Abstract
The ankyrin repeat and SOCS box (ASB) family is composed of 18 proteins and belongs to the suppressor of cytokine signaling (SOCS) box protein superfamily. The ASB proteins function as the substrate-recognition subunits of ECS-type (ElonginBC-Cullin-SOCS-box) Cullin RING E3 ubiquitin ligase (CRL) complexes that specifically transfer ubiquitin to cellular proteins targeting them for degradation by the proteasome. ASB9 binds to creatine kinase (CK) and targets it for degradation, however the way in which ASB9 interacts with CK is not yet known. We present complete characterization of the binding of ASB9 to CK. One ASB9 molecule binds to a dimer of CK. The binding affinity of ASB9(1-252) was extremely tight and no dissociation could be observed. Deletion of the N-terminal 34 amino acids forming ASB9(35-252) resulted in weakening of the binding so that a binding affinity of 2.6 nM could be measured. Amide hydrogen/deuterium exchange (HDXMS) experiments showed that both ASB9(1-252) and ASB9(35-252) protected the same region of CK, residues 182-203, which forms one side of the active site. The HDXMS experiments indicated that the N-terminal disordered region and first ankyrin repeat of ASB9 are protected from exchange in the complex. Molecular docking yielded a structural model consistent with all of the data that suggested the N-terminal residues of ASB9(1-252) may lie in one CK active site. This model was corroborated by enzymatic activity assays and mutational analysis.
Proteasome-dependent protein degradation occurs when ubiquitin is transferred to the ε-amine of lysine residues within the doomed protein (1, 2). The transfer of ubiquitin requires a three-enzyme system comprised of an E1 ubiquitin-activating enzyme, an E2-ubiquitin-conjugating enzyme, and an E3 ubiquitin ligase. It is the E3 ligase that binds the doomed protein, brings it together with the E2 enzyme, and catalyzes the transfer of ubiquitin. Over 600 human E3 ligases have been identified. The most well-characterized is the SCF family, for which structures have been available for over 10 years (3). The Cullin–RING E3 ligases (CRL) are the largest family of E3 ligases in eukaryotes and have separate substrate-binding and catalytic subunits (4, 5). The substrate-recognition protein binds to the N-terminal domain of the Cullin subunit (Cul1–5 or Cul7) and a RING protein (Rbx1 or Rbx2), which in turn recruits the E2-ubiquitin conjugate, binds to the C-terminal domain. Neddylation of the Cullin C-terminal domain is thought to alter the conformation so that the substrate and ubiquitin are brought into proximity (6).
The ankyrin repeat and SOCS box (ASB) family is composed of 18 proteins and belongs to the suppressor of cytokine signaling (SOCS) box protein superfamily. The ASB proteins interact with Cul5-Rbx2 to form a functional E3 ubiquitin ligase (7). ASB family members function as the substrate-recognition subunits of ECS-type (ElonginBC-Cullin-SOCS-box) Cullin RING E3 ubiquitin ligase (CRL) complexes that specifically transfer ubiquitin to cellular proteins targeting them for degradation by the proteasome. The quaternary multi-subunit complex formed by ASB9, Elongin B, Elongin C (EloBC), and Cullin 5 was recently characterized (8), and the structure of ASB9 bound to Elongins B and C was has also been reported (9). However, the interactions of the ASB subunits with their substrates and the assembly of the ASB subunits within ECS-type ubiquitin ligases remain poorly understood.
The Elongin BC, Cullin-5 and Rbx2 proteins are the same for each ASB-containing CRL so it is thought that the ASB protein is responsible for binding the target protein, and that each ASB binds to a different target protein. The ASBs are composed of an N-terminal ankyrin repeat domain (ARD) with different numbers of ankyrin repeats, and a C-terminal SOCS box domain (7). ASB proteins bind and recognize their specific substrate through the ARD. The CRL containing ASB9 binds to creatine kinase (CK) and targets it for degradation (10, 11), however the way in which ASB9 interacts with CK is not yet known. The structure of ASB9 (residues 37-294) bound to Elongin BC (9) and the structure of ASB9 (residues 19-252) (12, 13) both show similar architectures of the well-folded ARD of ASB9. Interestingly, a sequence C-terminal to the ARD appears to form part of an additional ankyrin repeat. A structural model for the ASB9-CK interaction was proposed based on the way in which other ankyrin repeat proteins bind their targets, however the experimental evidence for this model would also have been consistent with a variety of interaction modes (13).
ASB9 is predominantly expressed in the kidney and testes, and it has been shown to bind to and ubiquitinate brain-type cytosolic creatine kinase (CKB) (10) and ubiquitous mitochondrial creatine kinase (uMtCK) (11). Recent evidence suggests that ASB9 could be a biomarker for human breast cancer (14), and it has also been linked to colorectal cancer (15). The reduction of uMtCK expression by siRNA led to increased cell death, and reduced proliferation, migration and invasion in HCC cell lines. Transient overexpression of ASB9 reduced uMtCK protein levels in HCC cells, suggesting that the increased uMtCK levels which correlate with poor prognosis, may be due to reduced ASB9 expression (16).
We present complete characterization of the binding of ASB9 to CK, including measurement of the binding affinity and stoichiometry. In addition, we performed amide hydrogen/deuterium exchange (HDXMS) to determine the binding site (17), and we present a structural model consistent with all of the data.
Experimental Procedures (Materials and Methods)
Cloning, Expression and Purification
ASB9(1-252) and ASB9(35-252) were cloned into pHis8 vector with an N-terminal His8 tag. ASB9(1-252)D32A was generated by site directed mutagenesis from the ASB9(1-252) construct. All ASB9 proteins were expressed in BL21(DE3) cells, grown to an OD600 of 0.7 and induced with 0.4 mM IPTG overnight at 18°C in M9ZN media (M9 media supplemented with 10g/L NZ amine). The cell pellet was resuspended in phosphate buffer (pH 8.0, 50 mM sodium phosphate, 300 mM NaCl) containing 10 mM Imidazole and 0.5 mM PMSF, sonicated on ice, and centrifuged for 30 minutes at 12,000 rpm. The supernatant was loaded over a 10 mL Ni-NTA (His-Pur, Pierce) column at 4° C, washed with phosphate buffer containing 50 mM imidazole and eluted with phosphate buffer containing 250mM imidazole. Fractions containing ASB9 were pooled and dialyzed into pH 8.5, 20 mM tris, 200 mM NaCl, 0.5 mM EDTA and 1 mM DTT. 2 mL aliquots of the dialyzed protein were flash frozen in liquid nitrogen and stored for at -80° C and further purified on a Superdex-200 (S-200) gel filtration column before each experiment (GE Healthcare).
CKB was cloned in pET11a and expressed in BL21(DE3) cells, grown to an OD600 of 0.7 and induced with 0.4 mM IPTG overnight at 18°C in M9ZN media (M9 media supplemented with 10g/L NZ amine). The protein was purified over a blue sepharose column as described previously (18). The cells were resuspended in buffer A (pH 6.0, 10 mM MES, 20 mM KCl, 1 mM DTT) containing 0.5 mM PMSF, sonicated on ice and and centrifuged for 30 minutes at 12,000 rpm. The supernatant was loaded on to a 50 mL blue sepharose column at 4° C, washed with 5 column volumes of l buffer A and CKB was eluted with 2 column volumes of buffer B (pH 8.0, 10 mM TES, 20 mM KCl, and 1 mM DTT, pH 8.0). Fractions containing the enzyme were pooled and dialyzed into 50 mM HEPES, 0.1 mM EDTA, 1 mM DTT, pH 7.0 and aliquots were stored at -80° C. CKB fractions were also subjected to a S-200 gel filtration column (GE Healthcare) run and the eluted protein was concentrated in a 10 kD molecular weight cutoff Amicon Ultra centrifugal filter (Millipore).
The complex (CKB+ASB9) was formed by mixing a 10 μM solution of ASB9 with a 15 μM (monomer concentration) solution of CKB to achieve a 1.14 ASB9:CKB monomer excess. The mixture was incubated for 2 hrs at 4oC and purified by S200 size exclusion chromatography.
Activity assay
Creatine kinase activity was determined by using a coupled enzyme assay as described previously (19). The creatine kinase reaction is coupled to pyruvate kinase and lactate dehydrogenase reactions and the conversion of NADH to NAD is observed at 340 nm to monitor the amount of creatine phosphate formed. Assays were performed at 30° C in pH 9.0 triethanolamine buffer (20). The assay mixture contained 0.2 mM NADH, 0.4 mM phosphoenolpyruvate, 5 mM ATP, 6 mM magnesium acetate, 13 mM potassium acetate, 28-56 units/mL pyruvate kinase, and 54-108 units/mL lactate dehydrogenase. The creatine kinase concentration was 3 nM (monomer) and the creatine concentration was varied from 10 to 100 mM. The assay mixture was incubated at 30° C for 3 min and the reaction was initiated by the addition of enzyme. The velocity of the reaction is calculated using an extinction coefficient of 6290 M−1 cm−1 for NADH. For assays testing the role of ASB9 binding on CKB activity, the complex (CKB+ASB9) was added to achieve a CKB concentration of 3 nM. Vmax and KM values were determined by fitting the data to the Michalis Menten kinetic model in Kaleidagraph (Synergy, Inc).
Stoichiometry of the ASB9-CK complex
Multi Angle Light Scattering (MALS) analysis was performed to determine the stoichiometry of the ASB9-CK complex. In sequential runs, ASB9 alone, CK alone, and the ASB9-CK complex were analyzed. The proteins were buffer exchanged into 20 mM Tris pH 8.0, 100 mM NaCl and 100 μL of protein sample was analyzed on analytical size-exclusion chromatography using a GE Healthcare Superdex 200 10/300 GL column with a flow of .4 mL/min. The column flowed directly into a miniDAWN TREOS™ MALS instrument (Wyatt Technology) and an Optilab™ UT-rEX (Wyatt Technology). The Astra™ 6 (Wyatt Technologies) was used to determine the weight average molar mass of the eluting peaks using the intensity of the Rayleigh scattering as a function of scattering angle (LS) along with the buffer-subtracted refractive index (dRI) (21).
Measurement of binding thermodynamics
All Isothermal Calorimetry (ITC) experiments were performed on a VP-ITC calorimeter (MicroCal, Inc.). ASB9 constructs and CKB were purified by size exclusion chromatography on an S200 column in pH 8.5, 50 mM bistrispropane, 200 mM sodium chloride, immediately prior to use. Protein concentrations were determined by measuring the absorbance at 280 nM and calculated using extinction coefficients as determined by amino acid analysis (ASB9 constructs ε = 24400 M−1cm−1, CKB ε = 33700 M−1cm−1). Experiments were performed at 30° C, around 25, 10-μL injections of 50 μM CKB dimer were made into 5 μM ASB9 in the cell. Isotherms were analyzed using Origin software (OriginLab) as described before (22) and fit to a single binding site model.
Mesaurement of binding kinetics
For surface plasmon resonance (SPR) experiments, CKB was cloned in pMCSG51 to add a biotinylation tag (Midwest Center for Structural Genomics). This plasmid has an N-terminal HIS tag to aid purification, followed an AVI tag that can be biotinylated on a specific lysine using E.coli biotin ligase (BirA), that is co-expressed with the gene of interest. Biotinylated CKB was purified using a combination of Ni-NTA (using the phosphate buffers as the ASB9 constructs described above) and size exclusion chromatography over a S200 column. SPR experiments were performed on a Biacore 3000 (GE Healthcare). Biotinylated CKB was immobilized on streptavidin (SA) chips in a high-salt buffer (pH 8.0 50 mM HEPES, 500 mM NaCl, 0.5 mM sodium azide, 0.005% tween20). Biotinylated CKB (300 RU) was immobilized and data from FC1 (no RUs immobilized) were automatically subtracting FC 1 at a data collection rate set to high. The running buffer used for the binding experiments was pH 8.0 50 mM HEPES, 200 mM NaCl, 0.5 mM sodium azide, 0.005% tween20. It is to be noted that ASB9 constructs have to be freshly purified by size exclusion chromatography and maintained at 4 °C for binding to be observed. Injections were made using the kinject mode at 20 μL/ min, with a 240 s association time and 300 s dissociation time for ASB9(35-252). ASB9(35-252) concentration was varied from 0 -20 nM. The data were analyzed using the Bia Evaluation 4.1 software with a simple 1:1 Langmuir binding model.
Hydrogen/deuterium exchange mass spectrometry (HDXMS)
HDXMS was performed on CKB, ASB9(1-252), ASB9(35-252) and CKB in complex with ASB9(1-252) and ASB9(35-252). Complexes were made by adding an excess of ASB9 to CKB and vice versa and incubating the complexes for 30 min at 4° C. HDXMS experiments were conducted at Lilly, Inc. San Diego using a Waters nanoACQUITY UPLC system with H/DX technology as described previously (23, 24) with the exception that the quench buffer contained 100 mM phosphate, 2 M guanidine HCl with 320 mM TCEP (pH 2.4). Each sample (5 μL) was mixed with 55 μL of D2O buffer (containing 0.1X PBS) for several deuteration times (10 s to 10 min) at 15 °C. The exchange was quenched for 2 min at 1°C with an equal volume of quench buffer. A portion of the quenched sample (50 μL) was injected on to an online pepsin column (Applied Biosystems, Poroszyme Immobilized Pepsin cartridge). The resulting peptic peptides were then separated on C18 column (Waters, Acquity UPLC BEH C18, 1.7um, 1.0x50mm) fitted with a Vanguard trap column using a 3%–85% acetonitrile (containing 0.1 % formic acid) gradient over 12 min at a flowrate of 40 μL/ min. The separated peptides were directed into a Waters SYNAPT G2s quadrupole time-of-flight (qTOF) mass spectrometer. The mass spectrometer was set to collect data in the MSE, ESI+ mode; mass acquisition range of 255.00 - 1950.00 (m/z); scan time of 0.4 second. Continuous lock mass correction was accomplished with infusion of the LeuEnk peptide every 30 seconds (mass accuracy of 1ppm for calibration standard). The peptides were identified using PLGS 2.5 (Waters, Inc.). The relative deuterium uptake for each peptide was calculated by comparing the centroids of the mass envelopes of the deuterated samples with the undeuterated controls using DynamX 2.0 (Waters Corporation).
Molecular Modeling
The extended structure of the ASB9 intrinsically disordered N-terminal domain, ASB9(1-35), was generated based on the amino acid sequence in the program Maestro. Eleven parallel simulations were performed using the AMBER ff99SB force field (25) in a TIP3P water box (26). The simulated system contained a single copy of the 35-residue ASB9 N-terminus in an orthorhombic water-box of dimensions 67Å, 63.63Å, and 64.36Å, with periodic boundary conditions. The N-terminus and C-terminus of ASB9 were capped with acetyl and methyl groups respectively. The total system contained 61,016 atoms, including a low (0.15 M) concentration of sodium chloride in the water solvent. Simulations were performed with NVIDIA GK110 (GeForce GTX Titan) GPUs using the CUDA version of PMEMD in AMBER12 (27). The ASB9(1-35) system was relaxed using 86,000 steps of energy minimization followed by one restrained 250 ps heating step up to 303K. Three consecutive restrained 250 ps equilibration steps were performed. The restraint force was applied to the backbone atoms in these equilibration steps starting at 3.0 kcal/Å2mol for the first step, then 2.0 kcal/Å2mol for the second step and 1.0 kcal/Å2mol for the last step before the performance run. Long-range electrostatics were calculated using the particle mesh Ewald (PME) method (28), with a 10 Å cutoff and a 1.2 Å grid spacing. Ten simulations were performed at 303K for 25 ns each, for a total of 250ns of sampling. The trajectory structures were clustered using the GROMOS algorithm (29) in the GROMACS 4.5.5 software (30), with a backbone RMSD cutoff of 3.5Å for ASB91-35. The top 8 (90% of total ensemble) cluster centroids were analyzed and the most elongated and compact cluster centroids were appended onto the crystal structure of the ARD of ASB9.
The molecular model of ASB9(1-252) was built from the 2.2 Å crystal structure of the splice variant 2 of the ARD of ASB9 from hASB9-2 (PDB code 3D9H). The top 8 cluster centroids of the ASB9(1-35) simulation (representing 90% of total ensemble) were analyzed and the most elongated and compact cluster centroids were appended onto the crystal structure of the ARD of ASB9 in Maestro. The two models of ASB9(1-252), one with an elongated N-terminus and one with a compact N-terminus, were docked into two models of CK brain-type (CKB): the crystal structure of CKB (PDB code 3DRB, which has one open and one closed monomer) (31), and a model of the open-open CKB crystal structure generated in VMD by overlaying two crystal structures over one another. In VMD the RMSD trajectory tool extension was used to align two CKB crystal structures.
The ZDOCK server was used to dock both models of ASB9 into both models of CKB (http://zdock.umassmed.edu/)(32). The top ten structures of each of the four dockings were compared based on their ZDOCK score (1103-1778 points), the consistency of the binding interface determined from the RMSD of the docked orientation from the top 10 docked complexes, and the steric overlap of the SOCS box domain with CKBB when ASB9-ElonginBC crystal structure (3ZNG) (8) is overlaid with the docked complexes.
Results
Affinity of the ASB9-CK complex requires two regions of ASB9
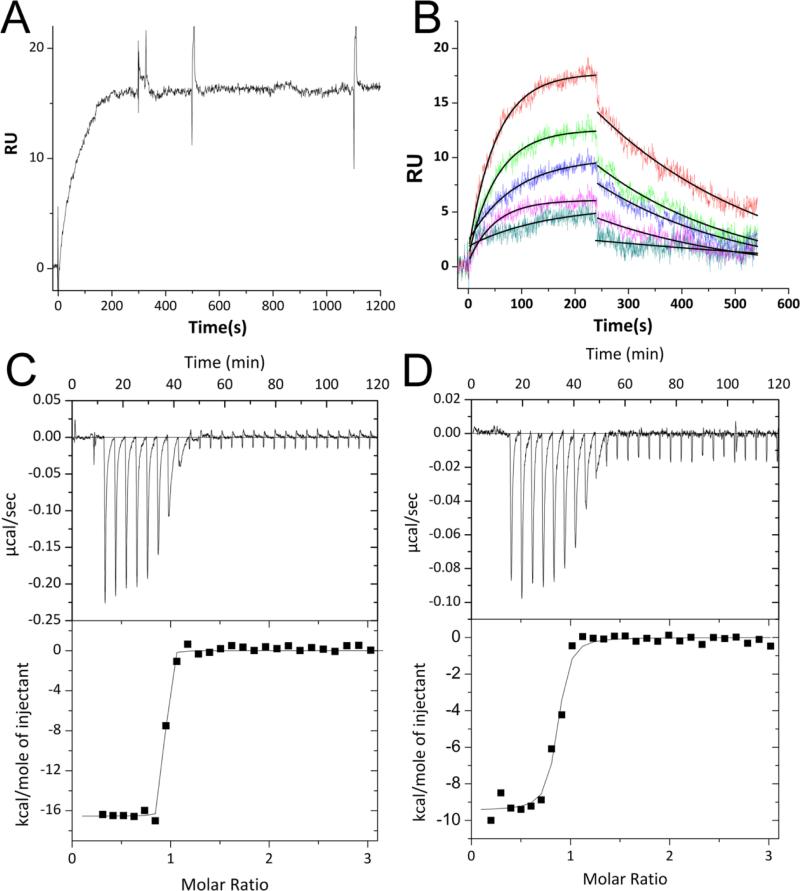
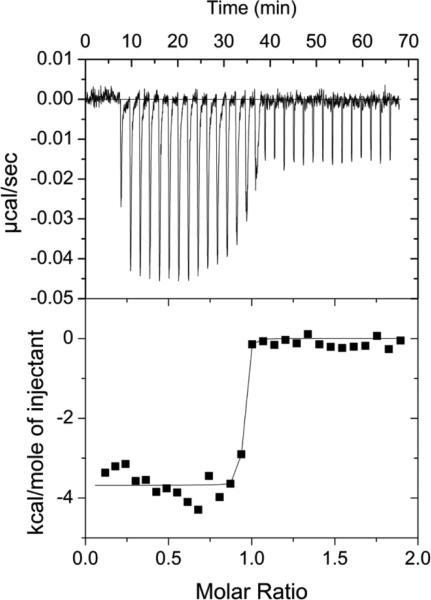
The affinity of the ASB9-CK complex was measured both by surface SPR and ITC. For these experiments, the binding affinity of the ARD of ASB9 (ASB9(35-252)) was compared to the affinity of a construct containing both the ARD and the N-terminal 34 residues which are not seen in the crystal structures, ASB9(1-252). Both SPR and ITC revealed that the binding affinity between ASB9(1-252) and CK is extremely high. For ASB9(1-252), no dissociation was observed, even when 3M Guanidine HCl was included in the dissociation buffer in the SPR experiments, (Figure 1A). ITC experiments corroborated this observation. Although the c-value was too high to measure the Kd, the ΔH of the interaction, was large, -16.5 kcal/mol, and could be determined with high precision (Figure 1C). Sensorgrams collected with immobilized CKB and ASB9(35-252) from 0-14 nM revealed a kd of 3.5e−3 s−1 and a ka of 1.36e6M−1s−1 yielding a KD = 2.6 × 10−9 M (Figure 1B). Although the c-value for this interaction was still high, the ΔH of the interaction, was only -9.8 kcal/mol for the truncated version (Figure 1D). These results strongly suggested a bipartite interaction of ASB9 with CK.
Figure 1.
Measurement of binding kinetics and thermodynamics. A) An SPR experiment in which 30 nM ASB9(1-252) was flowed over immobilized CKB at 20 μL/min. Regeneration was attempted by adding 3 M guanidine HCl to the regeneration solution at 500 s, but no dissociation was observed. B) An SPR experiment in which 8, 9, 10, 12, and 14 nM ASB9(35-252) was flowed over immobilized CKB at 20 μL/min. Regeneration was carried out with buffer containing 0.5 M guanidine HCl and 10 mM ATP. The curves were fit globally using a 1:1 Langmuir binding model to yield the binding constants: kd = 3.5e−3 s−1, ka = 1.36e6 M−1 s−1 and KD = 2.6 × 10−9 M. C) ITC thermogram and fit for 50 μM CKB (dimer concentration) (in the syringe) binding to 5 μM ASB9(1-252) (in the cell). Because the binding was so tight, only the ΔH could be determined from the data ΔH = -16.5 ± 0.2 kcal/mol (N=0.9). D) ITC thermogram and fit for 50 μM CKB (dimer concentration) (in the syringe) binding to 5 μM ASB9(35-252). For this truncated version of ASB9, ΔH = -9.9 ± 0.2 kcal/mol (N=0.78).
Stoichiometry of the ASB9-CK complex
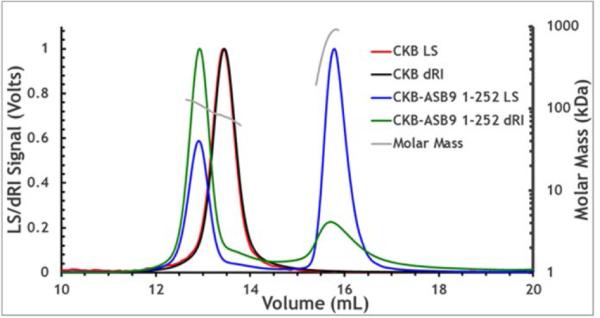
CK is a dimeric protein, and therefore it was necessary to determine whether one molecule of ASB9 bound to a dimer of CK, or whether each monomer of CK could bind an ASB9 molecule. Size exclusion chromatography and analytical ultracentrifugation experiments both suggested a stoichiometry of one ASB9:one CK dimer, but due to the elongated shape of ASB9, the results were inconclusive. MALS analysis of a mixture of CK with an excess of ASB9 showed conclusively that the stoichiometry was one ASB9 bound to one dimer of CK (Figure 2). The experiment was performed withn an excess of ASB9, which eluted at the expected volume for monomeric ASB9.
Figure 2.
Multiple angle light scattering (MALS) and refractive index curves for CKB and the CKB-ASB9 complex are shown. The calculated molecular mass for CKB=85.3 kDa, ASB9(1-252)=29 kDa, CKB+ ASB9(1-252) =114 kDa (one CKB dimer + one ASB molecule). Both light scattering (LS) and refractive index with buffer subtracted (dRI) were measured and are plotted for each protein sample.
Partial inhibition of CK activity by ASB9
The enzymatic activity of CK to form creatine phosphate was measured in a coupled assay (19). In the absence of ASB9, the Vmax was 3.36 ± 0.07 × 10−7 Ms−1 (for comparison to literature values this translated to kcat = 6.7×103 min−1) and the KM was 2.1± 0.2 mM. When the complex of ASB9 (1-252) with CK was assayed, partial inhibition was observed so that the Vmax of the complex was 2.1 ± 0.06 × 10−7 Ms−1 and the KM was 3.4 ± 0.3 mM (Figure 3). This result was consistent with the MALS result which showed one ASB9 bound to a dimer of CK, likely leaving one active site available in the CK dimer. The complex of ASB9(35-252) with CK was also assayed and this complex had full CK activity hinting that the N-terminus of ASB9 may occlude the active site of one monomer of CK in the ASB9(1-252)-CK complex.
Figure 3.
Enzyme activity of CKB (■) and CKB+ASB9(1-252) (●) at 3 nM enzyme concentration and 30° C. The KM was 2.1 ± 0.2 mM and the Vmax was 3.36 ± 0.07 × 10−7 Ms−1 (for comparison to literature values this translated to kcat = 6.7×103 min−1). ASB9(1-252) binding to CKB partially inhibits the activity of CKB increasing the KM slightly to 3.4 ± 0.3 mM and decreasing the Vmax to 2.1 ± 0.06 × 10−7 Ms−1.
HDXMS Mapping of the interface between ASB9 and CK
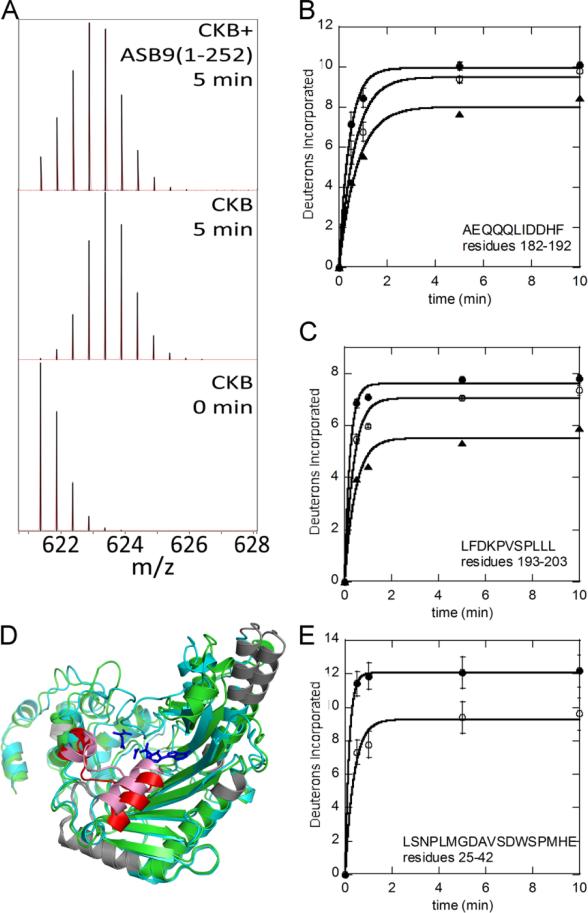
To obtain information about where ASB9 and CK come in contact, amide hydrogen/deuterium exchange experiments (HDXMS) were performed in which the proteins were exposed to deuterated buffer for short periods of time to map the surface-exposed amides in the individual proteins and in the complex (17). A Synapt G2S mass spectrometer was used for the study yielding amide exchange measurements across nearly the entire sequence of both proteins (Supplementary Figure 1). These experiments revealed only one region of CKB, residues 182-203, was protected upon binding to ASB9(1-252) and ASB9(35-252). This region was covered by two peptides: 182-192 and 193-203 both of which showed decreased exchange in the complex (Figure 4A-C). The protected residues 182-203 mapped to a helix-coil-helix region that is located right in front of the substrate binding pocket of the CK active site (Figure 4D). The helix, residues 182-189, undergoes a conformational change and closes upon substrate binding as shown by the overlay of the two monomers from the dimeric CK crystal structure (PDB code 3DRB).
Figure 4.
Amide hydrogen deuterium exchange (HDXMS) results. A) The peptide mass envelope for the CKB peptide ‘LFDKPVSPLLL’ from CKB alone or in complex with ASB9(1-252) after 5 min of exchange showing a clear decrease in deuterium uptake upon binding to ASB9. B) and C) D2O uptake curves for CKB peptides ‘AEQQQLIDDHF‘ and ‘LFDKPVSPLLL‘ for CKB (●), CKB+ASB9(35-252) (O) and CKB+ASB9(1-252) (▲). D) Overlay of human apo CKB (open, grey) and CKB (closed, cyan) bound to Mg – ADP, NO3 (shown in blue sticks) and creatine (shown as blue sticks) structures. The region of CKB that shows a difference in deuterium incorporation upon binding to ASB9 is colored red in the open conformer and pink in the closed conformer. CKB regions that had no coverage are shown in grey. E. Deuterium uptake plot for the ASB9 peptide ‘LSNPLMGDAVSDWSPMHE‘ for ASB9(1-252) (●), ASB9(1-252)+CKB (O).
The HDXMS results for ASB9 showed subtle changes throughout the protein, however, the main site of protection appeared to the N-terminal residues 25-42 (Figure 4E). A full analysis of the HDXMS on ASB9 will be presented in a separate study.
Molecular Modeling
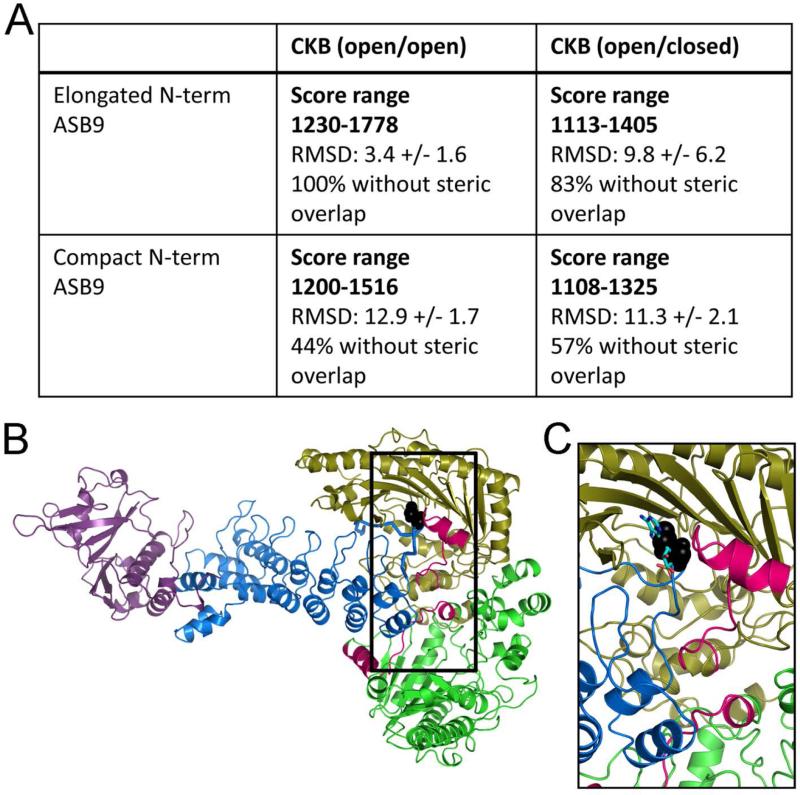
Although a model of the ASB9-CK interaction has already been proposed (13), this model was created from the crystal structure of ASB9 in which the first 35 residues were not observed, likely due to the N-terminus being disordered. As our binding studies implicated the N-terminus of ASB9 as a major contributor to the binding affinity, we sought to model the disordered N-terminus and predict a model that included these residues. Multiple simulations of the N-terminal residues, ASB9(1-35), were performed and the structures were clustered. Two clusters emerged from these simulations, one in which the N-terminus was extended and another in which it was folded back on itself. Representative models from these two cluster families were then attached to ASB9 to form a model of the full-length protein to create two different models of the full-length ASB9. To explore whether it is the open state of CK or the closed state, two different models of the CK dimer were used. One corresponded to CK crystal structure (3DRB) in which the A chain has an open active site and the B chain has the active site closed (and an ADP bound, which was removed for the docking). A second model, in which both active sites were open was created starting again from the 3DRB structure but replacing the B chain (closed structure) with a replica of the A chain (open structure). The two different ASB9 models were docked against these two CK models and the results of each trial were scored using a combination of the actual docking score from ZDOCK, and the consistency of the docked orientation from the top 10 docked complexes. Only when the CK dimer in which both active sites were open was docked with ASB9 that had an extended N-terminus were consistent solutions with good ZDOCK scores obtained. The consistent solution from this docking trial mainly had the N-terminus and first ankyrin repeat of ASB9 docked in between the two monomers of CK with the extended N-terminus laying in one of the CK active sites as was shown by a second scoring described in the Methods section (Figure 5).
Figure 5.
Best docked model of the ASB9-CK complex. A) Table of results from docking of two different structural models of ASB9, one with an extended and the other with a collapsed N-terminus were docked with CKB structures that either had open or closed active sites. The solutions with the best ZDOCK score were obtained from the docking of the ASB9 with an extended N-terminus and an open CKB. The solutions were also overlapped with the ASB9-EloBC crystal structure (3ZKJ) and the percentage of structures that did not have steric clashes between the SOCS-EloBC and CKB structures is reported in the table as well. B) Docked model of ASB9(1-252)-CKB. The regions of CK (green and olive) that were shown to be protected upon ASB9 (blue) binding by HDXMS are marked in bright pink. Aspartate32 within the disordered N-terminus in ASB9 that binds in the ATP binding pocket of CK is shown in black spheres. Elongin B/C, which bind at the other end of the ASB9 ARD are shown in purple. C) Zoomed in view of the active site of the CK monomer in which the N-terminus of ASB9 docks. The pose is the same as in B but the ATP has been added back into the structure to show the superposition of Asp32 with the ATP.
Role of Asp32 of ASB9
The molecular docking revealed a possible role for Asp32 of ASB9 in inhibition of CK activity. The docked structure showed Asp32 taking the place of one of the phosphates of ATP in the active site (Figure 5C). To test the importance of this interaction, we prepared the D32A mutant of ASB9 and compared its activity to the wild type. ITC experiments revealed the mutant protein interacted with CK with a ΔH = -3.7 kcal/mol, even lower than the binding enthalpy for the N-terminal truncation ASB9(35-252), (Figure 6). Activity assays showed that the D32A mutant partially inhibited the activity of CK. Whereas ASB9(1-252) inhibited the Vmax of CK by 38±4%, ASB9(D32A) inhibited the Vmax by 22±3% and ASB9(35-252) did not inhibit the activity at all.
Figure 6.
ITC thermogram and fit for 50 μM CKB (dimer concentration) (in the syringe) binding to 5 μM ASB9(1-252, D32A). For this mutant version of ASB9, ΔH = -3.7 ± 0.08 kcal/mol.
Discussion
In order to build-up a functional model of how ASB9 interacts with CK, we performed a number of experiments and interpreted these in light of a docked molecular model. First, we found that one molecule of ASB9 interacts with a dimer of CK in an asymmetric manner. The N-terminus of ASB9 and the first ankyrin repeat nestle into the cleft between the two monomers of CK. The HDXMS data reveal a single region of CK that is protected from exchange in the complex, residues 182-203. This helix-strand-helix forms one lip of the CK active site and is known to undergo a conformational change, being open in the apo enzyme and closed in the substrate-bound form (31).
Second, comparison of the full length ASB9(1-252) with an N-terminally truncated mutant, ASB9(35-252) revealed that both bound tightly, but the N-terminal residues made the affinity even higher. Interestingly, the N-terminal truncation, although it binds CK tightly, does not inhibit enzyme activity and even the full-length ASB9 only partially inhibits CK activity. Due to the stoichiometry of the complex (1ASB9:1dimer of CK), one active site remains open in the complex and therefore only partial inhibition is seen. Some studies have suggested that only one active site of CK functions at a time (31), and our studies reveal that the ASB9-CK complex retains much of the CK activity. The partial inhibition seen is consistent with the docked model, which shows the disordered N-terminus of ASB9 lying in one active site with Asp32 interacting with the positively charged residues that normally interact with ATP. The D32A mutation shows partial inhibition consistent with weakening of this probably disordered interaction that would inhibit enzyme activity. Thus it is possible that CK could exist in cells as the ASB9 complex and still retain significant activity.
Third, we found that ASB9(1-252) interacts with CK so tightly that dissociation was not observed even in the presence of denaturant. This result is reminiscent of the interaction of IκBα with NFκB, for which dissociation is also not observed (22, 33). As with IκBα, this result suggests that proteasomal degradation is the only way to dissociate the complex (34). The result also suggests that the ASB9-CK complex may be a stable subcomponent of the complete ECS-type CR E3 ligase. Studies of the other protein-protein interactions within the complex will reveal whether any of the other components weaken this interaction, or whether the complex remains intact until proteasomal degradation occurs.
Our model of the ASB9-CK complex differs significantly from that predicted previously when only the crystallographically-ordered portion of ASB9 was used in the docking (13). We observed that the N-terminal residues contributed substantially to the binding based on ITC, SPR, and enzymatic assays. Therefore, we sought a way to predict some possible structures of the disordered N-terminus of ASB9 in order to generate a more realistic docked model (13). Remarkably, the docked model we obtained, with the N-terminus included, was completely consistent with HDXMS data showing where the two proteins made surface contact. The ASB9 C-terminus, which must interact with Elongin BC and the rest of the ECS-type CR E3 ligase, protrudes from the center of the CK dimer placing the CK at the tip of the long ARD, almost like a lollypop on the end of a stick. Our model suggests that a simple hinging motion in the ECS-type CR E3 ligase would allow contact of the CK with the ubiquitin at the other end of the E3 ligase complex. Such a model is only one of several possible mechanisms for ubiquitin transfer requiring further study.
Supplementary Material
Acknowledgments
Funding source statement: DB is supported by an American Heart postdoctoral award, 14POST18970079. JS is supported by a NIH Molecular Biophysics Training Grant, T32 GM008326-23. This work was funded in part through a NIH Director's New Innovator Award (DP2-OD007237) to REA and by P01 GM071862 to EAK. Support from the National Biomedical Computation Resource (NBCR, P41 GM103426) to RA is also gratefully acknowledged.
Abbreviations and Textual Footnotes
- CKB
Creatine kinase brain type
- uMtCK
ubiquitous mitochondrial creatine kinase
Footnotes
Supporting Information Available
Supplementary Figure 1 showing all of the deuterium uptake curves for CK alone and in complex with ASB9(1-252) and ASB9(35-252). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 2.Schulman BA, Harper JW. Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways. Nat Rev Mol Cell Biol. 2009;10:319–331. doi: 10.1038/nrm2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng N, Schulman BA, Song L, Miller JJ, Jeffrey PD, Wang P, Chu C, Koepp DM, Elledge SJ, Pagano M, Conaway RC, Conaway JW, Harper JW, Pavletich NP. Structure of the Cul1–Rbx1–Skp1–F boxSkp2 SCF ubiquitin ligase complex. Nature. 2002;416:703–709. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
- 4.Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman ES, Schulman BA, Zheng N. Structural assembly of cullin-RING ubiquitin ligase complexes. Curr Opin Struct Biol. 2010;20:714–721. doi: 10.1016/j.sbi.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duda DM, Borg LA, Scott DC, Hunt HW, Hammel M, Schulman BA. Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation. Cell. 2008;134:995–1006. doi: 10.1016/j.cell.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohroki J, Nishiyama T, Nakamura T, Masuho Y. ASB proteins interact with Cullin5 and Rbx2 to form E3 ubiquitin ligase complexes. FEBS Lett. 2005;579:6796–6802. doi: 10.1016/j.febslet.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Thomas JC, Matak-Vinkovic D, Van Molle I, Ciulli A. Multimeric Complexes among Ankyrin-Repeat and SOCS-box Protein 9 (ASB9), ElonginBC, and Cullin 5: Insights into the Structure and Assembly of ECS-type Cullin-RING E3 Ubiquitin Ligases. Biochemistry. 2013;52:5236–5246. doi: 10.1021/bi400758h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muniz JR, Guo K, Kershaw NJ, Ayinampudi V, von Delft F, Babon JJ, Bullock AN. Molecular architecture of the ankyrin SOCS box family of Cul5-dependent E3 ubiquitin ligases. J Mol Biol. 2013;425:3166–3177. doi: 10.1016/j.jmb.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debrincat MA, Zhang JG, Willson TA, Silke J, Connolly LM, Simpson RJ, Alexander WS, Nicola NA, Kile BT, Hilton DJ. Ankyrin repeat and suppressors of cytokine signaling box protein asb-9 targets creatine kinase B for degradation. J Biol Chem. 2007;282:4728–4737. doi: 10.1074/jbc.M609164200. [DOI] [PubMed] [Google Scholar]
- 11.Kwon S, Kim D, Rhee JW, Park J-A, Kim D-W, Kim D-S, Lee Y, Kwon H-J. ASB9 interacts with ubiquitous mitochondrial creatine kinase and inhibits mitochondrial function. BMC Biology. 2010;8:1–23. doi: 10.1186/1741-7007-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fei X ZY, Gu X, Qiu R, Mao Y, Ji C. Protein Pept, and 16:333–335, L. (2009) Crystallization and preliminary X-ray analysis of the splice variant of human ankyrin repeat and suppressor of cytokine signaling box protein 9 (hASB9-2). Protein Pept Lett. 2009;2009;1616(3):333–5. 333–335. doi: 10.2174/092986609787601688. [DOI] [PubMed] [Google Scholar]
- 13.Fei X, Gu X, Fan S, Yang Z, Li F, Zhang C, Gong W, Mao Y, Ji C. Crystal Structure of Human ASB9-2 and Substrate-Recognitionof CKB. Protein J. 2012;31:275–284. doi: 10.1007/s10930-012-9401-1. [DOI] [PubMed] [Google Scholar]
- 14.Zhong L, Ge K, Zu JC, Zhao LH, Shen WK, Wang JF, Zhang XG, Gao X, Hu W, Yen Y, Kernstine KH. Autoantibodies as potential biomarkers for breast cancer. Breast Cancer Res. 2008;10:R40–41−R40-48. doi: 10.1186/bcr2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tokuoka M, Miyoshi N, Hitora T, Mimori K, Tanaka F, Shibata K, Ishii H, Sekimoto M, Doki Y, Mori M. Clinical significance of ASB9 in human colorectal cancer. Int. J. Oncol. 2010;37:1105–1111. doi: 10.3892/ijo_00000762. [DOI] [PubMed] [Google Scholar]
- 16.Uranbileg B, Enooku K, Soroida Y, Ohkawa R, Kudo Y, Nakagawa H, Tateishi R, Yoshida H, Shinzawa S, Moriya K, Ohtomo N, Nishikawa T, Inoue Y, Tomiya T, Kojima S, Matsuura T, Koike K, Yatomi Y, Ikeda H. High ubiquitous mitochondrial creatine kinase expression in hepatocellular carcinoma denotes a poor prognosis with highly malignant potential. Int J Cancer. 2014;134:2189–2198. doi: 10.1002/ijc.28547. [DOI] [PubMed] [Google Scholar]
- 17.Mandell JG, Falick AM, Komives EA. Identification of protein-protein interfaces by decreased amide proton solvent accessibility. Proc Nat Acad Sci USA. 1998;95:14705–14710. doi: 10.1073/pnas.95.25.14705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen LH, White CB, Babbitt PC, McLeish MJ, Kenyon GL. A comparative study of human muscle and brain creatine kinases expressed in Escherichia coli. J PROTEIN CHEM. 2000;19:59–66. doi: 10.1023/a:1007047026691. [DOI] [PubMed] [Google Scholar]
- 19.Tanzer ML, Gilvarg C. Creatine and creatine kinase measurement. J BIOL CHEM. 1959;234:3201–3204. [PubMed] [Google Scholar]
- 20.Wang PF, McLeish MJ, Kneen MM, Lee G, Kenyon GL. An unusually low p K a for Cys282 in the active site of human muscle creatine kinase. Biochemistry. 2001;40:11698–11705. doi: 10.1021/bi011208f. [DOI] [PubMed] [Google Scholar]
- 21.Trathnigg B. Determination of MWD and Chemical Composition of Polymers by Chromatographic Techniques. Prog. Polym. Sci. 1995;20:615–650. [Google Scholar]
- 22.Bergqvist S, Croy CH, Kjaergaard M, Huxford T, Ghosh G, Komives EA. Thermodynamics reveal that helix four in the NLS of NF-kappaB p65 anchors IkappaBalpha, forming a very stable complex. J Mol Biol. 2006;360:421–434. doi: 10.1016/j.jmb.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dembinski H, Wismer K, Balasubramaniam D, Gonzalez HA, Alverdi V, Iakoucheva LM, Komives EA. Predicted disorder-to-order transition mutations in IκBα disrupt function. Phys Chem Chem Phys. 2014;16:6480–6485. doi: 10.1039/c3cp54427c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang J NP, Kairys V, Venclovas C, Engen JR, Beuning PJ. Conformational analysis of processivity clamps in solution demonstrates that tertiary structure does not correlate with protein dynamics. Structure. 2014;22:572–581. doi: 10.1016/j.str.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornak V, Abel R, Okur A, Strockbine B, Roitberg A, Simmerling C. Comparison of Multiple Amber Force Fields and Development of Improved Protein Backbone Parameters. Proteins: Struct., Funct., Bioinf. 2006;65:712–725. doi: 10.1002/prot.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorgensen WL. Revised TIPS for simulations of liquid water and aqueous solutions. J Chem Phys. 1982;77:4156–4163. [Google Scholar]
- 27.Salomon-Ferrer R, Goetz AW, Poole D, Le Grand S, Walker RC. Routine microsecond molecular dynamics simulations with AMBER - Part II: Particle Mesh Ewald. J Chem Theory Comput. 2013;9:3878–3888. doi: 10.1021/ct400314y. [DOI] [PubMed] [Google Scholar]
- 28.Darden T, York D, Pedersen L. Particle mesh Ewald: An Nlog(N) method for Ewald sums in large systems. J Chem Phys. 1993;98:10089–10092. [Google Scholar]
- 29.Baron R, McCammon JA. Dynamics, hydration, and motional averaging of a loop-gated artificial protein cavity: the W191G mutant of cytochrome c peroxidase in water as revealed by molecular dynamics simulations. Biochemistry. 2007;46:10629–10642. doi: 10.1021/bi700866x. [DOI] [PubMed] [Google Scholar]
- 30.Hess B, Kutzner C, Van Der Spoel D, Lindahl E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput. 4:435–447. doi: 10.1021/ct700301q. 20008. [DOI] [PubMed] [Google Scholar]
- 31.Eder M, Schlattner U, Becker A, Wallimann T, Kabsch W, Fritz-Wolf K. Crystal structure of brain-type creatine kinase at 1.41 A resolution. Protein Sci. 1999;8:2258–2269. doi: 10.1110/ps.8.11.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierce BG, Wiehe K, Hwang H, Kim BH, Vreven T, Weng Z. ZDOCK Server: Interactive Docking Prediction of Protein-Protein Complexes and Symmetric Multimers. Bioinformatics. 2014;30:1771–1773. doi: 10.1093/bioinformatics/btu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alverdi V, Hetrick B, Joseph S, Komives E. Direct observation of a transient ternary complex during IκBα-mediated dissociation of NF-κB from DNA. Proc Natl Acad Sci U S A. 2014;111:225–230. doi: 10.1073/pnas.1318115111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferreiro DU, Komives EA. Molecular Mechanisms of System Control of NF-kappaB Signaling by IkappaBalpha. Biochemistry. 2010;49:1560–1567. doi: 10.1021/bi901948j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.