Abstract
Background
Food protein-induced enterocolitis syndrome (FPIES) is a gastrointestinal hypersensitivity disorder with a poorly understood pathophysiology and no biomarkers to aid in diagnosis.
Objective
To investigate humoral and cellular responses to casein in children with milk-FPIES, including the role of casein-specific (cs) IgA and T-cell mediated TGF-β responses.
Patients and methods
Thirty-one children previously diagnosed with milk-FPIES were challenged with milk. Twelve age-matched children with FPIES to other foods and 6 milk-tolerant children without a history of FPIES were used as controls. Casein-specific IgE, IgG, IgG4 and IgA were measured in serum and TGF-β levels in supernatants of casein-stimulated PBMCs.
Result
Twenty-six children with milk-FPIES reacted (active milk-FPIES) and five tolerated milk (milk-FPIES-resolved) during food challenge. All of them had significantly lower levels of csIgG, csIgG4 and csIgA than control children (p-value<0.001). There were no TGF-β responses in supernatants of active milk-FPIES children.
Conclusion
Children with milk-FPIES have low levels of csIgG, csIgG4 and csIgA. In particular, children with active FPIES to cow’s milk have deficient T-cell mediated TGF-β responses to casein, rendering TGF-β a promising biomarker in identifying children who are likely to experience FPIES reactions to this allergen. Prospective studies are needed to validate these findings, elucidate their role in FPIES pathophysiology and establish the diagnostic utility of TGF-β in milk-induced FPIES.
Keywords: milk allergy, casein, immunoglobulin A, IgA, IgG4, transforming growth factor β, TGF-β, peripheral blood mononuclear cell, PBMCs, food challenge
INTRODUCTION
Food protein-induced enterocolitis syndrome (FPIES) is a non-IgE mediated food hypersensitivity disorder primarily affecting formula-fed infants and young children (1). The clinical features of FPIES encompass chronic non-specific gastrointestinal symptoms including intermittent emesis and diarrhea with continuous exposure to a triggering food, as well as severe symptoms on re-exposure to a trigger following dietary exclusion, including repetitive, projectile emesis, abdominal distention, lethargy, dehydration, bloody diarrhea, metabolic acidosis, hypotension and hypovolemic shock (2). Severe symptoms are common and may be the first manifestation of FPIES. The clinical presentation mimics gastrointestinal infections and other gastrointestinal disorders (e.g. intussusception, volvulus, pyloric stenosis), sepsis, food poisoning, metabolic and neurologic disorders and must be differentiated from other gastrointestinal food allergy entities, such as proctocolitis and eosinophilic gastroenteropathies. Weight loss and failure to thrive may further complicate the differential diagnosis and contribute to poor recognition and mis-diagnosis of FPIES (3).
As a consequence of the limited understanding of the underlying pathophysiology, there are no biomarkers specific for FPIES (4). Currently the diagnosis of FPIES is based on the history, clinical symptoms, exclusion of other gastrointestinal pathology with similar non-specific symptoms and oral food challenges (OFC) (5). Although OFC may be omitted in the case of a convincing history and absence of symptoms after eliminating the causative food (1), they are necessary for less clear-cut presentations and for evaluating FPIES resolution. Elimination of the causative food is the only therapeutic approach. OFC for FPIES is considered a high risk procedure and should be performed in hospital setting, with fluid resuscitation available.
FPIES has been described predominantly in cow’s milk-formula-fed infants and extremely rarely during exclusive breastfeeding, even though cow’s milk proteins are being passed in the maternal milk (6). It is not clear how exclusive breastfeeding prevents FPIES. It has been suggested that breast milk IgA, either alone or as a complex with secreted antigens, may play a protective role (7) by minimizing the potentially harmful antigenicity of these milk proteins in the immature gastrointestinal tract during early life.
Considering the potentially important role of IgA, we hypothesized that this antibody class and the major regulatory cytokine for IgA, TGF-β, which can induce IgA isotype switch and B cell activation (8, 9), may play a role in the pathophysiology of FPIES.
SUBJECTS AND METHODS
Subjects
Children previously diagnosed with milk FPIES underwent an oral food challenge (OFC) in the inpatient Mount Sinai Clinical Research Unit to evaluate for resolution, usually 12–18 months following the most recent FPIES reaction to milk. During OFC, milk protein (0.06–0.6 g/kg body weight, based on the severity of the reactions described in the personal history) was administered in three equal portions over an hour. Pre-determined diagnostic criteria should be all met to define a positive OFC; these were emesis, diarrhea and increase in blood polymorphonuclear leukocyte count > 3,500 cells/mm3 peaking at 6 hours after the first symptoms (10). Following a negative (asymptomatic) OFC, children were observed for 4 hours, whereas following a positive (symptomatic) challenge they were treated and observed until stable, usually discharged within 6 hours. Blood samples were obtained immediately before the OFC (CBC, serum for humoral studies), 4 hours after the negative OFC, and 6 hours after the positive OFC (CBC T cell studies). Three control groups were also included: children with FPIES to foods other than milk (other-food-FPIES group); children with IgE-mediated milk allergy who had developed tolerance to regular milk and atopic children who never experienced milk allergy, regularly consume milk and without history of FPIES to any food (negative controls). Eligible subjects were all referred children to the inpatient Mount Sinai Clinical Research Unit with a diagnosis of milk-FPIES and available serum samples. The study was approved by the Mount Sinai Institutional Review Board and informed consent was obtained from the study subjects’ parents or legal guardians.
Serum casein-specific IgE and IgG4 levels
Casein-specific (cs) IgE (limits of assay 0.1–100 kUA/L), IgG (0.02–2 mgA/mL) and IgG4 (0–300 μgA/L) levels were measured with the UniCAP system (ThermoFisher Scientific, Portage, MI).
Serum total IgA and casein-specific IgA ELISA
Total and csIgA concentrations were determined by sandwich ELISA based on a previously described technique (11). Briefly, 96-well Immulon 4HBX plates (Fisher Scientific, Pittsburgh, PA) were coated overnight at 4°C with an α-chain specific goat F(ab)′2 anti-human IgA (InvivoGen, San Diego, CA) antibody at 2 μg/ml for generating the standard curve (range 0.391–50 ng/ml) and for total IgA measurements, and with casein (Sigma-Aldrich, St Louis, Mo) at 5 μg/ml for detection and measurement of csIgA, all diluted in 0.05 mol/L carbonate-bicarbonate buffer (pH 9.6). After washing (x3) with phosphate-buffered saline containing 0.05% Tween-20 (PBS-T), the plates were blocked with 2% BSA in PBS-T (blocking buffer) at 31°C for 60 minutes. Standard [native monomeric IgA1 antibodies isolated from human plasma (GenWay Biotech Inc, San Diego, CA)] and serum samples (prepared in a series of two-fold dilutions in blocking buffer starting from 1:40 for specific IgA and 1:16×104 for total IgA) were pipetted in triplicates of 100 μl and incubated at 31°C for 2 hours. After washing, 100μl/well of goat anti-human IgA – HRP Fc specific (Antibodies Online, Atlanta, GA), diluted 1:2000 in blocking buffer, were applied for 60 minutes at 31°C. Following washing (x6) with PBS-T, 100 μl/well of 2,2′azinobis(3-ethylbenzthiazolinesulfonic acid) peroxidase substrate (ABTS, KPL, Inc, Gaithersburg, MD) were added and allowed to develop at 31°C for 60 minutes. Absorbance values were read at 405 nm using SoftMax Pro© software.
Peripheral blood mononuclear cell casein stimulation and proliferation assay
Peripheral blood mononuclear cells (PBMCs) were obtained 4–6 hours following an OFC, from heparinized blood, by centrifugation in Ficoll-Paque (GE Healthcare, Uppsala, Sweden) and stained with carboxyfluorescein diacetate succinimidyl ester (CFSE, 2.78 μg/ml, AnaSpec Inc, San Jose, Calif) for 10 minutes. Excessive CFSE was removed by washing with medium (AIM-V, Invitrogen, Grand island, NY). A total of 2 × 106 PBMCs were cultured for 7 days with endotoxin-free α, β and κ caseins (total concentration of 50μg/ml) or anti-CD3/CD28 expander beads (Invitrogen Dynal AS, Oslo, Norway) as positive control according to the manufacturer’s protocol, and medium alone in the presence of interleukin-2 (IL-2, 20ng/ml, R&D Systems Inc, Minneapolis, MN) at 37°C. After centrifugation at 300g for 5 minutes, supernatants were collected and stored at −80°C until used.
TGF-β bioassay
Analysis of bioactive TGF-β was performed as previously described (12) with a few modifications, utilizing the mouse fibroblast cell line MFB-F11 [mouse fibroblasts isolated from mouse Tgfb1−/− embryos transfected with a reporter plasmid consisting of TGF-β-responsible Smad-binding elements coupled to a secreted alkaline phosphatase reporter gene (SBE-SEAP)]. Briefly, MFB-F11 cells were seeded at 30.000 cells/100μl (per well) in 96-well flat bottom tissue culture plates (BD Falcon, Franklin Lakes, NJ) and cultured for 24 hours at 37°C in a 5% CO2 atmosphere in Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with penicillin/streptomycin and 10% fetal bovine serum (Invitrogen, Carlsbad, CA). Afterwards, cells were washed twice and incubated with 50μl/well AIM-V for 2 hours before recombinant TGF-β standards (range 2pg/ml to 1ng/ml, eBioscience San Diego, CA) and supernatants from the stimulated (as described above) PBMCs cultures (samples) were added in 50μl volume. Samples were diluted 1:2 to 1:16 to measure bioactive TGF-β and from 1:16 to 1:128 to measure latent TGF-β (samples were activated by adding 2.5μl of 6M HCI into 50μl of sample at room temperature and incubated for 10 minutes and then neutralized with 2.5μl of 6M NaOH). Cultured supernatants were collected 24 hours later. SEAP activity was measured with the luminometer FLUOstar OPTIMA (BMG Labtechnologies, Offenburg, Germany) using the Great EcsAPe™ SEAP Chemiluminescence Kit 2.0 (Clontech, Mountain View, CA) according to the manufacturer’s instructions.
Statistical analysis
The distributions of the variables of interest were assessed with the Shapiro-Wilk test. Descriptive statistics are presented as median (inter-quartile range) for non-normally distributed variables. The Wilcoxon rank-sum test and Kruskal Wallis tests were used to compare continuous variables and Pearson’s X2 test to compare categorical variables among studied groups. All reported p-values are based on 2-sided tests and compared with a significance level of 5%. Stata 9.1 for Windows (Stata Corp LP, College Station, TX) was used for all statistical calculations and plots.
RESULTS
Among 31 children with a previous diagnosis of milk-FPIES, 26 reacted during milk OFC (milk-FPIES group) and 5 did not react (milk-FPIES-resolved group). Prior to the milk OFC, these children remained on a diet restricted of milk protein. Additional control subjects included twelve children tolerant to milk, but with FPIES to other foods (other-food-FPIES group) and 6 milk-tolerant atopic children without a history of FPIES (negative controls). These two last groups had cow’s milk in their diet on a regular basis. All these groups were matched for age and gender (Table 1), and had casein-specific IgE <0.35 IU/ml.
Table 1.
Demographic characteristics and serum antibody levels in children with FPIES (milk-FPIES: children with FPIES to milk, milk-FPIES-resolved: children with milk FPIES that resolve this intolerance, other-food-FPIES: children with FPIES to foods other than milk) and negative controls (children who never experienced any type of milk intolerance).
| Milk-FPIES (n=26) | Milk-FPIES-resolved (n=5) | Other-food FPIES (n=12) | p-value1 | Negative controls (n=6) | p-value2 | |
|---|---|---|---|---|---|---|
| Age (years) | 3.5 (2.3–5.7) | 2 (2–3.1) | 3.81 (2.7–6.8) | 0.864* | 3.6 (2–6) | 0.741* |
| Gender (male) | 14 | 3 | 9 | 0.432§ | 4 | 0.675§ |
| Total IgE (kUA/L) | 15.8 (3.7–36) | 80.8 (22.5–98.7) | 34 (10–133) | 0.293* | 45.7 (15.1–67) | 0.451 |
| Total IgA (mg/ml) | 0.78 (0.4–1.136) | 0.359 (0.26–0.45) | 0.557 (0.42–1.01) | 0.446* | 0.4 (0.3–1.1) | 0.570* |
| Casein specific IgG (mgA/L) | 3.9 (2.8–5.8) | 5.8 (1.9–7.2) | 14.3 (8.9–36.5) | <0.001* | 19.8 (7.5–35) | - |
| Casein specific IgG4 (μgA/L) | 0.17 (0.08–0.26) | 0.3 (0.1–1.9) | 3.3 (0.3–11.5) | 0.012* | 5.02 (2.53–9.5) | - |
values present in median (IQR), n: number of subjects
comparison among three first columns
comparison among the negative controls and the three first columns
p-values based on Kruskal-Wallis rank test
p-value based on X2 test
An additional group of 12 children with IgE-mediated milk allergy 8.3±2.2 years old, were followed until they became tolerant (after 1.3±0.8 years). Serum samples were obtained at baseline just before the oral food challenge to which they reacted, while they had remained on a milk-elimination diet, and 10 months after they became tolerant to milk when they had included milk products in their diet on a regular basis.
Humoral responses
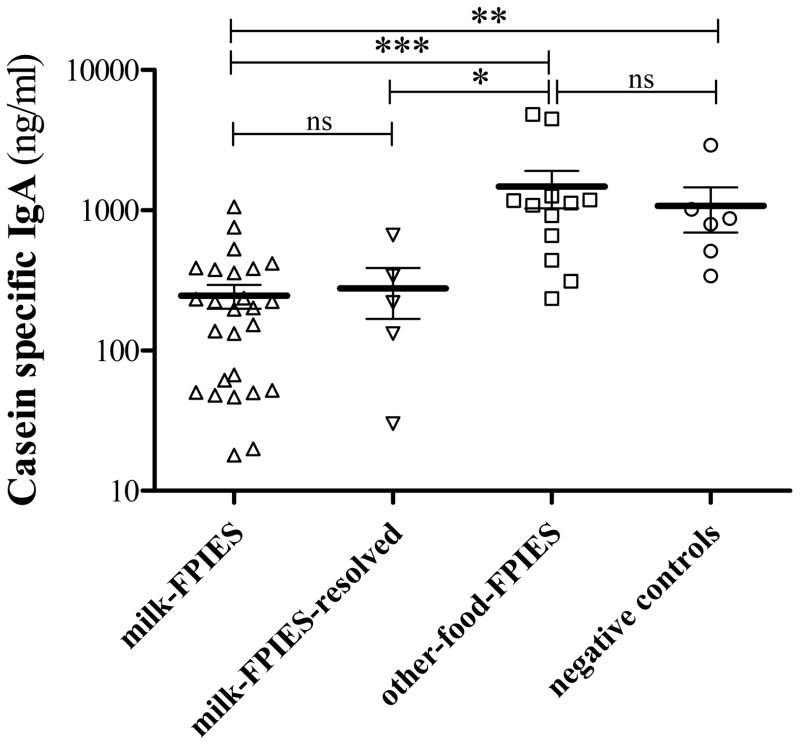
Serum csIgG, csIgG4 and csIgA antibody levels were significantly higher in the other-food-FPIES as compared with the milk-FPIES and milk-FPIES-resolved groups. Casein-specific-IgA levels in negative controls were comparable to other-food-FPIES children and significantly higher than in the milk-FPIES group (Table 1, Figure 1).
Figure 1.
Comparison of casein-specific (cs) IgA among the milk-FPIES, milk-FPIES-resolved, other-food-FPIES and negative control children.
*: 0.05 ≤ P < 0.01,
**: 0.01 ≤ P < 0.001,
***: P ≤ 0.001,
ns: non-significant
Assessing potential IgA dependence on milk exposure
In the group of children with IgE-milk allergy [median casein-specific IgE (interquartile range): 8.4(2.3–21.1) IU/ml, csIgA levels were comparable between baseline measurements (when the children were reactive to milk and thus were completely avoiding milk) and measurements 10 months after they became tolerant to regular milk when milk was being ingested regularly in their diet [median (IQR) csIgA: 195.9(62–344) ng/ml vs 219.4(144.5–394.9) ng/ml respectively, P=0.875] (Table 2).
Table 2.
Casein-specific IgA levels in children with milk-FPIES and control groups [other-food-FPIES, children with IgE-mediated milk allergy when they were maintained on a milk-free diet (Baked milk allergic, baseline) and the same children 10 months after they became tolerant to regular milk and have been ingesting milk on a regular basis (tolerant). Apart from negative controls and other-food-FPIES patients all other groups had comparable casein-specific IgA levels.
| Milk-FPIES (n=26) | Milk-FPIES-resolved (n=5) | Other-food FPIES (n=12) | Negative Controls (n=6) | Baked milk allergic (n=12) | ||
|---|---|---|---|---|---|---|
| baseline | tolerant | |||||
| Casein specific IgA (ng/ml) | 198.8 (52–377.4) | 220.2 (132.2–342.3) | 1108.1 (550.7–1222.8) | 836.1 (510.9–1013.5) | 195.9 (62–344) | 219.4 (144.5–394.9) |
values present in median (IQR), n: number of subjects, IQR: interquartile range
TGF-β in supernatants of casein-stimulated PBMCs
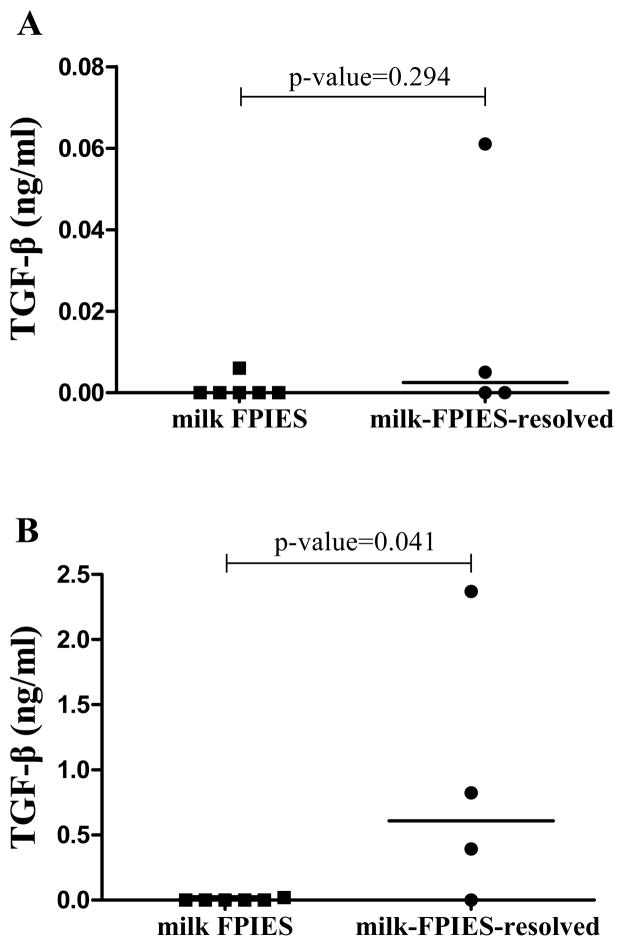
Bioactive and latent TGF-β (inactive precursor of bioactive TGF-β) were measured in supernatants of casein-stimulated PBMCs from six milk-FPIES and four milk-FPIES-resolved children with available blood samples. There were neither bioactive nor latent TGF-β responses [median (IQR): 0 (0–0) ng/ml, ] in stimulated supernatants from children with milk-FPIES, while latent TGF-β was significantly higher in milk-FPIES-resolved group (P=0.041) (Figure 2 and Table 3)
Figure 2.
Comparison of (A) bioactive and (B) latent TGF-β (inactive precursor of bioactive TGF-β) measured in supernatants of casein-stimulated PBMCs from children with FPIES to milk (milk-FPIES) and children with FPIES to cow’s milk that just passed a food challenge to cow’s milk, namely just resolved their intolerance (milk-FPIES-resolved) (values are adjusted for the media-alone TGF-β measurements).
Table 3.
Bioactive and latent TGF-β (inactive precursor of bioactive TGF-β) measured in supernatants of casein-stimulated PBMCs from children with FPIES to milk (milk-FPIES) and children with FPIES to cow’s milk that just passed a food challenge to cow’s milk, namely just resolved their intolerance (milk-FPIES-resolved).
| Milk-FPIES (n=6) |
Milk—FPIES-resolved (n=4) |
p-value | |
|---|---|---|---|
| Bioactive TGF-β (ng/ml) | 0 (0 – 0) | 0.003 (0 – 0.03) | 0.294 |
| Activated latent TGF-β (ng/ml) | 0 (0 – 0) | 0.61 (0.19 – 1.59) | 0.041 |
Values present in median (IQR)
IQR: interquartile range
p-values are based on Mann Whitney test
DISCUSSION
We report the novel observation of deficient serum csIgA levels and T-cell casein-mediated immunological responses in children with FPIES to milk. We also propose a potentially useful biomarker (TGF-β responses of casein-stimulated PBMCs) that may differentiate between children with persistent FPIES to milk and children with resolved FPIES to milk. We also found that both csIgG and csIgG4 levels were lower in children with FPIES to milk compared to the other-food-FPIES control subjects.
There are only a few, small published studies in which the potential role of IgA in FPIES has been examined (13, 14). Details regarding milk-specific IgA titers in patients and controls are only provided in the study by Shek et al(13), in which eleven children with milk-FPIES had similar serum α-, β- and κ-csIgA antibody levels with milk-tolerant children.
In the same study (13), the children with milk FPIES had significantly lower serum α-, β- and κ-csIgG4 antibody levels than milk-tolerant children, in keeping with the low csIgG4 and low csIgG levels we found. It has been shown that allergen specific-IgG4 antibodies increase with exposure to the particular allergen (15–18). In FPIES, it not clear if the low IgG4 levels can be explained by lack of exposure due to the milk-free diet. It can be argued that levels of csIgA may also be affected by exposure to the dietary milk protein. To account for this argument we assessed children with IgE-mediated milk allergy when they were completely avoiding milk, and 10 months after they had became milk tolerant and have introduced milk in their diet. There were no differences in csIgA to justify change in csIgA due to exposure. While we cannot exclude with 100% certainty the possibility of minor dietary transgressions that did not induce symptoms but might have induced csIgA in children with resolved milk-FPIES, none of these children reported accidental milk ingestions within 12 to 18 months prior to the study OFC.
Both IgG4 and IgA exhibit allergen-specific inhibitory activity in ex vivo and in vitro biologic assays such as basophil histamine release assays (19, 20). In vivo inhibitory activity has been shown directly or indirectly in several studies (21–24). Low levels of csIgG4 and csIgA could explain the reactions occurring in FPIES due to impaired neutralization capability of the gut microenvironment.
TGF-β are a group of cytokines that control many biological processes. TGF-β is initially released in precursor form known as a latent form that must be activated in the local microenvironment (e.g. pH changes) to become biologically active. This system is so sensitive that small amounts of latent TGF-β activation may generate maximum cellular responses, rendering latent TGF-β a type of extracellular sensor responding to specific signals by releasing bioactive TGF-β (25). In the TGF-β assay used in the current study, latent TGF-β does not signal unless it becomes activated. To measure TGF-β, we artificially activated it by acid treatment. PBMCs from milk-FPIES children showed deficient TGF-β responses upon casein stimulation since not only bioactive but also latent TGF-β could not be detected with a very sensitive assay.
Signaling via the TGF-β receptor is crucial for in vivo generation of IgA responses (26). Although based on a small sample, the deficient TGF-β responses of casein-stimulated PBMCs from milk-FPIES children found in the current study provide a potential explanation for the decreased serum levels of csIgA in these children. It could be argued that lack of exposure would result in fewer circulating casein-specific T-cells in the PBMC population examined. However, lack of casein-specific precursor cells in the PBMCs examined is unlikely if we consider the results from another study that showed that PBMCs from peanut-allergic children, were able to be stimulated with peanut extract, even after using half of the PBMCs (approximately one million as opposed to two millions in the current study) (27). These observations render TGF-β a potential biomarker for FPIES diagnosis, but of course they need to be further studied in larger cohorts of patients.
Our findings provide support for the potential role of TGF-β in FPIES pathogenesis suggested also by the undetectable TGF-β1 expression and the depressed expression of the TGF-β type I receptor found on the epithelial and mononuclear cells in the lamina propria of duodenal biopsies from most of the children with FPIES (28). TGF-β has been shown to regulate the intestinal epithelial integrity by maintaining and recovering the barrier function of human enterocytes (29, 30). Thus, a disruption of the epithelial barrier function, an enhanced antigen access to the submucosa and a subsequent activation of antigen-specific lymphocytes could be explained by deficient TGF-β responses.
In conclusion, children with milk-FPIES have low serum levels of csIgA, csIgG and csIgG4. In keeping with the low serum csIgA levels, the PBMCs from the same patients show TGF-β deficient responses upon casein stimulation which may play an important role in FPIES pathophysiology and could be used as a potential biomarker of persistent milk-FPIES. Prospective studies are needed to validate these findings in larger cohorts of children, recognize their role in the FPIES pathophysiology and establish the potential diagnostic utility in milk-induced FPIES.
Acknowledgments
The project was supported in part by Grant Number #UL1TR000067 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), by The Louis and Rachel Rudin Foundation Inc and by Food Allergy Initiative (now Food Allergy Research & Education).
ABBREVIATIONS
- TGF-β
transforming growth factor β
- OFC
oral food challenge
- cs
casein-specific
- PBMCs
peripheral blood mononuclear cells
- CBC
cell blood count
Footnotes
CONFLICT OF INTEREST
All authors do not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
References
- 1.Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. The Journal of allergy and clinical immunology. 2010;126:S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leonard SA, Nowak-Wegrzyn A. Clinical diagnosis and management of food protein-induced enterocolitis syndrome. Current opinion in pediatrics. 2012;24:739–45. doi: 10.1097/MOP.0b013e3283599ca1. [DOI] [PubMed] [Google Scholar]
- 3.Mehr S, Kakakios A, Frith K, Kemp AS. Food protein-induced enterocolitis syndrome: 16-year experience. Pediatrics. 2009;123:e459–64. doi: 10.1542/peds.2008-2029. [DOI] [PubMed] [Google Scholar]
- 4.Caubet JC, Nowak-Wegrzyn A. Current understanding of the immune mechanisms of food protein-induced enterocolitis syndrome. Expert review of clinical immunology. 2011;7:317–27. doi: 10.1586/eci.11.13. [DOI] [PubMed] [Google Scholar]
- 5.Nowak-Wegrzyn A, Assa’ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS. Work Group report: oral food challenge testing. The Journal of allergy and clinical immunology. 2009;123:S365–83. doi: 10.1016/j.jaci.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 6.Monti G, Castagno E, Liguori SA, et al. Food protein-induced enterocolitis syndrome by cow’s milk proteins passed through breast milk. The Journal of allergy and clinical immunology. 2011;127:679–80. doi: 10.1016/j.jaci.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Lake AM. Food-induced eosinophilic proctocolitis. Journal of pediatric gastroenterology and nutrition. 2000;30 (Suppl):S58–60. doi: 10.1097/00005176-200001001-00009. [DOI] [PubMed] [Google Scholar]
- 8.Kim PH, Kagnoff MF. Transforming growth factor beta 1 increases IgA isotype switching at the clonal level. J Immunol. 1990;145:3773–8. [PubMed] [Google Scholar]
- 9.Kim PH, Kagnoff MF. Transforming growth factor-beta 1 is a costimulator for IgA production. J Immunol. 1990;144:3411–6. [PubMed] [Google Scholar]
- 10.Nowak-Wegrzyn A. Food Protein-Induced Enterocolitis and Enteropathies. In: Metcalfe DD, Sampson HA, Simon RA, editors. Food Allergy: Adverse Reactions to Foods and Food Additives, 4th edition. Blackwell Publishing; 2008. pp. 195–210. [Google Scholar]
- 11.Konstantinou GN, Nowak-Wegrzyn A, Bencharitiwong R, Bardina L, Sicherer SH, Sampson HA. Egg-white-specific IgA and IgA2 antibodies in egg-allergic children: is there a role in tolerance induction? Pediatr Allergy Immunol. 2014;25:64–70. doi: 10.1111/pai.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tesseur I, Zou K, Berber E, Zhang H, Wyss-Coray T. Highly sensitive and specific bioassay for measuring bioactive TGF-beta. BMC cell biology. 2006;7:15. doi: 10.1186/1471-2121-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shek LP, Bardina L, Castro R, Sampson HA, Beyer K. Humoral and cellular responses to cow milk proteins in patients with milk-induced IgE-mediated and non-IgE-mediated disorders. Allergy. 2005;60:912–9. doi: 10.1111/j.1398-9995.2005.00705.x. [DOI] [PubMed] [Google Scholar]
- 14.McDonald PJ, Goldblum RM, Van Sickle GJ, Powell GK. Food protein-induced enterocolitis: altered antibody response to ingested antigen. Pediatric research. 1984;18:751–5. doi: 10.1203/00006450-198408000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Stapel SO, Asero R, Ballmer-Weber BK, et al. Testing for IgG4 against foods is not recommended as a diagnostic tool: EAACI Task Force Report. Allergy. 2008;63:793–6. doi: 10.1111/j.1398-9995.2008.01705.x. [DOI] [PubMed] [Google Scholar]
- 16.Lemon-Mule H, Sampson HA, Sicherer SH, Shreffler WG, Noone S, Nowak-Wegrzyn A. Immunologic changes in children with egg allergy ingesting extensively heated egg. The Journal of allergy and clinical immunology. 2008;122:977–83. e1. doi: 10.1016/j.jaci.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Kim JS, Nowak-Wegrzyn A, Sicherer SH, Noone S, Moshier EL, Sampson HA. Dietary baked milk accelerates the resolution of cow’s milk allergy in children. The Journal of allergy and clinical immunology. 2011;128:125–31. e2. doi: 10.1016/j.jaci.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowak-Wegrzyn A, Bloom KA, Sicherer SH, et al. Tolerance to extensively heated milk in children with cow’s milk allergy. The Journal of allergy and clinical immunology. 2008;122:342–7. 47 e1–2. doi: 10.1016/j.jaci.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 19.Nouri-Aria KT, Wachholz PA, Francis JN, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J Immunol. 2004;172:3252–9. doi: 10.4049/jimmunol.172.5.3252. [DOI] [PubMed] [Google Scholar]
- 20.Philips JR, Brouwer W, Edwards M, Mahler S, Ruhno J, Collins AM. The effectiveness of different rat IgG subclasses as IgE-blocking antibodies in the rat basophil leukaemia cell model. Immunology and cell biology. 1999;77:121–6. doi: 10.1046/j.1440-1711.1999.00801.x. [DOI] [PubMed] [Google Scholar]
- 21.Francis JN, James LK, Paraskevopoulos G, et al. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. The Journal of allergy and clinical immunology. 2008;121:1120–25. e2. doi: 10.1016/j.jaci.2008.01.072. [DOI] [PubMed] [Google Scholar]
- 22.Mothes N, Heinzkill M, Drachenberg KJ, et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy. 2003;33:1198–208. doi: 10.1046/j.1365-2222.2003.01699.x. [DOI] [PubMed] [Google Scholar]
- 23.Strait RT, Mahler A, Hogan S, Khodoun M, Shibuya A, Finkelman FD. Ingested allergens must be absorbed systemically to induce systemic anaphylaxis. The Journal of allergy and clinical immunology. 2011;127:982–9. e1. doi: 10.1016/j.jaci.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarze J, Cieslewicz G, Joetham A, et al. Antigen-specific immunoglobulin-A prevents increased airway responsiveness and lung eosinophilia after airway challenge in sensitized mice. American journal of respiratory and critical care medicine. 1998;158:519–25. doi: 10.1164/ajrccm.158.2.9801014. [DOI] [PubMed] [Google Scholar]
- 25.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. Journal of cell science. 2003;116:217–24. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 26.Cazac BB, Roes J. TGF-beta receptor controls B cell responsiveness and induction of IgA in vivo. Immunity. 2000;13:443–51. doi: 10.1016/s1074-7613(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 27.Pascal M, Konstantinou GN, Masilamani M, Lieberman J, Sampson HA. In silico prediction of Ara h 2 T cell epitopes in peanut-allergic children. Clin Exp Allergy. 2013;43:116–27. doi: 10.1111/cea.12014. [DOI] [PubMed] [Google Scholar]
- 28.Chung HL, Hwang JB, Park JJ, Kim SG. Expression of transforming growth factor beta1, transforming growth factor type I and II receptors, and TNF-alpha in the mucosa of the small intestine in infants with food protein-induced enterocolitis syndrome. The Journal of allergy and clinical immunology. 2002;109:150–4. doi: 10.1067/mai.2002.120562. [DOI] [PubMed] [Google Scholar]
- 29.Planchon SM, Martins CA, Guerrant RL, Roche JK. Regulation of intestinal epithelial barrier function by TGF-beta 1. Evidence for its role in abrogating the effect of a T cell cytokine. J Immunol. 1994;153:5730–9. [PubMed] [Google Scholar]
- 30.Suenaert P, Maerten P, Van Assche G, et al. Effects of T cell-induced colonic inflammation on epithelial barrier function. Inflammatory bowel diseases. 2010;16:1322–31. doi: 10.1002/ibd.21211. [DOI] [PubMed] [Google Scholar]