Abstract
The Chinese herbs Herba Epimedii, Fructus Ligustri Lucidi and Rhizoma Polygonati were injected into Parkinson's disease mice established via intraperitoneal injection of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine hydrochloride. The selective monoamine oxidase B inhibitor selegiline was used as a positive control drug. After successive administration for 4 weeks, Herba Epimedii could downregulate the expression of caspase-3 and increase the brain-derived neurotrophic factor level, as well as increase tyrosine hydroxylase activity in the substantia nigra of Parkinson's disease mouse models. Rhizoma Polygonati could downregulate the expression of caspase-3 and FasL, and increase neural growth factor and brain-derived neurotrophic factor levels. Fructus Ligustri Lucidi could downregulate caspase-3 expression. Rhizoma Polygonati and Fructus Ligustri Lucidi did not produce obvious effects on tyrosine hydroxylase activity. Herba Epimedii and Fructus Ligustri Lucidi yielded similar effects on apoptosis-promoting factors to those elicited by selegiline. Herba Epimedii and Rhizoma Polygonati significantly increased the levels of neurotrophic factors compared with selegiline. Herba Epimedii significantly increased tyrosine hydroxylase activity compared with selegiline. It is indicated that the kidney-tonifying Chinese herbal preparation can downregulate the expression of apoptosis-promoting factors, increase neurotrophic factors levels in the substantia nigra and striatum, as well as increase tyrosine hydroxylase activity in the substantia nigra of Parkinson's disease mouse models, thereby exerting a stronger or similar neuroprotective effects compared with selegiline.
Keywords: kidney-tonifying Chinese herbal preparation, Herba Epimedii, Fructus Ligustri Lucidi, Rhizoma Polygonati, Parkinson's disease, substantia nigra and striatum, substantia nigra neuron, neural regeneration
INTRODUCTION
The main pathological features of Parkinson's disease (PD) are degeneration and loss of dopaminergic neurons in the substantia nigra. The dopaminergic neuronal degeneration and loss is closely associated with activation of caspase-3[1,2] and decrease in expression of brain-derived neurotrophic factor (BDNF)[3]. Caspase activation and decrease in BDNF expression are the early signals of dopaminergic neuronal apoptosis. The caspases are a group of enzymes that are one of the main executors of the apoptotic process. In health, the caspases exist within cells as inactive zymogens. Under certain induction conditions (e.g., Fas combined with FasL), they are activated in a cascade, which eventually leads to the activation of caspase-3 (a key executor of apoptosis), resulting in cellular apoptosis[4]. In the process of caspase-3 activation, the apoptosis-inhibiting protein Bcl-2 inhibits activation of caspase-3 through pro-apoptotic protease-activating factor 1 (Apaf-1). Seventy percent of dopaminergic neurons in the substantia nigra (compact part) express BDNF simultaneously, which can postpone neuronal degeneration and apoptosis, thereby postponing the progression of PD[3]. BDNF is a protein discovered in 1982 that promotes nerve growth[5]. Just like other neurotrophic factors such as nerve growth factor (NGF)[6] and glial cell-derived neurotrophic factor (GDNF)[7], BDNF has an important role in maintaining neuronal function, promoting the regeneration and repair of injured neurons, and preventing degenerative changes in nerve cells[8,9,10]. BDNF deficiency can cause neuronal apoptosis. It has been reported that BDNF and BDNF mRNA expression is significantly decreased in the substantia nigra and striatum of PD patients[3]. Injection of NGF-treated PC-12 cells and GDNF into the striatum of PD rats can improve behavioral symptoms and increase the survival of dopaminergic neurons and number of tyrosine hydroxylase (TH)-positive neurons in the substantia nigra and striatum[11]. These findings suggest that increasing levels of neurotrophic factors in the brain can postpone the progression of dopaminergic neuronal apoptosis.
The pathogenesis of PD is complex. There has been no effective means to radically halt PD progression, only to treat the symptoms. L-dihydroxyphenylalanine is the primary drug for PD. It has remarkable curative effects, in particular within the first 3-5 years of treatment, but long-term medication results in less satisfactory effects. About 50–80% of patients present with fluctuating symptoms (primarily characterized by the on-off phenomenon, end-of-dose phenomenon, and up-down phenomenon), dyskinesia, and psychiatric symptoms over 0.3–18 years (mean, 4.1 years)[12]. Use of dopamine receptor agonists and monoamine oxidase B inhibitors can lead to adverse effects, including desensitization and insomnia, although their long-term application can avoid the L-dihydroxyphenylalanine failure syndrome or long-term L-dihydroxyphenylalanine syndrome[12]. Therefore, based on conventional treatments, novel therapeutic drugs with few side effects and new effect targets should be developed further.
At present, traditional Chinese medicine has not become dominant in treatment of PD. The traditional Chinese medicine theory believes that the treatment for PD should involve tonifying (i.e., nourishing and invigorating) the kidney and benefiting the bone marrow. Under the guidance of traditional Chinese medicine theory, physicians treated PD patients using kidney-tonifying Chinese herbal preparations (KTCHP) (Radix Polygoni Multiflori, Fructus Lycii, Rhizoma Polygonati, Herba Epimedii and Herba Cistanches) compared the outcome with those from the simple Western medicine group[13,14]. Results demonstrated that the KTCHP produced better curative effects than simple Western medicine. Modern pharmacological evidence[14,15,16] suggests that the main effective constituent of KTCHP, icariin (a polysaccharide from Fructus Ligustri Lucidi and Rhizoma Polygonati), can effectively remove the hydroxy radical, superoxide anion radical, and active oxygen; increase antioxidase activity; prevent cells being damaged by oxygen free radicals; strengthen immunological function; promote metabolism; and postpone aging. According to PD pathogenesis based on traditional Chinese medicine theory, clinical application, and modern pharmacological studies, we selected the Chinese herbs Herba Epimedii, Fructus Ligustri Lucidi and Rhizoma Polygonati as treatment drugs, and used selegiline (selective monoamine oxidase B inhibitor) as the positive control drug. Selegiline improves the symptoms of PD by: inhibiting the reuptake of monoamine oxidase B and dopamine; hampering 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) transformation into 1-methyl-4-phenylpyridinium ion (MPP+); and preventing MPTP neurotoxicity to protect neurons[17,18]. We detected the expression of apoptosis-promoting factors and neurotrophic factors in the substantia nigra and striatum in a mouse model of PD. We also investigated the possible pathway of KTCHP in PD treatment to provide experimental evidence for the clinical application of KTCHP.
RESULTS
Quantitative analysis of experimental animals
One-hundred male C57BL/6J mice were selected, and 10 of 100 were used as controls. The other 90 mice were intraperitoneally injected with 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine hydrochloride (MPTP·HCI) to establish PD models. Fifty PD model mice were selected and randomly divided into five groups (n = 10): Herba Epimedii, Fructus Ligustri Lucidi, Rhizoma Polygonati, selegiline and distilled water were administered, respectively. Finally, 60 mice were involved in result analysis.
Comparison of the expression of FasL, Fas, caspase-3, and Bcl-2 in the substantia nigra and striatum after drug administration among groups
Four weeks after drug administration, Herba Epimedii and Fructus Ligustri Lucidi could downregulate caspase-3 expression in the substantia nigra and striatum of PD mice (35.69% and 47.96%, respectively). Rhizoma Polygonati could downregulate the expression of caspase-3 and FasL in the substantia nigra and striatum of PD mice (43.41% and 61.92%, respectively). Herba Epimedii, Fructus Ligustri Lucidi, and Rhizoma Polygonati showed similar effects with respect to apoptosis-related factors as selegiline. Herba Epimedii, Fructus Ligustri Lucidi, and Rhizoma Polygonati did not produce effects on the expression of anti-apoptotic factor Bcl-2 (Figure 1).
Figure 1.
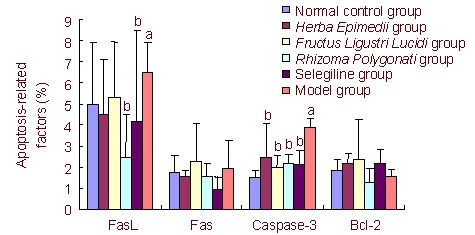
Level of apoptosis-related factors in the substantia nigra and striatum after drug administration (flow cytometry, %).
Data are presented as mean ± SD. aP < 0.05, vs. normal control group; bP < 0.05, vs. model group.
One-way analysis of variance was used for data comparison between groups. Least significant difference or Games-Howell post-hoc tests were used for multiple comparisons among groups.
The percentage of expression of FasL-, Fas-, caspase-3-, and Bcl-2-positive cells was calculated through use of CellQuest software.
Comparison of levels of NGF, BDNF and GDNF in the substantia nigra and striatum of PD mice after drug administration among groups
The following effects were noted 4 weeks after drug administration. Herba Epimedii increased the BDNF level in the substantia nigra-striatum (36.33%). Rhizoma Polygonati could increase BDNF and NGF levels (53.06% and 15.91%, respectively) in the substantia nigra and striatum. Herba Epimedii and Rhizoma Polygonati significantly increased the levels of neurotrophic factors compared with selegiline (P < 0.05). Fructus Ligustri Lucidi yielded no obvious effects on endogenous neurotrophic factors in the substantia nigra-striatum of PD mice. Herba Epimedii, Fructus Ligustri Lucidi, and Rhizoma Polygonati had no increase effect on GDNF (Figure 2).
Figure 2.
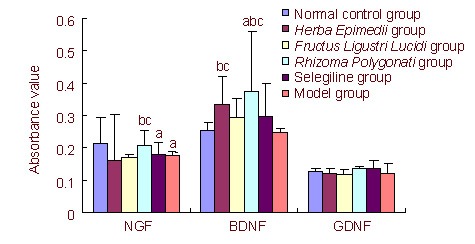
Content of nerve-related neurotrophic factors in the substantia nigra and striatum tissue after drug administration (enzyme-linked immunosorbent assay).
NGF: Neural growth factor; BDNF: brain-derived neurotrophic factor; GDNF: glial cell-derived neurotrophic factor. Data are presented as mean ± SD. aP < 0.05, vs. normal control group; bP < 0.05, vs. model group; cP < 0.05, vs. selegiline group.
One-way analysis of variance was used for data comparison between groups. Least significant difference or Games-Howell post-hoc tests were used for multiple comparisons among groups.
Comparison of TH activity in the substantia nigra-striatum of PD mice after drug administration among groups
Four weeks after drug administration, Herba Epimedii could increase TH activity until the level observed in the substantia nigra of normal mice, showing similar effects to those seen in the selegiline group (P < 0.05). Fructus Ligustri Lucidi and Rhizoma Polygonati exhibited no obvious effects on TH activity (Table 1).
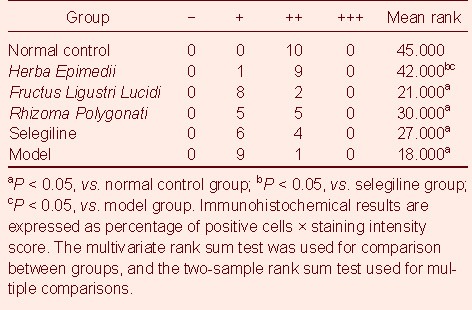
Table 1.
Activity of tyrosine hydroxylase in the substantia nigra of Parkinson's disease mouse models in each group
The following results were noted 4 weeks after drug administration. The normal control group showed the highest number of TH-positive cells and darkest staining. The Herba Epimedii group showed a similar number of TH-positive cells and staining degree to the normal control group. There was no obvious difference in the number of TH-positive cells and staining degree among the Fructus Ligustri Lucidi, Rhizoma Polygonati, selegiline and model groups, but the number of TH-positive cells was smaller and staining slighter in these four groups compared with that seen in the normal control group (Figure 3).
Figure 3.
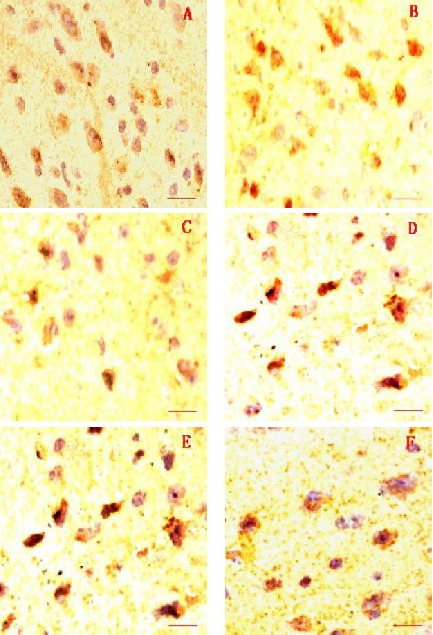
Immunohistochemical staining of the substantia nigra by tyrosine hydroxylase. Scale bars: 2 µm.
(A) Normal control group; (B) Herba Epimedii group; (C) Fructus Ligustri Lucidi group; (D) Rhizoma Polygonati group; (E) selegiline group; (F) model group.
The number of tyrosine hydroxylase-positive cells in the Herba Epimedii group was similar with that in the normal control group, but that was higher than that in the Fructus Ligustri Lucidi, Rhizoma Polygonati, selegiline, and model groups.
Morphological changes in substantia nigra neurons after drug administration in each group
Transmission electron microscopy results showed that, in the normal control group, substantia nigra neurons had intact cell membranes and organelles, abundant mitochondria and Golgi complexes, few lysosomes, and slightly stained chromatin scattering in the center of the nucleus. In the model group, substantia nigra neurons had the manifestations of apoptosis. This included reduced cell body, a decreased ratio of nuclear to chromatin, pyknosis, chromatin margination, and expanded endoplasmic reticula with vacuoles. In the Herba Epimedii, Fructus Ligustri Lucidi, Rhizoma Polygonati, and selegiline groups, substantia nigra neurons had slightly alleviated manifestations, including pyknosis and chromatin margination; expanded endoplasmic reticula were not obvious, but there were more lysosomes than in the model group (Figure 4).
Figure 4.
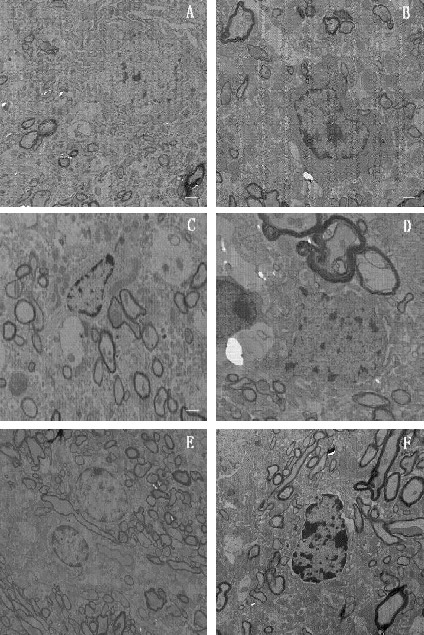
Transmission electron microscopy images of substantia nigra neurons from each group. Scale bars: 2 µm.
(A) Normal control group; (B) Herba Epimedii group; (C) Fructus Ligustri Lucidi group; (D) Rhizoma Polygonati group; (E) selegiline group; (F) model group.
In the normal control group, an intact cell membrane and organelles were observed. In the Herba Epimedii, Fructus Ligustri Lucidi, Rhizoma Polygonati, selegiline, and model groups, pyknosis of different degrees and chromatin margination were observed.
DISCUSSION
There is evidence that apoptosis-inhibiting factor Bcl-2 expression is downregulated in MPTP-induced PD mouse models[19,20]. Moreover, cell apoptosis is primarily induced by degradation of the substrate of caspases. Therefore, blocking the activation of caspases has become an important means to treat PD or hamper PD progression[4]. Studies have shown that a single drug or preparation for tonifying the liver and kidney or tonifying the spleen and kidney can upregulate expression of Bcl-2 and downregulate expression of caspase-3, Fas and FasL in the brains of experimental animals, thereby reducing neuronal apoptosis[5,10]. The present study demonstrated that: Herba Epimedii and Fructus Ligustri Lucidi can downregulate caspase-3 expression in the substantia nigra and striatum of PD mice; Rhizoma Polygonati can downregulate expression of caspase-3 and FasL in the substantia nigra and striatum of PD mice; Herba Epimedii, Fructus Ligustri Lucidi, and Rhizoma Polygonati exhibited no obvious effects on Bcl-2 expression. These results are consistent with previous findings that tonic traditional Chinese medicine can downregulate expression of caspase-3 and FasL in rat models of high fat diet or focal cerebral ischemia/reperfusion[21,22,23].
A clinical study has demonstrated that a liver- and kidney-nourishing Chinese herbal preparation shows satisfactory curative effects with respect to improving the symptoms of PD[15]. The present study demonstrated that: Herba Epimedii increased the BDNF level in the substantia nigra and striatum of PD mice; Rhizoma Polygonati increased levels of BDNF and NGF in the substantia nigra and striatum of PD mice, whereas Fructus Ligustri Lucidi did not produce obvious effects on endogenous neurotrophic factors. The endogenous neurotrophic factors NGF, BDNF and GDNF show important effects on the development, differentiation and survival of midbrain dopaminergic neurons[24,25].
Therefore, KTCHP may exert effects in PD patients by enhancing the levels of neurotrophic factors in the brain. The satisfactory effects of KTCHP on PD treatment are also related to the elevation of TH activity in brain tissue. For example, one study[26] showed that Bushen Huoxue Yin may treat PD by increasing TH activity in rat brain tissue. Another study[27] showed that cistanche total glycosides (the efficacious component of KTCHP Herba Cistanches (Roucongrong, Desert-living Cistanche)) can obviously inhibit the reduction in nigral dopaminergic neurons and the decrease in striatal TH activity in a mouse model of MPTP-induced PD. The present study demonstrated that Herba Epimedii could increase TH activity in the substantia nigra to normal levels in a mouse model of PD, whereas Fructus Ligustri Lucidi and Rhizoma Polygonati did not produce such obvious effects. The observation that Herba Epimedii can increase TH activity in the substantia nigra in a mouse model of PD is consistent with previous findings that KTCHP can increase TH activity in the brain[15,27]. In addition, transmission electron microscopy results showed that, after administration of Herba Epimedii, Fructus Ligustri Lucidi, and Rhizoma Polygonati, neuronal apoptosis induced by PD was alleviated, indicating that these three herbs exhibit protective effects on neurons damaged in PD.
Taken together, KTCHP can downregulate the expression of apoptosis-related factors in the substantia nigra-striatum of PD mice, increase the levels of neurotrophic factors, increase TH activity, decrease nigral neuronal apoptosis, and protect dopaminergic neurons. However, there are various herbal components in KTCHP; the precise effective components need further investigation.
MATERIALS AND METHODS
Design
A randomized controlled animal experiment.
Time and setting
The experiment was completed at the Laboratory Animal Center, Fujian University of Traditional Chinese Medicine (FUTCM), China from April to October 2009.
Materials
Animals
One-hundred male C57BL/6J mice (SPF grade; age, 7 weeks; 20 ± 2 g)[28,29] were provided by Shanghai SLAC Laboratory Animal Company Limited, Shanghai, China (license No. SCXK (Hu) 2007-0005). These mice were raised at the Laboratory Animal Center of FUTCM. After induction of PD, they were housed (5 mice/cage) at 20–22°C and at a relative humidity of 40–60%, in a 12-hour light/dark cycle (lights on between 7:00 a.m. and 7:00 p.m.). Complete granulated animal feeds were provided by the Laboratory Animal Center at the FUTCM. All experimental procedures were conducted in conformity with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Drugs
Herba Epimedii (the dried aerial part of Epimedium sagittatum Maxim.) is produced in Sichuan Province, China. Fructus Ligustri Lucidi (the dried fruit of Ligustrum lucidum Ait.), is produced in Zhejiang Province, China. Rhizoma Polygonati (the dried rhizome of Polygonatum sibiricum Red.), is produced in Hebei Province, China. These drugs were provided by the Fujian Medical Group (Fujian, China).
Herba Epimedii, Fructus Ligustri Lucidi, and Rhizoma Polygonati were identified and determined by the Laboratory of Pharmacology of Chinese Herbs, FUTCM. The total flavonoids of Herba Epimedii were determined by icariin level (12.4%)[30]. Polysaccharide from Fructus Ligustri Lucidi was determined by anhydrous dextrose (C6H12O6; 13.2%) using ultraviolet spectrophotometry (Chinese Pharmacopoeia Commission, 2005). The levels of oleanolic acid in Fructus Ligustri Lucidi and icariin level in Herba Epimedii as determined by high-performance liquid chromatography (HPLC)[30] were 1.37% and 2.67%, respectively (Figure 5). These results confirmed that the herbs corresponded to the quality criteria of the Chinese Pharmacopoeia in 2005[30] and could be used for subsequent experiments.
Figure 5.
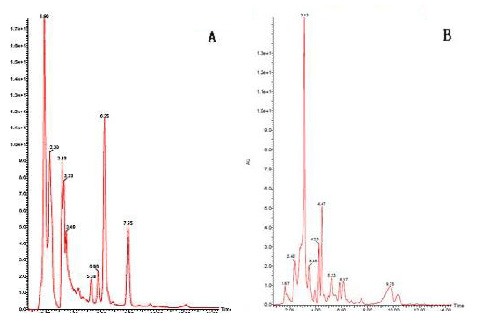
High-performance liquid chromatography (HPLC) maps of samples Herba Epimedii and Fructus Ligustri Lucidi.
(A) HPLC map of sample Herba Epimedii.
(B) HPLC map of sample Fructus Ligustri Lucidi.
Selegiline (State Drug Approval Document Number H20040632; 5 mg × 10 tablets/box) was produced by Nanjing Sike Pharmaceutical Company Limited (Nanjing, China).
Herba Epimedii (36 g), Fructus Ligustri Lucidi (36 g), and Rhizoma Polygonati (48 g) were respectively added into a decoction vessel containing 1 L distilled water (YSH-2, Dongling Science and Technology Company Limited, Taiyuan, China). After 60-minutes soaking, the mixture of drug and water was boiled, and decocted under slow heating for 30 minutes. Distilled water (500 mL) was added for another 15–20 minutes of decoction. Before and after this decoction was one step of liquor filtering. The liquor obtained by double-filtering was decocted to 300 mL. Finally, liquor was concentrated to 120, 120, and 160 mg/mL for Herba Epimedii, Fructus Ligustri Lucidi, and Rhizoma Polygonati, respectively. After filtration, the liquor was bottled, sealed under sterile conditions, and preserved at 4°C for later use.
Selegiline (12 mg) was prepared in 300 mL solution with distilled water (concentration, 0.04 mg/mL). This was followed by bottle filling, sealing under sterile conditions, and preservation at 4°C.
Methods
Modeling preparation and interventions
C57BL/6J mice were administered (i.p.) with MPTP·HCI (Sigma-Aldrich (Shanghai) Trading Company, Shanghai, China), 25 mg/kg once, then once every 2 days, on 18 occasions within 5 weeks[28,29]. At least 4 hours after each administration, numbers of autonomic activities and time-to-stabilize bar were determined through the use of a multifunctional mouse autonomic activity recorder (YLS-1A, Huaibei Zhenghua Biological Instruments Co., Ltd., Anhui Province, China) and a stabilizer bar fatigue testing machine (YLS-4C, Huaibei Zhenghua Biological Instruments Co., Ltd.)[31,32]. After the final detection, the autonomic activities within 5 weeks were statistically analyzed. Mice that presented a 40% decrease in the number of autonomic activities and time-to-stabilize bar at 5 weeks compared with those at 1 week were considered to be successfully induced with PD. Mice from the Herba Epimedii, Fructus Ligustri Lucidi, Rhizoma Polygonati, selegiline groups were administered via the intragastric route corresponding to liquor or aqueous solution. Model group mice and normal controls were administered distilled water. Each mouse received 0.5 mL liquor or aqueous solution twice a day for 4 successive weeks. Administrations were at 8:00 a.m. and 4:00 p.m. every day. The dose for Herba Epimedii, Fructus Ligustri Lucidi, Rhizoma Polygonati, and selegiline was 6, 6, 8, and 2 mg/kg per day, respectively (equal to the most common dose in adults).
Sample collection and observation indices
After 4-week administration, mice were anesthetized by intraperitoneal injection of 4% chloral hydrate (0.1 mL/10 g). The brain was rapidly harvested using scissors. Fatty tissue on the surface of the cerebral cortex was rinsed with physiological saline. After referring to the Mouse Brain[33], the substantia nigra and striatum were severed in an ice bath. The right substantia nigra and striatum tissue of six mice in each group were chopped into paste. After addition of phosphate-buffered saline (PBS), the resultant mixture was prepared into a monocellular solution through the use of 200-mesh nylon mesh (Anrui Wujin Mesh Industry Factory, Anping County, Hebei Province, China). After supernatant removal by centrifugation and subsequent addition of PBS, cells (1 × 106/mL) were used for the detection of expression of FasL, Fas, caspase-3, and Bcl-2. The left substantia nigra and striatum tissue (0.1 g) was homogenized after addition of 1 mL physiological saline for detection of the levels of NGF, BDNF and GDNF in the supernatant. Bilateral substantia nigra of the remaining four mice in each group was severed in an ice bath. The substantia nigra of one mouse was fixed in 2.5% glutaraldehyde and preserved at 4°C for transmission electron microscopy observation, and that of the other three mice was fixed in 10% formalin for 24 hours, washed with running water for 10 minutes, and fixed with 70% ethanol for immunohistochemical detection.
Detection of the expression of FasL-, Fas-, caspase-3-, Bcl-2 by flow cytometry
A 200-μL substantia nigra-striatum mononuclear cell suspension from each mouse was equally portioned into two tubes. Phycoerythrin-labeled Fasl antibody (5 μL, BD Bioscience Pharmingen Incorporated, San Diego, CA, USA) and 5 μL homotype control (BD Bioscience Pharmingen Incorporated) were added into each tube. After 15-minute preservation at room temperature in the dark, 2 mL PBS was added. The resultant mixture was centrifuged at 1 000 r/min for 5 minutes. After removal of the supernatant, 500 μL PBS was added for re-suspension. A FACSCalibur multicolor flow cytometer (BD Bioscience Pharmingen Incorporated) equipped with a 488-nm laser was used to detect 10 000 sample cells. The percentage of expression of FasL-positive cells was calculated through use of CellQuest software (Cell Quest, Tampa, FL, USA). The percentage expression of Fas-, caspase-3-, and Bcl-2-positive cells was determined using similar methods.
Determination of levels of NGF, BDNF, and GDNF by enzyme-linked immunosorbent assay (ELISA)
The standard sample solution and cleaning solution were made according to the instructions provided by the manufacturer of the NGF kit (Shanghai Xitang Biotechnology Company Limited, Shanghai, China). A 96-well ELISA plate was used. According to kit instructions, eight tubes served as standard tubes and were numbered; 200 μL sample dilution was added into each tube. We carefully followed the manufacturer instructions for creating a standard curve. Subsequently, 100 μL homogenate supernatant of the substantia nigra and striatum of each mouse was added to the ELISA wells, incubated at 37°C for 120 minutes in the incubator (DHP-9052, Yiheng Instruments, Shanghai, China), washed thrice, and dried. After addition of 100 μL anti-NGF-biotin antibody to each well, the resultant solution was incubated for 60 minutes at 37°C, washed six times, and dried. Enzyme-labeled antibody working solution (100 μL) was added to each well, incubated for 30 minutes at 37°C, washed six times, and dried. Substrate working solution (100 μL) was added to each well and incubated for 15 minutes at 37°C. Termination solution (100 μL) was added to each well and the absorbance value determined through the use of an automatic ELISA reader (XL800, BioTex, Incorporated, Houston, TX, USA). Levels of BDNF and GDNF were determined using the same procedures as those employed for NGF (BDNF and GDNF kits provided by Shanghai Xitang Biotechnology Company Limited).
Determination of TH activity by immunohistochemistry
The substantia nigra tissue block was gradually dehydrated in a series of ethanol solutions (70%, 95%, and 100%). It was then cleared with xylene twice (45-minutes each), soaked in a mixture of paraffin and xylene (1:1) for 30 minutes in one tube and then 90 minutes in another tube, embedded in paraffin, and sliced into 4-μm sections. These sections were then dried, de-waxed with xylene twice (20-minutes each) and hydrated with 100% ethanol. They were rinsed with 95% ethanol twice, (3-minutes each), with 85% ethanol for 3 minutes, 70% ethanol for 3 minutes, and with distilled water for 3 minutes. After heat retrieval using citrate buffer solution, sections were rinsed with double-distilled water for 5 minutes and with PBS twice (5 minutes each). After addition of PBS-diluted TH antibody (500: 1; rabbit anti-mouse; Abcam, Cambridge, England) at 50 μL/section, sections were incubated at 4°C overnight. They were then balanced at room temperature for 10 minutes, rinsed with PBS twice (10 minutes each), followed by another 5 minutes of washing in PBS. After addition of secondary antibody (goat anti-rabbit; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) at 50 μL/section, sections were incubated for 30 minutes at 37°C. They were rinsed with PBS thrice (5 minutes each), developed with diaminobenzidine (DAB; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) for 10 minutes, and soaked for 5 minutes with running water to terminate the reaction. They were counterstained with hematoxylin for 5 seconds, dehydrated, cleared, and mounted with neutral gum. They were then oven-dried and photographed under a microscope. At the same time, PBS served as the primary antibody for the negative control. The positive reaction of TH could be identified by the brown or yellow-brown granules within the cytoplasm of cells. Ten sections from each group were randomly selected. Under an optical microscope (BX41, Olympus, Shinjuku-ku, Tokyo, Japan) at a magnification of × 200, 10 non-overlapping visual fields from each section were selected for photographing. Immunohistochemical sections were analyzed through the use of Image Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA). The mean number of cells was used for calculating the ratio of positive cells in the visual field/total cells in the visual field). A score of: 0 indicated a ratio < 5%; 1 represented a ratio of 5–25%; 2 suggested a ratio of 26-50%; 3 demonstrated a ratio of > 50%. The staining intensity was scored from 0 to 3. That is: 0, basically non-colored and staining region similar to that of the background; 1, slightly colored, staining region slightly darker than of the background; 2, moderately colored, staining region obviously darker than the background; 3, intensively colored, brown-yellow staining region. Immunohistochemical results were expressed as percentage of positive cells × staining intensity score. That is: 0, negative results (−); 1–3, weakly positive (+); 4–6, moderately positive (++); 7–9, strongly positive (+++)[34].
Observation of substantia nigra neuronal morphology by transmission electron microscopy
Substantia nigra tissue blocks were fixed with 2.5% glutaraldehyde for ≥ 2 hours, rinsed thrice with PBS, and fixed with 1% osmic acid for 1.5 hours. They were rinsed thrice with PBS, dehydrated by 50% ethanol for 10 minutes, 70% ethanol saturated with uranyl acetate at 4°C overnight, 90% ethanol for 10 minutes, 90% ethanol and 90% acetone for 10 minutes, 90% acetone for 10 minutes, and 100% acetone thrice (10 minutes each). Subsequently, tissue blocks were soaked in a mixture of 100% acetone and epoxy resin 618 (1: 1) for 1.5 hours, in 100% acetone 618 at 35°C for 3 hours, at 35°C for 12 hours, at 45°C for 12 hours, and at 60°C for 3 days. Thereafter, tissue blocks were sliced into 70–80 nm sections using an ultramicrotome (EM UC6, Leica, Solms, Germany). They were stained with uranyl acetate and lead citrate for 8–10 minutes and rinsed with distilled water. They were observed under a transmission electron microscope (H7650, Hitachi, Tokyo, Japan), and photographed using a charge-coupled device (CCD) camera (SIS, Taipei, Taiwan, China).
Statistical analyses
Data were processed using SPSS 13.0 software (SPSS, Chicago, IL, USA). ELISA and flow cytometry results are expressed as mean ± SD. One-way analysis of variance was used for data comparison between groups. Least significant difference or Games-Howell post-hoc tests were used for multiple comparisons among groups. The level of significance was set at α = 0.05.
Immunohistochemical results are expressed as percentage of positive cells × staining intensity score. The multivariate rank sum test was used for comparison between groups, and the two-sample rank sum test used for multiple comparisons. The level of significance was set at α = 0.05.
Acknowledgments:
We thank the staff of the Laboratory of Cell Biology, Academy of Integrative Medicine, Fujian University of Traditional Chinese Medicine for technical guidance.
Footnotes
Conflicts of interest: None declared.
Funding: This study was supported by the Natural Science Foundation of Fujian Province, No. 2009J06018.
Ethical approval: This project had full ethical approval from the Animal Ethics Committee, Fujian University of Traditional Chinese Medicine, China.
(Edited by Wang L)
REFERENCES
- [1].Hartmann A, Hunot S, Michel PP, et al. Caspase-3: A vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson's disease. Proc Natl Acad Sci U S A. 2000;97:2875–2880. doi: 10.1073/pnas.040556597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].von Coelln R, Kügler S, Bähr M, et al. Rescue from death but not from functional impairment: caspase inhibition protects dopaminergic cells against 6-hydroxydopamine-induced apoptosis but not against the loss of their terminals. J Neurochem. 2001;77:263–273. doi: 10.1046/j.1471-4159.2001.t01-1-00236.x. [DOI] [PubMed] [Google Scholar]
- [3].Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer's and Parkinson's disease brain. Brain Res Brain Res Rev. 2000;33:199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- [4].Kumar S. Caspase function in programmed cell death. Cell Death Differ. 2007;14:32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- [5].Rose CR, Blum R, Kafitz KW, et al. From modulator to mediator: rapid effects of BDNF on ion channels. Bioessays. 2004;26:1185–1194. doi: 10.1002/bies.20118. [DOI] [PubMed] [Google Scholar]
- [6].Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- [7].Lin LF, Doherty DH, Lile JD, et al. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- [8].Studer L, Spenger C, Seiler RW, et al. Effects of brain-derived neurotrophic factor on neuronal structure of dopaminergic neurons in dissociated cultures of human fetal mesencephalon. Exp Brain Res. 1996;108:328–336. doi: 10.1007/BF00228106. [DOI] [PubMed] [Google Scholar]
- [9].Williams LR, Varon S, Peterson GM, et al. Continuous infusion of nerve growth factor prevents basal forebrain neuronal death after fimbria fornix transection. Proc Natl Acad Sci U S A. 1986;83:9231–9235. doi: 10.1073/pnas.83.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Reis RA, Cabral da Silva MC, Loureiro dos Santos NE, et al. Sympathetic neuronal survival induced by retinal trophic factors. J Neurobiol. 2002;50:13–23. doi: 10.1002/neu.10008. [DOI] [PubMed] [Google Scholar]
- [11].Wolff JA, Fisher LJ, Xu L, et al. Grafting fibroblasts genetically modified to produce L-dopa in a rat model of Parkinson disease. Proc Natl Acad Sci U S A. 1989;86:9011–9014. doi: 10.1073/pnas.86.22.9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen SD. Beijing: People's Medical Publishing House; 2006. Parkinson's Disease. [Google Scholar]
- [13].Chen JZ, Huang C, Li XM, et al. Research clew and practice of treating Parkinsonism with method of tonifying liver and kidney. Zhonghua Yiyao Zazhi. 2004;19:687. [Google Scholar]
- [14].Zhang ZM, Ge B, Xu AX, et al. Antisenile effect of polysaccharides from Fructus ligustri lucidi. Zhongguo Yaolixue yu Dulixue Zazhi. 2006;20:108–111. [Google Scholar]
- [15].Yang MH, Wang HM, Liu Y. Effects of Bushen Huoxue Yin on tyrosine hydroxylase and retinoid-related nuclear orphan receptor 1 mRNA of rats with Parkinson disease. Zhongguo Zhongxiyi Jiehe Jijiu Zazhi. 2009;16:72–74. [Google Scholar]
- [16].Chen Y, Sun X. Pharmaceutical progress of Parkinson's disease. Zhongyao Xinyao yu Linchuang Yaoli. 2010;21:328–330. [Google Scholar]
- [17].Choudhury ME, Moritoyo T, Yabe H, et al. Zonisamide attenuates MPTP neurotoxicity in marmosets. J Pharmacol Sci. 2010;114:298–303. doi: 10.1254/jphs.10120fp. [DOI] [PubMed] [Google Scholar]
- [18].He XJ, Uetsuka K, Nakayama H. Neural progenitor cells are protected against MPTP by MAO-B inhibitors. Neurotoxicology. 2008;29:1141–1146. doi: 10.1016/j.neuro.2008.05.009. [DOI] [PubMed] [Google Scholar]
- [19].Hartmann A, Mouatt-Prigent A, Vila M, et al. Increased expression and redistribution of the antiapoptotic molecule Bcl-xL in Parkinson's disease. Neurobiol Dis. 2002;10:28–32. doi: 10.1006/nbdi.2002.0494. [DOI] [PubMed] [Google Scholar]
- [20].Wang XB, Zhang XC. Mechanisms of Caspase activation. Xiandai Shengwu Yixue Jinzhan. 2006;6:53–55. [Google Scholar]
- [21].Sun JB, Lu XG, Tang JS. The effect of compound recipe TFR combined with exercise on the activity of FAS to diet-induced hypercholesteremia rats. Huaiyin Shifan Xueyuan Xuebao: Ziran Kexue Ban. 2008;7:64–68. [Google Scholar]
- [22].Zhao YL, Qu YZ, Wang ZR. Effects of astragalus on neuron apoptosis and expression of apoptosis-associated gene after cerebral ischemia/reperfusion. Zhongguo Zhongxiyi Jiehe Jijiu Zazhi. 2005;12:341–343. [Google Scholar]
- [23].Hu MH, Zhou YM, Zhu WW, et al. Effect of Jianpi Bushen Huoxue Decoction on myeloid element Fas and FasL in patients with aplastic anemia. Fujian Zhongyiyao. 2007;38:9–10. [Google Scholar]
- [24].Zhou T, Xu B, Que H, et al. Neurons derived from PC12 cells have the potential to develop synapses with primary neurons from rat cortex. Acta Neurobiol Exp (Wars) 2006;66:105–112. doi: 10.55782/ane-2006-1596. [DOI] [PubMed] [Google Scholar]
- [25].Perrier AL, Tabar V, Barberi T, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yang B. Pharmacological Research Advance of Icariin. Zhongguo Yaofang. 2009;20:1915–1917. [Google Scholar]
- [27].Li WW, Yang R, Cai DF. Protective effects of Cistanche total glycosides on dopaminergic neuron in substantia nigra of model mice of Parkinson's disease. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2008;28:248–251. [PubMed] [Google Scholar]
- [28].Petroske E, Meredith GE, Callen S, et al. Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience. 2001;106:589–601. doi: 10.1016/s0306-4522(01)00295-0. [DOI] [PubMed] [Google Scholar]
- [29].Meredith GE, Totterdell S, Potashkin JA, et al. Modeling PD pathogenesis in mice: advantages of a chronic MPTP protocol. Parkinsonism Relat Disord. 2008;14(Suppl 2):S112–115. doi: 10.1016/j.parkreldis.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].2005 ed. Beijing: Chemical Industry Press; 2005. Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia, Part I. [Google Scholar]
- [31].Yao QH, Zhang H, Gao GD. Comparison of behavioral tests in MPTP-induced Parkinson's disease in mice. Zhongguo Shiyan Dongwu Xuebao. 2006;16:264–270. [Google Scholar]
- [32].Bai YZ, Xia ZQ, Hu YE. Stability of the behavioral impairment of a MPTP induced chronic mouse model of Parkinson's disease. Zhongguo Xinwei Yixue Kexue. 2007;16:484–486. [Google Scholar]
- [33].Franklin KB, Paxinos G. 3rd ed. San Diego: Academic Press; 2007. The Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- [34].Zhang X. Shanghai: Second Military Medical University of Chinese PLA; 2008. Expression of Survivin and Bcl-2 in Endometriosis and Their Significance. [Google Scholar]


