase enzyme activity in T-cells in MDS patients
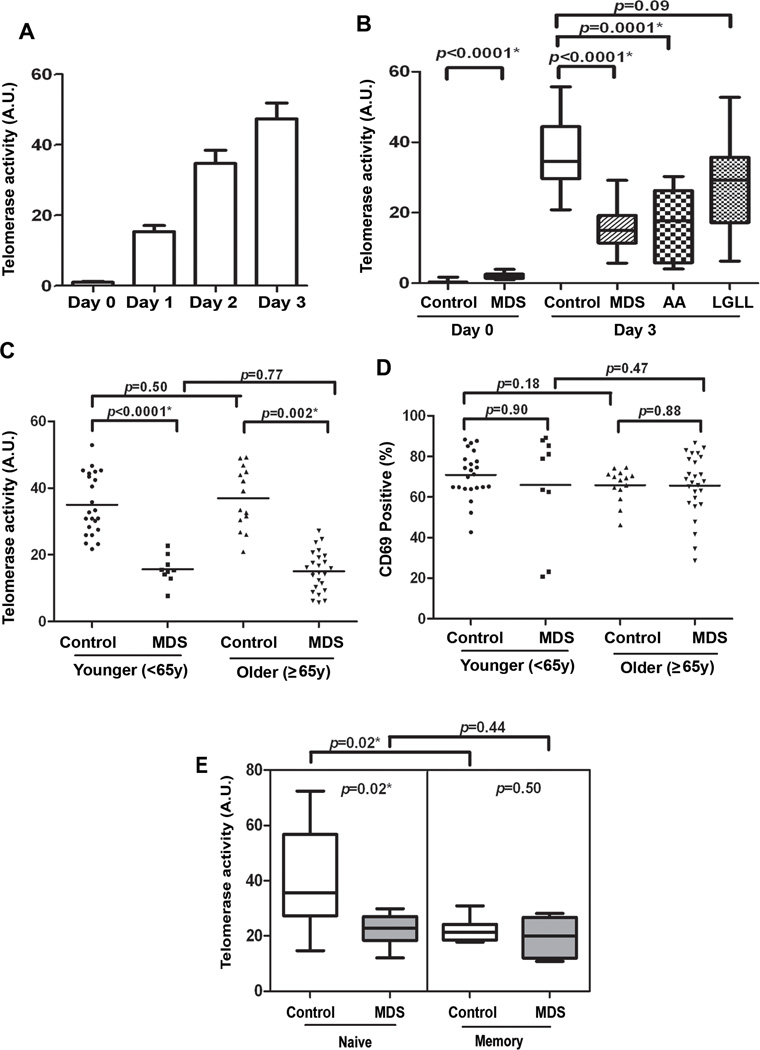
Telomeric repeats may be lost in the naïve compartment if there is a defect in telomere repair as there is an exorbitant demand for proliferation in the naïve compartment after thymic involution (21). In T-cells, telomerase activity is generally absent in resting cells and induced upon TCR activation (21). To assess the activity of telomerase in cases and controls, the TCR was stimulated with anti-CD3/anti-CD28-conjugated beads and the amount of enzyme activity determined by the TRAP assay. Preliminary assays were conducted to confirm the kinetics of telomerase activity and a 3-day stimulation period was found to be optimal (Fig. 4A). Results were compared at day 0 (ie, basal activity) and after 3 days of stimulation (ie, inducible activity) in cases and controls. Basal telomerase activity was significantly higher in MDS cases compared to controls, but the levels were still very low compared to that of stimulated cells (Fig. 4B). After stimulation, the amount of telomerase activity induced in MDS T-cells was significantly less (median=15.0, 95% CI, 13.4–17.2) than healthy controls (median=34.6, 95% CI, 33.6–39.3) (Wilcoxon p<0.0001) after adjustment for age and sex using log-transformed values of telomerase activity (p<0.0001). Aplastic anemia (AA) and MDS are clinically and epidemiologically linked bone marrow failure syndromes (6) and large granular lymphocyte (LGL) leukemia is related to MDS by virtue of an association with cytopenias and clonal T-lymphocyte expansion (11). As shown in supplemental Fig. 3, telomere length in purified T-cells from patients with LGL leukemia was significantly shorter than control. We compared inducible telomerase activity in purified T-cells in LGL leukemia, MDS and aplastic anemia cases and found similar levels of activity in LGL leukemia and controls, as shown in Fig. 4B, but low telomerase activity in both of the highly-related bone marrow failure diseases.
Figure 4. Impaired induction of telomerase activity in CD3+ T-cells in MDS patients.
(A) Preliminary analyses were conducted with CD3+ T-cells from healthy donors to identify the optimal time for telomerase induction using the TRAP assay on day 0, day 1, day 2, and day 3. CD3+ T-cells were separated from peripheral blood by negative selection and unstimulated (day 0) and day 3 stimulated cells were analyzed. Stimulated cells were collated after incubation with anti-CD3/CD28-conjugated beads. (B) The case-control differences in telomerase activity are shown for controls, MDS, aplastic anemia (AA) and large granular lymphocyte leukemia (LGLL). CD3+ T-cells were negatively separated from peripheral blood of MDS patients (n=35) and healthy donor (n=42), AA patients (n=8) and from patients with LGL leukemia (n=17) to test for specificity. (C and D) Telomerase activity (TRAP assays) and CD69 expression were compared in cases and control grouped by age <65y and ≥65y. (D) To determine that the T-cells are generally responsive to stimulation, we examined the surface expression of CD69, which is a well-known early activation-associated antigen (25) by staining with PE-conjugated anti-human-CD69 and analysis by flow cytometry. (E) From MDS patients (n=8, gray box) and age-matched healthy controls (n=8, white box), telomerase activity using TRAP assays were examined in purified sorted populations of naïve and memory cells. The sorting method and telomere length are shown in Figure 3 for these cells. The change in telomerase activity, difference post-pre activation in T-cells is shown. hTERT activity data was normally distributed and case-control differences were compared using a T test. p-values for are shown at the top of each comparison. All tests were two-sided and associations were considered statistically significant at a significance level of p<0.05.
We then compared the telomerase activity in older and younger MDS case and control groups (Fig. 4C) and observed a significant difference in both in cases (p<0.0001 younger group and p=0.002 older group). No difference in telomerase activity was observed in the younger and older controls (p=0.50) indicating that telomerase function is preserved although telomere length shortens with age. To ensure that T-cells from patients were adequately responsive to TCR stimulation, we measured the surface expression of CD69; an inducible early activation antigen (25). In contrast to the impairment in telomerase activity, BrdU incorporation (Fig. 2C), and population doubling (Fig. 2D), the expression of CD69 was similar in younger (p=0.90) and older (p=0.88) cases and controls on day 3 (Fig. 4D) suggesting that the telomerase defect is not due to a generalized loss in TCR signaling responses.
Given the data on telomere length in naïve and memory cells, telomerase activity was examined among these cell populations. Inducible telomerase activity was greatest in control naïve cells and significantly less in memory cells (p=0.02, Fig. 4E). Moreover, the case:control difference in telomerase activity was present in naïve cells (p=02) while the memory T-cell population showed no difference (p=0.50, Fig. 4E) compared to controls indicating that a primary telomerase deficiency underlies telomere loss in the naïve T-cell compartment and that the defect is not related to prior antigen exposure.
hTERT transcriptional deficiency responsible for impaired telomerase function
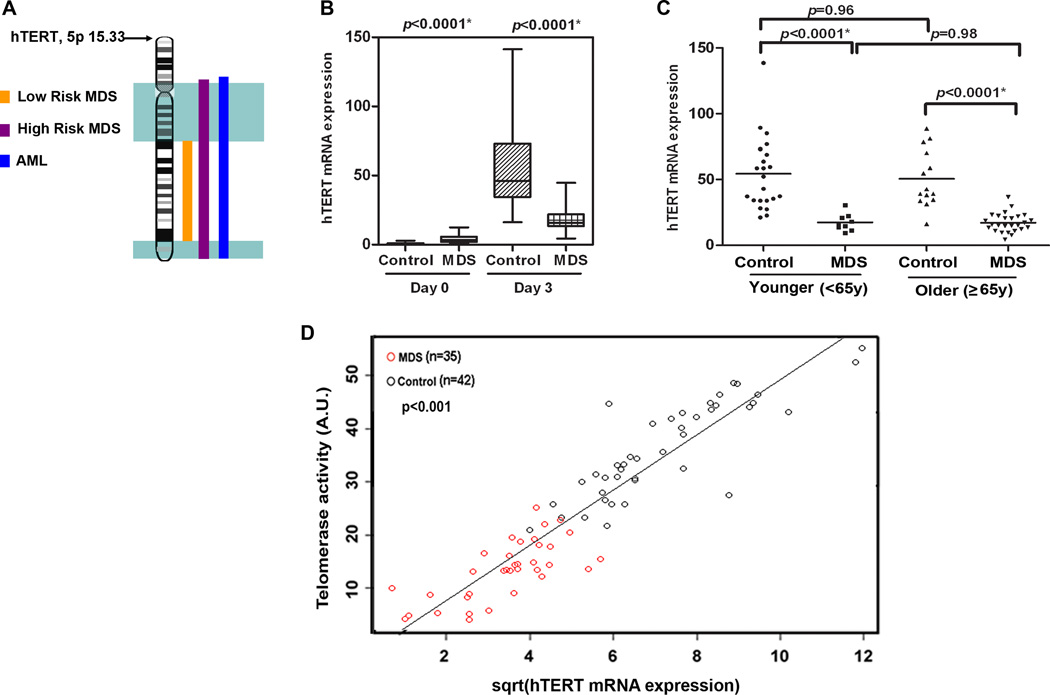
Transcriptional induction of hTERT mRNA represents the rate-limiting step for telomerase regulation (26). Interstitial deletions on chromosome 5 occur in MDS and refined mapping of the region was recently examined using SNP-A. Abnormalities including deletions and uniparental disomy (UPD) were reported to occur in 142 (12%) of 1,155 patients with MDS, MDS/myeloproliferative neoplasms, and AML (27). The hTERT gene maps to chromosome 5p15.33, which is non-overlapping with regions spanning commonly deleted regions on 5q (Fig. 5A). SNP-A was then performed on purified T-cells with data compared to an internal control series (n = 1,003) and the Database of Genomic Variants. No CNV or UPD were detected on chromosome 5 in purified T-cells (data not shown). To assess transcriptional regulation, basal and inducible hTERT mRNA expression was examined on day 0 and 3 after stimulation. As expected in control cells, there is little to no hTERT mRNA without stimulation (Fig. 5B). Basal hTERT mRNA expression was higher in MDS cases (p<0.0001) compared to controls, but the inducible amount was significantly lower in cases (median=15.7, 95% CI, 14.9–20.5 vs. median=46.0, 95% CI, 46.8–64.6 in controls) (p<0.0001) (Fig. 5B) and this case-control difference was independent of age and sex using square-root transformed hTERT data. Comparing the younger and older patient and control subset, the hTERT deficiency was observed across both age groups (p<0.0001 in younger vs. older cases and controls, Fig. 5C).
Figure 5. Telomerase insufficiency linked to defective hTERT transcription.
(A) Region on chromosome 5q that is commonly deleted in low risk (yellow) and high risk (pink) MDS and AML (blue) as well as the position of hTERT on 5p15.33. An aliquot of the purified CD3+ T-cells used in other assays from MDS patients (n=35) and healthy donors (n=42) were used to examine inducible hTERT mRNA expression as quantified by QRT-PCR on day 0 and day 3 after stimulation normalized to 18s ribosomal RNA. (B) Box and whisker plots of the relative mRNA expression on day 0 and day 3 after stimulation are shown. (C) Differences in inducible hTERT expression in younger and older groups of MDS cases and controls based on age <65y and ≥ 65y. (D) Inducible telomerase activity on Y-axis vs inducible hTERT mRNA expression in MDS patients (red circles) and healthy donors (black circles) reveals that these two variables were strongly correlated (r=0.89, p<0.001) by the Spearman rank correlation coefficient. Telomerase activity by the TRAP assay (data shown in Fig. 4) was normally distributed and square-root transformed hTERT mRNA expression was used for these analyses. Results were considered significant when p<0.05. Case-control differences for the hTERT expression in purified T-cells were compared using a Wilcoxon signed rank test with p-values shown at the top of each panel.
The amount of inducible hTERT mRNA expression was then correlated to the level of inducible telomerase activity in T-cells, as shown in Fig. 5D using square-root transformation to normalize hTERT data (r=0.89, p<0.001). The results suggest that there is a mechanistic link between telomerase deficiency and hTERT transcriptional impairment as indicated by the close correlation between these two events.
Sequencing of hTERT promoter
There are three main regions required for induction of hTERT expression consisting of a sequence from −203 to +55, corresponding to the promoter core, an activating region −1397 and −798, and an inhibitory region between −798 and −400. Several transcription factor binding sites are located in these regions, as shown in supplemental Figure 4. No mutations were observed by direct sequencing of cloned hTERT promoter DNA from five MDS patients with 4–5 clones tested per patient.
No correlation between clinical classification and telomere repair
Measurements including telomere length, hTERT mRNA, and inducible telomerase activity were correlated among patients to IPSS score, WHO subtype and cytogenetics, as shown in Table 2. There was no statistically significant association between these telomere variables and disease stratification, although patients with higher-risk (int-2 + high) IPSS classification demonstrated a trend for shorter telomere length (p=0.1), lower induction of telomerase activity (p=0.06), and less hTERT mRNA expression (p=0.12) in T-cells.
Table 2.
Clinical characteristics and telomerase measurements in CD3+ T- cells myelodysplastic syndrome (MDS) cases.
| N=35 | RTL* | hTERT$ | TA# | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | n | % | mean (SD) | p-value | mean (SD) | p-value | mean (SD) | p-value |
| IPSS** score | ||||||||
| Low+int-1 | 21 | 60 | 1.08 (0.10) | 14.42 (1.53) | 14.43 (1.31) | |||
| Int-2+high | 14 | 40 | 1.36 (0.13) | 0.10 | 10.45 (1.97) | 0.12 | 10.96 (1.14) | 0.06 |
| Cytogenetics | ||||||||
| Normal | 15 | 43 | 1.07 (0.08) | 12.72 (2.00) | 12.59 (1.36) | |||
| Abnormal | 20 | 57 | 1.36 (0.12) | 0.07 | 13.38 (1.71) | 0.80 | 13.62 (1.35) | 0.60 |
| WHO## MDS subtype | ||||||||
| MDSU+RARS | 6 | 17 | 1.45 (0.23) | 17.39 (4.95) | 13.05 (2.85) | |||
| RCMD | 15 | 43 | 1.11 (0.09) | 12.58 (1.72) | 13.74 (1.53) | |||
| RAEB1+RAEB2 | 14 | 40 | 1.25 (1.46) | 0.33 | 11.76 (1.62) | 0.31 | 12.55 (1.35) | 0.85 |
RTL=Relative telomere length;
hTERT= Human telomerase reverse transcriptase
TA=Telomerase activity;
IPSS=International Prognostic Scoring System;
WHO=World Health Organization
Proliferation defect is related to telomerase deficiency not telomere length
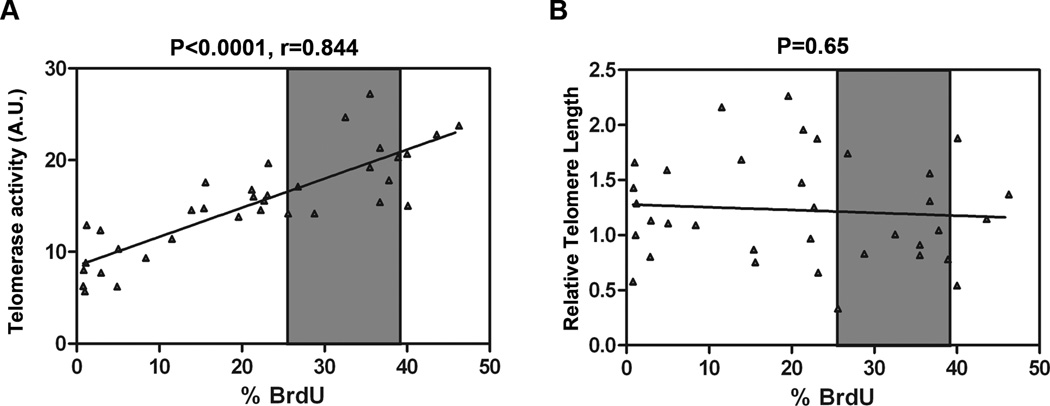
Telomere length must be critically short to force cell cycle arrest. It is possible that telomerase deficiency has important consequences that more broadly impact cell fate as treatment with RNA interference (siRNA) to hTERT resulted in an immediate suppression in replication potential and apoptosis without impacting telomere length8. The proportion of T-cells in MDS patients that underwent S-phase transition (i.e., % BrdU positive) was examined in relationship to telomerase activity and to telomere length. A mechanistic link between replication potential and telomerase activity is suggested by the close correlation (r=0.844, p<0.0001) between BrdU incorporation and telomerase enzymatic activity, but unrelated to telomere length (p=0.65) (Fig. 6 A and B)
Figure 6. Correlation between proliferation defect and telomerase activity.
Telomerase activity and telomere length were significantly reduced in all MDS patients relative to control but some patients retained the ability to undergo S-phase transition. (A) Inducible telomerase activity on Y-axis (as a continuous variable) vs % BrdU in MDS patients reveals that these two variables were strongly correlated (r=0.844, p<0.0001) by the Spearman rank correlation coefficient. (B) BrdU incorporation was not related to telomere length (as a continuous variable) in MDS patients (telomere length Y-axis vs % BrdU on X-axis). Gray shaded area indicates the 95% CI for % BrdU in healthy controls. Results were considered significant when p<0.05.
Discussion
Evidence indicates that accelerated telomere shortening occurs within the myeloid progenitor and stem cell compartments in patients with MDS (16, 28–32), while telomere length is preserved in stromal cells (33), suggesting that the defect is acquired within the hematopoietic compartment. Few studies have directly examined telomerase activity in MDS, and those that have were limited to unstimulated myeloid cells (29, 30). This study is the first to investigate the mechanism for pre-mature telomere attrition in MDS T-cells. For normal telomerase regulation, we show the need for TCR stimulation and demonstrate how this process is disrupted in all MDS patients compared to controls.
Telomerase plays a complex role during tumorigenesis and in the regulation of normal homeostasis. Normal telomerase activity coupled to short telomeres in LGL leukemia and older control individuals suggests that telomere repair is an incomplete restorative process. Replication in MDS T-cells was closely correlated to the activity of telomerase rather than to telomere length indicating that telomerase broadly impacts cell fate. Telomere repair is predominantly functional within naïve cells so the telomerase deficiency selectively affected the naïve compartment and potentially contributed to reduced numbers of naïve T-cells. Oligoclonal memory expansion and limited repertoire diversity are prominent, unexplained features of the disease (23). In order to maintain the total number of T-cells in the periphery, expansion of memory clones may fill the void left by the declining naïve cell compartment. We found evidence of excessive activation-induced cell death in MDS T-cells consistent with abnormalities in this pathway.
Naïve T-cells appear to prematurely reach replicative senescence possibly impacted by age and accelerated proliferation after thymic involution. A telomerase deficiency in naïve T-cells hinders their regeneration which may contribute to the accumulation of senescent cells in MDS. Some MDS patients have increased regulatory T-cells (34, 35), and enhanced liberation of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α)(36, 37), Fas ligand (4, 38), and other proapoptotic cytokines that may impact hematopoiesis and T-cell function (3, 5). Since senescent T-cells remain viable but produce more inflammatory cytokines and demonstrate non-MHC-restricted cytotoxicity through acquired natural-killer receptors (39), the telomere defect in naïve cells may contribute indirectly to the inflammatory cytokine milieu in MDS.
Telomerase activity in MDS correlates with the amount of hTERT mRNA and our results are consistent with a deficiency in hTERT mRNA production. Although interstitial deletions of chromosome 5 occur in this disease (27), UPD and deletions in the region of the hTERT gene on 5p were not detected in T-cells. Signaling events leading to the transcriptional induction of CD69 expression are intact indicating that the hTERT defect is selective. The minimal core promoter (located at −40 to −775) is required for expression and has a NFAT1-binding site flanked by two SP1 binding sites (26), as determined by luciferase and electrophoretic mobility shift assays (EMSA) using serial deletions of the 5’-UTR of the hTERT gene. Mutations in the core promoter region of the 5’-hTERT UTR were not detected in MDS patients (supplemental Fig. 4). Defects in telomere repair have been reported in inflammatory diseases such as rheumatoid arthritis (RA) and the defect is manifest by hTERT transcriptional repression (10, 40). In cancer and normal cells, mediators of hTERT transcription include cAMP responsive element binding protein (CREB), estradiol (E2) estrogen receptor alpha and beta (ERα and ERβ) c-Myc, β-catenin/TCF4, HIF-1, signal transducers and activators of transcription (STAT)-3 and interferon regulatory factor 1 (IRF-1)(41, 42). These transcription factors act downstream of well defined signaling cascades such as Ras, PI3K/Akt, NF-κB, MAP kinase and GSK-3β (41). At this point, It is unclear which pathways are blocked in MDS T-cells. Examination of hTERT deficient T-cells in RA demonstrated that apoptosis induction is dependent on upregulation of DNA damage sensing enzymes DNA-dependent protein kinase (DNA-PKcs), activation of pro-apoptotic BH3-only proteins Bim/Bmf, and activation of the MAPK family member JNK, but was independent of pATM and p53 (43). Increased expression of Bim/Bmf would be expected to induce apoptosis by stimulating the release of pro-apoptotic Bcl-2-family proteins like Bax/Bak resulting in the activation of downstream caspases (44). Pharmacological inhibition of JNK with TLK199, a glutathione analog that inhibits JNK kinase activity, is now under investigation in MDS (45). Stabilization of apoptosis through inhibition of this pathway should be further verified. A strong case can be made for a mechanism involving suppression of hTERT through changes in specific signaling events that limits the cellular of yield of T-cells by inducing cell death.
While multiple congenital and acquired mutations have been reported in hTERT, hTERC, and shelterin proteins (6, 46–48); this is the first report implicating aberrant inducible hTERT transcriptional regulation in MDS or in bone marrow failure. Telomere length in peripheral blood leukocytes from MDS patients was independent of genotype in samples screened for hTERT polymorphisms and mutations in codons 202, 279, 305, 412, 441 and 1062(16). Codons 1062, 279 and 412 polymorphisms were previously shown to be more prevalent in aplastic anemia (16, 47). Generally, myeloid and lymphoid cells in MDS are considered genetically divergent populations with the clonal population arising exclusively within a multipotent myeloid progenitor that gives rise to abnormal granulocytes, megakaryocytes and erythrocytes (49). A telomere defect within both the myeloid and lymphoid populations could be explained if the disease initiating event originates within a pluripotent stem cell with myeloid and lymphoid populating capacity. The origin of MDS within a true stem cell population is a matter of debate (49). Especially in MDS myeloid cells, critically short telomeres may be recognized as DNA damage resulting in the recruitment and activation of the DNA-damage response pathway, (i.e., DNA-PKcs or p53 pathway) stimulating apoptosis and increasing genetic events (50). According to studies in solid tumors and in patients with congenital or acquired mutations in telomere components, individuals with shorter telomeres are at higher risk for developing malignancies due to genomic instability (32).
The current study defines MDS as a member of the telomere repair disorders. Opportunities to improve MDS diagnosis and to design novel therapeutic interventions may be achieved from a better understanding of telomere abnormalities in T-cells and in the myeloid hematopoietic/stem cell compartment contributing to malignant transformation in MDS.
Supplementary Material
Acknowledgements
Funding for this project was provided by NCI R01 grant CA129952. Flow cytometry was supported by the H. Lee Moffitt Cancer Center Flow Cytometry Core Facility and statistical analysis was performed with assistance from Jimmy J. Fulp and Dr. Dung-Tsa Chen from the H. Lee Moffitt Cancer Center Biostatistics Program.
Footnotes
Disclosure of Conflicts of Interest: Authors of this paper have no financial interest to disclose.
References
- 1.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997 Mar 15;89(6):2079–2088. [PubMed] [Google Scholar]
- 2.Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011 Jun 30;364(26):2496–2506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calado RT. Immunologic aspects of hypoplastic myelodysplastic syndrome. Semin Oncol. 2011 Oct;38(5):667–672. doi: 10.1053/j.seminoncol.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lepelley P, Grardel N, Erny O, Iaru T, Obein V, Cosson A, et al. Fas/APO-1 (CD95) expression in myelodysplastic syndromes. Leuk Lymphoma. 1998 Jul;30(3–4):307–312. doi: 10.3109/10428199809057543. [DOI] [PubMed] [Google Scholar]
- 5.Raza A, Alvi S, Borok RZ, Span L, Parcharidou A, Alston D, et al. Excessive proliferation matched by excessive apoptosis in myelodysplastic syndromes: the cause-effect relationship. Leuk Lymphoma. 1997 Sep;27(1–2):111–118. doi: 10.3109/10428199709068277. [DOI] [PubMed] [Google Scholar]
- 6.Young NS. Telomere biology and telomere diseases: implications for practice and research. Hematology Am Soc Hematol Educ Program. 2010;2010:30–35. doi: 10.1182/asheducation-2010.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ju Z, Zhang J, Gao Y, Cheng T. Telomere dysfunction and cell cycle checkpoints in hematopoietic stem cell aging. Int J Hematol. 2011 Jul;94(1):33–43. doi: 10.1007/s12185-011-0882-z. [DOI] [PubMed] [Google Scholar]
- 8.Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008 May 1;111(9):4446–4455. doi: 10.1182/blood-2007-08-019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young NS. Pathophysiologic mechanisms in acquired aplastic anemia. Hematology Am Soc Hematol Educ Program. 2006:72–77. doi: 10.1182/asheducation-2006.1.72. [DOI] [PubMed] [Google Scholar]
- 10.Fujii H, Shao L, Colmegna I, Goronzy JJ, Weyand CM. Telomerase insufficiency in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009 Mar 17;106(11):4360–4365. doi: 10.1073/pnas.0811332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wlodarski MW, Gondek LP, Nearman ZP, Plasilova M, Kalaycio M, Hsi ED, et al. Molecular strategies for detection and quantitation of clonal cytotoxic T-cell responses in aplastic anemia and myelodysplastic syndrome. Blood. 2006 Oct 15;108(8):2632–2641. doi: 10.1182/blood-2005-09-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sloand EM, Kim S, Fuhrer M, Risitano AM, Nakamura R, Maciejewski JP, et al. Fas-mediated apoptosis is important in regulating cell replication and death in trisomy 8 hematopoietic cells but not in cells with other cytogenetic abnormalities. Blood. 2002 Dec 15;100(13):4427–4432. doi: 10.1182/blood-2002-01-0096. [DOI] [PubMed] [Google Scholar]
- 13.Sloand EM, Melenhorst JJ, Tucker ZC, Pfannes L, Brenchley JM, Yong A, et al. T-cell immune responses to Wilms tumor 1 protein in myelodysplasia responsive to immunosuppressive therapy. Blood. 2011 Mar 3;117(9):2691–2699. doi: 10.1182/blood-2010-04-277921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Germing U, Strupp C, Kuendgen A, Isa S, Knipp S, Hildebrandt B, et al. Prospective validation of the WHO proposals for the classification of myelodysplastic syndromes. Haematologica. 2006 Dec;91(12):1596–1604. [PubMed] [Google Scholar]
- 15.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003 Feb 1;361(9355):393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 16.Rollison DE, Epling-Burnette PK, Park JY, Lee JH, Park H, Jonathan K, et al. Telomere length in myelodysplastic syndromes. Leuk Lymphoma. 2011 Jun 3; doi: 10.3109/10428194.2011.568648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McFarland RD, Douek DC, Koup RA, Picker LJ. Identification of a human recent thymic emigrant phenotype. Proc Natl Acad Sci U S A. 2000 Apr 11;97(8):4215–4220. doi: 10.1073/pnas.070061597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson MW, Sverstiuk A, Hendel H, Cheung TW, Zagury JF, Rappaport J. Analysis of telomere length and thymic output in fast and slow/non-progressors with HIV infection. Biomed Pharmacother. 2000 Feb;54(1):21–31. doi: 10.1016/s0753-3322(00)88637-0. [DOI] [PubMed] [Google Scholar]
- 19.McDaniel JM, Zou JX, Fulp W, Chen DT, List AF, Epling-Burnette PK. Reversal of T-cell tolerance in myelodysplastic syndrome through lenalidomide immune modulation. Leukemia. 2012 Jun;26(6):1425–1429. doi: 10.1038/leu.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poulin JF, Viswanathan MN, Harris JM, Komanduri KV, Wieder E, Ringuette N, et al. Direct evidence for thymic function in adult humans. J Exp Med. 1999 Aug 16;190(4):479–486. doi: 10.1084/jem.190.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goronzy JJ, Fujii H, Weyand CM. Telomeres, immune aging and autoimmunity. Exp Gerontol. 2006 Mar;41(3):246–251. doi: 10.1016/j.exger.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Maciejewski JP, Hibbs JR, Anderson S, Katevas P, Young NS. Bone marrow and peripheral blood lymphocyte phenotype in patients with bone marrow failure. Exp Hematol. 1994 Oct;22(11):1102–1110. [PubMed] [Google Scholar]
- 23.Zou JX, Rollison DE, Boulware D, Chen DT, Sloand EM, Pfannes LV, et al. Altered naive and memory CD4+ T-cell homeostasis and immunosenescence characterize younger patients with myelodysplastic syndrome. Leukemia. 2009 Jul;23(7):1288–1296. doi: 10.1038/leu.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell JJ, Murphy KE, Kunkel EJ, Brightling CE, Soler D, Shen Z, et al. CCR7 expression and memory T cell diversity in humans. J Immunol. 2001 Jan 15;166(2):877–884. doi: 10.4049/jimmunol.166.2.877. [DOI] [PubMed] [Google Scholar]
- 25.Amlot PL, Tahami F, Chinn D, Rawlings E. Activation antigen expression on human T cells. I. Analysis by two-colour flow cytometry of umbilical cord blood, adult blood and lymphoid tissue. Clin Exp Immunol. 1996 Jul;105(1):176–182. doi: 10.1046/j.1365-2249.1996.d01-722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chebel A, Rouault JP, Urbanowicz I, Baseggio L, Chien WW, Salles G, et al. Transcriptional activation of hTERT, the human telomerase reverse transcriptase, by nuclear factor of activated T cells. J Biol Chem. 2009 Dec 18;284(51):35725–35734. doi: 10.1074/jbc.M109.009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jerez A, Gondek LP, Jankowska AM, Makishima H, Przychodzen B, Tiu RV, et al. Topography, clinical, and genomic correlates of 5q myeloid malignancies revisited. J Clin Oncol. 2012 Apr 20;30(12):1343–1349. doi: 10.1200/JCO.2011.36.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boultwood J, Fidler C, Kusec R, Rack K, Elliott PJ, Atoyebi O, et al. Telomere length in myelodysplastic syndromes. Am J Hematol. 1997 Dec;56(4):266–271. doi: 10.1002/(sici)1096-8652(199712)56:4<266::aid-ajh12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Counter CM, Gupta J, Harley CB, Leber B, Bacchetti S. Telomerase activity in normal leukocytes and in hematologic malignancies. Blood. 1995 May 1;85(9):2315–2320. [PubMed] [Google Scholar]
- 30.Gurkan E, Tanriverdi K, Baslamisli F. Telomerase activity in myelodysplastic syndromes. Leuk Res. 2005 Oct;29(10):1131–1139. doi: 10.1016/j.leukres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Sashida G, Ohyashiki JH, Nakajima A, Sumi M, Kawakubo K, Tauchi T, et al. Telomere dynamics in myelodysplastic syndrome determined by telomere measurement of marrow metaphases. Clin Cancer Res. 2003 Apr;9(4):1489–1496. [PubMed] [Google Scholar]
- 32.Lange K, Holm L, Vang Nielsen K, Hahn A, Hofmann W, Kreipe H, et al. Telomere shortening and chromosomal instability in myelodysplastic syndromes. Genes Chromosomes Cancer. 2010 Mar;49(3):260–269. doi: 10.1002/gcc.20737. [DOI] [PubMed] [Google Scholar]
- 33.Marcondes AM, Bair S, Rabinovitch PS, Gooley T, Deeg HJ, Risques R. No telomere shortening in marrow stroma from patients with MDS. Ann Hematol. 2009 Jul;88(7):623–628. doi: 10.1007/s00277-008-0649-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kordasti SY, Afzali B, Lim Z, Ingram W, Hayden J, Barber L, Matthews K, et al. IL-17-producing CD4(+) T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br J Haematol. 2009 Apr;145(1):64–72. doi: 10.1111/j.1365-2141.2009.07593.x. [DOI] [PubMed] [Google Scholar]
- 35.Kordasti SY, Ingram W, Hayden J, Darling D, Barber L, Afzali B, et al. CD4+CD25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS) Blood. 2007 Aug 1;110(3):847–850. doi: 10.1182/blood-2007-01-067546. [DOI] [PubMed] [Google Scholar]
- 36.Campioni D, Secchiero P, Corallini F, Melloni E, Capitani S, Lanza F, et al. Evidence for a role of TNF-related apoptosis-inducing ligand (TRAIL) in the anemia of myelodysplastic syndromes. Am J Pathol. 2005 Feb;166(2):557–563. doi: 10.1016/S0002-9440(10)62277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawanobori M, Yamaguchi S, Hasegawa M, Inoue M, Suzuki K, Kamiyama R, et al. Expression of TNF receptors and related signaling molecules in the bone marrow from patients with myelodysplastic syndromes. Leuk Res. 2003 Jul;27(7):583–591. doi: 10.1016/s0145-2126(02)00095-4. [DOI] [PubMed] [Google Scholar]
- 38.Brazil JJ, Gupta P. Constitutive expression of the Fas receptor and its ligand in adult human bone marrow: a regulatory feedback loop for the homeostatic control of hematopoiesis. Blood Cells Mol Dis. 2002 Jul-Aug;29(1):94–103. doi: 10.1006/bcmd.2002.0539. [DOI] [PubMed] [Google Scholar]
- 39.Henel G, Singh K, Cui D, Pryshchep S, Lee WW, Weyand CM, et al. Uncoupling of T-cell effector functions by inhibitory killer immunoglobulin-like receptors. Blood. 2006 Jun 1;107(11):4449–4457. doi: 10.1182/blood-2005-06-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schonland SO, Lopez C, Widmann T, Zimmer J, Bryl E, Goronzy JJ, et al. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc Natl Acad Sci U S A. 2003 Nov 11;100(23):13471–13476. doi: 10.1073/pnas.2233561100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daniel M, Peek GW, Tollefsbol TO. Regulation of the human catalytic subunit of telomerase (hTERT) Gene. 2012 May 1;498(2):135–146. doi: 10.1016/j.gene.2012.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Toh L, Lau P, Wang X. Telomerase reverse transcriptase (TERT) is a novel target of Wnt/beta-catenin pathway in human cancer. J Biol Chem. 2012 Jul 31; doi: 10.1074/jbc.M112.368282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shao L, Goronzy JJ, Weyand CM. DNA-dependent protein kinase catalytic subunit mediates T-cell loss in rheumatoid arthritis. EMBO Mol Med. 2010 Oct;2(10):415–427. doi: 10.1002/emmm.201000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuroda J, Taniwaki M. Involvement of BH3-only proteins in hematologic malignancies. Crit Rev Oncol Hematol. 2009 Aug;71(2):89–101. doi: 10.1016/j.critrevonc.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Gore SD, Hermes-DeSantis ER. Future directions in myelodysplastic syndrome: newer agents and the role of combination approaches. Cancer Control. 2008 Oct;15(Suppl):40–49. doi: 10.1177/107327480801504s05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fibbe WE. Telomerase mutations in aplastic anemia. N Engl J Med. 2005 Apr 7;352(14):1481–1483. doi: 10.1056/NEJMe058015. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005 Apr 7;352(14):1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 48.Ly H, Calado RT, Allard P, Baerlocher GM, Lansdorp PM, Young NS, et al. Functional characterization of telomerase RNA variants found in patients with hematologic disorders. Blood. 2005 Mar 15;105(6):2332–2339. doi: 10.1182/blood-2004-09-3659. [DOI] [PubMed] [Google Scholar]
- 49.Nimer SD. MDS: a stem cell disorder--but what exactly is wrong with the primitive hematopoietic cells in this disease? Hematology Am Soc Hematol Educ Program. 2008:43–51. doi: 10.1182/asheducation-2008.1.43. [DOI] [PubMed] [Google Scholar]
- 50.Briatore F, Barrera G, Pizzimenti S, Toaldo C, Casa CD, Laurora S, et al. Increase of telomerase activity and hTERT expression in myelodysplastic syndromes. Cancer Biol Ther. 2009 May;8(10):883–889. doi: 10.4161/cbt.8.10.8130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.