Abstract
Obstructive sleep apnea (OSA) is a prevalent disorder associated with a multitude of adverse outcomes when left untreated. There is significant heterogeneity in the evaluation and management of OSA resulting in variation in cost and outcomes. Thus, the goal for developing these measures was to have a way to evaluate the outcomes and reliability of the processes involved with the standard care approaches used in the diagnosis and management of OSA. The OSA quality care measures presented here focus on both outcomes and processes. The AASM commissioned the Adult OSA Quality Measures Workgroup to develop quality care measures aimed at optimizing care for adult patients with OSA. These quality care measures developed by the Adult OSA Quality Measures Workgroup are an extension of the original Centers for Medicare & Medicaid Services (CMS) approved Physician Quality Reporting System (PQRS) measures group for OSA. The measures are based on the available scientific evidence, focus on public safety, and strive to improve quality of life and cardiovascular outcomes for individual OSA patients.
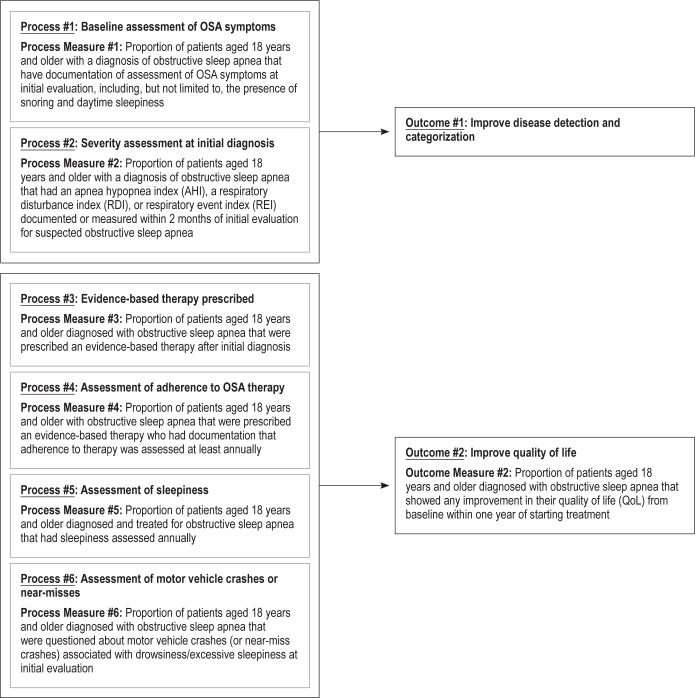
The three outcomes that were selected were as follows: (1) improve disease detection and categorization; (2) improve quality of life; and (3) reduce cardiovascular risk. After selecting these relevant outcomes, a total of ten process measures were chosen that could be applied and assessed for the purpose of accomplishing these outcomes.
In the future, the measures described in this document may be reported through the PQRS in addition to, or as a replacement for, the current OSA measures group. The overall objective for the development of these measures is that implementation of these quality measures will result in improved patient outcomes, reduce the public health burden of OSA, and provide a measurable standard for evaluating and managing OSA.
Citation:
Aurora RN, Collop NA, Jacobowitz O, Thomas SM, Quan SF, Aronsky AJ. Quality measures for the care of adult patients with obstructive sleep apnea. J Clin Sleep Med 2015;11(3):357–383.
Obstructive sleep apnea (OSA) is one of the most prevalent sleep disorders, affecting approximately to 3% to 7% of men and 2% to 5% of women in the general population.1–4 When polysomnographic criteria alone are considered, the prevalence rate increases dramatically to 24% in men and 9% in women.3 Despite the fact that OSA is a common disease, it remains considerably underdiagnosed, with 75% to 80% of cases remaining unidentified.5,6
The implications of untreated OSA are significant from the individual patient, healthcare, and economic perspectives. For the affected individual, OSA is associated with a number of nocturnal symptoms, as well as with difficulty in daytime functioning secondary to daytime sleepiness, irritability, fatigue, and decreased cognitive functioning.1 In fact, untreated OSA has been shown to significantly reduce quality of life.7,8 Furthermore, untreated OSA (especially severe OSA) is associated with a multitude of adverse health outcomes including cardiovascular disease,9 disorders of glucose metabolism including insulin resistance and diabetes,10,11 stroke,12 and an increased risk of death.13 Another compelling motivation for early case identification and treatment of OSA is the higher prevalence of traffic accidents noted in persons with untreated OSA.14–16 From an economic perspective, the healthcare costs and resource utilization of undiagnosed OSA is staggering, running into billions of dollars per year,17,18 similar to other chronic disorders. The financial burden of OSA-related motor vehicle crashes alone is enormous. Furthermore, therapy for OSA seems to reduce comorbidities associated with OSA as well as healthcare costs and utilization.19,20
Despite the individual health and economic imperatives to diagnose and treat OSA, there is substantial heterogeneity in how OSA patients are managed. In large part, this may be a function of the different pathways used to diagnose a patient with OSA. For example, the initial evaluation for OSA may begin in the primary care provider's office. The primary care provider may be confronted with patient symptoms such as snoring, excessive daytime somnolence, and pauses in breathing. In this paradigm of care, the primary care provider may then request a diagnostic sleep test and manage the patient without specialist input. Alternatively, the patient may be referred directly to a sleep specialist before or after the diagnostic sleep test for further evaluation. The evaluation by the sleep specialist is typically more detailed and includes a measure of sleepiness, a thorough physical exam with attention to the upper airway and a comprehensive differential diagnosis for the patient's sleep-related complaints. After evaluating the patient, the sleep specialist may co-manage the patient with the referring healthcare provider or return the patient back to the healthcare provider for all further care. Another factor explaining practice variation is the multiple medical disciplines included in the specialty of sleep medicine. In addition to the primary care provider and the sleep specialist, care for the patient with OSA may involve pulmonologists, otorhinolaryngologists, neurologists, psychiatrists, cardiologists, bariatric surgeons, anesthesiologists, obstetricians/gynecologists, and others. Given the aforementioned factors involved in the coordination and management of care of the OSA patient, considerable variability in practice patterns can occur, resulting in suboptimal or non-cost-effective care.
Where there is substantial variation in medical practice, and consequently differences in cost and outcomes, measurement of adherence to evidence-based management guidelines can help highlight the most significant opportunities for improvement and reduce unintended variations in medical practice. This is particularly applicable to the diagnosis and treatment of OSA, because it is known that treatment can reduce costs,19,20 and in many cases, improve quality of life21 and reduce morbidity from symptoms and comorbid conditions.22–25 To this end, the AASM commissioned five Workgroups to develop quality care measures aimed at optimizing care for patients suffering from sleep-related disorders, including adult and pediatric OSA, restless legs syndrome, narcolepsy, and insomnia.26 These quality care measures focus on both outcomes, that is, what happens to a patient as a result of the care received, and processes, or the steps taken by a healthcare provider in the care of an individual patient. Both outcomes and processes are important in the care of the patient. Outcomes are often more directly relevant to the patient, whereas processes tend to be less influenced by factors outside an individual provider's control, such as patient preference. All of the adult OSA outcomes and processes detailed in this report were developed by the Adult OSA Quality Measures Workgroup and received final approval from the AASM Quality Measures Task Force and the AASM Board of Directors. The quality measures developed by the Adult OSA Quality Measures Workgroup are an extension of the original Centers for Medicare & Medicaid Services (CMS) approved Physician Quality Reporting System (PQRS) measures group for OSA.27 These were used for the basis of developing additional measures with the goals of standardizing and enhancing the management of OSA patients, evaluating how well this care is being delivered, and using the data collected to prospectively improve the quality of that care. These new measures are harmonized with the prior PQRS measures, are meaningful and based on scientific evidence, include outcome measures, and focus on public safety and prevention/treatment of cardiovascular disease, the latter two being National Quality Strategy (NQS) priorities.28 Nonetheless, it is acknowledged that with evolution in the practice of sleep medicine, these quality measures should be regularly reevaluated to assure the measurement of the most up-to-date standards of care.
METHODS
Literature Review
As described in the parent paper26, a comprehensive search was conducted to identify any publications that addressed sleep apnea in terms of quality care or measures. All searches were limited to articles published between 2002–2013, pertaining to humans, and in the English language. Publication types such as news, letters, editorials, and case reports were excluded. A total of 795 articles were retrieved for review using this search.
An additional search was conducted to identify clinical practice guidelines, measures, systematic reviews, meta-analyses, and consensus recommendations published by the AASM or other organizations or groups in the National Guidelines Clearinghouse, the National Quality Measures Clearinghouse, PubMed, EMBASE, PsycInfo, and the Cochrane Library pertaining to obstructive sleep apnea (and all associated MeSH terms). All searches were limited to articles published between 2002–2013, pertaining to humans and adults, and in the English language. Publication types other than the ones listed above were excluded. A total of 136 articles resulted from this additional search. Workgroup members also performed “pearling,” where references from full articles found through the literature search were examined to identify any additional relevant evidence, resulting in another 5 articles.
The titles and abstracts of all articles were each reviewed by two Workgroup members. Any disagreements were resolved by a third Workgroup member. Full articles of publications thought to be relevant were obtained and reviewed in full to identify and provide support for the drafted quality measures.
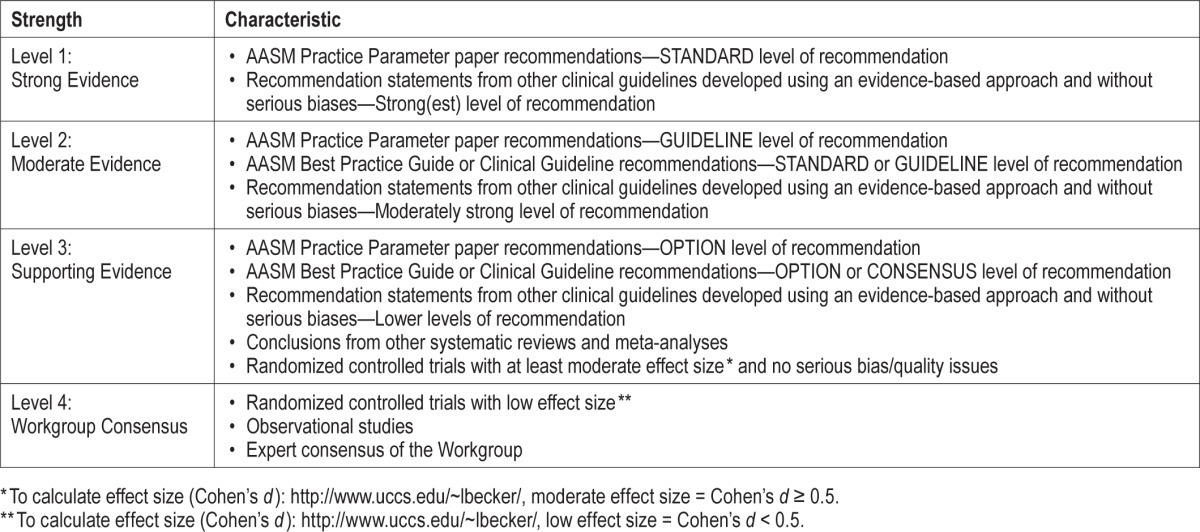
Workgroup members graded the available evidence from the literature searches for the strength of association between the proposed process and the desired outcome. For this, they used the grading scheme shown in the Table 1.
Table 1.
Strength of association between process measure and desired outcome.
Measure Selection
In considering the development of measures for adult OSA, the Workgroup sought to identify 1–3 outcomes that were patient-oriented, easily applicable, and well supported by the current literature and expert opinion. Process measures were then to be developed to correspond with each desired outcome yet not create significant burden for the patient, healthcare provider, or administrative staff. It was decided that a total of 2–10 process measures would be needed to achieve all of the outcomes.
Initially, consideration was given to the following outcomes: improvement in quality of life, improvement in functional status, and cardiovascular risk reduction. After much thought and discussion, the Workgroup opted to include and refine the previous OSA measures, developed in conjunction with the American Medical Association's Physician Consortium for Performance Improvement (AMA-PCPI), which aimed to improve disease detection and categorization and focus on the following new outcomes: improvement in quality of life and cardiovascular risk reduction. Initially, the following process measures were chosen as there was consensus by the Work-group members on their importance: (1) quality of life assessment by the healthcare provider, (2) snoring assessment, (3) nocturnal oxygen saturation assessment after therapy is implemented, (4) measurement of weight, (5) blood pressure monitoring at every visit, (6) a history of near-miss motor vehicle crashes, sustained motor vehicle crashes and workplace accidents, (7) assessment of sleepiness via a questionnaire or scale, and (8) objective treatment adherence data. Subjective adherence data could be used if objective adherence data is not available.
During the face-to-face meeting of all the Workgroups, it was decided that nocturnal oxygen saturation assessment after therapeutic intervention would be excluded. This process measure could potentially be burdensome to both the health-care provider and patient, and there was not enough standardization with regards to acceptable oxygen saturation levels to make it a meaningful measure. Other process measures were further honed over time. For example, the Workgroup agreed that because weight is a significant risk factor for OSA, a discussion about weight management would be important in overweight and obese patients. Additionally, given the strong association between OSA and hypertension, it would be important to address an elevated blood pressure reading with the patient. There was also concern that requiring that a quality of life (QoL) questionnaire be completed within one year of implementing treatment might be burdensome. The Workgroup and all of the BOD liaisons were surveyed, and the majority opinion was that it would be important to have a standardized, measurable approach to assessing quality of life, given that the tools to do this are widely available. However, the choice of questionnaire used was not specified to give flexibility to the provider, hopefully reducing any burden. The final process and outcome measures are depicted in Figure 1. The technical specifications associated with each of these quality measures can be found in the Appendix. These specifications outline how to calculate an individual provider's performance in meeting these measures using a combination of diagnostic and CPT codes and chart review.
Figure 1. Adult OSA quality measures driver diagram.
QUALITY MEASURES
Outcome 1 – Improve Disease Detection and Categorization
Description
Outcome 1, which is not a measured outcome but rather a broad goal of care, is to improve detection and categorization of OSA.
It is estimated that 3% to 7% of men and 2% to 5% of women in the adult general population have OSA.1–4 Nevertheless, despite increasing awareness by both the public and clinicians, OSA remains considerably underdiagnosed and treated.5,6 Furthermore, the therapeutic approaches used to treat people with OSA can vary according to severity.29–31 Thus, it is important to document disease severity in people in whom the diagnosis is made. To address these issues, the Workgroup chose 2 process measures (#1–2) focused on ascertaining OSA symptoms and severity that should result in improvement in disease detection and categorization.
Supporting Evidence and Rationale
Recognition of OSA is critically dependent on ascertainment of clinical symptoms. Any improvement in the diagnosis of OSA will flow from clinicians determining whether symptoms of OSA are present in their patients. The 2 most common symptoms of OSA are snoring and excessive daytime sleepiness.32,33 Thus, assessment of these symptoms and others will result in increased diagnostic testing for OSA and should be a useful process measure to increase disease detection.
Current classification of severity of OSA relies exclusively on the results from determination of event frequency.34 This can only be obtained using polysomnography or home sleep apnea testing. In either case, people with more severe disease have been shown to be at greater risk for cardiovascular disease, neurocognitive impairment, and other adverse health outcomes.9–16 A more aggressive therapeutic approach may be pursued in such individuals in contrast to those with milder disease. Thus, the Workgroup decided it was important to have documentation of disease severity as a process measure for this outcome.
Issues Addressed During Development
Although the Workgroup agreed that “Improving Disease Detection and Categorization” is an important outcome measure, it was recognized that directly measuring and tracking this outcome would be challenging because of socioeconomic, hereditary, cultural, and other influences beyond the control of a healthcare facility or clinician, and that any change in this outcome might take a considerable amount of time to be realized.26 However, it was felt that Process Measure #1 (Baseline Assessment of OSA Symptoms) and Process Measure #2 (Severity Assessment at Initial Diagnosis) would directly impact this outcome and would be reasonable surrogates.
Process Measure 1 – Baseline Assessment of OSA Symptoms
Description
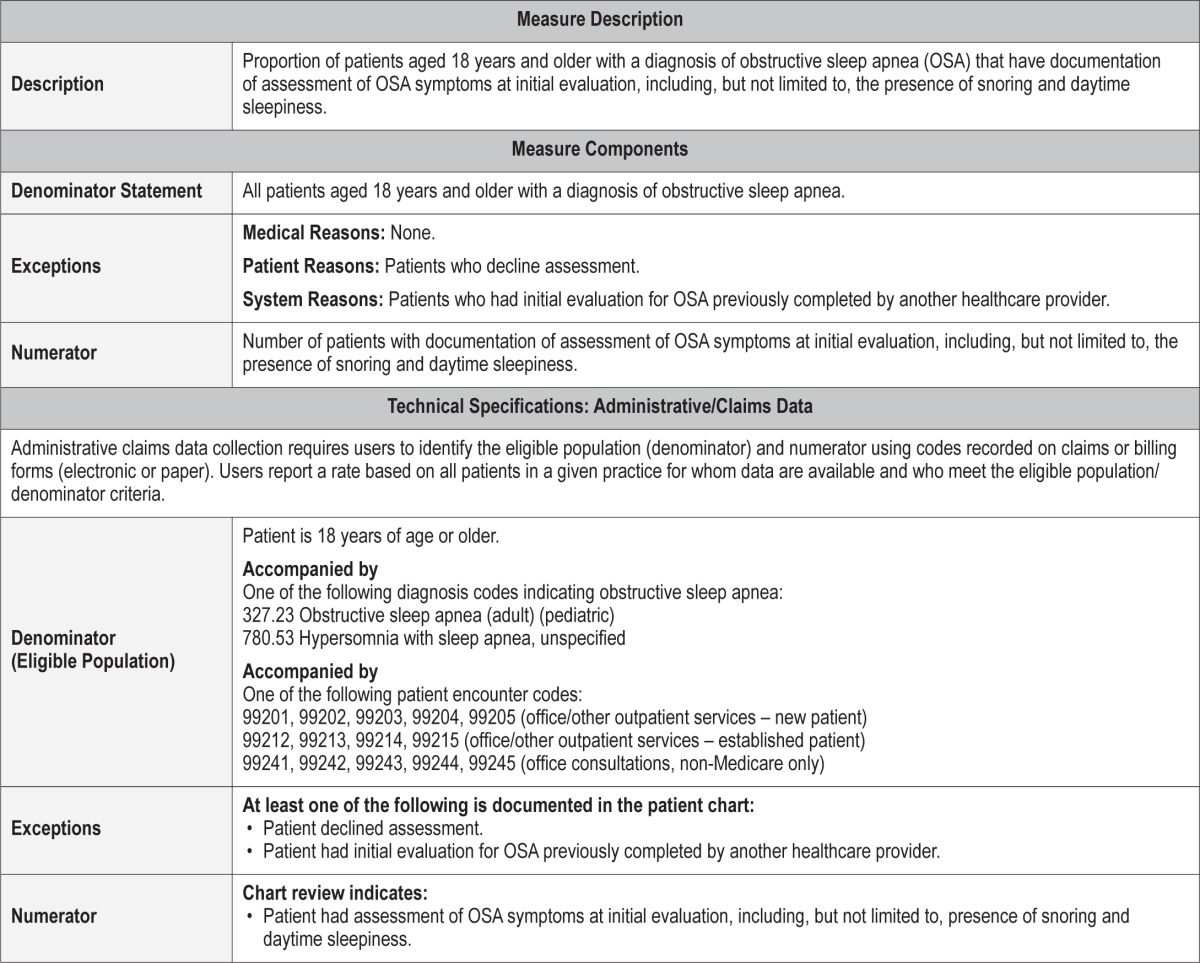
Proportion of patients aged 18 years and older with a diagnosis of obstructive sleep apnea (OSA) with documentation of assessment of OSA symptoms at initial evaluation, including the presence of snoring and daytime sleepiness.
Exceptions and Exception Justifications
Medical Reasons: None.
Patient Reasons: Patients who decline assessment.
System Reasons: Patients who had initial evaluation for OSA previously completed by another healthcare provider.
If the patient refuses to be evaluated, then their information will not be available for inclusion. Additionally, if the patient has had this information obtained on a previous visit with another healthcare provider, then it would pose unnecessary burden to repeat this process.
Supporting Evidence and Rationale
It is well-recognized that OSA is an underdiagnosed disorder that can pose significant economical and public health burdens if left untreated. Assessing OSA-related symptoms is an important first step in reducing the burden of undiagnosed disease. Clinical history and physical exam remain the cornerstone of initial disease detection. Thus, to improve disease detection, it is critical that all patients with suspected OSA be asked about OSA-related nocturnal and daytime symptoms. Process Measure 1 specifies that adult patients aged 18 years and older with a suspected diagnosis of OSA should have documentation of their presenting symptoms including, but not limited to, snoring and daytime sleepiness, at the time of initial evaluation for OSA. Both snoring and daytime sleepiness are relatively prevalent symptoms in those with OSA. It is estimated that snoring occurs in up to 30% to 50% of adults over the age of 50, and subjective sleepiness occurs in more than 30% of adults.35 Patients diagnosed with obstructive sleep apnea should be regularly assessed for the development and progression of both these symptoms as well as the patient's specific presenting symptoms to help guide therapeutic decisions. For example, continuous positive airway pressure (CPAP) settings may be modified in order to better treat the OSA which may improve snoring or daytime sleepiness.
Relationship to Desired Outcome
In order to improve detection and categorization of OSA, it is critical that all patients be asked about nocturnal and daytime symptoms that are associated with obstructive sleep apnea. Clinical history and physical exam remain the cornerstone of initial disease detection.
Opportunities for Improvement/Gaps
It is well-recognized that OSA is an underdiagnosed disorder and this lack of disease recognition poses significant economical and public health burdens. Assessing OSA-related symptoms is an important first step in reducing the burden of undiagnosed disease.
Issues Addressed During Development
None.
Process Measure 2 – Severity Assessment at Initial Diagnosis
Description
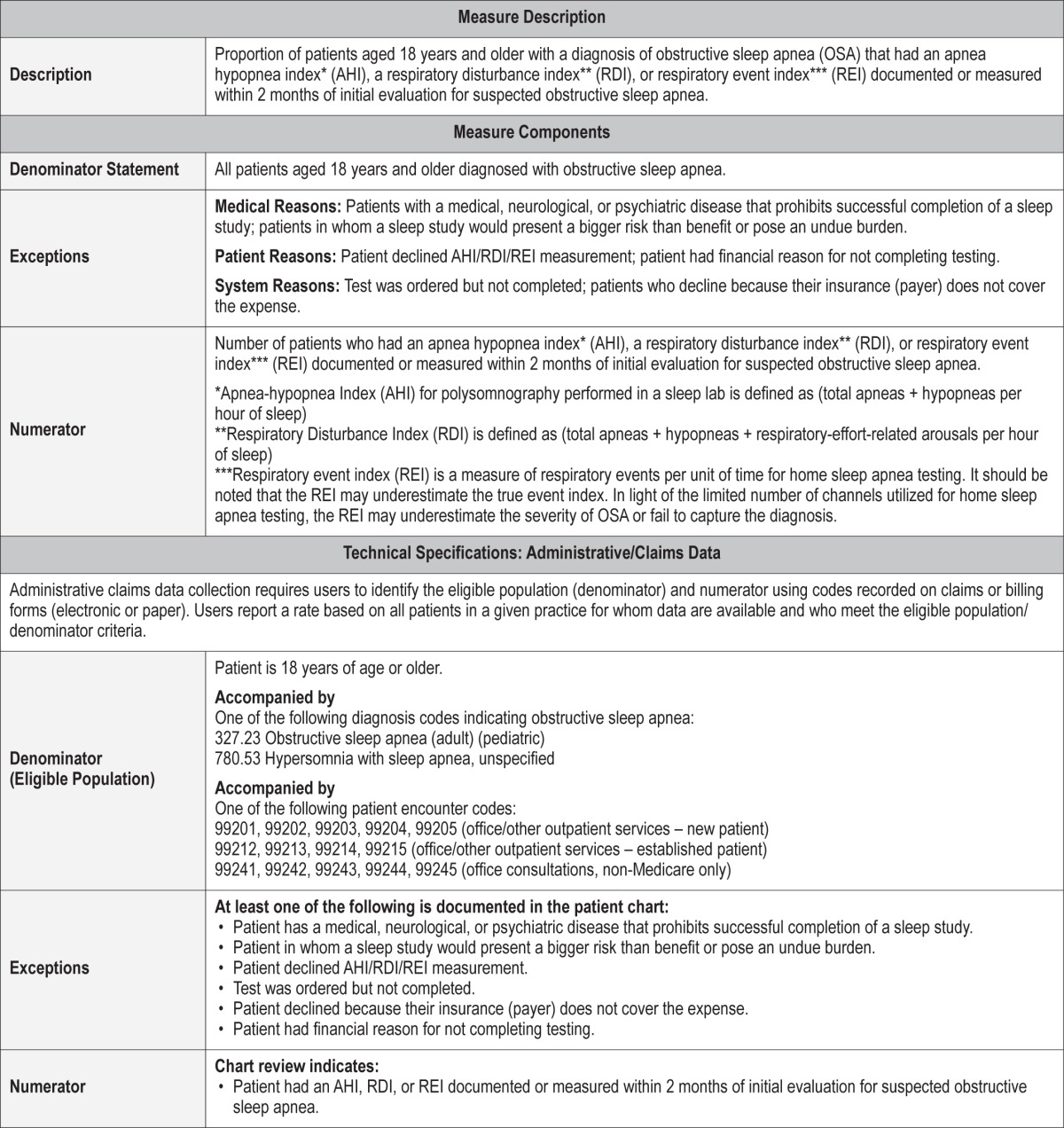
Proportion of patients aged 18 years and older with a diagnosis of obstructive sleep apnea that had an apnea hypopnea index* (AHI), a respiratory disturbance index** (RDI), or respiratory event index*** (REI) documented or measured within 2 months of initial evaluation for suspected obstructive sleep apnea.
*Apnea-hypopnea index (AHI) for polysomnography performed in a sleep lab is defined as (Total apneas + hypopneas per hour of sleep)36
**Respiratory disturbance index (RDI) is defined as (total apneas + hypopneas + respiratory-effort-related arousals per hour of sleep)36
***Respiratory event index (REI) is a measure of respiratory events per unit of time for home sleep apnea testing.37 It should be noted that, in light of the limited number of channels utilized for home sleep apnea testing, the REI may underestimate the severity of OSA or fail to capture the diagnosis because of underestimation of the true event index.
Exceptions and Exception Justifications
Medical Reasons: Patients with a medical, neurological, or psychiatric disease that prohibits successful completion of a sleep study; patients in whom a sleep study would present a bigger risk than benefit or pose an undue burden should not be included in the eligible population.
Patient Reasons: Patients who declined AHI/RDI/REI measurement; patients who have financial reasons for not completing testing.
System Reasons: Test was ordered but not completed; Patients who decline because their insurance (payer) does not cover the expense.
In patients that have physical or mental limitations, such as motor paralysis or intellectual disability, completing a sleep study may pose a significant burden on the patient and/or their family. If a patient refuses to have a sleep study, then this measure cannot be assessed. Also, paying for a sleep study may pose significant financial burden. These patients should be excluded from the assessment.
Supporting Evidence and Rationale
Expeditious diagnosis and treatment of OSA are important in reducing the burden associated with OSA-related comorbidities. However, history and physical exam alone are not sufficient to diagnose OSA. Objective testing with either in-laboratory polysomnography or home sleep apnea testing is necessary and required for the diagnosis of OSA and classification of disease severity.35,38,39 Determining OSA severity is important given that patients with moderate or severe OSA are at higher risk for cardiovascular diseases, neurocognitive dysfunction, lower quality of life, and other comorbid conditions.9,40,41 Thus, physicians evaluating patients with suspected sleep apnea should try to establish the patient's level of OSA severity in an expeditious manner as early case identification is important in prompt initiation of treatment and reduction of OSA-associated comorbidities.
Relationship to Desired Outcome
This process measure directly feeds into the outcome as OSA detection and categorization cannot occur without an objective evaluation of breathing during sleep.42 A sleep study is needed, as clinical evaluation alone has limited sensitivity and specificity.
Opportunities for Improvement/Gaps
Given the enormous burden of undiagnosed OSA, this measure provides an opportunity to address this burden by requiring study based disease diagnosis and categorization. It provides the opportunity to expedite appropriate treatment for OSA.
Issues Addressed During Development
There were some concerns raised that the requirement to obtain a sleep study within 2 months of initial evaluation was an insufficient amount of time and may not be possible in certain settings. However, after assessing the burden and benefits, the Workgroup was confident that 2 months was in fact an appropriate amount of time, given that the required sleep study could be an in-laboratory or home sleep apnea test (types I - IV).
Outcome Measure 2 – Improve Quality of Life
Description
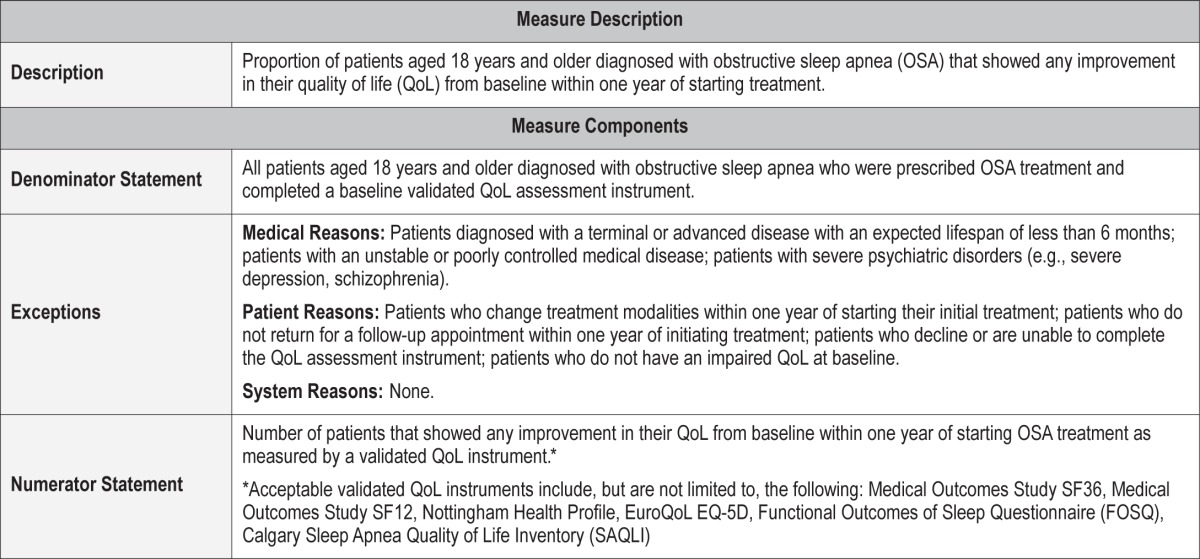
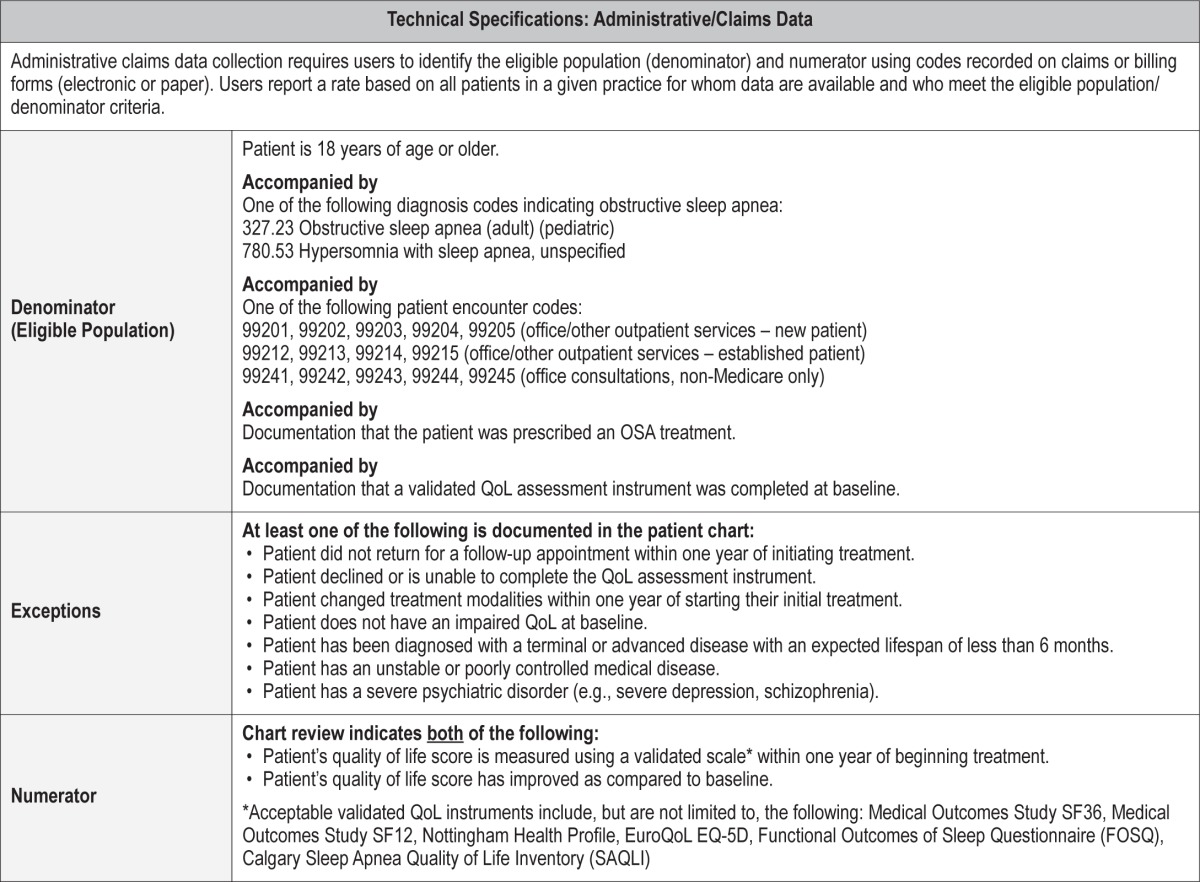
Proportion of patients aged 18 years and older diagnosed with obstructive sleep apnea (OSA) that showed any improvement in their quality of life (QoL) from baseline within one year of starting treatment.
Exceptions and Exception Justifications
Medical Reasons: Patients who have been diagnosed with a terminal or advanced disease with an expected lifespan of less than 6 months; patients with an unstable or poorly controlled medical disease; patients who have been diagnosed with severe psychiatric disorders (e.g., severe depression, schizophrenia).
Terminal illness will likely result in a deterioration in QoL and thus mask any improvement related to the treatment of OSA. Unstable comorbid illnesses will produce fluctuations in QoL that will confound any impact of OSA treatment. Severe psychiatric disorders are associated with reductions in QoL, which may mask changes related to OSA treatment. Furthermore, depressive symptoms may be caused by OSA. Simultaneous treatment of depression and OSA may make changes in QoL difficult to interpret.
Patient Reasons: Patients who change treatment modalities within one year of starting their initial treatment; patients who do not return for a follow-up appointment within one year of initiating treatment; patients who decline or are unable to complete the QoL assessment instrument; patients who do not have an impaired QoL at baseline.
If patients change treatment during the follow-up interval, there may be an inadequate length of time to determine whether an improvement in QoL has occurred. Furthermore, change in therapy is usually prompted by treatment failure or intolerance, and the therapy may actually lead to a temporary reduction in QoL. In addition, it will be difficult to assess which therapeutic intervention would be responsible for an improvement in QoL until the patient was stable on their “final” treatment. Some patients with OSA do not have an impairment in their QoL and treatment is initiated to mitigate the impact of OSA on cardiovascular risk. For these patients, there is no reason to expect any improvement in QoL. Exclusion of patients who refuse to participate or do not return for follow-up is self-explanatory.
System Reasons: None.
Supporting Evidence and Rationale
Quality of life is now considered one of the most fundamental patient-reported outcomes in healthcare. The duration and improvement in QoL is used to determine whether innovative and expensive therapies should be incorporated as standard therapies.43 For the vast majority of patients with OSA, a reduction in their QoL as reflected by symptoms such as intractable daytime sleepiness or fatigue, poor sleep quality, and inability to sleep in the same bedroom with their bed partner is the primary reason for seeking care. Thus, treatment for OSA should result in an improvement in the QoL of patients.
There is substantial evidence that OSA is associated with a reduction in QoL. The domains of physical functioning, general health, and vitality appear to be the most severely impacted.7 The prevalence of impaired quality of life ranges from 41% to 88% depending on QoL domain assessed and the assessment tool.44,45 Quality of life can be assessed using validated generic QoL instruments such as the Medical Outcomes Study SF-36 or SF-12. However, validated OSA specific instruments may be more sensitive and germane to people with OSA.46 Instruments such as the Calgary Sleep Apnea Quality of Life Inventory (SAQLI) or Functional Outcomes of Sleep Questionnaire (FOSQ) have been shown to be useful in identifying impaired QoL in persons with obstructive sleep apnea.
There have been a number of studies investigating the impact of treatment of OSA on QoL.46 In randomized controlled trials, QoL improves after adequate treatment of OSA with positive airway pressure,21,47 oral appliances,48 and upper airway surgery.49 All of these treatment modalities are commonly utilized for the treatment of OSA.
Acceptable validated QoL instruments include, but are not limited to, the following: Medical Outcomes Study SF-36,50 Medical Outcomes Study SF-12,51 Nottingham Health Profile,52 EuroQoL,53 EQ-5D,54 FOSQ,55 SAQLI.56 Although use of a validated QoL instrument will be expected to result in some amount of patient burden, as well as healthcare provider/staff effort to extract the data, use of a non-validated measure or a chart note indicating improved QoL will not provide any reliable or consistent measure of this outcome.
Opportunities for Improvement/Gaps
Although QoL is impaired in OSA and has been shown to improve with treatment, there are no studies documenting its assessment before and/or after initiating treatment in clinical populations.
Issues Addressed During Development
There were several issues that were considered in the development of this outcome measure. Foremost, was the feasibility of collecting the required information because ascertainment of quality of life is not normally done as part of typical clinical evaluations. This issue was discussed in the context of the practice burden required to collect the data as well. In addition, the question of whether there should be a standardized data collection instrument was debated. Feedback from stakeholders emphasized the potential burden of administering a questionnaire to fulfill this measure as well as greater specification of the exceptions. It was acknowledged that an additional limitation was the inherent subjectivity of quality of life assessments, although this is mitigated by use of validated collection instruments. Nevertheless, despite the potential difficulty in collecting data for this measure, the Workgroup decided that improvement in quality of life offered one of the best markers of effective treatment for OSA as well as alignment with national priorities.
Process Measure 3 – Evidence-Based Therapy Prescribed
Description
Proportion of patients aged 18 years and older diagnosed with obstructive sleep apnea that were prescribed an evidence-based therapy after initial diagnosis.
Exceptions and Exception Justifications
Medical Reasons: None.
Patient Reasons: Patients who do not wish to be prescribed therapy; patients who do not return for follow-up after initial diagnosis.
System Reasons: Patients whose insurance (payer) does not cover the expense.
A healthcare provider would not be able to prescribe therapy to patients who refuse treatment prescription or who do not return for a follow-up visit after initial diagnosis.
Supporting Evidence and Rationale
In order to improve quality of life for patients who have OSA, clinicians should employ an evidence-based therapy for OSA. No one treatment modality is universally accepted or used by all patients, and several treatment modalities are supported by evidence demonstrating improved alertness and quality of life in OSA patients. Thus, the clinician may consider various treatment options and match the modality appropriately to the patient's features and wishes.
Multiple treatment modalities are supported by evidence demonstrating improved alertness and quality of life in OSA patients. Evidence-based treatments include PAP therapy (including continuous positive airway pressure [CPAP], bilevel positive airway pressure [BPAP], and auto-titrating positive airway pressure [APAP]), oral appliances, upper airway surgery, and positional therapy. While weight loss may be beneficial for many OSA patients, it was not included as it was considered a useful adjunctive treatment, rather than an active modality of therapy.
Randomized controlled and observational studies support positive airway pressure therapy for improved alertness and quality of life in patients with severe OSA and sleepy patients with mild to moderate OSA.21,57 [Level 3, 4] The AASM practice parameters recommend CPAP for improving self-reported sleepiness in patients with OSA (Standard) [Level 1] and for improving quality of life in patients with OSA.58 (Option) [Level 3]
For oral appliance therapy, there are randomized trials, placebo controlled or in parallel cohort with CPAP for improved alertness and quality of life. [Level 357,59 and 460,61] The benefits were demonstrated for some patients with severe OSA as well.57
Surgical airway reconstruction is evidence-based,62 largely supported by cohort, case-series studies [Level 3, 4] and a randomized control study for quality of life and alertness effects.49,63–65 [Level 3]
Positional therapies for avoidance of supine sleep position in OSA patients selected for supine position dependency of the AHI are also evidence-based for improving quality of life and function, but evidence is from a few small case series and randomized studies.66–68 [Level 3, 4]
This process measure of prescribing evidence-based therapies may familiarize some clinicians with additional treatment modalities not previously considered and may provide support for some treatment modalities not considered a covered service by some insurance carriers.
Relationship to Desired Outcome
Multiple studies have demonstrated that untreated OSA is associated with lower quality of life, partially related to increased sleepiness and lower function.
Opportunities for Improvement/Gaps
Evidence-based treatments are not always employed and not all healthcare providers are familiar with all treatment modalities for OSA. Education of healthcare providers is encouraged.
Certain treatments for OSA may not be services covered by insurance carriers; thus support for these modalities is needed to modify coverage policy.
Issues Addressed During Development
Overall, there was agreement by the Workgroup that patients should be offered therapy for OSA for which there is evidence showing effectiveness.
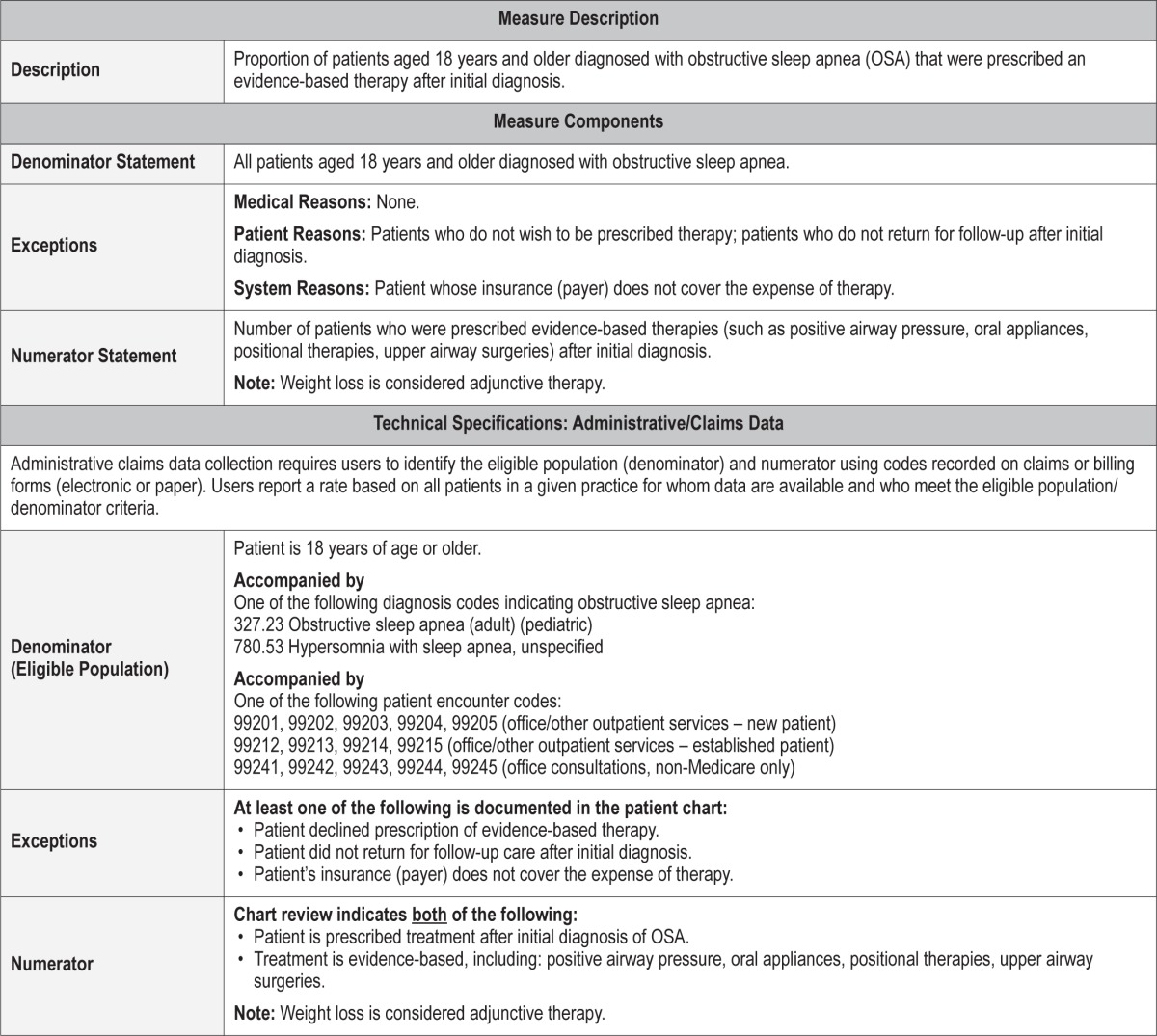
Process Measure 4 – Assessment of Adherence to OSA Therapy
Description
Proportion of patients aged 18 years and older with obstructive sleep apnea who were prescribed an evidence-based therapy who had documentation that adherence to therapy was assessed at least annually.
Exceptions and Exception Justifications
Medical Reasons: Patients who have been diagnosed with a terminal or advanced disease with an expected lifespan of less than 6 months; patients who underwent surgical treatment for OSA (e.g., bariatric, upper airway) and subsequently do not need ongoing assessment of adherence to therapy.
Patient Reasons: Patients who decline therapy; patients who do not return for follow-up care; patients unable to access/ afford therapy.
System Reasons: Patients who decline because their insurance (payer) does not cover the expense.
Patients diagnosed with a terminal or advanced disease with an expected lifespan of less than 6 months do not require long-term adherence assessment. Patients who do not comply with follow-up are unable to be assessed. The majority of treatment modalities for OSA are dependent on patient adherence. Upper airway surgery is dependent on acceptance, not adherence and thus was not included in this measure.
Supporting Evidence and Rationale
Untreated OSA is associated with sleepiness, lower quality of life and functional performance, and increased risk of motor vehicle crashes and workplace accidents. Evidence-based practice parameters support positive airway pressure, oral appliance therapy, and positional therapy for avoidance of supine sleep position as beneficial treatment modalities for OSA patients to improve quality of life.58,69,70 A measure of adherence is required to assess the efficacy of longitudinal therapy.
For CPAP, long-term adherence rates are estimated to be < 50% for 4-hour per night use.71 Adherence to CPAP is related to reduction in sleepiness.72 [Level 4]
Oral appliance mean adherence rate is estimated to be 50% to 70% by subjective reporting.73,74 Objective adherence monitoring should be available soon in the US for oral appliance therapy, but at present, objective adherence rates are largely unknown.75
Long-term adherence rates for positional therapy were reported to be < 30%, but data are lacking and adherence may depend on the particular device used for positional therapy.76
Treatment adherence assessment may allow for identification of patients who are no longer adherent or poorly adherent and thereby provide an opportunity for modifying a therapeutic intervention. A specific rate of usage or adherence to a treatment modality was not incorporated into this measure as the level of usage required for meaningful quality of life and alertness improvement has not been scientifically established and may vary between individuals.
Relationship to Desired Outcome
Untreated OSA is associated with sleepiness, lower quality of life and functional performance, and increased risk of motor vehicle crashes and workplace accidents.
Opportunities for Improvement/Gaps
By assessing OSA treatment adherence, patients who are no longer adherent or poorly adherent can be identified, thereby providing opportunity for modification of their therapeutic intervention or increased education.
Issues Addressed During Development
There was significant discussion regarding this process measure. Points of discussion included if and when subjective report of adherence was acceptable for PAP therapy. Additionally, it was discussed how other therapies for OSA such as oral appliances should be followed for adherence. It was determined that whenever possible, objective data should be obtained. When not available, subjective report will have to be used and will have to be appropriately documented by the patient's healthcare provider.
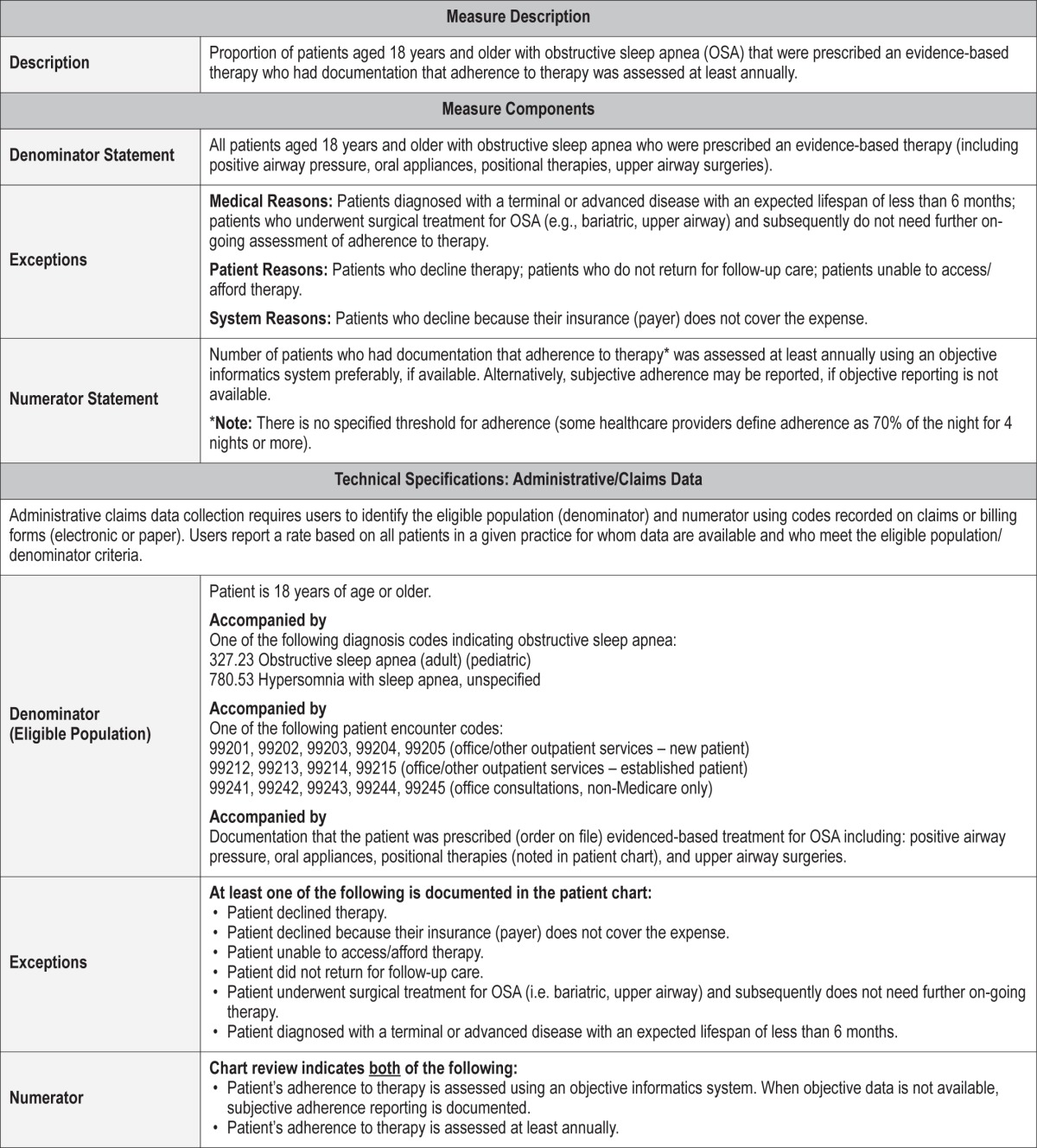
Process Measure 5 – Assessment of Sleepiness
Description
Proportion of patients aged 18 years and older diagnosed and treated for obstructive sleep apnea (OSA) who had sleepiness assessed annually.
Exceptions and Exception Justifications
Medical Reasons: Patients diagnosed with a terminal or advanced disease with an expected lifespan of less than 6 months; patients who underwent surgical treatment for OSA (i.e. bariatric, upper airway) and subsequently no longer meet the diagnostic criteria for OSA.
Patient Reasons: Patients who do not return for follow-up; patients who decline or are unable to respond to the assessment; patients who decline therapy; patients who are unable to access or afford therapy.
System Reasons: Patients who decline assessment because their insurance (payer) does not cover the expense.
Patients who do not return for follow-up for OSA cannot be included in the denominator. Also, if a patient declines or cannot respond to questions characterizing sleepiness, he/she cannot be included in the denominator.
Supporting Evidence and Rationale
Excessive daytime sleepiness is a common and debilitating symptom for many patients with OSA. It has been shown that daytime sleepiness is an important component of overall quality of life, and the presence of daytime sleepiness can significantly lower quality of life.77 There is a significant amount of Level 1 evidence supporting the monitoring of subjective sleepiness in patients who have initiated OSA therapy. The 2006 Practice Parameters on the use of CPAP and bilevel PAP for treating adult patients with sleep-related breathing disorders clearly state that “CPAP is indicated in improving self-reported sleepiness in patients with OSA (Standard).”58 [Level 1] Other therapies have also been shown to improve daytime sleepiness; therefore, patients receiving any OSA treatment should be followed for sleepiness. Several practice parameters and clinical guidelines put forth by the AASM show improvement in subjective sleepiness (measured primarily by ESS) with treatment of OSA both with CPAP therapy and oral appliances.38,39,58,73 Combined, these citations refer to a large number of papers (including randomized controlled trials) supporting the monitoring of subjective sleepiness in patients being treated for OSA. For this measure, healthcare providers can choose to follow sleepiness as part of the history-taking process or use a validated subjective sleepiness scale such as the Epworth Sleepiness Scale (ESS) or another sleepiness scale.
Relationship to Desired Outcome
Daytime sleepiness is an important component of overall quality of life perception and can significantly lower quality of life.77
Opportunities for Improvement/Gaps
Daytime sleepiness has been shown to negatively impact quality of life for many patients with OSA. Monitoring this important outcome would allow physicians to help target an area for improvement that seems to be important to patients.77
Issues Addressed During Development
None.
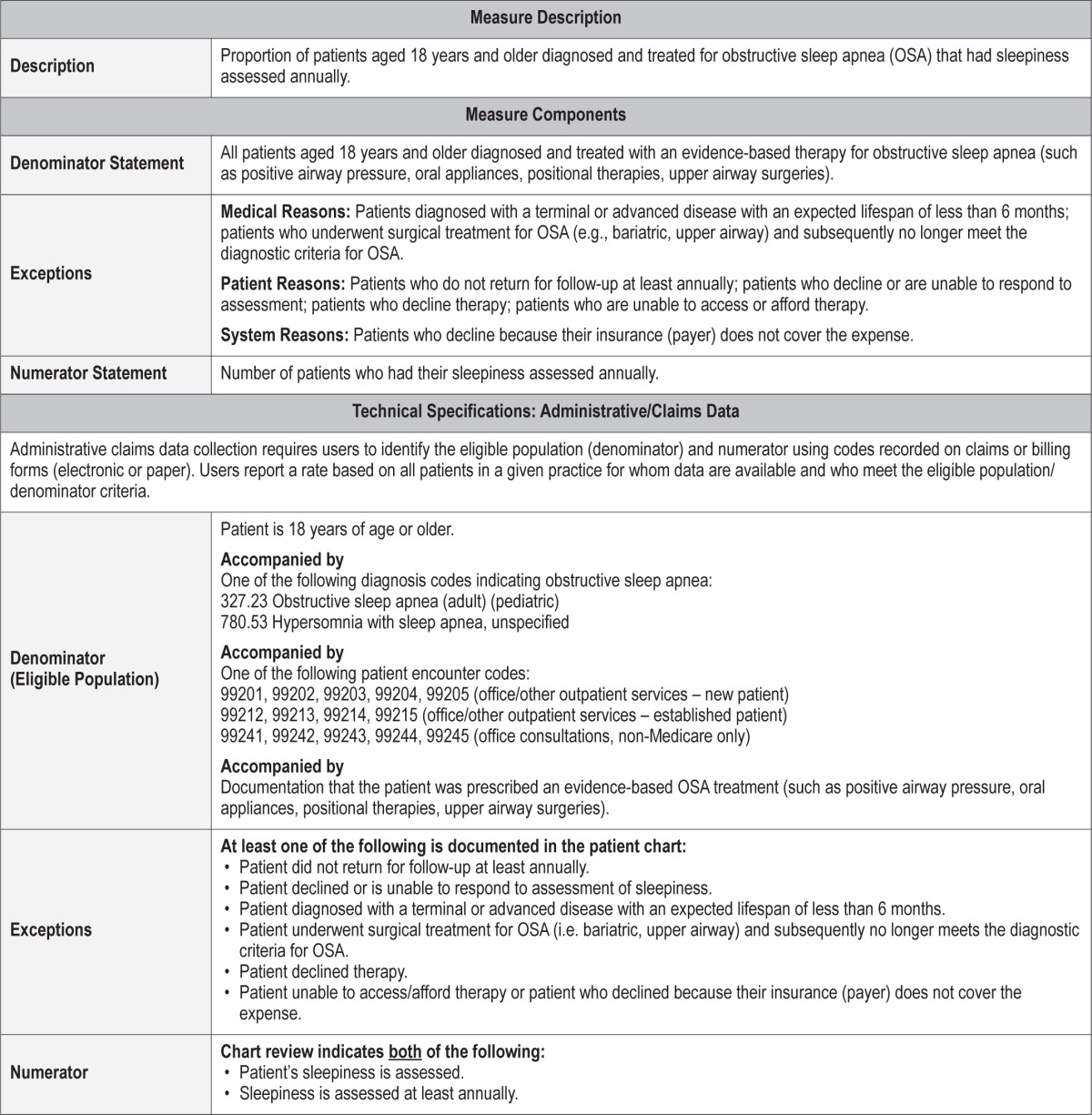
Process Measure 6 – Assessment of Motor Vehicle Crashes or Near-Miss Crashes
Description
Proportion of patients aged 18 years and older diagnosed with obstructive sleep apnea who were questioned about motor vehicle crashes (or near-miss crashes) associated with drowsiness/excessive sleepiness at initial evaluation.
Exceptions and Exception Justifications
Medical Reasons: None.
Patient Reasons: Patients who do not drive; patients who decline to respond.
System Reasons: None.
Patients who do not drive or who decline to respond cannot be assessed for this measure.
Supporting Evidence and Rationale
Assessment of motor vehicle crashes or near-miss crashes due to sleepiness in patients with OSA could be one of the most impactful process measures, as drowsy driving has significant public health and economic implications. This process measure provides the opportunity for healthcare providers to identify OSA patients at high risk for motor vehicle crashes. Two meta-analyses [Level 3] with a total of 10 studies showed sizeable protective effect of CPAP on traffic accidents (both simulated and real).78,79 Near-miss crashes or crashes secondary to sleepiness can lower quality of life by limiting the mobility and independence of patients with OSA. Furthermore, other people on the road are at risk for significant injury and death with a sleepy driver behind the wheel.
Relationship to Desired Outcome
Near-miss crashes or crashes secondary to sleepiness can lower quality of life by limiting mobility and independence of the patient with OSA. Additionally, other people are at risk around sleepy drivers, and if a motor vehicle crash does take place, their quality of life may also be compromised as a result.
Opportunities for Improvement/Gaps
This process measure provides the opportunity for health-care providers to identify OSA patients at high risk for motor vehicle crashes. The public healthcare and economic implications of this are significant as it could potentially improve safety on the road.
Issues Addressed During Development
There was significant consideration given to asking patients about motor vehicle crashes or near-miss motor vehicle crashes due to sleepiness at every visit. However, recognizing the burden of asking about motor vehicle crashes and near-miss motor vehicle crashes at every visit, the Workgroup decided that, at a minimum, the measure could be initiated with asking about motor vehicle crashes/near-miss motor vehicle crashes during the initial evaluation.
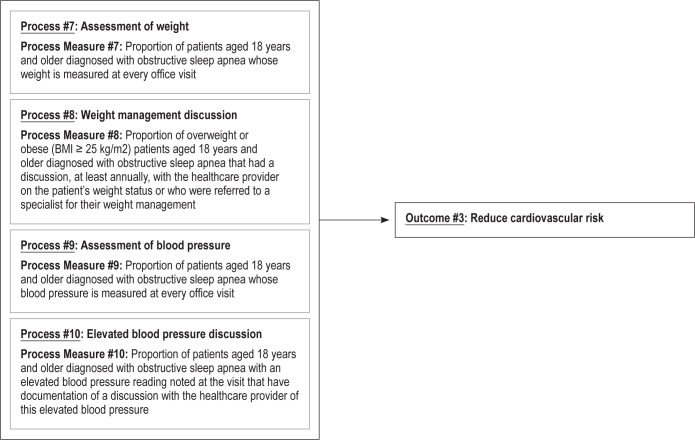
Outcome 3 – Reduce Cardiovascular Risk
Description
Outcome 3, which is not a measured outcome but rather a broad goal of care, is to reduce cardiovascular risk in patients with OSA.
Untreated OSA is associated with increased risk for cardiovascular disease,9 including hypertension,80 stroke,12 arrhythmias,81 coronary artery disease, and heart failure.82 A major impetus to treat OSA is to reduce cardiovascular risk. Since cardiovascular risk reduction is a long-term goal that can be difficult to measure, the Workgroup chose four process measures (#7–10) that will examine weight and blood pressure as ways to monitor cardiovascular risk.
Supporting Evidence and Rationale
A multitude of mechanisms have been implicated which link OSA to cardiovascular disease including intermittent deoxygenation/reoxygenation leading to oxidative stress, endothelial dysfunction, sympathetic dysfunction, inflammation, hypercoagulability, and metabolic dysregulation.83 It has been suggested that morbidity and mortality associated with OSA likely results from cardiovascular disease. Additionally, both hypertension and obesity frequently coexist with OSA and are known cardiovascular risk factors.4,80,84–87 Treatment of OSA has been shown to reduce some of the cardiovascular risks, specifically reduction in blood pressure88,89 and arrhythmias.90,91 In our process measures, therefore, we chose to target measurement of blood pressure and discussion of hypertension as key components to cardiovascular risk reduction.
Since obesity is present in > 50% of OSA patients,87 and obesity itself has adverse effects on the heart, including increase in cardiac output, increased risk of hypertension and diastolic dysfunction, and biventricular hypertrophy,92 we also chose to concentrate on weight. Reduction in BMI has been clearly shown to reduce OSA severity as well as reduce adverse effects on cardiac performance in obese patients.93,94 Therefore, we chose examination of weight and discussion of weight management as processes that can also assist in cardiovascular risk reduction.
Issues Addressed During Development
A number of ideas were considered for examining cardiovascular risk including documentation of improvement in oxygenation with treatment of OSA, the presence of cardiac arrhythmias, and the development of new cardiovascular disease states such as stroke, acute coronary syndrome, heart failure, etc., following OSA treatment. However, the burden of this documentation may have been too high on providers. Measuring weight and blood pressure and discussing weight and hypertension management options were felt to not only be important measures, but also ones that were already frequently performed in the assessment of OSA patients.
Process Measure 7 – Assessment of Weight
Description
Proportion of patients aged 18 years and older diagnosed with obstructive sleep apnea (OSA) whose weight is measured at every office visit.
Exceptions and Exception Justifications
Medical Reasons: Patients who are unable to get on the scale (e.g., wheelchair-bound); patients who are pregnant.
Patient Reasons: Patients who decline weight measurement. System Reasons: Patients who are unable to be weighed because the scale is not able to accommodate their weight; patients who have been seen and weighed within the past month.
If a patient cannot get on a scale, an accurate weight cannot be determined. A pregnant patient's weight often may not reflect the patient's non-pregnant weight and a recommendation of weight loss when pregnant may not be medically prudent. A patient also cannot be forced to undergo weighing. Some clinics either may not have a scale or may not have a scale that can accommodate weight larger than 300 pounds. A weight change occurring less than one month from the visit is unlikely to be large enough to make a difference in the patient's OSA risk.
Supporting Evidence and Rationale
Weight gain has been shown to be related to an increased risk for both developing and worsening OSA and, as a corollary, weight loss has been shown to reduce OSA severity.
It is known that obstructive sleep apnea is associated with being overweight. Although not all patients with OSA are overweight, the majority of patients with OSA are overweight. In addition, multiple studies have proven that weight loss can reduce OSA severity.70,95,96 [Levels 2, 4] Because of this strong relationship, it is important for healthcare providers managing OSA patients to evaluate the patient's weight to determine their level of risk.
Relationship to Desired Outcome
Weight measurement will allow both the healthcare providers and the patient to monitor changes in weight and should be discussed at each visit. Weight loss has been associated with reducing cardiovascular risk.
Opportunities for Improvement/Gaps
Given the reduction in severity of OSA following weight loss, measuring weight at each visit and discussing the results and setting goals, should help patients focus more on this important and often neglected aspect of treatment.
Issues Addressed During Development
In developing this measure we considered how frequently weight should be measured and if it was relevant to measure weight in non-overweight patients. We decided it was important to measure weight initially and then at some regular interval (typically at every office visit) to determine if weight gain or loss was occurring because of the impact of changes in weight on the treatment of this disorder. We also used the pregnancy exception that is present in the CMS quality reporting measure NQF 0421 BMI screening tool.
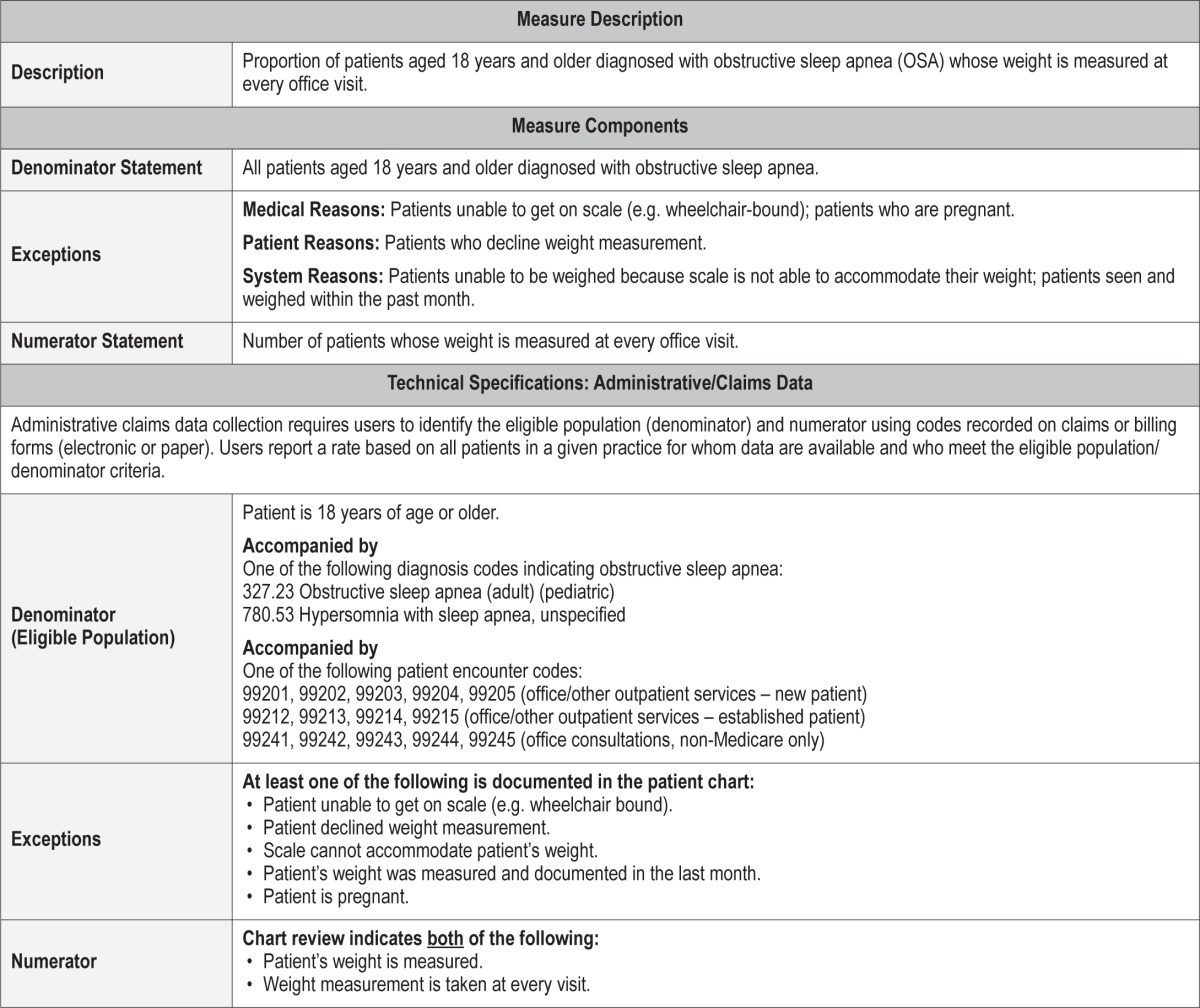
Process Measure 8 – Weight Management Discussion
Description
Proportion of overweight or obese (BMI ≥ 25 kg/m2) patients aged 18 years and older diagnosed with obstructive sleep apnea (OSA) who had a discussion at least annually with the healthcare provider on the patient's weight status, or who were referred to a specialist for their weight management
Exceptions and Exception Justifications
Medical Reasons: Patients who have been diagnosed with a terminal or advanced disease with an expected lifespan of less than 6 months; patients who are pregnant.
Patient Reasons: Patients who report they are currently in a weight management program.
System Reasons: None.
If a patient has an advanced or terminal disease, weight loss may have an adverse effect on their condition or be unreasonable to expect. Regarding pregnant patients, a recommendation of weight loss when pregnant may not be medically indicated. Patients currently in a weight management program have already met the purpose of this measure and do not need to be included.
Supporting Evidence and Rationale
Weight loss is a proven method for reducing the signs and symptoms of OSA.38,95–97 [Level 2, 3] For any patient with moderate to severe OSA, primary treatment for OSA should be initiated (e.g., CPAP, surgery, oral appliance); however, weight loss should be recommended as an adjunctive measure in those who are overweight or obese.
Although most patients who are overweight or obese have a desire to lose weight, many will not pursue specific weight reduction measures. It has been shown that overweight and obese patients are more likely to lose weight if their physician discusses their weight status and advises them on weight loss.98,99 [Level 3] The benefits of weight reduction should be discussed with all overweight or obese patients in order to draw attention to weight as a contributing factor and to have the patient begin to think about weight loss as a therapeutic strategy. Other strategies may be offered as well, including: recommendations for assisted self-management, including guidance on popular diets, providing information about commercial weight-loss programs, and referral to a weight management program or to a bariatric surgery program.100,101 [Level 4]
Relationship to Desired Outcome
The desired outcome is to assist overweight or obese OSA patients to enter into a meaningful weight loss program. Weight loss counseling is linked to reduced weight, reduced sleep disordered breathing, and reduced cardiovascular risk.
Opportunities for Improvement/Gaps
It is estimated that > 70% of OSA patients are overweight or obese and that if able to lose weight, the severity of OSA will diminish or completely resolve.102 A recent meta-analysis of patients undergoing bariatric surgery showed that of those with obstructive sleep apnea, > 75% had improvement or resolution of sleep apnea following the procedure and weight loss.103 [Level 2] However, most patients do not pursue weight loss and, in fact, a recent article suggested that many patients may gain weight after initiating CPAP.104 [Level 3] At a minimum, all overweight or obese patients should be offered counseling about weight reduction.
Issues Addressed During Development
Most discussion around this issue was the frequency to which weight loss counseling should occur. The Workgroup decided it was not unreasonable to have OSA patients seen annually in follow-up and that the healthcare provider should discuss weight management during that visit. Although many felt weight management may be solely the purview of primary care providers, given its important role in the pathophysiology of OSA, it was determined that this was a vital part of OSA management.
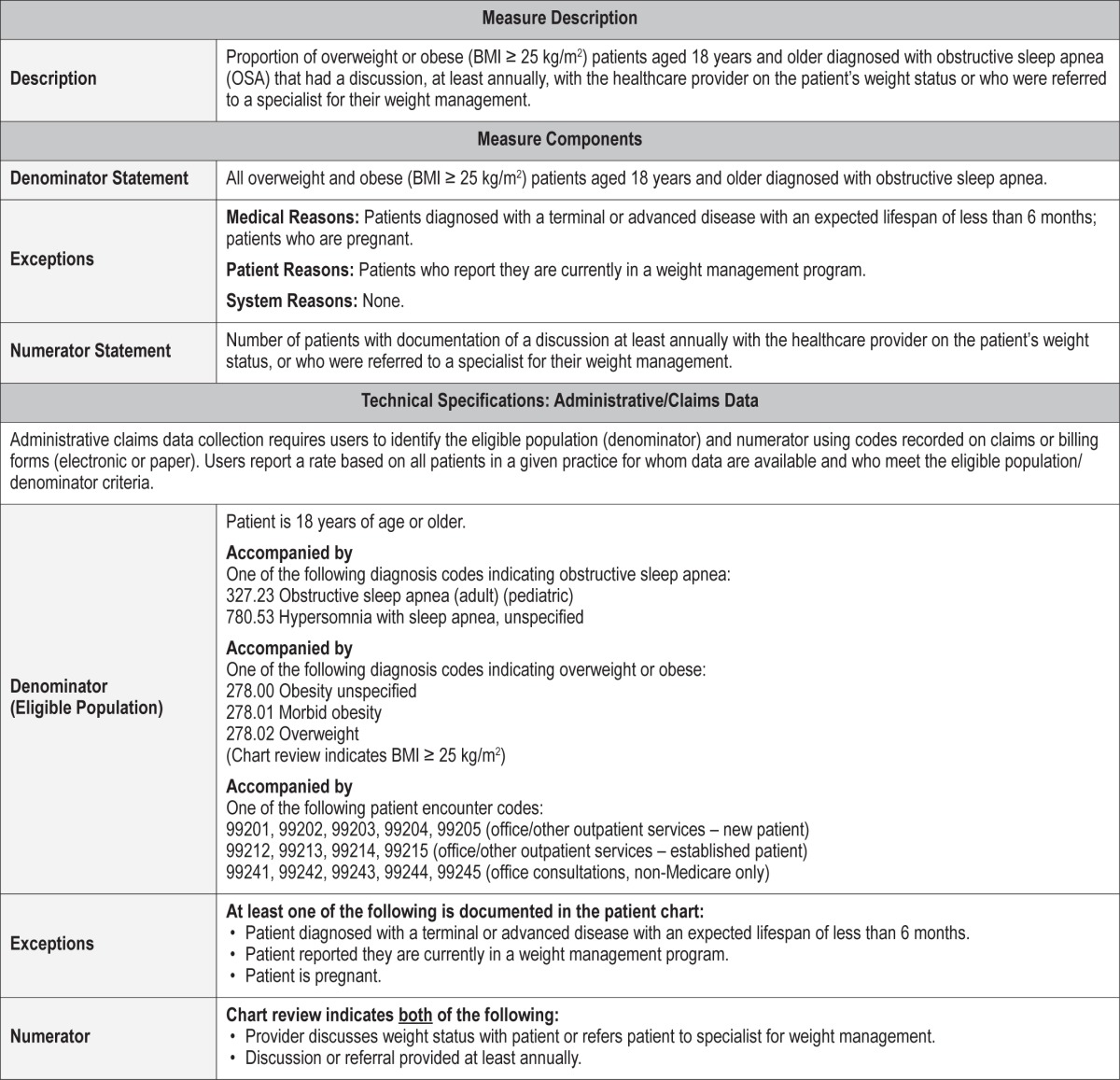
Process Measure 9 – Assessment of Blood Pressure
Description
Proportion of patients aged 18 years and older diagnosed with obstructive sleep apnea (OSA) whose blood pressure is measured at every office visit.
Exceptions and Exception Justifications
Medical Reasons: None.
Patient Reasons: Patients who have documented blood pressure measurement within the past 24 hours; patients who decline blood pressure measurement.
System Reasons: Blood pressure cuff is not available, not functional, or is the wrong size.
Supporting Evidence and Rationale
Obstructive sleep apnea is associated with increased prevalence and incidence of hypertension. The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JCN 7) lists OSA as a causal risk factor for hypertension.105 [Level 2] OSA is associated with cardiovascular events, including stroke106 [Level 2] and other new onset cardiovascular disease. [Level 4] Effective management of severe OSA reduces measures of blood pressure in hyper-tensive sleep apnea patients85,107,108 [Level 4] and may reduce blood pressure in patients with milder disease.109 [Level 4] As elevated blood pressure is an accepted major risk factor for cardiovascular disease, identifying the group at risk allows for the incidence of cardiovascular events to be reduced by effective treatment.85,107,108,110 [Level 4]
Relationship to Desired Outcome
Hypertension is a risk factor for cardiovascular and cerebrovascular disease. Symptomatic OSA is associated with increased risk of hypertension and stroke with potential risks to other cardiovascular morbidities such as heart failure, atrial fibrillation, peripheral vascular disease, heart attack, stroke, and increased risk of cardiovascular-related mortality.
Opportunities for Improvement/Gaps
Routine identification and monitoring of blood pressure increases awareness of healthcare providers and patients to the hypertension risk associated OSA. Effective treatment of symptomatic OSA has the potential of lowering cardiovascular morbidity.
Issues Addressed During Development
For this particular measure, discussion involved the need for blood pressure measurement at every visit and whether this imposes excessive burden to the medical staff and patient. Given the considerable evidence demonstrating an association between OSA and hypertension, it was determined that measurement of blood pressure at every visit presents an opportunity for early identification, intervention, and possible prevention of this OSA-related comorbidity.
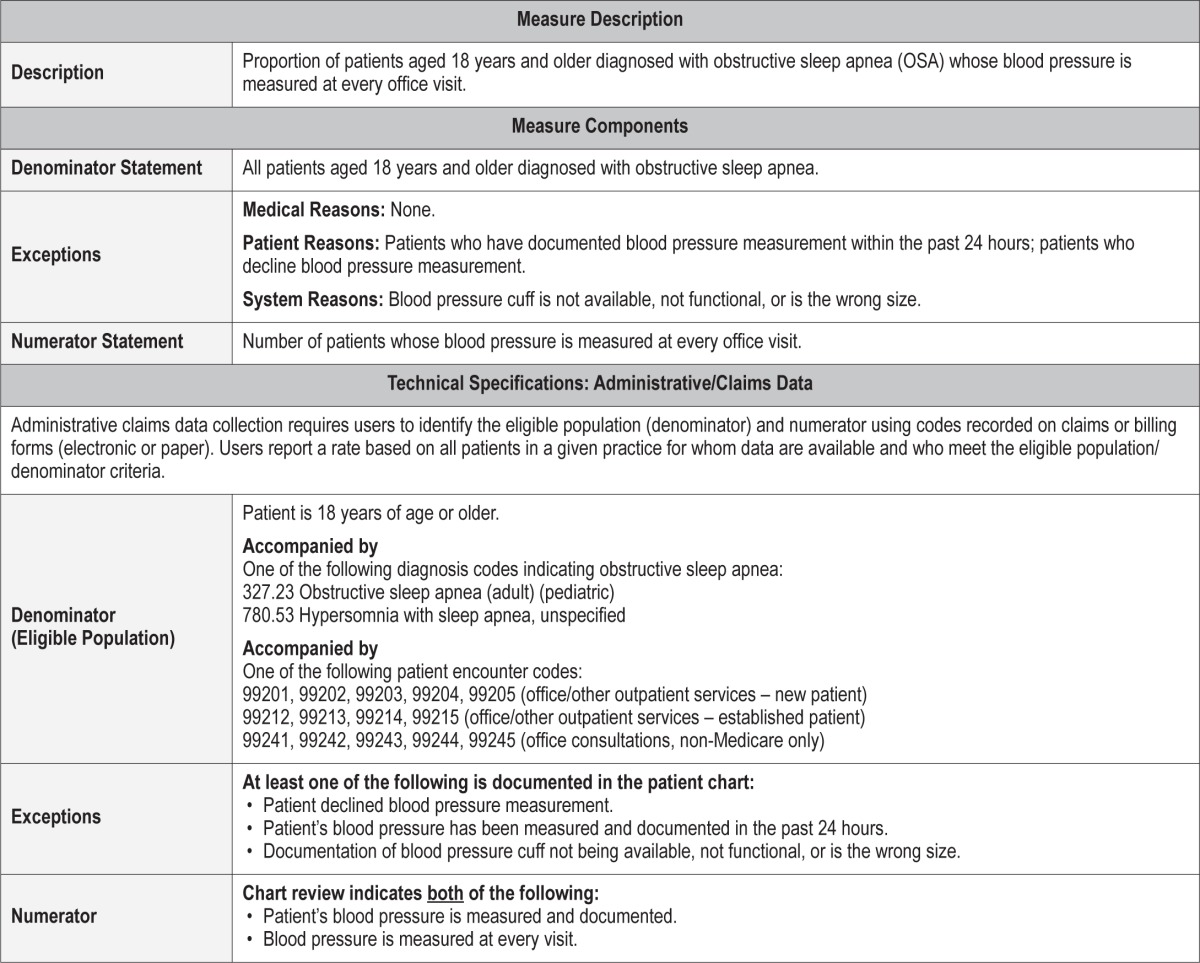
Process Measure 10 – Elevated Blood Pressure Discussion
Description
Proportion of patients aged 18 years and older diagnosed with obstructive sleep apnea (OSA) with an elevated blood pressure reading (according to the most recent Joint National Committee guideline for high blood pressure) noted at the visit who have documentation of a discussion with the healthcare provider of this elevated blood pressure
Exceptions and Exception Justifications
Medical Reasons: None.
Patient Reasons: None.
System Reasons: Patients who have had a discussion with another healthcare provider in the last 24 hours about their elevated blood pressure.
If there is documentation that the patient had their elevated blood pressure issue discussed within the last 24 hours, then readdressing their blood pressure within that time frame is unlikely to have added benefit or significantly change clinical outcomes.
Supporting Evidence and Rationale
Patients with obstructive sleep apnea demonstrate a higher prevalence and incidence of hypertension.80,109,111,112 [Level 2]
Treatment of symptomatic OSA may reduce blood pressure but does not eliminate hypertension risk.113 [Level 3] Clinical guidelines recommend that “those on chronic therapy should have monitoring for development of medical complications related to OSA.”38 [Level 3]
Relationship to Desired Outcome
Obstructive sleep apnea has been associated with increased risk of hypertension. Hypertension is considered a significant mediator of other sleep apnea cardiovascular and cerebrovascular comorbidities including congestive heart failure (CHF), coronary artery disease (CAD), stroke, and atrial fibrillation. In addition, effective treatment of obstructive sleep apnea has been demonstrated to reduce blood pressure.
Opportunities for Improvement/Gaps
Symptomatic OSA is identified with hypertension and cardiovascular diseases that are often associated with hypertension. In many patients, a treatment goal of OSA is to reduce cardiovascular risk. In patients with diagnosed hypertension, increased patient and healthcare provider awareness will improve care and health outcomes.
Issues Addressed During Development
For this process measure, there was discussion about how detailed the discussion should be and what should be addressed in the discussion. It was recommended that the discussion should be targeted to those who are noted to have an elevated blood pressure during their visit for OSA management. The discussion should make the patient aware of their elevated blood pressure and the need to monitor and manage their blood pressure.
IMPLEMENTATION STRATEGIES
The value of quality measures is well described and parallels the provision of quality patient care. However, healthcare professionals may feel that reporting additional measurements is too burdensome, time-consuming, or difficult to integrate into a busy medical practice. The Workgroup felt that the benefits of outcome measurement add value to the care of the sleep patient and encourages its integration into clinical practice. Practice transformation may be facilitated by the adoption of these measures in the electronic health record (EHR). This could be done directly or through an EHR “overlay” system or a template which could extract measures and transmit data. In the future, these measures may be reported through the Patient Quality Reporting System (PQRS) in addition to, or as a replacement for, the current sleep apnea measures group. Still some healthcare professionals may find it necessary to extract data manually from paper health records. The Workgroup acknowledges these challenges but also recognizes the importance of these efforts.
The obstructive sleep apnea literature is robust, particularly when compared to other sleep disorders. However, opportunities exist for research specific to the areas of patient-reported outcomes and quality of life measures. Concurrently, as scientific research advances, quality measures will need to be updated to reflect the most recent disease management paradigms. It should be noted that these measures were not risk adjusted to specific patient populations. As this process continues to mature, risk adjustment remains an additional challenge for future measures development.
Ultimately, integration of these outcome measures into sleep medicine practices will require provider education and acknowledgement that measuring patient outcomes equates with the shared goal of providing excellent patient care.
FUTURE DIRECTIONS
There are many rationales for treating OSA. Untreated OSA impairs daytime functioning, limits quality of life, accelerates multiple health risks, compromises public safety, and results in increased healthcare spending. The Adult OSA Workgroup believes that use of these quality measures will result in improved patient outcomes in these areas. However, for this to occur, there must be widespread adoption of these measures by all clinicians that frequently evaluate and treat patients with OSA. Clinicians must acknowledge the importance of these outcomes and strive to integrate them into their patient treatment plans. To facilitate this work flow, these measures should be incorporated into AASM center accreditation standards. In addition, payers should be urged to adopt these measures as part of their quality assessments of providers.
Quality measures are not static. The field of sleep medicine, and especially the evaluation and treatment of OSA, is evolving quickly. It will be important to determine whether these measures have improved patient outcomes and whether they are relevant in the years to come. For this to occur, results will need to be available so that appropriate analyses can be performed. Future quality measures may also need to be specific to individual therapeutic modalities to better validate the data obtained.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Collop is Editor-In-Chief of the Journal of Clinical Sleep Medicine and has received royalties from UpToDate. Dr. Jacobowitz has received research support from ImThera Medical Research. Dr. Thomas is an employee of the American Academy of Sleep Medicine. Dr. Quan is Editor Emeritus of the Journal of Clinical Sleep Medicine and has consulted for GCC (Global Corporate Challenge). Dr. Aronsky is employed by CareCentrix, Inc., a benefit management company and is a past member of the American Academy of Sleep Medicine Board of Directors. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The American Academy of Sleep Medicine (AASM) would like to thank B. Tucker Woodson, MD for his contributions to the initial discussions and development of these OSA quality measures. The AASM would also like to thank the following parties for their review of these measures, and for providing feedback and suggestions to improve the relevancy and utility of these measures in their field of practice: Imtiaz Ahmed, MD; Mohammad Amin, MD; Ruth Boland, RN; Loretta Colvin, MD; Naresh Dewan, MD; Bonnie Norris, RN; American Sleep Apnea Association (ASAA); American Association of Oral and Maxillofacial Surgeons (AAOMS), American College of Chest Physicians (ACCP), American Society of Anesthesiologists (ASA), American Society of Bariatric Physicians (ASBP), and the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS). The AASM did not seek or receive endorsement of these measures from any of the reviewers or the respective organizations who provided feedback. The authors carefully considered all feedback provided, and implemented as many suggestions as were feasible in the refining of these measures. We would also like to thank Carolyn Winter-Rosenberg, AASM Director of Coding and Compliance, for guidance in compiling the technical specifications associated with these quality measures.
APPENDIX
The following are the technical specifications for the adult OSA quality measures, which can be used to calculate an individual provider's performance in meeting these measures. Tracking and periodically reviewing this performance data will help providers identify opportunities for improvement within their own practices.
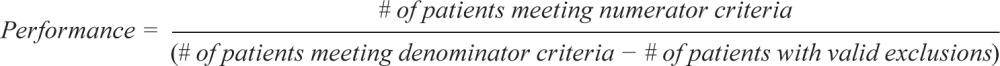 |
Outcome Measure #2: Improve quality of life
Process Measure #1: Baseline assessment of OSA symptoms
Process Measure #2: Severity assessment at initial diagnosis
Process Measure #3: Evidence-based therapy prescribed
Process Measure #4: Assessment of adherence to OSA therapy
Process Measure #5: Assessment of sleepiness
Process Measure #6: Assessment of motor vehicle crashes or near-miss crashes
Process Measure #7: Assessment of weight
Process Measure #8: Weight management discussion
Process Measure #9: Assessment of blood pressure
Process Measure #10: Elevated blood pressure discussion
REFERENCES
- 1.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stradling JR, Davies RJ. Sleep. 1: Obstructive sleep apnoea/hypopnoea syndrome: definitions, epidemiology, and natural history. Thorax. 2004;59:73–8. doi: 10.1136/thx.2003.007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 5.Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6:49–54. doi: 10.1007/s11325-002-0049-5. [DOI] [PubMed] [Google Scholar]
- 6.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–6. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin CM, Griffith KA, Nieto FJ, O'Connor GT, Walsleben JA, Redline S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24:96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- 8.Lopes C, Esteves AM, Bittencourt LR, Tufik S, Mello MT. Relationship between the quality of life and the severity of obstructive sleep apnea syndrome. Braz J Med Biol Res. 2008;41:908–13. doi: 10.1590/s0100-879x2008005000036. [DOI] [PubMed] [Google Scholar]
- 9.Lurie A. Cardiovascular disorders associated with obstructive sleep apnea. Adv Cardiol. 2011;46:197–266. doi: 10.1159/000325110. [DOI] [PubMed] [Google Scholar]
- 10.Aurora RN, Punjabi NM. Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. Lancet Respir Med. 2013;1:329–38. doi: 10.1016/S2213-2600(13)70039-0. [DOI] [PubMed] [Google Scholar]
- 11.Gharibeh T, Mehra R. Obstructive sleep apnea syndrome: natural history, diagnosis, and emerging treatment options. Nat Sci Sleep. 2010;2:233–55. doi: 10.2147/NSS.S6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horstmann S, Hess CW, Bassetti C, Gugger M, Mathis J. Sleepiness-related accidents in sleep apnea patients. Sleep. 2000;23:383–9. [PubMed] [Google Scholar]
- 15.Sassani A, Findley LJ, Kryger M, Goldlust E, George C, Davidson TM. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep. 2004;27:453–8. doi: 10.1093/sleep/27.3.453. [DOI] [PubMed] [Google Scholar]
- 16.Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340:847–51. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 17.AlGhanim N, Comondore VR, Fleetham J, Marra CA, Ayas NT. The economic impact of obstructive sleep apnea. Lung. 2008;186:7–12. doi: 10.1007/s00408-007-9055-5. [DOI] [PubMed] [Google Scholar]
- 18.The Harvard Medical School Division of Sleep Medicine. The price of fatigue: the suprising economic costs of unamanged sleep apnea. 2010. [cited 2014 August 8]; Available from: https://sleep.med.harvard.edu/file_download/100.
- 19.Albarrak M, Banno K, Sabbagh AA, et al. Utilization of healthcare resources in obstructive sleep apnea syndrome: a 5-year follow-up study in men using CPAP. Sleep. 2005;28:1306–11. doi: 10.1093/sleep/28.10.1306. [DOI] [PubMed] [Google Scholar]
- 20.Banno K, Manfreda J, Walld R, Delaive K, Kryger MH. Healthcare utilization in women with obstructive sleep apnea syndrome 2 years after diagnosis and treatment. Sleep. 2006;29:1307–11. doi: 10.1093/sleep/29.10.1307. [DOI] [PubMed] [Google Scholar]
- 21.Weaver TE, Mancini C, Maislin G, et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. Am J Respir Crit Care Med. 2012;186:677–83. doi: 10.1164/rccm.201202-0200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–23. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Rio F, Alonso-Fernández A, Armada E, et al. CPAP effect on recurrent episodes in patients with sleep apnea and myocardial infarction. Int J Cardiol. 2013;168:1328–35. doi: 10.1016/j.ijcard.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Kielb SA, Ancoli-Israel S, Rebok GW, Spira AP. Cognition in obstructive sleep apnea-hypopnea syndrome (OSAS): current clinical knowledge and the impact of treatment. Neuromolecular Med. 2012;14:180–93. doi: 10.1007/s12017-012-8182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun H, Shi J, Li M, Chen X. Impact of continuous positive airway pressure treatment on left ventricular ejection fraction in patients with obstructive sleep apnea: a meta-analysis of randomized controlled trials. PLoS One. 2013;8:e62298. doi: 10.1371/journal.pone.0062298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgenthaler TI, Aronsky AJ, Carden KA, Chervin RD, Thomas SM, Watson NF. Measurement of quality to improve care in sleep medicine. J Clin Sleep Med. 2015;11:279–91. doi: 10.5664/jcsm.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Academy of Sleep Medicine, Physician Consortium for Performance Improvement, National Committee for Quality Insurance. Obstructive Sleep Apnea Physician Performance Measurement Set. 2008. Sep 28, Available from: https://www.aan.com/practice/quality-measures/all-measures/
- 28.US Department of Health and Human Services. 2013 Annual Progress Report to Congress: National Strategy for Quality Improvement in Health Care. 2013. Jul, Available at: http://www.ahrq.gov/workingforquality/nqs/nqs2013annlrpt.htm.
- 29.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–47. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stephen GA, Eichling PS, Quan SF. Treatment of sleep disordered breathing and obstructive sleep apnea. Minerva Med. 2004;95:323–36. [PubMed] [Google Scholar]
- 31.Sunitha C, Kumar SA. Obstructive sleep apnea and its management. Indian J Dent Res. 2010;21:119–24. doi: 10.4103/0970-9290.62806. [DOI] [PubMed] [Google Scholar]
- 32.Guilleminault C, Dement WC. Clinical overview of the sleep apnea syndromes. In: Guilleminault C, Dement WC, editors. Sleep apnea syndromes. New York: A. R. Liss; 1978. [Google Scholar]
- 33.Mannarino MR, Di Filippo F, Pirro M. Obstructive sleep apnea syndrome. Eur J Intern Med. 2012;23:586–93. doi: 10.1016/j.ejim.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 34.American Academy of Sleep Medicine. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 35.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 36.Berry RB, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughn BV for the American Academy of Sleep Medicine. Darien, IL: American Academy of Sleep Medicine; 2014. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Version 2.1. [Google Scholar]
- 37.Collop NA, Tracy SL, Kapur V, et al. Obstructive sleep apnea devices for out-of-center (OOC) testing: technology evaluation. J Clin Sleep Med. 2011;7:531–48. doi: 10.5664/JCSM.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 39.Gay P, Weaver T, Loube D, Iber C Positive Airway Pressure Task Force, Standards of Practice Committee, American Academy of Sleep Medicine. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 40.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 41.Qaseem A, Holty JE, Owens DK, Dallas P, Starkey M, Shekelle P for the Clinical Guidelines Committee of the American College of Physicians. Management of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:471–83. doi: 10.7326/0003-4819-159-7-201310010-00704. [DOI] [PubMed] [Google Scholar]
- 42.Practice parameters for the use of portable recording in the assessment of obstructive sleep apnea. Standards of Practice Committee of the American Sleep Disorders Association. Sleep. 1994;17:372–7. [PubMed] [Google Scholar]
- 43.Marquis P, Caron M, Emery M, et al. The role of health-related quality of life data in the drug approval processes in the US and Europe: a review of guidance documents and authorizations of medicinal products from 2006 to 2010. Pharm Med. 2011;25:147–60. [Google Scholar]
- 44.D'Ambrosio C, Bowman T, Mohsenin V. Quality of life in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure--a prospective study. Chest. 1999;115:123–9. doi: 10.1378/chest.115.1.123. [DOI] [PubMed] [Google Scholar]
- 45.Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res. 1997;6:199–204. doi: 10.1046/j.1365-2869.1997.00043.x. [DOI] [PubMed] [Google Scholar]
- 46.Jing J, Huang T, Cui W, Shen H. Effect on quality of life of continuous positive airway pressure in patients with obstructive sleep apnea syndrome: a meta-analysis. Lung. 2008;186:131–44. doi: 10.1007/s00408-008-9079-5. [DOI] [PubMed] [Google Scholar]
- 47.Kuna ST, Gurubhagavatula I, Maislin G, et al. Noninferiority of functional outcome in ambulatory management of obstructive sleep apnea. Am J Respir Crit Care Med. 2011;183:1238–44. doi: 10.1164/rccm.201011-1770OC. [DOI] [PubMed] [Google Scholar]
- 48.Phillips CL, Cistulli PA, Grunstein RR. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879–87. doi: 10.1164/rccm.201212-2223OC. [DOI] [PubMed] [Google Scholar]
- 49.Woodson BT, Steward DL, Weaver EM, Javaheri S. A randomized trial of temperature-controlled radiofrequency, continuous positive airway pressure, and placebo for obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg. 2003;128:848–61. doi: 10.1016/S0194-59980300461-3. [DOI] [PubMed] [Google Scholar]
- 50.Ware J, Kosinski M, Keller S. SF-36 physical and mental health summary scales: a user's manual. Health Assessment Lab. 1994 [Google Scholar]
- 51.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 52.Hunt SM, McKenna SP, McEwen J, Backett EM, Williams J, Papp E. A quantitative approach to perceived health status: a validation study. J Epidemiol Community Health. 1980;34:281–6. doi: 10.1136/jech.34.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 54.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–43. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 55.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 56.Flemons WW, Reimer MA. Measurement properties of the calgary sleep apnea quality of life index. Am J Respir Crit Care Med. 2002;165:159–64. doi: 10.1164/ajrccm.165.2.2010008. [DOI] [PubMed] [Google Scholar]
- 57.Doff MH, Hoekema A, Wijkstra PJ, et al. Oral appliance versus continuous positive airway pressure in obstructive sleep apnea syndrome: a 2-year follow-up. Sleep. 2013;36:1289–96. doi: 10.5665/sleep.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kushida CA, Littner MR, Hirshkowitz M, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29:375–80. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 59.Petri N, Svanholt P, Solow B, Wildschiødtz G, Winkel P. Mandibular advancement appliance for obstructive sleep apnoea: results of a randomised placebo controlled trial using parallel group design. J Sleep Res. 2008;17:221–9. doi: 10.1111/j.1365-2869.2008.00645.x. [DOI] [PubMed] [Google Scholar]
- 60.Gotsopoulos H, Chen C, Qian J, Cistulli PA. Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:743–8. doi: 10.1164/rccm.200203-208OC. [DOI] [PubMed] [Google Scholar]
- 61.Naismith SL, Winter VR, Hickie IB, Cistulli PA. Effect of oral appliance therapy on neurobehavioral functioning in obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2005;1:374–80. [PubMed] [Google Scholar]
- 62.Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396–407. doi: 10.1093/sleep/33.10.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holty JE, Guilleminault C. Maxillomandibular advancement for the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2010;14:287–97. doi: 10.1016/j.smrv.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 64.Robinson S, Chia M, Carney AS, Chawla S, Harris P, Esterman A. Upper airway reconstructive surgery long-term quality-of-life outcomes compared with CPAP for adult obstructive sleep apnea. Otolaryngol Head Neck Surg. 2009;141:257–63. doi: 10.1016/j.otohns.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 65.Vicini C, Dallan I, Campanini A, et al. Surgery vs ventilation in adult severe obstructive sleep apnea syndrome. Am J Otolaryngol. 2010;31:14–20. doi: 10.1016/j.amjoto.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 66.Jokic R, Klimaszewski A, Crossley M, Sridhar G, Fitzpatrick MF. Positional treatment vs continuous positive airway pressure in patients with positional obstructive sleep apnea syndrome. Chest. 1999;115:771–81. doi: 10.1378/chest.115.3.771. [DOI] [PubMed] [Google Scholar]
- 67.Oksenberg A, Silverberg D, Offenbach D, Arons E. Positional therapy for obstructive sleep apnea patients: a 6-month follow-up study. Laryngoscope. 2006;116:1995–2000. doi: 10.1097/01.mlg.0000237674.66716.a7. [DOI] [PubMed] [Google Scholar]
- 68.Skinner MA, Kingshott RN, Filsell S, Taylor DR. Efficacy of the ‘tennis ball technique’ versus nCPAP in the management of position-dependent obstructive sleep apnoea syndrome. Respirology. 2008;13:708–15. doi: 10.1111/j.1440-1843.2008.01328.x. [DOI] [PubMed] [Google Scholar]
- 69.Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29:240–3. doi: 10.1093/sleep/29.2.240. [DOI] [PubMed] [Google Scholar]
- 70.Morgenthaler TI, Kapen S, Lee-Chiong T, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29:1031–5. [PubMed] [Google Scholar]
- 71.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29:244–62. doi: 10.1093/sleep/29.2.244. [DOI] [PubMed] [Google Scholar]
- 74.Hoffstein V. Review of oral appliances for treatment of sleep-disordered breathing. Sleep Breath. 2007;11:1–22. doi: 10.1007/s11325-006-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vanderveken OM, Dieltjens M, Wouters K, De Backer WA, Van de Heyning PH, Braem MJ. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax. 2013;68:91–6. doi: 10.1136/thoraxjnl-2012-201900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bignold JJ, Deans-Costi G, Goldsworthy MR, et al. Poor long-term patient compliance with the tennis ball technique for treating positional obstructive sleep apnea. J Clin Sleep Med. 2009;5:428–30. [PMC free article] [PubMed] [Google Scholar]
- 77.Silva GE, An MW, Goodwin JL, et al. Longitudinal evaluation of sleep-disordered breathing and sleep symptoms with change in quality of life: the Sleep Heart Health Study (SHHS) Sleep. 2009;32:1049–57. doi: 10.1093/sleep/32.8.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Antonopoulos CN, Sergentanis TN, Daskalopoulou SS, Petridou ET. Nasal continuous positive airway pressure (nCPAP) treatment for obstructive sleep apnea, road traffic accidents and driving simulator performance: a meta-analysis. Sleep Med Rev. 2011;15:301–10. doi: 10.1016/j.smrv.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 79.Tregear S, Reston J, Schoelles K, Phillips B. Continuous positive airway pressure reduces risk of motor vehicle crash among drivers with obstructive sleep apnea: systematic review and meta-analysis. Sleep. 2010;33:1373–80. doi: 10.1093/sleep/33.10.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 81.Vizzardi E, Sciatti E, Bonadei I, D'Aloia A, Curnis A, Metra M. Obstructive sleep apnoea-hypopnoea and arrhythmias: new updates. J Cardiovasc Med (Hagerstown) 2014 Jul 3; doi: 10.2459/JCM.0000000000000043. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 82.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Badran M, Ayas N, Laher I. Cardiovascular complications of sleep apnea: role of oxidative stress. Oxid Med Cell Longev. 2014;2014:985258. doi: 10.1155/2014/985258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown MA, Goodwin JL, Silva GE, et al. The impact of sleep-disordered breathing on body mass index (BMI): The Sleep Heart Health Study (SHHS) Southwest J Pulm Crit Care. 2011;3:159–68. [PMC free article] [PubMed] [Google Scholar]
- 85.Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307:2169–76. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med. 2005;165:2408–13. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 87.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 88.Becker HF, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 89.Schein AS, Kerkhoff AC, Coronel CC, Plentz RD, Sbruzzi G. Continuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea; a systematic review and meta-analysis with 1000 patients. J Hypertens. 2014;32:1762–73. doi: 10.1097/HJH.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 90.Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2013;62:300–5. doi: 10.1016/j.jacc.2013.03.052. [DOI] [PubMed] [Google Scholar]
- 91.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–94. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 92.Alpert MA, Lavie CJ, Agrawal H, Aggarwal KB, Kumar SA. Obesity and heart failure: epidemiology, pathophysiology, clinical manifestations, and management. Transl Res. 2014;164:345–56. doi: 10.1016/j.trsl.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 93.Haufe S, Utz W, Engeli S, et al. Left ventricular mass and function with reduced-fat or reduced-carbohydrate hypocaloric diets in overweight and obese subjects. Hypertension. 2012;59:70–5. doi: 10.1161/HYPERTENSIONAHA.111.178616. [DOI] [PubMed] [Google Scholar]
- 94.Rider OJ, Francis JM, Ali MK, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol. 2009;54:718–26. doi: 10.1016/j.jacc.2009.02.086. [DOI] [PubMed] [Google Scholar]
- 95.Fleetham J, Ayas N, Bradley D, et al. Canadian Thoracic Society 2011 guideline update: diagnosis and treatment of sleep disordered breathing. Can Respir J. 2011;18:25–47. doi: 10.1155/2011/506189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Veasey SC, Guilleminault C, Strohl KP, Sanders MH, Ballard RD, Magalang UJ. Medical therapy for obstructive sleep apnea: a review by the Medical Therapy for Obstructive Sleep Apnea Task Force of the Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep. 2006;29:1036–44. doi: 10.1093/sleep/29.8.1036. [DOI] [PubMed] [Google Scholar]
- 97.Randerath WJ, Verbraecken J, Andreas S, et al. Non-CPAP therapies in obstructive sleep apnoea. Eur Respir J. 2011;37:1000–28. doi: 10.1183/09031936.00099710. [DOI] [PubMed] [Google Scholar]
- 98.Pool AC, Kraschnewski JL, Cover LA, et al. The impact of physician weight discussion on weight loss in US adults. Obes Res Clin Pract. 2014;8:e131–9. doi: 10.1016/j.orcp.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rose SA, Poynter PS, Anderson JW, Noar SM, Conigliaro J. Physician weight loss advice and patient weight loss behavior change: a literature review and meta-analysis of survey data. Int J Obes (Lond) 2013;37:118–28. doi: 10.1038/ijo.2012.24. [DOI] [PubMed] [Google Scholar]
- 100.Rao G. Office-based strategies for the management of obesity. Am Fam Physician. 2010;81:1449–56. quiz 1429. [PubMed] [Google Scholar]
- 101.Tuomilehto HP, Seppä JM, Partinen MM, et al. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179:320–7. doi: 10.1164/rccm.200805-669OC. [DOI] [PubMed] [Google Scholar]
- 102.Resta O, Foschino-Barbaro MP, Legari G, et al. Sleep-related breathing disorders, loud snoring and excessive daytime sleepiness in obese subjects. Int J Obes Relat Metab Disord. 2001;25:669–75. doi: 10.1038/sj.ijo.0801603. [DOI] [PubMed] [Google Scholar]
- 103.Sarkhosh K, Switzer NJ, El-Hadi M, Birch DW, Shi X, Karmali S. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg. 2013;23:414–23. doi: 10.1007/s11695-012-0862-2. [DOI] [PubMed] [Google Scholar]
- 104.Quan SF, Budhiraja R, Clarke DP, et al. Impact of treatment with continuous positive airway pressure (CPAP) on weight in obstructive sleep apnea. J Clin Sleep Med. 2013;9:989–93. doi: 10.5664/jcsm.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.U.S. Department of Health and Human Services. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) National Institutes of Health: National Heart, Lung, and Blood Institute. 2004 NIH Publication No. 04-5230. [PubMed] [Google Scholar]
- 106.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 107.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 108.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 109.Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5:720–8. doi: 10.1161/CIRCOUTCOMES.111.964783. [DOI] [PubMed] [Google Scholar]
- 110.Iftikhar IH, Hays ER, Iverson MA, Magalang UJ, Maas AK. Effect of oral appliances on blood pressure in obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med. 2013;9:165–74. doi: 10.5664/jcsm.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Duran-Cantolla J, Aizpuru F, Martínez-Null C, Barbé-Illa F. Obstructive sleep apnea/hypopnea and systemic hypertension. Sleep Med Rev. 2009;13:323–31. doi: 10.1016/j.smrv.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 112.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 113.Alajmi M, Mulgrew AT, Fox J, et al. Impact of continuous positive airway pressure therapy on blood pressure in patients with obstructive sleep apnea hypopnea: a meta-analysis of randomized controlled trials. Lung. 2007;185:67–72. doi: 10.1007/s00408-006-0117-x. [DOI] [PubMed] [Google Scholar]