Abstract
Esophageal adenocarcinoma (EA) incidence is among the most rapidly increasing of any cancer type in the U.S., and prognosis is poor. Prevalence of the potential precursor lesion, Barrett's esophagus (BE), is also increasing. Candidates for safe and effective risk reduction strategies are needed, potentially including dietary components. In this qualitative review, we summarize recently published epidemiologic studies, in context of earlier work, on dietary intake and BE-EA outcomes. Potential cohort study/intervention trial candidates which could be increased to reduce BE-EA development include intake of: (1) fruits and vegetables; vegetables; fruit (EA only); (2) β-carotene and vitamins C and E; (3) folate (EA only); and (4) total fiber (EA only). Also, (5) red and processed meat intake could be targeted for dietary reduction/omission to reduce EA development. Few dietary constituents have been evaluated among EA patients to examine associations with mortality, thus interventions conducted among EA patients are premature.
Keywords: Barrett's esophagus, esophageal adenocarcinoma, survivorship, epidemiology, nutrition, dietary intake, vegetables, fruits, β-carotene, vitamin C, vitamin E, folate, total fiber, red meat
Introduction
Esophageal adenocarcinoma (EA) incidence is one of the most rapidly increasing of any cancer type in the United States (U.S.) [1] and prognosis is very poor (median survival is often less than a year) [2, 3]. EA appears to develop through a sequence of pathologic events [4], whereby chronic or severe gastroesophageal reflux disease (GERD) can cause ulceration of the normal squamous mucosa. This is followed, in some, by the development of Barrett's esophagus (BE) [5], the only known potential precursor of EA [6]. As EA often presents at an advanced stage, there are few effective treatment options [7]. Major EA risk factors include white race, male gender, GERD, obesity, and tobacco use [8]. Decreased tobacco use in the U.S. over the past few decades is attributed to personal and societal interventions [9]. Obesity prevalence has increased during this same time period [10]. Unfortunately, recidivism after weight loss is over 90% [11]. Thus, given the etiologic complexity of BE-EA continuum outcomes, additional risk reduction factors, or potential chemopreventives, are needed.
EA incidence rates vary 60-fold between high and low incidence countries [12]. Geographic differences do not appear to be due solely to genetic variation between racial/ethnic groups, as EA incidence rates in migrants from Asia, a low EA incidence country, to the U.S., a high EA incidence country, begin to approach those of European-Americans among the migrants as well as subsequent generations [13, 14, 15]. These epidemiologic observations have led to the hypothesis that geographic variability is in part due to differences in environmental exposures, including composition of diet, micronutrient intake, and dietary compounds [16, 17].
Our review synthesizes recent findings from population studies focused on dietary intake in relation to outcomes along the BE-EA continuum, published since 2010. By considering dietary associations along the BE-EA continuum (normal tissue → Barrett's esophagus → esophageal adenocarcinoma → mortality), our goal is to identify components of the diet with promise as potential interventions to reduce the likelihood of developing or dying from EA.
Our synthesis (see Table 1) uses the following considerations that are known to influence the interpretation of population research focused on dietary intake and BE-EA continuum outcomes: study design (the case-control approach is most commonly used, given the relative rarity of the study outcomes, although cohort studies are just now coming to fruition); study population (most studies primarily identify those at high-risk, older, white men in Western countries, because identifying the rarer low-risk subgroups of women and minorities is challenging); diet assessment method (most case-control and cohort studies have relied primarily on food frequency questionnaires (FFQ) to assess relative intake, although biomarkers or even repeat 24-hour recalls are currently considered superior approaches); dietary intake definitions (most consider micro-nutrients, macro-nutrients or food groups, with more recent efforts [18, 19, 20] considering dietary patterns, which may better mimic the complexity of dietary exposures, but are difficult to translate into public health messages [21]); BE-EA continuum outcome assessment (most studies focus on EA incidence, a few consider BE development, and almost none consider EA mortality, whereas consideration of the full BE-EA continuum would improve our ability to identify the timing of the exposure most optimal for implementing interventions); strength of the association (comparing the highest category of dietary intake exposure to the lowest); the extent to which a dietary component has been examined in a population setting (with the better-studied dietary components (five or more studies on the given compound) given higher consideration); and consistency of the findings (across epidemiologic studies and with laboratory-based experimental evidence).
Table 1.
Dietary intake and the risk of developing Barrett's Esophagus (BE) or Esophageal Adenocarcinoma (EA) – Summary of epidemiologic studies, with relative risks included.
| Dietary Intake Measure | BE Development | EA Incidence | EA Mortality |
|---|---|---|---|
|
|
|
|
|
| Summary: Frequency & Consistency* | Summary: Frequency & Consistency* | Summary: Frequency & Consistency* | |
| Author (YR) Direction of the Association** | Author (YR) Direction of the Association** | Author (YR) Direction of the Association** | |
| Fruits & Vegetables |
Better Studied: Likely ↓ Review Kubo (2010)[25] 3 case-control studies 39-73%↓ Case-Control Studies Pohl (2013)[27] 43%↓ (OR, 0.57) Ibiebele (2013)[28•] nondysplastic BE 46%↑ (OR, 1.46 [95% CI, 0.82-2.62]), dysplastic BE ≠ (OR, 1.08 [95% CI, 0.45-2.59]) |
Better Studied: Likely ↓ Meta-analysis Li (2014)[24•]5 studies total (4 case-control 39%↓, 1 cohort ≠) 32%↓(RR, 0.68 [95% CI, 0.49-0.93]) Alternative Exposure Assessment Ibiebele (2012)[84] dietary pattern 29%↑ (OR, 1.29 [95% CI, 0.77-2.14]) Jeurnink (2012)[85] variety score 24%↓ (HR, 0.76 [95% CI, 0.43-1.33]) |
No Studies To Date |
| Fruits |
Better Studied: Mixed Review Kubo (2010)[25] 2 case-control studies 24-43%↓ Case-Control Studies Ibiebele (2013)[28•] nondysplastic BE 83%↑ (OR, 1.83 [95% CI, 1.02-3.29]), dysplastic BE 19%↑ (OR, 1.19 [95% CI, 0.52-2.70]) Jiao (2013)[26••] 19%↓ (OR, 0.81 [95% CI, 0.47- 1.38]) Case-Cohort Study Keszei (2014)[29] ≠ (OR, 1.00 [95% CI, 0.65- 1.53]) |
Better Studied: Likely ↓ Meta-analysis Li (2014)[24•] 9 studies total (6 case-control 41%↓, 3 cohort ≠) 27%↓(RR, 0.73 [95% CI, 0.55-0.98]) Alternative Exposure Assessment Jeurnink (2012)[85] variety score 17%↓ (HR, 0.83 [95% CI, 0.46-1.49]) Navarro Silvera (2014)[20] non-citrus lower risk |
No Studies To Date |
| Vegetables |
Better Studied: Likely ↓ Review Kubo (2010)[25] 2 case-control studies 28-67%↓ Case-Control Studies Ibiebele (2013)[28•] nondysplastic BE 13%↑ (OR, 1.13 [95% CI, 0.65-1.96]), dysplastic BE 35%↓ (OR, 0.65 [95% CI, 0.29-1.46]) Jiao (2013)[26••] 39%↓ (OR, 0.61 [95% CI, 0.35- 1.06]) Case-Cohort Study Keszei (2014)[29] 34%↓ (OR, 0.66 [95% CI: 0.43- 1.01]) |
Better Studied: Likely ↓ Meta-analysis Li (2014)[24•] 9 studies total (6 case-control 25%↓, 3 cohort 24%↓) 24%↓ (RR, 0.76 [95% CI, 0.59-0.96]) Alternative Exposure Assessment Jeurnink (2012)[85] variety score 19%↓ (HR, 0.81 [95% CI, 0.46-1.43]) |
No Studies To Date |
| Vitamin A/Carotenoids (β-carotene, α-carotene, total carotenoids) |
Better Studied: Likely β-carotene ↓ Under Studied: Likely other carotenoids ↓ Case-Control Studies Kubo (2008)[34] β-carotene 44%↓ (OR, 0.56 [95% CI, 0.32-0.99]) Kubo (2008)[34]† β-carotene 43%↓ (OR, 0.57 [95% CI, 0.29-1.14]) Murphy (2010)[33]† total carotenoids 34%↓ (OR, 0.66 [95% CI, 0.39-1.13]) Jiao (2013)[26••]† α-carotene 12%↓ (OR, 0.88 [95% CI, 0.52-1.49]); β-carotene 36%↓ (OR, 0.64 [95% CI, 0.37-1.10]) Ibiebele (2013)[28•] β-carotene: nondysplastic BE ≠ (OR, 1.06 [95% CI, 0.64-1.74]), dysplastic BE 49%↓ (OR, 0.51 [95% CI, 0.23-1.11]) Ibiebele (2013)[28•]† β-carotene: nondysplastic BE ≠ (OR, 0.95 [95% CI, 0.58-1.56]), dysplastic BE 55%↓ (OR, 0.45 [95% CI, 0.20-1.00]) Alternative Exposure Assessment Fountoulakis (2004)[35] similar vitamin A plasma levels in BE cases and controls (2.40 vs. 2.23 µmol/L) |
Better Studied: Likely β-carotene ↓ Under Studied: Likely other carotenoids ↓ Meta-analysis Ge (2013)[31] β-carotene 4 studies (all case- control) 54%↓ (OR, 0.46 [95% CI, 0.36-0.58]) Case-Control Study Murphy (2010)[33]† total carotenoids 19%↓ (OR, 0.81 [95% CI, 0.49-1.34]) Ibiebele (2013)[28•] β-carotene 19%↓ (OR, 0.81 [95% CI, 0.53-1.22]) Ibiebele (2013)[28•]† β-carotene 14%↓ (OR, 0.86 [95% CI, 0.58-1.29]) |
No Studies To Date |
| Vitamin C |
Better Studied: Likely ↓ Case-Control Studies Kubo (2008)[34] 52%↓ (OR, 0.48 [95% CI, 0.26- 0.90]) Kubo (2008)[34]† 15%↓ (OR, 0.85 [95% CI, 0.45- 1.58]) Murphy (2010)[33]† 36%↓ (OR, 0.64 [95% CI, 0.36-1.13]) Jiao (2013)[26••]† 21%↓ (OR, 0.79 [95% CI, 0.47- 1.34]) Ibiebele (2013)[28•] nondysplastic BE 90%↑ (OR, 1.90 [95% CI, 0.99-2.86]), dysplastic BE 16%↑ (OR, 1.16 [95% CI, 0.53-2.53]) Ibiebele (2013)[28•]† nondysplastic BE ≠ (OR, 1.01 [95% CI, 0.60-1.69]), dysplastic BE 28%↓ (OR, 0.72 [95% CI, 0.34-1.53]) Alternative Exposure Assessment Fountoulakis (2004)[35] lower plasma/tissue levels in BE cases vs. controls (plasma: 47.31 vs. 59.57 µmol/L) |
Better Studied: Likely ↓ Meta-analysis Kubo (2007)[32] 4 studies (all case-control) 51%↓ (OR, 0.49 [95% CI, 0.39-0.62]) Case-Control Study Murphy (2010)[33]† 63%↓ (OR, 0.37 [95% CI, 0.21-0.66]) Ibiebele (2013)[28•] 21%↓ (OR, 0.79 [95% CI, 0.51-1.20]) Ibiebele (2013)[28•]† ≠ (OR, 0.98 [95% CI, 0.64- 1.49]) |
No Studies To Date |
| Vitamin E |
Better Studied: Likely ↓ Case-Control Studies Kubo (2008)[34] 75%↓ (OR, 0.25 [95% CI, 0.11- 0.59]) Kubo (2008)[34]† 33%↓ (OR, 0.67 [95% CI, 0.36- 1.24]) Murphy (2010)[33]† 15%↓ (OR, 0.85 [95% CI, 0.48-1.50]) Jiao (2013)[26••]† 54%↓ (OR, 0.46 [95% CI, 0.26- 0.83]) Ibiebele (2013)[28•] nondysplastic BE 24%↑ (OR, 1.24 [95% CI, 0.73-2.09]), dysplastic BE 43%↓ (OR, 0.57 [95% CI, 0.27-1.21]) Ibiebele (2013)[28•]† nondysplastic BE 24%↓ (OR, 0.76 [95% CI, 0.46-1.25]), dysplastic BE 42%↓ (OR, 0.58 [95% CI, 0.30-1.16]) Alternative Exposure Assessment Fountoulakis (2004)[35] similar plasma levels in BE cases and controls (33.55 vs. 34.90 µmol/L) |
Better Studied: Likely ↓ Review Kubo (2010)[25] 5 studies total: 1 cohort 27%↑, 4 case-control 10-87%↓ Case-Control Study Murphy (2010)[33]† 16%↓ (OR, 0.84 [95% CI, 0.48-1.47]) Ibiebele (2013)[28•] 57%↓ (OR, 0.43 [95% CI, 0.28-0.67]) Ibiebele (2013)[28•]† 36%↓ (OR, 0.64 [95% CI, 0.43-0.96]) |
No Studies To Date |
| Vitamin B (Folate, B6, B12, riboflavin) |
Under Studied: Likely Folate ↓ Under Studied: Likely B6 ↓ Under Studied: Likely B12 ↑ Under Studied: Likely Riboflavin ↓ Case-Control Studies Sharp (2013)[36]† folate 60%↓ (OR, 0.40 [95% CI, 0.21-0.75]), B6 69%↓ (OR, 0.31 [95% CI, 0.16-0.58]), B12 111%↑ (OR, 2.11 [95% CI, 1.12-3.98]), riboflavin 22%↓ (OR, 0.78 [95% CI, 0.42-1.44]) Jiao (2013)[26••] folate 42%↓ (OR, 0.58 [95% CI, 0.33-1.00]) Jiao (2013)[26••]† folate 48%↓ (OR, 0.52 [95% CI, 0.30-0.67]) Alternative Exposure Assessment Ekiz (2012)[38] lower serum folate and higher B12 levels in BE cases vs. controls (folate: 7.72 vs. 8.91 ng/mL, B12: 292.21 vs 272.63 pg/mL) |
Better Studied: Likely Folate ↓ Under Studied: Likely B6 ↓ Under Studied: Likely B12 ↑ Under Studied: Not Likely Riboflavin Review Kubo (2010)[25] 4 folate case-control studies 30-52%↓ Case-Control Studies Sharp (2013)[36] folate 44%↓ (OR, 0.56 [95% CI, 0.31-1.00]), B6 60%↓ (0.40 [95% CI, 0.22- 0.73]), B12 294%↑ (3.94 [95% CI, 2.17- 7.14]), riboflavin ≠ (1.09 [95% CI, 0.62-1.93]) Cohort Study Xiao (2014)[37] folate ≠ (HR, 1.00 [95% CI, 0.76- 1.31]), B6 ≠ (HR, 1.00 [95% CI, 0.76-1.32]), B12 ≠ (HR, 1.04 [95% CI, 0.80-1.34]) Alternative Exposure Assessment Ekiz (2012)[38] lower serum folate and no difference in B12 levels in EA cases vs. controls (folate: 7.09 vs. 8.91 ng/mL, B12: 334.07 vs 272.63 pg/mL) |
No Studies To Date |
| Selenium |
Better Studied: Mixed Case-Control Studies Kubo (2008)[34] 42%↓ (OR, 0.58 [95% CI, 0.26- 1.30]) Kubo (2008)[34]† 71%↓ (OR, 0.29 [95% CI, 0.09- 0.94]) Murphy (2010)[33]† ≠ (OR, 1.08 [95% CI, 0.64- 1.83]) Jiao (2013)[26••]†≠ (OR, 0.99 [95% CI, 0.58- 1.68]) Ibiebele (2013)[28•] nondysplastic BE 12%↑ (OR, 1.12 [95% CI, 0.68-1.84]),dysplastic BE ≠ (OR, 1.03 [95% CI, 0.47-2.26]) Ibiebele (2013)[28•]† nondysplastic BE 15%↑ (OR, 1.15 [95% CI, 0.70-1.88]), dysplastic BE ≠ (OR, 0.98 [95% CI, 0.47-2.03]) Alternative Exposure Assessment Steevens (2010)[43]toenail selenium concentration in case-cohort study ≠ (HR, 1.06 [95% CI, 0.71-1.57]; men: 31%↑ (HR, 1.31 [95% CI, 0.78-2.21]); women: 15%↓ (HR, 0.85 [95% CI, 0.46-1.57])) O'Rorke (2012)[39] toenail selenium concentration in case-control study 11%↓ (OR, 0.89 [95% CI, 0.37-2.12]) |
Better Studied: Mixed Case-Control Studies Murphy (2010)[33]† 20%↑ (OR, 1.20 [95% CI, 0.72-2.00]) Ibiebele (2013)[28•] 15%↑ (OR, 1.15 [95% CI, 0.76-1.73]) Ibiebele (2013)[28•]† ≠ (OR, 1.08 [95% CI, 0.71- 1.65]) Alternative Exposure Assessment Moe (2000)[42] decreased levels of dietary and serum selenium associated with increased risk of BE progression to EA Steevens (2010)[40]toenail selenium concentration in case-cohort study 24%↓ (HR, 0.76 [95% CI, 0.41-1.40]) O'Rorke (2012)[39] toenail selenium concentration in case-control study ≠ (OR, 0.94, [95% CI, 0.44-2.04]) Takata (2012)[41] serum selenium concentration in cohort study 40%↑ (HR, 1.40 [95% CI, 0.65-3.02]) |
No Studies To Date |
| Antioxidant Index |
Under Studied: Mixed Case-Control Studies Kubo (2008)[34] 70%↓ (OR, 0.30 [95% CI, 0.13- 0.71]) Kubo (2008)[34]† 65%↓ (OR, 0.35 [95% CI, 0.16- 0.79]) Murphy (2010)[33]† ≠ (OR, 0.95 [95% CI, 0.53- 1.71]) Jiao (2013)[26••]†40%↓ (OR, 0.60 [95% CI, 0.36- 1.00]) Ibiebele (2013)[28•] nondysplastic 28%↑ (OR, 1.28 [95% CI, 0.76-2.17]), dysplastic BE ≠ (OR, 1.02 [95% CI, 0.44-2.33]) Ibiebele (2013)[28•]† nondysplastic BE 11%↑ (OR, 1.11 [95% CI, 0.66-1.87]), dysplastic BE 28%↓ (OR, 0.72 [95% CI, 0.33-1.55]) |
Under Studied: Likely ↓ Case-Control Studies Murphy (2010)[33]† 43%↓ (OR, 0.57 [95% CI, 0.33-0.98]) Ibiebele (2013)[28•] 51%↓ (OR, 0.49 [95% CI, 0.30-0.80]) Ibiebele (2013)[28•]† 25%↓ (OR, 0.75 [95% CI, 0.40-1.15]) |
No Studies To Date |
| Vitamin Supplements | No Studies To Date |
Under Studied: Likely ↓ Cohort Studies Dawsey (2014)[44••]25%↓ (HR, 0.75 [95% CI, 0.49-1.16]) Dong (2008)[86] BE progression to EA 62%↓ (HR, 0.38 [95% CI, 0.15-0.99]) |
No Studies To Date |
| Vitamin A/β-carotene Supplements |
Under Studied: Likely ↓ Cross-Over Study Dutta (2012)[45] decreased GERD severity |
Under Studied: Not Likely Cohort Studies Dawsey (2014)[44••] vitamin A ≠ (HR, 0.91 [95% CI, 0.71-1.16]), β-carotene ≠ (HR, 0.96 [95% CI,0.76-1.20]) Dong (2008)[86] β-carotene: BE progression to EA ≠ (HR, 0.99 [95% CI, 0.34-2.94]) |
No Studies To Date |
| Vitamin C Supplements | No Studies To Date |
Under Studied: Mixed Cohort Studies Dawsey (2014)[44••] ≠ (HR, 0.93 [95% CI, 0.79- 1.10]) Dong (2008)[86] BE progression to EA 75%↓ (HR, 0.25 [95% CI, 0.11-0.58]) |
No Studies To Date |
| Vitamin E Supplements | No Studies To Date |
Under Studied: Mixed Cohort Studies Dawsey (2014)[44••] ≠ (HR, 0.95 [95% CI, 0.81- 1.13]) Dong (2008)[86] BE progression to EA 75%↓ (HR, 0.25 [95% CI, 0.10-0.60]) |
No Studies To Date |
| Vitamin B Supplements (Folic Acid) |
Under Studied: Likely ↑ Case-Control Study Jiao (2013)[26••]19%↑ (OR, 1.19 [95% CI, 0.76- 1.88]) |
Under Studied: Likely ↓ Cohort Study Dawsey (2014)[44••] 18%↓ (HR, 0.82 [95% CI, 0.60-1.13]) |
No Studies To Date |
| Selenium Supplements | No Studies To Date |
Under Studied: Mixed Cohort Studies Dawsey (2014)[44••] ≠ (HR, 1.02 [95% CI, 0.75- 1.38]) Dong (2008)[86] BE progression to EA 73%↓ (HR, 0.27 [95% CI, 0.03-2.21]) |
No Studies To Date |
| Flavonoids & Lignans |
Under Studied: Likely ↓ Case-Control Studies Jiao (2013)[26••] isoflavones 55%↓ (OR, 0.45 [95% CI, 0.25-0.81]) Petrick (2013)[56] anthocyanidins 51%↓ (OR, 0.49 [95% CI, 0.30-0.80]) |
Under Studied: Likely ↓ Case-Control Studies Bobe (2009)[50] total flavonoids 29%↓ (OR, 0.71 [95% CI, 0.36-1.42], anthocyanidins 53%↓ (OR, 0.47 [95% CI, 0.24-0.91]), flavan-3-ols 22%↑ (OR, 1.22 [95% CI, 0.60-2.49]), flavanones ≠ (OR, 0.99 [95% CI, 0.56-1.75]), flavones 19%↓ (OR, 0.81 [95% CI, 0.43- 1.51]), flavonols ≠ (OR, 0.98 [95% CI, 0.47- 2.01]), isoflavones 35%↓ (OR, 0.65 [95% CI, 0.36 1.18]), proanthocyanidins 11%↓ (OR, 0.89 [95% CI, 0.46-1.70]) Lin (2012)[53••] lignans 35%↓ (OR, 0.65 [95% CI, 0.38-1.12]) Petrick (2013)[51] anthocyanidins 57%↓ (OR, 0.43 [95% CI, 0.29-0.66]), isoflavones ↑ (OR not reported) Cohort Study Vermeulen (2013)[52] total flavonoids or any class ≠ (HR not reported) Clinical Assessment Study Kresty (2006)[48] berries lower oxidative stress markers in BE patients |
Under Studied: Likely ↓ Petrick (2013)[51] anthocyanidins 13%↓ |
| Total Fiber |
Under Studied: Likely ↓ Review Coleman (2013)[58••] 2 case-control studies 60-66%↓ |
Better Studied: Likely ↓ Meta-Analysis Coleman (2013)[58••] 8 studies (all case-control) 34%↓ (OR, 0.66 [95% CI, 0.44-0.98]) |
No Studies To Date |
| Fruit Fiber |
Under Studied: Likely ↓ Kubo (2009)[59] fruit and vegetable fiber combined: all BE 53%↓ (OR, 0.47 [95% CI, 0.25-0.88]), long-segment BE 70%↓ (OR, 0.30 [95% CI, 0.13-0.69]) |
Under Studied: Mixed Review Coleman (2013)[58••] 2 case-control studies 55%↓-70%↑ |
No Studies To Date |
| Vegetable Fiber |
Under Studied: Likely ↓ Kubo (2009)[59] fruit and vegetable fiber combined: all BE 53%↓ (OR, 0.47 [95% CI, 0.25-0.88]), long-segment BE 70%↓ (OR, 0.30 [95% CI, 0.13-0.69]) |
Under Studied: Likely ↓ Review Coleman (2013)[58••] 2 case-control studies 20-61%↓ |
No Studies To Date |
| Cereal Fiber |
Under Studied: Likely ↓ Kubo (2009)[59] all BE 27%↓ (OR, 0.73 [95% CI, 0.36-1.45]), long-segment BE 37%↓ (OR, 0.63 [95% CI, 0.27-1.51]) |
Under Studied: Likely ↓ Review Coleman (2013)[58••] 2 case-control studies 17-30%↓ |
No Studies To Date |
| Carbohydrates |
Under Studied: Not Likely Case-Control Study Mulholland (2009)[64] ≠ (OR, 1.02 [95% CI, 0.44- 2.35]) |
Better Studied: Likely ↓ Review Kubo (2010)[25] 6 case-control studies: 5 studies 16-66%↓, 1 study 90%↑ |
No Studies To Date |
| Sugar/Glycemic Index/Glycemic Load |
Under Studied: Likely Total Sugar ↑ Under Studied: Not Likely Glycemic Index Under Studied: Likely Glycemic Load ↓ Case-Control Study Mulholland (2009)[64] total sugar 12%↑ (OR, 1.12 [95% CI, 0.53-2.37]), glycemic index ≠ (OR, 0.93 [95% CI, 0.53-1.64]), glycemic load 21%↓ (OR, 0.79 [95% CI, 0.39-1.58]) |
Under Studied: Likely Total Sugar ↓ Under Studied: Likely Added Sugar ↑ Under Studied: Likely Glycemic Index ↑ Under Studied: Not Likely Glycemic Load Case-Control Study Mulholland (2009)[64] total sugar 57%↓ (OR, 0.43 [95% CI, 0.19-0.94]), glycemic index 52%↑ (OR, 1.52 [95% CI, 0.84-2.76]), glycemic load 14%↑ (OR, 1.14 [95% CI, 0.55-2.33]) Cohort Study Tasevska (2012)[65] added sugar 62%↑ (HR, 1.62 [95% CI, 1.07-2.45]) Alternative Exposure Assessment Chen (2002)[87] dessert dietary pattern 60%↑ (OR, 1.6 [95% CI, 0.4-6.9]) |
No Studies To Date |
| Total Meat |
Under Studied: Mixed Case-Control Studies Kubo (2009)[59] all BE 54%↓ (OR, 0.46 [95% CI, 0.21-1.01]), long-segment BE 75%↓ (OR, 0.25 [95% CI, 0.09-0.72]) O'Doherty (2011)[71] ≠ (OR, 0.95 [95% CI, 0.43- 2.08]) Jiao (2013)[72] 91%↑ (OR, 1.91 [95% CI, 1.07- 3.38]) Case-Cohort Study Keszei (2013)[70] men 21%↓ (HR, 0.79 [95% CI, 0.56-1.13]), women 28%↓ (HR, 0.72 [95% CI, 0.49-1.06]) |
Better Studied: Not Likely Meta-analysis Salehi (2013)[67•] 6 studies total (5 case-control, 1 cohort) ≠ (RR, 1.09 [95% CI, 0.92-1.29]) |
No Studies To Date |
| Red meat |
Under Studied: Mixed Case-Control Study O'Doherty (2011)[71] 11%↑ (OR, 1.11 [95% CI, 0.50-2.46]) Case-Cohort Study Keszei (2013)[70] men 19%↓ (HR, 0.81 [95% CI, 0.57-1.14]), women 23%↓ (HR, 0.77 [95% CI, 0.52-1.13]) |
Better Studied: Likely ↑ Meta-analysis Salehi (2013)[67•] 6 studies total (4 case-control, 2 cohort) 19%↑ (RR, 1.19 [95% CI, 0.98- 1.44]) Case-Cohort Study Keszei (2012)[73] men 43%↓ (HR, 0.57 [95% CI, 0.28-1.19]), women ≠ (HR, 1.09 [95% CI, 0.44-2.75]) |
No Studies To Date |
| Poultry |
Under Studied: Likely ↓ Case-Control Study O'Doherty (2011)[71] 44%↓ (OR, 0.56 [95% CI, 0.23-1.34]) Case-Cohort Study Keszei (2013)[70] men 11%↓ (HR, 0.89 [95% CI, 0.64-1.24]), women 16%↓ (HR, 0.84 [95% CI, 0.58-1.20]) |
Under Studied: Mixed Meta-analysis Salehi (2013)[67•] 3 studies total (2 case-control, 1 cohort) ≠ (RR, 1.02 0.52-2.00]) Jiang (2013)[66] 4 studies total (2 case-control, 2 cohort) 12%↓ (RR, 0.88 [95% CI, 0.52- 1.51]) |
No Studies To Date |
| Processed Meat |
Under Studied: Mixed Case-Control Study O'Doherty (2011)[71] 91%↑ (OR, 1.91 [95% CI, 0.81-4.49]) Case-Cohort Study Keszei (2013)[29] men ≠ (HR, 1.01 [95% CI, 0.68-1.48]), women ≠ (HR, 0.91 [95% CI, 0.62-1.34]) |
Under Studied: Likely ↑ Meta-analysis Salehi (2013)[67•] 6 studies total (4 case-control, 2 cohort) 37%↑ (RR, 1.37 [95% CI, 1.05- 1.78]) Case-Cohort Study Keszei (2012)[73] men ≠ (HR, 0.94 [95% CI, 0.46-1.89]), women 42%↓ (HR, 0.58 [95% CI, 0.22-1.50]) |
No Studies To Date |
| Iron (Total, Heme, Non-Heme) |
Under Studied: Likely Total Iron ↓ Under Studied: Mixed Heme Iron Under Studied: Likely Non-Heme Iron ↓ Case-Control Studies Corley (2008)[79] total iron 16% ↓ (OR, 0.84 [95% CI, 0.49-1.45]), dietary iron 63%↓ (OR, 0.37 [95% CI, 0.17-0.80]) O'Doherty (2010)[74] total iron 48%↓ (OR, 0.52 [95% CI, 0.26-1.02]), heme iron 66%↑ (OR, 1.66 [95% CI, 0.89-3.09]), non-heme iron 37%↓ (OR, 0.63 [95% CI, 0.34-1.17]) Case-Cohort Study Keszei (2013)[70] heme iron men 21%↓ (HR, 0.79 [95% CI, 0.55-1.14]), women 17% ↓ (HR, 0.83 [95% CI, 0.57-1.21]) Alternative Exposure Assessment Corley (2008)[79] serum ferritin 76% ↓ (OR, 0.24 [95% CI, 0.14-0.40]), serum iron 34%↓ (OR, 0.66 [95% CI, 0.41-1.04]) O'Doherty (2010)[74] serum ferritin 53%↓ (OR, 0.47 [95% CI, 0.23-0.97]), serum iron 25%↓ (OR, 0.75 [95% CI, 0.37-1.50]), serum transferrin 59%↓ (OR, 0.41 [95% CI, 0.20- 0.82]), toenail iron 18%↓ (OR, 0.82 [95% CI, 0.40-1.65]), total iron binding capacity 93%↑ (OR, 1.93 [95% CI, 0.95-3.93]) |
Better Studied: Mixed Total Iron Better Studied: Likely Heme Iron ↑ Under Studied: Likely Non-Heme Iron ↓ Review Kubo (2010)[25] 4 case-control studies 21-50%↓ Cohort Studies Cross (2010)[76] heme iron 47%↑ (HR, 1.47 [95% CI, 0.99-2.20]) Jakszyn (2013)[77] heme iron 67%↑ (HR, 1.67 [95% CI,1.05-2.68]) Case-Control Studies O'Doherty (2010)[74] total iron 50%↓ (OR, 0.50 [95% CI, 0.25-0.98]), heme iron 211%↑ (OR, 3.11 [95% CI, 1.46-6.61]), non-heme iron 48%↓ (OR, 0.52 [95% CI, 0.26-1.02]) Ward (2012)[75] total iron 67%↑ (OR, 1.67 [95% CI, 0.51-5.44]), heme iron 204%↑ (OR, 3.04 [95% CI, 1.20-7.72]), meat iron 167%↑ (OR, 2.67 [95% CI, 0.99-7.16]) Case-Cohort Study Keszei (2013)[78] heme iron men 19%↓ (HR, 0.81 [95% CI, 0.47-1.40]), women 61% ↓ (HR, 0.39 [95% CI, 0.15-1.01]) Alternative Exposure Assessment O'Doherty (2010)[74] toenail iron 60%↓ (OR, 0.40 [95% CI, 0.17-0.93]) |
No Studies To Date |
| Fish |
Under Studied: Mixed Case-Control Study O'Doherty (2011)[71] 39%↑ (OR, 1.39[95% CI, 0.62-3.11]) Case-Cohort Study Keszei (2013)[70] men ≠ (HR, 0.99 [95% CI, 0.70-1.41]), women 13%↑ (HR, 1.13 [95% CI, 0.76-1.69]) |
Better Studied: Mixed↓ Meta-analysis Han (2013)[82•] 6 studies total (5 case-control 14%↓, 1 cohort 22%↓) 14%↓ (RR, 0.86 [95% CI, 0.61-1.22]) |
No Studies To Date |
| Polyunsaturated Fatty Acids |
Under Studied: Mixed Case-Control Studies Kubo (2009)[59] all BE 51%↓ (OR, 0.49 [95% CI, 0.22-1.11]), long-segment BE 53%↓ (OR, 0.47 [95% CI, 0.17-1.27]) O'Doherty (2011)[71] ≠ (OR, 0.94 [95% CI, 0.40- 2.17]) |
Under Studied: Mixed Case-Control Study O'Doherty (2011)[71] 60%↑ (OR, 1.60 [95% CI, 0.73-3.49]) Cohort Study O'Doherty (2012)[83••] ≠ (HR, 0.91 [95% CI, 0.70-1.19]) |
No Studies To Date |
| Omega 3 |
Under Studied: Likely ↓ Case-Control Studies Kubo (2009)[59] all BE 54%↓ (OR, 0.46 [95% CI, 0.22-0.97]) long-segment BE 64%↓ (OR, 0.36 [95% CI, 0.14-0.90]) |
Under Studied: Not Likely Cohort Study O'Doherty (2012)[83••] ≠ (HR, 0.98 [95% CI, 0.75- 1.29]) |
No Studies To Date |
| Omega 6 | No Studies To Date | No Studies To Date | No Studies To Date |
Summary of the Evidence: Number of studies -- Under Studied (<5 studies); Better Studied (5+ studies); Consistency of the population studies -- Likely (most studies are consistent); Not Likely (most studies are null); Mixed (studies are not consistent);
Direction of the association (based on high versus low intake, unless otherwise stated): ↑ increased (risk estimates > 1.1); ↓ decreased (risk estimates < 0.9); ≠ no association (risk estimates = 0.9-1.1);
From food sources and supplements combined.
Fruits and Vegetables
Fruits and vegetables contain a variety of potentially anticarcinogenic substances, such as vitamins/minerals, fiber, and phytochemicals, which can act to decrease inflammation, oxidative stress, or cellular proliferation, or enhance apoptosis [22, 23].
For EA, fruits and vegetables combined together are better studied, with a recent meta-analysis reporting risk reductions of 32% at higher levels of intake. When fruits and vegetables were examined individually, a 27% reduced EA risk was seen for fruits and 24% for vegetables, and dose-response associations were evident. However, differences were noted by study type. In cohort studies, reduced EA risks were observed for vegetables, but not fruit, whereas in case-control studies reduced risks were found for both vegetables and fruits [24•].
For BE, fruits and vegetables combined were previously associated with a 39-73% decreased risk [25]. Two recent case-control studies, from Germany and the U.S., reported similar risk reductions of 19-43% for fruits, vegetables, or both [26••, 27]. However, two other recent studies, an Australian case-control study and a Dutch case-cohort study, found that BE risk was decreased by approximately 35% for vegetables, but not fruit [28•, 29].
Taken together, fruits and vegetables combined or vegetables alone are potential risk reduction strategies for EA and BE intervention trials.
Antioxidants and Other Vitamins/Minerals
In laboratory studies, certain vitamins, carotenoids, and minerals reduce oxidative DNA damage, which is implicated in the initiation process of carcinogenesis. For example, vitamin C protects cell membranes from oxidation, vitamin E protects against lipid peroxidation, β-carotene scavenges lipid free radicals, and selenium acts as an antimutagenic agent and protects DNA from oxidative damage [30].
Vitamins A/C/E
For EA, β-carotene and vitamin C are better studied, showing consistent risk reductions with higher intake in meta-analyses of 54% and 51%, respectively [31, 32]. Vitamin E is also better studied, with case-control studies showing EA risk reductions ranging from 10-87%, which was not confirmed in the single cohort study [25]. Two recent large case-control studies from Australia [28•] and Ireland [33] confirm EA risk reductions of similar magnitude for all three of these micronutrients.
For BE, β-carotene and vitamins C and E are also better studied, with most studies reporting risk reductions ranging from 15-75% [26••, 28•, 33, 34]. Two studies also examined α-carotene or total carotenoids, finding similar risk reductions [26••, 33]. However, an Australian case-control study found differences by type of BE, with reductions of 28-55% for dysplastic, but not nondysplastic BE, in association with β-carotene and vitamins C and E intake [28•]. In a biomarker study, lower plasma concentrations of vitamin C were observed among BE cases compared to controls, but no differences were noted for levels of vitamins A and E [35].
Taken together, β-carotene and vitamins C and E are potential risk reduction strategies for EA and BE intervention trials.
Vitamin B
For EA, folate is better studied, with previous studies reporting a 30-52% decreased risk associated with higher dietary folate intake [25]. A recent Irish case-control study found a similar EA reduction of 44% for folate [36], whereas a U.S. cohort study found no association [37]. Another recent investigation utilizing serum biomarkers found higher folate levels in controls versus EA cases [38]. For EA, vitamins B6 and B12 and riboflavin are under studied. A recent Irish case-control study reported a 60% decreased but nearly a 4-fold increased risk for vitamins B6 and B12, respectively [36], but no association was seen for EA in the U.S. cohort [37]. A study utilizing serum biomarkers found similar B12 levels in controls and EA cases [38]. No association was observed between EA and riboflavin [36].
For BE, the B vitamins are under studied. Two recent case-control studies, conducted in Ireland and the U.S., have reported 42-60% BE risk reductions for higher folate intake [26••, 36]. The Irish study also found 22-69% BE risk reductions for vitamin B6 and riboflavin, but BE risk was increased by 2-fold for higher intakes of vitamin B12 [36]. Utilizing serum biomarkers, a study found higher folate concentrations and lower vitamin B12 concentrations in controls versus BE cases [38].
Taken together, folate is a potential risk reduction strategy for EA intervention trials, but under studied for BE. Other B vitamins remain under studied for EA and BE, though there are suggestions that diets high in vitamin B12, may be associated with increased risk.
Selenium
For EA, selenium is better studied, but with mixed results. Two case-control studies from Australia [28•] and Ireland [33] reported a 15-20% increased risk associated with selenium intake. However, results differed when toenail selenium concentrations were examined; the Irish case-control study found no association [39], and a Dutch case-cohort study reported a 24% decreased risk [40]. In a cohort study of BE patients that examined EA-related outcomes, EA risk was increased 40% in association with higher serum selenium concentrations [41]. In contrast, a small cross-sectional survey reported serum selenium biomarkers were inversely associated with BE progression markers [42].
For BE, the association with selenium is also better studied, but with mixed results. In a California case-control study, dietary selenium was associated with a 42% decreased BE risk [34]. In contrast, three more recent case-control studies, conducted in Australia [28•], Ireland [33], and the U.S. [26••], found little or no association between selenium intake and BE. Toenail selenium levels were associated with modest 11% risk reductions in the Irish case-control study [39] and, among women only, in a Dutch case-cohort study [43].
Taken together, selenium cannot be recommended as a potential risk reduction strategy for BE or EA at this time.
Antioxidant Index
For EA, the antioxidant index (which is a summation score for vitamins C and E, β-carotene, and selenium) is under studied. The index was associated with a 25-51% risk reduction in two case-control studies from Australia and Ireland [28•, 33].
For BE, the antioxidant index is also under studied, and the results are mixed. Two case-control studies, in California HMO members and U.S. veterans, reported 40-70% BE risk reductions for high antioxidant index [34, 26••]. Another recent Australian study, found an 11-28% increased risk of nondysplastic, but not dysplastic, BE associated with the antioxidant index [28•], whereas an Irish case-control study found no association [33].
Taken together, vitamins C and E, β-carotene, and selenium combined is a promising risk reduction strategy, but more observational research is needed for EA and BE before implementation of potential intervention trials.
Vitamin Supplements
For EA, vitamin supplements are currently under studied. A U.S. retiree cohort study found that at least once a day multivitamin supplement use, compared to no use, was associated with 25% lower EA risk [44••]. Additionally, EA reductions of 15-32% were observed for at least monthly supplement use of iron, zinc, and folic acid; however, little or no association with EA was found for selenium, vitamin A, β-carotene, vitamin C, or vitamin E supplement use [44••]. In a U.S.-based BE patient cohort, progression to EA was decreased by 62% with daily or more multi-vitamin supplement use and by 75% with daily use of vitamins C and E and selenium supplements; but no association was reported with daily β-carotene supplement use [41].
For risk of BE development, vitamin supplements are under studied. One study of U.S. veterans reported a 19% increased BE risk with folic acid supplement use [26••]. A small case-crossover study found that β-carotene supplements reduced the severity of GERD symptoms [45].
Taken together, multivitamin supplements may be potential EA risk reduction strategies, but results are not yet convincing. Additional observational research on EA and BE is needed before implementation of intervention trials.
Polyphenols: Flavonoids and Lignans
Polyphenolic compounds, such as flavonoids and lignans, naturally occur in fruits, vegetables, and beverages of plant origin [46]. Experimental studies have shown that some flavonoid classes and lignans regulate cell cycle, proliferation, and apoptosis, and modulate carcinogen metabolism and inflammatory pathways, which have important chemopreventive effects against BE and EA [46, 47].
For EA, intake of total and six classes of flavonoids and lignans is under studied. Two small experimental or clinical studies [48, 46] have reported inverse associations with EA development, and one reported no association [49], for supplementation with flavonoids–freeze-dried berries high in anthocyanidins or apple-derived flavan-3-ols. Two additional larger U.S.-based case-control studies reported EA risk reductions of 53-57% with anthocyanidin intake [50, 51]. One of these case-control studies also reported an 11-35% decreased EA risk associated with flavone, isoflavone, total flavonoid and proanthocyanidin (polymers of flavan-3-ols) intake, little or no association with flavanones or flavonols, and a modest 22% increased EA risk associated with flavan-3-ol [50]. However, the other case-control study reported an increased EA risk with isoflavone intake [51]. A large European cohort study found no associations between EA incidence and any flavonoids [52]. A case-control study conducted in Sweden reported 35% decreased EA risk associated with dietary lignan intake [53••].
For mortality among EA patients, anthocyanidin intake was associated with a modest 13% decreased risk [51].
For BE development, flavonoids remain under studied. BE development was inhibited in two small experimental studies examining flavan-3-ol [54] and a synthetic flavone [55]. In two U.S.-based case-control studies, one reported a 51% decreased BE risk for anthocyanidin intake, more modest decreases for flavanone, flavonol, isoflavone, and lignan intake, but increased risk for flavones [56]. The other examined only isoflavone intake and found a 55% reduced BE risk [26••].
Taken together, specific classes of flavonoids or lignans may be potential risk reduction strategies for outcomes in the BE-EA continuum, but further observational research is needed before conducting intervention trials.
Fiber
Fiber-rich foods are known to have several anti-carcinogenic properties, including binding or diluting bile acids and thus reducing their carcinogenic activity and altering the glycemic response by slowing digestion and absorption of carbohydrates [57].
For EA, total fiber is better studied, showing EA risk reductions of 34%, as reported in a recent meta-analysis [58••]. However, specific types of fiber (e.g., fruit, vegetable, and cereal) remain under studied. Fiber from fruit has a mixed association, with one study showing a 55% decreased risk and the other reporting a 70% increased risk of EA, while vegetable and cereal fiber were associated with decreased EA risk of 17-61% [58••].
For BE, the association between BE and fiber is under studied but promising, with two studies reporting a 60-66% reduced risk with total fiber intake [58••]. One study reported BE risk reductions of 53% for fruit/vegetable fiber, and 27% for cereal fiber, which were slightly more pronounced for long-segment BE [59].
Taken together, total fiber is a potential risk reduction strategy for EA intervention trials, but under studied for BE. Additional observational research is needed for specific types of fiber.
Carbohydrates and Sugar Intake
Long-term consumption of a high-carbohydrate diet may lead to chronic hyperglycemia and hyperinsulinemia [60]. Insulin resistance, which is tethered to hyperinsulinemia, may promote carcinogenesis by stimulating cell proliferation and inhibiting apoptosis [60, 61, 62, 63]. Similar to carbohydrate intake, long-term consumption of a high-sugar diet may promote carcinogenesis by stimulating secretion of insulin and insulin-like growth factor 1 [60, 61, 62, 63].
Carbohydrates
For EA, carbohydrates are better studied, with most studies published before 2010 reporting 16-66% decreased risk [25], but no recent research on this topic has been published. However, experimental mechanistic studies suggest that carbohydrate intake could potentially increase EA risk [60, 61, 62, 63].
For BE, only one study has examined total carbohydrates, reporting no association [64].
Taken together, the BE/EA-carbohydrate intake association requires additional research, given the conflicting evidence between experimental and observational studies.
Sugar
For EA, sugar intake remains under studied. A recent U.S. cohort study reported an increased risk of 62% with high added sugar intake [65]. In contrast, an Irish case-control study observed reduced EA risk of 57% for total sugar [64]. The latter study also reported that EA risk was increased by 14 and 52% for the glycemic load and glycemic index, respectively [64].
For BE, sugar intake is under studied, with only the Irish case-control study reporting a modest 12% increased risk with total sugar, no association with glycemic index, and a 21% reduced risk associated with glycemic load [64].
Taken together, sugar intake remains under studied, and additional observational BE-EA continuum research is needed.
Meat and Iron
Meat may be positively associated with BE-EA continuum outcomes due to nutrient content (e.g., fat and iron), particularly in red meat, or the various carcinogenic compounds (e.g., nitrite/nitrate, heterocyclic amines, and polycyclic aromatic hydrocarbons), found particularly in processed, grilled and barbequed meats [25]. Poultry has also been investigated as a potential dietary risk reduction factor, as it is a source of selenium [66, 67•]. Iron has been considered as a potential risk factor, given EA is predominately a disease of white males and males typically have higher levels of iron saturation versus females, and whites typically have lower levels of iron deficiency compared to African-Americans [68, 69].
Total Meat
For EA, total meat is better studied, with a recent meta-analysis reporting little or no association [67•].
For BE, total meat intake is under studied, with mixed results. Two studies, one a California case-control and the other a Dutch case-cohort, reported 21-75% decreased BE risk for total meat [59, 70]. However, these results are inconsistent with two other recent case-control studies, including one from Ireland reporting no association [71] and one in U.S. veterans reporting a 91% increased BE risk [72].
Taken together, total meat is unlikely to be associated with EA, and more observational research is needed for BE.
Red Meat
For EA, high red meat intake was associated with a 19% increased risk in a recent meta-analysis [67•]. However, a more recently published Dutch case-cohort study found a 43% decreased EA risk among men whom consumed high levels of red meat compared to low- or non-consumers, but little or no association among women [73].
For BE, red meat intake is under studied and conflicting. An Irish case-control study reported an 11% increased risk [71], whereas a Netherlands case-cohort study reported 19 and 23% decreased risk for men and women, respectively [70].
Taken together, red meat is a potential EA risk factor that could be considered for intervention trials to decrease intake. However, for BE, additional observational studies are needed.
Poultry
For EA, poultry intake remains under studied, with conflicting results. Two recent meta-analyses have examined poultry intake, with one reporting no association [67•], and the other suggesting a 12% decreased EA risk [66].
For BE, a case-control study from Ireland and a case-cohort study from the Netherlands reported 11-44% decreased BE risk [71, 70].
Taken together, poultry intake may be a potential risk reduction strategy for BE, but additional observational research is needed for EA and BE.
Processed Meats
For EA, processed meat is better studied, with a recent meta-analysis reporting a 37% increased risk with higher intake [67•]. However, a Dutch case-cohort study found little or no association among men and a 42% decreased risk among women [73].
For BE, processed meat intake is under studied, with inconsistent results. The Irish case-control study reported a 91% increased risk [71], but the Netherlands case-cohort study reported little or no association [70].
Taken together, processed meat is a potential EA risk factor that could be considered for intervention trials to decrease intake. However, for BE, more observational research is needed.
Iron
For EA, iron is better studied. For dietary intake, most case-control studies reported 21-50% decreased EA risk with higher total iron intake [25, 74], but one Nebraskan case-control study observed a 67% increased risk [75]. Using biomarker iron concentrations in toenails, an Irish case-control study found a 60% decreased risk [74].
The association between iron intake and EA risk may also vary by type of iron: heme (better studied), which is primarily from meat, versus non-heme (under studied), which is primarily found in plants. Recent cohort [76, 77] and case-control studies [74, 75] report EA increases ranging from 47%-211% with heme iron intake. However, another case-cohort study reported 19 and 61% decreased risk among men and women, respectively [78]. The Irish case-control study is the only study that has examined non-heme iron and reported a 48% decreased EA risk [74].
For BE, iron intake is under studied. Two case-control studies in Ireland and in California report 16-48% decreased BE risk for total iron [74, 79]. The Irish study observed a 37% BE decreased risk for non-heme iron, but a 66% increased risk for heme iron intake [74]. In contrast, for heme iron, the Netherlands case-cohort study reported a 21% and 17% decreased BE risk in men and women, respectively [70]. The California and Ireland studies also examined serum and toenail measures of iron (i.e., iron ferritin, and transferrin), reporting 18-76% decreased BE risk [79, 74]. Total iron binding capacity, however, was associated with a 93% increased BE risk in the Irish study [74].
Taken together, total iron has been better studied for EA, but additional research is needed into heme versus non-heme iron intake in EA and BE.
Fish and Polyunsaturated Fatty Acids
Fish intake may be associated with reduced BE-EA continuum outcomes, as fish are rich in the long-chain ω-3 polyunsaturated fatty acids (PUFAs), which have been shown to regulate inflammation and tumor progression [80]. In contrast, ω-6 PUFAs, which are found in many non-marine oil-rich foods, have demonstrated carcinogenic properties, but are competitively inhibited by ω-3 [81].
Fish
For EA, fish intake is better studied, with a recent meta-analysis reporting EA risk reductions of 14% [82•], which included the null value. However, if the fish is fried, this cooking method could introduce oil containing adverse ω-6, and mask any beneficial associations with the ω-3-rich fish. Additional research with improved exposure assessment of considering fried versus non-fried fish is likely to clarify associations with EA.
For BE, fish intake is under studied, with one Irish case-control study and a Dutch case-cohort study among women, reporting 13-39% increased risk [71, 70]. No association was observed among men in the Netherlands study [70].
Taken together, additional observational research with improved exposure assessment of fish intake is needed for EA and BE.
PUFAs
For EA, PUFAs are under studied, with a case-control and a cohort study showing mixed results [71, 83••]. However, both studies only considered a combined measure of PUFAs, which may have resulted in masking important associations by type, e.g., beneficial ω-3 versus deleterious ω-6. Only a U.S.-based cohort study among retirees has reported little or no association between EA risk and ω-3 intake [83••].
For BE, PUFAs are under studied, with a California case-control study reporting a 51-64% reduced BE risk for overall PUFAs or ω-3 [59], which was not confirmed in an Irish case-control study [71].
Taken together, PUFAs are under studied and more observational research is needed for EA and BE.
Future Directions
As summarized in Figure 1 and Table 2, in this qualitative review, we identified five dietary intake components that are relatively better studied, and are potentially promising as potential risk reduction strategies for BE or EA. We recommend more formal evaluation in intervention trials (which would be more cost-effective than mounting new biomarker-based cohort studies) or in ongoing large cohort studies for which BE or EA are available as an outcome, for increasing intake of: (1) fruits & vegetables; vegetables; fruit (EA only); (2) β-carotene and vitamins C and E; (3) folate (EA only); and (4) total fiber (EA only). Also, (5) red and processed meat are better studied but likely to increase risk of EA only, and thus should be evaluated in an intervention study (or sufficiently large cohort) for removal/reduction in the diet.
Figure 1.
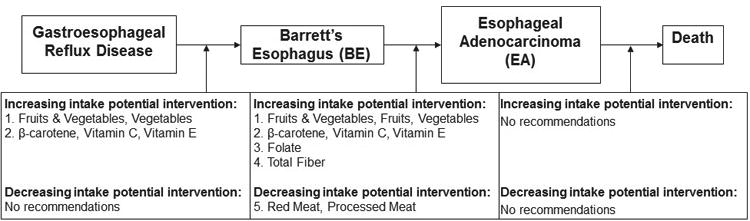
This review presents a summary of the associations between various dietary factors and the BE-EA continuum, to identify where there is potential to use these potential dietary risk reduction strategies to reduce EA progression. Summarized here are the dietary intake constituents that have been better studied and could be potentially utilized in intervention trials.
Table 2.
Dietary intake and the risk of developing Barrett's Esophagus (BE) or Esophageal Adenocarcinoma (EA) – Summary of epidemiologic studies of the dietary intake constituents that are better studied and could be potentially utilized in intervention trials.
| Dietary Intake Measure | BE Development | EA Incidence |
|---|---|---|
|
|
|
|
| Summary: Frequency & Consistency* | Summary: Frequency & Consistency* | |
| Author (YR) Direction of the Association** | Author (YR) Direction of the Association** | |
| Fruits & Vegetables |
Better Studied: Likely ↓ Review Kubo (2010)[25] 3 case-control studies 39-73%↓ Case-Control Studies Pohl (2013)[27] 43%↓ Ibiebele (2013)[28•] nondysplastic BE 46%↑, dysplastic BE ≠ |
Better Studied: Likely ↓ Meta-analysis Li (2014)[24•]5 studies total (4 case-control 39%↓, 1 cohort ≠) 32%↓ Alternative Exposure Assessment Ibiebele (2012)[84] dietary pattern 29%↑ Jeurnink (2012)[85] variety score 24%↓ |
| Fruits |
Better Studied: Mixed Review Kubo (2010)[25] 2 case-control studies 24-43%↓ Case-Control Studies Ibiebele (2013)[28•] nondysplastic BE 83%↑, dysplastic BE 19%↑ Jiao (2013)[26••] 19%↓ Case-Cohort Study Keszei (2014)[29] ≠ |
Better Studied: Likely ↓ Meta-analysis Li (2014)[24•] 9 studies total (6 case-control 41%↓, 3 cohort ≠) 27%↓ Alternative Exposure Assessment Jeurnink (2012)[85] variety score 17%↓ Navarro Silvera (2014)[20] non-citrus lower risk |
| Vegetables |
Better Studied: Likely ↓ Review Kubo (2010)[25] 2 case-control studies 28-67%↓ Case-Control Studies Ibiebele (2013)[28•] nondysplastic BE 13%↑, dysplastic BE 35%↓ Jiao (2013)[26••] 39%↓ Case-Cohort Study Keszei (2014)[29] 34%↓ |
Better Studied: Likely ↓ Meta-analysis Li (2014)[24•] 9 studies total (6 case-control 25%↓, 3 cohort 24%↓) 24%↓ Alternative Exposure Assessment Jeurnink (2012)[85] variety score 19%↓ |
| β-carotene |
Better Studied: Likely ↓ Case-Control Studies Kubo (2008)[34] 44%↓ Kubo (2008)[34]† 43%↓ Jiao (2013)[26••]†36%↓ Ibiebele (2013)[28•] nondysplastic BE ≠, dysplastic BE 49%↓ Ibiebele (2013)[28•]† nondysplastic BE ≠, dysplastic BE 55%↓ |
Better Studied: Likely ↓ Meta-analysis Ge (2013)[31] 4 studies (all case-control) 54%↓ Case-Control Study Ibiebele (2013)[28•] 19%↓ Ibiebele (2013)[28•]† 14%↓ |
| Vitamin C |
Better Studied: Likely ↓ Case-Control Studies Kubo (2008)[34] 52%↓ Kubo (2008)[34]† 15%↓ Murphy (2010)[33]† 36%↓ Jiao (2013)[26••]† 21%↓ Ibiebele (2013)[28•] nondysplastic BE 90%↑, dysplastic BE 16%↑ Ibiebele (2013)[28•]† nondysplastic BE ≠, dysplastic BE 28%↓ Alternative Exposure Assessment Fountoulakis (2004)[35] lower plasma/tissue levels in BE cases vs. controls |
Better Studied: Likely ↓ Meta-analysis Kubo (2007)[32] 4 studies (all case-control) 51%↓ Case-Control Studies Murphy (2010)[33]† 63%↓ Ibiebele (2013)[28•] 21%↓ Ibiebele (2013)[28•]† ≠ |
| Vitamin E |
Better Studied: Likely ↓ Case-Control Studies Kubo (2008)[34] 75%↓ Kubo (2008)[34]† 33%↓ Murphy (2010)[33]† 15%↓ Jiao (2013)[26••]† 54%↓ Ibiebele (2013)[28•] nondysplastic BE 24%↑, dysplastic BE 43%↓ Ibiebele (2013)[28•]† nondysplastic BE 24%↓, dysplastic BE 42%↓ Alternative Exposure Assessment Fountoulakis (2004)[35] similar plasma levels in BE cases and controls |
Better Studied: Likely ↓ Review Kubo (2010)[25] 5 studies total: 1 cohort 27%↑, 4 case-control 10-87%↓ Case-Control Studies Murphy (2010)[33]† 16%↓ Ibiebele (2013)[28•] 57%↓ Ibiebele (2013)[28•]† 36%↓ |
| Folate |
Under studied: Likely ↓ Case-Control Studies Sharp (2013)[36]† 60%↓ Jiao (2013)[26••]† 42%↓ Alternative Exposure Assessment Ekiz (2012)[38] lower serum folate levels in BE cases vs. controls |
Better Studied: Likely ↓ Review Kubo (2010)[25] 4 case-control studies 30-52%↓ Case-Control Study Sharp (2013)[36] 44%↓ Cohort Study Xiao (2014)[37] ≠ Alternative Exposure Assessment Ekiz (2012)[38] lower serum folate levels in EA cases vs. controls |
| Total Fiber |
Under studied: Likely ↓ Review Coleman (2013)[58••] 2 case-control studies 60-66%↓ |
Better Studied: Likely ↓ Meta-Analysis Coleman (2013)[58••] 8 studies (all case-control) 34%↓ |
| Red meat |
Under studied: Mixed Case-Control Study O'Doherty (2011)[71] 11%↑ Case-Cohort Study Keszei (2013)[70] men 19%↓, women 23%↓ |
Better Studied: Likely ↑ Meta-analysis Salehi (2013)[67•] 6 studies total (4 case-control, 2 cohort) 19%↑ Case-Cohort Study Keszei (2013)[73] men 43%↓, women ≠ |
| Processed Meat |
Under Studied: Mixed Case-Control Study O'Doherty (2011)[71] 91%↑ (OR, 1.91 [95% CI, 0.81-4.49]) Case-Cohort Study Keszei (2013)[29] men ≠ (HR, 1.01 [95% CI, 0.68-1.48]), women ≠ (HR, 0.91 [95% CI, 0.62-1.34]) |
Under Studied: Likely ↑ Meta-analysis Salehi (2013)[67•] 6 studies total (4 case-control, 2 cohort) 37%↑ (RR, 1.37 [95% CI, 1.05- 1.78]) Case-Cohort Study Keszei (2012)[73] men ≠ (HR, 0.94 [95% CI, 0.46-1.89]), women 42%↓ (HR, 0.58 [95% CI, 0.22-1.50]) |
Summary of the Evidence: Number of studies -- Under Studied (<5 studies); Better Studied (5+ studies); Consistency of the population studies -- Likely (most studies are consistent); Not Likely (most studies are null); Mixed (studies are not consistent);
Direction of the association (based on high versus low intake, unless otherwise stated): ↑ increased (risk estimates > 1.1); ↓ decreased (risk estimates < 0.9); ≠ no association (risk estimates = 0.9-1.1);
From food sources and supplements combined.
For all other dietary intake components discussed in this review (see Table 1), recommendations for evaluation in intervention studies cannot be made at this time, either because: (a) they remain un- or under studied; or (b) results are inconclusive or null despite being better studied. Additional observational research on these other dietary components is needed prior to their evaluation in an intervention trial.
Few studies have considered dietary intake among EA patients in relation to subsequent mortality; thus dietary intake intervention trials conducted among EA patients are premature for any of the dietary components reviewed here. Future observational research should also examine dietary intake in association with BE-EA continuum outcomes among women and minorities, which is largely unknown. This information would improve our ability to develop targeted risk reduction strategies.
Contributor Information
Jessica L. Petrick, Email: jessica.petrick@unc.edu.
Nan Li, Email: nanl@live.unc.edu.
Kathleen M. McClain, Email: kmccla@live.unc.edu.
Susan E. Steck, Email: ssteck@sc.edu.
Marilie D. Gammon, Email: gammon@unc.edu.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62(2):118–28. doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 2.Trivers KF, De Roos AJ, Gammon MD, Vaughan TL, Risch HA, Olshan AF, et al. Demographic and lifestyle predictors of survival in patients with esophageal or gastric cancers. Clin Gastroenterol Hepatol. 2005;3(3):225–30. doi: 10.1016/s1542-3565(04)00613-5. [DOI] [PubMed] [Google Scholar]
- 3.Crane SJ, Locke GR, 3rd, Harmsen WS, Zinsmeister AR, Romero Y, Talley NJ. Survival trends in patients with gastric and esophageal adenocarcinomas: a population-based study. Mayo Clin Proc. 2008;83(10):1087–94. doi: 10.4065/83.10.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaheen N, Ransohoff DF. Gastroesophageal reflux, barrett esophagus, and esophageal cancer: scientific review. JAMA. 2002;287(15):1972–81. doi: 10.1001/jama.287.15.1972. [DOI] [PubMed] [Google Scholar]
- 5.Riddell RH. The genesis of Barrett esophagus: has a histologic transition from gastroesophageal reflux disease-damaged epithelium to columnar metaplasia ever been seen in humans? Arch Pathol Lab Med. 2005;129(2):164–9. doi: 10.5858/2005-129-164-TGOBEH. [DOI] [PubMed] [Google Scholar]
- 6.Sharma P, McQuaid K, Dent J, Fennerty MB, Sampliner R, Spechler S, et al. A critical review of the diagnosis and management of Barrett's esophagus: the AGA Chicago Workshop. Gastroenterology. 2004;127(1):310–30. doi: 10.1053/j.gastro.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Mawhinney MR, Glasgow RE. Current treatment options for the management of esophageal cancer. Cancer Manag Res. 2012;4:367–77. doi: 10.2147/CMAR.S27593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falk GW. Risk factors for esophageal cancer development. Surg Oncol Clin N Am. 2009;18(3):469–85. doi: 10.1016/j.soc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Agaku IT, King BA, Dube SR Centers for Disease C, Prevention. Current cigarette smoking among adults - United States, 2005-2012. MMWR Morb Mortal Wkly Rep. 2014;63(2):29–34. [PMC free article] [PubMed] [Google Scholar]
- 10.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarlio-Lahteenkorva S, Rissanen A, Kaprio J. A descriptive study of weight loss maintenance: 6 and 15 year follow-up of initially overweight adults. Int J Obes Relat Metab Disord. 2000;24(1):116–25. doi: 10.1038/sj.ijo.0801094. [DOI] [PubMed] [Google Scholar]
- 12.Corley DA, Buffler PA. Oesophageal and gastric cardia adenocarcinomas: analysis of regional variation using the Cancer Incidence in Five Continents database. Int J Epidemiol. 2001;30(6):1415–25. doi: 10.1093/ije/30.6.1415. [DOI] [PubMed] [Google Scholar]
- 13.Schottenfeld D, Fraumeni JF. Cancer epidemiology and prevention. 3rd. Oxford; New York: Oxford University Press; 2006. [Google Scholar]
- 14.Kamineni A, Williams MA, Schwartz SM, Cook LS, Weiss NS. The incidence of gastric carcinoma in Asian migrants to the United States and their descendants. Cancer Causes Control. 1999;10(1):77–83. doi: 10.1023/a:1008849014992. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Demissie K, Lu SE, Rhoads GG. Cancer incidence among Korean-American immigrants in the United States and native Koreans in South Korea. Cancer Control. 2007;14(1):78–85. doi: 10.1177/107327480701400111. [DOI] [PubMed] [Google Scholar]
- 16.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83(10):2049–53. [PubMed] [Google Scholar]
- 17.Tzonou A, Lipworth L, Garidou A, Signorello LB, Lagiou P, Hsieh C, et al. Diet and risk of esophageal cancer by histologic type in a low-risk population. Int J Cancer. 1996;68(3):300–4. doi: 10.1002/(SICI)1097-0215(19961104)68:3<300::AID-IJC6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Bravi F, Edefonti V, Randi G, Ferraroni M, La Vecchia C, Decarli A. Dietary patterns and upper aerodigestive tract cancers: an overview and review. Ann Oncol. 2012;23(12):3024–39. doi: 10.1093/annonc/mds197. [DOI] [PubMed] [Google Scholar]
- 19.Kubo A, Levin TR, Block G, Rumore GJ, Quesenberry CP, Jr, Buffler P, et al. Dietary patterns and the risk of Barrett's esophagus. Am J Epidemiol. 2008;167(7):839–46. doi: 10.1093/aje/kwm381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarro Silvera SA, Mayne ST, Gammon MD, Vaughan TL, Chow WH, Dubin JA, et al. Diet and lifestyle factors and risk of subtypes of esophageal and gastric cancers: classification tree analysis. Ann Epidemiol. 2014;24(1):50–7. doi: 10.1016/j.annepidem.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc. 1996;96(10):1027–39. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- 23.Watzl B. Anti-inflammatory effects of plant-based foods and of their constituents. Int J Vitam Nutr Res. 2008;78(6):293–8. doi: 10.1024/0300-9831.78.6.293. [DOI] [PubMed] [Google Scholar]
- 24•.Li B, Jiang G, Zhang G, Xue Q, Zhang H, Wang C, et al. Intake of vegetables and fruit and risk of esophageal adenocarcinoma: a meta-analysis of observational studies. Eur J Nutr. 2014;53(7):1511–21. doi: 10.1007/s00394-014-0656-5. This recent meta-analysis provides the most current synthesis of the literature regarding fruit and vegetable intake and EA, suggesting a 32% decreased risk of EA with high intakes of fruits and vegetables. [DOI] [PubMed] [Google Scholar]
- 25.Kubo A, Corley DA, Jensen CD, Kaur R. Dietary factors and the risks of oesophageal adenocarcinoma and Barrett's oesophagus. Nutr Res Rev. 2010;23(2):230–46. doi: 10.1017/S0954422410000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.Jiao L, Kramer JR, Rugge M, Parente P, Verstovsek G, Alsarraj A, et al. Dietary intake of vegetables, folate, and antioxidants and the risk of Barrett's esophagus. Cancer Causes Control. 2013;24(5):1005–14. doi: 10.1007/s10552-013-0175-3. This is the first epidemiologic study to consider the association between isoflavones and BE, suggesting a 55% decreased risk of BE associated with high intake of isoflavones. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pohl H, Wrobel K, Bojarski C, Voderholzer W, Sonnenberg A, Rosch T, et al. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol. 2013;108(2):200–7. doi: 10.1038/ajg.2012.387. [DOI] [PubMed] [Google Scholar]
- 28•.Ibiebele TI, Hughes MC, Nagle CM, Bain CJ, Whiteman DC, Webb PM, et al. Dietary antioxidants and risk of Barrett's esophagus and adenocarcinoma of the esophagus in an Australian population. Int J Cancer. 2013;133(1):214–24. doi: 10.1002/ijc.28016. This is the first study to examine the association between dietary antioxidants and BE, accounting for dysplastic versus nondysplastic BE. [DOI] [PubMed] [Google Scholar]
- 29.Keszei AP, Schouten LJ, Driessen AL, Huysentruyt CJ, Keulemans YC, Goldbohm RA, et al. Vegetable, fruit and nitrate intake in relation to the risk of Barrett's oesophagus in a large Dutch cohort. Br J Nutr. 2014;111(8):1452–62. doi: 10.1017/S0007114513003929. [DOI] [PubMed] [Google Scholar]
- 30.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Ge XX, Xing MY, Yu LF, Shen P. Carotenoid intake and esophageal cancer risk: a meta-analysis. Asian Pac J Cancer Prev. 2013;14(3):1911–8. doi: 10.7314/apjcp.2013.14.3.1911. [DOI] [PubMed] [Google Scholar]
- 32.Kubo A, Corley DA. Meta-analysis of antioxidant intake and the risk of esophageal and gastric cardia adenocarcinoma. Am J Gastroenterol. 2007;102(10):2323–30. doi: 10.1111/j.1572-0241.2007.01374.x. [DOI] [PubMed] [Google Scholar]
- 33.Murphy SJ, Anderson LA, Ferguson HR, Johnston BT, Watson PR, McGuigan J, et al. Dietary antioxidant and mineral intake in humans is associated with reduced risk of esophageal adenocarcinoma but not reflux esophagitis or Barrett's esophagus. J Nutr. 2010;140(10):1757–63. doi: 10.3945/jn.110.124362. [DOI] [PubMed] [Google Scholar]
- 34.Kubo A, Levin TR, Block G, Rumore GJ, Quesenberry CP, Jr, Buffler P, et al. Dietary antioxidants, fruits, and vegetables and the risk of Barrett's esophagus. Am J Gastroenterol. 2008;103(7):1614–23. doi: 10.1111/j.1572-0241.2008.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fountoulakis A, Martin IG, White KL, Dixon MF, Cade JE, Sue-Ling HM, et al. Plasma and esophageal mucosal levels of vitamin C: role in the pathogenesis and neoplastic progression of Barrett's esophagus. Dig Dis Sci. 2004;49(6):914–9. doi: 10.1023/b:ddas.0000034548.89117.d6. [DOI] [PubMed] [Google Scholar]
- 36.Sharp L, Carsin AE, Cantwell MM, Anderson LA, Murray LJ, Group FS. Intakes of dietary folate and other B vitamins are associated with risks of esophageal adenocarcinoma, Barrett's esophagus, and reflux esophagitis. J Nutr. 2013;143(12):1966–73. doi: 10.3945/jn.113.174664. [DOI] [PubMed] [Google Scholar]
- 37.Xiao Q, Freedman ND, Ren J, Hollenbeck AR, Abnet CC, Park Y. Intakes of folate, methionine, vitamin B6, and vitamin B12 with risk of esophageal and gastric cancer in a large cohort study. Br J Cancer. 2014;110(5):1328–33. doi: 10.1038/bjc.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ekiz F, Ormeci N, Coban S, Karabulut HG, Aktas B, Tukun A, et al. Association of methylenetetrahydrofolate reductase C677T-A1298C polymorphisms with risk for esophageal adenocarcinoma, Barrett's esophagus, and reflux esophagitis. Dis Esophagus. 2012;25(5):437–41. doi: 10.1111/j.1442-2050.2011.01262.x. [DOI] [PubMed] [Google Scholar]
- 39.O'Rorke MA, Cantwell MM, Abnet CC, Brockman AJ, Murray LJ, Group FS. Toenail trace element status and risk of Barrett's oesophagus and oesophageal adenocarcinoma: results from the FINBAR study. Int J Cancer. 2012;131(8):1882–91. doi: 10.1002/ijc.27434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steevens J, van den Brandt PA, Goldbohm RA, Schouten LJ. Selenium status and the risk of esophageal and gastric cancer subtypes: the Netherlands cohort study. Gastroenterology. 2010;138(5):1704–13. doi: 10.1053/j.gastro.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Takata Y, Kristal AR, Santella RM, King IB, Duggan DJ, Lampe JW, et al. Selenium, selenoenzymes, oxidative stress and risk of neoplastic progression from Barrett's esophagus: results from biomarkers and genetic variants. PLoS One. 2012;7(6):e38612. doi: 10.1371/journal.pone.0038612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moe GL, Kristal AR, Levine DS, Vaughan TL, Reid BJ. Waist-to-hip ratio, weight gain, and dietary and serum selenium are associated with DNA content flow cytometry in Barrett's esophagus. Nutr Cancer. 2000;36(1):7–13. doi: 10.1207/S15327914NC3601_2. [DOI] [PubMed] [Google Scholar]
- 43.Steevens J, Schouten LJ, Driessen AL, Huysentruyt CJ, Keulemans YC, Goldbohm RA, et al. Toenail selenium status and the risk of Barrett's esophagus: the Netherlands Cohort Study. Cancer Causes Control. 2010;21(12):2259–68. doi: 10.1007/s10552-010-9651-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Dawsey SP, Hollenbeck A, Schatzkin A, Abnet CC. A prospective study of vitamin and mineral supplement use and the risk of upper gastrointestinal cancers. PLoS One. 2014;9(2):e88774. doi: 10.1371/journal.pone.0088774. This study is the largest study to date to examine the use of vitamins in association with EA, suggesting a 25% decreased risk of EA associated with daily intake of multivitamin supplements. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dutta SK, Agrawal K, Girotra M, Fleisher AS, Motevalli M, Mah'moud MA, et al. Barrett's esophagus and beta-carotene therapy: symptomatic improvement in GERD and enhanced HSP70 expression in esophageal mucosa. Asian Pac J Cancer Prev. 2012;13(12):6011–6. doi: 10.7314/apjcp.2012.13.12.6011. [DOI] [PubMed] [Google Scholar]
- 46.Pierini R, Kroon PA, Guyot S, Ivory K, Johnson IT, Belshaw NJ. Procyanidin effects on oesophageal adenocarcinoma cells strongly depend on flavan-3-ol degree of polymerization. Mol Nutr Food Res. 2008;52(12):1399–407. doi: 10.1002/mnfr.200700513. [DOI] [PubMed] [Google Scholar]
- 47.Lee LT, Huang YT, Hwang JJ, Lee AY, Ke FC, Huang CJ, et al. Transinactivation of the epidermal growth factor receptor tyrosine kinase and focal adhesion kinase phosphorylation by dietary flavonoids: effect on invasive potential of human carcinoma cells. Biochem Pharmacol. 2004;67(11):2103–14. doi: 10.1016/j.bcp.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 48.Kresty LA, Frankel WL, Hammond CD, Baird ME, Mele JM, Stoner GD, et al. Transitioning from preclinical to clinical chemopreventive assessments of lyophilized black raspberries: interim results show berries modulate markers of oxidative stress in Barrett's esophagus patients. Nutr Cancer. 2006;54(1):148–56. doi: 10.1207/s15327914nc5401_15. [DOI] [PubMed] [Google Scholar]
- 49.Aiyer HS, Li Y, Losso JN, Gao C, Schiffman SC, Slone SP, et al. Effect of freeze-dried berries on the development of reflux-induced esophageal adenocarcinoma. Nutr Cancer. 2011;63(8):1256–62. doi: 10.1080/01635581.2011.609307. [DOI] [PubMed] [Google Scholar]
- 50.Bobe G, Peterson JJ, Gridley G, Hyer M, Dwyer JT, Brown LM. Flavonoid consumption and esophageal cancer among black and white men in the United States. Int J Cancer. 2009;125(5):1147–54. doi: 10.1002/ijc.24421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petrick J, Steck S, Bradshaw P, Chow W, Engel L, He K, et al. Dietary flavonoid intake and risk of esophageal and gastric cancers (abstract) American Institute for Cancer Research nnual Research Conference on Food, Nutrition, Physical Activity and Cancer; Bethesda, MD: 2013. 2013. [Google Scholar]
- 52.Vermeulen E, Zamora-Ros R, Duell EJ, Lujan-Barroso L, Boeing H, Aleksandrova K, et al. Dietary flavonoid intake and esophageal cancer risk in the European prospective investigation into cancer and nutrition cohort. Am J Epidemiol. 2013;178(4):570–81. doi: 10.1093/aje/kwt026. [DOI] [PubMed] [Google Scholar]
- 53••.Lin Y, Yngve A, Lagergren J, Lu Y. Dietary intake of lignans and risk of adenocarcinoma of the esophagus and gastroesophageal junction. Cancer Causes Control. 2012;23(6):837–44. doi: 10.1007/s10552-012-9952-7. This is the only epidemiologic study to date to consider the association between lignan intake and EA, suggesting a 35% decreased risk of EA associated with high lignan intake. [DOI] [PubMed] [Google Scholar]
- 54.Song S, Krishnan K, Liu K, Bresalier RS. Polyphenon E inhibits the growth of human Barrett's and aerodigestive adenocarcinoma cells by suppressing cyclin D1 expression. Clin Cancer Res. 2009;15(2):622–31. doi: 10.1158/1078-0432.CCR-08-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lechpammer M, Xu X, Ellis FH, Bhattacharaya N, Shapiro GI, Loda M. Flavopiridol reduces malignant transformation of the esophageal mucosa in p27 knockout mice. Oncogene. 2005;24(10):1683–8. doi: 10.1038/sj.onc.1208375. [DOI] [PubMed] [Google Scholar]
- 56.Petrick J, Steck S, Bradshaw P, Engel L, He K, Vaughan T, et al. Dietary Flavonoid Intake and Risk of Barrett's Esophagus. Am J Epidemiol. 2013;177(11 Suppl):L02. doi: 10.1016/j.annepidem.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slavin JL. Mechanisms for the impact of whole grain foods on cancer risk. J Am Coll Nutr. 2000;19(3 Suppl):300S–7S. doi: 10.1080/07315724.2000.10718964. [DOI] [PubMed] [Google Scholar]
- 58••.Coleman HG, Murray LJ, Hicks B, Bhat SK, Kubo A, Corley DA, et al. Dietary fiber and the risk of precancerous lesions and cancer of the esophagus: a systematic review and meta-analysis. Nutr Rev. 2013;71(7):474–82. doi: 10.1111/nure.12032. This systematic review and meta-analysis provides the most current evidence for the association between fiber intake (including total, fruit, vegetable, and cereal fiber types) and BE-EA, suggesting a 34% decreased risk of EA associated with high levels of total fiber intake. [DOI] [PubMed] [Google Scholar]
- 59.Kubo A, Block G, Quesenberry CP, Jr, Buffler P, Corley DA. Effects of dietary fiber, fats, and meat intakes on the risk of Barrett's esophagus. Nutr Cancer. 2009;61(5):607–16. doi: 10.1080/01635580902846585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc. 2001;60(1):91–106. doi: 10.1079/pns200070. [DOI] [PubMed] [Google Scholar]
- 61.Greer KB, Thompson CL, Brenner L, Bednarchik B, Dawson D, Willis J, et al. Association of insulin and insulin-like growth factors with Barrett's oesophagus. Gut. 2012;61(5):665–72. doi: 10.1136/gutjnl-2011-300641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryan AM, Healy LA, Power DG, Byrne M, Murphy S, Byrne PJ, et al. Barrett esophagus: prevalence of central adiposity, metabolic syndrome, and a proinflammatory state. Ann Surg. 2008;247(6):909–15. doi: 10.1097/SLA.0b013e3181612cac. [DOI] [PubMed] [Google Scholar]
- 63.Duggan C, Onstad L, Hardikar S, Blount PL, Reid BJ, Vaughan TL. Association between markers of obesity and progression from Barrett's esophagus to esophageal adenocarcinoma. Clin Gastroenterol Hepatol. 2013;11(8):934–43. doi: 10.1016/j.cgh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mulholland HG, Cantwell MM, Anderson LA, Johnston BT, Watson RG, Murphy SJ, et al. Glycemic index, carbohydrate and fiber intakes and risk of reflux esophagitis, Barrett's esophagus, and esophageal adenocarcinoma. Cancer Causes Control. 2009;20(3):279–88. doi: 10.1007/s10552-008-9242-6. [DOI] [PubMed] [Google Scholar]
- 65.Tasevska N, Jiao L, Cross AJ, Kipnis V, Subar AF, Hollenbeck A, et al. Sugars in diet and risk of cancer in the NIH-AARP Diet and Health Study. Int J Cancer. 2012;130(1):159–69. doi: 10.1002/ijc.25990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang G, Li B, Liao X, Zhong C. Poultry and fish intake and risk of esophageal cancer: A meta-analysis of observational studies. Asia Pac J Clin Oncol. 2013 Aug 2; doi: 10.1111/ajco.12114. Epub. [DOI] [PubMed] [Google Scholar]
- 67•.Salehi M, Moradi-Lakeh M, Salehi MH, Nojomi M, Kolahdooz F. Meat, fish, and esophageal cancer risk: a systematic review and dose-response meta-analysis. Nutr Rev. 2013;71(5):257–67. doi: 10.1111/nure.12028. This is the most recent meta-analysis to examine all types of meat (including total, red, poultry, and processed meats) in association with EA, suggesting little or no association between EA risk and total meat or poultry and 19-37% increased EA risk assocoiated with red and processed meats. [DOI] [PubMed] [Google Scholar]
- 68.Barton JC, Acton RT, Dawkins FW, Adams PC, Lovato L, Leiendecker-Foster C, et al. Initial screening transferrin saturation values, serum ferritin concentrations, and HFE genotypes in whites and blacks in the Hemochromatosis and Iron Overload Screening Study. Genet Test. 2005;9(3):231–41. doi: 10.1089/gte.2005.9.231. [DOI] [PubMed] [Google Scholar]
- 69.Beutler E, West C. Hematologic differences between African-Americans and whites: the roles of iron deficiency and alpha-thalassemia on hemoglobin levels and mean corpuscular volume. Blood. 2005;106(2):740–5. doi: 10.1182/blood-2005-02-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keszei AP, Schouten LJ, Driessen AL, Huysentruyt CJ, Keulemans YC, van den Brandt PA. Meat consumption and the risk of Barrett's esophagus in a large Dutch cohort. Cancer Epidemiol Biomarkers Prev. 2013;22(6):1162–6. doi: 10.1158/1055-9965.EPI-13-0032. [DOI] [PubMed] [Google Scholar]
- 71.O'Doherty MG, Cantwell MM, Murray LJ, Anderson LA, Abnet CC Group FS. Dietary fat and meat intakes and risk of reflux esophagitis, Barrett's esophagus and esophageal adenocarcinoma. Int J Cancer. 2011;129(6):1493–502. doi: 10.1002/ijc.26108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiao L, Kramer JR, Chen L, Rugge M, Parente P, Verstovsek G, et al. Dietary consumption of meat, fat, animal products and advanced glycation end-products and the risk of Barrett's oesophagus. Aliment Pharmacol Ther. 2013;38(7):817–24. doi: 10.1111/apt.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keszei AP, Schouten LJ, Goldbohm RA, van den Brandt PA. Red and processed meat consumption and the risk of esophageal and gastric cancer subtypes in The Netherlands Cohort Study. Ann Oncol. 2012;23(9):2319–26. doi: 10.1093/annonc/mdr615. [DOI] [PubMed] [Google Scholar]
- 74.O'Doherty MG, Abnet CC, Murray LJ, Woodside JV, Anderson LA, Brockman JD, et al. Iron intake and markers of iron status and risk of Barrett's esophagus and esophageal adenocarcinoma. Cancer Causes Control. 2010;21(12):2269–79. doi: 10.1007/s10552-010-9652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ward MH, Cross AJ, Abnet CC, Sinha R, Markin RS, Weisenburger DD. Heme iron from meat and risk of adenocarcinoma of the esophagus and stomach. Eur J Cancer Prev. 2012;21(2):134–8. doi: 10.1097/CEJ.0b013e32834c9b6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cross AJ, Freedman ND, Ren J, Ward MH, Hollenbeck AR, Schatzkin A, et al. Meat consumption and risk of esophageal and gastric cancer in a large prospective study. Am J Gastroenterol. 2011;106(3):432–42. doi: 10.1038/ajg.2010.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jakszyn P, Lujan-Barroso L, Agudo A, Bueno-de-Mesquita HB, Molina E, Sanchez MJ, et al. Meat and heme iron intake and esophageal adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition study. Int J Cancer. 2013;133(11):2744–50. doi: 10.1002/ijc.28291. [DOI] [PubMed] [Google Scholar]
- 78.Keszei AP, Goldbohm RA, Schouten LJ, Jakszyn P, van den Brandt PA. Dietary N-nitroso compounds, endogenous nitrosation, and the risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Am J Clin Nutr. 2013;97(1):135–46. doi: 10.3945/ajcn.112.043885. [DOI] [PubMed] [Google Scholar]
- 79.Corley DA, Kubo A, Levin TR, Habel L, Zhao W, Leighton P, et al. Iron intake and body iron stores as risk factors for Barrett's esophagus: a community-based study. Am J Gastroenterol. 2008;103(12):2997–3004. doi: 10.1111/j.1572-0241.2008.02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang W, Zhu J, Lyu F, Panigrahy D, Ferrara KW, Hammock B, et al. omega-3 Polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer. Prostaglandins Other Lipid Mediat. 2014 Jul 11; doi: 10.1016/j.prostaglandins.2014.07.002. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pauwels EK, Kairemo K. Fatty acid facts, part II: role in the prevention of carcinogenesis, or, more fish on the dish? Drug News Perspect. 2008;21(9):504–10. doi: 10.1358/dnp.2008.21.9.1290819. [DOI] [PubMed] [Google Scholar]
- 82•.Han YJ, Li J, Huang W, Fang Y, Xiao LN, Liao ZE. Fish consumption and risk of esophageal cancer and its subtypes: a systematic review and meta-analysis of observational studies. Eur J Clin Nutr. 2013;67(2):147–54. doi: 10.1038/ejcn.2012.213. This meta-analysis provides the most complete and up-to-date synthesis of the literature on the association between fish consumption and EA, but is unable to distinguish cooking methods used in preparation which could influence this association. [DOI] [PubMed] [Google Scholar]
- 83••.O'Doherty MG, Freedman ND, Hollenbeck AR, Schatzkin A, Murray LJ, Cantwell MM, et al. Association of dietary fat intakes with risk of esophageal and gastric cancer in the NIH-AARP diet and health study. Int J Cancer. 2012;131(6):1376–87. doi: 10.1002/ijc.27366. This is the first epidemiologic study to consider a specific type of polyunsaturatted fatty acids (i.e., omega-3) and EA, suggesting little or no association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ibiebele TI, Hughes MC, Whiteman DC, Webb PM Australian Cancer S. Dietary patterns and risk of oesophageal cancers: a population-based case-control study. Br J Nutr. 2012;107(8):1207–16. doi: 10.1017/S0007114511004247. [DOI] [PubMed] [Google Scholar]
- 85.Jeurnink SM, Buchner FL, Bueno-de-Mesquita HB, Siersema PD, Boshuizen HC, Numans ME, et al. Variety in vegetable and fruit consumption and the risk of gastric and esophageal cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2012;131(6):E963–73. doi: 10.1002/ijc.27517. [DOI] [PubMed] [Google Scholar]
- 86.Dong LM, Kristal AR, Peters U, Schenk JM, Sanchez CA, Rabinovitch PS, et al. Dietary supplement use and risk of neoplastic progression in esophageal adenocarcinoma: a prospective study. Nutr Cancer. 2008;60(1):39–48. doi: 10.1080/01635580701586762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen H, Ward MH, Graubard BI, Heineman EF, Markin RM, Potischman NA, et al. Dietary patterns and adenocarcinoma of the esophagus and distal stomach. Am J Clin Nutr. 2002;75(1):137–44. doi: 10.1093/ajcn/75.1.137. [DOI] [PubMed] [Google Scholar]


