Abstract
Viral cellular immune surveillance is a dynamic and fluid system that is driven by finely regulated cellular processes including cytokines and other factors locally in the microenvironment and systemically throughout the body. It is questionable as to what extent the central nervous system (CNS) is an immune-privileged organ protected by the blood-brain barrier (BBB). Recent evidence suggests converging pathways through which viral infection, and its associated immune surveillance processes, may alter the integrity of the blood-brain barrier, and lead to inflammation, swelling of the brain parenchyma and associated neurological syndromes. Here, we expand upon the recent “gateway theory”, by which viral infection and other immune activation states may disrupt the specialized tight junctions of the BBB endothelium making it permeable to immune cells and factors. The model we outline here builds upon the proposition that this process may actually be initiated by cytokines of the IL-17 family, and recognizing the intimate balance between TH17 and TH9 cytokine profiles systemically. We argue that immune surveillance events, in response to viruses such as the Human Immunodeficiency Virus (HIV), cause a TH17/TH9 induced gateway through blood brain barrier, and thus lead to characteristic neuroimmune pathology. It is possible and even probable that the novel TH17/TH9 induced gateway, which we describe here, opens as a consequence of any state of immune activation and sustained chronic inflammation, whether associated with viral infection or any other cause of peripheral or central neuroinflammation. This view could lead to new, timely and critical patient-centered therapies for patients with neuroimmune pathologies across a variety of etiologies.
Abbreviations
BBB - blood brain barrier, BDV - Borna disease virus, CARD - caspase activation and recruitment domains, CD - clusters of differentiation, CNS - central nervous system, DAMP - damage-associated molecular patterns, DENV - Dengue virus, EBOV - Ebola virus, ESCRT - endosomal sorting complex required for transport-I, HepC - Hepatitis C virus, HIV - human immunodeficiency virus, IFN - interferon, ILn - interleukin-n, IRF-n - interferon regulatory factor-n, MAVS - mitochondrial antiviral-signaling, MBGV - Marburg virus, M-CSF - macrophage colony-stimulating factor, MCP-1 - monocyte chemotactic protein 1 (aka CCL2), MHC - major histocompatibility complex, MIP-α β - macrophage inflammatory protein-1 α β (aka CCL3 & CCL4), MIF - macrophage migration inhibitory factor, NVE - Nipah virus encephalitis, NK - natural killer cell, NLR - NLR, NOD - like receptor, NOD - nucleotide oligomerization domain, PAMP - pathogen-associated molecular patterns, PtdIns - phosphoinositides, PV - Poliovirus, RIG-I - retinoic acid-inducible gene I, RIP - Receptor-interacting protein (RIP) kinase, RLR - RIG-I-like receptor, sICAM1 - soluble intracellular adhesion molecule 1, STAT-3 - signal tranducer and activator of transcription-3, sVCAM1 - soluble vascular cell adhesion molecule 1, TANK - TRAF family member-associated NF- . B activator, TBK1 - TANK-binding kinase 1, TLR - Toll-like receptor, TNF - tumor necrosis factor, TNFR - TNF receptor, TNFRSF21 - tumor necrosis factor receptor superfamily member 21, TRADD TNFR-SF1A - associated via death domain, TRAF TNFR - associated factor, Tregs - regulatory T cellsubpopulation (CD4/8+CD25+FoxP3+), VHF - viral hemorrhagic fever.
Keywords: viral immune surveillance and evasion, M1 & M2 macrophages, Tregs, TH17, neuroinflammation, blood-brain barrier, “gateway theory”, TH17/TH9 BBB gateway model
Fundamentals of Viral Immunity
General Features:
Immune surveillance broadly consists of two principal branches: innate immunity includes those immune processes that are triggered by the recognition of a novel pathogen. Acquired immunity (i.e., antigen-dependent immunity) describes immune responses that are consequential to an antigen that was encountered previously. Both innate and acquired processes of immune surveillance are brought about principally either by humoral or cellular events. Humoral immunity provides protective immune surveillance by means of circulating soluble factors, such as cytokines, growth factors, complement factors and antibodies. These factors are detected and measured in a variety of bodily fluids (e.g., blood serum, cerebrospinal fluid, saliva, synovial fluid). Humoral immune factors are produced by cells, which principally belong to the immune system per se. Certain cell populations that are not immune cells by functional definition (e.g., fibroblasts, astrocytes) contribute to the production of humoral factors at local sites of inflammatory and immune responses. Cellular immunity consists of immune surveillance events that are brought about by concerted, regulated myeloid and lymphoid cell populations. There are two principal families of innate immunity cells: the natural killer (NK) cells, and the antigenpresenting cells, composed of myeloid derivatives, including dendritic cells and monocyte/macrophages. Cellular immune components of acquired immunity involve the lymphoid derivatives the T and B cells.
Immune cell populations are described and recognized by their functional status, and their phenol type. The latter is defined by glycoproteins that constitute the plasma membrane clusters of differentiation (CD). In most, but not all cases, CD׳s correspond to an identified function or functional structure, such as CD3 associated with the T cell receptor and marking all T cells. T cells express either CD4 or CD8 as their final stage of differentiation in the thymus. The majority of CD3+CD4+ cells are T cells endowed with the functional ability to assist cellular immunity to commence, expand, sustain and control a fully developed acquired immunity response. CD4 T cells are often referred to as helper T cells, despite the fact that a small proportion of CD4 T cells can be cytotoxic. The cytotoxic immune function is primarily managed by CD8+CD3+ T cells, which also produce humoral factors and contribute to assisting cellular immune processes. The CD4 moiety recognizes and binds to the major histo compatibility complex (MHC) Class II, whereas CD8 recognizes and binds to MHC Class I. Because of the fact that MHC Class I is ubiquitously expressed by every cell in the body, it follows that CD8 T cells provide immune surveillance of any cell that expresses foreign antigen on its membrane in association with MHC Class I, such as a tumor cells and virally infected cells.
Thus, this broad-stroke overview of immune surveillance mechanisms reveals that viral infections, in general, generate a CD4 response, which leads to the activation and maturation of B cells for the production of specific antibodies, and a CD8 response that directly targets the virally infected cells. The acquired viral immune response favors the recruitment of primed CD8 cells, which promptly activate their powerful cytotoxic actions to destroy virally infected cells. Over the last decade, the known spectrum of CD4+ and CD8+ T-cell effect or subsets has become broader, including their particular cytokine commitment, stage of differentiation, role in local immunity, and specific functional activity. Discrete subsets of CD4 T cells (e. g., TH1, TH2, TH17; T regulatory cells [ Tregs ]; CD45RA +CD4+ /CD8+; CD45R0 +CD4+ /CD8 +; CD25+Foxp3+CD4+/CD8+) work in complementary synergy and with the M1 and M2 macrophage activation states to mediate viral protection: a large arsenal of mechanisms, by which CD4+ and CD8+ T cells act to combat virus infection [1].
The TNF Receptor:
Many cytokines are involved in the process of inducing and regulating the immune-mediated clearing of a viral infection. Receptors and ligands of the tumor necrosis factor (TNF) family play important roles in controlling CD8 T cell activation and survival during a viral immune response. The role of specific TNF receptor (TNFR) family member in antiviral immunity depends on the stage of the immune response and can vary with the virus type and its virulence. TNFR Table 1 (see supplementary material), the death receptor, is critical in determining the cytotoxic response. The trimeric nature of this cytokine receptor cooperates with the tumor necrosis factor receptor type1-associated DEATH domain protein (i.e., TNFRSF1A-associated via death domain [TRADD]). TRADD media test hecytotoxic response. The TNFR-associated factor (TRAF) modulates primarily the inflammatory response. The receptorinteracting protein (RIP) kinases are a group of threonine/serine protein kinases with relatively conserved kinase domain that mediate broad-base dregulation of activation events.
CD120 is a member the TNFR family with a specific role in viral immune surveillance as it binds to the pro-inflammatory cytokine TNF -α. The CD137 (..) is expressed by activated T cells, but to a larger extenton CD8 rather than on CD4 T cells. CD27 is required for generating and sustaining T cell immunity. CD95is (.) the apoptotic death membrane receptor that engages the cytoplasmic pathways of programmed cell death, which is distinct from the mitochondrial pathway. CD358, the tumor necrosis factor receptor super family member 21 (TNFRSF21) (aka, death receptor-6) closely interacts with TRADD. The related TNFRSF25 does not have CD number yet, but is recognized to be endowed with the ability of facilitating the antigen-dependent response: costimulation of TNFRSF25 with a vaccine antigen sharply enhances vaccine-stimulated immunity. TNFRSF25 stimulation may be a critical driver of specific T cell mediated viral immunity [2].
Virus Recognition Receptors & Virus Infection:
The innate immune system plays an important role in viral immunity. Innate cells and humoral factors are essential for the initial detection of invading viruses and subsequent activation of acquired immunity. Three classes of receptors “sense” the foreign viral double-stranded RNA, the singlestranded RNA, and the DNA, and in response play essential roles in the production of type I interferons (IFNs) and proinflammatory cytokines Table 2 (see supplementary material).
Retinoic acid-inducible gene I (RIGI-I)-like receptors (RLRs) are cytoplasmic RNA helicase-like receptors that bind to intracellular viral double-stranded RNA. RIGI-I interacts with the IPS-1/MAVS/Cardif/VISA protein complex. The latter complex sits on the outer membrane of mitochondria, to signal the presence of virus-derived RNA, and induces type I IFN production. This activation event is regulated by the K63- linked polyubiquitin chain mediated by Riplet and TRIM25 ubiquitin ligases. Riplet is required for RIG-I RNA binding activity, and for TRIM25 to activate RIG-I. Riplet induces the release of RIG-I auto repression of its N-terminal caspase activation and recruitment domains (CARDs), which leads to the association of RIG-I with TRIM25 ubiquitin ligase and TBK1 protein kinase. TRIM25 mediates lysine 63-linked polyubiquitination of the RIG-I N-terminal CARD-like region. Both Riplet and TRIM25 are critical to RIG-I activation, but the exact molecular foot print of these interlocked mechanisms remains to be fully elucidated. Hepatitis C (HepC) virus NS3- 4A proteases appear to target Riplet and to prevent endogenous RIG-I polyubiquitination and association with TRIM25 and TBK1.RIG-I, upon sensing RNA, induces activation of the transcription factors NF-кB and interferon regulatory factor (IRF)-3 & -7 through the adaptor mitochondrial antiviral-signaling (MAVS) protein. MAVS proteins are CARDs-containing proteins that reside in the mitochondrial membrane, and play a crucial role in antiviral innate immunity. In point of fact, Hep C and other viruses can also use their proteases to cleave MAVS off the mitochondria, there by escaping immune surveillance [3].
Toll-likereceptors (TLRs) are single membrane-spanning noncatalyticrecep to rsexpressed by myeloidanddendriticsentinel cells. TLRsact as pattern recognition receptors (PRR) recognizing pathogen-associated molecular patterns (PAMPs), specific structurally conserved molecules derived from in vading pathogens, that activate in nateimmune cell responses. TLRs are criticalin the detection phase of viral invasion.TLRs areRLRs, and sense viral infection and activate transcription factors, including IRF3, leading to inductionof IFN production. The membrane phospholipid phosphatidy linositol-5- phosphate (PtdIns5P) is increase dupon viral infection and facilitates IFN production by binding to IRF3 and it supstream kinase TRAF family member-associated NF-kB activator (TANK)-bindingkinase 1 (TBK1) promoting TBK1-mediated IRF3 phosphorylation and activation. TBK1 is a serine/threonine kinase that playsan essential role in regulating in flammatory responses to viral pathogens. Following activation of TLRs by the viral components, TBK1 associates with TRAF3 and TANK to phosphory late IRF3 and IRF7, and them ulti functional ATP-dependent RNA helicase, DDX3X. This function leads to the homodimerization and nuclear translocation of the IRFs, and eventually transcriptiona lactivation of pro-inflammatory and antiviral genes including IFN-α and IFN-β. Inorder to establishsuchan antiviral state, TBK1 needs to interact with diversescaffolding molecules in the cytoskeleton (e.g., FADD, TRADD, MAVS, SINTBAD) to facilitate TBK1-mediated functions such as, phosphorylation of NF-kB inhibitors, phosphorylation of endosomal sorting complex required for transport-I (ESCRT-I) subunit VPS37C for retroviral budding, phosphorylation and activation of AKT1. Case in point, TBK1 mediates the phosphorylation of Borna disease virus (BDV) P protein, a multi-helical protein that regulates viral RNA synthesis and transports nucleotide oligomerization domain (NOD)-like receptors (NLRs), the intracellular sensors component for the recognition of pathogen-associated molecular patterns.
NLRs are important in the production of mature interleukin1-β (IL1-β) consequential to dsRNA stimulation, and are for thatreason, the basis for DNA vaccines. Small RNAs (sRNAs), a newly discovered universal class of powerful RNA-based regulatory biomolecules that regulate gene expression by basepairing with multiple downstream target mRNAs to prevent translation of mRNA into protein, also modulate immune regulation in general, and in viral immune surveillance in particular [4].
The Ebola virus (EBOV), for instance, infects dendritic cells, disables the interferon system, and disrupts henceforth the host antiviral immune surveillance response. The virus targets myeloid cells (i.e., monocytes/macrophages) and induces alterations in the blood clotting pathway, which together lead to significant increase in pro-inflammatory cytokines, including IL6, IL1-b and TNF-a, as well as nitric oxide, which damages the lining of blood vessels. The virus operates in a sequential fashion by first disabling the immune system, then the vascular system, during which observed symptoms can include hemorrhage, hypotension, drop in blood pressure, and catastrophic organ failure. These symptoms are usually followed by shock and death. The glycoprotein, protein from EBOV forms a trimeric complex, which binds the virus to the endothelial cell lining and the interior surface of blood vessels. The small glycoprotein also forms a dimeric protein that interferes with the signaling of neutrophils, allowing the virus to evade the immune system by inhibiting early steps of neutrophil activation. Neutrophils serve as carriers to transport the virus throughout the entire body, to places such as the lymph nodes, liver, lungs, spleen, and presumably other organs, including the central nervous system (CNS (vide infra) [5].
Immune “Escape”:
Following viral infection, IL10 levels are elevated, and so are the levels of soluble intracellular adhesion molecule (sICAM)- 1, and soluble vascular cell adhesion molecule (sVCAM)-1. This pattern reflect early excessive, and ultimately detrimental, endothelial activation in virally infected patients, with consequential increased plasminogen activator inhibitor 1 (PAI-1). The over-active endothelial response may contribute to hemorrhagic symptoms (cf., viral hemorrhagic fever caused by EBOV or Dengue virus [DENV]).The initial immune responses include a rapid rise in pro-inflammatory cytokines (e.g., IL6, IL1-β, TNF-α), which trigger a relatively short-lived initial burst of fever and inflammation and cellular immune migration factors (e.g., IL-8). Soon into the immune surveillance response, a slower process on cellular pathology ensues, which includes myeloid cell and endothelial cell infection and cytopathology, followed by a sharp rise in fever indicating systemic inflammatory responses. As the damage to the infected cells progresses, loss in vascular integrity leads to increased permeability of blood vessels with transudates increasingly rich in micronutrients, red blood cells (and eventually white blood cells in the case of EBOV), DENV and other VHFs. The organism is ultimately overwhelmed by a combination of inflammatory factors and virus-induced cell damage, which together can lead to death from liver and kidney failure complicated by septic shock [5, 6]. This follows type I interferon, which include IFN-α, IFN-β, IFN-к, IFN-δ, IFN-ε, IFN-τ, IFN-ω, and IFN-ζ, impairment. The EBOV and the Marbug virus (MBGV) escape immune surveillance by means of their respective viral protein, which inhibits the phosphorylation of IRF3/7 by TBK1, thus, masking the virus [7]. The HIV, by contrast, can escape from systemic immunity assessments simply by becoming hidden in macrophages and dendritic cells that transmigrate from the systemic compartment to organs and lymph nodes, such that lymphadenopathy is a clinically relevant sign and important guiding tool for detecting hidden HIV in asymptomatic individuals [8]. It is possible and even probable that Dengue, EBOV, MBGV and a host of other viruses also escape cellular immune surveillance by transmigrating to “hidden” lymphoid compartments including the CNS via infected cells.
The Microenvironment:
The microenvironment is greatly dependent on the intricate, fluid relationships that exist between different subpopulations of CD3+ cells, and the pattern of cytokines they produce. Two principal T cells-mediated cytokine patterns can be characterized on the basis of whether they foster T or B cell activation and proliferation. Whereas the human TH1 cytokines (e.g., IL2, INF-g, IL12) predominantly favor T cell activation, proliferation and maturation for cellular immunity toward parasites, virally infected cells and tumor cells, the human TH2 cytokine profile (e.g., IL4, IL5, IL10) favors the activation, proliferation and maturation of B cells and enhances humoral immunity and the production of antibodies. A third T cell population blunts cellular immunity: the regulatory T cell subpopulation (Tregs) characterized by triimmunofluorescence flow cytometry to express either CD4 or CD8, the a chain of the IL2 receptor, CD25, and FoxP3. Depending upon the microenvironment, TH1 populations might also engender a TH17 subpopulation, whose cytokine profile (e.g., IL17A, IL-17F, TNF-a, IL22, IL23, and IL9) lends to a state of sustained T cell-driven inflammation seen in autoimmune diseases and allergic reaction. TH2 cells may generate TH9 subpopulations characterized by elevated levels of IL9 and IL10, which down regulate TH1 activity. Tregs play a critical role in directing and regulating the dynamic plasticity required for balancing TH1/TH2, and the intimately related TH17/TH9 subpopulations [9, 10].
Cellular immune surveillance is a dynamic and fluid system that is driven by a finely balanced and delicately regulated equilibrium of cytokines. The microenvironment dictates and regulates whether or not there is a predominant TH1 and TH17, or TH2 and TH9 pattern of cytokines, and controls cellular immune surveillance to tumors [11], and viral infections. The TH1/TH2 and TH17/TH9 relationships are pivotal in maintaining the efficiency of anti-viral cellular immune surveillance. IL23 is also important for the microenvironment mediating the expansion of TH17 cells and the production of IL17A and IL9 [9, 10]. Should the hypothesis that certain viruses, such as HIV, MBGV, EBOV or DENV, impair the host׳s cellular immunity by altering the Tregsmediated regulation of TH1, TH2, TH17 and TH9 plasticity be proven true, then novel immune therapies could be designed and tested on virus-infected patients directed specifically at restoring the physiological homeostasis in TH1, TH2, TH17 and TH9 cytokines.
Myeloid derivatives, such as monocytes and macrophages, process foreign materials by phagocytosis, a process that has evolved in vertebrate immunology to recognize pathogens and damaged tissues through Toll receptors, which are pattern recognition receptors (PRRs) that recognize pathogenassociated molecular patterns (PAMP) and damage-associated molecular patterns (DAMP). In a classic pattern of cellular immune surveillance to viral infections, pathogens and cytohistological damaged tissues are detected through PAMP and DAMP within hours. This recognition event engenders a set of signals by resident macrophages. Levels of IFN-γ sharply rise, and anti-viral immune surveillance commences. Macrophages either turn on their killing program or “fight” against an invading pathogen, or they engage in a “fix” repairing, healing and remodeling programs. Depending on the microenvironment, macrophages can either elicit responses that include nitric oxide and oxygen radical production - the destructive M1 response, or produce factors that promote proliferation, angiogenesis, and matrix deposition -the reparative M2 response [11, 12]. IFN-g produced by natural killer cells and activated T-cells may be the most potent stimulus for the inducible nitric oxide synthase pathway for arginine catabolism.TGF-к1 stimulates the arginase M2 pathway, and is a potent inhibitor of the M1 pathway of arginine metabolism. Macrophages constitutively produce TGF-b, which is inversely related to their NO production, thus suggesting that TGF-b may act as an autocrine regulator for NO formation and M1 activity [12– 13].
The M1 and M2 states of macrophages activity represent a useful dichotomous functional classification that segregates the macrophage toxicity from its repairing physiological function. The M1 macrophage pattern reciprocally influences TH1 cytokines, including IL-12, which drive T-cells toward sustained inflammation (i.e., TH17), activation, proliferation and maturation; by contrast, the M2 state reciprocally favors TH2 patterns of cytokines to support humoral immunity, including B cell proliferation, maturation and production of antibodies [11–13].
In brief, the INF-g/TGF-b-1 and the TH1/TH2 balance correspond to the M1/M2 and the balance of tissue destruction (i.e., excessive nitric oxide and related cytotoxic compounds)/ tissue regeneration modality (i.e., arginase-mediated production of polyamines for DNA repair and L-proline and ornithine for cell and tissue repair) [13– 15]. The hypothesis can be brought forward that certain viral infections (e.g., DENV, HIV) selectively alter the M1/M2 balance because of their relative trophism to macrophages and dendritic cells− this novel hypothesis that could be tested by in vitro manipulations followed by tri- or tetra-immuno fluorescence flow cytometry. Should it be proven true, then the inference would follow that these and perhaps most viruses contribute to drive and sustain an M1 state simply because of chronic depletion of infected macrophages, and the need to generate new myeloid derivatives to combat the pathogens. A state of iNOS activation would ensue and profound cellular toxicity, leading to the physiological collapse that is evident in the advanced stages of virally infected patients. Novel immune-based therapies for these patients could then be developed that would be directed specifically at modulating the M1/M2 balance by attenuating M1 responses and favoring an M2 macrophage state might result in a positive treatment outcomes.
Neuroimmunology
Neurocognitive Pathology:
Many viral infections are associated with symptoms such as headache, encephalitis, meningitis, cerebral edema, and seizures, which suggests viral involvement in CNS pathology. Considering our current knowledge of viral mechanisms, reported complications from long-term survivors, and knowledge of the adverse effects of pro-inflammatory cytokines on neuronal function, the potential contributors to ongoing CNS dysfunction may include the persistent inflammation of the CNS through the release of cytokines, chemokines and recruitment of infected monocytes. These events lead to the disruption of the blood brain barrier (BBB), which increases its permeability to virus, immune and other cell types through deregulation of tight junction proteins, resulting the acquired neurological insults.
Inflammation and CNS:
Virus infection causes the initial activation of monocytes/ macrophages, which in turn releases cytokines that target the vascular system, particularly endothelial cells. While transient innate immune responses in the form of cytokines are beneficial to the host, the same essential spectrum of cytokines can lead to deregulation of homeostatic mechanisms, destruction of host tissues and apoptosis [16].
Macrophage-like cells have varied tissue distributions, and have different names depending on their anatomical sites. In the central nervous system, macrophages are called microglial cells whereas hepatic macrophages are referred to as Kuppfer cells. In the lungs they are recognized as the alveolar macrophages and in skin they are the Langerhans cells. Monocytes/macrophage subpopulations release large quantities of cytokines and chemokines in the CNS, following viral infection. A variety of pro-inflammatory proteins is released due to viral infection that include IL1β, IL6, IL8, IL15, IL16, the chemokine macrophage inflammatory protein (MIP- 1)-α and -β, monocyte chemotactic protein 1 (MCP1), macrophage colony-stimulating factor (M-CSF), macrophage migration inhibitory factor (MIF), IFN- β-induced protein 10 (IP-10), and eotaxin.
Cytokines have both atherogenic and prothrombotic effects that can influence neurological events such as ischemic stroke or vascular dementia. IL1β, IL2, IL6, and TNF-α have been directly implicated in the stimulation of the coagulation system. Abnormalities of the brain coagulation system have been implicated in traumatic brain injury [17]. Neuro- inflammation, mediated in part by chemokine activity and the release of proinflammatory cytokines, contributes to the breakdown of CNS microvascular endothelial cells that constitute the BBB, increasing the potential for continued viral invasion into the brain. While most studies have focused on the inflammatory response of microglia and astrocytes, perivascular cells also play a key role in brain inflammation. Pericytes of CNS are involved in recruitment of peripheral cells to the brain, which may directly induce neuronal damage, or promote microglial hyper-activation and inflammation [18].
Blood Brain Barrier (BBB) Disruption:
The BBB separates the brain from the circulatory system, thus maintaining a stable micro-environment. It is formed by specialized endothelial cells that are attached through tight junctions and adherence junctions, which function to separate the CNS from the circulation and restrict and prevent bloodborne molecules and peripheral cells from entering the CNS. Specialized endothelial cells line brain capillaries and form its structure. They transduce signals to and from the vascular system and brain. Both structure and function of the BBB is dependent upon the complex interplay between different surrounding cell types, including the endothelial cells, astrocytes, pericytes, and the extracellular matrix of the brain as well as the capillary blood. Tight junction proteins also provide the BBB with two functionally distinct sides: the luminal side facing the circulation and the abluminal side facing the CNS parenchyma, which are highly sensitive to major cytokines produced during immune response, including TNF-α, IL1-β, and IL6. Three sites have been identified with a physical barrier via tight junctions including the brain endothelium that forms the BBB, the arachnoid epithelium, which constitutes the middle layer of the meninges, and the choroid plexus epithelium, which secretes cerebrospinal fluid (CSF) [19–21].
The concerted cellular immune and inflammatory processes described above can disrupt the tight junctions of the BBB specialized endothelium thereby opening a gate, which enables the leakage and trans-vasation of activated immune cells and factors from the systemic circulation into the CNS and the brain parenchyma. A preliminary characterization of the molecular mechanisms of the BBB gateway proposes that it might be mediated in large part by NF-кB via the signal tranducer and activator of transcription-3 (STAT3) activation. Inflammatory cytokines, including IL-17, can act as a trigger to NF-кB-mediated transcriptions, and IL-6 as a target of NF-кB, play a critical role in opening the BBB gateway [21]. A role for certain other inflammatory modulators and chemokines has also been proposed in regulating the permeability of this BBBgate pathway [21– 23]. In animal models, exposure to an inflammatory stimulus at the time of an experimentally induced stroke leads to an identifiable Tregs response, which modulates the TH1 response, and an uncontrolled TH1 response to brain antigens is associated with higher neuropathologic scores [24– 27]. Case inpoint, IL-17 production by T cells contributes to ischemic brain injury up to 7 days following the stroke onset [28]. Based on current understanding of the role of TH17 and TH9, and specifically the TH17/TH9 balance in regulating viral immune surveillance mechanisms, and immune processes of chronic sustained inflammation, such as what is observed in peripheral or central neuroinflammation, it is possible and even probable that the “gateway theory” can be revised and expanded to involve and incorporate the role of TH17 and TH9 cytokines (Figure 1).
Figure 1.
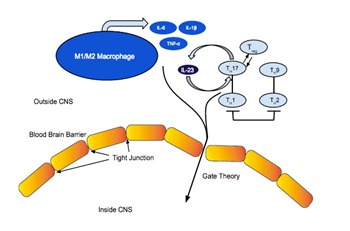
The TH17/TH9 BBB Gateway
nted by Arima, Kamimura, Ogura and collaborators (refs. 21-23). In their original description, these authors characterized a rodent NF-кB-mediated “inflammation amplifier”mediated by IL-17, which was hypothesized to lead to a localized gateway through the BBB. We propose those systemic inflammatory processes that involve macrophages in the M1 or the M2 states have differential effects upon the balance of TH17 and TH9 cytokines systemically, regulated in part by TH1 and TH2 cytokines. Together, these factors act locally on the tight junction of the BBB endothelium, and modulate the inflammation amplifier molecular cascade. The prevalence of M1 or M2 state of macrophage activation determines the porosity of the TH17/TH9 BBB gateway, which functions, we propose, at the molecular level quite as they described NF-кB-mediated gate theory of Arima, Kamimura, Ogura et al. In brief, the TH17/TH9 BBB gateway shown in the figure is gated, as it were, by the M1/M2 balance through its regulatory effects upon the molecular events of the inflammation amplifier, and thus mediates the extent of systemic inflammation that can permeate into the central nervous system. By acting on the TH17/TH9 BBB gateway, novel patient-centered therapies can now be developed to blunt NF-кB-mediated inflammation amplifier pathway, and block inflammation of the brain consequential to a variety of neuroimmune pathologies, from cranial nerve neuropathies (e.g., trigeminal neuralgia), to neuropathologies (e.g., Alzheimer׳s disease, multiple sclerosis), to viral infections of the brain including neuroAIDS. In the same vein, the etiology of Major Depression has now been hypothesized to be associated with some form, or some degree of neuroinflammation.
The degree to which CNS-specific TH17 cells contribute to injury in neurological disorders has yet to be explored. Nevertheless, should the hypothetical TH17/TH9 BBB gateway model proposed here prove correct, it could open timely and critical new therapeutic interventions in viral infections that penetrate the central nervous system, varied neuroimmune pathologies that involve local brain immune responses, following neurological injury, stoke, and a spectrum of neurological diseases, including central trigeminal neuralgia consequential to peripheral neuroinflammation.
Neurological Syndromes:
In 1967, cases of hemorrhagic fever occurred in Marburg. The Marburg virus (MBGV) and EBOV, as noted above, are both members of the family Filoviridae, and are very similar in terms of morphology, genome organization, and protein composition. The pathology of Marburg disease was investigated from gathering tissues from brain, spleen and liver from those infected and have been used to make inferences about the pathology of Ebola virus. Brain tissue derived from patients infected with the Marburg virus were noted to have brain swelling, increase in vascular permeability, associated reduced effective circulating blood volume, and interstitial edema in the brain. It was inferred that the pathologic alterations found in Ebola virus infection would share similar features to those found in Marburg virus infection, though an absence of comprehensive comparative studies remain.
EBOV infects the meninges, and most viral infections that involve the meninges manifest into progressive neurologic disorders. In general, disease progression and severity are determined in part by whether specific areas of CNS are involved, which is determined by viral tropism. Polioviruses (PV) preferentially infect motor neurons, and mumps infects epithelial cells of the choroid plexus. Encephalitis due to infection and post-infection encephalomyelitis, which may occur after measles or Nipah virus encephalitis (NVE), or conditions such as post-poliomyelitis syndrome, a putative persistent manifestation of PV infection, is examples of neurological syndromes that can follow due to viral infection of the CNS. Symptoms that signal encephalitis due to viral infection of the meninges include sudden fever, headache, vomiting, heightened sensitivity to light, stiffness of neck and back, confusion and impaired judgment, drowsiness, weakness of muscles, a clumsy and unsteady gait, and irritability. Even if the blood brain barrier is not impaired, neuroinflammatory mediators crossing the BBB may result in neurotoxic effects due to the accumulation of free radicals from the brain׳s cytotoxic response. Symptoms that require emergency treatment include loss of consciousness, seizures, muscle weakness, or sudden severe dementia. Chronic meningitis from Cryptococcus usually develops among patients with compromised immune systems (e.g., elderly, AIDS patients). CT or MRI is usually recommended first, before the lumbar puncture. Encephalitis and meningitis can cause cerebral edema, a dangerous condition where the brain's water content rises, causing an increased pressure in the skull [29, 30].
Conclusion
In conclusion, we propose that viral infection and other immune activation states disrupt the specialized tight junction of the BBB endothelium, thus making it permeable to immune cells and factors, including inflammatory cytokines, not only through IL-17-initiated and IL-6-mediated events, but also via the regulation by cytokines of the TH9 profile, which modulate TH17-mediated sustained inflammatory responses systemically [9, 10]. Expectations are that experimental and clinical immunology experiments will demonstrate that immune surveillance events to viruses, such as HIV, EBOV, DENV and others, disrupt the BBB and open a TH17/TH9 induced gateway. Together, these events contribute to neuroimmune pathology. It is possible and even probable that the “TH17/TH9 BBB gateway” we propose here opens as a consequence of any state of immune activation and sustained chronic neural inflammation, whether associated with viral infection or any other cause of peripheral or central neuroinflammation, including neuronal damage consequential to nerve ending constriction. The TH17/TH9 BBB gate we describe here will proffer a novel and useful model for improved understanding of psychoneuroendocrine-immune interactions.
Supplementary material
Acknowledgments
EBD-PBRN is registered with the US Agency for Healthcare Research & Quality (AHRQ) PBRN Resource Center as an affiliate primary care Practice-Based Research Network. The authors thank the past and present members of the Evidencebased research group who have contributed to the research presented here. The authors also thank the stakeholders of EBD-PBRN who have contributed many critical discussions of fundamental concepts. Support for this research was from Fulbright grant 5077 and UC Senate grants to FC; NIH/NIMH Career Development Award (K23 MH095661) grant to AT.
Footnotes
Citation:Chiappelli et al, Bioinformation 11(1): 047-054 (2014)
References
- 1.Strutt TM, et al. Immunol Rev. 2013;255:149. doi: 10.1111/imr.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wortzman ME, et al. Immunol Rev. 2013;255:125. doi: 10.1111/imr.12086. [DOI] [PubMed] [Google Scholar]
- 3.Oshiumi H, et al. PLoS Pathog. 2013;9:e1003533. doi: 10.1371/journal.ppat.1003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris JF, et al. Virulence. 2013;4:785. doi: 10.4161/viru.26119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansari AA. J Autoimmun. 2014:S0896. [Google Scholar]
- 6.Wong G, et al. Expert Rev Clin Immunol. 2014;10:781. doi: 10.1586/1744666X.2014.908705. [DOI] [PubMed] [Google Scholar]
- 7.Ramanan P, et al. Viruses. 2011;3:1634. doi: 10.3390/v3091634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tacchetti C, et al. Am J Pathol. 1997;150:533. [PMC free article] [PubMed] [Google Scholar]
- 9.Muranski P, Restifo NP. Blood. 2013;121:2402. doi: 10.1182/blood-2012-09-378653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komatsu N, et al. Nat Med. 2014;20:62. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 11.Oluwadara O, et al. Bioinformation. 2011;5:285. doi: 10.6026/97320630005285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez FO, Gordon S. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills CD. Crit Rev Immunol. 2001;21:399. [PubMed] [Google Scholar]
- 14.Pepper M, Jenkins MK. Nat Immunol. 2011;12:467. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anthony RM, et al. Nat Med. 2006;12:955. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosser DM, Edwards JP. Nat Rev Immunol. 2008;8:958. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nekludov M, et al. J Neurotrauma. 2007;24:174. doi: 10.1089/neu.2006.0173. [DOI] [PubMed] [Google Scholar]
- 18.Alcendor DJ, et al. J Neuroinflammation. 2012;9:95. doi: 10.1186/1742-2094-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbott NJ. Cell. Mol Neurobiol. 2000;20:131. doi: 10.1023/a:1007074420772. [DOI] [PubMed] [Google Scholar]
- 20.Wilson EH, et al. J Clin Invest. 2010;120:1368. doi: 10.1172/JCI41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamimura D, et al. Front Neurosci. 2013;7:204. doi: 10.3389/fnins.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogura H, et al. Biomed J. 2013;36:269. doi: 10.4103/2319-4170.113187. [DOI] [PubMed] [Google Scholar]
- 23.Arima Y, et al. Mediators Inflamm. 2013;2013:898165. doi: 10.1155/2013/898165. doi: 10.1155/2013/898165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker KJ, et al. J Cereb Blood Flow Metab. 2005;25:1634. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becke K, et al. Stroke. 2003;34:1809. doi: 10.1161/01.STR.0000078308.77727.EA. [DOI] [PubMed] [Google Scholar]
- 26.Zierath D, et al. Neurocrit Care. 2010;12:274. doi: 10.1007/s12028-009-9270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gee JM, et al. ExpTransl Stroke Med. 2009;1:3. [Google Scholar]
- 28.Frenkel D, et al. J Neurol Sci. 2005;233:125. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Whitley RJ, Gnann GW. Lancet. 2002;359:507. doi: 10.1016/S0140-6736(02)07681-X. [DOI] [PubMed] [Google Scholar]
- 30.Kauder SE, Racaniello VR. J Clin Invest. 2004;113:1743. doi: 10.1172/JCI21323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


