Abstract
Hyperreflexia and spasticity are chronic complications in spinal cord injury (SCI), with limited options for safe and effective treatment. A central mechanism in spasticity is hyperexcitability of the spinal stretch reflex, which presents symptomatically as a velocity-dependent increase in tonic stretch reflexes and exaggerated tendon jerks. In this study we tested the hypothesis that dendritic spine remodeling within motor reflex pathways in the spinal cord contributes to H-reflex dysfunction indicative of spasticity after contusion SCI. Six weeks after SCI in adult Sprague-Dawley rats, we observed changes in dendritic spine morphology on α-motor neurons below the level of injury, including increased density, altered spine shape, and redistribution along dendritic branches. These abnormal spine morphologies accompanied the loss of H-reflex rate-dependent depression (RDD) and increased ratio of H-reflex to M-wave responses (H/M ratio). Above the level of injury, spine density decreased compared with below-injury spine profiles and spine distributions were similar to those for uninjured controls. As expected, there was no H-reflex hyperexcitability above the level of injury in forelimb H-reflex testing. Treatment with NSC23766, a Rac1-specific inhibitor, decreased the presence of abnormal dendritic spine profiles below the level of injury, restored RDD of the H-reflex, and decreased H/M ratios in SCI animals. These findings provide evidence for a novel mechanistic relationship between abnormal dendritic spine remodeling in the spinal cord motor system and reflex dysfunction in SCI.
Keywords: H-reflex, hyperreflexia, Rac1, spasticity, spinal cord injury
hyperreflexia and spasticity, which arise in up to 60% of patients with spinal cord injury (SCI), can severely affect quality of life, contribute to chronic pain, and lead to musculoskeletal deformity (Skold et al. 1999; Walter et al. 2002). Although currently available drugs, such as baclofen, can provide some relief, these drugs have limited therapeutic utility and effectiveness. Thus there is a significant need for a more complete understanding of spasticity and for more effective treatment after SCI.
Central mechanisms that underlie pathological reflex control after injury or disease include the loss of cortical and local spinal inhibition, injury-induced plasticity, and increased motor neuron excitability (Bennett et al. 2001a; Boulenguez and Vinay 2009; Hultborn and Nielsen 2007; Hunanyan et al. 2013). Whereas plasticity between Ia afferents and α-motor neurons shapes the H-reflex response in an activity-dependent manner in human and rodent (Raisman 1994; Thompson et al. 2009), maladaptive changes can also contribute to pathological H-reflex function associated with spasticity, clinically defined as a velocity-dependent increase in tonic stretch reflexes with exaggerated tendon jerks, resulting from hyperexcitability of the spinal stretch reflex (Ashby et al. 1987; Lance 1980; Nielsen et al. 2007).
Dendritic spines, micron-sized structures that are sites of postsynaptic activity, regulate the efficacy of synaptic transmission and can thereby alter the electrical information passing through circuit pathways (Bourne and Harris 2007; Calabrese et al. 2006; Pongracz 1985; Segev and Rall 1988; Tan et al. 2009). Localized increases in synaptic strength through the de novo formation and development of postsynaptic dendritic spines constitute a persistent structural basis for learning and memory in the central nervous system (Xu et al. 2009; Yuste and Bonhoeffer 2001). In the present study, we assess the possibility that abnormalities in dendritic spine morphology on α-motor neurons contribute to the persistent dysfunctional state within the spinal motor reflex pathway after SCI.
Our previous studies and evidence in the literature demonstrate that dendritic spine morphology can change following disease or injury (Kim et al. 2006; Tan et al. 2008, 2012b, 2013). Importantly, adverse changes in spine morphology including 1) the elaboration from thin, filopodia-like spines to a mushroom shape, a morphology associated with increased synaptic strength and stability (Yuste and Majewska 2001), and 2) an increase in spine density along dendrites, which provides more sites for postsynaptic connections (Bonhoeffer and Yuste 2002), and a spatial redistribution of spines along dendrites to locations closer to the cell body (Kim et al. 2006; Ruiz-Marcos and Valverde 1969) have been shown to contribute to neuronal hyperexcitability (Tan et al. 2009). Although dendritic spine remodeling occurs in the motor cortex after SCI (Kim et al. 2006), no study has reported on dendritic spines located on spinal α-motor neurons. Moreover, it is unknown whether SCI-induced changes in dendritic spine morphologies can contribute to spasticity.
The activity of small GTP-binding protein Rac1 governs actin cytoskeleton reorganization to regulate dendritic spine morphology (Tashiro et al. 2000; Tashiro and Yuste 2004). Constitutively activated Rac1 increases the rate of dendritic spine turnover, spine density and stability, and spine volume (Nakayama and Luo 2000). In contrast, dominant negative Rac1 expression or administration of a Rac1-specific inhibitor NSC23766 disrupts dendritic spine formation and development (Tan et al. 2011; Tan and Waxman 2012; Tashiro et al. 2000; Tolias et al. 2007). Importantly, SCI increases Rac1 mRNA expression, with levels that can remain elevated for up to 3 mo or more (Dubreuil et al. 2003; Erschbamer et al. 2005). It is not known, however, if Rac1 activity contributes to dendritic spine remodeling and reflex dysfunction following SCI.
In this article we provide the first evidence of dendritic spine plasticity on α-motor neurons after SCI and demonstrate a structure-function relationship between dendritic spine dysgenesis and exaggerated spinal motor reflexes associated with spasticity. Six weeks after contusion SCI, animals exhibited increased H-reflex responsiveness (i.e., shown by reduced rate-dependent depression, or RDD). Histological assessment in these animals demonstrated that α-motor neurons located below the level of injury within the L4–L5 spinal segments had an increase in dendritic spine density, a significant redistribution of spines along dendrites, and increased dendritic spine head diameter, morphological profiles consistent with those shown to contribute to increased neuronal excitability (Pongracz 1985; Tan et al. 2009). In contrast, above the level of injury, we observed an absence of exaggerated H-reflex response, reduced dendritic spine densities, a close-to-normal distribution of spines, and normal dendritic spine length and head diameter. Inhibition of Rac1 disrupted SCI-induced dendritic spine profiles on α-motor neurons below the injury site, reduced vesicular glutamate transporter 1 (VGluT1) expression (a marker for excitatory primary afferent terminals), and decreased H-reflex responsiveness. Taken together, these observations provide evidence for a new perspective into mechanisms of neuroplasticity within the spinal reflex pathway and demonstrate a relationship between dendritic spine remodeling and reflex dysfunction after SCI. Targeting of molecular pathways that regulate spine structure could represent a novel avenue for managing spasticity after SCI.
MATERIALS AND METHODS
Animals and spinal cord injury.
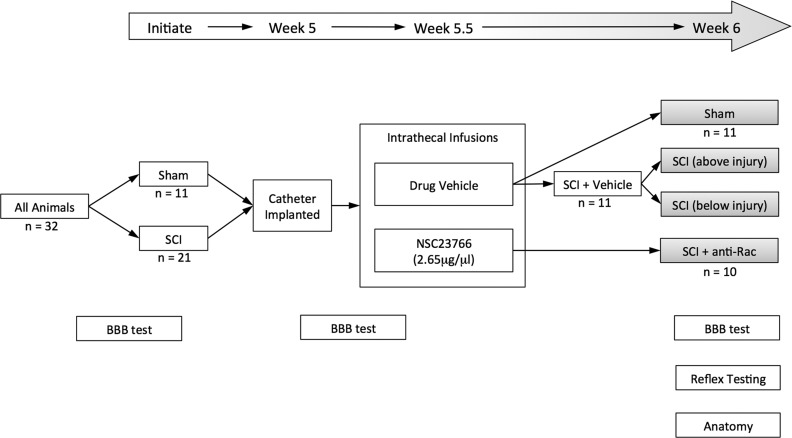
Experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All animal protocols were approved by the Yale University Institutional Animal Use Committee. Animals were housed under a 12:12-h light-dark cycle in a pathogen-free area with water and food provided ad libitum. A total of 32 adult male Sprague-Dawley rats (175–200 g; Harlan, Indianapolis, IN) underwent procedures to produce each treatment group (Fig. 1; study design: sham, n = 11; SCI + vehicle, n = 11; SCI + anti-Rac, n = 10). Animals were first divided into two treatment arms (Fig. 1). The first group received a contusive spinal cord injury at the 2nd lumbar spinal segment (L2): animals were anesthetized with a mixture of ketamine (80 mg/kg ip) and xylazine (5 mg/kg ip). A small laminectomy was carefully performed at the 12th thoracic vertebra (T12), which exposed the dorsal L2 spinal cord surface (Hebel and Stromberg 1976). We stabilized the spinal cord in an Infinite Horizon (IH) impactor device (Precision Systems and Instrumentation, Lexington KY) by clamping the rostral T11 and caudal T13 vertebral bodies with Adson stabilizing forceps attached to the IH stage (Scheff et al. 2003). The spinal contusion injury was performed with a metal rod (tip diameter 2.5 mm) that was applied to the spinal cord surface with an impact force of 170 kdyn (Rabchevsky et al. 2003; Scheff et al. 2003) (data shown in Fig. 2). For sham animals (without SCI), the same surgical procedure was followed, including placement of the animal within the IH stabilizing forceps, except no contusion injury was performed. Following all surgical procedures, the muscle, fascia, and skin were sutured in sequential layers with 4-0 monofilament sutures. Postoperative treatments included twice daily injections of 0.9% saline solution for rehydration (3.0 ml sc) and Baytril (0.3 ml, 3.5 mg/kg body wt sc, twice daily for 3 days) to prevent urinary tract infection.
Fig. 1.
Study design. All weight-matched animals underwent Basso, Beattie, and Bresnahan (BBB) locomotor testing to obtain baseline behavioral data. The number of animals (n values) in each group are shown. In week 1, animals were randomly assigned to receive sham or spinal cord injury (SCI) surgical procedures. In week 5, animals received intrathecal catheter implants. After 2–3 days of recovery, we performed pretreatment BBB testing and immediately administered intrathecal infusions of control vehicle or NSC23766 (twice a day for 3 days). At experimental endpoint at week 6, these treatments produced 4 comparator groups (gray-shaded boxes). Note that within SCI animals treated with vehicle (SCI + Veh), we assessed and compared data outcomes from above or below the injury site (i.e., forelimb and hindlimb, respectively). At endpoint, we also performed posttreatment BBB testing, H-reflex assessment, and histological analysis.
Fig. 2.
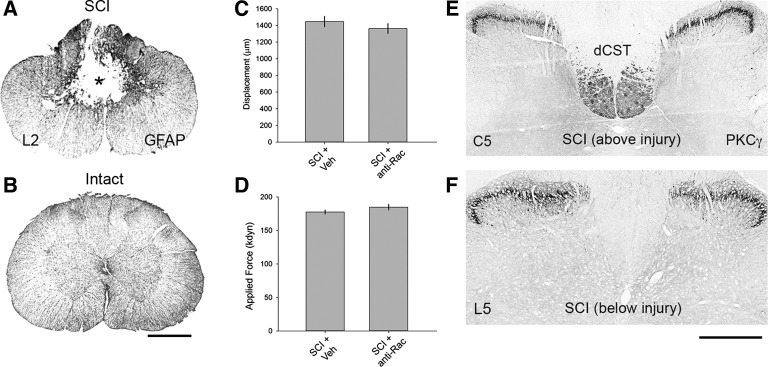
Spinal cord injury. A: contusion injury at L2 resulted in severe damage of the dorsal columns and gray matter, as shown by glial fibrillary acidic protein (GFAP) staining in coronal spinal cord tissue sections. Asterisk denotes lesion epicenter. B: intact spinal cord tissue from sham animal. C and D: biomechanical data provided by the Infinite Horizon (IH) impactor demonstrated no difference between vehicle (SCI + Veh)- and Rac1 inhibitor NSC23766-treated (SCI + anti-Rac) SCI groups. E: 6 wk after SCI (above injury), PKC-γ staining produced bilateral labeling of the dorsal corticospinal tract (dCST) and lamina I/II. F: at the lumbar level L5 (below injury), the absence of PKC-γ immunoreactivity in the dorsal column white matter tracts demonstrates significant disruption of the dCST. SCI did not affect PKC-γ staining in superficial laminae. Scale bars, 500 μm.
Behavior.
Two experimenters blinded to group assignment evaluated animals using the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale (Basso et al. 1995) for validation of injury equivalency across SCI animals, as well as to determine whether treatments had an effect on overall locomotor ability. The BBB score (1 = worst to 21 = best) consists of a combination of hindlimb movements, trunk position and stability, hindlimb stepping and coordination, paw placement, and tail position. Behavioral testing was performed at three time points (Fig. 1): 1) on naive animals before any surgical procedures, 2) within 1 wk after catheter implantation (and before any drug infusions at ∼5 wk post-SCI), and 3) immediately before experimental endpoint (6 wk post-SCI and sham surgeries). Before undergoing any testing, animals were allowed to acclimatize to the testing area for 60–90 min. During an experimental trial, animals were allowed to roam freely in the test field (enclosed 3 × 3-ft. flat surface), and a similar 4-min time frame of movement was assessed by the experimenters using the BBB scale, as previously described (Basso et al. 1995). After each trial, the surface was cleaned with soap and water and dried. For analysis, BBB scores from both right and left sides of animals were averaged, and data from the two experimenters were averaged within groups and then statistically compared across groups.
Intrathecal catheter implantation and drug delivery.
Five weeks after SCI or sham surgeries, all animals received ketamine-xylazine anesthesia (80 and 5 mg/kg ip, respectively). As described previously (Tan et al. 2012b), a small craniotomy was performed to expose the atlanto-occiptal membrane (between the occipital bone and vertebral column C1/atlas). A sterile 32-gauge catheter (ReCathCo, Allison Park, PA) was carefully inserted through a slit in the membrane and threaded intrathecally until the tip of the catheter reached the lumbar enlargement. The catheter was secured near the base of the skull with sutures placed through overlying muscle and skin. To prevent leakage and infection, we heat-sealed the exposed rostral tip of the catheter by pinching the end with a sufficiently heated and sterilized forceps. The location of the caudal end of the catheter was validated at the experimental endpoint after animals were killed. Animals were allowed to recover for 2–3 days after catheter implantation and then received one of two infusions through the catheter: 1) drug vehicle (0.9% sterile saline, 10-μl volume, twice daily for 3 days) or 2) NSC23766, a target-specific Rac1-GTPase inhibitor (EMD Chemicals, Darmstadt, Germany), at 2.65 μg/μl (5-μl volume, twice daily for 3 days) followed by a 5-μl sterile 0.9% saline flush. To measure the maximal effect of treatments, we performed experimental assessments within 1–2 days following the last infusion of vehicle or drug solution. We did not infuse NSC23766 drug in sham animals in this study, because we had previously already established that NSC23766 does not significantly affect higher-order electrophysiological or behavioral function in uninjured, control animals (Tan et al. 2012b). At the end of the study, this study design produced four comparator arms (Fig. 1, gray-shaded boxes): sham, SCI + vehicle [includes “SCI (above injury)” and “SCI (below injury)”], and SCI + anti-Rac.
Histology.
For Golgi-Cox staining with the use of a commercial kit and according to the manufacturer's instructions (FD Neurotechnologies, Ellicott, MD), a subpopulation of rats (sham, n = 5; SCI + vehicle, n = 4; SCI + anti-Rac, n = 5) from terminal electrophysiological recordings (see below) under ketamine-xylazine anesthesia were killed and processed. Spinal cord tissue (from the cervical enlargement, C4–C5, and lumbar enlargement, L4–L5) was quickly removed (<5 min), rinsed in distilled water, and immersed in the kit's impregnation solution. After the incubation period (∼3 wk), 200-μm-thick sections were cut on a vibratome (DTK-1000 microslicer; Ted Pella) and mounted on gelatinized glass slides. Sections were stained, rinsed in distilled water, dehydrated, cleared, and coverslipped with Permount medium. For immunohistochemistry, remaining rats were deeply anesthetized with ketamine-xylazine and transcardially perfused with 250 ml of 0.1 M phosphate buffer (PB) at 37°C followed by 300 ml of freshly prepared cold paraformaldehyde solution (4% in 0.1 M PB). The spinal cord was removed, postfixed for 2 h at room temperature, and cryoprotected by immersion in 30% sucrose in 0.1 M PB at 4°C. Frozen coronal sections from C4–C5, the injury site at L2, and L4–L5 were cut at 20-μm thickness using a cryostat (Leica, Bannockburn, IL). Sections were collected onto Superfrost Plus slides (Fischer Scientific, Pittsburgh, PA). Immunofluorescence staining methods were described previously (Tan et al. 2006). Sections were washed in blocking solution (0.1 M PBS, 0.1% Triton X-100, and 4% normal donkey serum) and incubated overnight at 4°C in mouse anti-VGluT (1:1,000; UC Davis/NIH NeuroMab facility), rabbit anti-glial fibrillary acidic protein (1:2,000; Abcam) or rabbit anti-PKC-γ antibody (Santa Cruz Biotechnology 1:1,000). After being washed in blocking solution, sections were incubated in the fluorescent secondary antibodies CY3 donkey anti-mouse (1:500; Jackson ImmunoResearch Laboratories) or Alexa Fluor 488 donkey anti-rabbit (1:2,000; Invitrogen). Sections were visualized and digitally imaged using a Nikon Eclipse 80i fluorescence microscope equipped with an HQ CoolSNAP camera (Roper Scientific, Tucson, Arizona) or a Nikon D-Eclipse C1 confocal microscopy system. MultiCapture mosaic images were digitally stitched with NIS Elements software (Nikon Instruments).
Dendritic spine visualization on motor neurons and analysis.
Investigators blinded to treatment conditions performed all imaging studies and analyses. To visualize neurons and ultrafine processes, we used a Golgi-staining method as previously described (Tan et al. 2008). For our purpose, we required the ability to fully reconstruct neuronal structure, which required that tissue be exposed to high-intensity light for long periods of time (up to 4 h per imaging session), which can quickly bleach or diminish other visualization tools (e.g., fluorophores). Golgi staining permits the identification and sampling of a relatively large number of neurons from cervical and lumbar levels within the same animal and provides robust visualization of the entire neuronal structure, including detailed resolution of dendritic spines. We were specifically interested in motor neuron pools that innervated muscle groups in the forelimb (i.e., extensor carpi radialis) and hindlimb (i.e., plantar muscle). To identify these α-motor neurons, we followed a screening workflow based on data from our previous study (Tan et al. 2012a) and those previously validated in rats (Crockett et al. 1987; Hashizume et al. 1988; Jacob 1998). We began with a broad sample population of neurons by identifying Golgi-stained α-motor neurons located in the ventral spinal cord in Rexed lamina IX and with soma diameters >25 μm (Hashizume et al. 1988; Jacob 1998). Above the injury site (C4–C5), we narrowed candidate neurons for analysis by selecting neurons from motor pools located in similar dorsolateral coordinates of α-motor neurons shown to innervate the extensor carpi radialis muscle group (∼1.5–2 mm deep, 1.5–2 mm lateral from midline), as we and others have demonstrated through intramuscular retrograde tracing studies (Sunshine et al. 2013; Tan et al. 2012a; Tosolini et al. 2013). Below the injury site (L4–L5), we narrowed our sampled α-motor neurons to those located in ventral motor pools with similar dorsolateral coordinates of motor pools known to innervate the plantar muscle (∼1.5–2.5 mm deep, 1.5–2.2 mm lateral from midline) as shown by retrograde tracing (Crockett et al. 1987; Jacob 1998). As a refinement step for analysis a priori, we only included α-motor neurons for analysis that had 1) dendrites and dendritic spines that were clearly and completely impregnated, 2) dendritic branches appearing as a continuous length for at least 350 μm within the tissue slice, and 3) at least one-half of the primary dendritic branches remaining within the thickness of the tissue section such that their endings were not cut and appeared to taper into a complete ending (see representative neuron in Fig. 3). To determine if there were any morphological differences across our sample neurons, we used NeuroExplorer software (MicroBrightField, Williston, VT) to measure maximum cell diameter, aspect ratio (Feret maximum/Feret minimum), form factor [(4π × area)/(perimeter)2], number of primary dendrites, and total dendritic branch length of each treatment arm and compared these morphometry values across treatment groups (Table 1). To refine our identification and measurements of dendritic spines, we used specific morphological characteristics (Kim et al. 2006; Tan et al. 2012b): we defined a spine neck as the structure between the base of the spine and the interface with the parent dendrite branch, and the base of the spine head where the appearance of the spine swells distally into a bulblike structure. Thin and mushroom-shaped spines were classified as follows: thin spines had head diameters that were less than or equal to the length of the spine neck, whereas mushroom spines had head diameters that were greater than the length of the spine neck. These criteria for two spine geometric categories were used because classification into only two spine shapes allowed us to use simple but very strict rules in classifying spine morphology. Although this approach prevented the discrimination of subtle variations in spine shape, it allowed the collection of a very large sample size, and we and others have described the physiological characteristics of thin and mushroom spine shapes on neuronal and circuit function (Bourne and Harris 2007; Holmes 1990; Tan et al. 2009). Note that these criteria do not imply the physiological characterization of the neurons we analyzed, but rather control for morphological diversity within the sampled spinal motor neuron population (Kitzman 2005; Tashiro and Yuste 2003).
Fig. 3.
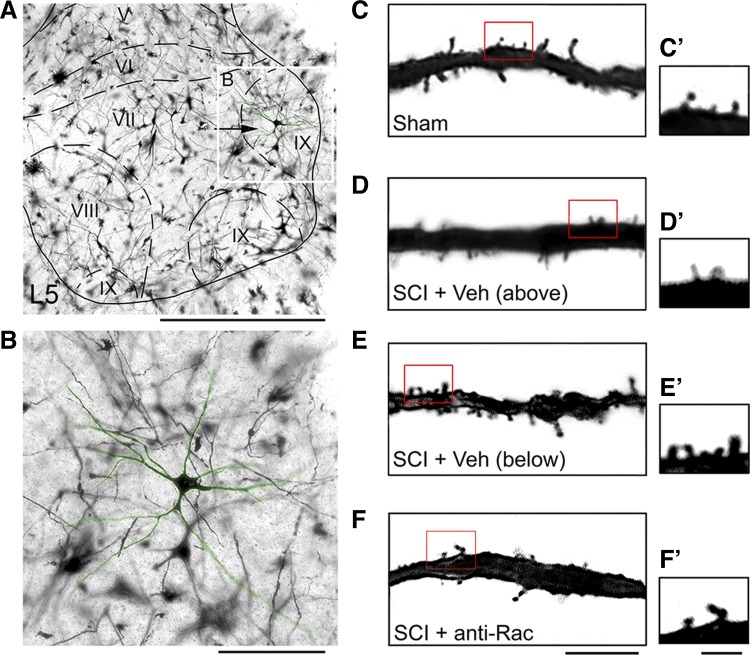
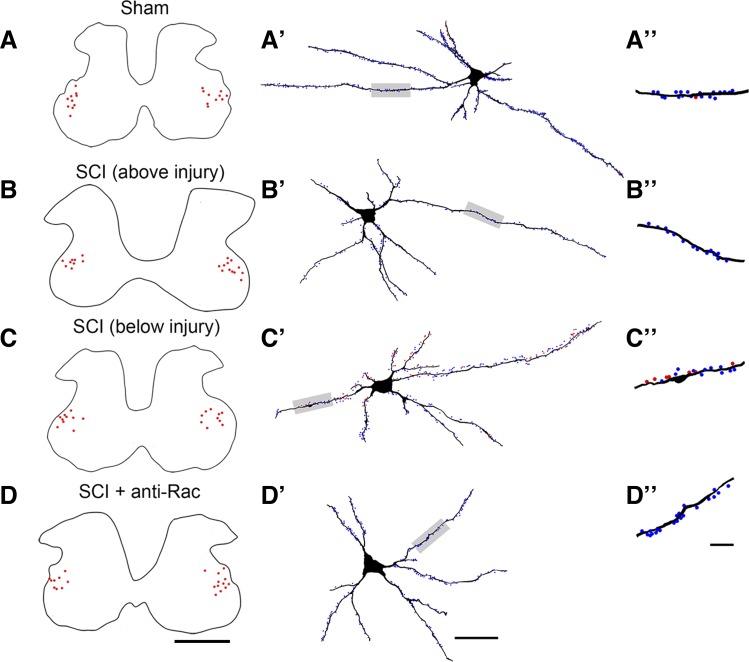
Golgi staining of spinal cord tissue reveals dendritic spines on motor neurons in the ventral gray matter. A: image of ventral gray matter with an identified α-motor neuron located in Rexed lamina IX (arrow and white box). B: high-power field of motor neuron shown in inset in A. Six weeks after sham and SCI procedures, representative images of dendritic branches show apparent differences in dendritic spine profiles from sham (C), SCI + Veh above the injury (D), SCI + Veh below the injury (E), and SCI + anti-Rac (F) treatment groups. C′–F′: high magnification of selected dendrite regions from C–F (red boxes). Scale bars: A, 500 μm; B, 100 μm; C–F, 10 μm; C′–F′, 2 μm.
Table 1.
Spinal cord motor neuron morphometry
| Maximum Cell Diameter, μm | Aspect Ratio | Form Factor | No. of Primary Dendrites | Total Dendrite Length, μm | |
|---|---|---|---|---|---|
| Sham | 54.6 ± 19.4 | 0.57 ± 0.17 | 0.49 ± 0.17 | 8.16 ± 3.69 | 1,073.0 ± 671.4 |
| SCI (above injury) | 46.7 ± 11.7 | 0.51 ± 0.16 | 0.50 ± 0.16 | 5.73 ± 1.95 | 815.10 ± 451.9 |
| SCI (below injury) | 51.4 ± 17.4 | 0.64 ± 0.13 | 0.62 ± 0.09 | 4.80 ± 1.23 | 739.30 ± 280.6 |
| SCI + anti-Rac | 46.1 ± 8.7 | 0.63 ± 0.11 | 0.60 ± 0.10 | 4.38 ± 1.15 | 1,085.8 ± 307.9 |
Values are means ± SD. SCI, spinal cord injury.
To digitally reconstruct motor neurons, we used a Neurolucida software suite (version 9.0; MicroBrightField) and a pen tablet (Intuos5 Touch; Wacom). We analyzed the completed three-dimensional reconstructions of motor neurons for spine density and distribution. Each imaging session consisted of a contour map (outline of the spinal cord section with location of identified neuron) and the motor neuron, which was traced in the X-, Y-, and Z-axes. Dendritic spine type were located and marked on each reconstructed dendritic branch (thin spines, blue; mushroom spines, red). Dendritic spine density was expressed as dendritic spine number per 10-μm dendrite length. To determine any changes in spatial distribution of dendritic spines relative to the cell body, we used a Sholl's analysis (Tan et al. 2008). Seven 50-μm-wide spherical bins were formed around each cell body, and spine density within each bin was averaged within each treatment group. For statistical comparison, spine density at dendrite branch locations within 50–150 μm (proximal bins) and 200–350 μm (distal bins) from the cell body were pooled and compared across treatment groups.
To determine changes in spine dimensions, five neurons were arbitrarily chosen from each treatment group and visible spines were measured for spine length and spine head diameter (Kim et al. 2006). Spine length was defined as the distance from the tip of the spine to the junction of the spine at the main dendrite branch. Spine head diameter was defined as the longest line drawn normal to the length of the parent dendrite branch. A total of 411 dendrites from 82 identified α-motor neurons (in 4–5 animals per treatment group) were included in our analyses [sham, 118 dendrites; SCI (below injury), 116 dendrites; SCI (above injury), 90 dendrites; SCI + anti-Rac, 87 dendrites].
Areal density of VGluT1 labeling.
To examine changes in the number of VGluT1-expressing synaptic terminals, we assessed areal density of VGluT1 expression using a modified approach described previously (Tan et al. 2012a). High-resolution digital photographs were taken at ×10 magnification and combined into a single mosaic image of the entire spinal cord at L4–L5. We photographed 10 sections per animal. Sections for analysis were chosen on the basis of tissue integrity (e.g., no major tears) and equivalent loss of PKC-γ immunoreactivity in the dorsal corticospinal tract (dCST) of the spinal cord dorsal columns. Sections were aligned according to the point of intersection between the gray matter above the central canal and the dorsal median septum. All images underwent threshold adjustments using equivalent contrast/brightness levels to highlight only VGluT1-expressing puncta (Photoshop; Adobe, San Jose, CA). Images were binarized and color-inverted for analysis. For color-coded heat maps in Fig. 9, binarized images were exported into MATLAB (The MathWorks, Natick, MA) and averaged using custom scripts, as described previously (Brus-Ramer et al. 2007; Friel and Martin 2007). For image analysis, mosaic images of each spinal cord coronal section were divided into three dorsoventral regions corresponding to the dorsal zone (∼lamina I–III), the intermediate zone (∼lamina IV–VI), and the remaining ventral horn of the gray matter (Tan et al. 2012a). Because we were only interested in VGluT1 in the gray matter, the white matter areas were digitally removed before analyses. Because VGluT1-expressing puncta were visually distinct from each other, without overlap, the number of VGluT1 puncta was easily counted in each region using ImageJ software (http://rsb.info.nih.gov/ij/index.html). Data from gray matter regions were pooled within groups and compared across experimental treatment groups.
Fig. 9.
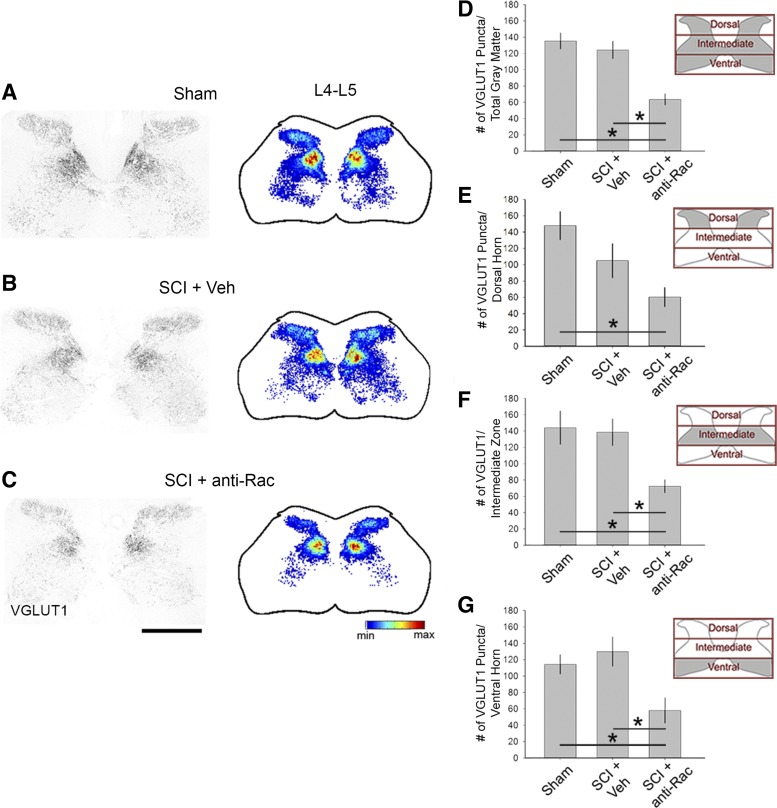
Excitatory terminals in the spinal cord gray matter. Vesicular glutamate transporter 1 (VGluT1)-immunopositive puncta appeared throughout all laminae of the spinal cord gray matter in the lumbar enlargement L4–L5 (A–C, left). Spatial heat maps (A–C, right; red = highest density, blue = lowest density) shows the overall areal density of VGluT1 expression in sham (A), SCI + Veh (B), and SCI + anti-Rac (C) treatment groups. Quantification of the VGluT1 puncta within the total gray matter region (D), dorsal horn (E), intermediate zone (F), and ventral horn (G) as represented in insets as gray shading demonstrates no significant change in SCI + Veh compared with sham. Treatment with the Rac1 inhibitor in SCI animals decreased VGluT1 areal density compared with SCI + Veh in the total gray matter, intermediate zone, and ventral horn only (*P < 0.05) with no significant change in the dorsal horn (P > 0.05). The areal density of VGluT1 decreased in SCI + anti-Rac1 compared with sham in the dorsal horn (*P < 0.05). Scale bar for A–C, 500 μm.
H-reflex testing.
Terminal electrophysiological experiments were performed 6 wk after SCI or sham surgeries. Because H-reflex responses in rats under ketamine anesthesia resemble those seen in unanesthetized humans (Ho and Waite 2002) and do not alter the time course of presynaptic inhibition, which may occur with other anesthetics (e.g., pentobarbital sodium) (Tang and Schroeder 1973), we anesthetized animals with an induction dose of ketamine (80 mg/kg ip) and xylazine (5 mg/kg ip) and maintained on ketamine alone (20 mg/kg ip) (Ho and Waite 2002; Hosoido et al. 2009). Core body temperature was monitored with a rectal thermometer and maintained at 38 ± 1°C with a circulating water heating pad placed under an absorbent pad. To record electromyogram (EMG) data, which included the muscle response (M-wave) and the monosynaptic reflex response (H-reflex) above and below the injury site in SCI animals, we used an established percutaneous needle preparation (Boulenguez and Vinay 2009; Lee et al. 2009; Schieppati 1987; Thompson et al. 1992a; Valero-Cabre et al. 2004). We chose to use this minimally invasive procedure because it is analogous to methods used to evoke and record H-reflex in humans (Palmieri et al. 2004; Schieppati 1987) and provides the opportunity to stimulate and record at all four limbs within the same animal without perturbing muscle or nerve tissue that would otherwise be disrupted from more invasive surgical electrode placement (e.g., cuff electrode implants). In addition, this recording approach maintains the integrity of the vascular system and optimizes the tissue preservation and collection methods for histological study (see above) that we performed following electrophysiological experiments. For stimulation, a pair of Teflon-insulated stainless steel fine-wire electrodes (0.002-in. bare metal diameter; A-M Systems, Carlsborg, WA) were threaded into a 32-gauge syringe needle. The wire ends were carefully bent into sharp barbs, the insulation was removed with heat (to expose tips ∼1 mm), and the needle and wire were then transcutaneously inserted until the wire tip was in close proximity to the mixed nerves of the deep radial nerve or tibial nerve, above or below the injury site in SCI animals, respectively. The needle was retracted and the wire remained in place. The second electrode was inserted similarly, spaced ∼2 mm apart from the first electrode. Stimulating electrode placement was adjusted until the intensity of square-wave stimulating pulses (0.2-ms duration continuously given at a rate of 1 every 3 s) required to induce subtle visible motor twitch responses (i.e., wrist extension/radial abduction or plantar flexion) was <1 mA (Lee et al. 2009; Valero-Cabre et al. 2004). For recording electrodes, we used insulated wire electrodes made of similar materials and exposed ∼2 mm of the wire tips using heat. To record EMG data from the forelimb (e.g., brachioradialis reflex), an electrode was inserted into the interosseous muscles between the fourth and fifth digit and a reference electrode was placed subcutaneously in the dorsolateral surface of the paw. To record EMG data from the hindlimb (e.g., plantar reflex), an electrode was inserted into the plantar muscles within the palmar/ventral surface of the hindpaw and proximal to the ankle region. A reference electrode was placed subcutaneously within the dorsolateral surface of the hindpaw. These reflexes were chosen on the basis of our pilot experiments and previous work that demonstrated EMG reflex response evoked in these muscles could be reproducibly recorded after SCI (Boulenguez et al. 2010; Kim et al. 2009; Valero-Cabre et al. 2004). Note that SCI-induced changes in the plantar reflex, primarily innervated by motor pools in L5, less from L4 (Crockett et al. 1987), have been shown to be similar to changes in reflexes elicited in other hindlimb muscles, i.e., tibialis anterior and gastrocnemius, which are also innervated by L4 and L5 (Lee et al. 2009; Valero-Cabre et al. 2004). EMG responses were filtered (10–1,000 Hz), amplified, and recorded for offline analysis using Spike2 (version 7.08; CED Software, Cambridge, UK). To identify optimal stimulation intensity for activating stable M-wave and H-reflex responses, square-wave pulses (0.2-ms duration) were applied at a rate of one every 3 s. The intensity of electrical stimulation was first adjusted to determine the minimum intensity to evoke an M-wave response ∼50% of the time and progressively increased until a stable M-wave and maximal H-reflex response could be observed.
To measure RDD of the H-reflex response, we performed a paired-pulse stimulation paradigm with a conditioning and test pulse that we applied at a range of interpulse intervals (10 to 2,000 ms). Three trials (10 sweeps/trial) with at least 30 s between trials were recorded for each interpulse interval. The M and H response wave amplitudes were quantified from averaged and rectified waveforms within each animal (Boulenguez et al. 2010; Tan et al. 2012a). For comparison across treatment groups, the maximum waveform amplitudes of the H and M responses to the test pulse were converted into a percentage of the maximum amplitude response to the conditioning pulse. M and H waveform amplitudes were measured from baseline to peak amplitude. To determine trial-to-trial consistency, the coefficients of variation (CoV) from the M and H waves were calculated by dividing the standard deviation by the mean maximum amplitude. After recording experiments, animals were killed for Golgi staining or immunohistological study as described above.
A number of studies have shown that dendritic spine morphology can change within minutes following cortical injury or activity (Majewska and Sur 2003; Mizrahi et al. 2004; Zhang et al. 2005). Moreover, others have shown that exogenous electrical stimulation can induce plasticity of the H-reflex, which can persistent for many hours or days (Chen et al. 2003, 2006). Although in this study our H-reflex testing was performed acutely (i.e., lasting ∼1 h per animal), we ensured that all animals, including both spinal cord-injured and uninjured sham animals, underwent similar H-reflex testing protocols to control for potential confounds of direct nerve stimulation.
Statistical analysis.
All statistical tests were performed at the α level of significance of 0.05 with two-tailed analyses using parametric or nonparametric tests, as appropriate. For comparisons of anatomical and functional changes above and below the injury site after SCI, we compared multiple comparisons of data collected within the same animals and with sham animals. To determine the appropriate statistical model to apply to these specific datasets, we incorporated two assumptions: 1) that the above- and below-injury data sets were dependent variables affected by the application of the independent treatment variable, SCI, and 2) as a consequence of SCI, a putative secondary pathway interaction arises between above- and below-injury spinal segments that can affect the above- or below-injury data sets (e.g., an emergent interaction following SCI that leads to differential effects on spinal cord tissue located above or below the SCI injury site). Given these assumptions, our data sets fit most closely with a one-way (or one factor) ANOVA statistical model, where SCI is the treatment factor applied to two dependent variables, above and below injury. Moreover, to control for multiple comparison errors with the additional comparison against the sham group, we applied a post hoc repeated-measures correction. Although we considered the application of a split-plot analytical model, this approach falsely assumes that the secondary, post-SCI interaction is a factor that is independent of the first-cause treatment factor, SCI. In summary, for comparing data sets gathered from above and below the injury site, we applied repeated-measures corrections (Dunn or Bonferroni post hoc tests) following ANOVA and Kruskal-Wallis one-way ANOVA on ranks analyses. As a note, previous reports have similarly applied ANOVA repeated-measures design to compare ipsilateral and contralateral sides of the spinal cord following unilateral nerve trauma or to compare regions above and below the injury site after SCI. The statistical design of these studies encompassed the assumed effect of a postinjury secondary, bidirectional interaction (Anderson et al. 1998; Chang et al. 2010; Tal and Bennett 1994; Tan et al. 2007, 2012a), which also may have emerged within our experimental SCI model. Data management and statistical analyses were performed using SigmaPlot (version 12.5; Systat Software) and Microsoft Office Excel (2011). Data in the text are means ± SD; data in graphs are plotted as means ± SE using SigmaPlot.
RESULTS
Contusion SCI disrupts the dorsal corticospinal tract.
In SCI animals, contusion injury at spinal cord segment L2 resulted in severe damage of the dorsal columns and gray matter (Fig. 2A), in contrast with sham animals (Fig. 2B). Histological examination of caudal spinal cord tissue (segments below L3) showed no visible tissue damage or glial scar tissue (not shown). The IH impactor device provided biomechanical measurements during each SCI procedure (Fig. 2, C and D). The cord surface displacements upon rod impact were not significantly different across both SCI groups, SCI + vehicle and SCI + anti-Rac (Fig. 2C; 1,419.8 ± 293.3 vs. 1,394.9 ± 266.2 μm, P > 0.05, t-test). Similarly, there was no difference in the actual applied force between the respective SCI groups (Fig. 2D; 172.4 ± 12.4 vs. 181.4 ± 19.7 kdyn, P > 0.05, ANOVA on ranks with Dunn's post hoc test). Applied impact force with the IH device predicts the amount of tissue sparing, which correlates closely with locomotor functional outcome (Scheff et al. 2003). PKC-γ immunoreactivity served as an anatomic marker to help confirm the injury magnitude of our SCI model (Bradbury et al. 2002; Sasaki et al. 2009; Tan et al. 2012a). In coronal spinal cord sections above the injury in the cervical enlargement, PKC-γ immunoreactivity symmetrically labeled the dCST and small-diameter cells located in laminae I/II (Fig. 2E) (Mori et al. 1990). Six weeks after SCI, below the injury level in the lumbar enlargement, PKC-γ staining of the dCST was bilaterally eliminated from the dorsal columns (Fig. 2F). PKC-γ staining profiles of the dCST and superficial laminae remained intact (data not shown) in cervical and lumbar enlargement tissues in sham animals.
Dendritic spine density changes on motor neurons after SCI.
Dendritic spines remodel in the motor cortex after SCI (Kim et al. 2006, 2008); however, it is not known whether SCI-induced dendritic spine dysgenesis occurs on α-motor neurons within the spinal cord. Injury-induced changes in dendritic spine morphology on nociceptive neurons in the dorsal horn have been shown to contribute to increased excitability associated with neuropathic pain (Tan et al. 2008, 2009, 2012b). To determine whether dendritic spine remodeling occurs on spinal cord α-motor neurons, we identified α-motor neurons (see materials and methods) and performed a morphological comparison of α-motor neurons across treatment groups (Fig. 3). We identified α-motor neurons located in lamina IX and within motor pools in the lateral regions of the ventral horn (Fig. 3, A and B). Six weeks after SCI, motor neurons had widely projecting dendritic trees containing numerous spines. Qualitative observations demonstrated marked differences in spine number across treatment arms (Fig. 3, C–F). To ensure equivalent sampling across groups, we assessed several morphological criteria and compared these values across treatment groups (Table 1). There were no statistically significant differences in maximum cell diameter, aspect ratio, form factor, number of primary dendrites, or total dendrite branch lengths (for all comparisons: P > 0.05). We therefore interpreted any differences in dendritic spine profiles across groups as not due to variations in neuronal sampling, but rather an effect of experimental treatments. As a note, the values for maximum cell diameter, form factor, and dendritic branch lengths were similar to measurements for α-motor neurons that were labeled by intramuscularly injected retrograde tracers (Bose et al. 2005; Crockett et al. 1987; Hashizume et al. 1988; Jacob 1998).
To obtain an accurate measure of dendritic spine profiles from ventral spinal cord tissue, we digitally reconstructed α-motor neurons using Neurolucida software (Fig. 4). We marked the location of sample neurons on a contour map of the spinal cord gray matter (Tan et al. 2008). α-Motor neurons from each treatment group (Fig. 4, A–D, red dots; n = 20–21 cells/group) were located in the ventrolateral regions of the gray matter, shown as a single representative contour from segmental level C5 (above injury) or L5 (below injury). Dendritic spines on traced motor neurons were marked along dendritic branches and color-coded with thin-shaped (blue) or mushroom-shaped (red) spines (Fig. 4, A′–D′).
Fig. 4.
Digital reconstructions of spinal cord motor neurons. To obtain an accurate profile of dendritic spines in motor neurons, we digitally reconstructed the entire branch structure of sampled neurons. A–D: contour traces from each group as indicated show the locations of all sampled motor neurons (red dots) within the gray matter (representative black trace). Density and distribution were measured from 3-dimensional neuron reconstructions from sham (A′), SCI + Veh above the injury (B′), SCI + Veh below the injury (C′), and SCI + anti-Rac (D′) treatment groups. A″–D″: ∼50-μm lengths of dendrites from neurons shown in A′–D′ (gray-shaded regions) show thin-shaped (blue dots) and mushroom-shaped spines (red dots). Scale bars: A–D, 500 μm; A′–D′, 50 μm; A″–D″, 10 μm.
A main objective of this study was to assess the contribution of SCI-induced changes in dendritic spines to reflex dysfunction; we therefore measured three morphological profiles of spines that have been associated with injury-induced neuronal hyperexcitability: 1) increased density of dendritic spines, particularly mature mushroom-shaped spines, 2) redistribution of spines toward dendritic branch locations close to the cell body, and 3) enlargement of the spine head diameter. Because spasticity often presents below the injury site following SCI and less commonly above (Skold et al. 1999), we measured dendritic spines on α-motor neurons in motor pools of the cervical (C4–C5; above injury) and lumbar (L4–L5; below injury) spinal segments that innervate forelimb and hindlimb musculature, respectively (see methods and materials).
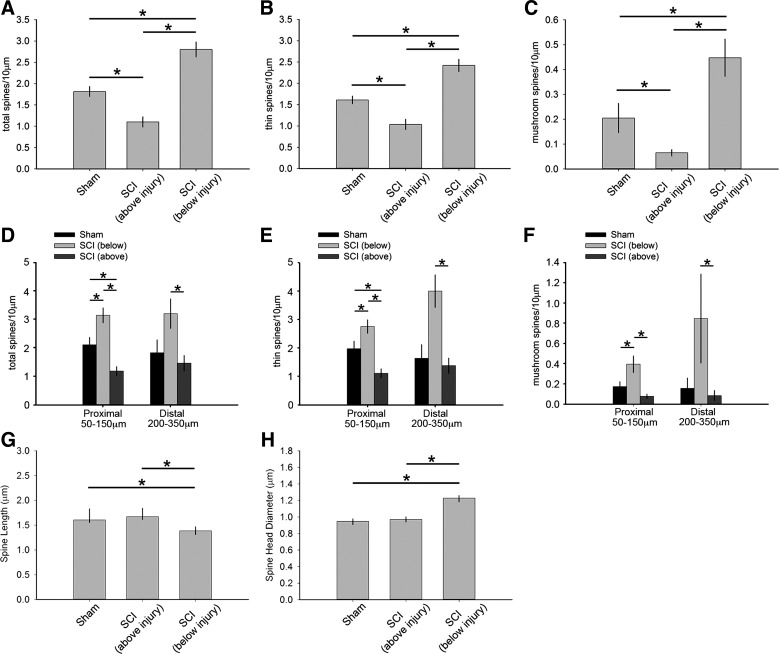
As shown in Fig. 5A, 6 wk after SCI, total dendritic spine density on motor neurons below the injury site increased compared with neurons from sham control and neurons above the injury site (P < 0.05; 2.80 ± 0.78 vs. 1.82 ± 0.52 vs. 1.10 ± 0.54 spines/10-μm dendrite, respectively; ANOVA on ranks with Dunn's post hoc test). In contrast, motor neurons above the injury site in the cervical enlargement had dendritic spine densities that decreased compared with neurons from sham (P < 0.05). A similar profile of dendritic spine density was also observed for thin-shaped dendritic spines (Fig. 5B): below the injury site there was a significant increase in thin spines compared with above the injury and sham (P < 0.05; 2.42 ± 0.63 vs. 1.03 ± 0.54 vs. 1.61 ± 0.40 spines/10-μm dendrite, respectively; ANOVA on ranks with Dunn's post hoc test). Importantly, there was a significant increase in the density of mature, mushroom-shaped spines located on α-motor neurons below the injury site compared with neurons from sham and neurons above the injury (P < 0.05; 0.45 ± 0.33 vs. 0.20 ± 0.26 vs. 0.06 ± 0.06 spines/10-μm dendrite, respectively; ANOVA on ranks with Dunn's post hoc test) (Fig. 5C). Note that the mushroom-shaped spine density observed below the injury site after SCI represents a more than 200–700% increase compared with mature-shaped spine densities above the injury site and sham control.
Fig. 5.
Quantitative analysis of dendritic spine profiles between sham and SCI animals above or below the injury. Analysis of dendritic spine profiles reveals differences in dendritic spine density (A–C), distribution (D–F), and shape (G and H). Total dendritic spine density (A), which includes all spine shapes, thin spine density (B) and mushroom spine density (C) decreased on motor neurons located above the injury after SCI compared with neurons from sham (*P < 0.05). In contrast, total spine density increased on motor neurons located below the injury compared with neurons from either sham or above the injury after SCI (*P < 0.05). Dendritic spine distribution for total (D), thin (E), and mushroom spines (F) differed across the comparator groups. At proximal regions in SCI animals, all spine densities increased below the injury compared with neurons from sham and above the injury (*P < 0.05). In contrast, neurons above the injury had lower total and thin spine density at proximal regions compared with neurons from sham and below the injury (*P < 0.05). Although mushroom spine density on motor neurons above the injury did not differ from that on neurons from sham at proximal regions (F), these neurons had significantly lower mushroom spine density than below the injury. At distal regions, motor neurons below the injury had greater spine density in all categories compared with motor neurons above the injury (*P < 0.05). There was no difference in any spine densities at distal regions on neurons from sham and above the injury in SCI animals. Dendritic spine shape analysis revealed no change in spine length (G) or spine head diameter (H) on motor neurons located above the injury in SCI animals compared with neurons from sham (*P < 0.05). Below the injury, these measurements demonstrated a decrease in spine length and an increase in spine head diameter compared with neurons from sham and above the injury (*P < 0.05).
Fig. 6.
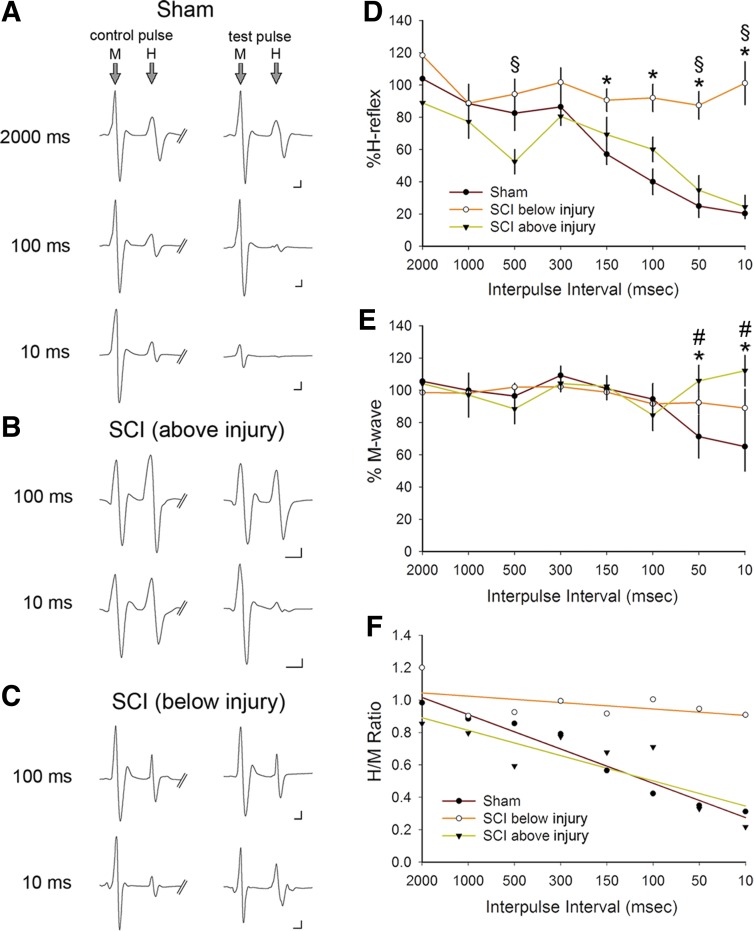
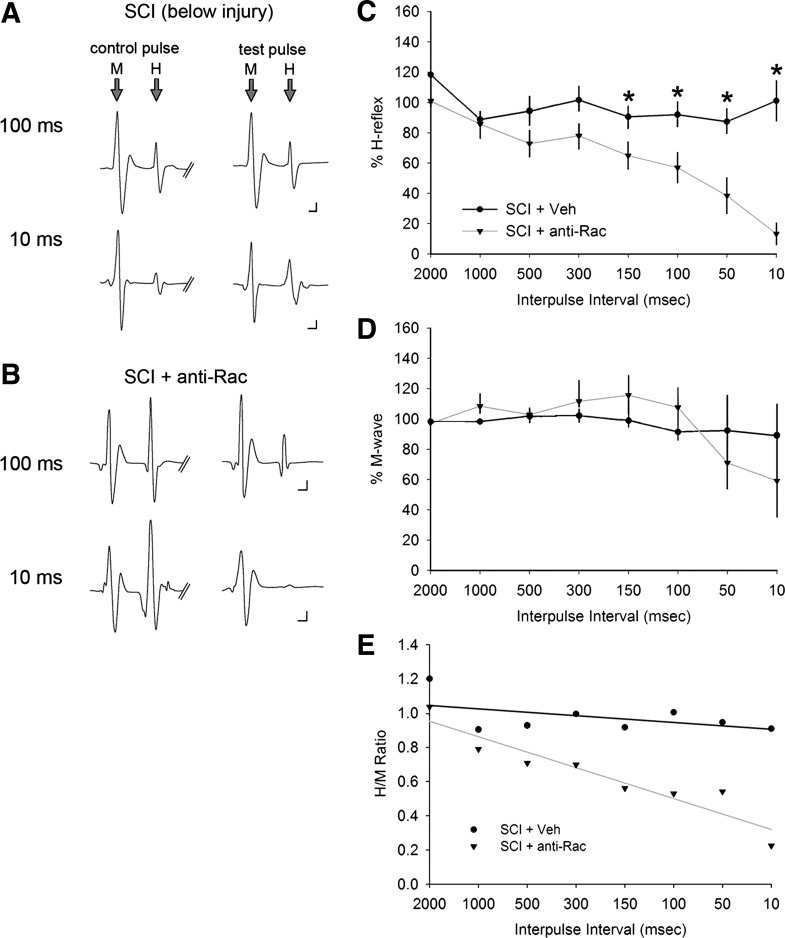
Rate-dependent depression (RDD) of the H-reflex and M-wave responses above and below SCI. As a physiological assessment of the monosynaptic H-reflex, we performed a paired-pulse stimulation protocol. Representative traces (averaged 10–20 traces) of the M and H responses to control (first) and test (second) pulse in sham (A), SCI above the injury (B), and SCI below the injury (C). The control and test pulses were separated with a range of interpulse latencies between 2,000 and 10 ms. Note that in sham animals, as the interpulse intervals decreased (e.g., increasing the rate of activity) between the test and control pulse, the amplitude of the M and H responses decreased. As shown in C, in SCI below the injury, RDD in amplitude of either the M or H response failed to appear. D and E: %H-reflex and %M-wave amplitudes are normalized values of the evoked stimulus response of the test and control pulse. D: after SCI, there was no significant difference in %H-reflex in SCI above the injury compared with sham at any interpulse interval. In contrast, in SCI animals below the injury, the %H-reflex significantly increased compared with sham at the shortest interpulse intervals between 100 and 10 ms (*P < 0.05), demonstrating a loss of RDD and increased excitability of the H-reflex. Similarly, %H-reflex below the injury was significantly greater than above the injury in SCI animals at 500-, 50-, and 10-ms interpulse intervals (§P < 0.05). E: % M-wave demonstrated a significantly increased response below the injury compared with the response in sham animals (*P < 0.05) and above the injury in SCI animals (#P < 0.05). F: the H/M ratio was calculated from M-wave and H-wave responses.
Dendritic spines redistribute toward proximal branches on motor neurons after SCI.
Excitatory afferent inputs located closer to the neuronal cell body can have a greater weighted impact on the overall electrical output of a neuron because of the closer proximity to the axon hillock (Pongracz 1985; Tan et al. 2009; Yuste and Urban 2004). To profile changes in dendritic spine distribution along motor neuron branch processes, we applied a Sholl's analysis and pooled spine densities within proximal regions close to the cell body (50–150 μm) and distal regions (200–350 μm) (see methods and materials) (Fig. 5, D–F).
On motor neurons below the injury site in SCI animals, total, thin-shaped, and mushroom-shaped dendritic spines increased on proximal dendrite branches compared with equivalent regions in sham and SCI neurons above the injury site (P < 0.05; for total spines: 3.1 ± 1.1 vs. 2.1 ± 1.1 vs. 1.2 ± 0.69, for thin spines: 2.7 ± 0.16 vs. 1.9 ± 0.05 vs. 1.1 ± 0.05, and for mushroom spines: 0.39 ± 0.04 vs. 0.17 ± 0.06 vs. 0.08 ± 0.01 spines/10-μm dendrite, respectively; 1-way ANOVA with Bonferroni's post hoc test) (Fig. 5, D–F). At distal regions, SCI did not change spine density of any category on motor neurons from below the injury compared with sham (P > 0.05). On the other hand, motor neurons below the injury had increased spine density for all categories compared with above the injury at distal regions (P < 0.05; for total spines: 3.2 ± 1.4 vs. 1.4 ± 0.9, for thin spines: 2.3 ± 1.4 vs. 1.4 ± 0.9, and for mushroom spines: 0.85 ± 0.9 vs. 0.08 ± 0.16 spines/10-μm dendrite, respectively; ANOVA on ranks with Dunn's post hoc test). There were no differences in any spine densities at distal regions on neurons from sham animals compared with neurons above the injury in SCI animals (P > 0.05).
Dendritic spine dimensions change on motor neurons after SCI.
To quantify the effects of SCI on spine length and spine head diameter, we analyzed 880–1,305 dendritic spines that were sampled from 4–7 motor neurons per group (see materials and methods; sham, n = 3 animals/5 neurons; above SCI level, n = 3 animals/7 neurons; below SCI level, n = 3 animals/4 neurons). As shown in Fig. 5G, dendritic spines below the injury site in SCI animals decreased in length compared with those above the injury site and in sham animals (P < 0.05; 1.38 ± 0.79 vs. 1.67 ± 0.97 vs. 1.60 ± 0.81 μm, respectively; 1-way ANOVA with Bonferroni's post hoc test). In contrast, spine head diameter increased after SCI below the injury site compared with that above the injury and in sham animals (P < 0.05; 1.22 ± 0.65 vs. 0.97 ± 0.57 vs. 0.95 ± 0.56 μm, respectively; 1-way ANOVA with Bonferroni's post hoc test) (Fig. 5H). Motor neurons above the injury site after SCI did not differ compared with sham in any dimension measured (P > 0.05).
H-reflex response increases below the injury site after SCI.
Dendritic spine morphology significantly influences synaptic function (i.e., in a structure-function relationship) (Pongracz 1985; Segev and Rall 1988, 1998). As we and others have shown (Leuner and Shors 2004; Majewska et al. 2000; Tan et al. 2009; Zhou et al. 2004), increased dendritic spine density, mature dendritic spine morphologies (e.g., mushroom shapes), and proximal redistribution of spine synapses can amplify neuronal excitability, enhance frequency-following ability, reduce noise-filtering capabilities, and attenuate inhibitory input. To determine the effect of SCI on reflex function in association with dendritic spine remodeling on α-motor neurons, we measured the H-reflex response in uninjured sham animals (i.e., in the hindlimb) and in SCI animals above and below the injury in the forelimb and hindlimb, respectively.
We also measured the M-wave, which reveals the electrical responsiveness of motor axons, its ability to conduct an action potential, and the electrochemical coupling of the efferent and muscle tissue (e.g., neuromuscular junction) (Hultborn and Nielsen 1995). In normal animals, the H-reflex undergoes activity RDD. A reduction in H-reflex RDD is a physiological indicator of spasticity (Boulenguez et al. 2010; Ho and Waite 2002; Lee et al. 2009; Taylor et al. 1984). To determine H-reflex and M-wave response in SCI animals, we electrically stimulated the deep radial nerve or tibial nerves and recorded reflex response from muscle in the forelimb (extensor carpi radialis) and hindlimb (plantar muscle). As a comparison, we measured evoked H- and M-responses from hindlimb muscle in uninjured sham animals. We chose these reflexes on the basis of preliminary studies and previously published work demonstrating that evoked reflex responses for these muscles could be reproducibly produced in adult rats after SCI (Boulenguez et al. 2010; Kim et al. 2009; Valero-Cabre et al. 2004). Importantly, previous studies have shown that changes in plantar reflex after SCI are similar to changes in reflexes elicited for other hindlimb muscles, i.e., tibialis anterior and gastrocnemius, which are also innervated by motor pools in L4–L5 (Lee et al. 2009; Valero-Cabre et al. 2004).
We used a paired-pulse stimulation paradigm: a control and test pulse, separated by a range of interpulse intervals from 2,000 to 10 ms (Fig. 6). A representative trace in sham produced from recordings of plantar muscle shows two evoked EMG waves: the M-response and the H-reflex (central loop pathway) (Fig. 6A). As the interval between the control and test pulse shortened from 2,000 to 10 ms, there was a marked depression of the H-reflex. The M-wave amplitude also decreased with shortening interpulse intervals, demonstrating RDD of motor neuron-to-muscle response. Figure 6, B and C, shows a qualitative example of SCI-induced reductions in H- and M-wave RDD from muscle recordings above and below the injury at 100- and 10-ms interpulse intervals. In SCI animals, the M-wave appeared to maintain stable amplitude even at shorter interpulse intervals.
We quantified the percentage change in H-reflex for uninjured sham (n = 7) and SCI animals (n = 6) over the range of interpulse intervals (Fig. 6D). In sham animals, the H-reflex maintained stable amplitude between 2,000 and 300 ms and with a steady decline at shorter interpulse intervals, similar to that observed in previous studies (Ho and Waite 2002; Hosoido et al. 2009; Tan et al. 2012a). The M-wave amplitude in sham animals remained stable through a wider range of interpulse intervals, 2,000 and 100 ms (%M-wave amplitude, 2,000 vs. 50 ms: P < 0.05; 1-way ANOVA with Bonferroni's post hoc test) (Fig. 6E). Therefore, the H-reflex depression response in sham animals is not due to the inability of muscle to respond to repeated stimulus activity. At shorter interpulse intervals (i.e., 3–5 ms), both the H-reflex and M-wave responses depressed at a much greater rate and, in most stimulus-recording trials, failed to appear in sufficient number for analysis (data not shown).
Six weeks after SCI, H-reflex measurements from evoked hindlimb reflex revealed a significant reduction in RDD (i.e., H-reflex amplitude stabilized) through the entire range of interpulse intervals tested (Fig. 6D). Between interpulse intervals from 50 to 150 ms, there was a significant increase in H-reflex response in SCI below the injury site compared with sham (P < 0.05; 1-way ANOVA with Bonferroni's post hoc test). Notably, SCI appeared to amplify the reflex response below the injury site at the shortest interpulse interval at 10 ms compared with sham (P < 0.05; 1-way ANOVA with Bonferroni's post hoc test), with amplitude responses greater than 100% of control amplitude. In contrast, within the same SCI animal, there continued to be significant RDD in recordings above the injury at 500, 50, and 10 ms (P < 0.05; 1-way ANOVA with Bonferroni's post hoc test) (Fig. 6D). H-reflex RDD above the injury was not significantly different from that in sham across all interpulse intervals (P > 0.05). These findings indicate that SCI-induced increases in H-reflex response only occurred below the level of the injury and from muscle innervated primarily by motor pools in spinal segment L4–L5. Over the range of interpulse intervals tested from 2,000 to 100 ms, the %M-wave amplitude remained close to 100% across all comparator groups (Fig. 6E). However, there was a significant increase in %M-wave amplitude above 100% of control in SCI above or below injury compared with sham, which depressed at 50 and 10 ms (P < 0.05; ANOVA on ranks with Dunn's post hoc test). The ratio of H-reflex to M-wave responses (H/M ratio) calculated from reflex data in SCI below the injury was larger than that in sham or in SCI above injury at all interpulse intervals (Fig. 6F). SCI below the injury site resulted in a more stabilized rate of decay for H/M ratio values, an indication of hyperreflexia and spasticity (Little and Halar 1985; Matthews, 1966; Nielsen et al. 2007).
To assess changes to H-reflex response fidelity, we calculated the CoV (SD/mean %H-reflex amplitude) for 50 and 10 ms for sham and SCI animals. At the 50-ms interpulse interval, the CoV after SCI below the injury was nearly 30–50% smaller than for SCI above the injury or for sham (SCI below injury, 0.20; SCI above injury, 0.58; sham, 0.85). Similarly, the CoV for SCI below the injury site was almost 40–60% smaller than for sham (SCI below injury, 0.37; SCI above injury, 0.66; sham, 0.92). Taken together, these values show that in addition to increasing H-reflex amplitude, SCI also increases the reliability of reflex activation below the injury site.
Inhibition of Rac1 disrupts dendritic spine remodeling.
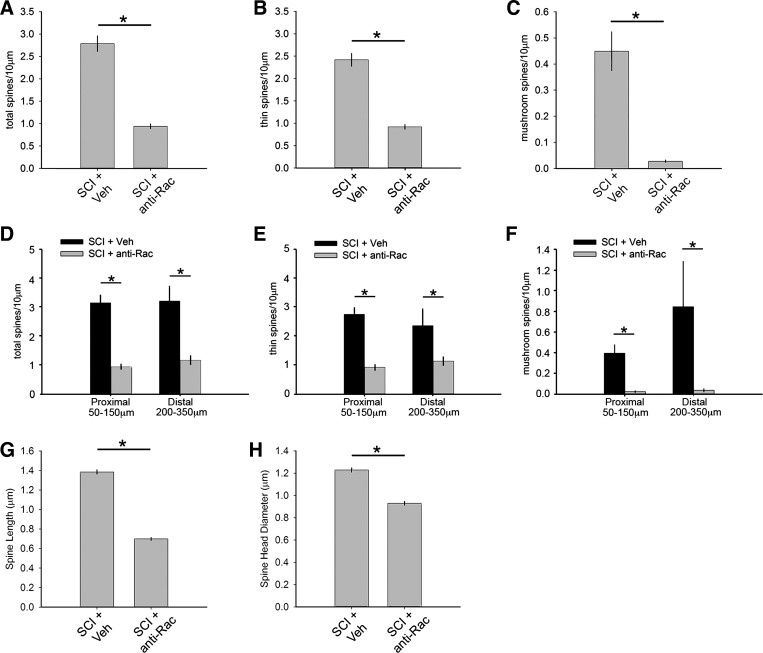
We reasoned that if abnormal dendritic spine profiles after SCI contributes to increased reflex excitability, then disruption of dendritic spine remodeling would reduce signs of spasticity. To determine whether disruption of dendritic spine remodeling on α-motor neurons in L4–L5 after SCI reduces spasticity, we assessed the effects of administering NSC23766, a specific Rac1-GTPase inhibitor. Treatment with NSC23766 resulted in a decrease in total, thin, and mushroom-shaped spine density compared with SCI + vehicle (P < 0.05; SCI + anti-Rac vs. SCI + vehicle: 0.95 ± 0.24 vs. 2.8 ± 0.78 total spines/10-μm dendrite, 0.92 ± 0.23 vs. 2.4 ± 0.63 thin spines/10-μm dendrite, and 0.03 ± 0.03 vs. 0.44 ± 0.33 mushroom spines/10-μm dendrite; ANOVA on ranks with Dunn's post hoc test) (Fig. 7, A–C). We also determined the effect of NSC23766 treatment on dendritic spine distribution by measuring spine density in proximal and distal locations along dendrites of α-motor neurons after SCI (Fig. 7, D–F). NSC23766 treatment significantly decreased spine density in proximal and distal regions and for all spine categories, including total, thin-shaped, and mushroom-shaped dendritic spines (P < 0.05; for proximal total spines: 3.1 ± 0.2 vs. 0.9 ± 0.4, for proximal thin spines: 2.7 ± 0.16 vs. 0.9 ± 0.4, for proximal mushroom spines: 0.4 ± 0.4 vs. 0.02 ± 0.02, for distal total spines: 3.2 ± 0.5 vs. 1.2 ± 0.5, for distal thin spines: 2.4 ± 0.5 vs. 1.1 ± 0.5, and for distal mushroom spines: 0.8 ± 0.9 vs. 0.04 ± 0.07 spines/10-μm dendrite; 1-way ANOVA with Bonferroni's post hoc test).
Fig. 7.
Rac1 inhibitory treatment disrupts dendritic spine morphology on motor neurons in the ventral horn after SCI. Treatment with NSC23766 in SCI animals (SCI + anti-Rac) resulted in a significant decrease in total (A), thin (B), and mushroom spine density (C) compared with SCI + Veh (*P < 0.05). D–F: assessment of dendritic spine distribution on motor neurons showed that NSC23766 treatment in SCI animals resulted in decreased spine density for all spine categories at both proximal and distal branch regions (*P < 0.05). NSC23766 treatment decreased SCI-induced spine length (G) and spine head diameter (H) compared with SCI + Veh (*P < 0.05).
Dendritic spines on α-motor neurons in SCI animals that were treated with NSC23766 decreased in spine length and head diameter compared with neurons in SCI + vehicle (P < 0.05; length, 0.7 ± 0.42 vs. 1.38 ± 0.79 μm; head diameter, 0.93 ± 0.51 vs. 1.23 ± 0.66 μm; ANOVA on ranks with Dunn's post hoc test) (Fig. 7, G and H). Together, these findings show that Rac1-inhibitor NSC23766 treatment can effectively disrupt SCI-induced dendritic spine remodeling on α-motor neurons.
Inhibition of dendritic spine remodeling reduces H-reflex excitability after SCI.
Previous work has demonstrated that intrathecal infusion of NSC23766 is efficacious in restoring close-to-normal dendritic spine profiles on nociceptive neurons within the dorsal horn after SCI and peripheral nerve injury (Tan et al. 2008, 2011). In these studies, treatment with NSC23766 also reduced neuronal hyperexcitability associated with central sensitization, demonstrating that Rac1-regulated dendritic spine remodeling can contribute to mechanisms underlying neuropathic pain (Tan and Waxman 2012). To determine whether disruption of Rac1-regulated dendritic spine profiles on α-motor neurons attenuates exaggerated H-reflex responsiveness after SCI, we used a paired-pulse stimulation paradigm as described above (Hultborn and Nielsen 1995; Tan et al. 2012a) (see Fig. 6). Representative EMG traces in SCI animals below the injury in the hindlimb demonstrates that stimulation produced both M-wave and H-reflex responses (Fig. 8). At the shortest interpulse interval of 10 ms, there was a notable reduction of the RDD. At the 10-ms interpulse interval, treatment with the Rac1 inhibitor NSC23766 appeared to restore RDD of the H-reflex in hindlimb EMG recordings (e.g., reduced H-reflex amplitude in response to the test pulse) (Fig. 8B).
Fig. 8.
Disruption of Rac1-regulated dendritic spines reduces SCI-induced H-reflex hyperexcitability. Representative traces show the M and H responses from paired-pulse testing in SCI + Veh below the injury (A) and SCI + anti-Rac (B) treatment groups. C: quantification of the %H-reflex response demonstrates that the H-reflex response in SCI + Veh animals exhibited reduced RDD (also see Fig. 6). Rac1 inhibitor treatment in SCI animals reduced the %H-reflex at 100, 50, and 10 ms compared with SCI + Veh (*P < 0.05). D: there was no significant difference in the %M-wave between SCI + Veh and SCI + anti-Rac. E: treatment with Rac1 inhibitor in SCI animals decreased the H/M ratio compared with SCI + Veh, as demonstrated by a steeper downward trend line.
Figure 8C shows the quantified changes in the H-reflex response in SCI + vehicle (n = 6) and SCI + anti-Rac1 (n = 5). Six weeks after SCI, in animals treated with control vehicle, the hindlimb H-reflex response demonstrated almost no RDD, with %H-reflex response remaining stable (e.g., close to 100%) across the range of interpulse intervals from 2,000 to 10 ms (for a comparison with sham, see Fig. 6). Treatment of SCI animals with the Rac1 inhibitor resulted in a restoration of RDD at the three shortest interpulse intervals of 100, 50, and 10 ms compared with SCI + vehicle (P < 0.05; at 100 ms, 56.9 ± 29.9% vs. 91.6 ± 8.5%; at 50 ms, 34.5 ± 35.3% vs. 92.4 ± 19.3%; at 10 ms, 13.3 ± 21.4% vs. 88.9 ± 32.9%; 1-way ANOVA with Bonferroni's post hoc test) (Fig. 8C). In comparisons across SCI animal groups, the %M-wave amplitude remained close to 100% between interpulse intervals of 2,000 and 100 ms (group means: for SCI + vehicle, 98.6 ± 1.5%; for SCI + anti-Rac1, 107.3 ± 11.4%) (Fig. 8E). The %M-wave response at shorter interpulse intervals, 50 and 10 ms, exhibited greater variability compared with that at longer stimulus intervals, but the difference was not statistically significant (P > 0.05).
We calculated the CoV of the %H-reflex at 50 and 10 ms across SCI animal groups treated with vehicle or the Rac1 inhibitor. At the 50- and 10-ms interpulse intervals, treatment with the Rac1 inhibitor in SCI animals resulted in a CoV that was nearly fourfold greater than in vehicle-treated SCI animals (SCI + vehicle, 0.27 and 0.37; SCI + anti-Rac, 0.9 and 1.6). Thus, in addition to restoring RDD, Rac1 inhibition also increased the variability of the reflex response. Figure 8E shows the plot for the H/M ratio. Treatment with the Rac1 inhibitor decreased the overall H/M ratio across all interpulse intervals tested between 2,000 and 10 ms, as shown by a downward shift in trend line slope.
VGluT1 bouton areal density in the gray matter does not increase after injury.
Synapse-associated protein markers (e.g., synaptophysin and PSD-95) increase after SCI, demonstrating the presence of injury-induced synaptic plasticity (Tan et al. 2008; Tan and Waxman 2012). Because upper motor tract injury and SCI can increase the excitability of spinal reflex pathways below the injury (Baastrup et al. 2010; Little and Halar 1985; Tan et al. 2012a), we next determined if excitatory inputs, particularly those of Ia afferents, change after SCI. VGluT1 is a widely used marker for excitatory Ia afferent terminations in the spinal cord (Alvarez et al. 2004, 2011; Kitzman 2007).
As shown in representative images in Fig. 9, A–D, immunopositive VGluT1 puncta were distributed throughout the spinal cord gray matter of the lumbar enlargement, L4–L5, for each analyzed treatment group (Fig. 9, A–D, left). To visualize the distribution of VGluT1 puncta in the gray matter, we compiled the VGluT1 staining profiles from multiple tissue sections from each treatment group and produced spatial heat maps (Fig. 9, A–C, right). Although the highest concentration of VGluT1-positive boutons appeared to correspond with Rexed laminae V/VI (Hantman and Jessell 2010; LaMotte et al. 1991), VGluT1 puncta were distributed throughout all laminae. The areal densities of VGluT1 boutons were calculated in the total gray matter (Fig. 9D) and within three regions: the dorsal horn, intermediate zone, and ventral horn (Fig. 9, E–G; also see insets). Six weeks after SCI, we observed no significant difference in the areal density of VGLUT boutons in the total gray matter compared with that in uninjured sham animals (P > 0.05). Similarly, there was no statistical difference following SCI + vehicle in the other three gray matter regions analyzed compared with uninjured sham (P > 0.05). In contrast, treatment with NSC23766 significantly decreased the areal density of VGluT1 compared with SCI + vehicle in the total gray matter, intermediate zone, and ventral horn (for total gray matter: 63.6 ± 37.1 vs. 124.5 ± 57.7, for intermediate zone: 72.4 ± 25.2 vs. 138.7 ± 51.3, and for ventral horn: 58.1 ± 48.4 vs. 129.8 ± 55.9 puncta; ANOVA on ranks with Dunn's post hoc test). There was no significant change in areal density of VGluT1 in the superficial dorsal horn following Rac1 inhibitor treatment in SCI animals compared with SCI + vehicle (P > 0.05).
Rac1 inhibition does not affect locomotor behavior.
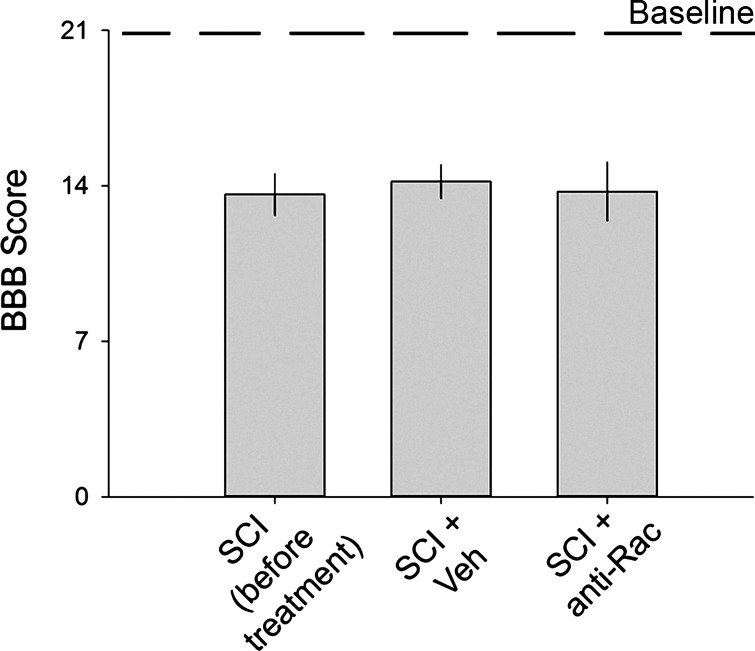
To rule out any differences in gross locomotor ability in SCI animals, we assessed postinjury locomotor behavior using the BBB locomotor scale (Basso et al. 1995, 1996) (Fig. 10). Blinded observers performed behavioral testing at three time points: on naive animals before any surgical procedures, within 1 wk after catheter implantation and before drug treatment, and at the 6-wk post-SCI endpoint (also see Fig. 1). All naive animals exhibited a baseline locomotor score of 21 (1 worst to 21 best). Five weeks after SCI and catheter implantation, before any treatments, animals exhibited a mean BBB score of 13.6 ± 3.8, demonstrating the expected locomotor ability in the late-SCI recovery phase (Basso et al. 1995). BBB testing of SCI animals after vehicle or drug delivery demonstrated no significant effect on locomotor ability with scores remaining unchanged between SCI animals treated with (n = 10) or without Rac1 inhibitor (n = 11) (P > 0.05, 14.2 ± 1.6 vs. 13.8 ± 2; ANOVA on ranks).
Fig. 10.
Locomotor testing. Blinded observers performed BBB testing on SCI animals at 3 time points: before any procedure (baseline), before treatment, and after treatment (SCI + Veh and SCI + anti-Rac). All naive animals exhibited a baseline locomotor score of 21. There were no significant differences in BBB scores across groups (P > 0.05).
DISCUSSION
Spinal cord circuits can reorganize, changing in structure and function after injury (Raisman 1991). Our present findings demonstrate robust changes in dendritic spine morphology on α-motor neurons after SCI, including an increase in dendritic spine density, a distribution of spines closer to the cell body, and the presence of more mature dendritic spines. These postsynaptic dendritic changes have been shown to accompany increased neuronal excitability after SCI (Rall et al. 1992; Segev and Rall 1998; Tan et al. 2009). In agreement, we observed a significant loss of H-reflex RDD below the injury (i.e., increased H/M ratio), indicative of spasticity (Boulenguez et al. 2010; Matthews 1966; Nielsen et al. 2007). Importantly, dendritic spines above the injury exhibited a nearly opposite morphological profile with decreased spine density and with distribution and shape that were more similar to control profiles. As expected, there was no change in H-reflex excitability above the level of injury. Overall, these results demonstrate that abnormal dendritic spine profiles below the level of injury accompany spasticity after SCI, and conversely, the lack of such spine profiles above the injury correspond with a lack of spinal reflex hyperexcitability.
To further elucidate the structure-function link between dendritic spine dysgenesis and hyperreflexia, we disrupted dendritic spine remodeling by targeting Rac1 signaling in SCI animals. We have previously shown that Rac1 inhibition disrupts dendritic spine remodeling in dorsal horn sensory neuron after SCI, nerve injury, and diabetes mellitus (Tan et al. 2008, 2011, 2012b). In the present study, we observed a decrease in spine density on α-motor neurons and a closer-to-normal distribution of dendritic spines following treatment with NSC23766, a Rac1 inhibitor. NSC23766 treatment also decreased spine length and head diameter, and partially restored normal H-reflex activity (i.e., increased RDD). With these results taken together, our study is the first to demonstrate robust dendritic spine reorganization on α-motor neurons in the ventral horn, which accompanies spasticity after SCI. We implicate Rac1 signaling as an important mediator in both the structural and functional changes within the spinal reflex pathway after injury.
Spasticity after SCI has been attributed to a variety of mechanisms within the spinal reflex arc (Nielsen et al. 2007; Roy and Edgerton 2012). Muscle spindle afferents may lose either presynaptic inhibition or reciprocal inhibition. Alternatively, loss of Renshaw interneuron activity, thought to mediate reciprocal inhibition, can trigger spasticity (Nielsen et al. 2007). Evidence obtained from intracellular recordings of spinal motoneurons also demonstrates increased motoneuron excitability, i.e., the ability to generate action potentials, including the appearance of plateau potentials and persistent inward sodium and calcium currents in rat motor neurons after SCI (Bennett et al. 2001b; Heckmann et al. 2005; Li et al. 2004), potassium chloride cotransporter KCC2 downregulation (Boulenguez et al. 2010; Vinay and Jean-Xavier 2008), and sodium channel misexpression (Harvey et al. 2006; Li and Bennett 2003). Inflammation (e.g., microgliosis) occurs in a number of nervous system injury models, including SCI (Craner et al. 2005; Hains and Waxman 2006), and may contribute to increasing excitability of neuronal populations within spinal circuits (Gwak and Hulsebosch 2009; Zhao et al. 2007b). Astrocyte activation after injury can potentially maintain hyperexcitability (Scholz and Woolf 2007). Finally, maladaptive plasticity such as “collateral” or “reactive” sprouting may contribute to altered spinal motor control (Boulenguez et al. 2010; Krenz and Weaver 1998; Nielsen et al. 2007; Raisman, 1994). Others have shown altered dendrite branch length on motor neurons in the spinal cord that accompanies spasticity after SCI (Kitzman 2005). Although dendritic spine morphologies change on pyramidal neurons in the motor cortex after SCI (Kim et al. 2006), the functional role for these spine alterations is not firmly understood. Computer simulations have attempted to predict the physiological contribution of dendritic spines on motor neurons (Rall et al. 1967); however, in vivo changes in dendritic spine structure have not been reported in spinal cord motor pools.
Dendritic spine morphology partly determines synaptic function and therefore provides a visual clue into how neural networks function (Calabrese et al. 2006; Segev and Rall 1998). Dendritic spines can reorganize rapidly following synaptic activity (e.g., activity-dependent plasticity) and increase in density, which provides new or stronger synapses (Halpain 2000). Abnormal dendritic spine morphologies have been reported in a wide spectrum of neuropsychiatric diseases, including posttraumatic stress disorder, substance dependence and addiction, autism spectrum disorders, and mental retardation (Halpain et al. 2005; Purpura 1974). Although adaptive plasticity between Ia afferents and spinal motor neurons can shape H-reflex response in both humans and rodents (Thompson et al. 2009; Wolpaw 1994), maladaptive plasticity can contribute to pathological H-reflex function associated with hyperreflexia and spasticity (Lance 1980; Nielsen et al. 2007). In chronic SCI, hyperexcitability of the spinal stretch reflex (e.g., H-reflex) is thought to underlie spasticity, which manifests as a velocity-dependent increase in tonic stretch reflexes, with uncontrollable “jerking” movement and abnormal muscle tone, whereby muscle continually contract (Ashby et al. 1987; Lance 1980; Nielsen et al. 2007; Skold and Woolf 1999). In our study, we observed significant SCI-induced changes in dendritic spine morphologies on α-motor neurons below the injury site that accompanied a loss of RDD and a stabilization of the H/M ratio over a broad range of nerve stimulation rates. Importantly, we observed only minor changes in M-wave response after SCI, indicating that changes in RDD and H/M ratio were primarily due to mechanistic changes within the spinal cord monosynaptic circuit.
Although sacrocaudal injuries might better replicate some aspects of clinical spasticity (Li and Bennett 2003; Ritz et al. 1992), these SCI models only allow studies of neurological deficits in tail musculature, which are absent in human. To permit sufficient locomotor ability for open-field behavioral assessment and H-reflex testing of hindlimb musculature, a parallel of leg muscle groups in human, we performed contusion SCI at spinal segment L2. As with all SCI animal studies, however, we encountered an observation suggesting that our injury model also cannot entirely reflect the human SCI condition. In contrast with human SCI at lower thoracic or upper lumbar segments, which generally produces some chronic negative motor signs, including flaccidity or lower limb weakness (Doherty et al. 2002), we observed increased spinal motor reflex activity associated with increase muscle tone 6 wk after injury. Thus it is important to mention that our contusion SCI model was utilized as a compromise to study spinal reflex function in hindlimb musculature.
We noted in our study that the RDD of the H-reflex did not exhibit depression at a similar rate compared with an earlier report of the effect of spinal contusion on RDD (Thompson et al. 1992b). Whereas Thompson et al. observed activity-rate depression of ∼85% of control at 5 Hz (Thompson et al. 1992a), we observed a similar loss of H-reflex activity at 10–20 Hz (i.e., 50- to 100-ms interpulse interval). A probable explanation for this discrepancy is due to the additional procedures that animals underwent before reflex testing in our study, including surgical implantation of intrathecal catheters and infusions of vehicle or drug solutions. Although no effect of catheter implantation and drug infusion has been observed in previous nociceptive testing in control animals (Tan et al. 2008), it is possible that these additional experimental procedures could have led to sensitization of afferents within the spinal reflex circuit. Nonetheless, the magnitude of activity-rate depression in sham and SCI animals in our study fell within other documented ranges (Hosoido et al. 2009; Tan et al. 2012a).
Exogenous electrical stimulation of the H-reflex circuit can directly induce changes in H-reflex response, which can persist for many hours or days (Chen et al. 2003, 2006). These previous studies demonstrate the presence of activity-dependent plasticity within the monosynaptic reflex system. In our current study, to establish a structure-function relationship between dendritic spine remodeling and H-reflex dysfunction after SCI, we were required to assess spine changes and H-reflex function within the same animals. This acute H-reflex function and dendritic spine assessment approach limited our ability to control for the possible confound that dendritic spine changes could also have resulted from EMG reflex testing, which required direct stimulation of muscle-sensory nerves. Although H-reflex testing only lasted about 1 h per animal and we ensured that all animals underwent similar testing protocols, we cannot exclude the possibility that each treatment group could have had a different capacity to respond to potential EMG testing-induced dendritic spine changes. We note that in uninjured sham animals, there was a greater proportion of thin-shaped dendritic spines compared with mushroom-shaped dendritic spines (see Figs. 4 and 5). Thin, filopodia-like dendritic spines are thought to represent newly formed or more plastic dendritic spines, whereas mushroom-shaped spines may represent more stable, mature spines (Bourne and Harris 2007, 2008). This observation in sham animals does suggest the possibility that EMG testing could have resulted in the de novo presence of more structurally responsive thin-shaped dendritic spines. Our current experiments, however, do not permit us to determine whether spine changes are a sole result of treatments (i.e., SCI, drug intervention, etc.) or a combination of treatments and the potential direct effect of EMG electrophysiological assessment, which could also have influenced spine morphologies.
Our findings raise the question of how altered dendritic spine morphologies below the level of injury contribute to hyperexcitable spinal reflex function. The dendritic spine profiles we observed after SCI below the injury can have direct biophysical effects on motor neuron excitability (Rall et al. 1967; Segev and Rall 1988; Tan et al. 2009). The distribution of spine synapses closer to motor neuron cell bodies can increase the overall impact of excitatory input and transmission. The increased head volume of mature, mushroom-shaped dendritic spines permits increased clustering of excitatory membrane receptors (e.g., AMPA receptors; Wiens et al. 2005). These properties can amplify synaptic transduction. Importantly, we observed an increase in mature spine geometries after SCI, which could increase input discretization (e.g., narrow excitatory postsynaptic potential waveforms) and allow trains of suprathreshold potentials to propagate at higher rates of activity with greater fidelity (Rall 1967; Rall et al. 1967). This transmission property could contribute to excessive firing in motor neurons (Tan et al. 2009; Yuste and Urban 2004) and facilitate supra- and subthreshold temporal summation (Carter et al. 2007; Gold and Bear 1994; Martiel et al. 1994). In our study, temporal summation would most likely have occurred at the shorter interpulse intervals during H-reflex testing. Consecutive transcutaneous electrical stimulations to facilitate withdrawal reflex have demonstrated maximal temporal facilitation at the 10- to 20-Hz range (Arendt-Nielsen et al. 1997, 2000), suggesting the presence of a temporally dependent integration mechanism within reflex pathways. Thus it is interesting to consider that SCI-induced dendritic spine changes lead to an amplification of presynaptic excitatory input and enhancement of a temporally dependent integration mechanism to increase the gain of H-reflex function after SCI.
We have implicated Rac1 in the pathological changes that contribute to chronic nociceptive hyperexcitability after SCI (Tan et al. 2008). Rac1 is a small-molecule GTPase that is involved in regulating dendritic spine morphology (Tashiro and Yuste 2008). In a mouse model of fragile X syndrome (FMR knockout), Rac1 dysfunction is associated with abnormal dendritic spine morphology and decreased pain (Chen et al. 2010; Lee et al. 2003; Price et al. 2007). In our model of SCI, we administered NSC23766, a Rac1-specific inhibitor that does not affect Cdc42 or Rho GTPases or affect the interaction of Rac1 to its downstream effector, PAK1 (Gao et al. 2004). Although our current study does not preclude off-target effects, we have previously shown that the Rac1 inhibitor disrupts the development and appearance of dendritic spines in vitro (Tan et al. 2011) and that NSC23766 treatment is effective in attenuating dendritic spine remodeling in the dorsal horn after SCI (Tan et al. 2008). Moreover, NSC23766 treatment does not significantly affect electrophysiological or behavioral signs of pain-reflex withdrawal function in normal, uninjured animals (Tan et al. 2012b). Although microglial activation has been implicated in altering the excitability of sensory neurons after injury (Tan et al. 2012a; Zhao et al. 2007a), administration of NSC23766 does not appear to affect the activation of microglia after nerve injury (Tan et al. 2011). In agreement with previous studies (Beauparlant et al. 2013; Kitzman 2007), we report that VGluT1 expression did not change after SCI, suggesting that the presence of H-reflex hyperexcitability is not due to increased excitatory presynaptic afferent inputs (Kitzman 2007). Although it is possible that Rac1 inhibition may have affected presynaptic elements, NSC23766 treatment only decreased VGluT1 areal density in the intermediate zone and ventral horn. This suggests that the Rac1-inhibitor had a topographically specific effect on deeper lamina only, where sensory-motor neuron populations exhibit dendritic spine plasticity after injury (Tan et al. 2008, 2011).
A major challenge in SCI research has been identifying the mechanisms that contribute to secondary complications such as spasticity and pain. Our previous work has demonstrated that dendritic spine remodeling in the dorsal horn contributes to hyperexcitability associated with neuropathic pain after SCI (Tan et al. 2008; Tan and Waxman 2012). In the present work we show that dendritic spine dysgenesis on α-motor neurons also contribute to the development of spasticity. Our data further show that Rac1 signaling participates in this dendritic spine remodeling and may provide a novel opportunity to specifically address the underlying pathophysiology of spasticity after SCI.
GRANTS
This work was supported by grants from Paralyzed Veterans of America (PVA) and the Department of Veterans Affairs (VA) Medical Research Service and Rehabilitation Research Service. A. M. Tan is funded by the PVA Research Foundation and VA Career Development Award 1 IK2 RX001123-01A2. The Center for Neuroscience and Regeneration Research is a Collaboration of PVA with Yale University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.P.B., S.L., and A.M.T. performed experiments; S.P.B. and A.M.T. analyzed data; S.P.B. and A.M.T. prepared figures; S.P.B., S.L., S.G.W., and A.M.T. approved final version of manuscript; S.G.W. and A.M.T. interpreted results of experiments; S.G.W. and A.M.T. edited and revised manuscript; A.M.T. conception and design of research; A.M.T. drafted manuscript.
ACKNOWLEDGMENTS
We thank Pamela Zwinger and Peng Zhao for excellent technical assistance.
REFERENCES
- Alvarez FJ, Titus-Mitchell HE, Bullinger KL, Kraszpulski M, Nardelli P, Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. I. Loss of VGLUT1/IA synapses on motoneurons. J Neurophysiol 106: 2450–2470, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol 472: 257–280, 2004. [DOI] [PubMed] [Google Scholar]
- Anderson LC, von Bartheld CS, Byers MR. NGF depletion reduces ipsilateral and contralateral trigeminal satellite cell reactions after inferior alveolar nerve injury in adult rats. Exp Neurol 150: 312–320, 1998. [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Graven-Nielsen T, Svensson P, Jensen TS. Temporal summation in muscles and referred pain areas: an experimental human study. Muscle Nerve 20: 1311–1313, 1997. [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Sonnenborg FA, Andersen OK. Facilitation of the withdrawal reflex by repeated transcutaneous electrical stimulation: an experimental study on central integration in humans. Eur J Appl Physiol 81: 165–173, 2000. [DOI] [PubMed] [Google Scholar]
- Ashby P, Mailis A, Hunter J. The evaluation of “spasticity”. Can J Neurol Sci 14: 497–500, 1987. [DOI] [PubMed] [Google Scholar]
- Baastrup C, Maersk-Moller CC, Nyengaard JR, Jensen TS, Finnerup NB. Spinal-, brainstem- and cerebrally mediated responses at- and below-level of a spinal cord contusion in rats: evaluation of pain-like behavior. Pain 151: 670–679, 2010. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12: 1–21, 1995. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 139: 244–256, 1996. [DOI] [PubMed] [Google Scholar]
- Beauparlant J, van den Brand R, Barraud Q, Friedli L, Musienko P, Dietz V, Courtine G. Undirected compensatory plasticity contributes to neuronal dysfunction after severe spinal cord injury. Brain 136: 3347–3361, 2013. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol 86: 1972–1982, 2001a. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol 86: 1955–1971, 2001b. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer T, Yuste R. Spine motility, phenomenology, mechanisms, and function. Neuron 35: 1019–1027, 2002. [DOI] [PubMed] [Google Scholar]
- Bose P, Parmer R, Reier PJ, Thompson FJ. Morphological changes of the soleus motoneuron pool in chronic midthoracic contused rats. Exp Neurol 191: 13–23, 2005. [DOI] [PubMed] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med 16: 302–307, 2010. [DOI] [PubMed] [Google Scholar]
- Boulenguez P, Vinay L. Strategies to restore motor functions after spinal cord injury. Curr Opin Neurobiol 19: 587–600, 2009. [DOI] [PubMed] [Google Scholar]
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol 17: 381–386, 2007. [DOI] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci 31: 47–67, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416: 636–640, 2002. [DOI] [PubMed] [Google Scholar]
- Brus-Ramer M, Carmel JB, Chakrabarty S, Martin JH. Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury. J Neurosci 27: 13793–13801, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese B, Wilson MS, Halpain S. Development and regulation of dendritic spine synapses. Physiology (Bethesda) 21: 38–47, 2006. [DOI] [PubMed] [Google Scholar]
- Carter AG, Soler-Llavina GJ, Sabatini BL. Timing and location of synaptic inputs determine modes of subthreshold integration in striatal medium spiny neurons. J Neurosci 27: 8967–8977, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YW, Tan A, Saab C, Waxman S. Unilateral focal burn injury is followed by long-lasting bilateral allodynia and neuronal hyperexcitability in spinal cord dorsal horn. J Pain 11: 119–130, 2010. [DOI] [PubMed] [Google Scholar]
- Chen LY, Rex CS, Babayan AH, Kramar EA, Lynch G, Gall CM, Lauterborn JC. Physiological activation of synaptic Rac>PAK (p-21 activated kinase) signaling is defective in a mouse model of fragile X syndrome. J Neurosci 30: 10977–10984, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XY, Chen L, Wolpaw JR. Conditioned H-reflex increase persists after transection of the main corticospinal tract in rats. J Neurophysiol 90: 3572–3578, 2003. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen XY, Jakeman LB, Chen L, Stokes BT, Wolpaw JR. Operant conditioning of H-reflex can correct a locomotor abnormality after spinal cord injury in rats. J Neurosci 26: 12537–12543, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craner MJ, Damarjian TG, Liu S, Hains BC, Lo AC, Black JA, Newcombe J, Cuzner ML, Waxman SG. Sodium channels contribute to microglia/macrophage activation and function in EAE and MS. Glia 49: 220–229, 2005. [DOI] [PubMed] [Google Scholar]
- Crockett DP, Harris SL, Egger MD. Plantar motoneuron columns in the rat. J Comp Neurol 265: 109–118, 1987. [DOI] [PubMed] [Google Scholar]
- Doherty JG, Burns AS, O'Ferrall DM, Ditunno JF Jr. Prevalence of upper motor neuron vs lower motor neuron lesions in complete lower thoracic and lumbar spinal cord injuries. J Spinal Cord Med 25: 289–292, 2002. [DOI] [PubMed] [Google Scholar]
- Dubreuil CI, Winton MJ, McKerracher L. Rho activation patterns after spinal cord injury and the role of activated Rho in apoptosis in the central nervous system. J Cell Biol 162: 233–243, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erschbamer MK, Hofstetter CP, Olson L. RhoA, RhoB, RhoC, Rac1, Cdc42, and Tc10 mRNA levels in spinal cord, sensory ganglia, and corticospinal tract neurons and long-lasting specific changes following spinal cord injury. J Comp Neurol 484: 224–233, 2005. [DOI] [PubMed] [Google Scholar]
- Friel KM, Martin JH. Bilateral activity-dependent interactions in the developing corticospinal system. J Neurosci 27: 11083–11090, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA 101: 7618–7623, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Bear MF. A model of dendritic spine Ca2+ concentration exploring possible bases for a sliding synaptic modification threshold. Proc Natl Acad Sci USA 91: 3941–3945, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat. Neuroscience 161: 895–903, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci 26: 4308–4317, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpain S. Actin and the agile spine: how and why do dendritic spines dance? Trends Neurosci 23: 141–146, 2000. [DOI] [PubMed] [Google Scholar]
- Halpain S, Spencer K, Graber S. Dynamics and pathology of dendritic spines. Prog Brain Res 147: 29–37, 2005. [DOI] [PubMed] [Google Scholar]
- Hantman AW, Jessell TM. Clarke's column neurons as the focus of a corticospinal corollary circuit. Nat Neurosci 13: 1233–1239, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol 96: 1141–1157, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume K, Kanda K, Burke RE. Medial gastrocnemius motor nucleus in the rat: age-related changes in the number and size of motoneurons. J Comp Neurol 269: 425–430, 1988. [DOI] [PubMed] [Google Scholar]
- Hebel R, Stromberg MW. Anatomy of the Laboratory Rat. Baltimore, MD: Williams and Wilkins, 1976. [Google Scholar]
- Heckmann CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 31: 135–156, 2005. [DOI] [PubMed] [Google Scholar]
- Ho SM, Waite PM. Effects of different anesthetics on the paired-pulse depression of the h reflex in adult rat. Exp Neurol 177: 494–502, 2002. [DOI] [PubMed] [Google Scholar]
- Holmes WR. Is the function of dendritic spines to concentrate calcium? Brain Res 519: 338–342, 1990. [DOI] [PubMed] [Google Scholar]
- Hosoido T, Motoyama S, Goto M, Mori F, Tajima T, Hirata H, Wada N. Characteristics of H- and M-waves recorded from rat forelimbs. Neurosci Lett 450: 239–241, 2009. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Nielsen JB. H-reflexes and F-responses are not equally sensitive to changes in motoneuronal excitability. Muscle Nerve 18: 1471–1474, 1995. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Nielsen JB. Spinal control of locomotion–from cat to man. Acta Physiol (Oxf) 189: 111–121, 2007. [DOI] [PubMed] [Google Scholar]
- Hunanyan AS, Petrosyan HA, Alessi V, Arvanian VL. Combination of chondroitinase ABC and AAV-NT3 promotes neural plasticity at descending spinal pathways after thoracic contusion in rats. J Neurophysiol 110: 1782–1792, 2013. [DOI] [PubMed] [Google Scholar]
- Jacob JM. Lumbar motor neuron size and number is affected by age in male F344 rats. Mech Ageing Dev 106: 205–216, 1998. [DOI] [PubMed] [Google Scholar]
- Kim BG, Dai HN, McAtee M, Bregman BS. Modulation of dendritic spine remodeling in the motor cortex following spinal cord injury: effects of environmental enrichment and combinatorial treatment with transplants and neurotrophin-3. J Comp Neurol 508: 473–486, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS. Remodeling of synaptic structures in the motor cortex following spinal cord injury. Exp Neurol 198: 401–415, 2006. [DOI] [PubMed] [Google Scholar]
- Kim HY, Wang J, Lee I, Kim HK, Chung K, Chung JM. Electroacupuncture suppresses capsaicin-induced secondary hyperalgesia through an endogenous spinal opioid mechanism. Pain 145: 332–340, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzman P. Alteration in axial motoneuronal morphology in the spinal cord injured spastic rat. Exp Neurol 192: 100–108, 2005. [DOI] [PubMed] [Google Scholar]
- Kitzman P. VGLUT1 and GLYT2 labeling of sacrocaudal motoneurons in the spinal cord injured spastic rat. Exp Neurol 204: 195–204, 2007. [DOI] [PubMed] [Google Scholar]
- Krenz NR, Weaver LC. Sprouting of primary afferent fibers after spinal cord transection in the rat. Neuroscience 85: 443–458, 1998. [DOI] [PubMed] [Google Scholar]
- LaMotte CC, Kapadia SE, Shapiro CM. Central projections of the sciatic, saphenous, median, and ulnar nerves of the rat demonstrated by transganglionic transport of choleragenoid-HRP (B-HRP) and wheat germ agglutinin-HRP (WGA-HRP). J Comp Neurol 311: 546–562, 1991. [DOI] [PubMed] [Google Scholar]
- Lance JW. Symposium synopsis. In: Spasticity: Disordered Motor Control, edited by Feldman RG, Young RR, and Koella WP. Chicago, IL: Year Book Medical, 1980, p. 485–494. [Google Scholar]
- Lee A, Li W, Xu K, Bogert BA, Su K, Gao FB. Control of dendritic development by the Drosophila fragile X-related gene involves the small GTPase Rac1. Development 130: 5543–5552, 2003. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Jakovcevski I, Radonjic N, Hoelters L, Schachner M, Irintchev A. Better functional outcome of compression spinal cord injury in mice is associated with enhanced H-reflex responses. Exp Neurol 216: 365–374, 2009. [DOI] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. New spines, new memories. Mol Neurobiol 29: 117–130, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol 90: 857–869, 2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91: 767–783, 2004. [DOI] [PubMed] [Google Scholar]
- Little JW, Halar EM. H-reflex changes following spinal cord injury. Arch Phys Med Rehabil 66: 19–22, 1985. [PubMed] [Google Scholar]
- Majewska A, Brown E, Ross J, Yuste R. Mechanisms of calcium decay kinetics in hippocampal spines: role of spine calcium pumps and calcium diffusion through the spine neck in biochemical compartmentalization. J Neurosci 20: 1722–1734, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska A, Sur M. Motility of dendritic spines in visual cortex in vivo: changes during the critical period and effects of visual deprivation. Proc Natl Acad Sci USA 100: 16024–16029, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiel JL, Mouchet P, Boissier MD. Modeling the integrative properties of dendrites: application to the striatal spiny neuron. Synapse 16: 269–279, 1994. [DOI] [PubMed] [Google Scholar]
- Matthews WB. Ratio of maximum H reflex to maximum M response as a measure of spasticity. J Neurol Neurosurg Psychiatry 29: 201–204, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi A, Crowley JC, Shtoyerman E, Katz LC. High-resolution in vivo imaging of hippocampal dendrites and spines. J Neurosci 24: 3147–3151, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Kose A, Tsujino T, Tanaka C. Immunocytochemical localization of protein kinase C subspecies in the rat spinal cord: light and electron microscopic study. J Comp Neurol 299: 167–177, 1990. [DOI] [PubMed] [Google Scholar]
- Nakayama AY, Luo L. Intracellular signaling pathways that regulate dendritic spine morphogenesis. Hippocampus 10: 582–586, 2000. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity–from a basic science point of view. Acta Physiol (Oxf) 189: 171–180, 2007. [DOI] [PubMed] [Google Scholar]
- Palmieri RM, Ingersoll CD, Hoffman MA. The Hoffmann reflex: methodologic considerations and applications for use in sports medicine and athletic training research. J Athl Train 39: 268–277, 2004. [PMC free article] [PubMed] [Google Scholar]
- Pongracz F. The function of dendritic spines: a theoretical study. Neuroscience 15: 933–946, 1985. [DOI] [PubMed] [Google Scholar]
- Price TJ, Rashid MH, Millecamps M, Sanoja R, Entrena JM, Cervero F. Decreased nociceptive sensitization in mice lacking the fragile X mental retardation protein: role of mGluR1/5 and mTOR. J Neurosci 27: 13958–13967, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purpura DP. Dendritic spine “dysgenesis” and mental retardation. Science 186: 1126–1128, 1974. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Sullivan PG, Fugaccia I, Scheff SW. Creatine diet supplement for spinal cord injury: influences on functional recovery and tissue sparing in rats. J Neurotrauma 20: 659–669, 2003. [DOI] [PubMed] [Google Scholar]
- Raisman G. Glia, neurons, and plasticity. Ann NY Acad Sci 633: 209–213, 1991. [DOI] [PubMed] [Google Scholar]
- Raisman G. Plasticity of adult central fibre tracts. Prog Brain Res 100: 195–201, 1994. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol 30: 1138–1168, 1967. [DOI] [PubMed] [Google Scholar]
- Rall W, Burke RE, Holmes WR, Jack JJ, Redman SJ, Segev I. Matching dendritic neuron models to experimental data. Physiol Rev 72: S159–S186, 1992. [DOI] [PubMed] [Google Scholar]
- Rall W, Burke RE, Smith TG, Nelson PG, Frank K. Dendritic location of synapses and possible mechanisms for the monosynaptic EPSP in motoneurons. J Neurophysiol 30: 1169–1193, 1967. [DOI] [PubMed] [Google Scholar]
- Ritz LA, Friedman RM, Rhoton EL, Sparkes ML, Vierck CJ Jr. Lesions of cat sacrocaudal spinal cord: a minimally disruptive model of injury. J Neurotrauma 9: 219–230, 1992. [DOI] [PubMed] [Google Scholar]
- Roy RR, Edgerton VR. Neurobiological perspective of spasticity as occurs after a spinal cord injury. Exp Neurol 235: 116–122, 2012. [DOI] [PubMed] [Google Scholar]
- Ruiz-Marcos A, Valverde F. The temporal evolution of the distribution of dendritic spines in the visual cortex of normal and dark raised mice. Exp Brain Res 8: 284–294, 1969. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Radtke C, Tan AM, Zhao P, Hamada H, Houkin K, Honmou O, Kocsis JD. BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. J Neurosci 29: 14932–14941, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE Jr. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma 20: 179–193, 2003. [DOI] [PubMed] [Google Scholar]
- Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol 28: 345–376, 1987. [DOI] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10: 1361–1368, 2007. [DOI] [PubMed] [Google Scholar]
- Segev I, Rall W. Computational study of an excitable dendritic spine. J Neurophysiol 60: 499–523, 1988. [DOI] [PubMed] [Google Scholar]
- Segev I, Rall W. Excitable dendrites and spines: earlier theoretical insights elucidate recent direct observations. Trends Neurosci 21: 453–460, 1998. [DOI] [PubMed] [Google Scholar]
- Skold C, Levi R, Seiger A. Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch Phys Med Rehabil 80: 1548–1557, 1999. [DOI] [PubMed] [Google Scholar]
- Sunshine MD, Cho FS, Lockwood DR, Fechko AS, Kasten MR, Moritz CT. Cervical intraspinal microstimulation evokes robust forelimb movements before and after injury. J Neural Eng 10: 036001, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain 57: 375–382, 1994. [DOI] [PubMed] [Google Scholar]
- Tan AM, Chakrabarty S, Kimura H, Martin JH. Selective corticospinal tract injury in the rat induces primary afferent fiber sprouting in the spinal cord and hyperreflexia. J Neurosci 32: 12896–12908, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Chang YW, Zhao P, Hains BC, Waxman SG. Rac1-regulated dendritic spine remodeling contributes to neuropathic pain after peripheral nerve injury. Exp Neurol 232: 222–233, 2011. [DOI] [PubMed] [Google Scholar]
- Tan AM, Choi JS, Waxman SG, Hains BC. Dendritic spine remodeling after spinal cord injury alters neuronal signal processing. J Neurophysiol 102: 2396–2409, 2009. [DOI] [PubMed] [Google Scholar]
- Tan AM, Colletti M, Rorai AT, Skene JH, Levine JM. Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J Neurosci 26: 4729–4739, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Petruska JC, Mendell LM, Levine JM. Sensory afferents regenerated into dorsal columns after spinal cord injury remain in a chronic pathophysiological state. Exp Neurol 206: 257–268, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Samad OA, Fischer TZ, Zhao P, Persson AK, Waxman SG. Maladaptive dendritic spine remodeling contributes to diabetic neuropathic pain. J Neurosci 32: 6795–6807, 2012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Samad OA, Liu S, Bandaru S, Zhao P, Waxman SG. Burn injury-induced mechanical allodynia is maintained by Rac1-regulated dendritic spine dysgenesis. Exp Neurol 248: 509–519, 2013. [DOI] [PubMed] [Google Scholar]
- Tan AM, Stamboulian S, Chang YW, Zhao P, Hains AB, Waxman SG, Hains BC. Neuropathic pain memory is maintained by Rac1-regulated dendritic spine remodeling after spinal cord injury. J Neurosci 28: 13173–13183, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Waxman SG. Spinal cord injury, dendritic spine remodeling, and spinal memory mechanisms. Exp Neurol 235: 142–151, 2012. [DOI] [PubMed] [Google Scholar]
- Tang AH, Schroeder LA. Spinal-cord depressant effects of ketamine and etoxadrol in the cat and the rat. Anesthesiology 39: 37–43, 1973. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex 10: 927–938, 2000. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Yuste R. Structure and molecular organization of dendritic spines. Histol Histopathol 18: 617–634, 2003. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Yuste R. Regulation of dendritic spine motility and stability by Rac1 and Rho kinase: evidence for two forms of spine motility. Mol Cell Neurosci 26: 429–440, 2004. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Yuste R. Role of Rho GTPases in the morphogenesis and motility of dendritic spines. Methods Enzymol 439: 285–302, 2008. [DOI] [PubMed] [Google Scholar]
- Taylor S, Ashby P, Verrier M. Neurophysiological changes following traumatic spinal lesions in man. J Neurol Neurosurg Psychiatry 47: 1102–1108, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Chen XY, Wolpaw JR. Acquisition of a simple motor skill: task-dependent adaptation plus long-term change in the human soleus H-reflex. J Neurosci 29: 5784–5792, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson FJ, Reier PJ, Lucas CC, Parmer R. Altered patterns of reflex excitability subsequent to contusion injury of the rat spinal cord. J Neurophysiol 68: 1473–1486, 1992a. [DOI] [PubMed] [Google Scholar]
- Thompson SW, Gerber G, Sivilotti LG, Woolf CJ. Long duration ventral root potentials in the neonatal rat spinal cord in vitro; the effects of ionotropic and metabotropic excitatory amino acid receptor antagonists. Brain Res 595: 87–97, 1992b. [DOI] [PubMed] [Google Scholar]
- Tolias KF, Bikoff JB, Kane CG, Tolias CS, Hu L, Greenberg ME. The Rac1 guanine nucleotide exchange factor Tiam1 mediates EphB receptor-dependent dendritic spine development. Proc Natl Acad Sci USA 104: 7265–7270, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosolini AP, Mohan R, Morris R. Targeting the full length of the motor end plate regions in the mouse forelimb increases the uptake of fluoro-gold into corresponding spinal cord motor neurons. Front Neurol 4: 58, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-Cabre A, Fores J, Navarro X. Reorganization of reflex responses mediated by different afferent sensory fibers after spinal cord transection. J Neurophysiol 91: 2838–2848, 2004. [DOI] [PubMed] [Google Scholar]
- Vinay L, Jean-Xavier C. Plasticity of spinal cord locomotor networks and contribution of cation-chloride cotransporters. Brain Res Rev 57: 103–110, 2008. [DOI] [PubMed] [Google Scholar]
- Walter JS, Sacks J, Othman R, Rankin AZ, Nemchausky B, Chintam R, Wheeler JS. A database of self-reported secondary medical problems among VA spinal cord injury patients: its role in clinical care and management. J Rehabil Res Dev 39: 53–61, 2002. [PubMed] [Google Scholar]
- Wiens KM, Lin H, Liao D. Rac1 induces the clustering of AMPA receptors during spinogenesis. J Neurosci 25: 10627–10636, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR. Acquisition and maintenance of the simplest motor skill: investigation of CNS mechanisms. Med Sci Sports Exerc 26: 1475–1479, 1994. [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462: 915–919, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci 24: 1071–1089, 2001. [DOI] [PubMed] [Google Scholar]
- Yuste R, Majewska A. On the function of dendritic spines. Neuroscientist 7: 387–395, 2001. [DOI] [PubMed] [Google Scholar]
- Yuste R, Urban R. Dendritic spines and linear networks. J Physiol (Paris) 98: 479–486, 2004. [DOI] [PubMed] [Google Scholar]
- Zhang S, Boyd J, Delaney K, Murphy TH. Rapid reversible changes in dendritic spine structure in vivo gated by the degree of ischemia. J Neurosci 25: 5333–5338, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Waxman SG, Hains BC. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J Neurosci 27: 2357–2368, 2007a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Waxman SG, Hains BC. Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J Neurosci 27: 8893–8902, 2007b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 44: 749–757, 2004. [DOI] [PubMed] [Google Scholar]