Abstract
Aim:
Defects in fatty acid metabolism contribute to the pathogenesis of insulin resistance and obesity. In this study, we investigated the effects of a novel compound yhhu981 on fatty acid metabolism in vitro and in vivo.
Methods:
The capacity to stimulate fatty acid oxidation was assessed in C2C12 myotubes. The fatty acid synthesis was studied in HepG2 cells using isotope tracing. The phosphorylation of AMPK and acetyl-CoA carboxylase (ACC) was examined with Western blot analysis. For in vivo experiments, ob/ob mice were orally treated with yhhu981 acutely (300 mg/kg) or chronically (150 or 300 mg·kg−1·d−1 for 22 d). On the last day of treatment, serum and tissue samples were collected for analysis.
Results:
Yhhu981 (12.5–25 μmol/L) significantly increased fatty acid oxidation and the expression of related genes (Sirt1, Pgc1α and Mcad) in C2C12 myotubes, and inhibited fatty acid synthesis in HepG2 cells. Furthermore, yhhu981 dose-dependently increased the phosphorylation of AMPK and ACC in both C2C12 myotubes and HepG2 cells. Compound C, an AMPK inhibitor, blocked fatty acid oxidation in yhhu981-treated C2C12 myotubes and fatty acid synthesis decrease in yhhu981-treated HepG2 cells. Acute administration of yhhu981 decreased the respiratory exchange ratio in ob/ob mice, whereas chronic treatment with yhhu981 ameliorated the lipid abnormalities and ectopic lipid deposition in skeletal muscle and liver of ob/ob mice.
Conclusion:
Yhhu981 is a potent compound that stimulates fatty acid oxidation, and exerts pleiotropic effects on lipid metabolism by activating AMPK.
Keywords: yhhu981, fatty acid oxidation, fatty acid synthesis, AMPK, compound C, sirtinol, EX527, STO609, AICAR, metformin, metabolism disorder, C2C12 myotubes, HepG2 cells, ob/ob mice
Introduction
Lipid metabolism disorder is one of the leading causes of insulin resistance. Among the peripheral tissues involved in energy metabolism, skeletal muscle plays an important role in lipid homeostasis1. In the presence of high concentrations of circulating non-esterified fatty acid (NEFA), lipid flux into skeletal muscle exceeds its oxidation capacity, leading to the ectopic accumulation of lipids and their metabolites, such as triglycerides, diacylglycerol and ceramide. These lipids are detrimental and ultimately impair insulin signaling pathways, contributing to skeletal muscle insulin resistance2. In the hyperglycemic state, elevated glucose levels alter the metabolic partitioning of fatty acids by shifting toward their esterification and away from their oxidation3. As a consequence, the direction of fatty acid metabolism switches from oxidation to synthesis, resulting in excess lipid accumulation in skeletal muscle4. Therefore, the fine-tuning of fatty acid oxidation represents a particularly attractive strategy for ameliorating skeletal muscle insulin resistance.
Fatty acid oxidation is regulated by multiple factors, of which AMP-activated protein kinase (AMPK) is the most important. Acting as a major cellular energy sensor, AMPK activation induces a variety of beneficial effects on glucose and lipid metabolism in peripheral tissues, such as skeletal muscle, liver and adipose tissue5,6. Research has demonstrated that AMPK increases fatty acid oxidation by directly inhibiting acetyl-CoA carboxylase (ACC) and by stimulating malonyl CoA decarboxylase (MCD), two enzymes responsible for malonyl CoA synthesis and degradation, respectively7,8. The result of these actions is to cause a net reduction in malonyl CoA levels, a release of the malonyl CoA-mediated inhibition of CPT-1, and an increase in fatty acid β-oxidation by mitochondria7,9. In addition, AMPK can regulate fatty acid oxidation by activating another downstream regulator, Sirtuin 1 (SIRT1). SIRT1 is a NAD+-dependent histone deacetylase that regulates life-span and lipid metabolism by deacetylating lysine residues on various transcription factors10. The activation of AMPK increases both the activity of SIRT1 by modulating nicotinamide phosphoribosyltransferase (NAMPT), an NAD+ synthetic enzyme, and the expression of SIRT1 by modulating the state of FOXO1 phosphorylation11,12. Thus, the beneficial metabolic changes induced by AMPK activation have attracted intense interest in developing AMPK activators as potential therapeutics for type 2 diabetes mellitus (T2DM) and obesity.
Recently, several compounds have been reported to improve fatty acid oxidation, primarily by activating the AMPK signaling pathway. 5-Aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (AICAR) is a commonly used AMPK activator. Once inside a cell, AICAR is metabolized into ZMP, an analog of AMP, leading to the activation of AMPK13. The beneficial effects of metformin, the first-line anti-diabetic drug, are mediated primarily through its effects on AMPK activation14. Moreover, berberine and resveratrol are two natural products that have been reported to exert beneficial effects as AMPK activators15,16. All of these compounds could stimulate AMPK activation, subsequently inhibiting ACC activity and leading to increased fatty acid oxidation. Furthermore, AICAR and resveratrol have been demonstrated to regulate fatty acid oxidation and mitochondrial function through the AMPK-SIRT1 pathway17,18.
In the present study, we aimed to identify novel molecules with the capacity to regulate lipid metabolism. Thus, we screened a library of synthetic small molecules for their activity to stimulate fatty acid oxidation in C2C12 myotubes and identified the potent compound yhhu981. This compound was further characterized in vitro and in vivo for its effects on lipid metabolism.
Materials and methods
Materials
AICAR (5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Sirtinol, EX527, STO-609, compound C and charcoal were purchased from Sigma (St Louis, MO, USA). Antibodies for phospho-AMPK(Thr172), AMPK, phospho-ACC (Ser79), ACC and GAPDH were from Cell Signaling Technology (Beverly, MA, USA). An anti-rabbit (IgG) secondary antibody conjugated to horseradish peroxidase was obtained from Bio-Rad Laboratories (Bio-Rad, CA, USA). The enhanced chemi-luminescence (ECL) plus Western Blotting Detection System was from GE Healthcare/Amersham Biosciences (Amersham, IL, USA). The vendors for all reagents and media used in cell experiments are as follows: Dulbecco's Modified Eagle's Medium (DMEM), Minimum Essential Medium (MEM) and Fetal Bovine Serum (FBS) were from GIBCO (Carlsbad, CO, USA); TRIzol reagent was from Invitrogen (Camarillo, CA, USA); the reverse transcription kit (DRR037A) and real-time PCR detection kit (DRR041A) were from Takara Biotechnology (Dalian) Co, Ltd (Dalian, China); the primers were synthesized by Sangon Biotech Co, Ltd (Shanghai, China); the triglyceride kit was from Nanjing Jiancheng Bioengineering Institute (Nanjing, China); the serum free fatty acid (NEFA) test kit was from Wako Chemicals GmbH (Neuss, Germany); the serum triacylglycerol (TG) kit was from Wenzhou Dongoujinma Reagent Co, Ltd (Wenzhou, China); the ultra-sensitive mouse insulin ELISA kit was from Crystal Chem Inc (Downers Grove, IL, USA). All other reagents and chemicals were of the highest grade available commercially.
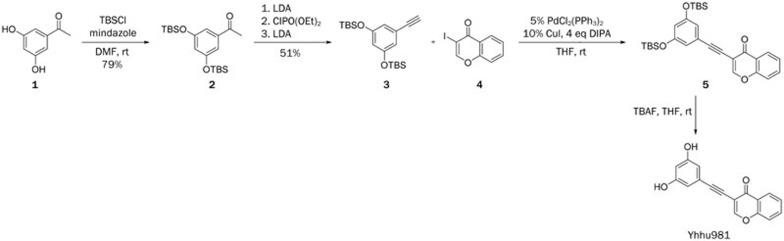
Synthetic route of yhhu981
Yhhu981 was prepared via the route shown in Scheme 1. Intermediates 2 and 3 were prepared according to a method previously reported in the literature19.
Scheme 1.
Synthetic route of yhhu981.
Compound 5. To a mixture of 3 (540 mg, 1.5 mmol), 4 (272 mg, 1 mmol), CuI (19 mg, 0.1 mmol) and PdCl2(PPh3)2 (35 mg, 0.05 mmol) in THF (5 mL) was added DIPEA (0.7 mL, 4 mmol) under nitrogen. The resulting mixture was stirred overnight at room temperature. The solvent was removed and the residue was purified by column, eluted with hexane: ethyl acetate (4:1) to give product 5 (395 mg, 78%) as a white solid.
Compound yhhu981. To a solution of 5 (250 mg, 0.5 mmol) in THF (5 mL) was added TBAF (260 mg, 1 mmol). The resulting mixture was stirred at room temperature for 3 h. The solvent was removed and the residue was purified by column, eluted with hexane: ethylacetate (2:1) to give product yhhu-981 (118 mg, 85%) as a white solid with purity >95%.
C2C12 cell differentiation and fatty acid oxidation
The skeletal muscle cell line C2C12 myoblasts were maintained in DMEM supplemented with 10% FBS at 37 °C with 95% air and 5% CO2. Differentiation into myotubes was performed as previously described20. Briefly, confluent cells were differentiated into myotubes by culturing with HG-DMEM containing 2% (v/v) horse serum (HS) for 5 d. Fatty acid oxidation was performed with differentiated C2C12 myotubes as previously described21. Briefly, C2C12 myotubes in 12-well plates were serum starved overnight. The cells were then incubated with 0.5 mL DMEM, supplemented with [9, 10(n)-3H] palmitic acid (PerkinElmer, 2.5 μCi/mL media) along with yhhu981 or the indicated treatment. The medium was collected and 0.2 mL of the cell supernatant was mixed with 0.9 mL charcoal slurry (10 g charcoal powder mixed with l00 mL 0.02 mol/L Tris-HCl buffer, pH 7.5) and shaken for 30 min at room temperature. Next, the samples were centrifuged at 13 000 r/min for 15 min, 0.2 mL of the supernatant was carefully withdrawn and added to 1 mL scintillation vials containing 0.8 mL of scintillation liquid and counted by liquid scintillation counting (PerkinElmer). The total cellular protein concentration was measured by the Bradford method (BioRad) and used for normalization.
HepG2 cell culture and fatty acid synthesis
The HepG2 cells were maintained in MEM supplemented with 10% FBS at 37 °C with 95% air and 5% CO2. Fatty acid synthesis was determined by measuring acetate incorporation into lipids. HepG2 cells were treated with various concentrations of yhhu981 or AICAR in serum-free MEM for 21 h, followed by co-incubation for 3 h in fresh medium containing [1-14C] acetate sodium salt (0.02 μCi/mL media) with or without indicated compound. Thereafter, cells were washed three times with ice-cold PBS and analyzed as previously described22.
Protein extraction and Western blot analysis
All protein extractions and Western blots were performed as previously described23. After treatment with yhhu981 or the other compounds, C2C12 myotubes or HepG2 cells were harvested by scraping into ice-cold lysis buffer (20 mmol/L Tris, pH 7.8, 137 mmol/L NaCl, 2.7 mmol/L KCl, 1 mmol/L MgCl2, 1% Triton X-100, 10 mmol/L NaF, 0.5 mmol/L Na3VO4, 10% glycerol, 1 μg/mL leupeptin, 0.2 mmol/L PMSF, 1 μg/mL aprotinin, 1 mmol/L EDTA, 1 mmol/L DTT, 5 mmol/L pyrophosphate sodium, 1 mmol/L benzamidine) and homogenated. The homogenates were rotated for 1 h and centrifuged (15 000×g for 10 min at 4 °C). The supernatant was collected and the total protein concentration was measured by the Bradford method. An equal amount of protein was loaded and resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes (Millipore, MA, USA), and blocked with 7.5% non-fat milk. Next, the membranes were blotted with primary antibodies against AMPK, phospho-AMPK (Thr172), ACC, phospho-ACC (Ser79), and GAPDH overnight at 4 °C, followed by a 2-h incubation with the horseradish peroxidase conjugated secondary antibody. The immunoreactive proteins were detected by ECL plus Western Blotting Detection System, and the Western blot signals were quantified by densitometry (BioRad) and normalized to total protein or GAPDH.
Quantitative real-time polymerase chain reaction
Total RNA was extracted from C2C12 myotubes using TRIzol reagent. Real-time reverse transcriptase polymerase chain reaction (RT-PCR) was performed via a two-step RT-PCR kit, followed by PCR using a SYBR® Premix Ex Taq™ II kit and ABI Prism 7500 Sequence Detection System (Life Technologies Corporation, California, USA). The primer sequences for all genes are listed here: Cpt1b forward: 5′-TGGATTCTGTGCGGCCCTTATTG-3′, reverse: 5′-TTTGCCTGGGATGCGTGTAGTGT-3′ Pgc1 forward: 5′-GAAGCGGGAGTCTGAAA-3′, reverse: 5′-GGTGTAACGGTAGGTGATG-3′ Mcad forward: 5′-GAGAAGAAGGGTGACGAGTATGT-3′, reverse: 5′-GGGTACTTTAGGATCTGGGTTAG-3′ Sirt1 forward: 5′-AGGGAACCTTTGCCTCATCTA-3′, reverse: 5′-GTGCCACTGTCACTGTTACTGC-3′ β-actin forward: 5′-TGCTGTCCCTGTATGCCTCTG-3′, reverse: 5′-TTGATGTCACGCACGATTTCC-3′. The 7500 Fast System Software was used for data analysis. β-actin mRNA was used as an endogenous control to normalize expression levels. The data were presented as the fold change relative to the endogenous control.
Animal experiments
B6.V-Lepob/Lepob mice (from Jackson Laboratory, Bar Harbor, ME, USA) were bred in the Shanghai Institute of Materia Medica (SIMM), Chinese Academy of Sciences. The animals were individually housed under constant temperature and humidity on a 12-h light-dark cycle with free access to water and food. The mice were assigned to four groups (n=8) based on fasting blood glucose and body weight and subjected to once daily oral gavage with vehicle (0.5% carboxymethyl cellulose, CMC), yhhu981 (150 or 300 mg/kg) or metformin (250 mg/kg) for 22 d. Body weights and food intake were measured daily during the whole treatment. At the end of treatment, blood was collected for glucose, insulin, NEFA, and triacylglycerol analysis. The gastrocnemius and liver were dissected for triacylglycerol measurement. The acute effect of yhhu981 (300 mg/kg) on the respiratory exchange ratio (RER) was measured by indirect calorimetry using the Oxymax system (Columbus Instruments, Columbus, OH, USA) as described24. All the animal studies were approved by the Animal Care and Use Committee, Shanghai Institute of Materia Medica, Chinese Academy of Sciences.
Determination of triacylglycerol content in gastrocnemius and liver
Triacylglycerol in the gastrocnemius muscle and liver was measured as previously described25. In brief, 2 mL each of muscle and liver tissue were homogenized in a Heptan-Isopropanol-Tween mixture (3:2:0.01 by volume), vortexed and allowed to separate into two phases, and then centrifuged at 500×g for 10 min at 4 °C. An equal volume of the organic phase was evaporated by vacuum drier until dry. Triacylglycerol was then measured using a triacylglycerol kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions. The triacylglycerol content was normalized to tissue weight and expressed as micromole per gram of tissue.
Statistical analysis
The data were expressed as the mean±SEM. All statistical analyses were performed using Prism 5 (GraphPad Software Incorporate, CA, USA). Comparisons were performed with Student's t-test or one-way ANOVA when appropriate. For ANOVA, if a significant variance was found, the Newman-Keuls test was used for post hoc analysis. The difference was deemed significant when P<0.05.
Results
Yhhu981 increased fatty acid oxidation in C2C12 myotubes
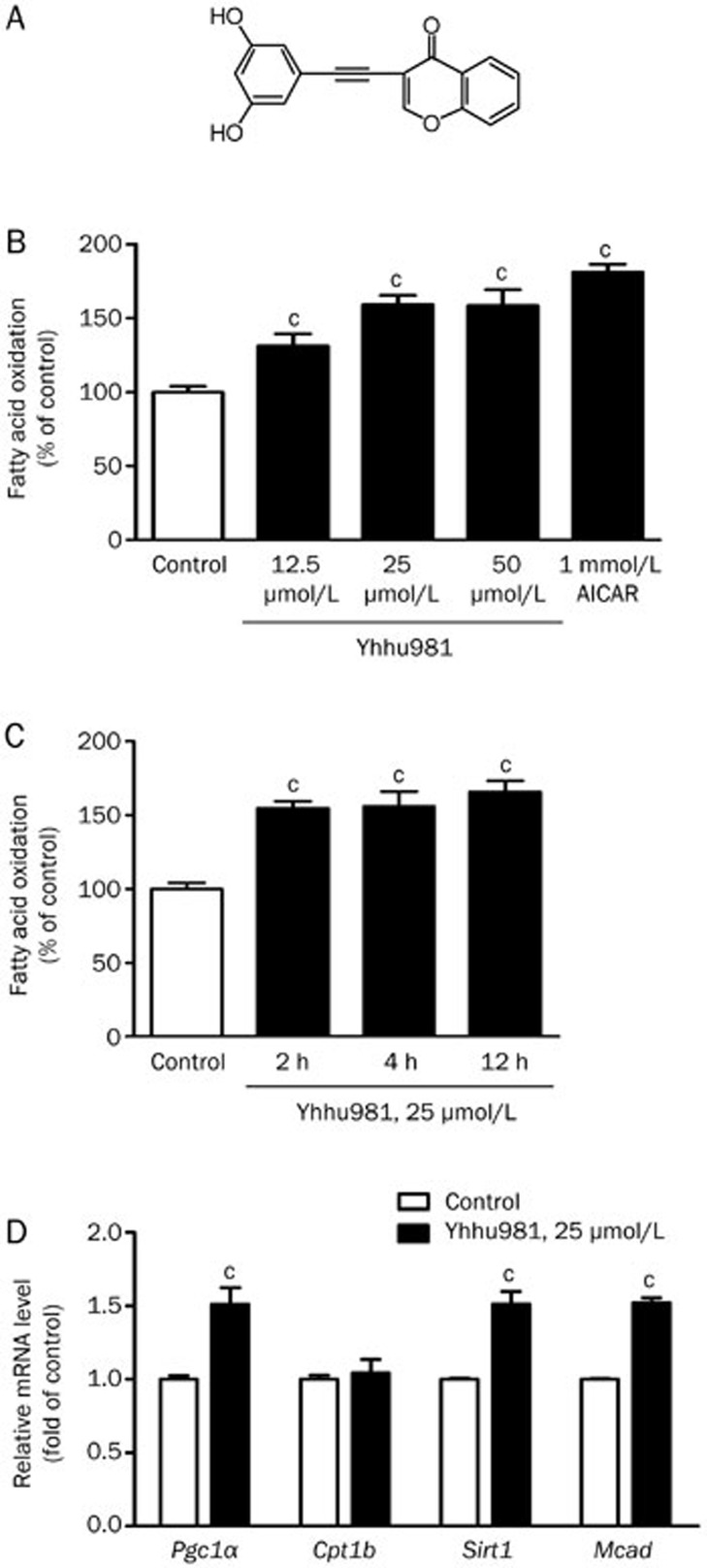
We screened a series of compounds for their ability to stimulate fatty acid oxidation in C2C12 myotubes, and yhhu981 was identified as the most potent candidate (Figure 1A). We tested the yhhu981 dose- and time-response of fatty acid oxidation in C2C12 myotubes. A significant increase in fatty acid oxidation was observed when yhhu981 was tested at 12.5 μmol/L (131%±7.76% of control, P<0.05), with a maximum response reached at 25 μmol/L (159%±6.17%, P<0.001, Figure 1B). The maximal response of fatty acid oxidation to yhhu981 reached at 2 h and sustained for more than 12 h (Figure 1C). At the concentrations tested, yhhu981 did not affect cell morphology or viability as assessed either by microscopic visualization or by MTT testing (data not shown). The effects of yhhu981 on fatty acid oxidation prompted us to investigate whether yhhu981 stimulates fatty acid oxidation by regulating gene expression. We employed real-time RT-PCR to profile several genes related to fatty acid oxidation. Our results showed that yhhu981 upregulated the expression of Sirt1, Pgc1a and Mcad in C2C12 myotubes by more than 1.5-fold. The expression of Cpt1b remained unchanged (Figure 1D).
Figure 1.
Yhhu981 stimulated fatty acid oxidation in C2C12 myotubes. (A) Structure of yhhu981. (B) C2C12 myotubes were incubated with yhhu981 at indicated concentrations in serum-free media for 2 h or (C) with yhhu981 (25 μmol/L) for the indicated periods. (D) C2C12 myotubes were treated with yhhu981 (25 μmol/L) for 24 h, total RNA was extracted and real-time PCR was used to measure the mRNA levels of genes involved in fatty acid oxidation. The expression of each gene in the control cells was set as 100%. The results represent the mean±SEM for more than three independent experiments in duplicates. cP<0.01 compared with control.
Yhhu981 activated the AMPK signaling pathway in C2C12 myotubes
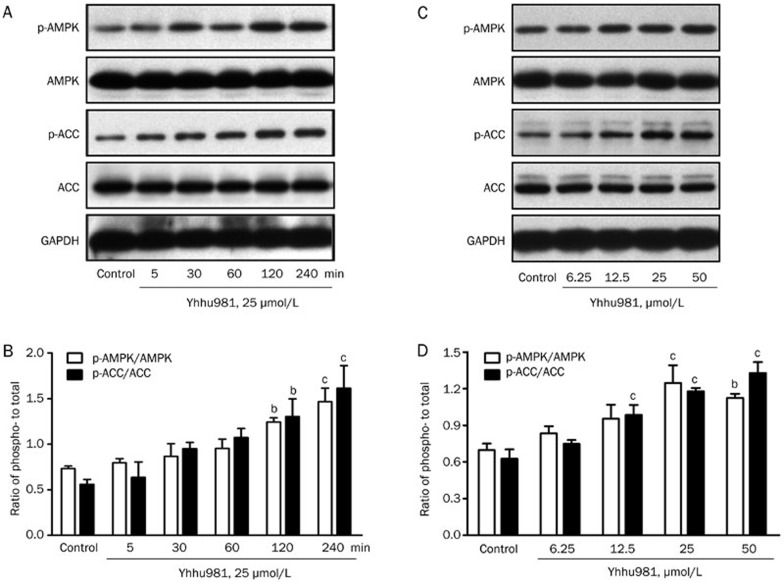
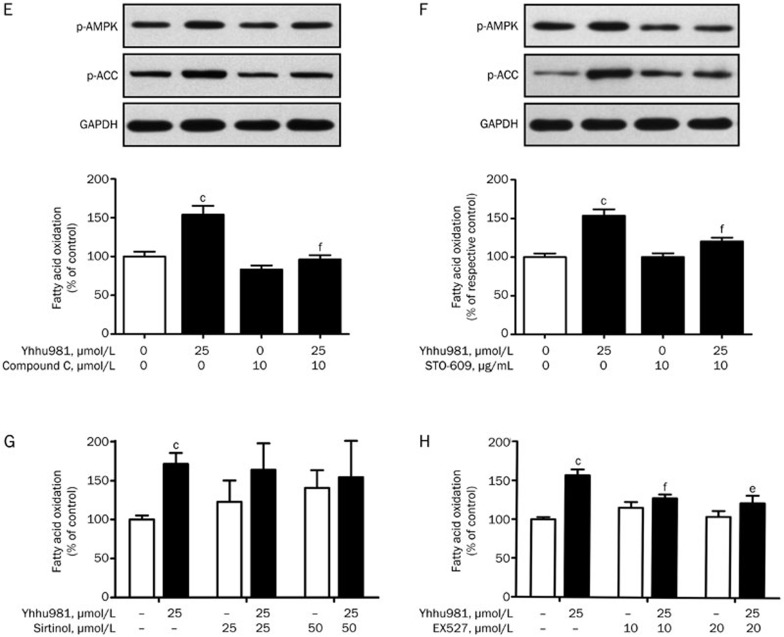
Thr172 phosphorylation serves as a marker for AMPK activation. We examined whether yhhu981 could activate AMPK and thereby promote fatty acid oxidation in C2C12 myotubes. Our results showed that yhhu981 stimulated AMPK Thr172 phosphorylation in a dose- and time-dependent manner. Consistent with an effect on AMPK activation, the phosphorylation of the AMPK substrate ACC was also significantly increased (Figure 2A–2D). AMPK activation is associated with improved lipid metabolism in rodents26,27,28. To determine whether AMPK activation mediates elevated fatty acid oxidation by yhhu981, we pre-incubated C2C12 myotubes with the AMPK inhibitor compound C for 30 min before co-incubation with yhhu981. Compound C abolished AMPK phosphorylation and blocked the yhhu981-induced increase in fatty acid oxidation (Figure 2E). It has been shown that CaMKK is an upstream kinase of AMPK29; therefore, we tested the CaMKK inhibitor STO-609 for its effects on the yhhu981-stimulated fatty acid oxidation. Pretreatment with STO-609 before incubation with yhhu981 resulted in an attenuation of yhhu981-stimulated AMPK phosphorylation and fatty acid oxidation (Figure 2F). Collectively, these results demonstrated that yhhu981 stimulated fatty acid oxidation in myotubes through the activation of the CaMKK-AMPK signaling pathway.
Figure 2A-2D.
Yhhu981 promoted fatty acid oxidation by activating the AMPK signaling pathway in C2C12 myotubes. C2C12 myotubes were exposed to 25 μmol/L yhhu981 for the indicated time period or indicated concentrations of yhhu981 for 2 h. (A, C) Protein samples were prepared and subjected to Western blot. (B, D) Ratio of phospho- to total-AMPK, ACC was presented as a bar graph.
Figure 2E-2H.
(E, F) C2C12 myotubes were pretreated with or without 10 μmol/L compound C or 10 μg/mL STO609 for 30 min and then co-treated with 25 μmol/L yhhu981 for another 2 h, followed by the measurement of fatty acid oxidation. The values of fatty acid oxidation in Figure 2E were normalized to the control group, and the values of fatty acid oxidation in Figure 2F were normalized to the respective control. (G, H) C2C12 myotubes were pretreated with SIRT1 inhibitors EX527 or sirtinol, then co-incubated with yhhu981 for another 2 h, followed by the measurement of fatty acid oxidation. The values of fatty acid oxidation in Figure 2G and 2H were normalized to the control group. The results represent the mean±SEM for three independent experiments. bP<0.05, cP<0.01 compared with related control. eP<0.05, fP<0.01 compared with yhhu981 treatment alone.
Previous reports showed that Sirt1 activation was related to its regulatory effect on fatty acid oxidation16,30,31. The induction of Sirt1 expression by yhhu981 prompted us to test whether Sirt1 is involved in the yhhu981-stimulated fatty acid oxidation. We pre-incubated C2C12 myotubes with the Sirt1 inhibitors EX527 or sirtinol before yhhu981 treatment32. No significant increase was observed in C2C12 myotubes treated with yhhu981 in the presence of sirtinol (Figure 2G). Moreover, EX527 significantly attenuated yhhu981-induced fatty acid oxidation (Figure 2H). These results suggested an important role for Sirt1 in yhhu981-stimulated fatty acid oxidation.
Effect of yhhu981 on fatty acid synthesis in HepG2 cells
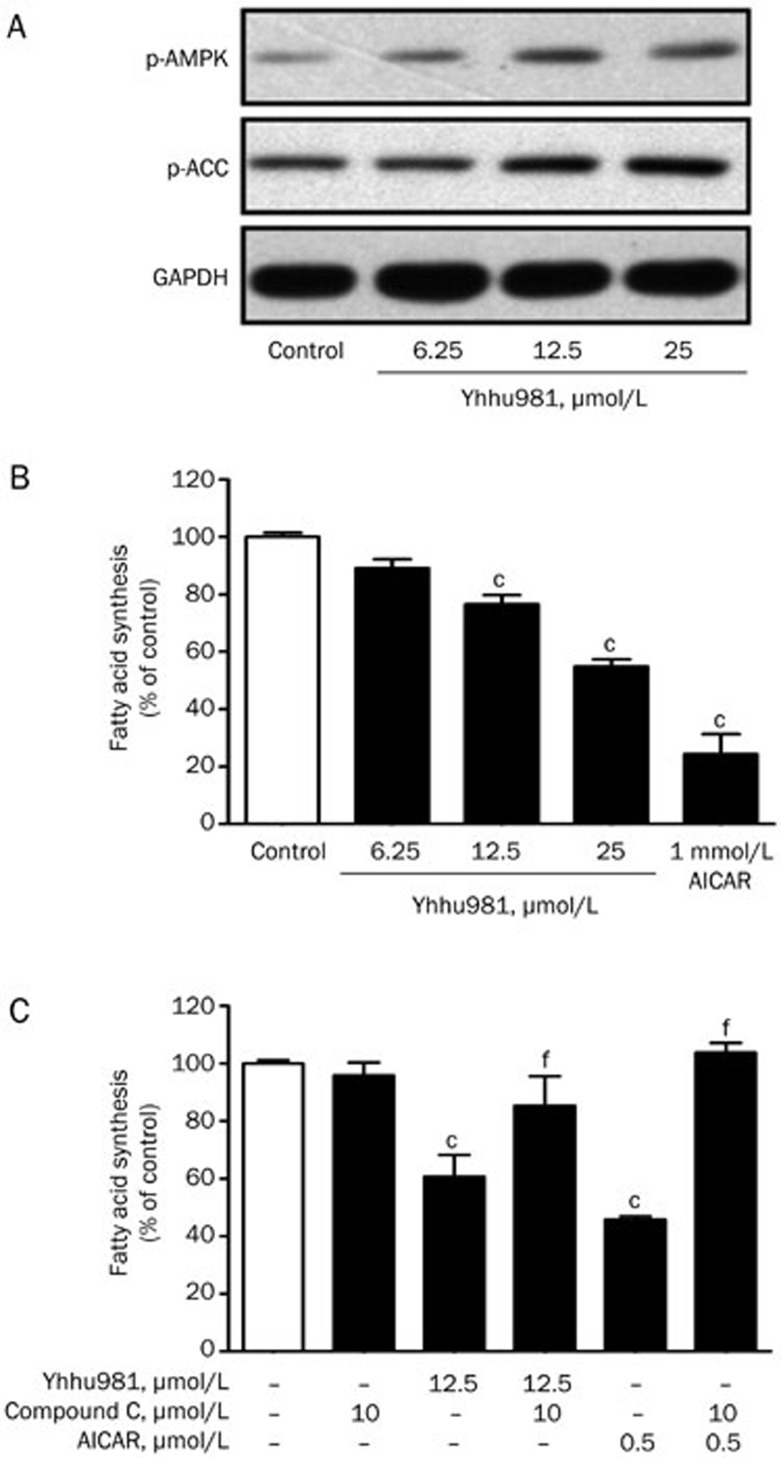
Liang et al reported that the activation of AMPK or Sirt1 alleviates ethanol-induced alcoholic fatty liver and improves hepatocyte lipid metabolism in mice33,34,35. We carried out a fatty acid synthesis assay in HepG2 hepatoma cells in the presence of increasing concentrations of yhhu981. Consistent with the results from C2C12 myotubes, yhhu981 dose-dependently increased the phosphorylation of AMPK (Figure 3A) and suppressed fatty acid synthesis (Figure 3B); the AMPK inhibitor compound C reversed the suppression of fatty acid synthesis by yhhu981 (Figure 3C). These results indicated that yhhu981 repressed fatty acid synthesis through a mechanism involving the activation of AMPK.
Figure 3.
Yhhu981 inhibited fatty acid synthesis in HepG2 cells. (A) HepG2 cells were incubated with yhhu981 at indicated concentration in serum-free media for 24 h, protein samples were prepared and subjected to Western blot. (B) HepG2 cells were incubated with yhhu981 at the indicated concentrations in serum-free media for 24 h, followed by the measurement of fatty acid synthesis. (C) HepG2 cells were pretreated with or without 10 μmol/L compound C for 30 min and then treated with 25 μmol/L yhhu981 or 1 mmol/L AICAR for another 24 h, followed by the measurement of fatty acid synthesis. AICAR treatment was used as a positive control. The results represent the mean±SEM for more than three independent experiments in duplicates. cP<0.01 compared with control. fP<0.01 compared with yhhu981 or AICAR treatment alone.
Acute effects of yhhu981 on respiration exchange ratio in ob/ob mice
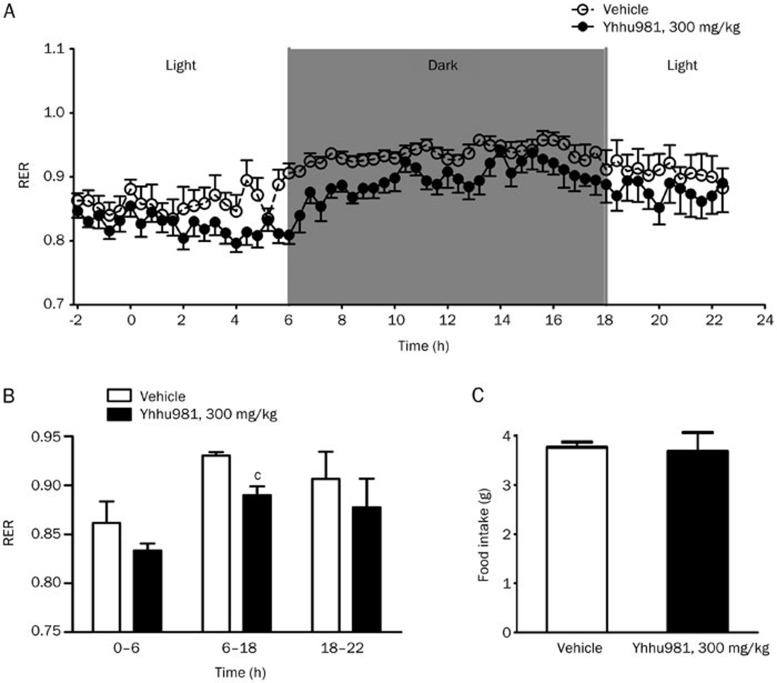
Based on the in vitro data, we examined whether yhhu981 could acutely affect whole-body metabolism in ob/ob mice. Mice were administered 300 mg/kg yhhu981 and monitored for the following 22 h. We observed a significant decrease in the respiratory exchange ratio (Figure 4A and 4B) without affecting food intake (Figure 4C), indicating a shift to fatty acid utilization.
Figure 4.
A single administration of yhhu981 decreased the respiration exchange ratio in ob/ob mice. Respiration exchange ratio (A, B) and food intake (C) were measured from −2 h (2 h before administration) to +22 h (22 h after administration) by indirect calorimetry using the Oxymax system. Data are presented as the mean±SEM. n=6. cP<0.01 compared with the vehicle group.
Chronic yhhu981 treatment improved fatty acid metabolism in ob/ob mice
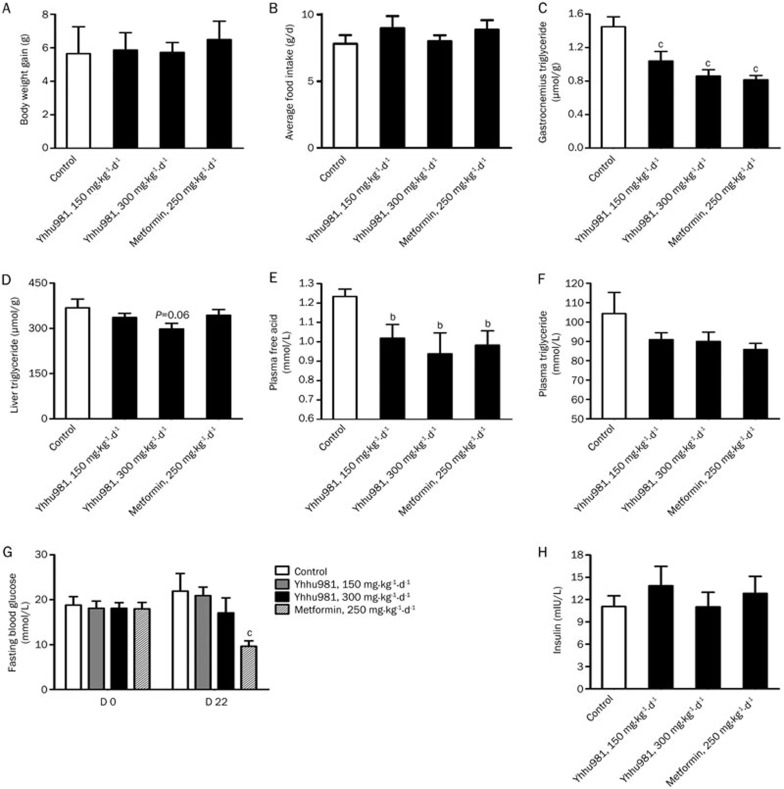
To test whether yhhu981 improves fatty acid metabolism in vivo after chronic treatment, we treated ob/ob mice with yhhu981 for 22 d. Yhhu981 treatment did not significantly change body weight (Figure 5A) or average food intake (Figure 5B), but significantly reduced the TG content in the gastrocnemius muscle (Figure 5C) and the serum NEFA levels (Figure 5E). The TG content in liver (Figure 5D), serum TG levels (Figure 5F) and fasting blood glucose (Figure 5G) all showed a trend towards lower levels, although the decrease did not reach statistical significance. However, the serum insulin level was not significantly changed (Figure 5H).
Figure 5.
Yhhu981 improved lipid metabolism in ob/ob mice. Ob/ob mice were subjected to gavage treatment once daily with vehicle (0.5% CMC), 150 mg·kg−1·d−1 or 300 mg·kg−1·d−1 compound yhhu981 or 250 mg·kg−1·d−1 metformin for 22 d. At the end of treatment, blood, liver and gastrocnemius were collected for posterior analysis. Body weight (A) and food intake (B) were recorded regularly during the treatment period. The content of triglyceride in the gastrocnemius (C) and liver (D) were determined by alkane-isopropyl alcohol-extract methods. Plasma free fatty acid (E) and triglyceride (F) were determined as described in Materials and methods. Fasting blood glucose concentrations (G) were measured before and after 22 d treatment. Insulin levels (H) were measured at the end of the treatment. Values are expressed as the mean±SEM for n=6–8 mice. bP<0.05, cP<0.01 vs control group.
Discussion
The regulation of fatty acid metabolism represents a critical strategy for improving metabolic disorders, such as insulin resistance and type 2 diabetes mellitus. Recently, a series of reported compounds were defined as fatty acid metabolic regulators. AICAR, an AMPK activator, stimulated fatty acid oxidation by the allosteric regulation of CPT-1, as well as by activating PPARα and PGC-136,37,38,39,40. Resveratrol, the first reported Sirt1 activator, increased fatty acid oxidation in skeletal muscle cells and adipocytes through the activation of AMPK16,41. Thus, a compound capable of regulating fatty acid metabolism may hold promise for the treatment of diabetes. In this study, we aimed to identify a new compound capable of potently regulating fatty acid oxidation in C2C12 myotubes. Yhhu981, a compound with a novel structure was found to stimulate fatty acid oxidation in C2C12 myotubes and to repress fatty acid synthesis in HepG2 cells. Further studies showed that yhhu981 reduced circulating NEFA levels and ectopic lipid deposition in skeletal muscle and liver of ob/ob mice.
In an attempt to uncover the mechanisms underlying yhhu981-stimulated fatty acid oxidation, we investigated the potential involvement of AMPK. AMPK plays a key role in the regulation of lipid metabolism through multiple signaling pathways, including by directly catalyzing its downstream substrates, and through the transcription of multiple genes42,43. In the present study, yhhu981 was found to stimulate fatty acid oxidation and upregulate the related genes, which was also accompanied by the activation of AMPK signaling pathways in C2C12 myotubes. More importantly, yhhu981-stimulated fatty acid oxidation was blunted by the AMPK inhibitor compound C and the CaMKK inhibitor STO-609. Collectively, these data suggest that yhhu981 stimulates fatty acid oxidation through the activation of the CaMKK-AMPK signaling pathway. SIRT1 plays a key role in regulating fatty acid oxidation and lipid metabolism in mammals35,44. The activation of SIRT1 has been considered as a promising approach to ameliorate lipid disorders45. Recently, reports showed that AMPK acted upstream of SIRT1 and increased its activity by modulating NAMPT, an NAD+ synthetic enzyme11,12. However, AMPK has also been shown to regulate SIRT1 expression by modulating the state of FOXO1 phosphorylation46,47. We did not detect direct SIRT1 activation by yhhu981 in the in vitro assay. However, yhhu981 induced the expression of Sirt1 in C2C12 myotubes. Furthermore, the selective SIRT1 inhibitors EX527 and sirtind reversed the stimulatory effect of yhhu981 on fatty acid oxidation. Given that the induction of Sirt1 by yhhu981 is downstream of AMPK, the results demonstrated that yhhu981 stimulates fatty acid oxidation by activating the CaMKK-AMPK-SIRT1 signaling pathway in myotubes11,48.
The liver is the major organ for buffering quick fatty acid storage and utilization. Hepatic fatty acid metabolism plays an important role in the regulation of whole-body energy homeostasis. The evidence that activation of AMPK in the liver or hepatocytes resulted in increased fatty acid oxidation and decreased lipogenesis holds considerable potential for ameliorating metabolic disorders49,50. Because yhhu981 was shown to stimulate AMPK signaling pathways in C2C12 myotubes, we further investigated its effects on hepatocytes. Consistently, yhhu981 increased the phosphorylation of AMPK and ACC in HepG2 cells, suggesting that the activation of AMPK has occurred. Moreover, yhhu981 dose-dependently inhibited fatty acid synthesis in HepG2 cells, and the AMPK inhibitor compound C reversed the inhibition caused by yhhu981. These results again demonstrated that yhhu981 suppressed fatty acid synthesis by activating AMPK in hepatocytes.
To investigate the physiological relevance of the above cellular observations, we treated ob/ob mice with yhhu981. Initially, we administered to mice a single dose of yhhu981, which did not significantly affect whole-body energy expenditure (data not shown); however, we did observe a reduction in the respiratory exchange ratio (RER) compared with vehicle throughout the monitored time after treatment, indicating a switch to whole-body fatty acid oxidation21. This elevated lipid utilization is consistent with the AMPK signaling pathway activation39 that we observed in the C2C12 and HepG2 cells treated with yhhu981. We next assessed the chronic effects of 22 d of yhhu981 treatment in ob/ob mice and phenotyped the mice for alterations in lipid metabolism. As a genetically obese rodent model, ob/ob mice displayed obesity, hyperglycemia, dyslipidemia and significant ectopic lipid deposition in both the liver and skeletal muscle. Yhhu981 treatment did not alter body weight or food intake, but significantly decreased circulating NEFA levels. In addition, yhhu981 reduced TG content in both the gastrocnemius muscle and the liver, indicating the amelioration of ectopic lipid accumulation. These observations in ob/ob mice are consistent with the enhanced fatty acid oxidation and diminished fatty acid synthesis that was uncovered in our in vitro study.
In conclusion, here we identified yhhu981 as a new compound that enhances free fatty acid oxidation in C2C12 myotubes. Further studies revealed the pleiotropic effects of yhhu981 on lipid metabolism by activating the AMPK signaling pathway. The lipid abnormalities and ectopic fat deposition in skeletal muscle and liver of ob/ob mice were ameliorated by chronic treatment with yhhu981. These results highlight the potential value of yhhu981 as a leading compound for the treatment of metabolic disorders and provide support for strategies that target the activation of free fatty acid oxidation for the prevention of lipid abnormalities and ectopic fat accumulation, which were associated with the development of metabolic syndrome and type 2 diabetes.
Author contribution
Ying LENG and You-hong HU designed research; Hong-liang ZENG, Su-ling HUANG, Fu-chun XIE, and Li-min ZENG performed research; Hong-liang ZENG, Su-ling HUANG, and Ying LENG wrote the paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81225022).
References
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–9. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Prentki M, Joly E, El-Assaad W, Roduit R. Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity: role in beta-cell adaptation and failure in the etiology of diabetes. Diabetes. 2002;51:S405–13. doi: 10.2337/diabetes.51.2007.s405. [DOI] [PubMed] [Google Scholar]
- Visiedo F, Bugatto F, Sanchez V, Cozar-Castellano I, Bartha JL, Perdomo G. High glucose levels reduce fatty acid oxidation and increase triglyceride accumulation in human placenta. Am J Physiol Endocrinol Metab. 2013;305:E205–12. doi: 10.1152/ajpendo.00032.2013. [DOI] [PubMed] [Google Scholar]
- Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859–65. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–83. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol. 2006;574:33–9. doi: 10.1113/jphysiol.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzamko N, Schertzer JD, Ryall JG, Steel R, Macaulay SL, Wee S, et al. AMPK-independent pathways regulate skeletal muscle fatty acid oxidation. J Physiol. 2008;586:5819–31. doi: 10.1113/jphysiol.2008.159814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg GR, Macaulay SL, Febbraio MA, Kemp BE. AMP-activated protein kinase--the fat controller of the energy railroad. Can J Physiol Pharmacol. 2006;84:655–65. doi: 10.1139/y06-005. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Stark MJ, Foster DW. Hepatic malonyl-CoA levels of fed, fasted and diabetic rats as measured using a simple radioisotopic assay. J Biol Chem. 1978;253:8291–3. [PubMed] [Google Scholar]
- Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–74. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–60. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–73. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill HM, Lally JS, Galic S, Thomas M, Azizi PD, Fullerton MD, et al. AMPK phosphorylation of ACC2 is required for skeletal muscle fatty acid oxidation and insulin sensitivity in mice. Diabetologia. 2014;57:1693–702. doi: 10.1007/s00125-014-3273-1. [DOI] [PubMed] [Google Scholar]
- Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–81. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- Brusq JM, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y, et al. Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine. J Lipid Res. 2006;47:1281–8. doi: 10.1194/jlr.M600020-JLR200. [DOI] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–63. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Mi SL, Hu N, Doser TA, Sun A, Ge J, et al. Mitochondrial aldehyde dehydrogenase 2 accentuates aging-induced cardiac remodeling and contractile dysfunction: role of AMPK, Sirt1, and mitochondrial function. Free Radic Biol Med. 2014;71:208–20. doi: 10.1016/j.freeradbiomed.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies KJ, Singh K, Saleem A, Hood DA. Sirtuin 1-mediated effects of exercise and resveratrol on mitochondrial biogenesis. J Biol Chem. 2013;288:6968–79. doi: 10.1074/jbc.M112.431155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Herrick IR, Atesin TA, Caruana PA, Kellenberger CA, Frontier AJ. Polarizing the Nazarov cyclization: the impact of dienone substitution pattern on reactivity and selectivity. J Am Chem Soc. 2008;130:1003–11. doi: 10.1021/ja077162g. [DOI] [PubMed] [Google Scholar]
- Epting CL, Lopez JE, Shen X, Liu L, Bristow J, Bernstein HS. Stem cell antigen-1 is necessary for cell-cycle withdrawal and myoblast differentiation in C2C12 cells. J Cell Sci. 2004;117:6185–95. doi: 10.1242/jcs.01548. [DOI] [PubMed] [Google Scholar]
- Rune A, Osler ME, Fritz T, Zierath JR. Regulation of skeletal muscle sucrose, non-fermenting 1/AMP-activated protein kinase-related kinase (SNARK) by metabolic stress and diabetes. Diabetologia. 2009;52:2182–9. doi: 10.1007/s00125-009-1465-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts AW, Ferguson K, Hennessy S, Vagelos PR. Regulation of lipid synthesis in cultured animal cells. J Biol Chem. 1974;249:5241–9. [PubMed] [Google Scholar]
- Hu X, Feng Y, Liu X, Zhao XF, Yu JH, Yang YS, et al. Effect of a novel non-thiazolidinedione peroxisome proliferator-activated receptor alpha/gamma agonist on glucose uptake. Diabetologia. 2007;50:1048–57. doi: 10.1007/s00125-007-0622-3. [DOI] [PubMed] [Google Scholar]
- Picard F, Gehin M, Annicotte J, Rocchi S, Champy MF, O'Malley BW, et al. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell. 2002;111:931–41. doi: 10.1016/s0092-8674(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–91. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–22. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin. 2008;29:698–706. doi: 10.1111/j.1745-7254.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403:139–48. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–6. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–51. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck B, Chen CY, Ho KK, Di Fruscia P, Myatt SS, Coombes RC, et al. SIRT inhibitors induce cell death and p53 acetylation through targeting both SIRT1 and SIRT2. Mol Cancer Ther. 2010;9:844–55. doi: 10.1158/1535-7163.MCT-09-0971. [DOI] [PubMed] [Google Scholar]
- Ajmo JM, Liang X, Rogers CQ, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G833–42. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Ajmo JM, Rogers CQ, You M. Resveratrol alleviates alcoholic fatty liver in mice. Alcohol Clin Exp Res. 2009;33:161a–61a. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–26. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher Z, Ruderman N, Tornheim K, Ido Y. Acute regulation of fatty acid oxidation and AMP-activated protein kinase in human umbilical vein endothelial cells. Circ Res. 2001;88:1276–82. doi: 10.1161/hh1201.092998. [DOI] [PubMed] [Google Scholar]
- Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273:E1107–12. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- Smith AC, Bruce CR, Dyck DJ. AMP kinase activation with AICAR simultaneously increases fatty acid and glucose oxidation in resting rat soleus muscle. J Physiol. 2005;565:537–46. doi: 10.1113/jphysiol.2004.081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Bruce CR, Dyck DJ. AMP kinase activation with AICAR further increases fatty acid oxidation and blunts triacylglycerol hydrolysis in contracting rat soleus muscle. J Physiol-London. 2005;565:547–53. doi: 10.1113/jphysiol.2004.081687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, Kim M, Park HS, Kim HS, Jeon MJ, Oh KS, et al. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochem Biophys Res Commun. 2006;340:291–5. doi: 10.1016/j.bbrc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Mercader J, Palou A, Bonet ML. Resveratrol enhances fatty acid oxidation capacity and reduces resistin and Retinol-Binding Protein 4 expression in white adipocytes. J Nutr Biochem. 2011;22:828–34. doi: 10.1016/j.jnutbio.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D. The AMP-activated protein kinase — fuel gauge of the mammalian cell. Eur J Biochem. 1997;246:259–73. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Seefeld K, Witters LA, Coleman RA. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J. 1999;338:783–91. [PMC free article] [PubMed] [Google Scholar]
- Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–58. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3:340–51. doi: 10.1038/nrd1344. [DOI] [PubMed] [Google Scholar]
- Rutanen J, Yaluri N, Modi S, Pihlajamaki J, Vanttinen M, Itkonen P, et al. SIRT1 mRNA expression may be associated with energy expenditure and insulin sensitivity. Diabetes. 2010;59:829–35. doi: 10.2337/db09-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–35. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen QW, Zhu MJ, Tong J, Ren J, Du M. Ca2+/calmodulin-dependent protein kinase kinase is involved in AMP-activated protein kinase activation by alpha-lipoic acid in C2C12 myotubes. Am J Physiol Cell Physiol. 2007;293:C1395–403. doi: 10.1152/ajpcell.00115.2007. [DOI] [PubMed] [Google Scholar]
- Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metabolism. 2006;3:403–16. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]