Abstract
Nitric oxide (NO) and hydrogen sulfide (H2S) are gasotransmitter molecules important in numerous physiological and pathological processes. Although these molecules were first known as environmental toxicants, it is now evident that that they are intricately involved in diverse cellular functions with impact on numerous physiological and pathogenic processes. NO and H2S share some common characteristics but also have unique chemical properties that suggest potential complementary interactions between the two in affecting cellular biochemistry and metabolism. Central among these is the interactions between NO, H2S, and thiols that constitute new ways to regulate protein function, signaling, and cellular responses. In this review, we discuss fundamental biochemical principals, molecular functions, measurement methods, and the pathophysiological relevance of NO and H2S.
Keywords: thiol, oxidation, redox, pulmonary, cardiovascular
historically considered only as industrial pollutants, nitric oxide (NO) and hydrogen sulfide (H2S) are now appreciated as two key physiologically produced signaling molecules that control multiple cellular functions. Appreciation that dysfunction in how these solvated gases affect these functions, through either deficient formation or downstream reactivity, has opened up new therapeutic avenues for a host of diseases in all major organ systems including the lung. Our understanding of NO homeostasis mechanisms are relatively advanced compared with H2S, primarily because of the latter's discovery as a biologically relevant entity being postdated by a decade or more compared with NO. Although they are chemically distinct, there are intriguing parallel mechanisms/concepts in NO and H2S biology that include overlapping functions, technical challenges in how each of these gases are measured in biological milieu, appreciation of the mechanisms and factors that modulate how metabolism of each of these is regulated, and therapeutic potential for either inhibiting or repleting NO or H2S. In this article, and within the framework outlined above, we provide a general overview of NO and H2S biology.
Nitric Oxide Formation: Enzymatic and Nonenzymatic Sources
Enzymatic sources.
Nitric oxide is synthesized by one of three different isoforms of nitric oxide synthase (NOS) that differ in enzymatic activity, in how they are regulated (transcriptional, translational, and posttranslational mechanisms), and in how they are expressed/compartmentalized in tissues as well as the subcellular level. These are called NOS I, II, and III, corresponding to the inducible (iNOS), neuronal (nNOS), and endothelial (eNOS) isoforms, typically with high, mid, and low activities, respectively. In the systemic and pulmonary vasculature, eNOS plays a key role in controlling blood flow and maintaining an antithrombotic and anti-inflammatory luminal surface. On the other hand, iNOS is induced by inflammatory stimuli in leukocytes, airway epithelial cells, and alveolar macrophages to mediate pathogen killing. However, production of NO at higher concentrations in this setting is also associated with collateral tissue damage mediated by increased oxidation, nitrosation, or nitration of biomolecules, which is emblematic of acute lung injury (ALI) and acute respiratory distress syndrome. However, this is only a general paradigm. The pleiotropic effects of NO are regulated only in part by high vs. low concentrations. It is evident that the cellular source (i.e., compartmentalization) of NO also plays a key role (97, 98, 123). For example iNOS expressed in polymorphonuclear leukocytes (PMNs) regulates sequestration of these cells in the lung vasculature during cecal ligation and puncture-induced sepsis but limits PMN infiltration into the alveolar space; on the other hand, NO derived from iNOS in lung parenchymal cells plays no role in this process (99). From a biochemical perspective, how iNOS-derived NO produced in PMNs elicit distinct effects compared with iNOS-derived NO produced in pulmonary endothelial cells is not clear and underscores the importance of compartmentalization with regard to the NO source and the local concentration of NO where produced. An additional consideration is that selective effects of NO may be mediated by distinct redox derivatives and associated products (e.g., nitrosation and nitrated epitopes), which can have distinct biological activities compared with NO per se. The parameters of concentration, localization, and redox conversion are also emerging as critical in the biological actions of H2S.
Nonenzymatic sources.
More recently, nonenzymatic sources of NO, via reduction of nitrite, have emerged as viable NO production processes in vivo. Widely used as a stable end-product for assessing NO formation, nitrite is now known to be an integral player in NO homeostasis pathways. Several mechanisms have been identified that operate under physiological and pathophysiological conditions that result in the one-electron reduction of nitrite to NO. Common features of these mechanisms include acceleration of nitrite-reduction as a function of decreasing pH and oxygen tension (30). Intriguingly, in the lung nitrite can elicit signaling responses at low biological (nM–μM) concentrations under normoxia as well. For example, airway epithelial cell repair was stimulated by nitrite via a mechanism that appeared to involve stimulation of oxidative signaling (122). Figure 1 shows a scheme generalizing NO formation pathways.
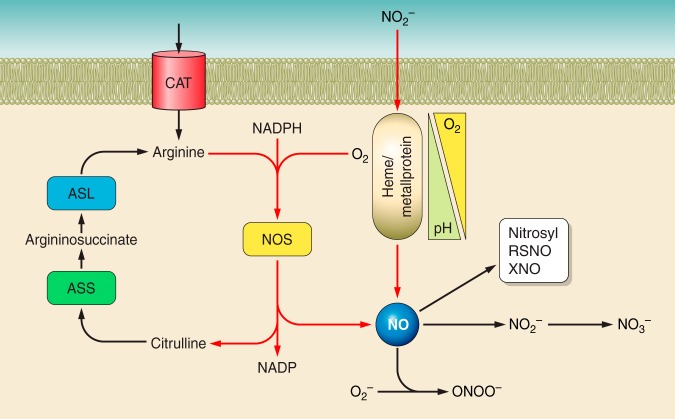
Fig. 1.
NO formation pathways: NO is synthesized by 1 of 3 nitric oxide synthases (NOS) that use l-arginine, oxygen, and NADPH (reducing equivalents) as substrates to produce NO and citrulline. Citrulline is recycled back to l-arginine via arginosuccinate synthase (ASS) and arginosuccinate lyase (ASL). Alternatively, nitrite can be reduced to NO in a hypoxia and pH-dependent manner via heme or metalloproteins that can couple lowering oxygen tensions and pH with a 1-electron reduction to NO. Once formed, NO can facilitate specific biological responses by nitrosylated heme proteins, by undergoing oxidation to facilitate nitrosation reactions resulting in protein S-nitrosothiols (RSNO) or nitrosamine (XNO) or undergoing rapid reactions with other free radicals (e.g., superoxide) to form peroxynitrite (ONOO−), which can mediate oxidation and nitration reactions. Ultimately, NO can be oxidized to nitrate, potentially via nitrite. Nitrate is then excreted or reduced back to nitrite by commensal nitrate-reducing bacteria in the oral cavity.
NO and Nitrite Therapeutics for Airway and Pulmonary Vascular Diseases
Unfortunately, the absence of a selective nitrite scavenger, or a way to selectively decrease nitrite levels in vivo, precludes definitive assessment of endogenous nitrite in NO homeostasis pathways. However, data from exogenous or therapeutic nitrite clearly demonstrates this possibility. In the lung, inhaled nitrite reversed pulmonary hypertension and was suggested to be more effective compared with inhaled NO. In preclinical models, inhaled nitrite did not lead to rebound hypertension once nitrite inhalation was stopped, which is a frequently observed complication associated with acute termination of inhaled NO gas (42). Putative mechanisms of nitrite action involve activation of pulmonary vascular smooth muscle soluble guanylate cyclase (sGC) and the canonical NO signaling cascade (10). Moreover, nitrite therapy also protects against pulmonary and vascular smooth muscle remodeling after injury and improves lung function after transplantation (4, 88, 111, 139). Additionally, nitrite therapy protects against ALI induced by mechanical ventilation and inhaled chemical toxicants, in the latter of which the risk of developing reactive airways is also reduced (40, 96, 101, 132). Improvement in both inflammatory and permeability components of ALI has been documented, suggesting that nitrite therapy prevents early events in the disease. Interestingly, inhaled NO, which to date is a primary therapeutic for treatment of pulmonary hypertension in the newborn, has also been shown to prevent ischemia-reperfusion injury in distal extrapulmonary tissues (26, 64, 65, 84, 117). In this case, the model proposed is that inhaled nitric oxide (iNO) increases the level of circulating nitrite (and perhaps other NO adducts, e.g., S-nitrosothiols) which then stimulate NO signaling in other tissues. In fact, NO repletion by nitrite or iNO has proven successful in multiple preclinical and clinical studies in different settings of acute and chronic inflammatory tissue injury and supports the model that biological pools of NO signaling equivalents exist in the form of nitrite in tissues that can be manipulated therapeutically.
H2S Formation: Enzymatic and Nonenzymatic Pathways
Enzymatic sources.
For nearly a decade, H2S has been discussed as the third biological gasotransmitter along with NO and carbon monoxide (CO) (126). Three distinct H2S-generating enzymes have been identified in mammalian systems (Fig. 2). The best-characterized H2S-producing enzyme, cystathionine β-synthase (CBS), is a pyridoxal-phosphate (PLP)-dependent enzyme that catalyzes the condensation between homocysteine and serine, giving rise to cystathionine. Owing to the similarity of structures, CBS also uses cysteine, instead of serine, to generate H2S (47). Another PLP-dependent enzyme, cystathionine γ-lyase (CSE), catalyzes the conversion of cystathionine to cysteine, but also utilizes homocysteine and cysteine to generate H2S (16, 138). Additionally, CSE and CBS are crucial hubs in transsulfuration pathways that pass sulfur between various sulfur containing amino acids (50). The third pathway comprises two enzymes found within the mitochondrion. Cysteine aminotransferase (CAT) first converts cysteine to 3-mercaptopyruvate, from which H2S is released by 3-mercaptopyruvate sulfurtransferase (3-MST). Importantly, this process involves two equivalents of reactive thiols or a reduced thioredoxin, with by-products of a disulfide or an oxidized thioredoxin.
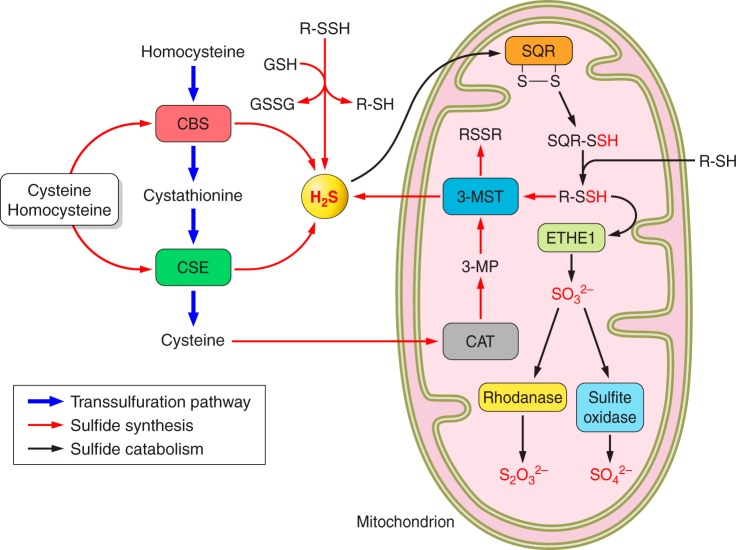
Fig. 2.
Sulfide metabolism and transsulfuration pathways cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) govern the flow of sulfur through homocysteine, cystathionine, and cysteine, which is known as the transsulfuration pathway. These two enzymes are also the major catalytic pathways for generating H2S from cysteine and homocysteine in the cytosol. The third pathway via cysteine aminotransferase (CAT) and 3-mercaptopyruvate sulfurtransferase (3-MST) may be more important in the mitochondrion and requires the transfer of sulfur from a persulfide (RSSH). On the other hand, persulfide can be a source of H2S at the expense of reductants such as glutathione (GSH) and thioredoxin [Trx (SH)2]. In the mitochondrion, H2S reduces sulfide quinone oxidoreductase (SQR), generating a persulfide that is further transferred to an acceptor (R-SH). This persulfide is then oxidized by persulfide dioxygenase (ETHE1) to SO32−, which is eventually converted to S2O32− and SO42− by rhodanase and sulfite oxidase, respectively.
All three enzymes, CBS, CSE, and 3-MST, have been documented to be expressed in the lungs, with possible variations between species and cell types (5, 14a, 83, 94, 124). The contribution of these enzymes on H2S production is affected by their expression levels and the availability of substrates. For example, CSE is ∼60-fold higher than CBS in the mouse liver and accounts >97% H2S production. In comparison, CBS is the predominant H2S-producing enzyme in the brain, where its expression is higher than that of CSE (51). Although cysteine and homocysteine are the substrates of both CSE and CBS, CSE is the primary source of H2S in homocysteinemia, whereas in the kidney, where the cysteine level is ∼10-fold higher than in the liver or the brain, CBS may predominate. Classically considered as a cytosolic enzyme, CBS is also found to localize in other compartments such as mitochondria (7, 112, 115a) and nuclei (1, 52). Its nuclear translocation is related to posttranslational modification by small ubiquitin-like modifier, which inhibits its activity (49). Moreover, CBS activity is regulated by other small molecules, including its activation by S-adenosylmethionine and glutathione (46, 86) and inhibition by NO and CO (28, 49, 115). Relatively less is known about regulation of CSE and 3-MST. Considered to be the major source of H2S in the cardiovascular system, CSE is expressed in both mouse lung endothelial cells and smooth muscle cells, with its effects on lung function poorly explored. 3-MST and CAT are found in most cell types but are less abundant than CBS and CSE. Although they are found in both cytosol and mitochondria (78, 83), their activity may be greater in mitochondria owing to higher concentrations of cysteine, the substrate for CAT (49). H2S has been proposed to be an endothelium-derived hyperpolarizing factor (EDHF), based on the deficiency of acetylcholine-induced vasorelaxation in CSE knockout mice (82, 125). However, the proposed mechanism is through KATP sulfhydration via CSE-derived H2S, which does not support a direct role of H2S as an EDHF (22).
Nonenzymatic sources.
Similar to NO, alternate pathways for H2S formation may exist in the absence of CBS, CSE, or 3-MST. An early study showed that human erythrocytes reduce elemental sulfur to H2S, with reducing equivalents provided by glutathione, NADH, and NADPH (102) (Eq. 1). Other forms of sulfane sulfur, where sulfur binding to another sulfur as in thiosulfate (S2O32−), polysulfide (RSnR), and persulfide (RSSH), may also be reduced to free H2S by low molecular weight reductants independent of enzymatic pathways (49, 70). The yield of H2S via this mechanism may be relatively low but could serve as storage depots of H2S equivalents, since sulfane sulfur can be generated by H2S autoxidation (Eq. 2) (70, 73) and by serial oxidation processes in the mitochondria (49). Figure 2 summarizes the transsulfuration pathway and H2S metabolism.
| (1) |
where GR is glutathione reductase.
| (2) |
NO and H2S Donors
NO and H2S donors are widely used experimental tools and potential therapeutics to model and interrogate biological functions. Donors allow introduction of defined amounts with defined rates of release of test compound. For NO, many different NO donors are commercially available that can expose biological systems to low levels of NO for long periods of time, to high levels of NO for short periods, and variations in between. In addition, NO donors display pH dependence and sensitivity to oxidants and reductants, which influence what NO derivatives are produced, and in the most recent generation also offer organelle targeting for NO release (56). These aspects of NO donors have been reviewed many times elsewhere. Therefore, we will focus our discussion on H2S donors, which is a rapidly evolving area.
To better understand the pros and cons of available sulfide donors, it is helpful to review the chemical properties of H2S. H2S exists in gaseous form at ambient temperature and pressure. At equilibrium between gas and aqueous phases, its solubility at 37°C is 80 mM (50). In aqueous solution, H2S dissolves into HS− and S2−, with the pKa of ∼7 and 12.2–15, respectively, at room temperature (87, 133) (Eq. 3). Accordingly, it is estimated that two-thirds of H2S dissolved in water is HS−, and one third H2S, with a negligible amount of S2−.
| (3) |
Equation 3 underscores the reversibility between H2S, HS−, and S2−. Importantly then in an open system, the equilibrium will be pulled to the left, leading to rapid decreases in solvated H2S levels; note that this is also the case with NO. An additional and important consideration is the permeability of H2S into lipid bilayers. Although H2S freely diffuses through cell membrane (75), HS− does not. This may affect the availability of H2S in the cytoplasm or other cellular compartments such as mitochondria, although these diffusion limitations may be altered by changes in pH resulting in conversion of HS− to H2S. Therefore, when using sulfide donors, it is important to consider its cellular accessibility vs. its volatility. Currently, available H2S donors fall into three categories: sulfide salts, natural compounds, and synthetic compounds.
Sulfide salts.
Sulfide salts, such as Na2S and NaHS, are the simplest H2S-generating compounds and most widely used in experimental studies. The release of H2S from Na2S and NaHS is instantaneous upon hydration and dissociation in aqueous solution. H2S release does not reach steady state, however, owing to volatilization of H2S into the gas phase, and thus H2S exposure is transient. The half-life of H2S in plasma is ∼20 min in humans and ∼5 min in mice (8) after a single bolus injection of Na2S, whereas the half-life in a cell culture model is also ∼5 min (89). Apart from volatility, H2S is also vulnerable to oxidation. It is recommended that stock solutions be made fresh and sealed in air-tight container with minimal head space and the concentration confirmed by validated methods. Because of these disadvantages, therapeutic potential of sulfide salts are highly limited.
Natural compounds.
Garlic consumption has been associated with health benefits for a long time and there are a variety of garlic-based products available and marketed accordingly. Allicin (diallyl thiosulfinate), the best known active ingredient in garlic extracts, decomposes into diallyl disulfide (DADS) and diallyl trisulfide (DATS) (3). These compounds have at least one disulfide bond and an allyl group whose double-bonded carbon is adjacent to the sulfur-bonded carbon. In the presence of GSH or other reduced thiols, a nucleophilic displacement of the α-carbon of this allyl group can generate a hydrodisulfide that is further reduced by GSH to H2S (6, 70). The requirement for cellular metabolism and reducing equivalents is an important consideration in assessing how much H2S is generated by these compounds and typically allows for slower and more sustained release of H2S compared with sulfide salts. Additionally, these natural products are lipophilic, allowing H2S formation to occur in all cell compartments. This hydrophobicity, however, is also a limitation since both DADS and DATS are immiscible in water, making delivery of these compounds challenging. Other natural compounds proposed to release H2S include isothiocyanates, such as sulforaphane from broccoli and allyl isothiocyanate from wasabi (53). However, further understanding of their mechanisms of action and role of H2S in health-promoting effects is required.
Synthetic compounds.
Several efforts have been made to develop synthetic H2S donors. GYY4137 is a derivative of Lawesson's reagent that releases H2S over several minutes via nucleophilic attack and after its dissociation in solution. GYY4137 is believed to release H2S in a prolonged manner, although some studies using amperometry have showed that the release rate was significantly reduced compared with NaHS (69). Characterization of GYY4137-dependent sulfide release in plasma has been reported (67, 69); however, the methylene blue method was used to measure H2S (89). This method for measuring H2S levels is questionable and we have observed that GYY4137 no longer increased free sulfide 30 min after application. Considering that a high dosage of GYY4137 is required for its physiological effects (400 μmol/l for a plateau concentration of <20 μmol/l in plasma, measured by methylene blue method), its efficiency of releasing H2S may be low and potential side effects of its metabolites at these concentrations should be considered. AP39, a sulfide donor with a mitochondria-targeting moiety, has been shown to increase intracellular H2S in these organelles as detected by the H2S probe 7-azido-4-methylcoumarin. It was also shown that AP39, at 100 nM, was able to prevent glucose oxidase-induced mitochondrial oxidative stress (113). Table 1 lists commonly used H2S donors and their properties.
Table 1.
Properties of some commonly used sulfide donors
| Compound | Solubility | Polarity | Mechanism | Duration |
|---|---|---|---|---|
| Na2S | 186 mg/ml | Hydrophilic | Hydration | Increase free H2S for ∼20 min in vivo; ∼5 min in vitro |
| Accelerated by lower pH and higher temperature | ||||
| GYY4137 | 3 mg/ml in water | Hydrophilic | Hydration | Release free H2S slower than Na2S |
| Nucleophilic attack | Accelerated by lower pH and higher temperature | |||
| DATS | Insoluble in water | Hydrophobic | Nucleophilic attack | Increase total H2S for hours |
| 10 mg/ml in DMF | Oxidation | May not increase free sulfide | ||
| Reduction | ||||
| AP39 | Not reported | Hydrophilic | Target mitochondria by triphenylphosphonium (TPP+) | AzMC staining was increased 1 h after treatment with 100 nM AP39 in bEnd.3 cells |
| Release H2S by dithiolethione |
H2S Therapeutics for Airway and Pulmonary Vascular Diseases
The last few years have seen a significant increase in the number of papers investigating the role of H2S in diverse lung diseases. Similar to NO, the perspectives vary, but the majority of studies indicate that endogenous or exogenous H2S protect against acute and chronic lung diseases. These include ALI, asthma, pulmonary fibrosis, chronic obstructive pulmonary disease, pulmonary hypertension, and neonatal lung development-associated diseases (27, 68, 74, 94, 100, 109, 120, 136, 137). However, specific molecular mechanisms for protection remain unclear. Like NO, it is unlikely that there is one unifying or common mechanism of action; that said, antioxidant effects, prevention of inflammatory tissue injury and regulating blood flow, and improvement of gas exchange are common proposed mechanisms. Thus the similarities between NO and H2S with respect to lung diseases are clear. It is not our goal to list and discuss all studies reporting on H2S and lung disease, but to compare H2S and NO and to note that to date there are no approved therapies for treatment of lung diseases with H2S-dependent approaches. The lungs are unique in that gas-based therapies offer direct access to the organ of interest, as exemplified by iNO. Interestingly, inhaled H2S has also been widely discussed as a therapeutic fueled by early studies demonstrating that this therapeutic modality induces a state of hibernation in small animals (9). However, it should be pointed out that H2S inhalation also causes lung injury in rats at exposure levels that are not considered high (80 ppm) (110). A key consideration in this context is oxygen. Both NO and H2S are oxidized by oxygen (Eqs. 2, 4, 5, and 6) (38, 44, 73, 119). This becomes more impactful when concentrations of NO, H2S, and O2 are high, as may be the case during inhalation and in hydrophobic cellular compartments. Autoxidation of NO will be exponential with respect to NO concentration (second order reaction with respect to NO) and thus doubling of NO levels will increase NO oxidation rates by fourfold. This not only accelerates NO decay but also increases formation of reactive nitrogen species, e.g., nitrogen dioxide that can elicit unique and cellular responses. Indeed, monitoring and minimizing nitrogen dioxide levels is a component of safety assessment with iNO therapy. Similarly, the effects of H2S are also modulated by O2. For example, H2S-dependent vasodilation of isolated aortic rings is markedly improved at lower vs. higher Po2, and induction of hibernation in rats by inhaled H2S is also more efficient at lower ambient oxygen tensions (21, 60). In part this relates to higher concentrations of H2S, but also, and like the situation with NO, one cannot exclude the potential for H2S oxidation products (e.g., sulfite) to elicit unique effects. Evaluating the role of oxygen and controlling H2S autoxidation are likely to be important in further developing H2S inhalation therapies, as well as understanding how H2S modulates signaling in environments where oxygen concentrations differ.
| (4) |
| (5) |
| (6) |
NO and H2S Toxicity
With respect to the above discussion highlighting the interest in NO and H2S therapeutics, it is important to remember that these molecules can be toxic at higher doses. With NO gas, administration is typically limited to 80 ppm and usually used at lower doses for pulmonary hypertension in the newborn. Primary toxicity concerns are formation of oxidant nitrogen dioxide and NO-dependent oxidation of oxyhemoglobin to methemoglobin. iNO delivery device design coupled with the low concentrations used clinically leads to minimal formation and exposure of patients to nitrogen dioxide. For example, our studies with liver transplant patients receiving 80 ppm for >4 h led to nitrogen dioxide concentrations of <1%. Moreover, methemoglobin levels remained <3% in these studies, significantly below levels at which oxygen delivery may be affected. On the other hand exposure to H2S at levels that led to “suspended animation” led to significant airway inflammation and epithelial injury in rats (110). In addition, exposure to H2S at significantly higher concentrations can occur during accidents (e.g., in geothermal and oil/gas industries) or chemical warfare and can result in injury to the respiratory, neuronal, cardiac, and other tissues (59). Key in this regard is that H2S toxicity is characterized by respiratory depression secondary to neuronal or direct ALI, which in turn likely results in ischemia. Furthermore, inflammation is likely a pivotal component to host responses to an H2S insult. Current therapies are limited to decreasing H2S concentrations by increasing concentrations of ferric hemoglobin or therapeutic administration of hydroxocobalamin, which binds and removes H2S (35). However, in light of new understanding on downstream signaling effects of H2S, we propose that a better understanding of H2S toxicity both during and postexposure is required, together with development of therapeutics that target downstream effects of H2S exposure. Thus we suggest that pulmonary toxicity to iNO under clinically relevant conditions is minimal, but for H2S further work is required for both exposure expected in the accidental/industrial setting and during therapeutic exposures.
NO and H2S Interactions
It is also worth noting that there is substantial interest in whether NO and H2S are interrelated and interregulated. Underscoring this perspective in part is the idea that NO and H2S metabolites may react with each other. However, much debate exists about this largely due to potential confounding reactions between NO donors or its products and H2S, independent of NO itself. For example, sodium nitroprusside (SNP) and S-nitroso-N-acetyl-d,l-penicillamine (SNAP) are used as NO donors (2, 128, 134). However, SNP can directly react with H2S (13, 23). Perhaps of more interest is the potential for H2S to react with S-nitrosothiols (RSNOs), although the precise nature of these reactions with both low and high molecular weight RSNOs remains under investigation with multiple mechanisms proposed (18, 25, 58a, 91, 116). It is also interesting to speculate that multiple mechanisms may offer added layers of control of H2S signaling. For example, H2S may denitrosate RSNO with production of nitroxyl (HNO) and hydrogen disulfide (HSSH), the latter of which may introduce a persulfide on the reactive thiol (49, 58a). Since −SNO and −SSH differ in size and the polarity, this further modification by H2S is likely to switch to a different protein conformation and function. One proposed scheme is illustrated in Fig. 3. Alternatively, the transient formation HSNO may mediate transnitrosation, donate NO, or generate nitrosopersulfide (SSNO−) (18, 25, 80). Finally, reactions between H2S- and NO-derived reactive nitrogen species including peroxynitrite should be considered. As with RSNOs, however, the specific reaction mechanisms are still being elucidated (14, 24).
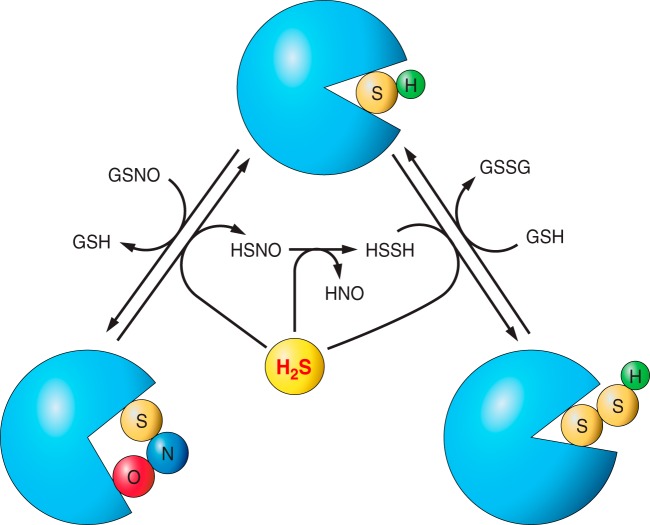
Fig. 3.
Potential mechanisms by which H2S regulates protein thiol posttranslational modifications S-nitrosation is a posttranslation modification. In the illustrated scheme, the formation of S-nitrosothiols (RSNOs) on a reactive thiol converts the protein from a “closed” conformation to an “open” one. RSNOs may serve as a target for H2S, which restores the protein to the closed form, generating thionitrous acid (HSNO) and hydrogen disulfide (HSSH) plus nitroxyl (HNO) in succession. However, HSNO may also mediate transnitrosation or undergo homolysis to NO, H2S, or nitrite (26). HSSH may further modify the active thiol to a persulfide resulting in a stabilized open or closed conformation, which may vary from case to case.
NO and H2S share many common protein targets for posttranslational modification. The biochemistry and biological functions of NO-dependent posttranslational modifications is widely appreciated, with the most prevalent being S-nitrosation, tyrosine nitration, and lipid nitration. H2S-dependent posttranslational modifications is an active area of investigation with mounting evidence suggesting that this is important to how H2S elicits diverse biological effects with H2S-thiol chemistry underlying this diversity. For example, S-nitrosation on GAPDH at Cys150 inhibits its glycolytic function, whereas H2S introduces persulfide (named sulfhydration) on the same cysteine residue that increases enzymatic activity (81). The p65 subunit of NF-κB can be S-nitrosated at Cys38, resulting in the dissociation of p50 and p65 subunits to inhibit its nuclear transcription (54). In comparison, H2S modifies Cys38 of p65 and favors its association with ribosomal protein S3, which increases transcription (93). H2S can also interact with protein S-guanylation. 8-Nitro-cGMP, a second messenger of reactive oxygen species and NO, induces S-guanylation. HS− is shown to denitrate 8-nitro-cGMP and form 9-SH-cGMP. The scavenging of excessive 8-nitro-cGMP by HS− is protective in myocardial infarction (85).
In addition, H2S can also attack protein disulfide bonds exemplified by the effects of H2S on the ATP-sensitive potassium channel (KATP). The regulatory sulfonylurea receptor (SUR) governs the KATP core (Kir 6.x). H2S breaks the disulfide bond formed by two extracellular cysteine residues, Cys6 and Cys26, of SUR1 to open Kir 6.1 (48). Similarly, the Cys1045-Cys1024 disulfide bond of vascular endothelial growth factor receptor 2 (VEGF-R2) acts as a molecular switch, the cleavage of which by H2S allows subsequent phosphorylation and activation of this receptor (114). These selected examples underscore the diversity of potential mechanisms by which H2S can regulate protein function and highlight a central role for H2S-thiol chemistry. This understanding further underscores the key role for H2S in redox signaling paradigms.
NO and H2S Measurements
A critical requirement to any insight regarding the role of NO and H2S in biology is accurate, reproducible, and sensitive methods to detect these species and their derivatives in biological matrixes, that importantly include protocols that maintain the NO− or H2S derivative in its native state during sample processing. Many articles and reviews have been written about these methods. We will focus our discussion here on overviewing the pros and cons and key considerations for commonly used approaches.
Nitric oxide.
A variety of methods have been well established to determine NO and its metabolites in biological samples. However, NO measurement is still challenging, and inappropriate sample collection and preservation may yield artificial results. In the blood, oxygenated ferrous hemoglobin binds to NO and oxidizes it to nitrate, terminating NO bioavailability. Deoxygenated ferrous hemoglobin also scavenges NO by forming a six-coordinate nitrosylheme species. Ferricyanide can effectively oxidize ferrous hemoglobin to methemoglobin, therefore preventing the destruction of NO in whole blood and red blood cell samples (31, 127). Meanwhile, ferricyanide also protects SNO-Hb from degradation in denaturing environment (31). Moreover, light exposure should be avoided to prevent homolysis of RSNOs. RSNO degradation is also catalyzed by trace levels of transition metals (e.g., Cu+) (108, 118), necessitating the presence of ion chelators [e.g., diethylenetriaminepentaacetic acid (DTPA)] in sample collection and storage media. Additionally, thiol blockade is also required to prevent reduced thiol-dependent reduction of RSNOs but also to prevent artifactual formation of NO-thiol adducts; N-ethylmaleimide is widely used for this indication. Finally, it is important to be aware of what a method is measuring and how to interpret it. For example, nitrate can be measured by multiple methods, yet it does not necessarily reflect NO bioavailability as outlined recently and discussed further next (57).
Griess reaction.
First described in 1879, the Griess reaction is now applied in various methods of NO measurement (20, 29). Under acidic conditions, nitrite reacts with sulfanilamide and N-(1-naphthyl)ethylenediamine to yield an azo product with characteristic increased absorbance at 540 nm. Both nitrite and nitrate are considered as end-oxidation products of NO and thus reflect total NO formation. However, dietary intake (e.g., green leafy vegetables, beets, and cured meats) contributes a considerable amount of nitrate, while nitrite and nitrate can be excreted in sweat and urine. Therefore, plasma measurements indicate a balance of endogenous NO formation, dietary intake, and clearance. Moreover, since nitrite is readily oxidized to nitrate in blood by hemoglobin, for an accurate assessment of NO formation, nitrate has to be reduced back to nitrite prior to the Griess assay with several reductant systems utilized including cadmium, zinc chloride, vanadium chloride or NADPH-dependent nitrate reductase. Note however, that NADPH inhibits Griess reaction necessitating its oxidation using lactate dehydrogenase or potassium ferricyanide (12, 34, 121). Similarly, nitroso compounds, such as RSNOs and N-nitrosamines (RNNOs), can be cleaved to yield NO by using UV light illumination or a reductant such as hydrogen bromide, iodide, and vanadium chloride. To distinguish RSNO from RNNO, mercuric chloride can be used to cleave RSNO to form nitrite, but leaving RNNO intact, which is known as the Saville reaction (12). Sensitivity of the original colorimetric method for the Griess assay was ∼0.5 μM for S-nitrosothiols, which is not low enough for detecting biological levels (33). However, combining the Griess assay with HPLC, flow injection analysis, and microgas analysis system, the detection range has been improved significantly and now these analyses are routinely applied to measuring biological sample. In addition, these approaches have the added value of being able to simultaneously quantitate nitrite and nitrate from the same sample.
Fluorescence probes.
Fluorescence probes not only enhance the sensitivity for NO measurement but also provide the potential for temporal and spatial resolution. As with the Griess assay, most fluorescence probes detect NO metabolites and not NO per se; this is an important consideration in data interpretation. For example, the aromatic diamino compound, 2,3-diaminonaphthalene, reacts with N2O3, a strong nitrosating agent derived from NO oxidation or nitrite acidification. The reaction results in the formation of a fluorescent product 2,3-naphthotriazole. This method is sensitive (10 nM of the product is detectable) (32) but suffers from limitations associated with an excitation wavelength of 375 nm (UV), which causes autofluorescence in the tissue, as well as poor solubility at neutral pH and potential cytotoxicity of the compound.
Diaminofluoresceins.
Diaminofluoresceins (DAFs) are a group of fluorescein derivatives that are more commonly used fluorescence probes for NO, although again this probe does not react with NO directly, but with NO oxidation products such as N2O3 or HNO that induce the formation of a fluorescent triazole ring. No interference was found in the presence of other oxidized forms of NO, including NO2− and NO3− or reactive species including H2O2 and peroxynitrite (61). The detection limit of DAF-2 is ∼5 nM and can be further improved by using sensitive analytical methods, such as HPLC and capillary electrophoresis (58). DAF-2 DA diffuses into cells, where it is hydrolyzed by esterases, leading to intracellular entrapment. Thus DAF-2 DA can be used to monitor intracellular NO, although it is important to verify equal loading of the dye between experimental groups. DAF-2 DA at a higher concentration may be toxic in some cell types (36). pH can be a potential interference, since the protonation of the phenolic group of DAFs abolishes their fluorescence. Their stability at certain pH is based on their individual pKa. DAF-1, DAF-2, and DAF-3 are stable when pH is above 7, whereas DAF-4, DAF-5, and DAF-6 can be only used when pH is above 9 (32). Tissue autofluorescence also limits its application.
Though technically allowing real-time detection of NO, the requirement for NO autoxidation necessitates additional discussion. The reaction between NO and oxygen is second order with respect to NO, thus limiting this reaction to areas in which local NO concentrations are high (e.g., hydrophobic compartments). In addition, the results will be dictated by where the dye compartmentalizes. This is also limited by the hydrophobicity of the dye. Moreover, different properties between the parent dye and product may result in redistribution after reaction with NO/O2. These considerations are important therefore when interpreting data since where the product is detected may not reflect precisely where NO was generated. Equally important is to ensure that sample collecting and processing do not alter distribution of NO metabolites and lead to artificial formation of them. Key factors here are light, presence of contaminating transition metal ions, and pH. The first two can promote decay of S-nitrosothiols to nitrite. Acidic conditions will favor the reverse and form S-nitrosothiols from nitrite. Since endogenous RSNOs are in the nanomolar range, and nitrite is typically present in the high nanomolar range, even a small artifact in acidified nitrite-dependent RSNO formation can lead to significant error in the latter's measurement. These issues are now widely appreciated in the NO field and use of metal chelating agents, blocking all reduced thiols immediately upon sample collection, avoiding low pH and excessive light exposure routine.
Chemiluminescence.
The reaction between NO and ozone produces an excited state for NO, followed by a spontaneous emission of light. In ozone-based chemiluminescent NO analyzing systems, an inert gas such as nitrogen or helium is used to carry NO to the ozone reaction cell and light emission measured. Although this method fails to provide temporal or spatial insight to NO metabolism, and requires reduction of NO derivatives (e.g., nitrite, S-nitrosothiols to NO), this detection approach does allow for direct NO measurement (39). Other existing compounds such as ethylene hydrocarbons and sulfur compounds may be excited at the same time, but they do not interfere with the measurement owing to distinct wavelength differences (77). Another substantial advantage of this method is its sensitivity (nM–pM) and flexibility (up to mM range).
Electron paramagnetic resonance.
This method allows for direct detection of free radical species including NO (37). However, the low levels of NO produced combined with a relatively poor sensitivity compared with other NO detection methods has limited the use of electron paramagnetic resonance (EPR) for NO detection in biological matrixes. Typically, EPR-based approaches employ the trapping of a radial species with a spin trap that forms a new and longer lived paramagnetic species. With NO, this is achieved by utilizing specific reaction of NO with iron centers. For example, NO bound to ferrous heme proteins utilizes not only the rapid and high affinity reactions between NO and deoxyferrous heme, but also the corresponding nitrosyl hemoprotein has a distinct EPR spectrum that can be detected with sensitivities of ∼200–300 nM. Key examples are hemoglobin and myoglobin, their presence in vivo offering a further advantage for NO measurement without requiring the exogenous addition of a spin trap. Other spin traps for NO detection include nitronyl nitroxides and iron-dithiocarbamate complexes, which collectively provide a range of compounds with distinct biophysical and cellular properties (e.g., solubility, subcellular compartmentalization) that could be useful for NO detection (37). A final advantage of EPR methods over others discussed herein is the ability to distinguish between 14N and 15N, and thus allowing selective use of isotopes to discern mechanisms of NO formation especially when there maybe more than one source of NO (enzymatic vs. nonenzymatic for example).
Electrochemical methods.
Most electrochemical NO sensors are based on the oxidation of NO to nitrite. Interference from nitrite, or other redox active species such as ascorbic acid, is avoided first by the use of selective gas-permeable membranes that prevent access of these species with the electrochemical cell. Second, electrocatalytic material, such as platinum, can be immobilized on the probe to reduce the potential required for NO oxidation. Platinization of the working probe also increases the surface area for NO oxidation, further increasing sensitivity with detection of 1 nM NO possible (66). Other approaches used include porphyrinic-based electrodes that couple NO binding to electrical current generation. More importantly, electrochemical NO sensors are able to provide spatial (at the cell level) and temporal resolution; however, frequent calibration is necessary for the measurement. Also, since the measurement is affected by the proximity of NO to the electrode, an equilibrium time is necessary during the measurement.
Biotin switch assay.
The chemiluminescence and electrochemical approaches measure NO concentrations but do not provide details on PTMs per se nor inform on the proteins on which these PTMs reside. A significant advancement was made with the development of the biotin switch assay that allows identification of protein S-nitrosothiols using a combination of thiol-blocking, RSNO-reducing strategies: immunoblotting with LC-MS-based protein identification (45). Several method-focused texts about the pros and cons of the biotin switch assay have been compiled and thus not discussed in this review (19, 55). We would like to underscore, however, how this approach can be used, in combination with quantitative approaches outlined above, to determine the position of an RSNO on a protein, and the amount of this modification. This is exemplified by recent studies showing that S-nitrosation of a specific cysteine in the ND3 subunit of Complex 1 in the electron transport chain confers resistance to cardiac ischemia-reperfusion injury (17).
Hydrogen sulfide.
H2S is volatile and is subject to auto-oxidation at atmospheric oxygen tensions. As discussed above, the pKa of H2S is ∼7. Therefore, air-tight containers and sampling protocols that ensure H2S gas is not lost because of venting are key. In addition, basic buffers (i.e., pH of 9.5) should be used to prevent the liberation of H2S gas. Since oxidation of H2S can be catalyzed by transition metals, DTPA is also recommended in preserving and reaction buffers. We have also found it important to use degased buffers and perform H2S reaction measurements in tightly controlled atmosphere conditions to prevent oxidation. In this way, we are able to preserve >90% H2S in the sample (106). Importantly, N-ethylmaleimide, which is often used to block thiols in NO measurement protocols, may interfere with H2S detection (106).
Methylene blue.
The methylene blue method was initially employed to detect sulfide contaminant in water. This method is based on the reaction between HS− and N,N-dimethyl-p-phenylenediamine generating methylene blue, which can be measured by its absorbance at 670 nm. In earlier studies of H2S in physiology and pathology, the methylene blue method was used to measure H2S in biological samples without careful validation. However, this method is no longer appropriate for H2S measurement in biological samples for several reasons including 1) the reaction occurs under acidic conditions in which biological pools of sulfide may be altered (e.g., acid labile sulfide); 2) methylene blue forms dimers and trimers that interfere with the absorbance of monomer at 670, therefore violating Beer's law; and 3) colored substances in the sample may interfere with methylene blue absorbance, thus leading to artificial readings. Therefore, methylene blue should not be used for sulfide measurement in biological samples.
Monobromobimane.
Monobromobimane (MBB) is an alkylating reagent that is widely used for thiol labeling (62). It reacts with H2S in alkaline conditions to yield the sulfide dibimane product (106). After reaction of excessive MBB and sulfide, the resultant fluorescent sulfide dibimane is measured by analytical approaches, such as HPLC and LC-MS/MS. Use of MBB in the evaluation of H2S in biological samples was first developed by Fahey and Togawa et al. and has been extensively explored by our group and others (83a, 95, 104, 106, 107, 117a, 130). This method presents several advantages over existing methods for H2S detection. Since the reaction occurs at alkaline condition (e.g., pH 9.5), H2S is not liberated from the acid-labile pool. By using HPLC, linear detection of sulfide-dibimane from 5 nM to 200 nM for small sample injection volumes of 10 μl is possible. In biological samples, MBB/HPLC also proves to be more sensitive and specific than the methylene blue method on the measurement of both endogenous and exogenous H2S (106). LC-MS/MS based measurement improves the assay's sensitivity and selectivity, allowing for detection of H2S at low concentrations present in cell culture studies (105). Moreover, results from our MS/MS show high consistency with HPLC with use of the same biological samples, indicating the validity of the MBB/HPLC method at physiological H2S levels. Perhaps most significant is the fact that, by using a careful sample workflow employing an acidic releasing buffer (pH 2.6) and a reductant, Tris(2-carboxyethyl)phosphine hydrochloride step, the acid-labile pool (e.g., iron-sulfur clusters) and the reductant-labile sulfur pool (e.g., sulfane sulfurs) can be released into the gas phase, allowing for measurement of distinct biochemical pools of H2S (107). We have previously reported the concentrations of free H2S, the acid-labile and the reductant-labile sulfur pools in mouse tissues (104). Total sulfide, which is the summation of all three pools, is highest in the aorta (6.11 nmol/mg protein) and lowest in the liver (1.64 nmol/mg protein). However, free sulfide is lowest in the lungs (0.108 nmol/mg protein) but highest in the kidneys (0.914 nmol/mg protein). Interestingly, the ratios of acid-labile sulfur and reductant-labile sulfur to free H2S are lowest in the kidneys (0.550 and 0.603 nmol/mg protein). In the adipose tissue where free H2S is as low as 0.146 nmol/mg protein, acid-labile sulfur and reductant-labile sulfur are 16.6- and 18.6-fold higher over free H2S. These findings highlight the complexity of dynamics between distinct biochemical pools of sulfide and possible roles of acid-labile and reductant-labile sulfur pools as biological H2S reservoirs. Recently, Ida and colleagues (43) identified persulfides (e.g., GSSH, CysSSH) and polysulfides (e.g., GSSSH, GSSSG) formed in mouse tissues using MBB and LC-MS/MS. The concentrations of these sulfane sulfurs were presented as micromolar. According to their supporting information, 100 mg mouse tissues were homogenized in 0.5 ml MBB solution. Therefore, we calculated (as shown in Table 2) the levels of reductant-labile sulfur, which were reported (persulfides and RSnR where n > 2) to be lower than what we observed. This is likely due to the fact that our chemical work flow for reductant labile sulfide also includes measurement of thiosulfate levels that were not measured using LC-MS methods in the report by Ida et al. However, similar GSSH levels were reported by Lu and colleagues (72) using MBB and LC-MS/MS. Together, comparison of these three studies suggests the existence of other reductant-labile sulfur in the tissue, such as protein persulfide/polysulfide and thiosulfate, could be biologically relevant forms of sulfide (43, 90).
Table 2.
Levels of persulfide/polysulfide and reductant labile sulfur
| Measurement | Heart | Lung | Liver | Brain |
|---|---|---|---|---|
| T Ida et al. (43) (nmol/mg tissue) | ||||
| GSSH | 0.27 | 0.02 | 0.3 | 0.77 |
| GSSSH | 0.0067 | 0.0008 | 0.002 | 0.0032 |
| CysSSH | 0.0195 | 0.001 | 0.005 | 0.011 |
| GSSSG | 0.0005 | 0.001235 | 0.00375 | 0.00035 |
| GSSSSG | 0.00075 | 0.00015 | 0.0003 | 0.00295 |
| CysSSSSCys | 0.0043 | 0.0045 | 0.006 | ND |
| CysSSSSSCys | 0.00525 | 0.0069 | ND | ND |
| Total | 0.307 | 0.034585 | 0.31705 | 0.7875 |
| C Lu et al. (72) (nmol/mg protein), GSSH | N/A | N/A | ∼0.2 | ∼0.5 |
| X Shen et al. (104) (nmol/mg protein), reductant labile sulfur | 0.4398 | 1.4508 | 0.4719 | 1.392 |
ND, not detectable; N/A, not measured.
Electrochemistry.
Similar to NO, H2S can be measured on the basis of its redox properties. The sulfide-specific ion-selective electrodes (ISEs) are well documented but used mostly in nonbiological samples. ISEs require an alkaline environment (pH > 11) that favors the accumulation of sulfide ion (S2−). In biological samples, however, this could lead to artificially high readings due to hydroxyl replacement of the cysteine sulfur (89). Alternatively, Kraus and colleagues (21, 60) developed a polarographic electrode with a H2S-permeable membrane, which allows the diffusion of H2S from biological samples at pH 7, into the electrode (pH 10). The polarographic electrode has the advantage of high sensitivity (10–20 nM H2S gas) and the capacity of real-time measurement (129). It may be possible to measurement different sulfide pools by altering sample pH. However, it is sensitive to temperature and pressure and requires frequent calibration.
Fluorescent/chemiluminescent probes.
Sulfide-specific fluorescent/chemiluminescent probes are a fast-developing tool in H2S research that generally provide the potential for real-time and subcellular measurements. These probes are usually designed according to the reducing and nucleophilic properties of sulfide (76). Current probes provide moderate to good specificity to sulfide compared with other biological thiols. To highlight a few, hydrosulfide naphthalimide-2 (HSN2) has an azido group that can be reduced by H2S into a fluorogenic amine (79). The fluorescence of HSN2 is increased 60-fold by 100 equivalents of H2S. Its specificity to H2S is maintained with the presence of 2,000 equivalents of GSH and cysteine. However, the activation of HSN2 takes 45 min and real-time measurement is not possible. Interestingly, Chen and Ai (15) reported a genetically coded azide in GFP, which they used to image endogenous H2S production in HEK 293T cells. This theoretically allows sulfide probing at target proteins. Guo and coworkers (71) also developed a ratiometric fluorescent probe, based on nucleophilic addition of H2S to the probe, that is membrane permeable and has little cytotoxicity, therefore allowing real-time H2S probing in living cells. Although azide-based fluorescent probes usually provide excellent selectivity for H2S over other thiols, excitation with higher power or extended exposure photoactivate these probes. To meet this challenge, Pluth, Bailey, and colleagues (96a) developed a chemiluminescent probe, CLSS-2, to minimize noise due to photoactivation. It is worth noting that most probes were tested in different conditions (sample concentration, buffer pH, oxygen pressure, etc.), which may alter biological sulfide pools. Selectivity and sensitivity of these probes need to be further investigated under physiological conditions and specific cell culture conditions.
Tag-switch technique.
Mounting evidence indicates that H2S elicits its biological effects by forming protein persulfides, termed sulfhydration or persulfidation (63, 81, 82, 103). However, because of similar reactivities of persulfides and thiols, it is challenging to identify protein persulfides specifically. The modified biotin switch assay developed by Snyder and colleagues (81) uses methyl methanethiosulfonate (MMTS) to mask free thiols but not persulfide, followed by pyridyldithiol-biotin compound labeling of persulfide. However, it is unknown how MMTS spares persulfide specifically. Actually, it has been shown that MMTS may also react with small molecular thiols such as GSH (92). Another method uses iodoacetic acid to label both persulfides and free thiols, after which persulfide adducts are reduced by dithiothreitol (DTT) (63). But the selectivity of DTT reduction also remains unclear. Recently, a thiol blocker, methylsulfonyl benzothiazole (MSBT), developed by Xian and colleagues (135) was applied in a tag-switch assay. MSBT reacts with protein persulfide and yields a highly activated disulfide, which distinguishes it from free thiol adducts and native protein disulfides, so that certain nucleophiles, such as cyanoacetate-biotin, can specifically label persulfides. Additionally, the MSBT based tag-switch technique also showed potential to provide spatial resolution in cell culture (135).
Conclusion
As a gasotransmitter, NO plays fundamental role in vascular function and redox biology. For decades, our understanding of NO biology has evolved through empirical medications, identification of sGC as a receptor that transduces NO signaling in various pathways, bioavailable metabolites, effects on protein posttranslational modifications, and novel therapeutic approaches. Although many refined tools have been developed to study NO biology as discussed in this review, not all of them are readily available in each and every laboratory. More importantly, careless choice of tools or misinterpretation of the data often leads to convoluted and false conclusions, especially when various commercial user-friendly kits are employed inappropriately. By comparison, the field of H2S biology is considerably further behind. Our knowledge on H2S chemistry and biology is confusing and contradictory on several aspects. The field is still trying to resolve basic questions such as how are H2S-producing enzymes regulated, what are physiological concentrations of H2S, and how does H2S elicit its various biological functions, all of which are limited by available tools and reagents for study. Although it is dangerous to ignore flaws in models and methods, especially when identified, it is also unwise to be frightened by difficulty. Without a doubt, the future NO and H2S research will yield interesting and important new discoveries in the years to come. Hopefully this review provides a good introduction to investigators new to the field of NO and H2S that will help them deftly approach study of these molecules in the biological systems.
DISCLOSURES
R. P. Patel and C. G. Kevil are coinventors on patents for use of nitrite salts for the treatment of cardiovascular conditions. C. G. Kevil has intellectual interest in hydrogen sulfide detection technology and is chief scientific officer and cofounder of Innolyzer LLC.
AUTHOR CONTRIBUTIONS
S.Y. and R.P.P. prepared figures; S.Y., R.P.P., and C.G.K. edited and revised manuscript; S.Y., R.P.P., and C.G.K. approved final version of manuscript; R.P.P. and C.G.K. drafted manuscript.
ACKNOWLEDGMENTS
This research was supported by the CounterACT Program, National Institutes of Health, Office of the Director, and the National Institute of Environmental Health Sciences, Grant Number 1U01ES023759 to R. P. Patel and by NIH HL11331 to C. G. Kevil and a Malcolm Feist Cardiovascular Predoctoral Fellowship to S. Yuan.
REFERENCES
- 1.Agrawal N, Banerjee R. Human polycomb 2 protein is a SUMO E3 ligase and alleviates substrate-induced inhibition of cystathionine beta-synthase sumoylation. PloS One 3: e4032, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali MY, Ping CY, Mok YY, Ling L, Whiteman M, Bhatia M, Moore PK. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br J Pharmacol 149: 625–634, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amagase H. Clarifying the real bioactive constituents of garlic. J Nutr 136: 716S–725S, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Baliga RS, Milsom AB, Ghosh SM, Trinder SL, Macallister RJ, Ahluwalia A, Hobbs AJ. Dietary nitrate ameliorates pulmonary hypertension: cytoprotective role for endothelial nitric oxide synthase and xanthine oxidoreductase. Circulation 125: 2922–2932, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baskar R, Li L, Moore PK. Hydrogen sulfide-induces DNA damage and changes in apoptotic gene expression in human lung fibroblast cells. FASEB J 21: 247–255, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci USA 104: 17977–17982, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharyya S, Saha S, Giri K, Lanza IR, Nair KS, Jennings NB, Rodriguez-Aguayo C, Lopez-Berestein G, Basal E, Weaver AL, Visscher DW, Cliby W, Sood AK, Bhattacharya R, Mukherjee P. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PloS One 8: e79167, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bir SC, Kolluru GK, McCarthy P, Shen X, Pardue S, Pattillo CB, Kevil CG. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1α and vascular endothelial growth factor-dependent angiogenesis. J Am Heart Assoc 1: e004093, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science 308: 518, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Blood AB, Schroeder HJ, Terry MH, Merrill-Henry J, Bragg SL, Vrancken K, Liu T, Herring JL, Sowers LC, Wilson SM, Power GG. Inhaled nitrite reverses hemolysis-induced pulmonary vasoconstriction in newborn lambs without blood participation. Circulation 123: 605–612, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med 43: 645–657, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler AR, Calsy-Harrison AM, Glidewell C, Sørensen PE. The pentacyanonitrosylferrate ion—V. The course of the reactions of nitroprusside with a range of thiols. Polyhedron 7: 1197–1202, 1988. [Google Scholar]
- 14.Carballal S, Trujillo M, Cuevasanta E, Bartesaghi S, Moller MN, Folkes LK, Garcia-Bereguiain MA, Gutierrez-Merino C, Wardman P, Denicola A, Radi R, Alvarez B. Reactivity of hydrogen sulfide with peroxynitrite and other oxidants of biological interest. Free Radic Biol Med 50: 196–205, 2011. [DOI] [PubMed] [Google Scholar]
- 14a.Chen YH, Wu R, Geng B, Qi YF, Wang PP, Yao WZ, Tang CS. Endogenous hydrogen sulfide reduces airway inflammation and remodeling in a rat model of asthma. Cytokine 45: 117–123, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Chen ZJ, Ai HW. A highly responsive and selective fluorescent probe for imaging physiological hydrogen sulfide. Biochemistry 53: 5966–5974, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, Banerjee R. H2S biogenesis by human cystathionine γ-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem 284: 11601–11612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, James AM, Cocheme HM, Reinhold J, Lilley KS, Partridge L, Fearnley IM, Robinson AJ, Hartley RC, Smith RA, Krieg T, Brookes PS, Murphy MP. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med 19: 753–759, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortese-Krott MM, Fernandez BO, Santos JL, Mergia E, Grman M, Nagy P, Kelm M, Butler A, Feelisch M. Nitrosopersulfide (SSNO−) accounts for sustained NO bioactivity of S-nitrosothiols following reaction with sulfide. Redox Biol 2: 234–244, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diers AR, Keszler A, Hogg N. Detection of S-nitrosothiols. Biochim Biophys Acta 1840: 892–900, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimitrios T. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: appraisal of the Griess reaction in the l-arginine/nitric oxide area of research. J Chromatogr B Analyt Technol Biomed Life Sci 851: 51–70, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, Lancaster JR Jr, Darley-Usmar VM, Kraus DW. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem 341: 40–51, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Edwards G, Feletou M, Weston AH. Hydrogen sulfide as an endothelium-derived hyperpolarizing factor in rodent mesenteric arteries. Circ Res 110: e13–e14; author reply e15–e16, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Filipovic MR, Eberhardt M, Prokopovic V, Mijuskovic A, Orescanin-Dusic Z, Reeh P, Ivanovic-Burmazovic I. Beyond H2S and NO interplay: hydrogen sulfide and nitroprusside react directly to give nitroxyl (HNO). A new pharmacological source of HNO. J Med Chem 56: 1499–1508, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Filipovic MR, Miljkovic J, Allgauer A, Chaurio R, Shubina T, Herrmann M, Ivanovic-Burmazovic I. Biochemical insight into physiological effects of H2S: reaction with peroxynitrite and formation of a new nitric oxide donor, sulfinyl nitrite. Biochem J 441: 609–621, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Filipovic MR, Miljkovic J, Nauser T, Royzen M, Klos K, Shubina T, Koppenol WH, Lippard SJ, Ivanovic-Burmazovic I. Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. J Am Chem Soc 134: 12016–12027, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox-Robichaud A, Payne D, Hasan SU, Ostrovsky L, Fairhead T, Reinhardt P, Kubes P. Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. J Clin Invest 101: 2497–2505, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.George TJ, Arnaoutakis GJ, Beaty CA, Jandu SK, Santhanam L, Berkowitz DE, Shah AS. Inhaled hydrogen sulfide improves graft function in an experimental model of lung transplantation. J Surg Res 178: 593–600, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gherasim C, Yadav PK, Kabil O, Niu WN, Banerjee R. Nitrite reductase activity and inhibition of H2S biogenesis by human cystathionine S-synthase. PloS One 9: e85544, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giustarini D, Rossi R, Milzani A, Dalle-Donne I. Nitrite and nitrate measurement by Griess reagent in human plasma: evaluation of interferences and standardization. Methods Enzymol 440: 361–380, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, Cannon RO 3rd, Kelm M, Wink DA, Espey MG, Oldfield EH, Pluta RM, Freeman BA, Lancaster JR Jr, Feelisch M, Lundberg JO. The emerging biology of the nitrite anion. Nat Chem Biol 1: 308–314, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Gladwin MT, Wang X, Reiter CD, Yang BK, Vivas EX, Bonaventura C, Schechter AN. S-Nitrosohemoglobin is unstable in the reductive erythrocyte environment and lacks O2/NO-linked allosteric function. J Biol Chem 277: 27818–27828, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Gomes A, Fernandes E, Lima JL. Use of fluorescence probes for detection of reactive nitrogen species: a review. J Fluoresc 16: 119–139, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Gow A, Doctor A, Mannick J, Gaston B. S-nitrosothiol measurements in biological systems. J Chromatogr B Analyt Technol Biomed Life Sci 851: 140–151, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grisham MB, Johnson GG, Lancaster JR Jr. Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol 268: 237–246, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Haouzi P, Chenuel B, Sonobe T, Klingerman CM. Are H2S-trapping compounds pertinent to the treatment of sulfide poisoning? Clin Toxicol (Phila) 52: 566, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Havenga MJ, van Dam B, Groot BS, Grimbergen JM, Valerio D, Bout A, Quax PH. Simultaneous detection of NOS-3 protein expression and nitric oxide production using a flow cytometer. Anal Biochem 290: 283–291, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Hawkins CL, Davies MJ. Detection and characterisation of radicals in biological materials using EPR methodology. Biochim Biophys Acta 1840: 708–721, 2014. [DOI] [PubMed] [Google Scholar]
- 38.Heinrich TA, da Silva RS, Miranda KM, Switzer CH, Wink DA, Fukuto JM. Biological nitric oxide signalling: chemistry and terminology. Br J Pharmacol 169: 1417–1429, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hetrick EM, Schoenfisch MH. Analytical chemistry of nitric oxide. Annu Rev Anal Chem (Palo Alto Calif) 2: 409–433, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Honavar J, Bradley E, Bradley K, Oh JY, Vallejo MO, Kelley EE, Cantu-Medellin N, Doran S, Dell'italia LJ, Matalon S, Patel RP. Chlorine gas exposure disrupts nitric oxide homeostasis in the pulmonary vasculature. Toxicology 321: 96–102, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro DB, Machado RF, Tarekegn S, Mulla N, Hopper AO, Schechter AN, Power GG, Gladwin MT. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat Med 10: 1122–1127, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Ida T, Sawa T, Ihara H, Tsuchiya Y, Watanabe Y, Kumagai Y, Suematsu M, Motohashi H, Fujii S, Matsunaga T, Yamamoto M, Ono K, Devarie-Baez NO, Xian M, Fukuto JM, Akaike T. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc Natl Acad Sci USA 111: 7606–7611, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ignarro LJ, Fukuto JM, Griscavage JM, Rogers NE, Byrns RE. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from l-arginine. Proc Natl Acad Sci USA 90: 8103–8107, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol 3: 193–197, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Janošík M, Kery V, Gaustadnes M, Maclean KN, Kraus JP. Regulation of human cystathionine β-synthase by S-adenosyl-l-methionine: evidence for two catalytically active conformations involving an autoinhibitory domain in the C-terminal region. Biochemistry 40: 10625–10633, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Jhee KH, Kruger WD. The role of cystathionine beta-synthase in homocysteine metabolism. Antioxid Redox Signal 7: 813–822, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Jiang B, Tang G, Cao K, Wu L, Wang R. Molecular mechanism for H2S-induced activation of KATP channels. Antioxid Redox Signal 12: 1167–1178, 2010. [DOI] [PubMed] [Google Scholar]
- 49.Kabil O, Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal 20: 770–782, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem 285: 21903–21907, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kabil O, Vitvitsky V, Xie P, Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid Redox Signal 15: 363–372, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kabil O, Zhou Y, Banerjee R. Human cystathionine beta-synthase is a target for sumoylation. Biochemistry 45: 13528–13536, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Kashfi K, Olson KR. Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras. Biochem Pharmacol 85: 689–703, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelleher ZT, Matsumoto A, Stamler JS, Marshall HE. NOS2 regulation of NF-κB by S-nitrosylation of p65. J Biol Chem 282: 30667–30672, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Kettenhofen NJ, Wang X, Gladwin MT, Hogg N. In-gel detection of S-nitrosated proteins using fluorescence methods. Methods Enzymol 441: 53–71, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kevil CG, Patel RP. S-nitrosothiol biology and therapeutic potential in metabolic disease. Curr Opin Investig Drugs 11: 1127–1134, 2010. [PMC free article] [PubMed] [Google Scholar]
- 57.Kim-Shapiro DB, Gladwin MT. Pitfalls in measuring NO bioavailability using NOx. Nitric Oxide 44C: 1–2, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim WS, Ye X, Rubakhin SS, Sweedler JV. Measuring nitric oxide in single neurons by capillary electrophoresis with laser-induced fluorescence: use of ascorbate oxidase in diaminofluorescein measurements. Anal Chem 78: 1859–1865, 2006. [DOI] [PubMed] [Google Scholar]
- 58a.King SB. Potential biological chemistry of hydrogen sulfide (H2S) with the nitrogen oxides. Free Radic Biol Med 55: 1–7, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klingerman CM, Trushin N, Prokopczyk B, Haouzi P. H2S concentrations in the arterial blood during H2S administration in relation to its toxicity and effects on breathing. Am J Physiol Regul Integr Comp Physiol 305: R630–R638, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koenitzer JR, Isbell TS, Patel HD, Benavides GA, Dickinson DA, Patel RP, Darley-Usmar VM, Lancaster JR Jr, Doeller JE, Kraus DW. Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. Am J Physiol Heart Circ Physiol 292: H1953–H1960, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem 70: 2446–2453, 1998. [DOI] [PubMed] [Google Scholar]
- 62.Kosower NS, Kosower EM. Thiol labeling with bromobimanes. Methods Enzymol 143: 76–84, 1987. [DOI] [PubMed] [Google Scholar]
- 63.Krishnan N, Fu C, Pappin DJ, Tonks NK. H2S-induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal 4: ra86, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lang JD Jr, Smith AB, Brandon A, Bradley KM, Liu Y, Li W, Crowe DR, Jhala NC, Cross RC, Frenette L, Martay K, Vater YL, Vitin AA, Dembo GA, Dubay DA, Bynon JS, Szychowski JM, Reyes JD, Halldorson JB, Rayhill SC, Dick AA, Bakthavatsalam R, Brandenberger J, Broeckel-Elrod JA, Sissons-Ross L, Jordan T, Chen LY, Siriussawakul A, Eckhoff DE, Patel RP. A randomized clinical trial testing the anti-inflammatory effects of preemptive inhaled nitric oxide in human liver transplantation. PLoS One 9: e86053, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lang JD Jr, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB, Cross RC, Frenette L, Kelley EE, Wilhite DW, Hall CR, Page GP, Fallon MB, Bynon JS, Eckhoff DE, Patel RP. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest 117: 2583–2591, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee Y, Oh BK, Meyerhoff ME. Improved planar amperometric nitric oxide sensor based on platinized platinum anode. 1. Experimental results and theory when applied for monitoring NO release from diazeniumdiolate-doped polymeric films. Anal Chem 76: 536–544, 2004. [DOI] [PubMed] [Google Scholar]
- 67.Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH, Li L, Moore PK, Deng LW. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PloS One 6: e21077, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li HD, Zhang ZR, Zhang QX, Qin ZC, He DM, Chen JS. Treatment with exogenous hydrogen sulfide attenuates hyperoxia-induced acute lung injury in mice. Eur J Appl Physiol 113: 1555–1563, 2013. [DOI] [PubMed] [Google Scholar]
- 69.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 117: 2351–2360, 2008. [DOI] [PubMed] [Google Scholar]
- 70.Li Q, Lancaster JR Jr. Chemical foundations of hydrogen sulfide biology. Nitric Oxide 35: 21–34, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu J, Sun YQ, Zhang J, Yang T, Cao J, Zhang L, Guo W. A ratiometric fluorescent probe for biological signaling molecule H2S: fast response and high selectivity. Chemistry 19: 4717–4722, 2013. [DOI] [PubMed] [Google Scholar]
- 72.Lu C, Kavalier A, Lukyanov E, Gross SS. S-sulfhydration/desulfhydration and S-nitrosylation/denitrosylation: A common paradigm for gasotransmitter signaling by H2S and NO. Methods 62: 177–181, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luther GW 3rd, Findlay AJ, Macdonald DJ, Owings SM, Hanson TE, Beinart RA, Girguis PR. Thermodynamics and kinetics of sulfide oxidation by oxygen: a look at inorganically controlled reactions and biologically mediated processes in the environment. Front Microbiol 2: 62, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Madurga A, Mizikova I, Ruiz-Camp J, Vadasz I, Herold S, Mayer K, Fehrenbach H, Seeger W, Morty RE. Systemic hydrogen sulfide administration partially restores normal alveolarization in an experimental animal model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 306: L684–L697, 2014. [DOI] [PubMed] [Google Scholar]
- 75.Mathai JC, Missner A, Kugler P, Saparov SM, Zeidel ML, Lee JK, Pohl P. No facilitator required for membrane transport of hydrogen sulfide. Proc Natl Acad Sci USA 106: 16633–16638, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Minor RL Jr, Myers PR, Guerra R Jr, Bates JN, Harrison DG. Diet-induced atherosclerosis increases the release of nitrogen oxides from rabbit aorta. J Clin Invest 86: 2109–2116, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miyamoto R, Otsuguro K, Yamaguchi S, Ito S. Contribution of cysteine aminotransferase and mercaptopyruvate sulfurtransferase to hydrogen sulfide production in peripheral neurons. J Neurochem 130: 29–40, 2014. [DOI] [PubMed] [Google Scholar]
- 79.Montoya LA, Pluth MD. Selective turn-on fluorescent probes for imaging hydrogen sulfide in living cells. Chem Commun (Camb) 48: 4767–4769, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Munro AP, Williams DLH. Reactivity of sulfur nucleophiles towards S-nitrosothiols. J Chem Soc Perkin Trans 2: 1794–1797, 2000. [Google Scholar]
- 81.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal 2: ra72, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109: 1259–1268, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nagahara N, Ito T, Kitamura H, Nishino T. Tissue and subcellular distribution of mercaptopyruvate sulfurtransferase in the rat: confocal laser fluorescence and immunoelectron microscopic studies combined with biochemical analysis. Histochem Cell Biol 110: 243–250, 1998. [DOI] [PubMed] [Google Scholar]
- 83a.Newton GL, Fahey RC. Determination of biothiols by bromobimane labeling and high-performance liquid chromatography. Methods Enzymol 251: 48–66, 1995. [DOI] [PubMed] [Google Scholar]
- 84.Ng ES, Jourd'heuil D, McCord JM, Hernandez D, Yasui M, Knight D, Kubes P. Enhanced S-nitroso-albumin formation from inhaled NO during ischemia/reperfusion. Circ Res 94: 559–565, 2004. [DOI] [PubMed] [Google Scholar]
- 85.Nishida M, Sawa T, Kitajima N, Ono K, Inoue H, Ihara H, Motohashi H, Yamamoto M, Suematsu M, Kurose H, van der Vliet A, Freeman BA, Shibata T, Uchida K, Kumagai Y, Akaike T. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat Chem Biol 8: 714–724, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Niu WN, Yadav PK, Adamec J, Banerjee R. S-glutathionylation enhances human cystathionine beta-synthase activity under oxidative stress conditions. Antioxid Redox Signal. 2014Jul 29. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nriagu JO. Stability of vivianite and ion-pair formation in the system Fe3(PO4)2–H3PO4–H2O. Geochim Cosmochim Acta 36: 459–470, 1972. [Google Scholar]
- 88.Okamoto T, Tang X, Janocha A, Farver CF, Gladwin MT, McCurry KR. Nebulized nitrite protects rat lung grafts from ischemia reperfusion injury. J Thorac Cardiovasc Surg 145: 1108–1116, 2013. [DOI] [PubMed] [Google Scholar]
- 89.Olson KR. A practical look at the chemistry and biology of hydrogen sulfide. Antioxid Redox Signal 17: 32–44, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Olson KR, Deleon ER, Gao Y, Hurley K, Sadauskas V, Batz C, Stoy GF. Thiosulfate: a readily accessible source of hydrogen sulfide in oxygen sensing. Am J Physiol Regul Integr Comp Physiol 305: R592–R603, 2013. [DOI] [PubMed] [Google Scholar]
- 91.Ondrias K, Stasko A, Cacanyiova S, Sulova Z, Krizanova O, Kristek F, Malekova L, Knezl V, Breier A. H2S and HS− donor NaHS releases nitric oxide from nitrosothiols, metal nitrosyl complex, brain homogenate and murine L1210 leukaemia cells. Pflügers Arch 457: 271–279, 2008. [DOI] [PubMed] [Google Scholar]
- 92.Pan J, Carroll KS. Persulfide reactivity in the detection of protein S-sulfhydration. ACS Chem Biol 8: 1110–1116, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paul BD, Snyder SH. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 13: 499–507, 2012. [DOI] [PubMed] [Google Scholar]
- 94.Perry MM, Hui CK, Whiteman M, Wood ME, Adcock I, Kirkham P, Michaeloudes C, Chung KF. Hydrogen sulfide inhibits proliferation and release of IL-8 from human airway smooth muscle cells. Am J Respir Cell Mol Biol 45: 746–752, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peter EA, Shen X, Shah SH, Pardue S, Glawe JD, Zhang WW, Reddy P, Akkus NI, Varma J, Kevil CG. Plasma free H2S levels are elevated in patients with cardiovascular disease. J Am Heart Assoc 2: e000387, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pickerodt PA, Emery MJ, Zarndt R, Martin W, Francis RC, Boemke W, Swenson ER. Sodium nitrite mitigates ventilator-induced lung injury in rats. Anesthesiology 117: 592–601, 2012. [DOI] [PubMed] [Google Scholar]
- 96a.Pluth MD, Bailey TS, Hamners MD, Montoya LA. Chemical tools for studying biological hydrogen sulfide. In: Biochalcogen Chemistry: The Biological Chemistry of Sulfur, Selenium, and Tellurium, edited by Bayse CA, Brumaghim JL. Washington, DC: American Chemical Society, 2013, p. 15–32. [Google Scholar]
- 97.Poon BY, Raharjo E, Patel KD, Tavener S, Kubes P. Complexity of inducible nitric oxide synthase: cellular source determines benefit vs. toxicity. Circulation 108: 1107–1112, 2003. [DOI] [PubMed] [Google Scholar]
- 98.Razavi HM, Wang L, Weicker S, Quinlan GJ, Mumby S, McCormack DG, Mehta S. Pulmonary oxidant stress in murine sepsis is due to inflammatory cell nitric oxide. Crit Care Med 33: 1333–1339, 2005. [DOI] [PubMed] [Google Scholar]
- 99.Razavi HM, Wang le F, Weicker S, Rohan M, Law C, McCormack DG, Mehta S. Pulmonary neutrophil infiltration in murine sepsis: role of inducible nitric oxide synthase. Am J Respir Crit Care Med 170: 227–233, 2004. [DOI] [PubMed] [Google Scholar]
- 100.Saito J, Mackay AJ, Rossios C, Gibeon D, Macedo P, Sinharay R, Bhavsar PK, Wedzicha JA, Chung KF. Sputum-to-serum hydrogen sulfide ratio in COPD. Thorax 69: 903–909, 2014. [DOI] [PubMed] [Google Scholar]
- 101.Samal AA, Honavar J, Brandon A, Bradley KM, Doran S, Liu Y, Dunaway C, Steele C, Postlethwait EM, Squadrito GL, Fanucchi MV, Matalon S, Patel RP. Administration of nitrite after chlorine gas exposure prevents lung injury: effect of administration modality. Free Radic Biol Med 53: 1431–1439, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Searcy DG, Lee SH. Sulfur reduction by human erythrocytes. J Exp Zool 282: 310–322, 1998. [DOI] [PubMed] [Google Scholar]
- 103.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, Snyder SH. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol Cell 45: 13–24, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shen X, Carlstrom M, Borniquel S, Jadert C, Kevil CG, Lundberg JO. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic Biol Med 60: 195–200, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shen X, Chakraborty S, Dugas TR, Kevil CG. Hydrogen sulfide measurement using sulfide dibimane: critical evaluation with electrospray ion trap mass spectrometry. Nitric Oxide 41: 97–104, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shen X, Pattillo CB, Pardue S, Bir SC, Wang R, Kevil CG. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic Biol Med 50: 1021–1031, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shen X, Peter EA, Bir S, Wang R, Kevil CG. Analytical measurement of discrete hydrogen sulfide pools in biological specimens. Free Radic Biol Med 52: 2276–2283, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singh RJ, Hogg N, Joseph J, Kalyanaraman B. Mechanism of nitric oxide release from S-nitrosothiols. J Biol Chem 271: 18596–18603, 1996. [DOI] [PubMed] [Google Scholar]
- 109.Spassov S, Pfeifer D, Strosing K, Ryter S, Hummel M, Faller S, Hoetzel A. Genetic targets of hydrogen sulfide in ventilator-induced lung injury—a microarray study. PLoS One 9: e102401, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stein A, Mao Z, Morrison JP, Fanucchi MV, Postlethwait EM, Patel RP, Kraus DW, Doeller JE, Bailey SM. Metabolic and cardiac signaling effects of inhaled hydrogen sulfide and low oxygen in male rats. J Appl Physiol 112: 1659–1669, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sugimoto R, Okamoto T, Nakao A, Zhan J, Wang Y, Kohmoto J, Tokita D, Farver CF, Tarpey MM, Billiar TR, Gladwin MT, McCurry KR. Nitrite reduces acute lung injury and improves survival in a rat lung transplantation model. Am J Transplant 12: 2938–2948, 2012. [DOI] [PubMed] [Google Scholar]
- 112.Szabo C, Coletta C, Chao C, Modis K, Szczesny B, Papapetropoulos A, Hellmich MR. Tumor-derived hydrogen sulfide, produced by cystathionine-beta-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci USA 110: 12474–12479, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Szczesny B, Modis K, Yanagi K, Coletta C, Le Trionnaire S, Perry A, Wood ME, Whiteman M, Szabo C. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide 41: 120–130, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tao BB, Liu SY, Zhang CC, Fu W, Cai WJ, Wang Y, Shen Q, Wang MJ, Chen Y, Zhang LJ, Zhu YZ, Zhu YC. VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal 19: 448–464, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taoka S, Banerjee R. Characterization of NO binding to human cystathionine beta-synthase: possible implications of the effects of CO and NO binding to the human enzyme. J Inorg Biochem 87: 245–251, 2001. [DOI] [PubMed] [Google Scholar]
- 115a.Teng H, Wu B, Zhao K, Yang G, Wu L, Wang R. Oxygen-sensitive mitochondrial accumulation of cystathionine β-synthase mediated by Lon protease. Proc Natl Acad Sci USA 110: 12679–12684, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Teng X, Scott Isbell T, Crawford JH, Bosworth CA, Giles GI, Koenitzer JR, Lancaster JR, Doeller JE, Kraus DW, Patel RP. Novel method for measuring S-nitrosothiols using hydrogen sulfide. Methods Enzymol 441: 161–172, 2008. [DOI] [PubMed] [Google Scholar]
- 117.Terpolilli NA, Kim SW, Thal SC, Kataoka H, Zeisig V, Nitzsche B, Klaesner B, Zhu C, Schwarzmaier S, Meissner L, Mamrak U, Engel DC, Drzezga A, Patel RP, Blomgren K, Barthel H, Boltze J, Kuebler WM, Plesnila N. Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circ Res 110: 727–738, 2012. [DOI] [PubMed] [Google Scholar]
- 117a.Togawa T, Ogawa M, Nawata M, Ogasawara Y, Kawanabe K, Tanabe S. High performance liquid chromatographic determination of bound sulfide and sulfite and thiosulfate at their low levels in human serum by pre-column fluorescence derivatization with monobromobimane. Chem Pharm Bull (Tokyo) 40: 3000–3004, 1992. [DOI] [PubMed] [Google Scholar]
- 118.Toubin C, Yeung DYH, English AM, Peslherbe GH. Theoretical evidence that CuI complexation promotes degradation of S-nitrosothiols. J Am Chem Soc 124: 14816–14817, 2002. [DOI] [PubMed] [Google Scholar]
- 119.Tsukahara H, Ishida T, Mayumi M. Gas-phase oxidation of nitric oxide: chemical kinetics and rate constant. Nitric Oxide 3: 191–198, 1999. [DOI] [PubMed] [Google Scholar]
- 120.Vadivel A, Alphonse RS, Ionescu L, Machado DS, O'Reilly M, Eaton F, Haromy A, Michelakis ED, Thebaud B. Exogenous hydrogen sulfide (H2S) protects alveolar growth in experimental O2-induced neonatal lung injury. PLoS One 9: e90965, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Verdon CP, Burton BA, Prior RL. Sample pretreatment with nitrate reductase and glucose-6-phosphate dehydrogenase quantitatively reduces nitrate while avoiding interference by NADP+ when the Griess reaction is used to assay for nitrite. Anal Biochem 224: 502–508, 1995. [DOI] [PubMed] [Google Scholar]
- 122.Wang L, Frizzell SA, Zhao X, Gladwin MT. Normoxic cyclic GMP-independent oxidative signaling by nitrite enhances airway epithelial cell proliferation and wound healing. Nitric Oxide 26: 203–210, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang LF, Patel M, Razavi HM, Weicker S, Joseph MG, McCormack DG, Mehta S. Role of inducible nitric oxide synthase in pulmonary microvascular protein leak in murine sepsis. Am J Respir Crit Care Med 165: 1634–1639, 2002. [DOI] [PubMed] [Google Scholar]
- 124.Wang P, Zhang G, Wondimu T, Ross B, Wang R. Hydrogen sulfide and asthma. Exp Physiol 96: 847–852, 2011. [DOI] [PubMed] [Google Scholar]
- 125.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92: 791–896, 2012. [DOI] [PubMed] [Google Scholar]
- 126.Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16: 1792–1798, 2002. [DOI] [PubMed] [Google Scholar]
- 127.Wang X, Bryan NS, MacArthur PH, Rodriguez J, Gladwin MT, Feelisch M. Measurement of nitric oxide levels in the red cell: validation of tri-iodide-based chemiluminescence with acid-sulfanilamide pretreatment. J Biol Chem 281: 26994–27002, 2006. [DOI] [PubMed] [Google Scholar]
- 128.Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, Moore PK. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem Biophys Res Commun 343: 303–310, 2006. [DOI] [PubMed] [Google Scholar]
- 129.Whitfield NL, Kreimier EL, Verdial FC, Skovgaard N, Olson KR. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am J Physiol Regul Integr Comp Physiol 294: R1930–R1937, 2008. [DOI] [PubMed] [Google Scholar]
- 130.Wintner EA, Deckwerth TL, Langston W, Bengtsson A, Leviten D, Hill P, Insko MA, Dumpit R, VandenEkart E, Toombs CF, Szabo C. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br J Pharmacol 160: 941–957, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yadav AK, Doran SF, Samal AA, Sharma R, Vedagiri K, Postlethwait EM, Squadrito GL, Fanucchi MV, Roberts LJ 2nd, Patel RP, Matalon S. Mitigation of chlorine gas lung injury in rats by postexposure administration of sodium nitrite. Am J Physiol Lung Cell Mol Physiol 300: L362–L369, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yadav PK, Yamada K, Chiku T, Koutmos M, Banerjee R. Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J Biol Chem 288: 20002–20013, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yong QC, Hu LF, Wang S, Huang D, Bian JS. Hydrogen sulfide interacts with nitric oxide in the heart: possible involvement of nitroxyl. Cardiovasc Res 88: 482–491, 2010. [DOI] [PubMed] [Google Scholar]
- 135.Zhang D, Macinkovic I, Devarie-Baez NO, Pan J, Park CM, Carroll KS, Filipovic MR, Xian M. Detection of protein S-sulfhydration by a tag-switch technique. Angew Chem Int Ed Engl 53: 575–581, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang J, Wang X, Chen Y, Yao W. Correlation between levels of exhaled hydrogen sulfide and airway inflammatory phenotype in patients with chronic persistent asthma. Respirology 19: 1165–1169, 2014. [DOI] [PubMed] [Google Scholar]
- 137.Zhang P, Li F, Wiegman CH, Zhang M, Hong Y, Gong J, Chang Y, Zhang JJ, Adcock I, Chung KF, Zhou X. Inhibitory effect of hydrogen sulfide on ozone-induced airway inflammation, oxidative stress and bronchial hyperresponsiveness. Am J Respir Cell Mol Biol 52: 129–137, 2015. [DOI] [PubMed] [Google Scholar]
- 138.Zhu W, Lin A, Banerjee R. Kinetic properties of polymorphic variants and pathogenic mutants in human cystathionine gamma-lyase. Biochemistry 47: 6226–6232, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zuckerbraun BS, Shiva S, Ifedigbo E, Mathier MA, Mollen KP, Rao J, Bauer PM, Choi JJ, Curtis E, Choi AM, Gladwin MT. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation 121: 98–109, 2010. [DOI] [PubMed] [Google Scholar]