Abstract
No single or combinatorial therapeutic approach has proven effective in decreasing morbidity or engendering a cure of metastatic cancer. In principle, conditionally replication-competent adenoviruses that induce tumor oncolysis through cancer-specific replication hold promise for cancer therapy. However, a single-agent approach may not be adequate to completely eradicate cancer in a patient because most cancers arise from abnormalities in multiple genetic and signal transduction pathways and targeting disseminated metastases is difficult to achieve. Based on these considerations, a novel class of cancer destroying adenoviruses have been produced, cancer terminator viruses (CTVs), in which cancer-specific replication is controlled by the progression-elevated gene-3 promoter and replicating viruses produce a second transgene encoding an apoptosis-inducing and immunomodulatory cytokine, either melanoma differentiation-associated gene-7/interleukin-24 (mda-7/IL-24) or interferon-γ. This review focuses on these viruses and ways to improve their delivery systemically and enhance their therapeutic efficacy.
1. INTRODUCTION
Cancer exacts an enormous global health burden, affecting every geo-graphical region and socioeconomic level and accounting for one in eight deaths worldwide, more than HIV/AIDS, tuberculosis, and malaria combined (Cancer Figurs & Facts, 2011). Cancer is characterized by uncontrolled cell growth with frequent spread of abnormal cells from a primary tumor site to distant sites in the body, often culminating in death. The etiology of this devastating disease includes both exogenous (tobacco, infectious organisms, chemicals, radiation) and endogenous factors (inherited mutations, epigenetic changes, hormones). Although, more than 60% of all cancer deaths occur in low- and middle-income countries, developed countries are also equally affected. According to recent statistics, in 2011, diagnosis of approximately 1.5 million new cancer cases was expected in the United States and about 15 thousand affected individuals were anticipated to die every day (Cancer Figurs & Facts, 2011).
A key challenge facing oncology researchers/clinicians in developing effective anticancer therapies is the molecular heterogeneity of tumors, which frequently increase during neoplastic progression. Major advances in genomics, transcriptomics, and proteomics continue to shed light on the underlying pathophysiology of tumors resulting in a shift from traditional cancer treatment, classically based on cytotoxics or antihormone therapies, and launching the concept and implementation of targeted gene therapy. In 1964, a trio of Nobel laureates (Edward Tatum, Joshua Lederberg, and Arthur Kornberg) suggested that it would be possible to cure often-fatal genetic disorders, like cystic fibrosis, muscular dystrophy, and multiple sclerosis, by replacing the defective gene with a functional one. In the past decades, tremendous efforts have been made to translate gene therapy of cancer into the clinical arena. The efficient delivery of therapeutic genes with appropriate targeted gene expression are crucial factors for promoting clinically effective gene therapies. Two broad approaches have been used to deliver DNA to cells, namely, viral vectors and nonviral vectors. Although the nonviral methods for gene therapy provide certain advantages including simple large-scale production and low-host immunogenicity, viral vectors are naturally evolved vehicles, thereby efficiently transferring their genes into host cells. This property of viruses makes them desirable agents for engineering viral vector-based systems to deliver therapeutic genes.
2. VIRAL VECTORS FOR CANCER GENE THERAPY
Viruses are obligate intracellular parasites and highly evolutionary conserved biological machines that very resourcefully gain access, transfer their DNA, and exploit the host cell machinery to ensure their replication (Thomas, Ehrhardt, & Kay, 2003). Use of the viral infection pathway, while circumventing the subsequent expression of viral genes that leads to replication and toxicity through deleting all or part of the coding regions of the viral genome, embodies virus based vectors with necessary properties for efficient gene transfer. Although a number of viruses have been developed as potential gene transfer vectors, based on the packing capacity oftransgenes, host range, tropism, and inflammatory potential (Wu & Zhou, 2011), interest has been focused on a few select viruses including adenoviruses (Ads), adeno-associated viruses, retroviruses, and herpes simplex virus type 1. A brief description of various virus vectors with their advantages/ disadvantages is presented in Table 1.1. Several additional virus vectors, derived from vaccinia virus, human cytomegalovirus, Epstein Barr virus, poxviruses, and foamy virus, have also been tested forgene therapy approaches in both basic and clinical research. In this review, we have integrated recent information related to Ad vector biology and methods to enhance their therapeutic potential.
Table. 1.1.
General characteristic of few commonly used viral vectors in cancer gene therapy
| Virus | Family | Genome/insert size/ particle size/envelope |
Advantages | Disadvantages |
|---|---|---|---|---|
|
| ||||
| Adenovirus | Adenoviridae | dsDNA, 36 kb/7.5 kb/ 90 nm/No |
Infects dividing and nondividing cells; nontoxic to infecting cells; efficient gene transfer; high-viral titers achievable |
Small insert size; postinfection of immune response leading to decreased infectivity to desired cells |
|
| ||||
| Herpes simplex virus |
Herpesviridae | dsDNA, 152 kb/ 40–50 kb/ 120–200 nm/Yes |
Infects dividing and nondividing cells; prolonged gene expression achievable; large insert size for transgene; high-viral titers achievable; sensitive to acyclovir/ ganciclovir |
Postinfection of immune response leading to decreased infectivity to desired cells; possibility of herpes encephalitis |
|
| ||||
| Retrovirus | Retroviridae | ssRNA, 7–11 kb/8 kb/ 10 nm/Yes |
High-transduction efficiency; easy to design; chronic infection; integration into host genome resulting into prolonged gene expression; viral proteins not expressed in the host, thereby limiting the chances of reconstitution of replication- competent virus |
Infects dividing cells only; low titers; random site integration; possibility of latent diseases, like malignancy or immunodeficiency |
|
| ||||
| Adeno- associated virus |
Paroviridae | ssDNA, 5 kb/5 kb/ 20 nm/No |
Infects dividing and nondividing cells; lack of pathogenicity; nontoxic to host cells; stable integration into the host cells at a specific site in the human chromosome 19 |
Low insert size; provoke humoral immunity on infection; large-scale production is labor intensive |
3. ADENOVIRUSES AS VECTORS FOR CANCER GENE THERAPY
Ads, a nonenveloped DNA virus, were first isolated from adenoid tissues in 1953 (Enders et al., 1956). This virus is associated with mild upper respiratory tract infections in humans. Based on hemagglutinin properties, 51 serotypes of Ads have been identified which are classified into six sub-groups (A–F). Because ofits association with mild disease and relatively easy genome to manipulate compared with other Ad serotypes, human serotype 5 (Ad.5) of species C has gained common use as a vector for gene therapy.
As a gene transfer vector, Ads have discrete advantages including high-transfection efficiency irrespective of the growth status of cells, inability to integrate into the host genome, easy capsid/genome modifications for retargeting tropism to different tissues or tumors, and efficient production of high virus yields. As of March 2011, ~25% of the gene therapy clinical trials included Ads as vectors (http://wiley.co.uk/genetherapy/clinica). Gendicine is the first commercialized gene therapy medicine for the treatment of cancer that uses a shuttling system based on Ad to carry a p53 gene to limit cancer growth (Zhaohui, 2005). However, the long-term clinical efficacy of this vector has been disappointing, despite over 300 clinical trials that have shown it to be well tolerated and efficient in gene transfer.
3.1. Biology of adenoviruses
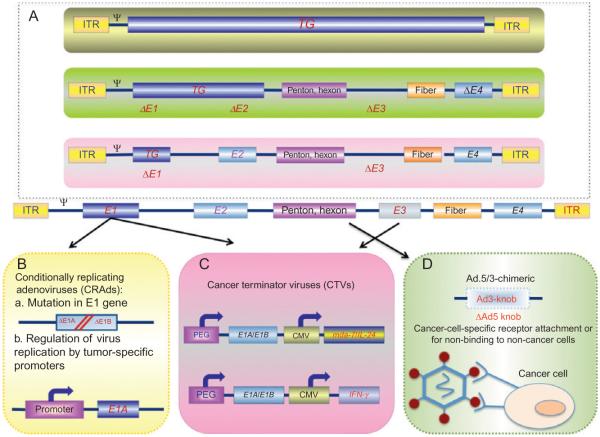
The Ad genome is composed of linear double stranded DNA roughly 36 kb long of which up to 30 kb can be exchanged with foreign DNA (Smith, 1995; Tooze, 1980). Gene expression is divided into four early (E1 through E4) transcriptional units, functioning in part as master transcriptional regulators, and late (L1 through L5) transcripts which code for structural proteins (Fig. 1.1). As a minimum, three regions of the viral genome can accept insertions or substitutions of DNA to generate helper independent viruses: a region in E1 (including E1A and E1B), a region in E3, and a short region between E4 and the end of genome. Since the E1A gene is indispensable for virus replication, in the first generation of Ads, the modification of the Ad genome was based on deletion of the entire E1A and/or partial deletions of E1B and E3 genes to produce replication incompetent vectors providing appropriate space to insert transgenes (Fig. 1.1). However, even in the absence of E1 gene products, there was low level viral replication of these vectors inducing CD4+- and CD8+-dependent immune responses leading to a reduced duration of gene expression in in vivo systems (Yang, Su, & Wilson, 1996; Yang & Wilson, 1995). To decrease toxicity and prolong gene expression, newer second generation Ad vectors have been created which lack E2A and contain mutations and/or deletions of the viral E4 gene (Dedieu et al., 1997; Yang et al., 1994). In another approach, “gutted” or “gutless” Ad vectors were formed by removing the complete viral coding regions leaving only the inverted terminal repeats (ITR). Therapeutic genes transmitted by gutless virus have been successfully transported to the liver of mouse models for various human diseases, such as hemophilia A and B, obesity, familial hypercholesterolemia, diabetes, and chronic viral hepatitis, with encouraging consequences in the context of long term expression with reduced and transient cellular immune responses (reviewed by Alba, Bosch, & Chillon, 2005). However, in most cases, stimulating a noticeable and durable antitumor response necessitates multiple administrations of replication-incompetent Ads, which can stimulate an immune response, promoting viral clearance (Sarkar, Su, & Fisher, 2006). In these contexts, conditionally replication-competent adenoviruses (CRCAs) are presently being evaluated because of their effectiveness in killing cancer cells by viral replication, thus requiring a reduced number of administrations (Fig. 1.1).
Figure 1.1.
Genetic modifications of the adenoviral genome result in oncolytic and cancer therapeutic viruses. Serotype 5 of adenovirus (Ad.5) is the most commonly used vector for gene therapy. In an effort to decrease the immune response, when delivered inside the human body, and increase cancer cell infectivity, a number of modifications in the Ad.5 genome are resulting in unique genetically modified Ad vectors (Box A). Further modifications, including mutations in the E1 region of Ad to permit cancer cell-specific replication or tumor-specific promoters driving the E1A gene, result in the development of conditionally replicating Ads (CRAds; Box B). Recombinant therapeutic Ads are being constructed in which nonessential genes of the Ad genome are deleted (e.g., E3 region) and replaced with therapeutic transgenes (e.g., mda-7/IL-24, IFN-γ). This type of “armed” oncolytic adenovirus is referred to as a cancer terminator virus (CTV; Box C). Modifications in the fiber domain of the Ad.5 genome have also been used to generate chimeric Ad.5/3 viruses (Box D). These viruses retain high infectivity in cancer cells that use the Coxsackie and adenovirus receptor (CAR) for entry of Ad.5 and also provide entry into cancer cells in a CAR-independent manner, thereby enhancing efficacy of these viruses for cancer gene therapy.
3.2. Conditionally replication-competent adenoviruses for cancer therapy
Considering their therapeutic potential, CRCAs exemplify a new class of anticancer agents. Ironically, although advances in genetic engineering have allowed the development of replication-defective Ad vectors and the long history of their use as simple delivery vehicles for therapeutic genes (Gomez Manzano et al., 1996; Roth & Cristiano, 1997), replication-competent wild type strains were actually the first to be delivered safely to patients (Smith, Huebner, Rowe, Schatten, & Thomas, 1956). Now more than half century later, replication competent Ads are once again in the limelight and are being analyzed as therapeutic agents (Heise, Williams, Xue, Propst, & Kirn, 1999; Kirn & McCormick, 1996). Moreover, through advanced genetic engineering CRCAs are now being used with the intention of maximizing tumor selective replication.
The most important consideration when using a CRCA is to guarantee cancer cell-specific replication. To achieve this objective, a number of distinctive strategies have been considered and are currently being tested. One of the tactics for optimizing tumor selectivity is to delete gene functions that are critical for efficient viral replication in normal cells, but not in tumor cells. For example, the functions of the p53 gene product are compromised both in tumors and in Ad infected cells. Since wild type Ads contain the E1B-55kd gene that is responsible for p53 binding and inactivation, it was presumed that the mutant Ad with deletion of E1B-55kd gene would be unable to inactivate p53 in normal cells and would thus be ineffective in replicating proficiently. In contrast, cancer cells missing functional p53 would be anticipated to be sensitive to viral replication and cytolysis. Based on this assumption, E1B-55Kd deficient Ads were generated (ONYX 015 or dl1520) that were predicted to replicate in p53 mutant cells, a common feature in many cancer cells, but not in p53 wild type (normal) cells. Accordingly, it was predicted that p53 mutant tumor cells would be eliminated in a replication dependent manner both in vitro and in vivo (Bischoff et al., 1996). However, the p53-dependent specificity became controversial and several studies confirmed that numerous tumor cell lines with normal p53 gene sequences were also somewhat sensitive to the effects of dl1520 (Hall, Dix, O’Carroll, & Braithwaite, 1998; Heise et al., 1997; Turnell, Grand, & Gallimore, 1999). Recent data confirmed that it is the loss of E1B-55Kd mediated late viral RNA export, rather than p53 inactivation that restricts ONYX-015 replication in primary cells (O’Shea et al., 2004). Cancer cells that support replication of ONYX-015 provide the RNA export function of the E1B-55Kd protein. In an alternate approach, exploiting the retinoblastoma protein pathway in normal cells, Fueyo et al. engineered an Ad mutant by removing the Rb-binding CR2 region of E1A (dl922/947) (Fueyo et al., 2000) that selectively permits Ads to propagate in human cancer cells deficient in the Rb pathway. A number of additional modifications in dl922/947 have been created and assessed for cancer-selective activity (Johnson et al., 2002; Kim, Cho, Kim, Jung, & Yun, 2002; Lee et al., 2002).
The second strategy to develop CRAds is to limit the expression of E1A gene product to tumor tissues through the use of tumor- or tissue-specific promoters. Ideally, when a specific promoter controls critical regulators of viral replication, replication would be limited to tissues where this particular promoter is active. E1A, as the key regulator of Ad replication by regulating S-phase entry, offers the natural choice to control virus replication via a specific promoter (Whyte, Ruley, & Harlow, 1988). Other Ad genes, such as E1B, E2, and E4, under a distinct promoter can also be used to develop conditionally replicating viruses (Nettelbeck, 2003). Tissue- or tumor-specific promoters can replace endogenous viral sequences in order to direct viral replication to a particular target tissue. For example, the prostate specific an tigen (PSA) promoter/enhancer element has been inserted upstream of the E1A gene, thereby creating a prostate specific oncolytic virus (Rodriguez et al., 1997) and a Phase I clinical trial by intraprostatic injection following radiotherapy has established safety and viral dose-dependent reduction in PSA levels (DeWeese et al., 2001). However, no objective clinical response in terms of tumor regression was observed. To increase the specificity, a second prostate specific enhancer sequence was subsequently inserted upstream of the E1B gene causing improved selectivity over the first-generation virus (Heise & Kirn, 2000). Human telomerase is very active in more than 85% of primary cancers regardless of their anatomic origin and its activity correlates closely with human telomerase reverse transcriptase (hTERT) expression (Fujiwara, Urata, & Tanaka, 2007). Since the hTERT promoter is preferentially activated in human cancer cells, recombinant viruses were produced in which E1A expression was under regulatory control of the hTERT promoter. These Ads show restricted replication to telomerase positive tumor cells and efficiently lyse the target tumor cells (Fujiwara, Shirakawa, & Kagawa, 2011; Huang, Savontaus, Shinozaki, Sauter, & Woo, 2003; Irving et al., 2004; Kim et al., 2003; Lanson, Friedlander, Schwarzenberger, Kolls, & Wang, 2003; Wirth et al., 2003; Zou et al., 2004). Survivin is often overexpressed in brain tumors and its promoter has been employed to drive E1A to treat malignant gliomas (Van Houdt et al., 2006). β-Catenin-responsive promoters have also been used to drive Ad early genes in cancers with an activated Wnt signaling pathway, such as colorectal and liver cancers (Fuerer & Iggo, 2002). To target malignant melanoma, Nettelbeck et al., developed a novel Ad (Ad. TyrΔ2Δ24) by replacing the Ad E1A promoter with the promoter/enhancer of the melanocyte and melanoma specific tyrosinase gene and by establishing mutations in the E1A gene to prevent the mutant protein from interacting with and inactivating pRb and p300, thus prohibiting viral replication in normal cells (Nettelbeck, Rivera, Balague, Alemany, & Curiel, 2002). Ad.TyrΔ2Δ24 has been further improved by incorporating the tyrosinase promoter to drive the E4 gene (Ad.2Xtyr) and the efficacy of this approach was documented in in vitro organotypic raft cultures (Banerjee et al., 2004). In a similar respect, the cyclooxygenase-2 promoter was used to control the expression of E1A of Ad and the oncolytic activity of such viruses was verified in various tumors, including cervical, ovarian, and pancreatic cancers (Hoffmann & Wildner, 2006). MUC1/DF3 is a transmembrane mucin protein normally expressed on the apical borders of secretory epithelial cells and the expression of this promoter is specific to multiple tumors, including breast, myeloma, and pancreatic cancers (Fukazawa et al., 2010). This promoter has been used to drive lacZ or human somatostatin receptor subtype 2 (hSSTR2) for targeting breast and pancreatic cancers, respectively (Chen et al., 1995). A similar approach has been followed using various cancer selective promoters such as carcinoembryonic antigen (Li et al., 2003), alpha fetoprotein (AFP) (Hallenbeck et al., 1999), and their preclinical applications have been tested and found to be effective in diverse animal models (Li et al., 2003; Rodriguez et al., 1997).
Although oncolytic Ads have been found to be safe in clinical trials, as a single agent they engender very restricted clinical responses and it is only in combination with radiation therapy or chemotherapy that they show noticeable effects. In these contexts, arming these viruses with additional agents offers potential to augment their oncolytic properties. Ad.TKRC, an E1B-55Kd deleted Ad (ONYX-015) in which HSV-Tk (herpes simplex virus thymidine kinase) was connected to E1A by an internal ribosome entry site, showed enhanced growth inhibition of colon cancer xenografts in nude mice in the presence of gancyclovir (Wildner, Blaese, & Morris, 1999). Kanai and colleagues engineered a recombinant Ad expressing the HSV-Tk gene under the human α-fetoprotein enhancer and promoter domain to target hepatocellular cancers (Kanai et al., 1996). In another study, tBid, a truncated BH3-interacting domain death agonist, driven by the AFP promoter was packaged in an Ad and cancer specific antitumoral activity was established both in vitro and in vivo (Miao et al., 2006). The bacterial nitroreductase gene was inserted at the site of the E1B-55Kd deletion to generate CRCA-NTR (PS1217H6), showing increased efficacy in the pres ence of the prodrug CD1954 (Chen et al., 2004). Ad.5-CD/TKrep that delivers a cytosine deaminase/herpes simplex virus-1 thymidine kinase fusion gene in an ONYX-015 backbone efficiently decreased serum PSA in a Phase I clinical trial in prostate cancer patients (Freytag et al., 2002). However, these clinical responses were further augmented when this viral approach was combined with radiotherapy (Freytag et al., 2003). In addition to suicide genes, immunomodulatory genes, such as granulocyte-macrophage colony-stimulating factor and the cofactor B7-1, have been combined in a replicating Ad backbone documenting antitumor activity and conferring long-lasting immunity against a tumor rechallenge in a syngeneic mouse melanoma model (Choi et al., 2006). Oncolytic Ads, in which replication is driven by the hTERT promoter, have been engineered to express interferon-γ (IFN-γ) causing regression of liver cancer xenografts in both immunocompetent and immunodeficient animals (Su et al., 2006). Apoptosis inducing genes have also been incorporated in a replicating Ad backbone such as Ad.HS4. AFP.E1a/TNF related apoptosis inducing ligand (TRAIL), in which an HS4 insulator containing α-fetoprotein promoter driving E1A expression is coupled with TRAIL expression, which displayed enhanced oncolysis of hepatocellular carcinomas (Ye et al., 2005).
Multiple approaches are currently being evaluated to increase stringent cancer specific replication, and various structural permutations and combinations with diverse agents with potent cancer-inhibitory and -destroying actions are being evaluated. To enable cancer-specific replication, we employed a unique cancer specific promoter, effective in all cancers studied so far, to regulate expression of the E1A gene, and to enhance the therapeutic potential of these viruses, we also engineered these Ads to produce apoptosis-inducing and immune-modulating genes and named these viruses “cancer terminator viruses” (CTVs) (Ye et al., 2005). In the remainder of this review, we focus on the recent advances in our comprehension of the mode of action of the CTVs and their potential applications as part of unique and promising cytokine based gene therapy strategy for effectively treating human cancers.
4. CANCER TERMINATOR VIRUSES: EFFICACIOUS REAGENTS FOR CANCER GENE THERAPY
We postulated that inducing tumor cell lysis (through viral replication) combined with induction of apoptosis by means of a secreted cytokine (such as melanoma differentiation-associated gene-7 (mda-7)/interleukin-24 (IL-24) or IFN-γ) would provide a potent tumor- and metastasis-destroying agent when combined into one therapeutic reagent or platform. The CTV is a first-generation single therapeutic type 5 adenovirus reagent employing a cancer-specific promoter derived from the rodent progression-elevated gene-3 promoter (PEG-Prom) that can specifically initiate viral replication specifically in tumor cells, but not in normal cells. To further improve the efficacy of this CTV, we engineered the CRCA to produce a tumor-specific killing molecule, either the novel therapeutic IL-10 family member cytokine gene mda-7/IL 24 or the immune modulating cytokine IFN-γ. Attractively, both the newly generated virus and its encoded therapeutic molecules enter the circulation and “‘seek and destroy” other tumor cells, whether in the local tumor environment or disseminated throughout the body (metastatic), without harming normal healthy cells. In principle, these unique features of the CTV would make it particularly useful for patients whose cancer has stopped responding to other treatments and has metastasized.
4.1. Development of the CTV
4.1.1 mda-7/IL-24: A novel cancer therapeutic cytokine gene
Dedifferentiation is one of the hallmarks of cancer (Sachs, 1987) and induction of differentiation, causing cancer cells to revert back to normal phenotypes, is considered as a potential anticancer therapeutic strategy (Borden, Lotan, Levens, Young, & Waxman, 1993; Fisher, Prignoli, Hermo, Weinstein, & Pestka, 1985; Jiang, Lin, Su, Goldstein, & Fisher, 1995; Jiang et al., 1996; Sachs, 1978, 1987, 1990). When human melanoma cells are treated with a combination of fibroblast interferon (IFN-β) and the protein kinase C activator mezerein terminal differentiation results which is characterized by acquisition of morphological, biochemical, and molecular attributes of normal melanocytes along with an irreversible loss in proliferative capacity and tumorigenic activity (Fisher et al., 1986, 1985; Staudt, Depass, Sarkar, & Fisher, 2009; Staudt & Dittmer, 2007). To detect the molecules potentially regulating terminal differentiation, a subtraction hybridization approach was performed between proliferating and terminally differentiated human melanoma cells resulting in the cloning of a spectrum of genes called melanoma differentiation-associated (mda) genes (Jiang et al., 1995; Jiang, Shah, & Hilt, 1993; Staudt et al., 2009).
mda-7, a novel gene at the time of its initial cloning by Jiang and Fisher in 1993 (Fisher et al., 1986, 1985; Staudt et al., 2009; Staudt & Dittmer, 2007), belongs to the IL-10 family of cytokines and is also designated as IL-24 based on the presence of an IL-10 signature motif, chromosomal localization in a cytokine cluster at 1q32–33, and functional properties (Caudell et al., 2002; Fisher et al., 2003; Sauane et al., 2003). The mda-7/IL-24 cDNA encodes a protein of 206 amino acids with a predicted size of ~24 kDa that contains a 49-amino acid signal peptide. The protein has three glycosylation sites at amino acids 95, 109, and 126, and the secreted product is heavily glycosylated showing multiple higher molecular weight bands in Western blots (Emdad et al., 2009). The observation that MDA-7/IL-24 protein is expressed in normal human melanocytes as well as in cells of hematopoietic lineages, while its expression is progressively lost during melanoma progression, suggests a potential tumor suppressor function of mda-7/IL-24 (Huang et al., 2001). Indeed, a series of studies, employing a replication incompetent adenovirus expressing mda-7/IL-24 (Ad.mda-7) or recombinant MDA 7/IL-24 protein, confirmed that mda-7/IL-24 induces apoptosis in a diverse group of tumors including melanoma, glioblastoma, leukemia, and carcinomas of breast, kidney, cervix, colorectum, liver, lung, ovary, and prostate without causing any detrimental effects to normal cells, including melanocytes, astrocytes, fibroblasts, and mesothelial and epithelial cells (Chada et al., 2004; Dash, Bhutia, et al., 2010; Emdad et al., 2009; Fisher, 2005; Fisher et al., 2003; Gopalkrishnan, Sauane, & Fisher, 2004; Gupta et al., 2006; Inoue et al., 2006; Lebedeva et al., 2007; Lebedeva, Su, et al., 2003; Liang et al., 2011; Sauane et al., 2003). In addition to antitumor activity, Yang et al. recently demonstrated that MDA-7/IL-24 is also involved in regulation of human myeloid leukemic cell differentiation (Yang et al., 2011). The molecular mechanism underlying cancer specific apoptosis induction by mda-7/IL-24 involves accumulation of MDA-7/IL-24 protein in the endoplasmic reticulum (ER) where it interacts with the chaperone protein BiP/GRP78 resulting in the activation of the ER stress or unfolded protein response (Sauane et al., 2004). Additionally, treatment with Ad.mda-7 also results in generation of ceramides and reactive oxygen species in mitochondria and these events culminate into activation of p38 MAP kinase (MAPK) pathway and induction of growth arrest and DNA damage (GADD)-inducible genes leading to a predominantly intrinsic pathway of apoptosis induction (Sarkar et al., 2002; Su et al., 2003). Recently, in prostate cancer MDA-7/IL-24 was identified to interact with clusterin and modulate the relative levels of soluble and nuclear isoforms promoting growth arrest at the G2/M phase and apoptosis (Bhutia et al., 2011). Depending upon the cell type, mda-7/IL-24 can either induce toxic autophagy (Park et al., 2008) or initial protective autophagy (Bhutia et al., 2010) that switches to apoptosis (reviewed by Dash, Bhutia, et al., 2010; Emdad et al., 2009). In addition to cancer specific apoptosis induction, mda-7/IL-24 embodies several attributes that make it an ideal gene therapeutic for cancer. Secreted MDA-7/IL-24 protein binds to its cognate IL-20R1/IL-20R2 or IL-20R1/IL 22R1 receptors on the cell surface and induces expression of endogenous mda-7/ IL-24 by stabilizing mda-7/IL-24 mRNA (Sauane et al., 2008). This phenomenon is observed both in normal and in cancer cells, and since normal cells are resistant to the toxic effects of MDA-7/IL-24, they might serve as a continuous source of MDA-7/IL-24, induced by exogenous MDA-7/IL-24, to kill neighboring and distant cancer cells therefore significantly magnifying the anticancer effect. Additionally, MDA-7/IL-24 inhibits tumor angiogenesis, synergizes with radiation, chemotherapy, and monoclonal antibody therapies and stimulates an antitumor immune response and together these events mount a potent “bystander” response so that MDA-7/IL-24 can eradicate both primary tumors to which it is delivered as well as distant tumors resembling metastases (Chada et al., 2006; Cunningham et al., 2005; Fisher, 2005; Gupta et al., 2006; Ramesh et al., 2003; Sarkar et al., 2007; Sauane et al., 2008; Su, Emdad, Sarkar, et al., 2005; Tong et al., 2005).
The findings gleaned from in vitro studies and mouse models have been confirmed in patients with advanced cancers (both carcinomas and melanomas) in a Phase I clinical trial using a replication incompetent serotype 5 adenovirus expressing MDA-7/IL-24, Ad.mda-7 (INGN 241) (Cunningham et al., 2005; Eager, Harle, & Nemunaitis, 2008; Fisher et al., 2003; Lebedeva et al., 2007; Tong et al., 2005). This study documented that MDA-7/IL-24 was well tolerated and demonstrated evidence of significant (~44%) clinical activity. Similar to the preclinical studies, potent “bystander” antitumor activity was also apparent in this clinical study. Infection of a small percentage (10–30%) of tumor cells with Ad.mda-7 resulted in detectable MDA-7/IL-24 protein levels and increased tumor cell apoptosis many centimeters from the site of original injection. A majority of the patients showed a marked increase in CD3+ and CD8+ T cells as well as transient increases in circulating cytokines, such as IL-6 and TNF-α, indicating activation of an antitumor immune response (Tong et al., 2005). These provocative results provide direct support for using mda-7/IL-24 in developing a potentially effective gene-based therapy for cancer.
4.1.2 Progression-elevated gene-3 promoter, a unique cancer-specific gene promoter for controlling therapeutic gene expression
While mda-7/IL-24 is a tumor suppressor, progression-elevated gene-3 (PEG-3) is an oncogene that was cloned by subtraction hybridization in our laboratory employing E11, a mutant adenovirus type 5 (H5ts125)-transformed rat embryo cell clone that forms small, slow-growing, compact tumors, and E11-NMT, a clone of E11 passaged through nude mice (Su, Gopalkrishnan, Narayan, Dent, & Fisher, 2002) that forms rapidly growing, highly aggressive tumors (Babiss, Zimmer, & Fisher, 1985; Fisher, Bozzone, & Weinstein, 1979; Su, Shi, & Fisher, 1997). Rodent PEG-3 is a C-terminal truncated mutant form of the growth arrest and DNA damage inducible gene-34 (GADD-34), a frequent event in rodent tumorigenesis, and functions as a dominant negative inhibitor of the apoptosis inducing function of GADD-34 (Hollander, Poola Kella, & Fornace, 2003; Su, Emdad, Sauane, et al., 2005; Su et al., 1997). PEG-3 is overexpressed in E11 NMT cells versus E11 cells and also in normal cloned rat embryo fibroblasts (CREF) displaying a transformed/tumorigenic phenotype upon expression of diverse oncogenes, including Ha-ras, v-src, human papilloma virus type-18-transforming genes, and a specific mutant of adenovirus 5 (H5hr1), relative to parental CREF cells (Su et al., 1997). Overexpression of PEG-3, by a replication-incompetent adenovirus (Ad.PEG-3), induces genomic instability which is associated with modulation of expression of genes regulating centrosomal duplication (Emdad et al., 2005), and an aggressive phenotype characterized by increased invasion, angiogenesis, and metastasis and associated with elevated metalloproteinases, MMP2 and MMP9, and vascular endothelial growth factor, in human tumor cells, but not in normal cells (Su, Shi, & Fisher, 2000; Su et al., 2001). In association with the oncogenic properties of PEG-3, the promoter region of the PEG-3 gene (PEG-Prom) showed 8- to 10-fold more activity in CREF transformed with either Ha-ras or v-raf than the parental CREF cells, and a minimum region of the PEG-Prom that extends from −118 to + 194 (when the transcription initiation site is regarded as + 1) was shown to be sufficient for the increased activity associated with transformation and cancer progression (Su et al., 2000, 2001). This region contains a binding site for polyoma enhancer activator-3 (PEA-3) at −104 and for activator protein-1 (AP-1) at + 8, and sequence-specific mutational analysis revealed that both of these transcription factors are important for regulating the basal and oncogene-induced activity of the PEG-Prom (Su et al., 2000, 2001). Transgenes, such as luciferase, green fluorescence protein (GFP), or herpes thymidine kinase-1 (HSV Tk), driven under PEG-Prom, are expressed uniquely in cancer cells and not in normal cells confirming the cancer specific activity of the PEG-Prom (Su, Emdad, Sauane, et al., 2005). A recent study using metastatic breast carcinoma or melanoma models has demonstrated that, upon intravenous administration, PEG-Prom facilitates transgene expression in all the cancer cells dispersed throughout the mouse body with no expression detected in normal cells, thus facilitating detection of tumor metastasis by in vivo imaging (Bhang, Gabrielson, Laterra, Fisher, & Pomper, 2011).
Based on the above considerations, exploiting the cancer specific expression property of the PEG-Prom, CTVs were generated in which viral replication is controlled by a minimal active region of this promoter. As indicated above, to enhance the therapeutic potential of these CTVs, we further modified/engineered these Ads to produce either mda-7/IL-24 (Ad.PEG-E1A-mda-7 or Ad.CTV-m7) or IFN-γ (Ad.PEG-E1A-IFN-γ or Ad.CTV-γ) under the control of a cytomegalovirus (CMV) promoter.
4.1.3 CTV construction
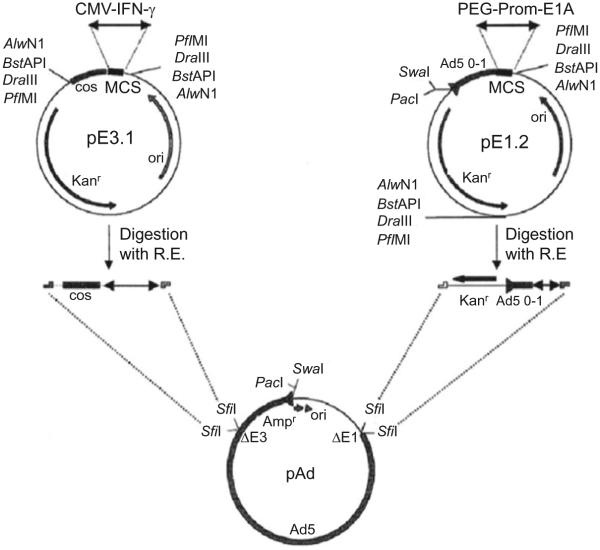
To generate CTVs, two shuttle vectors, pE1.2 and pE3.1, were used (Sarkar, Su, Vozhilla, Park, Randolph, et al., 2005) with the PEG-Prom driving the E1A gene (PEG-Prom E1A) and the CMV promoter controlling the therapeutic gene (mda-7/IL-24 or IFN-γ) were inserted into the multiple cloning sites of the vectors pE1.2 and pE3.1, respectively (Fig. 1.2). These transgene cassettes were inserted into the Ad backbone in a four-fragment ligation exploiting the presence of unique restriction enzyme sites in the shuttle vectors and in the Ad plasmid. The ligation product was transformed into Escherichia coli and clones were selected for resistance to ampicillin (ampicillin-resistance gene provided by adenoviral plasmid) and kanamycin (kanamycin-resistance gene provided by the fragment from the shuttle vector). The cosmid DNA was amplified by standard large-scale preparation using a CsCl gradient, digested with PacI restriction enzyme, and transfected into human embryonic kidney-293 cells for in vivo recombination. The Ad was purified and viral titer was determined by measuring absorbance at 260 nm and using BD AdenoX rapid titer kit (BD Biosciences).
Figure 1.2.
Constructing a CTV using recombinant DNA techniques. The cancer-specific PEG-Prom-driven E1A gene (PEG-Prom-E1A) and the CMV promoter-driven mda-7/IL-24 or IFN-γ gene (CMV-mda-7 or CMV-IFN-γ) were cloned into the multiple cloning site of the shuttle vectors pE1.2 and pE3.1, respectively. The cassettes containing the promoter and the transgene were further digested by different restriction enzymes as shown in the figure and ligated into SfiI-digested adenoviral vector. Taken from Sarkar, Su, Vozhilla, Park, Randolph, et al. (2005).
4.2. Preclinical studies with CTVs
The relative therapeutic efficiency of the CTVs, both Ad.PEG-E1A-mda-7 (Ad.CTV-m7) and/or Ad.PEG-E1A-IFN-γ (Ad.CTV-γ), was evaluated in various cancer models (Sarkar et al., 2006, 2008, 2005; Sarkar, Su, Vozhilla, Park, Randolph, et al., 2005). As a control Ad.CMV-E1A-mda-7 or Ad.CMV-E1A-IFN-γ, in which viral replication is controlled by the CMV promoter and which also expresses mda-7/IL-24 or IFN-γ, respectively; Ad.CMV-E1A and Ad.PEG-E1A, in which viral replication is controlled by the CMV promoter or the PEG-Prom, respectively; and Ad.CMV-mda-7 and Ad.PEG-mda-7 or Ad.CMV-IFN-γ and Ad.PEG-IFN-γ, replication-incompetent Ad in which the CMV or the PEG-Prom drives mda-7/IL-24 or IFN-γ expression, respectively, were used. In this particular section, we focus on the results obtained from these preclinical studies.
4.2.1 Ad.CTV-m7 cures primary and distant cancers
Breast cancer is a common female cancer accounting for 32% of all cancers in women (Jemal, Siegel, Xu, & Ward, 2010) and to compare the therapeutic efficacy of Ad.CTV-m7, experiments were performed in both immortal mammary epithelial cells and aggressive breast cancer cell lines (Sarkar, Su, Vozhilla, Park, Gupta, et al., 2005). In in vitro experiments, infection with Ad.CTV-m7-induced viral replication and generated significant MDA-7/IL-24 production only in cancer cell lines, but not in normal immortal mammary epithelial cells, firmly establishing the cancer cell-selective replication of Ad and mda-7/IL-24 expression. All the Ads, except for Ad.vec, resulted in significant apoptosis in the breast cancer cell lines. Infection with the replication-competent Ads resulted in predominantly necrosis as evidenced by an increase in late apoptotic cells, whereas infection with Ad.CMV-mda-7 and Ad.PEG-mda-7 resulted in predominantly apoptosis as demonstrated by an increase in early apoptotic cells. Substantiating the in vitro observations, in vivo assays also established that Ad.CTV-m7 is more efficacious in eradicating not only the primary tumor but also the distant tumors. Experimentally, T47D tumor xenografts were established in both flanks of nude mice and once palpable tumors of ~75 mm3 developed, Ad.CTV-m7 or other Ad were injected intratumorally only in the left flank tumor. No injections were given to the right sided tumors. Although Ad.CMV-E1A or Ad.PEG-E1A inhibited the growth of injected tumors on the left flank, they had little inhibitory effect on tumors on the right side, which was not statistically significant. Ad.CMV-mda-7 or Ad.PEG-mda-7 eradicated tumors on the left flanks and significantly inhibited tumor growth on the right flanks. Interestingly, injection of Ad.CTV-m7 resulted in complete eradication of not only the injected left-sided tumors but also the uninjected right-sided tumors (comparable with a metastasis), providing confidence that this strategy could prove amenable for successfully treating aggressive cancers.
Successful application of this dual cancer-specific targeting strategy in breast cancer provided an impetus for testing the CTVs in other human neo-plasms to evaluate potential for eradicating additional primary tumors and metastatic disease. Prostate cancer is one the most important health problems in industrialized countries (Di Lorenzo & De Placido, 2006; Sternberg, 2002) and is refractory to conventional anticancer treatments because of frequent overexpression of antiapoptotic proteins, including Bcl-2 and/or Bcl-xL (Colombel et al., 1993; Krajewska et al., 1996; Krajewski et al., 1994). Although parental prostate cancer cells are highly susceptible to Ad.mda-7-induced apoptosis, stable overexpression of Bcl-2 and Bcl-xL renders prostate cancer cells resistant to the apoptotic effects of Ad.mda-7 (Lebedeva, Sarkar, et al., 2003). To explore the efficacy of the CRCA based cancer gene therapy approach for eradicating resistant prostate cancer cells, Ad.CTV-m7 was injected into xenografts derived from DU-145-Bcl-xL cells in athymic nude mice. Similar to the breast cancer study, tumors were established in both flanks of the mice and therapeutic Ads were injected only into the left flank tumors. Ad.CTV-m7-mediated tumor regression was compared with other Ads, including Ad.CMV-E1A-mda-7, Ad.CMV-E1A, or Ad.PEG-E1A, over a period of 6 weeks. This set of experiments revealed that intratumoral injection of Ad.CTV-m7 could completely eradicate primary and distant tumors (comparable to a metastasis), whereas Ad.CMV-mda-7 or Ad.PEG-mda-7 only displayed marginal effects on the growth of both left and right sided tumors. These results correlate with the resistance profile of DU-145-Bcl-xL cells to mda-7/IL-24.
The efficacy of Ad.CTV-m7 was also determined in melanoma (Sarkar et al., 2008), an important public health issue because of rising prevalence in Caucasian populations (Jemal et al., 2010), using a series of melanoma cells including normal immortal human melanocytes. The functionality of all Ads, both replicating and nonreplicating, in cancer/noncancer cells was confirmed by the expression of both E1A and MDA-7/IL-24 protein. In vitro cell viability and apoptosis assays established the cancer-specific activity of mda-7/IL-24. In vivo assays were performed using nude mice containing established human melanoma subcutaneous xenografts on both right and left flanks of the animal. After palpable tumors developed, different Ads were administered to the tumors on the left flank only. No injections were given to the right sided tumors. The experiment was terminated after 8 weeks since control- and Ad.vec-injected animals reached maximum allowable tumor volumes. Similar to both breast and prostate cancer, injecting Ad. CTV-m7 completely eliminated not only primary treated tumors but also distant nontreated tumors (established in the opposite flank), thereby implementing a cure in these immune incompetent animals.
Cancer is a progressive disorder involving multiple genetic abnormalities. As such, combinatorial approaches are mandatory to effectively eradicate this disease and combining cancer-specific Ad replication with the multipronged antitumor effects of mda-7/IL-24 represents an appealing strategy (Sarkar et al., 2008). This assumption is supported by the observations that, while Ad.mda-7 could effectively suppress growth of noninjected distant xenografts over time, these tumors started to regrow, and only Ad.CTV-m7 could completely eliminate the distant tumors eliciting a “cure.” However, the precise mechanism(s) underlying these observations are yet to be defined. Hypothetically, the Ad.CTV-m7 induced “cure” in animals might be the summation of the direct apoptosis-inducing properties of MDA-7/IL-24, the indirect antitumor actions of MDA-7/IL-24 (inhibiting angiogenesis and stimulating an antitumor immune response), and in situ Ad replication (directly promoting cytolysis) in tumors.
4.2.2 Eradication of therapy-resistant pancreatic cancer by Ad.PEG-E1A-IFN-γ (Ad.CTV-g)
Based on difficulty in early diagnosis of pancreatic ductal adenocarcinoma (PDAC), this disease is often noticeable after metastasis has occurred and this is one of the reasons it has the shortest average survival time in contrast to all other cancer types (Bardeesy & DePinho, 2002; Jaffee, Hruban, Canto, & Kern, 2002; Russo, Butler, Ove, & Blackstock, 2007). Surgical resection remains the only viable choice for achieving a cure, but only 15–20% of patients, newly diagnosed with pancreatic cancer, are candidates for surgical resection and, regrettably, the majority of resections result in recurrent disease (Donahue & Reber, 2010). Although intensively examined, there are no effective or even palliative therapies for PDAC, and thus developing effective therapeutics remains a top priority. The cancer-specific activity and therapeutic effectiveness of the Ad.CTV-γ were evaluated in four pancreatic cancer cell lines, MIA Paca-2, PANC-1, AsPC-1, and BxPC-3, and two normal immortal cell lines, FM-516-SV, normal human melanocytes immortalized by SV40 TAg, and IM-PHFA, primary human fetal astrocytes immortalized by the catalytic subunit of human telomerase (Sarkar, Su, Vozhilla, Park, Randolph, et al., 2005). Monitoring IFN-γ production by ELISA and E1A protein levels by Western blotting after Ad infection confirmed the functionality of these constructs. This study also established that PEG-Prom allows restricted Ad replication in cancer cells, protecting normal cells from growth inhibition because of Ad replication. Mechanistically, Ad.CTV-γ induced predominantly necrosis as evidenced by an increase in late apoptotic cells, whereas infection with Ad.CMV-IFN-γ and Ad.PEG-IFN-γ resulted predominantly in apoptosis as evidenced by an increase in early apoptotic cells. To expand on these in vitro studies, in vivo assays were done using nude mice containing established AsPC 1 subcutaneous xenografts on both their right and left flanks. Intratumoral injections with Ad.CTV-γ on the left flank tumors produced marked regression within three injections and completely eradicated tumors on both flanks following seven injections, whereas Ad.CMV-E1A or Ad.PEG-E1A inhibited the growth of tumors on the left flank, but had little inhibitory effect on the tumors on the right side, which was not statistically significant. Ad.CMV-IFN-γ or Ad.PEG-IFN-γ appreciably inhibited tumor growth on both flanks, emphasizing the importance of immune stimulation, but these treatment regimens did not completely destroy the tumors. However, intratumoral injection of Ad.CTV-γ completely eradicated the primary tumor and dramatically inhibited or eliminated (four out of five animals) the distant tumor (comparable with a metastasis) providing confidence that this strategy may prove amenable for successfully treating aggressive cancers. Further experiments confirmed that Ad.CTV-γ enters the circulation and replicates in the tumors of the right side and/or the secreted IFN-γ induces an antitumor immune response by stimulating natural killer cells that are present in the nude mice. These findings indicate that the replication-competent Ad have the ability to migrate and replicate at distant sites in the animals. In this context, the ability of the PEG-Prom in Ad.CTV-γ to drive replication is extremely important for ensuring Ad replication in cancer cells, while sparing harmful effects in normal cells.
In addition to the significant direct and indirect antitumor effects of therapeutic genes, this particular strategy has an additional advantage over other combinatorial approaches because of the employment of the PEG-Prom. Most current strategies ensuring cancer selectivity of CRCAs exploit genetic abnormalities in cancer cells such as their p53, RB, or PKR status (Cascallo, Capella, Mazo, & Alemany, 2003; Heise et al., 2000; Johnson et al., 2002). This considerably limits the general application of such strategies in diverse cancer indications, which may lack these alterations, and clinical application of these CRCAs alone often fails to generate any discernable clinical response. In contrast, the minimal region of the PEG-Prom, which was used to construct the CTVs, is controlled by two transcription factors, AP-1 and PEA-3 (Su, Sarkar, Emdad, et al., 2005); the presence of either of these sites is sufficient to ensure cancer selectivity of the PEG-Prom. Since either AP-1 or PEA-3 is overexpressed in a high percentage of cancers, the universal utility of the PEG-Prom to assure cancer selectivity and, therefore, limit nonspecific cytotoxicity is comparable only to that of the promoter of the human telomerase (hTERT) gene (Huang et al., 2003).
Recently, a number of scientific reports have described oncolytic Ads armed with the mda-7/IL-24 gene, for example, SG600-IL24, an oncolytic Ad harboring mda-7/IL-24 selectively induces apoptosis in different hepatocarcinoma cell lines without affecting a normal liver cell line (Xue et al., 2010). In another approach, an E1B-55Kd deleted oncolytic Ad car rying mda-7/IL-24 was developed (ZD-55-IL-24) that exhibited better therapeutic efficacy compared with ONXY-015 against hepatic cancer (Xiao et al., 2010). This Ad was more potent in combination with dichloroacetate (Xiao et al., 2010) or cisplatin (Wu et al., 2009). To target liver, Cao et al. developed an α-fetoprotein controlled oncolytic Ad carry ing mda-7/IL-24 and demonstrated improved antitumor effects on hepatic cancer (Cao et al., 2011).
5. APPROACHES ENHANCING THERAPEUTIC OUTCOMES
Compared to currently available vector approaches, Ad vectors possess the greatest capacity to achieve in vivo infection of tumors (Mathis, Stewart, Zhu, & Curiel, 2006). Despite this capacity, broad clinical efficacy of Ad based treatment regimens remains imperfect because of suboptimal infectivity. The discrepancy between preclinical and clinical studies using type Ad.5 systems can frequently be attributed to differences in the expres sion of the primary Ad.5 receptor, the Coxsackie and adenovirus receptor (CAR), in primary tumors relative to their cell line counterparts (Kim, Zinn et al., 2002). In addition, other hurdles limiting successful cancer gene/protein therapy include appropriate methods for effectively delivering anti-cancer therapeutics intravenously without nonspecific trapping in the liver, clearance/neutralization by the immune system, or eliciting immune responses. In this section, we focus on several approaches that can significantly improve therapeutic outcomes.
5.1. Improvement of adenoviral vectors to enhance transduction
One approach to circumvent the low efficiency of Ad infection of tumor cells exploits “tropism modification” in which the virus capsid proteins that normally interact with CAR are modified, permitting CAR-independent infectivity of tumor cells. Understanding structural homology between Ad.5 and other Ad serotypes and the cellular entry pathways for Ads has facilitated the development of chimeric vectors in which whole fibers or even the distal knob domains from various Ad serotypes, whose native tropism is associated with receptors other than CAR, are engineered into Ad.5 (Fig. 1.3). In this respect, based on differences in receptor use (Krasnykh, Mikheeva, Douglas, & Curiel, 1996), replacement of the Ad.5 fiber knob domains with the Ad serotype 3 (Ad.3) fibers resulted in enhanced cytopathogenicity of an Ad virotherapy agent toward primary melanoma cells, which was at least four orders of magnitude greater than wild-type Ad.5 (Rivera et al., 2004; Volk et al., 2003). This transductional enhancement was similar for melanoma, ovarian cancer, renal cancer, squamous carcinoma, prostate, and colorectal cancer (Haviv et al., 2002; Kanerva et al., 2002; Kawakami et al., 2003; Rein et al., 2005). Intriguingly, shortening of the Ad.5 fiber shaft in an Ad.5/3 chimera also significantly reduced liver tropism and facilitated enhanced replicative ability compared with its Ad.5 counterpart (Yamamoto & Curiel, 2010). Importantly, Ad.5/3 also infects high-CAR-expressing tumor cells with equal efficacy or even improved activity when compared with Ad.5, thereby providing an expanded scope of utility for Ad.5/3, in both low- and high-CAR-expressing tumor cells. To enhance delivery of mda-7/IL-24, we have developed a tropism modified chimeric adenovirus expressing mda-7/IL-24 (Ad.5/3-mda-7) and explored its efficacy in the context of prostate cancer (Dash, Dmitriev, et al., 2010). Experiments were performed to directly compare the infectivity of Ad.5/3 chimeric viruses (Ad.5/3-mda-7) versus Ad.5 (Ad.5-mda-7) viruses in the context of prostate cancer using two cell lines with variable levels of CAR on their surface. Infection of PC-3 (low CAR) cells with Ad.5-mda-7 was significantly less effective in reducing cell proliferation and viability than Ad.5/3-mda-7. This differential effect correlated with a reduced level of MDA-7/IL-24 protein being produced in PC-3 cells infected with Ad.5-mda-7 versus Ad.5/3-mda-7. At the molecular level, compared to Ad.5-mda-7, Ad.5/ 3-mda-7 efficiently induced apoptosis as evidenced by increased cleavage of PARP, downregulation of antiapoptotic proteins Bcl-2 and Bcl-xL, as well as activation of caspase 3/7. Interestingly, Ad.5/3-mda-7, but not Ad.5-mda-7 efficiently activated the p38 MAPK pathway, known to have a crucial role in mediating mda-7/IL-24 effects, and inhibited the prosurvival ERK pathway suggesting that chimerism maintains the bona fide downstream effects exerted by mda-7/IL-24. Correlating with the in vitro data, Ad.5/3-mda-7 also showed a profound enhanced antitumor activity (Dash, Dmitriev, et al., 2010) as compared to Ad.5-mda-7 in PC-3 xenograft models using nude mice. These findings provide definitive evidence for enhanced therapeutic efficacy of the Ad.5/3-mda-7 virus versus Ad.5-mda-7 in prostate cancer cells with reduced CAR.
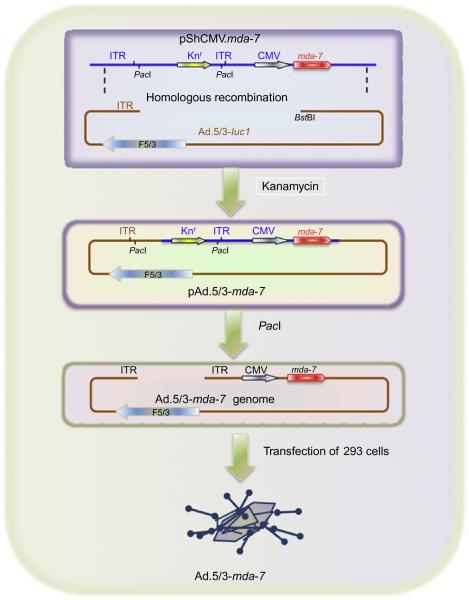
Figure 1.3.
Constructing a tropism-modified adenovirus (Ad.5/3) carrying the mda-7/IL-24 gene. The genome of Ad.5/3.mda-7 was generated by homologous recombination between the linearized plasmid pShCMV.mda-7 and 8st81-digested genomic DNA of Ad.5/3-Lucl, and kanamycin selection resulted in the pAd.5/3-mda-7 genome, where the CMV promoter in place of the early viral El region drives mda-7/IL-24 expression. This plasmid was digested with Pad to release viral ITRs and transfected in A549 cells to rescue the Ad.5/3-mda-7. Taken from Dash, Dmitriev, et al. (2010).
Dash, Dmitriev, et al. (2010) recently demonstrated that Ad.5/3-mda-7 in combination with BI-97C1 (Sabutoclax), which targets Mcl-1, sensitizes prostate cancer cells to mda-7/IL-24 mediated cytotoxicity, thereby enhancing therapeutic efficacy. Evidence was provided using two animal models, one employing immune deficient athymic nude mice xenografted with human prostate tumor cells and one using a spontaneous immuno-competent transgenic mouse model of prostate cancer (Hi-Myc mouse) (Ellwood-Yen et al., 2003). Nude mice bearing subcutaneous human prostate tumors established on both flanks were treated with the combination of mda-7/IL-24 (Ad.5/3-mda-7) and BI-97C1. Tumor growth was markedly inhibited by the combination treatment compared to treatment with either agent alone. This observation was further validated in Hi-Myc mice (Dash et al., 2011). To avoid sequestering of the virus in the liver and clearance of the virus by the immune system by Hi-Myc mice, complement-treated microbubbles (will be discussed in the next section) encapsulated Ad.5/3-mda-7 was delivered intravenously followed by sonoporation in the prostatic area. BI-97C1 was administered intraperitoneally. Decreased Ki-67 and increased TUNEL expression accompanied with tumor growth inhibition (based on size of the prostate gland) was observed in sections of the prostates of Hi-Myc mice treated with Ad.5/3-mda-7 and BI-97C1 (Sabutoclax) as compared to each single agent alone, signifying a sensitiza tion role of BI-97C1 (Sabutoclax). Further results provide definitive evidence for enhanced therapeutic efficacy of Ad.5/3-mda-7 versus Ad.5-mda-7 in ovarian carcinoma, malignant glioma, renal carcinoma, and colorectal carcinoma with reduced CAR (Azab et al., 2011; Eulitt et al., 2011; Hamed et al., 2010). Using serotype chimerism, a novel CTV (Ad.5/3-CTV) has now been created in collaboration with Drs. I.P. Dmitriev and D.T. Curiel (WUSM, St. Louis, MO) by replacing the Ad.5 fiber knob with the Ad.3 fiber knob resulting in enhanced infection of tumor cells in a CAR-independent manner and their therapeutic efficacy against cancer from various anatomic origins is currently under investigation (unpublished data).
5.2. Ultrasound-targeted microbubble destruction: A novel, safe, and efficient approach for delivery of therapeutic agents
A major challenge for effective cancer therapy is the ability to specifically deliver therapeutics directly into diseased tissue. Ultrasound (US) contrast agents (microbubbles) have recently been underscored as a potential candidate for improving delivery of molecules to target tissues (Howard et al., 2006; Larina et al., 2005; Lawrie et al., 2000; Ng & Liu, 2002). It contains high molecular weight gasses with less solubility and diffusivity, which increases microbubble endurance and allows passage through the microcirculation. Microbubbles can be injected in peripheral veins and can survive for several minutes within the bloodstream (Goldberg, Liu, & Forsberg, 1994; Howard et al., 2006). The ideal microbubble diameters are most likely between 2.5 and 4 mm. This is small enough to avert entrapment within the pulmonary capillary bed (ranging from 5 to 8 μm in diameter) but large enough to entrap and shield viral vectors such as Ads from the surrounding milieu. The gas-filled microspheres effectively lower the energy threshold for nonthermal cavitation. Ultrasound-targeted microbubble destruction (UTMD) enables focal release of entrapped materials as well as the creation of small shock waves that intensify cellular permeability (Pitt, Husseini, & Staples, 2004). In addition, the microbubbles safeguard the agents from rapid degradation by the immune system, thus allowing for intravenous injection rather than direct target organ delivery by catheter-based tactics or operative bed injections (Howard et al., 2006). This is especially important in cancer therapy of potentially inaccessible tumors because the microbubbles may also limit the amount of inflammatory response to the agents and should allow repeated injections. Howard et al. (2006) for the first time evaluated the feasibility of microbubbles for site-specific gene delivery in both in vitro and in vivo systems. The contrast agents were tested for their ability to enclose and to protect an Ad vector carrying the GFP marker gene (Ad. GFP) into the microbubbles. Systemic delivery of Ad.GFP enclosed in microbubbles resulted in specific targeting of the GFP transgene. Both fluorescence microscopy and GFP immunohistochemistry demonstrated US-guided specific transduction in the targeted tissue only, with no uptake in heart, lungs, or liver using complement pretreated Ad.GFP micro bubbles.
To confirm the ability of the US contrast agent to deliver viruses efficiently and specifically to defined sites in vivo, prostate tumor xenografts were established in both flanks of athymic nude mice by injecting each site with DU-145, aggressive human prostate carcinoma cells. The tumor-bearing nude mice were then injected in their tail vein with contrast agent that was reconstituted with Ad.GFP or water as control, and a portable SonoSite Micro-Maxx US platform (SonoSite, Inc., Bothell, WA) equipped with a L25 linear array transducer set at 0.7 Mechanical Index, 1.8 MPa for 10 min, was used to sonoporate only the tumor implanted on the right side. Immunoblotting of total protein extracts from different organs such as kidney, liver, lung, heart, and tumors from both right and left flanks showed expression of GFP only in the left tumors, the tumors that were exposed to US (Greco et al., 2010), confirming microbubble mediated specific delivery. This study further explored the systemic delivery of Ad.CTV-m7 by microbubbles. Experimentally, to confirm the ability of microbubbles to deliver viruses efficiently and specifically, tumor xenografts were established in both flanks of athymic nude mice by injecting each site with DU-145 human prostate carcinoma cells overexpressing antiapoptotic proteins, Bcl-2 or Bcl-xL, DU-Bcl-xL. The tumor-bearing nude mice were then injected via tail vein with 100 µL of Targestar-P contrast agent that was reconstituted with Ad.CTV-m7 treated with complement and only the tumor implanted on the left side was sonoporated (Greco et al., 2010). The US-guided delivery completely eradicated not only targeted therapy-resistant tumors, but also nontargeted distant tumors (established in the opposite flank) because of the “bystander” antitumor effect, thereby implementing a “cure” in athymic nude mice (Greco et al., 2010). Very recently, we also confirmed the utility of the UTMD approach in a spontaneous model of prostate can cer, the l-li Myc mouse (Dash et al., 2011; Greco et al., 2010).
6. CONCLUSIONS AND FUTURE DIRECTIONS
Despite meaningful advances in early detection and treatment of localized cancers, no single or combinatorial therapeutic strategy has proven effective in lessening morbidity or establishing a cure of metastatic disease. Notably, it is projected that 90% of cancer associated mortality may be due to disseminated tumor growth that impairs vital organ(s) function. There are multiple challenges and barriers to effective therapy of metastatic cancers. One is to be able to accurately locate metastases. A second impediment to effective therapy is to deliver the therapeutic to the metastases. A third limitation is the need for a therapeutic gene product or reagent that can circulate through the body to find metastases. The fourth limitation is to provide a means of protecting the therapeutic from destruction by the immune system and nonspecific trapping in the liver or other organ sites not harboring metastases. Although we are still not close to achieving the objective of pro ducing the perfect cancer gene therapeutic, the CTV approach is appealing because it provides (i) cancer-specific replication based on expression of the PEG-Prom either in primary or distant sites and (ii) selective local and systemic killing of cancer cells by means of the cancer-specific apoptosis-inducing cytokine mda-7/IL-24 or IFN-γ (Dash, Dmitriev, et al., 2010). The UTMD approach provides a means of delivering the CTV in the circulation in a protected “stealth” form with targeted delivery to sites of tumors and metastases (Dash, Dmitriev, et al., 2010). Additionally, engineering modifications in Ad tropism to enhance infectivity provides a way of increasing the efficacy of CTV delivery into tumor cells with release of mda-7/IL-24 or IFN-γ into the tumor vasculature and the circulation (Fig. 1.4). In addition, detection and therapy of metastases have proven dif ficult and inefficient for many reasons, which emphasize the inadequacies of conventional diagnostic and therapeutic approaches. Very recently, it was confirmed that systemic delivery of the PEG-Prom linked to and regulating an imaging construct would enable tumor specific expression of reporter genes, not only within a primary tumor but also in associated metastases in a manner broadly applicable to tumors of different tissue origin or subtype (Bhang et al., 2011). This approach holds potential for locating the enemy throughout the body. It is anticipated that, with the summation of these approaches, we will be one step closer to evoking a complete eradication of both primary and distant metastatic tumors, with minimal toxicity to normal organs, leading to a potential “cure” in cancer patients.
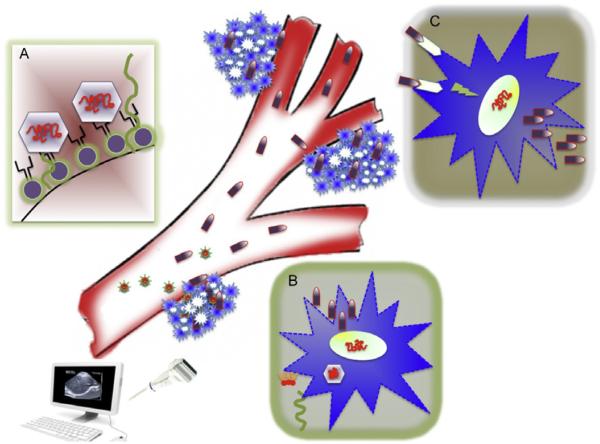
Figure 1.4.
Delivery of Cancer Terminator Viruses (CTVs) systemically following complexing with microbubbles (MB) coupled with ultrasound-targeted MB destruction, the UTMD approach. Complexes of CTVs with MBs are delivered intravenously (Box A, Ad incorporated in the lipid shell of MBs), which are released at the primary tumor site by the application of ultrasound (Box B, sonoporation of MBs in the tumor with an ultrasound probe). After intracellular entry, the CTVs replicate selectively in tumor cells, resulting in robust production of mda-7/IL-24 that when translated into MDA-7/IL-24 protein cause ER stress and “unfolded protein stress response” and cancer cell death. MDA-7/IL-24 is subsequently released into the circulatory system and due to virtue of its “bystander activity” (Box C, binding of MDA-7/IL-24 with IL-20R1/IL-20R2 or IL-20R1/IL-22R1 and promoting intracellular signaling leading to autocrine production of MDA-7/IL-24), which would be anticipated to induce tumor-specific apoptosis of primary and distant tumors, antiangiogenic effects in primary and distant tumor vasculatures, and immune modulatory effects targeting tumors for immune destruction. It is believed to be the “summation” of these multifaceted antitumor properties of MDA-7/IL-24 that promotes selective destruction of both primary and distant tumors.
ACKNOWLEDGMENTS
The present studies were supported in part by NIH grants R01 CA097318 (P. B. F.), R01 CA127641 (P. B. F.), P01 CA104177 (P. B. F. and P. D.), R01 CA108520 (P. D. and P. B. F.), R01 CA138540 (D. S.), R03 MH093195 (P. B. F.), DOD W81XWH 10 PCRP SIDA (P. B. F. and X. Y. W.), DOD W81XWH-11-1-0186 (S. S. and P. B. F.), the National Foundation for Cancer Research (NFCR) (P. B. F.), and the Samuel Waxman Cancer Research Foundation (P. B. F. and D. S.). D. S. and X. Y. W. are Harrison Scholars in VCU Massey Cancer Center and D. S. is a Blick Scholar in VCU School of Medicine. P. B. F. holds the Thelma Newmeyer Corman Chair in Cancer Research in the VCU School of Medicine.
REFERENCES
- Alba R, Bosch A, Chillon M. Gutless adenovirus: Last-generation adenovirus for gene therapy. Gene Therapy. 2005;12(Suppl. 1):S18–S27. doi: 10.1038/sj.gt.3302612. [DOI] [PubMed] [Google Scholar]
- Azab B, Dash R, Das SK, Bhutia SK, Shen XN, Quinn BA, et al. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) in combination with the Apogossypol derivative BI-97C1 (Sabutoclax) improves therapeutic efficacy in low CAR colorectal cancer cells. Journal of Cellular Physiology. 2011;227(5):2145–2153. doi: 10.1002/jcp.22947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss LE, Zimmer SG, Fisher PB. Reversibility of progression of the transformed phenotype in Ad5-transformed rat embryo cells. Science. 1985;228(4703):1099–1101. doi: 10.1126/science.2581317. [DOI] [PubMed] [Google Scholar]
- Banerjee NS, Rivera AA, Wang M, Chow LT, Broker TR, Curiel DT, et al. Analyses of melanoma-targeted oncolytic adenoviruses with tyrosinase enhancer/promoter-driven E1A, E4, or both in submerged cells and organotypic cultures. Molecular Cancer Therapeutics. 2004;3(4):437–449. [PubMed] [Google Scholar]
- Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nature Reviews. Cancer. 2002;2(12):897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- Bhang HEC, Gabrielson KL, Laterra J, Fisher PB, Pomper MG. Tumor-specific imaging through progression elevated gene-3 promoter-driven gene expression. Nature Medicine. 2011;17(1):123–U302. doi: 10.1038/nm.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutia SK, Das SK, Kegelman TP, Azab B, Dash R, Su ZZ, et al. mda-7/IL-24 differentially regulates soluble and nuclear clusterin in prostate cancer. Journal of Cellular Physiology. 2011;227:1805–1813. doi: 10.1002/jcp.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutia SK, Dash R, Das SK, Azab B, Su ZZ, Lee SG, et al. Mechanism of autophagy to apoptosis switch triggered in prostate cancer cells by antitumor cytokine melanoma differentiation-associated gene 7/interleukin-24. Cancer Research. 2010;70(9):3667–3676. doi: 10.1158/0008-5472.CAN-09-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274(5286):373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- Borden EC, Lotan R, Levens D, Young CW, Waxman S. Differentiation therapy of cancer: Laboratory and clinical investigations. Cancer Research. 1993;53(17):4109–4115. [PubMed] [Google Scholar]
- Cancer Figurs and Facts 2012 January 4, 2012. http://www.cancer.org.
- Cao X, Wei R, Liu X, Zeng Y, Huang H, Ding M, et al. Cancer targeting gene-viro-therapy specific for liver cancer by alpha-fetoprotein-controlled oncolytic adenovirus expression of SOCS3 and IL-24. Acta Biochimica et Biophysica Sinica (Shanghai) 2011;43(10):813–821. doi: 10.1093/abbs/gmr071. [DOI] [PubMed] [Google Scholar]
- Cascallo M, Capella G, Mazo A, Alemany R. Ras-dependent oncolysis with an adenovirus VAI mutant. Cancer Research. 2003;63(17):5544–5550. [PubMed] [Google Scholar]
- Caudell EG, Mumm JB, Poindexter N, Ekmekcioglu S, Mhashilkar AM, Yang XH, et al. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. The Journal of Immunology. 2002;168(12):6041–6046. doi: 10.4049/jimmunol.168.12.6041. [DOI] [PubMed] [Google Scholar]
- Chada S, Mhashilkar AM, Liu Y, Nishikawa T, Bocangel D, Zheng M, et al. mda-7 gene transfer sensitizes breast carcinoma cells to chemotherapy, biologic therapies and radiotherapy: Correlation with expression of bcl-2 family members. Cancer Gene Therapy. 2006;13(5):490–502. doi: 10.1038/sj.cgt.7700915. [DOI] [PubMed] [Google Scholar]
- Chada S, Sutton RB, Ekmekcioglu S, Ellerhorst J, Mumm JB, Leitner WW, et al. MDA-7/IL-24 is a unique cytokine—Tumor suppressor in the IL-10 family. International Immunopharmacology. 2004;4(5):649–667. doi: 10.1016/j.intimp.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Chen L, Chen D, Manome Y, Dong Y, Fine HA, Kufe DW. Breast cancer selective gene expression and therapy mediated by recombinant adenoviruses containing the DF3/MUC1 promoter. The Journal of Clinical Investigation. 1995;96(6):2775–2782. doi: 10.1172/JCI118347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Green NK, Reynolds GM, Flavell JR, Mautner V, Kerr DJ, et al. Enhanced efficacy of Escherichia coli nitroreductase/CB1954 prodrug activation gene therapy using an E1B-55K-deleted oncolytic adenovirus vector. Gene Therapy. 2004;11(14):1126–1136. doi: 10.1038/sj.gt.3302271. [DOI] [PubMed] [Google Scholar]
- Choi KJ, Kim JH, Lee YS, Kim J, Suh BS, Kim H, et al. Concurrent delivery of GM-CSF and B7-1 using an oncolytic adenovirus elicits potent antitumor effect. Gene Therapy. 2006;13(13):1010–1020. doi: 10.1038/sj.gt.3302759. [DOI] [PubMed] [Google Scholar]
- Colombel M, Symmans F, Gil S, O’Toole KM, Chopin D, Benson M, et al. Detection of the apoptosis-suppressing oncoprotein bc1-2 in hormone-refractory human prostate cancers. The American Journal of Pathology. 1993;143(2):390–400. [PMC free article] [PubMed] [Google Scholar]
- Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, et al. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: A phase I study. Molecular Therapy. 2005;11(1):149–159. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Dash R, Azab B, Quinn BA, Shen X, Wang XY, Das SK, et al. Apogossypol derivative BI-97C1 (Sabutoclax) targeting Mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(21):8785–8790. doi: 10.1073/pnas.1100769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R, Bhutia SK, Azab B, Su ZZ, Quinn BA, Kegelmen TP, et al. mda-7/IL-24: A unique member of the IL-10 gene family promoting cancer-targeted toxicity. Cytokine & Growth Factor Reviews. 2010;21(5):381–391. doi: 10.1016/j.cytogfr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R, Dmitriev I, Su ZZ, Bhutia SK, Azab B, Vozhilla N, et al. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) improves therapeutic efficacy in low CAR prostate cancer cells. Cancer Gene Therapy. 2010;17(7):447–456. doi: 10.1038/cgt.2009.91. [DOI] [PubMed] [Google Scholar]
- Dedieu JF, Vigne E, Torrent C, Jullien C, Mahfouz I, Caillaud JM, et al. Long-term gene delivery into the livers of immunocompetent mice with E1/E4-defective adenoviruses. Journal of Virology. 1997;71(6):4626–4637. doi: 10.1128/jvi.71.6.4626-4637.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWeese TL, van der Poel H, Li S, Mikhak B, Drew R, Goemann M, et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Research. 2001;61(20):7464–7472. [PubMed] [Google Scholar]
- Di Lorenzo G, De Placido S. Hormone refractory prostate cancer (HRPC): Present and future approaches of therapy. International Journal of Immunopathology and Pharmacology. 2006;19(1):11–34. [PubMed] [Google Scholar]
- Donahue TR, Reber HA. Pancreatic surgery. Current Opinion in Gastroenterology. 2010;26(5):499–505. doi: 10.1097/MOG.0b013e32833d1174. [DOI] [PubMed] [Google Scholar]
- Eager R, Harle L, Nemunaitis J. Ad-MDA-7; INGN 241: A review of preclinical and clinical experience. Expert Opinion on Biological Therapy. 2008;8(10):1633–1643. doi: 10.1517/14712598.8.10.1633. [DOI] [PubMed] [Google Scholar]
- Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4(3):223–238. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- Emdad L, Lebedeva IV, Su ZZ, Gupta P, Sauane M, Dash R, et al. Historical perspective and recent insights into our understanding of the molecular and biochemical basis of the antitumor properties of mda-7/IL-24. Cancer Biology & Therapy. 2009;8(5):391–400. doi: 10.4161/cbt.8.5.7581. [DOI] [PubMed] [Google Scholar]
- Emdad L, Sarkar D, Su ZZ, Boukerche H, Bar-Eli M, Fisher PB. Progression elevated gene-3 (PEG-3) induces pleiotropic effects on tumor progression: Modulation of genomic stability and invasion. Journal of Cellular Physiology. 2005;202(1):135–146. doi: 10.1002/jcp.20097. [DOI] [PubMed] [Google Scholar]
- Enders JF, Bell JA, Dingle JH, Francis T, Jr., Hilleman MR, Huebner RJ, et al. Adenoviruses: Group name proposed for new respiratory-tract viruses. Science. 1956;124(3212):119–120. doi: 10.1126/science.124.3212.119. [DOI] [PubMed] [Google Scholar]
- Eulitt PJ, Park MA, Hossein H, Cruikshanks N, Yang C, Dmitriev IP, et al. Enhancing mda-7/IL-24 therapy in renal carcinoma cells by inhibiting multiple protective signaling pathways using sorafenib and by Ad5/3 gene delivery. Cancer Biology & Therapy. 2011;10(12):1290–1305. doi: 10.4161/cbt.10.12.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Research. 2005;65(22):10128–10138. doi: 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- Fisher PB, Bozzone JH, Weinstein IB. Tumor promoters and epidermal growth factor stimulate anchorage-independent growth of adenovirus-transformed rat embryo cells. Cell. 1979;18(3):695–705. doi: 10.1016/0092-8674(79)90124-7. [DOI] [PubMed] [Google Scholar]
- Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, et al. mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: From the laboratory into the clinic. Cancer Biology & Therapy. 2003;2(4 Suppl. 1):S23–S37. [PubMed] [Google Scholar]
- Fisher PB, Hermo H, Jr., Solowey WE, Dietrich MC, Edwalds GM, Weinstein IB, Langer JA, Pestka S, Giacomini P, Kusama M, et al. Effect of recombinant human fibroblast interferon and mezerein on growth, differentiation, immune interferon binding and tumor associated antigen expression in human melanoma cells. Anticancer Res. 1986;6(4):765–774. [PubMed] [Google Scholar]
- Fisher PB, Prignoli DR, Hermo H, Jr., Weinstein IB, Pestka S. Effects of combined treatment with interferon and mezerein on melanogenesis and growth in human melanoma cells. Journal of Interferon Research. 1985;5(1):11–22. doi: 10.1089/jir.1985.5.11. [DOI] [PubMed] [Google Scholar]
- Freytag SO, Khil M, Stricker H, Peabody J, Menon M, DePeralta-Venturina M, et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Research. 2002;62(17):4968–4976. [PubMed] [Google Scholar]
- Freytag SO, Stricker H, Pegg J, Paielli D, Pradhan DG, Peabody J, et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Research. 2003;63(21):7497–7506. [PubMed] [Google Scholar]
- Fuerer C, Iggo R. Adenoviruses with Tcf binding sites in multiple early promoters show enhanced selectivity for tumour cells with constitutive activation of the wnt signalling pathway. Gene Therapy. 2002;9(4):270–281. doi: 10.1038/sj.gt.3301651. [DOI] [PubMed] [Google Scholar]
- Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces antiglioma effect in vivo. Oncogene. 2000;19(1):2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Shirakawa Y, Kagawa S. Telomerase-specific oncolytic virotherapy for human gastrointestinal cancer. Expert Review of Anticancer Therapy. 2011;11(4):525–532. doi: 10.1586/era.10.200. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Urata Y, Tanaka N. Telomerase-specific oncolytic virotherapy for human cancer with the hTERT promoter. Current Cancer Drug Targets. 2007;7(2):191–201. doi: 10.2174/156800907780058835. [DOI] [PubMed] [Google Scholar]
- Fukazawa T, Matsuoka J, Yamatsuji T, Maeda Y, Durbin ML, Naomoto Y. Adenovirus-mediated cancer gene therapy and virotherapy (Review) International Journal of Molecular Medicine. 2010;25(1):3–10. [PubMed] [Google Scholar]
- Goldberg BB, Liu JB, Forsberg F. Ultrasound contrast agents: A review. Ultrasound in Medicine & Biology. 1994;20(4):319–333. doi: 10.1016/0301-5629(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Gomez Manzano C, Fueyo J, Kyritsis AP, Steck PA, Roth JA, McDonnell TJ, et al. Adenovirus-mediated transfer of the p53 gene produces rapid and generalized death of human glioma cells via apoptosis. Cancer Research. 1996;56(4):694–699. [PubMed] [Google Scholar]
- Gopalkrishnan RV, Sauane M, Fisher PB. Cytokine and tumor cell apoptosis inducing activity of mda-7/IL-24. International Immunopharmacology. 2004;4(5):635–647. doi: 10.1016/j.intimp.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Greco A, Di Benedetto A, Howard CM, Kelly S, Nande R, Dementieva Y, et al. Eradication of therapy-resistant human prostate tumors using an ultrasound-guided site-specific cancer terminator virus delivery approach. Molecular Therapy. 2010;18(2):295–306. doi: 10.1038/mt.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Emdad L, Lebedeva IV, Sarkar D, Dent P, Curiel DT, et al. mda-7/IL-24: Multifunctional cancer-specific apoptosis-inducing cytokine. Pharmacology and Therapeutics. 2006;111(3):596–628. doi: 10.1016/j.pharmthera.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AR, Dix BR, O’Carroll SJ, Braithwaite AW. p53-dependent cell death/apoptosis is required for a productive adenovirus infection. Nature Medicine. 1998;4(9):1068–1072. doi: 10.1038/2057. [DOI] [PubMed] [Google Scholar]
- Hallenbeck PL, Chang YN, Hay C, Golightly D, Stewart D, Lin J, et al. A novel tumor-specific replication-restricted adenoviral vector for gene therapy of hepatocellular carcinoma. Human Gene Therapy. 1999;10(10):1721–1733. doi: 10.1089/10430349950017725. [DOI] [PubMed] [Google Scholar]
- Hamed HA, Yacoub A, Park MA, Eulitt PJ, Dash R, Sarkar D, et al. Inhibition of multiple protective signaling pathways and Ad.5/3 delivery enhances mda-7/IL-24 therapy of malignant glioma. Molecular Therapy. 2010;18(6):1130–1142. doi: 10.1038/mt.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Haviv YS, Blackwell JL, Kanerva A, Nagi P, Krasnykh V, Dmitriev I, et al. Adenoviral gene therapy for renal cancer requires retargeting to alternative cellular receptors. Cancer Research. 2002;62(15):4273–4281. [PubMed] [Google Scholar]
- Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A, et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nature Medicine. 2000;6(10):1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- Heise C, Kirn DH. Replication-selective adenoviruses as oncolytic agents. The Journal of Clinical Investigation. 2000;105(7):847–851. doi: 10.1172/JCI9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nature Medicine. 1997;3(6):639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- Heise CC, Williams AM, Xue S, Propst M, Kirn DH. Intravenous administration of ONYX-015, a selectively replicating adenovirus, induces antitumoral efficacy. Cancer Research. 1999;59(11):2623–2628. [PubMed] [Google Scholar]
- Hoffmann D, Wildner O. Restriction of adenoviral replication to the transcriptional intersection of two different promoters for colorectal and pancreatic cancer treatment. Molecular Cancer Therapeutics. 2006;5(2):374–381. doi: 10.1158/1535-7163.MCT-05-0374. [DOI] [PubMed] [Google Scholar]
- Hollander MC, Poola-Kella S, Fornace AJ., Jr. Gadd34 functional domains involved in growth suppression and apoptosis. Oncogene. 2003;22(25):3827–3832. doi: 10.1038/sj.onc.1206567. [DOI] [PubMed] [Google Scholar]
- Howard CM, Forsberg F, Minimo C, Liu JB, Merton DA, Claudio PP. Ultrasound guided site specific gene delivery system using adenoviral vectors and commercial ultrasound contrast agents. Journal of Cellular Physiology. 2006;209(2):413–421. doi: 10.1002/jcp.20736. [DOI] [PubMed] [Google Scholar]
- Huang EY, Madireddi MT, Gopalkrishnan RV, Leszczyniecka M, Su Z, Lebedeva IV, et al. Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation associated (mda-7) gene with cancer specific growth suppressing and apoptosis inducing properties. Oncogene. 2001;20(48):7051–7063. doi: 10.1038/sj.onc.1204897. [DOI] [PubMed] [Google Scholar]
- Huang TG, Savontaus MJ, Shinozaki K, Sauter BV, Woo SL. Telomerase-dependent oncolytic adenovirus for cancer treatment. Gene Therapy. 2003;10(15):1241–1247. doi: 10.1038/sj.gt.3301987. [DOI] [PubMed] [Google Scholar]
- Inoue S, Shanker M, Miyahara R, Gopalan B, Patel S, Oida Y, et al. MDA-7/IL-24 based cancer gene therapy: Translation from the laboratory to the clinic. Current Gene Therapy. 2006;6(1):73–91. doi: 10.2174/156652306775515574. [DOI] [PubMed] [Google Scholar]
- Irving J, Wang Z, Powell S, O’Sullivan C, Mok M, Murphy B, et al. Conditionally replicative adenovirus driven by the human telomerase promoter provides broad-spectrum antitumor activity without liver toxicity. Cancer Gene Therapy. 2004;11(3):174–185. doi: 10.1038/sj.cgt.7700666. [DOI] [PubMed] [Google Scholar]
- Jaffee EM, Hruban RH, Canto M, Kern SE. Focus on pancreas cancer. Cancer Cell. 2002;2(1):25–28. doi: 10.1016/s1535-6108(02)00093-4. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: A Cancer Journal for Clinicians. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Jiang H, Fisher PB. Use of a sensitive and efficient subtraction hybridization protocol for the identification of genes differentially regulated during the induction of differentiation in human melanoma cells. Molecular and Cellular Differentiation. 1993;1(3):285–299. [Google Scholar]
- Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11(12):2477–2486. [PubMed] [Google Scholar]
- Jiang H, Shah S, Hilt DC. Organization, sequence, and expression of the murine S100 beta gene. Transcriptional regulation by cell type-specific cis-acting regulatory elements. The Journal of Biological Chemistry. 1993;268(27):20502–20511. [PubMed] [Google Scholar]
- Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, Fisher PB. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(17):9160–9165. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Shen A, Boyle L, Kunich J, Pandey K, Lemmon M, et al. Selectively replicating adenoviruses targeting deregulated E2F activity are potent, systemic antitumor agents. Cancer Cell. 2002;1(4):325–337. doi: 10.1016/s1535-6108(02)00060-0. [DOI] [PubMed] [Google Scholar]
- Kanai F, Shiratori Y, Yoshida Y, Wakimoto H, Hamada H, Kanegae Y, et al. Gene therapy for alpha-fetoprotein-producing human hepatoma cells by adenovirus-mediated transfer of the herpes simplex virus thymidine kinase gene. Hepatology. 1996;23(6):1359–1368. doi: 10.1002/hep.510230611. [DOI] [PubMed] [Google Scholar]
- Kanerva A, Mikheeva GV, Krasnykh V, Coolidge CJ, Lam JT, Mahasreshti PJ, et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clinical Cancer Research. 2002;8(1):275–280. [PubMed] [Google Scholar]
- Kawakami Y, Li H, Lam JT, Krasnykh V, Curiel DT, Blackwell JL. Substitution of the adenovirus serotype 5 knob with a serotype 3 knob enhances multiple steps in virus replication. Cancer Research. 2003;63(6):1262–1269. [PubMed] [Google Scholar]
- Kim J, Cho JY, Kim JH, Jung KC, Yun CO. Evaluation of E1B gene-attenuated replicating adenoviruses for cancer gene therapy. Cancer Gene Therapy. 2002;9(9):725–736. doi: 10.1038/sj.cgt.7700494. [DOI] [PubMed] [Google Scholar]
- Kim E, Kim JH, Shin HY, Lee H, Yang JM, Kim J, et al. Ad-mTERT-delta19, a conditional replication-competent adenovirus driven by the human telomerase promoter, selectively replicates in and elicits cytopathic effect in a cancer cell-specific manner. Human Gene Therapy. 2003;14(15):1415–1428. doi: 10.1089/104303403769211637. [DOI] [PubMed] [Google Scholar]
- Kim M, Zinn KR, Barnett BG, Sumerel LA, Krasnykh V, Curiel DT, et al. The therapeutic efficacy of adenoviral vectors for cancer gene therapy is limited by a low level of primary adenovirus receptors on tumour cells. European Journal of Cancer. 2002;38(14):1917–1926. doi: 10.1016/s0959-8049(02)00131-4. [DOI] [PubMed] [Google Scholar]
- Kirn DH, McCormick F. Replicating viruses as selective cancer therapeutics. Molecular Medicine Today. 1996;2(12):519–527. doi: 10.1016/s1357-4310(97)81456-6. [DOI] [PubMed] [Google Scholar]
- Krajewska M, Krajewski S, Epstein JI, Shabaik A, Sauvageot J, Song K, et al. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. The American Journal of Pathology. 1996;148(5):1567–1576. [PMC free article] [PubMed] [Google Scholar]
- Krajewski S, Krajewska M, Shabaik A, Wang HG, Irie S, Fong L, et al. Immunohistochemical analysis of in vivo patterns of Bcl-X expression. Cancer Research. 1994;54(21):5501–5507. [PubMed] [Google Scholar]
- Krasnykh VN, Mikheeva GV, Douglas JT, Curiel DT. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. Journal of Virology. 1996;70(10):6839–6846. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanson NA, Jr., Friedlander PL, Schwarzenberger P, Kolls JK, Wang G. Replication of an adenoviral vector controlled by the human telomerase reverse transcriptase promoter causes tumor-selective tumor lysis. Cancer Research. 2003;63(22):7936–7941. [PubMed] [Google Scholar]
- Larina IV, Evers BM, Ashitkov TV, Bartels C, Larin KV, Esenaliev RO. Enhancement of drug delivery in tumors by using interaction of nanoparticles with ultrasound radiation. Technology in Cancer Research & Treatment. 2005;4(2):217–226. doi: 10.1177/153303460500400211. [DOI] [PubMed] [Google Scholar]
- Lawrie A, Brisken AF, Francis SE, Cumberland DC, Crossman DC, Newman CM. Microbubble-enhanced ultrasound for vascular gene delivery. Gene Therapy. 2000;7(23):2023–2027. doi: 10.1038/sj.gt.3301339. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Emdad L, Su ZZ, Gupta P, Sauane M, Sarkar D, et al. mda-7/IL-24, novel anticancer cytokine: Focus on bystander antitumor, radiosensitization and antiangiogenic properties and overview of the phase I clinical experience (Review) International Journal of Oncology. 2007;31(5):985–1007. [PubMed] [Google Scholar]
- Lebedeva IV, Sarkar D, Su ZZ, Kitada S, Dent P, Stein CA, et al. Bcl-2 and Bcl-x(L) differentially protect human prostate cancer cells from induction of apoptosis by melanoma differentiation associated gene-7, mda-7/IL-24. Oncogene. 2003;22(54):8758–8773. doi: 10.1038/sj.onc.1206891. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Sarkar D, Kitada S, Dent P, Waxman S, et al. Melanoma differentiation associated gene-7, mda-7/interleukin-24, induces apoptosis in prostate cancer cells by promoting mitochondrial dysfunction and inducing reactive oxygen species. Cancer Research. 2003;63(23):8138–8144. [PubMed] [Google Scholar]
- Lee B, Choi J, Kim J, Kim JH, Joo CH, Cho YK, et al. Oncolysis of human gastric cancers by an E1B 55 kDa-deleted YKL-1 adenovirus. Cancer Letters. 2002;185(2):225–233. doi: 10.1016/s0304-3835(02)00279-3. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen Y, Dilley J, Arroyo T, Ko D, Working P, et al. Carcinoembryonic antigen-producing cell-specific oncolytic adenovirus, OV798, for colorectal cancer therapy. Molecular Cancer Therapeutics. 2003;2(10):1003–1009. [PubMed] [Google Scholar]
- Liang J, Huang RL, Huang Q, Peng Z, Zhang PH, Wu ZX. Adenovirus-mediated human interleukin 24 (MDA-7/IL-24) selectively suppresses proliferation and induces apoptosis in keloid fibroblasts. Annals of Plastic Surgery. 2011;66(6):660–666. doi: 10.1097/SAP.0b013e3181e05039. [DOI] [PubMed] [Google Scholar]
- Mathis JM, Stewart PL, Zhu ZB, Curiel DT. Advanced generation adenoviral virotherapy agents embody enhanced potency based upon CAR-independent tropism. Clinical Cancer Research. 2006;12(9):2651–2656. doi: 10.1158/1078-0432.CCR-06-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Chen GG, Chun SY, Yun JP, Chak EC, Ho RL, et al. Adenovirus-mediated tBid overexpression results in therapeutic effects on p53-resistant hepatocellular carcinoma. International Journal of Cancer. 2006;119(8):1985–1993. doi: 10.1002/ijc.22040. [DOI] [PubMed] [Google Scholar]
- Nettelbeck DM. Virotherapeutics: Conditionally replicative adenoviruses for viral oncolysis. Anti-Cancer Drugs. 2003;14(8):577–584. doi: 10.1097/00001813-200309000-00001. [DOI] [PubMed] [Google Scholar]
- Nettelbeck DM, Rivera AA, Balague C, Alemany R, Curiel DT. Novel oncolytic adenoviruses targeted to melanoma: Specific viral replication and cytolysis by expression of E1A mutants from the tyrosinase enhancer/promoter. Cancer Research. 2002;62(16):4663–4670. [PubMed] [Google Scholar]
- Ng KY, Liu Y. Therapeutic ultrasound: Its application in drug delivery. Medicinal Research Reviews. 2002;22(2):204–223. doi: 10.1002/med.10004. [DOI] [PubMed] [Google Scholar]
- O’Shea CC, Johnson L, Bagus B, Choi S, Nicholas C, Shen A, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6(6):611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Park JS, Qiao L, Su ZZ, Hinman D, Willoughby K, McKinstry R, et al. PERK-dependent regulation of MDA-7/IL-24-induced autophagy in primary human glioma cells. Autophagy. 2008;4(4):513–515. doi: 10.4161/auto.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt WG, Husseini GA, Staples BJ. Ultrasonic drug delivery—A general review. Expert Opinion on Drug Delivery. 2004;1(1):37–56. doi: 10.1517/17425247.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh R, Mhashilkar AM, Tanaka F, Saito Y, Branch CD, Sieger K, et al. Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Research. 2003;63(16):5105–5113. [PubMed] [Google Scholar]
- Rein DT, Breidenbach M, Kirby TO, Han T, Siegal GP, Bauerschmitz GJ, et al. A fiber-modified, secretory leukoprotease inhibitor promoter-based conditionally replicating adenovirus for treatment of ovarian cancer. Clinical Cancer Research. 2005;11(3):1327–1335. [PubMed] [Google Scholar]
- Rivera AA, Davydova J, Schierer S, Wang M, Krasnykh V, Yamamoto M, et al. Combining high selectivity of replication with fiber chimerism for effective adenoviral oncolysis of CAR-negative melanoma cells. Gene Therapy. 2004;11(23):1694–1702. doi: 10.1038/sj.gt.3302346. [DOI] [PubMed] [Google Scholar]
- Rodriguez R, Schuur ER, Lim HY, Henderson GA, Simons JW, Henderson DR. Prostate attenuated replication competent adenovirus (ARCA) CN706: A selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Research. 1997;57(13):2559–2563. [PubMed] [Google Scholar]
- Roth JA, Cristiano RJ. Gene therapy for cancer: What have we done and where are we going? Journal of the National Cancer Institute. 1997;89(1):21–39. doi: 10.1093/jnci/89.1.21. [DOI] [PubMed] [Google Scholar]
- Russo S, Butler J, Ove R, Blackstock AW. Locally advanced pancreatic cancer: A review. Seminars in Oncology. 2007;34(4):327–334. doi: 10.1053/j.seminoncol.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Sachs L. Control of normal cell differentiation and the phenotypic reversion of malignancy in myeloid leukaemia. Nature. 1978;274(5671):535–539. doi: 10.1038/274535a0. [DOI] [PubMed] [Google Scholar]
- Sachs L. Cell differentiation and bypassing of genetic defects in the suppression of malignancy. Cancer Research. 1987;47(8):1981–1986. [PubMed] [Google Scholar]
- Sachs L. The control of growth and differentiation in normal and leukemic blood cells. Cancer. 1990;65(10):2196–2206. doi: 10.1002/1097-0142(19900515)65:10<2196::aid-cncr2820651006>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Lebedeva IV, Gupta P, Emdad L, Sauane M, Dent P, et al. Melanoma differentiation associated gene-7 (mda-7)/IL-24: A ‘magic bullet’ for cancer therapy? Expert Opinion on Biological Therapy. 2007;7(5):577–586. doi: 10.1517/14712598.7.5.577. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Fisher PB. Unique conditionally replication competent bipartite adenoviruses-cancer terminator viruses (CTV): Efficacious reagents for cancer gene therapy. Cell Cycle. 2006;5(14):1531–1536. doi: 10.4161/cc.5.14.3095. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K, et al. mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(15):10054–10059. doi: 10.1073/pnas.152327199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Park ES, Vozhilla N, Dent P, Curiel DT, et al. A cancer terminator virus eradicates both primary and distant human melanomas. Cancer Gene Therapy. 2008;15(5):293–302. doi: 10.1038/cgt.2008.14. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Vozhilla N, Park ES, Gupta P, Fisher PB. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(39):14034–14039. doi: 10.1073/pnas.0506837102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Vozhilla N, Park ES, Randolph A, Valerie K, et al. Targeted virus replication plus immunotherapy eradicates primary and distant pancreatic tumors in nude mice. Cancer Research. 2005;65(19):9056–9063. doi: 10.1158/0008-5472.CAN-05-1261. [DOI] [PubMed] [Google Scholar]
- Sauane M, Gopalkrishnan RV, Lebedeva I, Mei MX, Sarkar D, Su ZZ, et al. MDA-7/IL-24: Novel cancer growth suppressing and apoptosis inducing cytokine. Cytokine & Growth Factor Reviews. 2003;14(1):35–51. doi: 10.1016/s1359-6101(02)00074-6. [DOI] [PubMed] [Google Scholar]
- Sauane M, Lebedeva IV, Su ZZ, Choo HT, Randolph A, Valerie K, et al. Melanoma differentiation associated gene-7/interleukin-24 promotes tumor cell-specific apoptosis through both secretory and nonsecretory pathways. Cancer Research. 2004;64(9):2988–2993. doi: 10.1158/0008-5472.can-04-0200. [DOI] [PubMed] [Google Scholar]
- Sauane M, Su ZZ, Gupta P, Lebedeva IV, Dent P, Sarkar D, et al. Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(28):9763–9768. doi: 10.1073/pnas.0804089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AE. Viral vectors in gene therapy. Annual Review of Microbiology. 1995;49:807–838. doi: 10.1146/annurev.mi.49.100195.004111. [DOI] [PubMed] [Google Scholar]
- Smith RR, Huebner RJ, Rowe WP, Schatten WE, Thomas LB. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer. 1956;9(6):1211–1218. doi: 10.1002/1097-0142(195611/12)9:6<1211::aid-cncr2820090624>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Staudt MR, Depass AL, Sarkar D, Fisher PB. Model cell culture system for defining the molecular and biochemical events mediating terminal differentiation of human melanoma cells. Journal of Cellular Physiology. 2009;218(2):304–314. doi: 10.1002/jcp.21602. [DOI] [PubMed] [Google Scholar]
- Staudt MR, Dittmer DP. The Rta/Orf50 transactivator proteins of the gamma-herpesviridae. Current Topics in Microbiology and Immunology. 2007;312:71–100. doi: 10.1007/978-3-540-34344-8_3. [DOI] [PubMed] [Google Scholar]
- Sternberg CN. Highlights of contemporary issues in the medical management of prostate cancer. Critical Reviews in Oncology/Hematology. 2002;43(2):105–121. doi: 10.1016/s1040-8428(02)00023-9. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Emdad L, Sarkar D, Randolph A, Valerie K, Yacoub A, et al. Potential molecular mechanism for rodent tumorigenesis: Mutational generation of Progression Elevated Gene-3 (PEG-3) Oncogene. 2005;24(13):2247–2255. doi: 10.1038/sj.onc.1208420. [DOI] [PubMed] [Google Scholar]
- Su Z, Emdad L, Sauane M, Lebedeva IV, Sarkar D, Gupta P, et al. Unique aspects of mda-7/IL-24 antitumor bystander activity: Establishing a role for secretion of MDA-7/IL-24 protein by normal cells. Oncogene. 2005;24(51):7552–7566. doi: 10.1038/sj.onc.1208911. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Gopalkrishnan RV, Narayan G, Dent P, Fisher PB. Progression elevated gene-3, PEG-3, induces genomic instability in rodent and human tumor cells. Journal of Cellular Physiology. 2002;192(1):34–44. doi: 10.1002/jcp.10114. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Lebedeva IV, Sarkar D, Gopalkrishnan RV, Sauane M, Sigmon C, et al. Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene. 2003;22(8):1164–1180. doi: 10.1038/sj.onc.1206062. [DOI] [PubMed] [Google Scholar]
- Su C, Peng L, Sham J, Wang X, Zhang Q, Chua D, et al. Immune gene-viral therapy with triplex efficacy mediated by oncolytic adenovirus carrying an interferon-gamma gene yields efficient antitumor activity in immunodeficient and immunocompetent mice. Molecular Therapy. 2006;13(5):918–927. doi: 10.1016/j.ymthe.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Sarkar D, Emdad L, Duigou GJ, Young CS, Ware J, et al. Targeting gene expression selectively in cancer cells by using the progression-elevated gene-3 promoter. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(4):1059–1064. doi: 10.1073/pnas.0409141102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Shi Y, Fisher PB. Subtraction hybridization identifies a transformation progression-associated gene PEG-3 with sequence homology to a growth arrest and DNA damage-inducible gene. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(17):9125–9130. doi: 10.1073/pnas.94.17.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Shi Y, Fisher PB. Cooperation between AP1 and PEA3 sites within the progression elevated gene-3 (PEG-3) promoter regulate basal and differential expression of PEG-3 during progression of the oncogenic phenotype in transformed rat embryo cells. Oncogene. 2000;19(30):3411–3421. doi: 10.1038/sj.onc.1203666. [DOI] [PubMed] [Google Scholar]
- Su Z, Shi Y, Friedman R, Qiao L, McKinstry R, Hinman D, et al. PEA3 sites within the progression elevated gene-3 (PEG-3) promoter and mitogen-activated protein kinase contribute to differential PEG-3 expression in Ha-ras and v-raf oncogene transformed rat embryo cells. Nucleic Acids Research. 2001;29(8):1661–1671. doi: 10.1093/nar/29.8.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nature Reviews Genetics. 2003;4(5):346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, et al. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): Biologic outcome in advanced cancer patients. Molecular Therapy. 2005;11(1):160–172. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Tooze J. Molecular biology of tumor viruses (Part 2) Cold Spring Harbor Laboratory; New York: 1980. [Google Scholar]
- Turnell AS, Grand RJ, Gallimore PH. The replicative capacities of large E1B-null group A and group C adenoviruses are independent of host cell p53 status. Journal of Virology. 1999;73(3):2074–2083. doi: 10.1128/jvi.73.3.2074-2083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houdt WJ, Haviv YS, Lu B, Wang M, Rivera AA, Ulasov IV, et al. The human survivin promoter: A novel transcriptional targeting strategy for treatment of glioma. Journal of Neurosurgery. 2006;104(4):583–592. doi: 10.3171/jns.2006.104.4.583. [DOI] [PubMed] [Google Scholar]
- Volk AL, Rivera AA, Kanerva A, Bauerschmitz G, Dmitriev I, Nettelbeck DM, et al. Enhanced adenovirus infection of melanoma cells by fiber-modification: Incorporation of RGD peptide or Ad5/3 chimerism. Cancer Biology & Therapy. 2003;2(5):511–515. doi: 10.4161/cbt.2.5.440. [DOI] [PubMed] [Google Scholar]
- Whyte P, Ruley HE, Harlow E. Two regions of the adenovirus early region 1A proteins are required for transformation. Journal of Virology. 1988;62(1):257–265. doi: 10.1128/jvi.62.1.257-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildner O, Blaese RM, Morris JC. Therapy of colon cancer with oncolytic adenovirus is enhanced by the addition of herpes simplex virus-thymidine kinase. Cancer Research. 1999;59(2):410–413. [PubMed] [Google Scholar]
- Wirth T, Zender L, Schulte B, Mundt B, Plentz R, Rudolph KL, et al. A telomerase-dependent conditionally replicating adenovirus for selective treatment of cancer. Cancer Research. 2003;63(12):3181–3188. [PubMed] [Google Scholar]
- Wu YM, Zhang KJ, Yue XT, Wang YQ, Yang Y, Li GC, et al. Enhancement of tumor cell death by combining cisplatin with an oncolytic adenovirus carrying MDA-7/IL-24. Acta Pharmacologica Sinica. 2009;30(4):467–477. doi: 10.1038/aps.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TL, Zhou D. Viral delivery for gene therapy against cell movement in cancer. Advanced Drug Delivery Reviews. 2011;63(8):671–677. doi: 10.1016/j.addr.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Xiao L, Li X, Niu N, Qian J, Xie G, Wang Y. Dichloroacetate (DCA) enhances tumor cell death in combination with oncolytic adenovirus armed with MDA-7/IL-24. Molecular and Cellular Biochemistry. 2010;340(1–2):31–40. doi: 10.1007/s11010-010-0397-6. [DOI] [PubMed] [Google Scholar]
- Xue XB, Xiao CW, Zhang H, Lu AG, Gao W, Zhou ZQ, et al. Oncolytic adenovirus SG600-IL24 selectively kills hepatocellular carcinoma cell lines. World Journal of Gastroenterology. 2010;16(37):4677–4684. doi: 10.3748/wjg.v16.i37.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Curiel DT. Current issues and future directions of oncolytic adenoviruses. Molecular Therapy. 2010;18(2):243–250. doi: 10.1038/mt.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BX, Duan YJ, Dong CY, Zhang F, Gao WF, Cui XY, et al. Novel functions for mda-7/IL-24 and IL-24 delE5: Regulation of differentiation of acute myeloid leukemic cells. Molecular Cancer Therapeutics. 2011;10(4):615–625. doi: 10.1158/1535-7163.MCT-10-0863. [DOI] [PubMed] [Google Scholar]
- Yang Y, Nunes FA, Berencsi K, Gonczol E, Engelhardt JF, Wilson JM. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nature Genetics. 1994;7(3):362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- Yang Y, Su Q, Wilson JM. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. Journal of Virology. 1996;70(10):7209–7212. doi: 10.1128/jvi.70.10.7209-7212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wilson JM. Clearance of adenovirus-infected hepatocytes by MHC class I-restricted CD4 + CTLs in vivo. The Journal of Immunology. 1995;155(5):2564–2570. [PubMed] [Google Scholar]
- Ye X, Lu Q, Zhao Y, Ren Z, Ren XW, Qiu QH, et al. Conditionally replicative adenovirus vector carrying TRAIL gene for enhanced oncolysis of human hepatocellular carcinoma. International Journal of Molecular Medicine. 2005;16(6):1179–1184. [PubMed] [Google Scholar]
- Zhaohui P. Current status of gendicine in China: Recombinant human Ad-p53 agent for treatment of cancers. Human Gene Therapy. 2005;16:1016–1027. doi: 10.1089/hum.2005.16.1016. [DOI] [PubMed] [Google Scholar]
- Zou W, Luo C, Zhang Z, Liu J, Gu J, Pei Z, et al. A novel oncolytic adenovirus targeting to telomerase activity in tumor cells with potent. Oncogene. 2004;23(2):457–464. doi: 10.1038/sj.onc.1207033. [DOI] [PubMed] [Google Scholar]