Three transcription factors form a sequential transcriptional regulatory cascade which is involved in rice response to the infection of Magnaporthe oryzae.
Abstract
Blast caused by fungal Magnaporthe oryzae is a devastating disease of rice (Oryza sativa) worldwide, and this fungus also infects barley (Hordeum vulgare). At least 11 rice WRKY transcription factors have been reported to regulate rice response to M. oryzae either positively or negatively. However, the relationships of these WRKYs in the rice defense signaling pathway against M. oryzae are unknown. Previous studies have revealed that rice WRKY13 (as a transcriptional repressor) and WRKY45-2 enhance resistance to M. oryzae. Here, we show that rice WRKY42, functioning as a transcriptional repressor, suppresses resistance to M. oryzae. WRKY42-RNA interference (RNAi) and WRKY42-overexpressing (oe) plants showed increased resistance and susceptibility to M. oryzae, accompanied by increased or reduced jasmonic acid (JA) content, respectively, compared with wild-type plants. JA pretreatment enhanced the resistance of WRKY42-oe plants to M. oryzae. WRKY13 directly suppressed WRKY42. WRKY45-2, functioning as a transcriptional activator, directly activated WRKY13. In addition, WRKY13 directly suppressed WRKY45-2 by feedback regulation. The WRKY13-RNAi WRKY45-2-oe and WRKY13-oe WRKY42-oe double transgenic lines showed increased susceptibility to M. oryzae compared with WRKY45-2-oe and WRKY13-oe plants, respectively. These results suggest that the three WRKYs form a sequential transcriptional regulatory cascade. WRKY42 may negatively regulate rice response to M. oryzae by suppressing JA signaling-related genes, and WRKY45-2 transcriptionally activates WRKY13, whose encoding protein in turn transcriptionally suppresses WRKY42 to regulate rice resistance to M. oryzae.
Plants have evolved sophisticated mechanisms to respond to pathogen invasion. The plant immune system is considered to have four components: pathogen recognition, signal transduction, downstream defense-related gene activation, and cross talk among different signaling pathways (Helliwell and Yang, 2013). Many genes are involved in this defense system and can be divided into two classes, the receptor genes and the defense-responsive genes (Kou and Wang, 2010). The receptor genes include race-specific disease resistance (R) genes and host pattern recognition receptor (PRR) genes. The encoding proteins of defense-responsive genes function downstream of R or PRR proteins as either activators or repressors in defense responses. Plant-pathogen interaction usually results in transcriptional reprogramming of a large number of defense-responsive genes (Eulgem, 2005). This indicates that transcriptional regulators play crucial roles in pathogen-induced defense responses.
Different types of transcription factors have been reported to be involved in plant-pathogen interactions, which include auxin/indole acetic acid, basic-domain Leu-zipper, ethylene-responsive element binding, MYB, NAC (for no apical meristem [NAM], Arabidopsis [Arabidopsis thaliana] transcription activation factor [ATAF], and cup-shaped cotyledon [CUC]), Whirly, and WRKY (Singh et al., 2002; Desveaux et al., 2005; Qu and Zhu, 2006; Eulgem and Somssich, 2007; Kazan and Manners, 2009; Puranik et al., 2012). As one of the largest transcription factor families in plants, WRKY transcription factors, which bind to W-box (W) or W-like box-type cis elements or other cis elements, such as the barley (Hordeum vulgare) sugar-responsive element (core sequence: AA/TAA) and rice (Oryza sativa) pathogen response element4 element (TACTGCGCTTAGT; Sun et al., 2003; Cai et al., 2008; Yuan and Wang, 2012), play important roles in plant-pathogen interactions. Loss-of-function and gain-of-function studies have demonstrated that WRKY transcription factors act in a complex signaling network as both positive and negative regulators of disease resistance (Chen and Chen, 2002; Li et al., 2004; Zheng et al., 2006; Eulgem and Somssich, 2007; Oh et al., 2008). WRKY proteins function as either transcriptional activators or repressors (Pandey and Somssich, 2009). Interestingly, some WRKY genes carry a set of W or W-like boxes in their own promoters, suggesting the complex interaction of these transcription factors with each other (Eulgem and Somssich, 2007; Qiu et al., 2009; Tao et al., 2009). In addition, some WRKY proteins can bind to their own promoters to regulate their own gene expression (Robatzek and Somssich, 2002; Pandey and Somssich, 2009; Xiao et al., 2013). The autoregulation or cross regulation of different WRKY members may ensure quick and efficient defense signaling amplification (Eulgem and Somssich, 2007).
The rice WRKY gene family consists of 98 to 102 paralogs (Ross et al., 2007). Systematic analyses of WRKY gene expression in rice-pathogen interactions have revealed that many WRKY genes are rapidly induced or repressed upon pathogen infection, suggesting that these WRKY genes may contribute to the regulation of rice response to pathogen infection (Ryu et al., 2006; Bagnaresi et al., 2012; Wei et al., 2013). In addition, 13 rice WRKY genes from 12 loci have been characterized to be involved in rice-pathogen interactions. Among the 13 genes, 11 are involved in the interaction of rice with Magnaporthe oryzae, which causes blast diseases not only in rice but also in barley (Li and Wang, 2013). Among the 11 genes, WRKY13, WRKY22, WRKY30, WRKY45-1 (named WRKY45 in Shimono et al., 2007), WRKY45-2, WRKY47, WRKY53, WRKY55/WRKY31, and WRKY104/WRKY89 positively regulate rice resistance to M. oryzae, whereas WRKY28 and WRKY76 negatively regulate rice resistance to M. oryzae (Cheng and Wang, 2014). Rice WRKY13 is a quantitative trait locus that also confers resistance to Xanthomonas oryzae pv oryzae (Xoo), which causes bacterial blight disease (Qiu et al., 2007, 2008; Hu et al., 2008). In addition, WRKY45-2 also promotes rice resistance to Xoo and X. oryzae pv oryzicola (Xoc), which causes bacterial streak disease (Tao et al., 2009). These results suggest that WRKYs play important roles in rice response to M. oryzae, and some of these WRKYs are also involved in rice response to the infection of other pathogens.
However, the relationship of different WRKYs in the rice defense response to the same pathogen or the positions of different WRKYs in the rice defense signaling pathway to the same pathogen remain largely unknown. In this respect, WRKY13 is relatively more intensively studied than other characterized WRKY genes involved in rice-pathogen interactions. WRKY13 is a transcriptional repressor (Xiao et al., 2013). It can bind to the promoters of both WRKY45-1/WRKY45 and WRKY45-2, which are alleles encoding proteins with a 10-amino acid difference, in the rice-Xoo interaction, suggesting that WRKY13 may regulate the functions of the WRKY45 alleles in defense signaling (Tao et al., 2009; Xiao et al., 2013). WRKY45-1/WRKY45 is a transcriptional activator (Shimono et al., 2007), but the transcription function of WRKY45-2 is unknown. Activation of WRKY13 transcriptionally influenced a set of WRKY genes, including WRKY42, in rice resistance to Xoo; in addition, the WRKY13 protein binds to the WRKY42 promoter in yeast (Saccharomyces cerevisiae) cells (Qiu et al., 2009).
To elucidate whether WRKY42 is a player in rice disease resistance, and to elucidate its relationship with WRKY13 and WRKY45-2 in defense signaling, we modulated WRKY42 expression and generated WRKY13 WRKY42 and WRKY13 WRKY45-2 double transgenic lines. Our results show that WRKY42 negatively regulates rice resistance to M. oryzae by functioning downstream of WRKY13 and WRKY45-2 in the defense signaling pathway. WRKY45-2 WRKY13 WRKY42 forms a transcriptional regulatory cascade to regulate rice-M. oryzae interaction.
RESULTS
Fungal and Bacterial Pathogen Infection Influenced WRKY42 Expression
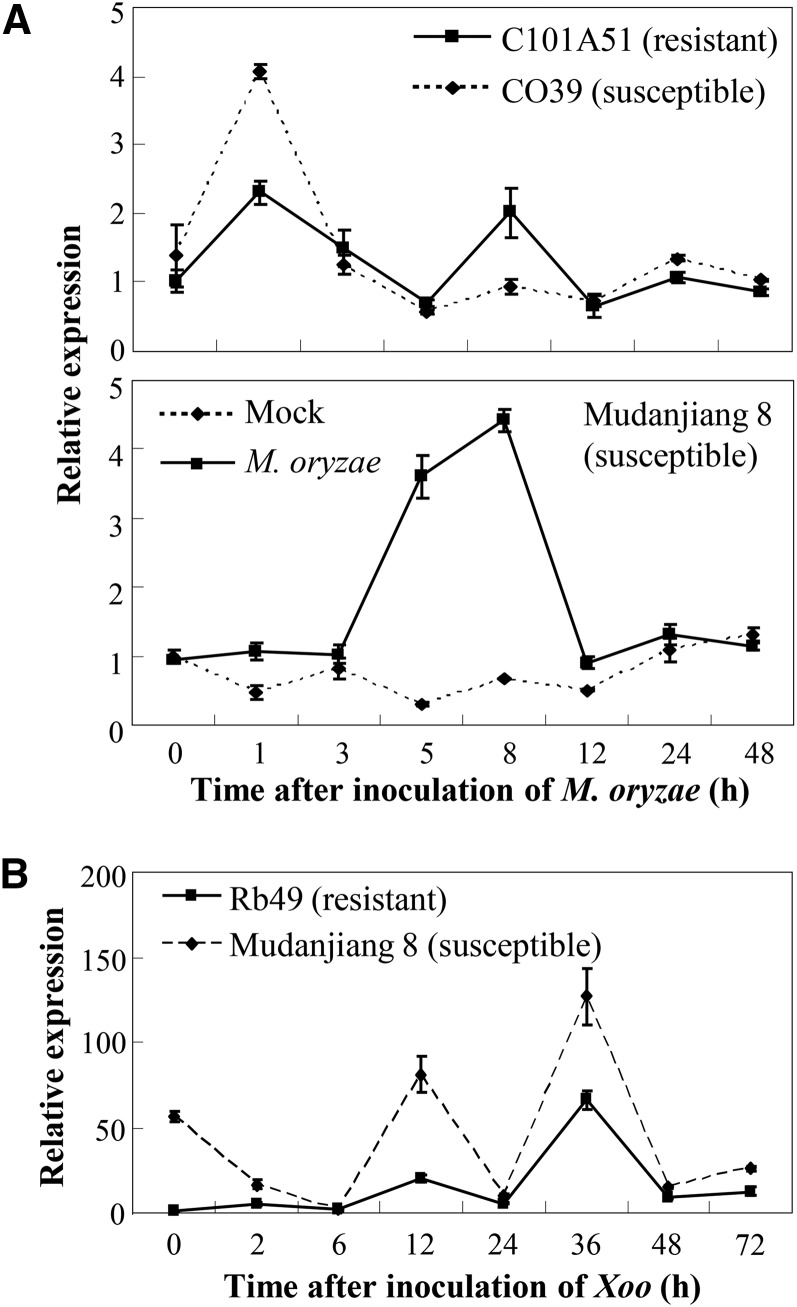
To study the potential function of WRKY42 in the rice defense response to the infection of fungal pathogen M. oryzae and bacterial pathogen Xoo, we first examined the expression patterns of WRKY42 in two pairs of resistant and susceptible rice lines, with each pair having the same genetic background. The first pair of rice lines was C101A51 and CO39. C101A51 carrying the R gene Pi2 was resistant (disease index 5.6 ± 3.9) to M. oryzae isolate Enshi2-2 (N2-2), whereas CO39 was highly susceptible to N2-2 (disease index 80.6 ± 9.6). N2-2 infection influenced WRKY42 expression (Fig. 1A). Its expression was rapidly induced in both resistant and susceptible plants at 1 h after inoculation. However, the expression level of WRKY42 was approximately 2-fold higher in susceptible CO39 than in resistant C101A51 at 1 h after inoculation. Furthermore, WRKY42 expression showed a second induction at 8 h after inoculation in C101A51 but not in CO39, which might be due to the R gene Pi2. We further examined WRKY42 expression in susceptible (disease index 43.3 ± 4.6) japonica rice var Mudanjiang 8, which was used as a recipient of transgenes in our transformation experiments. WRKY42 expression reached the highest level at 8 h after inoculation with N2-2, whereas mock inoculation (control) with water did not affect WRKY42 expression (Fig. 1A).
Figure 1.
Fungal (A) and bacterial (B) pathogen infections influenced WRKY42 expression. Rice lines C101A51, CO39, and Mudanjiang 8 were inoculated with fungal M. oryzae isolate N2-2 at the three- to four-leaf stage. Mudanjiang 8 was also mock inoculated with water as control. Rice lines Rb49 and Mudanjiang 8 were inoculated with bacterial Xoo strain PXO61 at the booting (panicle development) stage. Data represent means (three replicates) ± sd. 0 H, Before inoculation of pathogen.
The second pair of rice lines was Rb49 and Mudanjiang 8. Rb49 carrying the PRR-like gene Xa3/Xa26 is resistant to Xoo strain PXO61, and Mudanjiang 8 is susceptible to PXO61 (Sun et al., 2004; Cao et al., 2007). PXO61 inoculation induced WRKY42 expression in both resistant and susceptible rice lines (Fig. 1B). However, the expression level of WRKY42 was obviously higher in susceptible Mudanjiang 8 than in resistant Rb49. The rapidly induced expression of WRKY42 in rice lines suggests that this gene may be involved in rice-pathogen interaction.
Modulating WRKY42 Expression Influenced Rice Resistance to the Infection of M. oryzae But Not Xoo and Xoc
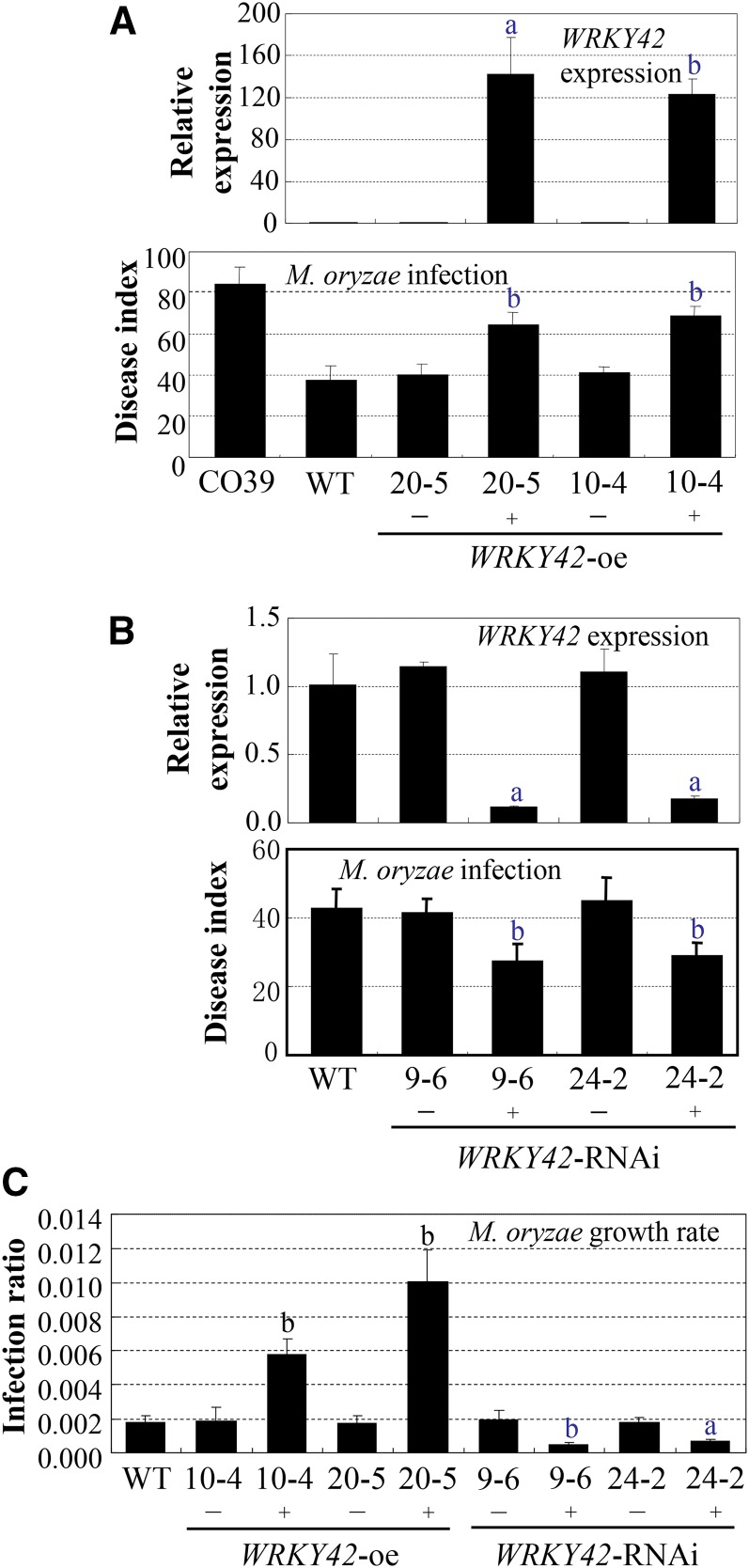
To explore the role of WRKY42 in rice-pathogen interactions, we modulated its expression. Twenty-seven WRKY42-overexpressing (oe; D153UM; also named WRKY42-oe in the following text) and 29 WRKY42-suppressing (D153RM or RNA interference [RNAi]; also named WRKY42-RNAi in the following text) independent transformants were generated in the genetic background of rice var Mudanjiang 8. The homozygous WRKY42-oe (WRKY42-oe+) and WRKY42-RNAi (WRKY42-RNAi+) lines and wild-type siblings (WRKY42-oe− or WRKY42-RNAi−) segregated from the same T0 plants were analyzed. The WRKY42-oe plants showed increased susceptibility (P < 0.01) to M. oryzae, with disease indexes ranging from 64.6 ± 6.2 to 68.9 ± 4.7 (high susceptibility) compared with 37.8 ± 6.8 (moderate susceptibility) for wild-type Mudanjiang 8 (Fig. 2A). The increased susceptibility of WRKY42-oe+ plants was associated with the overexpression of WRKY42 (Fig. 2A). The WRKY42-oe− plants had a level of susceptibility (disease indexes ranged from 40.4 ± 5.0 to 41.1 ± 2.9; moderate susceptibility) to M. oryzae similar to that of wild-type plants. In contrast, the WRKY42-RNAi+ plants showed enhanced resistance (P < 0.01) to M. oryzae, with disease indexes ranging from 27.3 ± 5.0 to 28.7 ± 4.0 (moderate resistance) compared with 42.6 ± 6.0 (moderate susceptibility) for wild-type Mudanjiang 8 (Fig. 2B). The enhanced resistance of the WRKY42-RNAi+ plants was associated with the suppressed expression of WRKY42 (Fig. 2B). Conversely, the WRKY42-RNAi− plants had a level of susceptibility (disease indexes ranged from 41.2 ± 4.4 to 44.9 ± 6.9; moderate susceptibility) to M. oryzae similar to that of wild-type plants. The fungal growth rate in WRKY42-oe+ plants was significantly higher (P < 0.01) than that in wild-type plants measured at 6 d after inoculation, whereas the fungal growth rate in WRKY42-RNAi+ plants was significantly lower (P < 0.01; Fig. 2C). The growth rate of M. oryzae in wild-type siblings showed no significant difference compared with the wild type.
Figure 2.
Modulating WRKY42 expression influenced rice response to M. oryzae infection. Homozygous (T3 generation) WRKY42-oe and WRKY42-suppressing (RNAi) plants were inoculated with M. oryzae isolate N2-2 at the three- to four-leaf stage. WT, Wild-type (Mudanjiang 8); +, positive transgenic plant; −, wild-type siblings segregated from the same T0 plants. Bar represents mean (three replicates for gene expression and fungal growth and 17–29 plants for disease index) ± sd. A, Increased susceptibility of WRKY42-oe plants to M. oryzae was associated with overexpression of WRKY42. CO39 was the rice line used as susceptible control. B, Enhanced resistance of WRKY42-RNAi plants to M. oryzae was associated with suppressed expression of WRKY42. C, Growth of M. oryzae in the leaves of plants at 6 d after infection. The “a” and “b” indicate that a significant difference between WRKY142-transgenic plants and wild-type Mudanjiang 8 was detected at P < 0.05 and P < 0.01, respectively.
Although Xoo infection influenced the expression of WRKY42, the WRKY42-oe and WRKY42-RNAi plants showed no obvious difference in disease level when compared with wild-type plants after Xoo infection (Supplemental Figs. S1 and S2). These transgenic plants also showed no difference when compared with wild-type plants after Xoc infection (Supplemental Fig. S3). These results suggest that WRKY42 negatively regulates rice resistance to M. oryzae but not Xoo or Xoc.
WRKY42 Affected Jasmonic Acid-Dependent Signaling in Rice after M. oryzae Infection
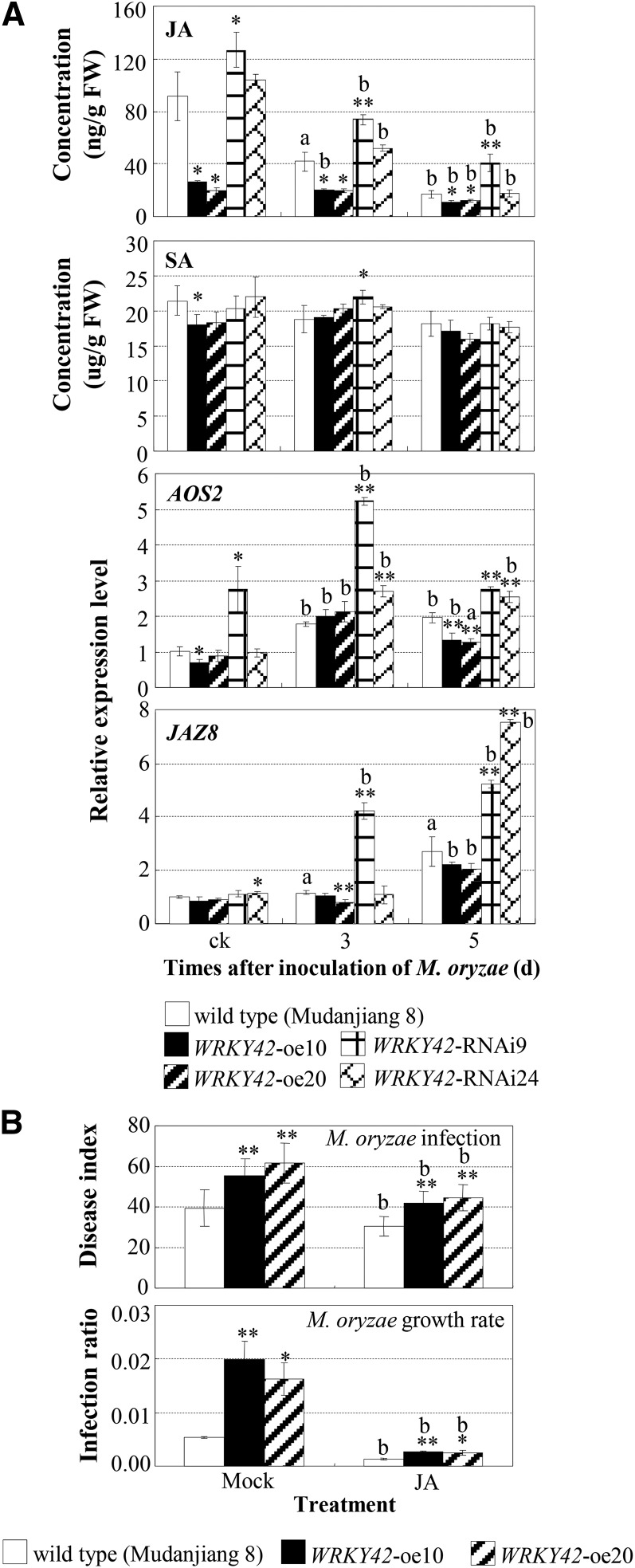
Jasmonic acid (JA) and salicylic acid (SA) are important defense signaling-related molecules. To examine whether WRKY42-involved rice-M. oryzae interaction was related to these phytohormones, we quantified JA and SA in rice plants. The JA contents in different rice plants appeared to be negatively associated with the transcript levels of WRKY42 and with the increased susceptibility to M. oryzae infection. Modulating WRKY42 expression influenced the level of JA but not SA (Fig. 3A). M. oryzae infection reduced JA levels in both wild-type and WRKY42-transgenic plants. However, compared with wild-type plants, WRKY42-oe plants showed reduced JA levels and WRKY-RNAi plants showed increased JA levels (Fig. 3A). Consistent with the JA content, the expression of Allene Oxide Synthase2 (AOS2), encoding an allene oxide synthase that is a key enzyme in the JA biosynthetic pathway in rice (Mei et al., 2006), was slightly suppressed in WRKY42-oe plants but was increased in WRKY42-RNAi plants compared with the wild type (Fig. 3A). The expression of JAZ8, encoding a jasmonate zinc-finger protein expressed in inflorescence meristem-domain (JAZ) protein functioning in JA-dependent signaling (Liu et al., 2012; Yamada et al., 2012), showed a pattern similar to that of AOS2 in WRKY42-transgenic plants. These results suggested that the negative regulation of rice resistance to M. oryzae by WRKY42 may be at least partly related to the decreased JA level and JA-dependent signaling.
Figure 3.
The negative regulation of rice resistance to M. oryzae by WRKY42 was associated with suppressed JA signaling. Plants were inoculated with M. oryzae isolate N2-2 without treatment (ck) or after being treated with 200 μm JA or the solution not containing JA (mock) at the three- to four-leaf stage. Bars represent mean (three replicates for hormone concentration and gene expression and 12–15 plants for disease index) ± sd. The letters a and b indicate a significant difference was detected between inoculated and noninoculated ck plants or JA- and mock-treated plants at P < 0.05 and P < 0.01, respectively. Asterisks indicate a significant difference was detected between wild-type and WRKY42-transgenic plants subjected to the same treatment at **P < 0.01 and *P < 0.05. A, WRKY42-transgenic plants showed changes in levels of JA and JA synthesis-related and signaling-related genes but not in the level of SA. B, Exogenous application of JA enhanced rice resistance to M. oryzae.
To further examine the inference that WRKY42-suppressed resistance was associated with reduced JA level, we treated WRKY42-oe plants with exogenous application of JA. Studies have demonstrated that exogenous application of JA enhanced rice resistance to M. oryzae (Mei et al., 2006; Riemann et al., 2013). Consistent with previous reports, JA treatment enhanced resistance to M. oryzae not only in wild-type plants but also in WRKY42-oe plants (Fig. 3B). However, overexpressing WRKY42 compromised the effect of JA on rice resistance to M. oryzae. The JA-treated WRKY42-oe plants showed 26% reduced (P < 0.01) disease symptoms, whereas the JA-treated wild-type plants only showed 22% reduced disease symptoms. The fungal growth rates in JA-treated or mock-treated plants were further examined in rice leaves. The results showed that the fungal growth rate was significantly decreased (P < 0.01) in both WRKY42-oe+ and wild-type plants after JA treatment. The JA-treated WRKY42-oe plants showed 85% reduced (P < 0.05) fungal growth rate, whereas the JA-treated wild-type plants only showed 76% reduced fungal growth rate. These results suggested that overexpression of WRKY42 increased susceptibility to M. oryzae, which was associated with suppressed JA signaling.
WRKY42 Had the Characteristics of a Transcription Factor
The predicted encoding protein of WRKY42 consists of 253 amino acids and putatively harbors a nuclear localization signal, a WRKY domain, and zinc finger motif, which are the characteristics of a typical type II WKRY protein (Supplemental Fig. S4; Ross et al., 2007). To determine whether WRKY42 functioned as a transcription factor, we first analyzed its subcellular localization. The fusion genes of yellow fluorescent protein (YFP)-WRKY42 and cyan fluorescent protein (CFP)-Ghd7 (for Grain number, plant height, and heading date7) were cotransformed into rice protoplasts. Rice Ghd7 has been used as a marker since it was reported as a transcription factor localized in the nucleus (Xue et al., 2008). The yellow fluorescence signal produced by YFP-WRKY42 overlapped with the cyan fluorescence signal produced by CFP-Ghd7 (Supplemental Fig. S5), suggesting that WRKY42 localizes in the nucleus.
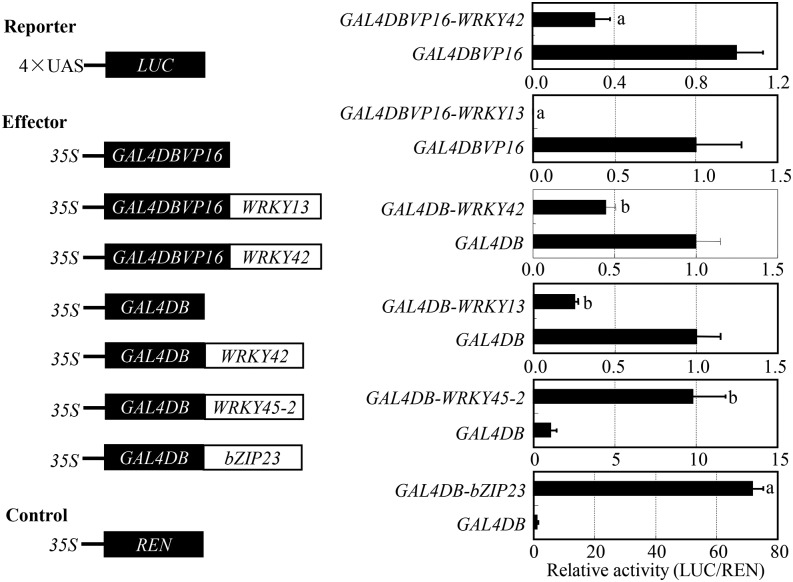
In the transactivation activity assay, the complete WRKY42, the N-terminal part of WRKY42 (harboring the nuclear localization signal), and the C-terminal part of WRKY42 (harboring the WRKY domain and the zinc finger motif) did not show activity of transactivation in yeast cells as compared with rice transcription activator basic leucine zipper23 (OsbZIP23), which was the positive control (Supplemental Fig. S6; Xiang et al., 2008). Because some WRKY transcription factors, such as rice WRKY13 (Xiao et al., 2013), function as transcription repressors, we further assayed the transcriptional activity of WRKY42 using the galectin4 DNA binding domain (GAL4DB)/upstream activating sequence (UAS)/luciferase (LUC) and GAL4DB-VP16 (Herpes simplex virus activation domain)/UAS/LUC transient expression systems in rice protoplasts. The relative activity of reporter LUC in the presence of GAL4DB-VP16 WRKY42 was significantly reduced (P < 0.05) compared with that of control (existence of GAL4DB-VP16 effector; Fig. 4). The LUC activity in the presence of the transcriptional repressor control, GAL4DB-VP16 WRKY13, was also significantly (P < 0.05) reduced compared with control. The LUC activity in the presence of GAL4DB WRKY42 and GAL4DB WRKY13 was also significantly reduced (P < 0.01), whereas the LUC activity in the presence of the positive control GAL4DB bZIP23, expressing a transcriptional activator, was significantly increased (P < 0.05; Fig. 4). These results suggest that WRKY42 is a transcriptional repressor. Considering the results presented in Figure 3A, WRKY42 may suppress rice resistance to M. oryzae by directly or indirectly suppressing JA synthesis-related and/or signaling-related genes.
Figure 4.
WRKY42 and WRKY45-2 functioned as a transcriptional repressor and activator, respectively. The relative LUC activities in rice protoplasts after transformation with reporter plasmid and different effector plasmids were presented. bZIP23, which is a transcriptional activator, and WRKY13, which is a transcriptional repressor, were used as the positive control. REN was used as the internal control to standardize the difference of the transformation ratio. Data represent mean (three biological repeats with each repeat having three replicates) ± sd. The letters a and b indicate a significant difference was detected between the effector and empty vector (GAL4DB-VP16 or GAL4DB) at P < 0.05 and P < 0.01, respectively.
WRKY13 Bound to WRKY42 Promoter in Rice and Suppressed WRKY42 Expression
Rice WRKY13, as a transcriptional repressor, confers resistance to both M. oryzae and Xoo (Qiu et al., 2007, 2008; Xiao et al., 2013). WRKY13 interacts with the promoter of WRKY42 in yeast cells (Qiu et al., 2009). To further characterize the relationship of WRKY13 protein and WRKY42 expression, we performed a series of analyses.
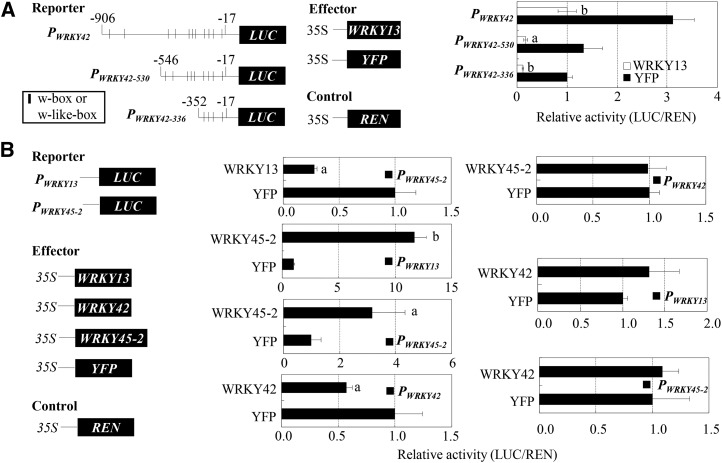
First, the WRKY13 and WRKY42 promoter were transiently expressed in rice protoplasts using a chimeric reporter/effector assay. In this assay, the effector plasmid, which carried WRKY13 driven by the Cauliflower mosaic virus 35S promoter, was cotransformed with the reporter plasmid, which carried the LUC reporter gene driven by the full-length or truncated WRKY42 promoter into protoplasts (Fig. 5A). Compared with the LUC activity in protoplasts carrying control effector YFP and LUC reporter gene, the LUC activities in the protoplasts carrying WRKY13 effector and LUC reporter gene driven by the PWRKY42, PWRKY42-530, or PWRKY42-336 promoter were reduced (P < 0.05) by approximately 3-, 8-, and 9-fold, respectively. These results suggest that WRKY13 can suppress LUC expression by interacting with the full-length or truncated WRKY42 promoters.
Figure 5.
Transient transcriptional regulatory activities of WRKY42, WRKY13, and WRKY45-2 analyzed by the chimeric reporter/effector assay in rice protoplasts. REN was used as the internal control to standardize the difference of the transformation ratio. Data represent mean (three biological repeats with each repeat having three replicates) ± sd. The letters a and b indicate a significant difference was detected between effector WRKY13, WRKY42, or WRKY45-2 and control effector YFP at P < 0.05 and P < 0.01, respectively. A, WRKY13 directly suppressed LUC driven by full-length or truncated WRKY42 promoters. B, WRKY13 and WRKY45 regulated each other’s promoter, and WRKY42 and WRKY45-2 regulated their own promoters.
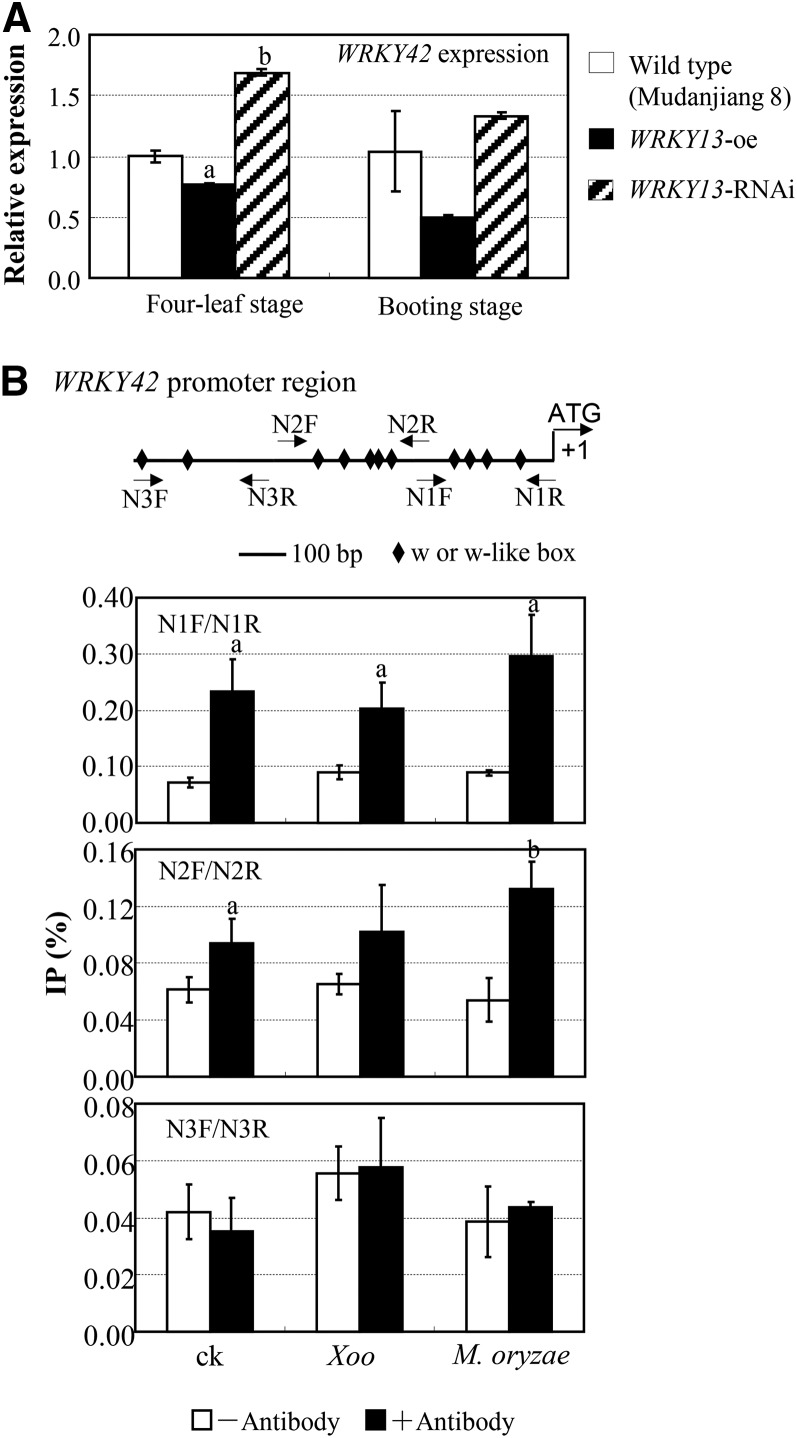
Second, to ascertain whether stably expressed WRKY13 had an effect on the expression of WRKY42, we generated WRKY13-RNAi plants in the genetic background of Mudanjiang 8. The WRKY13-oe plants, which showed enhanced resistance to M. oryzae and Xoo, also had the Mudanjiang 8 background (Qiu et al., 2007). The WRKY13-RNAi plants of both the T0 and T1 generation showed increased susceptibility to Xoo compared with wild-type plants (Supplemental Fig. S7), which is similar to the WRKY13-RNAi plants with the genetic background of an indica var Minghui 63 (Qiu et al., 2009). The increased susceptibility of these WRKY13-RNAi plants to Xoo was associated with reduced WRKY13 transcripts (Supplemental Fig. S7). The WRKY13-RNAi plants also showed significantly increased (P < 0.05) susceptibility to M. oryzae as compared with wild-type Mudanjiang 8, and the increased susceptibility of these plants to M. oryzae was associated with suppressed expression of WRKY13 (Supplemental Fig. S8). The WRKY13-RNAi plants showed increased expression of WRKY42, whereas the WRKY13-oe plants showed reduced expression of WRKY42 (Fig. 6A). These results suggest that WRKY13 suppresses WRKY42 in plants (Fig. 6A).
Figure 6.
The interaction of WRKY13 and WRKY42. Bars represent mean (three replicates) ± sd. A, Transcriptionally modulating WRKY13 influenced WRKY42 expression. The letters a and b indicate that a significant difference between WRKY13-transgenic and wild-type plants was detected at P < 0.05 and P < 0.01, respectively. B, WRKY13 protein bound to the promoter regions of WRKY42 analyzed by ChIP assay. Samples were from rice var Mudanjiang 8 that was untreated (ck), at 1 d after inoculation with Xoo, or at 1 d after inoculation with M. oryzae. The qPCR was conducted before immunoprecipitation (input), after IP without anti-WRKY13 antibody (–), or after IP with anti-WRKY13 antibody (+). The presented percentage of PCR product from IP is relative (IP/input %) to that from input. The letters a and b indicate that a significant difference was detected between the PCR products from IP with and without anti-WRKY13 antibody at P < 0.05 and P < 0.01, respectively. ATG, Start codon.
Finally, to determine whether WRKY13 protein directly interacted with WRKY42 promoter in rice during rice-pathogen interaction, we performed chromatin immunoprecipitation (ChIP) assay using anti-WRKY13 antibody (Supplemental Fig. S9). Three segments of the WRKY42 promoter, which harbor W and W-like boxes, were analyzed (Fig. 6B; Supplemental Fig. S10). After immunoprecipitation (IP) with anti-WRKY13 antibody, significant enrichment of the first (N1F/N1R) and second (N2F/N2R) DNA segments from untreated, Xoo-inoculated, and M. oryzae-inoculated samples was detected by quantitative PCR (qPCR) as compared with the corresponding controls (samples after IP without anti-WRKY13 antibody; Fig. 6B). Compared with other treated samples, there was more enrichment of the N1F/N1R than of the N2F/N2R segments in both pathogen-infected and control samples. However, the enrichment levels of the N1F/N1R in M. oryzae-infected and control samples were similar, whereas higher enrichment of the N2F/N2R was detected in the M. oryzae-infected sample compared with the control sample. No significant or obvious enrichment of the third DNA segment (N3F/N3R) was detected, although it also harbors W-like boxes (Fig. 6B; Supplemental Fig. S10). Together, these results suggest that WRKY13 may suppress WRKY42 expression by preferentially binding to certain regions of the WRKY42 promoter even without pathogen infection, and WRKY13 may further suppress WRKY42 by binding to N2F/N2R in the rice-M. oryzae interaction.
WRKY13, WRKY42, and WRKY45-2 Functioned in a Complex Regulation Loop
A previous study revealed that suppressing WRKY13 induces WRKY45-2 expression, whereas transcriptionally modulating WRKY45-2 influences WRKY13 expression (Qiu et al., 2009; Tao et al., 2009). To further ascertain the transcriptional regulation relationship of WRKY13, WRKY42, and WRKY45-2, we first examined the feature of WRKY45-2 as a transcription factor using the GAL4DB/UAS/LUC transient expression system in rice protoplasts. The activity of reporter LUC in the presence of GAL4DB WRKY45-2 significantly increased (P < 0.01) compared with that of control (GAL4DB effector; Fig. 4). The LUC activity in the presence of the transcriptional activator control, GAL4DB bZIP23, also significantly increased (P < 0.05) compared with control. These results suggest that WRKY45-2 is a transcriptional activator.
In the chimeric reporter/effector assay, LUC activity in rice protoplasts carrying the WRKY13 effector and LUC gene driven by the WRKY45-2 promoter was significantly reduced (P < 0.05) compared with that of the control protoplasts carrying the YFP effector and LUC gene driven by the WRKY45-2 promoter (Fig. 5B). The LUC activity in the protoplasts carrying the WRKY45-2 effector and LUC gene driven by the WRKY13 promoter significantly increased (P < 0.01) compared with the control. Although WRKY13 suppressed WRKY42 (Fig. 5A), the LUC activities in the protoplasts carrying the WRKY45-2 effector and LUC gene driven by the WRKY42 promoter, carrying the WRKY42 effector and LUC gene driven by the WRKY13 promoter, or carrying the WRKY42 effector and LUC gene driven by the WRKY45-2 promoter showed no significant difference compared with corresponding controls (Fig. 5B). Together, these results suggest that WRKY45-2 and WRKY13 may regulate each other in a forward feedback loop; WRKY13 may directly suppress WRKY45-2, whereas WRKY45-2 may directly activate WRKY13. However, it appears that WRKY42 does not directly regulate the expression of WRKY13 and WRKY45-2, and WRKY45-2 does not directly regulate WRKY42 expression.
In addition to transcriptional regulation of WRKY42 and WRKY45-2, WRKY13 has the ability to suppress its own gene (Cai et al., 2008; Xiao et al., 2013). In the chimeric reporter/effector transient expression system, the LUC activity in rice protoplasts carrying the WRKY45-2 effector and LUC gene driven by the WRKY45-2 promoter significantly increased compared with the control protoplasts carrying the YFP effector and LUC gene driven by the WRKY45-2 promoter (Fig. 5B). The LUC activity in rice protoplasts carrying the WRKY42 effector and LUC gene driven by the WRKY42 promoter was significantly suppressed compared with control. These results suggest that WRKY45-2 and WRKY42 can also regulate their own gene expression, which may be important for the balance of defense signaling transduction.
Transcriptional Modulating of WRKY42 or WRKY13 Affected WRKY13-Mediated or WRKY45-2-Mediated Disease Resistance
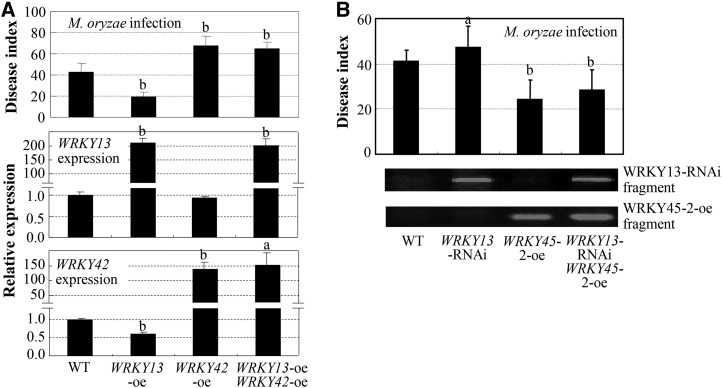
To analyze the relationship of WRKY13, WRKY42, and WRKY45-2 in the defense signaling pathway against M. oryzae, we generated double transgenic lines by crossing the transgenic lines of the three WRKY genes. The WRKY13-oe WRKY42-oe double transgenic line (disease index 64.8 ± 6.4) was highly susceptible to M. oryzae compared with WRKY13-oe plants (disease index 19.4 ± 4.4) but showed a similar level of susceptibility to M. oryzae as the WRKY42-oe plants (disease index 67.6 ± 9.1; Fig. 7A). The WRKY13-RNAi WRKY45-2-oe double transgenic line (disease index 29.2 ± 8.9) showed slightly increased susceptibility to M. oryzae compared with the WRKY45-2-oe plants (disease index 24.4 ± 9.8) but showed reduced susceptibility to M. oryzae compared with the WRKY13-RNAi plants (disease index 47.2 ± 9.5; Fig. 7B). Furthermore, transcriptional modulating of WRKY42 did not influence WRKY13 expression (Supplemental Fig. S11). Together, these results suggest that WRKY42, WRKY13, and WRKY45-2 may function in the same defense transduction pathway in which WRKY45-2 functions in the upstream, followed by WRKY13 and WRKY42, leading to resistance to M. oryzae.
Figure 7.
Rice double-gene transgenic lines showed different responses to M. oryzae compared with their parent plants (single-gene transgenic lines). Rice plants were inoculated with M. oryzae isolate N2-2 at the four-leaf stage. Bars represent mean (three replicates for gene expression and 21–27 plants for disease index) ± sd. The letters a and b indicate that a significant difference between transgenic and wild-type (WT; Mudanjiang 8) plants was detected at P < 0.05 and P < 0.01, respectively. A, WRKY13-oe WRKY42-oe double transgenic line showed increased susceptibility compared with WRKY13-oe plants. B, WRKY13-RNAi WRKY45-2-oe double transgenic line showed increased susceptibility to M. oryzae compared with WRKY45-2-oe plants and reduced susceptibility compared with WRKY13-RNAi plants.
DISCUSSION
A large number of WRKYs have been reported to regulate rice response to M. oryzae (Cheng and Wang, 2014). However, the relationship of these WRKYs in rice resistance to the same disease is largely unknown. The present results add another gene, WRKY42, to the list of WRKY defense regulators in rice-M. oryzae interaction. Furthermore, the present results suggest that the WRKY42 gene with the previously characterized WRKY13 and WRKY45-2 form a WRKY transcriptional regulatory cascade in rice response to M. oryzae invasion.
WRKY42, as a Transcriptional Repressor, Negatively Regulates Rice Disease Resistance by Suppressing JA-Dependent Signaling
WRKY proteins can function as either transcriptional activators or transcriptional repressors (Pandey and Somssich, 2009). Among the 13 characterized rice WRKY proteins involved in rice-pathogen interactions, only six have been examined for their transcriptional activity in rice cells (Cheng and Wang, 2014). WRKY45-1/WRKY45 and WRKY53 show transactivation activity (Chujo et al., 2007; Shimono et al., 2007), whereas WRKY13, WRKY28, WRKY71, and WRKY76 are transcriptional repressors (Chujo et al., 2008, 2013; Xiao et al., 2013; Yokotani et al., 2013). Interestingly, the four repressors belong to group II WRKYs, which harbor one WRKY motif and one C2H2-type zinc-finger motif, and WRKY53 and WRKY45-1/WRKY45 belong to group I WRKYs, which harbor two WRKY motifs and two C2H2-type zinc-finger motifs, and group III WRKYs, which harbor one WRKY motif and one C2HC-type zinc-finger motif, respectively, based on the classification of rice WRKY proteins (Wu et al., 2005). WRKY42 also belongs to group II. The present results suggest that WRKY42 appears to be a transcriptional repressor. This inference is proposed based on the evidence that WRKY42 did not display transactivation activity in yeast cells and rice protoplasts but expressed transcriptional repressor activity in rice protoplasts (Fig. 4; Supplemental Fig. S6). Thus, future studies are needed to determine whether all rice group II WRKYs involved in rice-pathogen interactions function as transcriptional repressors.
Rice resistance to M. oryzae is dependent on JA or SA. Increasing the expression of AOS2, which is required for JA synthesis, enhanced the rice resistance to M. oryzae (Mei et al., 2006). Mutation of Allene Oxide Cyclase, which is also a JA synthesis-related gene, increased rice susceptibility to M. oryzae (Riemann et al., 2013). Benzothiadiazole, the analog of SA, serves as a defense activator and enhances rice resistance to M. oryzae (Shimono et al., 2007). JA- and SA-dependent defense signaling frequently interact with each other either synergistically or antagonistically (Durrant and Dong, 2004). However, the present results showed that WRKY42-regulated rice-M. oryzae interaction was associated with reduced JA levels but not with SA levels (Fig. 3A). The reduced JA level was related to the suppressed expression of JA synthesis-related and signaling-related genes. Exogenous application of JA had a larger effect on WRKY42-oe plants than on the wild-type plants in reducing the susceptibility to M. oryzae (Fig. 3B). These results suggest that WRKY42 may negatively regulate rice resistance to M. oryzae by direct or indirect suppression of the JA synthesis-related genes, which in turn suppresses JA-dependent defense signaling.
WRKY42 Functions in a WRKY Transcriptional Regulatory Cascade in Rice Resistance to M. oryzae
A set of WRKY proteins is frequently involved in plant resistance to a pathogen in a given species (Pandey and Somssich, 2009). Modulating the expression of a WRKY gene frequently influences the expression of other WRKY genes. For example, both AtWRKY11 and AtWRKY17 negatively regulate basal resistance in Arabidopsis (Arabidopsis thaliana), and the two WRKYs also influence the expression of each other (Journot-Catalino et al., 2006). Rice WRKY13 positively regulates a broad-spectrum resistance to both bacterial and fungal pathogens (Qiu et al., 2007). Activation of WRKY13 influenced the expression of at least nine WRKY genes in rice response to Xoo infection (Qiu et al., 2009). However, the relationship of different WRKYs in the plant defense response to the same pathogen remains largely unknown. Only a few studies revealed that a WRKY gene directly regulates the expression of another WRKY gene in plant-pathogen interactions. Arabidopsis AtWRKY46 is involved in disease resistance, and two defense-related WRKYs, AtWRKY8 an AtWRKY48, directly activate AtWRKY46 (Hu et al., 2012; Gao et al., 2013). Rice WRKY13 directly suppresses WRKY45-1/WRKY45, which negatively regulates rice resistance to Xoo, in the rice-Xoo interaction (Tao et al., 2009; Xiao et al., 2013).
The present results suggest that WRKY13 directly suppresses WRKY42, which functions downstream of WRKY13 in the rice defense signaling pathway to M. oryzae. This inference is supported by the following evidence. First, WRKY13 suppressed the function of WRKY42 promoter in rice protoplasts (Fig. 5A). Second, transcriptional activation of WRKY13 suppressed the WRKY42 expression, and suppressing WRKY13 increased WRKY42 expression, whereas transcriptional modulation of WRKY42 did not influence WRKY13 expression (Fig. 6A; Supplemental Fig. S11). Third, WRKY13 selectively bound to two regions that harbor W and W-like boxes known for WRKY protein binding in the WRKY42 promoter in rice (Fig. 6B). Finally, the WRKY13 WRKY42 double transgenic line showed the phenotype of the WRKY42 transgenic line in the rice response to M. oryzae (Fig. 7A). This inference is consistent with the fact that WRKY42 negatively regulates rice resistance only to M. oryzae, but WRKY13 positively regulates rice resistance to both M. oryzae and Xoo (Qiu et al., 2007). However, WRKY13 functions in association with activation of SA-dependent signaling and suppression of JA-dependent signaling (Qiu et al., 2007, 2008), whereas WRKY42 only suppresses JA signaling. This inconsistency may be due to the fact that both SA- and JA-dependent signaling are involved in rice resistance to M. oryzae (Mei et al., 2006; Shimono et al., 2007; Riemann et al., 2013). The SA- and JA-dependent defense pathways may function antagonistically; however, the two pathways may have crisscross roles at different processes in rice resistance to M. oryzae just like in rice resistance to Xoo (Liu et al., 2012).
A previous study has shown that WRKY13 binds to the promoter of WRKY45-2, which confers broad-spectrum resistance to both bacterial and fungal pathogens (Tao et al., 2009). The present results suggest that WRKY45-2 can also bind to the promoter of WRKY13 (Supplemental Fig. S12). WRKY45-2 is a transcriptional activator that can directly promote the expression of the reporter gene driven by the WRKY13 promoter (Figs. 4 and 5B). In addition, WRKY13 can directly suppress the expression of the reporter gene driven by the WRKY45-2 promoter (Fig. 5B). These results suggest that WRKY45-2 and WRKY13 mutually regulate the expression of each other's gene.
The WRKY13-RNAi WRKY45-2-oe double transgenic line showed increased susceptibility to M. oryzae compared with WRKY45-2-oe plants, but showed enhanced resistance to M. oryzae compared with WRKY13-RNAi plants (Fig. 7B). WRKY45-2 confers resistance to three pathogen species, M. oryzae, Xoc, and Xoo, and WRKY13 only confers resistance to M. oryzae and Xoo (Qiu et al., 2007; Tao et al., 2009). Furthermore, the present results have revealed that WRKY45-2 is a transcriptional activator, but WRKY13 is a transcriptional repressor (Xiao et al., 2013). These results suggest that WRKY45-2 may function upstream of WRKY13 in rice resistance to M. oryzae. WRKY45-2 functions in association with activation of JA- but not SA-dependent signaling (Tao et al., 2009). The previous studies and present results further suggest that the SA- and JA-dependent signaling may have crisscross roles in rice resistance to M. oryzae. However, WRKY45-2-mediated resistance to M. oryzae may also require a subpathway that is WRKY13 independent. This hypothesis is supported by evidence that the WRKY13-RNAi WRKY45-2-oe double transgenic line showed a disease index between those of WRKY13-RNAi and WRKY42-2-oe plants (Fig. 7B). Furthermore, WRKY13 may regulate WRKY45-2 by negative feedback regulation.
CONCLUSION
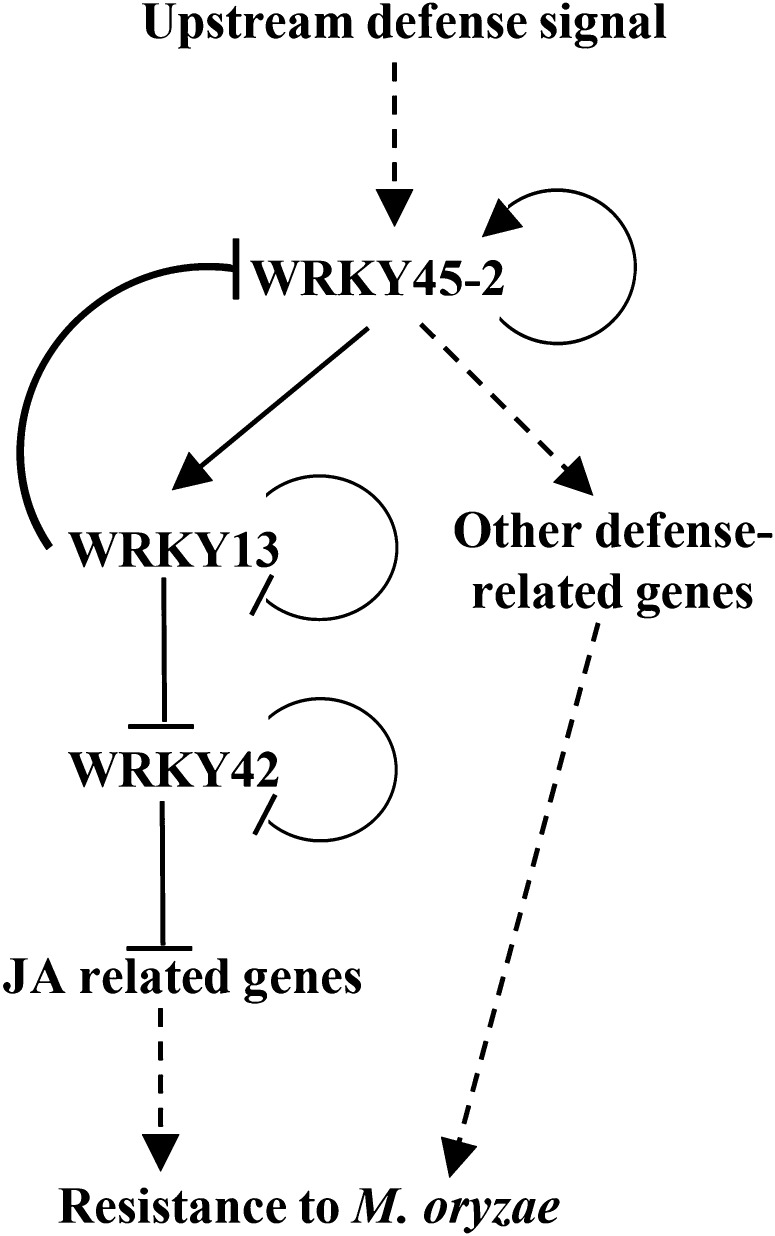
The present results suggest that WRKY45-2, WRKY13, and WRKY42 form a sequential transcriptional regulatory cascade, which is required in rice resistance to M. oryzae (Fig. 8). The function of this cascade may be balanced via the negative feedback regulation of WRKY45-2 by WRKY13, and it also may be balanced by the autoregulation of the three WRKY proteins on their own genes. Because WRKY45-2 and WRKY13 are negatively involved in the regulation of rice response to abiotic stresses (Qiu et al., 2008; Tao et al., 2011), this cascade may provide rapid amplification of pathogen-induced defense signaling and balancing adaptation to different environmental stimuli by reprogramming the transcriptome.
Figure 8.
Model of rice WRKY45-2 WRKY13 WRKY42 transcriptional regulatory cascade involving defense signaling against M. oryzae. Solid arrows and T lines indicate promotion and inhibition of gene expression, respectively. Dotted arrows indicate predicted actions.
MATERIALS AND METHODS
Rice Materials and Treatment
Indica rice (Oryza sativa ssp. indica) C101A51 and CO39 are near-isogenic lines with the genetic background of CO39 (Jiang and Wang, 2002). Transgenic line Rb49 carries the PRR-like gene Xa3/Xa26 driven by its native promoter and has the genetic background of japonica rice ssp. japonica var Mudanjiang 8 (Sun et al., 2004; Xiang et al., 2006). Transgenic lines WRKY13-oe (D11UM7-2) and WRKY45-2-oe (D114UM4) were also used in this study. WRKY13 and WRKY45-2 genes from indica rice var Minghui 63 driven by the maize (Zea mays) ubiquitin promoter were separately transformed into Mudanjiang 8 to generate WRKY13-oe and WRKY45-2-oe plants (Qiu et al., 2007; Tao et al., 2009). Plant treatment with JA was performed as reported previously (Ke et al., 2014). Rice plants at the three- to four-leaf stage were sprayed with 200 μm JA in 0.1% (v/v) methanol and 0.015% (v/v) Tween 20 or 0.1% (v/v) methanol and 0.015% (v/v) Tween 20 (mock treatment). The sprayed plants were kept in sealed plastic shade for 2 d before further treatment.
Vector Construction and Rice Transformation
To make an overexpressing construct of rice WRKY42, the genomic DNA fragment harboring the complete coding region (1,187 nucleotides) of this gene was obtained from rice var Minghui 63 by PCR amplification using primers 153U3F and 153U3R (Supplemental Table S1), and the PCR product was digested with KpnI and inserted into vector pU1301, which contained a maize ubiquitin gene promoter in the multiple cloning site (Cao et al., 2007). To construct the RNAi vectors for WRKY42 and WRKY13, the 313-nucleotide fragment of WRKY42 and the 366-nucleotide fragment of WRKY13 amplified from the complementary DNA (cDNA) of rice var Mudanjiang 8 using primer pairs 153R4F/153R4R and WRKY1322F/WRKY1322R (Supplemental Table S1), respectively, were separately inserted into the pDS1301 vector (Yuan et al., 2007). These constructs were transferred into Agrobacterium tumefaciens strain EHA105. A. tumefaciens-mediated transformation was performed using the callus derived from mature embryos of rice var Mudanjiang 8 (Lin and Zhang, 2005).
Pathogen Inoculation
To evaluate fungal blast disease, rice plants were inoculated with isolate N2-2 of Magnaporthe oryzae at the three- to four-leaf stage by the spraying method (Chen et al., 2003). The inoculated plants were first treated in the growth chamber at 28°C in darkness for 36 h, and then under alternating light and darkness every 12 h with 95% humidity. Disease was scored for individual plants using a scale rating system (scale of 0–9) at 7 d after inoculation (Tao et al., 2009). Disease index was calculated with the individual leaf ratings using the following formula: disease index = [sum of numerical ratings from all leaves/(number of leaves assessed × maximum lesion rating)] × 100. The average disease index of all plants was presented. A disease index of ≥0 and ≤5 indicates high resistance, >5 and ≤15 indicates resistance, >15 and ≤30 indicates moderate resistance, >30 and ≤45 indicates moderate susceptibility, >45 and ≤60 indicates susceptibility, and >60 indicates high susceptibility. The fungal growth rate in rice leaves was determined by detecting the ratio of the Pot gene of M. oryzae and the ubiquitin gene of rice via qPCR using primer pairs PotF and PotR (Supplemental Table S1; Duan et al., 2014). The PCR product level of the rice ubiquitin gene amplified using primers UbiqF and UbiqR (Supplemental Table S1) was used to standardize the DNA sample for analyzing fungal growth rate.
To examine bacterial blight disease, rice plants were inoculated with Xoo strain PXO61 by the leaf-clipping method (Chen et al., 2002). Disease was scored by measuring the percentage of the lesion area (lesion length/leaf length) at 2 weeks after inoculation.
To examine bacterial streak disease, rice plants were inoculated with Xoc strain RH3 by using a penetration method (Ke et al., 2014). Disease was scored by measuring the lesion length at 2 weeks after inoculation.
Gene Expression
For quantitative reverse-transcriptase PCR, total RNA was isolated from rice leaves. To examine the influence of M. oryzae infection on gene expression, whole leaves were used for RNA isolation. To examine the influence of bacterial blight infection on gene expression, 3-cm leaf fragments next to bacterial infection sites were used for RNA isolation. The qPCR was performed as described previously using primers listed in Supplemental Table S2 (Qiu et al., 2007). The expression level of the rice actin gene was used to standardize the RNA sample for each quantitative reverse-transcriptase PCR. Because the gene primers used in this study have different amplification efficiency, the expression level of each gene in transgenic or treated plants was calculated relative to that in wild-type or untreated plants.
Database Search
The known motif and domain of the WRKY42 protein were determined by motif scanning (http://hits.isb-sib.ch).
Hormone Quantification
Whole leaves were used for phytohormone quantification. Samples were prepared, and JA and SA were quantified using an ultrafast liquid chromatography/electrospray ionization/tandem mass spectrometry system as described previously (Liu et al., 2012).
Protein Subcellular Localization
To determine the subcellular localization of WRKY42, the full coding region of WRKY42 was amplified from the cDNA of rice var Mudanjiang 8 using primers 1536F and 1536R (Supplemental Table S1) and fused to the coding region of YFP driven by the 35S promoter in the pM999 vector (kindly provided by Jian Xu, Huazhong Agricultural University, Wuhan, China). Rice Ghd7 has been used as a marker since it was reported as a transcription factor localized in the nucleus (Xue et al., 2008). 35S:YFP-WRKY42 and 35S:CFP-Ghd7, kindly provided by Lei Wang of Huazhong Agricultural University, were cotransformed and transiently expressed in rice protoplasts prepared from an etiolated shoot of japonica rice var Zhonghua 11 by polyethylene glycol treatment (Ning et al., 2010). After 24 h of transformation, the fluorescence signal was observed using a confocal microscope.
Transactivation and Transsuppression Activity Assays
The transactivation activity of WRKY42 was analyzed in both yeast (Saccharomyces cerevisiae) cells and rice protoplasts using the known rice transcription activator OsbZIP23 as the positive control. The yeast assay was performed as described previously (Deng et al., 2012). In brief, the complete coding region, the N-terminal region, and the C-terminal region of WRKY42 were amplified using primer pairs 1532F/R, 153Y1F/R, and 153Y2F/R, respectively (Supplemental Table S1). The PCR products were respectively ligated into the pGAL4-BD vector. The pGAL4-BD-OsbZIP23 vector was kindly provided by Xiang et al. (2008). These recombinant plasmids and empty vector (negative control) were respectively transformed into yeast strain Y187. Yeast transformants were screened by culture in synthetic dropout medium-Trp-Leu-Ade medium.
For study of the transactivation or transsuppression activity of WRKY42 and WRKY45-2, the GAL4DB/UAS/LUC or GAL4DB-VP16/UAS/LUC transient assays were performed in rice protoplasts according to the process reported previously (Jing et al., 2013; Weng et al., 2014). This system contains three vectors: the effector, reporter, and control. The complete coding region of WRKR42 or WRKY45-2 was fused with GAL4DB or GAL4DB-VP16 driven by the 35S promoter as the effector. The LUC linked to four tandem repeats of UAS, which harbors DNA sequence for GAL4DB binding (4×UAS-LUC), was the reporter. The Renilla LUC (REN) driven by the 35S promoter was the control for standardizing the difference of the transformation ratio. The three vectors were cotransferred into rice protoplasts. The LUC activity was detected with a luminescence kit using the LUC assay substrate (Promega). The expression level of reporter gene LUC was determined by counting the ratio of LUC to REN. The transcriptional activity of the target transcription factor was calculated relative to that of the empty vector.
To study the role of a transcription factor in the target gene, the chimeric reporter/effector transient expression assay was performed in rice protoplasts according to the process reported previously (Gao et al., 2013). In this assay, LUC was driven by the target promoter as the reporter, the target transcription factor gene driven by the 35S promoter was used as the effector, and the YFP driven by the 35S promoter was used as the effector control. The reporter and effector vectors were cotransferred into rice protoplasts with the control REN vector.
Protein-DNA Interaction
ChIP assay was performed as described previously (Tao et al., 2009). Samples were collected from the shoot of rice var Mudanjiang 8 at the three- to four-leaf stage. Sonicated chromatin fragments were immunoprecipitated with WRKY13-specific antibody (Tao et al., 2009), which was custom synthesized by NewEast Biosciences. The IP chromatin was analyzed by qPCR using primer pairs N1F/N1R, N2F/N2R, and N3F/N3R (Supplemental Table S1). The non-IP and sonicated chromatin was used as the total input DNA control. The percentage of immunoprecipitation (IP%) was used to compare different samples by calculating the ratio of the amount of target PCR product from IP relative to that from the input. The IP% was calculated by using the following formula: IP% = 2−ΔCt (normalized ChIP), in which ΔCycle threshold (ΔCt) = Ct [ChIP] − (Ct [input] − log2 input dilution factor) and input dilution factor = (fraction of the input chromatin saved)−1 in the qPCR, according to a previous report (Haring et al., 2007).
The interaction of the WRKY protein with the DNA regulatory element was determined by a yeast one-hybrid assay as described previously (Chen et al., 2014). In brief, the full-length cDNA of WRKY protein obtained by PCR amplification was ligated into the pB42AD vector (Clontech). The target cis-acting DNA fragment obtained by PCR amplification of the promoter region of the target gene was ligated into the p8op-lacZ vector containing the β-galactosidase reporter. The two recombinant vectors were cotransformed into yeast strain EGY48. Transformants were grown on synthetic dropout medium/-Uracil-Trp plates containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside for blue color development.
Statistical Analysis
The correlation analysis between disease area and gene expression level was performed using the CORREL analysis in Excel (Microsoft). The significant differences between control and treatment of the samples were analyzed by the pairwise t test in the Excel program.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers EF143611 (WRKY13), AK110587 (WRKY42), and GQ331927 (WRKY45-2).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. WRKY42-oe plants showed no resistance to Xoo strain PXO61 inoculation.
Supplemental Figure S2. WRKY42-RNAi plants showed no resistance to Xoo strain PXO61 inoculation.
Supplemental Figure S3. WRKY42 transgenic plants showed no resistance to Xoc strain RH3 inoculation.
Supplemental Figure S4. The schematic diagram of rice WRKY42 protein structure.
Supplemental Figure S5. WRKY42 colocalized with transcription factor Ghd7 in the nucleus of rice protoplast.
Supplemental Figure S6. WRKY42 displayed no transactivation activity in yeast cells.
Supplemental Figure S7. Increased susceptibility to Xoo is associated with suppressed expression of WRKY13.
Supplemental Figure S8. Modulating WRKY13 expression influenced rice response to M. oryzae infection.
Supplemental Figure S9. The specificity of anti-WRKY13 antibody was examined.
Supplemental Figure S10. The W displayed in the promoter region of WRKY42.
Supplemental Figure S11. Transcriptionally modulating WRKY42 did not influence WRKY13 expression.
Supplemental Figure S12. Interaction of the three WRKY genes detected by yeast one-hybrid.
Supplemental Table S1. PCR primers used for construction of vectors, gene structure analysis, and ChIP analysis.
Supplemental Table S2. Primers used for qPCR in gene expression analysis.
Supplementary Material
Glossary
- R
race-specific disease resistance
- PRR
pattern recognition receptor
- Xoo
Xanthomonas oryzae pv oryzae
- Xoc
X. oryzae pv oryzicola
- RNAi
RNA interference
- JA
jasmonic acid
- SA
salicylic acid
- ChIP
chromatin immunoprecipitation
- qPCR
quantitative PCR
- cDNA
complementary DNA
- IP
immunoprecipitation
- IP%
percentage of immunoprecipitation
Footnotes
This work was supported by the National Natural Science Foundation of China (grant no. 31330062), the National Program on the Development of Basic Research in China (grant no. 2011CB100700), and the National Program of High Technology Development of China (grant no. 2012AA10A303).
Articles can be viewed without a subscription.
References
- Bagnaresi P, Biselli C, Orrù L, Urso S, Crispino L, Abbruscato P, Piffanelli P, Lupotto E, Cattivelli L, Valè G (2012) Comparative transcriptome profiling of the early response to Magnaporthe oryzae in durable resistant vs susceptible rice (Oryza sativa L.) genotypes. PLoS ONE 7: e51609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M, Qiu D, Yuan T, Ding X, Li H, Duan L, Xu C, Li X, Wang S (2008) Identification of novel pathogen-responsive cis-elements and their binding proteins in the promoter of OsWRKY13, a gene regulating rice disease resistance. Plant Cell Environ 31: 86–96 [DOI] [PubMed] [Google Scholar]
- Cao Y, Ding X, Cai M, Zhao J, Lin Y, Li X, Xu C, Wang S (2007) The expression pattern of a rice disease resistance gene xa3/xa26 is differentially regulated by the genetic backgrounds and developmental stages that influence its function. Genetics 177: 523–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chen Z (2002) Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor. Plant Physiol 129: 706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Li B, Li G, Charron JB, Dai M, Shi X, Deng XW (2014) Arabidopsis phytochrome A directly targets numerous promoters for individualized modulation of genes in a wide range of pathways. Plant Cell 26: 1949–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wang S, Xing Y, Xu C, Hayes PM, Zhang Q (2003) Comparative analyses of genomic locations and race specificities of loci for quantitative resistance to Pyricularia grisea in rice and barley. Proc Natl Acad Sci USA 100: 2544–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wang S, Zhang Q (2002) New gene for bacterial blight resistance in rice located on chromosome 12 identified from minghui 63, an elite restorer line. Phytopathology 92: 750–754 [DOI] [PubMed] [Google Scholar]
- Cheng H, Wang S (2014) The important player of rice-pathogen interactions: WRKY-type transcription factors. Scientia Sinica Vitae 44: 784–793 [Google Scholar]
- Chujo T, Kato T, Yamada K, Takai R, Akimoto-Tomiyama C, Minami E, Nagamura Y, Shibuya N, Yasuda M, Nakashita H, et al. (2008) Characterization of an elicitor-induced rice WRKY gene, OsWRKY71. Biosci Biotechnol Biochem 72: 240–245 [DOI] [PubMed] [Google Scholar]
- Chujo T, Miyamoto K, Shimogawa T, Shimizu T, Otake Y, Yokotani N, Nishizawa Y, Shibuya N, Nojiri H, Yamane H. , et al (2013) OsWRKY28, a PAMP-responsive transrepressor, negatively regulates innate immune responses in rice against rice blast fungus. Plant Mol Biol 82: 23–37 [DOI] [PubMed] [Google Scholar]
- Chujo T, Takai R, Akimoto-Tomiyama C, Ando S, Minami E, Nagamura Y, Kaku H, Shibuya N, Yasuda M, Nakashita H, et al. (2007) Involvement of the elicitor-induced gene OsWRKY53 in the expression of defense-related genes in rice. Biochim Biophys Acta 1769: 497–505 [DOI] [PubMed] [Google Scholar]
- Deng H, Liu H, Li X, Xiao J, Wang S (2012) A CCCH-type zinc finger nucleic acid-binding protein quantitatively confers resistance against rice bacterial blight disease. Plant Physiol 158: 876–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desveaux D, Maréchal A, Brisson N (2005) Whirly transcription factors: defense gene regulation and beyond. Trends Plant Sci 10: 95–102 [DOI] [PubMed] [Google Scholar]
- Duan L, Liu H, Li X, Xiao J, Wang S (2014) Multiple phytohormones and phytoalexins are involved in disease resistance to Magnaporthe oryzae invaded from roots in rice. Physiol Plant 152: 486–500 [DOI] [PubMed] [Google Scholar]
- Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42: 185–209 [DOI] [PubMed] [Google Scholar]
- Eulgem T. (2005) Regulation of the Arabidopsis defense transcriptome. Trends Plant Sci 10: 71–78 [DOI] [PubMed] [Google Scholar]
- Eulgem T, Somssich IE (2007) Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol 10: 366–371 [DOI] [PubMed] [Google Scholar]
- Gao X, Chen X, Lin W, Chen S, Lu D, Niu Y, Li L, Cheng C, McCormack M, Sheen J, et al. (2013) Bifurcation of Arabidopsis NLR immune signaling via Ca²⁺-dependent protein kinases. PLoS Pathog 9: e1003127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring M, Offermann S, Danker T, Horst I, Peterhansel C, Stam M (2007) Chromatin immunoprecipitation: optimization, quantitative analysis and data normalization. Plant Methods 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell EE, Yang Y (2013) Molecular strategies to improve rice disease resistance. Methods Mol Biol 956: 285–309 [DOI] [PubMed] [Google Scholar]
- Hu KM, Qiu DY, Shen XL, Li XH, Wang SP (2008) Isolation and manipulation of quantitative trait loci for disease resistance in rice using a candidate gene approach. Mol Plant 1: 786–793 [DOI] [PubMed] [Google Scholar]
- Hu Y, Dong Q, Yu D (2012) Arabidopsis WRKY46 coordinates with WRKY70 and WRKY53 in basal resistance against pathogen Pseudomonas syringae. Plant Sci 185-186: 288–297 [DOI] [PubMed] [Google Scholar]
- Jiang J, Wang S (2002) Identification of a 118-kb DNA fragment containing the locus of blast resistance gene Pi-2(t) in rice. Mol Genet Genomics 268: 249–252 [DOI] [PubMed] [Google Scholar]
- Jing Y, Zhang D, Wang X, Tang W, Wang W, Huai J, Xu G, Chen D, Li Y, Lin R (2013) Arabidopsis chromatin remodeling factor PICKLE interacts with transcription factor HY5 to regulate hypocotyl cell elongation. Plant Cell 25: 242–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journot-Catalino N, Somssich IE, Roby D, Kroj T (2006) The transcription factors WRKY11 and WRKY17 act as negative regulators of basal resistance in Arabidopsis thaliana. Plant Cell 18: 3289–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan K, Manners JM (2009) Linking development to defense: auxin in plant-pathogen interactions. Trends Plant Sci 14: 373–382 [DOI] [PubMed] [Google Scholar]
- Ke Y, Liu H, Li X, Xiao J, Wang S (2014) Rice OsPAD4 functions differently from Arabidopsis AtPAD4 in host-pathogen interactions. Plant J 78: 619–631 [DOI] [PubMed] [Google Scholar]
- Kou Y, Wang S (2010) Broad-spectrum and durability: understanding of quantitative disease resistance. Curr Opin Plant Biol 13: 181–185 [DOI] [PubMed] [Google Scholar]
- Li H, Wang S (2013) Disease resistance. In Zhang Q, Wing RA, eds, Plant Genetics and Genomics: Crops and Models. Genetics and Genomics of Rice, Vol 5 Springer, Heidelberg, pp 161−175 [Google Scholar]
- Li J, Brader G, Palva ET (2004) The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 16: 319–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YJ, Zhang Q (2005) Optimising the tissue culture conditions for high efficiency transformation of indica rice. Plant Cell Rep 23: 540–547 [DOI] [PubMed] [Google Scholar]
- Liu H, Li X, Xiao J, Wang S (2012) A convenient method for simultaneous quantification of multiple phytohormones and metabolites: application in study of rice-bacterium interaction. Plant Methods 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei C, Qi M, Sheng G, Yang Y (2006) Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression, and host resistance to fungal infection. Mol Plant Microbe Interact 19: 1127–1137 [DOI] [PubMed] [Google Scholar]
- Oh SK, Baek KH, Park JM, Yi SY, Yu SH, Kamoun S, Choi D (2008) Capsicum annuum WRKY protein CaWRKY1 is a negative regulator of pathogen defense. New Phytol 177: 977–989 [DOI] [PubMed] [Google Scholar]
- Pandey SP, Somssich IE (2009) The role of WRKY transcription factors in plant immunity. Plant Physiol 150: 1648–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puranik S, Sahu PP, Srivastava PS, Prasad M (2012) NAC proteins: regulation and role in stress tolerance. Trends Plant Sci 17: 369–381 [DOI] [PubMed] [Google Scholar]
- Qiu D, Xiao J, Ding X, Xiong M, Cai M, Cao Y, Li X, Xu C, Wang S (2007) OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol Plant Microbe Interact 20: 492–499 [DOI] [PubMed] [Google Scholar]
- Qiu D, Xiao J, Xie W, Cheng H, Li X, Wang S (2009) Exploring transcriptional signalling mediated by OsWRKY13, a potential regulator of multiple physiological processes in rice. BMC Plant Biol 9: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu D, Xiao J, Xie W, Liu H, Li X, Xiong L, Wang S (2008) Rice gene network inferred from expression profiling of plants overexpressing OsWRKY13, a positive regulator of disease resistance. Mol Plant 1: 538–551 [DOI] [PubMed] [Google Scholar]
- Qu LJ, Zhu YX (2006) Transcription factor families in Arabidopsis: major progress and outstanding issues for future research. Curr Opin Plant Biol 9: 544–549 [DOI] [PubMed] [Google Scholar]
- Riemann M, Haga K, Shimizu T, Okada K, Ando S, Mochizuki S, Nishizawa Y, Yamanouchi U, Nick P, Yano M, et al. (2013) Identification of rice Allene Oxide Cyclase mutants and the function of jasmonate for defence against Magnaporthe oryzae. Plant J 74: 226–238 [DOI] [PubMed] [Google Scholar]
- Robatzek S, Somssich IE (2002) Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev 16: 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Liu Y, Shen QJ (2007) The WRKY gene family in rice (Oryza sativa). J Integr Plant Biol 49: 827–842 [Google Scholar]
- Ryu HS, Han M, Lee SK, Cho JI, Ryoo N, Heu S, Lee YH, Bhoo SH, Wang GL, Hahn TR. , et al (2006) A comprehensive expression analysis of the WRKY gene superfamily in rice plants during defense response. Plant Cell Rep 25: 836–847 [DOI] [PubMed] [Google Scholar]
- Shimono M, Sugano S, Nakayama A, Jiang CJ, Ono K, Toki S, Takatsuji H (2007) Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. Plant Cell 19: 2064–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Foley RC, Oñate-Sánchez L (2002) Transcription factors in plant defense and stress responses. Curr Opin Plant Biol 5: 430–436 [DOI] [PubMed] [Google Scholar]
- Sun C, Palmqvist S, Olsson H, Borén M, Ahlandsberg S, Jansson C (2003) A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter. Plant Cell 15: 2076–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Cao Y, Yang Z, Xu C, Li X, Wang S, Zhang Q (2004) Xa26, a gene conferring resistance to Xanthomonas oryzae pv. oryzae in rice, encodes an LRR receptor kinase-like protein. Plant J 37: 517–527 [DOI] [PubMed] [Google Scholar]
- Tao Z, Kou Y, Liu H, Li X, Xiao J, Wang S (2011) OsWRKY45 alleles play different roles in abscisic acid signalling and salt stress tolerance but similar roles in drought and cold tolerance in rice. J Exp Bot 62: 4863–4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z, Liu H, Qiu D, Zhou Y, Li X, Xu C, Wang S (2009) A pair of allelic WRKY genes play opposite roles in rice-bacteria interactions. Plant Physiol 151: 936–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Ou B, Li J, Zhao Y, Guo D, Zhu Y, Chen Z, Gu H, Li C, Qin G. , et al (2013) Transcriptional profiling of rice early response to Magnaporthe oryzae identified OsWRKYs as important regulators in rice blast resistance. PLoS ONE 8: e59720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng X, Wang L, Wang J, Hu Y, Du H, Xu C, Xing Y, Li X, Xiao J, Zhang Q (2014) Grain Number, Plant Height, and Heading Date7 is a central regulator of growth, development, and stress response. Plant Physiol 164: 735–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KL, Guo ZJ, Wang HH, Li J (2005) The WRKY family of transcription factors in rice and Arabidopsis and their origins. DNA Res 12: 9–26 [DOI] [PubMed] [Google Scholar]
- Xiang Y, Cao Y, Xu C, Li X, Wang S (2006) Xa3, conferring resistance for rice bacterial blight and encoding a receptor kinase-like protein, is the same as Xa26. Theor Appl Genet 113: 1347–1355 [DOI] [PubMed] [Google Scholar]
- Xiang Y, Tang N, Du H, Ye H, Xiong L (2008) Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant Physiol 148: 1938–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Cheng H, Li X, Xiao J, Xu C, Wang S (2013) Rice WRKY13 regulates cross talk between abiotic and biotic stress signaling pathways by selective binding to different cis-elements. Plant Physiol 163: 1868–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Xing Y, Weng X, Zhao Y, Tang W, Wang L, Zhou H, Yu S, Xu C, Li X, et al. (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40: 761–767 [DOI] [PubMed] [Google Scholar]
- Yamada S, Kano A, Tamaoki D, Miyamoto A, Shishido H, Miyoshi S, Taniguchi S, Akimitsu K, Gomi K (2012) Involvement of OsJAZ8 in jasmonate-induced resistance to bacterial blight in rice. Plant Cell Physiol 53: 2060–2072 [DOI] [PubMed] [Google Scholar]
- Yokotani N, Sato Y, Tanabe S, Chujo T, Shimizu T, Okada K, Yamane H, Shimono M, Sugano S, Takatsuji H, et al. (2013) WRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance. J Exp Bot 64: 5085–5097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B, Shen X, Li X, Xu C, Wang S (2007) Mitogen-activated protein kinase OsMPK6 negatively regulates rice disease resistance to bacterial pathogens. Planta 226: 953–960 [DOI] [PubMed] [Google Scholar]
- Yuan T, Wang S (2012) Pathogen-responsive cis-elements. In Mérillon JM, Ramawat KG, eds, Progress in Biological Control. Plant Defence: Biological Control, Vol 12 Springer, Heidelberg, pp 363–378 [Google Scholar]
- Zheng Z, Qamar SA, Chen Z, Mengiste T (2006) Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J 48: 592–605 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.