Abstract
Background
Injecting drug use has historically been the principal driver of the HIV epidemic in the Northeast states of India. However, recent data indicate growing numbers of people who inject drugs (PWID) in North and Central Indian cities.
Methods
We conducted face-to-face surveys among PWID in 7 Northeast and 8 North/Central Indian cities using respondent-driven sampling. We used a rapid HIV testing protocol to identify seropositive individuals and multi-assay algorithm to identify those with recent infection. We used multi-level regression models that incorporated sampling weights and had random intercepts for site to assess risk factors for prevalent and incident (recent) HIV infection.
Results
We surveyed 14,481 PWID from 15 Indian cities between January and December 2013. Participants reported high rates of needle/syringe sharing. The median (site range) estimated HIV prevalence and incidence were 18.1% (5.9, 44.9) and 2.9 per 100 person-years (0, 12.4), respectively. HIV prevalence was higher in Northeast sites while HIV incidence was higher in North/Central sites. The odds of prevalent HIV were over 3-fold higher in women than men. Other factors associated with HIV prevalence or incidence included duration since first injection, injection of pharmaceutical drugs, and needle/syringe sharing.
Conclusions
The burden of HIV infection is high among PWID in India, and may be increasing in cities where injecting drug use is emerging. Women who inject drugs were at substantially higher risk for HIV than men, a situation that may be mediated by dual injection-related and sexual risks.
Keywords: injecting drug use, India, HIV prevalence, HIV incidence, harm reduction, needle and syringe exchange
Introduction
The HIV epidemic in India has been propelled primarily by heterosexual transmission [1, 2]. Indian public health measures targeting female sex workers and high-risk heterosexual populations have yielded meaningful progress in the control of the HIV epidemic [3]. However, in light of the low prevalence of HIV in the general Indian population, the importance of monitoring trends among key populations – notably people who inject drugs (PWID) and men who have sex with men – has been emphasized as an essential need [1, 2].
Estimates of the number of PWID in India vary 5-fold [4, 5] and geographic differences are substantial. Historically, injecting drug use has been the principal driver of the HIV epidemic in Northeast states, where proximity to the `golden triangle’ of heroin production (Myanmar, Thailand and Laos) has fueled much higher rates of injecting drug use than in other areas of the country [6, 7]. Recent reports have drawn attention to increases in drug injecting in North and Central Indian states, with injection of buprenorphine and other pharmaceuticals predominating [8, 9]. High HIV prevalence rates have generally been reported among PWID in Northeast Indian states and in major cities [10–13], however, there are less data available on HIV burden among PWID in the North and Central regions of India. As part of baseline data collection for a cluster-randomized trial (ClinicalTrials.gov Identifier: NCT01686750), we used respondent-driven sampling to collect demographic, behavioral and laboratory data among PWID from 15 cities in India.
Methods
Setting and recruitment
We conducted a cross-sectional survey among PWID from sites in 15 cities (Figure 1), including 7 sites in the Northeast region (Aizawl, Churachandpur, Dimapur, Gangtok, Imphal, Lunglei and Moreh), where injecting drug use is long established, and 8 sites in North and Central India (Amritsar, Bhubaneshwar, Bilaspur, Chandigarh, Kanpur, Ludhiana, Mumbai, and New Delhi), where increases in injecting drug use have variably been reported. At each site, we partnered with non-governmental organizations that provide services to PWID and conducted preliminary ethnographic work. We used respondent-driven sampling to recruit PWID, with the goal of recruiting 1000 participants from each site [14–16]. We initiated recruitment at each site with two or three `seeds’ – individuals identified in the ethnographic phase as well-connected in the PWID communities.
Figure 1.
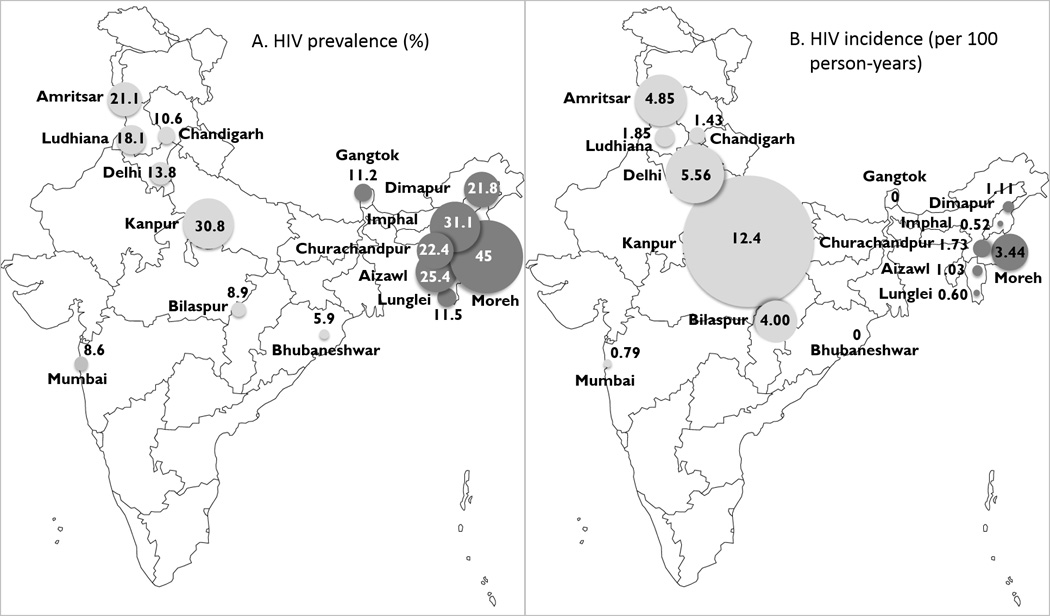
Estimated HIV prevalence (A) and incidence (B) among people who inject drugs recruited from 15 Indian cities. Circle diameters are proportional to site point estimates. Northeast sites are shown in dark grey and North/Central cities are shown in light grey.
Individuals were eligible to participate if they were 1) 18 years or older, 2) reported injecting drug use in the prior 2 years, 3) provided verbal informed consent, and 4) presented a valid recruitment coupon (except for the seeds). Each participant who completed the study was given two coupons to recruit other individuals from his or her network. Participants were reimbursed for participating in the study and for each eligible participant they recruited. Coupons were barcoded to track recruitment chains and imprinted with a holographic image to hinder duplication. We also used a biometric system that converted fingerprint images to unique hexadecimal codes to prevent duplicate enrollment [16].
Procedures
Trained interviewers administered a face-to-face structured survey that captured information on demographic factors, network size, drug (injecting and non-injecting) and alcohol use [17], use of needle and syringe exchange services, use of opioid substitution treatment, sexual behaviors, and history of prior HIV testing. Blood was drawn for HIV testing and processed for additional testing and for storage at the YR Gaitonde Centre for AIDS Research and Education (YRGCARE) laboratory in Chennai, India. Following the survey, participants were offered rapid HIV testing with pre- and post-test counseling. Participants were invited to return to the study site approximately two weeks after the survey to collect reimbursement for successfully recruiting other participants by coupon and to receive results of CD4 cell counts (if HIV-positive). HIV-positive participants were provided with referrals to local HIV treatment services.
Laboratory methods
On-site HIV testing was conducted with a protocol using three rapid HIV testing kits: Alere™ Determine™ HIV-1/2 (Alere Medical Co., Ltd., Chiba, Japan), First Response HIV card test 1–2.0 (PMC Medical India Pvt Ltd, Daman, India), and Signal Flow Through HIV 1+2 Spot/Immunodot Test kit, (Span Diagnostics Ltd, Surat, India). Western blot tests were used to characterize samples with indeterminate results with the three rapid kits. In HIV-positive participants, we measured absolute CD4 cell count with the FlowCARE™ PLG CD4 (CD45-FITC/CD4-PE) assay (Beckman Coulter, Brea, CA, USA) and HIV RNA with RealTime HIV-1 assay (Abbott Laboratories, Abbott Park, Illinois, USA).
Among samples from HIV-positive participants, we characterized recent HIV infection according to a multi-assay algorithm that has been validated for HIV subtype C [18] - the predominant subtype in India [19]. The algorithm included four assays: CD4 cell count, HIV RNA level, Aware™ BED™ EIA HIV-1 Incidence Test (Calypte Biomedical Corporation, Portland, OR, USA), and an avidity modified GS HIV-1/HIV-2 PLUS O EIA kit (Biorad Laboratories, Redmond, CA, USA) using diethyl amine as the chaotropic agent. HIV-positive subjects were considered recently infected if the CD4 count >200 cells/mm3, HIV RNA >400 copies/mL, BED-CEIA <1.0 normalized optical density, and avidity index <80%.
Statistical analysis
We assessed the occurrence of equilibrium during recruitment (the tendency for covariate proportions to stabilize with successive recruitment waves) and homophily (the tendency of participants to recruit individuals with similar characteristics) according to HIV status and other factors [15]. We excluded data from `seed’ participants from analyses. We used the Volz-Heckathorn estimator, which weights estimates for network size (number of PWID in the city whom the participant saw in the prior 30 days), to calculate site-level estimates of demographic, behavioral, and clinical characteristics [20]. HIV prevalence was defined by dividing the number of HIV-positive participants by the total population at each site incorporating Volz-Heckathorn weights. Annualized HIV incidence (I) was estimated at each site using the following equation:
Where w is the number of HIV-positive subjects determined to have recent infection by the multi-assay algorithm, n is the number of HIV-negative subjects, and μ is the window period in years (0.56), which was based on prior optimization for HIV serotype C [18]. Incidence estimates were not weighted.
We compared participant characteristics from the 7 Northeast sites with 8 sites in North and Central India. Estimates for continuous and categorical variables were expressed as the median of site medians and the median site percentage, respectively, with site-level ranges. We identified correlates of prevalent HIV using multi-level logistic regression models and correlates of recent HIV infection using multi-level Poisson regression models, both of which included random-intercepts for each site (to account for clustering) and incorporated sample size-scaled Volz-Heckathorn sampling weights. For the prevalence analysis, we considered primarily lifetime risk factors and for the recent HIV infection analysis we focused on risk behaviors reported in the prior six months. Factors that were associated with HIV prevalence or incidence at p<0.10 in univariate analysis were considered for inclusion in multivariate models. With the exception of age and region, which were included regardless of statistical significance, only those variables associated with the outcome at p<0.05 were retained in the final multivariable models. We tested for interactions between covariates by combining terms in regression models. Additionally, we assessed the sensitivity of our conclusions to the weighting scheme by also fitting unweighted models and models weighted the Salganik-Heckathorn estimator [21]. We used RDS Analyst Software version 0.1 (http://hpmrg.org) and STATA version 12.0 (STATA Corp., College Station, Texas, USA) for analyses.
Ethical oversight
This study was approved by the institutional review boards of YRGCARE in Chennai, India and Johns Hopkins Medicine in Baltimore. Study survey participants provided verbal informed consent.
Results
We conducted surveys among PWID in 15 Indian sites between January and December 2013. A total of 14,481 PWID were recruited - approximately 1000 participants per site (Table 1) - with the exception of Moreh, where we discontinued recruitment after enrolling 459 participants because of civil unrest. A typical respondent-driven sampling recruitment diagram is shown in Figure S1 (supplemental digital content). Across sites, the median (range) time to complete recruitment was 135 days (52, 200) and the median (range) number of recruitment waves was 22, (12, 50) (Table S1, supplemental digital content). In general, homophily values for HIV status were low, meaning that participants were only marginally more likely than chance to recruit someone with the same HIV status as they.
Table 1.
Characteristicsa of people who inject drugs recruited from 15 Indian cites in 2013
| Northeast Cites | North or Central Cities | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AIZ | CCP | DIM | GTK | IMP | LGL | MOR | DEL | MUM | AMR | CDH | LUD | BBE | BIL | KAN | |
| Sample size (including “seeds") | 1002 | 1002 | 1002 | 1003 | 1002 | 1002 | 459 | 1001 | 1001 | 1001 | 998 | 1002 | 1002 | 1002 | 1002 |
| Age, years (median) | 26 | 29 | 30 | 28 | 34 | 24 | 32 | 30 | 30 | 27 | 29 | 27 | 32 | 27 | 34 |
| Male (%) | 81.3 | 77.3 | 85.8 | 93.3 | 87.7 | 87.9 | 76.8 | 100 | 96.5 | 98.8 | 99.6 | 99.8 | 99.9 | 99.5 | 99.3 |
| Education (%) | |||||||||||||||
| Primary school or less | 6.2 | 26.6 | 33.7 | 17.3 | 28.9 | 4.9 | 39.4 | 69.3 | 61.4 | 42.0 | 33.8 | 33.9 | 32.0 | 30.1 | 63.3 |
| Secondary school | 63.0 | 49.7 | 51.3 | 38.7 | 38.2 | 68.9 | 55.6 | 27.6 | 33.0 | 45.1 | 48.3 | 40.2 | 46.6 | 46.7 | 27.5 |
| High school and above | 30.9 | 23.6 | 15.0 | 44.0 | 32.8 | 26.2 | 5.0 | 3.1 | 5.7 | 12.9 | 17.9 | 25.9 | 21.3 | 23.2 | 9.2 |
| Monthly income, Indian rupees (median)b | 2500 | 4000 | 5000 | 7000 | 6000 | 2000 | 6000 | 5000 | 6000 | 4500 | 6000 | 6000 | 6000 | 5000 | 5000 |
| Marital status (%) | |||||||||||||||
| Unmarriedc | 73.0 | 58.7 | 40.5 | 58.9 | 43.8 | 82.3 | 44.3 | 69.4 | 65.4 | 52.2 | 53.0 | 62.7 | 40.1 | 43.1 | 58.6 |
| Currently married or living with partner | 27.0 | 41.3 | 59.5 | 41.1 | 56.2 | 17.8 | 55.7 | 30.6 | 34.6 | 47.8 | 47.0 | 37.4 | 59.9 | 56.9 | 41.4 |
| Age at first injection, years (median) | 18 | 22 | 20 | 18 | 21 | 18 | 25 | 23 | 23 | 21 | 21 | 21 | 23 | 21 | 26 |
| Injection in past 6 months (%) | 92.0 | 98.5 | 68.6 | 83.2 | 98.2 | 91.6 | 88.2 | 97.4 | 89.3 | 87.3 | 83.0 | 80.3 | 91.1 | 95.7 | 99.1 |
| Drugs injected in past 6 months (%) | |||||||||||||||
| Heroin | 57.2 | 97.0 | 23.7 | 11.1 | 98.2 | 2.5 | 87.7 | 45.2 | 88.5 | 35.1 | 3.2 | 4.6 | 19.8 | 0.6 | 14.9 |
| Buprenorphine | 0.1 | 1.2 | 2.5 | 3.9 | 0.3 | 0.3 | 0 | 75.7 | 0.6 | 64.6 | 60.0 | 71.5 | 7.2 | 89.9 | 67.2 |
| Cocaine | 0.4 | 0.2 | 0.5 | 0 | 0 | 0.4 | 0 | 1.9 | 0 | 0.8 | 1.0 | 0.3 | 0.4 | 0.2 | 0.1 |
| Stimulants | 0 | 0 | 0 | 0 | 0.6 | 0 | 0 | 0.7 | 0 | 0.2 | 0.4 | 0 | 0.5 | 0 | 0 |
| Sedatives/anti-anxiety | 1.9 | 0.6 | 23.6 | 23.5 | 0.4 | 1.0 | 0.3 | 20.3 | 0.1 | 1.2 | 9.6 | 17.1 | 0.9 | 0.6 | 3.8 |
| Other pharmaceutical opioidsd | 47.8 | 5.7 | 62.9 | 82.7 | 1.4 | 88.7 | 0.2 | 7.7 | 0.7 | 6.9 | 10.5 | 3.3 | 39.8 | 0.9 | 43.4 |
| Needle or syringe sharing (%) | |||||||||||||||
| Ever | 72.8 | 62.8 | 36.3 | 56.8 | 80.4 | 52.9 | 37.2 | 37.5 | 37.0 | 49.0 | 38.4 | 24.5 | 31.0 | 19.9 | 72.1 |
| Past 6 months | 46.7 | 52.8 | 20.2 | 36.8 | 71.0 | 30.6 | 31.3 | 32.3 | 31.6 | 40.0 | 29.5 | 19.7 | 25.6 | 16.4 | 69.1 |
| Last time injected | 29.6 | 9.3 | 28.6 | 41.6 | 61.2 | 5.3 | 35.8 | 10.8 | 34.9 | 42.3 | 15.0 | 20.1 | 19.1 | 3.0 | 38.1 |
| Alcohol usee (%) | |||||||||||||||
| None/non-hazardous use | 54.5 | 54.2 | 33.2 | 71.0 | 77.5 | 72.0 | 80.8 | 50.7 | 88.5 | 52.5 | 44.5 | 77.8 | 35.8 | 44.9 | 72.6 |
| Hazardous use | 17.8 | 22.8 | 15.8 | 12.5 | 13.0 | 18.4 | 13.2 | 23.9 | 5.5 | 18.6 | 16.4 | 10.6 | 26.3 | 34.5 | 15.8 |
| Dependence | 27.7 | 23.0 | 51.0 | 16.5 | 9.4 | 9.6 | 6.0 | 25.5 | 6.0 | 28.9 | 39.1 | 11.5 | 37.9 | 20.6 | 11.6 |
| Ever sex with a man, male or transgender only (%) | 3.5 | 0.8 | 2.3 | 2.7 | 2.7 | 1.8 | 0.7 | 12.3 | 7.4 | 11.7 | 12.8 | 2.5 | 2.8 | 4.1 | 8.3 |
| Unprotected heterosexual sex in the past 6 months (%) | 57.9 | 35.8 | 55.8 | 54.1 | 40.5 | 36.3 | 49.2 | 26.7 | 24.2 | 51.8 | 37.6 | 36.9 | 63.1 | 62.8 | 33.6 |
| History of HIV testing | |||||||||||||||
| Ever | 71.4 | 44.9 | 52.3 | 34.3 | 58.6 | 51.1 | 57.0 | 29.2 | 61.5 | 57.2 | 40.4 | 40.8 | 82.3 | 24.3 | 7.9 |
| In past 12 monthsf | 42.7 | 20.0 | 31.7 | 12.7 | 16.8 | 29.3 | 32.6 | 22.5 | 38.7 | 44.2 | 30.2 | 28.4 | 76.3 | 17.6 | 4.4 |
| Use needle exchange program | |||||||||||||||
| Ever | 53.0 | 44.5 | 13.4 | 36.3 | 28.0 | 36.1 | 52.9 | 42.1 | 73.8 | 38.9 | 23.7 | 59.2 | 44.2 | 7.7 | 6.8 |
| Past 6 months | 36.2 | 42.1 | 7.6 | 19.9 | 15.1 | 31.0 | 50.5 | 40.4 | 67.8 | 33.8 | 17.7 | 52.8 | 39.6 | 7.2 | 6.3 |
| Use opioid substitution program | |||||||||||||||
| Ever | 23.7 | 10.8 | 11.9 | 6.5 | 33.0 | 6.4 | 12.8 | 27.0 | 48.7 | 43.3 | 23.6 | 18.9 | 25.1 | 0 | 6.4 |
| Past 6 months | 15.9 | 5.1 | 6.9 | 2.2 | 15.3 | 3.6 | 3.9 | 22.1 | 39.8 | 36.8 | 15.5 | 16.4 | 23.3 | 0 | 1.6 |
| Ever incarcerated (%) | 70.2 | 46.1 | 55.4 | 34.6 | 51.9 | 54.2 | 48.8 | 29.2 | 84.5 | 54.7 | 43.6 | 28.4 | 90.0 | 31.9 | 19.7 |
| HIV Prevalence (%) | 25.4 | 22.4 | 21.8 | 11.2 | 31.1 | 11.5 | 44.9 | 13.8 | 8.6 | 21.1 | 10.6 | 18.1 | 5.9 | 8.9 | 30.8 |
| HIV annual incidenceg (%) | 1.0 | 1.7 | 1.1 | 0 | 0.5 | 0.6 | 3.4 | 5.6 | 0.8 | 4.9 | 1.4 | 1.9 | 0 | 4.0 | 12.4 |
AIZ – Aizawl, CCP – Churachandpur, DIM – Dimapur, GTK – Gangtok, IMP – Imphal, LGL – Lunglei, MOR – Moreh, DEL – New Delhi, MUM – Mumbai, AMR – Amritsar, CDH – Chandigarh, LUD - Ludhiana, BBE – Bhubaneshwar, BIL – Bilaspur, KAN – Kanpur
Estimates weighted with the Volz-Heckathorn method (20).
Conversion rate for Indian rupee to US dollar approximately 60:1.
Unmarried includes those never married, not living with long-term partners, widowed, divorced, and separated.
Pharmaceutical opioids include morphine, pentazocine, and propoxyphene.
Hazardous use defined by score ≥ 8 on Alcohol Use Disorder Identification Test (AUDIT) and dependence defined by AUDIT score ≥ 15 (17).
Among subjects not self-reporting HIV-positive status.
See text for methods.
Regional and city variability
Characteristics of PWID in Northeast sites are compared with North/Central sites with weighted site-level estimates (medians and percentages) (Table 2). Female PWID were almost exclusively recruited from Northeast sites. The vast majority of participants across both regions reported injecting in the preceding 6 months. Heroin was the predominant drug injected in the Northeast, whereas buprenorphine was the predominant drug injected in North/Central sites, with minorities injecting other pharmaceuticals or heroin. However, there were exceptions to regional trends in drugs injected. For example, in the Northeast, injection of non-buprenorphine pharmaceutical opioids predominated in the sites at Dimapur, Gangtok, and Lunglei. Additionally, 85% of PWID in the large port city of Mumbai reported heroin injection in the prior 6 months, compared with much lower rates in other North/Central sites. Injection of cocaine or other stimulants was rare across all sites. Needle and syringe sharing was common and generally similar in Northeast and North/Central sites, although there was a wide range across sites (range 19.9% to 80.4% reporting ever sharing needle/syringe).
Table 2.
Characteristicsa of people who inject drugs from cities in the Northeast and North/Central India in 2013
| Northeast citiesb (Median city value [range])d |
North/Central citiesc (Median city value [range])d |
|
|---|---|---|
| Age, years | 29 (24 – 34) | 30 (27 – 34) |
| Sex | ||
| Male | 85.8 (76.8 – 93.3) | 99.5 (96.5 – 100) |
| Female | 14.2 (6.7 – 23.3) | 0.5 (0 – 3.5) |
| Education | ||
| Primary school or less | 26.6 (4.9 – 39.4) | 37.9 (30.1 – 69.3) |
| Middle school | 51.3 (38.2 – 68.9) | 42.7 (27.5 – 48.3) |
| High school graduate or beyond | 26.2 (5.0 – 44.0) | 15.4 (3.1 – 25.9) |
| Monthly income, Indian Rupeese | 3500 (2000 – 7000) | 5500 (5000 – 6000) |
| Marital status | ||
| Unmarriedf | 58.7 (40.5 – 82.3) | 55.8 (40.1 – 69.4) |
| Currently married or living with partner | 41.3 (17.8 – 59.5) | 44.2 (30.6 – 59.9) |
| Age at first injection drug use, years | 20 (18 – 25) | 22 (21 – 26) |
| Injection in past 6 months | 91.5 (68.6 – 98.5) | 90.2 (80.3 – 99.1) |
| Drugs injected in past 6 months | ||
| Heroin | 57.2 (2.5 – 98.2) | 17.3 (0.6 – 88.5) |
| Buprenorphine | 0.3 (0.02 – 3.9) | 65.9 (0.6 – 89.9) |
| Cocaine | 0.2 (0 – 0.5) | 0.3 (0 – 1.9) |
| Stimulants | 0.01 (0 – 0.6) | 0.1 (0 – 0.7) |
| Sedatives/antianxiety | 1.0 (0.3 – 23.6) | 2.5 (0.07 – 20.3) |
| Other pharmaceutical opioidsg | 47.8 (0.2 – 88.7) | 7.3 (0.7 – 43.4) |
| Needle or syringe sharing | ||
| Ever | 56.8 (36.3 – 80.4) | 37.3 (19.9 – 72.1) |
| Past 6 months | 36.8 (20.2 – 71.0) | 30.6 (16.4 – 69.1) |
| Last time injected | 29.6 (5.3 – 61.2) | 19.6 (3.0 – 42.3) |
| Alcohol useh | ||
| None or non-hazardous use | 71.0 (33.2 – 80.8) | 51.6 (35.8 – 88.5) |
| Hazardous use | 15.8 (12.5 – 22.8) | 17.5 (5.5 – 34.5) |
| Dependence | 16.5 (6.0 – 51.0) | 23.0 (6.0 – 39.1) |
| Unprotected heterosexual sex in past 6 months | 49.2 (35.8 – 57.9) | 37.2 (24.2 – 63.1) |
| Ever sex with a man (male or transgender only) | 2.3 (0.7 – 3.5) | 7.8 (2.5 – 12.8) |
| Ever incarcerated | 51.9 (34.6, 70.2) | 37.7 (19.7, 90.0) |
| History of HIV testing | ||
| Ever | 52.3 (34.3 – 71.4) | 40.6 (7.9 – 82.3) |
| In past 12 monthsi | 29.3 (12.7 – 42.7) | 29.3 (4.4 – 76.3) |
| Use needle exchange program | ||
| Ever | 36.3 (13.4 – 53.0) | 40.5 (6.8 – 73.8) |
| Past 6 months | 31.0 (7.6 – 50.5) | 36.7 (6.3 – 67.8) |
| Use opioid substitution program | ||
| Ever | 11.9 (6.4 – 33.0) | 24.4 (0 – 48.7) |
| Past 6 months | 5.1 (2.2 – 15.9) | 19.3 (0 – 39.8) |
| HIV prevalence | 22.3 (11.2 – 44.9) | 12.2 (5.9 – 30.8) |
| HIV annual incidence, per 100 person-yearsj | 1.0 (0 – 3.4) | 2.9 (0–12.4) |
Estimates weighted with the Volz-Heckathorn method (20).
Northeast cities include Aizawl, Churachandpur, Dimapur, Gangtok, Imphal, Lunglei and Moreh (n=6,457).
North/Central cities include Amritsar, Bhubaneshwar, Bilaspur, Chandigarh, Kanpur, Ludhiana, Mumbai, and New Delhi (n=7,993).
Continuous variables shown as the median city median (city range) and categorical variables shown as the median city percentage (city range).
Conversion rate for Indian Rupee to US Dollar approximately 60:1.
Unmarried includes those never married, not living with long-term partners, widowed, divorced, and separated.
Pharmaceutical opioids include morphine, pentazocine, and propoxyphene.
Hazardous use defined by score ≥ 8 on Alcohol Use Disorder Identification Test (AUDIT) and dependence defined by AUDIT score ≥ 15 (17).
Among subjects not self-reporting HIV-positive status.
See text for methods. Incidence estimates are unweighted.
While access to key PWID services was broadly similar in Northeast and North/Central sites, there was striking heterogeneity by site. For example, the percentage of PWID reporting ever being tested for HIV ranged from 7.9% (Kanpur) to 82.3% (Bhubaneshwar). Similarly, no participants in Bilaspur reported ever receiving opioid substitution treatment compared with 48.7% in Mumbai. Trends were similar when considering unweighted estimates (Table S2, supplemental digital content) and estimates weighted using the Salganik-Heckathorn estimator (data not shown).
HIV prevalence
Across the 15 sites, a total of 2,905 participants tested positive for HIV, for a median weighted site-level HIV prevalence of 18.1% (range 5.9%, 44.9%). Site-level estimates of HIV prevalence and incidence are shown in Table 1 and Figure 1. Unweighted site-level prevalence estimates and 95% confidence intervals are shown in Table S3 (supplemental digital content). Compared with the Northeast sites, HIV prevalence was lower in North/Central sites, although not statistically significantly so after adjustment for other factors (Table 3).
Table 3.
Factors associated with prevalent HIV infection among persons who inject drugs in India
| Unadjusted Odds Ratio (95% CI) |
Adjusted Odds Ratio (95% CI) |
|
|---|---|---|
| Age (per 10 year increase) | 1.41 (1.01, 1.96) | 1.02 (0.75, 1.40) |
| Sex | ||
| Male | 1 | 1 |
| Female | 3.19 (1.98, 5.14) | 3.24 (2.22, 4.72) |
| Education | ||
| Primary school or less | 1 | 1 |
| Secondary school | 0.79 (0.67, 0.93) | 0.84 (0.69, 1.03) |
| High school graduate or beyond | 0.55 (0.39, 0.77) | 0.64 (0.49, 0.84) |
| Marital Status | ||
| Unmarrieda | 1 | |
| Currently married or living with partner | 1.13 (0.84, 1.51) | -- |
| Time since first injection (per 10 year increase) | 1.84 (1.44, 2.36) | 1.72 (1.42, 2.07) |
| Drug categories injected (ever) | ||
| Heroin only | 1 | 1 |
| Pharmaceuticals only (including buprenorphine) | 1.64 (0.97, 2.77) | 1.60 (0.98, 2.60) |
| Both heroin and pharmaceuticals | 1.84 (1.24, 2.73) | 1.70 (1.19, 2.42) |
| Ever injected cocaine or stimulants | 1.19 (0.66, 2.15) | -- |
| Ever shared needle or syringe | 3.19 (2.35, 4.34) | 3.15 (2.28, 4.36) |
| Alcohol useb | ||
| None or non-hazardous use | 1 | 1 |
| Hazardous use | 0.57 (0.43, 0.76) | 0.57 (0.43, 0.75) |
| Dependence | 0.50 (0.29, 0.86) | 0.48 (0.32, 0.71) |
| Number of lifetime sex partners (per 5 partner increase) | 1.01 (0.99, 1.02) | -- |
| Ever exchange sex for money or goods | 1.49 (0.99, 2.25) | -- |
| Ever sex with a man (among males only) | 1.03 (0.67, 1.58) | -- |
| Ever used needle exchange programc | 1.47 (1.05, 2.06) | -- |
| Ever used opioid substitution treatment | 1.59 (1.25, 2.01) | -- |
| Ever incarcerated | 2.86 (1.92, 4.26) | 2.35 (1.58, 3.51) |
| Region | ||
| Northeast | 1 | 1 |
| North and central | 0.53 (0.28, 1.00) | 0.75 (0.34, 1.62) |
CI, confidence interval
Unmarried includes those never married, not living with long-term partners, widowed, divorced, and separated.
Hazardous use defined by score ≥ 8 on Alcohol Use Disorder Identification Test (AUDIT) and dependence defined by AUDIT score ≥ 15 (17).
Ever use of needle exchange program did not remain statistically significant after adjustment for other correlates; therefore, it was not included in the multivariate model.
HIV prevalence was substantially higher in women than men. In the Northeast sites, where women accounted for greater than 5% of PWID recruited, HIV prevalence was 53.2% in women compared with 18.2% among men (P<0.01). In a multivariate model that included all sites, women had over 3-fold higher odds of HIV-infection compared with men (Table 3). Higher educational attainment was negatively associated with prevalent HIV infection. Duration since initiation of injecting and history of sharing needles or syringes were also strongly associated with increased odds of prevalent HIV infection. Participants who injected pharmaceuticals or both heroin and pharmaceuticals had increased odds of HIV compared with those that exclusively injected heroin. Hazardous or higher levels of alcohol use were negatively associated with HIV infection. History of incarceration was associated with more than 2-fold increased odds of prevalent HIV. A history of exchanging sex for money or goods had a borderline statistically significant association with higher HIV prevalence in univariate analysis, but not in multivariate analysis. The odds ratios for commercial sex and HIV prevalence were similar in men and women (1.22 and 1.56, respectively). Use of opioid substitution treatment or needle exchange services were associated with increased odds of prevalent HIV in univariate analysis, but not after adjustment for other factors.
HIV incidence
Of 2,905 HIV positive subjects, 155 (5.3%) met laboratory criteria for recent infection. In contrast to HIV prevalence, HIV incidence tended to be higher in North/Central sites compared with Northeast sites (median [range] 2.9 per 100 person-years [0, 12.4] versus 1.0 per 100 person-years [0, 3.4], respectively), although the difference was not statistically significant. The four sites with the highest HIV incidence among PWID were in the North/Central region and one of these sites (Kanpur) had an HIV incidence that was over twice as high as the next highest site (Figure 1).
Considering individual-level factors and risk of recent HIV infection, there was no association between sex and recent HIV infection in univariate analysis (Table 4). However, there was evidence of effect modification between sex and marital/partner status, whereby a married or cohabitating status was associated with a substantially increased risk of recent infection in women, but had a protective or null association in men (P=0.003 and P=0.018 for interaction terms in univariate and multivariate models, respectively). While recent injecting frequency was not significantly associated with the risk of recent HIV infection, using needles/syringes after larger numbers of individuals in the prior 30 days was associated with recent HIV infection in a dose-dependent manner. Compared with reporting no sex partners in the prior 6 months, having 1 or 2 to 3 partners were associated with significantly lower risks of recent HIV infection. Moreover, this protective association between sex partners and recent HIV infection was similar and statistically significant in both men and women (data not shown). The magnitude of the association changed little in the multivariate model, suggesting that having sex partners was not simply a surrogate for less risky injection practices. Similar to associations seen with prevalent HIV infection, injection of both heroin and pharmaceutical drugs was associated with increased risk of recent infection compared with injecting heroin alone and high levels of alcohol use were associated with lower risk of recent HIV infection compared with no or low levels of alcohol use.
Table 4.
Factors associated with recent HIV infectiona among persons who inject drugs in India
| Unadjusted risk ratio (95% CI) |
Adjusted risk ratio (95% CI) |
|
|---|---|---|
| Age (per 10 year increase) | 0.56 (0.32, 0.98) | 0.62 (0.32, 1.21) |
| Marital Status by sexb | ||
| Male | ||
| Unmarried | 1 | 1 |
| Married/living with partner | 0.45 (0.26, 0.78) | 1.08 (0.44, 2.65) |
| Female | ||
| Unmarried | 1 | 1 |
| Married/living with partner | 23.72 (2.30, 244.1) | 26.23 (2.70, 254.5) |
| Education | ||
| Primary school or less | 1 | |
| Secondary school | 0.73 (0.48, 1.10) | -- |
| High school graduate or beyond | 0.76 (0.38, 1.52) | |
| Injection frequency in past 6 months | ||
| None | 1 | |
| Less than daily | 0.95 (0.32, 2.77) | -- |
| Daily | 1.82 (0.58, 5.69) | |
| Drug categories injected (past 6 months) | ||
| Heroin only | 1 | 1 |
| Pharmaceuticals only (including buprenorphine) | 1.39 (0.65, 2.97) | 1.32 (0.65, 2.68) |
| Both heroin and pharmaceuticals | 2.29 (1.10, 4.76) | 2.28 (1.14, 4.57) |
| Number of persons from whom participant accepted a used needle/syringe in past 30 days | ||
| 0 | 1 | 1 |
| 1 | 1.74 (0.92, 3.28) | 1.44 (0.70, 2.98) |
| 2 – 5 | 2.35 (1.05, 5.24) | 2.05 (0.89, 4.72) |
| 6 or more | 3.52 (1.98, 6.27) | 3.19 (2.01, 5.05) |
| Alcohol usec | ||
| None or non-hazardous use | 1 | 1 |
| Hazardous use | 0.47 (0.29, 0.76) | 0.51 (0.35, 0.73) |
| Dependence | 0.60 (0.36, 1.01) | 0.76 (0.50, 1.15) |
| Number of sex partners in past 6 months | ||
| None | 1 | 1 |
| 1 | 0.35 (0.22, 0.56) | 0.34 (0.20, 0.59) |
| 2 – 3 | 0.35 (0.12, 1.01) | 0.32 (0.12, 0.83) |
| 4 or more | 1.04 (0.20, 5.49) | 1.04 (0.21, 5.08) |
| Sex with a man in past 6 months (among males only) | 0.47 (0.11, 2.07) | -- |
| Used needle exchange program past 6 months | 2.06 (0.92, 4.62) | -- |
| Used opioid substitution treatment past 6 months | 0.84 (0.51, 1.37) | -- |
| Incarcerated in past 6 months | 2.07 (0.94, 4.57) | |
| Region | ||
| Northeast | 1 | 1 |
| North and central | 2.03 (0.62, 6.67) | 2.07 (0.61, 6.99) |
CI, confidence interval
See text for methods of characterizing recent HIV infection.
Interaction term between sex and marital status statistically significant in univariate (P=0.003) and multivariate analysis (P=0.018).
Hazardous use defined by score ≥ 8 on Alcohol Use Disorder Identification Test (AUDIT) and dependence defined by AUDIT score ≥ 15 (17).
Discussion
This study - which collected data from 14,481 participants using respondent-driven sampling across sites in 15 Indian cities - is among the largest systematic epidemiological assessments of HIV burden among PWID in any country. We found high levels of lifetime and recent needle and syringe sharing, varying access to risk reduction services and HIV testing, and a high burden of HIV, with a median weighted HIV prevalence of 18.1% across these sites. We were particularly interested in comparing Northeast sites, where there is a longstanding link between injecting drug use and HIV, to North/Central sites, where injecting drug use has emerged in recent years [8, 9]. HIV prevalence rates were higher in the Northeast region compared with the North/Central region. However, estimated HIV incidence rates tended to be higher in North/Central sites compared with Northeast sites, with the four highest incidence rates in North/Central sites, suggesting a growing HIV epidemic among PWID in these regions.
An important caveat to interpreting regional differences is high between-site variability, both across and within regions. Site-level HIV prevalence among PWID ranged from 5.9% to 44.9%, prior HIV testing ranged from 7.9% to 82%, and ever use of opioid substitution treatment ranged from 0% to 48.7%. This heterogeneity challenges the ability to provide meaningful nation-wide estimates of HIV-related factors among PWID. However, our data suggest that the burden of HIV among PWID in India may be higher than previously estimated [1].
PWID in the North/Central site in Kanpur, Uttar Pradesh had an HIV prevalence of 31% and an HIV incidence of 12.4 per 100 person-years, a value over twice as high as any other site. The scale of the HIV epidemic among PWID in Kanpur was surprising, as there were limited data on PWID in this city previously. However, a recent media report indicated that Kanpur had the third largest number of registered cases for drug-trafficking in 2012, following Mumbai and Delhi, supporting our finding of high HIV burden in this medium-sized city [22]. Access to needle/syringe exchange and opioid substitution treatment were extremely low in Kanpur, and the rate of HIV testing was the lowest of all sites surveyed.
Participant-level correlates of HIV infection in our study were consistent with prior work [10, 12] and included duration since first injection and lifetime history of sharing needles or syringes. Correspondingly, in the analysis of factors associated with recent HIV infection we found that using needles/syringes after larger numbers of people in the previous 6 months was associated with recent HIV infection in a dose-response relationship, underscoring the importance of curtailing needle/syringe sharing in the public health approach to HIV prevention among PWID. Lifetime history of incarceration was also strongly associated with higher HIV prevalence, a risk factor which has been reported by other groups [23, 24].
We found that female PWID, who were nearly exclusively encountered in the Northeast, had a greater than 3-fold higher odds of HIV than male PWID, supporting the findings of Mahanta and coworkers [10]. Preliminary ethnographic work by our team in the Northeast revealed that female PWID are rejected by their families to a greater degree than male PWID, which may contribute to the vulnerability of these women. Although we did not find women to be at statistically significantly higher risk for recent HIV infection than men, we found evidence of effect modification, whereby having a spouse or domestic partner was associated with significantly increased risk of recent infection in women but not in men, suggesting that transmission of HIV from a partner (either sexually or via injection behaviors) is a greater risk to female than to male PWID. Previous work has highlighted the high risk of sexual HIV transmission from men who inject drugs to their wives [24, 25]. Moreover, in a separate analysis from this study population, we found that female PWID had a significantly lower prevalence of hepatitis C infection compared with men (adjusted odds ratio 0.32; 95% CI 0.12, 0.83), suggesting that the higher prevalence of HIV infection among women is not explained by more risky injecting practices [26]. However, we did not find strong evidence that self-reported data on sexual HIV risk behaviors (e.g. history of commercial sex work or number of recent sex partners) explained the observed HIV prevalence difference in male and female PWID.
Unexpectedly, we found that having up to three sex partners in the prior 6 months was associated with a lower risk of recent HIV infection compared with having no recent sex partners - a finding that was similar and statistically significant in both men and women. Opioid addiction is consistently linked to hypogonadism and decreased libido [27]. So recent sex partners may correlate with less intense or risky drug use, although we could find no clear indicator of such an association in the data. In both the analyses of HIV infection overall and of recent HIV infection, hazardous levels of alcohol use had a protective association with HIV infection. Higher levels of alcohol use may be a marker of less frequent or less risky injecting behavior. In a longitudinal study of PWID in Chennai, we found that cessation of injecting was associated with increases in hazardous levels of alcohol use [28].
Our study has notable strengths. First, we used respondent-driven sampling, a strategy that is suited for `hidden’ populations and permits weighting to produce unbiased estimates of factors of interest in the target population. Second, we systematically surveyed PWID from sites in 15 Indian cities over the course of one year, which facilitated cross-site comparisons that were not confounded by methodological differences or temporal trends. Third, we recruited approximately 1000 PWID from each site, which provided precise estimates of factors of interest, even after accounting for the design effect of respondent-driven sampling compared with simple random sampling. Finally, our central reference laboratory used state-of-the-art methods to characterize recent HIV infection across sites, permitting HIV incidence estimates.
A limitation of our study was that we did not randomly select sites and regions for sampling and, consequently, our data should not be considered nationally representative. Notably, we did not survey Southern cities, although our group has conducted epidemiologic work among PWID in Chennai previously [12, 28]. Additionally, we selected sites where we believed recruiting 1000 PWID would be feasible. A second limitation, is that regional differences in HIV recombinant forms may have affected incidence estimates derived from our multi-assay algorithm. HIV-1 subtype C predominates in India, but recombinant forms, which are rare overall, are more common in the Northeast [19, 29]. However, most Indian recombinants described to date [29] have had a subtype C- or A-derived envelope gene, which have identical performance characteristics in the multi-assay algorithm we used [18]. Finally, we did not collect detailed network-level risk data.
In this respondent-driven sampling survey of 14,481 PWID from 15 Indian cities, we found high rates of lifetime and recent needle and syringe sharing and an HIV prevalence above 10% in all but 3 sites. HIV prevalence tended to be lower, but HIV incidence higher, in North/Central sites, where injecting drug use has emerged more recently than in the Northeast, where injecting drug use is endemic. These data suggest that increases in injecting drug use outside of the Northeast pose an emerging challenge to successful HIV control efforts in India. The finding that injecting pharmaceutical agents (with or without heroin) was associated with higher HIV risk compared with injecting heroin alone, may suggest new public health approaches focusing on injected pharmaceuticals. Although rare outside of the Northeast, women who inject drugs were at substantially higher risk for HIV than men. Risk reduction strategies targeting women who inject drugs are needed.
Supplementary Material
Acknowledgements
This project was funded by the National Institute on Drug Abuse (R01 DA032059, K24 DA035684). Other author support was provided by the Johns Hopkins Center for AIDS Research (P30 AI094189), the National Institute of Mental Health (R01 MH 89266), and by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases. The authors want to acknowledge the support and contributions of our partnering non-governmental organizations and to thank the participants for their contributions to the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author contributions: G.M.L, S.S.S, A.K.S., D.D.C., and S.H.M. designed and obtained funding for the study. S.S.S, A.K.S., A.A., S.I., M.S.K. and S.S. implemented study procedures and contributed expertise on the Indian HIV epidemic among people who inject drugs. S.I. and O.L. contributed laboratory expertise to the study. A.M.M., E.O., and S.H.M analyzed data for the study or provided input on statistical analyses. G.M.L. drafted the manuscript. All authors reviewed and approved the final manuscript.
References
- 1.National AIDS Control Organisation. Department of AIDS Control, Ministry of Health & Family Welfare, Government of India. Annual Report 2012–13. 2013 Available at http://www.naco.gov.in.
- 2.Chandrasekaran P, Dallabetta G, Loo V, Rao S, Gayle H, Alexander A. Containing HIV/AIDS in India: the unfinished agenda. Lancet Infect Dis. 2006;6:508–521. doi: 10.1016/S1473-3099(06)70551-5. [DOI] [PubMed] [Google Scholar]
- 3.Arora P, Kumar R, Bhattacharya M, Nagelkerke NJ, Jha P. Trends in HIV incidence in India from 2000 to 2007. Lancet. 2008;372:289–290. doi: 10.1016/S0140-6736(08)61105-8. [DOI] [PubMed] [Google Scholar]
- 4.Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372:1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 5.Aceijas C, Friedman SR, Cooper HL, Wiessing L, Stimson GV, Hickman M. Estimates of injecting drug users at the national and local level in developing and transitional countries, and gender and age distribution. Sex Transm. Infect. 2006;82(Suppl 3):iii10–iii17. doi: 10.1136/sti.2005.019471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorabjee J, Samson L. A multi-centre rapid assessment of injecting drug use in India. Int J Drug Policy. 2000;11:99–112. doi: 10.1016/s0955-3959(99)00058-4. [DOI] [PubMed] [Google Scholar]
- 7.Medhi GK, Mahanta J, Akoijam BS, Adhikary R. Size estimation of injecting drug users (IDU) using multiplier method in five districts of India. Subst Abuse Treat Prev Policy. 2012;7:9. doi: 10.1186/1747-597X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambekar A, Tripathi BM. Size estimation of injecting drug use in Punjab & Haryana. UNAIDS. 2008 Available at http://saathii.org/saathiiorc/index.php?option=com_docman&task=doc_download&gid=175&Itemid=5.
- 9.Yardley J. Indian State Finds Itself in Tight Grip of Addiction. [accessed 6/24/2014];The New York Times, New York. 2012 available at http://www.nytimes.com/2012/04/19/world/asia/drug-addiction-is-a-growing-problem-in-punjab.html?pagewanted=all&_r=0. [Google Scholar]
- 10.Mahanta J, Borkakoty B, Das HK, Chelleng PK. The risk of HIV and HCV infections among injection drug users in northeast India. AIDS Care. 2009;21:1420–1424. doi: 10.1080/09540120902862584. [DOI] [PubMed] [Google Scholar]
- 11.Mahanta J, Medhi GK, Paranjape RS, Roy N, Kohli A, Akoijam BS, et al. Injecting and sexual risk behaviours, sexually transmitted infections and HIV prevalence in injecting drug users in three states in India. AIDS. 2008;22(Suppl 5):S59–S68. doi: 10.1097/01.aids.0000343764.62455.9e. [DOI] [PubMed] [Google Scholar]
- 12.Solomon SS, Srikrishnan AK, Mehta SH, Vasudevan CK, Murugavel KG, Thamburaj E, et al. High prevalence of HIV, HIV/hepatitis C virus coinfection, and risk behaviors among injection drug users in Chennai, India: a cause for concern. J Acquir. Immune. Defic. Syndr. 2008;49:327–332. doi: 10.1097/QAI.0b013e3181831e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baveja UK, Chattopadhya D, Khera R, Joshi PM. A cross sectional serological study of the co-infection of hepatitis B virus, hepatitis C virus and human immunodeficiency virus amongst a cohort of IDUs at Delhi. Indian J Med Microbiol. 2003;21:280–283. [PubMed] [Google Scholar]
- 14.Abdul-Quader AS, Heckathorn DD, McKnight C, Bramson H, Nemeth C, Sabin K, et al. Effectiveness of respondent-driven sampling for recruiting drug users in New York City: findings from a pilot study. J. Urban. Health. 2006;83:459–476. doi: 10.1007/s11524-006-9052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heckathorn DD. Respondent-driven sampling II: Deriving valid population estimates from chain-referral samples of hidden populations. Social Problems. 2002;49:11–34. [Google Scholar]
- 16.Solomon SS, Lucas GM, Celentano DD, Sifakis F, Mehta SH. Beyond Surveillance: A Role for Respondent-driven Sampling in Implementation Science. Am J Epidemiol. 2013;178:260–267. doi: 10.1093/aje/kws432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saunders JB, Aasland OG, Babor TF, de lF, Jr, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 18.Laeyendecker O, Kulich M, Donnell D, Komarek A, Omelka M, Mullis CE, et al. Development of Methods for Cross-Sectional HIV Incidence Estimation in a Large, Community Randomized Trial. PLoS One. 2013;8:e78818. doi: 10.1371/journal.pone.0078818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemelaar J, Gouws E, Ghys PD, Osmanov S. Isolation W-UNfH, Characterisation. Global trends in molecular epidemiology of HIV-1 during 2000–2007. AIDS. 2011;25:679–689. doi: 10.1097/QAD.0b013e328342ff93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volz E, Heckathorn DD. Probability Based Estimation Theory for Respondent Driven Sampling. Journal of Official Statistics. 2008;24:79–97. [Google Scholar]
- 21.Salganik MJ, Heckathorn DD. Sampling and estimation in hidden populations using respondent-driven sampling. Sociological Methodology. 2004;2004;3434:193–239. [Google Scholar]
- 22.Delhi, Mumbai at the top of the list of Indian cities active in drug trade. [accessed July 2014];Times of India. 2014 Jun 22; Available at http://timesofindia.indiatimes.com/india/drug-trade/articleshow/37028283.cms?intenttarget=nos. [Google Scholar]
- 23.Beyrer C, Malinowska-Sempruch K, Kamarulzaman A, Kazatchkine M, Sidibe M, Strathdee SA. Time to act: a call for comprehensive responses to HIV in people who use drugs. Lancet. 2010;376:551–563. doi: 10.1016/S0140-6736(10)60928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panda S, Kumar MS, Lokabiraman S, Jayashree K, Satagopan MC, Solomon S, et al. Risk factors for HIV infection in injection drug users and evidence for onward transmission of HIV to their sexual partners in Chennai, India. J Acquir. Immune. Defic. Syndr. 2005;39:9–15. doi: 10.1097/01.qai.0000160713.94203.9b. [DOI] [PubMed] [Google Scholar]
- 25.Solomon SS, Mehta SH, Latimore A, Srikrishnan AK, Celentano DD. The impact of HIV and high-risk behaviours on the wives of married men who have sex with men and injection drug users: implications for HIV prevention. J Int AIDS Soc. 2010;13(Suppl 2):S7. doi: 10.1186/1758-2652-13-S2-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon SS, Mehta SH, Srikrishnan AK, Solomon S, McFall AM, Laeyendecker O, et al. Burden of hepatitis C virus disease and access to hepatitis C virus services in people who inject drugs in India: a cross-sectional study. Lancet Infect Dis. 2015;15:36–45. doi: 10.1016/S1473-3099(14)71045-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vuong C, Van Uum SH, O'Dell LE, Lutfy K, Friedman TC. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev. 2010;31:98–132. doi: 10.1210/er.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta SH, Sudarshi D, Srikrishnan AK, Celentano DD, Vasudevan CK, Anand S, et al. Factors associated with injection cessation, relapse and initiation in a community-based cohort of injection drug users in Chennai, India. Addiction. 2012;107:349–358. doi: 10.1111/j.1360-0443.2011.03602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neogi U, Bontell I, Shet A, De Costa A, Gupta S, Diwan V, et al. Molecular epidemiology of HIV-1 subtypes in India: origin and evolutionary history of the predominant subtype C. PLoS One. 2012;7:e39819. doi: 10.1371/journal.pone.0039819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


