Abstract
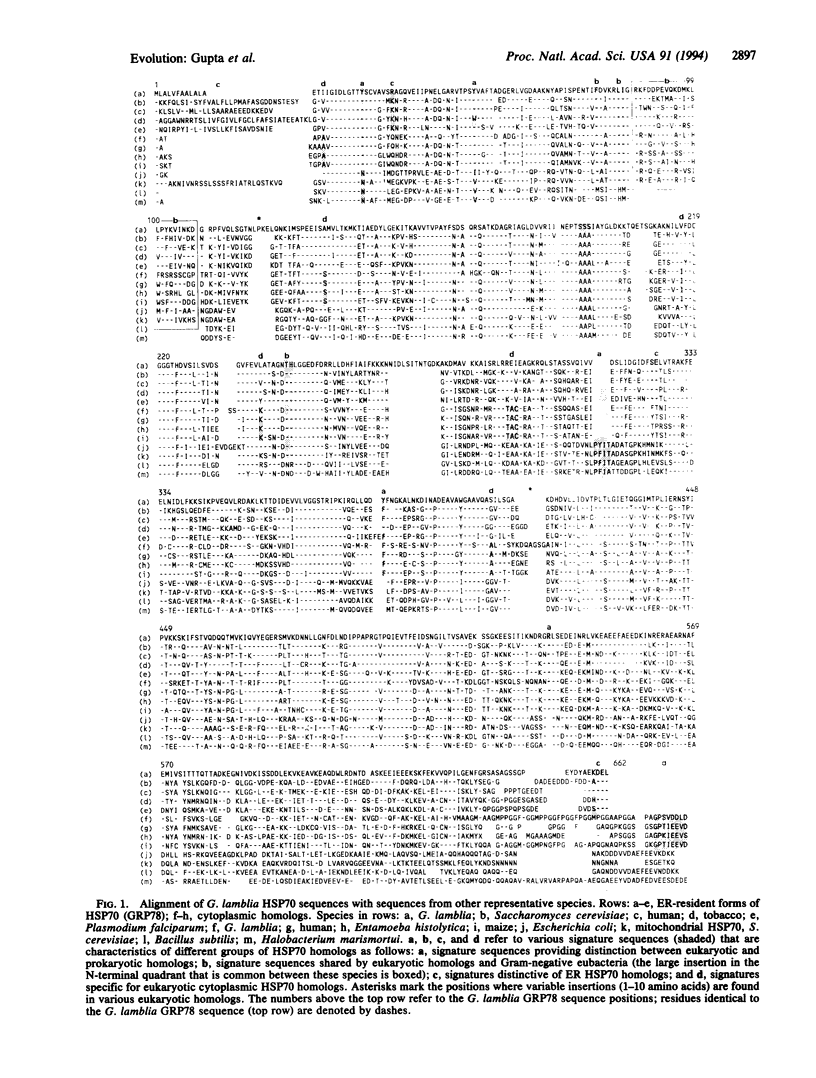
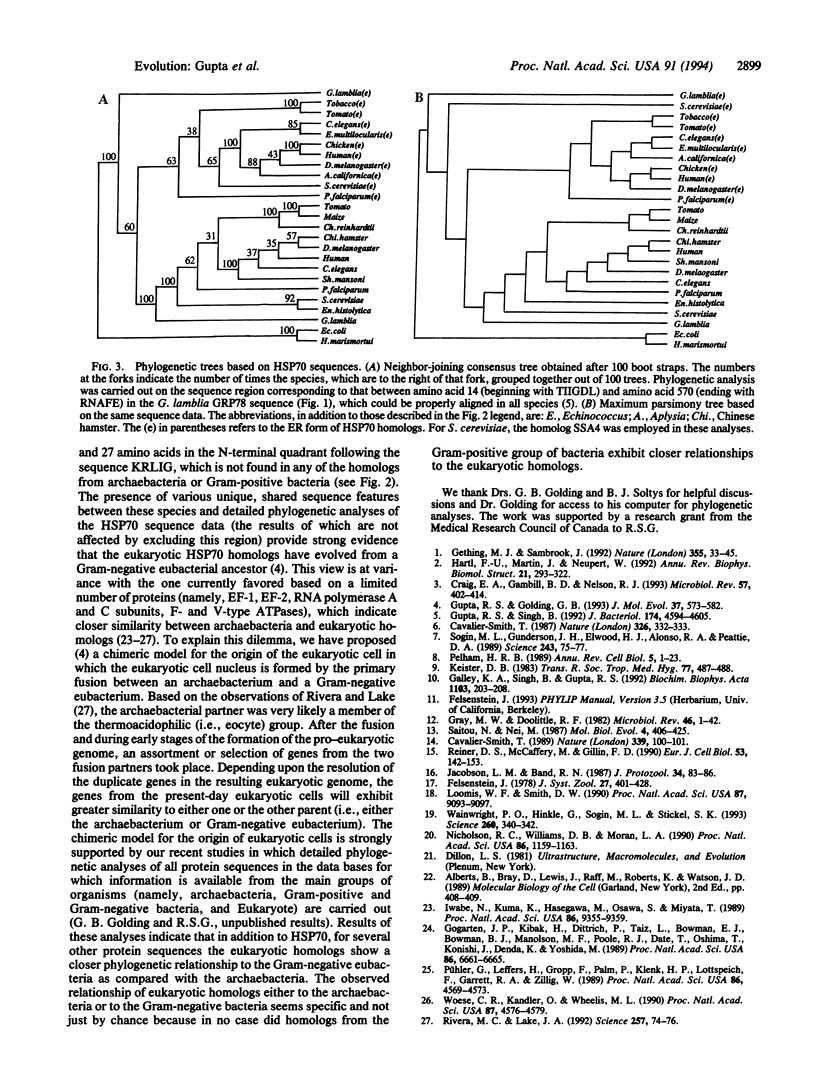
The genes for two different 70-kDa heat shock protein (HSP70) homologs have been cloned and sequenced from the protozoan Giardia lamblia. On the basis of their sequence features, one of these genes corresponds to the cytoplasmic form of HSP70. The second gene, on the basis of its characteristic N-terminal hydrophobic signal sequence and C-terminal endoplasmic reticulum (ER) retention sequence (Lys-Asp-Glu-Leu), is the equivalent of ER-resident GRP78 or the Bip family of proteins. Phylogenetic trees based on HSP70 sequences show that G. lamblia homologs show the deepest divergence among eukaryotic species. The identification of a GRP78 or Bip homolog in G. lamblia strongly suggests the existence of ER in this ancient eukaryote. Detailed phylogenetic analyses of HSP70 sequences by boot-strap neighbor-joining and maximum-parsimony methods show that the cytoplasmic and ER homologs form distinct subfamilies that evolved from a common eukaryotic ancestor by gene duplication that occurred very early in the evolution of eukaryotic cells. It is postulated that because of the essential "molecular chaperone" function of these proteins in translocation of other proteins across membranes, duplication of their genes accompanied the evolution of ER or nucleus in the eukaryotic cell ancestor. The presence in all eukaryotic cytoplasmic HSP70 homologs (including the cognate, heat-induced, and ER forms) of a number of autapomorphic sequence signatures that are not present in any prokaryotic or organellar homologs provides strong evidence regarding the monophyletic nature of eukaryotic lineage. Further, all eukaryotic HSP70 homologs share in common with the Gram-negative group of eubacteria a number of sequence features that are not present in any archaebacterium or Gram-positive bacterium, indicating their evolution from this group of organisms. Some implications of these findings regarding the evolution of eukaryotic cells and ER are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavalier-Smith T. Eukaryotes with no mitochondria. 1987 Mar 26-Apr 1Nature. 326(6111):332–333. doi: 10.1038/326332a0. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Gambill B. D., Nelson R. J. Heat shock proteins: molecular chaperones of protein biogenesis. Microbiol Rev. 1993 Jun;57(2):402–414. doi: 10.1128/mr.57.2.402-414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley K. A., Singh B., Gupta R. S. Cloning of HSP70 (dnaK) gene from Clostridium perfringens using a general polymerase chain reaction based approach. Biochim Biophys Acta. 1992 Mar 24;1130(2):203–208. doi: 10.1016/0167-4781(92)90529-9. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gogarten J. P., Kibak H., Dittrich P., Taiz L., Bowman E. J., Bowman B. J., Manolson M. F., Poole R. J., Date T., Oshima T. Evolution of the vacuolar H+-ATPase: implications for the origin of eukaryotes. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6661–6665. doi: 10.1073/pnas.86.17.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. W., Doolittle W. F. Has the endosymbiont hypothesis been proven? Microbiol Rev. 1982 Mar;46(1):1–42. doi: 10.1128/mr.46.1.1-42.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. S., Golding G. B. Evolution of HSP70 gene and its implications regarding relationships between archaebacteria, eubacteria, and eukaryotes. J Mol Evol. 1993 Dec;37(6):573–582. doi: 10.1007/BF00182743. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Singh B. Cloning of the HSP70 gene from Halobacterium marismortui: relatedness of archaebacterial HSP70 to its eubacterial homologs and a model for the evolution of the HSP70 gene. J Bacteriol. 1992 Jul;174(14):4594–4605. doi: 10.1128/jb.174.14.4594-4605.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F. U., Martin J., Neupert W. Protein folding in the cell: the role of molecular chaperones Hsp70 and Hsp60. Annu Rev Biophys Biomol Struct. 1992;21:293–322. doi: 10.1146/annurev.bb.21.060192.001453. [DOI] [PubMed] [Google Scholar]
- Iwabe N., Kuma K., Hasegawa M., Osawa S., Miyata T. Evolutionary relationship of archaebacteria, eubacteria, and eukaryotes inferred from phylogenetic trees of duplicated genes. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9355–9359. doi: 10.1073/pnas.86.23.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister D. B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77(4):487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Smith D. W. Molecular phylogeny of Dictyostelium discoideum by protein sequence comparison. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9093–9097. doi: 10.1073/pnas.87.23.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson R. C., Williams D. B., Moran L. A. An essential member of the HSP70 gene family of Saccharomyces cerevisiae is homologous to immunoglobulin heavy chain binding protein. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1159–1163. doi: 10.1073/pnas.87.3.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Pühler G., Leffers H., Gropp F., Palm P., Klenk H. P., Lottspeich F., Garrett R. A., Zillig W. Archaebacterial DNA-dependent RNA polymerases testify to the evolution of the eukaryotic nuclear genome. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4569–4573. doi: 10.1073/pnas.86.12.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner D. S., McCaffery M., Gillin F. D. Sorting of cyst wall proteins to a regulated secretory pathway during differentiation of the primitive eukaryote, Giardia lamblia. Eur J Cell Biol. 1990 Oct;53(1):142–153. [PubMed] [Google Scholar]
- Rivera M. C., Lake J. A. Evidence that eukaryotes and eocyte prokaryotes are immediate relatives. Science. 1992 Jul 3;257(5066):74–76. doi: 10.1126/science.1621096. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H., Elwood H. J., Alonso R. A., Peattie D. A. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989 Jan 6;243(4887):75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- Wainright P. O., Hinkle G., Sogin M. L., Stickel S. K. Monophyletic origins of the metazoa: an evolutionary link with fungi. Science. 1993 Apr 16;260(5106):340–342. doi: 10.1126/science.8469985. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]




