Abstract
Pluripotent human embryonic stem cells (hESCs) acquire mesenchymal characteristics during the epithelial-mesenchymal transition (EMT) process. Here, we report a simple and an efficient isolation method for mesenchymal stem cells (MSCs) from hESCs undergoing EMT using a commercialized porous membrane transwell culture insert. Suspension culture of hESC colonies results in the formation of embryoid bodies, which adhered on the upper compartment of 8 μm porous membrane in the presence of EMG2-MV media. The population migrating through the permeable membrane to the lower compartment not only exhibited EMT markers but also expressed high levels of a panel of typical MSC surface antigen markers, and demonstrated multipotent differentiation capability. In addition, they have a prolonged proliferation capacity without characteristics and chromosomal changes. Furthermore, the isolated MSCs significantly enhanced cardiac functions in a rat model of myocardial infarction (MI) as measured by the left ventricle wall thickness (MI control, 32.9%±3.2% vs. hESCs-MSCs, 38.7%±2.4%), scar length (MI control, 46.1%±2.5% vs. hESCs-MSCs, 41.8%±1.3%), fibrosis area (MI control, 34.3%±1.6% vs. hESCs-MSCs, 28.9%±3.5%), and capillary density. Our findings demonstrate an ease with which hESCs-MSCs can be effectively isolated using the porous membrane, which overcomes the lack of availability of MSCs for therapeutic applications in various diseased animal models.
Introduction
Clinical applications of mesenchymal stem cells (MSCs) derived from various sources have proved to be safe, and they contribute to functional recoveries in a number of diseases and medical conditions.1 MSCs are typically characterized by the expression of multiple surface antigens in the light of CD105, CD73, CD166, HLA Class I, CD44, CD 146, and CD90; whereas antigens of the hematopoietic lineage (CD45, CD34, CD14, CD31, CD19, and HLA-DR) are not found in MSCs.2 In addition, multipotent MSCs are capable of differentiating into cells of mesenchyme lineage such as adipocytes, chondrocytes, and osteocytes.3 MSCs were first isolated from bone marrow but other sources such as adipose tissue, cord blood, and placenta have been known to harbor MSCs.4,5 Despite a multiple source of MSCs, their isolation procedures are often invasive and exhibit a limited proliferative capacity, which pose major hurdles for wider clinical applications of MSCs.
Human embryonic stem cells (hESCs) have been considered an alternative cellular source of MSCs.6,7 Pluripotent hESCs differentiate into almost all types of cells in the body, and with a capacity for an unlimited self-renewal, hESCs are an attractive cellular source in the field of regenerative cell therapy.8,9 hESCs undergo epithelium-mesenchyme transition (EMT) to adapt mesenchymal characteristics either in the presence of growth factors or during spontaneous differentiation.10,11 In recent years, protocols for generating MSCs-like cells from hESCs have been developed. These include the selection of spontaneously differentiated progeny of hESCs, and induce them to differentiate in the presence of various growth factors,12 co-culture with mouse-derived stromal cells (OP9 cells), and monolayer differentiation in the presence of commercialized differentiation media,13 However, these protocols are either time consuming (>30 days) or involve complicated and labor-intensive sorting procedures.14
In this study, we developed a simple induction and efficient purification procedure for MSC populations derived from hESCs using commercialized transwell cell culture inserts. The inserts consisted of a cell-permeable membrane with 8 μm pores, which is a widely used tool for invasion and migration assay of various cell types.12
Materials and Methods
hESC culture
Undifferentiated hESC line H9 was cultured according to protocols from WiCell Research Institute. As previously reported,15,16 hESCs cell line H9 was cultured on mouse embryonic fibroblasts feeder layers in DMEM/F-12 medium supplemented with 20% knockout serum replacement, 1 mM glutamine, 0.1 mM β-mercaptoethanol, 0.1 mM nonessential amino acids, and 4 ng/mL human recombinant bFGF (all supplements were purchased from Invitrogen Corporation) at 37°C in 5% CO2 and 95% humidity.
Isolation of hESC-derived MSCs using a porous membrane and their subsequent in vitro expansion
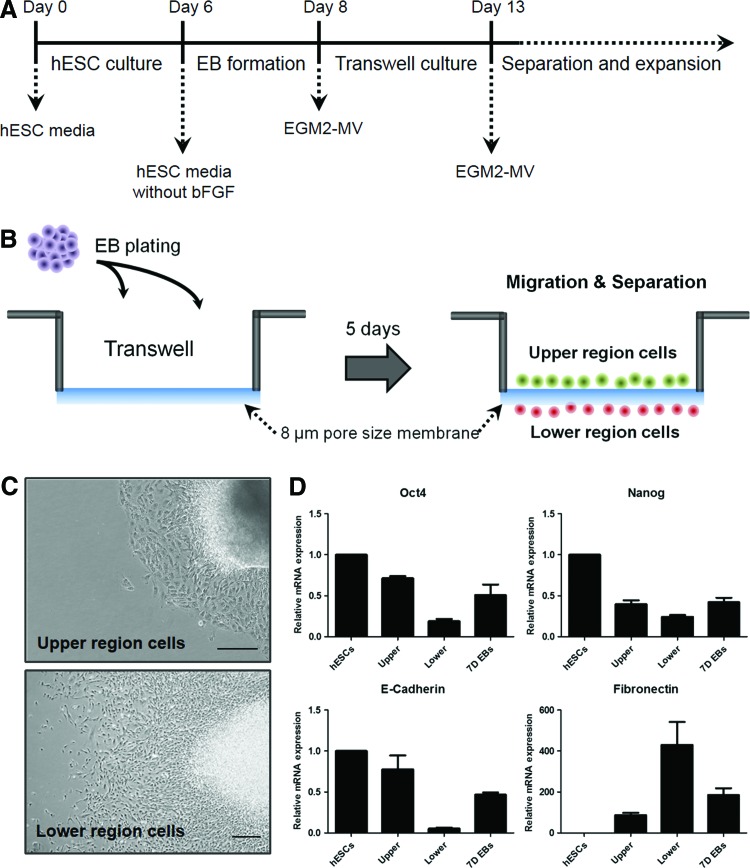
For embryoid body (EB) formation, hESC colonies were removed from the feeder layers by dispase treatment (1 mg/mL in serum-containing medium; Roche). The harvested hESC colonies were grown in suspension culture for 2 days with the same hESC culture medium except bFGF. The porous membrane transwell inserts with 8 μm pores were used to isolate MSC-like cells. The upper compartment of the inserts was coated with 0.1% gelatin (manufacturer), and EBs were attached in EGM2-MV (Lonza) for 5 days. The migrated cells to the lower compartment of the inserts formed colonies, which were gently scraped and subcultured onto a new 60 mm dish in the same media. The isolated MSCs were maintained in EMG2-MV according to the general method,3 and were passaged for approximately 20 times in vitro (Fig. 1A).
FIG. 1.
Isolation of hESC-MSCs by porous membrane. (A) Experimental scheme for porous membrane-based differentiation. (B) Schematic diagram depicting the use of a porous membrane for hESC-MSCs isolation. hESCs were differentiated in EGM2-MV for 5 days. (C) Morphology of cells in the upper and lower compartments of the transwell; images were taken by an inverted microscope. Scale bar, 500 μm. (D) Quantitative real-time polymerase chain reaction analysis of pluripotency and EMT-related gene markers in undifferentiated hESCs, cells in the upper and lower compartments and D7 EBs. Results are shown as mean±standard deviations. EBs, embryoid bodies; EMT, epithelial-mesenchymal transition; hESC, human embryonic stem cell; MSCs, mesenchymal stem cells. Color images available online at www.liebertpub.com/tec
Quantitative real-time polymerase chain reaction
For quantitative real-time polymerase chain reaction (qRT-PCR) analysis, the following samples were harvested using Tryp LE (Gibco). Total RNA of each sample was extracted using TRIzol reagent (Invitrogen) according to previous reports,17 and 3 μg of total RNA was transcribed into cDNA using Super Script III reverse transcriptase (Invitrogen) and oligo d(T) (Invitrogen). Using SYBR Green PCR master mix (Invitrogen), 1 μL of the synthesized first strand cDNA was added to the master mix with an equal volume of primer pairs (Macrogen) in a total reaction volume of 20 μL. The reaction was performed using the ABI 7300 qRT-PCR system (Applied Biosystems). All measurements were taken in triplicate and normalized to levels of GAPDH (an endogenous housekeeping gene), and relative gene expression was calculated using delta–delta CT method. The list of primers used in this experiment is shown in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/tec).
Flow cytometry
For flow cytometry analysis, the cells were dissociated by incubating with Tryp LE for 40 s at 37°C, and were washed with phosphate-buffered saline (PBS) containing 2% fetal bovine serum (FBS; Gibco). 1.5×105 cells were then incubated with each of the following conjugated monoclonal antibodies: CD90-PE, CD73-PE, CD105-PE, CD44-PE, HLA-ABC-APC, HLA-DR-PE, PDGFR-α-PE, PDGFR-β-PE, CD45-FITC, CD133-PE, SMA-PE, KDR-PE, Tie-2-PE, VE-CAD-PE, PECAM-1-PE, N-CAM-FITC, SSEA4-PE, and CD34-APC (R&D) for 30 min at room temperature. After incubation, cells were washed and resuspended in PBS. Data were analyzed with the FACS Calibur cytometer and Cell Quest software (Becton Dickinson).
Multipotent differentiation of the isolated cells
Adipogenic differentiation and cytochemical staining
To induce adipogenic differentiation, fifth- to seventh-passage cells were treated with adipogenic medium (IMDM supplemented with 0.5 mM 3-isobutyl-1-methylxanthine [IBMX; Sigma-Aldrich], 1 M hydrocortisone [Sigma-Aldrich], 0.1 mM indomethacin [Sigma-Aldrich], and 10% FBS). For oil-red O staining, cells were fixed with 4% formaldehyde, stained with oil-red O (Sigma-Aldrich) for 10 min, and then counterstained with Mayer hematoxylin (Sigma-Aldrich) for 1 min.
Osteogenic differentiation and cytochemical staining
To induce osteogenic differentiation, cells were treated with osteogenic medium (IMDM supplemented with 0.1 μM dexamethasone [Sigma-Aldrich], 10 mM glycerol phosphate [Sigma-Aldrich], and 0.2 mM ascorbic acid [AsA; Sigma-Aldrich]) for 3 weeks. For evaluation, differentiated cells were fixed with 4% formaldehyde and stained with 1% Alizarin-red S (Sigma-Aldrich) solution in water for 10 min. In addition, cells were also evaluated by von Kossa staining using 1% silver nitrate (Sigma-Aldrich) under UV light for 45 min, followed by 3% sodium thiosulfate (Sigma-Aldrich) for 5 min, and then counterstained with van Gieson (Sigma-Aldrich) for 5 min.
Chondrogenic differentiation and histological analysis
To induce chondrogenic differentiation, cells were treated with high-glucose DMEM (Invitrogen) supplemented with 0.1 μM dexamethasone, 50 μg/mL AsA, 10 ng/mL TGF-β1 (R&D), and 50 mg/mL ITS premix (Becton Dickinson). Chondrogenic differentiation was evaluated after pellets were fixed in 4% formaldehyde, dehydrated in serial ethanol dilutions, and embedded in paraffin blocks. The blocks were cut, and sections were stained with Alcian blue (Sigma-Aldrich).
Myogenic differentiation
The isolated MSCs were cultured in alpha minimum essential medium (MEM) (Invitrogen) with 20% FBS for 2 weeks. Differentiated myocytes were stained using Vimentin (Millipore), smooth muscle actin (SMA; Abcam), and alpha-actin (Abcam), TuJ1 (Abcam), Brachyury (Abcam), AFP (Abcam). The images were visualized using an LSM 510 META confocal microscope (Carl Zeiss, Inc.) after counter staining with 2 μg/mL DAPI (Sigma-Aldrich).
Myocardial infarction and cell transplantation
All animal experiments were approved by the CHA University Institutional Animal Care and Use Committee (IACUC # 120005) and were performed under the guidance of the National Research Council's Guidelines for the Care and Use of Laboratory Animals. Six-week-old male athymic nude mice (body weight 20–25 g; Orient Bio, Inc.) were anesthetized with rompun (20 mg/kg) and ketamine (100 mg/kg). Myocardial infarction (MI) was induced by ligation of the left coronary artery. Immediately after the left coronary artery occlusion, the isolated MSCs and PBS (sham control) were injected into the border zone surrounding the infarct (two injections of 1×105 cells, n=6).
Infarct size and microvessel density measurement
As previously reported,18 the heart sections were stained with Masson's trichrome, and the images were digitized using Image J software (NIH Image, National Institutes of Health). The infarct size was measured by calculating three variables (fibrosis area, scar length, and wall thickness), which were expressed as percentages. To measure microvessel density, the tissue sections were stained using SMA (Abcam), and the SMA-positive vessels in the ischemic heart region were counted under a light microscope. Five fields from seven samples were randomly selected for counting.
Karyotype analysis
hESCs-MSCs were incubated in culture media with 0.1 μg/mL colcemid (KaryoMax colcemid solution; Invitrogen) for 3–4 h. The harvested cells were resuspended in hypotonic solution (0.1% sodium citrate; Sigma) and were incubated for 20 min at 37°C. The sample was then fixed in methanol:acetic acid (3:1, v/v), and the chromosomes were visualized using the G-band standard staining (Giemsa; Merck).
Statistical analysis
Data are represented as mean value±SD for statistical comparison. Significance of differences was assessed by an unpaired student's t-test, where p<0.05 was considered significant. Histograms of the obtained data were fitted using the GraphPad Prism program (version 5). All experiments were independently performed thrice, each in triplicate.
Results
Isolation of hESCs-MSCs using a porous membrane
The general scheme to isolate MSC-like cells from hESCs using 8 μm porous membrane transwell cultural inserts is described (Fig. 1A, B). Briefly, small clumps of hESCs colonies were cultured in suspension to form EBs. The resulting EBs were cultured in suspension for 2 days, which were then plated onto the upper compartment of the culture inserts in EGM2-MV media. Five days after plating EBs, some of the cells emerging from the attached EBs appeared to migrate through the permeable membrane to the lower compartment of the inserts (Fig. 1B), while epithelial-like cells remained in the upper compartment (Fig. 1C). Morphological analysis revealed that the migrated cells in the lower compartments were proliferating as a colony of cells (Fig. 1C). To assess whether the migrated cells acquired the mesenchymal characteristics through the EMT-like processes, we performed qRT-PCR to analyze the expression of pluripotency and EMT markers. Downregulation of pluripotency genes (Oct4, E-cadherin, and Nanog) and a concurrent upregulation of EMT gene marker Fibronectin suggest the generation of MSC-like populations through the EMT process (Fig. 1D).
Characterization of the isolated hESCs derived MSCs
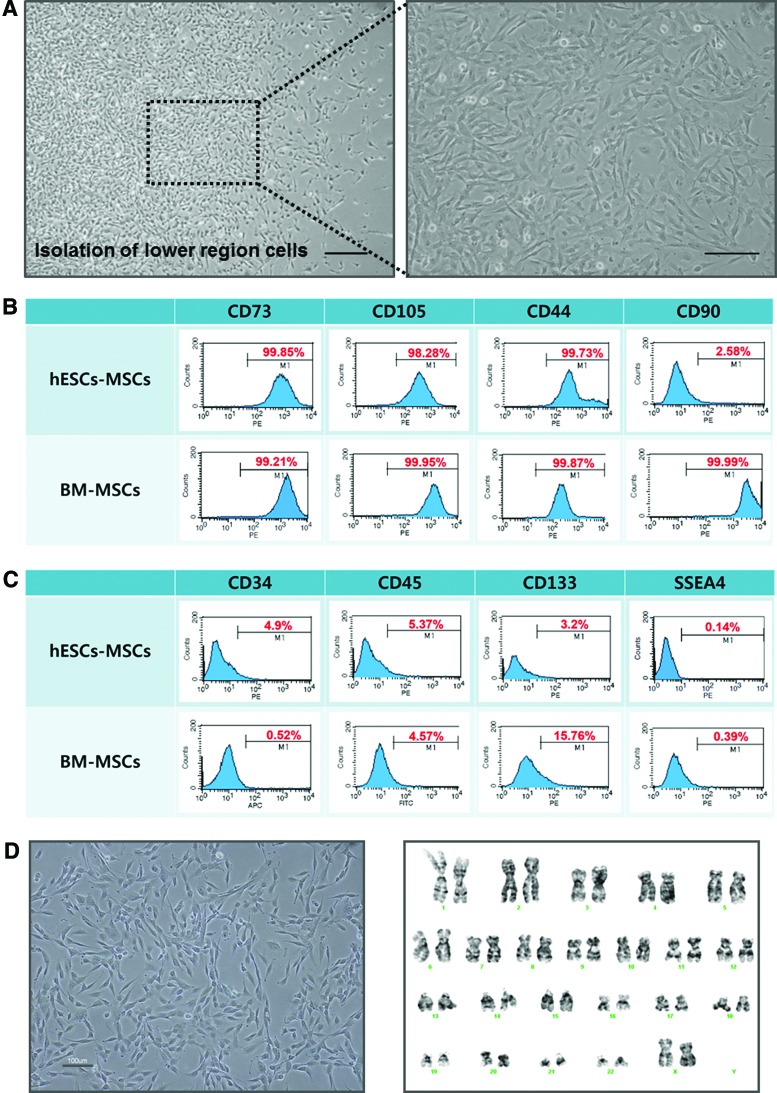
The migrated cells exhibited the typical MSC morphological features of spindle and fibroblast-like cells at 7 days after replating on the gelatin-coated surface (Fig. 2A). To characterize whether the isolated cells using the inserts are MSC-like cells, the expression of various MSC markers was analyzed using FACS. The isolated cells expressed high levels of the typical MSC surface markers CD73, 105, and 44 in a similar manner to bone marrow-derived mesenchymal stem cells (BM-MSCs), but the expression of another MSC surface marker CD90 was weakly expressed in relation to BM-MSCs (Fig. 2B). The expression levels of the surface markers for hematopoietic (CD34, 45, and 133) and undifferentiated (SSEA4) cells were generally low for both the migrated cells and BM-MSCs (Fig. 2C). In addition, the markers for endothelial cells markers (SMA, KDR, Tie-2, VE-CAD, and PECAM-1), MHC class I (HLA-ABC) and II (HLA-DR), and neural cells (NCAM) were expressed at low levels by the migrated cells (Supplementary Fig. S1A–C), The expression of PDGFR-α was also low, but the migrated cells expressed PDGFR-β at a high level (Supplementary Fig. S1D). The isolated cells were capable for a stable long-term proliferation as they were passaged for approximately 24 times without any characteristic and chromosomal changes (Fig. 2D).
FIG. 2.
Flow cytometry analysis of hESC-MSCs. (A) Morphological characteristics of hESC-MSCs. Scale bar, 200 μm. (B) P6 hESCs-MSCs were analyzed for specific surface antigen markers for hMSCs (CD73, CD105, CD44, and CD90), (C) hematopoietic lineage (CD34, CD45, and CD133), and undifferentiated cells (SSEA4). Bone marrow-derived mesenchymal stem cells (BM-MSCs) served as a positive control. (D) Morphological and karyotypic analysis of P24 hESC-MSCs. Karyotype was performed by G-band method, which was found to be 46, XX normal female. Color images available online at www.liebertpub.com/tec
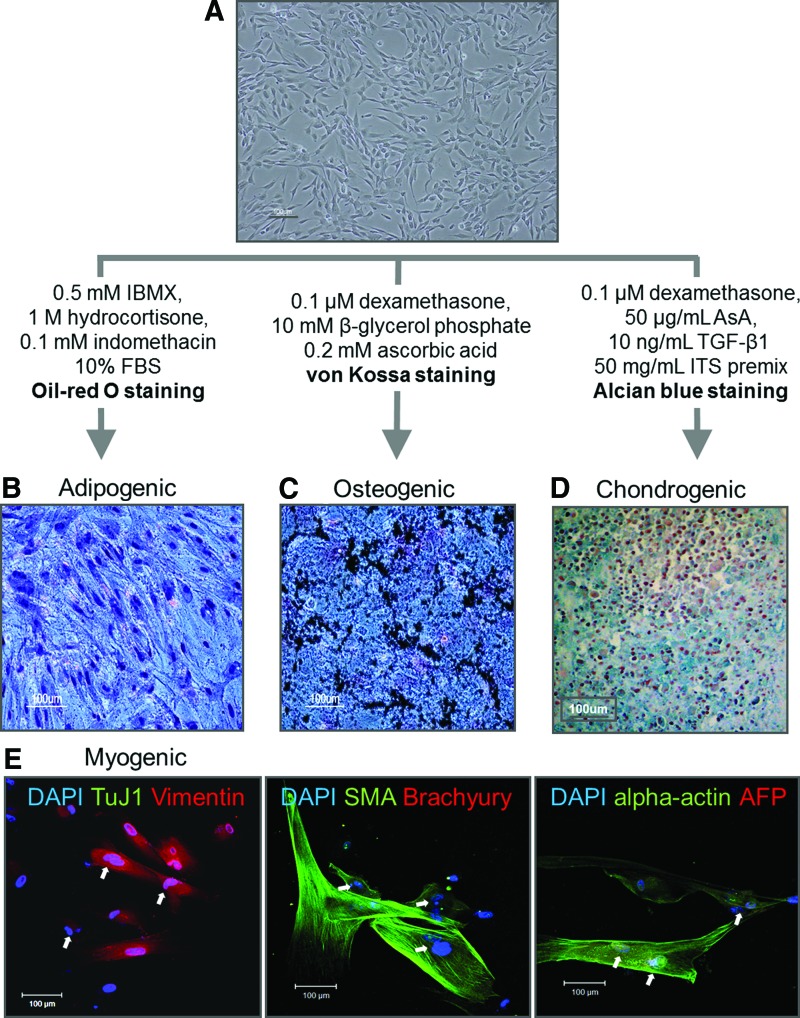
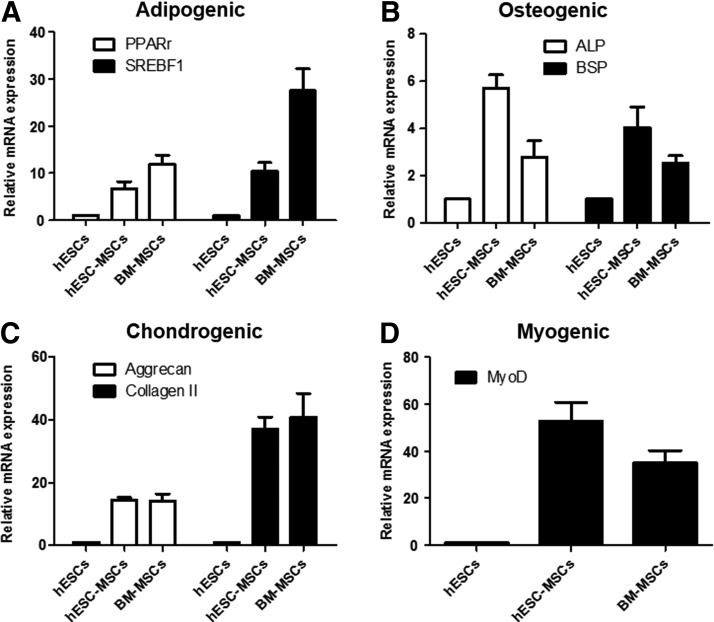
Next, differentiation potential was investigated by comparing differentiation efficiency and through qPCR analysis for specific marker expression. hESCs-MSCs (passage No.24) was used to assess multilineage differentiation capability (Fig. 3A). Twenty-one days after inducing adipogenic differentiation, lipid vesicles were visualized by Oil red O staining (Fig. 3B), but they were smaller in size compared with those differentiated from BM-MSC (Supplementary Fig. 2A). Although adipogenic markers such as PPARγ and SREBF1 expression was confirmed, the differentiation efficiency was relatively lower than that of BM-MSCs (Fig. 4A). Osteogenic differentiation was observed in the presence of beta-glycerol phosphate. After 3 weeks of induction, von Kossa staining revealed that calcium deposits were observed in the matrix (Fig. 3C), and the differentiation efficiency was similar to the positive control BM-MSCs (Supplementary Fig. S2A). Interestingly, stronger expression patterns of osteogenic specific markers ALP and BSP were observed (Fig. 4B). Chondrogenic differentiation was also efficient with more than 90% of cells producing proteoglycans in extracellular matrix in a comparable manner to BM-MSC as detected by Alcian Blue staining (Fig. 3D and Supplementary Fig. S2A). Chondrogenic-specific markers Aggrecan and Collagen II gene were also strongly expressed (Fig. 4C). In addition, myogenic differentiation of hESC-derived MSCs was successfully achieved using alpha MEM medium supplemented with 20% FBS. After 2 weeks of induction, myocytes expressing Vimentin, SMA, and alpha-actin were generated. In addition, the presence of multi-nucleated cells further supports myogenic lineage differentiation as it is a characteristic for myotubes (white arrows), but they were negative for the expression of an ectoderm (TuJ1), early mesoderm (Brachyury), and endoderm (AFP) similar to BM-MSCs (Fig. 3E and Supplementary Fig. S2B). Moreover, strong expression of MyoD gene was observed (Fig. 4D), providing evidence which indicates that MSCs isolated from hESCs using porous membranes are capable of multipotent differentiation.
FIG. 3.
Differentiation of hESC-MSCs into various mesenchymal derivatives. (A) Morphology of hESC-MSCs at passage 24. (B) Adipogenic differentiation in the presence of dexamethasone, insulin, and isobutylxanthine. Adipogenic characterization by Oil Red O staining. (C) Osteogenic differentiation in the presence of beta-glycerolphosphate, dexamethasone, and ascorbic acid. Osteogenic characterization by von Kossa staining. (D) Chondrogenic differentiation in the presence of TGF-b3 and ascorbic acid. Chondrogenic characterization by Alcian Blue staining. Scale bar, 100 μm. (E) Myogenic differentiation. Immunocytochemistry for Vimentin and TuJ1 (left), SMA and Brachyury (middle), and α-actin and AFP (right). Multi nucleated cells are depicted by white arrows. Scale bar, 100 μm. Color images available online at www.liebertpub.com/tec
FIG. 4.
qRT-PCR analysis of hESC-MSCs and BM-MSCs derivatives. (A) Expression level of adipogenic (PPARγ and SREBF1), (B) osteogenic (ALP and BSP), (C) chondrogenic (Aggrecan and Collagen II), and (D) myogenic-specific marker genes (MyoD) in undifferentiated hESC (control), hESC-MSCs, and BM-MSCs. The results were presented as mean±standard deviations.
Evaluating therapeutic application of hESC-MSC for functional recovery of MI
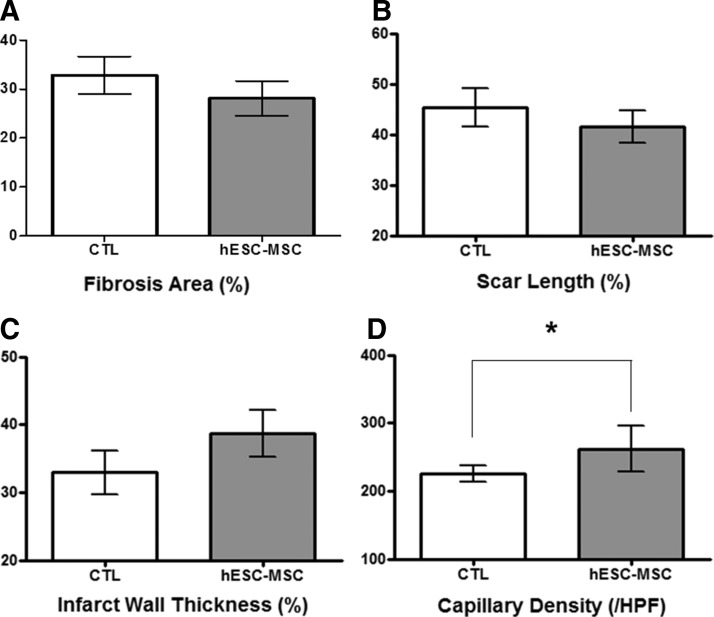
Therapeutic application of the isolated hESCs-MSCs was investigated in a mice model of MI. To confirm potential functional recoveries after transplantation, we compared the structural differences in the fibrosis area, scar length, and left ventricle (LV) wall thickness between groups that received PBS (sham control) and hESCs-MSCs. For the fibrosis area, the group that received hESCs-MSCs displayed a reduction in the size compared with the MI control group (MI control, 34.3%±1.6% vs. hESCs-MSCs, 28.9%±3.5%) (Fig. 5A). For the scar length, the hESCs-MSCs group displayed a decrease when compared with the MI control group (MI control, 46.1%±2.5% vs. hESCs-MSCs, 41.8%±1.3%) (Fig. 5B). In addition, hESCs-MSCs group exhibited an improved LV wall thickness as the wall was thicker compared with the sham control group (MI control, 32.9%±3.2% vs. hESCs-MSCs, 38.7%±2.4%) (Fig. 5C). Furthermore, immunohistochemistry was performed to examine the functionality of the transplanted cells. When measuring the microvessel density (per high power field), the vessels in the hESC-MSCs were significantly increased than the MI control group (Fig. 5D). These results suggest that it is feasible for hESCs-MSCs to enhance regenerative capabilities in most of the measured areas.
FIG. 5.
Improvement in the ischemic myocardium from cell transplantation. The infarct size was measured with Image J, which were expressed in percentages. (A) Comparison of the fibrosis area, (B) the scar length, and (C) wall thickness in LV. (D) Quantification of microvessel density (SMA positive) per high power field (/HPF) for each group (*p<0.05 vs. MI control). LV, left ventricle; MI, myocardial infarction.
Discussion
A large-scale production of MSCs is desirable for the development of cellular therapies for various medical conditions and diseases. However, obtaining MSCs in a large quantity is challenging due to invasive procedures and the limited proliferative capacity.19 In attempts to overcome this problem, hESCs have been used to differentiate MSCs.14,20 hESCs have the potential for being an unlimited cellular source for generating MSCs, but current protocols require to be streamlined for a clinical scale.21 We have demonstrated a feasibility of the commercialized porous membrane transwell culture inserts for a simple and efficient isolation of MSCs derived from hESCs.
hESCs colonies exhibit epithelial characteristics, and their cell–cell contacts are held by E-cadherin.12 On differentiation, the cells lose the epithelial characterizations and undergo a transition to adopt mesenchymal phenotypes such as migratory ability, fibroblastic morphology, and the expression of ECM molecules.11,12 When differentiating hESCs were cultured on the upper compartment of the inserts, cells undergoing EMT processes passed through an 8 μm pore-sized permeable membrane to the lower compartment (Fig. 1C). Previously, it was demonstrated that differentiated progeny from hESCs had permeated through 8 μm pore-sized membrane expressing markers associated with cell migration, but the migrated cells were not fully characterized.12 Since it has been demonstrated that MSCs can migrate through a membrane with the same pore size,22 it was postulated that the migrated cells may exhibit MSCs characteristics. Further analysis revealed that the cells in the lower compartments of the inserts expressed a panel of MSCs surface antigen markers, and were negative for hematopoietic markers (Fig. 2B). It is worth noting that the isolated cells expressed a very low level of a typical MSC marker CD90 (Fig. 2B). The absence of CD90 expression in MSCs has been reported, especially in the presence of angiogenic factors.23 Due to the fact that hESCs were initially induced to differentiate into MSCs in angiogenic factors containing EMG2-MV medium, we hypothesize that CD90 expression may have been lost during the initial derivation stage of MSCs. To support this, we cultured the isolated MSCs in normal culture conditions in alpha MEM containing 20% FBS,13 and observed (Supplementary Fig. S3A) that CD90 antigen was abundantly expressed along with CD74, 105, and 44 (Supplementary Fig. S3B) while markers for hematopoietic (CD34, 45, and 133) and undifferentiated (SSEA4) cells remained at a low level (Supplementary Fig. S3C). In addition, it is widely accepted that small variations exist in surface antigen expressions among MSCs of different origins.24 The exact function of CD90 is unknown, but due to the fact that the isolated cells satisfied many MSC criteria, we postulate that CD90 expression may be dispensable for functioning of MSCs. Indeed, the isolated cells being capable of a long-term proliferation without an aberrant karyotype indicates a potential large-scale expansion of MSCs for therapeutic applications.
Conclusion
Taking advantage of cells' acquired migratory property during the EMT induction, we were able to obtain a highly homogenous hESC-MSC population using a 8-um porous membrane transwell. This offers a simple and efficient purification technique for MSCs derived from hESCs, which could serve as an unlimited source for MSCs. Furthermore, the isolated hESC-MSCs exhibited prolonged proliferative capacities, which has an advantage compared with BM-MSCs for a large scale up. Our newly developed system could provide a means to overcome the limited supply of MSCs for the development of cell-based therapies for various diseases in the future.
Supplementary Material
Acknowledgments
This study was supported by grants (PJ.009014) (PJ.0095602) from the Next-Generation BioGreen 21 Program, Rural Development Administration, Republic of Korea, and supported by a Grant (2011–0019487) from the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government.
Disclosure Statement
No competing financial interests exist.
References
- 1.Beltrami A.P., Cesselli D., Bergamin N., Marcon P., Rigo S., Puppato E., D'Aurizio F., Verardo R., Piazza S., Pignatelli A., Poz A., Baccarani U., Damiani D., Fanin R., Mariuzzi L., Finato N., Masolini P., Burelli S., Belluzzi O., Schneider C., and Beltrami C.A.Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow). Blood 110,3438, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Conget P.A., and Minguell J.J.Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol 181,67, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., and Marshak D.R.Multilineage potential of adult human mesenchymal stem cells. Science 284,143, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H., Alfonso Z.C., Fraser J.K., Benhaim P., and Hedrick M.H.Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13,4279, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz-Prado S., Muinos-Lopez E., Hermida-Gomez T., Rendal-Vazquez M.E., Fuentes-Boquete I., de Toro F.J., and Blanco F.J.Multilineage differentiation potential of cells isolated from the human amniotic membrane. J Cell Biochem 111,846, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Karlsson C., Emanuelsson K., Wessberg F., Kajic K., Axell M.Z., Eriksson P.S., Lindahl A., Hyllner J., and Strehl R.Human embryonic stem cell-derived mesenchymal progenitors—potential in regenerative medicine. Stem Cell Res 3,39, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Li O., Tormin A., Sundberg B., Hyllner J., Le Blanc K., and Scheding S.Human embryonic stem cell-derived mesenchymal stroma cells (hES-MSCs) engraft in vivo and support hematopoiesis without suppressing immune function: implications for off-the shelf ES-MSC therapies. PLoS One 8,e55319, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., and Jones J.M.Embryonic stem cell lines derived from human blastocysts. Science 282,1145, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Moon S.H., Kim J.M., Hong K.S., Shin J.M., Kim J., and Chung H.M.Differentiation of hESCs into mesodermal subtypes: vascular-, hematopoietic- and mesenchymal-lineage cells. Int J Stem Cells 4,24, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hay E.D.An overview of epithelio-mesenchymal transformation. Acta Anat 154,8, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Boyd N.L., Robbins K.R., Dhara S.K., West F.D., and Stice S.L.Human embryonic stem cell-derived mesoderm-like epithelium transitions to mesenchymal progenitor cells. Tissue Eng Part A 15,1897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee E.J., Lee H.N., Kang H.J., Kim K.H., Hur J., Cho H.J., Lee J., Chung H.M., Cho J., Cho M.Y., Oh S.K., Moon S.Y., Park Y.B., and Kim H.S.Novel embryoid body-based method to derive mesenchymal stem cells from human embryonic stem cells. Tissue Eng Part A 16,705, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Barberi T., Willis L.M., Socci N.D., and Studer L.Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med 2,e161, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lian Q., Lye E., Suan Yeo K., Khia Way Tan E., Salto-Tellez M., Liu T.M., Palanisamy N., El Oakley R.M., Lee E.H., Lim B., and Lim S.K.Derivation of clinically compliant MSCs from CD105+, CD24- differentiated human ESCs. Stem Cells 25,425, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Park S.J., Moon S.H., Lee H.J., Lim J.J., Kim J.M., Seo J., Yoo J.W., Kim O.J., Kang S.W., and Chung H.M.A comparison of human cord blood- and embryonic stem cell-derived endothelial progenitor cells in the treatment of chronic wounds. Biomaterials 34,995, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Moon S.H., Kang S.W., Park S.J., Bae D., Kim S.J., Lee H.A., Kim K.S., Hong K.S., Kim J.S., Do J.T., Byun K.H., and Chung H.M.The use of aggregates of purified cardiomyocytes derived from human ESCs for functional engraftment after myocardial infarction. Biomaterials 34,4013, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Moon S.H., Ban K., Kim C., Kim S.S., Byun J., Song M.K., Park I.H., Yu S.P., and Yoon Y.S.Development of a novel two-dimensional directed differentiation system for generation of cardiomyocytes from human pluripotent stem cells. Int J Cardiol 168,41, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong K.S., Byun K.H., Seo J., Lee H.J., Choi J.J., Kim K.S., Choi Y., Moon S.H., and Chung H.M.Modification to the injection needle to a screw needle improves effective cell delivery in acute myocardial infarction. Biotechnol Lett 36,859, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Odorico J.S., Kaufman D.S., and Thomson J.A.Multilineage differentiation from human embryonic stem cell lines. Stem Cells 19,193, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Olivier E.N., Rybicki A.C., and Bouhassira E.E.Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells 24,1914, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Hwang N.S., Varghese S., Lee H.J., Zhang Z., Ye Z., Bae J., Cheng L., and Elisseeff J.In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc Natl Acad Sci U S A 105,20641, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruster B., Grace B., Seitz O., Seifried E., and Henschler R.Induction and detection of human mesenchymal stem cell migration in the 48-well reusable transwell assay. Stem Cells Dev 14,231, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Campioni D., Rizzo R., Stignani M., Melchiorri L., Ferrari L., Moretti S., Russo A., Bagnara G.P., Bonsi L., Alviano F., Lanzoni G., Cuneo A., Baricordi O.R., and Lanza F.A decreased positivity for CD90 on human mesenchymal stromal cells (MSCs) is associated with a loss of immunosuppressive activity by MSCs. Cytometry Part B Clin Cytom 76,225, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Kolf C.M., Cho E., and Tuan R.S.Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther 9,204, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.