Jasmonates reduce root damage by belowground herbivores, but enhanced jasmonate biosynthesis improves herbivore growth.
Abstract
Induced defenses play a key role in plant resistance against leaf feeders. However, very little is known about the signals that are involved in defending plants against root feeders and how they are influenced by abiotic factors. We investigated these aspects for the interaction between rice (Oryza sativa) and two root-feeding insects: the generalist cucumber beetle (Diabrotica balteata) and the more specialized rice water weevil (Lissorhoptrus oryzophilus). Rice plants responded to root attack by increasing the production of jasmonic acid (JA) and abscisic acid, whereas in contrast to in herbivore-attacked leaves, salicylic acid and ethylene levels remained unchanged. The JA response was decoupled from flooding and remained constant over different soil moisture levels. Exogenous application of methyl JA to the roots markedly decreased the performance of both root herbivores, whereas abscisic acid and the ethylene precursor 1-aminocyclopropane-1-carboxylic acid did not have any effect. JA-deficient antisense 13-lipoxygenase (asLOX) and mutant allene oxide cyclase hebiba plants lost more root biomass under attack from both root herbivores. Surprisingly, herbivore weight gain was decreased markedly in asLOX but not hebiba mutant plants, despite the higher root biomass removal. This effect was correlated with a herbivore-induced reduction of sucrose pools in asLOX roots. Taken together, our experiments show that jasmonates are induced signals that protect rice roots from herbivores under varying abiotic conditions and that boosting jasmonate responses can strongly enhance rice resistance against root pests. Furthermore, we show that a rice 13-lipoxygenase regulates root primary metabolites and specifically improves root herbivore growth.
The ability to resist herbivory is essential for plant survival in nature. Most plants rely on a combination of physical, chemical, and biochemical defenses to reduce herbivore damage. A common requirement for the deployment of plant resistance factors is their tight regulation to avoid autotoxicity and excessive fitness costs (Bergelson et al., 1996; Robert et al., 2013). Plant stress hormones, including jasmonates, have emerged as universal regulators in this context (Howe and Jander, 2008). Jasmonates are synthesized from α-linoleic acid, which originates from galactolipids of plastid membranes (Bonaventure et al., 2011; Qi et al., 2011a). Linoleic acid is oxidized at the C-13 position by 13-lipoxygenases (13-LOX; Glauser et al., 2009; Zhou et al., 2009) before being converted to 12-oxophytodienoic acid (OPDA) by an allene oxide synthase and an allene oxide cyclase (AOC; Hamberg and Fahlstadius, 1990). OPDA is released from the plastid into the peroxisome, where it is reduced by OPDA reductase (Schaller et al., 2000) and converted to jasmonic acid (JA) through three rounds of β-oxidation (Schneider et al., 2005). The most active form of JA is jasmonic acid Ile conjugate (JA-Ile; Fonseca et al., 2009), which is produced by a jasmonate:amino acid synthetase (JAR; Guranowski et al., 2007) and binds to the Skp1/Cullin/F-box Coronatine insensitive1 (SCFCOI1) complex (Katsir et al., 2008). The activated SCF complex then ubiquinates jasmonate ZIM domain proteins, which are degraded and thereby, release bound JA-responsive transcription factors that subsequently bind to their respective promoters and activate gene transcription (Thines et al., 2007). Many other hormones, including abscisic acid (ABA), ethylene (ET), and salicylic acid (SA), can modulate jasmonate signaling (Van der Does et al., 2013), and the resulting signaling network enables plants to respond in a tissue-, development-, and environment-specific manner (Pieterse et al., 2009; Erb et al., 2012b; Meldau et al., 2012).
Using a combination of jasmonate biosynthesis and jasmonate perception mutants as well as exogenous application of JA and methyl jasmonic acid (MeJA), it was shown that jasmonates regulate the accumulation of defensive proteins (Farmer and Ryan, 1992), toxic secondary metabolites (Schweizer et al., 2013), primary metabolites (Machado et al., 2013), volatile organic compounds that attract natural enemies (Christensen et al., 2013), and physical defenses (Qi et al., 2011b). Taken together, this results in a dramatic susceptibility of JA-deficient plants to insect herbivores (Howe and Jander, 2008; Abe et al., 2013) as well as other members of the meso- and macrofauna, including crustaceans (Farmer and Dubugnon, 2009), reptiles (Mafli et al., 2012), and mollusks (Falk et al., 2014). Among the most dramatic examples in this context are detritus feeders that switch their lifestyle and become herbivorous when encountering JA-defective Arabidopsis (Arabidopsis thaliana) plants (Farmer and Dubugnon, 2009).
Compared with the well-documented role of plant stress hormones above ground, little is known about their importance in protecting plant roots against belowground herbivores (Erb et al., 2012a, 2012b). Possibly because of the lower proportion of α-linoleic acid in root plastid membranes (Li et al., 2003), plant roots typically display a much weaker herbivore- and wound-induced jasmonate burst than the leaves (Tretner et al., 2008; Hasegawa et al., 2011), leading to speculation that other induced signals may be more important for induced herbivore defenses below ground (Erb et al., 2012a, 2012b). However, several lines of indirect evidence point to a potential role of jasmonates as root resistance factors. First, the COI1 mutant of Arabidopsis was found to be more susceptible to fungus gnats (Bradysia impatiens), which feed on leaves, roots, and detritus (McConn et al., 1997). Second, the application of JA and MeJA reduced rice water weevil (Lissorhoptrus oryzophilus) oviposition and larval numbers feeding on rice (Oryza sativa; Hamm et al., 2010), pupation rates of cabbage fly (Delia radicum) larvae feeding on broccoli (Brassica oleracea ssp. italica; Pierre et al., 2012), and numbers of phyllorexa (Daktulosphaira vitifoliae) eggs on grape (Vitis vinifera) vine roots. Third, JA and MeJA were found to induce a variety of root defenses, including sesquiterpenes in maize (Zea mays; Erb et al., 2011), glucosinolates in Brassica spp. (Pierre et al., 2012), nicotine in Nicotiana spp. (Baldwin, 1989), and defense-related transcripts in Beta vulgaris (Puthoff and Smigocki, 2007), Arabidopsis (Hasegawa et al., 2011), and Brassica oleracea (Tytgat et al., 2013). Despite this indirect evidence, few studies have systematically investigated the importance of induced phytohormones, including jasmonates, for root herbivore resistance, although root-feeding insects have a strong impact on plant fitness and yield in agriculture and are potentially important drivers of plant community structure in nature (Hunter, 2001; Rasmann and Agrawal, 2008).
Abiotic factors may modulate the role of phytohormones in belowground plant-insect interactions. The deprivation of oxygen through water saturation, for instance, is a frequent and in most cases, soil-specific event (Erb and Lu, 2013) that leads to changes in ET and ABA signaling in the roots (Castonguay et al., 1993; Voesenek et al., 1993). These changes may then modulate the expression of herbivore defense traits. Furthermore, the oxidation of fatty acids may be reduced under anoxic conditions. Assessing the plant-mediated impact of flooding on root resistance remains challenging, because herbivore fitness is directly impacted by the moisture levels in the rhizosphere (Hoback et al., 2002; Johnson et al., 2010). One of the few studies that investigated the impact of flooding on root herbivore resistance so far found that Swingle citrumelo (Citrus paradisi × Poncirus trifoliata) roots that were submerged for 20 d before being exposed to Diaprepes abbreviatus were more susceptible to this root weevil (Li et al., 2006). Whether this flooding-induced susceptibility is caused by changes in plant defensive signaling remains to be determined.
To elucidate the importance of jasmonates and other stress hormones in root-herbivore interactions under different abiotic conditions, we carried out a series of experiments in rice. Rice is a suitable model for this purpose, because several genes involved in jasmonate biosynthesis have been identified (Zhou et al., 2009; Qi et al., 2011a; Riemann et al., 2013; Svyatyna et al., 2014), and jasmonate-deficient genotypes are available (Riemann et al., 2003; Zhou et al., 2009). Furthermore, rice plants are frequently attacked by root herbivores in the field, including the major pest rice water weevil whose larvae feed on rice roots under flooded conditions and obtain their oxygen directly through the root aerenchyma (Saito et al., 2005), as well as other generalist herbivores, such as the cucumber beetle (Diabrotica balteata), which is not a major pest of rice but has been reported to feed on rice occasionally. Finally, the fact that rice plants grow and develop well under both flooded and nonflooded conditions allows for biologically relevant insights into the impact of flooding on defensive signaling (Voesenek and Bailey-Serres, 2013). Using the rice system, we addressed the following questions. (1) How does root herbivore attack alter the phytohormonal signature in rice roots? (2) Is this response modulated by the flooding status of the soil? (3) Do jasmonates influence plant resistance against root-feeding insects under flooded and nonflooded conditions? By combining different herbivores that feed under flooded and nonflooded conditions with jasmonate-deficient rice genotypes and phytochemical analyses, our experiments provide clear evidence for an important role of jasmonates in protecting rice roots against root-feeding arthropods under variable abiotic conditions. Furthermore, we uncover an unexpected role of a 13-LOX in the herbivore-induced reconfiguration of the root primary metabolism and herbivore growth patterns.
RESULTS
Root Herbivory and Wounding Induce Root Jasmonate Signaling Independent of Flooding
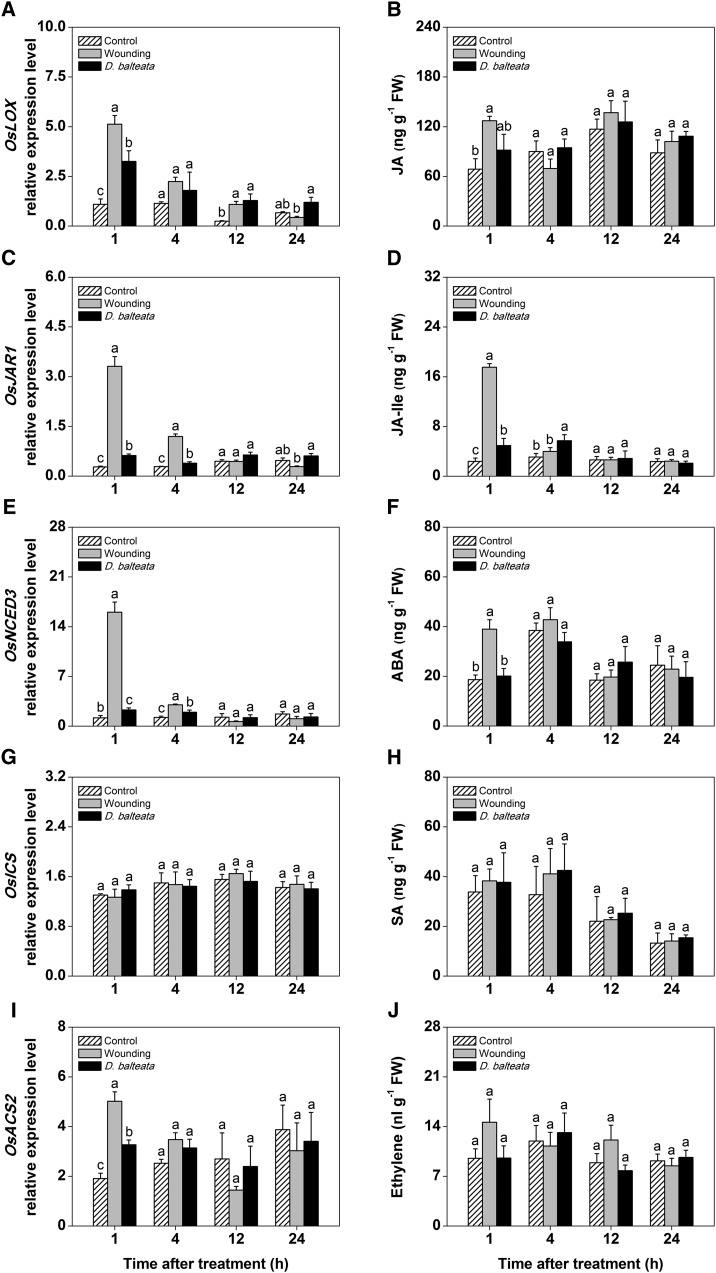
To profile the defensive response of rice roots to herbivore attack under different abiotic conditions, we conducted three independent experiments. In the first experiment, we grew rice seedlings in sand that was moistened daily from the bottom of the pots, resulting in a relative humidity of 10% to 20% in the rhizosphere. Rice plants were then infested with first-instar larvae of the generalist root herbivore cucumber beetle, which feeds on a variety of host plants, including rice (PaDIL PH-PaDIL, 2013). A second batch of plants was wounded in the roots by applying manual pressure to the outside of the pots, and a third batch of plants was left untreated. The manual pressure created friction between the sand particles and thereby, wounded the roots. Although the extent of damage caused by this treatment is difficult to quantify, we estimate that up to 50% of the root surface of the larger roots was wounded. We harvested plants 1, 4, 12, and 24 h after wounding or the beginning of cucumber beetle infestation and measured the concentrations of major stress hormones as well as the expression of corresponding biosynthetic marker genes in the roots. The following hormone biosynthetic genes were measured (Table I): a 13-lipoxygenase gene involved in JA biosynthesis (OsHI-LOX; Zhou et al., 2009), a JA-Ile synthase gene (OsJAR1; Riemann et al., 2008), a 9-cis-epoxycarotenoid dioxygenase gene involved in ABA biosynthesis (OsNCED3; Hwang et al., 2010), an isochorismate synthase gene involved in SA biosynthesis (OsICS; Yuan et al., 2009), and a 1-aminocyclopropane-1-carboxylic acid synthase gene involved in ET biosynthesis (OsACS2; Helliwell et al., 2013). In wounded plants, we observed a rapid and transient induction of JA, JA-Ile, ABA, and their corresponding biosynthetic genes (Fig. 1, A–F). Such rapid induction is typical of plant response to wounding and sufficient for inducing many resistance traits (Howe and Jander, 2008). SA was unaffected by the different treatments (Fig. 1, G and H). The ET biosynthetic gene OsACS2 was induced without an apparent increase in ET levels (Fig. 1, I and J). The induction in cucumber beetle-attacked plants was lower than in wounded plants. On a phytohormonal level, only JA-Ile was induced 1 and 4 h after the beginning of infestation (Fig. 1D). Furthermore, OsLOX, OsJAR1, OsNCED3, and OsACS2 expressions were induced 1 to 4 h after herbivore attack. Based on root biomass removal data in a long-term infestation experiment (see below), we estimate that cucumber beetle attacks over 24 h resulted in removal of about 5% of root biomass. Compared with this feeding rate, wounding treatment was likely more severe.
Table I. Primers used for quantitative real-time PCR of target genes.
| Gene | Gene Identifier | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|---|
| OsHI-LOX | Os08g39840 | CCGAGCTTGACGCGAAGA | GATCGTCGTCGTCCACATTGT |
| OsJAR1 | Os05g50890 | AAGGTTTGTGAACCCATCAAACAGC | AATAATACTTTGCAGCACTTGTTACG |
| OsNCED3 | Os03g44380 | CCCCTCCCAAACCATCCAAACCGA | TGTGAGCATATCCTGGCGTCGTGA |
| OsICS | Os09g19734 | ACCAATTATGTTTCCGATCAATCA | CGTCGCCTTCTTGGATTTATG |
| OsACS2 | Os04g48850 | CACCCCGAGGCATCCAT | ATTGGCGATCCTCTTGAACTG |
| OsACT | Os03g50885 | TGGACAGGTTATCACCATTGGT | CCGCAGCTTCCATTCCTATG |
Figure 1.
Jasmonates are induced by mechanical wounding and cucumber beetle attack under nonflooded conditions. Induction patterns of phytohormones and corresponding biosynthetic genes in rice roots that were wounded or infested with the generalist herbivore cucumber beetle at different time points are shown (n = 5–6; + se). All plants were grown in moist sand. The biosynthesis of jasmonates (A–D), ABA (E and F), SA (G and H), and ET (I and J) was profiled. Different letters indicate significant differences among treatments within time points (P < 0.05). FW, Fresh weight.
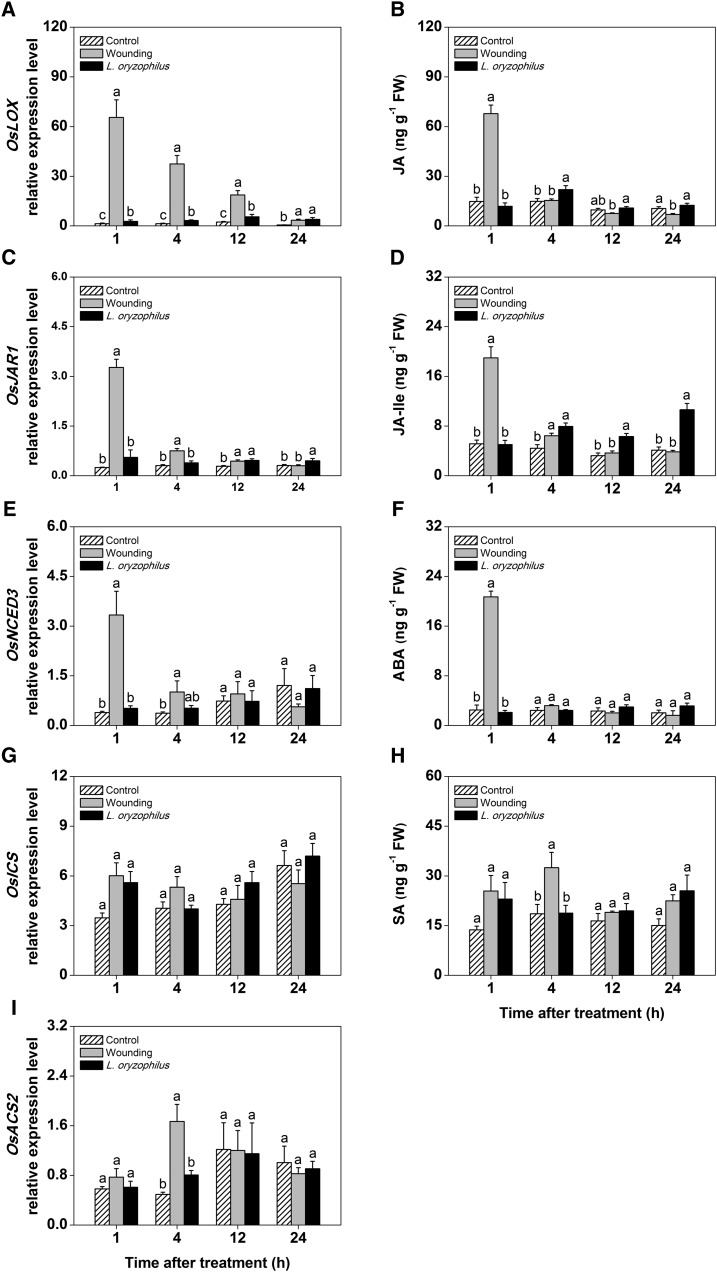
In the second experiment, rice plants were grown under the same conditions, but humidity levels in the soil were increased to 100% by flooding the pots with tap water 2 weeks before infestation. For this experiment, we used the flooding-tolerant larvae of the rice water weevil to infest the roots. The same time course as in the first experiment was used. Overall, wound-induced signaling was comparable with nonflooded conditions (Fig. 2), including a wound-induced burst of JA, JA-Ile, and ABA. Rice water weevil-infested plants had elevated levels of JA at 4 h and higher levels of JA-Ile 4 to 24 h after the beginning of infestation compared with uninfested controls (Fig. 2, B and D). Expression of OsHI-LOX and OsJAR1 was increased over the full infestation period, whereas OsACS2 was induced 4 h after the beginning of infestation only. The ET biosynthetic gene OsACS2 was induced at 4 h after wounding and herbivore infestation. Note that ET measurements were not possible in this experiment, because the available ET sensor system was not suited to operate under elevated humidity levels.
Figure 2.
Jasmonates are induced by mechanical wounding and rice water weevil attack under flooded conditions. Induction patterns of phytohormones and corresponding biosynthetic genes in rice roots that were wounded or infested with the generalist herbivore rice water weevil at different time points are shown (n = 5; + se). All plants were grown in submerged substrate. The biosynthesis of jasmonates (A–D), ABA (E and F), SA (G and H), and ET (I) was profiled. Different letters indicate significant differences among treatments within time points (P < 0.05). FW, Fresh weight.
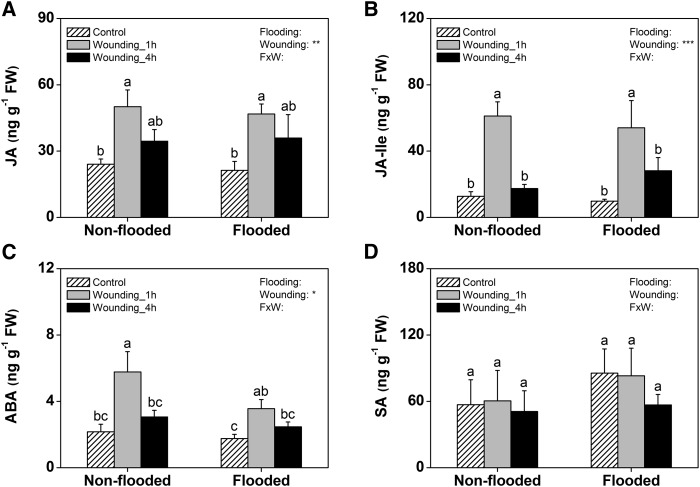
In a third experiment, we directly compared the wound induction of stress hormones in flooded and nonflooded rice plants that were grown and treated at the same time. Plants were analyzed 1 and 4 h after the wounding event. No significant differences in the elicitation of JA, JA-Ile, ABA, or SA were observed between flooded and nonflooded roots (Fig. 3). Taken together, these experiments illustrate that wounding and root herbivory induce jasmonate and ABA signaling in the roots in a flooding-independent manner.
Figure 3.
The wound response of rice roots is not altered by flooding. Wound induction patterns of JA (A), JA-Ile (B), ABA (C), and SA (D) in rice roots growing in flooded and nonflooded substrate are shown 1 and 4 h postinduction (n = 5; + se). The results of two-way ANOVAs are indicated. Different letters indicate significant differences among treatments within time points (P < 0.05). FW, Fresh weight; FxW, flooding × wounding. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Exogenous Jasmonate Application Specifically Increases Root Resistance and Decreases Herbivore Performance
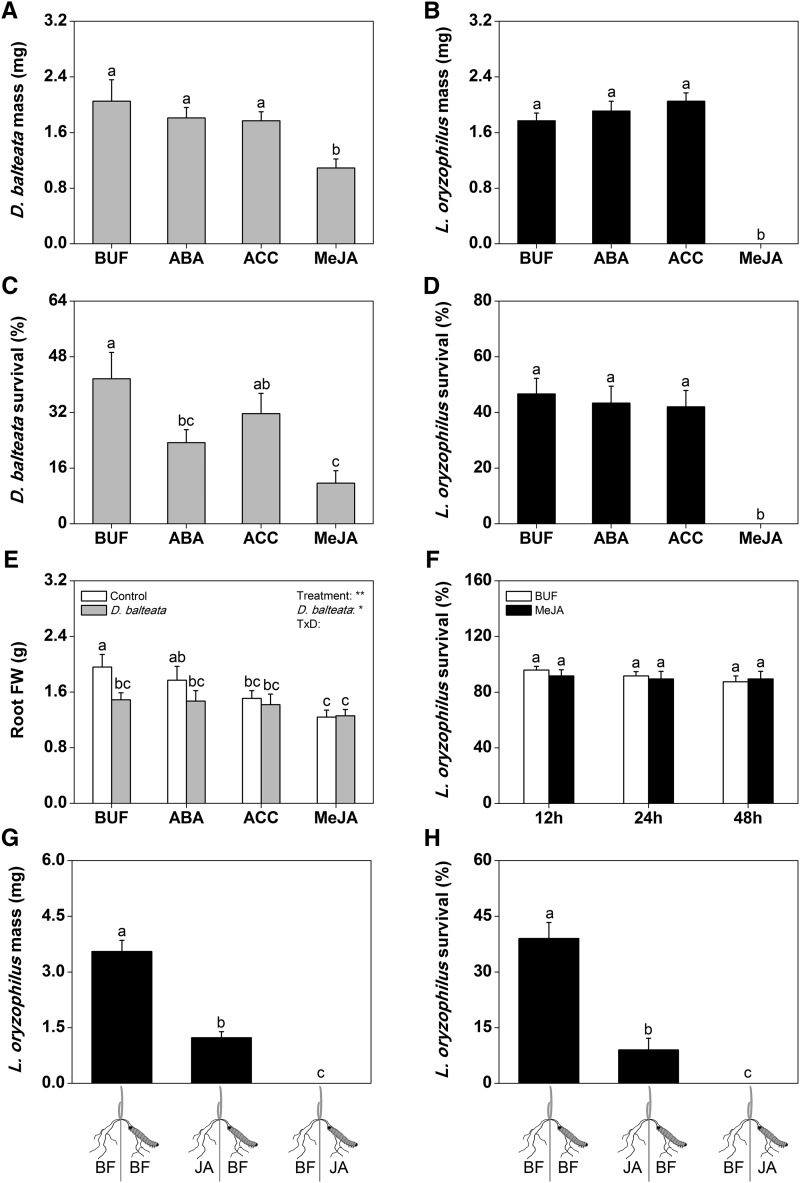
To get insights into the impact of phytohormone signaling on rice root resistance, we treated rice seedlings with the three hormones that responded on a transcriptional level to wounding and root herbivore attack: ABA, ET (using the ET precursor aminocyclopropane-1-carboxylic acid [ACC]), and JA (using MeJA). All compounds were applied to the roots one time at a concentration of 100 µm (Hashimoto et al., 2004). The treated plants were infested with either neonates of cucumber beetle or rice water weevil 24 h later, and herbivore performance was measured 7 and 20 d postinfestation, respectively. Although ACC and ABA did not affect herbivore growth (Fig. 4, A–D), MeJA reduced cucumber beetle growth by 50% and rice water weevil growth by 100%. The survival rate of both herbivores was markedly reduced by MeJA. No rice water weevil larvae feeding on MeJA-treated rice roots survived (Fig. 4D). ABA treatment reduced the survival of cucumber beetle but not rice water weevil. Root biomass was reduced by cucumber beetle feeding in untreated plants but not hormone-treated roots (Fig. 4E). To exclude any direct negative effects of MeJA on rice water weevil survival, we incubated larvae in water containing 100 µm MeJA for 48 h. No significant mortality of MeJA-submerged larvae was observed (Fig. 4F). We further conducted a split-root experiment, in which we treated one-half of the root system with MeJA, infested these roots and the other one-half of the roots with rice water weevil, and measured rice water weevil performance after 20 d. MeJA reduced herbivore growth and survival irrespective of whether the larvae were in direct contact with the chemical or not (Fig. 4, G and H). Taken together, these experiments show that MeJA induces resistance to root-feeding herbivores through plant-mediated effects.
Figure 4.
Jasmonates increase root resistance against herbivores. Growth and survival of cucumber beetle (n = 10) and L. oryzophilus (n = 15) larvae on rice roots treated with buffer solution (BUF), 100 µm ABA, the ET precursor ACC, or MeJA are shown (A–D; ± se). We also recorded root fresh weights of control and cucumber beetle-infested plants treated with the different hormones (E; n = 10–15; ± se). Plants infested with cucumber beetle were grown under nonflooded conditions and infested for 7 d, whereas plants infested with L. oryzophilus were flooded and infested for 20 d. To test whether MeJA is toxic to root herbivores, we incubated L. oryzophilus larvae with 100 µm MeJA for 48 h and measured their survival (F; n = 8; ± se), and we applied 100 µm MeJA to L. oryzophilus-infested and uninfested roots of plants in a split-root setup with buffer (BF) treatment of the remaining roots and measured growth and survival 20 d postinfestation (G and H; n = 10; ± se). Different letters indicate significant differences among treatments (P > 0.05). The results of a two-way ANOVA are shown for E. FW, Fresh weight; TxD, treatment × cucumber beetle. *, P < 0.05; **, P < 0.01.
Jasmonate-Deficient Rice Plants Are More Susceptible to Root Herbivore Attack
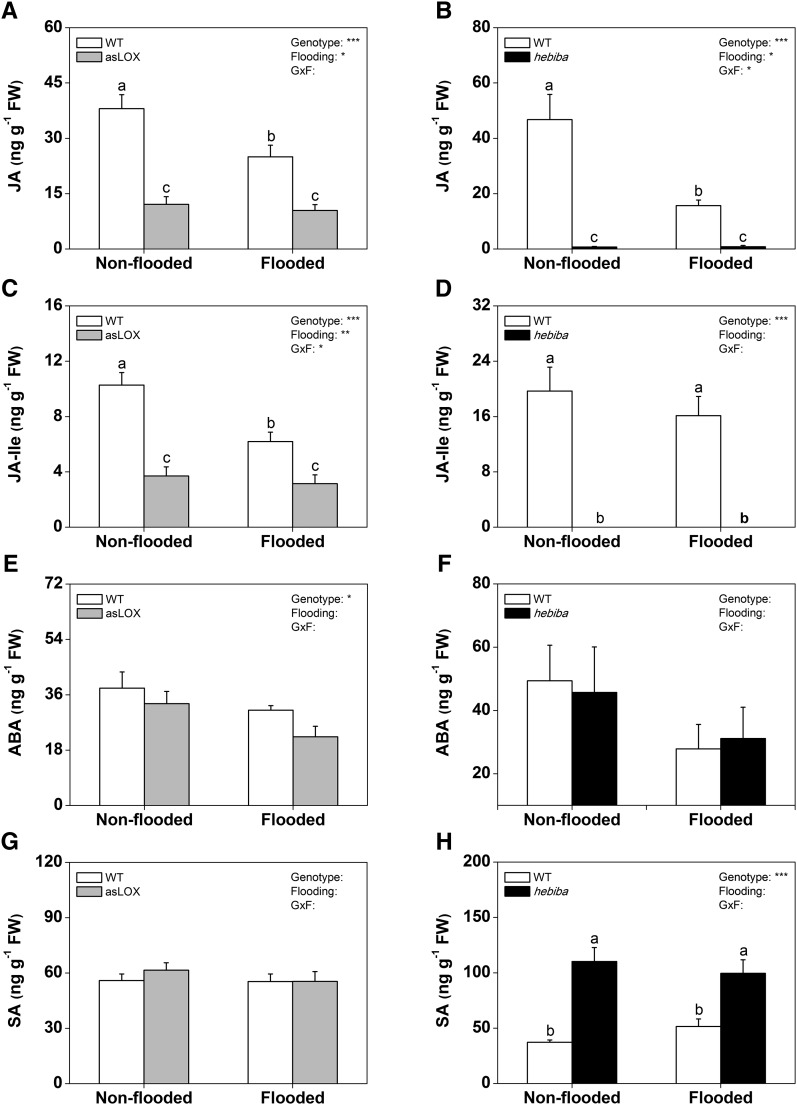
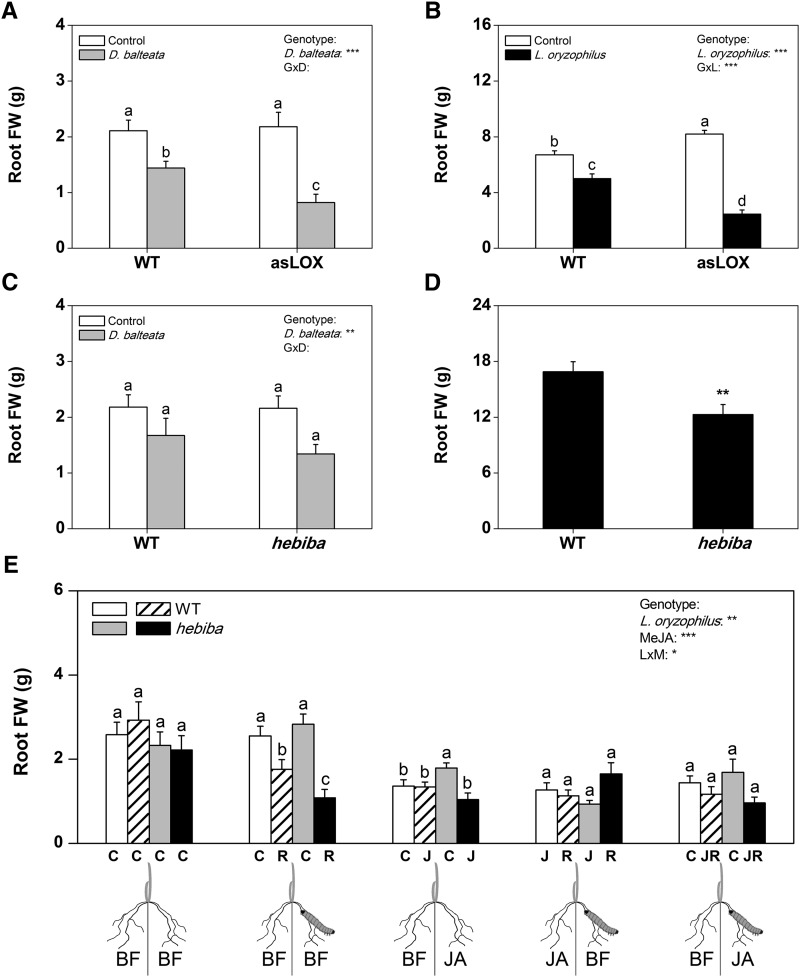
To test whether endogenous jasmonates are required for rice resistance against root-feeding insects, we measured root herbivore-induced tissue loss in two jasmonate-deficient rice genotypes. Antisense 13-lipoxygenase (asLOX) plants are silenced in the expression of OsHI-LOX, a class II 13-LOX that is required for JA biosynthesis (Zhou et al., 2009). The hebiba line is defective in the OsAOC gene and therefore, jasmonate deficient as well (Riemann et al., 2003). In both genotypes, we measured significantly lower levels of JA and JA-Ile in wound-induced roots 1 h postinduction under both flooded and nonflooded conditions (Fig. 5), confirming that the biosynthesis of JA in the roots is regulated by the same enzymes as in the shoots. The hebiba mutant had strongly increased SA levels, whereas asLOX plants showed a suppression of ABA under flooded conditions (Fig. 5). Similar patterns were found for rice water weevil-attacked roots 20 d after the beginning of infestation (Supplemental Fig. S1) with two differences. First, JA-Ile levels were less strongly suppressed in asLOX plants. Second, ABA levels were significantly lower in both jasmonate-deficient lines compared with wild-type plants. When infested with root feeders, asLOX plants suffered more from attack by cucumber beetle and rice water weevil than wild-type controls. Root biomass removal was more than doubled in this genotype (Fig. 6, A and B). Similar patterns were observed for the hebiba genotype, although the effect was only significant for rice water weevil 20 d postinfestation (Fig. 6, C and D; Supplemental Fig. S2). To further explore the role of jasmonate signaling in root herbivore resistance, we performed a split-root experiment using wild-type and hebiba mutant plants. One-half of the roots was left untreated or induced with 100 µm MeJA as described above. The other one-half was left untreated, induced with MeJA, and/or infested with rice water weevil for 20 d. This experiment allowed us to separate local and systemic effects of herbivory on root growth and assess the impact of MeJA application on rice water weevil without the confounding effect of environmental alterations that could be caused by the application of the chemical to the rhizosphere. Plants were infested with rice water weevil 24 h after MeJA application. Again, root fresh mass was more strongly reduced by rice water weevil feeding on the hebiba line than on wild-type plants (Fig. 6E). The reduction of root mass only occurred on the locally infested side. MeJA treatment reduced root growth on both the locally infested and the uninfested sides of wild-type and hebiba plants. In the hebiba mutant, the local effect of MeJA was stronger than when the other side of the roots (the systemic side) was treated. MeJA treatment of both genotypes led to a complete disappearance of rice water weevil feeding damage, irrespective of whether the local (herbivore-infested) or systemic (herbivore-free) side of the root system was treated. Taken together, these results illustrate that jasmonates increase rice root resistance against arthropod herbivores and can reduce root damage by belowground feeders.
Figure 5.
Wound-induced jasmonates are suppressed in asLOX and hebiba plants. Levels of JA (A and B), JA-Ile (C and D), ABA (E and F), and SA (G and H) in wild-type (WT) and jasmonate-deficient asLOX and hebiba plants are shown. Plants were wounded under nonflooded and flooded conditions and harvested after 1 h in two independent experiments (n = 6; + se). Different letters indicate significant differences among treatments (P > 0.05). The results of two-way ANOVAs are shown. FW, Fresh weight; GxF, genotype × flooding; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure 6.
Jasmonate-deficient plants lose more root biomass under herbivore attack. Root biomass removal by cucumber beetle and rice water weevil feeding on wild-type or jasmonate-deficient asLOX and hebiba rice root systems was measured (A–D). Plants infested with cucumber beetle were grown under nonflooded conditions and infested for 7 d, whereas plants infested with rice water weevil were flooded and infested for 20 d (n = 12; ± se). Furthermore, a split-root experiment was conducted that compared root biomass removal on control plants (C) and rice water weevil-infested plants (R) that were treated with buffer solution (BF) or 100 µm MeJA on the locally infested or systemic side of the system (E; n = 10; ± se). Plants were infested for 20 d and treated 24 h before infestation. Different letters and asterisks indicate significant differences among genotypes and treatments (P > 0.05). For controls in D, see Supplemental Figure S2 (separate experiment). FW, Fresh weight; GxD, genotype × cucumber beetle; GxL, genotype × rice water weevil; J, MeJA; LxM, rice water weevil × MeJA; WT, wild type; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Silencing of OsHI-LOX Specifically Decreases Herbivore Performance
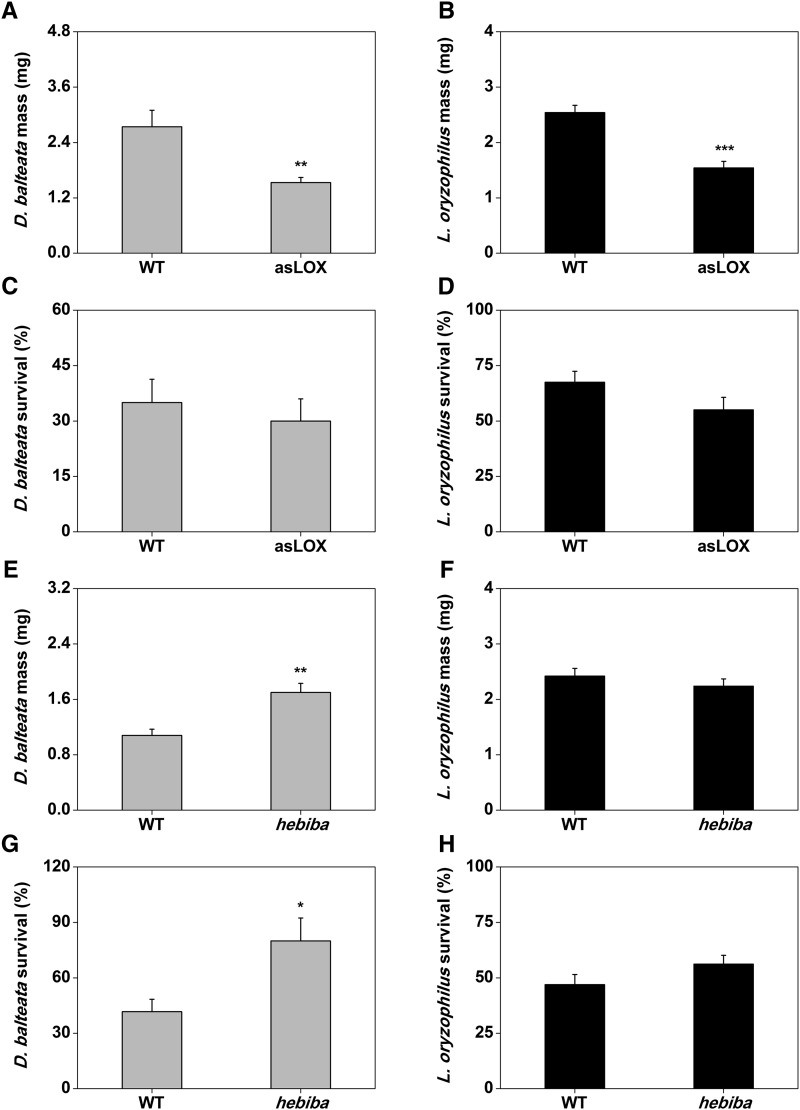
Based on the fact that root herbivores remove more biomass on jasmonate-deficient root systems, we expected their growth and performance to increase on these plants. To test this hypothesis, we measured mass and survival of cucumber beetle and rice water weevil after 7 and 20 d of feeding on the different rice genotypes. Surprisingly, despite the higher root damage, both herbivores gained significantly less mass on asLOX plants compared with the wild type (Fig. 7, A and B). In contrast, the growth of both herbivores was not negatively affected in the hebiba mutant, and cucumber beetle gained significantly more weight on hebiba plants and was more likely to survive compared with the wild type (Fig. 7, E–G). Taken together, these results show that the LOX gene OsHI-LOX improves herbivore performance.
Figure 7.
Herbivores feed more but grow less on asLOX plants. Larval mass and survival of cucumber beetle (A, C, E, and G) and rice water weevil (B, D, F, and H) feeding on wild-type or jasmonate-deficient asLOX and hebiba rice root systems were evaluated. Plants infested with cucumber beetle were grown under nonflooded conditions and infested for 7 d, whereas plants infested with rice water weevil were flooded and infested for 20 d (n = 12; + se). Asterisks indicate significant differences between genotypes. WT, Wild type; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
OsHI-LOX Maintains Suc Supply to Herbivore-Attacked Roots
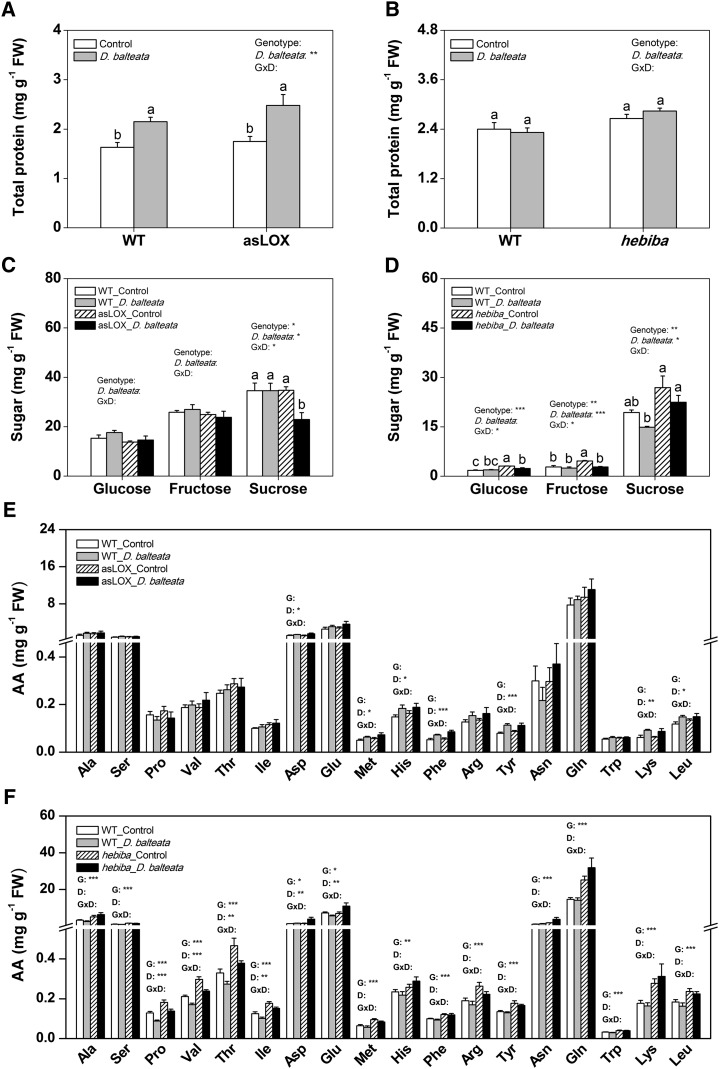
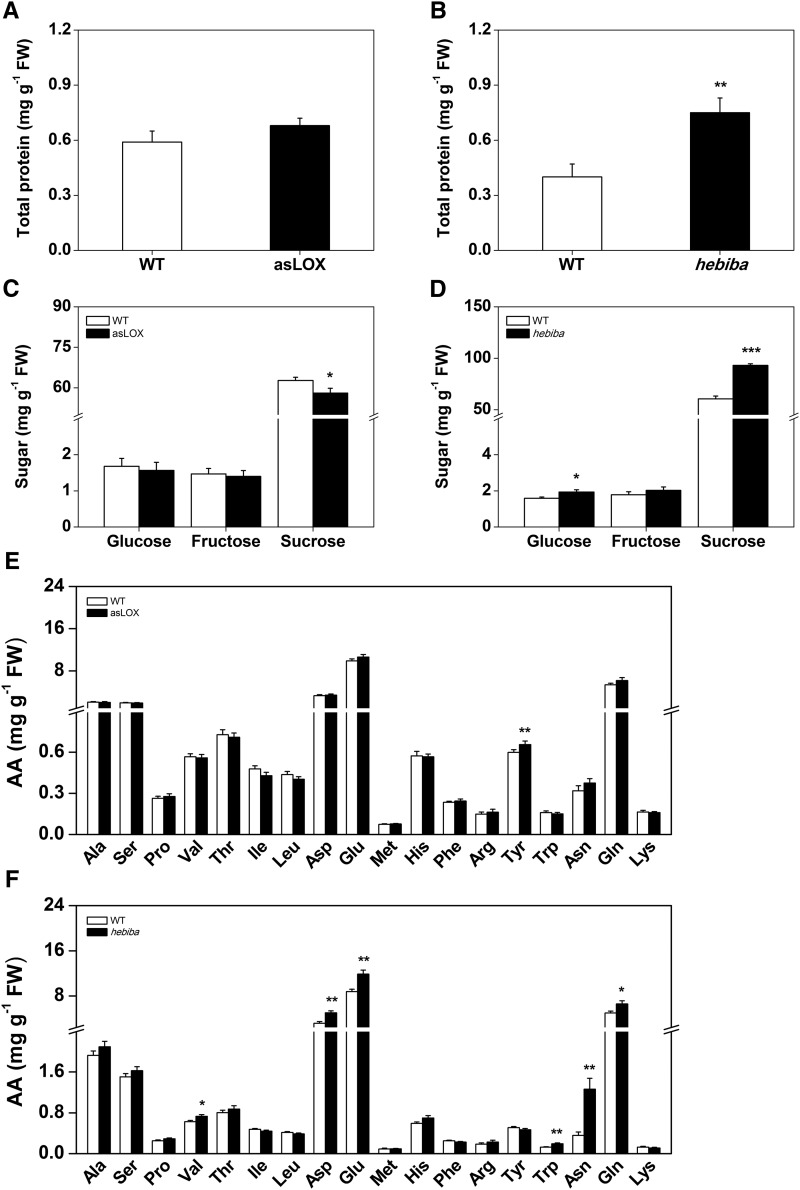
Based on the fact that herbivores cause more damage but grow less on asLOX roots, we hypothesized that these plants may be impoverished in primary metabolites that are essential for the growth of the herbivores. As a consequence, the root feeders may have tried to compensate for the lack of nutrients by feeding more voraciously on the roots (Raubenheimer and Simpson, 1993). To test this hypothesis, we determined constitutive cucumber beetle- and rice water weevil-induced total protein, free amino acid, fatty acid, and sugar levels in the roots (Figs. 8 and 9; Supplemental Figs. S3 and S4). Total protein levels are important for herbivore performance, because most herbivores are nitrogen limited (Ritchie, 2000). Free amino acids, sugars, and fatty acids are known to stimulate feeding of many root herbivores (Johnson and Nielsen, 2012). Under nonflooded conditions, protein and fatty acid levels were not altered in the jasmonate-deficient lines (Fig. 8, A and B; Supplemental Fig. S4). Free amino acid levels were increased in hebiba but not asLOX plants (Fig. 8, E and F). Cucumber beetle attack reduced Suc levels specifically in asLOX plants (Fig. 8, C and D). Under flooded conditions, protein, free amino acid, and sugar levels were enhanced in the hebiba mutant but not asLOX plants (Fig. 9). Similar to nonflooded conditions, Suc levels were reduced in rice water weevil-attacked asLOX roots but not wild-type plants (Fig. 9; Supplemental Fig. S3). Taken together, these results illustrate that jasmonate-deficient lines are not constitutively impoverished in major primary nutrients used by herbivores. On the contrary, nutrient levels were even increased in the hebiba mutant under flooded conditions. asLox plants, by contrast, displayed consistently decreased Suc concentrations under herbivore attack, which suggests that they regulate root primary metabolism in a jasmonate-independent manner.
Figure 8.
Silencing a 13-LOX destabilizes metabolite homeostasis in cucumber beetle-attacked roots. Concentrations of total protein (A and B), soluble sugars (C and D), and free amino acids (E and F) in wild-type and jasmonate-deficient asLOX and hebiba rice root systems under attack by cucumber beetle were measured 7 d postinfestation (n = 8; + se). Plants infested with cucumber beetle were grown under nonflooded conditions. The results of two-way ANOVAs testing for genotype and root treatment effects are shown. Different letters and asterisks indicate significant differences among treatments and genotypes (P < 0.05). AA, Amino acid; D, cucumber beetle; FW, fresh weight; G, genotype; GxD, genotype × cucumber beetle; WT, wild type; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure 9.
Silencing a 13-LOX destabilizes metabolite homeostasis in rice water weevil-attacked roots. Concentrations of total protein (A and B), soluble sugars (C and D), and free amino acids (E and F) in wild-type and jasmonate-deficient asLOX and hebiba rice root systems under attack by rice water weevil under flooded conditions 20 d postinfestation were measured (n = 8; + se). Asterisks indicate significant differences between genotypes (Student’s t test, P < 0.05). For noninfested controls, see Supplemental Figure S3 (separate experiment). AA, Amino acid; FW, fresh weight; WT, wild type; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
Our experiments reveal that root herbivore attack induces jasmonate signaling in the roots and that this response is independent of the flooding status of the soil. In addition, we show that jasmonates improve plant resistance against root-feeding insects, with contrasting effects of two different jasmonate biosynthesis genes on herbivore performance.
Although jasmonates are well known as positive regulators of plant resistance against aboveground herbivores (Howe and Jander, 2008), their role in belowground interactions has not been studied in detail (Rasmann and Agrawal, 2008; Erb et al., 2012a, 2012b). We present three lines of evidence that illustrate that jasmonates also increase root resistance against belowground herbivores. First, the jasmonate pathway was induced by wounding and herbivore attack in the roots. Second, the exogenous application of MeJA strongly increased resistance against root herbivores. Third, jasmonate-deficient rice genotypes were more susceptible to root attack than their wild-type controls. Therefore, our study shows that jasmonates, in addition to protecting rice plants against leaf feeders (Zhou et al., 2009) and root knot nematodes (Nahar et al., 2011), are of vital importance for root herbivore resistance.
Although the role of jasmonates as defense regulators seems to be conserved across different tissues, our results also illustrate a number of root-specific signaling patterns. First, as reported before in other plant systems (Tretner et al., 2008; Hasegawa et al., 2011), the herbivore-induced jasmonate burst was found to be significantly reduced in the roots. Root herbivore attack in this study increased JA by 30% only, whereas rice shoots attacked by the rice striped stem borer Chilo suppressalis, for instance, increase their jasmonate levels 2- to 3-fold within 3 h (Lu et al., 2011; Qi et al., 2011a). Therefore, our study adds to the growing evidence that the herbivore-induced jasmonate burst in roots is attenuated compared with leaves (Erb et al., 2012a, 2012b). A second aspect that suggests root-specific defensive signaling is the fact that, contrary to what is known from herbivore-attacked plant leaves (De Vos et al., 2005; Qi et al., 2011a), no induction of ET or SA was found in herbivore-attacked roots. In leaves, ET acts synergistically with JA to deploy herbivore defenses (Schmelz et al., 2003; von Dahl and Baldwin, 2007; Zhu et al., 2011), and SA negatively regulates JA responses (for review, see Pieterse et al., 2012). Evidently, these two hormones are not deployed in a similar fashion to regulate JA-dependent root responses to herbivory in rice. Given the important role of ET in root branching and architecture (Růzicka et al., 2007), it is possible that plants benefit from stabilizing ET levels to avoid the secondary effects of wounding on essential root processes. The absence of SA induction, however, may boost the deployment of JA-dependent defenses (Gilardoni et al., 2011) and thereby, partially compensate for the lower inducibility of JA in the roots. The fact that jasmonates are, nevertheless, closely linked to the other phytohormones below ground is illustrated by the fact that roots of JA-deficient genotypes had higher SA levels and lower ABA levels under herbivore attack. The antagonism between JA and SA is well documented (Gilardoni et al., 2011; Robert-Seilaniantz et al., 2011; Thaler et al., 2012), but we are not aware of any study showing a direct positive effect of JA on ABA biosynthesis. The fact that this pattern was not consistently present in wounded plants but only occurred under herbivore attack points to a herbivore-specific plant response. This conception is that JA and ABA signaling are integrated at several downstream nodes, including the JA-regulated ABA receptor PYL4 (Lackman et al., 2011) and the MYC2 transcription factor (Kazan and Manners, 2013; Dinh et al., 2013). Evidently, direct interactions between JA and ABA biosynthesis need to be considered as an additional layer of regulation in root signaling in the future. How the constitutive and induced hormonal cross talk between JA, ABA, and SA biosynthesis influences plant-biotic interactions in the rhizosphere, including, for instance, plant colonization by mycorrhizal fungi (Gutjahr and Paszkowski, 2009), remains to be determined.
An important factor that needs to be taken into account when studying defensive signaling in the rhizosphere is that the most variable abiotic factors may be different from those in aboveground environments. Whereas light and temperature vary profoundly above ground, humidity levels vary dramatically in soils between locations and over the growing season (Eagleson, 1978; Stout et al., 2002). Both plants and herbivores have adapted to flooded and nonflooded conditions (Saito et al., 2005; Voesenek and Bailey-Serres, 2013), and the interaction between abiotic and biotic factors may determine the outcome of their interaction (Erb and Lu, 2013). In the case of rice, the jasmonate pathway seems to be remarkably resistant to abiotic disturbance. The wound- and herbivore-induced jasmonate burst was comparable under flooded and nonflooded conditions, and the protective effects of jasmonates were present independent of the humidity level in the soil. This finding illustrates that the massive reprogramming of rice roots under flooded conditions (Lee et al., 2009; Sasidharan et al., 2013) is decoupled from jasmonate signaling, which may enable rice plants to mount a defensive response independently of soil humidity. Similar results were found in the case of arbuscular mycorrhization, which was not directly impacted by flooding but only by flooding-induced changes in root anatomy and architecture (Vallino et al., 2014). Management schemes aiming at reducing damage by rice water weevil (for instance, Way et al., 2006) are, therefore, unlikely to suffer from any unwanted side effects of flooding on plant resistance.
The dramatic plant-mediated effect of MeJA on rice water weevil larval survival indicates the potential of defense elicitors to control one of the most important global rice pests (Hamm et al., 2010). Clearly, manipulation of the jasmonate response before the onset of herbivore attack could result in a significant increase of plant resistance that goes beyond the root herbivory-induced response. An important outstanding question in this context is which defenses in rice are actually regulated by jasmonates. Proteinase inhibitors are important for rice resistance against leaf herbivores (Lu et al., 2011; Qi et al., 2011a). However, we did not detect any proteinase inhibitor activity in induced rice roots (data not shown), suggesting that other defenses are up-regulated by jasmonates. Untargeted metabolite profiling may help to identify these resistance factors in the future (Marti et al., 2013).
Our study reveals a striking discrepancy between feeding activity and herbivore growth in asLOX rice plants. Contrary to what has been documented for the leaves of many plants, including rice, maize, and Nicotiana attenuata (Wang et al., 2008; Zhou et al., 2009; Christensen et al., 2013), both investigated herbivores grew less when feeding on asLOX plants. The effect of OsHI-LOX seems to be independent of JA signaling, because the application of MeJA did not increase herbivore growth and herbivore growth was increased rather than decreased in the hebiba mutant, which has significantly lower JA and JA-Ile levels than the asLOX line. A recent study in Arabidopsis documented that the root-expressed 9-LOX LOX5 positively regulates the growth of the leaf-feeding aphid Myzus persicae (Nalam et al., 2012). Nalam et al. (2012) attributed this positive effect to either the increased supply of 9-hydroxyoctadecadienoic acid, an oxylipin that aphids are not able to synthesize themselves, or other unknown LOX5-mediated physiological changes in the plant that affect aphid susceptibility (Nalam et al., 2012). In the case of cucumber beetle and rice water weevil, a direct negative nutritional effect of reduced 13-LOX activity is unlikely, because linoleic and linolenic acids from the roots should be sufficient to satisfy the dietary lipid needs of the insects (Dadd, 1973), and we did not detect major differences in root fatty acid levels between wild-type and asLOX plants. As an alternative hypothesis, we tested whether asLOX plants are impoverished in other primary metabolites, which may lead to overcompensatory feeding (Raubenheimer and Simpson, 1993) and possibly, self-intoxication (Slansky and Wheeler, 1992). Primary metabolite profiling revealed no striking differences in total protein and free amino acid levels in asLOX plants. However, Suc levels were significantly and specifically reduced in herbivore-attacked asLOX plants. This finding illustrates that OsHI-LOX expression affects the levels of certain primary metabolites under herbivore attack and indicates that the opposing trends in herbivore growth and consumption on asLOX plants may be the result of a (herbivore-induced) reduction in the nutritional quality of rice roots. Whether herbivore growth differences can be explained by Suc alone or whether the levels of other primary metabolites (including, for instance, starch) are the causal factors remains to be determined. Also, the possibility that OsHI-LOX regulates toxic secondary metabolites should be considered. Generally, our findings illustrate that the performance of root herbivores may not necessarily be a good measure for plant resistance, because plants may suffer more root damage, despite lower herbivore performance. This aspect should be taken into account in future studies aiming at understanding the mechanistic basis of herbivore resistance in plants.
In general, jasmonates affected both investigated herbivores in a similar manner, with the exception of herbivore growth on the hebiba mutant, which was increased for cucumber beetle but not rice water weevil. This could point to a certain degree of defense tolerance in the more specialized rice water weevil, although the exogenous application of MeJA strongly affected this herbivore as well. In this context, it is noteworthy that hebiba plants had higher levels of protein, sugars, and free amino acids under flooded conditions but not under nonflooded conditions. It remains unclear how these differences influenced herbivore growth in these experiments. The interpretation of the hebiba-specific results is complicated by the fact that the hebiba mutant contains a 170-bp deletion that comprises other genes apart from AOC (Riemann et al., 2013). Experiments with additional jasmonate mutants, including coleoptile photomorphogenesis2 (cmp2; Riemann et al., 2013), may help to investigate the observed patterns in more detail.
CONCLUSION
Our study illustrates that jasmonates increase root resistance against herbivores and provides first insights, to our knowledge, into root-specific aspects of hormonal defense signaling in plants. Although jasmonates are at the center of defense responses in rice roots, such as in leaves, the phytohormonal response seems to be different in the roots, and additional studies are warranted to understand how the different signaling components influence jasmonate-dependent defenses below ground. Because roots have a different suite of biotic and abiotic influences to respond to than the leaves, it is likely that their hormonal networks have been rewired accordingly.
MATERIAL AND METHODS
Plant Growth
The following rice (Oryza sativa) lines were used for experiments: an asLOX line and its corresponding wild-type Xiushui 11 (Zhou et al., 2009) and the hebiba mutant and its corresponding wild-type Nihonmasari (Riemann et al., 2003). Seeds were germinated in water for 3 d and individually potted in a bottom-pierced plastic pot (diameter of 10 cm and height of 8 cm) with 0.7- to 1.2-mm sand and clay granules. Plants were grown in a greenhouse (26°C ± 2°C, 14-h-light/10-h-dark cycle, 55% relative humidity), daily irrigated with tap water, and fertilized two times per week with 10 mL of 0.1% (w/v) Ferty 3 (Planta Düngemittel; EUFLOR GmbH). Seedlings were transferred to either nonflooded or flooded conditions 15 d after potting. Nonflooded plants were irrigated daily by soaking the bottom of the pots for 2 to 3 h. Flooded plants had their pots submerged in water permanently. For the split-root experiments, rice roots of 14-d-old seedlings were carefully washed and equally divided into two Magenta vessels (width × length × height: 77 × 77 × 97 mm) filled with sand and clay granules. Thirty-day-old plants were used for all experiments.
Insects
Two species of root feeders, the rice-feeding rice water weevil Lissorhoptrus oryzophilus and the generalist cucumber beetle (Diabrotica balteata), were used in the herbivore experiments. Adult rice water weevil were collected from flooded rice fields at the Louisiana State University Agricultural Center Rice Research Station in 2012 and 2013 and maintained in the laboratory as described (Cosme et al., 2011). Neonates were reared and used to infest rice plants. Eggs of cucumber beetle were obtained from an in-house colony. Freshly hatched neonates were kept on germinated maize (Zea mays) seedlings (var Biotop; Maisadour Semences) in a climate chamber (25°C ± 2°C, 14-h-light/10-h-dark cycle, 60% relative humidity) until use.
Plant Treatments
Mechanical wounding was performed by applying manual pressure to the outside of pots 25 times. The manual pressure slightly deformed the pots, which led to friction between the roots and the substrate and resulted in evenly distributed damage across the root system. To standardize damage, treatments were performed by the same person, and all pots were squeezed until their diameter was reduced by approximately 2 cm. Root herbivore infestation was performed under different abiotic conditions according to the lifestyle of herbivores: nonflooded plants were infested with 10 second-instar cucumber beetle larvae, and flooded plants were infested with 10 second-instar rice water weevil larvae. Nonmanipulated plants were used as controls for wounding and herbivore attack. For exogenous phytohormone applications, MeJA, ABA, and ACC were dissolved in 0.2% (v/v) ethanol buffer and supplied to the plants at a final concentration of 100 μm in the growth substrate. Control plants were applied with an equal volume of ethanol buffer.
Quantitative Real-Time PCR
For quantitative real-time PCR (qRT-PCR) analysis, freshly harvested roots were ground into a fine powder in liquid nitrogen, and an aliquot of 100 mg was used for RNA extraction. The rest of the plant material was stored for phytohormone analysis (see below). Total RNA was isolated using the RNeasy Plant Mini Kit (Qiagen) according to the manufacturer’s instructions; 1 mg of total RNA was reverse transcribed using the SuperScript III Reverse Transcriptase (Invitrogen; Life Technologies GmbH). qRT-PCR was performed on Mx3000P (Stratagene) using Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (Agilent Technologies). A rice actin gene, OsACT (The Institute for Genomic Research identification Os03g50885), was used as an internal standard to normalize complementary DNA concentrations. The primers and probes used for qRT-PCR are provided in Table I. Each treatment at each time point was replicated six times.
Analysis of JA, JA-Ile, ABA, SA, and ET
Phytohormone extraction (n = 6–8) was performed by adding 1 mL of ethyl acetate containing 200 ng of D2-JA and 40 ng of D6-ABA, D4-SA, and JA-Ile-13C6 to 100 mg of ground root tissue. All samples were then vortexed for 10 min and centrifuged at 13,000 rpm for 20 min at 4°C. Supernatants were collected and evaporated using a vacuum concentrator to dry at 30°C. Residues were resuspended in 500 μL of MeOH:H2O (70:30, v/v) and centrifuged at 13,000 rpm for 10 min. The supernatants were then collected and measured using an API 3200 high-performance liquid chromatography-tandem mass spectrometry System (Applied Biosystems) as previously described (Vadassery et al., 2012). To measure ET, freshly harvested rice roots were weighed and immediately incubated in a 2-mL rubber-capped vial for 2 h at room temperature. ET concentrations (n = 6) were analyzed using an ETD 300 Ethylene Analyzer (Sensor Sense) using a stop and flow approach according to the instruction manual. Flow rate was maintained at 2 L h−1, and the period of measurement was set for 10 min. Note that ET was not measured in flooded plants, because the system is not suited to operate under high-humidity (i.e. flooded) conditions.
Determination of Total Protein, Free Amino Acids, Fatty Acids, and Soluble Sugars
Total protein extraction and determination were performed using an adapted Bradford assay as described (n = 8; van Dam et al., 2001). To determine free amino acids and soluble sugars, 100 mg of ground root tissue (n = 8) was extracted by adding 1 mL of water and vortexing for 10 min. After centrifugation at 13,000 rpm for 20 min at 4°C, 50 μL of supernatant was transferred into 450 μL of Millar-Q water spiked with internal standards of amino acids and analyzed by API 3200 as described by Docimo et al. (2012). Meanwhile, 50 μL of supernatant was transferred into 450 μL of Millar-Q water for soluble sugar measurement. Samples were analyzed using the API 3200 high-performance liquid chromatography-tandem mass spectrometry as described (Falk et al., 2014). In a separate experiment, we measured fatty acid concentrations in control and cucumber beetle-infested wild-type and asLOX roots 7 d postinfestation (n = 9–10). Fatty acid methyl esters (FAMEs) were prepared from 25 mg of rice roots ground in liquid nitrogen with a protocol adapted from Li et al. (2006). Briefly, frozen samples were resuspended in 1 mL of 5% (v/v) H2SO4 in MeOH containing butylated hydroxytoluene (50 µg per sample), and glyceryl triheptadecanoate (12.5 µg per sample; Sigma) was used as an internal standard. Transesterification was carried out at 85°C for 60 min in a dry block (digital heat block; VWR) and neutralized after cooling with 1.5 mL of 0.9% (w/v) NaCl. FAMEs were extracted three times with 2 mL of hexane and resuspended in 100 µL of heptane. FAMEs (2 µL; split ratio 1:25) were separated with a gas chromatograph (7890A; Agilent Technologies) equipped with a DB-23 capillary column (30 m × 0.25 mm × 0.25 µm; Agilent Technologies). FAMEs were quantified relative to the internal standard, and FAME relative response factors were determined from a four-level calibration curve (r2 > 0.999).
Herbivore Performance
Several independent experiments were conducted to determine cucumber beetle and rice water weevil performance and feeding under nonflooded and flooded conditions. We evaluated the effects of phytohormones on herbivore performance. To this end, nonflooded and flooded Xiushui 11 plants were treated by applying ABA, ACC, MeJA, or buffer solution as described above. Twenty-four hours later, 10 freshly hatched neonates of cucumber beetle or rice water weevil were placed onto the sand surface and allowed to feed on the roots of individual seedlings. Hormone-treated plants without herbivore infestation were used as controls. Final herbivore mass, numbers of surviving larvae, and fresh weight of rice roots were measured 7 and 20 d after cucumber beetle or rice water weevil infestation, respectively (n = 12). The time intervals were chosen to account for the different developmental rates of the two herbivores. Roots of control and cucumber beetle-infested plants were weighed after the experiment. To determine whether MeJA has direct negative effects on rice water weevil survival, six first-instar larvae were incubated in a 10-mL test tube containing 2 mL of MeJA or buffer solution as described above for 48 h. The number of surviving larvae in each tube was counted at 12, 24, and 48 h after the start of incubation (n = 8). Furthermore, we grew wild-type plants (genotype Nihonmasari) in a split-root setup, in which either the local or the systemic side was treated with MeJA followed by local rice water weevil infestation. Growth and survival of the root herbivore were measured as above. In a second series of experiments, we determined herbivore performance on the two JA-deficient rice lines and their corresponding controls. Herbivore and plant performances were determined as described above (n = 10). We also measured rice water weevil performance on the hebiba and Nihonmasari genotypes using the split-root system. Plants were treated with a buffer control solution, infested with rice water weevil on the local side, treated with exogenous MeJA on the local side, treated with both MeJA and rice water weevil on the local side, or treated with MeJA on the local side and rice water weevil on the systemic side. After 20 d of infestation, root mass was determined (n = 10).
Data Analysis
Data were analyzed using SigmaPlot 12.0 (http://www.sigmaplot.com/). Multiple comparisons were analyzed by ANOVAs followed by Duncan’s multiple-range test. Differences between two rice genotypes were analyzed by Student’s t tests. The normality of data was tested using the Kolmogorov-Smirnov test (P < 0.05), and the equality of error variances was tested by Levene’s test (P < 0.05). In the case of nonnormality and/or unequal variances, data were transformed before ANOVAs.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Jasmonates regulate salicylic and abscisic acid in the roots of root herbivore-attacked plants.
Supplemental Figure S2. Comparison of root biomass on wild-type and JA-deficient hebiba plants under flooded conditions.
Supplemental Figure S3. Constitutive soluble sugars in wild-type or JA-deficient asLOX and hebiba plants under flooded conditions.
Supplemental Figure S4. Fatty acid concentrations in control and cucumber beetle-infested wild-type and asLOX roots.
Supplementary Material
Acknowledgments
We thank Stefan Meldau for constructive comments on an earlier version of this article, Michael Reichelt for providing assistance for the primary metabolite analyses, and Dominique Darimont for helping with RNA extraction.
Glossary
- ABA
abscisic acid
- ACC
aminocyclopropane-1-carboxylic acid
- ET
ethylene
- FAME
fatty acid methyl ester
- JA
jasmonic acid
- JA-Ile
jasmonic acid Ile conjugate
- MeJA
methyl jasmonic acid
- OPDA
12-oxophytodienoic acid
- qRT-PCR
quantitative real-time PCR
- SA
salicylic acid
Footnotes
This work was supported by the Max Planck Society and a Marie Curie Intra-European Fellowship (273107 to M.E.).
References
- Abe H, Tateishi K, Seo S, Kugimiya S, Hirai MY, Sawada Y, Murata Y, Yara K, Shimoda T, Kobayashi M (2013) Disarming the jasmonate-dependent plant defense makes nonhost Arabidopsis plants accessible to the American serpentine leafminer. Plant Physiol 163: 1242–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin IT. (1989) Mechanism of damage-induced alkaloid production in wild tobacco. J Chem Ecol 15: 1661–1680 [DOI] [PubMed] [Google Scholar]
- Bergelson J, Purrington CB, Palm CJ, López-Gutiérrez JC (1996) Costs of resistance: a test using transgenic Arabidopsis thaliana. Proc Biol Sci 263: 1659–1663 [DOI] [PubMed] [Google Scholar]
- Bonaventure G, Schuck S, Baldwin IT (2011) Revealing complexity and specificity in the activation of lipase-mediated oxylipin biosynthesis: a specific role of the Nicotiana attenuata GLA1 lipase in the activation of jasmonic acid biosynthesis in leaves and roots. Plant Cell Environ 34: 1507–1520 [DOI] [PubMed] [Google Scholar]
- Castonguay Y, Nadeau P, Simard RR (1993) Effects of flooding on carbohydrate and ABA levels in roots and shoots of alfalfa. Plant Cell Environ 16: 695–702 [Google Scholar]
- Christensen SA, Nemchenko A, Borrego E, Murray I, Sobhy IS, Bosak L, DeBlasio S, Erb M, Robert CAM, Vaughn KA, et al. (2013) The maize lipoxygenase, ZmLOX10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J 74: 59–73 [DOI] [PubMed] [Google Scholar]
- Cosme M, Stout MJ, Wurst S (2011) Effect of arbuscular mycorrhizal fungi (Glomus intraradices) on the oviposition of rice water weevil (Lissorhoptrus oryzophilus). Mycorrhiza 21: 651–658 [DOI] [PubMed] [Google Scholar]
- Dadd RH. (1973) Insect nutrition: current developments and metabolic implications. Annu Rev Entomol 18: 381–420 [DOI] [PubMed] [Google Scholar]
- De Vos M, Van Oosten VR, Van Poecke RM, Van Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Métraux JP, Van Loon LC, Dicke M, et al. (2005) Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microbe Interact 18: 923–937 [DOI] [PubMed] [Google Scholar]
- Dinh ST, Baldwin IT, Galis I (2013) The HERBIVORE ELICITOR-REGULATED1 gene enhances abscisic acid levels and defenses against herbivores in Nicotiana attenuata plants. Plant Physiol 162: 2106–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docimo T, Reichelt M, Schneider B, Kai M, Kunert G, Gershenzon J, D’Auria JC (2012) The first step in the biosynthesis of cocaine in Erythroxylum coca: the characterization of arginine and ornithine decarboxylases. Plant Mol Biol 78: 599–615 [DOI] [PubMed] [Google Scholar]
- Eagleson PS. (1978) Climate, soil, and vegetation. 6. Dynamics of the annual water balance. Water Resour Res 14: 749–764 [Google Scholar]
- Erb M, Balmer D, De Lange ES, Von Merey G, Planchamp C, Robert CA, Röder G, Sobhy I, Zwahlen C, Mauch-Mani B, et al. (2011) Synergies and trade-offs between insect and pathogen resistance in maize leaves and roots. Plant Cell Environ 34: 1088–1103 [DOI] [PubMed] [Google Scholar]
- Erb M, Glauser G, Robert CA (2012a) Induced immunity against belowground insect herbivores- activation of defenses in the absence of a jasmonate burst. J Chem Ecol 38: 629–640 [DOI] [PubMed] [Google Scholar]
- Erb M, Lu J (2013) Soil abiotic factors influence interactions between belowground herbivores and plant roots. J Exp Bot 64: 1295–1303 [DOI] [PubMed] [Google Scholar]
- Erb M, Meldau S, Howe GA (2012b) Role of phytohormones in insect-specific plant reactions. Trends Plant Sci 17: 250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk KL, Kästner J, Bodenhausen N, Schramm K, Paetz C, Vassão DG, Reichelt M, von Knorre D, Bergelson J, Erb M, et al. (2014) The role of glucosinolates and the jasmonic acid pathway in resistance of Arabidopsis thaliana against molluscan herbivores. Mol Ecol 23: 1188–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Dubugnon L (2009) Detritivorous crustaceans become herbivores on jasmonate-deficient plants. Proc Natl Acad Sci USA 106: 935–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA (1992) Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell 4: 129–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R (2009) (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol 5: 344–350 [DOI] [PubMed] [Google Scholar]
- Gilardoni PA, Hettenhausen C, Baldwin IT, Bonaventure G (2011) Nicotiana attenuata LECTIN RECEPTOR KINASE1 suppresses the insect-mediated inhibition of induced defense responses during Manduca sexta herbivory. Plant Cell 23: 3512–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser G, Dubugnon L, Mousavi SAR, Rudaz S, Wolfender JL, Farmer EE (2009) Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J Biol Chem 284: 34506–34513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guranowski A, Miersch O, Staswick PE, Suza W, Wasternack C (2007) Substrate specificity and products of side-reactions catalyzed by jasmonate:amino acid synthetase (JAR1). FEBS Lett 581: 815–820 [DOI] [PubMed] [Google Scholar]
- Gutjahr C, Paszkowski U (2009) Weights in the balance: jasmonic acid and salicylic acid signaling in root-biotroph interactions. Mol Plant Microbe Interact 22: 763–772 [DOI] [PubMed] [Google Scholar]
- Hamberg M, Fahlstadius P (1990) Allene oxide cyclase: a new enzyme in plant lipid metabolism. Arch Biochem Biophys 276: 518–526 [DOI] [PubMed] [Google Scholar]
- Hamm JC, Stout MJ, Riggio RM (2010) Herbivore- and elicitor-induced resistance in rice to the rice water weevil (Lissorhoptrus oryzophilus Kuschel) in the laboratory and field. J Chem Ecol 36: 192–199 [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Sogabe Y, Asano T, Nakagawa T, Nakamura H, Kodama H, Ohta H, Yamaguchi K, Mueller MJ, Nishiuchi T (2011) Gene expression analysis of wounding-induced root-to-shoot communication in Arabidopsis thaliana. Plant Cell Environ 34: 705–716 [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Kisseleva L, Sawa S, Furukawa T, Komatsu S, Koshiba T (2004) A novel rice PR10 protein, RSOsPR10, specifically induced in roots by biotic and abiotic stresses, possibly via the jasmonic acid signaling pathway. Plant Cell Physiol 45: 550–559 [DOI] [PubMed] [Google Scholar]
- Helliwell EE, Wang Q, Yang Y (2013) Transgenic rice with inducible ethylene production exhibits broad-spectrum disease resistance to the fungal pathogens Magnaporthe oryzae and Rhizoctonia solani. Plant Biotechnol J 11: 33–42 [DOI] [PubMed] [Google Scholar]
- Hoback WW, Clark TL, Meinke LJ, Higley LG, Scalzitti JM (2002) Immersion survival differs among three Diabrotica species. Entomol Exp Appl 105: 29–34 [Google Scholar]
- Howe GA, Jander G (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59: 41–66 [DOI] [PubMed] [Google Scholar]
- Hunter MD. (2001) Out of sight, out of mind: the impacts of root-feeding insects in natural and managed systems. Agric For Entomol 3: 3–9 [Google Scholar]
- Hwang SG, Chen HC, Huang WY, Chu YC, Shii CT, Cheng WH (2010) Ectopic expression of rice OsNCED3 in Arabidopsis increases ABA level and alters leaf morphology. Plant Sci 178: 12–22 [Google Scholar]
- Johnson SN, Gregory PJ, McNicol JW, Oodally Y, Zhang X, Murray PJ (2010) Effects of soil conditions and drought on egg hatching and larval survival of the clover root weevil (Sitona lepidus). Appl Soil Ecol 44: 75–79 [Google Scholar]
- Johnson SN, Nielsen UN (2012) Foraging in the dark - chemically mediated host plant location by belowground insect herbivores. J Chem Ecol 38: 604–614 [DOI] [PubMed] [Google Scholar]
- Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA (2008) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci USA 105: 7100–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan K, Manners JM (2013) MYC2: the master in action. Mol Plant 6: 686–703 [DOI] [PubMed] [Google Scholar]
- Lackman P, González-Guzmán M, Tilleman S, Carqueijeiro I, Pérez AC, Moses T, Seo M, Kanno Y, Häkkinen ST, Van Montagu MC, et al. (2011) Jasmonate signaling involves the abscisic acid receptor PYL4 to regulate metabolic reprogramming in Arabidopsis and tobacco. Proc Natl Acad Sci USA 108: 5891–5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Chen PW, Lu CA, Chen S, Ho TH, Yu SM (2009) Coordinated responses to oxygen and sugar deficiency allow rice seedlings to tolerate flooding. Sci Signal 2: ra61. [DOI] [PubMed] [Google Scholar]
- Li C, Liu G, Xu C, Lee GI, Bauer P, Ling HQ, Ganal MW, Howe GA (2003) The tomato Suppressor of prosystemin-mediated responses2 gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal for defense gene expression. Plant Cell 15: 1646–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Syvertsen JP, McCoy CW, Stuart RJ, Schumann AW (2006) Water stress and root injury from simulated flooding and Diaprepes abbreviatus root weevil larval feeding in citrus. Soil Sci 171: 138–151 [Google Scholar]
- Lu J, Ju H, Zhou G, Zhu C, Erb M, Wang X, Wang P, Lou Y (2011) An EAR-motif-containing ERF transcription factor affects herbivore-induced signaling, defense and resistance in rice. Plant J 68: 583–596 [DOI] [PubMed] [Google Scholar]
- Machado RA, Ferrieri AP, Robert CAM, Glauser G, Kallenbach M, Baldwin IT, Erb M (2013) Leaf-herbivore attack reduces carbon reserves and regrowth from the roots via jasmonate and auxin signaling. New Phytol 200: 1234–1246 [DOI] [PubMed] [Google Scholar]
- Mafli A, Goudet J, Farmer EE (2012) Plants and tortoises: mutations in the Arabidopsis jasmonate pathway increase feeding in a vertebrate herbivore. Mol Ecol 21: 2534–2541 [DOI] [PubMed] [Google Scholar]
- Marti G, Erb M, Boccard J, Glauser G, Doyen GR, Villard N, Robert CAM, Turlings TC, Rudaz S, Wolfender JL (2013) Metabolomics reveals herbivore-induced metabolites of resistance and susceptibility in maize leaves and roots. Plant Cell Environ 36: 621–639 [DOI] [PubMed] [Google Scholar]
- McConn M, Creelman RA, Bell E, Mullet JE, Browse J (1997) Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci USA 94: 5473–5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldau S, Erb M, Baldwin IT (2012) Defence on demand: mechanisms behind optimal defence patterns. Ann Bot (Lond) 110: 1503–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar K, Kyndt T, De Vleesschauwer D, Höfte M, Gheysen G (2011) The jasmonate pathway is a key player in systemically induced defense against root knot nematodes in rice. Plant Physiol 157: 305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalam VJ, Keeretaweep J, Sarowar S, Shah J (2012) Root-derived oxylipins promote green peach aphid performance on Arabidopsis foliage. Plant Cell 24: 1643–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PaDIL PH-PaDIL (2013) Banded Cucumber Beetle, Diabrotica balteata Leconte (Coleoptera: Chrysomelidae: Galerucinae). http://www.padil.gov.au/pests-and-diseases/Pest/Main/135549 (October 22, 2013)
- Pierre PS, Dugravot S, Cortesero AM, Poinsot D, Raaijmakers CE, Hassan HM, van Dam NM (2012) Broccoli and turnip plants display contrasting responses to belowground induction by Delia radicum infestation and phytohormone applications. Phytochemistry 73: 42–50 [DOI] [PubMed] [Google Scholar]
- Pieterse CM, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SC (2012) Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol 28: 489–521 [DOI] [PubMed] [Google Scholar]
- Pieterse CM, Leon-Reyes A, Van der Ent S, Van Wees SC (2009) Networking by small-molecule hormones in plant immunity. Nat Chem Biol 5: 308–316 [DOI] [PubMed] [Google Scholar]
- Puthoff DP, Smigocki AC (2007) Insect feeding-induced differential expression of Beta vulgaris root genes and their regulation by defense-associated signals. Plant Cell Rep 26: 71–84 [DOI] [PubMed] [Google Scholar]
- Qi J, Zhou G, Yang L, Erb M, Lu Y, Sun X, Cheng J, Lou Y (2011a) The chloroplast-localized phospholipases D α4 and α5 regulate herbivore-induced direct and indirect defenses in rice. Plant Physiol 157: 1987–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi T, Song S, Ren Q, Wu D, Huang H, Chen Y, Fan M, Peng W, Ren C, Xie D (2011b) The Jasmonate-ZIM-domain proteins interact with the WD-Repeat/bHLH/MYB complexes to regulate Jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell 23: 1795–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmann S, Agrawal AA (2008) In defense of roots: a research agenda for studying plant resistance to belowground herbivory. Plant Physiol 146: 875–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raubenheimer D, Simpson S (1993) The geometry of compensatory feeding in the locust. Anim Behav 45: 953–964 [Google Scholar]
- Riemann M, Haga K, Shimizu T, Okada K, Ando S, Mochizuki S, Nishizawa Y, Yamanouchi U, Nick P, Yano M, et al. (2013) Identification of rice Allene Oxide Cyclase mutants and the function of jasmonate for defence against Magnaporthe oryzae. Plant J 74: 226–238 [DOI] [PubMed] [Google Scholar]
- Riemann M, Muller A, Korte A, Furuya M, Weiler EW, Nick P (2003) Impaired induction of the jasmonate pathway in the rice mutant hebiba. Plant Physiol 133: 1820–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann M, Riemann M, Takano M (2008) Rice JASMONATE RESISTANT 1 is involved in phytochrome and jasmonate signalling. Plant Cell Environ 31: 783–792 [DOI] [PubMed] [Google Scholar]
- Ritchie ME. (2000) Nitrogen limitation and trophic vs. abiotic influences on insect herbivores in a temperate grassland. Ecology 81: 1601–1612 [Google Scholar]
- Robert CA, Erb M, Hiltpold I, Hibbard BE, Gaillard MD, Bilat J, Degenhardt J, Cambet-Petit-Jean X, Turlings TC, Zwahlen C (2013) Genetically engineered maize plants reveal distinct costs and benefits of constitutive volatile emissions in the field. Plant Biotechnol J 11: 628–639 [DOI] [PubMed] [Google Scholar]
- Robert-Seilaniantz A, Grant M, Jones JDG (2011) Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu Rev Phytopathol 49: 317–343 [DOI] [PubMed] [Google Scholar]
- Růzicka K, Ljung K, Vanneste S, Podhorská R, Beeckman T, Friml J, Benková E (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19: 2197–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Hirai K, Way MO (2005) The rice water weevil, Lissorhoptrus oryzophilus Kuschel (Coleoptera: curculionidae). Appl Entomol Zool (Jpn) 40: 31–39 [Google Scholar]
- Sasidharan R, Mustroph A, Boonman A, Akman M, Ammerlaan AM, Breit T, Schranz ME, Voesenek LA, van Tienderen PH (2013) Root transcript profiling of two Rorippa species reveals gene clusters associated with extreme submergence tolerance. Plant Physiol 163: 1277–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller F, Biesgen C, Müssig C, Altmann T, Weiler EW (2000) 12-Oxophytodienoate reductase 3 (OPR3) is the isoenzyme involved in jasmonate biosynthesis. Planta 210: 979–984 [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Alborn HT, Tumlinson JH (2003) Synergistic interactions between volicitin, jasmonic acid and ethylene mediate insect-induced volatile emission in Zea mays. Physiol Plant 117: 403–412 [DOI] [PubMed] [Google Scholar]
- Schneider K, Kienow L, Schmelzer E, Colby T, Bartsch M, Miersch O, Wasternack C, Kombrink E, Stuible HP (2005) A new type of peroxisomal acyl-coenzyme A synthetase from Arabidopsis thaliana has the catalytic capacity to activate biosynthetic precursors of jasmonic acid. J Biol Chem 280: 13962–13972 [DOI] [PubMed] [Google Scholar]
- Schweizer F, Fernández-Calvo P, Zander M, Diez-Diaz M, Fonseca S, Glauser G, Lewsey MG, Ecker JR, Solano R, Reymond P (2013) Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 25: 3117–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slansky F, Wheeler GS (1992) Caterpillars' compensatory feeding response to diluted nutrients leads to toxic allelochemical dose. Entomol Exp Appl 65: 171–186 [Google Scholar]
- Stout MJ, Stout MJ, Rice WC, Ring DR (2002) The influence of plant age on tolerance of rice to injury by the rice water weevil, Lissorhoptrus oryzophilus (Coleoptera: Curculionidae). Bull Entomol Res 92: 177–184 [DOI] [PubMed] [Google Scholar]
- Svyatyna K, Jikumaru Y, Brendel R, Reichelt M, Mithöfer A, Takano M, Kamiya Y, Nick P, Riemann M (2014) Light induces jasmonate-isoleucine conjugation via OsJAR1-dependent and -independent pathways in rice. Plant Cell Environ 37: 827–839 [DOI] [PubMed] [Google Scholar]
- Thaler JS, Humphrey PT, Whiteman NK (2012) Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci 17: 260–270 [DOI] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J (2007) JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448: 661–665 [DOI] [PubMed] [Google Scholar]
- Tretner C, Huth U, Hause B (2008) Mechanostimulation of Medicago truncatula leads to enhanced levels of jasmonic acid. J Exp Bot 59: 2847–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat TO, Verhoeven KJ, Jansen JJ, Raaijmakers CE, Bakx-Schotman T, McIntyre LM, van der Putten WH, Biere A, van Dam NM (2013) Plants know where it hurts: root and shoot jasmonic acid induction elicit differential responses in Brassica oleracea. PLoS ONE 8: e65502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadassery J, Reichelt M, Hause B, Gershenzon J, Boland W, Mithöfer A (2012) CML42-mediated calcium signaling coordinates responses to Spodoptera herbivory and abiotic stresses in Arabidopsis. Plant Physiol 159: 1159–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallino M, Fiorilli V, Bonfante P (2014) Rice flooding negatively impacts root branching and arbuscular mycorrhizal colonization, but not fungal viability. Plant Cell Environ 37: 557–572 [DOI] [PubMed] [Google Scholar]
- van Dam NM, Horn M, Mares M, Baldwin IT (2001) Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. J Chem Ecol 27: 547–568 [DOI] [PubMed] [Google Scholar]
- Van der Does D, Leon-Reyes A, Koornneef A, Van Verk MC, Rodenburg N, Pauwels L, Goossens A, Körbes AP, Memelink J, Ritsema T, et al. (2013) Salicylic acid suppresses jasmonic acid signaling downstream of SCFCOI1-JAZ by targeting GCC promoter motifs via transcription factor ORA59. Plant Cell 25: 744–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek L, Banga M, Thier RH, Mudde CM, Harren F, Barendse G, Blom C (1993) Submergence-induced ethylene synthesis, entrapment, and growth in two plant species with contrasting flooding resistances. Plant Physiol 103: 783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LA, Bailey-Serres J (2013) Flooding tolerance: O2 sensing and survival strategies. Curr Opin Plant Biol 16: 647–653 [DOI] [PubMed] [Google Scholar]
- von Dahl CC, Baldwin IT (2007) Deciphering the role of ethylene in plant-herbivore interactions. J Plant Growth Regul 26: 201–209 [Google Scholar]
- Wang L, Allmann S, Wu J, Baldwin IT (2008) Comparisons of LIPOXYGENASE3- and JASMONATE-RESISTANT4/6-silenced plants reveal that jasmonic acid and jasmonic acid-amino acid conjugates play different roles in herbivore resistance of Nicotiana attenuata. Plant Physiol 146: 904–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way MO, Reay-Jones FP, Stout MJ, Tarpley L (2006) Effects of nitrogen fertilizer applied before permanent flood on the interaction between rice and rice water weevil (Coleoptera: Curculionidae). J Econ Entomol 99: 2030–2037 [DOI] [PubMed] [Google Scholar]
- Yuan Y, Chung JD, Fu X, Johnson VE, Ranjan P, Booth SL, Harding SA, Tsai CJ (2009) Alternative splicing and gene duplication differentially shaped the regulation of isochorismate synthase in Populus and Arabidopsis. Proc Natl Acad Sci USA 106: 22020–22025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Qi J, Ren N, Cheng J, Erb M, Mao B, Lou Y (2009) Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J 60: 638–648 [DOI] [PubMed] [Google Scholar]
- Zhu Z, An F, Feng Y, Li P, Xue L, A M, Jiang Z, Kim JM, To TK, Li W, et al. (2011) Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc Natl Acad Sci USA 108: 12539–12544 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.