Abstract
In order to interpret/integrate data obtained with different functional neuroimaging modalities (e.g. fMRI, EEG/MEG, PET/SPECT, fNIRS), forward-generative models of a diversity of brain mechanisms at the mesoscopic level are considered necessary. For the cerebral cortex, the brain structure with possibly the most relevance for functional neuroimaging, a variety of such biophysical models has been proposed over the last decade. The development of technological tools to investigate in vitro the physiological, anatomical and biochemical principles at the microscopic scale in comparative studies formed the basis for such theoretical progresses. However, with the most recent introduction of systems to record electrical (e.g. miniaturized probes chronically/acutely implantable in the brain), optical (e.g. two-photon laser scanning microscopy) and atomic nuclear spectral (e.g. nuclear magnetic resonance spectroscopy) signals using living laboratory animals, the field is receiving even greater attention. Major advances have been achieved by combining such sophisticated recording systems with new experimental strategies (e.g. transgenic/knock-out animals, high resolution stereotaxic manipulation systems for probe-guidance and cellular-scale chemical-delivery). Theoreticians may now be encouraged to re-consider previously formulated mesoscopic level models in order to incorporate important findings recently made at the microscopic scale. In this series of reviews, we summarize the background at the microscopic scale, which we suggest will constitute the foundations for upcoming representations at the mesoscopic level. In this first part, we focus our attention on the nerve ending particles in order to summarize basic principles and mechanisms underlying cellular metabolism in the cerebral cortex. It will be followed by two parts highlighting major features in its organization/working-principles to regulate both cerebral blood circulation and neuronal activity, respectively. Contemporary theoretical models for functional neuroimaging will be revised in the fourth part, with particular emphasis in their applications, advantages/limitations and future prospects.
Keywords: Functional neuroimaging, Cerebral cortex, Micro-architecture, Cellular metabolism
Prologue
The brain is mainly composed of a restless collection of neuronal and glial cells incessantly working (i.e. receiving and distributing signals) to preserve functional integrity, where even transient limitations in energy supply can critically harm the function of these cells (Sokoloff, 1991). Energy usage and oxygen consumption are tightly coupled in the brain due to oxidative D-glucose (Glc) breakdown. Both energy and oxygen mobilization by tissue can be accessed through different neuroimaging modalities. For example, fluorine-18 (F-18), tagged to 2-fluoro-2-deoxy-D-glucose (FDG), has been commonly used in PET studies as a Glc analog with clinical and research applications. In cardiac imaging, FDG has been combined with N-13 ammonia to study flow-metabolism mismatch. Other common radioactive labeled compounds in PET studies are 15O and 18F-fluoromisonidazole, which permits tracing oxygen consumption and hypoxic cells, respectively. By using fMRI, stimulus induced changes of cerebral metabolic rate of oxygen (CMRO2) can be estimated from cerebral blood flow (CBF) and blood oxygenation level-dependent (BOLD) signals when contrasted with the changes during hypercapnia and hyperoxia1, called the ‘calibrated BOLD’, approach. BOLD signal calibration can be strengthened by comparison with CMRO2 measurement (Hyder et al., 2001). Alternative methods measuring brain metabolic activity with nuclear magnetic resonance (NMR) scanners not based on hemodynamics have been developed: most notably 17O2 gas measuring oxygen consumption by using spectroscopy and echo-planar imaging. These modalities, which are analogous to PET, permit targeting intra- and extra-cellular contrast agents. Additionally, NMR spectroscopy is used to measure in vivo directly metabolite flows and their related functional neuroenergetics (Rothman et al., 2002). For that end, biological molecules are isotopically labelled (1H, 13,14C, 31P and 15 N nucleus) to determine concentrations and synthesis rates of individual chemical compounds [e.g. amino acids (glutamate, GABA), acetate, aspartate, lactate, Glc, α-D-glucose 6-phosphate (G6P), glutamine] from their spectral content. The chemical shift refers to the dependency of the resonance frequency of an NMR active nucleus as a function not only of the local magnetic field strength, but also of the chemical environment. Novel applications of neuroimaging have also been developed to study the activity and synthesis of other neurotransmitters in the human brain. For example, [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol could be used to study dopamine D2/3 receptors through a PET camera. In the same way, we can study the activity of serotonin 5-HT transporter by using [11C]N, N-dimethyl-2-(2-amino-4-cyanophenylthio) benzylamine, as well as its synthesis through α-[11C]Methyl-L-tryptophan and 5-Hydroxy-L-[β-11C]tryptophan tracers.
However, a wide range of neuroimaging methods have been routinely used to map task-induced changes in the neuronal activity indirectly reflected in variations of the local cerebral perfusion (i.e. functional hyperemia). For many years now, researchers have been trying to elucidate the basic principles underlying this phenomenon and several hypotheses have been formulated so far (see a review by Iadecola 2004). From them the metabolic and neurogenic hypotheses, which arise from answering the question of whether this phenomenon is entirely due to a metabolic deficit or if it is a simple act of consumerism in the neurons’ societies set off by their signaling, have been for sometime now the most trustworthy. More recently new methodologies have come to light to study activity in default networks that are active when the brain is “at rest2”. An implicit component of the BOLD signal, sometimes associated with a marked initial dip, reflects directly the oxygen consumption fraction by the capillary bed, an activity that could also be observed through fNIRS (i.e. oxy, de-oxy and total hemoglobin content in brain vessels). Nowadays, we know that a negative component in the BOLD signal could indicate an unbalanced response between the CBF and the oxygen consumption, probably associated with implicit GABAergic activity in the default networks (Northoff et al., 2007), which could modulate brain metabolism (Nasrallah et al., 2007). As a consequence, in the last few years, looking for signatures relating changes in the CBF to the states of synchronization of local neuronal networks have attracted the interest of many researchers, bringing back the debate about how neurons communicate with the vasculature. It is our belief that in order to regulate dynamically the global and local cerebral perfusion in the brain both metabolic and neurogenic mechanisms must coexist. Actually, it has been recently claimed that hemodynamics could also play a role in information processing through modulation of neural activity (Moore and Cao, in press), in what is named the “hemo-neural hypothesis”. Therefore, data interpretation and/or integration require the understanding of brain metabolism at various levels since the neuroimaging signals do not usually measure energy, oxygen and metabolite flows directly. In the best case that we could be able to observe these magnitudes directly in the brain from neuroimaging, we will need to understand how they are related to the underlying neuronal activity. The purpose of this review is to cover different aspects of cellular metabolism and their implications on neuroimaging signals.
Introduction
Cells constitute open systems that constantly exchange different forms of energy with the environment. They use the major portion of the available internal energy to do work (e.g. chemical, electric, mechanical) necessary for their vital functions, while a small amount is irreversibly released in the form of heat. Examples of vital functions that directly involve cellular work are endocytic cycling (i.e. endo- and exo-cytosis), mediated/active trans-membrane transport, synthesis and transport of macro-molecules, maintaining/modifying cell structure (i.e. assembly and disassembly of elements of the cytoskeleton), locomotion, adhesion and proliferation. All these forms of cellular work comprise an abundant number of competing/cooperating chemical reactions with an energetically unfavorable endergonic/exergonic balance. The cells maintain integrity and function by regular delivery of Gibb’s free energy from the environment, which is stored in basic nutrients (e.g. carbohydrates, glycerol, amino acids and fatty acids). In order to extract the energy stored in nutrients, the cells carry out respiration, a process regulated by a network of dynamically interacting enzymes.
The entire process by which a cell modifies the principles of action in several of these enzymes to adjust the energy extraction rate from nutrients as a function of the overall cellular work is termed cellular metabolism. It comprises of both anabolic and catabolic mechanisms in domains strategically separated by membranes or phospholipid bilayers, e.g. cytosol, the mitochondrial matrix, the endoplasmic reticulum lumen. The course of metabolism inside a single cell is influenced by different types of substance traffic (i.e. signal transduction) among cell domains and also between the cell and its surroundings, often involving metabolite shuttles and second messenger pathways. The energy extracted from nutrients is ultimately converted into adenosine triphosphate (ATP). ATP is a multifunctional nucleotide, which is considered to be the intracellular energetic currency. However, there are many ways, other than ATP, for a cell to store internal energy (e.g. chemical potentials in any by-product from nutrient catabolism, glycogen polymers, energy-rich electron donors, electrochemical potentials across membranes created by both ions and mitochondrial translocated H+) and they all transform dynamically from one form to another depending on the energetic deficit in the cell and its interaction with nearby cells. Since physiological processes in the cells take place at different temporal scales, ranging from those occurring within a few milliseconds to others which are long-lasting, the time constants for accessibility to these energetic batteries differ.
The metabolism of carbohydrates in all cell types is accomplished through three pathways (i.e. glycolysis, the TCA-cycle3 and oxidative-phosphorylation), each of which is involved in metabolite synthesis/degradation in a compartmentalized way at both multi- and single-cellular levels. Most of the enzymes in these three metabolic pathways catalyze substrate/product formation in an independent manner. However, it is possible that to ensure a high degree of efficacy, certain enzymes catalyzing interdependent reactions may be arranged in multi-enzyme complexes, the study of which is termed metabolomics (McKenna et al., 2006a). How are the fluxes through multi-enzyme systems controlled? As pointed out by Hofmeyr and Cornish-Bowden (2000), “cellular metabolism is a molecular economy that is functionally organised into supply and demand blocks linked by metabolic products and cofactor cycles”. Therefore, specific blocks might exert particular influence on the others. For example, in some cases a main block controls the flux, while others determine the degree to which the concentration of the linking metabolite(s) is(are) maintained in a homeostatic sense. Several approaches, most of them being somehow related to the engineering discipline known as sensitivity analysis, have been proposed by studying the kinetic behaviors of multi-enzyme systems, e.g. metabolic control theory (Hofmeyr 1997) and flux-oriented theory (Crabtree and Newsholme 1987). These two approaches are particular cases of a previously formulated general theory of metabolic control, the biochemical systems theory (Savageau 1976). Although such mathematical theories place much more emphasis on predicting how complex biochemical systems will behave when the conditions are changed, in our review the discussions will be formulated having implicitly in mind similar kinetic and thermodynamic aspects of regulations to those introduced in these theories, e.g. control and elasticity coefficients of supply and demand. In this context, we can postulate that several of these enzymes will be positioned at strategic check points of the metabolic pathways to detect irregularities and accordingly modify metabolic function (e.g. allosteric modulation, post-translational modification), to ensure homeostasis.
Homeostasis, as defined by Hochachka and McClelland (1997), refers to the maintenance of a relatively constant internal milieu in the face of changing environmental conditions or changing physiological function. For instance, ATP concentration remains unvarying even while its turnover rate has changed by two orders of magnitude. Hence, ATP is considered one of the most universally “homeostatic” substances. However, there are other intermediates during cellular metabolism that are regulated within narrow ranges, i.e. during rest-work transitions their concentrations do not vary by more than 0.5-to threefold over the resting condition. Therefore, the demands of homeostasis prevail versus metabolic regulation. The [s]4 stability paradox- why most metabolite concentrations are homeostatic over large changes in pathway fluxes- constitutes a major problem in cellular metabolism and two models have been proposed for metabolic regulation so far (Hochachka, 2003): a) the classical [the cells behave like a watery bag of enzymes] and b) the alternative [3-dimensional order and structure of cells constrain metabolite movement and conversion]. Even though the classical model is consistent with the metabolite homeostasis, it fails while providing a global explanation for the [s] stability paradox. In the alternative model, intracellular movement of metabolites are assisted by macromolecular motors running on actin (e.g. unconventional myosins) or tubulin (e.g. dyneins, kinesins) tracks and the convection system acts as an over-riding aid mechanism which facilitates the enzyme-substrate encounter. Hochachka (2003) called attention to the fact that classical and alternative models of metabolic regulation have operated as ‘two solitudes’,, each considering the other incompatible with its own experimental modus operandi. Cellular metabolism is robustly organized among species, resulting in a network with heterogeneous scale-free design, where a few hubs play protagonist roles (Jeong et al., 2000; Table 1, supplementary material I). Tracing cellular metabolic pathways and elucidating the associated principles for enzyme induction/inhibition (i.e. transcriptional regulation) constituted a major challenge in the 20th century; this topic is beyond the scope of the current review (see Desvergne et al., 2006 for more detail).
The human brain accounts for only 2% of the body’s weight but 20% of its resting energy usage ([Sokoloff, 1991] and [Clark and Sokoloff, 1999]). The high energy demand of the brain is partly because the neural processing of information is metabolically expensive. It was suggested early on that almost all cerebral energy consumption is derived from Glc oxidation (Siesjo, 1978). Perhaps, this hypothesis toned very well with the idea of a blood-brain barrier having only influx transport mechanisms for Glc (i.e. the Glc carrier, GLUT1). Nowadays, we know that several amino acid carriers (e.g. L-system for large neutral amino acids, LAT1) and transporters for nucleosides/nucleobases coexist with GLUT1 in the membranes of endothelial cells, which serve to supply different nutrients to the brain ([Leybaert, 2005] and [Abbott et al., 2006]). At present, there is a consensus that metabolism in neurons and glial cells (e.g. astrocytes) is accomplished straightforwardly by having Glc as the preferable nutrient and minimizing the amount of substance traffic. Glc oxidation yields a far higher number of ATP than its alternate non-oxidative path (Siesjo, 1978). Thus total Glc consumption (CMRglc) includes oxidative (CMRglc(ox)) and non-oxidative (CMRglc(non-ox)) components and the oxygen-to-glucose index (OGI) reflects the degree of oxidative vs. non-oxidative Glc breakdown. In the cerebral cortex, the OGI at seizure may be as low as 4 because of high CMRglc(non-ox), whereas at rest it can be close to the theoretical stoichiometric value of 6 because CMRglc(non-ox) can be quite negligible (Siesjo, 1978). Departure of OGI from 6 would indicate some extra lactate, which has been observed during bicuculline-induced seizures in rats [by means of 1H-observed, 13C-edited NMR spectroscopy, Patel et al., 2004]. While the early PET study of Fox et al. (1988) showed a very large drop in OGI with stimulation, later studies during sensory stimulation in humans show much smaller drops in OGI (Shulman et al., 2001a).
A few examples of vital functions in neurons and astrocytes requiring cellular work and hence direct internal energy usage are: a) the glutamate/GABA5-glutamine cycle, b) vesicularization, docking and exocytosis of neurotransmitters from vesicles, c) the trans-membrane ATPases in both types of cells, d) actin-filament growth in dendrites of spiny neurons, e) phospholipid and glycolipid metabolism, f) axoplasmic transport. Nonetheless, endothelial cells (EC) and smooth muscle cells (SMC) in the brain express selective transport systems for several hexoses and amino acids. Different types of cross-talk between these cells involving multiple intracellular signaling cascades have been reported, but this remains an issue for investigation. Additionally, understanding the mechanisms by which secreted mediators (e.g. nitric oxide, NO) and cytokines modulate hexoses and amino acid transporters in EC and SMC is very limited ([Mann et al., 2003] and [Mehta and Malik, 2006]). Therefore, the metabolic demands of vital functions in both EC (e.g. regulation/maintenance of cell-cell adherent junctions) and SMC (e.g. interactions between myosin and actin-filaments) are not discussed in this review.
Elucidating the principles of neuronal and astrocytic metabolism has been a challenge for many years now. To that end, several in vitro (e.g. slices, cells cultured or acutely isolated from brain tissues) and in vivo experimental models have been developed. For example, the isolated nerve ending particle (i.e. synaptosome) preparation has been used successfully to study many principles of the metabolic pathways in the brain (Erecińska et al., 1996). In the synaptosomes, the majority of cellular mechanisms with impact on energy usage and production are present. While it is tempting to link results from one type of study to another, we recommend care in data interpretation from the perspective of homeostasis. In the in vitro case concentrations are usually measured transiently with perturbations (e.g. adding substrates), whereas in the in vivo case fluxes are measured typically under natural conditions (e.g. steady-state paradigm). In this review, the particulars for the above mentioned metabolic pathways as well as the most recently discovered metabolite shuttles in the nerve ending and astrocytic processes will be discussed, with special emphasis on the main branches and check points that have been discovered for neurons and astrocytes in the cerebral cortex. Understanding the working principle of cellular metabolism in the cerebral cortex would have direct implications for modeling several functional neuroimaging modalities (e.g. fMRI, PET, SPECT, fNIRS). We recommend to those readers not familiar with basic concepts used henceforth to consult previously published reviews on the cellular metabolism in the central nervous system (e.g. [Erecińska et al., 1996], [Ames, 2000], [McKenna et al., 2006b] and [Hertz et al., 2007]).
The glycolytic pathway
The glycolytic pathway involves breakdown of Glc, a monosaccharide, into pyruvate (Pyr). A segment appended to this pathway brings about the glycogen-shunt, from where a polysaccharide (i.e. the glycogen polymer) can enter the glycolytic pathway. Breakdown steps at different levels (e.g. glycogen polymers → hexoses → trioses → glyceric acids) can be either impeded or hastened through the activity of many enzymes in this pathway, which constitute check points located in different cellular compartments. In this section, we will review the main biochemical signaling implicated in the regulation of these principal enzymes.
Regulation mechanisms of glycolysis
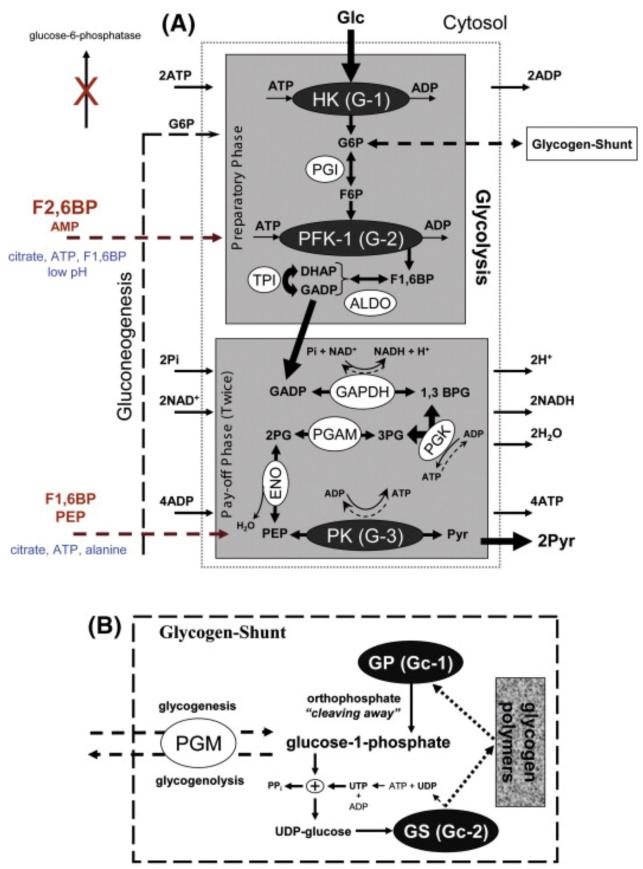
Glycolysis6 occurs in the cytosol of cells and is referred to as the primary pathway of cellular metabolism ultimately coupled to respiration. Glycolysis comprises two phases: preparatory and pay-off. In the preparatory (or investment) phase, two ATP are used to convert one Glc into two D-glyceraldehyde 3-phosphate (GADP) molecules. During the pay-off phase, a GADP molecule is used to produce two ATP and one reduced nicotinamide adenine dinucleotide (NADH) cofactor. This phase runs twice, once for each GADP molecule. Despite several enzymes being involved in the glycolytic pathway (Fig. 1, A), only hexokinase (HK), phosphofructokinase-1 (PFK-1) and pyruvate kinase (PK) are considered to play a regulatory role. In addition, it has been demonstrated recently that glycolysis in the brain is in some way altered by the activity of glycogen polymers; a mechanism dominated by the glycogen-shunt (Fig. 1, B). In the glycogen-shunt, the enzymes glycogen synthase (GS) and glycogen phosphorylase (GP) adjust the storage (i.e. glycogenesis) and the mobilization (i.e. glycogenolysis) of glycogen polymers allosterically.
Fig. 1.
A) Diagram of the preparatory and pay-off phases in glycolysis. The preparatory phase comprises four steps: a first phosphorylation [enzyme (HK), substrates (Glc, ATP), products (G6P, ADP), comments (a-it is the control point G-1, b-HK isozymes have direct access to mitochondrial ATP through specific binding to porins)], an isomerase reaction [enzyme (PGI), substrate (G6P), product (F6P), comment (it is a reversible and not normally favorable reaction driven by the concentration of F6P)], a second phosphorylation [enzyme (PFK-1), substrates (F6P, ATP), products (F1,6BP, ADP), comments (a-it is the control point G-2, the most important in glycolysis, b-during gluconeogenesis, a pathway crucial in developing brain, the reverse conversion must be performed by fructose 1,6 bisphosphatase)], an aldol reaction [enzyme (ALDO), substrate (F1,6BP), products (DHAP, GADP), comment (DHAP and GADP are rapidly and reversibly interconverted by TPI, a step essential to produce energy efficiently)]. The pay-off phase comprises five steps: a redox reaction [enzyme (GAPDH), substrates (GADP, NAD+, Pi), products (1,3BPG, NADH, H+), comment (the highly exergonic oxidation of GADP drives the endergonic transferring of Pi to an intermediate to finally form 1,3BPG, a product with high phosphoryl-transfer potential)], a first substrate-level phosphorylation [enzyme (PGK), substrates (1,3BPG, ADP), products (3PG, ATP), comment (it is the break-even point in glycolysis)], a mutase reaction [enzyme (PGAM), substrate (3PG), product (2PG), comment (a mutase does not change the oxidation state of the carbons in the compound)], a hydration [enzyme (ENO), substrates (2PG), product (PEP, H2O), comment (there are several enolase isozymes in humans)], a second substrate-level phosphorylation [enzyme (PK), substrates (PEP, ADP), products (Pyr, ATP), comment (it is the control point G-3)]. The negative and positive allosteric effectors are highlighted in blue and orange, respectively. B) The glycogen shunt hypothesis. Glycogenesis: The PGM, an isomerase, synthezises glucose-1-phosphate from G6P. The enzyme GS is responsible for the synthesis of glycogen polymers. This pathway utilizes UDP-glucose as the activated Glc donor. Glycogenolysis: Phosphorolysis by enzyme GP is the cleaving away of a bond by orthophosphate, and thus degradation of glycogen polymers to glucose-1-phosphate; which can then be isomerized to G6P by PGM iso-energetically. Abbreviations: ALDO → fructose 1,6 bisphosphate aldolase, DHAP → dihydroxyacetone phosphate, TPI → triose-phosphate isomerase, GAPDH → glyceraldehyde phosphate dehydrogenase, NAD+ → nicotinamide adenine dinucleotide (oxidized form), 1,3BPG → 1,3-bisphosphoglycerate, 3PG → 3-phosphoglycerate, 2PG → 2-phosphoglycerate, PGAM → phosphoglycerate mutase, ENO → enolase.
The influx of Glc into cells is regulated by a family of HK enzymes, constituting the first check point in the glycolytic pathway (G-1). The benefit of the immediate phosphorylation of Glc in the cytoplasm by HK is to facilitate the cells taking up extracellular Glc, which occurs in a concentration-dependent manner through specific trans-membrane carriers (e.g. GLUT-1, 45 kDa form, in astrocytes; GLUT-3 in neurons). Glc is continuously delivered from blood to the extracellular milieu through the GLUT-1 (55 kDa) trans-membrane carrier, which is highly expressed by vascular ECs in the brain. Although issues such as substrate binding affinity and dissociation constant for these trans-membrane carriers have been very well studied (see a review by Simpson et al., 2007), little is understood thus far about how they are regulated by signal transduction. The HK enzyme adds a charged phosphate group to Glc to form G6P, an irreversible chemical reaction with Mg2+ as cofactor. There are no trans-membrane transporters for G6P, so it cannot leak out of the cells. Several HK isozymes have been found in mammalian brain, all of them distinguished in terms of location, kinetic characteristics with respect to Glc and operating conditions by product inhibition. However, most of these isozymes share a high affinity for Glc, even at very low concentrations, and strong inhibition by G6P (i.e. isozymes I, II and III). In contrast, glucokinase, an HK isozyme with no product inhibition and a very low affinity for Glc, has been mainly detected in brain areas containing glucose-sensing neurons (i.e. ventromedial nucleus and arcuate nucleus of the hypothalamus). However, glucokinase must be inoperative at the actual levels of Glc in the brain tissues during euglycemia, which are much lower than in the plasma. As far as it is known, the brain has no glucose-6-phosphatase, so G6P cannot be transformed back into Glc, the last step of gluconeogenesis.
The glycolytic pathway continues with the isomerization of G6P into β-D-fructose 6-phosphate (F6P) by phosphoglucose isomerase (PGI); and subsequently with the transformation of F6P into β-D-fructose 1,6-bisphosphate (F1,6BP) by the allosteric enzyme PFK-1. PFK-1 is not only inhibited by its product F1,6BP, but also by ATP, citrate and a low pH; the latter may aid in preventing the accumulation of cytosolic H+, in part caused by glycolytic activity itself. The enzyme has two sites with different affinities for ATP which is both a substrate and an allosteric inhibitor. Citrate is the first product of the TCA-cycle that occurs in the matrix of the mitochondrion. Several mitochondrial tricarboxylate carriers, possessing citrate transport activity, have been found in the brain. However, most of these tricarboxylate carriers are rather ubiquitous and strongly expressed in the mitochondria of neurons, although a number of studies have reported some more specific for glial cells. The allosteric inhibition of PFK-1 by citrate would serve to drop off the glycolytic pathway if the TCA-cycle is overworking. In addition, PFK-1 is activated by high-levels of adenosine monophosphate (AMP), but the most potent activator is β-D-fructose 2,6-bisphosphate (F2,6BP). For all these reasons, PFK-1 is considered the key regulatory point (G-2) in the glycolytic pathway. By using specific polyclonal antibodies, Almeida et al. (2004) found that expression of liver (L) and muscle (M) PFK-1 isoforms in neurons and astrocytes were very similar, but brain PFK-1 isoform (C) had approximately fourfold greater expression in astrocytes than in neurons.
In the brain, F2,6BP is reversibly produced from F6P by the homodimeric and bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFK-27) (Kessler and Eschrich, 2001), a reaction consuming one ATP (see a review by Rider et al., 2004). It is known that during fasting the concentration of F2,6BP is low such that PFK-1 activity is reduced. There are four PFK-2.(1-4) isoforms, which differ in their regulatory properties and kinase-bisphosphatase activity ratio. Among them, PFK-2.(3) exhibits the highest kinase-bisphosphatase activity ratio; hence, it is able to generate more F2,6BP at a given substrate concentration. Using reverse transcription polymerase chain reaction analysis of PFK-2 isoforms, Almeida et al. (2004) demonstrated that PFK-2.(3) had greater expression in astrocytes than in neurons. They showed data suggesting a rapid and cGMP8-independent up-regulation of the glycolytic pathway in astrocytes by NO, which does not occur in neurons. Exogenously applied NO produced a time-dependent decrease of F6P and an increase of F1,6BP concentration in astrocytes, which may be associated with enhancement of PFK-1 (C) isoform activity. The NO-mediated up-regulation of glycolysis in astrocytes was a direct consequence of the inhibition of mitochondrial oxidative-phosphorylation, specifically cytochrome c. These authors verified that the phosphorylation of the PFK-2.(3) isoform was mediated by the AMP-activated protein kinase (AMPK). AMP constitutes a crucial signal to maintain energetic equilibrium in the cell. AMPK-mediated phosphorylation events switch cells from active ATP consumption to active ATP production. This kind of event is referred to as a short-term regulatory process and may serve the cells, in this case the astrocytes, as protection against apoptosis. In any cell type NO could act as a signal molecule which regulates not only oxygen consumption in the mitochondrion but also reactive oxygen species production, both having implications for brain metabolism and cerebral blood flow control. As discussed later, NO may be also one of the pivotal mediators triggering the lactate shuttle from astrocytes to neurons. The cellular/tissue physiology and molecular mechanism underlying NO regulation of mitochondrial oxygen consumption have been recently discussed in a two-part review ([Giulivi et al., 2006] and [Cooper and Giulivi, 2007]).
The chemical reaction catalyzed by PFK-1, where Mg2+ is a cofactor, is energetically very favorable; hence, F1,6BP is forced to proceed down the glycolytic pathway almost irreversibly after this step (see details in Fig. 1, A). Except the final substrate-level phosphorylation by the allosteric enzyme PK, none of the intermediate enzymatic reactions are relevant for the discussion in this review. PK represents the last check point (G-3) in the glycolytic pathway. Pyr and ATP are irreversibly produced in this enzymatic reaction, where Mg2+ is a cofactor. Similar to PFK-1, PK is inhibited by ATP and citrate, having a comparable retarding effect on glycolysis when the cell is energetically charged or the mitochondrial TCA-cycle is saturated. In contrast, high levels of F1,6BP and phosphoenolpyruvate (PEP) might speed up the pay-off phase by activating PK. It has been reported that alanine also inhibits PK, which could be an important factor to maintain the ammonia homeostasis in the brain, as discussed latterly. A deficit of adenosine di-phosphate (ADP), indirectly related to high levels of ATP, also inhibits phosphoglycerate kinase (PGK), which could suggest a regulatory role for this enzyme. However, its impact on glycolysis is questionable, because when cells are short of ADP, and ATP is no longer required, PK drives the glycolytic pathway to completion.
The glycogen-shunt proposition
As mentioned above, G6P could have two fates, either being converted into F6P by PGI, and thus continuing along the glycolytic pathway, or otherwise being stored as glycogen polymer. Additionally, G6P may be oxidatively metabolized by G6P dehydrogenase which initiates the pentose shunt pathway. Glycogen polymers have been observed in the brain in vivo ([Öz et al., 2003] and [Gruetter et al., 2003]). Glycogen polymers are considered as one of the largest energy reservoirs in the brain, and could be used as an emergency fuel supply during severe energetic stress resulting not only from a pathological condition (e.g. hypoglycemia, cerebral ischemia) but also from high neuronal activity in a healthy brain (Gibbs et al., 2006). In such circumstances, GP rapidly catalyzes the phosphorolytic cleavage of about 90% of residues to glucose-1-phosphate, which is reversibly isomerized to G6P by phosphoglucose mutase (PGM). GP is an enzyme that is regulated by both phosphorylation and allosteric factors. The brain contains two of the three existing isoforms of the enzyme, i.e. the brain and the muscle types. The reversible phosphorylation of GP by phophorylase kinase converts the enzyme from a less activated, allosterically regulated form b to a more active, allosterically unresponsive form a. The phosphorylated enzyme is less sensitive to allosteric inhibitors. The dephosphorylation of GP is carried out by the enzyme called phosphoprotein phosphatase 1. From immunocytochemical studies, it has been shown that GP is mainly expressed in astrocytes (Cataldo and Broadwell, 1986). GP converts to form a through either the activation of the phosphorylase kinase by intense astrocytic Ca2+signaling or the cyclic AMP (cAMP) cascade of hormonal origin. In addition, GP is inhibited by G6P overloading. GP is considered a checkpoint (Gc-1) of the glycogen-shunt, not only for these reasons but also due to its direct activation by AMP and inhibition by ATP, both being allosteric effectors that to some extent reflect the cellular energy charge.
As mentioned before, the theoretical stoichiometric value for OGI is 6 for complete oxidation of Glc, but this value is not observed in the human brain even in awake resting conditions. Therefore, it was assumed that a fraction of Glc in the brain might be metabolized through glycogen polymers, which could increase with an enhancement of neuronal activity (Shulman et al., 2001b), a phenomenon mainly occurring in astrocytes. In recent work, Brown et al. (2005) used isofagomine, a novel inhibitor of GP, to block glycogen polymer degradation in the mouse optic nerve. They observed an acceleration of compound action potential failure after addition of isofagomine during both aglycemia and high-intensity stimulation conditions. Moreover, the amount of glycogen polymers found in neocortical astrocytes was more than twice that in cerebellar astrocytes (Sickmann et al., 2005). Conversely, the percent labeling of glycogen from [U-13C]glucose in cultured astrocytes from cerebellum was higher than in those originated from neocortex, indicating a higher glycogen turnover in the cerebellar astrocytes. In both cell types, its labeling was surprisingly reduced after inhibiting the degradation pathway of glycogen polymers, which might implicate the presence of some inhibitory feedback mechanism on the synthetic pathway. Labeling of intracellular lactate from [U-13C]glucose was threefold lower in neocortical astrocytes exposed to isofagomine compared to controls, but no change was observed in the labeling of extracellular lactate. The presence of isofagomine did not affect the labeling of citrate in either extra- or intra-cellular domains; however, a small but significant change was reported for glutamine. For example, the percent labeling of extracellular glutamine was slightly decreased in neocortical astrocytes exposed to the GP inhibitor, indicating an importance of glycogen turnover in the synthesis of releasable glutamine. These findings pointed to a compartmentalization of these metabolites in astrocytes, markedly exhibited for lactate, which might be driven by glycogen polymer turnover, glycolysis and TCA-cycle activity (Sickmann et al., 2005).
On the other hand, GS is an allosteric enzyme catalyzing the formation of glycogen polymers from glucose-1-phosphate, and is considered to be the other checkpoint in the glycogen-shunt (Gc-2). GS is allosterically activated by G6P and inhibited by physiological concentrations of ATP, ADP and inorganic phosphate (Pi). Like GP, allosteric controls of GS are overridden by reversible phosphorylation. GS is likewise phosphorylated and dephosphorylated by phosphorylase kinase and phosphoprotein phosphatase 1, respectively. GS could be inhibited by protein kinase A (PKA) phosphorylation during times of high stress or low Glc levels. One ATP equivalent is required to synthesize a glycogen polymer from glucose-1-phosphate, which makes this pathway energetically inefficient. Neurons have large expression of GS (Vilchez et al., 2007), while GP is poorly expressed (Cataldo and Broadwell, 1986). Brain glycogen is contained predominantly in astrocytes, a cell type which express both GP and GS ([Cataldo and Broadwell, 1986] and [Ignacio et al., 1990]). However, phosphorylase kinase subunits (Phka1, Phkg2) are expressed exclusively in astrocytes (Pfeiffer et al., 1992). A challenge for the coming years will be to understand both inter-and intra-cellular mechanisms underlying the compartmentalization of glycogen polymers in the brain.
The glycogen-shunt is considered by some authors as a fight-or-flight response, which is in concordance with the fact that several hormones, peptides and neurotransmitters could also regulate storage and mobilization of glycogen polymers (e.g. glucagon, insulin, adrenaline, noradrenaline, serotonin, histamine, vasointestinal peptide). However, their mechanisms of action in the brain are not yet completely understood. Recent studies based on the use of GP-specific inhibitors [i.e. iminosugars such as isofagomine and 1,4-dideoxy-1,4-imino-D-arabinitol (DAB)] have provided evidence that glycogen turnover is essential to maintain, e.g. normal glutamatergic activity (i.e. glutamate release and uptake, Sickmann et al., 2007). Moreover, using the same tool Gibbs et al. (2006) have shown that glycogen turnover is required for memory consolidation in young chickens. Altogether, this points to an important function of the glycogen path and in particular its turnover in brain function.
The breakdown of glycogen polymers into G6P, which involves few steps, is primarily dependent on glycogen polymer storage, while Glc breakdown is intrinsically linked to regulation of Glc uptake. An important area of research into the glycolytic pathway which needs further study is the heterogeneous degree of cell-specific Glc uptake, where both neuronal and astrocytic compartments are involved. As for Glc breakdown in these respective compartments, the roles of several ions also require further investigation. Glycogen polymer breakdown preferentially implicates the astrocytic compartment but its activation would plausibly seem to be linked to the neuronal compartment. The OGI may be an important link mediating the balance between the glycolytic pathway and glycogen-shunt (Shulman et al., 2001b).
The astrocyte-neuron lactate shuttle
The hypothetical involvement of a three carbon metabolite that mediates communication between neuronal and astrocytic events implicates lactate as the molecule. While this proposal has many interesting implications as discussed below; most importantly, this idea changes the classical viewpoint of lactate as an end-point of the glycolytic pathway. Rather, according to this proposal, lactate can subsequently be oxidized in a different compartment where the enzyme lactate dehydrogenase (LDH) may play an important role. Experimental studies providing evidence for and against this working hypothesis are discussed in this section.
Major findings and implications
The fate of the major product of glycolysis, the cytosolic Pyr, depends on several factors, some of which possibly being related to the cellular energy charge and others to the action of metabolite shuttles to maintain neurotransmitter, ammonia and carbon homeostasis among domains in the cell and in its surroundings. For instance, Pyr in the cytosol could be reduced to lactate by the enzyme LDH. This phenomenon has been called anaerobic glycolysis as it was thought to occur in several tissues, but only when oxygen is depleted during prolonged vigorous cellular activity. Several LDH isozymes have so far been characterized in mammals; however, most of them are formed by combining the LDH-1 (heart type) and LDH-5 (muscle type) subunits. The reversible chemical reaction catalyzed by the LDH enzyme is:
The kinetic characteristics of these two subunits are very dissimilar. Early studies showed that product inhibition is predominantly noncompetitive in both subunits, with the peculiarity of a marked difference in the extent of the inhibition by lactate of Pyr reduction in terms of the dissociation constant (LDH- 5 < < LDH- 1), but very little difference in the inhibition by Pyr of lactate oxidation. However, at a wide range of Pyr and lactate concentrations, substrate inhibition has been reported to be insignificant in both subunits, although the substrate binding affinity for LDH-5 is greater than for LDH-1. Bittar et al. (1996), using polyclonal antibodies against these two subunits, demonstrated that neurons and astrocytes were both stained by an antibody against LDH-1, but the inmunoreactivity against LDH-5 was exclusively restricted to astrocytic populations. In this context, it may be worth considering that mature cultured astrocytes express all five LDH isoenzymes (Nissen and Schousboe, 1979). Moreover, as an indication that expression of LDH-1 is not a prerequisite for effective metabolism of lactate it should be noted that cerebellar neurons in culture, which utilize lactate as an energy substrate (Bak et al., 2006a), do not express LDH-1 (Schousboe et al., 1993). A year after Bittar et al.,’s discovery, Bergles and Jahr (1997) provided evidence of specific carriers in the membrane of hippocampal astrocytes that use the Na+ electrochemical potential as a driving force to cotransport glutamate, so guaranteeing its rapid clearance from the synaptic cleft in preparation for the next neurotransmission.
The major implication for functional neuroimaging of these findings emerged when several subsequent studies using NMR spectroscopy (Hyder et al., 2006 and references therein) demonstrated a tight stoichiometric coupling (≈ 1:1) between total glutamate-glutamine cycling and the neuronal Glc oxidation rate, measured as one-half of the rate of TCA-cycle in neurons, in the cerebral cortex of anesthetized rats under a range of brain activities from deep isoelectric to awake. However, a fraction of around 15% of the Glc oxidized in neurons was not coupled to the total glutamate-glutamine cycle. This fraction might represent ATP needed for non-cycling activities. Based on these observations, Magistretti et al. (1999) suggested that the glutamate-glutamine cycle, a two ATP-consuming dual task (i.e. one ATP is used to convert glutamate into glutamine by glutamine synthetase and the other to reestablish the Na+ gradient by the activation of the Na+K+-ATPase), might trigger lactate production by glycolysis in the astrocytes during the course of neuronal activity, so stimulating them to take up extracellular Glc (see a review by Pellerin and Magistretti, 2004a). Lactate so produced may be released for oxidation in adjacent neurons. As pointed out by Hertz et al. (2007) in an extensive review on astrocytic metabolism it is not at all clear where the oxidation of lactate derived from Glc and/or glycogen metabolism may occur. Moreover, during activation, glutamatergic activity in neurons is better preserved by Glc metabolism than by lactate metabolism (Bak et al., 2006a). An increase of lactate under steady state conditions has been reported in several studies. Lactate9 may be taken up into neurons through the monocarboxylate transporter MCT-2, which has the highest affinity for its substrate (Fig. 2). An extensive overproduction of lactate must efflux into the circulating blood.
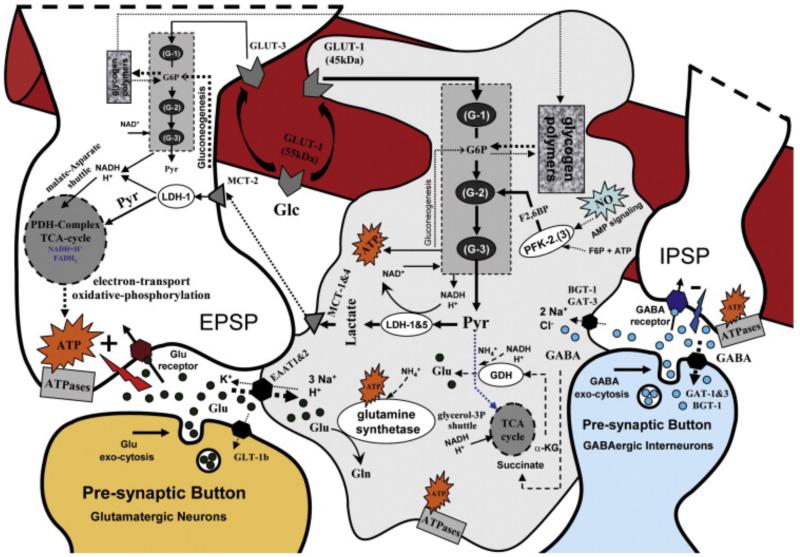
Fig. 2.
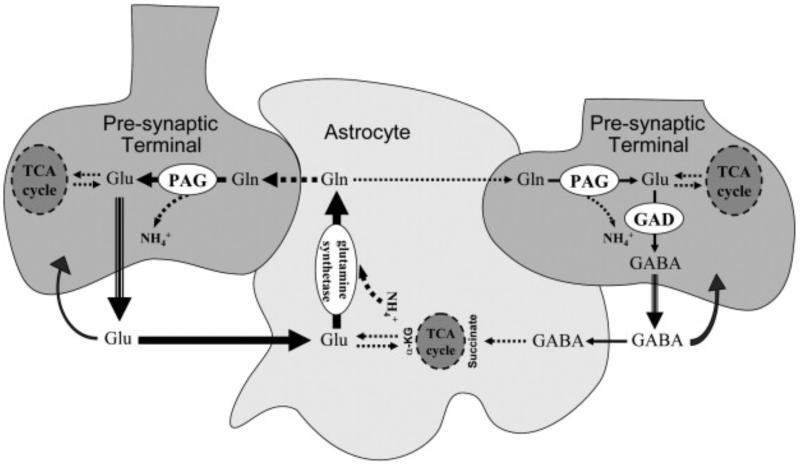
Schematic representation of the glycolysis/glycogen-shunt (grey even/mosaic squares) and TCA-cycle (grey circles) pathways in nerve ending particles and astrocytes, as well as the respective single/multi cellular compartmentalization for the metabolite/ion flows (arrows). The impact of each pathway in neurons and astrocytes is symbolized by the size of the squares/circles. Glc is continuously delivered from capillaries (red) to the extracellular milieu through GLUT-1 (55 kDa). Cells take up extracellular Glc through GLUT-1, 45 kDa form (astrocytes) and GLUT-3 (neurons). In different proportions, Pyr and ATP are produced from glycolysis in both cell types. The astrocytes-neuron lactate shuttle is facilitated by the differential presence of LDH-1 and LDH-1&5 in neurons and astrocytes, respectively. This shuttle may be favored by the existence of monocarboxylate transporters MCT-2 and MCT-1&4 in neurons and astrocytes, respectively. NO may catalyze the glycolysis in astrocytes through PFK-2.(3). Neurotransmitters [e.g. glutamate (Glu), GABA] released into the synaptic cleft in the course of pre-synaptic neuronal activity will freely diffuse toward neuronal postsynaptic buttons and nearby astrocytic processes. They will cause either excitatory (EPSP) or inhibitory (IPSP) postsynaptic potentials by receptor-specific flows of ions in the postsynaptic neurons. After the genesis of EPSP/IPSP, ionic gradients will be reestablished by way of transmembrane ATPases, which consume large amounts of ATP. In the postsynaptic button, the required ATP is produced from Pyr through the TCA-cycle and electron-transport/oxidative-phosphorylation pathways. The major portion of the lasting extracellular glutamate is promptly taken up into astrocytes via EAAT1&2, although pre- and post-synaptic terminals of glutamatergic neurons could also take up smaller amounts of extracellular glutamate via GLT1-b and EAAT3, respectively. In contrast, the pre-synaptic terminals of GABAergic interneurons take up most of the extracellular GABA via transporters GAT-1&3 and BGT-1. However, it is known that a small fraction is taken up by astrocytes via transporters GAT-3 and BGT-1 to contribute to the overall carbon and ammonia homeostasis in the nerve ending. GABA inside the astrocytes could be catabolized to succinate to enter the TCA-cycle. The transferred carbon will flow out of the TCA-cycle as α-KG, which could then be converted into glutamate by either glutamate dehydrogenase (GDH) or an aminotransferase. Astrocytic glutamate is converted to glutamine (Gln) by glutamine synthetase, a chemical reaction consuming one ATP. Several ions (e.g. Na+, K+, H+, Cl−) are co/anti transported with glutamate and GABA while these neurotransmitters are taken up by astrocytes. ATP are required by different ATPases to reestablish the ionic equilibrium concentrations. Pyr could enter the TCA-cycle in astrocytes to supply needed carbons (dotted blue line).
Energetic budget for excitation and inhibition
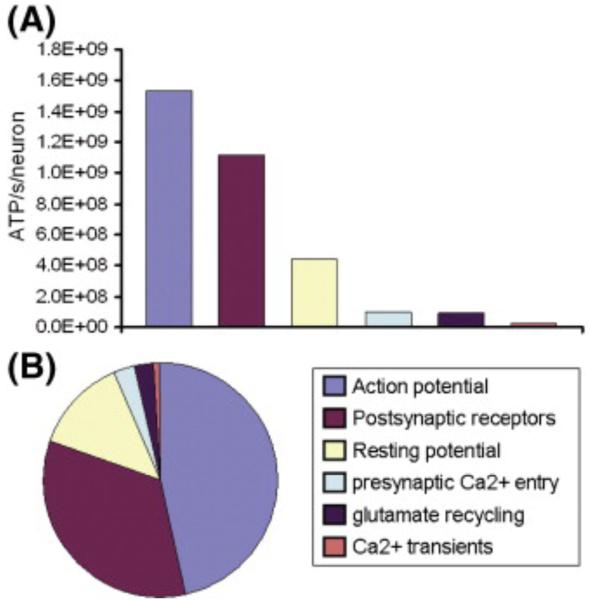
Magistretti et al. (1999) emphasized that if their hypothesis holds, the major fraction of Glc usage in the cerebral cortex measured by PET and fMRI would be directly related to excitatory glutamate release. They did not discard the possibility that other neurotransmitters (e.g. GABA) could also elicit a metabolic demand, but argued that in such a case their contribution should be properly quantified. The last suggestion initiated a vigorous discussion about the amount of Glc required to be oxidized in order to fuel inhibitory activity. Even though many questions were clarified by using different techniques [e.g. Waldvogel et al., 2000 (fMRI and transcranial magnetic stimulation), Chatton et al., 2003 (microspectrofluorimetry)], the debate continued for several years, particularly as exact quantitative values of each metabolic pathway were not available. Activity in GABAergic interneurons might be enhanced by the level of anesthesia, a situation causing some difficulties in the interpretation of results obtained from in vivo preparations. Several researchers were concerned about the contribution of metabolic pathways that were not measured in these previous studies (e.g. oxidation of Pyr in astrocytes by TCA-cycle) as well as the distinction from the observed data of those metabolite flows that were intrinsically mixed. For example, even though released GABA is mainly taken up by neurons using a trans-membrane transport mechanism, a small portion could also be recycled through astrocytes (Schousboe et al., 2004a); this might indirectly affect the observed rate of glutamate-glutamine cycling. Among the most significant questions raised were: a-what fraction of the total oxidized Glc in neurons corresponds to direct Glc uptake and what comes from astrocyte-neuron lactate shuttling?, b-how do GABAergic and glutamatergic neurons use the total oxidized Glc?, and c-what proportion of the total glutamate-glutamine cycle is due to GABA recycling through astrocytes? Some of these questions have received an explanation from recent studies. For example, distinctions between glutamatergic and GABAergic fluxes require measurement of 13C turnover from Glc and acetate into glutamate, glutamine, and GABA (Pfeuffer et al., 1999). The ingredients necessary for a dynamic metabolic modeling in such case are discussed in a review by Henry et al. (2006). By using 13C NMR spectroscopy in anesthetized rats, explicitly combining [1-13C]glucose and [2-13C]acetate infusion with three-compartment metabolic modeling, Patel et al. (2005) showed recently that total neurotransmitter cycling (Vcyc(tot)) could be resolved into separate glutamatergic (Vcyc(glu)) and GABAergic (Vcyc(GABA)) components. Similarly, neuronal Glc oxidation (CMRglc(ox),N) could also be separated into glutamatergic (CMRglc(ox), glutamate) and GABAergic (CMRglc(ox), GABA) components. These authors documented that the GABA-glutamine cycle comprised 23% of total neurotransmitter cycling and the contribution of GABAergic neurons to total Glc oxidation in the cerebral cortex is around 18%. Hyder et al. (2006) showed that neurons and astrocytes produce at least 88% and 8% of the total oxidative ATP, respectively. They also found a partitioned Glc uptake of ~ 26% by neurons and of ~ 74% by glia, which is ~ 30% less than predicted in previous studies. It should be emphasized that other authors (e.g. Hertz et al., 2007) have provided evidence that astrocytes account for a degree of oxidative metabolism which reflects their relative volume in the brain. Therefore, many issues still remain to be deciphered in the coming years.
The notion of an astrocyte-neuron lactate shuttle, strategically triggered by the glutamate-glutamine cycle to provide large amounts of energy for demanding neurons in close proximity, is still being debated ([Chih et al., 2001], [Dienel and Hertz, 2001], [Gjedde and Marrett, 2001], [Marcaggi and Attwell, 2004], [Hertz, 2004], [Bonvento et al., 2005], [Hertz et al., 2007] and [Simpson et al., 2007]). For example, Chih et al. (2001) have revisited some critical aspects and compared it with the classical neuroenergetics viewpoint. Based on a theoretical model for the kinetics of Glc and lactate transporters in the brain, Simpson et al. (2007) have suggested that Glc diffuses through the basal lamina and interstitium to neurons. These authors have also claimed that neurons are responsible not only for most of Glc uptake and metabolism, but also for the generation of the lactate transients observed during neuronal activity. We would like to highlight here that astrocytes apparently meet the requirements for that purpose as discussed in Magistretti and Pellerin (1999a): a) they are ideally positioned between neurons and vessels, b) they possess certain specialized processes that cover the surface of intraparenchymal capillaries allowing them to take up Glc easily, while other processes, enriched in high-affinity trans-membrane glutamate transporters EAAT1&2 as well as in metabotropic glutamate receptors mGluR, are embedded within synapses; thus they would be ideally situated to take up glutamate from the synaptic cleft, c) these latter processes also possess trans-membrane GABA transporters BGT-110 and GAT-3 (Schousboe and Kanner, 2002), d) they are rich in lactate MCT-1 and MCT-4 monocarboxylate transporters.
By using two-photon laser scanning fluorescence microscopy, Kasischke et al. (2004) observed a biphasic response in the endogenous NADH signaling during focal neuronal activity in hippocampal slice preparations. They showed two additive and anticolocalized monophasic NADH responses, a rapid initial dip and a lengthy overshoot, with the latter often colocalized with astrocytes and their processes. The initial dip was assumed to have a neuronal origin and the authors speculated that it comprised a first consumption of NADH by oxidative-phophorylation so as to produce the required ATP, followed by its replenishment through dehydrogenase activity in the TCA-cycle. We believe that LDH-1 activity, as a result of the astrocyte-neuron lactate shuttle, might also contribute to the delayed neuronal NADH signal. By contrast, the late overshoot in astrocytes was thought to be caused by an increase of glycolytic NADH, which is used at the end to reduce Pyr into lactate. The complete annihilation of the initial dip and attenuation (~ 39%) of the overshoot after applying a glutamate receptor antagonist (6-cyano-7-nitroquinoxaline-2,3-dione) makes it more difficult to interpret the nature of the total NADH signal, although the authors justified the latter results by hypothesizing an entirely dendritic (i.e. postsynaptic) origin of the neuronal NADH component. In this context, the finding by Almeida et al. (2004) could suggest that the ATP deficit in astrocytes could be caused not only by its direct consumption in the glutamate-glutamine cycle, but also by a temporal cessation of its production inside the mitochondrion, which is mediated by NO signaling. Although Kasischke et al. (2004) have reported that such spatiotemporal partitioning of the glycolytic and TCA-cycle pathways between astrocytes and neurons are supportive of the astrocyte-neuron lactate shuttle (Pellerin and Magistretti, 2004b), none of the key components of the model (i.e., lactate or Pyr) were ever assayed in the study. Subsequent studies using optical measurement in hippocampal slices (Brennan et al., 2006) and 1H NMR spectroscopy in human brain (Mangia et al., 2007) demonstrate that it is too simplistic to depict NADH transients as recruitment of glycolytic metabolism.
Also, using NMR spectroscopy, Chen et al. (2005) have shown an increase of the endogenous GABA levels in the α-chloralose anesthetized rat brain after acute administration of vigabatrin or gabaculine, which are suicide inhibitors of GABA transaminase (GABA-T11). These inhibitors are structural analogues of GABA which are highly specific for the degrading enzyme GABA-T (Sarup et al., 2003). An elevated concentration of endogenous GABA could facilitate transporter-mediated GABA release; and hence elicit activity-dependent reinforcement of inhibition. These authors found a decrease in the amplitude of the BOLD signal in the rat somatosensory cortex during forepaw stimulation which significantly correlated with the rise of endogenous GABA. Given that an increase of exogenous GABA levels might result in dilation of pial vessels, via GABAA receptors (Fergus and Lee, 1997), such a negative effect on the BOLD response remains to be investigated. One possibility is that acute reinforcement of inhibition may also cause a dropping off in cortical excitability; hence, an indirect decrease in the CBF as a result of a fall in the production of astrocyte-derived vasodilator factors. However, further secondary effects might be associated with a strengthening or reduction of GABAergic inhibition. GABAergic interneurons are key elements in the complex cortical wiring and their activation might affect functional neuroimaging through a diversity of mechanisms other than just a suppression of cortical excitability or an alteration of the total metabolic cost due to GABA/glutamine cycling.
For example, vasomotions of diverse origins (reviewed in part II) could be induced by specialized GABAergic interneurons (Cauli et al., 2004). Furthermore, the fact that neuronal NO synthase (NOS) is largely expressed in axon terminals (comment: perhaps also in minor quantities at dendritic boutons) of GABAergic interneurons (Wang et al., 2005), which are minimally separated from penetrating arterioles/capillaries by astrocytic processes, could have a dual role in preventing neuronal apoptosis: a) to facilitate the delivery of oxygen and Glc to tissue by increasing the CBF (i.e. neurovascular coupling) and b) to enhance the cellular glycolytic respiration in astrocytes to fuel nearby neurons (i.e. neurometabolic coupling). NO released by GABAergic interneurons might not only induce strong dilations of SMCs (Estrada and DeFelipe, 1998), but may also impinge on cerebral metabolism by switching on/off the glycolytic pathway in astrocytes (Almeida et al., 2004). Finally, McKenna and Sonnewald (2005) suggested that exogenous GABA could substitute for glutamate as an energy source for astrocytes. Indeed GABA has been shown to support oxidative metabolism in cultured astrocytes (Yu and Hertz, 1983). However, a recent study revealed that metabolism in the cerebral cortex could be stimulated, dampened or even unaltered by local inhibitions (Nasrallah et al., 2007). Therefore, the effect of GABAergic modulation on brain metabolism and blood circulation must be carefully examined in the future by separating its direct and indirect components.
The metabolic pathways and mechanisms for substance traffic discussed up to now succeed in explaining the fate of carbon atoms; however, they implicitly include an astrocyte-neuron detoxification pathway of glutamine synthesis, an issue that will be discussed in detail shortly. Alternative approaches have also been formulated in previous works to account for global homeostasis in the brain through an ammonia (NH4+) detoxification (or anaplerotic) pathway for the synthesis of glutamine as well as an astrocytic pathway for neuronal glutamate repletion (see a review by Rothman et al., 2002). Recently, the astrocyte-neuron lactate shuttle was extended by Waagepetersen et al. (2000) to include an intracellular mechanism for ammonia homeostasis in glutamatergic neurons and astrocytes, denoted the lactate-alanine shuttle. The lactate-alanine shuttle and the associated glutamate-glutamine cycle have been shown to coexist in cerebellar cocultures, but they were uncoupled and only the latter seemed activity-dependent (Bak et al., 2005). These authors used alanine, glutamine and ammonia as precursors and applied mass spectrometry to analyze cell extracts.
Astrocytic metabolic waves
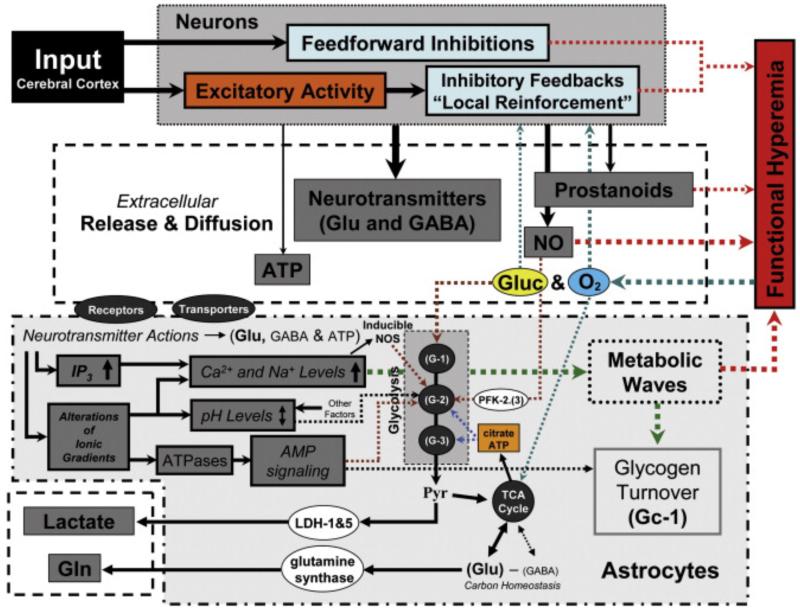
Regarding the question of whether glycogen polymers could participate in astrocytic glycolysis, Sickmann et al. (2005) provided evidence that lactate originating from glycogen polymers constitutes an alternative source of fuel, although its use by adjacent neurons depends on the availability of Glc. During sustained stimulation, a rapid accumulation of astrocytic intracellular Ca2+ could promote the phosphorylation of GP to its form a, resulting in uncontrollable cleavage of glycogen polymers, and consequently, an increase of lactate in the extracellular milieu. Astrocytes appear to function as a network for concerted neurometabolic coupling through the generation of intercellular Na+- and Ca2+-mediated metabolic waves (Bernardinelli et al., 2004). Despite some evidence having been provided therefor (Hirase et al., 2004), the existence of such Ca2+ waves and their propagation through the astrocytic networks still remains to be rigorously demonstrated in vivo. Astrocytes express a plethora of metabotropic receptors that can couple to second messenger systems [e.g. norepinephrine, glutamate, GABA, acetylcholine, histamine, adenosine, and ATP], which have been demonstrated to induce Ca2+elevations in glial cells in brain slice preparations (see list of references in Haydon and Carmignoto 2006). Most studies reporting metabotropic receptors have been performed in culture; hence, it is not yet determined whether these receptors exist in astrocytes under in vivo conditions. It is hypothesized that release of ATP and glutamate by astrocytes in the extracellular space represents a main signaling mechanism of the Ca2+ waves, whereas the Na+ waves may result from the activation of Na+-glutamate transporters EAAT1&2 during an elevation of extracellular glutamate levels. The Na+ waves give rise to a spatially-correlated increase in Glc uptake. Extracellular glutamate could also bind to the metabotropic glutamate receptor mGluR on astrocytes, which might induce a phospholipase C-dependent accumulation of inositol trisphosphate (IP3) that stimulates the release of Ca2+ from IP3-sensitive internal stores. It is also conjectured that a large quantity of extracellular ATP could bind to P2 purinoceptors (i.e. P2Y receptors) in nearby astrocytes, a mechanism that could facilitate Ca2+ wave propagation among neighboring disconnected astrocytes. It has been reported that some Ca2+ oscillations are restricted to portions of the processes of individual astrocytes, called microdomains. The existence of these microdomains confirms that astrocytes are functionally compartmentalized, as discussed below. Ca2+ signaling in astrocytes could induce a Ca2+-dependent synthesis of NO through inducible NOS, which in turn could stimulate the Ca2+ influx pathway, a mechanism thought to be also responsible for the refilling of internal Ca2+ stores in astrocytes (Li et al., 2003).
Because the astrocyte-neuron lactate shuttle can be shown to be linked to many important inter-cellular communicating pathways, it can be appreciated why this proposal has been difficult to confirm experimentally. While there are results in support of the idea, there are findings which question the validity of the shuttle hypothesis. However an apparent caution in interpretation of these varied results is differences expected between in vitro and in vivo preparations. Nevertheless, an important area of research which could potentially shed novel insights into the astrocyte-neuron lactate shuttle is NAD+/NADH balance. Furthermore, the involvement of this shuttle comprising enzymes which could send signals to the vasculature could have implications for the interpretation of several functional neuroimaging modalities.
TCA-cycle and oxidative-phosphorylation
An understanding of the single- and multi-cellular compartmentalization of metabolites, as well as the mechanisms for their exchange among domains, is needed for a reliable interpretation of a number of functional neuroimaging modalities (Waagepetersen et al., 2003). In order to evaluate this issue, in what follows, we will review the TCA-cycle and the associated intracellular malate-aspartate and the glycerol 3-phosphate shuttles. The majority of ATPs yielded from Glc oxidation are generated by breakdown of carbon skeletons in the TCA-cycle and electron donors located inside the mitochondria. The biochemical mechanisms underlying these energy substrates as well as major enzymatic check points inside the TCA-cycle and electron-transport/oxidative-phosphorylation pathway will be discussed in this section.
The PDH-complex, the lobby for the TCA-cycle
Pyr is actively transported12 into the mitochondrion, a process inhibited by cyano-OH-cinnamate. Moreover, Pyr molecules after entering the mitochondrion will encounter two metabolizing routes: their irreversible carboxylation by the pyruvate carboxylase [i.e. Pyr→oxaloacetate (OAA)] and their oxidative decarboxylation by the pyruvate dehydrogenase (PDH)-complex [i.e. Pyr→acetyl-coenzyme A (Acetyl-CoA)]. It has been pointed out that the former is vital for the synthesis of citrate occurring in distinct domains of the cells; however, its main role might be either to incorporate extra OAA needed by the TCA-cycle or to begin the gluconeogenesis if there is a excess of Pyr. The latter is thought of as a “transition reaction” to prepare the Pyr for its oxidation in the TCA-cycle. In addition, this process is necessary for net synthesis of glutamine (Waagepetersen et al., 2007).
As aforementioned, the PDH-complex pathway and the TCA-cycle occur sequentially inside the mitochondrial matrix. Depending on the energy requirement in the cell, the PDH-complex (Fig. 3, top) produces Acetyl-CoA from Pyr, a step implicating utilization and formation of a number of cofactors. Acetyl-CoA cannot leave the mitochondrion because of its very large size; thus the PDH-complex maintains a positive flow of carbon toward the TCA-cycle. The PDH-complex is active in a desphosphorylated state and becomes inactive when it is phosphorylated by the PDH-kinase, an enzymatic reaction requiring ATP. The activity of the PDH-kinase is enhanced by energy-rich molecules (e.g. NADH, ATP, Acetyl-CoA); hence, the flow of carbon into the TCA-cycle is diminished when the cell is charged energetically. Once the energy levels in the cell start to fall, NAD+, coenzyme A (CoA-SH) and ADP will accumulate, which has a negative allosteric effect on the activity of the PDH-kinase. Also, high levels of Pyr and Ca2+ inhibit this enzyme. Inactive PDH-complexes are uninterruptedly dephosphorylated by the PDH-phosphatase; however, the activity of this enzyme rises when Ca2+and Mg2+ are present. Additionally, two products of the PDH-complex, i.e. NADH and Acetyl-CoA, reduce the affinity of the complex for Pyr, which constitutes a negative allosteric effect. For all the abovementioned, the PDH-complex represents the first checkpoint of this pathway (H-1).
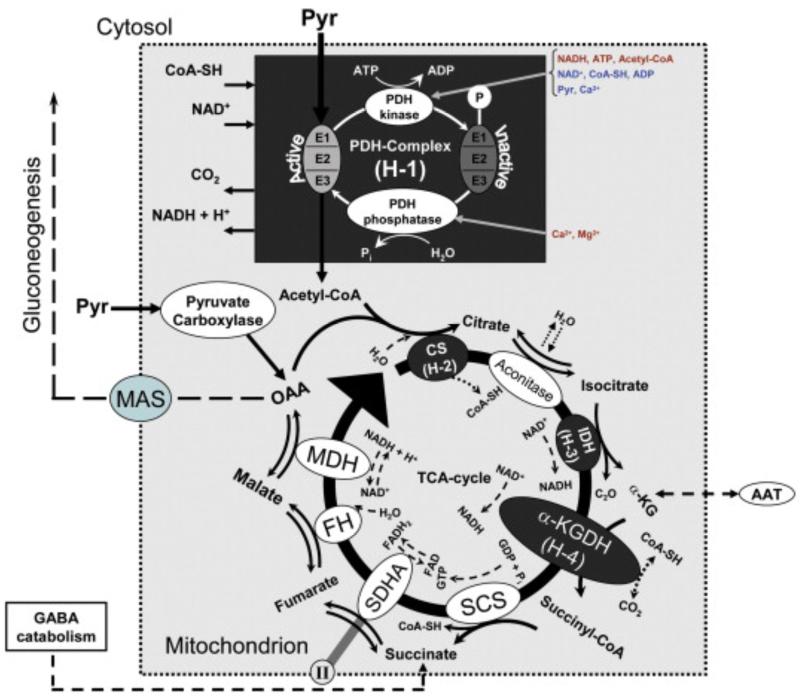
Fig. 3.
Diagram of the PDH-complex/TCA-cycle. The PDH-complex comprises just one step: a decarboxylation [enzymes (PDH “E1”, dihydrolipoyl transacetylase “E2”, dihydrolipoyl dehydrogenase “E3”), substrates (Pyr, CoA-SH, NAD+), products (Acetyl-CoA, CO2, NADH), comment (there are multiples copies of the enzymes E1, E2 and E3, depending on species)]. In order to sense the cellular energy charge, two enzymes (i.e. the PDH-kinase and the PHD-phosphatase) are endowed with a variety of allosteric modulators and covalent modifiers. These enzymes compete to determine the state of phosphorylation of the PDH-complex, and consequently to regulate its activity. The negative and positive allosteric effectors to the PDH-complex are highlighted in blue and orange, respectively. The TCA-cycle comprises eight steps: a condensation [enzyme (CS), substrates (OAA, Acetyl-CoA, H2O), products (citrate, CoA-SH), comment (it is also referred to as the first committed step in the cycle)], an isomerization [enzyme (aconitinase), substrate (citrate), product (isocitrate), comment (H2O is used for a sequential dehydration and hydration, with the cis-Aconitate as the intermediate)], a first oxidative decarboxylation [enzyme (IDH), substrates (isocitrate, NAD+), products (α-KG, NADH, CO2), comment (isocitrate is firstly oxidized to oxalosuccinate, which in turn decarboxylates to α-KG)], a second oxidative decarboxylation [multienzyme complex (α-KGDH), substrates (α-KG, NAD+, CoA-SH), products (Succinyl-CoA, NADH, CO2), comment (it is very exergonic)], a substrate-level phosphorylation [enzyme (SCS), substrates (Succinyl-CoA, GDP, Pi), products (succinate, GTP, CoA-SH), comments (a- a hydrogen ion bound to Pi enters the TCA-cycle, represented in the stoichiometry of the overall chemical reaction, b- GTP is finally used in a trans-phosphorylation catalyzed by the mitochondrial nucleoside diphosphokinase to phosphorylate ADP, producing ATP and generating GDP)], a first dehydrogenation [enzyme (SDHA), substrates (succinate, FAD), products (fumarate, FADH2), comment (SDHA is tightly bound to the mitochondrion inner membrane through the protein subunits SDHB, SDHC, and SDHD, which all constitutes the complex II of the electron-transport chain)], a hydration [enzyme (FH), substrates (fumarate, H2O), product (malate)], a second dehydrogenation [enzyme (MDH), substrates (malate, NAD+), products (OAA, NADH, H+), comment (it is highly endergonic; however, the exergonic character of the upcoming condensation drives OAA formation by mass action principals)]. In spite of the last seven steps in the TCA-cycle being reversible, the cycle always flows in a clockwise direction (black curved arrow). The reason for that is the irreversible character of the condensation with a thermodynamic equilibrium in favor of the products. Glutamate can enter the TCA-cycle by either oxidative deamination catalyzed by GDH or transamination via the aspartate aminotransferase (AAT), an enzymatic reaction producing aspartate from OAA. Abbreviations: IDH → isocitrate dehydrogenase, α-KGDH → α-Ketoglutarate dehydrogenase, SCS → succinyl-CoA synthetase, GDP → guanosine diphosphate, SDHA → succinate dehydrogenase, FAD → flavin adenine dinucleotide (oxidized form), FH → fumarase, MDH → malate dehydrogenase.
AMP could inhibit the activity of Acetyl-CoA carboxylase (ACC), thereby increasing Acetyl-CoA levels inside the mitochondrion. ACC catalyzes the biotin-dependent conversion of Acetyl-CoA, HCO3 and ATP to malonyl-CoA. However, its role in brain is poorly understood. When the levels of mitochondrial Pyr are sufficiently high and the cell is energetically charged, Pyr carboxylation into OAA may occur. In that situation, OAA could be transported back into the cytosol by the malate-aspartate shuttle (MAS)13 (McKenna et al., 2006c) for its decarboxylation and simultaneous phosphorylation to produce PEP, which is catalyzed by the PEP carboxykinase. This is also referred to as the first step in gluconeogenesis, but as mentioned above, the brain lacks glucose-6-phosphatase; hence, the final product of this upstream pathway will be G6P, which then could be stored as glycogen polymer. Note that any intermediate sub-product of the gluconeogenic pathway could potentially be converted again into Pyr by glycolysis whenever fuel is required by the cell.
The PDH-complex pathway is followed by the TCA-cycle14, a series of endergonic/exergonic chemical reactions involving not only the catabolism of energy-rich molecules but also providing precursors for many components that are utilized in the overall cellular metabolism. A synopsis of the TCA-cycle is: “two carbons are oxidized to CO2 and the energy from the involved chemical reactions is stored in the form of guanosine triphosphate (GTP) and in energy-rich electron donors, i.e. NADH and reduced flavin adenine dinucleotide (FADH2)”. Despite the complexity of the whole TCA-cycle, there are only three main checkpoints. The first (H-2) is the condensation of OAA to citrate by citrate synthase (CS). The activity of CS is inhibited by mitochondrial citrate. This product inhibitory feedback in the TCA-cycle in combination with the allosteric inhibition of glycolytic enzymes PFK-1 and PK by cytosolic citrate could be of great consequence for the crosstalk between both cell domains to coordinate these two metabolic pathways. Additionally, CS regulates its activity, thus controlling the flow of carbons into the TCA-cycle, by allosteric effects depending on the cellular energy charge; i.e. inhibition through succinyl-CoA synthetase (Succinyl-CoA), ATP, NADH and activation in the presence of high levels of ADP. Likewise, isocitrate dehydrogenase (IDH) catalyzes the rate-limiting step in the cycle by both negative (e.g. NADH, ATP) and positive (isocitrate, ADP, AMP, Ca2+) allosteric effectors. α-Ketoglutarate (α-KG), a product of IDH, is an important metabolite in driving the MAS. For these reasons, IDH (H-3) also constitutes a checkpoint in the TCA-cycle. α-Ketoglutarate dehydrogenase (α-KGDH), which represents the last checkpoint (H-4), is very similar to the PDH-complex in the intricacy of its protein makeup, cofactors and mechanisms of action. Its activity is inhibited by ATP and activated by NAD+, both allosterically. Also, Succinyl-CoA and NADH are negative product effectors for this enzyme. It has been reported that the activity of this enzyme increases in the presence of Ca2+. The mitochondrial NAD+/NADH ratio constitutes one of the major regulators of the TCA-cycle and its value is strongly affected by the level of oxygen as a result of the electron-transport/oxidative-phosphorylation pathway. Fig. 3 (bottom) summarizes the entire TCA-cycle, the final result of which is the production of three NADH, one FADH2, two H+ and one GTP. At the end, two carbon atoms that entered the TCA-cycle are released in the form of CO2. It must be noted, however, that the carbon atoms that are released as CO2 originate from OAA and not from Acetyl-CoA. This is important for the understanding of labeling studies using [13C]acetate or –Glc and subsequent NMR spectrometry to monitor metabolism and TCA-cycle activity (see McKenna et al., 2006b).
The electron-transport/oxidative-phosphorylation
The purpose of the electron-transport/oxidative-phosphorylation pathway is to create ATP from high-energy electron donors located inside the mitochondrial matrix. Electrons enter the electron-transport chain from NADH and FADH2 donors in complex I and II, respectively. These electrons are transferred to coenzyme Q (CoQ15), the reduced form of which (ubiquinol, CoQH2) carries them through the mitochondrial inner membrane to complex III. From complex III, electrons are transferred to a peripheral membrane protein, cytochrome c, which takes them into complex IV. Several studies have reported a potent reversible inhibition of cytochrome c by nanomolar concentration of NO, particularly in synaptosomes. Under special conditions, NO could inhibit mitochondrial respiration by 85-90% (references in Almeida et al., 2004). Complex IV uses the electrons to reduce O2 to H2O. The entire route represents a multi-step redox process that involves several enzymes, most of them crossing the mitochondrial inner membrane acting like ferryboats to deliver H+ to the outer chamber, named proton pumps. A proton pump creates gradients in both the pH and the electric charge, establishing an electrochemical potential that acts as a kind of reservoir of stored energy for the cell. Finally, chemiosmosis16 is accomplished by complex V. Although the electron-transport chain is very efficient, some electrons are prematurely leaked to oxygen, resulting in the formation of reactive oxygen species. The last pathway of cellular metabolism, i.e. the electron-transport/oxidative-phosphorylation, is primarily regulated by the availability of ADP. Uncoupling proteins have the capability to shift the regulatory control towards the availability of NADH. Several compounds have been found to inhibit complex I (e.g. rotenone, amytal), complex II (e.g. malonate, 3-nitropropionic acid (3-NP)), complex III (e.g. antimycin A), complex IV (e.g. cyanide, CO, azide) and complex V (e.g. oligomycin).
Modulation of some enzymes from the glycolytic pathway by TCA-cycle intermediates seems to suggest potential important interactions between the rate of generation of three carbon metabolites and their subsequent oxidation. Furthermore, action of several ions on intermediates of the TCA-cycle remains understudied. Also the potential involvement of vascular signaling molecules (e.g. endothelium-derived factors) on electron transport chain enzymes is a research area which has implications for further understanding of the complex nature of functional neuroimaging.
Principal intracellular metabolite shuttles of NADH
Continuation of glycolysis requires maintenance of the cytosolic NAD+/NADH ratio, thus the NADH formed in glycolysis needs to be re-oxidized. The cells can either reduce Pyr to lactate, oxidizing NADH to regenerate NAD+ in the cytosol or shuttle the reduced equivalent into the mitochondrial matrix for re-oxidation. In the case of the first mentioned scenario, the end product of Glc metabolism is lactate which is an unattractive choice from an energy point of view. Mitochondrial re-oxidation demands an operative shuttle for transferring the reduced equivalent since the inner mitochondrial membrane is impermeable to NADH. It is therefore of crucial importance that shuttle mechanisms can operate. In brain, two such mechanisms may be of functional significance, namely the MAS and the glycerol 3-phosphate shuttle (McKenna et al., 2006c). In what follows, these two shuttles (schematically presented in Fig. 4) will be discussed in detail.
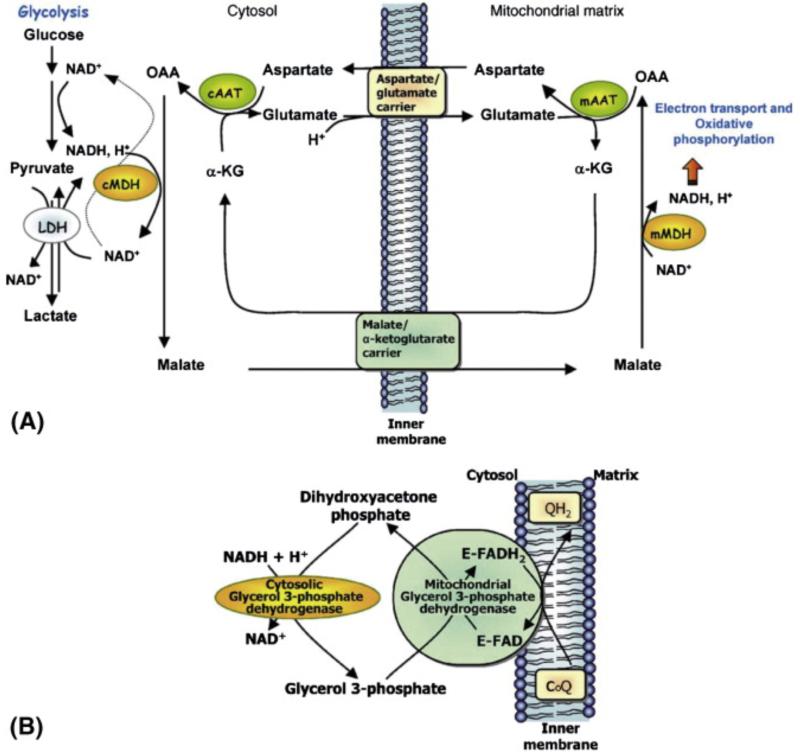
Fig. 4.
Principal intracellular metabolite shuttles. A) The MAS for transferring reducing equivalents from the cytosol to the mitochondria. Electrons from glycolysis or from oxidation of lactate to Pyr are transferred from NADH, H+as OAA is converted to malate by cytosolic MDH (cMDH). Malate enters the mitochondrial matrix via the malate/α-ketoglutarate carrier in exchange for α-KG. Electrons are transferred to the electron-transport chain as malate is oxidized to OAA by mitochondrial MDH (mMDH). OAA is subsequently converted to aspartate by transamination with glutamate via mitochondrial AAT (mAAT). The aspartate exits the mitochondria via the aspartate/glutamate carrier (AGC1, aralar1) in an electrogenic exchange for glutamate and a proton. In the cytosol, aspartate is converted to OAA by transamination with α-KG via cytosolic AAT (cAAT) completing the shuttle. B) The glycerol 3-phosphate shuttle for transferring reducing equivalents from the cytosol to the mitochondria. Electrons are transferred from NADH when dihydroxyacetone phosphate is reduced to glycerol 3-phosphate. Glycerol 3-phosphate is reoxidized to dihydroxyacetone phosphate by mitochondrial glycerol 3-phosphate dehydrogenase that is bound to an FAD prosthetic group on the outer side of the inner mitochondrial membrane and electrons are transferred to CoQ and subsequently enter the electron transport chain. Less energy is produced when electrons transferred into the mitochondria via the glycerol 3-phosphate shuttle enter the electron transport chain since FAD is the acceptor rather than NAD. Adapted from drawings/legends of Figs. (1) and (2) in McKenna et al. (2006c).
The malate-aspartate shuttle
The operation of the MAS (Fig. 4, A) requires the concerted action of enzymes (i.e. AAT and MDH) and membrane transporters (i.e. aspartate/glutamate and malate/α-ketoglutarate carriers). The two enzymes need to exist in cytoplasmic (c) and mitochondrial (m) isoforms as well (McKenna et al., 2006c). The aspartate/glutamate carrier exists in two forms, aralar 1 (AGC1) which is Ca2+sensitive and citrin (AGC2). The former is present in tissues such as muscle and brain whereas the latter may not be present in the adult brain but is expressed in liver and kidney (Del Arco et al., 2002). It has been argued that AGC1 is much more extensively expressed in neuronal mitochondria than in astroglial mitochondria but the latter cell type may express AGC2 when maintained in culture (Ramos et al., 2003). If indeed AGC1 is absent from astrocytes in the brain, one would expect that oxidative metabolism of Glc in this cellular compartment might be severely hampered (see below), in keeping with the astrocyte-neuron lactate shuttle (Pellerin and Magistretti, 1994). However, a transcriptomic analysis of acutely isolated astrocytes from adult mice has clearly demonstrated the presence of aralar1 mRNA (Lovatt et al., 2007). A non-operational MAS is clearly at odds with the demonstration of a significant oxidative metabolism of Glc in astrocytes in the brain as well as in cultured astrocytes (Hertz et al., 2007) and the significant (30%) de-novo synthesis of glutamine from Glc observed in-vivo (Öz et al., 2004). The alternative glycerol 3-phosphate shuttle described below may be of importance for understanding this apparent discrepancy.
The glycerol 3-phosphate shuttle
The operation of the glycerol 3-phosphate shuttle (Fig. 4, B) is based on the concerted action of cytosolic and mitochondrial isoforms of glycerol 3-phosphate dehydrogenase, the former using NAD+/NADH as coenzyme and the latter using FAD/FADH2 in this capacity. As a result, reducing equivalents are transferred to CoQ in the mitochondrial matrix; hence, cytosolic generated NADH will result in only 2 ATP molecules finally generated from the oxidative phosphorylation process in the mitochondrial matrix (McKenna et al., 2006c). The operation of this shuttle is somewhat controversial since the cytosolic and mitochondrial isoforms of glycerol 3-phosphate dehydrogenase appear to have different cellular localizations, the cytosolic form being expressed in glial cells while the mitochondrial form is neuronal ([Leveille et al., 1980] and [Nguyen et al., 2003]). However, a recent study of the transcriptome of acutely isolated astrocytes from adult mouse brain has presented evidence that both these enzymes are likely to be present in astrocytes as well as in neurons (Lovatt et al., 2007). This is in keeping with several studies in cultured astrocytes and glutamatergic neurons providing evidence that this shuttle mechanism may well be operating ([McKenna et al., 1993], [Atlante et al., 1999] and [Waagepetersen et al., 2001]).
Experimental approaches for studying shuttle activity
Obviously, as discussed above, it is of fundamental importance for Glc metabolism whether or not the shuttle mechanisms are working. This can at least to some extent be probed using pharmacological tools such as aminooxyacetic acid (AOAA), 3-NP or phenylsuccinate, all of which directly or indirectly inhibit the MAS (McKenna et al., 2006c). However, due to lack of specificity, particularly for AOAA, these tools are not easy to use, as discussed in detail by McKenna et al. (2006c). In the case of the glycerol 3-phosphate shuttle, it is even more complicated because no pharmacological tools are currently available to inhibit this shuttle mechanism (McKenna et al., 2006c). Since the cytosolic and mitochondrial redox levels will be affected by the operation of the shuttle mechanisms, it is of great interest for imaging studies based on NADH or FAD fluorescence changes to utilize pharmacological tools to monitor the functional importance of these shuttles in situ. While numerous studies have been performed utilizing NAD(P)H and FAD fluorescence for detection of metabolic activity in different brain tissue preparations (e.g. [Jobsis et al., 1971], [Schuchmann et al., 2001], [Kann et al., 2003], [Shuttleworth et al., 2003], [Kasischke et al., 2004], [Murakami et al., 2004] and [Brennan et al., 2006]), the functional imaging technique has been utilized less frequently (e.g. [Strong et al., 1996], [Kunz et al., 2002], [Shibuki et al., 2003] and [Foster et al., 2005]). It is, however, likely that such sophisticated techniques will provide much more detailed information about activity dependent metabolism in discrete brain regions in the future.
The operation of an NADH shuttle is obligatory for oxidative Glc metabolism and thus a determining factor for the functional importance of a possible astrocyte-neuron lactate shuttle. Hence, the absence of an NADH shuttle necessitates the operation of the astrocyte-neuron lactate shuttle while an operational NADH shuttle highly diminishes the need of lactate production and translocation. As delineated above strong evidence for support of an operational NADH shuttle in astrocytes is available.
The glutamate/GABA-glutamine cycle
Glutamatergic and to a less extent GABAergic neurotransmission are terminated by astrocytic uptake. This, together with the lack of neuronal capability of de-novo neurotransmitter synthesis, demands return of a precursor, i.e. glutamine, from astrocytes. These events are the basis of the glutamate/GABA-glutamine cycle. The operation of the cycle can be divided into two major parts, i.e. transfer of the carbon skeletons of the amino acids and the concomitant transfer of the ammonia nitrogen. These two parts will be considered separately henceforth.
Chemical neurotransmission can only be maintained if a mechanism is present in the pre-synaptic nerve ending by which released neurotransmitter can be regenerated. This may be accomplished by reuptake of released neurotransmitter followed by vesicular packaging and/or de-novo biosynthesis prior to vesicular storage. In case de-novo biosynthesis plays a major quantitative role, the enzymatic machinery necessary for this process is expected to be expressed in the particular nerve ending. Indeed, in certain cases, e.g. in cholinergic synapses, the biosynthetic enzyme (choline acetyl transferase) is even a marker for such pre-synaptic nerve endings.
Cellular distribution of enzymes
In the case of the amino acid neurotransmitters, e.g. glutamate and GABA, the scenario is quite complicated. While the GABA biosynthetic enzyme glutamate decarboxylase (GAD) is almost exclusively localized in GABAergic neurons as first demonstrated by Saito et al. (1974), the primary enzyme responsible for glutamate synthesis, phosphate-activated glutaminase (PAG) is more ubiquitously expressed (see Waagepetersen et al., 2007) albeit with a more prominent expression in glutamatergic structures (Laake et al., 1999). It should be noted, however, that glutamate biosynthesis can take place using α-KG as a precursor and either an amino acid transferase (e.g. AAT, alanine aminotransferase) or a dehydrogenase (e.g. GDH, reductive amination), as the enzyme. Regardless of the exact pathway, neurons are unable to perform de-novo net synthesis of these two neurotransmitters from Glc due to the lack of the key enzyme pyruvate carboxylase, being exclusively expressed in astrocytes, a cell specific localization also valid for another key enzyme glutamine synthetase ([Norenberg and Martinez-Hernandez, 1979], [Yu et al., 1983] and [Shank et al., 1985]). Such distribution is consistent with the operation between neurons and astrocytes of a glutamate-glutamine cycle (Fig. 5) which was first proposed based on metabolic relationships between glutamate and glutamine demonstrating that these two amino acids were likely to be present in at least two distinct metabolic pools representing primarily neurons and astrocytes, respectively ([Van den Berg and Garfinkel, 1971], [Benjamin and Quastel, 1972], [Berl and Clarke, 1983] and [Ottersen et al., 1992]). GABAergic neurotransmission relies also to a considerable extent on neuron-glial exchange of glutamine, thus the cycle should be named the glutamate/GABA-glutamine cycle.
Fig. 5.
Illustration of the glutamate/GABA-glutamine cycle. The glutamate (Glu) released by a glutamatergic pre-synaptic terminal (left) is mainly taken up into astrocytes, although a small portion could flood back into the terminal through the transporter GLT-1b. In the GABAergic synapse (right), the released GABA is taken up into both the pre-synaptic terminal and the astrocytes, with the former having higher affinity for the neurotransmitter. Inside the astrocyte, GABA is catabolized to succinate before entering the TCA-cycle. The carbon skeleton of this amino acid exits the TCA-cycle as α-KG, which is then transformed to glutamate. The glutamate is amidated to glutamine (Gln) by glutamine synthetase, an enzymatic reaction that benefits adjacent tissues by consuming and detoxifying free ammonia. The synthesized glutamine returns to the pre-synaptic terminals of both glutamatergic and GABAergic neurons, with a preference for the former. In both terminals, glutamine is used to regenerate glutamate and ammonia via phosphate-activated glutaminase (PAG). The regenerated glutamate in the GABAergic pre-synaptic terminal is converted back into GABA via glutamate decarboxylase (GAD). The more relevant a pathway is, the more thick is its arrow.
Transport in the glutamate/GABA-glutamine cycle
In order for the tissue to carry out the transfer processes in the GABA/glutamate-glutamine cycle, specific transporters for glutamate, GABA and glutamine must be present and differentially expressed in neuronal and astrocytic plasma membranes ([Schousboe et al., 2004a] and [Bak et al., 2006b]) (Fig. 2).
By far the majority of vesicularly-released glutamate is taken up into astrocytes via the abundantly expressed high affinity glutamate transporters EAAT1 (or GLAST) and EAAT2 (or GLT-1) ([Gegelashvili and Schousboe, 1997] and [Danbolt, 2001]). In addition to these astrocytic glutamate transporters, a transporter named EAAT3 (or EAAC1) is expressed primarily in postsynaptic neurons (Danbolt, 2001). The presence of a pre-synaptic high affinity glutamate transporter has been controversial although these have been unequivocally demonstrated in cultured neurons ([Drejer et al., 1982] and [Drejer et al., 1983]) and have subsequently been shown to play a functional role in the maintenance of release of neurotransmitter glutamate in glutamatergic neurons (Waagepetersen et al., 2005). For example, attempts have been made to assign this role to a second isoform of EAAT2, called GLT-1b (Chen et al., 2002).
In the case of GABA, it appears that in contrast to neurotransmitter glutamate which is primarily taken up into astrocytic elements (see above), the inhibitory neurotransmitter is preferentially taken up into the pre-synaptic GABAergic nerve endings ([Hertz and Schousboe, 1987], [Schousboe et al., 2004a], [Schousboe et al., 2004b] and [Schousboe et al., 2004c]) leading to a considerable recycling of neurotransmitter GABA. This would seem to have implications for the development of anticonvulsant drugs acting as inhibitors of GABA uptake. A close correlation has been demonstrated between potency as an inhibitor of astroglial GABA uptake and potency as an anticonvulsant in a mouse audiogenic seizure model, whereas no such correlation was found for inhibition of neuronal GABA transport (White et al., 2002). In this context, it is of interest that inhibition of BGT-1 seems particularly important for an anticonvulsant efficacy (White et al., 2005). A more detailed discussion of this issue can be found elsewhere ([Schousboe et al., 2004b], [Schousboe et al., 2004c] and [Cooper and Giulivi, 2007]). The glutamate/GABA-glutamine cycle includes transfer of glutamine from the astrocytic compartment to either glutamatergic or GABAergic neurons in which it serves as a precursor for neurotransmitter synthesis. It appears that uptake of glutamine in pre-synaptic neurons is mainly mediated by the System A transporter family whereas release from astrocytes is mediated by the system N and L transporter families (see Fig. 2 in Bak et al., 2006b). Interestingly, system N can mediate glutamine transport in both the outward and the inward directions (Bröer et al., 2002).
Carbon transfer
The original version of the glutamate-glutamine cycle (Van den Berg and Garfinkel, 1971) was concerned only with the fate of the carbon skeletons of the amino acids. The cycle was also mainly considered to operate in a stoichiometrical manner (Cotman et al., 1981), a notion challenged by the demonstration of a considerable astrocytic oxidative metabolism of glutamate via entry into the TCA-cycle ([Yu et al., 1982] and [McKenna et al., 1996]). Actually, glutamate may contribute significantly to the oxidative energy metabolism as discussed in detail by Hertz et al. (2007).
Nitrogen transfer
The glutamate/GABA-glutamine cycle deals with the transfer of the amino acid carbon skeleton between neurons and astrocytes but essentially leaves the transfer of ammonia nitrogen to facilitated diffusion. It seems unlikely that this would be the preferred process. Alternatively, this nitrogen could be transferred as the amino group in an amino acid and two such mechanisms have been proposed, i.e. the branched chain amino acid (BCAA) ([Yudkoff, 1997] and [Lieth et al., 2001]) and alanine-lactate ([Waagepetersen et al., 2007] and [Zwingmann et al., 2000]) shuttles. With regard to the latter, it has recently been shown that it may not be stimulated by an increase in glutamatergic neuronal activity (Bak et al., 2005). This seems somewhat peculiar as one would expect the glutamate-glutamine cycle, which is activity-dependent, to be coupled to the alanine-lactate shuttle. However, it should be pointed out that with regard to the BCAA shuttle mechanism it has been demonstrated that [15N]valine metabolism in cultured astrocytes is activity-dependent (Bak et al., 2007). Hence, this supports a functional role for the BCAA shuttle. Regardless of the nitrogen shuttle mechanism, a prerequisite for functioning is that the GDH17-catalyzed reaction occurs as a reductive amination. Normally in the brain this reaction proceeds in the opposite direction, i.e. oxidative deamination mainly due to the high Km value of this enzyme for ammonia (Zaganas et al., 2001). However, the enzyme in glutamatergic neurons has a higher activity and a lower Km than the enzyme in other types of neurons (Zaganas et al., 2001) which together with a high ammonia concentration in the mitochondrial microenvironment created by the high PAG activity in these neurons may facilitate reductive amination (Bak et al., 2006b).
The glutamate/GABA-glutamine cycle, was suggested decades ago and it has been investigated ever since. Three major issues with great impact on our understanding of this cycle are still to be unraveled, particularly with regard to regulation in time and regional microenvironment: i) the extent of astrocytic versus neuronal clearance of neurotransmitter, ii) the stoichiometry of the cycle determined by the extent of complete oxidative degradation of the neurotransmitter, iii) transfer of ammonia nitrogen between the neuronal and astrocytic compartments.
The energy budget for cellular work
In this section, we will discuss how internal energy is allocated to various subcellular processes in the cerebral cortex, making special emphasis in two related aspects: how the fuel budget is distributed when cells do different types of work and how energy availability could impinge on neuronal information processing.
For a long time it was assumed – based on rather old calculations from Creutzfeldt (1975) – that neuronal signaling requires only a negligible fraction of cerebral energy. This view of low energetic cost for neuronal signaling was further supported by early PET data suggesting that negligible energy increments were needed for function (Fox et al., 1988). The new consensus ([Shulman and Rothman, 1998] and [Attwell and Laughlin, 2001]) differs from such a prior prevailing view. The majority of the energy used in the brain is expended on signaling, and most of that energy is employed by neurons on reversing the ionic fluxes caused by their activity (Attwell and Laughlin, 2001). In the whole brain (i.e. grey matter plus white matter), if the Na+K+-ATPase is blocked, or coma induced, the energy usage is approximately halved ([Kety, 1957], [Sokoloff, 1960], [Siesjo, 1978], [Astrup et al., 1981], [Ames and Li, 1992], [Ames et al., 1992] and [Rolfe and Brown, 1997]). The residual energy consumption (~ 10 μmol ATP/g/min in rodent) presumably sustains basic cellular non-signaling processes or “housekeeping” tasks. Attwell and Laughlin (2001) took a bottom-up modeling approach to estimate an energy budget for the grey matter of rodent cortex (differences with primates are discussed in Attwell and Iadecola, 2002), which suggests that most energy consumption is associated with synaptic currents and action potential propagation (34% and 47% of the total signaling energy use, respectively). Attwell and Laughlin hypothesized that synaptic energy cost is high and is determined by two parameters: the probability that an action potential releases neurotransmitter at a pre-synaptic terminal and the number of postsynaptic channels activated by the neurotransmitter at the postsynaptic bouton. They suggested that the remaining signaling-related ATP consumption is distributed as follows: maintaining the neuronal and glial resting potentials 13%, pre-synaptic Ca2+ entry 3%, glutamate recycling 3% and Ca2+ transients < 1% (Fig. 6). The large differences in energy usage for each process stem from the different numbers of ions or molecules involved. Also, these authors predicted a total energy use for grey matter of 40 μmol ATP/g/min of which 30 μmol ATP/g/min was signaling-related energy, and perhaps 10 μmol ATP/g/min was housekeeping energy. This agrees with the measured value of 33–50 μmol ATP/g/min (Sokoloff et al., 1977), and suggests that 75% of energy expenditure in the grey matter is devoted to signaling with the remaining 25% spent on housekeeping tasks. Fig. 6 suggests that for the rodent brain the input and output synaptic events are roughly balanced energetically. The impact of these results on functional neuroimaging was discussed in Attwell and Iadecola (2002).
Fig. 6.
Adapted from Attwell and Laughlin (2001). Distribution of signaling-related ATP usage among different cellular mechanisms when the mean firing rate of neurons is 4 Hz. (A) The ATP use per second per neuron maintaining resting potentials, propagating action potentials through a neuron, and driving pre-synaptic Ca2+ entry, glutamate recycling, and postsynaptic ion fluxes, are shown. (B) Action potential propagation uses 47% of the total signaling energy use, while synaptic currents (postsynaptic receptors) use 34%, maintaining the neuronal and glial resting potentials uses 13%, pre-synaptic Ca2+entry uses 3%, glutamate recycling 3% and Ca2+ transients < 1% of the signaling-related ATP consumption.
As most of the brain,’s energy is used on reversing ion fluxes which generate the action potentials and synaptic currents, there will be evolutionary pressure for metabolically efficient wiring patterns and neural codes. The brain grey matter has a higher energy usage than the whole brain due to the high signaling-related energy demand which exists in grey matter ([Sokoloff et al., 1977], [Siesjo, 1978], [Kennedy et al., 1978], [Rolfe and Brown, 1997] and [Clark and Sokoloff, 1999]). The brain,’s information processing rate is limited by its energy supply. The energy available to the brain for signaling, and its cellular distribution, limits the time scale of information processing, in both axons and dendrites, to the millisecond range (Attwell and Gibb, 2005). The time scale of processing of sub-threshold signals is partly limited by the membrane time constant τm, which is the product of membrane resistance and capacitance, and is usually within the range of around 1–30 milliseconds. In order to process higher frequency synaptic signals τm must be smaller, in which case the resistance or capacitance of the plasma membrane must be decreased. However, decreasing the resistance/capacitance by inserting more “leakage” channels into the plasma membrane will result in larger ion fluxes, which would lead to a higher energy expenditure on maintaining the resting potential (Attwell and Laughlin, 2001). Consequently, it is not possible to increase the upper frequency limit for information processing with synaptic potentials, because that would decrease the energy available for expenditure on action potentials. This would decrease the highest frequency at which action potentials can occur and lower the temporal resolution with which signals can be encoded in axons. Thus, a balance between energy expenditure on resting potential and action potentials must be maintained. Similarly, increasing energy use by raising the frequency of action potentials would decrease the energy available to maintain the resting potential, thus requiring a higher membrane time constant, which would slow the possible processing speed of synaptic signals (Attwell and Gibb, 2005).
The α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors used for rapid information transfer between neurons have kinetics which are matched to the millisecond time scale imposed by the brain,’s energy supply – activation by glutamate and deactivation once the glutamate is removed both occur in milliseconds. The fast unbinding rate for glutamate implies that there is a low glutamate affinity for AMPA receptors (Attwell and Gibb, 2005). In order to make use of these fast kinetics, glutamate must be cleared from the synaptic cleft rapidly, requiring synapse diameters of less than 1μm for fast glutamate diffusion out of the cleft, a rapid initial glutamate removal step by transporters and a high transporter density surrounding synapses (Attwell and Gibb, 2005). In contrast, the kinetic properties of N-methyl-D-aspartic acid (NMDA) receptors are not set by energetic factors. NMDA receptors are responsible for coincidence detection and so need to remain activated for longer than the AMPA receptors. Thus, they have a slow unbinding rate for glutamate and are high affinity receptors (Attwell and Gibb, 2005).
Shulman et al. (2001b) suggested that 80% of energy use is correlated with glutamate passing through the glutamate-glutamine cycle and, therefore, excitatory active signaling processes ([Sibson et al., 1998] and [Shen et al., 1999]). Astrocyte glutamate transporters have been proposed to coordinate Glc and O2 usage in the central nervous system, linking energy production to synaptic glutamate release ([Magistretti and Pellerin, 1999a], [Magistretti and Pellerin, 1999b] and [Voutsinos-Porche et al., 2003]). In this context, it is also important to note that glutamate taken up by astrocytes is not only converted to glutamine. It is to a large extent oxidatively metabolized to CO2 ([Yu et al., 1982] and [McKenna et al., 1996]) producing a considerable amount of energy in the form of ATP (Hertz et al., 2007). Smith et al. (2002) combined measurements of neuronal spiking frequencies with BOLD signals in rat cortical layer IV to show that localized changes in brain energy metabolism (ΔCMRO2/CMRO2) were proportional to changes in excitatory neurotransmitter flux (ΔVcyc/Vcyc) and approximately equal to changes in neuronal spiking frequency (Δν/ν). Combining these results with those of Sibson et al. (1998) leads to ΔCMRO2/CMRO2 ≈ Δν/ν ≈ ΔVcyc/Vcyc, thus relating both electrical and neurotransmitter responses to the energetics of glutamatergic neurons (Smith et al., 2002). Although direct measurement of tissue oxygen with Clark-type electrodes do not directly reflect CMRO2 (Popel, 1989), these measurements could potentially provide novel insights into the neurophysiological basis of neuroimaging signals because a transient dip in the signal suggests a momentary excess of oxygen demand over oxygen supply at the location of the oxygen electrode (Ances et al., 2001). However these measurements have been shown to be either well (Thompson et al., 2003) or poorly (Viswanathan and Freeman, 2007) correlated with spiking activity in a region specific manner within the visual cortex. These findings are in partial agreement with an earlier study by Logothetis et al. (2001), which suggested that there is a slightly better correlation of the BOLD signal with local field potentials rather than spiking activity. Another issue impacting on the interpretation of neuroimaging signals is that whereas oxygen consumption by operating neurons is localized to the active area, the associated CBF increase appears to occur over a larger area (Devor et al., 2005). An important consideration in all of the apparent discrepancies discussed above is the variation of spatial resolution and sensitivity of the different methods used, which are usually assumed to be similar.
An interesting question concerns the relative contributions of excitatory and inhibitory synapses. Traditionally, inhibitory processes were thought to require either very little, or no, energy (Waldvogel et al., 2000), although several authors recently presented conflicting results ([Tagamets and Horwitz, 2001], [Caesar et al., 2003] and [Thomsen et al., 2004]). For a few years, this hypothesis was debated, but recently Patel et al. (2005) have shown that energy consumption increases with activity in both excitatory and inhibitory cells, and suggested that the contribution of GABAergic neurons to the total Glc oxidative metabolism in the cerebral cortex must not be disregarded. Because inhibition plays an essential role for the synchronization of neuronal populations, the basis for understanding how ongoing/induced brain oscillations affect functional neuroimaging is now beginning to be established. Recent comparative studies in cats and monkeys are helping to clarify this important issue ([Leopold et al., 2003], [Niessing et al., 2005] and [Maandag et al., 2007]). It has been suggested that incoming sensory information produces only small changes to ongoing activity in cortical neurons ([Arieli et al., 1996], [Kenet et al., 2003] and [Fiser et al., 2004]), implying that sensory input alters cortical energy use only slightly. Smith et al. (2002) found that maximum O2 consumption values and spike frequency in response to forepaw stimulation were approximately the same for two different levels of basal activity (set by two different depths of anesthesia). Baseline activity has become of considerable interest for the functional imaging community (Gusnard and Raichle, 2001), in part because of its somewhat apparently paradoxical energetic basis (Shulman et al., 1999) and in part because of the incomplete assignments of the energetic contributions to the negative BOLD signal (Shulman et al., 2007). An interesting question, revised in the fifth part of this series of reviews, is what is meant by ‘baseline,’ in terms of brain function and how does baseline activity relate to transient changes in activity (the ‘activations,’ frequently seen in functional neuroimaging) (Raichle and Gusnard, 2002)?
In summary, the majority of energy expenditure in the grey matter is devoted to signaling, and most of that energy is employed by neurons on reversing the ion fluxes caused by their synaptic currents and action potentials. The energy supply to the brain has limited the time scale of information processing in the brain to the millisecond range which, in turn, has determined the kinetics of the AMPA receptors (Attwell and Gibb, 2005). Understanding the cellular and subcellular distribution of energy use in the grey matter is relevant to interpreting functional neuroimaging signals, and in this sense, we are still not clear whether these signals indirectly reflect cellular works associated with principal neuron firing or with the overall synaptic activity.
Conclusions
Although Roy and Sherrington (1890) first suggested a coupling between cerebral energy consumption and neuronal activity over a hundred years ago, the exact relationship remains unclear today. If energy resources are to be allocated flexibly among regions according to neuronal demand, it requires a local control of cerebral tissue perfusion18, an effect that is exploited by several modalities of functional neuroimaging (e.g. fMRI, PET/SPECT, fNIRS). CBF is increased to areas where neurons are more active, a phenomenon named functional hyperemia. The physiological mechanisms underlying the origin of both functional hyperemia (Fig. 7, red-dashed lines/arrows) and spontaneous vasomotions (caused by signaling crosstalk between ECs and SMCs) will be discussed in part II of this series of reviews. In the course of neuronal activity, small activated brain areas are rapidly overperfused (i.e. they receive more blood than would be expected to meet the urgent metabolic needs, i.e. Glc and oxygen consumption). Both excitatory (orange) and inhibitory (cyan) specialized neuronal populations are endowed with numerous mechanisms for fight-or-flight CBF responses, probably corresponding to what Hirase (2005) has termed the phasic modulation pathway. These specific neurons create transient changes in the CBF either through axonal processes directly targeting those neuron-astrocyte-vascular tripartite functional units (Cohen et al., 1996) in close proximity to a variety of neurotransmitters (Cauli et al., 2004) or by the remote release of vasoactive substances (e.g. prostanoids and NO) to their surroundings, which can rapidly diffuse toward the vascular structures. In contrast, a signaling cascade (e.g. Na+- and Ca2+-mediated metabolic waves) inside the astrocytic gap-connected network may take part in functional hyperemia for events occurring on different time scales (Haydon and Carmignoto 2006), e.g. whenever the input is prolonged in time, a pathway that, as suggested by Hirase (2005), could underlie a tonic modulation. As discussed in the text, metabolic waves might be triggered by neurotransmitter action (e.g. glutamate, GABA, ATP) on a variety of receptors and transporters located on the plasma membrane of astrocytes. During prolonged input, at some point the cerebral cortex will run out of disposable fuel (i.e. Glc), a negative phenomenon that could be counterbalanced by glycogen turnover. Mobilization of glycogen polymers, which are mainly stored in the astrocytes, will be rapidly provoked by an AMP signaling cascade (Gc-1). Also, Ca2+ waves are thought to catalyze the phosphorolytic cleavage of residues to glucose-1-phosphate by converting GP into the allosterically unresponsive and active form a.
Fig. 7.
A schematic representation of the metabolic events triggered by an increase of the neuronal activity in an elemental cortical area (discussed in detail in preceding sections). These metabolic events may be selectively activated depending of the characteristics (e.g. duration and intensity) of the input to the cerebral cortex (black box) as well as the particular cortical region. The pathways directly implicated on the functional hyperemia (red box) are highlighted with red-dashed lines/arrows. The neuronal mass, including excitatory and inhibitory sub-populations, are represented by a dark-grey box (point-like border). The astrocytic gap-connected networks are represented by a light-grey box (point/line-like border). The extracellular milieu is enclosed by dashed-line boxes.
Both, excitatory and inhibitory synapses are dynamically created/eliminated while activity-dependent gene programs are running in the course of brain plasticity. Such neurotransmission adjustments would affect the original status of the carbon skeleton in each cell type. In the cerebral cortex, neurotransmitter recycling via astrocytes may underlie an inter-cellular regulatory mechanism of carbon homeostasis, which perhaps comprises signaling crosstalk of net carbon flow between neurons and astrocytes. However, such mechanisms associated with metabolite flows at a multi-cellular level remain to be determined. The fact that glutamine synthesized by astrocytes can be dispatched to both glutamatergic and GABAergic neurons according to their neurotransmitter (or carbon) requirements might suggest the astrocytic TCA-cycle as a chief candidate in the multi-cellular crosstalk. In this context, portions of Pyr enter the TCA-cycle in astrocytes to supply needed carbons for a balanced glutamate/GABA-glutamine cycle, additionally providing these cells with access to ATP energy. However, a part of astrocytic Pyr is believed to be reduced to lactate by LDH1&5 in order to supply the bulk of energy to nearby neurons. A local increase in the levels of Glc and O2 in the brain tissue follows functional hyperemia, which may facilitate maintenance of both the glycolytic (via G-1) and TCA-cycle pathways. Through checkpoint G-2, NO and AMP signaling may induce the activation of glycolysis. By allosteric inhibition in checkpoints G-2 and G-3, citrate and ATP could put off the glycolytic pathway if the TCA-cycle is overworking. The role of cytosolic pH levels in glycolysis via G-2 needs to be clarified in future studies.
We would like to conclude this review by referring to the role that neuron-astrocyte metabolic coupling may play in synaptic plasticity via glycogen mobilization (see a recent review by Magistretti 2006). Variations in the expression of genes involved in glial glycogen metabolism have been observed during the sleep-wake cycle. During daytime, a huge number of new synapses are thought to be created, which brings about an increase in total brain energy usage. In order to compensate such a metabolic cost for memory consolidation, the synaptic connections must be reorganized and adjusted homogeneously in the whole brain during sleep, which might help reduce the extra energy requirements due to synaptic reinforcement (Tononi and Cirelli, 2006). Recent discoveries motivate the neuroscience community to initiate new avenues of research in this direction. A number of neuroactive molecules (e.g. adenosine, noradrenaline and certain cytokines), regulating the expression of key genes [e.g. protein targeting to glycogen (PTG), GS, GP] involved in glycogen metabolism, are currently being identified in the brain of some laboratory animals (Magistretti 2006). Captivatingly, the exposure to any of these signaling compounds results in the cyclic-AMP-dependent induction of expression of the transcription factor C/EBP, of GS and of PTG. In vivo studies indicate the presence of a circadian rhythm for the expression of PTG mRNA (Petit et al., 2002). These authors have also reported a marked induction of PTG mRNA expression following sleep deprivation, which was found to be reversible.
Acknowledgments
We thank the attendees to sessions IV at BCW200619 for the interesting discussions, which represent the source of inspiration for the present review. We extend our gratitude to David Attwell who read over the manuscript and recommended certain modifications during the preparation of “The energy budget for cellular work” section. A Schousboe and HS Waagepetersen received financial support from the Danish Medical Research Council (22-04-0314, 22-04-0250, 271-05-0057), The Lundbeck and The Alfred Benzon Foundations. The authors thank Kamil Uludag for kindly revising the manuscript. J Riera has been supported by JST/RISTEX; R&D promotion scheme for regional proposals promoted by TAO; the 21st Century Center of Excellence (COE) Program (Ministry of Education, Culture, Sports, Science and Technology) entitled “A Strategic Research and Education Center for an Integrated Approach to Language, Brain and Cognition,,” and the Management Expenses Grants of Tohoku University. C Howarth is in the Wellcome Trust 4 year PhD programme in Neuroscience at UCL. F Hyder was supported by grants from National Institutes of Health (R01 MH-067528, R01 DC-003710, P30 NS-52519).
Abbreviations
- fMRI
functional magnetic resonance imaging
- EEG/MEG
electro/magneto encephalography
- PET
positron emission tomography
- SPECT
single photon emission computed tomography
- fNIRS
functional near infrared spectroscopy
Footnotes
BOLD signal calibration by hyperoxia requires additionally measuring PaO2 (partial pressure of oxygen in arterial blood).
Sometimes referred to as mind-wandering states.
TCA-cycle stands for tricarboxylic acid cycle.
The symbol [s] refers to the substrate concentration in a chemical reaction.
The gamma aminobutyric acid (GABA) is the glutamate conjugate inhibitory neurotransmitter. Glutamate is a precursor of GABA.
It is also known as the Embdem-Meyerhoff pathway.
It is sometimes named PFK-2/FBPase-2.
cGMP stands for cyclic guanosine monophosphate.
Lactate is negatively charged at physiological pH.
BGT-1 stands for betaine-GABA transporter. It is a betaine transporter with a lower affinity for GABA.
GABA could enter the TCA-cycle after its catabolization to succinate by the sequential actions of GABA-T and succinic semialdehyde dehydrogenase (SSADH).
Pyr is a polar molecule.
The MAS is reversible, although the aspartate/glutamate carrier is energetically favorable for the efflux of aspartate from and the entry of glutamate into the mitochondrion.
It is also known as the citric acid cycle or the Krebs cycle.
CoQ10 is the type found in the mitochondria of humans.
Chemiosmosis is the creation of ATP from using the H+ motive force as a source of energy.
GDH is located in the mitochondria. It is an important branch-point enzyme between carbon and nitrogen metabolism.
Perfusion of cerebral tissues may also aid in the local thermoregulation (Trubel et al., 2006).
The Fifth Brain Connectivity Workshop, Sendai, Japan, May 17th-20th 2006. http://www.idac.tohoku.ac.jp/BCW2006/
References
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Almeida A, Moncada S, Bolanos JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat. Cell Biol. 2004;6:45–51. doi: 10.1038/ncb1080. [DOI] [PubMed] [Google Scholar]
- Ames CNS energy metabolism as related to function. Brains Res. Rev. 2000;34:42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- Ames A, III, Li YY. Energy requirements of glutamatergic pathways in rabbit retina. J. Neurosci. 1992;12:4234–4242. doi: 10.1523/JNEUROSCI.12-11-04234.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A, III, Li YY, Heher EC, Kimble CR. Energy metabolism of rabbit retina as related to function: high cost of Na+ transport. J. Neurosci. 1992;12:840–853. doi: 10.1523/JNEUROSCI.12-03-00840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Buerk DG, Greenberg JH, Detre JA. Temporal dynamics of the partial pressure of brain tissue oxygen during functional forepaw stimulation in rats. Neurosci. Lett. 2001;306:106–110. doi: 10.1016/s0304-3940(01)01868-7. [DOI] [PubMed] [Google Scholar]
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- Astrup J, Sorensen PM, Sorensen HR. Oxygen and glucose consumption related to Na+-K+ transport in canine brain. Stroke. 1981;12:726–730. doi: 10.1161/01.str.12.6.726. [DOI] [PubMed] [Google Scholar]
- Atlante A, Gagliardi S, Marra E, Calissano P, Passarella S. Glutamate neurotoxicity in rat cerebellar granule cells involves cytochrome c release from mitochondria and mitochondrial shuttle impairment. J. Neurochem. 1999;73:237–246. doi: 10.1046/j.1471-4159.1999.0730237.x. [DOI] [PubMed] [Google Scholar]
- Attwell D, Gibb A. Neuroenergetics and the kinetic design of excitatory synapses. Nat. Rev. Neurosci. 2005;6:841–849. doi: 10.1038/nrn1784. [DOI] [PubMed] [Google Scholar]
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25(12):621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Bak LK, Johansen ML, Schousboe A, Waagepetersen HS. Among the branched-chain amino acids only valine metabolism is up-regulated in astrocytes during glutamate exposure. J. Neurosci. Res. 2007;85:3465–3470. doi: 10.1002/jnr.21347. [DOI] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Sonnewald U, Waagepetersen HS. Glucose is necessary to maintain neurotransmitter homeostasis during synaptic activity in cultured glutamatergic neurons. J. Cereb. Blood Flow Metab. 2006;26:1285–1297. doi: 10.1038/sj.jcbfm.9600281. [DOI] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- Bak LK, Sickmann HM, Schousboe A, Waagepetersen HS. Activity of the lactate-alanine shuttle is independent of glutamate-glutamine cycle activity in cerebellar neuronal-astrocytic cultures. J. Neurosci. Res. 2005;79:88–96. doi: 10.1002/jnr.20319. [DOI] [PubMed] [Google Scholar]
- Benjamin AM, Quastel JH. Locations of amino acids in brain slices from the rat. Tetrodotoxin-sensitive release of amino acids. Biochem. J. 1972;128:631–646. doi: 10.1042/bj1280631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl S, Clarke DD. The metabolic compartmentation concept. In: Hertz L, Kvamme E, McGeer EG, Schousboe A, editors. Glutamine, Glutamate and GABA in The Central Nervous System. New York: 1983. pp. 205–217. [Google Scholar]
- Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- Bernardinelli Y, Magistretti PJ, Chatton J-Y. Astrocytes generate Na+-mediated metabolic waves. PNAS. 2004;101(41):14937–14942. doi: 10.1073/pnas.0405315101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar PG, Charnay Y, Pellerin L, Bouras C, Magistretti PJ. Selective distribution of lactate dehydrogenase isoenzymes in neurons and astrocytes of human brain. J. Cereb. Blood Flow Metab. 1996;16(6):1079–1089. doi: 10.1097/00004647-199611000-00001. [DOI] [PubMed] [Google Scholar]
- Bonvento G, Herard A-S, Voutsinos-Porche B. The astrocyte-neuron lactate shuttle: a debated but still valuable hypothesis for brain imaging. J. Cereb. Blood Flow Metab. 2005;25:1394–1399. doi: 10.1038/sj.jcbfm.9600127. [DOI] [PubMed] [Google Scholar]
- Brennan AM, Connor JA, Shuttleworth CW. NAD(P)H fluorescence transients after synaptic activity in brain slices: predominant role of mitochondrial function. J. Cereb. Blood Flow Metab. 2006;26:1389–1406. doi: 10.1038/sj.jcbfm.9600292. [DOI] [PubMed] [Google Scholar]
- Brown AM, Sickmann HM, Fosgerau K, Lund TM, Schousboe A, Waagepetersen HS, Ransom BR. Astrocyte glycogen metabolism is required for neural activity during aglycemia or intense stimulation in mouse white matter. J. Neurosci. Res. 2005;79:74–80. doi: 10.1002/jnr.20335. [DOI] [PubMed] [Google Scholar]
- Bröer A, Albers A, Setiawan I, Edwards RH, Chaudhry FA, Lang F, Wagner CA, Bröer S. Regulation of the glutamine transporter SN1 by extracellular pH and intracellular sodium ions. J. Physiol. 2002;539:3–14. doi: 10.1113/jphysiol.2001.013303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar K, Gold L, Lauritzen M. Context sensitivity of activity dependent increase in cerebral blood flow. PNAS. 2003;100:4239–4244. doi: 10.1073/pnas.0635075100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J. Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Broadwell RD. Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. II. Choroid plexus and ependymal epithelia, endothelia and pericytes. J. Neurocytol. 1986;15:511–524. doi: 10.1007/BF01611733. [DOI] [PubMed] [Google Scholar]
- Chatton JY, Pellerin L, Magistretti PJ. GABA uptake into astrocytes is not associated with significant metabolic cost: Implications for brain imaging of inhibitory transmission. PNAS. 2003;100:12456–12461. doi: 10.1073/pnas.2132096100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Silva AC, Yang J, Shen J. Elevated endogenous GABA level correlates with decreased fMRI signals in the rat brain during acute inhibition of GABA transaminase. J. Neurosci. Res. 2005;79:383–391. doi: 10.1002/jnr.20364. [DOI] [PubMed] [Google Scholar]
- Chen W, Aoki C, Mahadomrongkul V, Gruber CE, Wang GJ, Blitzblau R, Irwin N, Rosenberg PA. Expression of a variant form of the glutamate transporter GLT1 in neuronal cultures and in neurons and astrocytes in the rat brain. J. Neurosci. 2002;22:2142–2152. doi: 10.1523/JNEUROSCI.22-06-02142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih C-P, Lipton P, Robert EL. Do active cerebral neurons really use lactate rather than glucose. Trends Neurosci. 2001;24:573–578. doi: 10.1016/s0166-2236(00)01920-2. [DOI] [PubMed] [Google Scholar]
- Clark DD, Sokoloff L. In: Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. Lippincott; Philadelphia: 1999. pp. 637–670. [Google Scholar]
- Cohen Z, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of the brain microcirculation. Prog. Neurobiol. 1996;50:335–362. doi: 10.1016/s0301-0082(96)00033-0. [DOI] [PubMed] [Google Scholar]
- Cooper CE, Giulivi C. Nitric oxide regulation of mitochondrial oxygen consumption II: molecular mechanism and tissue physiology. Am. J. Physiol. Cell Physiol. 2007;292:C1993–C2003. doi: 10.1152/ajpcell.00310.2006. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Foster A, Lanthorn T. An overview of glutamate as a neurotransmitter. Adv. Biochem. Psychopharmacol. 1981;27:1–27. [PubMed] [Google Scholar]
- Crabtree B, Newsholme EA. The derivation and interpretation of control coefficients. Biochem J. 1987;247:113–120. doi: 10.1042/bj2470113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt OD. Neurophysiological correlates of different functional states of the brain. In: Ingvar DH, Lassen NA, editors. Alfred Benzon Symposium VII. Academic Press; New York: 1975. pp. 21–46. [Google Scholar]
- Danbolt NC. Glutamate uptake. Progr. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Morcillo J, Martinez-Morales JR, Galian C, Martos V, Bovolenta P, Satrustegui J. Expression of the aspartate/glutamate mitochondrial carriers aralar1 and citrin during development and in adult rat tissues. Eur. J. Biochem. 2002;269:3313–3320. doi: 10.1046/j.1432-1033.2002.03018.x. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol. Rev. 2006;86:465–514. doi: 10.1152/physrev.00025.2005. [DOI] [PubMed] [Google Scholar]
- Devor A, Ulbert I, Dunn AK, Narayanan SN, Jones SR, Andermann ML, Boas DA, Dale AM. Coupling of the cortical hemodynamic response to cortical and thalamic neuronal activity. PNAS. 2005;102:3822–3827. doi: 10.1073/pnas.0407789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Hertz L. Glucose and lactate metabolism during brain activation. J. Neurosci. Res. 2001;66:824–838. doi: 10.1002/jnr.10079. [DOI] [PubMed] [Google Scholar]
- Drejer J, Larsson OM, Schousboe A. Characterization of uptake and release processes for D- and L-aspartate in primary cultures of astrocytes and cerebellar granule cells. Neurochem. Res. 1983;8:231–243. doi: 10.1007/BF00963923. [DOI] [PubMed] [Google Scholar]
- Drejer J, Larsson OM, Schousboe A. Characterization of glutamate uptake into and release from astrocytes and neurons cultured from different brain regions. Exp. Brain Res. 1982;47:259–269. doi: 10.1007/BF00239385. [DOI] [PubMed] [Google Scholar]
- Erecińska M, Nelson D, Silver IA. Metabolic and energetic properties of isolated nerve ending particles (synaptosomes) Biochim. Biophys. Acta. 1996;1277:13–34. doi: 10.1016/s0005-2728(96)00103-x. [DOI] [PubMed] [Google Scholar]
- Estrada C, DeFelipe J. Feature article nitric oxide-producing neurons in the neocortex: morphological and functional relationship with intraparenchymal microvasculature. Cereb. Cortex. 1998;8:193–203. doi: 10.1093/cercor/8.3.193. [DOI] [PubMed] [Google Scholar]
- Fergus A, Lee KS. GABAergic regulation of cerebral microvascular tone in the rat. J. Cereb. Blood Flow Metab. 1997;17:992–1003. doi: 10.1097/00004647-199709000-00009. [DOI] [PubMed] [Google Scholar]
- Fiser J, Chiu C, Weliky M. Small modulation of ongoing cortical dynamics by sensory input during natural vision. Nature. 2004;431:573–578. doi: 10.1038/nature02907. [DOI] [PubMed] [Google Scholar]
- Foster KA, Beaver CJ, Turner DA. Interaction between tissue oxygen tension and NADH imaging during synaptic stimulation and hypoxia in rat hippocampal slices. Neuropharmacology. 2005;37:645–657. doi: 10.1016/j.neuroscience.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Schousboe A. High-affinity glutamate transporters: Regulation of expression and activity. Mol. Pharmacol. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Marrett S. Glycolysis in neurons, not astrocytes, delays oxidative metabolism of human visual cortex during sustained checkerboard stimulation in vivo. J. Cereb. Blood Flow Metab. 2001;21:1384–1392. doi: 10.1097/00004647-200112000-00002. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Anderson DG, Hertz L. Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia. 2006;54:214–222. doi: 10.1002/glia.20377. [DOI] [PubMed] [Google Scholar]
- Giulivi C, Kato K, Cooper CE. Nitric oxide regulation of mitochondrial oxygen consumption I: cellular physiology. Am. J. Physiol. Cell Physiol. 2006;291:C1225–C1231. doi: 10.1152/ajpcell.00307.2006. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001;2:685–693. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Adriany G, Choi IY, Henry PG, Lei H, Öz G. Localized in vivo 13C NMR spectroscopy of the brain. NMR Biomed. 2003;16:313–338. doi: 10.1002/nbm.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Henry PG, Adriany G, Deelchand D, Gruetter R, Marjanska M, Oz G, Seaquist ER, Shestov A, Ugurbil K. In vivo 13C NMR spectroscopy and metabolic modeling in the brain: a practical perspective. Magn. Reson Imaging. 2006;24(4):527–539. doi: 10.1016/j.mri.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Hertz L. The astrocyte-neuron lactate shuttle: a challenge of a challenge. J. Cereb. Blood Flow Metab. 2004;24:1241–1248. doi: 10.1097/00004647-200411000-00008. [DOI] [PubMed] [Google Scholar]
- Hertz L, Schousboe A. Primary cultures of GABAergic and glutamatergic neurons as model systems to study neurotransmitter functions. I. Differentiated cells. In: Vernadakis A, Privat A, Lauder JM, Timiras PS, Giacobini E, editors. Model Systems of Development and Aging of the Nervous System. Nijhoff Publ. Comp.; Boston: M.: 1987. pp. 19–31. [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J. Cereb. Blood Flow. Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Hirase H. A multi-photon window onto neuronal-glial-vascular communication. Trends Neurosci. 2005;28:217–219. doi: 10.1016/j.tins.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Hirase H, Qian L, Barthó P, Buzsáki G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2004;2(4):0494–0499. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW. Intracellular convection, homeostasis and metabolic regulation. J Exp. Biol. 2003;206:2001–2009. doi: 10.1242/jeb.00402. [DOI] [PubMed] [Google Scholar]
- Hochachka PW, McClelland GB. Cellular metabolic homeostasis during large-scale change in ATP turnover rates in muscles. J. Exp. Biol. 1997;200(2):381–386. doi: 10.1242/jeb.200.2.381. [DOI] [PubMed] [Google Scholar]
- Hofmeyr JHS. Anaerobic energy metabolism in yeast as a supplydemand system. In: Cornish-Bowden A, editor. New beer in an old bottle. Universidad de Valencia; Valencia, Spain: 1997. pp. 225–242. [Google Scholar]
- Hofmeyr JHS, Cornish-Bowden A. Regulating the cellular economy of supply and demand. FEBS Lett. 2000;476:47–51. doi: 10.1016/s0014-5793(00)01668-9. [DOI] [PubMed] [Google Scholar]
- Hyder F, Kida I, Behar KL, Kennan RP, Maciejewski PK, Rothman DL. Quantitative functional imaging of the brain: towards mapping neuronal activity by BOLD fMRI. NMR Biomed. 2001;14:413–431. doi: 10.1002/nbm.733. [DOI] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J. Cereb. Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Ignacio PC, Baldwin BA, Vijayan VK, Tait RC, Gorin FA. Brain isozyme of glycogen phosphorylase: immunohistological localization within the central nervous system. Brain Res. 1990;529:42–49. doi: 10.1016/0006-8993(90)90809-p. [DOI] [PubMed] [Google Scholar]
- Jeong H, Tombor B, Albert R, Oltvai ZN, Barabási AL. The large-scale organization of metabolic networks. Nature. 2000;407:651–654. doi: 10.1038/35036627. [DOI] [PubMed] [Google Scholar]
- Jobsis FF, O’Connor M, Vitale A, Vreman H. Intracellular redox changes in functioning cerebral cortex. I. Metabolic effects of epileptiform activity. J. Neurophysiol. 1971;34:735–749. doi: 10.1152/jn.1971.34.5.735. [DOI] [PubMed] [Google Scholar]
- Kann O, Schuchmann S, Buchheim K, Heinemann U. Coupling of neuronal activity and mitochondrial metabolism as revealed by NAD(P)H fluorescence signals in organotypic hippocampal slice cultures of the rat. Neuroscience. 2003;119:87–100. doi: 10.1016/s0306-4522(03)00026-5. [DOI] [PubMed] [Google Scholar]
- Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305:99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A. Spontaneously emerging cortical representations of visual attributes. Nature. 2003;425:954–956. doi: 10.1038/nature02078. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Sakurada O, Shinohara M, Jehle J, Sokoloff L. Local cerebral glucose utilization in the normal conscious macaque monkey. Ann. Neurol. 1978;4:293–301. doi: 10.1002/ana.410040402. [DOI] [PubMed] [Google Scholar]
- Kessler R, Eschrich K. Splice isoforms of ubiquitous 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase in human brain. Mol. Brain Res. 2001;87:190–195. doi: 10.1016/s0169-328x(01)00014-6. [DOI] [PubMed] [Google Scholar]
- Kety SS. In: The general metabolism of the brain in vivo. In Metabolism of the nervous system. Richter D, editor. Pergamon; London: 1957. pp. 221–237. [Google Scholar]
- Kunz D, Winkler K, Elger CE, Kunz WS. Functional imaging of mitochondrial redox state. Methods Enzymol. 2002;352:135–150. doi: 10.1016/s0076-6879(02)52014-0. [DOI] [PubMed] [Google Scholar]
- Laake JH, Takumi Y, Eidet J, Torgner IA, Roberg B, Kvamme E, Ottersen OP. Postembedding immunogold labelling reveals subcellular localization and pathway-specific enrichment of phosphate activated glutaminase in rat cerebellum. Neuroscience. 1999;88:1137–1151. doi: 10.1016/s0306-4522(98)00298-x. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Murayama Y, Logothetis NK. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb. Cortex. 2003;13:422–433. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- Leveille PJ, McGinnis JF, Maxwell DS, de Vellis J. Immunocytochemical localization of glycerol-3-phosphate dehydrogenase in rat oligodendrocytes. Brain Res. 1980;196:287–305. doi: 10.1016/0006-8993(80)90397-2. [DOI] [PubMed] [Google Scholar]
- Leybaert L. Neurobarrier coupling in the brain: a partner of neurovascular and metabolic coupling? J. Cereb. Blood Flow Metab. 2005;25:2–16. doi: 10.1038/sj.jcbfm.9600001. [DOI] [PubMed] [Google Scholar]
- Li N, Sul J-Y, Haydon PG. A calcium-induced calcium influx factor, nitric oxide, modulates the refilling of calcium stores in astrocytes. J. Neurosci. 2003;23(32):10302–10310. doi: 10.1523/JNEUROSCI.23-32-10302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieth E, LaNoue KF, Berkich DA, Xu B, Ratz M, Taylor C, Hutson SM. Nitrogen shuttling between neurons and glial cells during glutamate synthesis. J. Neurochem. 2001;76:1712–1723. doi: 10.1046/j.1471-4159.2001.00156.x. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JHC, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J. Neurosci. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maandag NJG, Coman D, Sanganahalli BG, Herman P, Smith AJ, Blumenfeld H, Shulman RG, Hyder F. Energetics of neuronal signaling and fMRI activity. Proc. Natl. Acad. Sci. USA. 2007;104:20546–20551. doi: 10.1073/pnas.0709515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J. Exp. Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Phi. Trans R. Soc. B. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Astrocytes couple synaptic activity to glucose utilization in the brain. News Physiol. Sci. 1999;14:177–182. doi: 10.1152/physiologyonline.1999.14.5.177. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- Mangia S, Tkac I, Gruetter R, Van de Moortele PF, Maraviglia B, Ugurbil K. Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from 1H NMR spectroscopy in the human visual cortex. J. Cereb. Blood Flow Metab. 2007;27:1055–1063. doi: 10.1038/sj.jcbfm.9600401. [DOI] [PubMed] [Google Scholar]
- Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol. Rev. 2003;83:183–252. doi: 10.1152/physrev.00022.2002. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Role of glial amino acid transporters in synaptic transmission and brain energetics. Glia. 2004;47:217–225. doi: 10.1002/glia.20027. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Sonnewald U. GABA alters the metabolic fate of [U-13C]glutamate in cultured cortical astrocytes. J. Neurosci. Res. 2005;79:81–87. doi: 10.1002/jnr.20309. [DOI] [PubMed] [Google Scholar]
- McKenna M, Hopkins IB, Lindauer SL, Bamford P. Aspartate aminotransferase in synaptic and nonsynaptic mitochondria: Differential effect of compounds that influence transient hetero-enzyme complex (metabolon) formation. Neurochem. Int. 2006;48:629–636. doi: 10.1016/j.neuint.2005.11.018. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Gruetter R, Sonnewald U, Waagepetersen HS, Schousboe A. Energy metabolism of the brain. In: Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. In Basic Neurochemistry: Molecular, Cellular, and Medical Aspects. Lippincott-Raven; Philadelphia: 2006. pp. 531–557. [Google Scholar]
- McKenna MC, Waagepetersen HS, Schousboe A, Sonnewald U. Neuronal and astrocytic shuttle mechanisms for cytosolic-mitochondrial transfer of reducing equivalents: Current evidence and pharmacological tools. Biochem. Pharmacol. 2006;71:399–407. doi: 10.1016/j.bcp.2005.10.011. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Sonnewald U, Huang X, Stevenson J, Zielke HR. Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J. Neurochem. 1996;66:386–395. doi: 10.1046/j.1471-4159.1996.66010386.x. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Tildon JT, Stevenson JH, Boatright R, Huang S. Regulation of energy metabolism in synaptic terminals and cultured rat brain astrocytes: differences revealed using aminoocxyacetate. Dev. Neurosci. 1993;15:320–329. doi: 10.1159/000111351. [DOI] [PubMed] [Google Scholar]
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Moore CI, Cao R. The hemo-neural hypothesis: on the role of blood flow in information processing. J Neurophysiol. 2007 Oct 3; doi: 10.1152/jn.01366.2006. in press. doi:10.1152/jn.01366.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H, Kamatani D, Hishida R, Takao T, Kudoh M, Kawaguchi T, Tanaka R, Shibuki K. Short-term plasticity visualized with flavorprotein autofluorescence in the somatosensory cortex of anaesthetized rats. Eur. J. Neurosci. 2004;19:1352–1360. doi: 10.1111/j.1460-9568.2004.03237.x. [DOI] [PubMed] [Google Scholar]
- Nasrallah FA, Griffin JL, Balcar VJ, Rae C. Understanding your inhibitions: modulation of brain cortical metabolism by GABAB receptors. J. Cereb. Blood Flow Metab. 2007;27:1510–1520. doi: 10.1038/sj.jcbfm.9600453. [DOI] [PubMed] [Google Scholar]
- Nguyen NH, Bråthe A, Hassel B. Neuronal uptake and metabolism of glycerol and the neuronal expression of mitochondrial glycerol-3-phosphate dehydrogenase. J. Neurochem. 2003;85:831–842. doi: 10.1046/j.1471-4159.2003.01762.x. [DOI] [PubMed] [Google Scholar]
- Nissen C, Schousboe A. Activity and isoenzyme pattern of lactate dehydrogenase in astroblasts cultured from brains of newborn mice. J. Neurochem. 1979;32:1787–1792. doi: 10.1111/j.1471-4159.1979.tb02292.x. [DOI] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RAW. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, Boeker H, Grimm S, Boesiger P. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat. Neurosci. 2007;10(12):1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Zhang N, Walberg F. Metabolic compartmentation of glutamate and glutamine: morphological evidence obtained by quantitative immunocytochemistry in rat cerebellum. Neuroscience. 1992;46:519–534. doi: 10.1016/0306-4522(92)90141-n. [DOI] [PubMed] [Google Scholar]
- Öz G, Berkich DA, Henry P-G, Xu Y, LaNoue K, Hutson SM, Gruetter R. Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J. Neurosci. 2004;24:11273–11279. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öz G, Henry PG, Seaquist ER, Gruetter R. Direct, noninvasive measurement of brain glycogen metabolism in humans. Neurochem. Int. 2003;43:323–329. doi: 10.1016/s0197-0186(03)00019-6. [DOI] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL. The contribution of GABA to glutamate_glutamine cycling and energy metabolism in the rat cortex in vivo. PNAS. 2005;102(15):5588–5593. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Mason GF, Kanamatsu T, Rothman DL, Shulman RG, Behar KL. Glutamatergic neurotransmission and neuronal glucose oxidation are coupled during intense neuronal activation. J. Cereb. Blood Flow Metab. 2004;24:972–985. doi: 10.1097/01.WCB.0000126234.16188.71. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, Tkac I, Choi IY, Merkle H, Ugurbil K, Garwood M, Gruetter R. Localized in vivo 1H NMR detection of neurotransmitter labeling in rat brain during infusion of [1-13C] D-glucose. Magn. Reson. Med. 1999;41:1077–1083. doi: 10.1002/(sici)1522-2594(199906)41:6<1077::aid-mrm1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. The Neuroscientist. 2004;10(1):53–62. doi: 10.1177/1073858403260159. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Let there be (NADH) light. Science. 2004;305:50–52. doi: 10.1126/science.1100428. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. PNAS. 1994;15:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J-M, Tobler I, Allaman I, Borbély AA, Magistretti PJ. Sleep deprivation modulates brain mRNAs encoding genes of glycogen metabolism. Eur. J. Neurosci. 2002;16:1163–1167. doi: 10.1046/j.1460-9568.2002.02145.x. [DOI] [PubMed] [Google Scholar]
- Pfeiffer B, Meyermann R, Hamprecht B. Immunohistochemical co-localization of glycogen phosphorylase with the astroglial markers glial fibrillary acidic protein and S-100 protein in rat brain sections. Histochemistry. 1992;97:405–412. doi: 10.1007/BF00270387. [DOI] [PubMed] [Google Scholar]
- Popel AS. Theory of oxygen transport to tissue. Crit. Rev. Biomed. Eng. 1989;17:257–321. [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Gusnard DA. Appraising the brain’s energy budget. PNAS. 2002;99:10237–10239. doi: 10.1073/pnas.172399499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos M, Del Arco A, Pardo B, Martinez-Serrano A, Martinez-Morales JR, Kobayashi K, Yasuda T, Bogonez E, Bovolenta P, Saheki T, Satrustegui J. Developmental changes in the Ca2+-regulated mitochondrial aspartate-glutamate carrier aralar1 in brain and prominent expression in the spinal cord. Brain Res. Dev. Brain Res. 2003;143:33–46. doi: 10.1016/s0165-3806(03)00097-x. [DOI] [PubMed] [Google Scholar]
- Rider MH, Bertrand L, Vertommen D, Michels PA, Rousseau GG, Hue L. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis. Biochem. J. 2004;381:561–579. doi: 10.1042/BJ20040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- Rothman DL, Hyder F, Sibson N, Behar KL, Mason GF, Shen J, Petroff OAC, Shulman RG. In vivo magnetic resonance spectroscopy studies of the glutamate and GABA neurotransmitter cycles and functional neuroenergetics. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The fifth generation of progress. American College of Neuropsychopharmacology; 2002. Chapter 25. [Google Scholar]
- Roy C, Sherrington C. On the regulation of the blood supply of the brain. J. Physiol. 1890;11:85–100. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Barber R, Wu J, Matsuda T, Roberts E, Vaughn JE. Immunohistochemical localizaation of glutamate decarboxylase in rat cerebellum. PNAS. 1974;71:269–273. doi: 10.1073/pnas.71.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarup A, Larsson OM, Schousboe A. GABA transporters and GABA-transaminase as drug targets. Curr. Drug Targ. CNS Neurol. Dis. 2003;2:269–277. doi: 10.2174/1568007033482788. [DOI] [PubMed] [Google Scholar]
- Savageau MA. Biochemical systems analysis: a study of function and design in molecular biology. Addison-Wesley, Reading; Massachusetts: 1976. [Google Scholar]
- Schousboe A, Sarup A, Bak LK, Waagepetersen HS, Larsson OM. Role of astrocytic transport processes in glutamatergic and GABAergic neurotransmission. Neurochem. Int. 2004;45:512–527. doi: 10.1016/j.neuint.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Sarup A, Larsson OM, White HS. GABA transporters as drug targets for modulation of GABAergic activity. Biochem. Pharmacol. 2004;68:1557–1563. doi: 10.1016/j.bcp.2004.06.041. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Larsson OM, Sarup A, White HS. Role of the betaine/GABA transporter (BGT-1/GAT2) for the control of epilepsy. Eur. J. Pharmacol. 2004;500:281–287. doi: 10.1016/j.ejphar.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Kanner B. In: GABA transporters: functional and pharmacological properties. In: Glutamate and GABA Receptors and Transporters. Egebjerg J, Schousboe A, Krogsgaard-Larsen P, editors. Taylor & Francis Publ.; London, U.K.: 2002. pp. 337–349. [Google Scholar]
- Schousboe I, Tønder N, Zimmer J, Schousboe A. A developmental study of lactate dehydrogenase isozyme and aspartate aminotransferase activity in organotypic rat hippocampal slice cultures and primary cultures of mouse neocortical and cerebellar neurons. Int. J. Dev. Neurosci. 1993;11:765–772. doi: 10.1016/0736-5748(93)90065-l. [DOI] [PubMed] [Google Scholar]
- Schuchmann S, Kovacs R, Kann O, Heinemann U, Buchheim K. Monitoring NAD(P)H autofluorescence to assess mitochondrial metabolic functions in rat hippocampal-entorhinal cortex slices. Brain Res. Protoc. 2001;7:267–276. doi: 10.1016/s1385-299x(01)00080-0. [DOI] [PubMed] [Google Scholar]
- Shank RP, Bennett GS, Freytag SO, Campbell GL. Pyruvate carboxylase: astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain Res. 1985;329:364–367. doi: 10.1016/0006-8993(85)90552-9. [DOI] [PubMed] [Google Scholar]
- Shen J, Petersen KF, Behar KL, Brown P, Nixon TW, Mason GF, Petroff OA, Shulman GI, Shulman RG, Rothman DL. Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. PNAS. 1999;96:8235–8240. doi: 10.1073/pnas.96.14.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuki K, Hishida R, Murakami H, Kudoh M, Kawaguchi T, Watanabe M, Watanabe S, Kouuchi T, Tanaka R. Dynamic imaging of somatosensory cortical activity in the rat visualized by flavoprotein autofluorescence. J. Physiol. 2003;549:919–927. doi: 10.1113/jphysiol.2003.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL. Interpreting functional imaging studies in terms of neurotransmitter cycling. PNAS. 1998;95:11993–11998. doi: 10.1073/pnas.95.20.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Hyder F. A BOLD search for baseline. Neuroimage. 2007;36:277–281. doi: 10.1016/j.neuroimage.2006.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Lactate efflux and the neuroenergetic basis of brain function. NMR Biomed. 2001;14:389–396. doi: 10.1002/nbm.741. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging. PNAS. 2001;98:6417–6422. doi: 10.1073/pnas.101129298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Hyder F. Stimulated changes in localized cerebral energy consumption under anesthesia. PNAS. 1999;96:3245–3250. doi: 10.1073/pnas.96.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth CW, Brennan AM, Connor JA. NAD(P)H fluorescence imaging of postsynaptic neuronal activation in murine hippocampal slices. J. Neurosci. 2003;23:3196–3208. doi: 10.1523/JNEUROSCI.23-08-03196.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. PNAS. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sickmann HM, Schousboe A, Fosgerau K, Waagepetersen HS. Compartmentation of lactate originating from glycogen and glucose in cultured astrocytes. Neurochem. Res. 2005;30(10):1295–1304. doi: 10.1007/s11064-005-8801-4. [DOI] [PubMed] [Google Scholar]
- Sickmann HM, Schousboe A, Bouman SD, Waagepetersen HS. A functioning glycolysis is important for maintenance of glutamate transport in cultured astrocytes. J. Neurochem. 2007;102(Suppl. 1):285. [Google Scholar]
- Siesjo B. Brain energy metabolism. Wiley; New York: 1978. [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J. Cereb. Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. PNAS. 2002;99:10765–10770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L. Relationship between functional activity and energy metabolism in the nervous system: whether, where and why? In: Lassen NA, Ingvar DH, Raichle ME, Friberg L, editors. Brain work and mental activity. Munksgaard; Copenhagen: 1991. pp. 52–64. [Google Scholar]
- Sokoloff L. The metabolism of the central nervous system in vivo. In: Field J, Magoun HW, Hall VE, editors. Handbook of Physiology, Section I, Neurophysiology. Vol. 3. American Physiological Society; Washington D.C.: 1960. pp. 1843–1864. [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Strong AJ, Harland SP, Meldrum BS, Whittington DJ. The use of in vivo fluorescence image sequences to indicate the occurrence and propagation of transient focal depolarizations in cerebral ischemia. J. Cereb. Blood Flow Metab. 1996;16:367–377. doi: 10.1097/00004647-199605000-00003. [DOI] [PubMed] [Google Scholar]
- Tagamets MA, Horwitz B. Interpreting PET and fMRI measures of functional neural activity: The effects of synaptic inhibition on cortical activation in human imaging studies. Brain Res. Bull. 2001;54:267–273. doi: 10.1016/s0361-9230(00)00435-4. [DOI] [PubMed] [Google Scholar]
- Thompson JK, Peterson MR, Freeman RD. Single-neuron activity and tissue oxygenation in the cerebral cortex. Science. 2003;299:1070–1072. doi: 10.1126/science.1079220. [DOI] [PubMed] [Google Scholar]
- Thomsen K, Offenhauser N, Lauritzen M. Principal neuron spiking: neither necessary nor sufficient for cerebral blood flow in rat cerebellum. J. Physiol. 2004;560:181–189. doi: 10.1113/jphysiol.2004.068072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Trubel HK, Sacolick LI, Hyder F. Regional temperature changes in the brain during somatosensory stimulation. J. Cereb. Blood Flow Metab. 2006;26:68–78. doi: 10.1038/sj.jcbfm.9600164. [DOI] [PubMed] [Google Scholar]
- Van den Berg CJ, Garfinkel D. A simulation study of brain compartments: Metabolism of glutamate and related substances in mouse brain. Biochem. J. 1971;23:211–218. doi: 10.1042/bj1230211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchez D, Ros S, Cifuentes D, Pujadas L, Valles J, Garcia-Fojeda B, Criado-Garcia O, Fernandez-Sanchez E, Medraño-Fernandez L, Domínguez J, Garcia-Rocha M, Soriano E, Rodriguez de Cordoba S, Guinovart JJ. Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat. Neurosci. 2007;10(11):1407–1413. doi: 10.1038/nn1998. [DOI] [PubMed] [Google Scholar]
- Viswanathan A, Freeman RD. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat. Neurosci. 2007;10(10):1308–1312. doi: 10.1038/nn1977. [DOI] [PubMed] [Google Scholar]
- Voutsinos-Porche B, Bonvento G, Tanaka K, Steiner P, Welker E, Chatton JY, Magistretti PJ, Pellerin L. Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron. 2003;37:275–286. doi: 10.1016/s0896-6273(02)01170-4. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Qu H, Sonnewald U, Shimamoto K, Schousboe A. Role of glutamine and neuronal glutamate uptake in glutamate homeostasis and synthesis during vesicular release in cultured glutamatergic neurons. Neurochem. Int. 2005;47:92–102. doi: 10.1016/j.neuint.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Sonnewald U, Schousboe A. Compartmentation of glutamine, glutamate and GABA metabolism in neurons and astrocytes: functional implications. The neuroscientist. 2003;9(5):398–403. doi: 10.1177/1073858403254006. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Qu H, Schousboe A, Sonnewald U. Elucidation of the quantitative significance of pyruvate carboxylation in cultured cerebellar neurons and astrocytes. J. Neurosci. Res. 2001;66:763–770. doi: 10.1002/jnr.10061. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Sonnewald U, Larsson OM, Schousboe A. A possible role of alanine for ammonia transfer between astrocytes and glutamatergic neurons. J. Neurochem. 2000;75(2):471–479. doi: 10.1046/j.1471-4159.2000.0750471.x. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Sonnewald U, Schousboe A. Glutamine, glutamate and GABA: Metabolic aspects. In: Lajtha A, editor. Handbook of Neurochemistry and Molec. Neurobiol. 3rd ed. Vol. 6. Springer-Verlag; Berlin Heidelberg: 2007. pp. 1–21. [Google Scholar]
- Waldvogel D, van Gelderen P, Muellbacher W. The relative metabolic demand of inhibition and excitation. Nature. 2000;406:995–998. doi: 10.1038/35023171. [DOI] [PubMed] [Google Scholar]
- Wang H, Hitron IM, Iadecola C, Pickel VM. Synaptic and vascular associations of neurons containing cyclooxygenase-2 and nitric oxide synthase in rat somatosensory cortex. Cereb. Cortex. 2005;15:1250–1260. doi: 10.1093/cercor/bhi008. [DOI] [PubMed] [Google Scholar]
- White HS, Watson WP, Hansen S, Slough S, Sarup A, Bolvig T, Petersen G, Larsson OM, Clausen RP, Frølund B, Krogsgaard-Larsen P, Schousboe A. First demonstration of a functional role for CNS betaine/GABA transporter (mGAT2) based on synergistic anticonvulsant action among inhibitors of mGAT1 and mGAT2. J. Pharmacol. Exp. Ther. 2005;312:866–874. doi: 10.1124/jpet.104.068825. [DOI] [PubMed] [Google Scholar]
- White HS, Sarup A, Bolvig T, Kristensen AS, Petersen G, Nelson N, Pickering DS, Larsson OM, Frølund B, Krogsgaard-Larsen P, Schousboe A. Correlation between anticonvulsant activity and inhibitory action on glial GABA uptake of the highly selective mouse GAT1 inhibitor 3-hydroxy-4-amino-4,5,6,7-tetrahydro-1,2-benzisoxazole (exo-THPO) and its N-alkylated analogs. J. Pharmacol. Exp. Ther. 2002;302:636–644. doi: 10.1124/jpet.102.034819. [DOI] [PubMed] [Google Scholar]
- Yu ACH, Hertz L. Metabolic sources of energy in astrocytes. In: Hertz L, Kvamme E, McGeer EG, Schousboe A, editors. Glutamine, Glutamate and GABA in the Central Nervous System. Alan R. Liss, Inc.; New York: 1983. pp. 431–438. [Google Scholar]
- Yu ACH, Drejer J, Hertz L, Schousboe A. Pyruvate carboxylase activity in primary cultures of astrocytes and neurons. J. Neurochem. 1983;41:1484–1487. doi: 10.1111/j.1471-4159.1983.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Yu ACH, Schousboe A, Hertz L. Metabolic fate of 14C-labeled glutamate in astrocytes in primary cultures. J. Neurochem. 1982;39:954–960. doi: 10.1111/j.1471-4159.1982.tb11482.x. [DOI] [PubMed] [Google Scholar]
- Yudkoff M. Brain metabolism of branched-chain amino acids. Glia. 1997;21:92–98. doi: 10.1002/(sici)1098-1136(199709)21:1<92::aid-glia10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Zaganas I, Waagepetersen HS, Georgopoupolos P, Sonnewald U, Plaitakis A, Schousboe A. Differential expression of glutamate dehydrogenase in cultured neurons and astrocytes from mouse cerebellum and cerebral cortex. J. Neurosci. Res. 2001;66:909–913. doi: 10.1002/jnr.10058. [DOI] [PubMed] [Google Scholar]
- Zwingmann C, Richter-Landsberg C, Brand A, Leibfritz D. NMR spectroscopic study on the metabolic fate of [3-(13)C]alanine in astrocytes, neurons, and cocultures: implications for glia-neuron interactions in neurotransmitter metabolism. Glia. 2000;3:286–303. doi: 10.1002/1098-1136(200012)32:3<286::aid-glia80>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]