Abstract
This study examines the activity and tolerability of a regimen combining vorinostat and rituximab in patients with indolent B-cell non-Hodgkin lymphoma. A total of 28 patients with newly diagnosed or relapsed/refractory follicular, marginal zone, or mantle cell lymphoma, with 4 or less prior therapies were eligible for this open-label phase II study. Oral vorinostat 200 mg was administered twice daily on days 1–14 along with 375 mg/m2 of intravenous rituximab on day 1 of a 21-day cycle, continuing until disease progression or unacceptable toxicity. Primary end point was objective response rate, with secondary end points of progression-free survival, time to progression, duration of response, safety, and tolerability. Median follow up was 25.6 months and median number of vorinostat cycles was 11.5. Overall response rate was 46% for all patients, 67% for previously untreated, and 41% for relapsed/refractory patients. Median progression-free survival was 29.2 months for all patients, 18.8 months for previously treated patients, and not reached for untreated patients. The regimen was well tolerated over long treatment periods with the most common grade 3/4 adverse events being asymptomatic thrombosis, neutropenia, thrombocytopenia, lymphopenia, and fatigue. The vorinostat/rituximab combination exhibits activity in indolent B-cell non-Hodgkin lymphoma with an acceptable safety profile and durable responses. Re-treatment was effective in 2 of 3 relapsing responders. This phase II clinical trial was registered at clinicaltrials.gov identifier: 00720876.
Introduction
The ability to administer drugs aimed at specific epigenetic targets, and thus alter a cancer cell’s epigenetic programming, has led to therapeutic advances in several tumor types. In particular, in lymphoid malignancies, the efficacy of vorinostat, an orally bioavailable synthetic hydroxamic acid class histone deacetylase (HDAC) inhibitor with both histone and protein deacetylase inhibitory activity1–3 has led to FDA approval for treatment of T-cell lymphomas.
The indolent lymphomas are a group of B-lymphoid malignancies with a propensity towards slower growth that remain incurable following progressively shorter cycles of response and recurrence. Given the pre-clinical activity of HDAC inhibitors in lymphoid neoplasms, and the desire to discover alternatives to repeated courses of chemotherapy, with all the attendant long-term complications, we previously reported on a trial of the HDAC inhibitor vorinostat as a single agent in follicular, marginal zone and mantle cell non-Hodgkin lymphoma (NHL). In a phase II study of oral vorinostat 200 mg given twice daily for 14 days of a 3-week cycle, we demonstrated activity in patients with relapsed/refractory follicular and marginal zone lymphoma. The overall response rate (ORR) for follicular lymphoma patients, including grade III follicular lymphoma, was 47% [4 complete responses (CRs) and 4 partial responses (PRs) out of 17 patients], with a 22% ORR in marginal zone lymphoma.4 Most striking was the duration of the response, with a median duration of 27 months for responders. We chose this vorinostat schedule due to the short half-life of the drug, and because of issues of tolerability with larger single daily doses. These results were recently confirmed by a pan-Asian multicenter trial, utilizing the same dosing schedule, with an ORR of 49% in relapsed/refractory follicular lymphoma and a median PFS of 20 months.5
Rituximab is a chimeric IgG1 monoclonal antibody targeting the CD20 surface antigen present on B-cell lymphomas that has dramatically enhanced response rates and progression-free survival duration in follicular lymphoma, and has significant single-agent activity.6,7 The original trial using rituximab in rituixmab-naïve patients showed an ORR of 48%, CR of 6%, and median time to progression of 13.0 months.8 Given that pre-clinical data suggest synergy between HDAC inhibitors and rituximab,9,10 and the promising responses seen in our single-agent vorinostat study, we investigated the combination of vorinostat and rituximab in the same population of previously untreated patients with indolent lymphoma. In addition, we investigated the efficacy and tolerability of this combination as a re-treatment strategy for patients who had achieved remission and later relapsed while off treatment.
In a mouse model of allogeneic stem cell transplantation, vorinostat as part of the pre-transplant conditioning regimen showed a marked effect on multiple circulating peripheral blood cytokines.11 Therefore, in this clinical trial, we measured peripheral blood cytokine levels before and after the initial cycle of rituximab plus vorinostat in order to gauge response to the drug and evaluate possible associations of cytokine levels with response outcomes.
Methods
This trial was approved by the City of Hope Institutional Review Board and all patients gave their consent in accordance with the Declaration of Helsinki.
Patient eligibility
Patients over the age of 18 with histologically or cytologically confirmed indolent non-Hodgkin lymphoma were eligible including: untreated, relapsed, or refractory follicular lymphomas of any grade, marginal zone B-cell lymphoma (nodal and extranodal), and mantle cell lymphoma. Patients may have had up to 4 prior regimens, not including steroids alone, rituximab alone, or local radiation.
Treatment and evaluation
One cycle of therapy consisted of oral vorinostat 200 mg twice daily for 14 days followed by a 7-day break, and intravenous rituximab 375 mg/m2 on day 1 of a 21-day cycle. Radiological assessment by computed tomography (CT) and/or positron emission tomography (PET) scan was performed at baseline and after every three cycles. Patients with measurable responses or stable disease were allowed to continue vorinostat and rituximab until progression. Patients who achieved CR received two further cycles of vorinostat and rituxmab and stopped treatment. Patients who stopped treatment in CR were allowed to resume the regimen in the event of relapse.
Toxicity was assessed on days 1 and 8 of each cycle, and graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Treatment was held for patients who presented with non-hematologic toxicity of grade 3 or 4, until toxicity resolved to less than grade 2. These patients resumed treatment with vorinostat at a reduced dose of 100 mg daily. If grade 3 or greater toxicity occurred at the reduced dose, the patient was taken off treatment.
Initially, CT or PET scans were performed after every three cycles of treatment to assess response. After one year of treatment, if the patient had achieved PR on two consecutive CT or PET scans, subsequent radiographic assessment was performed after every four cycles. If a patient achieved CR for more than one year, subsequent radiographic studies were performed every six months rather than every three cycles.
Bone marrow biopsy for the presence of marrow involvement was performed at baseline and every three cycles if the base-line examination revealed marrow involvement. Responses were assessed according to the 2007 Cheson criteria.12,13
Study design
The primary study objective was to evaluate the anti-tumor activity of vorinostat plus rituximab as assessed by overall response rate (ORR=CR+PR). The secondary study objectives were to evaluate the toxicity profile of vorinostat plus rituximab, time to progression, and progression-free survival. The Simon 2-stage optimal design was used to assess the overall response rate. In the first stage, 17 patients were enrolled, with the design specifying that if 4 or more patients achieved CR or PR, accrual would continue to a total of 33 patients, with 10 or more responses regarded as evidence of sufficient activity to warrant further investigation.
Correlatives
The effects of treatment on systemic blood levels of immune cytokines were evaluated by cytokine bead array analysis using Luminex X-MAP bead array technology (Luminex, Austin, TX, USA). Whole blood was drawn peripherally on day 1 pre-treatment and on day 14 of cycle 1.
Results
Patients’ characteristics
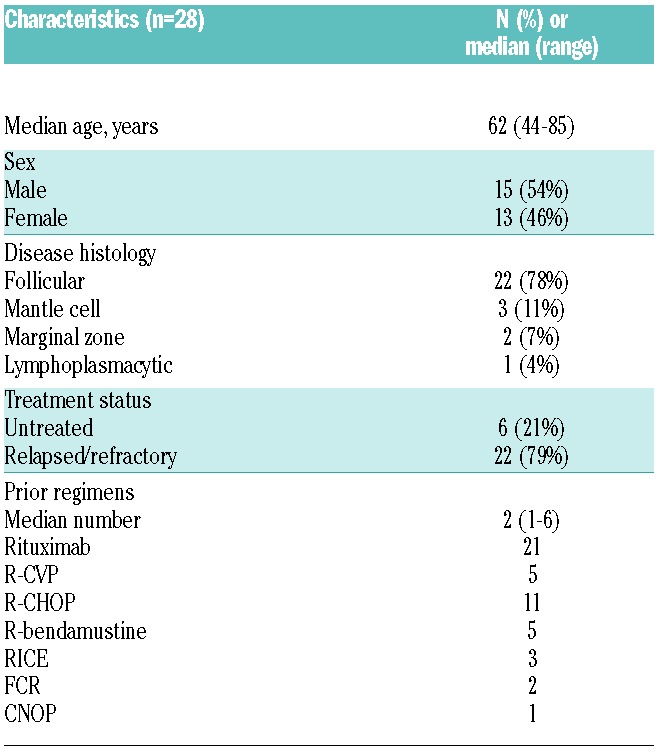
A total of 30 patients were enrolled in the study between July 2008 and January 2013 (Table 1). Two patients were discovered to be ineligible shortly after starting therapy and were excluded from the analysis: one patient was discovered to have angiosarcoma upon repeat biopsy, and another patient was taking a contraindicated drug. In the 28 eligible patients (13 female, 15 male), the median age at treatment was 62 years (range 44–85). Disease histologies included mantle cell (MCL) in 3 patients, marginal zone lymphoma (MZL) in 2 patients, lymphoplasmacytic lymphoma in one patient, and follicular lymphoma (FL) in 22 patients. Grades 1, 2, and 3A follicular lymphomas were included. Twenty-two patients had received prior therapy and 6 patients were previously untreated. Of the untreated patients, one patient had stage 4 MZL and 5 patients had grade I-II FL (2 with stage 3–4 disease and 3 with stage 1–2 disease, 2 of whom had B symptoms). The median number of prior therapies was 2 (range 1–6) in previously treated patients. Previous treatments included rituximab (n=21), R-CVP (rituximab, cyclophosphamide, vincristine, prednisolone) (n=5), R-CHOP (rituximab, cyclophosphamide, adriamycin, vincristine, prednisolone) (n=11), R-bendamustine (n=5), RICE (rituximab, ifosphosphamide, cyclophosphamide, etoposide) (n=3), FCR (fludarabine, cyclophosphamide, rituximab) (n=2), CNOP (cyclophosphamide, mitoxantrone, vincristine, prednisolone) (n=1), and bortezomib-containing regimens (n=4).
Table 1.
Patients’ characteristics.
Efficacy
Twenty-eight of the 30 patients were evaluable for response. The median number of cycles received was 11.5 (range 1–69). Median duration of treatment was 17.8 months (95%CI: 6.2–46.8). Response rates for the overall population and each disease histology are shown in Table 2. ORR was 46% (13/28), with 10 complete responses (36%) and 3 partial responses (11%) reported. By histology, the ORR for FL was 50% (11 of 22, 9 CR). For MCL, one of 3 patients attained a PR, and for MZL, one of 2 patients achieved CR. The ORR and CR rates for previously untreated patients were 67% (4 of 6, all CR). ORR for relapsed/refractory patients was 41% (9 of 22), with a CR rate of 27% (6 of 22). Median PFS was 29.2 months (95%CI: 14.4–51) for all patients, 18.8 months (95%CI: 8.5–51.9) for previously treated patients, and not yet reached for untreated patients. The median follow up for surviving patients was 26.8 months (0.7–62.0). Figure 1A shows the progression-free survival (PFS) for all patients (n=28), estimated at two years as 55% (95%CI: 32–73). When patients were stratified according to whether or not they responded to treatment, 2-year PFS for CR/PR patients was 90.0% (95%CI: 47.3–98.5) and for other patients this was 26.9% (95%CI: 6.7–53.0) (Figure 1B). For the subset of 22 patients with follicular lymphoma, estimated 2-year PFS was 61% (95%CI: 34–79). Patients with follicular lymphoma in CR/PR had 2-year PFS of 87.5% (95%CI: 38.7–98.1), while other patients had 35.8% (95%CI: 8.8–64.8) (Figure 1C). The 2-year PFS for previously untreated patients was 100% (95% CI not established), and for previously treated patients was 43% (95%CI: 19–65).
Table 2.
Patient best response.
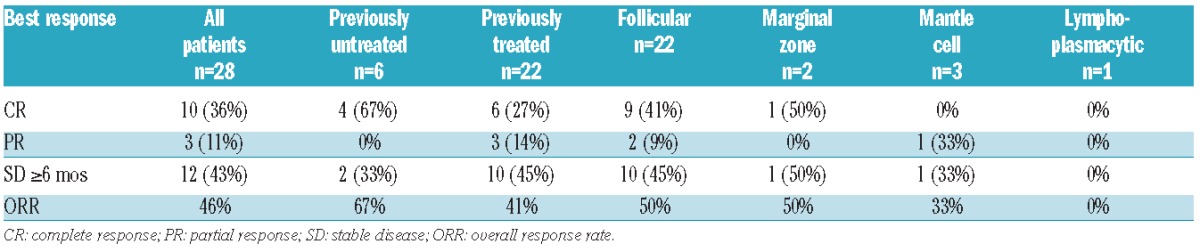
Figure 1.
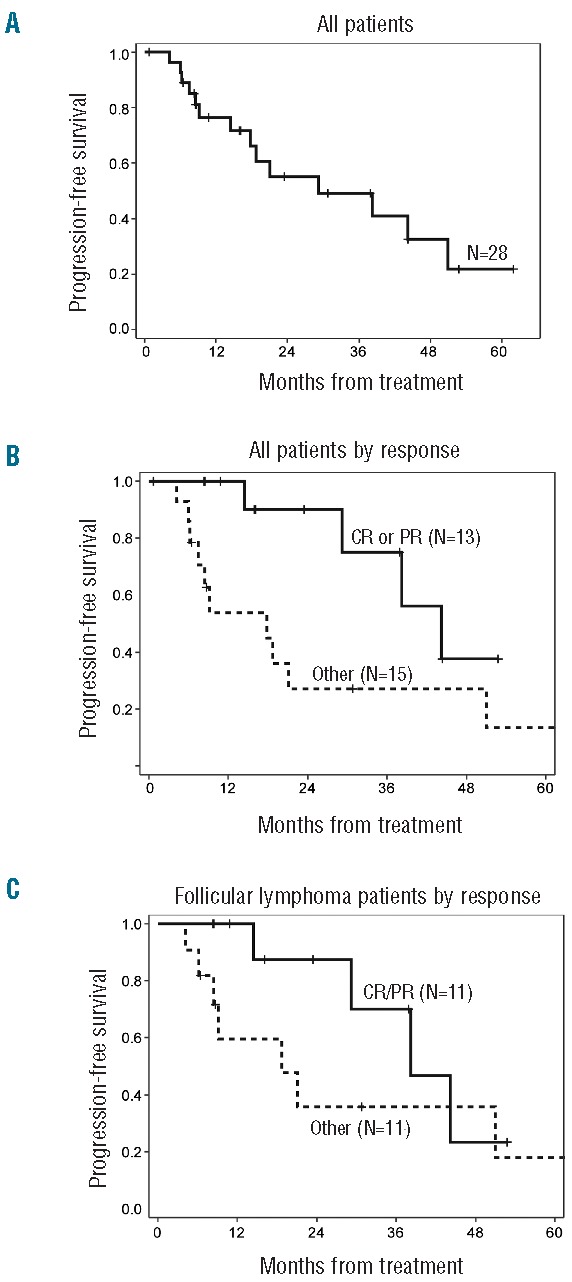
Kaplan-Meier estimation of progression-free survival (PFS) defined as time from initial protocol treatment to progression, relapse, or death from any cause, whichever occurred first. Median follow up for surviving patients was 26.8 months. PFS is shown: (A) for all patients (n=28), (B) for all patients stratified by response, and (C) for patients with follicular lymphoma (n=22) stratified by response.
Patients who achieved CR were allowed to discontinue treatment and resume treatment if lymphoma progressed. Six of 10 CR patients were progression free with a median follow up of 27.0 months (range 8–53 months). Four patients relapsed and resumed re-treatment. Of these 4 re-treated patients, 2 achieved a second CR. The third re-treated patient was initially treated for 23.6 months until achieving CR, with response duration of 14.6 months. At relapse this patient returned to therapy for another 10.5 months with stable disease, after which the patient developed concurrent transformation to both Hodgkin lymphoma and diffuse large B-cell lymphoma (DLBCL) (biopsy proven). The fourth patient had been in re-treatment for less than three cycles at the time the data was locked for analysis for this manuscript, and was not, therefore, evaluable for response. Figure 2 shows the impressive duration of response and response to re-treatment. Figure 2 also illustrates that this combination treatment exhibits a cumulative benefit to disease response. All patients who eventually achieved CR, initially achieved stable disease (SD), and after continued treatment converted to CR, or PR then CR. Of non-responders (no CR or PR), 12 patients achieved SD for at least nine cycles with one patient maintaining SD for 69 cycles. Median duration of SD was 8.9 months (range 4.1–62.0 months). The disease control rate for more than nine cycles (CR + PR + SD >9 cycles) was 89% (25 of 28). Nine patients were taken off study for disease progression. Five patients were taken off study for reasons other than progression (2 patient choice, 1 to transplant, 1 for violation, and 1 physician choice). The median time to treatment failure for all patients was 17.8 months (95%CI: 6.2–47).
Figure 2.
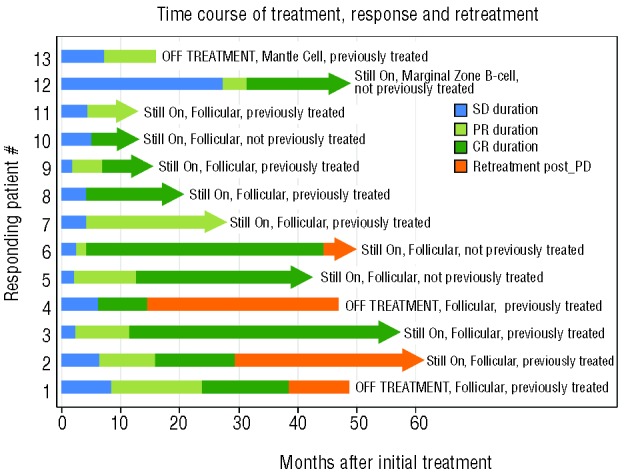
Treatment time course in responding patients. Timing of treatment is shown for the 13 responding patients with duration of first response: stable disease (SD) (blue); duration of partial response (PR) (light green); duration of complete response (CR) (dark green). Duration of re-treatment after progressive disease (PD) (is shown in orange). Patients’ previous treatment status is indicated on the right and an arrow indicates that the patient was on treatment at the time of data analysis.
Treatment and toxicities
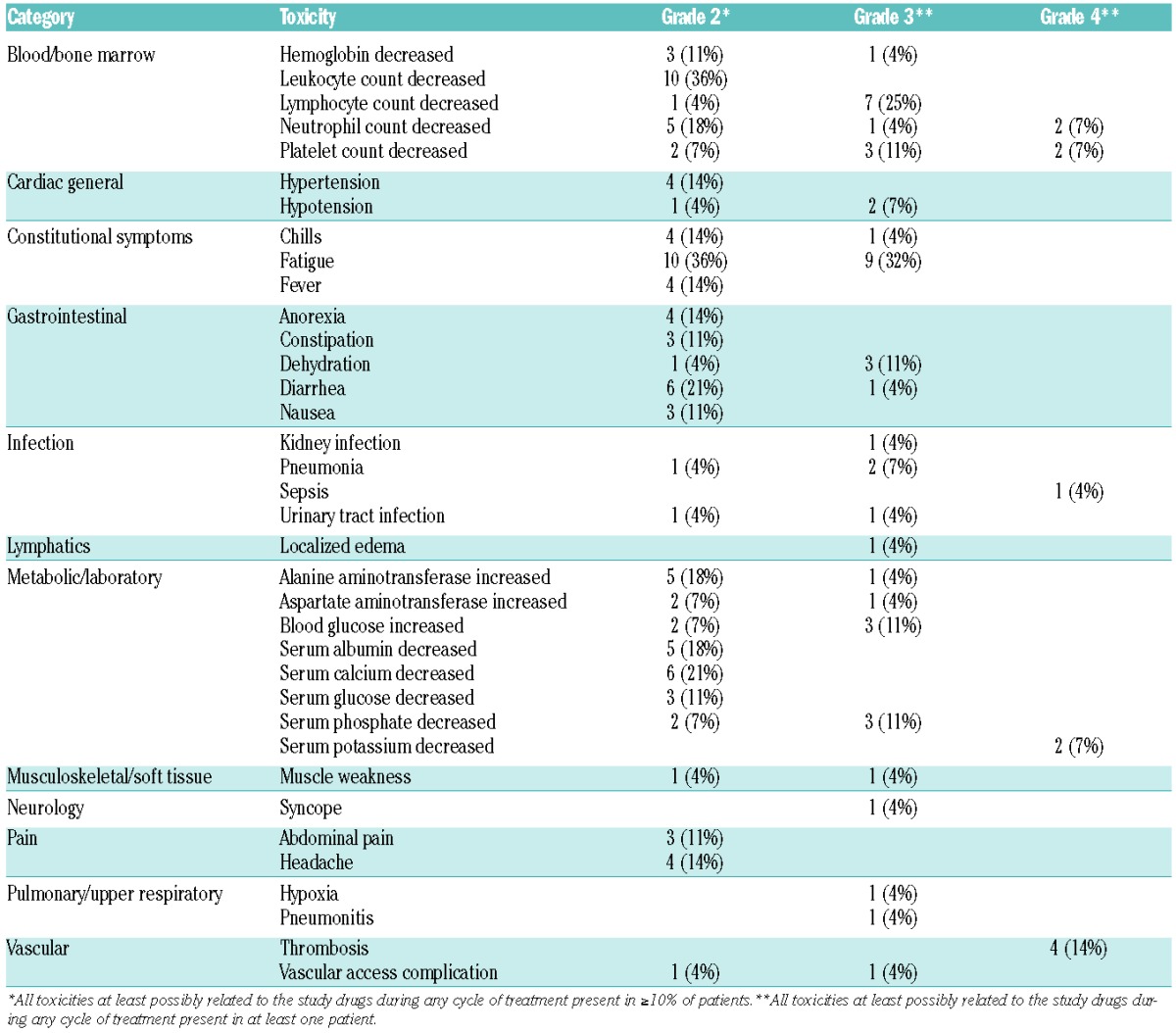
Overall, the combination was well tolerated with few grade 3/4 events. Table 3 shows CTCAE v.3.0 grade 2, 3, and 4 toxicities that were at least possibly attributable to the study drug. Grade 4 toxicities included neutropenia (n=1), incidental asymptomatic thrombosis (n=4), and thrombocytopenia (n=2). Grade 3 toxicities possibly attributable to the study drug, seen in more than 20% of patients, included fatigue (n=9, 32%) and lymphopenia (n=7, 25%). The thromboses were non-clinical pulmonary emboli discovered incidentally on staging CT scans, and resulted in amendment of the study to include prophylaxis. Initially 81 mg of aspirin was used, but after consultation with the sponsor, prophylaxis was changed to 40 mg enoxaparin. No further thromboses were identified after prophylaxis was implemented.
Table 3.
Toxicity graded according to Common Terminology Criteria for Adverse Events version 3.0.
Correlative assays
We have previously demonstrated11 that even low doses of vorinostat dramatically decrease pro-inflammatory cytokines such as IL-2, IFN-γ, TNF-α, and IL-6. This suppression of cytokine production may be of clinical importance for the effects of histone deacetylase inhibition on symptoms such as itching or the B symptoms of lymphoma, as well as potentially impacting lymphoma growth. The effects of treatment on systemic levels of immune cytokines were evaluated by cytokine bead array analysis using Luminex X-MAP bead array technology. We found 8 of the 16 cytokine markers showed statistical decrease (P<0.05) on day 14 as compared to day 0 (IL1RA, IL2R, IL12p40p70, MIG, EOTAXIN, MCP1, EGF, and FGFBasic), and none showed a significant increase (Figure 3). However, the cytokine measurements on day 0 and day 14 did not distinguish between responders and non-responders.
Figure 3.
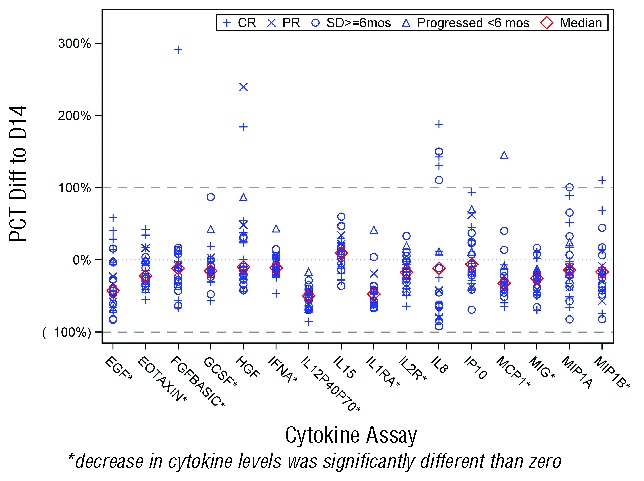
Changes in cytokine levels in 14 days of treatment. Systemic levels of 16 cytokines (IL-1RA, IL-2R, IL-8, IL-12p40/p70, IL-15, IFN-α, MIP-1α, MIP-1β, IP-10, MIG, Eotaxin, MCP-1, G-CSF, EGF, FGF-basic, and HGF). were compared between day 1 pre-treatment and day 14 post-treatment and results are depicted graphically for each cytokine assay (x-axis) with each patient plotted as percent difference between day 1 and day 14 cytokine levels (y-axis). Individual patients are plotted with disease response indicated by the symbols depicted in the legend. Median for each assay is indicated by the red diamond.
Discussion
Vorinostat is an oral HDAC inhibitor with histone and protein deacetylase activity, currently FDA approved for cutaneous T-cell lymphoma. In our initial phase II study in indolent lymphomas, vorinostat demonstrated efficacy as single-agent therapy against relapsed/refractory follicular lymphoma, including grade 3 follicular lymphoma.4 Given its efficacy as a single agent in patients with relapsed/refractory indolent NHL, we conducted a trial of vorinostat plus rituximab for patients with both newly diagnosed and relapsed/refractory indolent NHL. We hypothesized that this regimen would be well tolerated and effective in the up-front as well as the relapsed/refractory setting, given the responses seen in the single-agent study. Furthermore, this study also addressed the question of whether the regimen would continue to be active for re-treatment after progression.
Given that this is a single arm study, it was not designed to show improved efficacy over the original single agent rituximab or vorinostat trials.4,8 However, the results of this trial do suggest that the combination regimen may have an enhanced response rate as compared to single-agent vorinostat or rituximab. For single-agent vorinostat, the overall response rate was 29%, with 5 CRs and 5 PRs, whereas for the combination, the overall response rate was 46%, with a higher proportion of the patients achieving complete remissions (10 CR, 3 PR). While the overall response rate for the follicular lymphoma group was similar, the CR rate was higher in the combination study (4 CR and 4 PR in the single agent study, 9 CR and 3 PR in the combination study). For single-agent rituximab, ORR was 48% and CR 6% in the rituixmab-naïve population, whereas for the combination in untreated patients, ORR and CR were both 67%. Of note, we observed one PR in a patient with mantle cell lymphoma, whereas there were no formal responses in mantle cell in the original vorinostat study. It is worthy of note that re-treatment was effective in 2 of 3 evaluable patients who had originally responded and then progressed, with the third patient progressing a second time with a novel presentation of combined DLBCL and Hodgkin lymphoma.
Overall, the combination regimen was well tolerated, with many patients able to remain on outpatient therapy for an extended number of cycles, as was also the case for single-agent vorinostat on this dosing schedule. Very little additional toxicity was seen with the addition of rituximab; however, 4 cases of asymptomatic subsegmental pulmonary emboli were found during the scans performed for assessment of response. None of the patients suffered repeat episodes, nor were there clinical sequelae. The original vorinostat single-agent and rituximab single-agent studies do not show increased incidence of thrombosis. It is unclear if subsegmental emboli of this sort are clinically meaningful or require treatment; however, for reasons of patient safety, we subsequently instituted preventative anticoagulation on all patients and no further cases of thrombosis were seen.
Although vorinostat does not target a specific pathway, there are several potential mechanistic explanations for the activity of vorinostat and rituximab in B-cell lymphomas. Overexpression of cytokines by immune cells in the tumor microenvironment of follicular lymphoma can stimulate tumor growth and survival.14 Preclinical data in animals suggest that vorinostat has a potent effect on multiple proinflammatory cytokines, which may be relevant in particular to follicular lymphoma.11 For this reason, we tested patients pre- and post-treatment to assess the feasibility of this assay as a biomarker for vorinostat activity and efficacy. Of 30 cytokines assayed, 16 gave interpretable results, of which 8 showed statistically consistent downregulation after treatment with vorinostat and rituximab. Although the downregulation of cytokines did not correlate with formal response, it is possible that addition of further time points or additional cytokines would enhance the correlation with response, as has been seen for Hodgkin lymphoma.15 The exact mechanism of enhanced response in vitro upon combination of epigenetic agents with rituximab is unclear, although such enhanced activity has been noted in prior reports.9,10 There is some suggestion that the vorinostat suppression of MYC already reported by our group16 may be involved in the enhanced response to rituximab, similar to the sensitization to rituximab seen with CYCLON inhibition of MYC-over-expressing tumors by Emalid et al.17 However, further work is necessary given the multiple downstream activities of both rituximab and vorinostat.
In summary, this study demonstrates that the combination of vorinostat and rituximab is an effective and well-tolerated regimen in the up-front, relapsed, and re-treatment settings. This combination appears promising and could be expanded to a randomized phase II or III setting, However, this trial was initiated five years ago, and recent advances have produced a variety of biological agents and targeted therapy for the treatment of indolent non-Hodgkin’s lymphoma. Lenalidomide, an immune modulator, has been used as single agent in patients with relapsed indolent NHL and showed an overall response rate of 23% and CR rate of 7%.18 Bortezomib, a proteasome inhibitor, has been used with rituximab in patients with follicular lymphoma showing an overall response rate of 49%.19 Ibrutinib, a Bruton tyrosine kinase inhibitor, is undergoing clinical trial evaluation for indolent NHL and Fowler et al. presented preliminary results at ASH 2012 showing an ORR of 54.5%.20 CAL-101 or idelalisib, a PI3K inhibitor, has recently been tested in a phase II study for patients with relapsed/refractory indolent NHL showing a response rate of 57% and CR rate of 6%.21 Many of these novel targeted agents demonstrate reasonable activity but have low CR rates and short duration of response, and there is room for improvement. The majority of these agents are well tolerated and thus amenable to combination strategies. Rational combination of these novel drugs (lenalidomide, bortezomib, bendamustine, idelasib, or ibrutinib) with vorinostat and rituximab should be explored given the promising activity, prolonged duration of response, and long-term tolerability of the vorinostat / rituximab regimen.
Acknowledgments
We would like to thank the City of Hope staff and nurses without whom this work would not be possible. RC is a K12 Calabresi Career Development Scholar.
Footnotes
Funding
This clinical trial was supported by Merck. Data collection and analysis was partially supported by the City of Hope Comprehensive Cancer Center grant NIH P30 CA33572. RC is supported by the National Cancer Institute of the National Institutes of Health under award number K12CA001727 and CCITLA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Ellis L, Hammers H, Pili R. Targeting tumor angiogenesis with histone deacetylase inhibitors. Cancer Lett. 2009;280(2):145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frew AJ, Johnstone RW, Bolden JE. Enhancing the apoptotic and therapeutic effects of HDAC inhibitors. Cancer Lett. 2009;280(2):125–133. [DOI] [PubMed] [Google Scholar]
- 3.Maeda T, Towatari M, Kosugi H, Saito H. Up-regulation of costimulatory/adhesion molecules by histone deacetylase inhibitors in acute myeloid leukemia cells. Blood. 2000;96(12):3847–3856. [PubMed] [Google Scholar]
- 4.Kirschbaum M, Frankel P, Popplewell L, et al. Phase II study of vorinostat for treatment of relapsed or refractory indolent non-Hodgkin’s lymphoma and mantle cell lymphoma. J Clin Oncol. 2011;29(9):1198–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogura M, Ando K, Suzuki T, et al. A multi-centre phase II study of vorinostat in patients with relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Br J Haematol. 2014;165(6):768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colombat P, Salles G, Brousse N, et al. Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: clinical and molecular evaluation. Blood. 2001;97(1):101–106. [DOI] [PubMed] [Google Scholar]
- 7.Martinelli G, Hsu Schmitz S-F, Utiger U, et al. Long-Term Follow-Up of Patients With Follicular Lymphoma Receiving Single-Agent Rituximab at Two Different Schedules in Trial SAKK 35/98. J Clin Oncol. 2010;28(29):4480–4484. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825–2833. [DOI] [PubMed] [Google Scholar]
- 9.Shi W, Han X, Yao J, Yang J, Shi Y. Combined effect of histone deacetylase inhibitor suberoylanilide hydroxamic acid and anti-CD20 monoclonal antibody rituximab on mantle cell lymphoma cells apoptosis. Leuk Res. 2012;36(6):749–755. [DOI] [PubMed] [Google Scholar]
- 10.Zhao WL, Wang L, Liu YH, et al. Combined effects of histone deacetylase inhibitor and rituximab on non-Hodgkin’s B-lymphoma cells apoptosis. Exp Hematol. 2007; 35(12):1801–1811. [DOI] [PubMed] [Google Scholar]
- 11.Li N, Zhao D, Kirschbaum M, et al. HDAC inhibitor reduces cytokine storm and facilitates induction of chimerism that reverses lupus in anti-CD3 conditioning regimen. Proc Natl Acad Sci USA. 2008; 105(12):4796–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheson BD. The International Harmonization Project for response criteria in lymphoma clinical trials. Hematol Oncol Clin North Am. 2007;21(5):841–854. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4): 1244–1253. [DOI] [PubMed] [Google Scholar]
- 14.Epron G, Ame-Thomas P, Le Priol J, et al. Monocytes and T cells cooperate to favor normal and follicular lymphoma B-cell growth: role of IL-15 and CD40L signaling. Leukemia. 2012;26(1):139–148. [DOI] [PubMed] [Google Scholar]
- 15.Harrison SJ, Hsu AK, Neeson P, et al. Early thymus and activation-regulated chemokine (TARC) reduction and response following panobinostat treatment in patients with relapsed/refractory Hodgkin lymphoma following autologous stem cell transplant. Leuk Lymphoma. 2014; 55(5):1053–1060. [DOI] [PubMed] [Google Scholar]
- 16.Kretzner L, Scuto A, Dino PM, et al. Combining histone deacetylase inhibitor vorinostat with aurora kinase inhibitors enhances lymphoma cell killing with repression of c-Myc, hTERT, and microRNA levels. Cancer Res. 2011; 71(11):3912–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emadali A, Rousseaux S, Bruder-Costa J, et al. Identification of a novel BET bromodomain inhibitor-sensitive, gene regulatory circuit that controls Rituximab response and tumour growth in aggressive lymphoid cancers. EMBO Mol Med. 2013;5(8):1180–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witzig TE, Wiernik PH, Moore T, et al. Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin’s Lymphoma. J Clin Oncol. 2009;27(32):5404–5409. [DOI] [PubMed] [Google Scholar]
- 19.de Vos S, Goy A, Dakhil SR, et al. Multicenter randomized phase II study of weekly or twice-weekly bortezomib plus rituximab in patients with relapsed or refractory follicular or marginal-zone B-cell lymphoma. J Clin Oncol. 2009;27(30):5023–5030. [DOI] [PubMed] [Google Scholar]
- 20.Fowler NH, Advani RH, Sharman J, et al. The Bruton’s Tyrosine Kinase Inhibitor Ibrutinib (PCI-32765) Is Active and Tolerated in Relapsed Follicular Lymphoma. ASH Annual Meeting Abstracts. 2012; 120:156. [Google Scholar]
- 21.Gopal AK, Kahl BS, de Vos S, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]


