Abstract
Various drugs and surgical procedures have been utilized for the treatment of trigeminal neuralgia (TN). Despite numerous available approaches, the results are not completely satisfying. The need for more contemporaneous drugs to control the pain attacks is a common experience. Moreover, a number of patients become drug resistant, needing a surgical procedure to treat the neuralgia. Nonetheless, pain recurrence after one or more surgical operations is also frequently seen. These facts reflect the lack of the precise understanding of the TN pathogenesis. Classically, it has been related to a neurovascular compression at the trigeminal nerve root entry-zone in the prepontine cistern. However, it has been evidenced that in the pain onset and recurrence, various neurophysiological mechanisms other than the neurovascular conflict are involved. Recently, the introduction of new magnetic resonance techniques, such as voxel-based morphometry, diffusion tensor imaging, three-dimensional time-of-flight magnetic resonance angiography, and fluid attenuated inversion recovery sequences, has provided new insight about the TN pathogenesis. Some of these new sequences have also been used to better preoperatively evidence the neurovascular conflict in the surgical planning of microvascular decompression. Moreover, the endoscopy (during microvascular decompression) and the intraoperative computed tomography with integrated neuronavigation (during percutaneous procedures) have been recently introduced in the challenging cases. In the last few years, efforts have been made in order to better define the optimal target when performing the gamma knife radiosurgery. Moreover, some authors have also evidenced that neurostimulation might represent an opportunity in TN refractory to other surgical treatments. The aim of this work was to review the recent literature about the pathogenesis, diagnosis, and medical and surgical treatments, and discuss the significant advances in all these fields.
Keywords: microvascular decompression, percutaneous balloon compression, gamma knife radiosurgery, surgical treatment, magnetic resonance imaging, therapy
Introduction
Trigeminal neuralgia (TN) is a facial pain syndrome characterized by paroxysmal, shock-like pain attacks located in the somatosensory distribution of the trigeminal nerve. The prevalence of TN in the general population is 0.015%.1 Facial pain has a considerable impact on quality of life. It has been recently shown that TN is the most frequent type of facial pain2 and that, among facial pain syndromes, the overall incidence of TN has remained constant3 ranging from 12.6/100,000/year2 to 27/100,000/year.3 TN is uncommon in population younger than 40 years (overall incidence of 0.2/100,000/year) and increases in incidence with advancing age, occurring in 25.9/100,000/year in individuals older than 80 years.4 TN appears to be slightly more common among women and has both classical and symptomatic (~15% of cases) subtypes with the former most often associated with a neurovascular conflict of the trigeminal nerve in the prepontine cistern.5 The right side is more frequently involved.6 When TN occurs in young age or presents with bilateral symptoms, lack of triggered pain, absence of a refractory period, or an abnormal neurologic examination, secondary causes such as multiple sclerosis (MS) should be suspected.5 Bilaterality may be seen in 5% of classical cases, but even in these cases, synchronous pain is not observed. Patients with bilateral TN often have a positive family history.7 In patients affected by MS, prevalence is higher, ranging from 1%8 to 6.3%.9 In these patients, in addition to the episodic pain, a constant pain component is often complained. Although in these patients, pain is mainly unilateral, bilateral involvement can occur up to 31% of patients.10
Advances in diagnosis
Clinical criteria for TN
The International Headache Society recently defined strict clinical criteria for TN diagnosis.11 According to these criteria, a diagnosis of TN can be made when at least three attacks of unilateral facial pain occur fulfilling these criteria: 1) occurring in one or more divisions of the trigeminal nerve, with no radiation beyond the trigeminal distribution and 2) pain with at least three of the following four characteristics: a) recurring in paroxysmal attacks lasting from a fraction of a second to 2 minutes; b) severe intensity; c) electric shock-like, shooting, stabbing, or sharp in quality; and d) precipitated by innocuous stimuli to the affected side of the face. Important criteria for clinical diagnosis are also the lack of evident neurologic deficit and a pain that cannot be attributed to another disorder. Moreover, to rationalize the different subtypes of facial pain, a new classification scheme that divides facial pain into several distinct categories has been recently introduced.12
More specifically, in this new classification,12 it has been proposed to differentiate TN into: 1) type 1 (previously referred to as classic or typical TN), which is an idiopathic episodic pain with the previously reported clinical characteristics, lasting several seconds, with pain-free intervals between attacks and 2) type 2, describing idiopathic trigeminal facial pain that is aching, throbbing, or burning for more than 50% of the time and is constant in nature (constant background pain being the most significant attribute) with a minor component of sharp, episodic pain. It has also been theorized that TN type 1 can progress toward TN type 2 and that in this second type, the likelihood of detecting a structural abnormality such as a tumor or a vascular malformation is higher.12
Nonetheless, the neurophysiological recording of trigeminal reflexes represents a useful and reliable test for the TN diagnosis, according to the European Federation of Neurological Societies (EFNS) guidelines on neuropathic pain assessment13 and the American Academy of Neurology–EFNS guidelines on TN management (Table 1).14,15 In cases of symptomatic TN, neurophysiological testing of trigeminal reflexes seems to provide the same sensitivity (95%) and specificity (93%) as magnetic resonance imaging (MRI).16 Advances in MRI have been playing an important role in the diagnostic setting, especially in the presurgical evaluation of TN patient in order to identify secondary causes of TN and/or the neurovascular conflict. Studies have been published17,18 on the usefulness of three-dimensional fast imaging employing steady-state acquisition and magnetic resonance angiography (MRA) in surgical planning and prediction of surgical findings during micro-vascular decompression (MVD). A correlation more than 95% between this method and surgical findings has been demonstrated.17,18
Table 1.
AAN–EFNS guidelines on TN management
| Topic | Recommendations |
|---|---|
| Diagnosis | For patients with TN without non-trigeminal neurological symptoms, routine imaging may be considered to identify STN (Level C) |
| Younger age of onset, involvement of the first division of the trigeminal nerve, unresponsiveness to treatment, and abnormal trigeminal-evoked potentials should be disregarded as useful for disclosing STN (Level B) | |
| Determining the presence of trigeminal sensory deficits or bilateral involvement of the trigeminal nerves should be considered useful to distinguish STN from CTN | |
| However, the absence of these features should be disregarded as useful for distinguishing STN from CTN (Level B) | |
| Measuring trigeminal reflexes in a qualified electrophysiological laboratory should be considered useful for distinguishing STN form CTN (Level B) | |
| Pharmacological treatment | CBZ is established as effective (Level A) and OXC is probably effective (Level B) for controlling pain in CTN |
| Baclofen, lamotrigine, and pimozide may be considered to control pain in patients with CTN (Level C) | |
| Topical ophthalmic anesthesia is probably ineffective in controlling pain in patients with CTN (Level B) | |
| Surgical treatment | For patients with TN refractory to medical therapy, early surgical therapy may be considered (Level C) |
| Percutaneous procedures on the Gasserian ganglion, gamma knife, and MVD may be considered (Level C) | |
| MVD may be considered over other surgical techniques to provide the longest duration of pain freedom (Level C) |
Abbreviations: AAN, American Academy of Neurology; EFNS, European Federation of Neurological Societies; TN, trigeminal neuralgia; STN, symptomatic trigeminal neuralgia; CTN, classical trigeminal neuralgia; CBZ, carbamazepine; OXC, oxcarbazepine; MVD, microvascular decompression.
Pathophysiological theories
Classically, TN has been related to a neurovascular compression in the prepontine cistern at the nerve root entry-zone due to an abnormal artery or vein, arteriovenous malformation, vestibular schwannoma, meningioma, epidermoid cyst, tuberculoma, various other cysts and tumors, aneurysm, vessels aggregation, and arachnoiditis.19–21 MS, diabetes mellitus, odontogenic inflammatory diseases, and otolaryngological pathology, such as sinusitis, have also been proposed as causes of TN.22–31
From a pathogenic point of view, TN shows a high complexity related to the involvement of various underlying neurophysiological mechanisms. Activation of peripheral receptor, transmission and projection of nociceptive information, and convergence of nociceptive afferents onto common central neurons,32 as well as the interaction of a multitude of neurotransmitters and neuromodulators, may play a key role in the perception of pain.33
Trigeminal convergence-projection theory
In the trigeminal convergence-projection theory, it has been hypothesized that continuous or recurrent nociceptive inputs from head and neck converge on spinal trigeminal nucleus (subnucleus caudalis), where the release of neurotransmitters and vasoactive substances may be promoted.34,35 This release decreases the threshold of adjacent second-order neurons that receive input from sites other than nociceptive sources. The signals from these excited second-order neurons may be transmitted to the thalamus, limbic system, and somatosensory cortex and interpreted as pain.36
Bioresonance hypothesis
Recently, the bioresonance hypothesis for TN pathogenesis has been proposed. This theory states that when the vibration frequency of a structure surrounding the trigeminal nerve becomes close to its natural frequency, the resonance of the trigeminal nerve occurs. The bioresonance can damage trigeminal nerve fibers and lead to the abnormal transmission of the impulse, which may finally result in facial pain.37
Ignition hypothesis
According to the ignition hypothesis, based on recent advances in the understanding of the electrical behavior of injured sensory neurons and on findings from histopathologic observations obtained from patients undergoing MVD, injury of trigeminal afferent neurons in the trigeminal root or ganglion makes these axons and axotomized somata hyperexcitable, giving rise to pain paroxysms as a result of synchronized afterdischarge activity.38
Pathogenesis possibilities from brain imaging studies
New insights about the pathogenesis of TN have been coming from new MRI studies, such as voxel-based morphometry,39 diffusion tensor imaging (DTI),40 three-dimensional time- of-flight (TOF) MRA, and fluid-attenuated inversion recovery DTI-sequences.41 Moreover, it has been evidenced that using functional MRI (fMRI), changes in brain activity associated with stimulation of the cutaneous trigger zone in patients with TN can be analyzed. Recently, Moisset et al42 showed that painful stimuli in TN patients were associated with significantly increased activity in the spinal trigeminal nucleus, thalamus, primary and secondary somatosensory cortices, anterior cingulate cortex, insula, premotor/motor cortex, prefrontal areas, putamen, hippocampus, and brain-stem and that non-painful stimulation of the trigger zone activated all but three of these structures (spinal trigeminal nucleus, brainstem, and anterior cingulate cortex). This wide involvement of different neural structures also during non-painful stimulation of the trigger zone suggests a state of maintained sensitization of the trigeminal nociceptive systems. Interestingly, after a successful surgical treatment, the activation of the operated side was confined only to primary and secondary somatosensory cortices.42 A gray matter volume reduction in TN patients was found, using voxel-based morphometry, in the primary somatosensory and orbitofrontal cortices, as well as in the secondary somatosensory cortex, thalamus, insula, cerebellum, and dorsolateral prefrontal cortex. Gray matter volume decreased within the anterior cingulate cortex, parahippocampus, and temporal lobe and correlated with increasing disease duration in TN, reflecting adaptation mechanism to chronic pain with regard to neuronal plasticity.39 Similarly, using DTI, a lower fractional anisotropy, reflecting an abnormal tissue microstructure, was found in TN patients’ trigeminal nerves and in white matter in the brain, suggesting that trigeminal nerve structural abnormalities occur in TN, even if not apparent on gross imaging.40 To investigate microstructural tissue changes of trigeminal nerve in patients with unilateral TN, Liu et al41 used TOF MRA and fluid-attenuated inversion recovery DTI-sequences, and measured fractional anisotropy, mean diffusivity, axial diffusivity, and radial diffusivity on the involved trigeminal nerve. They found that the affected side showed significantly decreased fractional anisotropy and increased radial diffusivity, suggesting that demyelination without significant axonal injury is an important factor in TN pathogenesis.41 Moreover, these new MRI techniques together with trigeminal tractography have also been utilized to identify microstructural changes in the trigeminal nerve after radiosurgery and possibly monitor the response to this treatment. In patients submitted to radiosurgery, a drop in fractional anisotropy values at the target with no significant change outside the target was evidenced, demonstrating highly focal changes after treatment. Radial diffusivity also changed markedly, suggesting that radiosurgery primarily affects myelin. Fractional anisotropy changes were detected regardless of trigeminal nerve enhancement, suggesting more sensitivity of tractography than conventional gadolinium-enhanced post-treatment MRI. In subjects with long-term follow-up, recovery of fractional anisotropy/radial diffusivity correlated with pain recurrence.43
Advances in medical therapy
Historical and current medical therapy
Phenytoin was the first drug used for TN with reported positive effects.44 However, according to the recent EFNS guidelines,13 two drugs are considered as first-line therapy in TN: carbamazepine (CBZ; 200–1,200 mg/day) and oxcarbazepine (OXC; 600–1,800 mg/day). The effectiveness of CBZ was demonstrated in several studies.45–50 Specifically, CBZ has been found to reduce both the frequency and intensity of painful paroxysms and was equally efficacious on spontaneous and trigger-evoked attacks.45 Nevertheless, frequent adverse event has been reported during CBZ therapy, especially in elderly patients.51–53 Thus, OXC is often used as initial treatment for TN54 due to accepted greater tolerability and decreased potential drug interactions.55 Three randomized controlled trials, comparing OXC (600–1,800 mg/day) to CBZ in TN patients,56,57 reported a reduction in the number of attacks and pain assessments equally good for both CBZ and OXC with more than 80% of patients responding to these drugs. Other drugs have been used in TN: baclofen was found to be superior to placebo in reducing the number of pain attacks.58 Lamotrigine,59 pimozide,60 and tocainide61 were reported to have good efficacy on pain attacks control. Lamotrigine in combination with CBZ or phenytoin was also found to be more effective than placebo.59,62 In patients having already undergone trigeminal surgery or taking concurrent medications, tizanidine was found to be better than placebo, but its effect decayed within 1–3 months.63
Emerging medical therapy
It is common experience that TN can be difficult to treat and can recur after surgical treatments in patients under therapy with more drugs used in combination. Thus, new therapeutic modalities have been tried. More specifically, according to a recent overview,64 gabapentin combined with regular ropivacaine injections into trigger sites improved pain control and quality of life, and pregabalin was found to be effective at 1 year follow-up in TN patients. Recently, Hu et al65 systematically reviewed the therapeutic efficacy and safety of injection of botulinum toxin type A (BTX-A) in TN and found a response in approximately 70%–100% of patients with mean pain intensity and frequency reduced by approximately 60%–80% with no major adverse events reported. On these bases, they concluded that BTX-A may be effective in treatment of TN. These results are in agreement with Cruccu and Truini,66 who recently reviewed the literature on the medical management of refractory TN and found that there is increasing evidence that BTX-A injections are efficacious and may be offered to patients before surgery or to patients unwilling to undergo surgery. Although it represents a promising treatment of TN with favorable risk-to-benefit ratio, to investigate the optimal dose of BTX-A treatment, the duration of therapeutic efficacy, the side effects, and the time and indications for repeat injection, further well-designed, randomized, controlled, double-blinded trials are needed. As recently evidenced, a problem in TN is the treatment of the acute crisis, where local anesthesia, such as ropivacaine, injected into a trigger area, an 8% spray of lidocaine, and the intravenous infusion of fosphenytoin can provide temporary pain relief.64
Advances in surgical therapy
Various surgical approaches have been proposed for the treatment of drug-resistant TN. MVD is performed with the objective to resolve the neurovascular conflict between an abnormal vessel and the trigeminal nerve. On the other hand, percutaneous destructive procedures, involving a trans foramen ovale approach to the retrogasserian portion of the trigeminal nerve and gamma knife radiosurgery (GKRS) aiming at damaging the trigeminal nerve root with a high and concentrated dose of radiation, have been developed during the past years. While there is a wide literature about the surgical treatment of TN,67,68 the difficulty to evaluate the quality of published surgical reports is an emerging problem69 as recently evidenced by international guidelines and systematic reviews.67,70
MVD
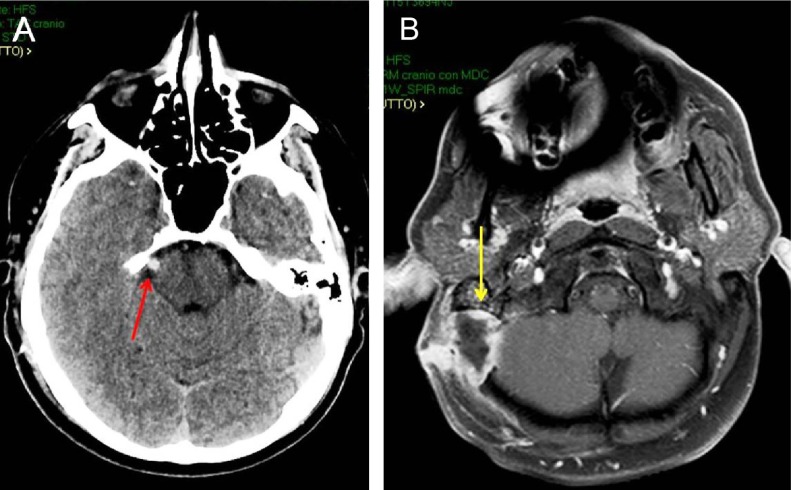
MVD is based on the assumption that a compression of trigeminal nerve by an abnormal vascular loop is the direct cause of TN.71 Obviously, preoperatory radiological studies are mandatory in order to identify the abnormal vessels and the conflict with the nerve. Recently, three-dimensional fast imaging employing steady-state acquisition sequence that produces a very high-resolution T2-weighted MRI with an excellent contrast between structures, including cerebrospinal fluid, trigeminal nerve and adjacent blood vessels, and TOF MRA have been introduced. MVD has become one of the most common treatments for TN providing long pain relief. Unfortunately, not all patients achieve a good outcome after MVD.14 The reported pain-free duration without medication after MVD ranges from 0.6 years to 10 years.72 After 5 years, the percentage of patients free of pain ranges from 58% to 78%.73,74 It has been reported that patients with typical TN and immediate postoperative remission have more often an excellent/good postoperative outcome, being the immediate postoperative remission an independent predictive factor for good long-term outcome.74 Unfortunately, as recently evidenced, no randomized controlled trials of reasonable quality have investigated the role of MVD in the TN treatment.64,70 Moreover, little is reported in the literature about the quality of life after MVD, but it has been evidenced as patients undergoing primary surgery with no recurrence and no complications show no evidence of depression and are very satisfied after MVD.75 Described complications after this procedure are infections (Figure 1), facial palsy, facial numbness, cerebrospinal fluid leak, and hearing deficit with a mortality of 0.1%.76 Obviously, complications and side effects reduce satisfaction mainly after the partial sensory rhizotomy, which causing a sensory loss can lead to keratitis and eating difficulties, decreasing satisfaction of these patients compared to MVD patients.75 It is a common experience that in some cases, identifying the neurovascular conflict cannot be easy during surgery. Recently, some authors reported the use of endoscope as a significant aid in patients with a bony ridge obscuring the view of the fifth nerve, with a very distal vascular compression, or if a combination of both occurs.76,77 Broggi et al76 reported 8.5% of cases in whom conflict was not clearly visible with the microscope but revealed and solved with the endoscope. A fully endoscopic MVD has been described78 with pain outcome and rate of complications very similar to microscopic MVD.77,79
Figure 1.
Postoperative CT scan of a 40-year-old man submitted to MVD for right TN (A; red arrow). Brain axial MRI after gadolinium administration (B) 2 months after MVD, showing an abscess at the site of operation (yellow arrow).
Abbreviations: CT, computed tomography; MVD, microvascular decompression; TN, trigeminal neuralgia; MRI, magnetic resonance imaging.
Percutaneous balloon compression
Percutaneous balloon compression (PBC) was introduced in the clinical setting by Mullan et al79,80 and has been extensively used in the treatment of TN due to low cost, simplicity, and the advantage of being the only percutaneous procedure performed with the patient under general anesthesia. There is a general consensus about the usefulness of PBC either in general population67 or in MS patients.26,68 PBC offers a good rate of immediate postoperative pain relief ranging from 80% to 90%67,81,82 and a pain-free time without medication that ranges from 2 years to 3 years.82,83 However, no reports about the long-term quality of life of these patients are available in the literature. Some authors suggested84,85 a lower efficacy in patients previously treated with other surgical procedures, in cases with positive history of MS and when a pear-like shape of the balloon at the operation is not obtained.86 Complications can include numbness, dysesthesia, and, more rarely, masseter weakness that usually resolves within some months; meningitis and cranial nerve deficits are less common.82,87,88 There are no standardized criteria concerning the compression time and the compression pressure. While experimental animal models suggest that a long compression time is associated with better outcome,89 these data are not confirmed in the clinical setting where there are consistent data that a longer compression time does not affect the pain relief and only increase the complication rate.86,90,91 Moreover, higher balloon pressures have been associated with higher rates of dysesthesia,severe numbness, and masseter weakness.92 The variability of Meckel’s cave size has been advocated as another factor involved in the efficacy of the procedure; thus, cannulas of different sizes have been designed.93 Technical failure to cannulate the foramen ovale using fluoroscopy can be a significant problem in some cases, but recently, intraoperative computed tomography with integrated neuronavigation has been safely used in reoperated patients due to prior failure under fluoroscopy.94
Glycerol rhizotomy
The injection of glycerol in the trigeminal cistern determines pain relief in patients with TN due to demyelination and axonal fragmentation.95,96 Since its introduction,97,98 this technique has remained relatively unchanged with a reported initial pain relief more than 90%99 and a rate of pain-free patients at 3 years of almost 50%.100,101 There are evidences that the success of glycerol rhizotomy depends on some degree of sensory loss postoperatively96,102,103 and that the chance of good outcome would be increased if facial pain was present during glycerol injection.96 Dysesthesias, corneal numbness, masseter weakness, and herpes labialis have been reported as frequent complications of this procedure.87,96,101 Recently, Goodwin et al104 performed a MVD with injection of glycerol to the inferior third cisternal portion of the nerve, anterior to the root entry-zone, in 14 patients without neurovascular conflict on pre-operative MRI, reporting an 80% of good response at 3 months follow-up.
Radiofrequency thermocoagulation
Radiofrequency thermocoagulation is based on the attempt to electrocoagulate the trigeminal nerve and Gasserian ganglion rootlets.105,106 An initial pain relief more than 90% with a recurrence rate of up to 25% has been reported.107,108 The reported side effects, such as masticatory weakness, dysesthesia, and corneal numbness, seem to be related to significant individual variation of somatotopic organization of trigeminal nerve fibers and the irreversible damage of small, unmyelinated pain fibers.109,110 To overcome these limitations, a quadripolar electrode improving the accuracy of somatotopic identification, decreasing the lesion size, and reducing the unwanted injury has been developed.111 A decrease in the incidence of masseter weakness and undesirable paresthesias and an improvement in the immediate pain relief rate using a curved tip electrode has previously been reported.112 Moreover, the use of neuronavigator and computer tomography to improve needle localization seems to be associated with lower complications and recurrence rate compared to standard fluoroscopy in recent studies.113,114 Pulsed radiofrequency had been introduced with the aim to reduce the incidence of side effects; however, as recently reported in a prospective, randomized, double-blinded study comparing the effect of pulsed radiofrequency and conventional radiofrequency, although none of the patients in the pulsed radiofrequency group showed paresthesia, pain relief was not satisfactory as it was expected.115
Gamma knife radiosurgery
GKRS has been used as a treatment modality in several centers for patients with concurrent medical illness who were poor candidates for MVD or who refuse more invasive surgery.116,117 Usually, the root entry-zone of the trigeminal nerve is used as a target and the dose protocols range from 70 Gy to 100 Gy.118–121 Nonetheless, considering that the underlying mechanisms are not fully understood, to date there is still uncertainty about the exact target and optimum dose to be used. In many clinical target volume definitions, the root entry-zone of the trigeminal nerve situated at 2–3 mm from the brainstem surface is chosen.122 In a recent study, the radiosurgical target appeared to affect the duration of pain relief with the target closer to the brainstem, providing extended pain relief. However, the proximal radiosurgical target was also associated with an increased risk of mild to moderate facial numbness.123 Alternative targets include the trigeminal nuclei in the brainstem or the centromedian nucleus of the thalamus.124 In general, it has been reported that higher doses of radiation are related to better outcomes, but complications increase at doses greater than 90 Gy.120,125 In published long-term follow-up studies, the mean maximal radiation dose was approximately 80 Gy, and complications included facial numbness, which affected approximately 10% of the treated patients.126–128 Moreover, permanent dysesthesias and anesthesia dolorosa affecting the quality of life have been reported.129 Although GKRS achieves relatively good outcomes on initial pain relief, the results suggest a rate of late failure, particularly among patients who performed GKRS following prior surgery.130 Little et al126 reported that 75% of patients with no previous surgery achieved long-term pain relief at 7 years compared with only 10% of patients with previous surgery. GKRS requires a delay before pain relief occurs. For this reason, some authors suggest that patients with extreme pain in need of fast relief should undergo other procedures.131 Recently, it has been evidenced that overall pain relief following GKRS was comparable in patients with and without evidence of vascular compression on MRI. In the subgroup analysis of those with MRI evidence of vessel impingement of the affected trigeminal nerve, pain relief correlated with a higher dose to the point of contact between the impinging vessel and the trigeminal nerve.132 Nonetheless, in a recent prospective cohort study comparing GKRS and MVD, the last one was significantly superior to GKRS in maintaining a pain-free status and provided similar early and superior longer-term patient satisfaction rates compared to GKRS.133
Neuromodulation, really a chance for TN?
Two kinds of neuromodulation have been reported as optional treatments for chronic pain, refractory to conventional medical and surgical treatment: motor cortex stimulation (MCS) and deep brain stimulation (DBS). Chronic stimulation of the precentral cortex for the treatment of pain was first reported by Tsubokawa et al134 in 1991, and several studies have documented excellent results of using MCS for the treatment of trigeminal neuropathic pain, with 75%–100% of patients achieving good to excellent pain relief.135–139 Nevertheless, these studies, mostly focusing on the use of MCS in pain syndrome, report few patients with idiopathic TN,137–139 with a limited follow-up.139 On the other hand, DBS has been applied in the treatment of medically and surgically refractory chronic pain since 1997.135,140–143 According to an interesting hypothesis, one of its main target, the posterior hypothalamus (pHyp), controls relationship between the neuropsychological circuits involved in pain behavior and the neurovegetative system. Franzini et al144 reported the first series of chronic pHyp stimulation, and since then, many authors have proposed it to treat severe pain syndromes. In a systematic review,145 the same authors reported that none of the four patients suffering from refractory neuropathic trigeminal pain benefited from the procedure, whereas all five patients affected with refractory TN due to MS and undergoing pHyp DBS experienced a significant decrease in pain attacks within the first trigeminal branch. Nevertheless, better results were obtained in chronic cluster headache and short, unilateral neuralgiform headache attacks with conjunctival injection and tearing. As for MCS, the limited number of studies and the relatively short follow-up make difficult to fully evaluate the efficacy of neuromodulation procedures. However, neurostimulation might represent an opportunity in TN refractory to other surgical treatments.
Conclusion
The treatment of TN is a challenge both for neurologists and neurosurgeons. The lack of a full comprehension of the complex pathogenesis at the basis of TN remains a key factor explaining the results that are not always satisfying with the medical therapy. Progress has been made in the recent years both for the pathogenesis and surgical treatment due to implementation of neuroradiological techniques. Surgery has also taken advantage from the introduction of the endoscope and neuronavigation in the operating room. New drugs, such as BTX-A, may be offered to patients before surgery or to patients unwilling to undergo surgery. Better definition of GKRS targets would improve the results of this technique. Neurostimulation might represent an opportunity in patients refractory to other surgical treatments, but further studies are needed due to the few cases treated.
Footnotes
Disclosure
The authors reported no conflicts of interest.
References
- 1.Penman J. Trigeminal neuralgia. In: Vinken PJ, Bruyn GW, editors. Handbook of Clinical Neurology. Vol. 5. Amsterdam: North-Holland Publishing Company; 1968. pp. 296–322. [Google Scholar]
- 2.Koopman JS, Dieleman JP, Huygen FJ, de Mos M, Martin CG, Sturkenboom MC. Incidence of facial pain in the general population. Pain. 2009;147(1–3):122–127. doi: 10.1016/j.pain.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Hall GC, Carroll D, McQuay HJ. Primary care incidence and treatment of four neuropathic pain conditions: a descriptive study, 2002–2005. BMC Fam Pract. 2008;9:26. doi: 10.1186/1471-2296-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katusic S, Beard CM, Bergstralh E, Kurland LT. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945–1984. Ann Neurol. 1990;27(1):89–95. doi: 10.1002/ana.410270114. [DOI] [PubMed] [Google Scholar]
- 5.Gronseth G, Cruccu G, Alksne J, et al. Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology. 2008;71(15):1183–1190. doi: 10.1212/01.wnl.0000326598.83183.04. [DOI] [PubMed] [Google Scholar]
- 6.Maarbjerg S, Gozalov A, Olesen J, Bendtsen L. Trigeminal neuralgia – a prospective systematic study of clinical characteristics in 158 patients. Headache. 2014;54(10):1574–1582. doi: 10.1111/head.12441. [DOI] [PubMed] [Google Scholar]
- 7.Pollack IF, Jannetta PJ, Bissonette DJ. Bilateral trigeminal neuralgia: a 14-year experience with microvascular decompression. J Neurosurg. 1988;68(4):559–565. doi: 10.3171/jns.1988.68.4.0559. [DOI] [PubMed] [Google Scholar]
- 8.Ruge D, Brochner D, Davis L. A study of the treatment of 637 patients with trigeminal neuralgia. J Neurosurg. 1958;15(5):528–536. doi: 10.3171/jns.1958.15.5.0528. [DOI] [PubMed] [Google Scholar]
- 9.Putzki N, Pfriem A, Limmroth V, et al. Prevalence of migraine, tension-type headache and trigeminal neuralgia in multiple sclerosis. Eur J Neurol. 2009;16(2):262–267. doi: 10.1111/j.1468-1331.2008.02406.x. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96–111. doi: 10.1016/j.pain.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Headache Classification Committee of the International Headache Society (IHS) The international classification of headache disorders, 3rd edition (beta version) Cephalalgia. 2013;33(9):629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 12.Eller JL, Raslan AM, Burchiel KJ. Trigeminal neuralgia: definition and classification. Neurosurg Focus. 2005;18(5):E3. doi: 10.3171/foc.2005.18.5.4. [DOI] [PubMed] [Google Scholar]
- 13.Cruccu G, Sommer C, Anand P, et al. EFNS guidelines on neuropathic pain assessment: revised 2009. Eur J Neurol. 2010;17(8):1010–1018. doi: 10.1111/j.1468-1331.2010.02969.x. [DOI] [PubMed] [Google Scholar]
- 14.Cruccu G, Gronseth G, Alksne J, et al. American Academy of Neurology Society; European Federation of Neurological Society AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol. 2008;15(10):1013–1028. doi: 10.1111/j.1468-1331.2008.02185.x. [DOI] [PubMed] [Google Scholar]
- 15.Bowsher D. Trigeminal neuralgia: a symptomatic study on 126 successive patients with and without prevoius intervention. Pain Clin. 2000;12:93–101. [Google Scholar]
- 16.Cruccu G, Biasiotta A, Galeotti F. Diagnostic accuracy of trigeminal reflex testing in trigeminal neuralgia. Neurology. 2006;66(1):139–141. doi: 10.1212/01.wnl.0000191388.64530.8f. [DOI] [PubMed] [Google Scholar]
- 17.Zeng Q, Zhou Q, Liu Z, Li C, Ni S, Xue F. Preoperative detection of the neurovascular relationship in trigeminal neuralgia using three-dimensional fast imaging employing steady-state acquisition (FIESTA) and magnetic resonance angiography (MRA) J Clin Neurosci. 2013;20(1):107–111. doi: 10.1016/j.jocn.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Q, Liu ZL, Qu CC, Ni SL, Xue F, Zeng QS. Preoperative demonstration of neurovascular relationship in trigeminal neuralgia by using 3D FIESTA sequence. Magn Reson Imaging. 2012;30(5):666–671. doi: 10.1016/j.mri.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Kano H, Awan NR, Flannery TJ, et al. Stereotactic radiosurgery for patients with trigeminal neuralgia associated with petroclival meningiomas. Stereotact Funct Neurosurg. 2011;89(1):17–24. doi: 10.1159/000321187. [DOI] [PubMed] [Google Scholar]
- 20.Jamjoom AB, Jamjoom ZA, al-Fehaily M, el-Watidy S, al-Moallem M, Nain-Ur-Rahman Trigeminal neuralgia related to cerebellopontine angle tumors. Neurosurg Rev. 1996;19(4):237–241. doi: 10.1007/BF00314838. [DOI] [PubMed] [Google Scholar]
- 21.Guo Z, Ouyang H, Cheng Z. Surgical treatment of parapontine epidermoid cysts presenting with trigeminal neuralgia. J Clin Neurosci. 2011;18(3):344–346. doi: 10.1016/j.jocn.2010.07.110. [DOI] [PubMed] [Google Scholar]
- 22.Marinković S, Todorović V, Gibo H, et al. The trigeminal vasculature pathology in patients with neuralgia. Headache. 2007;47(9):1334–1339. doi: 10.1111/j.1526-4610.2007.00933.x. [DOI] [PubMed] [Google Scholar]
- 23.Siqueira SR, Teixeira MJ, Siqueira JT. Clinical characteristics of patients with trigeminal neuralgia referred to neurosurgery. Eur J Dent. 2009;3(3):207–212. [PMC free article] [PubMed] [Google Scholar]
- 24.Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001;124(pt 12):2347–2360. doi: 10.1093/brain/124.12.2347. [DOI] [PubMed] [Google Scholar]
- 25.Sarlani E, Grace EG, Balciunas BA, Schwartz AH. Trigeminal neuralgia in a patient with multiple sclerosis and chronic inflammatory demyelinating polyneuropathy. J Am Dent Assoc. 2005;136(4):469–476. doi: 10.14219/jada.archive.2005.0202. [DOI] [PubMed] [Google Scholar]
- 26.Montano N, Papacci F, Cioni B, Di Bonaventura R, Meglio M. Percutaneous balloon compression for the treatment of trigeminal neuralgia in patients with multiple sclerosis. Analysis of the potentially prognostic factors. Acta Neurochir (Wien) 2012;154(5):779–783. doi: 10.1007/s00701-012-1301-9. [DOI] [PubMed] [Google Scholar]
- 27.Urban PP, Forst T, Lenfers M, Koehler J, Connemann BJ, Beyer J. Incidence of subclinical trigeminal and facial nerve involvement in diabetes mellitus. Electromyogr Clin Neurophysiol. 1999;39(5):267–272. [PubMed] [Google Scholar]
- 28.Roberts AM, Person P, Chandran NB, Hori JM. Further observations on dental parameters of trigeminal and -atypical facial neuralgias. Oral Surg Oral Med Oral Pathol. 1984;58(2):121–129. doi: 10.1016/0030-4220(84)90123-3. [DOI] [PubMed] [Google Scholar]
- 29.al-Gailani M, Haidar Z. Trigeminal neuralgia: an unusual case of dental origin. Odontostomatol Trop. 1987;10(3–4):225–227. [PubMed] [Google Scholar]
- 30.Sawaya RA. Trigeminal neuralgia associated with sinusitis. ORL J Otorhinolaryngol Relat Spec. 2000;62(3):160–163. doi: 10.1159/000027738. [DOI] [PubMed] [Google Scholar]
- 31.Lin YW, Lin SK, Weng IH. Fatal paranasal sinusitis presenting as trigeminal neuralgia. Headache. 2006;46(1):174–178. doi: 10.1111/j.1526-4610.2006.00316_5.x. [DOI] [PubMed] [Google Scholar]
- 32.Svensson E. Pain mechanisms in myogenous temporomandibular disorders. Pain Forum. 1997;6:158–165. [Google Scholar]
- 33.Siddall PJ, Cousins MJ. Pain mechanisms and management: an update. Clin Exp Pharmacol Physiol. 1995;22:679–688. doi: 10.1111/j.1440-1681.1995.tb01921.x. [DOI] [PubMed] [Google Scholar]
- 34.Sessle BJ, Hu JW. Mechanisms of pain arising from articular tissues. Can J Physiol Pharmacol. 1991;69:617–626. doi: 10.1139/y91-092. [DOI] [PubMed] [Google Scholar]
- 35.Sessle BJ, Ho JW, Yu XM. New trends in referred pain and hyperalgesia, pain research and clinical management. In: Vecchiet D, Albe-Fessard D, Limblom U, editors. Brainstem Mechanisms of Referred Pain and Hyperalgesia in the Orofacial and Temporomandibular Region. 7th ed. Amsterdam: Elsevier; 1993. pp. 59–71. [Google Scholar]
- 36.Bonica JL. The Management of Pain. 2nd ed. Malvern, PA: Lea and Febiger; 1990. p. 180. [Google Scholar]
- 37.Jia DZ, Li G. Bioresonance hypothesis: a new mechanism on the pathogenesis of trigeminal neuralgia. Med Hypotheses. 2010;74(3):505–507. doi: 10.1016/j.mehy.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 38.Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain. 2002;18(1):4–13. doi: 10.1097/00002508-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Obermann M, Rodriguez-Raecke R, Naegel S, et al. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage. 2013;74:352–358. doi: 10.1016/j.neuroimage.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 40.Aguiar de Sousa D, Geraldes R, Gil-Gouveia R, et al. New daily persistent headache and radiologically isolated syndrome. J Neurol. 2013;260(8):2179–2181. doi: 10.1007/s00415-013-7015-y. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Li J, Butzkueven H, et al. Microstructural abnormalities in the trigeminal nerves of patients with trigeminal neuralgia revealed by multiple diffusion metrics. Eur J Radiol. 2013;82(5):783–786. doi: 10.1016/j.ejrad.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 42.Moisset X, Villain N, Ducreux D, et al. Functional brain imaging of trigeminal neuralgia. Eur J Pain. 2011;15(2):124–131. doi: 10.1016/j.ejpain.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Hodaie M, Chen DQ, Quan J, Laperriere N. Tractography delineates microstructural changes in the trigeminal nerve after focal radiosurgery for trigeminal neuralgia. PLoS One. 2012;7(3):e32745. doi: 10.1371/journal.pone.0032745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sindrup SH, Jensen TS. Pharmacotherapy of trigeminal neuralgia. Clin J Pain. 2002;18(1):22–27. doi: 10.1097/00002508-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Campbell FG, Graham JG, Zilkha KJ. Clinical trial of carbamazepine (tegretol) in trigeminal neuralgia. J Neurol Neurosurg Psychiatry. 1966;29(3):265–267. doi: 10.1136/jnnp.29.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Killian JM, Fromm GH. Carbamazepine in the treatment of neuralgia. Use of side effects. Arch Neurol. 1968;19(2):129–136. doi: 10.1001/archneur.1968.00480020015001. [DOI] [PubMed] [Google Scholar]
- 47.Nicol CF. A four year double blind study of tegretol in facial pain. Headache. 1969;9(1):54–57. doi: 10.1111/j.1526-4610.1969.hed0901054.x. [DOI] [PubMed] [Google Scholar]
- 48.Rockcliff BW, Davis EH. Controlled sequential trials of carbamazepine in trigeminal neuralgia. Arch Neurol. 1996;15(2):129–136. doi: 10.1001/archneur.1966.00470140019003. [DOI] [PubMed] [Google Scholar]
- 49.Wiffen PJ, Derry S, Moore RA, Kalso EA. Carbamazepine for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;4:CD005451. doi: 10.1002/14651858.CD005451.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zakrzewska JM, Linskey ME. Trigeminal neuralgia. Clin Evid (Online) 2014;10:1207. [PMC free article] [PubMed] [Google Scholar]
- 51.McQuay H, Carroll D, Jadad AR, Wiffen P, Moore A. Anticonvulsant drugs for management of pain: a systematic review. BMJ. 1995;311(7012):1047–1052. doi: 10.1136/bmj.311.7012.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiffen P, Collins S, McQuay H, Carroll D, Jadad A, Moore A. Anticonvulsant drugs for acute and chronic pain. Cochrane Database Syst Rev. 2005;20(3):CD001133. doi: 10.1002/14651858.CD001133.pub2. [DOI] [PubMed] [Google Scholar]
- 53.Wiffen P, McQuay H, Moore R. Carbamazepine for acute and chronic pain. Cochrane Database Syst Rev. 2005;3:CD005451. doi: 10.1002/14651858.CD005451. [DOI] [PubMed] [Google Scholar]
- 54.Jensen TS. Anticonvulsants in neuropathic pain: rationale and clinical evidence. Eur J Pain. 2002;6(suppl A):61–68. doi: 10.1053/eujp.2001.0324. [DOI] [PubMed] [Google Scholar]
- 55.Kutluay E, McCague K, D’Souza J, Beydoun A. Safety and tolerability of oxcarbazepine in elderly patients with epilepsy. Epilepsy Behav. 2003;4(2):175–180. doi: 10.1016/s1525-5050(03)00037-4. [DOI] [PubMed] [Google Scholar]
- 56.Beydoun A. Clinical use of tricyclic anticonvulsants in painful neuropathies and bipolar disorders. Epilepsy Behav. 2002;3(3S):S18–S22. doi: 10.1016/s1525-5050(02)00017-3. [DOI] [PubMed] [Google Scholar]
- 57.Beydoun A. Safety and efficacy of oxcarbazepine: results of randomized, double-blind trials. Pharmacotherapy. 2000;20(8 pt 2):152S–158S. doi: 10.1592/phco.20.12.152s.35254. [DOI] [PubMed] [Google Scholar]
- 58.Fromm GH, Terrence CF, Chattha AS. Baclofen in the treatment of trigeminal neuralgia: double-blind study and long-term follow-up. Ann Neurol. 1984;15(3):240–244. doi: 10.1002/ana.410150306. [DOI] [PubMed] [Google Scholar]
- 59.Zakrzewska JM, Chaudhry Z, Nurmikko TJ, Patton DW, Mullens EL. Lamotrigine (Lamictal) in refractory trigeminal neuralgia: results from a double-blind placebo controlled crossover trial. Pain. 1997;73(2):223–230. doi: 10.1016/S0304-3959(97)00104-8. [DOI] [PubMed] [Google Scholar]
- 60.Lechin F, van der Dijs B, Lechin ME, et al. Pimozide-therapy for trigeminal neuralgia. Arch Neurol. 1989;46(90):960–963. doi: 10.1001/archneur.1989.00520450030015. [DOI] [PubMed] [Google Scholar]
- 61.Lindstrom P, Lindblom U. The analgesic effect of tocainide in trigeminal neuralgia. Pain. 1987;28(1):45–50. doi: 10.1016/0304-3959(87)91058-X. [DOI] [PubMed] [Google Scholar]
- 62.Wiffen PJ, Derry S, Moore RA. Lamotrigine for acute and chronic pain. Cochrane Database Syst Rev. 2011;2:CD006044. doi: 10.1002/14651858.CD006044.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fromm GH, Aumentado D, Terrence CF. A clinical and experimental investigation of the effects of tizanidine in trigeminal neuralgia. Pain. 1993;53(3):265–271. doi: 10.1016/0304-3959(93)90222-B. [DOI] [PubMed] [Google Scholar]
- 64.Zakrzewska JM, Linskey ME. Trigeminal neuralgia. BMJ. 2014;348:g474. doi: 10.1136/bmj.g474. [DOI] [PubMed] [Google Scholar]
- 65.Hu Y, Guan X, Fan L, et al. Therapeutic efficacy and safety of botulinum toxin type A in trigeminal neuralgia: a systematic review. J Headache Pain. 2013;14:72. doi: 10.1186/1129-2377-14-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cruccu G, Truini A. Refractory trigeminal neuralgia. Non-surgical treatment options. CNS Drugs. 2013;27(2):91–96. doi: 10.1007/s40263-012-0023-0. [DOI] [PubMed] [Google Scholar]
- 67.Tatli M, Satici O, Kanpolat Y, Sindou M. Various surgical modalities for trigeminal neuralgia: literature study of respective long-term outcomes. Acta Neurochir (Wien) 2008;150(3):243–255. doi: 10.1007/s00701-007-1488-3. [DOI] [PubMed] [Google Scholar]
- 68.Montano N, Papacci F, Cioni B, Di Bonaventura R, Meglio M. What is the best treatment of drug-resistant trigeminal neuralgia in patients affected by multiple sclerosis? A literature analysis of surgical procedures. Clin Neurol Neurosurg. 2013;115(5):567–572. doi: 10.1016/j.clineuro.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 69.Akram H, Mirza B, Kitchen N, Zakrzewska JM. Proposal for evaluating the quality of reports of surgical interventions in the treatment of trigeminal neuralgia: the surgical trigeminal neuralgia score. Neurosurg Focus. 2013;35(3):E3. doi: 10.3171/2013.6.FOCUS13213. [DOI] [PubMed] [Google Scholar]
- 70.Zakrzewska JM, Akram H. Neurosurgical interventions for the treatment of classical trigeminal neuralgia. Cochrane Database Syst Rev. 2011;9:CD007312. doi: 10.1002/14651858.CD007312.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jannetta PJ. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg. 1967;26(1):159–162. doi: 10.3171/jns.1967.26.1part2.0159. [DOI] [PubMed] [Google Scholar]
- 72.Gu W, Zhao W. Microvascular decompression for recurrent trigeminal neuralgia. J Clin Neurosci. 2014;21(9):1549–1553. doi: 10.1016/j.jocn.2013.11.042. [DOI] [PubMed] [Google Scholar]
- 73.Broggi G, Ferroli P, Franzini A, Servello D, Dones I. Microvascular decompression for trigeminal neuralgia: comments on a series of 250 cases, including 10 patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2000;68(1):59–64. doi: 10.1136/jnnp.68.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oesman C, Mooij JJ. Long-term follow-up of microvascular decompression for trigeminal neuralgia. Skull Base. 2011;21(5):313–322. doi: 10.1055/s-0031-1284213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zakrzewska JM, Lopez BC, Kim SE, Coakham HB. Patient reports of satisfaction after microvascular decompression and partial sensory rhizotomy for trigeminal neuralgia. Neurosurgery. 2005;56(6):1304–1311. doi: 10.1227/01.neu.0000159883.35957.e0. discussion 1312. [DOI] [PubMed] [Google Scholar]
- 76.Broggi M, Acerbi F, Ferroli P, Tringali G, Schiariti M, Broggi G. Microvascular decompression for neurovascular conflicts in the cerebello-pontine angle: which role for endoscopy? Acta Neurochir (Wien) 2013;155(9):1709–1716. doi: 10.1007/s00701-013-1824-8. [DOI] [PubMed] [Google Scholar]
- 77.Sandell T, Ringstad GA, Eide PK. Usefulness of the endoscope in microvascular decompression for trigeminal neuralgia and MRI-based prediction of the need for endoscopy. Acta Neurochir (Wien) 2014;156(10):1901–1909. doi: 10.1007/s00701-014-2171-0. [DOI] [PubMed] [Google Scholar]
- 78.Halpern CH, Lang SS, Lee JY. Fully endoscopic microvascu-lar decompression: our early experience. Min Invasive Surg. 2013;2013:739432. doi: 10.1155/2013/739432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mullan S, Duda EE, Patronas NJ. Some examples of balloon technology in neurosurgery. J Neurosurg. 1980;52(3):321–329. doi: 10.3171/jns.1980.52.3.0321. [DOI] [PubMed] [Google Scholar]
- 80.Mullan S, Lichtor T. Percutaneous microcompression of the trigeminal ganglion for trigeminal neuralgia. J Neurosurg. 1983;59(6):1007–1012. doi: 10.3171/jns.1983.59.6.1007. [DOI] [PubMed] [Google Scholar]
- 81.Bergenheim AT, Asplund P, Linderoth B. Percutaneous retrogasserian balloon compression for trigeminal neuralgia: review of critical technical details and outcomes. World Neurosurg. 2013;79(2):359–368. doi: 10.1016/j.wneu.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 82.Brown JA, McDaniel MD, Weaver MT. Percutaneous trigeminal nerve compression for treatment of trigeminal neuralgia: results in 50 patients. Neurosurgery. 1993;32(4):570–573. doi: 10.1227/00006123-199304000-00012. [DOI] [PubMed] [Google Scholar]
- 83.Baabor MG, Perez-Limonte L. Percutaneous balloon compression of the gasserian ganglion for the treatment of trigeminal neuralgia: personal experience of 206 patients. Acta Neurochir Suppl. 2011;108:251–254. doi: 10.1007/978-3-211-99370-5_39. [DOI] [PubMed] [Google Scholar]
- 84.Jellish WS, Benedict W, Owen K, Anderson D, Fluder E, Shea JF. Perioperative and long-term operative outcomes after surgery for trigeminal neuralgia: microvascular decompression vs percutaneous balloon ablation. Head Face Med. 2008;4:11. doi: 10.1186/1746-160X-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Skirving DJ, Dan NG. A 20-year review of percutaneous balloon compression of the trigeminal ganglion. J Neurosurg. 2001;94(6):913–917. doi: 10.3171/jns.2001.94.6.0913. [DOI] [PubMed] [Google Scholar]
- 86.Montano N, Papacci F, Cioni B, Di Bonaventura R, Meglio M. The role of percutaneous balloon compression in the treatment of trigeminal neuralgia recurring after other surgical procedures. Acta Neurol Belg. 2014;114(1):59–64. doi: 10.1007/s13760-013-0263-x. [DOI] [PubMed] [Google Scholar]
- 87.Lopez BC, Hamlyn PJ, Zakrzewska JM. Systematic review of ablative neurosurgical techniques for the treatment of trigeminal neuralgia. Neurosurgery. 2004;54(4):973–982. doi: 10.1227/01.neu.0000114867.98896.f0. discussion 982–983. [DOI] [PubMed] [Google Scholar]
- 88.Lichtor T, Mullan JF. A 10-year follow-up review of percutaneous microcompression of the trigeminal ganglion. J Neurosurg. 1990;72(1):49–54. doi: 10.3171/jns.1990.72.1.0049. [DOI] [PubMed] [Google Scholar]
- 89.Li F, Han S, Ma Y, Yi F, Xu X, Liu Y. Optimal duration of percutaneous microballoon compression for treatment of trigeminal nerve injury. Neural Reg Res. 2014;9(2):179–189. doi: 10.4103/1673-5374.125347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meglio M, Cioni B. Percutaneous procedures for trigeminal neuralgia: microcompression versus radiofrequency thermocoagulation. Personal experience. Pain. 1989;38(1):9–16. doi: 10.1016/0304-3959(89)90066-3. [DOI] [PubMed] [Google Scholar]
- 91.Park SS, Lee MK, Kim JW, Jung JY, Kim IS, Ghang CG. Percutaneous balloon compression of trigeminal ganglion for the treatment of idiopathic trigeminal neuralgia: experience in 50 patients. J Korean Neurosurg Soc. 2008;43(4):186–189. doi: 10.3340/jkns.2008.43.4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zanusso M, Curri D, Landi A, Colombo F, Volpin L, Cervellini P. Pressure monitoring inside Meckel’s cave during percutaneous micro-compression of gasserian ganglion. Stereotact Funct Neurosurg. 1991;56(1):37–43. doi: 10.1159/000099391. [DOI] [PubMed] [Google Scholar]
- 93.Goerss SJ, Atkinson JL, Kallmes DF. Variable size percutaneous balloon compression of the gasserian ganglion for trigeminal neuralgia. Surg Neurol. 2009;71(3):388–390. doi: 10.1016/j.surneu.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 94.Georgiopoulos M, Ellul J, Chroni E, Constantoyannis C. Minimizing technical failure of percutaneous balloon compression for trigeminal neuralgia using neuronavigation. ISRN Neurol. 2014;2014:630418. doi: 10.1155/2014/630418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kondziolka D, Lunsford LD. Percutaneous retrogasserian glycerol rhizotomy for trigeminal neuralgia: technique and expectations. Neurosurg Focus. 2005;18(5):E7. doi: 10.3171/foc.2005.18.5.8. [DOI] [PubMed] [Google Scholar]
- 96.Pollock BE. Percutaneous retrogasserian glycerol rhizotomy for patients with idiopathic trigeminal neuralgia: a prospective analysis of factors related to pain relief. J Neurosurg. 2005;102(2):223–228. doi: 10.3171/jns.2005.102.2.0223. [DOI] [PubMed] [Google Scholar]
- 97.Bennett MH, Lunsford LD. Percutaneous retrogasserian glycerol rhizotomy for tic douloureux, part 2: results and implications of trigeminal evoked potential studies. Neurosurgery. 1984;14(4):431–435. doi: 10.1227/00006123-198404000-00007. [DOI] [PubMed] [Google Scholar]
- 98.Lunsford LD, Bennett MH. Percutaneous retrogasserian glycerol rhizotomy for tic douloureux, part 1: technique and results in 112 patients. Neurosurgery. 1984;14(4):424–430. doi: 10.1227/00006123-198404000-00006. [DOI] [PubMed] [Google Scholar]
- 99.Mahajan VK, Ranjan N, Sharma S, Sharma NL. Spontaneous tooth exfoliation after trigeminal herpes zoster: a case series of an uncommon complication. Indian J Dermatol. 2013;58(3):244. doi: 10.4103/0019-5154.110878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.North RB, Kidd DH, Piantadosi S, Carson BS. Percutaneous retrogasserian glycerol rhizotomy: predictors of success and failure in treatment of trigeminal neuralgia. J Neurosurg. 1990;72(6):851–856. doi: 10.3171/jns.1990.72.6.0851. [DOI] [PubMed] [Google Scholar]
- 101.Slettebø H, Hirschberg H, Lindegaard KF. Long-term results after percutaneous retrogasserian glycerol rhizotomy in patients with trigeminal neuralgia. Acta Neurochir (Wien) 1993;122(3–4):231–235. doi: 10.1007/BF01405534. [DOI] [PubMed] [Google Scholar]
- 102.Blomstedt PC, Bergenheim AT. Technical difficulties and perioperative complications of retrogasserian glycerol rhizotomy for trigeminal neuralgia. Stereotact Funct Neurosurg. 2002;79(3–4):168–181. doi: 10.1159/000070830. [DOI] [PubMed] [Google Scholar]
- 103.Burchiel KJ. Percutaneous retrogasserian glycerol rhizolysis in the management of trigeminal neuralgia. J Neurosurg. 1988;69(3):361–366. doi: 10.3171/jns.1988.69.3.0361. [DOI] [PubMed] [Google Scholar]
- 104.Goodwin CR, Yang JX, Bettegowda C, et al. Glycerol rhizotomy via a retrosigmoid approach as an alternative treatment for trigeminal neuralgia. Clin Neurol Neurosurg. 2013;115(12):2454–2456. doi: 10.1016/j.clineuro.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 105.Sweet WG. Proceedings: analgesia dolorosa after differential retrogasserian thermal or mechanical rhizotomy: tactics employed to decrease its influence. J Neurol Neurosurg Psychiatry. 1975;38(4):407. doi: 10.1136/jnnp.38.4.407-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu JK, Apfelbaum RI. Treatment of trigeminal neuralgia. Neurosurg Clin N Am. 2004;15(3):319–334. doi: 10.1016/j.nec.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 107.Karol EA, Agner C. Technological advances in the surgical management of trigeminal neuralgia. Crit Rev Neurosurg. 1999;9(2):70–78. doi: 10.1007/s003290050113. [DOI] [PubMed] [Google Scholar]
- 108.Karol EA, Sanz OP, Gonzalez La Riva FN, Rey RD. A micrometric multiple electrode array for the exploration of gasserian and retrogasserian trigeminal fibers: preliminary report: technical note. Neurosurgery. 1993;33(1):154–158. doi: 10.1227/00006123-199307000-00027. [DOI] [PubMed] [Google Scholar]
- 109.Kanpolat Y, Onol B. Experimental percutaneous approach to the trigeminal ganglion in dogs with histopathological evaluation of radiofrequency lesions. Acta Neurochir Suppl (Wien) 1980;30:363–366. doi: 10.1007/978-3-7091-8592-6_44. [DOI] [PubMed] [Google Scholar]
- 110.Smith HP, McWhorter JM, Challa VR. Radiofrequency neurolysis in a clinical model: neuropathological correlation. J Neurosurg. 1981;55(2):246–253. doi: 10.3171/jns.1981.55.2.0246. [DOI] [PubMed] [Google Scholar]
- 111.Karol EA, Karol MN. A multiarray electrode mapping method for percutaneous thermocoagulation as treatment of trigeminal neuralgia: technical note on a series of 178 consecutive procedures. Surg Neurol. 2009;71(1):11–17. doi: 10.1016/j.surneu.2007.09.021. discussion 17–18. [DOI] [PubMed] [Google Scholar]
- 112.Tobler WD, Tew JM, Jr, Cosman E, Keller JT, Quallen B. Improved outcome in the treatment of trigeminal neuralgia by percutaneous stereotactic rhizotomy with a new, curved tip electrode. Neurosurgery. 1983;12(3):313–317. doi: 10.1227/00006123-198303000-00011. [DOI] [PubMed] [Google Scholar]
- 113.Gusmão S, Oliveira M, Tazinaffo U, Honey CR. Percutaneous trigeminal nerve radiofrequency rhizotomy guided by computerized tomography fluoroscopy. Technical note. J Neurosurg. 2003;99(4):785–786. doi: 10.3171/jns.2003.99.4.0785. [DOI] [PubMed] [Google Scholar]
- 114.Xu SJ, Zhang WH, Chen T, Wu CY, Zhou MD. Neuronavigator-guided percutaneous radiofrequency thermocoagulation in the treatment of intractable trigeminal neuralgia. Chin Med J (Engl) 2006;119(18):1528–1535. [PubMed] [Google Scholar]
- 115.Erdine S, Ozyalcin NS, Cimen A, Celik M, Talu GK, Disci R. Comparison of pulsed radiofrequency with conventional radiofrequency in the treatment of idiopathic trigeminal neuralgia. Eur J Pain. 2007;11(3):309–313. doi: 10.1016/j.ejpain.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 116.McNatt SA, Yu C, Giannotta SL, Zee CS, Apuzzo ML, Petrovich Z. Gamma knife radiosurgery for trigeminal neuralgia. Neurosurgery. 2005;56(6):1295–1301. doi: 10.1227/01.neu.0000160073.02800.c7. discussion 1301–1303. [DOI] [PubMed] [Google Scholar]
- 117.Pollock BE, Phuong LK, Gorman DA, Foote RL, Stafford SL. Stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2002;97(2):347–353. doi: 10.3171/jns.2002.97.2.0347. [DOI] [PubMed] [Google Scholar]
- 118.Kondziolka D, Lunsford LD, Flickinger JC, et al. Stereotactic radio-surgery for trigeminal neuralgia: a multiinstitutional study using the gamma unit. J Neurosurg. 1996;84(6):940–945. doi: 10.3171/jns.1996.84.6.0940. [DOI] [PubMed] [Google Scholar]
- 119.Kondziolka D, Perez B, Flickinger JC, Habeck M, Lunsford LD. Gamma knife radiosurgery for trigeminal neuralgia: results and expectations. Arch Neurol. 1998;55(12):1524–1529. doi: 10.1001/archneur.55.12.1524. [DOI] [PubMed] [Google Scholar]
- 120.Régis J, Métellus P, Lazorthes Y, Porcheron D, Peragut JC. Effect of gamma knife on secondary trigeminal neuralgia. Stereotact Funct Neurosurg. 1998;70(suppl 1):210–217. doi: 10.1159/000056424. [DOI] [PubMed] [Google Scholar]
- 121.Young RF, Vermulen S, Posewitz A. Gamma knife radiosurgery for the treatment of trigeminal neuralgia. Stereotact Funct Neurosurg. 1998;70(suppl 1):192–199. doi: 10.1159/000056422. [DOI] [PubMed] [Google Scholar]
- 122.Lettmaier S. Radiosurgery in trigeminal neuralgia. Phys Med. 2014;30(5):592–595. doi: 10.1016/j.ejmp.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 123.Xu Z, Schlesinger D, Moldovan K, et al. Impact of target location on the response of trigeminal neuralgia to stereotactic radiosurgery. J Neurosurg. 2014;120(3):716–724. doi: 10.3171/2013.10.JNS131596. [DOI] [PubMed] [Google Scholar]
- 124.Keep MF, DeMare PA, Ashby LS. Gamma knife surgery for refractory postherpetic trigeminal neuralgia: targeting in one session both the retrogasserian trigeminal nerve and the centromedian nucleus of the thalamus. J Neurosurg. 2005;102(suppl):276–282. doi: 10.3171/jns.2005.102.s_supplement.0276. [DOI] [PubMed] [Google Scholar]
- 125.Pollock BE, Phuong LK, Foote RL, Stafford SL, Gorman DA. High-dose trigeminal neuralgia radiosurgery associated with increased risk of trigeminal nerve dysfunction. Neurosurgery. 2001;49(1):58–62. doi: 10.1097/00006123-200107000-00008. discussion 62–64. [DOI] [PubMed] [Google Scholar]
- 126.Little AS, Shetter AG, Shetter ME, Bay C, Rogers CL. Long-term pain response and quality of life in patients with typical trigeminal neuralgia treated with gamma knife stereotactic radiosurgery. Neurosurgery. 2008;63(5):915–923. doi: 10.1227/01.NEU.0000327689.05823.28. discussion 923–924. [DOI] [PubMed] [Google Scholar]
- 127.Riesenburger RI, Hwang SW, Schirmer CM, et al. Outcomes following single-treatment gamma knife surgery for trigeminal neuralgia with a minimum 3-year follow-up. J Neurosurg. 2010;112(4):766–771. doi: 10.3171/2009.8.JNS081706. [DOI] [PubMed] [Google Scholar]
- 128.Urgosik D, Liscak R, Novotny J, Jr, Vymazal J, Vladyka V. Treatment of essential trigeminal neuralgia with gamma knife surgery. J Neurosurg. 2005;102(suppl):29–33. doi: 10.3171/jns.2005.102.s_supplement.0029. [DOI] [PubMed] [Google Scholar]
- 129.Lopez BC, Hamlyn PJ, Zakrzewska JM. Stereotactic radiosurgery for primary trigeminal neuralgia: state of the evidence and recommendations for future reports. J Neurol Neurosurg Psychiatry. 2004;75(7):1019–1024. doi: 10.1136/jnnp.2003.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee JK, Choi HJ, Ko HC, Choi SK, Lim YJ. Long term outcomes of gamma knife radiosurgery for typical trigeminal neuralgia-minimum 5-year follow-up. J Korean Neurosurg Soc. 2012;51(5):276–280. doi: 10.3340/jkns.2012.51.5.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mathieu D, Effendi K, Blanchard J, Séguin M. Comparative study of gamma knife surgery and percutaneous retrogasserian glycerol rhizotomy for trigeminal neuralgia in patients with multiple sclerosis. J Neurosurg. 2012;117(suppl):175–180. doi: 10.3171/2012.6.GKS12987. [DOI] [PubMed] [Google Scholar]
- 132.Sheehan JP, Ray DK, Monteith S, et al. Gamma Knife radiosurgery for trigeminal neuralgia: the impact of magnetic resonance imaging-detected vascular impingement of the affected nerve. J Neurosurg. 2010;113(1):53–58. doi: 10.3171/2009.9.jns09196. [DOI] [PubMed] [Google Scholar]
- 133.Linskey ME, Ratanatharathorn V, Peñagaricano J. A prospective cohort study of microvascular decompression and gamma knife surgery in patients with trigeminal neuralgia. J Neurosurg. 2008;109(suppl):160–172. doi: 10.3171/JNS/2008/109/12/S25. [DOI] [PubMed] [Google Scholar]
- 134.Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien) 1991;52:137–139. doi: 10.1007/978-3-7091-9160-6_37. [DOI] [PubMed] [Google Scholar]
- 135.Levy R, Deer TR, Henderson J. Intracranial neurostimulation for pain control: a review. Pain Phisician. 2010;13(2):157–165. [PubMed] [Google Scholar]
- 136.Fontaine D, Hamani C, Lozano A. Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: critical review of the literature. J Neurosurg. 2009;110(2):251–256. doi: 10.3171/2008.6.17602. [DOI] [PubMed] [Google Scholar]
- 137.Raslan AM, Nasseri M, Bahgat D, Abdu E, Burchiel KJ. Motor cortex stimulation for trigeminal neuropathic or deafferentation pain: an institutional case series experience. Stereotact Funct Neurosurg. 2011;89(2):83–88. doi: 10.1159/000323338. [DOI] [PubMed] [Google Scholar]
- 138.Tanei T, Kajita Y, Noda H, et al. Efficacy of motor cortex stimulation for intractable central neuropathic pain: comparison of stimulation parameters between post-stroke pain and other central pain. Neurol Med Chir (Tokyo) 2011;51(1):8–14. doi: 10.2176/nmc.51.8. [DOI] [PubMed] [Google Scholar]
- 139.Buchanan RJ, Darrow D, Monsivais D, Nadasdy Z, Gjini K. Motor cortex stimulation for neuropathic pain syndromes: a case series experience. Neuroreport. 2014;25(9):715–717. doi: 10.1097/WNR.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 140.Broggi G, Franzini A, Leone M, Bussone G. Update on neurosurgical treatment of chronic trigeminal autonomic cephalalgias and atypical facial pain with deep brain stimulation of posterior hypothalamus: results and comments. Neurol Sci. 2007;28(suppl 2):S138–S145. doi: 10.1007/s10072-007-0767-3. [DOI] [PubMed] [Google Scholar]
- 141.Rasche D, Rinaldi PC, Young RF, Tronnier VM. Deep brain stimulation for the treatment of various chronic pain syndromes. Neurosurg Focus. 2006;21(6):E8. doi: 10.3171/foc.2006.21.6.10. [DOI] [PubMed] [Google Scholar]
- 142.Franzini A, Marras C, Tringali G, et al. Chronic high frequency stimulation of the posteromedial hypothalamus in facial pain syndromes and behaviour disorders. Acta Neurochir Suppl. 2007;97(pt 2):399–406. doi: 10.1007/978-3-211-33081-4_45. [DOI] [PubMed] [Google Scholar]
- 143.Cordella R, Franzini A, La Mantia L, Marras C, Erbetta A, Broggi G. Hypothalamic stimulation for trigeminal neuralgia in multiple sclerosis patients: efficacy on the paroxysmal ophthalmic pain. Mult Scler. 2009;15(11):1322–1328. doi: 10.1177/1352458509107018. [DOI] [PubMed] [Google Scholar]
- 144.Franzini A, Ferroli P, Leone M, Broggi G. Stimulation of the posterior hypothalamus for treatment of chronic intractable cluster headaches: first reported series. Neurosurgery. 2003;52(5):1095–1099. discussion 1101. [PubMed] [Google Scholar]
- 145.Franzini A, Messina G, Cordella R, Marras C, Broggi G. Deep brain stimulation of the posteromedial hypothalamus: indications, long term results, and neurophysiological considerations. Neurosurg Focus. 2010;29(2):E13. doi: 10.3171/2010.5.FOCUS1094. [DOI] [PubMed] [Google Scholar]