Abstract
Genetic elements that cheat Mendelian segregation by biasing transmission in their favor gain a significant fitness benefit. Several examples of sex-ratio meiotic drive, where one sex chromosome biases its own transmission at the cost of the opposite sex chromosome, exist in animals and plants. While the distorting sex chromosome gains a significant advantage by biasing sex ratio, the autosomes, and especially the opposite sex chromosome, experience strong selection to resist this transmission bias. In most well-studied sex-ratio meiotic drive systems, autosomal and/or Y-linked resistance has been identified. We specifically surveyed for Y-linked resistance to sex-ratio meiotic drive in Drosophila affinis by scoring the sex ratio of offspring sired by males with a driving X and one of several Y chromosomes. Two distinct types of resistance were identified: a restoration to 50/50 sex ratios and a complete reversal of sex ratio to all sons. We confirmed that fathers siring all sons lacked a Y chromosome, consistent with previously published work. Considerable variation in Y-chromosome morphology exists in D. affinis, but we showed that morphology does not appear to be associated with resistance to sex-ratio meiotic drive. We then used two X chromosomes (driving and standard) and three Y chromosomes (susceptible, resistant, and lacking) to examine fertility effects of all possible combinations. We find that both the driving X and resistant and lacking Y have significant fertility defects manifested in microscopic examination of testes and a 48-hr sperm depletion assay. Maintenance of variation in this sex-ratio meiotic drive system, including both the X-linked distorter and the Y-resistant effects, appear to be mediated by a complex interaction between fertility fitness and transmission dynamics.
Keywords: sex-ratio meiotic drive, genetic conflict, sex chromosomes, Drosophila affinis, genetics of sex
SEX-RATIO meiotic drive (SRMD) is a phenomenon that has been studied for nearly as long as Drosophila (Morgan et al. 1925). While usually associated with Drosophila, it has also been studied in several other Dipterans (medfly, housefly, stalk-eyed fly, mosquitoes), lepidopterans, lemmings, mice, and two plant species (Jaenike 2001). It occurs when one sex chromosome, usually the X, is able to disable sperm carrying the opposite sex chromosome therefore skewing the ratio of sex chromosomes in gametes and thus the offspring sex ratio. Traditionally an evolutionary curiosity, the role that SRMD might play in several evolutionary and ecological processes suggests that, in some species, it may be a considerable force driving phenotypic, behavioral, and molecular evolution.
SRMD has been implicated in processes as diverse as speciation (Frank 1991; Hurst and Pomiankowsi 1991; Tao et al. 2001; Phadnis and Orr 2009; McDermott and Noor 2010), changes in patterns of linkage disequilibrium (Dyer et al. 2007), mating system evolution (Price et al. 2008; Pinzone and Dyer 2013), extinction (Hamilton 1967), and interspecific competition (James and Jaenike 1990; Unckless and Clark 2014). Since Y chromosomes have fitness close to zero when paired with a sex-ratio X (XSR), those Y-chromosome genotypes able to decrease offspring sex-ratio bias will be selectively favored when the frequency of XSR is appreciable (Clark 1987; Hall 2004). Furthermore, when the population sex ratio is skewed toward females, both the autosomes and Y chromosome will be selected to restore Fisherian sex ratios (Fisher 1930). Given these pressures, SRMD systems are likely to lead to strong selection for resistance.
One model for SRMD is that the driving X (denoted as XSR, while the standard X is denoted XST) produces a toxin that targets a sequence on the Y chromosome or is carried by Y-bearing sperm (reviewed in Jaenike 2001). This toxin then disrupts development of the Y-bearing sperm, leading to nearly 100% X-bearing sperm in the ejaculate (Burt and Trivers 2009). Resistance to SRMD could take at least two different forms. First, Y-bearing sperm could become resistant to the effects of the driving X. If the toxin model is correct, a simple way to become resistant would be to delete the target region of the Y chromosome. In contrast, a Y chromosome or autosome could evolve to actively suppress the molecular mechanism causing the drive. In many cases, it is difficult to distinguish between these two possibilities, but this conceptual framework may be informative. Both resistant and suppressing Y chromosomes should sweep to fixation if they carry sufficiently small cost in fertility or viability (Clark 1987; Hall 2004).
Previously published studies have reported Y-linked resistance to SRMD in several species (Drosophila paramelanica, D. mediopunctata, D. quinaria, and D. simulans) (Stalker 1961; Carvalho et al. 1997; Jaenike 1999; Montchamp-Moreau et al. 2001; Branco et al. 2013), while other studies found no evidence for Y-linked resistance in D. pseudoobscura. Jaenike (1999) surveyed 61 wild-caught strains of D. quinaria for autosomal or Y-linked resistance and found that 9 led to offspring sex ratios significantly less female-biased than the rest. He further dissected the genetic basis of resistance in six of those lines and found evidence for Y linkage in three of them. Carvalho et al. (1997) estimated that 10–20% of Y chromosomes in D. mediopunctata were at least partially resistant to drive. Two surveys were conducted for two of the distinct X-linked drivers in D. simulans. First, 107 Y chromosomes from several populations were assayed against the Paris sex-ratio system, and resistance was found segregating in most populations (Montchamp-Moreau et al. 2001). Branco et al. (2013) surveyed 78 Y-chromosome replacement lines against the Winters sex-ratio system and found sex ratios ranging from 63 to 98% female.
Empirical examples of resistant and suppressing Y chromosomes suggest that they are likely to carry some cost since they exist at intermediate frequency. Understanding the connection between fitness effects of the driving X and a resistant Y provides greater insight into the sex-ratio system and these previous studies do not consider empirical estimates of fitness costs.
The ancestral karyotype of Drosophila consists of five rod-shaped chromosomes and a dot-shaped chromosome, each assigned a “Muller element” A–F (White 1973). Ancestors of the obscura group experienced a fusion of their X chromosome (Muller element A) and an autosome (Muller element D, chromosome 3L in D. melanogaster) resulting in X linkage of ∼40% of their genome (White 1973; Powell 1997). In D. pseudoobscura, it appears that some of the Y chromosome is homologous to Muller element D—the new arm of the X chromosome (Carvalho and Clark 2005). There is widespread Y-chromosome morphological diversity in the obscura group of Drosophila. Dobzhansky (1935) noted four different types in D. pseudoobscura (his race A) and three types in D. persimilis (his race B). In D. athabasca, Miller and Roy (1964) found four. In D. affinis, Miller found four different morphologies with a considerable range in size in addition to males lacking a Y chromosome but who were fertile (Voelker and Kojima 1971). Fertility in XO males is rare in Drosophila (Voelker and Kojima 1971), and in D. affinis, Voelker and Kojima (1972) found a significant fitness cost in males lacking a Y, though the severity of the cost depended on genetic background.
This fertility of XO males provides an interesting angle on the mechanism of sex-ratio meiotic drive. If XSR-bearing sperm are somehow targeting Y-bearing sperm based on Y-derived sequence or protein, XSRO males might be resistant to drive. In D. affinis, not only do XSRO males sire sons, they sire no daughters (Voelker 1972). This led Jaenike (2001) to suggest a mechanism for this reversal in sex ratio. He posits that given the relatively recent divergence between neo-X and Y sequence, the Y-linked target might also be present on the X chromosome, but in fewer copies. In XSRY males, the toxin is preferentially absorbed by the Y-bearing sperm, and therefore, the X-bearing sperm are not appreciably affected. If males lack a Y chromosome (as in XSRO males), the only target is the XSR chromosome, and therefore the sex-ratio chromosome kills itself, leading to only O-bearing sperm surviving and exclusively male offspring. In the context of D. affinis, XSRO males are likely resistant because they cannot have targets on their missing Y chromosome. Because the sex ratio of offspring sired by XSRO males is exclusively sons, the Jaenike (2001) model would therefore imply that XSRY males producing sex ratios that are ∼50% female carry suppressors because if they lacked a target like XSRO males, we would expect a strongly male-biased sex ratio.
Here we report the existence of segregating Y chromosomes that are resistant to SRMD. We also confirm the results of Voelker (1972) finding that XSRO males sire only sons. We then examine the connection between Y-chromosome morphology and resistance as well as sperm developmental abnormalities in six different genotypes (standard and sex-ratio X against susceptible Y, resistant Y, and O). Finally, we dissect the male fertility fitness consequences of each of these six genotypes and infer severe costs of the resistant Y and O.
Methods
Fly stocks and husbandry
All lines were derived from wild-caught flies, except one laboratory strain (referred to as the genome strain or 141.02) that was obtained from Brian Charlesworth. Wild flies were captured in April and May 2011 using a sweep net over fruit baits or compost piles in Rochester, New York or Athens, Georgia. Flies were maintained on standard glucose agar food (8.2% glucose, 8.2% yeast, 1% agar, 1.2% acid mix) supplemented with a cotton roll for pupation. Each wild-caught male was mated to a virgin 141.02 female and the sex ratio of their offspring was noted.
Generating X- and Y-replacement lines
To isolate the sex-ratio X chromosomes, males siring mostly daughters were mated to their daughters in an attempt to create homozygous females. Those F2 females were mated either to their grandfather (the original wild-caught male) or to a 141.02 male. The females mated to their grandfather will produce mostly daughters, some of which should be homozygous for the driving X chromosome. Those mated to the 141.02 male should produce equal numbers of males and females, but if the mother was homozygous for the driver, all males should carry it and so their offspring should show the sex-ratio skew (Supporting Information, Figure S1). This basic design was repeated with putatively homozygous females mated to either males thought to have the SR chromosome or 141.02 standard males until all males in each generation sired all daughters. Because there is presumably some cost to the driver in females, this process took several generations. During this process, however, the autosomes and Y chromosome from 141.02 were being moved into the sex-ratio line. In this way, the SR X chromosome was introgressed onto the 141.02 background for >20 generations.
Nineteen Y-chromosome replacement lines were generated by mating either wild-caught males or sons of wild-caught females to 141.02 virgin females. Each subsequent generation, sons were backcrossed to 141.02 virgin females. These backcrosses continued for at least six generations, then stocks were maintained without further backcrossing.
Assaying Y-linked resistance to drive
Y-chromosome replacement males were crossed to (homozygous) XSR-chromosome replacement females to create males carrying the XSR chromosome, a unique Y chromosome, and 141.02 autosomes. Seven-day-old virgin males were mated to virgin 141.02 females (4–11 pairs per Y-replacement line) in two blocks and offspring sex ratios were recorded for each cross. We omitted all crosses producing <10 offspring, except those from line 62, which never produced >10 (see below).
To determine the effect of the Y chromosome on offspring sex ratio we performed logistic regression on sex ratio (number male and female) in R (R Development Core Team 2014), with block and Y-replacement line as independent variables. We performed a Tukey test post hoc to compare sex ratios between individual Y-replacement lines. In this case, we added a male and female to the count for each male because the post hoc test was otherwise unable to distinguish these lines as different from others. P-values were adjusted for multiple tests using the false discovery rate method.
Y-chromosome karyotype variation
Larval brains were dissected from third instar larvae of lines 114, 162, 177, 159, 98, XO, and 141.02 (the genome strain). Brains were treated with a hypotonic solution of 0.5% sodium citrate and then fixed in 1.8% formaldehyde in 45% acetic acid, squashed, and stained with 4′,6-diamidino-2-phenylindole (DAPI). Mitotic figures in larval neuroblasts were visualized on a Zeiss Axioplan epifluorescence microscope at ×100, and images were captured with a Hamamatsu Orca-ER digital camera and Micro-Manager software.
Fluorescence microscopy of testes
Testes from 5-day-old sex-ratio (6) and standard wild-type (4) males were dissected in Ringer’s solution and fixed for 6 min in 4% paraformaldehyde in 1× phosphate-buffered saline solution. Spermatogenic cysts were visualized on a Zeiss Axioplan epifluorescence microscope at ×10 and ×40, and images were captured with a Hamamatsu Orca-ER digital camera and Micro-Manager software.
Electron microscopy of sperm bundles
Sperm bundles from six genotypes were imaged using TEM. Genotypes used were all combinations of standard and sex-ratio X chromosome and standard susceptible Y chromosome (Ysus), resistant Y chromosome (Yres), and O (lacking Y chromosome). The Y-resistant line used was YAF-159. Testes of ∼10–14 day old males were dissected in fixative following Noguchi et al. (2008) and imaged on a Hitachi 7650 transmission electron microscope (Tokyo). For each genotype, 5–10 males were dissected and images from several testes were used.
Forty-eight-hour sperm depletion
For each of the six genotypes (XSTYsus, XSTYres, XSTO, XSRYsus, XSRYres, and XSRO), virgin males were aged 7–9 days and then transferred (without anesthesia) to a vial with 10 virgin 141.02 females. Males were allowed to mate with females for 48 hr, then males were removed and females were placed in vials individually and allowed to oviposit for 7 days. After 7 days, females were discarded and a cotton roll was added to each vial for pupation. Offspring counts and sex ratio were noted for each individual female and from the original vial that housed the male and all females. This procedure was performed in two blocks of 10 males per genotype.
Virility was measured in three ways. In all cases, models consider X chromosome, Y chromosome, their interaction, and block. First, we noted the number of females of 10 possible that produced offspring. We refer to this as the number of successful matings and analyzed the data using a generalized linear model assuming a Poisson error distribution, since the mean (4.61) and variance (4.88) were approximately the same. The second measure of virility was the maximum number of offspring produced by one of the 10 females mated to a particular male. We assume this is the first mating and refer it to it as such, though it is possible that some males produce more offspring from subsequent matings. Finally, we consider total offspring sired summed over all matings. For both the number of offspring sired from the first mating and the total number of offspring sired, we used a generalized linear model with a negative binomial error distribution as implemented in the MASS package in R.
Results
Variation for Y-linked resistance to drive
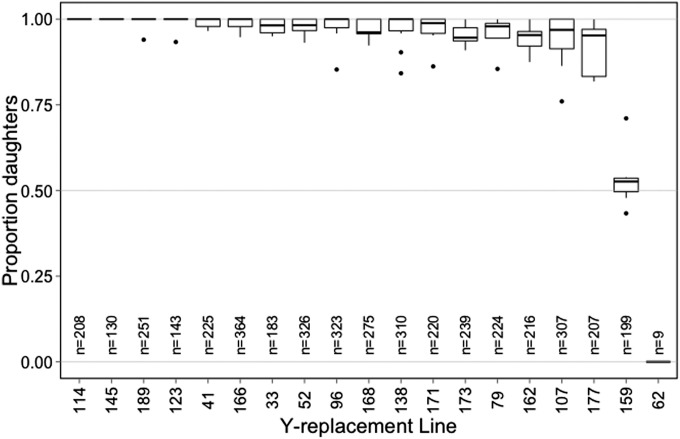
When paired with the driving X chromosome (XSR), most Y chromosomes resulted in nearly all daughters and therefore showed no evidence for resistance to sex-ratio meiotic drive. There were two notable exceptions: line 159 males produced just over 50% daughters and line 62 males produced only sons (Figure 1). Overall, Y-replacement line was a highly significant predictor of sex ratio (P < 0.0001), while block was not (P = 0.22) (Table 1).
Figure 1.
Two Y-replacement lines show resistance to sex-ratio distortion from XSR. Boxplots for sex ratio of offspring sired by males with XSR and one of several Y chromosomes. Boxes represent first and third quartile and median (bold line), whiskers extend to 1.5 times the interquartile range for each genotype, and outliers are denoted by open circles. Total offspring sired by each genotype is also noted.
Table 1. ANOVA table for the logistic regression of Y-replacement line resistance to sex-ratio meiotic drive.
| Factor | d.f. | Deviance | Residual d.f. | Residual deviance | P |
|---|---|---|---|---|---|
| Y-line | 18 | 440.62 | 107 | 185.76 | <0.0001 |
| Block | 1 | 1.8 | 125 | 626.38 | 0.18 |
| Null | 32.41 | 126 | 628.18 |
Upon post hoc pairwise comparisons (Table S1), lines 159 and 62 were significantly different from the other lines (P < 0.0001 in all comparisons). Lines 159 and 62 were also significantly different from each other (P = 0.048). Several other comparisons were also significant (114 vs. 107, P = 0.029; 166 vs. 107, P = 0.015; 52 vs. 107, P = 0.048; 162 vs. 114, P = 0.048; 166 vs. 162, P = 0.039; 173 vs. 166, P = 0.047).
There are therefore at least three distinct Y-chromosome phenotypes. First, the susceptible Y (Ysus) which, when paired with the sex-ratio X (XSR), yielded offspring sex ratios quite close to one. The second phenotype was characterized by only line 159, whose Y chromosome produced roughly equal numbers of males and females when paired with XSR. Though we do not definitely know whether this Y chromosome resists drive or suppresses it, we will refer to it as a resistant Y chromosome (Yres). The third phenotype was also found in a single line, 62, which produced only sons—though not very many. This is consistent with the XSRO phenotype observed by Voelker (1972) and we have confirmed that line 62 lacks a Y chromosome (see below). A set of lines (162, 107, 177) appears to produce a slightly less female-biased sex ratio than other lines, but we do not explore those further here.
Y-chromosome morphological variation and its relation to offspring sex ratio
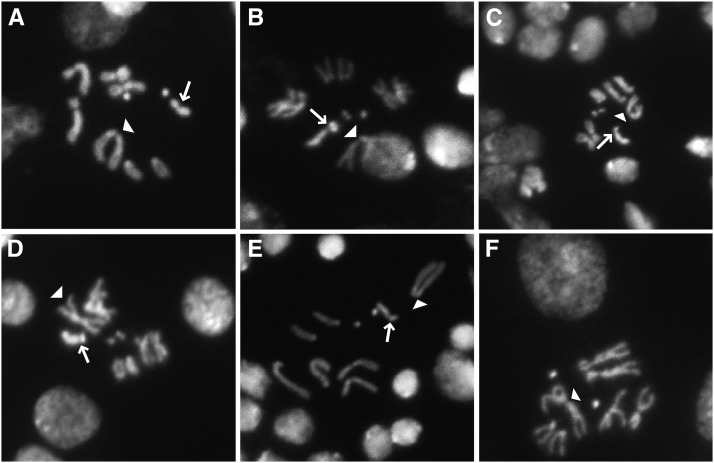
We selected several lines from across the distribution of offspring sex ratios to survey for Y-morphological variation with the assumption that resistant and suppressing Y chromosomes might be morphologically distinct from susceptible Y chromosomes. Chromosome images derived from brain squashes reveal clear heterogeneity in Y-chromosome morphology, but it was not associated with resistance to drive (Figure 2).
Figure 2.
Mitotic figures from male larval neuroblasts in: (A) genome strain (141.02, susceptible), (B) line 114 (susceptible), (C) line 162 (slightly resistant), (D) line 177 (slightly resistant), (E) line 159 (resistant), and (F) XO (line 62). The arrows mark Y chromosomes and the arrowheads mark X chromosomes. Note the lack of a Y chromosome in the XO line. There are at least three Y chromosome types: the small submetacentric type of the genome strain and 159, the J-shaped Y of lines 162 and 177, and no Y chromosome as in XO (line 62).
Two Y-replacement lines that consistently sired almost exclusively daughters (141.02 and 114, Figure 2, A and B, respectively) appeared different morphologically. Two Y-replacement lines showing slightly less female biased sex ratios (162 and 177, Figure 2, C and D, respectively) were also morphologically distinct from each other. The suppressing Y chromosome from line 159 (Figure 2E) looks morphologically similar to 141.02 (Figure 2A), which is completely susceptible to drive. Finally, line 62 (Figure 2F), which produced all sons, showed no trace of a Y chromosome, making it XO. Interestingly, another line (98) seemed to lose its Y chromosome during the course of the experiment. In the first block, of the assay for Y resistance, this line was completely susceptible to drive. In the second block, some males were susceptible and others sired only sons, suggesting the line was then polymorphic for the presence of the Y. The experiment was then repeated in a third block, and all males produced only sons. The absence of a Y chromosome was then confirmed by karyotyping (not shown in Figure 2) as described above, and this line was omitted from further analysis.
Defects in fertility evident in sperm bundles
Germline stem cells in male members of the obscura group of Drosophila undergo five mitotic and two meiotic divisions yielding 128 sperm cells per cyst (Philip 1944). After meiosis, spermatids differentiate into mature sperm without any further cell divisions—a process referred to as spermiogenesis. During spermiogenesis, round spermatid heads undergo a massive remodeling to elongate into the needle-shaped head of a mature sperm. During remodeling, histones are replaced by small sperm-specific protamines in a process called the histone-to-protamine transition (Rathke et al. 2007). A fully fertile male is therefore expected to have nearly 128 needle-shaped maturing spermatids per cyst. We compared the various stages of spermatogenesis of XSTYsus and XSRYsus males to determine the timing and nature of the defect.
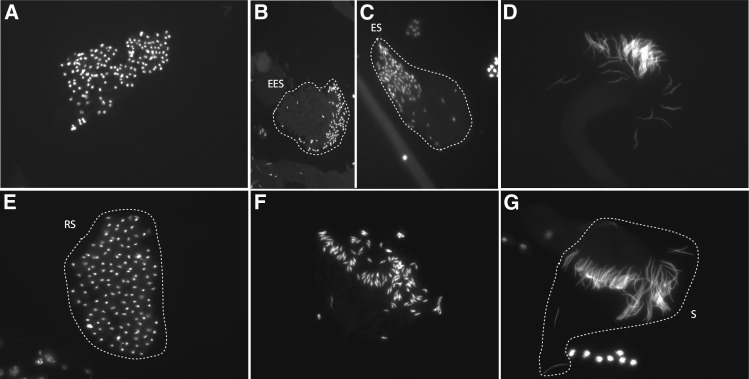
We first examined spermatogenesis in testes of XSTYsus and XSRYsus males for any gross abnormalities using fluorescence microscopy. Premeiotic and meiotic cysts in both XSTYsus and XSRYsus testes contained the correct number of well-formed nuclei. While XSTYsus postmeiotic spermatid bundles through all stages of spermiogenesis had ∼128 well-formed nuclei, the number of nuclei in XSRYsus postmeiotic cysts declined beginning prior to early elongation and continued as spermiogenesis proceeded (Figure 3). The only postmeiotic cysts with 128 nuclei in XSRYsus testes were at the round spermatid stage (Figure 3A). Throughout elongation, the number of properly formed nuclei decreased to 64–76 fully differentiated spermatids (Figure 3, B–D). Therefore, we found evidence for a postmeiotic defect in spermatogenesis in XSRYsus testes. While we cannot exclude the possibility that the lesion occurs in an earlier stage of spermatogenesis, the earliest visible defect occurs during postmeiotic spermatid differentiation, during the histone-to-protamine transition. Consistent with this timing, we saw a range of mal-shaped spermatid head phenotypes among XSRYsus males beyond the missing nuclei (Figure S2).
Figure 3.
Spermatogenesis in XSRYsus testes appears to proceed normally through meiosis. (A) Cysts containing round spermatids that have just completed meiosis tend to have ∼128 nuclei. Cysts containing spermatids beginning to elongate have <128 spermatids: (B) 112 early elongation spermatids (EESs; outlined) and (C) 98 elongating spermatids (ESs; outlined). (D) Fully differentiated sperm at individualization have ∼64 nuclei. (E) Representative wild-type cysts from XSTYsus testes showing ∼128 postmeiotic round spermatids (RSs; outlined), (F) ∼128 elongating nuclei, and (G) ∼128 fully differentiated sperm (S; outlined) at individualization.
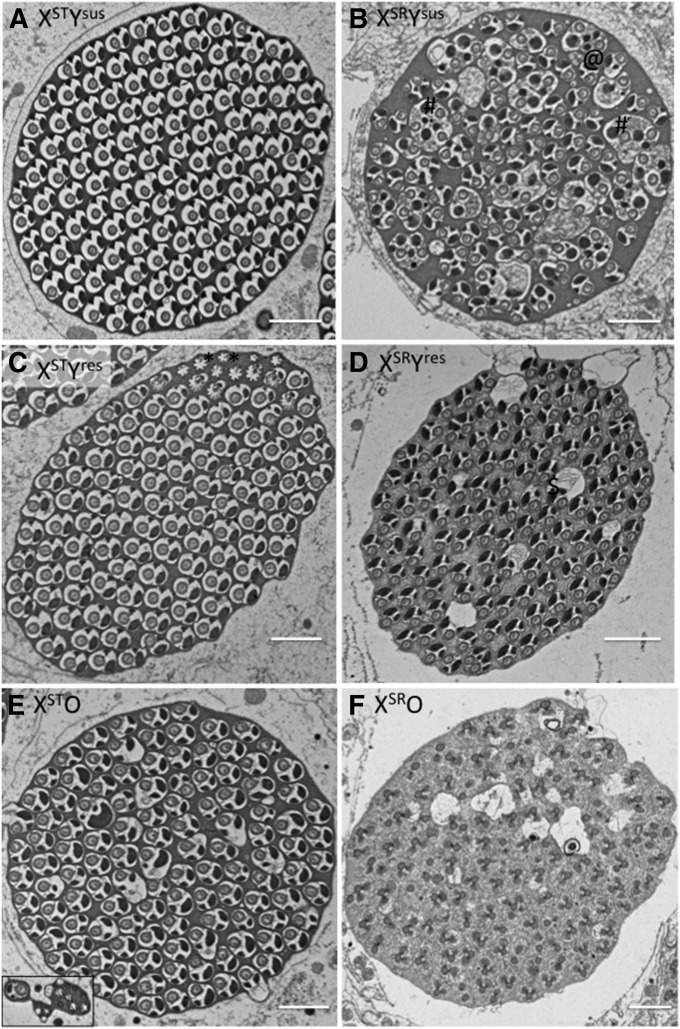
To characterize the pathology of the sex-ratio phenotype in more detail, we examined spermatogenic cysts in the testes of all six genotypes (XSTYsus, XSTYres, XSTO, XSRYsus, XSRYres, and XSRO) using electron microscopy. We first examined the number of normal spermatids per cyst. As expected, cysts of XSTYsus males contained close to 128 spermatids (Figure 4, Figure S3, Table 2; n = 5, mean = 123.4). Cysts of XSTYres males contained slightly fewer spermatids (n = 4, mean = 119.25), while those of XSTO males appeared bimodal with two having a single healthy spermatid and four containing 118 or more (n = 6, mean = 81). Males carrying the sex-ratio X chromosome (XSR) provide a useful comparison as we can both begin to understand the pathology of the sex-ratio system and the physiological basis of suppression. Males with the driving X and susceptible Y (XSRYsus) had cysts with roughly half as many mature spermatids (n = 4, mean = 65.75). One cyst contained >64 (half of the total 128 cysts) with 69. Cysts of XSRYres males contained similar numbers to those males with the standard X and either resistant or susceptible Y (n = 3, mean = 120), while we never found a single normal looking spermatid in XSRO males (n = 6, mean = 0) consistent with the very small number of sons sired by these males.
Figure 4.
Electron micrographs of sperm bundle testes from six genotypes. (A) Standard male with susceptible Y chromosome showing ∼128 normally developing spermatids. (B) Sex-ratio males with the susceptible Y chromosome (XSRYsus) show ∼64 normally developing spermatids and several that do not appear to have individualized. @, double spermatids; #, groups of spermatids that have failed to individualize, and ↑, axonemes without cytoplasm or nebenkern. (C and D) Sperm bundles from XSTYres and XSRYres males appear similar to each other with ∼120 normally developing spermatids. *, either caudal ends of sperm tails or incompletely developed sperm tails and %, spermatids surrounded by vacuoles. (E) Sperm bundles from XSTO males, which look either normal or are almost completely void of normal spermatids (inset), and (F) sperm bundles from XSRO males had no normally developing spermatids. Bars, 1 μm.
Table 2. Sperm cyst counts in each of the six genotypes.
| Genotype | n | Mean/median | SD | Values |
|---|---|---|---|---|
| XSTYsus | 5 | 123.4/123 | 0.55 | 123, 123, 123, 124, 124 |
| XSTYres | 4 | 119.25/120 | 7.80 | 109, 120, 120, 128 |
| XSTO | 6 | 81/118 | 62.02 | 1, 1, 118, 118, 124, 124 |
| XSRYsus | 4 | 65.75/66 | 3.77 | 60, 62, 63, 69 |
| XSRYres | 3 | 120/125 | 11.36 | 107, 125, 128 |
| XSRO | 3 | 0/0 | 0 | 0, 0, 0 |
In addition to raw counts of normal spermatids, the electron micrographs also provide us with some insight into pathologies of each of the genotypes. For example, while all cysts of XSTYsus males appeared normal, some cysts of XSTYres males contained a significant number of what are either less developed sperm or caudal tails of sperm (the latter of which may not have any effect on fertility, denoted by * in Figure 4C, top right corner). Cysts of sex-ratio males (XSRYsus) showed three distinct pathologies. First, some spermatids appear to have individualized in pairs forming a double spermatid (denoted by @ in Figure 4B). Surprisingly, these spermatids seem to have undergone individualization as they lack excess cytoplasm and are membrane bound, but contain two axonemes and two nebenkerns. Second, several spermatids appear to have failed to undergo individualization (denoted by # in Figure 4B). These spermatids contain excess cytoplasm and several axonemes and nebenkerns. This pattern is consistent with those found in other male meiotic drive systems (Tokuyasu et al. 1972; Tao et al. 2007). Finally, there are a small number of axonemes that appear to be without cytoplasm or nebenkern in the sperm bundle (black arrow in Figure 4B). These three phenotypes vary in relative proportions from sperm bundle to sperm bundle but exist in all sperm bundles examined.
Figure 4D, which shows cysts of XSRYres males, depicts several spermatids that are surrounded by vacuoles and might be in the process of degradation (denoted as % in Figure 4D). Cysts of XSRO males have no normal spermatids, consistent with their near sterility. The pathology of these spermatids appears different from those above, with axonemes present, but not membrane bound, and no sign of differentiated nebenkern.
Both the driving X and resistant Y chromosomes carry fitness costs
Fertility effects of the driving X chromosome (XSR) and resistant (or suppressing) Y chromosomes were measured in three ways: total number of successful matings out of 10 possible females, offspring sired in the “first” mating, and total offspring sired across all females. Note that we assess total successful matings by counting the number of females that produce at least one viable larva. It is therefore possible that males may have mated with a female but their sperm failed to fertilize any eggs, which would be counted as a nonmating. Second, we assume that males will transfer the most sperm, and therefore sire the most offspring during their first mating. We therefore classify first mating as that which produces the most offspring.
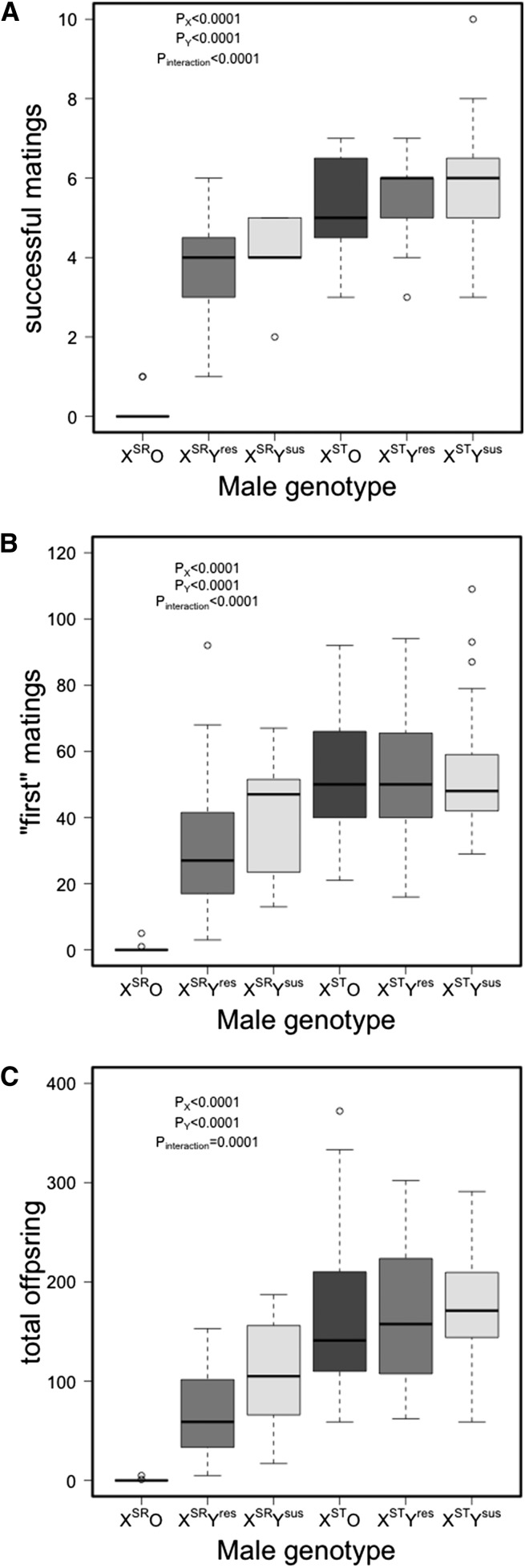
Across all genotypes, males mated an average of 4.61 (median = 5) times with the number of matings ranging from 0 (n = 13 and all XSRO) to 10 (n = 1 XSTYsus). Figure 5A shows the numbers of successful matings for each genotype. X-chromosome identity, Y-chromosome identity, and their interaction were all significant (P < 0.0001, Table S2). Post hoc comparisons reveal that significant differences between Ysus and O (P < 0.0001), Yres and O (P < 0.0001), but not between Ysus and Yres (P = 0.75) with the Ysus males having slightly more successful matings. Comparing individual genotypes post hoc, we found a significant difference between XSRO and all other genotypes (P < 0.0001 in all cases), and between XSTYres and XSRYres (P = 0.026) with XSTYres males having more successful matings.
Figure 5.
Sperm depletion (48 hr) across six genotypes (XSR and XST by Ysus, Yres, and O). (A) Number of females producing offspring per male (out of 10); (B) maximum number of offspring produced from one mating (presumably the first mating); and (C) total number of offspring produced from all matings. Boxes represent first and third quartile and median (bold line), whiskers extend to 1.5 times the interquartile range for each treatment, and outliers are denoted by open circles. Boxes are shaded by Y-chromosome state (dark shading, susceptible; medium shading, O; and light shading, resistant) for ease of comparison.
The X chromosome, Y chromosome, and interaction between X and Y were all significant predictors of the number of offspring sired in the “first” mating (P < 0.0001 in all cases, Figure 5B, Table S3). Similar to the number of successful matings, males with Yres and Ysus sired significantly more offspring in their first mating (P < 0.0001 in both cases) but did not differ significantly from each other (P = 0.24). The XSRO males sired significantly fewer offspring in the first mating than all others in a post hoc test (P < 0.0001 for all comparisons). The only other post hoc comparison of note was that XSRYres sired significantly fewer (P = 0.002) offspring in the first mating than XSRYsus.
Perhaps the best measure of fertility fitness for an individual male is the total offspring sired summed over the 10 available females. The identity of both X and Y and interaction between X and Y have a significant impact on total offspring (Figure 5C; Table S4; P < 0.0001 in all cases). In this case, however, Ysus males sired significantly more offspring than both Yres (P = 0.019) and O males (P < 0.0001). In comparisons of individual genotypes, we now see telling differences that might help explain the maintenance of variation for drive resistance and the presence of the driver itself. First, considering the susceptible Y chromosome, sex-ratio males (XSRYsus) sired fewer total offspring than standard males (XSTYsus, P = 0.017). The same is true when considering other Y chromosomes (P < 0.0001 in both cases). Males carrying the resistant Y chromosome sired fewer offspring than those carrying the susceptible Y chromosome (P < 0.0001) on a standard X background. Surprisingly, however, males lacking a Y chromosome (XSTO) sired only slightly fewer offspring (P = 0.99) than those with a Y chromosome (XSTYsus), a result inconsistent with previously published reports of the inferiority of males lacking a Y chromosome (Voelker and Kojima 1972). Once again, XSRO males sired significantly fewer offspring than males of all other genotypes. No other comparisons showed significant differences.
Discussion
Several species in the obscura group of Drosophila show striking intraspecific Y-chromosome morphological variation. Sex-ratio meiotic drive is also common within the group and it is therefore tempting to suggest that a link exists between the two. However, in a survey of Y-replacement lines, the single Y chromosome that is nearly completely resistant is indistinguishable from one that is susceptible. Furthermore, a complete lack of Y chromosome has a severe effect on the outcome of SRMD by inverting the sex ratio completely such that only sons are produced. From our initial screen, we find three distinct Y-chromosome phenotypes: susceptible, resistant, and O. We paired each with both the standard and driving X chromosomes to create six genotypes (XSTYsus, XSTYres, XSTO, XSRYsus, XSRYres, and XSRO) to investigate the sex-ratio phenotype more fully.
Four different morphological variants of the Y chromosome plus its absence (the O) were described by Miller and Stone (1962) without an obvious geographical signal. The maintenance of five genotypes in a haploid system is quite difficult without invoking frequency-dependent selection or strong local or seasonal adaptation (Charlesworth and Charlesworth 2010). Clark (1987) found that an X-linked drive system could lead to stable maintenance of two Y chromosomes, and it seems reasonable that multiple X-linked drivers could maintain multiple Y chromosomes. Voelker (1972) reported the existence of two different driving X chromosomes in D. affinis. We have isolated several driving X chromosomes from the wild, and one (XSR2) appears to be distinct from XSR in at least three ways. First, a phylogenetic analysis of a single X-linked gene indicates XSR2 is more closely related to the genome strain (141.02) than to other drivers (Table S5). Second, we crossed females homozygous for XSR2 to our Yres line, and males from this cross were not resistant to drive, siring nearly 100% female offspring (in four crosses we recovered 33/34, 56/59, 24/24, and 31/32 females). Finally, the testes of XSR2Ysus males also show a defect in postmeiotic spermatid development. However, rather than missing nuclei, preindividualization postmeiotic spermatid cysts contained ∼50% unelongated spermatid heads (Figure S2). In our limited sample, the second driver (XSR2) was only identified once compared to four that appear similar to the initial sex-ratio X chromosome (XSR). This suggests that the drive system in D. affinis might be quite complex with Y chromosomes being completely resistant to one driver while being completely susceptible to another driver. Such a system might begin to explain the apparent persistence of Y-chromosome morphological diversity within natural populations—a more complex equilibrium may exist between the three X chromosomes (two sex ratio and one standard) and the several Y chromosomes. It is also possible, of course, that an equilibrium has not been reached in D. affinis, and that some morphs are slowly declining to extinction. Finally, it is possible that Y chromosomes are continuously broken, regenerating the smaller morphs that may have a competitive disadvantage otherwise.
Two means of maintaining variation are through context-dependent and background-dependent fitness (Charlesworth and Charlesworth 2010). The six genotypes (2 X by 3 Y) show both differences in sperm development and significant fertility effects of X, Y, and their interaction. In both development and fertility, the standard X by susceptible Y genotype (XSTYsus) was at least as fit as the others. The susceptible Y paired with the sex-ratio X chromosome (XSRYsus) has essentially zero fitness, as nearly no males were produced. The lack of a Y chromosome had little fitness cost when paired with a standard X chromosome (XSTO), though there was some evidence that XSTO had some nearly empty sperm cysts. Sex-ratio males lacking the Y chromosome (XSRO) sired only sons but were nearly sterile and therefore had little fitness advantage over the susceptible Y when paired with a driving X. Both the relatively high fitness of XSTO males and the near sterility of XSRO males is in contrast with previous work (Voelker 1972; Voelker and Kojima 1972) which found a severe cost of the O in cage experiments (selection coefficient of 0.24 to 0.38), but they also found that XSRO males sired many more sons (but still nearly no daughters). Though the cage experiments are quite different from our 48-hr sperm depletion assay, it seems likely that genetic background (autosomes and/or X chromosome) may influence the fitness of males lacking a Y chromosome. The resistant Y chromosome appears to carry a fertility cost—perhaps explaining its failure to sweep through the population. Even though XSRYres males sired fewer offspring overall than XSRYsus males, the fitness of the Y chromosome depends only on sons produced and this is obviously much more in XSRYres than XSRYsus males.
Using previously published models, we can predict the ability of each of our Y chromosomes to invade a population. First, assume that the frequency of drive in the population is 10% (R. L. Unckless, personal observation). Stocks that are homozygous for XSR are healthy and easy to maintain, so a nonquantitative conclusion is that the fitness cost of XSR is not severe. Since we do not know the fitness costs of drivers in females, we assume either additive costs or purely recessive costs and calculate based on other known parameters. Using fitted estimates of the total offspring count in our 48-hr sperm depletion analysis, and assuming additive costs in females, and that all costs are to fertility, not viability, then with the model proposed by Clark (1987), a stable frequency of 9% for sex-ratio X will be obtained if XSR has a 36.8% fitness cost in males, a 10.5% cost in heterozygous females, and a 21% cost in homozygous females. Assuming that XSTO males have a fitness cost of 10%, invasion of O can only occur if XSRO males have a fitness cost of ∼20% or less. But the data clearly show a much more pronounced fitness cost to XSRO males, making it very difficult by this simple model to explain invasion of the O state. Furthermore, Voelker and Kojima (1972) calculated large fitness costs (24–38%) to XSTO males in cage experiments, making the O invasion even less likely. With all else equal, assuming fitness costs in females are recessive, even if recessive (XSRXSR) females were sterile (which they are not), the equilibrium frequency of the driver in males would be ∼11%. In this case, the fitness costs to XSRO males would need to be less than ∼50% for invasion of the O.
This leads us to believe that the O is maintained not by selection for resistance to drive, but by haploid mutation selection balance at a frequency of P = μ/s, where μ is the rate of nondisjunction leading to loss of Y (or X) and s is the selection coefficient against the O. Ignoring the role of meiotic drive, then if the rate of nondisjunction is 0.1% (the rate estimated for D. quinaria; Jaenike 1999), and if s is on the order of 0.2, the expected frequency of O in a natural population would be 0.5%. We strongly suspect that both our Y-replacement lines that are XO lost their Y chromosomes while in culture (sex ratios were initially not male biased in the presence of XSR), and this would be consistent with a frequency in the wild of 1/200 males. It is curious, however, that two lines would lose their Y chromosomes during the tens of generations since capture. Low effective population sizes in lab culture would facilitate the O drifting to fixation after the Y was lost in an individual, but would also result in many fewer nondisjunction events summed across the vial. So for the O to arise and fix in two different lab stocks, it seems likely that natural rates of nondisjunction are actually quite high for D. affinis.
With the same parameters as discussed above, and assuming (based on fitted data from the 48-hr sperm depletion assays) a fitness cost of 8% in XSTYres males, 36.7% in XSRYres males, and restoration of 50% female sex ratios sired by XSRYres males and additive fitness costs in females, the resistant Y cannot invade either. In this case, the cost in XSRYres males would need to be almost zero for invasion. If fitness costs on females were recessive, the XSRYres males would need to be <25%. We can imagine at least three reasons that the resistant Y is found in populations. First, as discussed above, fitness costs might be background dependent, possibly leading to an overestimate of fitness costs in our experiment. Second, a driver at higher frequency in the past would select for a resistant Y with exactly these fitness costs. Given the magnitude of fitness differences imposed by the sex-ratio drive system, this magnitude of changes in fitness seems quite plausible over short time scales. Finally, equilibrium frequencies in this model are sensitive to slight changes in parameter values, and therefore by tweaking fitness values by a few percent, invasion may be possible.
The model discussed above ignores sperm competition, which is likely important in many sex-ratio systems (Taylor and Jaenike 2002). Though of interest, to our knowledge there is no published model that takes into account both Y-linked resistance and sperm competition, and constructing and analyzing such a model is beyond the scope of this paper. Furthermore, we have no knowledge of the effect of the resistant Y chromosome on sperm competition when paired with either XSR or XST, so we therefore do not explore sperm competition further at this time.
An examination of sperm development is largely consistent with fertility defects seen in the 48-hr sperm depletion assay. The most extreme case is XSRO males, who never had a single normal spermatid. Interestingly, however, their sperm cysts looked distinct from pathologies seen with other genotypes. In addition to the membraneless bundles of XSRO males, we identified three other likely pathological phenotypes. First several spermatids appear to be underdeveloped (* in Figure 4C and inset in Figure 4E). It is unclear whether these are developing incorrectly or just slow to develop, but in the case of XSTO males, these make up the majority of the cyst. Sex-ratio males (XSRYsus) have both spermatids that appear to be degraded (@ in Figure 4B) and spermatids that appear paired with each other. Finally, resistant sex-ratio males (XSRYres) appear to have several vacuoles or in the process of degrading spermatids. Thus it appears that the pathologies associated with several of the genotypes are different. This may even be true within a single male’s sperm bundles. This suggests that the actual defect in Y-bearing sperm is inflicted earlier—likely during or directly after meiosis—and that there are several ways development can go wrong after that point.
Supplementary Material
Acknowledgments
We thank Eduardo Gonzalez and Alix Gresov for assistance with stock maintenance and experiments. Brian Charlesworth provided our standard lab strain of D. affinis. John Jaenike and Kelly Dyer assisted with Drosophila collections. All electron microscropy was performed at the Microscopy Core Facility at the University of Rochester. We also thank two anonymous reviewers who provided valuable comments and suggestions. This work was supported by National Institutes of Health (NIH) grant R01-GM064590 to A.G.C., NIH grant F32-HD071703 to R.L.U., and NIH grant F32-GM105317 to A.M.L.
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.173948/-/DC1.
Communicating editor: D. J. Begun
Literature Cited
- Branco A. T., Tao Y., Hartl D. L., Lemos B., 2013. Natural variation of the Y chromosome suppresses sex ratio distortion and modulates testis-specific gene expression in Drosophila simulans. Heredity 111: 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt A., Trivers R., 2009. Genes in Conflict: The Biology of Selfish Genetic Elements, Harvard University Press, Cambridge, MA. [Google Scholar]
- Carvalho A. B., Vaz S. C., Klaczko L. B., 1997. Polymorphism for Y-linked suppressors of sex-ratio in two natural populations of Drosophila mediopunctata. Genetics 146: 891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A. B., Clark A. G., 2005. Y chromosome of D. pseudoobscura is not homologous to the ancestral Drosophila Y. Science 307: 108–110. [DOI] [PubMed] [Google Scholar]
- Charlesworth, B., and D. Charlesworth, 2010 Elements of Evolutionary Genetics, Roberts and Company Publishers, Greenwood Village, CO. [Google Scholar]
- Clark A. G., 1987. Natural selection and Y-linked polymorphism. Genetics 115: 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T., 1935. The Y Chromosome of Drosophila Pseudoobscura. Genetics 20: 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer K. A., Charlesworth B., Jaenike J., 2007. Chromosome-wide linkage disequilibrium as a consequence of meiotic drive. Proc. Natl. Acad. Sci. USA 104: 1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. A., 1930. The Genetical Theory of Natural Selection, The Claredon Press, Oxford. [Google Scholar]
- Frank S. A., 1991. Divergence of meiotic drive-suppression systems as an explanation for sex-biased hybrid sterility and inviability. Evolution 45: 262–267. [DOI] [PubMed] [Google Scholar]
- Hall D. W., 2004. Meiotic drive and sex chromosome cycling. Evolution 58: 925–931. [DOI] [PubMed] [Google Scholar]
- Hamilton W. D., 1967. Extraordinary sex ratios. A sex-ratio theory for sex linkage and inbreeding has new implications in cytogenetics and entomology. Science 156: 477–488. [DOI] [PubMed] [Google Scholar]
- Hurst L. D. and A. Pomiankowski, 1991. Causes of sex-ratio bias may account for unisexual sterility in hybrids: a new explanation of Haldane’s rule and related phenomena. Genetics 128: 841–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenike J., 1999. Suppression of sex-ratio meiotic drive and the maintenance of Y-chromosome polymorphism in Drosophila. Evolution 53: 164–174. [DOI] [PubMed] [Google Scholar]
- Jaenike J., 2001. Sex chromosome meiotic drive. Annu. Rev. Ecol. Syst. 32: 25–49. [Google Scholar]
- James A. C., Jaenike J., 1990. “Sex ratio” meiotic drive in Drosophila testacea. Genetics 126: 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott S. R., Noor M. A. F., 2010. The role of meiotic drive in hybrid male sterility. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365: 1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. D. and L. E. Stone, 1962. Reinvestigation of karyotype in Drosophila affinis Sturtevant and related species. J. Hered. 53: 12–24. [DOI] [PubMed] [Google Scholar]
- Miller D. D. and R. Roy, 1964. Further data on Y chromosome types in Drosophila athabasca. Can. J. Genet. Cytol. 259: 334–348. [DOI] [PubMed] [Google Scholar]
- Montchamp-Moreau C., Ginhoux V., Atlan A., 2001. The Y chromosomes of Drosophila simulans are highly polymorphic for their ability to suppress sex-ratio drive. Evolution 55: 728–737. [DOI] [PubMed] [Google Scholar]
- Morgan T. H., Bridges C. B., Sturtevant A. H., 1925. The genetics of Drosophila. Bibliogr. Genet. 2: 1–262. [Google Scholar]
- Noguchi T., Lenartowska M., Rogat A. D., Frank D. J., Miller K. G., 2008. Proper cellular reorganization during Drosophila spermatid individualization depends on actin structures composed of two domains, bundles and meshwork, that are differentially regulated and have different functions. Mol. Biol. Cell 19: 2363–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadnis N., Orr H. A., 2009. A single gene causes both male sterility and segregation distortion in Drosophila hybrids. Science 323: 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip U., 1944. Crossing-over in the males of Drosophila subobscura. Nature 153: 222–223. [Google Scholar]
- Pinzone C. A., Dyer K. A., 2013. Association of polyandry and sex-ratio drive prevalence in natural populations of Drosophila neotestacea. Proc. Biol. Sci. 280: 20131397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, J. R., 1997 Progress and Prospects in Evolutionary Biology: The Drosophila Model, Oxford University Press, New York. [Google Scholar]
- Price T. A. R., Hodgson D. J., Lewis Z., Hurst G. D. D., Wedell N., 2008. Selfish genetic elements promote polyandry in a fly. Science 322: 1241–1243. [DOI] [PubMed] [Google Scholar]
- Rathke C., Baarends W. M., Jayaramaiah-Raja S., Bartkuhn M., Renkawitz R., et al. , 2007. Transition from a nucleosome-based to a protamine-based chromatin configuration during spermiogenesis in Drosophila. J. Cell Sci. 120: 1689–1700. [DOI] [PubMed] [Google Scholar]
- Stalker H. D., 1961. The genetic systems modifying meiotic drive in Drosophila paramelanica. Genetics 46: 177–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Hartl D. L., Laurie C. C., 2001. Sex-ratio segregation distortion associated with reproductive isolation in Drosophila. Proc. Natl. Acad. Sci. USA 98: 13183–13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Araripe L., Kingan S. B., Ke Y., Xiao H., et al. , 2007. A sex-ratio meiotic drive system in Drosophila simulans. II: an X-linked distorter. PLoS Biol. 5: 2576–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. E., Jaenike J., 2002. Sperm competition and the dynamics of X chromosome drive: stability and extinction. Genetics 160: 1721–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T., Peacock W. J., Hardy R. W., 1972. Dynamics of spermiogenesis in Drosophila melanogaster. I. Individualization process. Z. Zellforsch. Mikrosk. Anat. 124: 479–506. [DOI] [PubMed] [Google Scholar]
- R Development Core Team, 2014 R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing, Vienna. Available at: www.R-project.org/.
- Unckless R. L., Clark A. G., 2014. Sex-ratio meiotic drive and interspecific competition. J. Evol. Biol. 27: 1513–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker R. A., 1972. Preliminary characterization of “sex ratio” and rediscovery and reinterpretation of “male sex ratio” in Drosophila affinis. Genetics 71: 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker R. A., Kojima K. I., 1971. Fertility and fitness of XO males in Drosophila I. Qualitative study. Evolution 25: 119–128. [DOI] [PubMed] [Google Scholar]
- Voelker R. A., Kojima K., 1972. Fertility and fitness of XO males in Drosophila. II. Quantiative analysis. Evolution 26: 560–573. [DOI] [PubMed] [Google Scholar]
- White M., 1973. Animal Cytology and Evolution, Cambridge University Press, Cambridge. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.