Abstract
We have previously shown that impulsivity in rats is linked to decreased dopamine D2/3 receptor availability in the ventral striatum. In the present study, we investigated, using longitudinal positron emission tomography (PET), the effects of orally administered methylphenidate (MPH), a first-line treatment for attention deficit hyperactivity disorder, on D2/3 receptor availability in the dorsal and ventral striatum and related these changes to impulsivity. Rats were screened for impulsive behavior on a five-choice serial reaction time task. After a baseline PET scan with the D2/3 ligand [18F]fallypride, rats received 6 mg/kg MPH, orally, twice each day for 28 d. Rats were then reassessed for impulsivity and underwent a second [18F]fallypride PET scan. Before MPH treatment, we found that D2/3 receptor availability was significantly decreased in the left but not the right ventral striatum of high-impulse (HI) rats compared with low-impulse (LI) rats. MPH treatment increased impulsivity in LI rats, and modulated impulsivity and D2/3 receptor availability in the dorsal and ventral striatum of HI rats through inverse relationships with baseline levels of impulsivity and D2/3 receptor availability, respectively. However, we found no relationship between the effects of MPH on impulsivity and D2/3 receptor availability in any of the striatal subregions investigated. These findings indicate that trait-like impulsivity is associated with decreased D2/3 receptor availability in the left ventral striatum, and that stimulant drugs modulate impulsivity and striatal D2/3 receptor availability through independent mechanisms.
Keywords: addiction, attention-deficit hyperactivity disorder, dopamine, methylphenidate, nucleus accumbens, positron emission tomography
Introduction
Converging evidence from neuroimaging, clinical psychopharmacology, and animal models implicates dysregulated dopaminergic and norepinephrinergic neurotransmission in the pathophysiology of attention deficit hyperactivity disorder (ADHD), the prototypical impulse control disorder (Biederman, 2005; Arnsten, 2006; Dalley et al., 2011). Methylphenidate (MPH), which acts by increasing extrasynaptic dopamine (DA) and norepinephrine (NE) levels by blocking their reuptake (Zetterström et al., 1988), has been the first-line pharmaceutical therapy for ADHD (Wilens, 2008). Although its pharmacological action has been well characterized, the precise neurobiological mechanisms underlying the therapeutic effects of MPH remain unclear. Recent findings suggest that only specific neurocognitive processes in domains such as impulse control and attention are affected by MPH, and that these interact with the drug in a baseline performance-dependent manner (Dews and Wenger, 1977; Sahakian and Robbins, 1977; Robbins and Sahakian, 1979; Turner et al., 2003; Clatworthy et al., 2009; DeVito et al., 2009). Such effects, which depend on optimizing catecholamine levels in the brain, are hypothesized to follow an inverted U-shaped function (Clatworthy et al., 2009; van der Schaaf et al., 2013).
Recently, we reported (Caprioli et al., 2013) a similar baseline-dependent effect of cocaine in a preclinical animal model of impulsivity. We reported that impaired response inhibition in a rodent model of impulsivity is associated with a deficiency in DA D2/3 receptor availability in the left ventral striatum, and that prior response-contingent exposure to cocaine both restored D2/3 receptor availability in this region and improved impulse control. This evidence directly supports the baseline dependency hypothesis at the neurobiological level in the striatum, and this may be relevant to recent findings (Volkow et al., 2007a,b, 2009, 2012). Indeed, a set of well powered case-control positron emission tomography (PET) studies in adult medication-naive ADHD patients, found ADHD to be associated with reduced D2/3 receptor availability in the nucleus accumbens and caudate (Volkow et al., 2007a,b, 2009), and that treatment response was associated with increased DA transmission in the ventral striatum (Volkow et al., 2012). Thus, the clinical efficacy of stimulant drugs such as MPH in ADHD may depend, in part, on restoring D2/3 receptor signaling in the ventral striatum of impulsive individuals.
In the present study, we therefore investigated the effects of repeated oral administration of MPH on D2/3 receptor availability in the ventral striatum of high-impulsive (HI) rats on the five-choice serial reaction time task (5-CSRTT). Impulsivity in this task is measured by the number of anticipatory responses to an imminent visual signal and is analogous to false alarms on the analogous continuous performance test in humans (Robbins, 2002). We used PET and the selective high-affinity D2/3 receptor antagonist [18F]fallypride (Mukherjee et al., 1995) to investigate D2/3 receptor availability in the ventral and dorsal striatum, both before, and following chronic exposure of rats to MPH. In parallel, we investigated the relationship between behavioral impulsivity in selected low-impulsive (LI) versus HI rats and MPH-evoked changes in D2/3 receptor availability in the ventral and dorsal striatum.
Materials and Methods
Subjects.
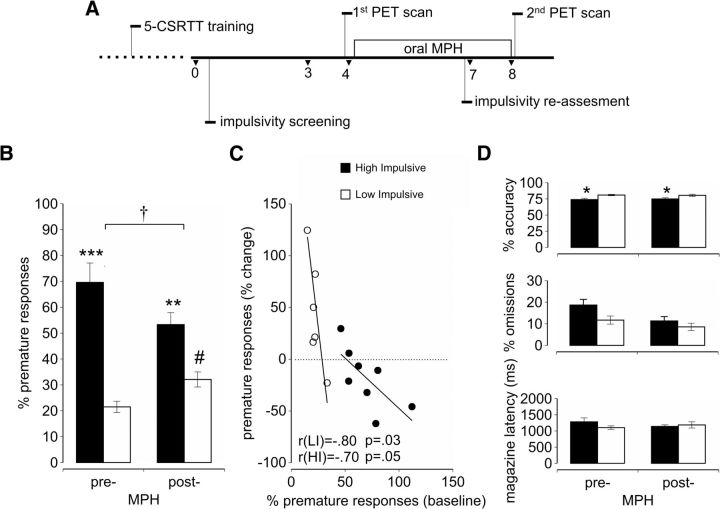
Ninety-six adult male Lister-hooded rats (Charles River), weighing 250–275 g and 2–3 months of age at the beginning of behavioral training, were used. These were housed in groups of four in enclosed ventilation chambers during the initial training and selection of HI and LI rats. Upon completion of the screening and for the remaining period of the study, rats were singly housed (n = 8 HI rats; n = 7 LI rats), similar to our previous study (Caprioli et al., 2013). Rats were singly housed because MPH has been shown to disrupt social behavior in adolescent and young adult rats (Beatty et al., 1982; Arakawa, 1994; Vanderschuren et al., 2008). The holding room was humidity and temperature controlled (22°C), and rats were maintained under a reversed 12 h light/dark cycle (white lights off/red lights on at 7:00 A.M.). Food was restricted to maintain body weights at 85–90% of free-feeding weights. Water was available ad libitum. The present experiment conformed to the United Kingdom Animals (Scientific Procedures) Act of 1986 and local ethical guidelines. A timeline of experimental procedures is shown in Figure 1.
Figure 1.
Effects of methylphenidate on sustained attention and impulsivity in selected LI versus HI rats. A, Timeline of the experimental procedure in rats expressing differential levels of impulsive behavior on the 5-CSRTT. The dashed line refers to 5-CSRTT training, which took ∼3 months. Values shown are weeks. B, Effects of prior oral MPH administration on impulsivity in LI (white bars) and HI (black bars) rats on the 5-CSRTT. Pre-cocaine administration values are averaged across 3 weekly spaced L-ITI sessions. It can be seen in B that impulsivity was altered both in HI and LI rats (group × MPH interaction: F(1,13) = 7.59, † p < 0.05) during the challenge sessions. The increase in impulsivity post-MPH was significant in LI rats (#p = 0.044). C, Correlation plots showing the relationship between relative changes in impulsivity from baseline produced by MPH. It can be seen in C that the effect of MPH on increasing impulsivity was greatest in LI rats showing the lowest baseline level of impulsivity (r = −0.80; p = 0.03), whereas MPH decreased impulsivity to a greater extent in those HI rats showing the highest baseline level of impulsivity (r = −0.7; p = 0.05). D, Differences in accuracy, omissions, and magazine latency before and after the oral MPH dosing between HI and LI rats (*p < 0.05, **p < 0.01, ***p < 0.001 HI vs LI).
Five-choice serial reaction time task.
The 5-CSRTT apparatus has been described in detail previously (Bari et al., 2008). The training procedure used in the present study was identical to that previously described (Caprioli et al., 2013). In brief, rats were trained on the 5-CSRTT over ∼60 daily sessions (6 sessions per week) to detect the location of a brief visual stimulus (0.7 s) presented on a random basis in one of the five recesses. Each session consisted of 100 discrete trials and lasted ∼30 min. Training was considered complete when rats responded to the target stimuli of duration 0.7 s with an accuracy of 75% and omissions on <20% of trials. Trials were initiated by subjects entering the magazine. After a fixed intertrial interval (ITI) of 5 s, a visual stimulus was presented in a single aperture. Rats were rewarded with a single pellet if they correctly located the position of the target stimulus (a “correct” response). A failure to respond within a limited hold period of 5 s was deemed an “omission” and was signaled by a 5 s time-out period and a loss of food reward on that trial. Similar feedback was given on trials where rats responded in an adjacent aperture (an “incorrect” response) or before the onset of the light stimulus (a “premature” response). Behavioral performance was assessed by the following: choice accuracy, (percentage correct responses/(correct + incorrect trials); premature responding, (percentage premature responses/(correct + incorrect + omission trials); omissions, (percentage omission trials/(correct + incorrect + omission trials); latency to collect food, (time from nose-poke response to entering the magazine, in milliseconds); and correct response latency, (time to make a response in the correct aperture after the onset of the light stimulus). Once rats had acquired the 5-CSRTT, they were ranked for impulsivity during a 3 week screening period. Each week consisted of 5 consecutive days of testing with days 1, 2, 4, and 5 comprising sessions of 100 discrete trials each and an ITI of 5 s [short ITI (S-ITI)]. During day 3, the ITI was increased to 7 s to increase the frequency of premature responses [long ITI (L-ITI)]. HI animals were defined as those exhibiting a level of premature responding of >50 on all three L-ITI sessions. LI rats were selected from the remaining rats and responded prematurely on <30% of trials during the L-ITI sessions.
Long-term methylphenidate treatment.
Methylphenidate hydrochloride (Sigma) was dissolved in Ribena (GlaxoSmithKline) and administered orally (6 mg/ml/kg) twice a day (10:00 A.M. and 5:00 P.M.). The oral route of administration was used to model the normal manner in which this drug is administered clinically (Kuczenski and Segal, 2002; Swanson and Volkow, 2009). Two days before the first oral dosing of MPH, rats were trained to consume the Ribena solution from a 1 ml syringe. Chronic MPH exposure was maintained for 7 d a week for 4 consecutive weeks (Fig. 1). During this period, rats were assessed for performance on the 5-CSRTT at 8:00 A.M. when rats were in the drug-free state (i.e., 15 h after the last MPH administration). During the last 7 d of MPH treatment, rats were challenged with three L-ITI sessions, each spaced 3 d apart.
Analysis of methylphenidate and ritalinic acid.
Four nonimpulsive rats were used to quantify plasma levels of MPH and its metabolite ritalinic acid. Rats were orally administered Ribena spiked with 6 mg/kg MPH, as described above, and after 10 min were anesthetized with 5% isoflurane. General anesthesia was maintained via the delivery of 1.5% isoflurane in medical air. Blood samples were taken from a tail vein (0.5 ml) at 5, 15, 30, 60, 90, and 120 min following MPH administration. Blood was allowed to clot at room temperature (24°C) before being centrifuged at 2000 rpm for 10 min. Plasma was aspirated from the centrifuged sample and stored at −80°C before the determination of MPH and ritalinic acid using HPLC-tandem mass spectrometry (MS/MS). Calibration standards (range, 0.01–5 mg/L) were prepared with MPH and ritalinic acid spiked in blank rat plasma. Quality control samples were similarly prepared at 0.05, 1.00, and 1.50 mg/L. Calibration standards and triplicate controls were carried through with each batch of analysis. Samples were prepared by adding 50 μl of test specimen, calibrators, or controls to 2 ml of 100 mm phosphate buffer, pH 6.0, and spiking with the internal standard (10 μl of 20% acetonitrile in 0.1% aqueous formic acid containing 100 pg trideuterated-MPH). The solutions were then placed in an ultrasonic bath for 10 min before extracting the drugs by adding to a preconditioned Strata Screen-C GF 200 mg/6 ml solid phase extraction column. Ritalinic acid was eluted first with hexane/ethyl acetate (50:50) followed by MPH using dichloromethane/isopropanol/ammonia (78:20:2). The eluates were evaporated to dryness at 30°C and reconstituted in 100 μl of methanol/water before injection into an HPLC-MS/MS spectrometer for analysis. HPLC separation was achieved using a FORTIS 3 μm C18 150 × 3 mm column with detection using an API 3200 MS/MS system with a TurboIon spray interface. The HPLC consisted of a Shimadzu system with mobile phase A consisting of water containing 0.01% ammonia and mobile phase B consisting of methanol containing 0.01% ammonia with 10 ml of isopropanol added per liter. The gradient run was 20–100% mobile phase B over 10 min. Positive multiple reaction monitoring was used to monitor the chromatography column eluent [MPH (MRM 234–84), ritalinic acid (MRM 220–84) and d3-MPH (MRM 237–84)]. The lower limit of detection was 0.008 mg/L (signal-to-noise ratio, 10) and the lower limit of quantification was 0.01 mg/L. The overall precision (between batch quality control specimens run on 4 separate days) was 2–11% within the calibration range used.
Positron emission tomography.
HI and LI rats were scanned using [18F]fallypride PET on the following two occasions: before the first oral MPH dose and 2 d after the last MPH dose, which occurred within 48 h of the last L-ITI session on the 5-CSRTT (Fig. 1). The scanning procedure has been described in detail previously (Caprioli et al., 2013). In brief, before the injection of tracer, single-mode transmission data were acquired using a rotating 68Ge/68Ga point source (∼20 MBq) to provide measured attenuation correction. For all scans, [18F]fallypride was injected intravenously over 30 s, followed by a 15 s heparin-saline flush. The injected [18F]fallypride activity (5.3–66.9 MBq) was adjusted so that the total mass of labeled and unlabeled fallypride injected was 0.5 nmol/kg. Dynamic data were acquired in list mode for 180 min and subsequently binned into sinograms for the following time frames: 6 × 10 s, 3 × 20 s, 6 × 30 s, 10 × 60 s, 10 × 120 s, and 29 × 300 s. Corrections were applied for randoms, dead time, normalization, attenuation, and decay. Fourier rebinning (Defrise et al., 1997) was used to compress the four-dimensional sinograms to three dimensions before reconstruction with two-dimensional filtered back projection with a Hann window cutoff at the Nyquist frequency. The image voxel size was 0.95 × 0.95 × 0.80 mm, with an array size of 128 × 128 × 95. The reconstructed images were converted to kilobecquerels per milliliter using global and slice factors determined from imaging a uniform phantom filled with a [18F]fluoride solution.
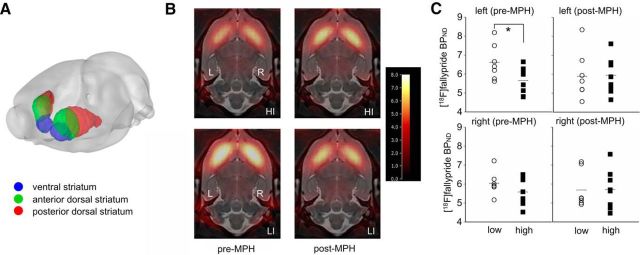
Thirty-two T2-weighted MR brain scans from previous [18F]fallypride PET studies in Lister-hooded rats were used to create a high-resolution MR brain template with SyN (Avants et al., 2008), part of the Advanced Normalization Tools (ANTS) package. A PET template was then created by applying the spatial normalization parameters from the above template creation process to late [18F]fallypride images (average image 120–180 min after injection) that had been manually coregistered to their corresponding MR scan. Late [18F]fallypride images from the 30 PET scans in this study were affine registered to the PET template using ANTS, and each transformation was used to reslice the corresponding dynamic [18F]fallypride PET image set to template space. Finally, the MR template used in a previous study (Caprioli et al., 2013; Fig. 2A) was spatially normalized to the new MR template described above, with the resulting transformation applied to the previously defined regions of interest to align them to the new template space.
Figure 2.
MPH-induced effects on D2/3 receptor availability in the left ventral striatum of HI and LI rats. A, Three-dimensional depiction of regions of interest showing the ventral striatum (blue), anterior dorsal striatum (green), and posterior dorsal striatum (red). B, Horizontal section through [18F]fallypride BPND maps for HI and LI rats overlaid on the coregistered MR template (L, left; R, right). The images are 7 mm below the dorsal brain surface and have a BPND threshold = 8. C, [18F]fallypride BPND in the left and right ventral striatum of LI (circle symbols, n = 7) and HI (square symbols, n = 8) rats before (pre-MPH) and after (post-MPH) oral administration. It can be seen that [18F]fallypride BPND is significantly reduced in the left ventral striatum of HI rats compared with LI rats before MPH exposure (*p < 0.05) and that MPH reduces the contrast in D2/3 receptor availability between LI and HI rats in the left ventral striatum.
D2/3 receptor availability was quantified using nondisplaceable binding potential (BPND) (Innis et al., 2007), determined from reference tissue-based kinetic analysis, with the cerebellum acting as the reference region. The borders of the reference region drawn on the MR template excluded the outermost lamina of the cerebellar cortex to avoid partial volume error from uptake in the Purkinje cell layer. Regional and voxelwise BPND values were estimated from the distribution volume ratio (DVR; BPND = DVR − 1) determined using the reference tissue input Logan plot (Logan et al., 1996) with data fitted from 90 to 180 min postinjection.
Statistical analysis.
Behavioral data were subjected to ANOVA (SPSS version 17.0) using a general linear model. Homogeneity of variance was verified using Levene's test. For repeated-measures analyses, Mauchly's test of sphericity was applied, and the degrees of freedom were corrected to more conservative values using the Huynh-Feldt ε for any terms involving factors in which the sphericity assumption was violated. Differences in BPND between HI and LI rats were evaluated using repeated-measures ANOVA. Significantly meaningful interactions (p < 0.1) were further analyzed by simple main effects using the pooled sum of square error term (Cochran and Cox, 1957). A significance level of α = 0.05 was used to interpret the main effects and post hoc tests. Pearson product moment correlations were used to assess the strength of the association between the following: (1) the change in BPND ((post-MPH − pre-MPH)/(pre-MPH) × 100) and baseline BPND (pre-MPH scan); and (2) the change in premature responses ((post-MPH − pre-MPH)/(pre-MPH) × 100) and baseline BPND (pre-MPH scan). All figures show group means ± SEM.
Results
Segregation of high- and low-impulsivity groups
The behavioral performance of LI and HI rats on the 5-CSRTT is summarized in Table 1. The percentages of premature responses for HI (n = 8) and LI (n = 7) rats, averaged across the three L-ITI sessions before the commencement of MPH dosing, were (mean ± SEM) 69.6 ± 7.5% and 21.5 ± 2.2%, respectively. HI rats were more impulsive than LI rats regardless of the ITI being set to 5 s (baseline 1 to baseline 10; S-ITI; p = 0.002) or 7 s (L-ITI; p < 0.001). Among the various behavioral variables recorded only attentional accuracy was significantly impaired in HI rats compared with LI rats during the L-ITI sessions (p = 0.014). Although omissions appeared to be increased in HI rats compared with LI rats, this contrast was not significant (p = 0.054).
Table 1.
Summary of the effects of oral MPH administration on the behavioral performance of LI and HI rats on the 5-CSRTT
| Pre-MPH |
Post-MPH |
|||
|---|---|---|---|---|
| LI (n = 7) | HI (n = 8) | LI (n = 7) | HI (n = 8) | |
| S-ITI sessions | ||||
| Premature (%) | 2.9 ± 0.5 | 8.6 ± 1.2* | 2.7 ± 0.6 | 4.4 ± 0.7 |
| Accuracy (%) | 83.0 ± 1.7 | 80.4 ± 1.7 | 83.4 ± 1.0 | 79.8 ± 2.0 |
| Omissions (%) | 12.5 ± 2.2 | 9.0 ± 1.4 | 7.5 ± 1.1 | 6.7 ± 1.2 |
| Magazine latency (ms) | 1169.4 ± 91.9 | 1156.4 ± 58.4 | 1213.8 ± 85.5 | 1159.5 ± 51.2 |
| Correct latency (ms) | 751.6 ± 38.0 | 626.0 ± 41.8 | 842.4 ± 50.9 | 688.1 ± 40.0 |
| L-ITI sessions | ||||
| Premature (%) | 21.5 ± 2.2 | 69.6 ± 7.5** | 32.1 ± 2.9 | 53.3 ± 4.6* |
| Accuracy (%) | 80.9 ± 1.0 | 73.9 ± 2.1*** | 80.5 ± 1.7 | 74.9 ± 1.9*** |
| Omissions (%) | 12.7 ± 1.9 | 18.7 ± 2.6 | 8.6 ± 1.7 | 11.3 ± 2.0 |
| Magazine latency (ms) | 1103.9 ± 54.0 | 1281.9 ± 122.7 | 1186.6 ± 96.7 | 1140.1 ± 49.0 |
| Correct latency (ms) | 671.7 ± 38.6 | 601.6 ± 32.5 | 721.8 ± 63.2 | 603.0 ± 26.0 |
Data are reported as the mean ± SEM.
*p < 0.01;
**p < 0.001;
***p < 0.05 (LI vs HI).
Interactive effects of methylphenidate on impulsivity in LI and HI rats
Twenty-one days after the commencement of daily MPH dosing, rats were reassessed for impulsivity and attentional performance on the 5-CSRTT. It can be seen in Figure 1B that MPH produced divergent effects on impulsivity in the two impulsivity subgroups, with impulsivity appearing to decrease in HI rats but increase in LI rats (group × MPH interaction: F(1,13) = 7.59, p = 0.016). Although post hoc t tests failed to reveal a significant decrease in impulsivity, at a group level, following MPH treatment in HI rats (p = 0.095), the increase in impulsivity observed in LI rats was significant (p = 0.044). Thus, following exposure to MPH, the initial contrast in impulsivity between LI and HI rats was greatly diminished. Importantly, it can be seen in Figure 1C that MPH increased impulsivity in LI rats in an inverse relationship to the baseline level of impulsivity, while impulsivity decreased in HI rats, with the magnitude of the decrease being positively correlated to the baseline level of impulsivity. Thus, the effect of MPH on increasing impulsivity was greatest in LI rats showing the lowest baseline level of impulsivity (r = −0.80; p = 0.03), whereas MPH decreased impulsivity to a greater extent in those HI rats showing the highest baseline level of impulsivity (r = −0.7; p = 0.05). However, MPH did not restore the attentional inaccuracy of HI rats, which remained significantly impaired relative to LI rats (main effect of group: F(1,13) = 3.62, p = 0.049; group × MPH interaction: F(1,13) = 0.37, p = 0.55). Moreover, there were no significant effects of MPH on omissions or magazine latencies on the 5-CSRTT (Fig. 1D; Table 1).
Modulation of D2/3 receptor availability in the ventral striatum by MPH
Consistent with our recent study (Caprioli et al., 2013), the availability of D2/3 receptors was significantly reduced in the left (t(13) = 2.25, p = 0.043), but not the right (t(13) = 1.29, p = 0.219), ventral striatum of drug-naive HI rats compared with LI rats (pre-/post-MPH × hemisphere × group interaction: F(1,13) = 5.75, p = 0.05; group × hemisphere interaction: F(1,13) = 4.047, p = 0.066; Fig. 2C). Following 28 d of exposure to MPH, the difference in D2/3 receptor availability between LI and HI rats in the left ventral striatum was no longer evident (t(13) = 0.09, p = 0.930). The normalizing effect of MPH on D2/3 receptor availability in the left ventral striatum appeared to be explained by a near-significant reduction in D2/3 BPND in the LI rats (t(6) = 2.292, p = 0.062) rather than an increase in D2/3 BPND in this region of HI rats (t(7) = −0.495, p = 0.636). Exposure of LI and HI rats to MPH had no significant effect on D2/3 receptor availability in the right ventral striatum.
Baseline-dependent effects of MPH on striatal D2/3 receptors in HI rats
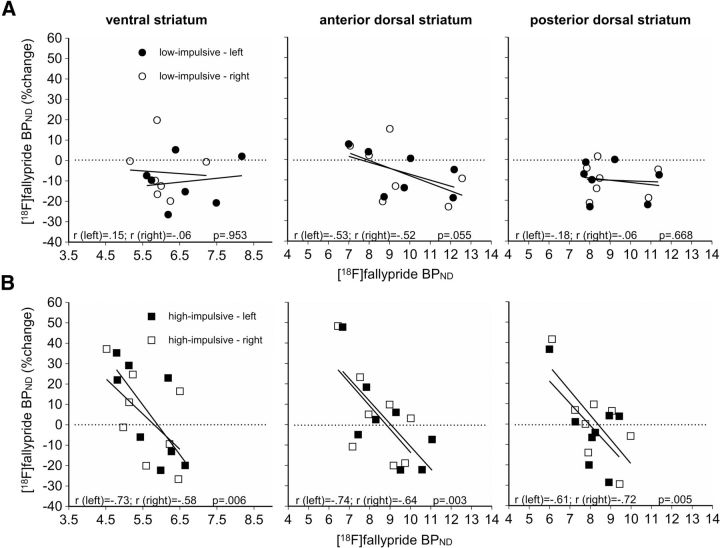
We found no significant group differences in D2/3 receptor availability between LI and HI rats in the dorsal striatum, either at baseline (pre-MPH) or following MPH treatment (data not shown). However, when we compared the change in [18F]fallypride BPND before and after drug administration, we found that MPH both increased and decreased [18F]fallypride BPND in the ventral and dorsal striatum in HI rats, depending on the baseline availability of D2/3 receptors (Fig. 3). In the ventral striatum, we observed a strong inverse relationship between the percentage change in [18F]fallypride BPND and baseline [18F]fallypride BPND in both the left hemisphere (rleft = −0.73, p < 0.01) and right hemisphere (rright= −0.58, p < 0.01). Baseline-dependent effects of MPH on D2/3 receptor availability were also observed in the anterior and posterior dorsal striata of HI rats. For all regions of interest, the relationship was strongly inversely related to baseline D2/3 receptor availability (anterior dorsal striatum HI rats: rleft = −0.74, <0.01; rright = −0.64, p < 0.01; posterior dorsal striatum HI rats: rleft = −0.61, p < 0.01, rright = −0.72, p < 0.01). In contrast, we did not observe baseline-dependent effects on D2/3 receptor availability in any of the striatal areas investigated in LI rats.
Figure 3.
A, B, Relationship between the percentage change in [18F]fallypride BPND in the ventral and dorsal striatum before and after the exposure of LI (A) and HI (B) rats to MPH as a function of baseline (i.e., pre-MPH) [18F]fallypride BPND. The results show that the effects of MPH on D2/3 receptor availability depend inversely on baseline [18F]fallypride BPND values in the anterior and posterior regions of the dorsal striatum, as well as in the ventral striatum of HI but not LI rats. The horizontal dotted line depicts no net effect of MPH on [18F]fallypride BPND values. Pearson product moment correlation coefficients and p values are given in each panel.
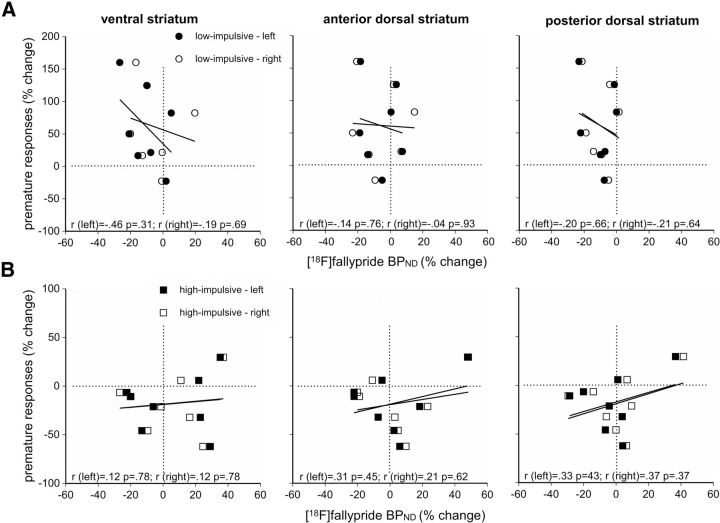
We next compared the relative changes in D2/3 receptor availability and impulsivity produced by MPH treatment (Fig. 4). We found no significant relationship between these parameters in any of the striatal subregions examined for either LI or HI rats.
Figure 4.
A, B, Correlation plots showing the relationship between relative changes in impulsivity and [18F]fallypride BPND in the ventral and dorsal striatum of LI (A) and HI (B) rats produced by MPH. The results indicate that the effects of MPH on impulsivity and D2/3 receptor availability are independent. The vertical dotted line depicts no net effect of MPH on [18F]fallypride BPND. Pearson product moment correlation coefficients and p values are given in each panel.
Discussion
This study investigated striatal D2/3 receptor availability in highly impulsive rats and the mechanisms underlying the therapeutic effects of long-term oral MPH treatment. We found that repeated oral MPH administration was sufficient to produce bidirectional effects on impulsivity that depended on the baseline level of impulsivity. Thus, in LI rats, MPH increased impulsivity, whereas in HI rats it reduced impulsivity in animals exhibiting the highest baseline level of impulsivity, consistent with an underlying rate-dependent mechanism. Our results indicate that the rate dependency model held for LI and HI rats but with clear differences in the underlying regulatory parameters, which is suggestive of a nonunitary process. Since the baseline-dependent effects of MPH on impulsivity and D2/3 receptors were dissociable, we conclude that D2/3 receptors may not play a major contribution to the effects of MPH on impulsivity. These data are consistent with findings showing that MPH modulates performance in humans in a baseline-dependent manner both in healthy controls and in subjects in whom ADHD has been diagnosed (del Campo et al., 2013).
We found a strong inverse relationship between baseline D2/3 BPND and the change in this parameter after MPH treatment in HI rats. However, no such relationship was found for LI rats in any of the striatal subregions examined. These findings correspond with our earlier findings in rats self-administering cocaine, which had the similar effect of modulating D2/3 BPND in a manner dependent on baseline D2/3 BPND values (Caprioli et al., 2013). However, in our previous study, cocaine also modulated D2/3 receptors in the dorsal striatum. The more pervasive effects of cocaine on D2/3 receptors throughout the ventral and dorsal striata may be due to differences in the route of administration (intravenous vs oral), response-contingent cocaine vs response noncontingent MPH, differing quantities of cocaine and MPH, and higher relative efficacy of cocaine over MPH. This may explain why cocaine produced a more substantial reduction in impulsivity in the HI subgroup compared with MPH in the present study (Caprioli et al., 2013).
The mechanism underlying the observed rate-dependent modulation of impulsivity following MPH treatment is unknown but, as discussed above, may be distinct for LI and HI rats. Although for LI rats there was no obvious relationship between baseline D2/3 BPND and the change in this parameter following MPH treatment, at a group level, MPH had the trend effect of reducing D2/3 BPND values in the left ventral striatum, a deficit associated with increased impulsivity on this task (Dalley et al., 2007) and localized to the nucleus accumbens shell (Besson et al., 2010; Jupp et al., 2013). The reduction in D2/3 receptor availability in LI rats may reflect a downregulation of D2/3 receptors, but since [18F]fallypride competes with DA in binding to D2/3 receptors, it could also reflect an increase in synaptic DA release, possibly due to sensitization of the mesolimbic DA systems following repeated MPH treatment (Shuster et al., 1982; Gaytan et al., 1997). However, sensitization of the locomotion response does not appear to develop after long-term oral MPH treatment (McNamara et al., 1993; Kuczenski and Segal, 2002). Furthermore, no simple relationship exists between hyperactivity and impulsivity on the 5-CSRTT (Dalley et al., 2007; Molander et al., 2011; Moreno et al., 2013).
In HI rats, MPH had the dual effect of decreasing impulsivity and modulating striatal D2/3 receptor availability according to the principal of rate dependency (Dews and Wenger, 1977). However, neither parameter significantly covaried after MPH treatment, suggesting that the modifying effects of MPH on impulsivity are separable from effects on D2/3 receptor regulation. That the measure of impulsivity is not directly related to changes in D2/3 receptor availability is possibly due to other actions of MPH, especially for example on NE. Thus, atomoxetine, which reduces impulsivity in HI rats, blocks reuptake of NE and has no effect on subcortical DA (Bymaster et al., 2002). Moreover, this drug exerts at least some of its anti-impulsive effects within the shell region of the nucleus accumbens (Economidou et al., 2012). Alternatively, the reduction in impulsivity in MPH-treated HI rats may include actions at the level of the nucleus accumbens core. Thus, previously, we have shown that HI rats exhibit a reduced density of markers associated with dendritic spines in this region and GABA synthesis (Caprioli et al., 2014), abnormalities that were mainly restricted to the left hemisphere, similar to the locus of deficient D2/3 receptor availability in HI rats (Caprioli et al., 2013). Since in the present study MPH had the greatest beneficial effects in the most impulsive animals, these effects may be mediated by a restoration of the structural and functional integrity of GABAergic medium spiny neurons in the nucleus accumbens core, as previously hypothesized (Caprioli et al., 2014). The origin of the hemispheric imbalance in D2/3 receptors in HI rats is unknown but may arise from genetic and/or environmental factors affecting trophic signaling during development (Concha et al., 2012). Left/right asymmetries in the midbrain DA systems have been reported in rats (Carlson and Glick, 1989; Afonso et al., 1993; Rodriguez et al., 1994) and healthy humans (Tomer et al., 2008), as well as in individuals with ADHD (Volkow et al., 2007a, 2009; del Campo et al., 2013).
An analogous PET study in rats found that treating rats with oral MPH for 8 months, initiated during the periadolescent period, increased D2/3 availability in the striatum (Thanos et al., 2007). By contrast, striatal D2/3 availability decreased 2 months after starting MPH treatment. These findings demonstrate that the MPH-induced changes in striatal D2/3 receptors depend on treatment length and developmental stage (Rodriguez et al., 2010; Gill et al., 2012). The mechanisms underlying these changes in D2/3 receptors are unknown but may involve alterations in synaptic DA and/or the pool of receptors available for binding in the striatum. However, research in nonhuman primates demonstrates that long-term treatment with extended-release MPH for 1 year has no effect on the DA transporter or D2/3 receptors in the striatum (Soto et al., 2012; Gill et al., 2012). This discrepancy with rodent studies may be species specific or a consequence of differing doses of MPH and/or length of treatment. In the context of the present study, it may also reflect the fact that animals in the study by Gill et al., 2012 were not preselected for impulsivity-related traits. This may be relevant, as it has been shown that treating adults with ADHD for 1 year decreases striatal D2/3 receptor availability, as assessed using PET (Volkow et al., 2012).
There are several limitations of the present study that merit discussion. First, although we dosed MPH orally and assessed the levels of serum MPH and its metabolite ritalinic acid, as endorsed by others (Volkow and Insel, 2003; Gill et al., 2012), peak MPH levels were in excess of the typical therapeutic range of MPH of 8–10 ng/ml (Swanson and Volkow, 2002; Table 2). However, consistent with other research (Patrick et al., 1984; Robb et al., 2014), MPH was rapidly cleared with an elimination half-life of 30–50 min. Thus, although serum levels of MPH were initially high, these soon declined to clinically relevant values after the administration of MPH and well before the next dose. Nevertheless, with twice-daily dosing and consequent fluctuations in serum MPH levels, our results are difficult to extrapolate to studies in humans that use extended-release oral formulations (Robb et al., 2014). A second consideration is that the primary objective of our research was to investigate the long-term effects of MPH on impulse control and D2/3 receptors in the striatum. The design of our study thus excluded the analysis of short-term treatment with low doses of MPH, which increase NE and DA availability selectively in the prefrontal cortex (Berridge et al., 2006) and facilitate cognitive functions relevant to ADHD (Andrzejewski et al., 2014). Third, our conclusions are based on relatively small group sizes (n = 7–8). Nevertheless, we have now reported in three independent studies reduced striatal D2/3 receptor availability in the ventral striatum of HI rats. In addition, we observed qualitatively similar changes from baseline following the administration of MPH and cocaine (Caprioli et al., 2013), using a within-subjects longitudinal design.
Table 2.
Summary of the serum concentration of oral MPH and ritalinic acid obtained from four nonimpulsive rats after a single oral dose of MPH (6 mg/kg)
| Collection time | Mean (ng/ml) |
SD (ng/ml) |
Minimum (ng/ml) |
Maximum (ng/ml) |
||||
|---|---|---|---|---|---|---|---|---|
| MPH | RA | MPH | RA | MPH | RA | MPH | RA | |
| 5 min | 112.5 | 107.5 | 20.6 | 42.7 | 90.0 | 60.0 | 130.0 | 160.0 |
| 15 min | 102.5 | 142.5 | 22.2 | 20.6 | 90.0 | 120.0 | 130.0 | 170.0 |
| 30 min | 87.5 | 145.0 | 22.2 | 20.8 | 70.0 | 120.0 | 120.0 | 170.0 |
| 60 min | 9.5 | 67.5 | 7.50 | 15.0 | 4.0 | 50.0 | 20.0 | 80.0 |
| 90 min | 2.2 | 35.0 | 0.91 | 5.78 | 1.0 | 30.0 | 3.0 | 40.0 |
| 120 min | 1.0 | 13.0 | 0.11 | 8.71 | 1.0 | 2.0 | 1.0 | 20.0 |
RA, Ritalinic acid.
In conclusion, our results confirm that deficits in impulsive control are associated with reduced D2/3 receptor availability in the left ventral striatum, as previously reported (Dalley et al., 2007; Caprioli et al., 2013), and in three independent studies in ADHD patients (Volkow et al., 2007a, 2009; del Campo et al., 2013). Although further research is needed to test the full dose–response curve of MPH on impulsivity and D2/3 receptor availability, we have now shown how different psychomotor stimulant drugs produce baseline-dependent effects on D2/3 receptor availability in the ventral striatum. In addition, we have demonstrated that the therapeutic effects of MPH on impulsivity are unlikely to arise as a direct consequence of changes in the regulation of D2/3 receptors in the ventral striatum, a conclusion supported by research in adults with ADHD (Volkow et al., 2012). Nevertheless, by restoring levels of D2/3 receptors in the ventral striatum of HI rats, MPH may diminish the risk of addiction in addiction-prone highly impulsive rats (Belin et al., 2008; Economidou et al., 2009).
Footnotes
This work was funded by Medical Research Council Grant G0701500, and by a joint award from the Medical Research Council (Grant G1000183) and the Wellcome Trust (Grant 093875/Z/10/Z) in support of the Behavioural and Clinical Neuroscience Institute at the University of Cambridge. We also acknowledge funding from the Medical Research Council in support of the ICCAM addiction cluster in the United Kingdom (G1000018). B.J. is supported by grants from the AXA Research Fund and the Australian National Health and Medical Research Council (Grant 1016313).
The authors declare no conflicting financial interests.
References
- Afonso D, Santana C, Rodriguez M. Neonatal lateralization of behavior and brain dopaminergic asymmetry. Brain Res Bull. 1993;32:11–16. doi: 10.1016/0361-9230(93)90312-Y. [DOI] [PubMed] [Google Scholar]
- Andrzejewski ME, Spencer RC, Harris RL, Feit EC, McKee BL, Berridge CW. The effects of clinically relevant doses of amphetamine and methylphenidate on signal detection and DRL in rats. Neuropharmacology. 2014;79:634–641. doi: 10.1016/j.neuropharm.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa O. Effects of methamphetamine and methylphenidate on single and paired rat open-field behaviors. Physiol Behav. 1994;55:441–446. doi: 10.1016/0031-9384(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry. 2006;67(Suppl 8):7–12. [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc. 2008;3:759–767. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Dodge AM, Dodge LJ, White K, Panksepp J. Psychomotor stimulants, social deprivation and play in juvenile rats. Pharmacol Biochem Behav. 1982;16:417–422. doi: 10.1016/0091-3057(82)90445-2. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Besson M, Belin D, McNamara R, Theobald DE, Castel A, Beckett VL, Crittenden BM, Newman AH, Everitt BJ, Robbins TW, Dalley JW. Dissociable control of impulsivity in rats by dopamine d2/3 receptors in the core and shell subregions of the nucleus accumbens. Neuropsychopharmacology. 2010;35:560–569. doi: 10.1038/npp.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry. 2005;57:1215–1220. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Caprioli D, Hong YT, Sawiak SJ, Ferrari V, Williamson DJ, Jupp B, Adrian Carpenter T, Aigbirhio FI, Everitt BJ, Robbins TW, Fryer TD, Dalley JW. Baseline-dependent effects of cocaine pre-exposure on impulsivity and D2/3 receptor availability in the rat striatum: possible relevance to the attention-deficit hyperactivity syndrome. Neuropsychopharmacology. 2013;38:1460–1471. doi: 10.1038/npp.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Sawiak SJ, Merlo E, Theobald DE, Spoelder M, Jupp B, Voon V, Carpenter TA, Everitt BJ, Robbins TW, Dalley JW. Gamma aminobutyric acidergic and neuronal structural markers in the nucleus accumbens core underlie trait-like impulsive behavior. Biol Psychiatry. 2014;75:115–123. doi: 10.1016/j.biopsych.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JN, Glick SD. Cerebral lateralization as a source of interindividual differences in behavior. Experientia. 1989;45:788–798. doi: 10.1007/BF01954054. [DOI] [PubMed] [Google Scholar]
- Clatworthy PL, Lewis SJ, Brichard L, Hong YT, Izquierdo D, Clark L, Cools R, Aigbirhio FI, Baron JC, Fryer TD, Robbins TW. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci. 2009;29:4690–4696. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran WG, Cox GM. Experimental designs. Ed 2. New York: Wiley; 1957. [Google Scholar]
- Concha ML, Bianco IH, Wilson SW. Encoding asymmetry within neural circuits. Nat Rev Neurosci. 2012;13:832–843. doi: 10.1038/nrn3371. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Defrise M, Kinahan PE, Townsend DW, Michel C, Sibomana M, Newport DF. Exact and approximate rebinning algorithms for 3-D PET data. IEEE Trans Med Imaging. 1997;16:145–158. doi: 10.1109/42.563660. [DOI] [PubMed] [Google Scholar]
- del Campo N, Fryer TD, Hong YT, Smith R, Brichard L, Acosta-Cabronero J, Chamberlain SR, Tait R, Izquierdo D, Regenthal R, Dowson J, Suckling J, Baron JC, Aigbirhio FI, Robbins TW, Sahakian BJ, Müller U. A positron emission tomography study of nigro-striatal dopaminergic mechanisms underlying attention: implications for ADHD and its treatment. Brain. 2013;136:3252–3270. doi: 10.1093/brain/awt263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Blackwell AD, Clark L, Kent L, Dezsery AM, Turner DC, Aitken MR, Sahakian BJ. Methylphenidate improves response inhibition but not reflection-impulsivity in children with attention deficit hyperactivity disorder (ADHD) Psychopharmacology. 2009;202:531–539. doi: 10.1007/s00213-008-1337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews PB, Wenger GR. Rate-dependency of the behavioral effects of amphetamine. In: Thompson T, Dews PB, editors. Advances in behavioral pharmacology. Vol 1. San Diego, CA: Academic; 1977. pp. 167–227. [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry. 2009;65:851–856. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Economidou D, Theobald DE, Robbins TW, Everitt BJ, Dalley JW. Norepinephrine and dopamine modulate impulsivity on the five-choice serial reaction time task through opponent actions in the shell and core sub-regions of the nucleus accumbens. Neuropsychopharmacology. 2012;37:2057–2066. doi: 10.1038/npp.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytan O, al-Rahim S, Swann A, Dafny N. Sensitization to locomotor effects of methylphenidate in the rat. Life Sciences. 1997;61:PL101–PL107. doi: 10.1016/S0024-3205(97)00598-5. [DOI] [PubMed] [Google Scholar]
- Gill KE, Pierre PJ, Daunais J, Bennett AJ, Martelle S, Gage HD, Swanson JM, Nader MA, Porrino LJ. Chronic treatment with extended release methylphenidate does not alter dopamine systems or increase vulnerability for cocaine self-administration: a study in nonhuman primates. Neuropsychopharmacology. 2012;37:2555–2565. doi: 10.1038/npp.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jupp B, Caprioli D, Saigal N, Reverte I, Shrestha S, Cumming P, Everitt BJ, Robbins TW, Dalley JW. Dopaminergic and GABA-ergic markers of impulsivity in rats: evidence for anatomical localisation in ventral striatum and prefrontal cortex. Eur J Neurosci. 2013;37:1519–1528. doi: 10.1111/ejn.12146. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- McNamara CG, Davidson ES, Schenk S. A comparison of the motor-activating effects of acute and chronic exposure to amphetamine and methylphenidate. Pharmacol Biochem Behav. 1993;45:729–732. doi: 10.1016/0091-3057(93)90532-X. [DOI] [PubMed] [Google Scholar]
- Molander AC, Mar A, Norbury A, Steventon S, Moreno M, Caprioli D, Theobald DE, Belin D, Everitt BJ, Robbins TW, Dalley JW. High impulsivity predicting vulnerability to cocaine addiction in rats: some relationship with novelty preference but not novelty reactivity, anxiety or stress. Psychopharmacology. 2011;215:721–731. doi: 10.1007/s00213-011-2167-x. [DOI] [PubMed] [Google Scholar]
- Moreno M, Economidou D, Mar AC, López-Granero C, Caprioli D, Theobald DE, Fernando A, Newman AH, Robbins TW, Dalley JW. Divergent effects of D(2)/(3) receptor activation in the nucleus accumbens core and shell on impulsivity and locomotor activity in high and low impulsive rats. Psychopharmacology. 2013;228:19–30. doi: 10.1007/s00213-013-3010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, Yang ZY, Das MK, Brown T. Fluorinated benzamide neuroleptics–III. Development of (S)-N-[(1-allyl-2-pyrrolidinyl)methyl]-5-(3-[18F]fluoropropyl)-2, 3-dimethoxybenzamide as an improved dopamine D-2 receptor tracer. Nucl Med Biol. 1995;22:283–296. doi: 10.1016/0969-8051(94)00117-3. [DOI] [PubMed] [Google Scholar]
- Patrick KS, Ellington KR, Breese GR. Distribution of methylphenidate and p-hydroxymethylphenidate in rats. J Pharmacol Exp Ther. 1984;231:61–65. [PubMed] [Google Scholar]
- Robb AS, Findling RL, Childress AC, Berry SA, Belden HW, Wigal SB. Efficacy, safety, and tolerability of a novel methylphenidate extended-release oral suspension (MEROS) in ADHD. J Atten Disord. 2014 doi: 10.1177/1087054714533191. doi: 10.1177/1087054714533191. Advance online publication. Retrieved 17 January 2015. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Sahakian BJ. “Paradoxical” effects of psychomotor stimulant drugs in hyperactive children from the standpoint of behavioural pharmacology. Neuropharmacology. 1979;18:931–950. doi: 10.1016/0028-3908(79)90157-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez JS, Morris SM, Hotchkiss CE, Doerge DR, Allen RR, Mattison DR, Paule MG. The effects of chronic methylphenidate administration on operant test battery performance in juvenile rhesus monkeys. Neurotoxicol Teratol. 2010;32:142–151. doi: 10.1016/j.ntt.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Martin L, Santana C. Ontogenic development of brain asymmetry in dopaminergic neurons. Brain Res Bull. 1994;33:163–171. doi: 10.1016/0361-9230(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Robbins TW. Are the effects of psychomotor stimulant drugs on hyperactive children really paradoxical? Med Hypotheses. 1977;3:154–158. doi: 10.1016/0306-9877(77)90065-2. [DOI] [PubMed] [Google Scholar]
- Shuster L, Hudson J, Anton M, Righi D. Sensitization of mice to methylphenidate. Psychopharmacology. 1982;77:31–36. doi: 10.1007/BF00436096. [DOI] [PubMed] [Google Scholar]
- Soto PL, Wilcox KM, Zhou Y, Kumar A, Ator NA, Riddle MA, Wong DF, Weed MR. Long-term exposure to oral methylphenidate or dl-amphetamine mixture in peri-adolescent rhesus monkeys: effects on physiology, behavior, and dopamine system development. Neuropsychopharmacology. 2012;37:2566–2579. doi: 10.1038/npp.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Pharmacokinetic and pharmacodynamic properties of stimulants: implications for the design of new treatments for ADHD. Behav Brain Res. 2002;130:73–78. doi: 10.1016/S0166-4328(01)00433-8. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Psychopharmacology: concepts and opinions about the use of stimulant medications. J Child Psychol Psychiatry. 2009;50:180–193. doi: 10.1111/j.1469-7610.2008.02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacol Biochem Behav. 2007;87:426–433. doi: 10.1016/j.pbb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Tomer R, Goldstein RZ, Wang GJ, Wong C, Volkow ND. Incentive motivation is associated with striatal dopamine asymmetry. Biol Psychol. 2008;77:98–101. doi: 10.1016/j.biopsycho.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Relative lack of cognitive effects of methylphenidate in elderly male volunteers. Psychopharmacology. 2003;168:455–464. doi: 10.1007/s00213-003-1457-3. [DOI] [PubMed] [Google Scholar]
- van der Schaaf ME, Fallon SJ, Ter Huurne N, Buitelaar J, Cools R. Working memory capacity predicts effects of methylphenidate on reversal learning. Neuropsychopharmacology. 2013;38:2011–2018. doi: 10.1038/npp.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Trezza V, Griffioen-Roose S, Schiepers OJ, Van Leeuwen N, De Vries TJ, Schoffelmeer AN. Methylphenidate disrupts social play behavior in adolescent rats. Neuropsychopharmacology. 2008;33:2946–2956. doi: 10.1038/npp.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Insel TR. What are the long-term effects of methylphenidate treatment? Biol Psychiatry. 2003;54:1307–1309. doi: 10.1016/j.biopsych.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS, Logan J, Ma Y, Schulz K, Pradhan K, Wong C, Swanson JM. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007a;64:932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn J, Fowler JS, Telang F, Solanto MV, Logan J, Wong C, Ma Y, Swanson JM, Schulz K, Pradhan K. Brain dopamine transporter levels in treatment and drug naive adults with ADHD. Neuroimage. 2007b;34:1182–1190. doi: 10.1016/j.neuroimage.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma Y, Pradhan K, Wong C, Swanson JM. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Kollins SH, Wigal TL, Newcorn JH, Telang FW, Fowler JS, Logan J, Wong CT, Swanson JM. Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. J Neurosci. 2012;32:841–849. doi: 10.1523/JNEUROSCI.4461-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE. Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2008;28:S46–S53. doi: 10.1097/JCP.0b013e318173312f. [DOI] [PubMed] [Google Scholar]
- Zetterström T, Sharp T, Collin AK, Ungerstedt U. In vivo measurement of extracellular dopamine and DOPAC in rat striatum after various dopamine-releasing drugs; implications for the origin of extracellular DOPAC. Eur J Pharmacol. 1988;148:327–334. doi: 10.1016/0014-2999(88)90110-0. [DOI] [PubMed] [Google Scholar]