Abstract
Mitochondrial function and specifically its implication in cellular redox/oxidative balance is fundamental in controlling the life and death of cells, and has been implicated in a wide range of human pathologies. In this context, mitochondrial therapeutics, particularly those involving mitochondria-targeted antioxidants, have attracted increasing interest as potentially effective therapies for several human diseases. For the past 10 years, great progress has been made in the development and functional testing of molecules that specifically target mitochondria, and there has been special focus on compounds with antioxidant properties. In this review, we will discuss several such strategies, including molecules conjugated with lipophilic cations (e.g., triphenylphosphonium) or rhodamine, conjugates of plant alkaloids, amino-acid- and peptide-based compounds, and liposomes. This area has several major challenges that need to be confronted. Apart from antioxidants and other redox active molecules, current research aims at developing compounds that are capable of modulating other mitochondria-controlled processes, such as apoptosis and autophagy. Multiple chemically different molecular strategies have been developed as delivery tools that offer broad opportunities for mitochondrial manipulation. Additional studies, and particularly in vivo approaches under physiologically relevant conditions, are necessary to confirm the clinical usefulness of these molecules. Antioxid. Redox Signal. 22, 686–729.
I. Mitochondria
Mitochondria are self-replicating organelles that evolved from an α-proteobacterium endocytosed by an ancestral eukaryotic cell through the process of endosymbiosis. As a consequence of this remote autonomy, several mitochondrial features result in their resembling prokaryotes: mitochondria preserve their own genome, formed by a circular DNA molecule (mitochondrial DNA [mtDNA]) that encodes 13 mitochondrial electron transport chain (ETC) complex subunits, the two RNA subunits of the mitochondrial ribosomes and 22 tRNAs (219); they are enclosed by a double membrane with an unusual lipid composition, and possess autonomous protein synthesis machinery. Why mitochondria have retained a part of their genome is still a matter of debate; historically, the most widely touted hypothesis regarding the retention of mitochondrial genes has centered on the encoded proteins' extreme hydrophobicity, which prevents their efficient import into the organelle (245). Virtually all eukaryotic cells contain mitochondria; however, their number, size, shape, distribution, and metabolism vary largely in accordance with cell-type and cell-cycle stage. While highly metabolic tissues, such as liver, cardiac and skeletal muscle, and the brain, contain several thousands of mitochondria per cell, cells in somatic tissues with low energy demands possess only a few dozen mitochondria. Mitochondria also vary considerably between different species in terms of their metabolism, mitochondrial proton conductance, membrane protein, and lipid composition/packaging. These characteristics are related to the energetic capacity of mitochondria and are crucial for many evolutionary phenomena such as endothermy, the ability for flight, adaptability to different climates, or lifespan. Mitochondria are essential for the maintenance of cellular homeostasis. They generate and supply most of the cell's energy through the mechanism of oxidative phosporylation (OXPHOS) in the inner mitochondrial membrane (IMM). This multi-step process is mediated by the electron transfer through four large multi-protein complexes (I–IV) coupled to the ATP synthase, also denominated as complex V. Mitochondria are complex structures of more than 1000 proteins and besides their role in the generation of energy, these organelles participate in other crucial cell processes, such as Ca2+ homeostasis, thermogenesis, urea cycle and heme biosynthesis, apoptosis, and reactive oxygen species (ROS) production (Fig. 1) (3).
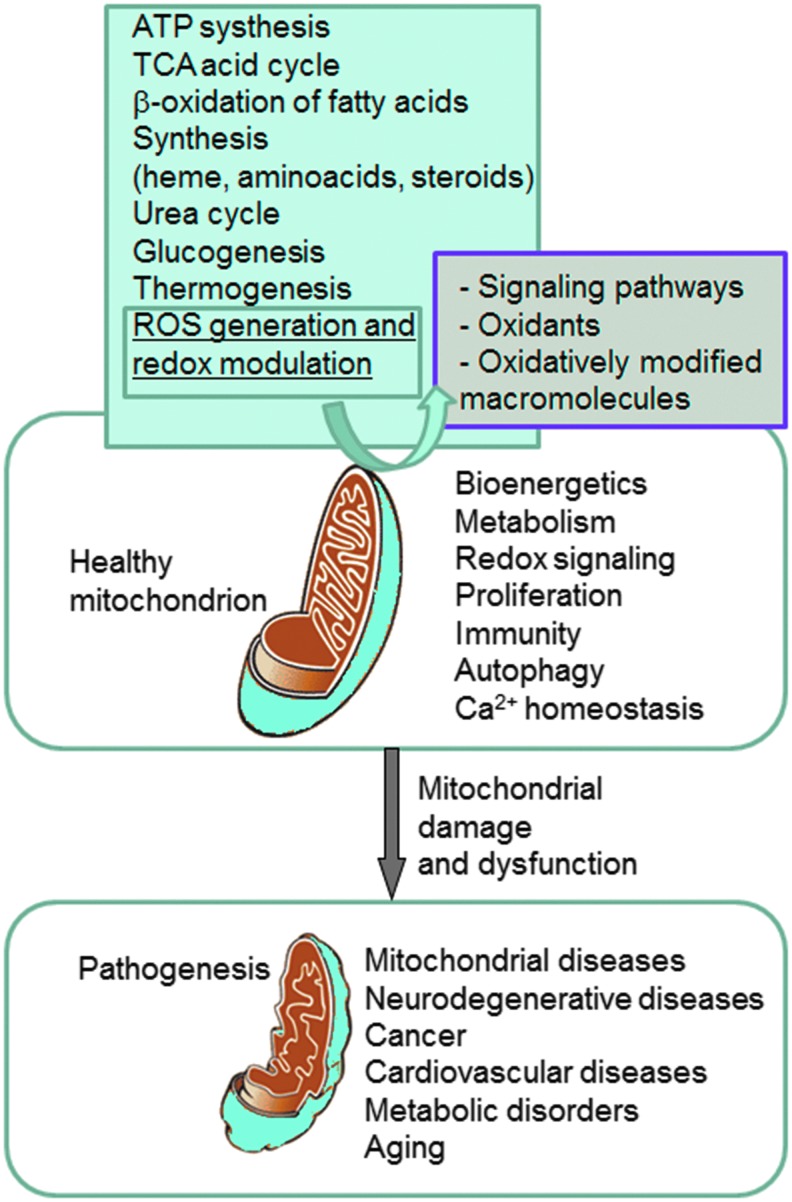
FIG. 1.
Representation of the involvement of mitochondria in health and disease. Mitochondria play a fundamental role in cell physiology; these organelles are involved in a variety of processes, including bioenergetics, various metabolic pathways, including crucial anabolic and catabolic reactions, such as ATP synthesis, the TCA cycle, and biosynthetic processes, and govern fundamental cellular actions, including proliferation, immunity, and autophagy. Mitochondrial damage and malfunction have been related to the pathogenesis of a large number of human pathologies, such as mitochondrial diseases, neurodegenerative diseases, cancer, cardiovascular diseases, metabolic disorders, and aging. The participation of mitochondria in the redox equilibrium and redox signaling of the cell is also pivotal. Modification of the redox state and increased ROS production within mitochondria have major consequences for both mitochondrial and extramitochondrial processes and, ultimately, modulate fundamental cellular phenomena such as autophagy and apoptosis. ROS, reactive oxygen species; TCA, tricarboxylic acid. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Within mitochondria, energy in the form of ATP is obtained in a reaction coupled with the reduction of O2 to form H2O. This process is mediated by the ETC in the IMM, which transfers electrons from the reduced co-factors (NADH and FADH2 using the tricarboxylic acid cycle and the β-oxidation of fatty acids) to the ultimate electron receptor O2. The transfer of electrons is coupled with the simultaneous transport of protons from the mitochondrial matrix across the IMM into the intermembrane space, thus generating a proton gradient between these two compartments, which is harnessed by the ATP synthase to produce ATP. Most of the O2 is completely consumed during this process; only a small part (1–2% in experiments with normal isolated mitochondria) leaks from complex I and III of the ETC in the form of superoxide anion (O2•−) (30, 114).
A. Implication of mitochondria in cellular redox homeostasis
The mitochondrion is believed to be the major intracellular source of ROS, with specific sites at the ETC complexes constituting the foremost origin (Fig. 2A) (30, 78, 114). O2•− seems to be the first radical to be generated, while other ROS are formed downstream, such as hydrogen peroxide (H2O2), which arises through the dismutation of O2•− mediated by manganese superoxide dismutase (MnSOD), and hydroxyl radical (•OH), which is created through the reduction of H2O2 in the presence of reduced transition metals (78). This radical is highly reactive and, thus, very harmful to molecules and cellular membranes. Besides the activity of MnSOD, O2•− can also be converted to H2O2 by other types of enzymes, including pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, which generates both O2•− and H2O2 via the oxidation pathway and can be considered a possible target for clinical research in the treatment of certain diseases that harbor mitochondrial dysfunction, such as neurodegenerative diseases (281). As a negatively charged molecule, O2•− has limited mobility across biologic membranes and its diffusion depends on anion channels in the membrane. Thus, O2•− is characterized by spatial specificity and can be confined within certain organelles, including mitochondria. Apart from complex I, O2•− is also released into the intermembrane space from Qo of complex III, where it is mostly dismuted by SOD1. This has important implications for cytosolic signaling in many tissues, including smooth and cardiac muscle, as matrix and intermembrane space/cytosol ROS may change in the opposite direction under certain physiological conditions. For example, Waypa et al. demonstrated that increases in mitochondrial ROS can trigger hypoxia-induced Ca2+ responses in pulmonary artery smooth muscle cells (346) and that acute hypoxia induces O2•− release from complex III of smooth muscle cells (347), constituting oxidant signals that diffuse into the cytosol and trigger increases in [Ca2+(i)] that cause acute hypoxic pulmonary vasoconstriction (348).
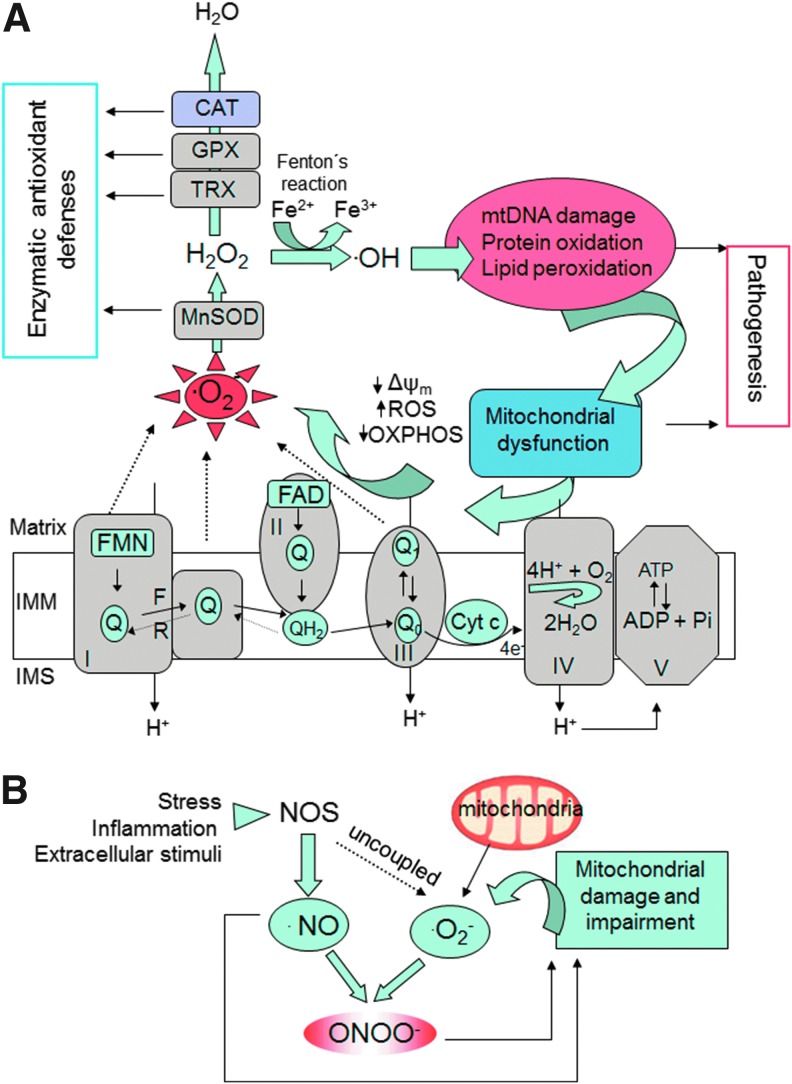
FIG. 2.
Mechanisms of oxidative stress involving mitochondria. (A) The mitochondrial ETC reoxidizes reduced cofactors (NADH and FADH2) using molecular oxygen as the final electron acceptor, and the energy released in this process is captured in the form of ATP. Several sites of the ETC (CI and CIII and the reverse electron flow at Complex II) generate O2•−. This radical is further converted into H2O2 by mitochondrial SOD. Other antioxidant enzymes within mitochondria involve TRX and GPX, and in certain tissues (liver, cardiac muscle) also CAT. Through the Fenton reaction, H2O2 is converted into •OH, a molecule that produces oxidative cell injury through DNA damage, carboxylation of proteins, and lipid peroxidation. Damaged mitochondria are dysfunctional and further produce free radicals, thus generating a “vicious cycle.” (B) Mechanisms of nitrosative stress. •NO is produced by the activity of intracellular NOS. •NO can be combined with O2•− to produce ONOO−, a molecule that acts as a strong oxidant and can damage many cellular structures and alter their function. Reactive nitrogen species such as ONOO− contribute to further mitochondrial dysfunction. •NO, nitric oxide; •OH, hydroxyl radical; CAT, catalase; ETC, electron transport chain; F, forward; GPX, glutathione peroxidase; H2O2, hydrogen peroxide; IMM, inner mitochondrial membrane; IMS, intermembrane space; NOS, nitric oxide synthase; O2•−, superoxide anion; ONOO−, peroxynitrite; OXPHOS, oxidative phosphorylation; R, reverse; SOD, superoxide dismutase; TRX, thioredoxin; Δψm, mitochondrial membrane potential. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Other studies have indicated that in pulmonary vascular smooth muscle cells mitochondrial matrix oxidant signals generated during hyperoxia, specifically H2O2, activate phosphodiesterase type 5 in a reaction that involves cGMP-dependent protein kinase (86). In general, this action has important implications for the source of mitochondria-derived ROS and differential targeting of matrix, intermembrane space, and cytosolic antioxidants.
Although not a free radical, H2O2 is a very important biological marker of oxidative stress due to its ability to cross cellular membranes. In addition, it can act as an intracellular messenger in different redox pathways. H2O2 can be converted to H2O by several enzymes, including glutathione peroxidase (GPX) and catalase (CAT).
Another enzyme with antioxidant activity is heme oxygenase (HO-1), which metabolizes heme to biliverdin, iron, and carbon monoxide (27). HO-1 is activated under oxidative stress conditions (hyperthermia, hypoxia, heavy metals, etc.) and has been shown to be beneficial in several diseases, including cardiovascular pathologies, acute kidney injury, liver disease, and diabetes (112).
Under physiological conditions, ROS are released primarily from mitochondrial ETC, but there are other important sources that need to be taken into account, such as peroxidases, NADPH oxidase in the membrane of leukocytes, and certain types of non-phagocytic cells, myeloperoxidase, xanthine oxidase, cyclooxygenase, lipoxygenase, cytochrome P450 monooxygenase, or nitric oxide synthase (NOS). Therefore, other intracellular compartments that can contribute to the cellular production of ROS, besides mitochondria, involve the plasma membrane, peroxisomes, and the endoplasmic reticulum (ER). The reactive species generated from all these sources can damage many intracellular structures, which is related to the development of different pathologies (269). For example, NADPH oxidase, well known to be involved in the inflammatory process, catalyzes the production of O2•− and is present mainly not only in leukocytes but also in other types of cells such as mesangial cells, epithelial cells, fibroblasts, chondrocytes, or endothelial cells, where it plays important regulatory functions (258). It has also been demonstrated that this enzyme plays a key role in the development of many diseases (90). In this sense, Wedgwood and Steinhorn have demonstrated that persistent pulmonary hypertension of the newborn (PPHN) increases p22 (phox) and Nox4 expression and activity, resulting in elevated H2O2 levels in the PPHN pulmonary artery. Increased H2O2 induces vasoconstriction via mechanisms involving SOD3 inactivation, and stimulates vascular remodeling via nuclear factor kappa B (NF-κB) activation and increased cyclin D1 expression (348). It is important to mention that the oxidants produced by immune cells have a dual function. On the one hand, they function as microbicidal agents by killing pathogens, and on the other hand, they can act as signaling molecules in different intracellular pathways (78, 90). In fact, ROS and reactive nitrogen species (RNS) can modulate several enzymes as well as membrane receptors, ion channels, lipid kinases and phosphatase transporters, and various transcription factors, including NF-κB, hypoxia-inducible factor alpha, and nuclear factor-E2-related factor 2 (Nrf-2) (78, 114). Furthermore, different proinflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor alpha (TNF-α) can be modulated by the levels of ROS, thus controlling the inflammatory response, and therefore the different leukocyte functions (adhesion, migration, and phagocytosis), and crucial cellular processes such as apoptosis and autophagy. Under oxidative stress conditions, the excessive production of ROS can damage vicinal cells, thereby contributing to tissue damage (270).
It should also be highlighted that redox signaling plays a role in the interplay between mitochondria and NADPH oxidases, and several reviews [for example, by Daiber (65)] have described different models of such signaling pathways: (i) NADPH oxidase activation can be triggered by angiotensin-II with subsequent opening of mitochondrial ATP-sensitive K channels in an ROS-dependent manner, leading to depolarization of mitochondrial membrane potential (Δψm) followed by mitochondrial ROS formation and respiratory dysfunction; (ii) Using pharmacological and genetic inhibitors, a role of mitochondrial ROS for the induction of NADPH oxidase via PKCɛ was demonstrated in a model of hypoxia-stimulated formation of mitochondrial ROS (mtROS); (iii) Cell death by serum withdrawal enhances ROS production in human 293T cells by stimulating both mitochondria and Nox1; and finally; (iv) Cross-talk between NADPH oxidases (serum-soluble gp91phox [Nox2]) and mitochondria has been observed in nitroglycerin-induced tolerance involving the mitochondrial permeability transition pore and ATP-sensitive K+ channels.
In addition to ROS, mitochondria produce RNS (Fig. 2B), which originate from nitric oxide (•NO), a gaseous molecule that can passively diffuse through mitochondrial membranes. One such RNS is peroxynitrite (ONOO−), a strong oxidant and nitrating agent formed by the reaction of O2•− with •NO. Both ROS and RNS (collectively known as RONS) are produced through aerobic metabolism, and therefore act as “sensors” of intracellular alterations in O2 concentration. Through this action, RONS play an important role as signaling molecules involved in diverse cell functions in normal physiological conditions such as programmed cell death, regulation of stress responses, and cell proliferation (30, 79). •NO is an important intracellular signaling molecule that, unlike many other messengers, is both freely diffusible across membranes and (comparatively) highly reactive. Moreover, it is capable of reacting with a large number of intracellular components at considerable distances from its site of synthesis. •NO is released as a by-product by several isoforms of NOS, including a neuronal isoform (NOS1), an inducible isoform (NOS2) expressed in several types of cells, and the endothelial constitutive isoform (NOS3). In addition, a mitochondrially located •NO synthase has been described, which is responsive to changes in Ca2+ concentration in the mitochondrial matrix and plays a very important role in the modulation of mitochondrial O2 consumption (286). •NO can inhibit O2 consumption by inhibiting cytochrome c oxidase (complex IV of ETC), therefore inducing ROS production (55, 100, 192). In addition, •NO can inhibit mitochondrial complex I (269), an action that has important consequences regarding the OXPHOS process and the redox state of mitochondria. Furthermore, •NO can modulate mitochondrial biogenesis through its ability to regulate ROS production and mitochondrial O2 consumption (217, 254, 360). Besides mitochondria, RNS can be generated in mammalian cells by peroxisomes (94) or the plasma membrane.
It has been proposed that endogenous hydrogen sulfide (H2S), a naturally occurring gassotransmitter similar to •NO, plays a particularly important role in the process of acute O2 sensing in blood vessels (224) and the mammalian carotid body (225). Buckler has demonstrated that H2S can inhibit mitochondrial function over a similar concentration range than cyanide, suggesting that the effects of H2S on background K channels are a consequence of inhibition of OXPHOS (32).
Importantly, mitochondria should be viewed not only as a generator of toxic ROS but rather as a crucial regulator of ROS signaling and balance (78). Indeed, there is evidence of the role of mitochondria-generated ROS as signaling molecules and participants in cellular adaptative mechanisms, including the phenomenon of preconditioning. For instance, mitochondrial preconditioning induced by cyanide in cultured brain endothelial and neuronal cells triggers a protective response mediated by mtROS, which prevents apoptotic cell death and creates resistance against glucotoxicity (56). In a recent paper, Yee et al. postulate that, in nematodes, sensing of mtROS by the apoptotic pathway can elicit protective mechanisms that promote survival under stressful conditions, independently of apoptosis, which points to increased mtROS generation as a cause of extended longevity (368). Regarding ROS as signaling molecules, increasing evidence indicates that redox-dependent protein modification is an important mechanism in signal transduction involving redox modification of specific protein moities such as iron–sulfide (Fe–S) centers or cysteine residues (41). Inside mitochondria, several targets have been described; for example, the mitochondrial ETC, which contains the biggest multi-Fe–S protein, NADH dehydrogenase. Moreover, destabilization of the Fe–S cluster in aconitase by O2•− inhibits this enzyme's activity, thereby limiting mitochondrial respiration. In addition, the peroxiredoxin (Prx) family of peroxidases, one of which (namely PrxIII) has been shown to be localized inside mitochondria, is believed to be involved in signaling loops through oxidation of a conserved cysteine residue present in the NH2-terminal region, the primary site of peroxide-induced oxidation. The importance of the maintenance of the cellular thiol/disulfide couples, which include thioredoxins (TRXs), Cys/CySS, and glutathione (GSH)/oxidized glutathione (GSSG), has led to a modification of the traditional concept of oxidative stress as an imbalance of prooxidants and antioxidants. Instead, the disruption of the existing thiol-dependent redox signaling and control mechanisms seems to be among the most sensitive and quantitatively relevant processes in oxidative stress (136). This “redox hypothesis,” an alternative to the “free radical hypothesis,” postulates that oxidizable thiols are common control elements for biologic processes and are functionally organized in redox circuits that are kinetically limited, insulated from each other, and highly responsive to redox conditions. It is the disruption of these circuits that causes oxidative stress. Since these redox mechanisms control a wide range of biologic processes that do not require macromolecular damage, including cell proliferation, apoptosis, or inflammation, it is conceivable that free radical scavenger trials have failed, because oxidative stress research has overemphasized the importance of free radical-induced damage to macromolecules as the underlying mechanism of pathogenesis (136). Moreover, the “redox hypothesis” is centered on redox compartmentalization and compartment-specific signaling, which can explain the characteristics of mitochondria versus those of other subcellular territories in the cellular redox metabolism. For instance, when HeLa cells are exposed to the cytokine TNF-α, compartmentalized, mitochondria-specific ROS generation occurs and results in TRX-2 oxidation, downstream signaling to cytoplasm with NF-κB activation and apoptosis; whereas TRX-1, the extra-mitochondrial TRX isoform, does not undergo oxidation (116).
While low to moderate levels of ROS are physiological and can be beneficial or necessary for cell survival, high ROS levels are detrimental and associated with cell death. Clearly, mitochondrial dysfunction and oxidative stress are critical factors in the pathogenesis of several diseases. Nevertheless, the mechanisms of mitochondria-induced injury differ depending on the type of disease and the participation of ROS in the pathogenic processes varies largely (oxidative stress can be a cause, an aggravator, or a part of the outcome in a particular pathology). Diverse mechanisms of mitochondrial dysfunction have been described, including OXPHOS impairment, mtDNA depletion, membrane permeabilization, and alteration of fatty acid oxidation; therefore, specific drugs, including antioxidants, may have different effects in different pathological settings.
There is much evidence to show that mtROS can have an important impact on extra-mitochondrial structures. Increased mtROS production has been attributed an integral role in the acute inotropic response of cardiomyocytes to β-adrenergic stimulation. This is of clinical relevance, as chronically sustained adrenergic stress is associated with the development of heart failure and cardiac arrhythmias (9). Moreover, stimulation of β-adrenergic receptors causes apoptosis in adult rat ventricular myocytes through ROS/JNK-dependent activation of the mitochondrial death pathway (263). In a rat model of ischemia/reperfusion, caspase-8 activation has been shown to increase mtROS and •NO production, resulting in S-nitrosylation of ryanodine receptor RyR2 and depletion of calstabin2 from the channel complex, causing a diastolic sarcoplasmic reticulum (SR) Ca2+ leak that leads to acute pathological left ventricular remodeling (87).
1. ROS implication in specific aspects of mitochondrial function
ROS are implicated in virtually all mitochondrial functions, from ATP generation, [Ca2+] buffering to induction of apoptosis. During recent years, the implication of specific mitochondrial functions such as mitochondrial dynamics (Fig. 3) and autophagy/mitophagy (Fig. 4) have been studied in different pathophysiological settings. In addition, both processes have been shown to be intimately connected to the redox balance in mitochondria through a complex and multi-way cause/effect relationship. For example, multiple reports describe that mitochondrial shape can influence cellular and physiological functions, such as ROS production, Ca2+ signaling, leukocyte migration, or lifespan (34, 35, 146). These actions can be explained by the specific mitochondrial localization and the relationship between mitochondrial shape and mitochondrial function. In fact, mitochondria are located where high amounts of ATP are required (34) and also where Ca2+ signaling needs tight regulation (126). Importantly, alterations in mitochondrial Ca2+ have been related to several pathologies, including neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS) (318), and aging (332). In recent years, it has been demonstrated that mitochondria actively interact with ER membranes through structure-denominated mitochondria-associated membranes, which enable direct and rapid exchange of lipids and Ca2+ between the two compartments. These complex ER–mitochondria interactions are emerging as a crucial hub for many cellular processes, such as Ca2+ signaling, mitochondrial dynamics, apoptosis, autophagy, and lipid biosynthesis/trafficking, with far-reaching implications for cell life and death (213). Mitochondrial localization and Ca2+ signaling in other areas of the cells have also been shown to be important and clinically relevant. In a recent elegantly performed study employing atomic force and electron microscopy, Dague et al. have shown that, after heart failure, cardiomyocytes exhibit overall sarcolemma disorganization with general loss of crests, accompanied by depletion of subsarcolemmal mitochondria (62).
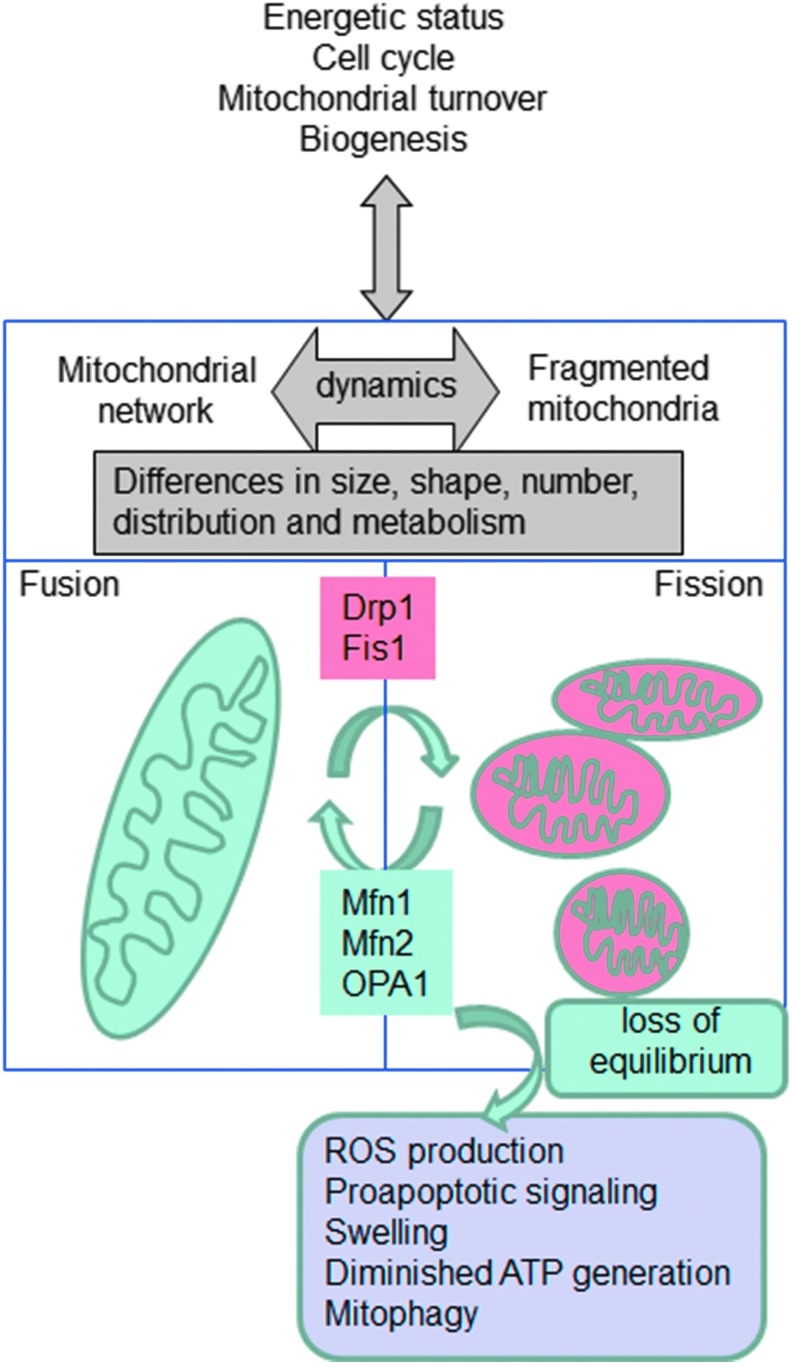
FIG. 3.
Schematic representation of the process of mitochondrial dynamics (fission and fusion) and the proteins that regulate it. Fragmented mitochondria display features of mitochondrial dysfunction such as compromised OXPHOS, swelling, and induction of apoptosis. Oxidative stress is both an inducer and a consequence of mitochondrial fission. Increased ROS levels within the cell can result in mitochondrial fragmentation, which disrupts normal mitochondrial cycling. In turn, damaged and fragmented mitochondria generate increased amounts of ROS. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
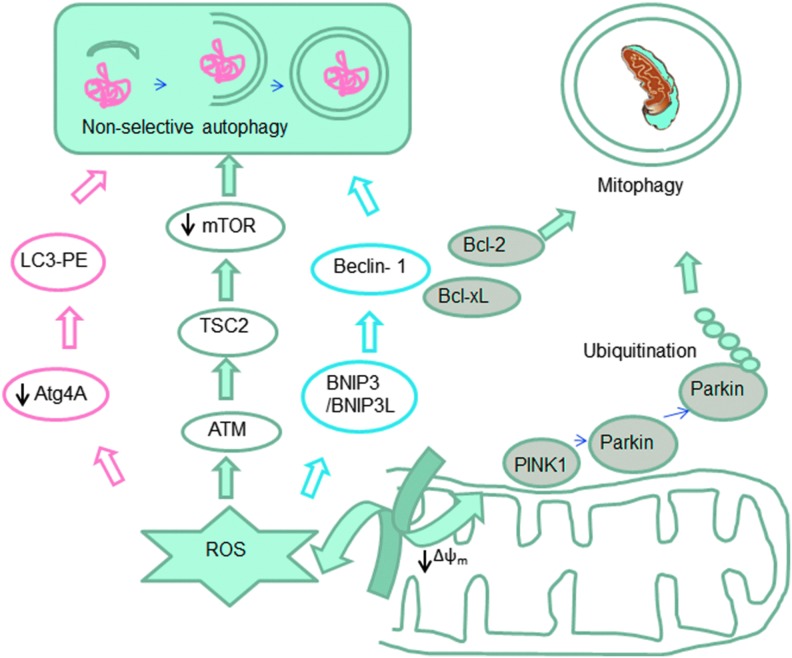
FIG. 4.
Mitochondrial implication in non-selective autophagy (bulk autophagy) and in selective mitochondrial removal through autophagy (mitophagy). The schematic diagram shows the implication of ROS in these processes. Oxidative stress in the cytoplasm triggers autophagy through several pathways. Oxidative stress in mitochondria together with other hallmarks of mitochondrial dysfunction (especially a drop in the mitochondrial membrane potential) induce selective degradation of mitochondria through a process called mitophagy. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
a. Mitochondrial dynamics
The permanent and dynamic balance between fission and fusion states is very important to the cellular homeostasis. Alterations in the mitochondrial network have been associated with several human pathologies, including cardiovascular diseases (CVDs), diabetes, and genetic mitochondrial diseases. Several members of the fusion-regulating machinery have been described in detail (74) and comprise dynamin-homologous GTPases that participate in fission, fusion, and tabulation of membranes. In mammalian cells, it has been described that mitochondrial fission depends on dynamin-related protein (Drp1), a cytoplasmic dynamin GTPase involved in the fragmentation of peroxisomes, ER, and mitochondria (283). Drp1 is located in the cytoplasm, and several mechanisms facilitate its translocation to mitochondria, where it is located at the outer mitochondrial membrane (OMM). Drp1 translocation is triggered by mitochondrial dysfunction as an inducer of fragmentation (127). In fact, in mitochondrial dysfunction, there is an increase in the cytosolic Ca2+ that activates calcineurin and dephosphorylates Ser-637 residue of Drp1 (37), thereby inducing its translocation. This process is crucial in different conditions, such as ischemia-reperfusion damage of the heart (338), and can be counteracted by peptide inhibitors to inhibit Drp1-dependent cell death and mitochondrial damage (36). Its importance in Huntington's disease has also been underlined, where hyperactivation of calcineurin, which dephosphorylates Drp1 at Ser-637, induces an increase in Drp1 translocation to mitochondria, thereby increasing apoptosis (58).
It has also been described that Ser-637 can be phosphorylated by calmodulin-dependent protein kinase Iα; in this case, Drp1 phosphorylation is related with its mitochondrial location (115), with important homeostatic consequences. Mitochondrial Drp1 is also stabilized by other mechanisms, such as SUMOylation, a post-translational modification involved in various cellular processes (344), in this way gaining protection from degradation by the ubiquitin-proteasome system. This has been recognized as an important site for the regulation of mitochondrial function in several diseases models, such as cardiomyopathy and ischemia/reperfusion.
Mitochondrial fission 1 protein (Fis1), a mitochondrial protein anchored to the OMM, is also an important regulator of mitochondrial dynamics. Mitochondrial fragmentation can be caused by both Fis1 overexpression and the enhanced expression of a dominant negative mutant of Drp1 (131). Fis1 has also demonstrated additional functions, such as inducing apoptosis via the ER pathway (5), and can trigger autophagy to remove damaged mitochondria (102), thus playing an important physiological role.
Two mitofusins (Mfn1 and Mfn2) control OMM fusion. During mitochondrial fusion, Mfn1 is responsible for mitochondrial tethering and Mfn2 participates at the end of the process of fusion (155). Furthermore, it has been proposed that Mfn2 correlates with the proliferation of vascular smooth muscle cells (42) and with the oxidative metabolism in muscle (16). Mfn2 can also control the shape of ER and tethers it to mitochondria (67). In other types of cells, such as fibroblasts, Mfn1 is required for fusion triggered by optic atrophy 1 protein (OPA1) (49). OPA1 can be anchored to the IMM, the maintenance of which is another important function of OPA1 (221). At least eight splice variants of OPA1 have been described, and they are subject to a complex post-translational cleavage (222).
In general, mitochondrial dynamics represent the link between the shape of the organelle and pathophysiological processes, and can be considered a key target in the treatment of multiple diseases. Recently, mitochondrial dynamics has also been associated with cancer, as the migration of cells during cancer cell invasion, a phenomenon that is crucial for the invading and metastasizing capacity of the tumor, is related to specific intracellular localization of mitochondria. In faster moving cells, mitochondria are located in between the nucleus and the leading edge of the migrating cells, and this asymmetric distribution is governed by mitochondrial fusion (OPA1) and fission (Drp1) proteins (69).
Mitochondrial dynamics and ROS are closely related, and this relation may be crucial for many human diseases. There is abundant evidence that mitochondrial fragmentation and increased ROS generation coincide; however, it is unclear whether ROS is a cause, consequence, and/or exacerbating mechanism of mitochondrial fission. On the one hand, mitochondrial network formation is considered a regulator of mtROS generation. For example, mitochondrial fragmentation is necessary for high glucose-induced ROS overproduction and respiration increase in cultured rat hepatocytes (372), which supports results obtained in human aortic endothelial cells cultured under high glucose conditions where enhanced mtROS production, impaired endothelial nitric oxide synthase (eNOS) activation, and loss of •NO bioavailability are prevented by inhibiting mitochondrial fission (291). On the other hand, mtROS influence fission/fusion processes; for instance, Makino et al. provided evidence that in mouse endothelial cells, ROS scavenging prevents glucose-induced mitochondrial fragmentation, suggesting that an increase in ROS triggers fission under these conditions (186).
b. Autophagy
Another process that merits attention is autophagy (Fig. 4), which is vital for maintenance of cellular homeostasis and generation of energy, fatty acids, and amino acids for macromolecular synthesis and tissue remodeling. This process involves an intracellular, lysosomal degradation of cellular components such as macromolecules, protein aggregates, and organelles. Three different types of autophagy have been described so far (macro-, micro-, and chaperone-mediated autophagy), although the most evaluated process in mammalian cells is macro-autophagy (203). It begins with the sequestration and isolation of the cytoplasmic cargo by the autophagosome, followed by the fusion of these vesicles with the lysosome, which leads to the formation of autolysosomes, where cellular components are degraded by lysosomal enzymes, with the consequent release of ROS (146, 203, 204).
Autophagy has been related to the development of different diseases, including diabetes, neurodegenerative and myodegenerative disorders, Crohn's disease, various liver diseases, and inherited diseases such as Huntington's disease (57). Initially, autophagy was related to cell death, but mounting evidence has pinpointed the cell-protective role of this process (15, 22, 284). In fact, different studies have demonstrated the existence of a complex relationship between autophagy and cell death that determines whether a cell will live or die in response to anticancer therapies (22). ROS and autophagy are intimately connected (Fig. 4) (147). For example, nutrient starvation-induced ROS generation plays a key role in autophagy in cancer cell lines U87 and HeLa cells (44). Other studies have demonstrated that ROS scavenging or SOD overexpression can modulate autophagy and cell death in the presence of different inhibitors of the ETC (43,44). In this sense, mtROS can modulate autophagy by different pathways, including alteration of the proteins that are indirect participants in the autophagic process. For instance, it has been postulated that ROS in general, and specifically H2O2 released from mitochondria, modulate the activity of Atg4, which is a key cysteine protease in the autophagic process. Atg4 cleaves the C-terminus arginine residue of Atg8, which enables the conjugation of phosphatidylethanolamine with Atg8 and the subsequent recruitment of this protein on the autophagosomal membrane, a fundamental step in the final maturation of the autophagosome. H2O2 can also oxidize Atg4, thus enabling autophagosome completion. Therefore, mtROS can act as signaling molecules that trigger autophagy as a cell survival mechanism (284). Other studies have shown that, in the presence of ROS, there is an upregulation of beclin 1, a protein involved in the initiation of autophagy (287, 365). Moreover, as mentioned earlier, H2O2 and O2•− can modulate the activity of different signaling pathways involved in autophagy. Several groups have demonstrated that ROS play a key role in the autophagy pathway in cancer cells. In fact, H2O2 can modulate Δψm, which leads to inhibition of the Akt/mTOR pathway that induces autophagy (375). Other studies have demonstrated that high levels of ROS induce autophagy that is dependent on p38 signaling in skeletal muscle or cardiomyocytes (193). In addition, Wong et al. have proposed that ROS and downstream activation of JNK and ERK pathways can be responsible for autophagy induction (355). Moreover, not only mtROS are involved in the autophagy process, as the release of ROS from NADPH oxidase has been shown to induce autophagy in immune cells.
Another kinase related to both autophagy and mitochondrial function is AMP-activated protein kinase (AMPK). Pharmacological AMPK activation has been shown to promote autophagy, and this effect is beneficial in several models of human diseases, such as a mouse model of Duchenne muscular dystrophy (234) or diabetic cardiomyopathy (120). The mechanism proposed for AMPK-mediated activation of autophagy involves direct phosphorylation of ULK1 (147).
Autophagy can regulate the mitochondrial network by eliminating damaged mitochondria. Kissova et al. have demonstrated that the Uth1p protein is responsible for the early selective elimination of mitochondria through autophagy under nutrient deprivation (149). This action has been defined as mitophagy, a term introduced by Lemasters to describe a type of macroautophagy where damaged mitochondria are joined by autophagosomes and removed to maintain mitochondrial homeostasis (165). In humans, the molecular mechanism that regulates mitophagy involves the parkin/PTEN-induced putative kinase protein 1 (PINK1) pathway. PINK1 is a mitochondrial kinase that is capable of identifying depolarized mitochondria. It undergoes voltage-dependent lysis and is removed from mitochondria in normal conditions; however, when mitochondria are impaired with the subsequent decrease in Δψm, PINK1 accumulates and recruits parkin (135, 214). Parkin can ubiquitinate proteins and recruit autophagic machinery to the damaged mitochondria for their removal (97). Many studies have pointed out that autophagy is a complex process which can be regulated by ROS at different points. Moderated levels of ROS can also induce mitophagy or autophagy to eliminate damaged mitochondria; on the contrary, high levels of ROS can modify signaling pathways and induce apoptosis (216). Importantly, there is evidence that mitochondria-targeted antioxidants can modulate autophagy, which will be discussed in detail.
In summary, mitochondria are a major source of ROS and RNS, species that can regulate mitochondrial activity through different mechanisms, including modulation of O2 consumption and OXPHOS, induction of mitochondrial membrane permeability transition, regulation of mitochondrial biogenesis, mitochondrial dynamics, and autophagy/mitophagy (23, 28, 218, 238). Another field of action involves the presence of oxidative stress and its consequences, such as lipid peroxidation, protein and DNA oxidation, which occur when there is a strong disruption of the cellular redox homeostasis (Fig. 2) (118).
2. Mitochondrial susceptibility to ROS/RNS damage
Mitochondria continuously generate ROS (7), and immediate and constant exposure to the latter renders mitochondrial components such as mtDNA, proteins, and membranes especially sensitive to such insult (Figs. 1 and 2). First, mtDNA accumulates much more oxidative damage than nuclear DNA due to its proximity to the source of ROS release, its less sophisticate repair systems, and the lack of histones that physically protect nuclear DNA from exogenous attacks (190). Currently, much research is focused on the proteins involved in the maintenance of the mitochondrial genome. One such protein is mitochondrial transcription factor A, a histone-like protein, member of a high mobility protein family and the first-identified mitochondrial transcription factor, which binds to mtDNA and is essential for its maintenance (139). Second, mitochondrial proteins are also particularly susceptible to oxidative damage because of the presence of Fe–S clusters in their structure that are susceptible to oxidant inactivation, and also of thiol residues that are vulnerable to S-nitrosation by RNS (63). In this sense, some mitochondrial proteins are known to be altered by redox modifications and ROS/RNS signaling, such as the enzyme aconitase (under aging conditions) (364) or α-synuclein and DJ-1 in Parkinson's disease (PD) (66). In addition, proteins integrating complexes of the ETC can be affected by ROS and RNS (63, 79). Damage to complex I, the most vulnerable ETC complex, increases ROS production, which leads to a vicious circle of further mitochondrial dysfunction. It is important to note that damage to complex I has a stronger impact on mitochondrial function than damage to other complexes, as mitochondria possess smaller amounts of complex I than other ETC complexes (212). Finally, it is known that phospholipids that integrate mitochondrial membranes are rich in unsaturated fatty acids, which are extremely vulnerable to lipid peroxidation by ROS. One of the lipids most affected by this oxidative damage is the IMM essential component cardiolipin, which is constituted exclusively by polyunsaturated fatty acids (PUFAs) and is also closely associated with mitochondrial ETC complexes (113). This ROS-induced damage to the IMM is particularly relevant, not only because this membrane contains ETC complexes but also because of a special feature, namely, elevated Δψm, which is necessary for maintaining its electrical properties. When IMM integrity and function are compromised by oxidative damage, respiratory energy is dissipated through the leakage of H+, which further compromises mitochondrial OXPHOS (85).
It is noteworthy that, in addition to mitochondria, other subcellular structures have been described as direct and specific targets of oxidative and nitrosative stress, including the ER, the plasma membrane, and the nucleus. For example, NAD(P)H oxidase has been associated with the SR of cardiac and skeletal muscle, and the O2•− generated by this enzyme can oxidate RyR and therefore influence Ca2+ release during excitation–contraction coupling by the SR (129). RyR belongs to a class of intracellular Ca2+ channels and is an important mediator of Ca2+-induced Ca2+ release in excitable cells such as muscles and neurons. RyR2 (cardiac muscle) contains≈33 free thiol residues, rendering it highly sensitive to the cellular redox state. Cysteine oxidation and S-nitrosylation facilitate RyR opening and SR Ca2+ leak, which can provoke arrhythmia, skeletal muscle weakness, and muscle remodeling. Modifications of several sarcomeric proteins have also been identified and associated with defects in contractile function (306). Oxidative stress has also been shown to cause impairment of intracellular proteolysis via covalent binding of 4-hydroxy-2-nonenal, a major end product of lipid peroxidation, to proteasomes (220).
3. Cellular antioxidant defense systems
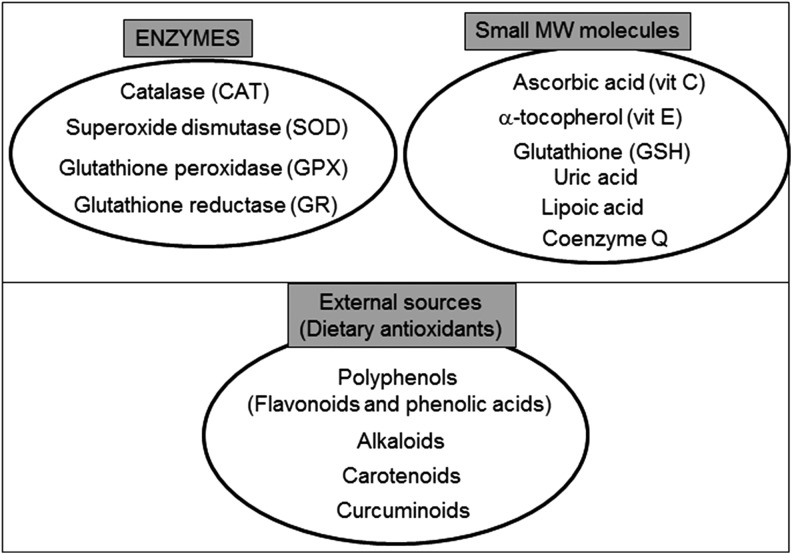
Excessive ROS production is usually compensated by the antioxidant defense system, thus maintaining the redox balance. Cellular antioxidants, including enzymes such as SOD and CAT and low-molecular-weight antioxidants such as vitamins C and E, and GSH, are the main orchestrators of this defensive barrier (Fig. 5) (78, 114).
FIG. 5.
The antioxidant machinery of a mammalian cell. Endogenous antioxidant defenses include enzymes and non-enzymatic small MW molecules. Many important antioxidants come from exogenous sources and can be delivered through the diet. MW, molecular weight.
The tripeptide GSH (γ-glutamylcysteinylglycine) is the principle thiol antioxidant and redox buffer of the cell and is present in different intracellular locations, such as the nucleus, mitochondria, ER, or cytosol. Together with its oxidized form (GSSG), it maintains the redox balance, a function that is crucial to the fine-tuning of the cellular redox environment between control situations and oxidative stress conditions (336). GSH participates in the maintenance of an adequate redox state of the nuclear proteins through the presence of protein sulfhydryls that are vital for DNA expression and repairment. The protective effect of GSH against oxidative stress is due to its role as a (i) participant in amino-acid transport through the plasma membrane; (ii) cofactor of several detoxifying enzymes against oxidative stress (e.g., GPX); (iii) direct scavenger of singlet oxygen and •OH; (iv) detoxifier of H2O2 and lipid peroxides through the enzymatic action of GPX; and (v) regenerator of antioxidants such α-tocopherol or ascorbic acid back to their active forms. This capacity of GSH for regeneration of antioxidants is related with the redox state of the GSH disulfide–GSH couple (GSSG/2GSH). High levels of GSSG can induce oxidative stress and damage of multiple enzymes (333). In fact, one of the best methods to evaluate oxidative stress inside cells is the assessment of GSSG or the GSSG/GSH ratio (327).
Mitochondria are usually protected from oxidative insults and damage by a complex, multilayer network of mitochondrial antioxidant systems (Fig. 5). H2O2 can be readily converted to water by mitochondrial GPX, which oxidizes GSH to GSSG, and glutathione reductase then reduces GSSG back to GSH. GSH is synthesized in the cytosol, and its import into mitochondria is mediated by a transporter. In addition to GSH, mitochondria have two other small thiol-disulfide oxidoreductases—TRX and glutaredoxin—that play important roles in thiol redox control. Another detoxification enzyme, CAT, is present only in certain types of mitochondria [murine heart (265) or liver (280)]. In addition to these antioxidant enzymes, mitochondria possess several low-molecular-weight antioxidants, including α-tocopherol and ubiquinol. These molecules are particularly effective in scavenging lipid peroxyl radicals and preventing the free radical chain reaction of lipid peroxidation.
Another group of molecules implicated in redox control in the cell (and inside mitochondria) are metallothioneins (MT). These small cysteine-rich, metal-binding proteins play important roles in many biological processes such as metal ion homeostasis and detoxification, protection of cells from oxidative stress, cell proliferation, and cell survival. Their expression can be induced by many stimuli including hormones, cytokines, metal ions, oxidating agents, and specifically mitochondrial dysfunction (278). The mitochondrial distribution of these proteins is still not fully known, but it seems that some mitochondria do not contain them (e.g., heart mitochondria); however, their presence in the mitochondrial intermembrane space in the liver has been demonstrated (367). Abundant in vitro and in vivo evidence has pinpointed the antioxidant properties of MT based on their ability to act as important maintainers of the cellular zinc pool and as free radical scavengers. Using MT-overexpressing transgenic or MT-null mice, it has been shown that MT confer protection against the oxidative damage induced by a diversity of oxidative conditions, including doxorubicin cardiotoxicity, ischemia/reperfusion, diabetes, and alcohol administration (278).
II. Non-Targeted Antioxidant Treatments
Oxidative stress has been related to the pathophysiology of a wide variety of diseases (275, 276), including cardiometabolic diseases (hypertension, stroke, diabetes, metabolic syndrome), neurodegenerative disorders (Alzheimer's disease [AD], PD, and others), Duchenne muscular dystrophy, chronic obstructive pulmonary disease (COPD), and cancer.
It is not surprising that research over recent years has focused on antioxidants as a potential therapy with which to restore the normal physiology in these pro-oxidant conditions. Although many initial studies in cells or animal models have been successful, clinical trials have produced disappointing results. For example, in rat models of hypertension and renal dysfunction, treatment with vitamins C and E prevented renal inflammation and decreased arterial pressure, thus improving renal function (323). However, vitamin E treatment in hemodialysis patients with chronic kidney disease did not reduce oxidative protein modifications and lipid peroxidation (181), although a reduction in oxidative stress markers (60, 185) and improvements in endothelial function (356) have been reported. Regarding the antioxidant prevention of CVD, Papparella et al. showed that vitamin C prevented cardiac damage in rats with zidovudine-induced CVD, exerting its beneficial effects through inhibition of NADPH oxidase activity (230). In contrast, several clinical trials, such as the long-term trial performed by Sesso et al., have found that vitamins C and E have no preventive effect with regard to cardiovascular events, including coronary revascularization, non-lethal myocardial infarction, stroke, or CVD death (54, 178, 288). Another example of antioxidant therapy attempt is that used in AD, in which lipid peroxidation and β-amyloid deposition have been found to be reduced in the brain of mice treated with vitamin E in the early stage of the disease (312). In contrast to these results, this antioxidant did not improve cognitive defects in AD patients (176), thus calling into question its potential benefits in this neurodegenerative disorder (249). In addition to vitamins E and C, β-carotene has been presented as promising, but proved to have null or even detrimental effects on CVD when administered to high-risk subjects (54, 260).
The effects of antioxidants in cancer treatment are controversial. In fact, in the majority of past clinical trials, supplementation with antioxidants had no impact on the risk of developing a variety of cancers and in some studies, antioxidant supplementation was actually associated with an increased risk of cancer. The most notable of these studies were the β-carotene and Retinol Efficacy Trial (CARET) trial and the ATBC Study, in which daily supplementation with β-carotene or a combination of β-carotene and vitamin A increased incidence of lung cancer and all-cause mortality in smokers. The CARET evaluated the effect of daily retinyl palmitate (25,000 IU) and β-carotene (30 mg) administration on the incidence of lung cancer, other cancers, and death in 18,314 participants at a high risk of developing lung cancer because of a history of asbestos exposure or smoking. The CARET was aborted ahead of schedule in January 1996, because participants who had been randomly assigned the active intervention were found to have a higher rate of CVD mortality, and displayed a 28% increase in incidence of lung cancer and a 17% increase in incidence of death compared with participants in the placebo group (103). In another study, the effect of vitamin E and β-carotene (ATBC) on the incidence of lung cancer and other cancers was studied, and no reduction was found in the incidence of lung cancer among male smokers after 5–8 years of dietary supplementation with α-tocopherol or β-carotene. In fact, this trial pointed to the possibility that these supplements have harmful as well as beneficial effects (322).
The Selenium and Vitamin E Cancer Prevention Trial, which randomized 35,533 healthy >55 year-old men (>50 years if African American), revealed that neither selenium nor vitamin E, alone or together, prevented prostate cancer in this heterogeneous population (82). Other studies have demonstrated that N-acetylcysteine (NAC) and vitamin E markedly increase tumor progression and reduce survival in mouse models of B-RAF- and K-RAS-induced lung cancer, and accelerate tumor growth by disrupting the ROS-p53 axis. Given that somatic mutations in p53 occur late in tumor progression, antioxidants may accelerate the growth of early tumors or precancerous lesions in high-risk populations such as smokers and patients with COPD who receive NAC to relieve mucus production (282).
Exercise promotes longevity and ameliorates type 2 diabetes mellitus and insulin resistance (143, 229, 343). However, exercise also increases mtROS (248). Antioxidants are widely used as nutritional supplements, but whether they affect the health-promoting effects of exercise is not clear. In this sense, Ristow et al. (266) evaluated the effects of a combination of vitamin E (400 IU/day) and vitamin C (1000 mg/day) on insulin sensitivity in pretrained (n=20) and untrained (n=19) healthy young men. In both groups, exercise increased parameters of insulin sensitivity (glucose infusion rate and plasma adiponectin) only in the absence of antioxidants. This was paralleled by an increased expression of ROS-sensitive transcriptional regulators of insulin sensitivity and ROS defense capacity, peroxisome-proliferator-activated receptor gamma (PPARγ), and PPARγ coactivators PGC1α and PGC1β, again only in the absence of antioxidants. Exercise also increased the expression of SOD1, SOD2, and GPX, and this effect was inhibited by antioxidant supplementation. Consistent with the concept of mitohormesis, exercise-induced ROS generation reduces insulin resistance and causes an adaptive response by which the endogenous antioxidant defense capacity is enhanced. Thus, supplementation with antioxidants may preclude the health-promoting effects of exercise in humans.
Due to its essential role in mitochondrial respiration as an endogenous co-enzyme of ETC proteins and an ROS scavenger, Coenzyme Q10 (CoQ10), otherwise known as ubiquinone, exerts antioxidant effects in different pathological situations. Persson et al. demonstrated restored mitochondrial O2 consumption and an improvement in mitochondrial and renal functions in db/db diabetic mice fed a diet supplemented with the aforementioned coenzyme (236). Since CoQ10 is present at high concentrations in the heart, its potential benefits for cardiac dysfunction have been widely tested in humans with discrepant results (18, 345). Similar dicrepances have been reported in animal models; whereas CoQ10 attenuated amyloid β-peptide-induced mitochondrial dysfunction in brain mitochondria isolated from diabetic Goto-Kakizaki rats (207), and its administration was not fully advantageous for heart mitochondrial function in this model (223). More hydrosoluble molecules than ubiquinone have been developed and tested in different diseases; for example, the short-chain quinone idebenone, a synthetic derivative of CoQ10, has been shown to prevent cardiac hypertrophy in Friedreich's ataxia (119) and to improve measures of cognitive scores in patients with AD (351). Recently, idebenone has been awarded a temporary authorization and awaits marketing authorization for the treatment of Leber's Hereditary Optic Neuropathy, an inherited mitochondrial disease (Raxone®; Santhera Pharmaceuticals) (150).
Another strategy to preserve redox balance and an adequate mitochondrial function is the induction of endogenous antioxidants, and the Nrf-2 antioxidant signaling pathway is among the most eligible ones for this purpose. In patients with multiple sclerosis, the induction of Nrf-2 has been shown to be neuroprotective and anti-inflammatory (172), whereas sulforaphane induces enzymes downstream of Nrf-2 in airway disease, thus highlighting its potential antioxidant properties (264). However, bardoxolone methyl, a potent inducer of Nrf-2, has proved to have toxic effects in humans, leading to the interruption of an ongoing Phase III clinical trial (the BEACON study) (314).
Another potent antioxidant used in the treatment of diseases is lipoic acid (LA), which can be rapidly absorbed by cells and has a protective role against oxidative stress in different animal models (110, 111, 310). These studies demonstrated that LA can accumulate in different parts of the cell, but only in small concentrations within mitochondria (117). LA has powerful antioxidant properties and is therefore suitable for the treatment of diseases related to oxidative stress; for example, short-term and long-term supplementation of LA (200–1800 mg/day) in type 2 diabetic patients have beneficial effects in maintenance of glycemic control (200). LA can exert beneficial effects by several mechanisms, including improvement in vascular endothelial cell function (257), decrease in inflammation (104), amelioration of lipid abnormalities (376), and protection against myocardial ischemia/reperfusion injury (341) or antihypertensive effects (83). Defects in LA biosynthesis in human subjects can lead to the development of mitochondrial diseases, as has been shown in children with a mutation of NFU1, which is necessary for maturation of proteins such as succinate dehydrogenase or lipoic synthase (215). An in vitro study has shown that LA exerts a protective effect in fibroblasts obtained from patients with AD, as evidenced by decreases in oxidative stress and apoptotic markers, and this action is enhanced when LA is combined with NAC (206).
NAC is another important antioxidant with beneficial effects in different conditions. For instance, it might exert beneficial actions mediated by an increase in intracellular GSH levels, a major antioxidant, in different tissues (e.g., the lung) (255), and in particular respiratory diseases, including COPD, which is characterized, in part, by chronic mucus production, leading to an enhanced risk of infection. The benefits of short- and long-term administration of NAC for bronchial hypersecretion in chronic bronchitis have been studied since the early 1980s (320). Other research has focused specifically on NAC and has acknowledged the beneficial actions of oral administration during treatment of 3–6 months in reducing exacerbations and improving symptoms of chronic bronchitis (307). Moreover, administration of NAC is beneficial in acetaminophen poisoning, where it acts rapidly by increasing hepatic GSH synthesis, therefore protecting against oxidative stress (279). A number of clinical studies performed to investigate the potential of NAC as a therapeutic agent have been conducted in type 2 diabetes. This is of particular interest, as diabetic patients are a target group with a high risk of CVD in whom aspirin is ineffective in primary prevention of cardiovascular complications. For example, 6-month treatment with a combination of l-arginine and NAC reduced blood pressure by 5 mmHg in hypertensive diabetic patients (191). Meanwhile, several other studies have focused on the impact of NAC on platelet function: for instance, Gibson et al. demonstrated that NAC could increase intraplatelet levels of GSH, decreasing ROS levels and reducing platelet activation in vitro (98). Another study has demonstrated the antiplatelet properties of NAC in a cohort of type 2 diabetes patients (329), and showed an inhibition of monocyte-platelet conjugation, a surrogate marker of cardiovascular risk, within 2 h of administration, an effect that was maintained after daily self-administration over a 1-week period. Finally, several clinical studies and case reports have demonstrated the utility of NAC in different neuropsychiatric conditions, such as bipolar disorder (19), addiction (106, 151), obsessive compulsive disorder (158), and schizophrenia (161), and as a neuroprotective agent in AD (1).
There is abundant evidence in animal models of the utility of NAC for treatment or prevention of ROS-associated pathologies. Oral NAC treatment is beneficial against RNS-dependent ventricular tachycardia. In fact, Fauconnier et al. (87) demonstrated that diastolic SR Ca2+ leak via RyR2 due to S-nitrosylation of the channel and that calstabin2 depletion from the channel complex triggers cardiac arrhythmias, a situation in which treatment with NAC has beneficial effects. In addition, it has been demonstrated that NAC protects from ventricular arrhythmias by attenuating reduced connexin 43 expression and function via both PKA- and Epac-dependent pathways, which converge through the inactivation of glycogen synthase kinase-3β (164). Other studies have shown the beneficial effects of NAC in diabetes (140) in which diabetic C57BL/KsJ-db/db mice were exposed to antioxidant treatment (NAC, vitamin C plus E, or both). NAC increased glucose-stimulated insulin secretion and moderately decreased blood glucose levels, whereas vitamins C and E were not effective when used alone and only slightly effective when used in combination with NAC. Histologic analyses of the pancreas revealed that the β-cell mass was significantly larger in the diabetic mice treated with antioxidants than in the untreated animals. It was speculated that the antioxidant treatment suppressed apoptosis in β-cells without changing the rate of β-cell proliferation, supporting the hypothesis that, in chronic hyperglycemia, oxidative stress-induced apoptosis causes reduction of β-cell mass. The antioxidant treatment also preserved the amounts of insulin mRNA and peptide, making the extent of insulin degranulation less evident. Furthermore, expression of pancreatic and duodenal homeobox factor-1, a β-cell-specific transcription factor, was more evident in the nuclei of islet cells after the antioxidant treatment. In another study, Cuzzocrea et al. (61) demonstrated, using an animal model, that NAC (20 mg/kg; 30 min before reperfusion and 1, 2, and 6 h after reperfusion) reduced the formation of post-ischemic brain edema and attenuated the increase of brain levels of malondialdehyde (MDA) and myeloperoxidase in the hippocampus caused by cerebral ischemia. NAC treatment increased survival and reduced hyperactivity linked to neurodegeneration induced by cerebral ischemia and reperfusion. These results suggest that NAC alleviates damage induced by transient cerebral ischemia or brain injuries.
Trolox (6-hydroxy-2,5,7,8- tetramethylchroman-2-carboxylic acid) is a water-soluble analogue of the free radical scavenger α-tocopherol. Due to its enhanced water solubility, Trolox may function more rapidly during acute oxidative stress (357), while α-tocopherol requires several days of pretreatment to exhibit antioxidant benefits. Moreover, it seems that Trolox can scavenge peroxyl radicals better than α-tocopherol. For example, Wu et al. (358) demonstrated that Trolox protects human hepatocytes and myocytes against ROS. Furthermore, Trolox can reduce hypoxia/reoxygenation-induced hepatic injury in the isolated perfused rat liver (163). In cancer studies, it has been demonstrated that Trolox can inhibit breast cancer cell-induced osteoclast differentiation and the invasive behavior of cancer cells through PGE2-dependent and independent mechanisms, thereby suppressing osteolytic bone metastasis in breast cancer (162). Another example of the beneficial effects of Trolox has been described by Du et al. (81), who demonstrated that chitosan nanoparticles, when used as drug carriers for the delivery of Trolox, exert a protective effect against hypoxia-mediated oxidative stress and can block the mitochondria-dependent apoptotic pathway through upregulation of Bcl-2 expression and inhibition of Bax activation and Caspase-3 expression.
Another important antioxidant is the paraoxonase (PON) family of enzymes, which also contribute to vascular antioxidant defenses and protect against coronary artery disease (CAD), among other conditions (14). It has been described that PON1 and PON3 enzymes are synthesized in the liver and are associated with high-density lipoprotein (HDL) fraction. In fact, the capacity of HDL for controlling levels of HDL and low-density lipoprotein (LDL) lipid peroxidation depends on its PON levels (14). On the contrary, deletion of the PON1 gene can enhance oxidative stress in aorta and in mouse macrophages (277) and apoE knockout mice overexpressing PON1 have been shown to be protected against the atherosclerotic process (331). PON2 is expressed in various types of cells, and some polymorphisms have been associated with CVD (168). PON2 has demonstrated beneficial effects by scavenging ROS in fibroblasts, endothelial cells, and vascular smooth muscle cells (124). Some authors have demonstrated that overexpression of PON2 in apoE knockout mice protects against atherosclerosis. For these reasons, the increase in the levels of PON enzymes could be beneficial in oxidative stress-related conditions. In this sense, it has also been suggested that PON1 is a better atherosclerotic risk predictor than HDL in type 2 diabetes patients (232). The authors in question demonstrated that the levels of HDL and PON1 were negatively correlated with various atherogenic indexes but that the strength of negative correlation was always greater for PON1. In addition, and by using multiple linear regression analysis, they found that the regression coefficient (β) was always higher for PON1 than for HDL when taking the atherogenic indices such as atherogenic index of plasma=Log(triglicerides/HDL), atherogenic coefficient=(total cholesterol [TC]−HDL)/HDL, Castelli's risk index I (CRI I)=TC/HDL, and II (CRIII)=LDL/HDL as outcome variables (232). Several meta-analyses have been published about the importance of PON1 levels in human diseases. For example, a meta-analysis of 47 studies with 9853 CAD patients and 11,408 control subjects published in 2012 confirmed that lower plasma PON1 activity was related to an increased risk of CAD (339).
Importantly, some classical pharmacological agents have been attributed antioxidant properties. This is the case of statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) and β-adrenergic blockers. Several actions of statins have been related to their antioxidant capacity. First, they can suppress NADPH oxidase activity and expression (12, 239, 350). In fact, Pignatelli et al. (239) observed the effect of statins on 30 hypercholesterolaemic patients and 20 controls by assessing Nox2 and urinary isoprostane, a marker of oxidative stress. They demonstrated that the said patients had higher levels of Nox2 and urinary isoprostane. After atorvastatin treatment for 30 days, both soluble gp91phox and isoprostane were markedly reduced. The authors concluded that statins exerted an antioxidant action through the inhibition of serum gp91phox. Second, they can prevent eNOS uncoupling by upregulation of GTP cyclohydrolase 1 (GTPCH1) expression and increased tetrahydrobiopterin (BH4) biosynthesis (12, 350). Third, statins can induce antioxidant enzymes such as SOD1, SOD3, and GPX. Finally, they enhance eNOS expression and activity. For all these reasons, statins have beneficial effects in different conditions associated with oxidative stress and mitochondrial impairment. Some β-adrenergic blockers have demonstrated similar properties. One study evaluated the β-blockers atenolol, labetalol, metoprolol, pindolol, propranolol, sotalol, timolol, and carvedilol for their putative scavenging activity for ROS and RNS, and demonstrated that some were effective scavengers (101), which makes them useful in preventing oxidative damage in hypertension and other CVD that frequently emerge in association with oxidative stress. The beneficial effects of β-blockers associated with their antioxidant property have also been shown in arrhythmia (carvedilol improved intracellular Ca2+ handling and contractile dysfunction by correcting defective interdomain interaction within the RyR in the failing heart) (205) or nephrotoxicity (carvedilol protected rats from gentamicin-induced nephrotoxicity) (157).
Finally, the important antioxidant molecule resveratrol (3,5,4′-trihydroxy-stilbene) deserves a mention. This polyphenolic compound, present in red grapes and other plants including oriental herbal plants (170), exhibits ROS-scavenging activity and acts on several redox enzymes. Resveratrol has been shown to inhibit the activity and expression of NADPH oxidase in cardiovascular tissues (359), to accelerate mtROS detoxification by upregulating SOD2 (319), and to reduce mitochondrial radical production (250). Furthermore, it induces several antioxidant enzymes, such as CAT, GPX1, and SOD isoforms (170). Resveratrol can also prevent eNOS uncoupling by upregulating GTPCH1 and BH4 biosynthesis, and can enhance eNOS activity and expression (170). In addition, resveratrol supplementation can restore high-fat diet-induced insulin secretion dysfunction by increasing mitochondrial function in the islets (153), and preserve mitochondrial function by stimulating mitochondrial biogenesis together with attenuation of oxidative stress in regulatory T-cells of mice fed a high-fat diet (337). The potential effect of resveratrol has been evaluated in different clinical trials in humans with metabolic diseases (324, 325, 334). In one study, resveratrol showed beneficial effects in insulin resistance type 2 diabetic patients (29). It can also decrease insulin and glucose levels, enhance mitochondrial function, and suppress inflammation in healthy obese men (324). Its beneficial effects have also been demonstrated in type 2 diabetes by decreasing cholesterol, blood pressure, and HbA1c levels (20), while it promoted insulin sensitivity in the elderly (59). However, other studies have found no beneficial effects of resveratrol on the metabolism of obese men (250) or control non-obese women (373). Such studies provide discrepant evidence regarding the role of SIRT1 in resveratrol treatment (122), pointing to differences in the methodologies employed and the subjects studied, as well as the dosages, the routes of administration, and the bioavailability of resveratrol. A bioavailability study of resveratrol found that it can cross the blood–brain barrier (BBB) and reach the brain tissue rapidly after systemic administration (340), an effect related to the therapeutic potential of this compound in stroke and neurodegenerative diseases. Most clinical trials have been performed with high doses (ranging from 75 mg to 5 g per day) of resveratrol and during weeks or months (247). In general, it is unknown whether long-term consumption of resveratrol or low levels of resveratrol-rich foods can prevent or ameliorate the onset of type 2 diabetes. In addition, pharmacokinetic studies have detected nanogram levels of resveratrol in plasma, providing evidence that other resveratrol metabolites (e.g., resveratrol-3-sulfate, or resveratrol-3-O-glucuronide, which are more abundant in the blood) are responsible for the health-benefiting effects (247). Therefore, future studies taking into account these metabolites and focusing on systemic physiology in addition to tissue-specific effects may help understand the metabolic effect of resveratrol and its potential therapeutic actions in metabolic diseases or related pathologies.
Although some achievements have been made, in general terms, the experience with antioxidant therapies has not met the initial expectations. Multiple reasons could explain the lack of effectiveness of antioxidants: inadequate dose and/or timing of antioxidant supplementation, poor bioavailability, heterogeneous microenvironments in which antioxidants have to exert their action, antioxidant status of the study population, and untargeted delivery of the antioxidant compound. Regarding supplemental dosages, some studies may have administrated insufficient amounts of the antioxidant compound for assessing its beneficial effects (208), whereas excessive concentrations could have produced pro-oxidant effects in others (68, 227, 244), thus raising concerns about safety. The duration of antioxidant administration should also be taken into account, with acute pathological conditions requiring immediate and shorter intervals than chronic diseases (123). In terms of the microenvironment in which antioxidants are supposed to act, the same molecule may exert antioxidant or pro-oxidant effects depending on different factors; for example, the pressure of O2 in the case of β-carotene (33), or the amount of iron in the case of vitamin C (which induces the generation of •OH under iron overload) (121). In addition, it is important to note that redox signaling is a crucial part of many physiological processes, and that disruption of the redox equilibrium by excessive or inappropriate administration of antioxidants may have negative effects. This may be another contributing factor to the controversial results of clinical trials with antioxidant therapies, and it needs to be considered in the future.
In this context, some oxygenated metabolites are believed to have beneficial effects. Indeed, this is the case of n-3 PUFAs, eicosapentaenoic acid, and docosahexaenoic acid (DHA). DHA is very susceptible to spontaneous oxidation by non-enzymatic oxidation pathways. As the dogma regarding lipid peroxides has always dictated that they are undesirable and toxic, the possibility that they may be involved in mediating the beneficial effect of n-3 PUFAs is counterintuitive. However, mounting evidence suggests that in many contexts, spontaneously oxidized PUFAs can be beneficial, due largely to the fact that they are highly reactive agonists for certain receptors. For example, recent reports have demonstrated that oxidized DHA has a high affinity for the PPAR family of transcription factors, which regulate many cellular processes, including cellular development, differentiation, metabolism, and tumorigenesis. Moreover, oxidized DHA has a greater PPAR-activating effect than any other PPAR ligand tested (8, 128). These findings could have broad clinical implications, as they indicate that DHA peroxidation in vivo could greatly enhance its potency as PPAR agonist—a class of widely prescribed drugs that treat a variety of pathologies, from high cholesterol and arrhythmia to type 2 diabetes. In addition, it is possible that a large portion of the electrophysiological effects attributed to n-3 PUFAs are dependent on their oxidation (137).
Another explanation of the reported lack of benefits of antioxidants is the antioxidant status of the population selected for the study (309). This became evident in the clinical trial performed by Meagher et al., in which vitamin E supplementation did not affect lipid peroxidation in healthy subjects with normal endogenous levels of vitamin E due to its routine intake through the populations's Western diet (195). Finally, the most likely cause for these disappointing results is that the untargeted administration of general antioxidants leads to insufficient local amounts in the target tissue (123, 249) and, more specifically, in the primary source site of free radicals, that is, the mitochondrion. Many studies over the past decade have focused on the development of mitochondria-targeted antioxidants, with highly promising results (268) that will be discussed in detail.
III. Strategies for Mitochondrial Pharmacology with Special Focus on Antioxidants
Taking into account the important role of mitochondria in human pathophysiology (Fig. 1), these organelles are an obvious pharmacological target. Multiple compounds have been developed to alleviate their dysfunction in different pathological situations (295), with varying targets and mechanisms of action, including maintenance of Ca2+ homeostasis, regulation of mitochondrial dynamics, stimulation of mitochondrial biogenesis, or regulation of apoptosis and, particularly, oxidative stress (210).
A. Unspecific approaches
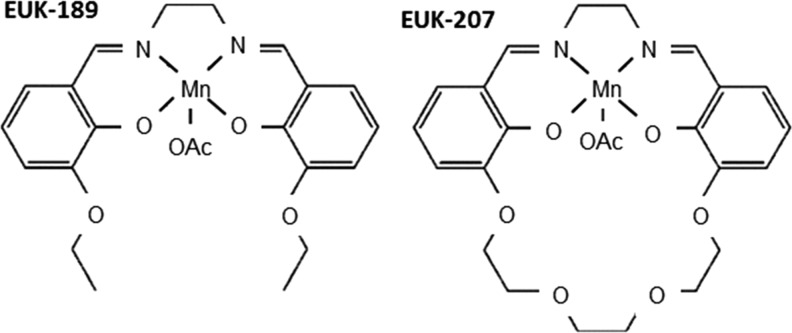
Although not designed to target mitochondria, several molecules display “mito-protective” activity; namely, an ability to attenuate mitochondrial injury (75). They are referred to as “synthetic catalytic scavengers or antioxidants” and were first investigated and developed by Eukarion (hence their code name EUK) (Fig. 6). These analogues of SOD-CAT mimetic have been shown to be beneficial in various models of oxidative stress; for example, salen Mn complexes were effective in a direct test of mammalian mitochondrial oxidative stress in SOD2 KO mice (196–198), in which EUK-8, EUK-134, and EUK-189 significantly extended the lifespan of the animals (160, 197). Beneficial effects have been reported, not only in the brain but also in other tissues (198). Treatment with salen Mn mimetics has been shown to exert protective effects against oxidative stress in animal models of several diseases, such as PD (235), ALS (138), AD (198), stroke (17), and age-associated cognitive impairment (174). Specific animal models of ROS-related injury have revealed the potential of molecules such as EUK-207 to mitigate radiation damage in the lungs (161), or EUK-189 to prolong survival after a lethal dose of total body radiation (304). Some of these compounds are orally bioavailable, whereas others have been proposed for topic or parenteral administration. The compound EUK-134 has been used as an active ingredient in cosmetics for about 10 years, and another EUK compound is a clinical candidate for the prevention and treatment of certain neurodegenerative diseases.
FIG. 6.
Structures of the salen Mn complexes EUK-189 and EUK-207. OAc acetoxy (CH3COO).
Several studies have demonstrated the beneficial effect of antioxidant compounds on the vasculature through protection against oxidative damage and consequent restoration of endothelial function (285). Novel approaches have been developed in this aspect, with certain compounds having been shown to render protection against these CVD and other related diseases by acting specifically on mitochondria. For example, diazoxide, an FDA-approved drug for the management of symptomatic hypoglycemia, has been identified as cardioprotective. Diazoxide is generally believed to inhibit succinate dehydrogenase, a mitochondrial complex II protein (51). This inhibition, which also takes place in the heart, can occur at concentrations that are often used to study cardioprotection, and therefore may constitute a mechanism involving partial uncoupling of OXPHOS and/or modulation of ROS production. However, there is contrasting evidence regarding its targets and mechanisms of action. It seems that diazoxide exerts an ambivalent effect on mtROS production, as shown in a mechanistic study using submitochondrial particles and intact rat heart mitochondria, in which, depending on the metabolic state and Δψm, diazoxide-mediated inhibition of complex II either promoted transient generation of signaling ROS at complex III (during preconditioning) or attenuated the production of deleterious ROS at complex I (during ischemia and reperfusion) (80). Whatever the underlying molecular mechanism(s) may be, it is clear that improved mitochondrial function is a principal cardioprotective effect of diazoxide (52).
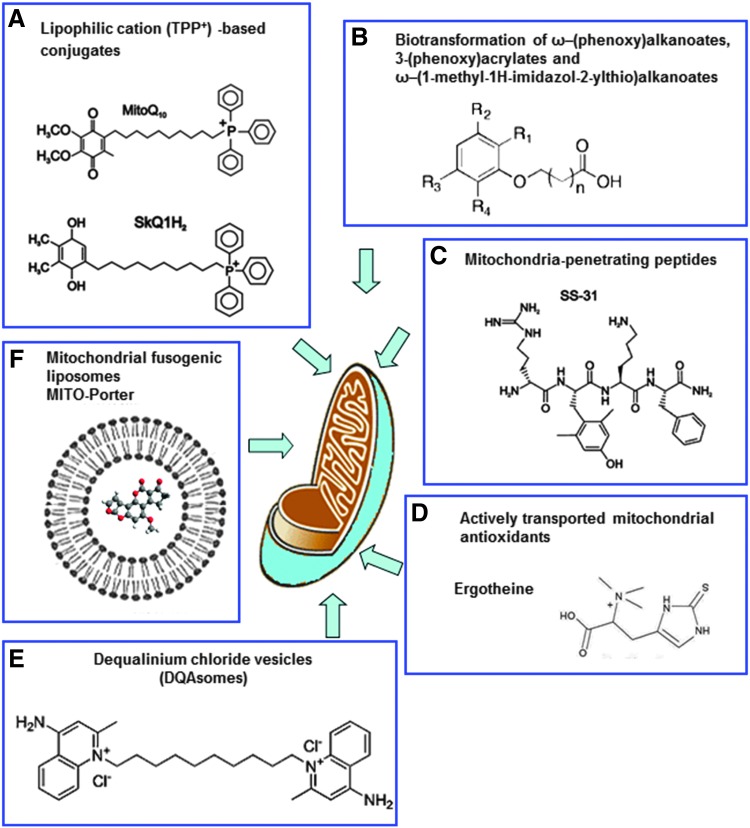
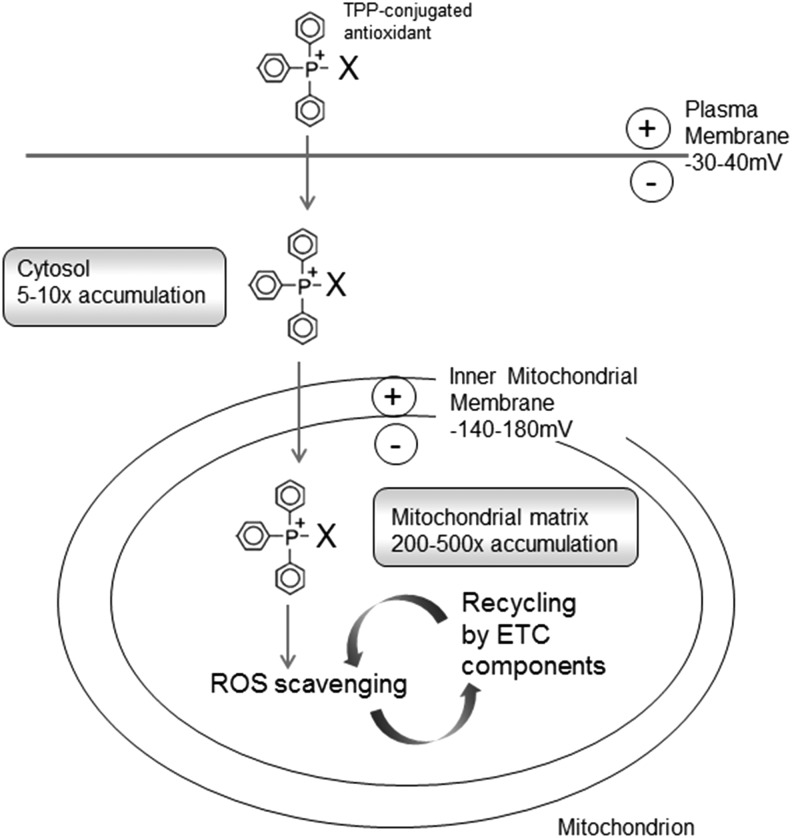
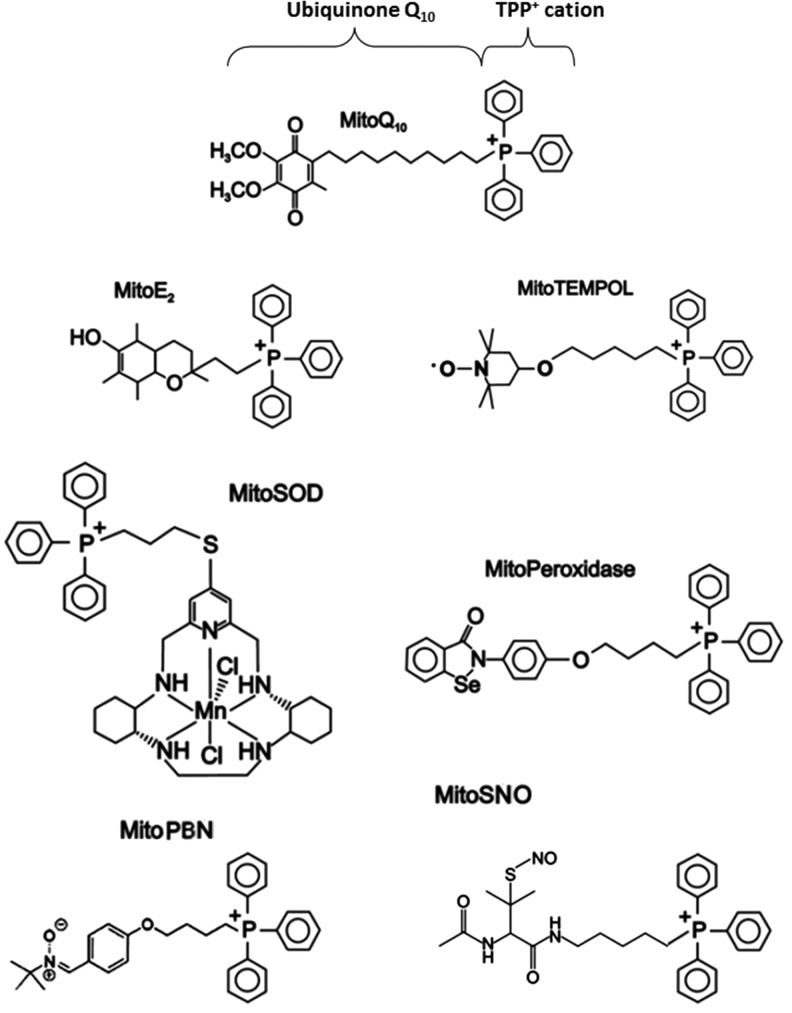
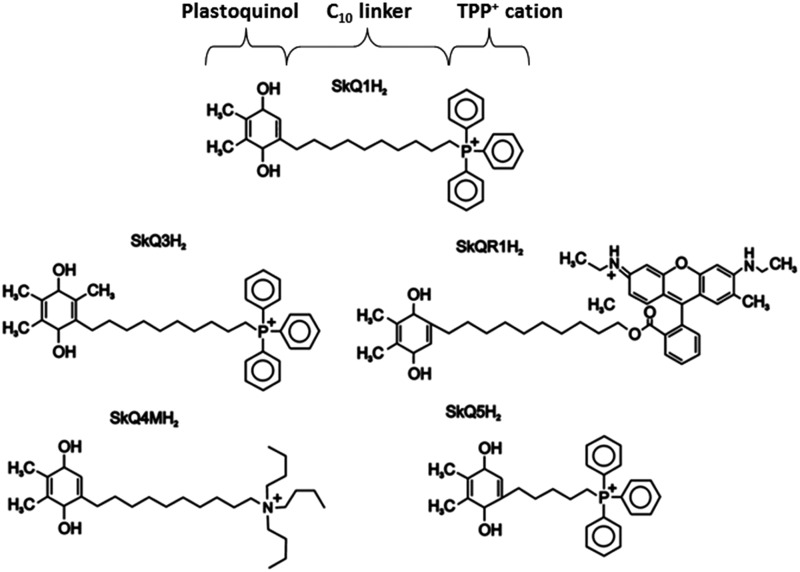
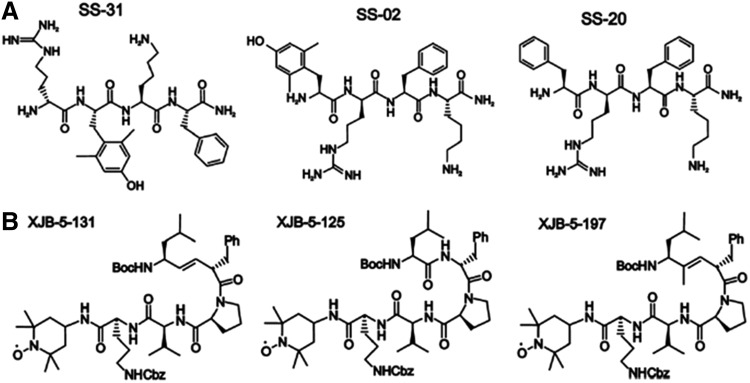
B. Targeted mitochondrial delivery
The delivery of drugs to specific subcellular territories is a promising avenue for therapeutics in many human diseases. It improves the therapeutic efficacy of compounds through accumulation of the drug near the specific target and reduction of the deleterious consequences of its off-target subcellular localization. Moreover, targeted delivery systems may improve certain characteristics of the drug and thus overcome the classical limitations of conventional drug administration, such as low bioavailability, insolubility, and drug resistance. Due to the crucial implication of mitochondria in human pathophysiology, the elaboration of methods and construction of vehicles for efficient selective drug delivery to these organelles is currently the focus of molecular pharmacological research. Even when the compound has the adequate physicochemical properties as to overcome the anatomical, immunological, and biochemical barriers to reach the target tissue and cells, it still has to cross several membranes to reach its final destination inside the mitochondrion. This greatly limits its action at the specific site inside this organelle; therefore, selective targeting of the drug to the mitochondrial localization is necessary. Mitochondrial drug targeting is a complex process that depends on the particular nature of these cellular compartments, including the presence of specific transporters located on the mitochondrial membrane. Diffusion through the IMM is difficult; therefore, mitochondria-targeted compounds need to be encapsulated inside a drug carrier. Moreover, this encapsulation and the subsequent release of the compound inside the mitochondrion must preserve the drug's pharmacological stability/activity. In recent years, great efforts have been made to find solutions for the development of efficient drug carriers. These molecules display several features: (i) ability for binding to the pharmacologically active form of the drug or a prodrug; (ii) an efficient transport system that carries the drug to the site of its action; (iii) specific and selective targeting to the mitochondrial compartment; and (iv) release of the drug inside the mitochondrion (Fig. 7). Current strategies for mitochondrial drug delivery include passive and active targeting (287). In the case of active targeting, specific interactions that take place at mitochondrial sites, including ligand-receptor associations and antigen-antibody binding, are exploited and take advantage of the compatibility between the physicochemical properties of the carrier molecule (electric charge, hydrophilicity, size, and mass) and those of the mitochondrial compartment. Due to mitochondrial morphological properties, passive targeting to these organelles is difficult; hence, active targeting strategies are being developed. Small molecules have been efficiently targeted to mitochondria in vivo by several targeting strategies; namely, through enclosure inside liposomes (287), conjugation to lipophilic cations (210), and incorporation into mitochondria-targeted peptides (315) (Table 1). So far, the molecules employed in these approaches involve relevant antioxidants as well as substrates and coenzyme components of the ETC, such as cytochrome c, B2, succinate, and vitamin B1, and proapoptotic proteins including those of the Bax/Bcl2 family and p53 (287).
FIG. 7.
Pharmacological characteristics of an “ideal” mitochondria-targeted antioxidant with clinical relevance. As a pharmaceutical agent, the mitochondria-targeted scavenger or redox-active molecule should be bioavailable, ideally on oral administration. After its absorption in plasma, it is delivered to target tissues (organs in which mitochondria play a prominent role, such as muscle, brain, or liver, are primary targets for treatment). The molecule readily enters the cell and accumulates inside mitochondria, where it ameliorates oxidative and nitrosative stress. BBB, blood brain barrier. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Table 1.
Current Most Relevant Strategies for Mitochondrial Targeting
| Strategy | Mechanism | Group | Agents | Conditions of reported beneficial actions |
|---|---|---|---|---|
| Conjugation to lipophilic cations | Use mitochondrial ΔΨm to accumulate within the mitochondrial matrix | Mito-compounds | MitoQ MitoTEMPO MitoTEMPOL MitoE MitoSNO |
Rotenone-treated fibroblasts (154) UVA- and H2O2-induced mtDNA damage in human dermal fibroblasts (228) β-cell lines RINm5F and HIT-T15 under glucotoxic stress (171) Endothelial cell model of sepsis (180) mtROS-mediated activation of AMPK in HSVEC cultures obtained from patients with CAD and type 2 diabetes (184) Tolerance to nitroglycerine (84, 96) Mitochondrial fission induced by chemical inhibition of mitochondrial ETC in HeLa cells and fibroblasts (240) Basal and induced autophagy in murine C2C12 myoblasts (257) Selective cancer cell cytotoxicity (259) Animal models of: • CVD: endotoxin-induced cardiac dysfunction (313), age-related arterial endothelial dysfunction (99), cardiac ischemia/reperfusion (2, 48), doxorubicin-induced cardiac toxicity (40, 70), hypertension (105, 194), cocaine-induced cardiac dysfunction (335), endothelial dysfunction and prevent vascular inflammation in Ang II-treated PGC-1α KO mice (156), cardiac renin-angiotensin system activation (301) • sepsis (180, 373) • diabetes, metabolic diseases, and obesity (38, 199, 252) • ethanol-induced micro- and macrohepatosteatosis (39) • neurodegeneration (342) Chronic hepatitis in HCV-infected patients (296) |
| Cationic plastoquinone derivatives | SkQ1 SkQR1 |
In vitro models of hemolysis (226) Kidney function in myoglobinuria (rhabdomyolysis) or ischemia/reperfusion rat model (241) Rat model (242) and in vitro model (243) of pyelonephritis Animal models of kidney failure (ischemia, rhabdomyolysis) and focal brain ischemia (243) Prolong lifespan of many organisms, including mammals (292) Treatment of eye diseases: cataract, glaucoma, macular degeneration, uveitis, and dry eye syndrome (292) Animal models of AD-like pathologies (141, 305) |
||
| Peptide-based targeting | Mitochondrial localization due to chemical structure | SS-peptides | SS-31 SS-02 SS-20 |
Hyperglycemia-induced damage in human retinal endothelial cells (169) Autophagic flux and proteolysis in C2C12 myoblasts under basal condition or subjected to autophagy triggers (257) Reactive chlorine species-mediated damage to cultured human fetal liver cells (352) Reperfusion-associated myocardial stunning in isolated perfused guinea pig heart (378) Animals models of: • ischemic brain injury (46), myocardial infarction (47), microvascular rarefaction (174), pressure overload-induced heart failure (64) • ALS (237), PD (365), AD (187, 261), oxaliplatin-induced neuropathy (328) • Obesity (11) • unilateral urethral obstruction (202) • cell and organ transplantation (316) |
| XJB peptides | XJB-5-131 XJB-5-125 XJB-5-197 |
Actinomycin D-induced O2•− generation, cardiolipin peroxidation, and apoptosis in mouse embryonic cells (134, 354) Radiation damage of cells in culture (133) Acute tissue ischemia in rat enterocytes exposed to lethal hemorrhagic shock (183) |
||
| Liposomes and liposome-like structures | Entry in the cell by endocytosis and fusion with the mitochondrial membrane | Colloidal dequalinium vesicles | DQAsome | Efficient and selective delivery of nucleic acids or antioxidants to mitochondria (71, 72, 333, 349, 374) |
| Liposome-based nano-carriers | MITO-Porter | Delivery of DNase I to the mitochondrial matrix (361) | ||
| Organelle-specific bioactivation reactions | Prodrug to drug conversion by mitochondria-specific enzymes | Alkanoate-based prodrugs exploiting fatty acid β-oxidation | ω-(phenoxy) alkanoates 3-(phenoxy)acrylates ω-(1-methyl-1H-imidazol-2-ylthio)alkanoates |
Cytoprotective effect in a hypoxia-reoxygenation model of rat cardiomyocytes (272) |
| Mn porphyrin-based targeting | Mimicking mitochondrial SOD (MnSOD) | Cationic Mn(III) N-substituted pyridyl- and N,N′-disubstituted imidazolyl porphyrins | MnTDE-2-ImP5+ MnTnHex-2-PyP5+ MnTnOct-2-PyP5+ |
Submitochondrial particles exposed to ONOO−-mediated oxidative stress (88) or against oxidative damage in mouse heart mitochondria (302) Anticancer effects in a large number of in vitro and in vivo models (326) Protection of photoreceptors and retinal capillaries exposed to proton radiation (326) Animals models of diabetes and CNS diseases (stroke, cerebral palsy, spinal cord injury, subarachnoid hemorrhage, AD, and chronic morphine tolerance) (326) |
Major characteristics are detailed, together with examples of compounds and settings in which they have been reported to have beneficial effects.
AD, Alzheimer's disease; ALS, amyotrophic lateral sclerosis; AMPK, AMP-activated protein kinase; CAD, coronary artery disease; CNS, central nervous system; CVD, cardiovascular disease; ETC, electron transport chain; H2O2, hydrogen peroxide; HCV, human hepatitis C virus; HSVEC, human saphenous vein endothelial cells; MitoQ, mitoquinone; MnSOD, manganese superoxide dismutase; mtDNA, mitochondrial DNA; mtROS, mitochondrial ROS; O2•−, superoxide anion; ONOO−, peroxynitrite; PD, Parkinson's disease; SkQ1, plastoquinonyl decyltriphenylphosphonium; SS, Szeto-Schiller; UVA, ultraviolet A; Δψm, mitochondrial membrane potential.
1. Lipophilic cations
Lipophilic cations take advantage of mitochondrial ΔΨm to facilitate their selective targeting and accumulation within the mitochondrial matrix (273). This process can be expressed by the Nernst equation, by which the uptake of these molecules increases ∼10-fold for every ∼60 mV of membrane potential, leading to significant uptake within mitochondria in vivo (246, 271) (Figs. 8 and 9). Massive mitochondrial targeting is enabled by the electrochemical gradient from the plasma membrane potential (−30 to −60 mV) to the IMM potential (Δψm), (−150 to −180 mV), which provides a potent force for the selective targeting of large lipophilic cations and their concentration inside the mitochondria (211, 295). This high negative membrane potential present in the mitochondria is not found in any other subcellular compartment, which offers a very selective molecule delivery to these organelles. Several lipophilic cations, including triphenylphosphonium (TPP+), rhodamine 123, flupirtine, MKT-077, and anthracyclins, exhibit selective mitochondrial accumulation on cellular entry (95).
FIG. 8.
Available strategies for mitochondrial targeting. (A) Conjugates of lipophilic cations such as TPP and small molecules or nano-carriers exemplified by MitoQ (TPP-bound version of ubiquinol) and SkQ1H2 (plastoquinonyl decylTPP). (B) Targeting through mitochondria-specific bioactivation reactions, which catalyze the conversion of a prodrug to a drug. (C) Mitochondria-targeted peptides, including SS peptides and XJB peptides. (D) Actively transported mitochondrial antioxidants such as ergotheine. (E) DQAsomes—vesicle-like aggregates formed by the dicationic mitochondriotropic compound, dequalinium chloride. (F) Liposome-based carrier such as MITO-porter. MitoQ, mitoquinone; SkQ1, plastoquinonyl decyltriphenylphosphonium; SS, Szeto-Schiller; TPP, triphenylphosphonium. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 9.
Targeting compounds to mitochondria by conjugation to the lipophilic cation TPP. TPP has a large, hydrophobic surface area that enables it to pass easily through phospholipid bilayers. The molecule first passes through the plasma membrane and accumulates in the cytosol driven by the plasma membrane potential (Δψp). This strategy further utilizes the large Δψm (negative inside) to drive accumulation of TPP+-linked bioactive compounds inside mitochondria for several hundred fold. The antioxidant properties of the conjugates are exploited inside the mitochondrial compartment, and the oxidized forms are then re-reduced (recycled) by ETC complexes.
The delivery of pharmacological agents into the cell through the use of lipophilic cations was first shown with the lipophilic cation rhodamine 123 in combination with the anticancer drug cisplatin (321). TPP+ and TPMP+ (its methylated form) are the most widely employed lipophilic cations for mitochondrial targeting of antioxidants (210, 211). An alternative lower-molecular weight spin trap bearing an N-arylpyridinium ion (267) has been used and positive results are obtained, though its efficacy needs further confirmation. The TPP moiety is driven by the plasma membrane potential, which allows rapid cellular uptake of bioactive molecules, followed by specific mitochondrial matrix accumulation. Once inside the mitochondrion, TPP+ molecules position themselves primarily on the mitochondrial matrix-facing surface of the phospholipid bilayer, with the functional group and the linker positioned within the IMM (211). The extent to which TPP+ molecules anchor themselves to the IMM depends on the hydrophobicity of the molecule and on the length of both the linker and the functional group. A large number of compounds have been generated through conjugation to the TPP moiety (Fig. 10), of which several are antioxidants, such as ubiquinone (13), tocopherol (298), LA and LA spin traps, (31), ebselen (91), resveratrol (21), nitrones (209), plastoquinone (293), and TEMPOL (330). These Mito-derived redox modulators are reported to diminish the level of a great variety of reactive species (e.g., H2O2, •NO, ONOO−, lipid peroxyl, and alkoxyl radicals). The accumulation of antioxidants within mitochondria though the IMM anchoring of their TPP+-conjugates has been proposed to control mitochondrial redox signaling and prevent membrane lipid peroxidation. Since lipid peroxidation is a crucial feature of mitochondrial oxidative damage, TPP+-conjugates that are effective against lipid peroxidation are the focus of current research. Lipophilic cations show great potential for the successful delivery of antioxidants to mitochondria; however, they present several disadvantages: (i) limited capacity (only low-molecular-weight molecules and electrically neutral chemicals can be successfully transferred); (ii) sublocalization (these compounds tend to localize in the mitochondrial matrix and the matrix-facing surface of IMM; thus, the targeting of important processes that occur on the outer leaflet of the IMM, the OMM, or the intermembrane space is largely limited or impossible); and (3) toxicity (at high concentrations, they can depolarize ΔΨm and compromise cell viability).
FIG. 10.
Chemical structures of mitochondria-targeted antioxidants based on lipophilic cations: TPP+-conjugates (Mito compounds).
The TPP-bound version of ubiquinol (mitoquinone [MitoQ]) is the most extensively evaluated and best understood mitochondria-targeted antioxidant (50, 210, 211, 298). The cellular uptake of MitoQ is far more rapid than that of methylTPP, presumably because its higher hydrophobicity reduces the activation energy available for passage across the plasma membrane. The longer a molecule's alkyl chain is, the greater the mitochondrial accumulation of MitoQ; in this context, MitoQ10, which has a 10-carbon atom-alkyl chain, shows optimum performance (Fig. 10). MitoQ uptake occurs primarily in mitochondria rather than in other cell compartments, as supported by the fact that its accumulation in cells is largely blocked when Δψm is disrupted by the uncoupler carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP). A further indication that the mitochondrial concentration of TPP-containing molecules within cells is membrane potential—dependent is the FCCP-sensitive protection afforded by MitoQ in a cell model of Friedreich's ataxia, in which FCCP does not alter the efficiency of the non-targeted compounds decylQ and idebenone (132). Within mitochondria, MitoQ is accumulated at the matrix-facing surface of the IMM, where complex II of the ETC recycles it into the active ubiquinol form (MitoQH2), which remains stable during long-term incubation (13, 210). This reduction is crucial for the compound to function as a reducing agent, as the totality of the effects of MitoQ can probably be attributed to accumulation of the antioxidant ubiquinol form. This form has been shown to be a highly effective antioxidant by reacting with ROS and also inhibiting ONOO− formation. The antioxidant property of the ubiquinol form of MitoQ is based on its oxidation into the ubiquinone form, which is then rapidly re-reduced by complex II, thus regaining its antioxidant efficacy. Given that MitoQ is generally adsorbed to the IMM, and the fact that its linker chain enables the penetration of the active antioxidant form, ubiquinol, deep into the membrane core, this molecule is believed to be effective as an antioxidant against mitochondrial lipid peroxidation (144, 294). The reactivity of MitoQ10 toward O2•− and other O2 radicals has been studied in detail (188). The ubiquinone form of MitoQ directly reacts with other ROS such as O2•−, although, as occurs with other ubiquinols, its reaction with H2O2 can be considered negligible. MitoQ10 reacts with O2•− in water and methanol with rate constants of 2.0×108 M−1 s−1 and 4.2×108 M−1 s−1, respectively, forming the semiquinone radical MitoQH, which then dismutes to MitoQ10 and the quinol MitoQH2. In addition, the reverse reaction of MitoQH with O2 displays rate constants of 2.9×107 M−1 s−1 and 7.3×106 M−1 s−1 in water and methanol, respectively. MitoQH2 rapidly reacts with ONOO− (whereby MitoQH is produced and undergoes dismutation), an action that is particularly effective against lipid peroxidation, which accounts for the protection it is reported to afford against ONOO−-induced damage (294). In short, in isolated mitochondria, MitoQ seems to fulfill most of the requirements of a successful mitochondria-targeted antioxidant.
Stable-free radical nitroxides with antioxidant properties (TEMPO derivatives) are another group of mitochondria-targeted antioxidants that have been described. The action of these compounds to metabolize ROS is atributed primarily to cyclic one- or two-electron transfer in three oxidation states: the oxammonium cation, the nitroxide, and the hydroxylamine (353). Nitroxides undergo a very rapid, one-electron reaction to the corresponding hydroxylamine, which has antioxidant activity and can be converted to nitroxides by H2O2 or other oxidants, such as transition metals. The nitroxide moiety is considered essential for full antioxidant activity, whereas substitution at the four-position affects potency. One-electron redox cycling of six-member ring nitroxides such as TEMPOL is enhanced by their ability to undergo reversible “boat-and-chair” conformational change; this is not possible with five-member ring nitroxides, which may be responsible for their lesser biological activity (353). MitoTEMPO, which possesses O2•− and alkyl radical scavenging properties, is a combination of the pleiotropic intracellular antioxidant piperidine nitroxide TEMPO (2,2,6,6-tetramethylpiperidin-1-yloxy) and the TPP cation. A related molecule is MitoTEMPOL, in which the conjugate molecule is 4-hydroxy-TEMPO (TEMPOL) and, therefore, less hydrophobic. TEMPOL is an amphilite nitroxide that concentrates on hydrophobic microdomains of cell membranes.
Antioxidants targeted to mitochondria have been extensively used in isolated preparations of these organelles and cells, with toxicity being one of the main issues addressed. The massive accumulation of lipophilic cations within isolated mitochondria has been shown to disrupt mitochondrial membrane integrity and uncouple OXPHOS, which occurs as a consequence of the adsorption of cations to the matrix surface of the IMM (262). In accordance with this idea, the disruption of the mitochondrial function occurs at lower concentrations when more hydrophobic TPP cations are employed, and the degree of this interference shows a positive correlation with the quantity of the compound adsorbed to the IMM (262). The effects of MitoQ on mitochondria that occur as a result of non-specific interference are assessed using decylTPP as a control compound, which has a similar hydrophobicity to that of MitoQ, but lacks the ubiquinol moiety and therefore does not display antioxidant properties. Mitochondrial function is disrupted by MitoQ and decylTPP at similar concentrations, which implies that some of the bioenergetic and redox effects detected in cell culture systems and ascribed to mitochondria-targeted antioxidants are, in fact, produced by the linker group and are independent of the antioxidant functional group (262). Another study has reported impairment of the mitochondrial efflux of Ca2+ in cell culture systems exposed to TPP+, an effect attributed to direct inhibition of Na+ (or H+)/Ca2+ exchanger (167). MitoQ can also act as a pro-oxidant by generating O2•− in accordance with the capacity of all ubiquinols, including that derived from MitoQ, to deprotonate in water and form ubiquinolate, which triggers O2•− formation from O2 (130). This pro-oxidant activity is limited if the ubiquinol moiety is maintained in the lipid phase, and may also largely depend on the concentration employed (50). In this sense, MitoQ has been shown to induce O2•− production, redox cycling at mitochondrial complex I, and apoptosis in isolated mitochondria from bovine aortic endothelial cells (77). More recent data with the same cell type have shown that MitoQ alters mitochondrial respiration in a concentration-dependent manner (100–300 nM) and that this effect is not primarily due to a protonophoric action but rather to a pro-oxidant action of MitoQ (92). Importantly, the cationic component, methylTPP did not modify the bioenergetic profile of the cells in question. In light of all this evidence, the effect of TPP compounds is likely to be dependent on cell type, as the bioenergetics of cells show a wide range of responses depending on their number of mitochondria and regulation. Obviously, such deleterious effects would limit the amounts of TPP+-conjugated targeted antioxidants that can be used; in this sense, when comparing cells to preparations of isolated mitochondria, it is imperative to keep in mind that, as a result of the presence of the plasma membrane potential, targeted antioxidants accumulate within cells at a concentration that is 5- to 10-fold greater than that in the extracellular environment (262). For cultured mammalian cells, this concentration generally ranges from 0.1 to 0.5 μM, but can vary considerably due to its dependence on cell density, cell type, and incubation conditions. However, inhibition of OXPHOS in cell culture as a result of the use of TPP+-conjugated compounds is obtained only at concentrations of these agents that are 10–100 times greater than those generated through oral therapy in animal models and human subjects (271).
a. In vitro results
MitoQ has been employed in a large range of both mitochondrial and cell models (211) (Table 1), and abundant evidence proves it is effective against mitochondrial oxidative damage. For example, detailed studies of the interaction of MitoQ with mtROS have been performed in rotenone-treated fibroblasts (154). MitoQ did not decrease O2•− production when this process was analyzed by dihydroethidium oxidation, but it prevented lipid peroxidation when it was detected by the fluorescent marker C11-BODIPY. This finding is in agreement with the model of MitoQ action described by studies with isolated mitochondria in which the main antioxidant action of MitoQ was the prevention of lipid peroxidation. The protective action of MitoQ against oxidative stress has also been shown with regard to another target of ROS-induced damage: mtDNA. In a more recent study involving human dermal fibroblasts, Oyewole et al. demonstrated that MitoQ alleviated ultraviolet A- and H2O2-induced mtDNA damage. Interestingly, tiron, an unrelated compound with ROS-scavenging properties, was shown to be more efficient than MitoQ, despite it being a non-selective antioxidant (228). Tiron permeabilizes the mitochondrial membrane and localizes to mitochondria; however, it is not a targeted drug, and its action is not limited to mitochondria. The authors of the study in question speculated that the fact that tiron rendered greater protection than MitoQ in this model may have been a result of the dual action of tiron as a radical scavenger and a metal chelator.
In another study, mitochondria-targeted antioxidants were employed in β-cells such as RINm5F and HIT-T15 under conditions of glucotoxic and glucolipotoxic stress typical of type 2 diabetes (171). β-cells under oxidative stress conditions manifested increased levels of mitochondrial antioxidant enzymes (such as MnSOD), enhanced expression of mitochondrial ETC complex subunits and lipogenic enzymes (such as fatty acid synthase, ATP-binding cassette transporter A1, and acetyl-CoA carboxylase), induction of apoptosis, incremented intracellular lipid droplet accumulation, ER stress, mitochondrial membrane depolarization, presence of oxidative stress, sterol regulatory element binding protein 1c, and NF-κB expression, in parallel with a reduction in citrate synthase activity, ATP concentration, and insulin release. These manifestations were related with mitochondrial oxidative stress and were prevented by administration of the mitochondria-targeted antioxidants MitoQ and MitoTEMPOL, thereby improving insulin secretion and promoting the survival of these cells. The anti-inflammatory effect of MitoQ has been investigated in an endothelial cell model of sepsis in which it was shown to reduce oxidative stress and protect against mitochondrial damage, as indicated by a lower rate of ROS formation and maintenance of Δψm. These effects were accompanied by suppressed proinflammatory cytokine release from cells, while production of the anti-inflammatory cytokine IL-10 was enhanced (180).
A recent report by Mackenzie et al. (184) indicated a novel, mtROS-mediated activation of AMPK in the endothelium of patients with type 2 diabetes and CAD. Human saphenous vein endothelial cell (HSVEC) cultures obtained from these patients revealed an increase in AMPK activity and enhanced mitochondrial H2O2 production with regard to that in healthy volunteers. Short-term treatment with MitoQ10 of HSVEC reduced AMPK activation, but without modifying basal mitochondrial H2O2 production (184).
A potential therapeutic effect of mitochondria-targeted antioxidants has also been demonstrated in models of tolerance to nitroglycerine (GTN), in which the deleterious effects of GTN on mitochondrial O2 consumption, aldehyde dehydrogenase 2 activity and of ROS on the mitochondria were prevented by MitoQ (84, 96). Nevertheless, whether this constitutes the primary mechanism by which MitoQ is protective in all cell types and forms of oxidative stress remains to be explored. A large body of evidence demonstrates that antioxidants are capable of specific modulation of the cell survival/cell death pathways, and specifically those related to mitochondrial function, including apoptosis, mitophagy, mitoptosis, and necrosis. Such processes reveal the special attention that mitochondria-targeted antioxidants merit. Moreover, in HeLa cells and fibroblasts, MitoQ has been shown to efficiently inhibit mitochondrial fission induced by application of specific chemical inhibitors of the mitochondrial ETC (myxothiazol and piericidin), which endorses the potential of this mitochondria-targeted agent in other mitochondrial processes such as mitochondrial dynamics (240).
Regarding autophagy, 0.5 mM MitoTEMPOL has been shown to diminish both basal and induced (pharmacological induction with rapamycin or induction by nutrient deprivation) autophagy in murine C2C12 myoblasts, thus pinpointing the role of mtROS in muscle autophagy (256). Moreover, the study in question also reported that MitoTEMPOL had a greater ability than the untargeted version of TEMPOL to alleviate the increase in ROS (mitochondrial O2•−) associated with autophagy.
It is important to bear in mind that the protective effects of antioxidants depend on cell type, number of mitochondria in the cells, and the extent to which cells depend on OXPHOS. Moreover, caution need to be taken for in vitro experimental conditions that often do not reflect the physiological redox environment. In this sense, there is evidence of a lack of beneficial effects of Mito-compounds in certain models. For instance, in a study evaluating the effect of palmitate on cell viability and mitochondrial respiratory function of murine C2C12 myoblasts, neither MitoQ (0.5 μM) nor MitoTEMPOL (10 μM) was capable of preventing cell death, whereas only MitoTEMPOL blocked palmitate-induced mtDNA damage. Indeed, MitoQ increased H2O2 production, and both antioxidants had a marked negative effect on cellular respiration (231).
The usefulness of Mito-compounds as anticancer drugs merits detailed analysis. Recent findings have highlighted the complex role of antioxidants in survival and cell death signaling and have shown that, though protective for normal tissue, Mito-compounds may exert cytotoxic effects in cancer cells. MitoQ was shown to be cytotoxic, inducing both autophagy and apoptosis in human breast cancer cells, an effect not observed with the untargeted ubiquinone (259). The mechanism suggested for this action involved the pro-oxidant capacity of MitoQ. Importantly, MitoQ is much more cytotoxic for cancer cells than for normal cells. In this latter publication, the GI50 (growth inhibition) value for MitoQ in the breat cancer cell lines MDA-MB-231 and MCF-7 was 296 and 113 nM, respectively, whereas it was >10 μM in normal mammary epithelial cells. There may be several reasons for the cytotoxic specificity of Mito-compounds in cancer versus healthy cells. First, cancer cells have a greater Δψm than healthy cells, which enhances mitochondrial accumulation of these agents. Second, cancer cells have higher levels of ROS and may be more susceptible to further tilting of the redox balance.
b. In vivo results
Mitochondria-targeted antioxidants have also been employed in in vivo studies (Table 1). In the case of MitoQ, the role it plays in in vitro settings of mitochondrial oxidative stress has been shown to be beneficial in many conditions; moreover, its protective actions have also been observed in animal models of cardiometabolic diseases, including ischemia/reperfusion, diabetes, and sepsis, and also in patients (297). Notably, it has been demonstrated that MitoQ is uptaken rapidly from the blood into cells (246). To be of acceptable therapeutic relevance (among other necessary properties), mitochondria-targeted antioxidants must reach and accumulate inside the mitochondria within the cells of patients, ideally after oral administration (Fig. 7). Taking into account that TPP cations can easily cross phospholipid bilayers, they should pass efficiently from the digestive system to the bloodstream and then from the plasma to most tissues. It has been demonstrated that, when simple alkylTPP compounds are injected into mice intravenously, they undergo rapid clearance from the plasma and accumulate significantly in different organs, including the heart, skeletal muscle, brain, liver, and kidney (299). Importantly, TPP-derived compounds in the form of tritiated methylTPP, MitoE2, or MitoQ were made bioavailable orally to mice through their drinking water, after which they were uptaken into the plasma and delivered to mitochondria-rich tissues such as the heart, brain, liver, kidney, and muscle (299). MethylTPP has been shown to be cleared from all organs at a similar rate by a first-order process with a half-life of about 1.5 days (299). The study in question confirmed the distribution of orally administered alkylTPP compounds to all organs due to their facile permeation through biological membranes. The non-specific toxicity of alkylTPP cations found in mitochondria and cells also occurs in vivo, and this is probably the major limiting factor regarding the amounts of the compound that can be safely administered. In crude toxicity assessments (299), intravenously administered methylTPP and MitoE2 showed no toxicity at 300 nmol (∼4–6 mg/kg), but were toxic at 500 nmol (∼6–10 mg/kg). MitoQ was tolerated marginally better, with no toxicity at 750 nmol (∼20 mg/kg), but with evident toxicity at 1000 nmol (∼27 mg/kg). When mice were administered 500 μM TPMP, MitoE2, or MitoQ via their drinking water, no toxic effects were observed over the course of the experiment, which lasted 43, 14, and 14 days for TPMP, MitoE2, and MitoQ10, respectively (299). These similar levels of toxicity are consistent with the hypothesis that the deleterious effects of these compounds are largely attributable to non-specific disruption of the mitochondria due to the accumulation of large amounts of the lipophilic cation. To summarize, it is possible to administer alkyltTPP compounds to animals orally, as they are uptaken from the gut into the circulation with reasonable bioavailability. They are then rapidly cleared from the plasma as they reach target tissues, where they accumulate in the mitochondria of cells. Having confirmed the viability of the long-term exposure to mitochondria-targeted antioxidants, the following step is to assess whether the amount of accumulated compound is sufficient to act as an antioxidant in vivo. MitoQ has proved effective in several animal models of oxidative stress, such as various CVD settings, including endotoxin-induced cardiac dysfunction (313), age-related arterial endothelial dysfunction (99), cardiac ischemia/reperfusion (2), and doxorubicin-induced cardiac toxicity (40) and was shown to confer protection against an increase in blood pressure in rats that spontaneously develop hypertension (105). In one study, rats were administered 500 μM MitoQ for 2 weeks via their drinking water, after which their hearts were isolated and exposed to ischemia–reperfusion injury in a Langendorff perfusion system. In that study, MitoQ provided greater protection against tissue damage, loss of heart function, and mitochondrial dysfunction than the control compounds that employed methylTPP or short-chain quinol (2). MitoQ-mediated prevention of the lipid peroxidation in the IMM seems to be the most likely cause for the protection observed in this experimental model (2). In another work, MitoQ protected against oxidative damage caused by nitroglycerin in the rat aorta in a model of nitrate tolerance (84). MitoQ also reversed age-related endothelial dysfunction in mice. Its acute (ex vivo) administration or chronic supplementation through the animals' drinking water completely restored carotid artery endothelium-dependent dilation in older mice by improving •NO bioavailability. The improvements in endothelial function were associated with the normalization of age-related increases in total and mitochondria-derived arterial O2•− production and oxidative stress (99). MitoQ has also been reported to prevent endotoxin-induced reductions in cardiac mitochondrial and contractile function in rodents (313). Drug-induced cardiotoxicity has also been assessed; for example, in a rat model of cardiomyopathy induced by doxorubicin, MitoQ significantly reversed the effects of this antineoplasic drug, allowing the left ventricular function and several mitochondrial parameters, such as cytochrome c activity and expression, to be recovered (40). The cardioprotective effect of Mito-compounds toward doxorubicin has also been shown through administration of MitoTEMPOL to immune-competent, spontaneously hypertensive rats. The underlying mechanism involved an increase of the pro-survival autophagy marker LC3-II and a decrease of the apoptosis marker caspase-3 in the heart (70). In the study in question, doxorubicin-treated rats were implanted with the breast tumor cell line SST-2 and of note, MitoTEMPOL caused significant tumor reduction and appeared to increase the antitumor effect of doxorubicin (70), thus reproducing the anticancer properties of Mito-compounds observed in vitro. Similarly, MitoQ prevented mitochondrial impairment in a rat model of cocaine-induced cardiac dysfunction (335). In addition, administration of MitoQ10 to young, stroke-prone, spontaneously hypertensive rats was shown to improve endothelial function, reduce cardiac hypertrophy, and protect against the development of hypertension (105). In vivo treatment with the mitochondria-targeted antioxidant MitoTEMPO has been found to partially correct endothelial dysfunction and prevent vascular inflammation in Ang II-treated PGC-1α KO mice (156). Similarly, MitoTEMPO has proved to be effective in a mouse model (ACE8/8) of cardiac renin–angiotensin system activation with elevated cardiac ROS, a high rate of spontaneous ventricular tachycardia, and sudden cardiac death secondary to a reduction in connexin 43 level. 2-week treatment with MitoTEMPO reduced sudden cardiac death, decreased spontaneous ventricular premature beats and ventricular tachycardia inducibility, reduced elevated mtROS to control levels, and prevented structural damage to mitochondria. Of note, general antioxidants, including sepiapterin (the precursor of BH4), TEMPOL, apocynin (nicotinamide adenine dinucleotide phosphate oxidase inhibitor), and allopurinol (a xanthine oxidase inhibitor), did not prevent ventricular tachycardia and sudden cardiac death, which highlights the importance of mitochondrial targeting versus non-targeting therapies (301). In addition, it has been demonstrated that apocynin does not inhibit all Nox isoforms (e.g., Nox4, which is inducible and may be involved in the pathology of chronic cardiac disease, among others) and must be metabolised intracellularly to be effective, and all cell types do not contain the relevant mechanisms. A very recent study by McLachlan et al. has assessed the combined effect of MitoQ10 and a low dose of the angiotensin receptor blocker losartan on stroke-prone, spontaneously hypertensive rats. The authors report an additive therapeutic benefit, with a significant attenuation of left ventricular hypertrophy and hypertension. In addition, MitoQ10 was shown to mediate a direct antihypertrophic effect on rat cardiomyocytes in vitro. These results endorse MitoQ10's potential as a novel therapeutic intervention in conjunction with current antihypertensive drugs (194).
MitoQ has also been shown to be beneficial in a rat model of sepsis, a pathological process characterized by a systemic dysregulated inflammatory response and oxidative stress, often leading to organ dysfunction, organ failure, and death. In lipopolysaccharide-peptidoglycan treated rats, treatment with MitoQ resulted in lower levels of biochemical markers of acute liver and renal dysfunction and an increase in Δψm in most organs (179). MitoE, another mitochondria-targeted antioxidant, has also demonstrated its utility in a rat model of pneumonia-related sepsis (369). The study in question confirmed the in vivo distribution of MitoE in the heart and liver at 24 h after its oral administration, and provided evidence that MitoE improves mitochondria-specific antioxidant defense and mitochondrial function (respiration and mitochondrial membrane integrity) in the heart, provides cardiac protection from sepsis, and suppresses sepsis-induced inflammation. Importantly, untargeted vitamin E also exhibited anti-inflammatory and protective effects, but to a lesser extent than the mitochondria-targeted molecule.
There is also in vivo evidence of the beneficial effects of MitoQ in metabolic pathologies, including diabetes and obesity. Chacko et al. demonstrated that MitoQ improved glomerular and tubular function in a Ins2(+/−) (AkitaJ) mouse model of type 1 diabetes when administered orally over a 12-week period (38). Creatinine levels were not significantly changed by MitoQ, but it reduced urinary albumin to levels similar to those exhibited by non-diabetic animals. In addition, it prevented the increase in the nuclear accumulation of the pro-fibrotic transcription factors β-catenin and phospho-Smad2/3. The same authors have also reported the efficacy of MitoQ in decreasing ethanol-induced micro- and macrohepatosteatosis in rats (39). A recently published study has demonstrated a protective role of mitochondria-targeted antioxidants in obesity-related comorbidities. Zucker obese fatty (ZOF) rats displayed higher levels of ROS in the smooth muscle cells and the aortic endothelium as well as increased UCP-2 and antioxidant enzyme activity in comparison with Zucker control rats. In ZOF rats, MitoQ reduced lipid peroxides to levels similar to those present in lean rats and also improved their metabolic profile, thereby restoring coronary collateral growth in response to ischemia to control levels (252). MitoQ has also been shown to render protective effects in animal models of metabolic syndrome and atherosclerosis (fat-fed ApoE−/−and ATM+/−/ApoE−/−mice, which are haploinsufficient for ataxia telangiectasia-mutated protein kinase, ATM). In one study, MitoQ prevented hypercholesterolemia (an increase in hypertriglyceridemia and adiposity related to metabolic syndrome) when administered orally for 14 weeks. It also reduced hepatic steatosis, hyperglycemia, and lipid and DNA oxidative damage (8-oxo-G) in different organs. Furthermore, a lower macrophage content together with diminished cell proliferation was observed within the plaques of fat-fed ATM+/−/ApoE−/−and ATM+/+/ApoE−/−mice after administration of MitoQ (199).
It is noteworthy that, in recent years, MitoQ has also been shown to be efficient in central nervous system (CNS) pathologies. In the case of AD, Ma et al. have reported that amyloid β-induced impairments in mice with hippocampal synaptic plasticity were reversed by decreasing mitochondrial O2·− through administration of MitoQ (182). MitoQ was shown to significantly reduce neuronal apoptosis and alleviate oxidative stress in rats exposed to Dichlorvos, a synthetic insecticide related to organophosphate pesticides known to induce neurodegeneration with clear signs of mitochondrial dysfunction, manifested as inhibition of mitochondrial complex I, cytochrome oxidase generation of ROS, and apoptotic cell death (342).
In conclusion, extensive evidence suggests that Mito-compounds are a promising approach to therapeutic intervention in numerous aspects of human pathophysiology.
c. MitoQ as a pharmacological agent
The design of MitoQ as a pharmacological agent has differed somewhat from that of most other drugs. In medicinal chemistry, compounds are typically developed to interact with a specific target in the biological system, such as a receptor-binding site. For this purpose, performing a preliminary screening is often necessary to confirm solubility, bioavailability, and capacity of a drug to cross phospholipid bilayers (173). Mitochondria-targeted antioxidants based on TPP lipophilic cations have several unusual properties. They are targeted to an organelle to modify a general, rather than a specific, process (namely oxidative damage), and have the peculiar pharmacological feature of being both relatively water soluble and membrane permeant. MitoQ, for example, is readily bioavailable and passes easily through biological membranes, even though its molar mass is relatively large for a pharmaceutical agent and has a high octanol—phosphate buffered saline partition coefficient. Therefore, if lipophilic cations such as MitoQ fulfil their potential as effective drugs, a new and different approach to the traditional view of medicinal chemistry and drug discovery would be endorsed. MitoQ is now being developed as a pharmaceutical agent by Antipodean Pharmaceuticals, Inc. In the search for a commercially satisfactory stable formulation, the creation of the compound has been based on the methane sulfonate counter anion and its decomposition is inhibited by complexation with β-cyclodextrin (50). This preparation is easily manufactured in tablet form and has passed conventional animal toxicity tests with no observable adverse effects at 10.6 mg/kg. In human studies, MitoQ has shown a positive pharmacokinetic behavior when administered orally at 80 mg (1 mg/kg), resulting in a plasma Cmax of 33.15 ng/ml and Tmax at ∼1 h, and 10% oral bioavailability. This formulation has good pharmaceutical characteristics and has been tested in two phase II clinical trials. In the first, which evaluated MitoQ10's ability to slow down the progression of PD, no difference was observed between placebo and experimental groups. In hepatitis C virus-infected patients who also had chronic liver hepatitis, MitoQ was found to decrease serum alanine transaminase, but had no effect on viral load (296).
d. Mito-compounds and nitrosative stress
•NO and RNS play a crucial role in oxidative stress-related disorders. A wide variety of •NO donors and S-nitrosating agents have been shown to protect the ischemic myocardium from infarction. Recently, mitochondria-selective S-nitrosating agents have been developed; MitoSNO, the best known, is the product of a covalent linking of an S-nitrosothiol to the lipophilic TPP cation (Fig. 10), which enables extensive and rapid accumulation within mitochondria (driven by Δψm), where it generates •NO and S-nitrosate thiol proteins. MitoSNO has been shown to provide protection in an in vivo rodent model of ischemia reperfusion, in which it reversed S-nitrosation of complex I by slowing down the reactivation of mitochondria during the crucial first minutes of reperfusion of ischemic tissue, thereby decreasing ROS production, oxidative damage, and tissue necrosis (48).
2. Cationic plastoquinone derivatives
Another group of synthetic cationic mitochondria-targeted antioxidants are SkQs-cationic plastoquinone derivatives containing positively charged phosphonium or rhodamine moieties connected to plastoquinone by decane or pentane linkers (Fig. 11). The prototypical example of these molecules is the plastoquinonyl decyltriphenylphosphonium (SkQ1). When SkQ1 electrophoretically enters the cell (assuming a plasma membrane potential of −60 mV) as a monovalent penetrating cation, it accumulates in the cytosol and the intermembrane space of mitochondria by a factor of 10, and then by factor 1000 when it enters, again electrophoretically, the mitochondrial matrix, which is 180 mV more negative than the intermembrane space. The membrane/water distribution coefficient, which is about 104 for SkQ1, also has a bearing. Hence, the total magnification of SkQ1 concentration in the inner leaflet of the IMM with regard to the extracellular space is expected to be 108. The relative accumulation may even be higher, as the SkQ1 cation forms a complex with cardiolipin anion, which seems to be the prime target of the antioxidant activity of SkQs (11). In a similar fashion to MitoQ, SkQs are rechargeable antioxidants, as their inactive oxidized forms are reduced (i.e., re-activated), in the case of SkQ by complex III of ETC (11). The efficacy of SkQs has been demonstrated in many in vitro and in vivo models (Table 1).
FIG. 11.
Chemical structures of mitochondria-targeted antioxidants based on lipophilic cations: Sk-compounds.
a. In vitro results
SkQ1 has been shown to be protective in in vitro models of hemolysis; it protected erythrocyte membrane lipids from peroxidation induced by the lipophilic free radical initiator 2,2-azobis-(2,4-dimethylvaleronitrile) and avoided hemolysis induced by the water-soluble free radical initiator 2,2-azobis-(2-methylpropionamidine)-dihydrochloride, but it had no antioxidant effect when the process was assessed by lipid peroxidation (226). The concentrations at which this occurred were approximately two to three orders of magnitude higher than those that have been proved active in a system of isolated mitochondria, which reflects the low ability of this compound to accumulate in erythrocytes due the low potential across the erythrocyte membrane (−10±3 mV) and emphasizes SkQ1 accumulation in mitochondria as a result of the electrophoretic transport. In addition, higher concentrations of SkQ1 displayed hemolytic abilities, likely due to its detergent-like effects that can cause disruption of the lipid membranes and not due to pro-oxidant activity, as Trolox was not capable of reversing it.
b. In vivo results and clinical studies
Several pathophysiological settings have been explored to test the utility of SkQ compounds in vivo. In a rat model, SkQR1 rescued kidney function from the deleterious effects of myoglobinuria (rhabdomyolysis) or ischemia/reperfusion (241), and the report suggested that SkQR1 exerted protective effects not only through direct scavenging of ROS but also by providing the kidney with elements of ischemic tolerance signaling mechanisms. Similarly, SkQR1 was shown to be beneficial in a rat model of acute pyelonephritis, where it inhibited leukocyte infiltration in the kidney and the spreading of the inflammatory process (242). SkQR1 recovered normal ROS levels in the leukocytes and expression of Bcl-2 in the kidney of pyelonephritic rats. Remarkably, treatment with SkQR1 during the first 2 days after infection resulted in significantly greater animal survival; therefore, the antioxidant protected the organisms from the development of sepsis. (SkQR1). Another study reported the nephroprotective action of SkQR1in two animal models of kidney failure (ischemia, rhabdomyolysis) and in an in vitro setting of pyelonephritis (243), as well as a neuroprotective role in a rat model of focal brain ischemia (243). Importantly, C12R, a conjugate of rhodamine with a linker chain of 12 carbon atoms, did not exhibit neuroprotective effects in this model of stroke, thereby showing that this biological activity could not be attributed to the part of the molecule not carrying the quinone residue. Moreover, SkQ1 has been reported to prolong the lifespan of many organisms, including mammals (292). It has proved to be particularly effective in animals suffering from eye diseases, retarding the development of (and in certain cases even reversing) symptoms of cataract, glaucoma, macular degeneration, uveitis, and dry eye syndrome (292). In the case of dry eye syndrome, clinical trials have shown that 3-week therapy with SkQ1 eliminates symptoms in 60% of patients (292). Indeed, SkQ1-containing eye drops (in the form of “Visomitin”) are commercially available in Russia since 2012, and clinical trials of SkQ1 as a medicine against age-related cataract and glaucoma are close to completion (292). Several recent studies have provided evidence of SkQ1 as a promising candidate for the treatment or prophylaxis of age-related and neurodegenerative disorders, particularly AD. The beneficial prophylactic effect of long-term treatment with SkQ1 has been shown in OXYS rats, senescence-accelerated rats that also develop AD-like pathology. SkQ1 reduced age-related alterations of behavior and spatial memory deficit and slowed down pathological accumulation of AβPP, Aβ and hyperphosphorylation of tau-protein (305). Another study revealed that an in vivo and in vitro injection of SkQ1 can compensate Aβ-induced oxidative damage of long-term synaptic plasticity (LTP) in the rat hippocampus. The neuroprotective activity of the analog of SkQ1 lacking plastoquinone (C12TPP) was found to be much lower than SkQ1, thus identifying the plastoquinone part of the SkQ1 molecule as being responsible for the LTP rescue (141).
A significant feature of SkQs is that they can be administered effectively in extremely low doses in in vivo treatments, thus significantly diminishing the probability of adverse side effects. However, similar to the majority of antioxidants, when their concentration is increased, which can represent overdose, SkQs become pro-oxidants. This has been shown in isolated yeast mitochondria, where lipophilic cations including SkQ1, SkQ3, and MitoQ acted as antioxidants, uncouplers, pro-oxidants, and detergents depending on the concentrations used (311). Fortunately, the window between the anti- and pro-oxidant activities of plastoquinone and its derivatives is large (30–1000 times), and is likely to be even wider than that for MitoQ.
c. Other derivatives
A similar strategy for mitochondria-targeted antioxidant therapy also involves the use of a series of novel compounds composed of natural constituents, such as conjugates of the plant alkaloids berberine and palmatine with the antioxidant plastoquinone (SkQBerb or SkQB and SkQPalm or SkQP). The antioxidant capacity of these molecules has been demonstrated in vitro using isolated mitochondria and cultured human cells (45), but their efficacy in vivo requires further assessment. Similarly, the penetrating cations decyltriphenylphosphonium and decylrhodamine 19 have been combined with thymoquinone, a plant antioxidant responsible for the pleiotropic favourable pharmacological effects of black cumin, resulting in the derivatives SkQT1 and SkQTR1, respectively (290). These molecules selectively accumulate within mitochondria and are recharged by reduction at ETC complex III in a similar fashion to SkQs. In vitro experiments have shown that these cationic derivatives of thymoquine display a high antioxidant activity at low concentrations. This is of relevance, as thymoquine itself is beneficial as a plant medicine in a large number of human pathologies, including several types of cancer, inflammation processes such as neuropathies, nephropathies, asthma, and sepsis. Moreover, its effect on cancer merits special mention, particularly SkQTR1, which is a better substrate for multidrug resistance (MDR) pumps than other SkQs. In normal cells, the amount of MDR pumps is negligible, which allows much higher concentrations of SkQTR1 in these cells than in tumor cells, an effect that may be exploited to preserve non-tumor tissue during anticancer treatments (89). In light of all these promising findings, SkQ-based molecules could constitute a new generation of mitochondrial medicines.
3. Peptide-based targeting
Szeto-Schiller (or SS)-peptides (378) and mitochondria-penetrating peptides (MPPs) (370) are peptide-based targeting agents for delivering antioxidants to mitochondria (Fig. 8). SS-peptides were developed by Szeto and Schiller and constitute a series of 4 small, cell-permeable antioxidant compounds (SS-19 H-Tyr-d-Arg-Phe-Lys-NH2, SS-02 H-Dmt-d-Arg-Phe-Lys-NH2, SS-31 H-d-Arg-Dmt-Lys-Phe-NH2, and SS-20 H-Phe-d-Arg-Phe-Lys-NH2) with three positive charges in homeostatic pH conditions (Fig. 12A). SS-peptides display a unique aromatic-cationic sequence motif, which alternates between basic and aromatic residues, enabling their efficient cell uptake, as it occurs in a non-saturable manner, independently of the energy status and without the need for peptide transporters. Cell studies performed in vitro have shown the rapid uptake of these molecules through the cellular membrane of a wide variety of cell types, including neurons, renal, epithelial, endothelial cells and human embryonic kidney cells. Within cells, SS-peptides accumulate 1000–5000-fold in mitochondria, where they localize at/bind to IMM (317, 377) through a mechanism that is not entirely clear. It is of particular importance that the specific mitochondrial uptake of these compounds does not take place as a result of the presence of ΔΨm (172, 315, 317). This is clearly an advantage, as mitochondrial membrane polarization is not altered and is not a self-limiting process. Therefore, SS-peptides have adequate characteristics that make them ideal for the treatment of many diseases related to mitochondrial dysfunction and oxidative stress, including CVD and cancer. They are small, readily soluble in water and easily synthetized, and are even resistant to peptidase degradation. The presence of a d-amino acid in the first and second positions renders them resistant to aminopeptidase activity, and, due to their structure, they can reach the mitochondria independently of tissue and location (373). In this sense, SS-02 has been shown to readily penetrate a monolayer of intestinal epithelial cells in both basolateral apical and apical-basolateral directions (251). The same compound has also been shown to cross BBB, a crucial aspect of any candidate drug in the development of strategies for neuroprotection.
FIG. 12.
Chemical structures of peptide-based mitochondria-targeted antioxidants. (A) SS peptides. (B) XJP peptides.
SS-peptides can scavenge H2O2 and ONOO− and inhibit lipid peroxidation, antioxidant actions attributed to a tyrosine or dimethyltyrosine residue in their structure, the latter of the two being more effective with regard to ROS scavenging. This moiety is believed to scavenge •OH and ONOO−, and possibly HOCl and peroxyl radicals, but a direct reaction with O2•− or H2O2 seems unlikely. The specific location of these residues does not appear to be significant, as SS-31 is as effective as SS-02 in scavenging H2O2 and inhibiting LDL oxidation (261). SS-peptides have been reported to be beneficial against the conditions of oxidative stress both in cellular models of disease and in isolated mitochondria, with SS-31 proving to be the most efficient (187, 352, 378).
a. In vitro studies
The protective effects of these mitochondria-targeted therapies have been demonstrated in major comorbidities associated with diabetes, such as some vasculopathies, namely nephropathy and retinopathy (Table 1). Li et al. described a protective effect of peptide SS-31 on hyperglycemia-induced damage in human retinal endothelial cells (HRECs) (169). They reported that SS-31 decreased ROS production (especially in mitochondria), impaired the translocation of cytochrome c from the mitochondrion to the cytosol, controlled Δψm, decreased the expression of caspase-3, and enhanced the expression of TRX-2 in HRECs. In addition, cultured C2C12 myoblasts under basal condition or subjected to triggers of autophagy (pharmacological induction with rapamycin or induction by nutrient deprivation) display diminished autophagic flux and decreased rate of proteolysis when exposed to 1 μM SS-31 (256).
b. In vivo studies
SS-31 has been found to protect cells from mitochondrial toxicity in rodent models of human diseases, including ischemic brain injury (47), myocardial infarction (46), and ALS (237). Other animal studies have confirmed the efficacy of SS peptides aimed at PD (366), AD (187), obesity (11), and unilateral urethral obstruction (202) and those employed for cell and organ transplantation (316), although their exact mechanism of action and whether it unequivocally includes an antioxidant activity is not always clear. This antioxidant tetrapeptide has also proved to be beneficial in a mouse model of oxaliplatin-induced neuropathy (328). In a recent study, concomitant SS-31 administration (chronic treatment) with repeated oxaliplatin administration attenuated both cold and mechanical hypersensitivity, whereas acute SS-31 administration after symptoms manifested themselves only reversed cold hypersensitivity (328). The authors concluded that SS-31 has the potential to prevent acute and chronic neuropathy but is helpful only in the treatment of acute neuropathy. SS-31 utility has also been shown in a rat model of microvascular rarefaction or loss of microvascular density, a phenomenon implicated in the progression from acute ischemic kidney injury to chronic kidney disease. Microvascular dropout is thought to begin with ischemic damage to endothelial mitochondria due to cardiolipin peroxidation, resulting in loss of cristae and the failure to regenerate ATP on reperfusion. In a recent study, Liu et al. showed that SS-31 prevented swelling of mitochondria and protected mitochondrial cristae in both endothelial and epithelial cells. This was associated with a significantly reduced loss of peritubular capillaries and cortical arterioles, interstitial inflammation, and fibrosis 4 weeks after ischemia (175). The mechanism proposed for this action of SS-31 is its selective binding to cardiolipin and inhibition of cardiolipin peroxidation by cytochrome c peroxidase activity. Actually, a recent report has explored in detail the interaction of SS-31 and cardiolipin (23). Using as a model liposomes and bicelles containing phosphatidylcholine alone or with cardiolipin, Birk et al. (23) proved that SS-31 selectively targeted cardiolipin and that this interaction occured only with liposomes and bicelles containing cardiolipin in a ratio of 1:1. Moreover, SS-31 modulated the interaction of cardiolipin with cytochrome c, inhibiting cytochrome c/cardiolipin complex peroxidase activity while protecting the ability of cyttochrome c to serve as an electron carrier. In fresh mitochondria, SS-31 increased state 3 respiration and ATP generation efficiency. Another mechanism responsible for the beneficial effects of SS-31 in vascular diseases has been related to its ability to reduce the expression of CD36, a multifunctional class B scavenger receptor that mediates free radical production and tissue injury in cerebral ischemia. In the work in question, C57BL/6 mice were subjected to transient middle cerebral artery occlusion and treatment with SS-31 immediately after reperfusion significantly attenuated ischemia-induced GSH depletion in the cortex and reduced infarct size. Importantly, the protective effect of SS-31 was absent in CD36 KO mice, indicating that SS-31 was acting through inhibition of CD36 (47).
In a mouse model of pressure overload (by transverse aortic constriction)-induced heart failure, SS-31 ameliorated cardiac malfunction and the proteomic remodeling associated with it (64). SS-20 was also tested, and its protective effect was found to be more modest and possibly related to the fact that SS-20, unlike SS-31, is not a direct ROS scavenger. Importantly, SS-31 administration largely prevented mitochondrial oxidative damage and the process of autophagy in the heart induced by chronic pressure overload. Several studies using neurons treated with Aβ25–35 peptide and primary neurons from Tg2576 mice (AβPP transgenic) have highlighted the potential of SS-31 for treatment of AD (261). Primary neurons from AβPP mice present decreased anterograde mitochondrial movement, increased mitochondrial fission and decreased fusion, abnormal mitochondrial and synaptic proteins, and defective mitochondrial function when compared with wild-type neurons. SS-31 protects neurons from Aβ toxicity by reducing Aβ-induced mitochondrial toxicity, increasing axonal transport of mitochondria and enhancing synaptic viability. It also decreased levels of mitochondrial fission proteins (Drp1, Fis1) and matrix protein CypD in neurons affected by AD. Moreover, it enhanced the number of healthy and intact mitochondria and increased synaptic outgrowth and neuronal branching. These are very promising findings that encourage the evaluation of SS-31 in clinical trials with AD patients.
c. Mitochondria-penetrating peptides
MPPs consist of between four and eight alternating positively charged and hydrophobic partly unnatural aminoacids. They are believed to be a promising targeting approach for the mitochondrial delivery of small molecules (biotin and trolox have already been tested), though it is yet to be determined whether MPPs are effective in the transport and targeting of larger cargos such as DNA or nano-carriers (125, 371). In terms of the accumulation of MPPs in the mitochondrial matrix, specific chemical properties, such as electric charge and hydrophobicity, are clearly considered relevant (371), although the exact mechanisms that enable MPP transport through the phospholipid bilayer, including the role of ΔΨm and other mitochondrial factors in this process, are yet to be determined.
d. XJB peptides
Novel mitochondria-targeted molecules also include XJB peptides, a series of hemigramicidin-TEMPO compounds (Fig. 12B). These molecules are composed of 4-NH2-TEMPO, a stable nitroxide radical that exhibits antioxidant properties in vitro, conjugated to a pentapeptide fragment from gramicidin S (Leu-d-Phe-Pro-Val-Orn), a natural membrane-active cyclopeptide antibiotic (93) that not only localizes to bacterial cell membranes but is also associated with mitochondrial membranes, probably as a result of its high affinity for the cardiolipin, abundant phospholipid in IMM. The most studied of all the XJB peptides is XJB-5-131, an electron- and ROS scavenger that can be targeted successfully to the mitochondrion and has been shown to inhibit actinomycin D-induced O2•− generation, cardiolipin peroxidation, and apoptosis in mouse embryonic cells (134, 354). Recently, XJB peptides have also rendered protection against radiation damage of cells in culture (133). In vivo, XJB-5-131 has proved its benefit in acute tissue ischemia, such as that detected in rat enterocytes exposed to lethal hemorrhagic shock (183).
4. Nanotechnology
Nano-preparations, or nano-particles (NP), are nano-sized, uniformly dispersed particles composed of biodegradable and biocompatible materials such as polymers, self-assembled lipids, or inorganic (metallic and magnetic) materials that are capable of carrying molecules with pharmacological activities such as peptides, proteins, nucleic acids, or small-molecular-weight drugs and prodrugs. There are several reasons that drug delivery systems based on NP are advantageous when compared with conventional drugs: (i) increased drug concentration at a specific site through passive and active targeting, thus reducing concentration-dependent side effects; (ii) better pharmacokinetics and pharmacodynamics properties; and (iii) improved cellular internalization and organelle-specific delivery. Recently, great interest has been focused on the development of specific delivery systems based on nano-materials designed to carry antioxidant agents. Selective targeting has been proposed in different strategies of mitochondrial medicine, including liposome-based vehicles (described in detail in section “Liposomal carriers”) and polymer-based, noble metal oxide-based, or carbon-based nano-antioxidants (107).
a. Colloidal dequalinium vesicles
One of the most extensively studied compounds among mitochondriotropic nano-carriers is the so called dequalinium-based liposome-like vesicle, or DQAsome (107, 361, 374). The antimicrobial and antineoplastic agent dequalinium (1,1′-(1,10-decamethylene-bis-[aminoquinaldinium])-chloride) is a dicationic amphiphilic molecule; it possesses two symmetrical cationic charge centers separated by a hydrophobic carbon chain (Fig. 8). DQAsome is generated through the self-assembly of dequalinium chloride into vesicle-like aggregates during sonication in aqueous suspension. DQAsomes penetrate cells through the process of endocytosis and, once inside cells, escape endosomal compartments through destabilization of endosomal membranes. It has been postulated that these delivery particles are attracted to mitochondria by electrostatic interactions, after which they fuse with the OMM. Several studies have demonstrated that DQAsomes can render efficient and selective delivery of nucleic acids to mitochondria (71, 349, 374), which opens an avenue for mitochondria-targeted gene therapy. Apart from nucleic acid delivery, DQAsomes have also been investigated for mitochondrial targeting of drugs (72, 333). However, this approach for delivery of either drugs or DNA into mitochondria needs further analysis, as liposomes designed from dequalinium analogues may have cytotoxic effects (333). Very recently, the mitochondrial delivery of DQAsome loaded with the antioxidant curcumin has been reported in Caco-2 cells (380). The same study did not detect any significant cytotoxicity in human epithelial A549 cells exposed for 72 h to DQAsomes at concentrations below 3 μM and postulated that curcumin-loaded DQAsomes may represent a promising inhalation formulation as a novel approach to the treatment of acute lung injury.
b. Liposomal carriers
Liposomal carriers (liposomes) are nano-carrier delivery systems that are capable of carrying hydrosoluble drugs in their core or lipid-soluble compounds in their outer membrane layers. An advantage of this strategy compared with chemically modified drugs is that active molecules are encapsulated without modifying their molecular structure, which preserves the drug's pharmacodynamics profile. When employed in antioxidant therapy, lisposomes are constituted by phosphatidylcholine, phosphatidylglycerol, and cholesterol, and further include molecular antioxidants, antioxidant enzymes, or a combination of various agents with antioxidant activities (308). It appears that antioxidants such as quercetin, the liposomally encapsulated NAC (4), and Siliphos (a complex formed by silybin and phospholipids) have therapeutic effects in a model of sepsis. Siliphos has been shown to be hepatoprotective in a rat model of steatosis (73) and to ameliorate liver enzyme levels in patients with non-alcoholic fatty liver disease (177) by enhancing mitochondrial function and through an insulin-sensitizing action.
In recent years, a Japanese group developed MITO-Porter (Fig. 8), a liposome-based nano-carrier that delivers cargo to mitochondria through a membrane fusion mechanism based on the multifunctional envelope-type nano-device, which consists of a condensed plasmidic DNA core and a lipid envelope that mimics envelope-type viruses (152). This mechanism seems to be able to transport proteins, functional nucleic acids, and small bioactive molecules (362). These delivery systems are useful in that they can transport encapsulated molecules of varying physicochemical characteristics or size. Mitochondrial delivery using MITO-Porter takes place over three steps: (i) delivery of the carrier from the extracellular space to the cytosol; (ii) intracellular trafficking of the carrier, including mitochondrial targeting; and (iii) mitochondrial delivery via membrane fusion (Fig. 13). This system of delivery allows large cargos to be delivered to mitochondria, provided they can be encapsulated in the MITO-Porter. Recently, the same group created a conjugate nano-carrier that targets mitochondria; it contains a mitochondria-targeting signal peptide and MITO-Porter to ensure selectivity for mitochondrial delivery (363). A dual function MITO-Porter (DF-MITO-Porter) that integrates the octaarginine (R8)-modified liposome for cytoplasmic delivery has also been created (361). High-density R8-modified liposomes are internalized primarily via macropinocytosis and are efficiently delivered to the cytosol in a similar fashion to an adenoviral vector (145). This exploits the characteristics of R8, a synthetic peptide that mimics the trans-activating transcriptional activator derived from the human immunodeficiency virus. DF-MITO-Porter includes a complexed particle of cargo coated with two mitochondria-fusogenic inner membranes and two endosome-fusogenic outer membranes. Modification of the outer envelope surface with a high density of R8 enables efficient internalization of the carrier into cells (first step). Once inside the cell, the carrier escapes from the endosome into the cytosol via membrane fusion, a process mediated by the outer endosome-fusogenic lipid membranes (second step). Indeed, a major limiting factor of nano-carrier systems for intracellular delivery is their escape from endosomes after endocytosis; only a small fraction of the endosome degrades spontaneously, which severely limits the possibility of the endocytosed material to reach the cytosol and, subsequently, the mitochondria. To overcome this limitation, nano-carriers are chemically designed to provoke the rupture of the endosome and to facilitate their release into the cytosol. After this step of intracellular trafficking, a third step consists of the binding of the carrier to mitochondria via R8, which is followed by fusion with the mitochondrial membrane (fourth step). This nano-carrier system, which can accomplish both efficient cytoplasmic delivery and mitochondrial macromolecule targeting, could open up new avenues of mitochondrial disease therapies. The efficacy of DF-MITO-Porter for the delivery of DNase I to the mitochondrial matrix has been demonstrated (361). The report in question described the process of construction of this particle, for which it was first necessary to determine the optimal conditions for complexation between DNase I protein and stearyl R8 through a process of three steps: (i) construction of the NP to contain an adequate amount of DNase I; (ii) coating of the NP obtained in step 1 with a mitochondria-fusogenic envelope; and (iii) further coating of the endosome-fusogenic envelope in a step-wise fashion. When DF-MITO-Porter was tested for cytotoxicity in HeLa cells, it was found that mitochondrial membrane fusion activity did not universally correlate with cytotoxicity (361). Rather, cytotoxicity was related to the composition of the inner envelope; among several lipid components tested, including sphingomyelin, cholesterol, cholesteryl hemisuccinate, cardiolipin, phosphatidic acid, phosphatidyl glycerol, phosphatidyl inositol, and phosphatidyl serine, sphingomyelin showed the best performance in terms of cell viability.
FIG. 13.
Stepwise schematic representation of the mitochondrial delivery of MITO-Porter encapsulated drugs. MITO-Porter enters cells via macropinocytosis, and its cytosolic delivery is enabled through the disruption of the macropinosome. Then, MITO-Porter is translocated to mitochondria via an electrostatic interaction of the MITO-Porter membrane component R8 with the mitochondrial membrane, and the liposomal cargo is delivered to mitochondria via mitochondrial membrane fusion. The lipid composition of MITO-Porter promotes both its fusion with the mitochondrial membrane and the release of its cargo into the intra-mitochondrial compartment.
Mixed complex molecules containing both liposomes and lipophilic cations have also been designed. Effective delivery into mitochondria has been achieved with antitumor drug-loaded liposomes surface-functionalized with TPP (24, 26).
5. Mild mitochondrial uncoupling
Modulation of mitochondrial function to diminish the production of ROS by this organelle is another possibility. It is known that high mtROS generation is positively correlated with high Δψm. Mitochondrial uncoupling is a physiological process by which proton leak across the IMM allows protons to bypass complex V (ATP synthase), thereby disabling ATP synthesis as a result of the ETC function. In the mammalian cell, this process is regulated by the activity of the specialized proteins located at the IMM known as uncoupling proteins (UCP). Mitochondrial uncoupling can also be induced chemically by addition of protonophores such as 2,4-dinitrophenol (DNP). There is evidence that dissipation of Δψm by means of mild uncoupling can significantly reduce mtROS production and therefore protect macromolecules, such as nucleic acids, lipid, and proteins, from oxidative damage. This is the basis of the “uncoupling to survive theory,” which associates mild mitochondrial uncoupling with the prolonging of the lifespan, an effect already shown in mice (303) and Caenorhabditis elegans (166). Therefore, mild mitochondrial uncoupling by activation of the physiological regulators of the uncoupling process or by addition of chemical uncouplers may represent a plausible strategy for selective intervention in mtROS generation and function. There is abundant evidence that genetic modulation of innate UCP activity may be a promising avenue for treatment; for example, overexpression of hUCP2 was found to partially rescue the phenotype of PD in mice (10, 53). However, findings with UCP transgenics are contradictory in general and difficult to extrapolate to human therapeutics. With this in mind, direct mitochondrial uncoupling using chemical uncouplers currently seems a more rational approach, and compounds already studied include DNP, FCCP, and carbonyl cyanide m-chlorophenyl hydrazone (CCCP). Leaving to one side the discrepancies in the reported data, these compounds have been shown to prolong lifespan in some animal models (107). However, we need to bear in mind that although DNP, CCCP, and FCCP may have foreseeable clinical applications, their use is largely limited by the narrow therapeutic range they exhibit. For instance, despite being banned as a legal weight loss drug, DNP is still sold for this purpose and has produced more than 60 recorded deaths, of which 12 have occurred over the past decade. Classic symptoms include tachycardia, tachypnoea, and hyperthermia, culminating with deadly cardiovascular collapse and cardiac arrest (109). In this sense, a safer profile is displayed by the uncoupler butylated hydroxytoluene (BHT), which has been reported to partially uncouple isolated rat mitochondria and rat thymocytes in vitro, even at very low concentrations (2×10−3 nM) (179). Although BHT showed antioxidant properties in vitro, mice treated with this compound did not have uncoupled mitochondria and did not present a significant decrease in ROS production or oxidative damage of proteins, which calls into question the assumption that in vitro results will be observed in in vivo settings (114). With the aim of improving mitochondrial selectivity and, thus, therapeutic safety, conventional chemical uncouplers such as BHT and DNP have been conjugated with the TPP cation to generate Mito-BHT (179) and Mito-DNP (20) (Fig. 14). MitoBHT was shown to be effective in terms of mitochondrial uncoupling in isolated rat mitochondria and cultured rat thymocytes, an effect that was believed to be due to enhancement of the proton leak through the adenine nucleotide translocator (179). In contrast, MitoDNP was ineffective in uncoupling isolated rat mitochondria, probably as a result of the absence of efflux of the deprotonated MitoDNP from the mitochondrial matrix back to the mitochondrial intermembrane space (25). Importantly, the mitochondria-targeted TPP-conjugated plastoquinone SkQ1, previously described in section “Cationic plastoquinone derivatives,” not only displays antioxidant properties but can also act as an uncoupler by facilitating fatty acid cycling in IMM, thus dissipating Δψm, an effect observed in both isolated rat and yeast mitochondria (289). Bringing mild uncoupling one step further, Quin et al. synthetized a series of novel caged mitochondrial uncouplers designed to sense redox state (specifically H2O2 levels) and to respond only when there is an excess of this oxidant in the mitochondria. When levels of H2O2 are high, these molecules release the chemical uncouplers DNP or FCCP, thus dissipating Δψm and, consequently, lowering mtROS production (253). The trigger mechanism for this release exploits the reaction of arylboronate esters with H2O2 in mildly alkaline media, which converts them into phenols (e.g., at pH 8.3, which is the approximate pH of the matrix of active mitochondria), an effect already employed to make a range of fluorescent sensors that are sensitive to and selective for H2O2 (300). After this conversion, the molecule is fragmented and the mitochondrial uncoupler is released. Although H2O2-activated uncouplers may be useful, their beneficial effect and practical utility need to be further assessed both in vitro and in vivo.
FIG. 14.
Chemical structures of TPP+-conjugated uncouplers: MitoBHT and MitoDNP. BHT, butylated hydroxytoluene; DNP, 2,4-dinitrophenol.
6. Other strategies
a. Organelle-specific bioactivation reactions
Another promising strategy for selective mitochondrial delivery of compounds is through an organelle-specific bioactivation reaction (6) (Fig. 8). This approach is based on the presence of bioactivating enzymes in subcellular territories such as mitochondria, which can be exploited to catalyze the conversion of a prodrug to a drug. Mitochondrial enzymes that are potential candidates for controlled xenobiotic transformations include mitochondrial monoamine oxidase B, cytochrome P450, and mitochondrial medium-chain acyl-CoA dehydrogenase (6).
One strategy uses the enzymes of mitochondrial β-oxidation, which catalyze the biotransformation of dietary and endogenously generated fatty acids along with a long list of xenobiotic alkanoic acids, including tianeptine, chlorophenoxybutyric acid, and 5-hydroxydecanoic acid. Mitochondrial targeting of antioxidants using mitochondrial β-oxidation has been studied in the case of alkanoate-based prodrugs (6). The transporters and enzymes that make up the fatty acid β-oxidation pathway for short and medium chain fatty acids are found only in mitochondria, which make the biotransformation of the aforementioned molecules selective for mitochondria. The potential of thia- and oxaalkanoate-based prodrugs has been assessed through studies of the biotransformation of ω-(phenoxy)alkanoates, 3-(phenoxy)acrylates and ω-(1-methyl-1H-imidazol-2-ylthio)alkanoates (Fig. 8). Importantly, 3- and 5-(1-methyl-1H-imidazol-2-ylthio)alkanoates have been shown to undergo a rapid biotransformation into the antioxidant methimazole and to exert a cytoprotective effect in a hypoxia-reoxygenation model of rat cardiomyocytes (272). In the same model, 3-(2,6-dimethylphenoxy)propanoic acid and 3-(2,6-dimethylphenoxy)acrylic acid also afforded cytoprotection. The protective effect of these compounds was blocked by etomoxir, an inhibitor of carnitine palmitoyl transferase I, the mitochondrial enzyme that enables their delivery into mitochondria. Very importantly, despite being reported as an efficient and versatile ROS scavenger (148), methimazole failed to provide cytoprotection for cardiomyocytes under conditions of hypoxia-reoxygenation when administered alone. This evidence supports the idea that antioxidants need to be specifically delivered into mitochondria so that they can reach the necessary “in situ” concentration to be active. Further studies are required to confirm the therapeutic potential of these and similar molecules in vivo.
b. Mn porphyrin-based cellular redox modulators
Mn porphyrin (MnP)-based cellular redox modulators also merit attention. These compounds have been designed to mimic mitochondrial SOD (MnSOD), an enzyme with a fundamental role in mitochondrial redox homeostasis. Such compounds belong to the class of cationic Mn(III) N-substituted pyridyl- and N,N′-disubstituted imidazolylporphyrins. Some of them have rate constants kcat (O2•−) that are almost identical in magnitude to those of SOD enzymes. The first potent porphyrin-based SOD mimics, MnTM-2-PyP5+ and MnTE-2-PyP5+, were developed about 20 years ago. Since then, several other molecules have been generated based on the same principle; namely MnTDE-2-ImP5+ and, more recently, MnTnHex-2-PyP5+ and MnTnOct-2-PyP5+, which are produced by lengthening the alkyl chains of meso pyridyl groups while maintaining their total cationic charge and, therefore, their redox activity. The structure of the cationic MnP molecule combines the following critical features that allow it to mimic both the action and site of endogenous MnSOD: (i) the redox active Mn site; (ii) a multiple cationic charge; and (iii) four alkyl chains (the longer the alkyl chains, the higher the accumulation of MnP in mitochondria) (201). Available data suggest that MnP accumulates inside mitochondria, specifically in the mitochondrial matrix. The reason for this selective accumulation may be their cationic charge that is attracted by the negative Δψm and the anionic phosphate groups of cellular membranes. Porphyrin accumulation was investigated in heart mitochondria of mice after intraperitoneal injections over 7 days; the lipophilic compound MnTnHex-2-PyP5+ accumulated several-fold more in mitochondria than in cytosol compared with the hydrophilic analog MnTE-2-PyP5+ (326). The complex reactivities of MnPs within the cells make further mechanistic studies necessary; however, it seems that apart from acting as scavengers of O2•−, synthetic SOD mimics have an impact on levels of O2•−-derived or related molecules such as •OH, ONOO−, NO2, CO3−, and lipid peroxyl radicals. Reduction of CO3− by MnPs and reactivity toward •NO has also been reported. Ferrer-Sueta et al. showed that ≥3 μM MnTE-2-PyP5+ protects submitochondrial particles against oxidative stress imposed by ONOO− (88). In another work, accumulation of MnTE-2-PyP5+ in mouse heart mitochondria was reported to be 5.1 μM, a level high enough to render protection of mitochondria against oxidative damage (302).
The reaction in vivo will ultimately depend on the levels of MnPs, ROS, and RONS, cellular reductants, including GSH, ascorbate, BH4, and flavoprotein components of the mitochondrial respiration, and their co-localization with MnPs. MnP-based SOD mimics have proved to be effective in many in vitro and in vivo models, including tumorigenesis (particularly in combination with radiation and chemotherapy), renal ischemia reperfusion, diabetes, and CNS injuries and disorders (stroke, cerebral palsy, spinal cord injury, subarachnoid hemorrhage, AD, and chronic morphine tolerance) (as thoroughly reviewed by Tovmasyan et al.). Some studies have produced relevant data for the pathogenesis and/or management of CVD and related states, such as cardiometabolic pathologies, including diabetes (326).
c. Technology of biodegradable polymers
The use of platform technology, specifically the technology of biodegradable polymers, constitutes another promising approach for targeting bioactive molecules to the mitochondrial matrix (189). This method involves a rationally designed system of polymeric NP and contains the combination of a targeted poly(d,l-lactic-co-glycolic acid)-block (PLGA-b)-poly(ethylene glycol) (PEG)-TPP polymer (PLGA-b-PEG-TPP) with either non-targeted PLGA-b-PEG-OH or PLGA-COOH. In particular, the construct PLGA-b-PEG-TPP NP shows great potential as a therapeutical agent for mitochondrial dysfunction-related metabolic diseases such as obesity.
d. Actively transported mitochondrial antioxidants
Exploiting actively transported mitochondrial antioxidants is another encouraging approach for specific targeting of these organelles (Fig. 8). Although natural antioxidants generally have a good safety profile, their use is limited because of their incapacity to penetrate mitochondria. However, there are several exceptions, and the antioxidant best understood in this regard is ergothioneine (EGT) (Fig. 8), a naturally occurring sulfur-containing amino acid that animals acquire through their diet. In humans, EGT has been shown to accumulate in specific tissues at millimolar concentrations (107), and rat studies have shown the accumulation of EGT in the mitochondrial fraction of hepatic cells (142). It has been postulated that the active transport of EGT through the plasma membrane is mediated by an organic cation transporter (OCTN1), is compound specific, and occurs in an Na+-dependent way (108). The mitochondrial localization of this transporter has also been confirmed (159). Several studies have shown the antioxidant properties of EGT in vitro, where it scavenges several ROS, including •OH, ONOO−, and hypochlorous acid (107), and can deactivate singlet oxygen (kΔ=2.3×107 M−1 s−1) at a rate higher than that achieved by other natural thiols, including GSH (274). In vitro studies have analyzed the potential of EGT to protect DNA against damage induced by various reactive species, and this has been related to its cytoprotective role, such as in the case of PC12 cells exposed to H2O2 (52). It seems that EGT acts by conferring protection to mitochondrial components that are vulnerable to ROS generated by the mitochondrial ETC. This is supported by the fact that H2O2 treatment of HeLa cells in which OCTN1 had been silenced provoked increased mtDNA damage (233). Furthermore, addition of EGT potentiated the protective effect of CoQ10, an antioxidant primarily found in mitochondria, in a study that assessed alloxan-induced lipid peroxidation of phosphatidylcholine liposomes (76). EGT also protects against metal-induced oxidative damage in accordance with its ability to react with bivalent metal cations such as Cu2+ and Fe2+ forming redox-inactive complexes (107). Of note, and unlike GSH, which generates ROS in the presence of Cu2+ through the formation of a redox-active Cu (I)-(GSH)2 complex, the association of EGT and Cu2+ is relatively stable and does not generate free radicals (379). In summary, EGT seems to possess a protective capacity against mtROS in vitro, though its actions in vivo await further assessment.
IV. Conclusions
Oxidative stress is implicated in a wide range of pathologies. Mitochondria generate most of the cell's energy by OXPHOS and are considered the main source of ROS in many cell types. Mitochondria-generated ROS are crucial signaling molecules and participants in many cellular adaptative mechanisms. However, when the redox balance is disrupted as a consequence of excessive ROS generation or insufficient scavenging, mtROS become harmful. The continuous generation of ROS inside mitochondria and the specific molecular/morphological characteristics of these organelles versus other parts of the cell (both mtDNA and proteins are particularly susceptible to oxidative damage) make this cellular compartment highly vulnerable to the impact of ROS. Oxidative stress leads to mitochondrial impairment, both of which are features of the pathophysiology of a wide variety of diseases, including cardiometabolic diseases, neurodegenerative disorders, cancer, and other age-related diseases.
For decades now, numerous studies have focused on the potential of non-targeted antioxidant therapy for restoring normal physiology in oxidative stress conditions. In general, studies in cells or animal models have been successful, but clinical trials have led to disappointing and contradictory results.
Taking into account the importance of their role in human pathophysiology, mitochondria stand out as a key pharmacological target. Multiple compounds have been developed to treat mitochondrial dysfunction in different pathological situations with varying targets and mechanisms of action. In the present review, we have explored the characteristics and the use of different mitochondria-targeting strategies, including lipophilic cations, cationic plastoquinone derivatives, liposomal carriers, peptide-based targeting, uncouplers, and other recently described compounds. The majority of these compounds have demonstrated their beneficial effects in different models of oxidative stress, and some of them have proved to be effective in clinical trials.
Moreover, multiple novel delivery tools have recently been developed and are currently under evaluation, which offers broad opportunities for mitochondrial modulation. Many of these approaches are promising according to the results obtained in vitro or in cell-free systems; however, additional in-depth studies, particularly in vivo, are lacking to ensure translation of findings to more clinically relevant settings. Specific animal disease models are necessary to address the ability of a given compound to prevent and/or treat oxidative stress-related dysfunction. Finally, human clinical trials under physiologically relevant conditions are crucial to assess the clinical relevance of these molecules.
Funding
This study was financed by grants PI13/1025, PI11/00327, CIBER CB06/04/0071, PROMETEOII/2014/035, and GV/2014/118 and by the European Regional Development Fund (ERDF). V.M.V. is recipient of a contract from the Ministry of Health of the Valencian Regional Government (CES10/030).
Abbreviations Used
- Δψm
mitochondrial membrane potential
- AD
Alzheimer's disease
- ALS
amyotrophic lateral sclerosis
- AMPK
AMP-activated protein kinase
- BBB
blood–brain barrier
- BH4
tetrahydrobiopterin
- BHT
butylated hydroxytoluene
- CAD
coronary artery disease
- CARET
β-Carotene and Retinol Efficacy Trial
- CAT
catalase
- CCCP
carbonyl cyanide m-chlorophenyl hydrazone
- CNS
central nervous system
- COPD
chronic obstructive pulmonary disease
- CoQ10
coenzyme Q10
- CRI I
Castelli's risk index I
- CVD
cardiovascular disease
- DF-MITO-Porter
dual function MITO-Porter
- DHA
docosahexaenoic acid
- DNP
2,4-dinitrophenol
- Drp1
dynamin-related protein
- EGT
ergothioneine
- ER
endoplasmic reticulum
- ETC
electron transport chain
- EUK
eukarion
- FCCP
carbonylcyanide-p-trifluoromethoxyphenylhydrazone
- Fis1
mitochondrial fission 1 protein
- GIR
glucose infusion rate
- GPX
glutathione peroxidase
- GSH
glutathione
- GSSG
oxidized glutathione
- GTN
nitroglycerine
- GTPCH1
GTP cyclohydrolase 1
- H2O2
hydrogen peroxide
- H2S
hydrogen sulphide
- HCV
human hepatitis C virus
- HDL
high density lipoprotein
- HO-1
heme oxygenase
- HRECs
human retinal endothelial cells
- HSVEC
human saphenous vein endothelial cell
- IL
interleukin
- IMM
inner mitochondrial membrane
- LA
lipoic acid
- LDL
low density lipoprotein
- LTP
long-term synaptic plasticity
- MDA
malondialdehyde
- MDR
multidrug resistance
- Mfn1
mitofusin 1
- Mfn2
mitofusin 2
- MitoQ
mitoquinone
- MnP
Mn porphyrin
- MnSOD
manganese superoxide dismutase
- MPPs
mitochondria-penetrating peptides
- MT
metallothioneins
- mtDNA
mitochondrial DNA
- mtROS
mitochondrial ROS
- MTS
mitochondria-targeting signal peptide
- MW
molecular weight
- NAC
N-acetyl cysteine
- NF-κB
nuclear factor kappa B
- •NO
nitric oxide
- NOS
nitric oxide synthase
- Nox2
serum soluble gp91phox
- NP
nano-particle
- Nrf-2
nuclear factor-E2-related factor 2
- O2•−
superoxide anion
- OCTN1
organic cation transporter
- •OH
hydroxyl radical
- OMM
outer mitochondrial membrane
- ONOO−
peroxynitrite
- OPA1
optic atrophy 1 protein
- OXPHOS
oxidative phosporylation
- PBS
phosphate buffered saline
- PEG
poly(ethylene glycol)
- PINK1
PTEN-induced putative kinase protein 1
- PLGA
poly(d,l-lactic-co-glycolic acid)
- PON
paraoxonase
- PPARγ
peroxisome-proliferator-activated receptor gamma
- PPHN
persistent pulmonary hypertension of the newborn
- Prx
peroxiredoxin
- PUFA
polyunsaturated fatty acid
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SCS
synthetic catalytic scavengers
- SkQ1
plastoquinonyl decyltriphenylphosphonium
- SOD
superoxide dismutase
- SR
sarcoplasmic reticulum
- SS
Szeto-Schiller
- TC
total cholesterol
- TCA
tricarboxylic acid
- TG
triglicerides
- TNF-α
tumor necrosis factor alpha
- TPP
triphenylphosphonium
- TRX
thioredoxin
- UCP
uncoupling proteins
- UVA
ultraviolet A
- ZOF
Zucker obese fatty
References
- 1.Adair JC, Knoefel JE, and Morgan N. Controlled trial of N-acetylcysteine for patients with probable Alzheimer's disease. Neurology 57: 1515–1517, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, and Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J 19: 1088–1095, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, and Walter P. Molecular Biology of the Cell, 4th Edition. New York: Garland Science, 2002 [Google Scholar]
- 4.Alipour M, Omri A, Smith MG, and Suntres ZE. Prophylactic effect of liposomal N-acetylcysteine against LPS-induced liver injuries. J Endotoxin Res 13: 297–304, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Alirol E, James D, Huber D, Marchetto A, Vergani L, Martinou JC, and Scorrano L. The mitochondrial fission protein hFis1 requires the endoplasmic reticulum gateway to induce apoptosis. Mol Biol Cell 17: 4593–4605, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anders MW. Putting bioactivation reactions to work: targeting antioxidants to mitochondria. Chem Biol Interact 192: 8–13, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, and Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119: 573–581, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson EJ, Thayne K, Harris M, Carraway K, and Shaikh SR. Aldehyde stress and upregulation of Nrf2 mediated antioxidant systems accompany functional adaptations in cardiac mitochondria from mice fed n3 polyunsaturated fatty acids. Biochem J 441: 359–366, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson DC, Fauconnier J, Yamada T, Lacampagne A, Zhang SJ, Katz A, and Westerblad H. Mitochondrial production of reactive oxygen species contributes to the β-adrenergic stimulation of mouse cardiomycytes. J Physiol 589: 1791–1801, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews ZB, Horvath B, Barnstable CJ, Elsworth J, Yang L, Beal MF, Roth RH, Matthews RT, and Horvath TL. Uncoupling protein-2 is critical for nigral dopamine cell survival in a mouse model of Parkinson's disease. J Neurosci 25: 184–191, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonenko YN, Avetisyan AV, Bakeeva LE, Chernyak BV, Chertkov VA, Domnina LV, Ivanova OY, Izyumov DS, Khailova LS, Klishin SS, Korshunova GA, Lyamzaev KG, Muntyan MS, Nepryakhina OK, Pashkovskaya AA, Pletjushkina OY, Pustovidko AV, Roginsky VA, Rokitskaya TI, Ruuge EK, Saprunova VB, Severina II, Simonyan RA, Skulachev IV, Skulachev MV, Sumbatyan NV, Sviryaeva IV, Tashlitsky VN, Vassiliev JM, Vyssokikh MY, Yaguzhinsky LS, Zamyatnin AA, Jr., and Skulachev VP. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program 1. Cationic plastoquinone derivatives: synthesis and in vitro studies. Biochemistry (Mosc) 73: 1273–1287, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Antoniades C, Bakogiannis C, Tousoulis D, Reilly S, Zhang MH, Paschalis A, Antonopoulos AS, Demosthenous M, Miliou A, Psarros C, Marinou K, Sfyras N, Economopoulos G, Casadei B, Channon KM, and Stefanadis C. Preoperative atorvastatin treatment in CABG patients rapidly improves vein graft redox state by inhibition of Rac1 and NADPH-oxidase activity. Circulation 122: 66–73, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Asin-Cayuela J, Manas AR, James AM, Smith RA, and Murphy MP. Fine-tuning the hydrophobicity of a mitochondria-targeted antioxidant. FEBS Lett 571: 9–16, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, and La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest 101: 1581–1590, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azad MB, Chen Y, and Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal 11: 777–790, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, Daugaard JR, Lloberas J, Camps M, Zierath JR, Rabasa-Lhoret R, Wallberg-Henriksson H, Laville M, Palacín M, Vidal H, Rivera F, Brand M, and Zorzano A. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem 278: 17190–17197, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Baker K, Bucay-Marcus C, Huffman C, Kruk H, Malfroy B, and Doctrow SR. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J Pharmacol Exp Ther 284: 215–221, 1998 [PubMed] [Google Scholar]
- 18.Belardinelli R, Mucaj A, Lacalaprice F, Solenghi M, Tiano L, and Littarru GP. Coenzyme Q10 improves contractility of dysfunctional myocardium in chronic heart failure. Biofactors 25: 137–145, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Berk L, Jorm AF, Kelly CM, Dodd S, and Berk M. Development of guidelines for caregivers of people with bipolar disorder: a Delphi expert consensus study. Bipolar Disord 13: 556–570, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Bhatt JK, Thomas S, and Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res 32: 537–541, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Biasutto L, Mattarei A, Marotta E, Bradaschia A, Sassi N, Garbisa S, Zoratti M, and Paradisi C. Development of mitochondria-targeted derivatives of resveratrol. Bioorg Med Chem Lett 18: 5594–5597, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Bincoletto C, Bechara A, Pereira GJ, Santos CP, Antunes F, Peixoto da-Silva J, Muler M, Gigli RD, Monteforte PT, Hirata H, Jurkiewicz A, and Smaili SS. Interplay between apoptosis and autophagy, a challenging puzzle: new perspectives on antitumor chemotherapies. Chem Biol Interact 206: 279–288, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Birk AV, Chao WM, Bracken C, Warren JD, and Szeto HH. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol 171: 2017–2028, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biswas S, Dodwadkar NS, Deshpande PP, and Torchilin VP. Liposomes loaded with paclitaxel and modified with novel triphenylphosphonium-PEG-PE conjugate possess low toxicity, target mitochondria and demonstrate enhanced antitumor effects in vitro and in vivo. J Control Release 159: 393–402, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blaikie FH, Brown SE, Samuelsson LM, Brand MD, Smith RA, and Murphy MP. Targeting dinitrophenol to mitochondria: limitations to the development of a self-limiting mitochondrial protonophore. Biosci Rep 26: 231–243, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Boddapati SV, D'Souza GG, Erdogan S, Torchilin VP, and Weissig V. Organelle-targeted nanocarriers: specific delivery of liposomal ceramide to mitochondria enhances its cytotoxicity in vitro and in vivo. Nano Lett 8: 2559–2563, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Bolisetty S, Traylor AM, Kim J, Joseph R, Ricart K, Landar A, and Agarwal A. Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury. J Am Soc Nephrol 21: 1702–1712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bourens M, Fontanesi F, Soto IC, Liu J, and Barrientos A. Redox and reactive oxygen species regulation of mitochondrial cytochrome c oxidase biogenesis. Antioxid Redox Signal 19: 1940–1952, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brasnyo P, Molnar GA, Mohas M, Marko L, Laczy B, Cseh J, Mikolás E, Szijártó IA, Mérei A, Halmai R, Mészáros LG, Sümegi B, and Wittmann I. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr 106: 383–389, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Brookes PS, Levonen AL, Shiva S, Sarti P, and Darley-Usmar VM. Mitochondria: regulators of signal transduction by reactive oxygen and nitrogen species. Free Radic Biol Med 33: 755–764, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Brown SE, Ross MF, Sanjuan-Pla A, Manas AR, Smith RA, and Murphy MP. Targeting lipoic acid to mitochondria: synthesis and characterization of a triphenylphosphonium-conjugated alpha-lipoyl derivative. Free Radic Biol Med 42: 1766–1780, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Buckler KJ. Effects of exogenous hydrogen sulphide on calcium signalling, background (TASK) K channel activity and mitochondrial function in chemoreceptor cells. Pflugers Arch 463: 743–754, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burton GW. and Ingold KU. Beta-carotene: an unusual type of lipid antioxidant. Science 224: 569–573, 1984 [DOI] [PubMed] [Google Scholar]
- 34.Campello S, Lacalle RA, Bettella M, Manes S, Scorrano L, and Viola A. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J Exp Med 203: 2879–2886, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campello S. and Scorrano L. Mitochondrial shape changes: orchestrating cell pathophysiology. EMBO Rep 11: 678–684, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cereghetti GM, Costa V, and Scorrano L. Inhibition of Drp1-dependent mitochondrial fragmentation and apoptosis by a polypeptide antagonist of calcineurin. Cell Death Differ 17: 1785–1794, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cereghetti GM, Stangherlin A, de Martins BO, Chang CR, Blackstone C, Bernardi P, and Scorrano L. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A 105: 15803–15808, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chacko BK, Reily C, Srivastava A, Johnson MS, Ye Y, Ulasova E, Agarwal A, Zinn KR, Murphy MP, Kalyanaraman B, and Darley-Usmar V. Prevention of diabetic nephropathy in Ins2(+/)(AkitaJ) mice by the mitochondria-targeted therapy MitoQ. Biochem J 432: 9–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chacko BK, Srivastava A, Johnson MS, Benavides GA, Chang MJ, Ye Y, Jhala N, Murphy MP, Kalyanaraman B, and Darley-Usmar VM. Mitochondria-targeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology 54: 153–163, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chandran K, Aggarwal D, Migrino RQ, Joseph J, McAllister D, Konorev EA, Antholine WE, Zielonka J, Srinivasan S, Avadhani NG, and Kalyanaraman B. Doxorubicin inactivates myocardial cytochrome c oxidase in rats: cardioprotection by Mito-Q. Biophys J 96: 1388–1398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen K, Craige SE, and Keaney JF., Jr.Downstream targets and intracellular compartmentalization in Nox signaling. Antioxid Redox Signal 11: 2467–2480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen KH, Guo X, Ma D, Guo Y, Li Q, Yang D, Li P, Qiu X, Wen S, Xiao RP, and Tang J. Dysregulation of HSG triggers vascular proliferative disorders. Nat Cell Biol 6: 872–883, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Azad MB, and Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ 16: 1040–1052, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, McMillan-Ward E, Kong J, Israels SJ, and Gibson SB. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J Cell Sci 120: 4155–4166, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Chernyak BV, Antonenko YN, Galimov ER, Domnina LV, Dugina VB, Zvyagilskaya RA, Ivanova OY, Izyumov DS, Lyamzaev KG, Pustovidko AV, Rokitskaya TI, Rogov AG, Severina II, Simonyan RA, Skulachev MV, Tashlitsky VN, Titova EV, Trendeleva TA, and Shagieva GS. Novel mitochondria-targeted compounds composed of natural constituents: conjugates of plant alkaloids berberine and palmatine with plastoquinone. Biochemistry (Mosc) 77: 983–995, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Cho J, Won K, Wu D, Soong Y, Liu S, Szeto HH, and Hong MK. Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coron Artery Dis 18: 215–220, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Cho S, Szeto HH, Kim E, Kim H, Tolhurst AT, and Pinto JT. A novel cell-permeable antioxidant peptide, SS31, attenuates ischemic brain injury by down-regulating CD36. J Biol Chem 282: 4634–4642, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, James AM, Cochemé HM, Reinhold J, Lilley KS, Partridge L, Fearnley IM, Robinson AJ, Hartley RC, Smith RA, Krieg T, Brookes PS, and Murphy MP. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med 19: 753–759, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cipolat S, Martins de Brito O, Dal Zilio B, and Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A 101: 15927–15932, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cochemé HM, Kelso GF, James AM, Ross MF, Trnka J, Mahendiran T, Asin-Cayuela J, Blaikie FH, Manas AR, Porteous CM, Adlam VJ, Smith RA, and Murphy MP. Mitochondrial targeting of quinones: therapeutic implications. Mitochondrion 7: 94–102, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Coetzee WA. Multiplicity of effectors of the cardioprotective agent, diazoxide. Pharmacol Ther 140: 167–175, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colognato R, Laurenza I, Fontana I, Coppedé F, Siciliano G, Coecke S, Aruoma OI, Benzi L, and Migliore L. Modulation of hydrogen peroxide-induced DNA damage, MAPKs activation and cell death in PC12 by ergothioneine. Clin Nutr 25: 135–145, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Conti B, Sugama S, Lucero J, Winsky-Sommerer R, Wirz SA, Maher P, Andrews Z, Barr AM, Morale MC, Paneda C, Pemberton J, Gaidarova S, Behrens MM, Beal F, Sanna PP, Horvath T, and Bartfai T. Uncoupling protein 2 protects dopaminergic neurons from acute 1,2,3,6-methyl-phenyl-tetrahydropyridine toxicity. J Neurochem 93: 493–501, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, and Manson JE. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Arch Intern Med 167: 1610–1618, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooper CE. and Giulivi C. Nitric oxide regulation of mitochondrial oxygen consumption II: molecular mechanism and tissue physiology. Am J Physiol Cell Physiol 292: 1993–2003, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Correia SC, Santos RX, Cardoso SM, Santos MS, Oliveira CR, and Moreira PI. Cyanide preconditioning protects brain endothelial and NT2 neuron-like cells against glucotoxicity: role of mitochondrial reactive oxygen species and HIF-1α. Neurobiol Dis 45: 206–218, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Cortes CJ. and La Spada AR. The many faces of autophagy dysfunction in Huntington's disease: from mechanism to therapy. Drug Discov Today 19: 963–971, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Costa V, Giacomello M, Hudec R, Lopreiato R, Ermak G, Lim D, Malorni W, Davies KJ, Carafoli E, and Scorrano L. Mitochondrial fission and cristae disruption increase the response of cell models of Huntington's disease to apoptotic stimuli. EMBO Mol Med 2: 490–503, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, Hawkins M, Cohen HW, and Barzilai N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci 67: 1307–1312, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cristol JP, Bosc JY, Badiou S, Leblanc M, Lorrho R, Descomps B, and Canaud B. Erythropoietin and oxidative stress in haemodialysis: beneficial effects of vitamin E supplementation. Nephrol Dial Transplant 12: 2312–2317, 1997 [DOI] [PubMed] [Google Scholar]
- 61.Cuzzocrea S, Mazzon E, Costantino G, Serraino I, Dugo L, Calabrò G, Cucinotta G, De Sarro A, and Caputi AP. Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br J Pharmacol 130: 1219–1226, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dague E, Genet G, Lachaize V, Guilbeau-Frugier C, Fauconnier J, Mias C, Payré B, Chopinet L, Alsteens D, Kasas S, Severac C, Thireau J, Heymes C, Honton B, Lacampagne A, Pathak A, Sénard JM, and Galés C. Atomic force and electron microscopic-based study of sarcolemmal surface of living cardiomyocytes unveils unexpected mitochondrial shift in heart failure. J Mol Cell Cardiol 74: 162–172, 2014 [DOI] [PubMed] [Google Scholar]
- 63.Dahm CC, Moore K, and Murphy MP. Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite: implications for the interaction of nitric oxide with mitochondria. J Biol Chem 281: 10056–10065, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Dai DF, Hsieh EJ, Chen T, Menendez LG, Basisty NB, Tsai L, Beyer RP, Crispin DA, Shulman NJ, Szeto HH, Tian R, MacCoss MJ, and Rabinovitch PS. Global proteomics and pathway analysis of pressure-overload-induced heart failure and its attenuation by mitochondrial-targeted peptides. Circ Heart Fail 6: 1067–1076, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta 1797: 897–906, 2010 [DOI] [PubMed] [Google Scholar]
- 66.Danielson SR. and Andersen JK. Oxidative and nitrative protein modifications in Parkinson's disease. Free Radic Biol Med 44: 1787–1794, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Brito OM. and Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610, 2008 [DOI] [PubMed] [Google Scholar]
- 68.de Oliveira BF, Veloso CA, Nogueira-Machado JA, and Martins Chaves M. High doses of in vitro beta-carotene, alpha-tocopherol and ascorbic acid induce oxidative stress and secretion of IL-6 in peripheral blood mononuclear cells from healthy donors. Curr Aging Sci 5: 148–156, 2012 [DOI] [PubMed] [Google Scholar]
- 69.Desai SP, Bhatia SN, Toner M, and Irimia D. Mitochondrial localization and the persistent migration of epithelial cancer cells. Biophys J 104: 2077–2088, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dickey JS, Gonzalez Y, Aryal B, Mog S, Nakamura AJ, Redon CE, Baxa U, Rosen E, Cheng G, Zielonka J, Parekh P, Mason KP, Joseph J, Kalyanaraman B, Bonner W, Herman E, Shacter E, and Rao VA. Mito-tempol and dexrazoxane exhibit cardioprotective and chemotherapeutic effects through specific protein oxidation and autophagy in a syngeneic breast tumor preclinical model. PLoS One 8: e70575, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.D'Souza GG, Boddapati SV, and Weissig V. Mitochondrial leader sequence-plasmid DNA conjugates delivered into mammalian cells by DQAsomes co-localize with mitochondria. Mitochondrion 5: 352–358, 2005 [DOI] [PubMed] [Google Scholar]
- 72.D'Souza GG, Cheng SM, Boddapati SV, Horobin RW, and Weissig V. Nanocarrier-assisted sub-cellular targeting to the site of mitochondria improves the pro-apoptotic activity of paclitaxel. J Drug Target 16: 578–585, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Di Sario A, Bendia E, Taffetani S, Omenetti A, Candelaresi C, Marzioni M, De Minicis S, and Benedetti A. Hepatoprotective and antifibrotic effect of a new silybin-phosphatidylcholine-vitamin E complex in rats. Dig Liver Dis 37: 869–876, 2005 [DOI] [PubMed] [Google Scholar]
- 74.Dimmer KS, Fritz S, Fuchs F, Messerschmitt M, Weinbach N, Neupert W, and Westermann B. Genetic basis of mitochondrial function and morphology in Saccharomyces cerevisiae. Mol Biol Cell 13: 847–853, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doctrow SR, Liesa M, Melov S, Shirihai OS, and Tofilon P. Salen Mn complexes are superoxide dismutase/catalase mimetics that protect the mitochondria. Curr Inorg Chem 2: 325–334, 2012 [Google Scholar]
- 76.Dong KK, Damaghi N, Kibitel J, Canning MT, Smiles KA, and Yarosh DB. A comparison of the relative antioxidant potency of L-ergothioneine and idebenone. J Cosmet Dermatol 6: 183–188, 2007 [DOI] [PubMed] [Google Scholar]
- 77.Doughan AK. and Dikalov SI. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid Redox Signal 9: 1825–1836, 2007 [DOI] [PubMed] [Google Scholar]
- 78.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–49, 2002 [DOI] [PubMed] [Google Scholar]
- 79.Dröse S, Brandt U, and Wittig I. Mitochondrial respiratory chain complexes as sources and targets of thiol-based redox-regulation. Biochim Biophys Acta 1844: 1344–1354, 2014 [DOI] [PubMed] [Google Scholar]
- 80.Dröse S, Hanley PJ, and Brandt U. Ambivalent effects of diazoxide on mitochondrial ROS production at respiratory chain complexes I and III. Biochim Biophys Acta 1790: 558–565, 2009 [DOI] [PubMed] [Google Scholar]
- 81.Du L, Miao X, Gao Y, Jia H, Liu K, and Liu Y. The protective effects of Trolox-loaded chitosan nanoparticles against hypoxia-mediated cell apoptosis. Nanomedicine 10: 1411–1420, 2014 [DOI] [PubMed] [Google Scholar]
- 82.Dunn BK, Richmond ES, Minasian LM, Ryan AM, and Ford LG. A nutrient approach to prostate cancer prevention: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Nutr Cancer 62: 896–918, 2010 [DOI] [PubMed] [Google Scholar]
- 83.El Midaoui A. and de Champlain J. Prevention of hypertension, insulin resistance, and oxidative stress by alpha-lipoic acid. Hypertension 39: 303–307, 2002 [DOI] [PubMed] [Google Scholar]
- 84.Esplugues JV, Rocha M, Nuñez C, Bosca I, Ibiza S, Herance JR, Ortega A, Serrador JM, D'Ocon P, and Victor VM. Complex I dysfunction and tolerance to nitroglycerin: an approach based on mitochondrial-targeted antioxidants. Circ Res 99: 1067–1075, 2006 [DOI] [PubMed] [Google Scholar]
- 85.Fariss MW, Chan CB, Patel M, Van Houten B, and Orrenius S. Role of mitochondria in toxic oxidative stress. Cationic antioxidants as a powerful tool against mitochondrial oxidative stress. Mol Interv 5: 94–111, 2005 [DOI] [PubMed] [Google Scholar]
- 86.Farrow KN1, Lee KJ, Perez M, Schriewer JM, Wedgwood S, Lakshminrusimha S, Smith CL, Steinhorn RH, and Schumacker PT. Brief hyperoxia increases mitochondrial oxidation and increases phosphodiesterase 5 activity in fetal pulmonary artery smooth muscle cells. Antioxid Redox Signal 17: 460–470, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fauconnier J, Meli AC, Thireau J, Roberge S, Shan J, Sassi Y, Reiken SR, Rauzier JM, Marchand A, Chauvier D, Cassan C, Crozier C, Bideaux P, Lompré AM, Jacotot E, Marks AR, and Lacampagne A. Ryanodine receptor leak mediated by caspase-8 activation leads to left ventricular injury after myocardial ischemia-reperfusion. Proc Natl Acad Sci U S A 108: 13258–13263, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferrer-Sueta G, Hannibal L, Batinic-Haberle I, and Radi R. Reduction of manganese porphyrins by flavoenzymes and submitochondrial particles: a catalytic cycle for the reduction of peroxynitrite. Free Radic Biol Med 41: 503–512, 2006 [DOI] [PubMed] [Google Scholar]
- 89.Fetisova EK, Avetisyan AV, Izyumov DS, Korotetskaya MV, Chernyak BV, and Skulachev VP. Mitochondria-targeted antioxidant SkQR1 selectively protects MDR (Pgp 170)-negative cells against oxidative stress. FEBS Lett 584: 562–566, 2010 [DOI] [PubMed] [Google Scholar]
- 90.Fialkow L, Wang Y, and Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med 42: 153–164, 2007 [DOI] [PubMed] [Google Scholar]
- 91.Filipovska A, Kelso GF, Brown SE, Beer SM, Smith RA, and Murphy MP. Synthesis and characterization of a triphenylphosphonium-conjugated peroxidase mimetic. Insights into the interaction of ebselen with mitochondria. J Biol Chem 280: 24113–24126, 2005 [DOI] [PubMed] [Google Scholar]
- 92.Fink BD, Herlein JA, Yorek MA, Fenner , Kerns RJ, and Sivitz WI. Bioenergetic effects of mitochondrial-targeted coenzyme Q analogs in endothelial cells. JPET 342: 709–719, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fink MP, Macias CA, Xiao J, Tyurina YY, Delude RL, Greenberger JS, Kagan VE, and Wipf P. Hemigramicidin-TEMPO conjugates: novel mitochondria-targeted antioxidants. Crit Care Med 35: 461–467, 2007 [DOI] [PubMed] [Google Scholar]
- 94.Fransen M, Nordgren M, Wang B, and Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochim Biophys Acta 1822: 1363–1373, 2012 [DOI] [PubMed] [Google Scholar]
- 95.Frantz MC. and Wipf P. Mitochondria as a target in treatment. Environ Mol Mutagen 51: 462–475, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garcia-Bou R, Rocha M, Apostolova N, Herance R, Hernandez-Mijares A, and Victor VM. Evidence for a relationship between mitochondrial complex I activity and mitochondrial aldehyde dehydrogenase during nitroglycerin tolerance: effects of mitochondrial antioxidants. Biochim Biophys Acta 1817: 828–837, 2012 [DOI] [PubMed] [Google Scholar]
- 97.Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, and Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 12: 119–131, 2010 [DOI] [PubMed] [Google Scholar]
- 98.Gibson KR, Winterburn TJ, Barrett F, Sharma S, MacRury S, and Megson IL. Therapeutic potential of N-acetylcysteine as an antiplatelet agent in patients with type-2 diabetes. Cardiovasc Diabetol 10: 1–8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, and Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol 592: 2549–2561, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gladwin MT. and Shiva S. The ligand binding battle at cytochrome c oxidase: how NO regulates oxygen gradients in tissue. Circ Res 104: 1136–1138, 2009 [DOI] [PubMed] [Google Scholar]
- 101.Gomes A, Costa D, Lima JL, and Fernandes E. Antioxidant activity of beta-blockers: an effect mediated by scavenging reactive oxygen and nitrogen species?. Bioorg Med Chem 14: 4568–4577, 2006 [DOI] [PubMed] [Google Scholar]
- 102.Gomes LC. and Scorrano L. High levels of Fis1, a pro-fission mitochondrial protein, trigger autophagy. Biochim Biophys Acta 1777: 860–866, 2008 [DOI] [PubMed] [Google Scholar]
- 103.Goodman GE, Thornquist MD, Balmes J, Cullen MR, Meyskens FL, Jr., Omenn GS, Valanis B, and Williams JH., Jr.The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst 96: 1743–1750, 2004 [DOI] [PubMed] [Google Scholar]
- 104.Goraca A, Huk-Kolega H, Piechota A, Kleniewska P, Ciejka E, and Skibska B. Lipoic acid—biological activity and therapeutic potential. Pharmacol Rep 63: 849–858, 2011 [DOI] [PubMed] [Google Scholar]
- 105.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cochemé HM, Murphy MP, and Dominiczak AF. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 54: 322–328, 2009 [DOI] [PubMed] [Google Scholar]
- 106.Gray KM, Watson NL, Carpenter MJ, and LaRowe SD. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict 19: 187–189, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gruber J, Fong S, Chen CB, Yoong S, Pastorin G, Schaffer S, Cheah I, and Halliwell B. Mitochondria-targeted antioxidants and metabolic modulators as pharmacological interventions to slow ageing. Biotechnol Adv 31: 563–592, 2013 [DOI] [PubMed] [Google Scholar]
- 108.Gründemann D, Harlfinger S, Golz S, Geerts A, Lazar A, Berkels R, Jung N, Rubbert A, and Schömig E. Discovery of the ergothioneine transporter. Proc Natl Acad Sci U S A 102: 5256–5261, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grundlingh J, Dargan PI, El-Zanfaly M, and Wood DM. 2,4-Dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death. J Med Toxicol 7: 205–212, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hagen TM, Ingersoll RT, Lykkesfeldt J, Liu J, Wehr CM, Vinarsky V, Bartholomew JC, and Ames AB. (R)-alpha-lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB J 13: 411–418, 1999 [DOI] [PubMed] [Google Scholar]
- 111.Hagen TM, Liu J, Lykkesfeldt J, Wehr CM, Ingersoll RT, Vinarsky V, Bartholomew JC, and Ames BN. Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. Proc Natl Acad Sci U S A 99: 1870–1875, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haines DD, Lekli I, Teissier P, Bak I, and Tosaki A. Role of haeme oxygenase-1 in resolution of oxidative stress-related pathologies: focus on cardiovascular, lung, neurological and kidney disorders. Acta Physiol (Oxf) 204: 487–501, 2012 [DOI] [PubMed] [Google Scholar]
- 113.Haines TH. and Dencher NA. Cardiolipin: a proton trap for oxidative phosphorylation. FEBS Lett 528: 35–39, 2002 [DOI] [PubMed] [Google Scholar]
- 114.Halliwell B. and Gutteridge JMC. Free Radicals in Biology and Medicine, 4th edition. Oxford, NY: Oxford University Press, 2007 [Google Scholar]
- 115.Han XJ, Lu YF, Li SA, Kaitsuka T, Sato Y, Tomizawa K, Nairn AC, Takei K, Matsui H, and Matsushita M. CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J Cell Biol 182: 573–585, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hansen JM, Zhang H, and Jones DP. Mitochondrial thioredoxin-2 has a key role in determining tumor necrosis factor-alpha-induced reactive oxygen species generation, NF-kappaB activation, and apoptosis. Toxicol Sci 91: 643–650, 2006 [DOI] [PubMed] [Google Scholar]
- 117.Haramaki N, Han D, Handelman GJ, Tritschler HJ, and Packer L. Cytosolic and mitochondrial systems for NADH- and NADPH-dependent reduction of alpha-lipoic acid. Free Radic Biol Med 22: 535–542, 1997 [DOI] [PubMed] [Google Scholar]
- 118.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol 11: 298–300, 1956 [DOI] [PubMed] [Google Scholar]
- 119.Hausse AO, Aggoun Y, Bonnet D, Sidi D, Munnich A, Rötig A, and Rustin P. Idebenone and reduced cardiac hypertrophy in Friedreich's ataxia. Heart 87: 346–349, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.He C, Zhu H, Li H, Zou MH, and Xie Z. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes 62: 1270–1281, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Herbert V, Shaw S, and Jayatilleke E. Vitamin C-driven free radical generation from iron. J Nutr 126: 1213S–1220S, 1996 [DOI] [PubMed] [Google Scholar]
- 122.Higashida K, Kim SH, Jung SR, Asaka M, Holloszy JO, and Han DH. Effects of resveratrol and SIRT1 on PGC-1alpha activity and mitochondrial biogenesis: a reevaluation. PLoS Biol 11: e1001603, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hood E, Simone E, Wattamwar P, Dziubla T, and Muzykantov V. Nanocarriers for vascular delivery of antioxidants. Nanomedicine (Lond) 6: 1257–1272, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Horke S, Witte I, Wilgenbus P, Kruger M, Strand D, and Forstermann U. Paraoxonase-2 reduces oxidative stress in vascular cells and decreases endoplasmic reticulum stress-induced caspase activation. Circulation 115: 2055–2064, 2007 [DOI] [PubMed] [Google Scholar]
- 125.Horton KL, Stewart KM, Fonseca SB, Guo Q, and Kelley SO. Mitochondria-penetrating peptides. Chem Biol 15: 375–382, 2008 [DOI] [PubMed] [Google Scholar]
- 126.Hoth M, Fanger CM, and Lewis RS. Mitochondrial regulation of store operated calcium signaling in T lymphocytes. J Cell Biol 137: 633–648, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ishihara N, Jofuku A, Eura Y, and Mihara K. Regulation of mitochondrial morphology by membrane potential, and DRP1-dependent division and FZO1-dependent fusion reaction in mammalian cells. Biochem Biophys Res Commun 301: 891–898, 2003 [DOI] [PubMed] [Google Scholar]
- 128.Itoh T, Fairall L, Amin K, Inaba Y, Szanto A, Balint BL, Nagy L, Yamamoto K, and Schwabe JW. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat Struct Mol Biol 15: 924–931, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jackson MJ, Pye D, and Palomero J. The production of reactive oxygen and nitrogen species by skeletal muscle. J Appl Physiol 102: 1664–1670, 2007 [DOI] [PubMed] [Google Scholar]
- 130.James AM, Smith RA, and Murphy MP. Antioxidant and prooxidant properties of mitochondrial Coenzyme Q. Arch Biochem Biophys 423: 47–56, 2004 [DOI] [PubMed] [Google Scholar]
- 131.James DI, Parone PA, Mattenberger Y, and Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem 278: 36373–36379, 2003 [DOI] [PubMed] [Google Scholar]
- 132.Jauslin ML, Meier T, Smith RA, and Murphy MP. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J 17: 1972–1974, 2003 [DOI] [PubMed] [Google Scholar]
- 133.Jiang J, Belikova NA, Hoye AT, Zhao Q, Epperly MW, Greenberger JS, Wipf P, and Kagan VE. A mitochondria-targeted nitroxide/hemigramicidin S conjugate protects mouse embryonic cells against gamma irradiation. Int J Radiat Oncol Biol Phys 70: 816–825, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jiang J, Kurnikov I, Belikova NA, Xiao J, Zhao Q, Amoscato AA, Braslau R, Studer A, Fink MP, Greenberger JS, Wipf P, and Kagan VE. Structural requirements for optimized delivery, inhibition of oxidative stress, and antiapoptotic activity of targeted nitroxides. J Pharmacol Exp Ther 320: 1050–1060, 2007 [DOI] [PubMed] [Google Scholar]
- 135.Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, and Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol 191: 933–942, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jones DP. and Go YM. Redox compartmentalization and cellular stress. Diabetes Obes Metab 12: 116–125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Judé S, Bedut S, Roger S, Pinault M, Champeroux P, White E, and Le Guennec JY. Peroxidation of docosahexaenoic acid is responsible for its effects on I TO and I SS in ratventricular myocytes. Br J Pharmacol 139: 816–822, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jung C, Rong Y, Doctrow S, Baudry M, Malfroy B, and Xu Z. Synthetic superoxide dismutase/catalase mimetics reduce oxidative stress and prolong survival in a mouse amyotrophic lateral sclerosis model. Neurosci Lett 304: 157–160, 2001 [DOI] [PubMed] [Google Scholar]
- 139.Kanki T, Nakayama H, Sasaki N, Takio K, Alam TI, Hamasaki N, and Kang D. Mitochondrial nucleoid and transcription factor A. Ann N Y Acad Sci 1011: 61–68, 2004 [DOI] [PubMed] [Google Scholar]
- 140.Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, Hanafusa T, Matsuzawa Y, Yamasaki Y, and Hori M. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes 48: 2398–2406, 1999 [DOI] [PubMed] [Google Scholar]
- 141.Kapay NA, Popova OV, Isaev NK, Stelmashook EV, Kondratenko RV, Zorov DB, Skrebitsky VG, and Skulachev VP. Mitochondria-targeted plastoquinone antioxidant SkQ1 prevents amyloid-β-induced impairment of long-term potentiation in rat hippocampal slices. J Alzheimers Dis 36: 377–383, 2013 [DOI] [PubMed] [Google Scholar]
- 142.Kawano H, Otani M, Takeyama K, Kawai Y, Mayumi T, and Hama T. Studies on ergothioneine. VI. Distribution and fluctuations of ergothioneine in rats. Chem Pharm Bull (Tokyo) 30: 1760–1765, 1982 [DOI] [PubMed] [Google Scholar]
- 143.Kelley DE. and Goodpaster BH. Effects of physical activity on insulin action and glucose tolerance in obesity. Med Sci Sports Exerc 31: S619–S623, 1999 [DOI] [PubMed] [Google Scholar]
- 144.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, and Murphy MP. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem 276: 4588–4596, 2001 [DOI] [PubMed] [Google Scholar]
- 145.Khalil IA, Kogure K, Futaki S, and Harashima H. High density of octaarginine stimulates macropinocytosis leading to efficient intracellular trafficking for gene expression. J Biol Chem 281: 3544–3551, 2006 [DOI] [PubMed] [Google Scholar]
- 146.Kiffin R, Bandyopadhyay U, and Cuervo AM. Oxidative stress and autophagy. Antioxid Redox Signal 8: 152–162, 2006 [DOI] [PubMed] [Google Scholar]
- 147.Kim J, Kundu M, Viollet B, and Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13: 132–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim H, Lee TH, Hwang YS, Bang MA, Kim KH, Suh JM, Chung HK, Yu DY, Lee KK, Kwon OY, Ro HK, and Shong M. Methimazole as an antioxidant and immunomodulator in thyroid cells: mechanisms involving interferon-gamma signaling and H(2)O(2) scavenging. Mol Pharmacol 60: 972–980, 2001 [DOI] [PubMed] [Google Scholar]
- 149.Kissova I, Deffieu M, Manon S, and Camougrand N. Uth1p is involved in the autophagic degradation of mitochondria. J Biol Chem 279: 39068–39074, 2004 [DOI] [PubMed] [Google Scholar]
- 150.Klopstock T, Metz G, Yu-Wai-Man P, Büchner B, Gallenmüller C, Bailie M, Nwali N, Griffiths PG, von Livonius B, Reznicek L, Rouleau J, Coppard N, Meier T, and Chinnery PF. Persistence of the treatment effect of idebenone in Leber's hereditary optic neuropathy. Brain 136: e230, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, and Kalivas PW. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry 65: 841–845, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kogure K, Akita H, Yamada Y, and Harashima H. Multifunctional envelope-type nano device (MEND) as a non-viral gene delivery system. Adv Drug Deliv Rev 60: 559–571, 2008 [DOI] [PubMed] [Google Scholar]
- 153.Kong W, Chen LL, Zheng J, Zhang HH, Hu X, Zeng TS, and Hu D. Resveratrol supplementation restores high-fat diet-induced insulin secretion dysfunction by increasing mitochondrial function in islet. Exp Biol Med 2014. [Epub ahead of print]; pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Koopman WJ, Verkaart S, Visch HJ, van der Westhuizen FH, Murphy MP, van den Heuvel LW, Smeitink JA, and Willems PH. Inhibition of complex I of the electron transport chain causes O2-mediated mitochondrial outgrowth. Am J Physiol Cell Physiol 288: 1440–1450, 2005 [DOI] [PubMed] [Google Scholar]
- 155.Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, and Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science 305: 858–862, 2004 [DOI] [PubMed] [Google Scholar]
- 156.Kröller-Schön S, Jansen T, Schüler A, Oelze M, Wenzel P, Hausding M, Kerahrodi JG, Beisele M, Lackner KJ, Daiber A, Münzel T, and Schulz E. Peroxisome proliferator-activated receptor γ, coactivator 1α deletion induces angiotensin II-associated vascular dysfunction by increasing mitochondrial oxidative stress and vascular inflammation. Arterioscler Thromb Vasc Biol 33: 1928–1935, 2013 [DOI] [PubMed] [Google Scholar]
- 157.Kumar KV, Shifow AA, Naidu MU, and Ratnakar KS. Carvedilol: a beta blocker with antioxidant property protects against gentamicin-induced nephrotoxicity in rats. Life Sci 66: 2603–2611, 2000 [DOI] [PubMed] [Google Scholar]
- 158.Lafleur DL, Pittenger C, Kelmendi B, Gardner T, Wasylink S, Malison RT, Sanacora G, Krystal JH, and Coric V. N-acetylcysteine augmentation in serotonin reuptake inhibitor refractory obsessive-compulsive disorder. Psychopharmacology (Berl) 184: 254–256, 2006 [DOI] [PubMed] [Google Scholar]
- 159.Lamhonwah AM. and Tein I. Novel localization of OCTN1, an organic cation/carnitine transporter, to mammalian mitochondria. Biochem Biophys Res Commun 345: 1315–1325, 2006 [DOI] [PubMed] [Google Scholar]
- 160.Langan AR, Khan MA, Yeung IW, Van Dyk J, and Hill RP. Partial volume rat lung irradiation: the protective/mitigating effects of Eukarion-189, a superoxide dismutase-catalase mimetic. Radiother Oncol 79: 231–238, 2008 [DOI] [PubMed] [Google Scholar]
- 161.Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, Bovet P, Bush AI, Conus P, Copolov D, Fornari E, Meuli R, Solida A, Vianin P, Cuénod M, Buclin T, and Do KQ. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology 33: 2187–2199, 2008 [DOI] [PubMed] [Google Scholar]
- 162.Lee JH, Kim B, Jin WJ, Kim JW, Kim HH, Ha H, and Lee ZH. Trolox inhibits osteolytic bone metastasis of breast cancer through both PGE2-dependent and independent mechanisms. Biochem Pharmacol 91: 51–60, 2014 [DOI] [PubMed] [Google Scholar]
- 163.Lee SM. and Cho TS. Effect of Trolox on hypoxia/reoxygenation-induced injury in isolated perfused rat liver. Arch Pharm Res 20: 471–475, 1997 [DOI] [PubMed] [Google Scholar]
- 164.Lee TM, Lin SZ, and Chang NC. Both PKA and Epac pathways mediate N-acetylcysteine-induced Connexin43 preservation in rats with myocardial infarction. PLoS One 8: e71878, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res 8: 3–5, 2005 [DOI] [PubMed] [Google Scholar]
- 166.Lemire BD, Behrendt M, DeCorby A, and Gásková D. C. elegans longevity pathways converge to decrease mitochondrial membrane potential. Mech Ageing Dev 130: 461–465, 2009 [DOI] [PubMed] [Google Scholar]
- 167.Leo S, Szabadkai G, and Rizzuto R. The mitochondrial antioxidants MitoE(2) and MitoQ(10) increase mitochondrial Ca(2+) load upon cell stimulation by inhibiting Ca(2+) efflux from the organelle. Ann N Y Acad Sci 1147: 264–274, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Leus FR, Zwart M, Kastelein JJ, and Voorbij HA. PON2 gene variants are associated with clinical manifestations of cardiovascular disease in familial hypercholesterolemia patients. Atherosclerosis 154: 641–649, 2001 [DOI] [PubMed] [Google Scholar]
- 169.Li J, Chen X, Xiao W, Ma W, Li T, Huang J, Liu X, Liang X, Tang S, and Luo Y. Mitochondria-targeted antioxidant peptide SS31 attenuates high glucose-induced injury on human retinal endothelial cells. Biochem Biophys Res Commun 404: 349–356, 2011 [DOI] [PubMed] [Google Scholar]
- 170.Li H, Xia N, and Förstermann U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide 26: 102–110, 2012 [DOI] [PubMed] [Google Scholar]
- 171.Lim S, Rashid MA, Jang M, Kim Y, Won H, Lee J, Woo JT, Kim YS, Murphy MP, Ali L, Ha J, and Kim SS. Mitochondria-targeted antioxidants protect pancreatic β-cells against oxidative stress and improve insulin secretion in glucotoxicity and glucolipotoxicity. Cell Physiol Biochem 28: 873–886, 2011 [DOI] [PubMed] [Google Scholar]
- 172.Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, Zeng W, Hronowsky X, Buko A, Chollate S, Ellrichmann G, Brück W, Dawson K, Goelz S, Wiese S, Scannevin RH, Lukashev M, and Gold R. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 134: 678–692, 2011 [DOI] [PubMed] [Google Scholar]
- 173.Lipinski CA, Lombardo F, Dominy BW, and Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46: 3–26, 2001 [DOI] [PubMed] [Google Scholar]
- 174.Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, and Baudry M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci U S A 100: 8526–8531, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu S, Soong Y, Seshan SV, and Szeto HH. Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation and fibrosis. Am J Physiol Renal Physiol 306: 970–980, 2014 [DOI] [PubMed] [Google Scholar]
- 176.Lloret A, Badía MC, Mora NJ, Pallardó FV, Alonso MD, and Viña J. Vitamin E paradox in Alzheimer's disease: it does not prevent loss of cognition and may even be detrimental. J Alzheimers Dis 17: 143–149, 2009 [DOI] [PubMed] [Google Scholar]
- 177.Loguercio C, Federico A, Trappoliere M, Tuccillo C, de Sio I, Di Leva A, Niosi M, D'Auria MV, Capasso R, Del Vecchio Blanco C, and Real Sud Group. The effect of a silybin-vitamin e-phospholipid complex on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci 52: 2387–2395, 2007 [DOI] [PubMed] [Google Scholar]
- 178.Lonn E, Yusuf S, Hoogwerf B, Pogue J, Yi Q, Zinman B, Bosch J, Dagenais G, Mann JF, and Gerstein HC. Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: results of the HOPE study and MICRO-HOPE substudy. Diabetes Care 25: 1919–1927, 2002 [DOI] [PubMed] [Google Scholar]
- 179.Lou PH, Hansen BS, Olsen PH, Tullin S, Murphy MP, and Brand MD. Mitochondrial uncouplers with an extraordinary dynamic range. Biochem J 407: 129–140, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lowes DA, Thottakam BM, Webster NR, Murphy MP, and Galley HF. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic Biol Med 45: 1559–1565, 2008 [DOI] [PubMed] [Google Scholar]
- 181.Lu L, Erhard P, Salomon RG, and Weiss MF. Serum vitamin E and oxidative protein modification in hemodialysis: a randomized clinical trial. Am J Kidney Dis 50: 305–313, 2007 [DOI] [PubMed] [Google Scholar]
- 182.Ma T, Hoeffer CA, Wong H, Massaad CA, Zhou P, Iadecola C, Murphy MP, Pautler RG, and Klann E. Amyloid β-induced impairments in hippocampal synaptic plasticity are rescued by decreasing mitochondrial superoxide. J Neurosci 31: 5589–5595, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Macias CA, Chiao JW, Xiao J, Arora DS, Tyurina YY, Delude RL, Wipf P, Kagan VE, and Fink MP. Treatment with a novel hemigramicidin-TEMPO conjugate prolongs survival in a rat model of lethal hemorrhagic shock. Ann Surg 245: 305–314, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mackenzie RM, Salt IP, Miller WH, Logan A, Ibrahim HA, Degasperi A, Dymott JA, Hamilton CA, Murphy MP, Delles C, and Dominiczak AF. Mitochondrial reactive oxygen species enhance AMP-activated protein kinase activation in the endothelium of patients with coronary artery disease and diabetes. Clin Sci (Lond) 124: 403–411, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mafra D.Santos FR, Lobo JC, de Mattos Grosso D, Barreira AL, Velarde LG, Abdalla DS, and Leite M., Jr.Alpha-tocopherol supplementation decreases electronegative low-density lipoprotein concentration [LDL(−)] in haemodialysis patients. Nephrol Dial Transplant 24: 1587–1592, 2009 [DOI] [PubMed] [Google Scholar]
- 186.Makino A, Scott BT, and Dillmann WH. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia 53: 1783–1794, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, and Reddy PH. Mitochondria-targeted antioxidants protect against amyloid-β toxicity in Alzheimer's disease neurons. J Alzheimers Dis 20: S609–S631, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Maroz A, Anderson RF, Smith RA, and Murphy MP. Reactivity of ubiquinone and ubiquinol with superoxide and the hydroperoxyl radical: implications for in vivo antioxidant activity. Free Radic Biol Med 46: 105–109, 2009 [DOI] [PubMed] [Google Scholar]
- 189.Marrache S. and Dhar S. Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. Proc Natl Acad Sci U S A 109: 16288–16293, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Martin LJ. Biology of mitochondria in neurodegenerative diseases. Prog Mol Biol Transl Sci 107: 355–415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Martina V, Masha A, Gigliardi VR, Brocato L, Manzato E, Berchio A, Massarenti P, Settanni F, Della Casa L, Bergamini S, and Iannone A. Long-term N-acetylcysteine and L-arginine administration reduces endothelial activation and systolic blood pressure in hypertensive patients with type 2 diabetes. Diabetes Care 31: 940–944, 2008 [DOI] [PubMed] [Google Scholar]
- 192.Mason MG, Nicholls P, Wilson MT, and Cooper CE. Nitric oxide inhibition of respiration involves both competitive (heme) and noncompetitive (copper) binding to cytochrome c oxidase. Proc Natl Acad Sci U S A 103: 708–713, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.McClung JM, Judge AR, Powers SK, and Yan Z. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol 298: C542–C549, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.McLachlan J, Beattie E, Murphy MP, Koh-Tan CH, Olson E, Beattie W, Dominiczak AF, Nicklin SA, and Graham D. Combined therapeutic benefit of mitochondria-targeted antioxidant, MitoQ10, and angiotensin receptor blocker, losartan, on cardiovascular function. J Hypertens 32: 555–564, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Meagher EA, Barry OP, Lawson JA, Rokach J, and FitzGerald GA. Effects of vitamin E on lipid peroxidation in healthy persons. JAMA 285: 1178–1182, 2001 [DOI] [PubMed] [Google Scholar]
- 196.Melov S, Coskun P, Patel M, Tuinstra R, Cottrell B, Jun AS, Zastawny TH, Dizdaroglu M, Goodman SI, Huang TT, Miziorko H, Epstein CJ, and Wallace DC. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc Natl Acad Sci U S A 96: 846–851, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Melov S, Doctrow SR, Schneider JA, Haberson J, Patel M, Coskun PE, Huffman K, Wallace DC, and Malfroy B. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J Neurosci 21: 8348–8353, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Melov S, Wolf N, Strozyk D, Doctrow SR, and Bush AI. Mice transgenic for Alzheimer disease beta-amyloid develop lens cataracts that are rescued by antioxidant treatment. Free Radic Biol Med 38: 258–261, 2005 [DOI] [PubMed] [Google Scholar]
- 199.Mercer JR, Yu E, Figg N, Cheng KK, Prime TA, Griffin JL, Masoodi M, Vidal-Puig A, Murphy MP, and Bennett MR. The mitochondria-targeted antioxidant MitoQ decreases features of the metabolic syndrome in ATM+/−/ApoE−/−mice. Free Radic Biol Med 52: 841–849, 2012 [DOI] [PubMed] [Google Scholar]
- 200.Miranda-Massari JR, Gonzalez MJ, Jimenez FJ, Allende-Vigo MZ, and Duconge J. Metabolic correction in the management of diabetic peripheral neuropathy: improving clinical results beyond symptom Control. Curr Clin Pharmacol 6: 260–273, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Miriyala S, Spasojevic I, Tovmasyan A, Salvemini D, Vujaskovic Z, St. Clair D, and Batinic-Haberle I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim Biophys Acta 1822: 794–814, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mizuguchi Y, Chen J, Seshan SV, Poppas DP, Szeto HH, and Felsen D. A novel cell-permeable antioxidant peptide decreases renal tubular apoptosis and damage in unilateral ureteral obstruction. Am J Physiol Renal Physiol 295: 1545–1553, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mizushima N. Autophagy: process and function. Genes Dev 21: 2861–2873, 2007 [DOI] [PubMed] [Google Scholar]
- 204.Mizushima N, Levine B, Cuervo AM, and Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 451: 1069–1075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mochizuki M, Yano M, Oda T, Tateishi H, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, and Matsuzaki M. Scavenging free radicals by low-dose carvedilol prevents redoxdependent Ca2+ leak viastabilization of ryanodine receptor in heart failure. J Am Coll Cardiol 49: 1722–1732, 2007 [DOI] [PubMed] [Google Scholar]
- 206.Moreira PI, Harris PL, Zhu X, Santos MS, Oliveira CR, Smith MA, and Perry G. Lipoic acid and N-acetyl cysteine decrease mitochondrial-related oxidative stress in Alzheimer disease patient fibroblasts. J Alzheimers Dis 12: 195–206, 2007 [DOI] [PubMed] [Google Scholar]
- 207.Moreira PI, Santos MS, Sena C, Nunes E, Seiça R, and Oliveira CR. CoQ10 therapy attenuates amyloid beta-peptide toxicity in brain mitochondria isolated from aged diabetic rats. Exp Neurol 196: 112–119, 2005 [DOI] [PubMed] [Google Scholar]
- 208.Morris MC. and Tangney CC. A potential design flaw of randomized trials of vitamin supplements. JAMA 305: 1348–1349, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Murphy MP, Echtay KS, Blaikie FH, Asin-Cayuela J, Cocheme HM, Green K, Buckingham JA, Taylor ER, Hurrell F, Hughes G, Miwa S, Cooper CE, Svistunenko DA, Smith RA, and Brand MD. Superoxide activates uncoupling proteins by generating carbon-centered radicals and initiating lipid peroxidation: studies using a mitochondria-targeted spin trap derived from alpha-phenyl-N-tert-butylnitrone. J Biol Chem 278: 48534–48545, 2003 [DOI] [PubMed] [Google Scholar]
- 210.Murphy MP. and Smith RA. Drug delivery to mitochondria: the key to mitochondrial medicine. Adv Drug Deliv Rev 41: 235–250, 2000 [DOI] [PubMed] [Google Scholar]
- 211.Murphy MP. and Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol 47: 629–656, 2007 [DOI] [PubMed] [Google Scholar]
- 212.Musatov A. and Robinson NC. Susceptibility of mitochondrial electron-transport complexes to oxidative damage. Focus on cytochrome c oxidase. Free Radic Res 46: 1313–1326, 2012 [DOI] [PubMed] [Google Scholar]
- 213.Naon D. and Scorrano L. At the right distance: ER-mitochondria juxtaposition in cell life and death. Biochim Biophys Acta 1843: 2184–2194, 2014 [DOI] [PubMed] [Google Scholar]
- 214.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, and Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 8: e1000298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Navarro-Sastre A, Tort F, Stehling O, Uzarska MA, Arranz JA, Del Toro M, Labayru MT, Landa J, Font A, Garcia-Villoria J, Merinero B, Ugarte M, Gutierrez-Solana LG, Campistol J, Garcia-Cazorla A, Vaquerizo J, Riudor E, Briones P, Elpeleg O, Ribes A, and Lill R. A fatal mitochondrial disease is associated with defective NFU1 function in the maturation of a subset of mitochondrial Fe-S proteins. Am J Hum Genet 89: 656–667, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nishida K, Yamaguchi O, and Otsu K. Crosstalk between autophagy and apoptosis in heart disease. Circ Res 103: 343–351, 2008 [DOI] [PubMed] [Google Scholar]
- 217.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, and Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299: 896–899, 2003 [DOI] [PubMed] [Google Scholar]
- 218.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, and Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310: 314–317, 2005 [DOI] [PubMed] [Google Scholar]
- 219.Ojala D, Montoya J, and Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature 290: 470–474, 1981 [DOI] [PubMed] [Google Scholar]
- 220.Okada K, Wangpoengtrakul C, Osawa T, Toyokuni S, Tanaka K, and Uchida K. 4-Hydroxy-2-nonenal-mediated impairment of intracellular proteolysis during oxidative stress. Identification of proteasomes as target molecules. J Biol Chem 274: 23787–23793, 1999 [DOI] [PubMed] [Google Scholar]
- 221.Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, and Lenaers G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem 278: 7743–7746, 2003 [DOI] [PubMed] [Google Scholar]
- 222.Olichon A, Elachouri G, Baricault L, Delettre C, Belenguer P, and Lenaers G. OPA1 alternate splicing uncouples an evolutionary conserved function in mitochondrial fusion from a vertebrate restricted function in apoptosis. Cell Death Differ 14: 682–692, 2007 [DOI] [PubMed] [Google Scholar]
- 223.Oliveira PJ, Seica R, Santos DL, Rolo AP, Sardao VA, Ferreira FM, Palmeira CM, Santos MS, and Moreno AJ. Vitamin E or coenzyme Q10 administration is not fully advantageous for heart mitochondrial function in diabetic goto kakizaki rats. Mitochondrion 3: 337–345, 2004 [DOI] [PubMed] [Google Scholar]
- 224.Olson KR. Hydrogen sulfide is an oxygen sensor in the carotid body. Respir Physiol Neurobiol 179: 103–110, 2011 [DOI] [PubMed] [Google Scholar]
- 225.Olson KR. and Whitfield NL. Hydrogen sulfide and oxygen sensing in the cardiovascular system. Antioxid Redox Signal 12: 1219–1234, 2010 [DOI] [PubMed] [Google Scholar]
- 226.Omarova EO. and Antonenko YN. Inhibition of oxidative hemolysis in erythrocytes by mitochondria-targeted antioxidants of SkQ series. Biochemistry (Mosc) 79: 139–145, 2014 [DOI] [PubMed] [Google Scholar]
- 227.Omaye ST, Krinsky NI, Kagan VE, Mayne ST, Liebler DC, and Bidlack WR. beta-carotene: friend or foe? Fundam Appl Toxicol 40: 163–174, 1997 [DOI] [PubMed] [Google Scholar]
- 228.Oyewole AO, Wilmot MC, Fowler M, and Birch-Machin MA. Comparing the effects of mitochondrial targeted and localized antioxidants with cellular antioxidants in human skin cells exposed to UVA and hydrogen peroxide. FASEB J 28: 485–494, 2014 [DOI] [PubMed] [Google Scholar]
- 229.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, and Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 20: 537–544, 12997 [DOI] [PubMed] [Google Scholar]
- 230.Papparella I, Ceolotto G, Berto L, Cavalli M, Bova S, Cargnelli G, Ruga E, Milanesi O, Franco L, Mazzoni M, Petrelli L, Nussdorfer GG, and Semplicini A. Vitamin C prevents zidovudine-induced NAD(P)H oxidase activation and hypertension in the rat. Cardiovasc Res 73: 432–438, 2007 [DOI] [PubMed] [Google Scholar]
- 231.Patková J, Anděl M, and Trnka J. Palmitate-induced cell death and mitochondrial respiratory dysfunction in myoblasts are not prevented by mitochondria-targeted antioxidants. Cell Physiol Biochem 33: 1439–1451, 2014 [DOI] [PubMed] [Google Scholar]
- 232.Patra SK, Singh K, and Singh R. Paraoxonase 1: a better atherosclerotic risk predictor than HDL in type 2 diabetes mellitus. Diabetes Metab Syndr 7: 108–111, 2013 [DOI] [PubMed] [Google Scholar]
- 233.Paul BD. and Snyder SH. The unusual amino acid L-ergothioneine is a physiologic cytoprotectant. Cell Death Differ 17: 1134–1140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pauly M, Daussin F, Burelle Y, Li T, Godin R, Fauconnier J, Koechlin-Ramonatxo C, Hugon G, Lacampagne A, Coisy-Quivy M, Liang F, Hussain S, Matecki S, and Petrof BJ. AMPK activation stimulates autophagy and ameliorates muscular dystrophy in the mdx mouse diaphragm. Am J Pathol 181: 583–592, 2012 [DOI] [PubMed] [Google Scholar]
- 235.Peng J, Stevenson FF, Doctrow SR, and Andersen JK. Superoxide dismutase/catalase mimetics are neuroprotective against selective paraquat-mediated dopaminergic neuron death in the substantial nigra: implications for Parkinson disease. J Biol Chem 280: 29194–29198, 2005 [DOI] [PubMed] [Google Scholar]
- 236.Persson MF, Franzen S, Catrina SB, Dallner G, Hansell P, Brismar K, and Palm F. Coenzyme Q10 prevents GDP-sensitive mitochondrial uncoupling, glomerular hyperfiltration and proteinuria in kidneys from db/db mice as a model of type 2 diabetes. Diabetologia 55: 1535–1543, 2012 [DOI] [PubMed] [Google Scholar]
- 237.Petri S, Kiaei M, Damiano M, Hiller A, Wille E, Manfredi G, Calingasan NY, Szeto HH, and Beal MF. Cell-permeable peptide antioxidants as a novel therapeutic approach in a mouse model of amyotrophic lateral sclerosis. J Neurochem 98: 1141–1148, 2006 [DOI] [PubMed] [Google Scholar]
- 238.Piantadosi CA. and Suliman HB. Redox regulation of mitochondrial biogenesis. Free Radic Biol Med 53: 2043–2053, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Pignatelli P, Carnevale R, Cangemi R, Loffredo L, Sanguigni V, Stefanutti C, Basili S, and Violi F. Atorvastatin inhibits gp91phox circulating levels in patients with hypercholesterolemia. Arterioscler Thromb Vasc Biol 30: 360–367, 2010 [DOI] [PubMed] [Google Scholar]
- 240.Pletjushkina OY, Lyamzaev KG, Popova EN, Nepryakhina OK, Ivanova OY, Domnina LV, Chernyak BV, and Skulachev VP. Effect of oxidative stress on dynamics of mitochondrial reticulum. Biochim Biophys Acta 1757: 518–524, 2006 [DOI] [PubMed] [Google Scholar]
- 241.Plotnikov EY, Chupyrkina AA, Jankauskas SS, Pevzner IB, Silachev DN, Skulachev VP, and Zorov DB. Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochim Biophys Acta 1812: 77–86, 2011 [DOI] [PubMed] [Google Scholar]
- 242.Plotnikov EY, Morosanova MA, Pevzner IB, Zorova LD, Manskikh VN, Pulkova NV, Galkina SI, Skulachev VP, and Zorov DB. Protective effect of mitochondria-targeted antioxidants in an acute bacterial infection. Proc Natl Acad Sci U S A 110: E3100–E3108, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Plotnikov EY, Silachev DN, Chupyrkina AA, Danshina MI, Jankauskas SS, Morosanova MA, Stelmashook EV, Vasileva AK, Goryacheva ES, Pirogov YA, Isaev NK, and Zorov DB. New-generation Skulachev ions exhibiting nephroprotective and neuroprotective properties. Biochemistry (Mosc) 75: 145–150, 2010 [DOI] [PubMed] [Google Scholar]
- 244.Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, and Lunec J. Vitamin C exhibits pro-oxidant properties. Nature 392: 559, 1998 [DOI] [PubMed] [Google Scholar]
- 245.Popot JL. and de Vitry C. On the microassembly of integral membrane proteins. Annu Rev Biophys Biophys Chem 19: 369–403, 1990 [DOI] [PubMed] [Google Scholar]
- 246.Porteous CM, Logan A, Evans C, Ledgerwood EC, Menon DK, Aigbirhio F, Smith RA, and Murphy MP. Rapid uptake of lipophilic triphenylphosphonium cations by mitochondria in vivo following intravenous injection: implications for mitochondria-specific therapies and probes. Biochim Biophys Acta 1800: 1009–1017, 2010 [DOI] [PubMed] [Google Scholar]
- 247.Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stødkilde-Jørgensen H, Møller N, Jessen N, Pedersen SB, and Jørgensen JO. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebocontrolled clinical trial of substratemetabolism, insulin sensitivity, and body composition. Diabetes 62: 1186–1195, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Powers SK. and Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243–1276, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Praticò D. Oxidative stress hypothesis in Alzheimer's disease: a reappraisal. Trends Pharmacol Sci 29: 609–615, 2008 [DOI] [PubMed] [Google Scholar]
- 250.Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, and Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15: 675–690, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Przyklenk K. Pharmacologic treatment of the stunned myocardium: the concepts and the challenges. Coron Artery Dis 12: 363–369, 2001 [DOI] [PubMed] [Google Scholar]
- 252.Pung YF, Rocic P, Murphy MP, Smith RA, Hafemeister J, Ohanyan V, Guarini G, Yin L, and Chilian WM. Resolution of mitochondrial oxidative stress rescues coronary collateral growth in Zucker obese fatty rats. Arterioscler Thromb Vasc Biol 32: 325–334, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Quin C, Robertson L, McQuaker SJ, Price NC, Brand MD, and Hartley RC. Caged mitochondrial uncouplers that are released in response to hydrogen peroxide. Tetrahedron 66: 2384–2389, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Radi R, Rodriguez M, Castro L, and Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys 308: 89–95, 1994 [DOI] [PubMed] [Google Scholar]
- 255.Rahman I. and MacNee W. Regulation of redox glutathione levels and gene transcription in lung inflammation: therapeutic approaches. Free Radic Biol Med 28: 1405–1420, 2000 [DOI] [PubMed] [Google Scholar]
- 256.Rahman M, Mofarrahi M, Kristof AS, Nkengfac B, Harel S, and Hussain SN. Reactive oxygen species regulation of autophagy in skeletal muscles. Antioxid Redox Signal 20: 443–459, 2014 [DOI] [PubMed] [Google Scholar]
- 257.Rahman ST, Merchant N, Haque T, Wahi J, Bhaheetharan S, Ferdinand KC, and Khan BV. The impact of lipoic acid on endothelial function and proteinuria in Quinapril-treated diabetic patients with stage I hypertension: results from the quality study. J Cardiovasc Pharmacol Ther 17: 139–145, 2012 [DOI] [PubMed] [Google Scholar]
- 258.Rains JL. and Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med 50: 567–575, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rao VA, Klein SR, Bonar SJ, Zielonka J, Mizuno N, Dickey JS, Keller PW, Joseph J, Kalyanaraman B, and Shacter E. The antioxidant transcription factor Nrf2 negatively regulates autophagy and growth arrest induced by the anticancer redox agent mitoquinone. J Biol Chem 285: 34447–34459, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Rapola JM, Virtamo J, Ripatti S, Huttunen JK, Albanes D, Taylor PR, and Heinonen OP. Randomised trial of alpha-tocopherol and beta-carotene supplements on incidence of major coronary events in men with previous myocardial infarction. Lancet 349: 1715–1720, 1997 [DOI] [PubMed] [Google Scholar]
- 261.Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, Shirendeb UP, Calkins MJ, Reddy AP, Mao P, and Manczak M. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer's disease: implications to mitochondria-targeted antioxidant therapeutics. Biochim Biophys Acta 1822: 639–649, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Reily C, Mitchell TF, Chacko BK, Benavides G, Murphy MP, and Darley-Usmar V. Mitochondrially targeted compounds and their impact on cellular bioenergetics. Redox Biol 1: 86–93, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Remondino A, Kwon SH, Communal C, Pimentel DR, Sawyer DB, Singh K, and Colucci WS. Beta-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ Res 92: 136–138, 2003 [DOI] [PubMed] [Google Scholar]
- 264.Riedl MA, Saxon A, and Diaz-Sanchez D. Oral sulforaphane increases phase II antioxidant enzymes in the human upper airway. Clin Immunol 130: 244–251, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Rindler PM, Plafker SM, Szweda LI, and Kinter M. High dietary fat selectively increases catalase expression within cardiac mitochondria. J Biol Chem 288: 1979–1990, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, and Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A 106: 8665–8670, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Robertson L. and Hartley RC. Synthesis of N-arylpyridinium salts bearing a nitrone spin trap as potential mitochondria-targeted antioxidants. Tetrahedron 65: 5284–5292, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Rocha M, Apostolova N, Herance JR, Rovira-Llopis S, Hernandez-Mijares A, and Victor VM. Perspectives and potential applications of mitochondria-targeted antioxidants in cardiometabolic diseases and type 2 diabetes. Med Res Rev 34: 160–189, 2014 [DOI] [PubMed] [Google Scholar]
- 269.Rocha M, Rovira-Llopis S, Bañuls C, Bellod L, Falcon R, Castello R, Morillas C, Herance JR, Hernandez-Mijares A, and Victor VM. Mitochondrial dysfunction and oxidative stress in insulin resistance. Curr Pharm Des 19: 5730–5741, 2013 [DOI] [PubMed] [Google Scholar]
- 270.Rocha M, Rovira-Llopis S, Herance JR, Bañuls C, Polo M, Blas-Garcia A, Hernandez-Mijares A, and Victor VM. The pivotal role of nitric oxide: effects on the nervous and immune systems. Curr Pharm Des 20: 4679–4689, 2014 [DOI] [PubMed] [Google Scholar]
- 271.Rodriguez-Cuenca S, Cochemé HM, Logan A, Abakumova I, Prime TA, Rose C, Vidal-Puig A, Smith AC, Rubinsztein DC, Fearnley IM, Jones BA, Pope S, Heales SJ, Lam BY, Neogi SG, McFarlane I, James AM, Smith RA, and Murphy MP. Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free Radic Biol Med 48: 161–172, 2010 [DOI] [PubMed] [Google Scholar]
- 272.Roser KS, Brookes PS, Wojtovich AP, Olson LP, Shojaie J, Parton RL, and Anders MW. Mitochondrial biotransformation of omega-(phenoxy)alkanoic acids, 3-(phenoxy)acrylic acids, and omega-(1-methyl-1H-imidazol-2-ylthio)alkanoic acids: a prodrug strategy for targeting cytoprotective antioxidants to mitochondria. Bioorg Med Chem 18: 1441–1448, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ross MF, Prime TA, Abakumova I, James AM, Porteous CM, Smith RA, and Murphy MP. Rapid and extensive uptake and activation of hydrophobic triphenylphosphonium cations within cells. Biochem J 411: 633–645, 2008 [DOI] [PubMed] [Google Scholar]
- 274.Rougee M, Bensasson RV, Land EJ, and Pariente R. Deactivation of singlet molecular oxygen by thiols and related compounds, possible protectors against skin photosensitivity. Photochem Photobiol 47: 485–489, 1988 [DOI] [PubMed] [Google Scholar]
- 275.Rovira-Llopis S, Bañuls C, Apostolova N, Morillas C, Hernandez-Mijares A, Rocha M, and Victor VM. Is glycemic control modulating endoplasmic reticulum stress in leukocytes of type 2 diabetic patients? Antioxid Redox Signal 21: 1759–1765, 2014 [DOI] [PubMed] [Google Scholar]
- 276.Rovira-Llopis S, Rocha M, Falcon R, de Pablo C, Alvarez A, Jover A, Hernandez-Mijares A, and Victor VM. Is myeloperoxidase a key component in the ROS-induced vascular damage related to nephropathy in type 2 diabetes? Antioxid Redox Signal 19: 1452–1458, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Rozenberg O, Shih DM, and Aviram M. Paraoxonase 1 (PON1) attenuates macrophage oxidative status: studies in PON1 transfected cells and in PON1 transgenic mice. Atherosclerosis 181: 9–18, 2005 [DOI] [PubMed] [Google Scholar]
- 278.Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, Stiborova M, Adam V, and Kizek R. The role of metallothionein in oxidative stress. Int J Mol Sci 14: 6044–6066, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Saito C, Zwingmann C, and Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology 51: 246–254, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Salvi M, Battaglia V, Brunati AM, La Rocca N, Tibaldi E, Pietrangeli P, Marcocci L, Mondovì B, Rossi CA, and Toninello A. Catalase takes part in rat liver mitochondria oxidative stress defense. J Biol Chem 282: 24407–24415, 2007 [DOI] [PubMed] [Google Scholar]
- 281.Satarkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, and Beal MF. Mitochondrial α-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci 24: 7779–7788, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, and Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med 6: 221ra15, 2014 [DOI] [PubMed] [Google Scholar]
- 283.Schrader M. Shared components of mitochondrial and peroxisomal division. Biochim Biophys Acta 1763: 531–541, 2006 [DOI] [PubMed] [Google Scholar]
- 284.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, and Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J 26: 1749–1760, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Schulz E, Anter E, and Keaney JF. Oxidative stress, antioxidants, and endothelial function. Curr Med Chem 11: 1093–1104, 2004 [DOI] [PubMed] [Google Scholar]
- 286.Sen S, Kawahara B, and Chaudhuri G. Mitochondrial-associated nitric oxide synthase activity inhibits cytochrome c oxidase: implications for breast cancer. Free Radic Biol Med 57: 210–220, 2013 [DOI] [PubMed] [Google Scholar]
- 287.Serviddio G, Bellanti F, Sastre J, Vendemiale G, and Altomare E. Targeting mitochondria: a new promising approach for the treatment of liver diseases. Curr Med Chem 17: 2325–2337, 2010 [DOI] [PubMed] [Google Scholar]
- 288.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, and Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA 300: 2123–2133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Severin FF, Severina II, Antonenko YN, Rokitskaya TI, Cherepanov DA, Mokhova EN, Vyssokikh MY, Pustovidko AV, Markova OV, Yaguzhinsky LS, Korshunova GA, Sumbatyan NV, Skulachev MV, and Skulachev VP. Penetrating cation/fatty acid anion pair as a mitochondria-targeted protonophore. Proc Natl Acad Sci U S A 107: 663–668, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Severina II, Severin FF, Korshunova GA, Sumbatyan NV, Ilyasova TM, Simonyan RA, Rogov AG, Trendeleva TA, Zvyagilskaya RA, Dugina VB, Domnina LV, Fetisova EK, Lyamzaev KG, Vyssokikh MY, Chernyak BV, Skulachev MV, Skulachev VP, and Sadovnichii VA. In search of novel highly active mitochondria-targeted antioxidants: thymoquinone and its cationic derivatives. FEBS Lett 587: 2018–2024, 2013 [DOI] [PubMed] [Google Scholar]
- 291.Shenouda SM1, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess MA, Levit A, Kim B, Hartman ML, Joseph L, Shirihai OS, and Vita JA. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 124: 444–453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Skulachev VP. Cationic antioxidants as a powerful tool against mitochondrial oxidative stress. Biochem Biophys Res Commun 441: 275–279, 2013 [DOI] [PubMed] [Google Scholar]
- 293.Skulachev VP, Anisimov VN, Antonenko YN, Bakeeva LE, Chernyak BV, Erichev VP, Filenko OF, Kalinina NI, Kapelko VI, Kolosova NG, Kopnin BP, Korshunova GA, Lichinitser MR, Obukhova LA, Pasyukova EG, Pisarenko OI, Roginsky VA, Ruuge EK, Senin II, Severina II, Skulachev MV, Spivak IM, Tashlitsky VN, Tkachuk VA, Vyssokikh MY, Yaguzhinsky LS, and Zorov DB. An attempt to prevent senescence: a mitochondrial approach. Biochim Biophys Acta 1787: 437–461, 2009 [DOI] [PubMed] [Google Scholar]
- 294.Smith RA, Adlam VJ, Blaikie FH, Manas AR, Porteous CM, James AM, Ross MF, Logan A, Cochemé HM, Trnka J, Prime TA, Abakumova I, Jones BA, Filipovska A, and Murphy MP. Mitochondria-targeted antioxidants in the treatment of disease. Ann N Y Acad Sci 1147: 105–111, 2008 [DOI] [PubMed] [Google Scholar]
- 295.Smith RA, Hartley RC, Cochemé HM, and Murphy MP. Mitochondrial pharmacology. Trends Pharmacol Sci 33: 341–352, 2012 [DOI] [PubMed] [Google Scholar]
- 296.Smith RA. and Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci 1201: 96–103, 2010 [DOI] [PubMed] [Google Scholar]
- 297.Smith RA. and Murphy MP. Mitochondria-targeted antioxidants as therapies. Discov Med 11: 106–114, 2011 [PubMed] [Google Scholar]
- 298.Smith RA, Porteous CM, Coulter CM, and Murphy MP. Selective targeting of an antioxidant to mitochondria. Eur J Biochem 263: 709–716, 1999 [DOI] [PubMed] [Google Scholar]
- 299.Smith RA, Porteous CM, Gane AM, and Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci U S A 100: 5407–5412, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Soh N. Recent advances in fluorescent probes for the detection of reactive oxygen species. Anal Bioanal Chem 386: 532–543, 2006 [DOI] [PubMed] [Google Scholar]
- 301.Sovari AA, Rutledge CA, Jeong EM, Dolmatova E, Arasu D, Liu H, Vahdani N, Gu L, Zandieh S, Xiao L, Bonini MG, Duffy HS, and Dudley SC. Mitochondria oxidative stress, connexin43 remodeling, and sudden arrhythmic death. Circ Arrhythm Electrophysiol 6: 623–631, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Spasojević I, Chen Y, Noel TJ, Yu Y, Cole MP, Zhang L, Zhao Y, St. Clair DK, and Batinić-Haberle I. Mn porphyrin-based superoxide dismutase (SOD) mimic, MnIIITE-2-PyP5+, targets mouse heart mitochondria. Free Radic Biol Med 42: 1193–1200, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Speakman JR, Talbot DA, Selman C, Snart S, McLaren JS, Redman P, Krol E, Jackson DM, Johnson MS, and Brand MD. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell 3: 87–95, 2004 [DOI] [PubMed] [Google Scholar]
- 304.Srinivasan V, Doctrow S, Singh VK, and Whitnall MH. Evaluation of EUK-189, a synthetic superoxide dismutase/catalase mimetic as a radiation countermeasure. Immunopharmacol Immunotoxicol 30: 271–290, 2008 [DOI] [PubMed] [Google Scholar]
- 305.Stefanova NA, Muraleva NA, Skulachev VP, and Kolosova NG. Alzheimer's disease-like pathology in senescence-accelerated OXYS rats can be partially retarded with mitochondria-targeted antioxidant SkQ1. J Alzheimers Dis 38: 681–694, 2014 [DOI] [PubMed] [Google Scholar]
- 306.Steinberg SF. Oxidative stress and sarcomeric proteins. Circ Res 112: 393–405, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Stey C, Steurer J, Bachmann S, Medici TC, and Tramer MR. The effect of oral N-acetylcysteine in chronic bronchitis: a quantitative systematic review. Eur Respir J 16: 253–262, 2000 [DOI] [PubMed] [Google Scholar]
- 308.Stone WL. and Smith M. Therapeutic uses of antioxidant liposomes. Mol Biotechnol 27: 217–230, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Suarna C, Wu BJ, Choy K, Mori T, Croft K, Cynshi O, and Stocker R. Protective effect of vitamin E supplements on experimental atherosclerosis is modest and depends on preexisting vitamin E deficiency. Free Radic Biol Med 41: 722–730, 2006 [DOI] [PubMed] [Google Scholar]
- 310.Suh JH, Shigeno ET, Morrow JD, Cox B, Rocha AE, Frei B, and Hagen TM. Oxidative stress in the aging rat heart is reversed by dietary supplementation with (R)-(alpha)-lipoic acid. FASEB J 15: 700–706, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Sukhanova EI, Trendeleva TA, and Zvyagilskaya RA. Interaction of yeast mitochondria with fatty acids and mitochondria-targeted lipophilic cations. Biochemistry (Mosc) 75: 139–144, 2010 [DOI] [PubMed] [Google Scholar]
- 312.Sung S, Yao Y, Uryu K, Yang H, Lee VM, Trojanowski JQ, and Praticò D. Early vitamin E supplementation in young but not aged mice reduces Abeta levels and amyloid deposition in a transgenic model of Alzheimer's disease. FASEB J 18: 323–325, 2004 [DOI] [PubMed] [Google Scholar]
- 313.Supinski GS, Murphy MP, and Callahan LA. MitoQ administration prevents endotoxin-induced cardiac dysfunction. Am J Physiol Regul Integr Comp Physiol 297: 1095–1102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Suzuki T, Motohashi H, and Yamamoto M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol Sci 34: 340–346, 2013 [DOI] [PubMed] [Google Scholar]
- 315.Szeto HH. Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J 8: 277–283, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal 10: 601–619, 2008 [DOI] [PubMed] [Google Scholar]
- 317.Szeto HH. and Schiller PW. Novel therapies targeting inner mitochondrial membrane—from discovery to clinical development. Pharm Res 28: 2669–2679, 2011 [DOI] [PubMed] [Google Scholar]
- 318.Tadic V, Prell T, Lautenschlaeger J, and Grosskreutz J. The ER mitochondria calcium cycle and ER stress response as therapeutic targets in amyotrophic lateral sclerosis. Front Cell Neurosci 8: 147, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Tanno M, Kuno A, Yano T, Miura T, Hisahara S, Ishikawa S, Shimamoto K, and Horio Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem 285: 8375–8382, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Tattersall AB, Bridgman KM, and Huitson A. Acetylcysteine (Fabrol) in chronic bronchitis—a study on general practice. J Int Med Res 11: 279–284, 1983 [DOI] [PubMed] [Google Scholar]
- 321.Teicher BA, Holden SA, and Cathcart KN. Efficacy of Pt(Rh-123)2 as a radiosensitizer with fractionated X rays. Int J Radiat Oncol Biol Phys 13: 1217–1224, 1987 [DOI] [PubMed] [Google Scholar]
- 322.The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers inmale smokers. N Engl J Med 330: 1029–1035, 1994 [DOI] [PubMed] [Google Scholar]
- 323.Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, and Manning RD., Jr.Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 293: 3388–3395, 2007 [DOI] [PubMed] [Google Scholar]
- 324.Timmers S, Hesselink MK, and Schrauwen P. Therapeutic potential of resveratrol in obesity and type 2 diabetes: new avenues for health benefits? Ann N Y Acad Sci 1290: 83–89, 2013 [DOI] [PubMed] [Google Scholar]
- 325.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, and Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 14: 612–622, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Tovmasyan A, Sheng H, Weitner T, Arulpragasam A, Lu M, Warner DS, Vujaskovic Z, Spasojevic I, and Batinic-Haberle I. Design, mechanism of action, bioavailability and therapeutic effects of mn porphyrin-based redox modulators. Med Princ Pract 22: 103–130, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Townsend DM, Tew KD, and Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother 57: 145–155, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Toyama S, Shimoyama N, Ishida Y, Koyasu T, Szeto HH, and Shimoyama M. Characterization of acute and chronic neuropathies induced by oxaliplatin in mice and differential effects of a novel mitochondria-targeted antioxidant on the neuropathies. Anesthesiology 120: 459–473, 2014 [DOI] [PubMed] [Google Scholar]
- 329.Treweeke AT, Winterburn TJ, MacKenzie I, Barrett F, Barr C, Rushworth GF, Dransfield I, MacRury SM, and Megson IL. N-acetylcysteine inhibits platelet–monocyte conjugation in patients with type 2 diabetes with depleted intraplatelet glutathione: a randomised controlled trial. Diabetologia 55: 2920–2928, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Trnka J, Blaikie FH, Smith RA, and Murphy MP. A mitochondria-targeted nitroxide is reduced to its hydroxylamine by ubiquinol in mitochondria. Free Radic Biol Med 44: 1406–1419, 2008 [DOI] [PubMed] [Google Scholar]
- 331.Tward A, Xia YR, Wang XP, Shi YS, Park C, Castellani LW, Lusis AJ, and Shih DM. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation 106: 484–490, 2002 [DOI] [PubMed] [Google Scholar]
- 332.Ureshino RP, Rocha KK, Lopes GS, Bincoletto C, and Smaili SS. Calcium signaling alterations, oxidative stress, and autophagy in aging. Antioxid Redox Signal 21: 123–137, 2014 [DOI] [PubMed] [Google Scholar]
- 333.Vaidya B, Paliwal R, Rai S, Khatri K, Goyal AK, Mishra N, and Vyas SP. Cell-selective mitochondrial targeting: a new approach for cancer therapy. Cancer Ther 7: 141–148, 2009 [Google Scholar]
- 334.Vang O. What is new for resveratrol? Is a new set of recommendations necessary? Ann N Y Acad Sci 1290: 1–11, 2013 [DOI] [PubMed] [Google Scholar]
- 335.Vergeade A, Mulder P, Vendeville-Dehaudt C, Estour F, Fortin D, Ventura-Clapier R, Thuillez C, and Monteil C. Mitochondrial impairment contributes to cocaine-induced cardiac dysfunction: prevention by the targeted antioxidant MitoQ. Free Radic Biol Med 49: 748–756, 2010 [DOI] [PubMed] [Google Scholar]
- 336.Victor VM, Rocha M, Herance R, and Hernandez-Mijares A. Oxidative stress and mitochondrial dysfunction in type 2 diabetes. Curr Pharm Des 17: 3947–3958, 2011 [DOI] [PubMed] [Google Scholar]
- 337.Wang B, Sun J, Ma Y, Wu G, Tian Y, Shi Y, and Le G. Resveratrol preserves mitochondrial function, stimulates mitochondrial biogenesis, and attenuates oxidative stress in regulatory T cells of mice fed a high-fat diet. J Food Sci 79: 1823–1831, 2014 [DOI] [PubMed] [Google Scholar]
- 338.Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, Li YR, and Li PF. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med 17: 71–78, 2011 [DOI] [PubMed] [Google Scholar]
- 339.Wang M, Lang X, Cui S, Zou L, Cao J, Wang S, and Wu X. Quantitative assessment of the influence of paraoxonase 1 activity and coronary heart disease risk. DNA Cell Biol 31: 975–982, 2012 [DOI] [PubMed] [Google Scholar]
- 340.Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, and Sun AY. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res 958: 439–447, 2002 [DOI] [PubMed] [Google Scholar]
- 341.Wang X, Yu Y, Ji L, Liang X, Zhang T, and Hai C. Alpha lipoic acid protects against myocardial ischemia/reperfusion injury via multiple target effects. Food Chem Toxicol 49: 2750–2757, 2011 [DOI] [PubMed] [Google Scholar]
- 342.Wani WY, Gudup S, Sunkaria A, Bal A, Singh PP, Kandimalla RJ, Sharma DR, and Gill KD. Protective efficacy of mitochondrial targeted antioxidant MitoQ against dichlorvos induced oxidative stress and cell death in rat brain. Neuropharmacology 61: 1193–1201, 2011 [DOI] [PubMed] [Google Scholar]
- 343.Warburton DE, Nicol CW, and Bredin SS. Health benefits of physical activity: the evidence. Can Med Ass J 174: 801–809, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wasiak S, Zunino R, and McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria Turing apoptotic cell death. J Cell Biol 177: 439–450, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Watson PS, Scalia GM, Galbraith A, Burstow DJ, Bett N, and Aroney CN. Lack of effect of coenzyme Q on left ventricular function in patients with congestive heart failure. J Am Coll Cardiol 33: 1549–1552, 1999 [DOI] [PubMed] [Google Scholar]
- 346.Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, and Schumacker PT. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res 99: 970–978, 2006 [DOI] [PubMed] [Google Scholar]
- 347.Waypa GB, Marks JD, Guzy RD, Mungai PT, Schriewer JM, Dokic D, Ball MK, and Schumacker PT. Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation. Am J Respir Crit Care Med 187: 424–432, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Wedgwood S. and Steinhorn RH. Role of reactive oxygen species in neonatal pulmonary vascular disease. Antioxid Redox Signal 21: 1926–1942, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Weissig V, D'Souza GG, and Torchilin VP. DQAsome/DNA complexes release DNA upon contact with isolated mouse liver mitochondria. J Control Release 75: 401–408, 2001 [DOI] [PubMed] [Google Scholar]
- 350.Wenzel P, Daiber A, Oelze M, Brandt M, Closs E, Xu J, Thum T, Bauersachs J, Ertl G, Zou MH, Förstermann U, and Münzel T. Mechanisms underlying recoupling of eNOS by HMG-CoA reductase inhibition in a rat model of streptozotocininduced diabetes mellitus. Atherosclerosis 198: 65–76, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Weyer G, Babej-Dolle RM, Hadler D, Hofmann S, and Herrmann WM. A controlled study of 2 doses of idebenone in the treatment of Alzheimer's disease. Neuropsychobiology 36: 73–82, 1997 [DOI] [PubMed] [Google Scholar]
- 352.Whiteman M, Spencer JP, Szeto HH, and Armstrong JS. Do mitochondriotropic antioxidants prevent chlorinative stress-induced mitochondrial and cellular injury? Antioxid Redox Signal 10: 641–650, 2008 [DOI] [PubMed] [Google Scholar]
- 353.Wilcox CS. and Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev 60: 418–469, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Wipf P, Xiao J, Jiang J, Belikova NA, Tyurin VA, Fink MP, and Kagan VE. Mitochondrial targeting of selective electron scavengers: synthesis and biological analysis of hemigramicidin-TEMPO conjugates. J Am Chem Soc 127: 12460–12461, 2005 [DOI] [PubMed] [Google Scholar]
- 355.Wong CH, Iskandar KB, Yadav SK, Hirpara JL, Loh T, and Pervaiz S. Simultaneous induction of non-canonical autophagy and apoptosis in cancer cells by ROS-dependent ERK and JNK activation. PLoS One 5: e9996, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MA, Ives SJ, Barrett-O'Keefe Z, and Richardson RS. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 59: 818–824, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Wu TW, Hashimoto N, Au JX, Wu J, Mickle DA, and Carey D. Trolox protects rat hepatocytes against oxyradical damage and the ischemic rat liver from reperfusion injury. Hepatology 13: 575–580, 1991 [PubMed] [Google Scholar]
- 358.Wu TW, Hashimoto N, Wu J, Cavey D, Li RK, Micle DA, and Weisel RD. The cytoprotective effect of Trolox demonstrated with three types of human cells. Biochem Cell Biol 68: 1189–1194, 1990 [DOI] [PubMed] [Google Scholar]
- 359.Xia N, Daiber A, Habermeier A, Closs EI, Thum T, Spanier G, Lu Q, Oelze M, Torzewski M, Lackner KJ, Münzel T, Förstermann U, and Li H. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J Pharmacol Exp Ther 335: 149–154, 2010 [DOI] [PubMed] [Google Scholar]
- 360.Xie YW, Kaminski PM, and Wolin MS. Inhibition of rat cardiac muscle contraction and mitochondrial respiration by endogenous peroxynitrite formation during posthypoxic reoxygenation. Circ Res 82: 891–897, 1998 [DOI] [PubMed] [Google Scholar]
- 361.Yamada Y, Furukawa R, Yasuzaki Y, and Harashima H. Dual function MITO-Porter, a nano carrier integrating both efficient cytoplasmic delivery and mitochondrial macromolecule delivery. Mol Ther 19: 1449–1456, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Yamada Y. and Harashima H. Mitochondrial drug delivery systems for macromolecule and their therapeutic application to mitochondrial diseases. Adv Drug Deliv Rev 60: 1439–1462, 2008 [DOI] [PubMed] [Google Scholar]
- 363.Yamada Y. and Harashima H. Enhancement in selective mitochondrial association by direct modification of a mitochondrial targeting signal peptide on a liposomal based nanocarrier. Mitochondrion 13: 526–532, 2013 [DOI] [PubMed] [Google Scholar]
- 364.Yan LJ, Levine RL, and Sohal RS. Oxidative damage during aging targets mitochondrial aconitase. Proc Natl Acad Sci U S A 94: 11168–11172, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Yang J, Wu LJ, Tashino S, Onodera S, and Ikejima T. Reactive oxygen species and nitric oxide regulate mitochondria-dependent apoptosis and autophagy in evodiamine-treated human cervix carcinoma HeLa cells. Free Radic Res 42: 492–504, 2008 [DOI] [PubMed] [Google Scholar]
- 366.Yang L, Zhao K, Calingasan NY, Luo G, Szeto HH, and Beal MF. Mitochondria targeted peptides protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Antioxid Redox Signal 11: 2095–2104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Ye B, Maret W, and Vallee BL. Zinc metallothionein imported into liver mitochondria modulates respiration. Proc Natl Acad Sci U S A 98: 2317–2322, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Yee C, Yang W, and Hekimi S. The intrinsic apoptosis pathway mediates the pro-longevity response to mitochondrial ROS in C. elegans. Cell 157: 897–909, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi Fanelli F, Patterson BW, and Klein S. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab 16: 658–664, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Yousif LF, Stewart KM, Horton KL, and Kelley SO. Mitochondria-penetrating peptides: sequence effects and model cargo transport. Chembiochem 10: 2081–2088, 2009 [DOI] [PubMed] [Google Scholar]
- 371.Yousif LF, Stewart KM, and Kelley SO. Targeting mitochondria with organelle-specific compounds: strategies and applications. Chembiochem 10: 1939–1950, 2009 [DOI] [PubMed] [Google Scholar]
- 372.Yu T, Robotham JL, and Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A 103: 2653–2658, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Zang QS, Sadek H, Maass DL, Martinez B, Ma L, Kilgore JA, Williams NS, Frantz DE, Wigginton JG, Nwariaku FE, Wolf SE, and Minei JP. Specific inhibition of mitochondrial oxidative stress suppresses inflammation and improves cardiac function in a rat pneumonia-related sepsis model. Am J Physiol Heart Circ Physiol 302: 1847–1859, 2012 [DOI] [PubMed] [Google Scholar]
- 374.Zhang E, Zhang C, Su Y, Cheng T, and Shi C. Newly developed strategies for multifunctional mitochondria-targeted agents in cancer therapy. Drug Discov Today 16: 140–146, 2011 [DOI] [PubMed] [Google Scholar]
- 375.Zhang H, Kong X, Kang J, Su J, Li Y, Zhong J, and Sun L. Oxidative stress induces parallel autophagy and mitochondria dysfunction in human glioma U251 cells. Toxicol Sci 110: 376–388, 2009 [DOI] [PubMed] [Google Scholar]
- 376.Zhang Y, Han P, Wu N, He B, Lu Y, Li S, Liu Y, Zhao S, Liu L, and Li Y. Amelioration of lipid abnormalities by α-lipoic acid through antioxidative and anti-inflammatory effects. Obesity (Silver Spring) 19: 1647–1653, 2011 [DOI] [PubMed] [Google Scholar]
- 377.Zhao K, Luo G, Zhao GM, Schiller PW, and Szeto HH. Transcellular transport of a highly polar 3+ net charge opioid tetrapeptide. J Pharmacol Exp Ther 304: 425–432, 2003 [DOI] [PubMed] [Google Scholar]
- 378.Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, and Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279: 34682–34690, 2004 [DOI] [PubMed] [Google Scholar]
- 379.Zhu BZ, Mao L, Fan RM, Zhu JG, Zhang YN, Wang J, Kalyanaraman B, and Frei B. Ergothioneine prevents copper-induced oxidative damage to DNA and protein by forming a redox-inactive ergothioneine-copper complex. Chem Res Toxicol 24: 30–34, 2011 [DOI] [PubMed] [Google Scholar]
- 380.Zupančič Š, Kocbek P, Zariwala MG, Renshaw D, Gul MO, Elsaid Z, Taylor KM, and Somavarapu S. Design and development of novel mitochondrial targeted nanocarriers, DQAsomes for curcumin inhalation. Mol Pharm 11: 2334–2345, 2014 [DOI] [PubMed] [Google Scholar]