Abstract
We have previously observed that Wnt signaling activates a fibrogenic program in adult muscle stem cells, called satellite cells, during aging. We genetically labeled satellite cells in a mouse model of Duchenne muscular dystrophy to follow their fate during the progression of the disease. We observed that a fraction of satellite cells had a reduced myogenic potential and showed enhanced expression of profibrotic genes compared to age-matched controls. By combining in vitro and in vivo results, we found that expression of transforming growth factor–β2 (TGFβ2) was induced in response to elevated canonical Wnt signaling in dystrophic muscles and that the resulting increase in TGFb activity affected the behavior of satellite cells in an autocrine or paracrine fashion. Indeed, pharmacological inhibition of the TGFb pathway in vivo reduced the fibrogenic characteristics of satellite cells. These studies shed new light on the cellular and molecular mechanisms responsible for stem cell dysfunction in dystrophic muscle and may contribute to the development of more effective and specific therapeutic approaches for the prevention of muscle fibrosis.
INTRODUCTION
Satellite cells represent a muscle-specific stem cell population that is responsible for adult skeletal muscle regeneration (1–3). In healthy muscles, satellite cells are maintained in a quiescent state and are juxtaposed close to the surface of the myofibers and beneath the basal lamina that surrounds each fiber (4). In response to muscle damage, satellite cells exit the quiescent state and begin to proliferate. Their progeny either differentiate and fuse into newly regenerated muscle fibers or renew the pool of satellite cells by becoming quiescent again (2). Quiescent satellite cells express Pax7, but undetectable amounts of the myogenic regulatory factor MyoD (3). Upon activation, the satellite cells rapidly express MyoD and subsequently myogenin before undergoing terminal differentiation (3). Recent cell ablation studies have demonstrated that muscle regeneration is abrogated in the absence of Pax7+ve satellite cells (5, 6).
Duchenne muscular dystrophy (DMD) is the most common inherited muscle disease of childhood (7). DMD is caused by mutations in the gene encoding the sarcolemmal protein dystrophin (8). In the absence of dystrophin, myofibers are particularly prone to degeneration, leading to repetitive rounds of fiber degeneration and regeneration (9). Over time, the regenerative ability of dystrophic muscles becomes impaired, and fibrotic tissue replaces the myofibers, leading to a severe reduction in muscle function (9). The cellular and molecular bases for the defective regenerative potential seen in the advanced stages of DMD are still largely unexplored (9, 10). Moreover, although there is evidence of an involvement of macrophages and T cells and a role for different members of the transforming growth factor–β (TGFβ) superfamily in the etiology of fibrosis, determinants of the accumulation of fibrotic tissue in dystrophic muscles remain poorly defined (11).
Defective regeneration and accumulation of fibrotic tissue also characterize aging muscle. Previous work from our laboratory has shown that a fraction of satellite cells isolated from aged muscle convert from a myogenic to a fibrogenic lineage in a Wnt-dependent manner and that this accounts in part for the declining regenerative potential of muscle with age (12). Given the even greater disruption of regenerative potential in dystrophic muscle, we hypothesized that the dystrophic setting could also affect the fate of satellite cells. This idea is supported by the observation that satellite cells obtained from mdx mice (a model of DMD) (13) or cultured from muscles of DMD patients are more likely to produce increased amounts of extracellular matrix (ECM) proteins compared to control satellite cells (14, 15). However, whether satellite cells undergo a conversion to an alternate lineage in the dystrophic environment in vivo is not known. Such a conversion would certainly have a negative impact on the efficacy of muscle regeneration.
Using an in vivo genetic lineage tracing strategy relying on the Cre/loxP system, we observed that a fraction of satellite cells in the mdx mouse lose their ability to follow a myogenic program and show increased expression of fibrotic genes. We present data suggesting a causal link between the canonical Wnt and TGFβ2 pathways, activation of which lead to the induction of fibrogenic features in satellite cells in dystrophic muscles and potentially to increased tissue fibrosis.
RESULTS
A fraction of satellite cells show an aberrant lineage decision in dystrophic mice
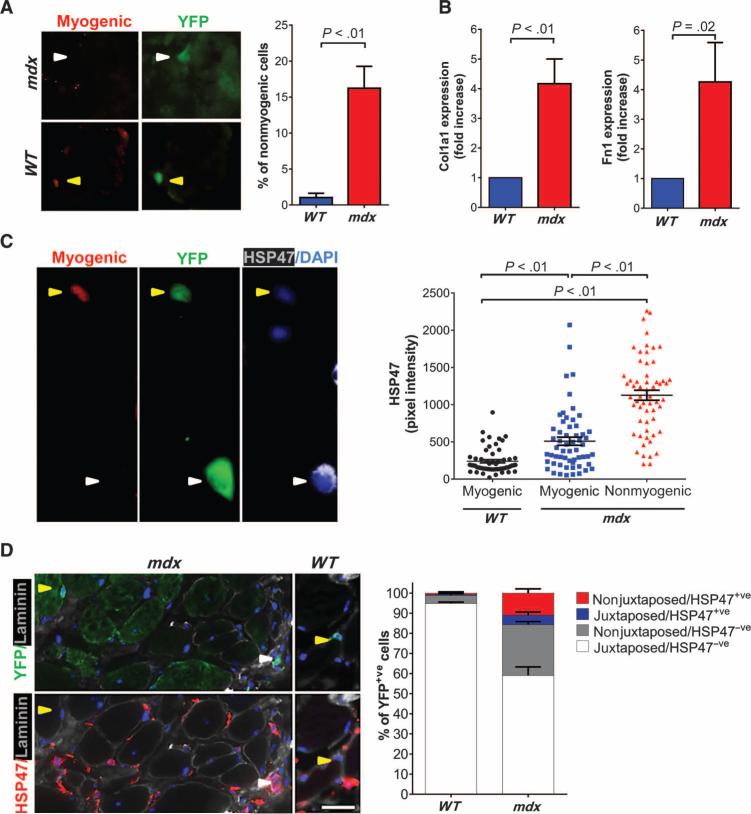
We have previously shown that the aging environment induces fibrogenic features in satellite cells (12). To evaluate if the fate of satellite cells is similarly affected by the dystrophic muscle environment, we traced the fate of satellite cells using the Pax7CreER;R26RYFP mouse strain. In this strain, CreER is knocked into the Pax7 locus, and the administration of tamoxifen (TMX) allows the fate of satellite cell progeny to be monitored through genetic lineage tracing using the yellow fluorescent protein (YFP) reporter gene (fig. S1A). We bred this strain with the mdx5Cv mouse model of DMD (16). Eleven months after TMX treatment, we evaluated satellite cells and their progeny (YFP+ve) for their myogenic potential. Sections from diaphragm, a muscle that is severely affected in mdx mice (17), were stained with antibodies recognizing YFP and the myogenic cell markers Pax7, MyoD, and myogenin. All of the myogenic markers were undetectable in a fraction (ranging from 7 to 20%) of YFP+ve cells in mdx5Cv diaphragms, whereas nearly all (>98%) of the YFP+ve cells from wild-type(WT)age-matchedcontrolsexpressed one or more of the myogenic markers (Fig. 1A). The analysis of diaphragm satellite cells in vitro confirmed this observation (fig. S1B). We observed an increase in the expression of Col1a1, Fn1, and other well-known fibrotic genes in YFP+ve cells purified by fluorescence-activated cell sorting (FACS) from mdx5Cv mice (Fig. 1B and fig. S1, C and D). An increase in the expression of Col1a1 and Fn1 and a decrease in the expression of the myogenic marker MyoD were confirmed when satellite cells were allowed to expand in vitro (fig. S1E). Moreover, Pax7/MyoD/myogenin−ve cells exhibited an increase in the intensity of staining for the collagen-specific molecular chaperone heat shock protein 47 (HSP47) (18, 19) (Fig. 1C). In diaphragms from mdx5Cv mice, a fraction of the YFP+ve cells were observed outside of its typical position (which is juxtaposed to the fiber beneath the basal lamina), with almost one-third of this cell fraction expressing HSP47 and surrounded by basal lamina (Fig. 1D and fig. S2A). The localization of satellite cells or their progeny in the interstitium was also confirmed using a LacZ-expressing reporter strain (fig. S2B). In contrast, only a small fraction (~4%) of satellite cell progeny was not juxtaposed to myofibers in age-matched WT mouse muscle (Fig. 1D and fig. S2C). Altogether, these observations suggest that during the progression of dystrophic disease, the progeny of satellite cells display a defective myogenic program and adopt a fibrogenic phenotype, both in terms of gene expression and anatomical localization. These changes would be expected to impair muscle regenerative potential and potentially contribute to the accumulation of ECM in the muscle interstitium.
Fig. 1. Influence of the dystrophic environment on muscle stem cells.
(A) Representative images of diaphragms from a 12-month-old Pax7CreER;R26RYFP;mdx5Cv and Pax7CreER;R26RYFP;WT mouse were stained with a cocktail of antibodies recognizing the myogenic markers Pax7, MyoD, and myogenin and with an antibody to YFP. The percentage of YFP+ve cells that were nonmyogenic was quantified (N = 4). Examples of nonmyogenic YFP+ve and myogenic YFP+ve cells are indicated by white and yellow arrowheads, respectively. (B) Reverse transcription polymerase chain reaction (RT-PCR) analysis of the expression of the fibrotic genes Col1a1 and Fn1 in YFP+ve cells purified from hindlimb muscles of 8- to 12-month-old Pax7CreER;R26RYFP;mdx5Cv mice (N = 4). Data are expressed as fold increase of the expression in cells isolated from Pax7CreER;R26RYFP;WT age-matched mice (N = 4). (C) Cells isolated from diaphragm muscles of 18-month-old Pax7CreER;R26RYFP;mdx5Cv and Pax7CreER;R26RYFP;WT mice were stained for Pax7, MyoD, myogenin, YFP, and HSP47. Examples of myogenic and nonmyogenic YFP+ve cells are indicated by yellow and white arrowheads, respectively. The intensity of the staining for HSP47 was quantified and correlated with the presence of the myogenic markers. The analysis was performed on at least 50 cells from three independent experiments. DAPI, 4′,6-diamidino-2-phenylindole. (D) Left: Diaphragms of 12-month-old Pax7CreER;R26RYFP;mdx5Cv and Pax7CreER;R26RYFP;WT mice were stained with antibodies against YFP, HSP47, and laminin and were counterstained with DAPI for nuclei. Shown are examples of YFP+ve cells that are closely associated with myofibers (yellow arrowhead) or physically separated from myofibers (white arrowhead). Scale bar, 25 μm. Right: The percentage of YFP+ve cells in different locations relative to the fiber (juxtaposed or nonjuxtaposed) and their corresponding expression of HSP47 was quantified in WT and mdx mouse muscle (N = 3 for each genotype). Note that the fraction of nonjuxtaposed HSP47+ve cells were significantly more abundant in mdx5Cv than in WT mouse muscles (P < 0.01; unpaired one-tailed t test).
Canonical Wnt signaling is elevated in mdx mouse muscles
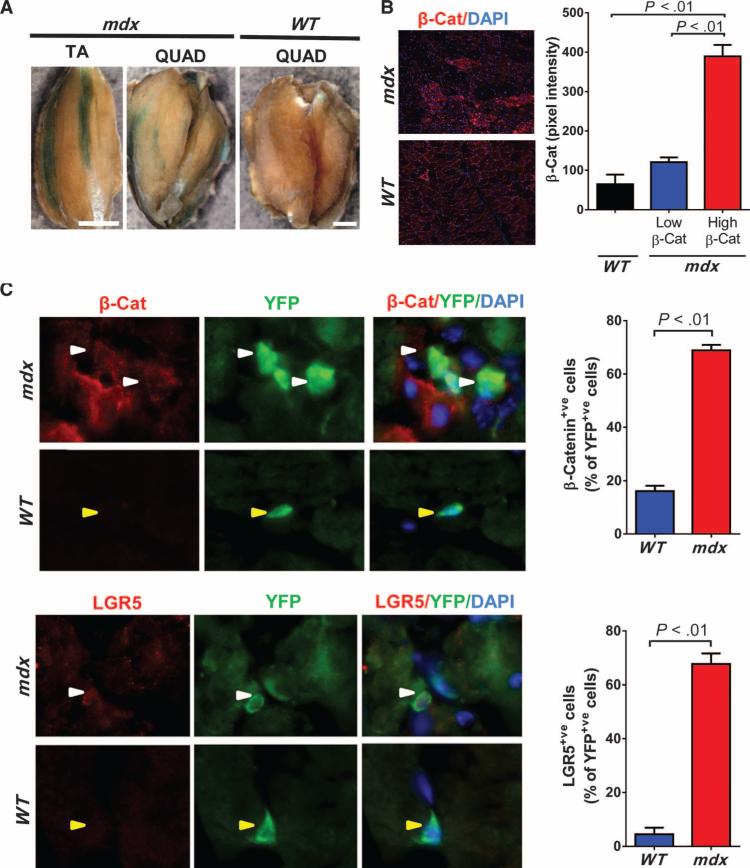
A critical molecular determinant of the fibrogenic conversion of satellite cells in the aged environment is the presence of factors that lead to enhanced Wnt signaling in the cells (12, 20). Moreover, a recent report has implicated increased Wnt signaling in the proliferation of stromal cells in mdx mouse muscle (21). With the aim to explore the Wnt pathway in more detail, we bred the Wnt reporter BAT-GAL mice onto the mdx5Cvbackground. Increased Wnt signaling, reflected by increased β-galactosidase (β-Gal) activity, was not homogeneous but was patchy, as has been described for the pattern of degeneration and regeneration in dystrophic muscle (22) (Fig. 2A). A similar patchy distribution of the canonical Wnt signaling pathway effector β-catenin (23) was confirmed by immunofluorescence(Fig. 2B). Furthermore, we observed anincreased number of cells expressing β-catenin and the downstream target of the canonical Wnt pathway, LGR5 (leucine-rich repeat-containing G protein–coupled receptor 5) (24), among the YFP+ve population from Pax7CreER;R26RYFP;mdx5Cv mice compared to Pax7CreER;R26RYFP;WT mice (Fig. 2C).
Fig. 2. Enhanced Wnt signaling in dystrophic muscles.
(A) Representative whole-mount X-gal staining of tibialis anterior (TA) and quadriceps (QUAD) muscles from 4-month-old BAT-GAL;mdx5Cv and BAT-GAL;WT mice. Scale bar, 2 mm. (B) β-Catenin (β-Cat) staining of muscles from 6-month-old mdx5Cv and WT mice (N = 3 for each genotype). The average intensity of the signal corresponding to β-catenin was evaluated in distinct regions of the muscles with high or low β-catenin+ve staining. Note that there was negligible β-catenin staining in muscles of WT mice. (C) The percentage of YFP+ve cells that were either β-catenin+ve or LGR5+ve was quantified in diaphragm sections of 12-month-old Pax7CreER;R26RYFP;mdx5Cv and Pax7CreER;R26RYFP;WT mice (N = 3 for each genotype). Examples of YFP+ve cells that were also either β-catenin+ve or LGR5+ve are indicated by white arrowheads; examples of YFP+ve cells that are also either β-catenin-ve or LGR5-ve are indicated by yellow arrowheads (DAPI nuclear stain, blue). P < 0.01; unpaired one-tailed t test.
TGFβ2 is induced by canonical Wnt signaling
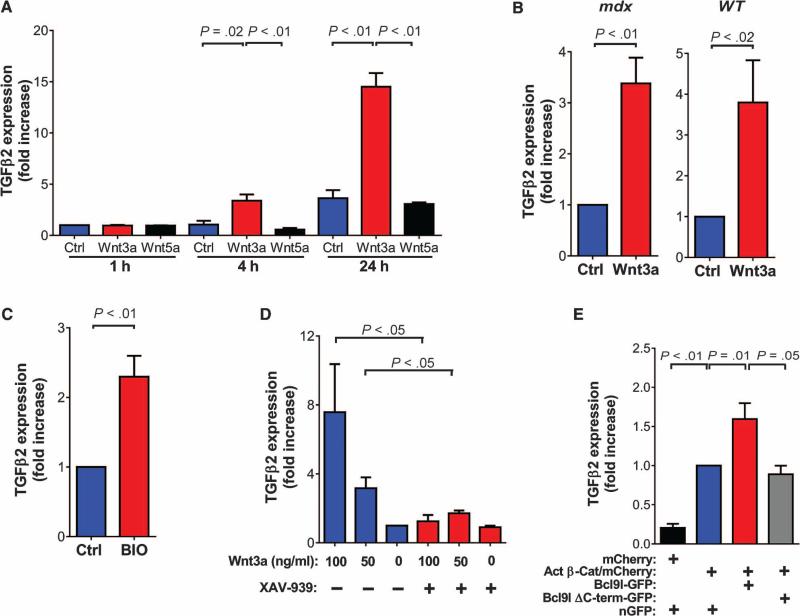
Previous work showed that exogenous Wnt3a added to satellite cell cultures can induce their conversion to a fibrogenic phenotype (12). To gain insight into the molecular events mediating this effect, we compared by microarray analysis the transcriptome of myoblasts cultured for 4 hours in the presence and absence of Wnt3a. This analysis led to the identification of 27 genes, including the well-known canonical target genes Axin2 and LGR5 (24, 25), which were induced ≥2.5 times in myogenic cells treated with Wnt3a compared to untreated myogenic cells (table S1). One of the most differentially expressed genes was TGFβ2, which was consistently induced >3.8 times in response to Wnt treatment when evaluated with three distinct probe sets. This observation was particularly intriguing because the TGFβ family of proteins is among the most well-studied mediators of tissue fibrosis in a wide range of conditions (26, 27). Quantitative RT-PCR revealed that, similar to Axin2, TGFβ2 was induced by canonical Wnt3a but not by noncanonical Wnt5a (Fig. 3A and fig. S3A). Primary myoblasts and YFP+ve cells isolated from dystrophic and WT mouse muscles displayed a strong Wnt3a-dependent induction of TGFβ2 expression (Fig. 3, A and B, and fig. S3, A and B). To further demonstrate that the Wnt-dependent induction of TGFβ2 expression occurred through the canonical Wnt pathway, we treated myoblasts with the GSK-3β (glycogen synthase kinase–3β) inhibitor BIO to activate the Wnt pathway. Induction of both Axin2 and TGFβ2 expression was observed (Fig. 3C and fig. S3C). In contrast, the addition of the tankyrase inhibitor XAV-939 at a concentration that could effectively block Wnt signaling markedly reduced the induction of TGFβ2 and Axin2 by Wnt3a in WT myoblasts (Fig. 3D and fig. S3, D and E). The induction of TGFβ2 by the canonical Wnt pathway was further confirmed by the overexpression of a stabilized form of β-catenin (28) (Fig. 3E and fig. S3F). Expression of the canonical Wnt signaling effector Bcl9l potentiated the action of β-catenin on TGFβ2 expression, whereas a mutant form, missing the C-terminal domain (29), was unable to induce the same effect (Fig. 3E and fig. S3, F and G). Several potential Wnt-responsive elements characterized by the T cell factor/lymphoid enhancer factor (TCF/LEF) binding consensus sequence were noted in the regulatory regions upstream and downstream of the transcription start site of the mouse Tgfβ2 gene (fig. S4A). Chromatin immunoprecipitation (ChIP) assays with an antibody recognizing β-catenin confirmed its binding to a region in the first intron of Tgfβ2, about 2000 base pairs downstream of the transcription start site (fig. S4B). This evidence, together with the rapid kinetics of induction of TGFβ2 by Wnt3a, suggests that TGFβ2 could be a direct target of canonical Wnt signaling.
Fig. 3. Canonical Wnt signaling induces TGFβ2 expression in myogenic cells.
(A) TGFβ2 was evaluated by RT-PCR in primary myoblasts (N = 3) after treatment with Wnt3a or Wnt5a (100 ng/ml). Data are expressed as fold increase of the expression in untreated control cells cultured for 1 hour (Ctrl). (B) TGFβ2 was evaluated by RT-PCR in YFP+ve cells purified by FACS from 12-month-old Pax7CreER;R26RYFP;mdx5Cv (N = 3) and Pax7CreER;R26RYFP;WT (N = 4) mice after treatment with Wnt3a (100 ng/ml). Data are expressed as fold increase of the expression in untreated control cells. (C and D) TGFβ2 was evaluated by RT-PCR in myoblasts treated for 24 hours (C) with BIO (1 μM) or (D) with XAV-939 (100 μM) in the presence of different concentrations of Wnt3a. Data are expressed as fold increase of the expression in untreated cells (N = 3 for all conditions). (E) TGFβ2 was evaluated by RT-PCR in myoblasts after transfection with the indicated vectors. The analysis was done on FACS-sorted mCherry+ve myoblasts to ensure a high efficiency of transfection. Data are expressed as fold increase in the expression in cells transfected with the active β-catenin (Act β-Cat)/mCherry– expressing plasmid (N = 3). P values are as indicated (unpaired one-tailed t test). nGFP, nuclear green fluorescent protein. C-term, C-terminal.
A Wnt-TGFβ2 axis controls the fate of muscle cells
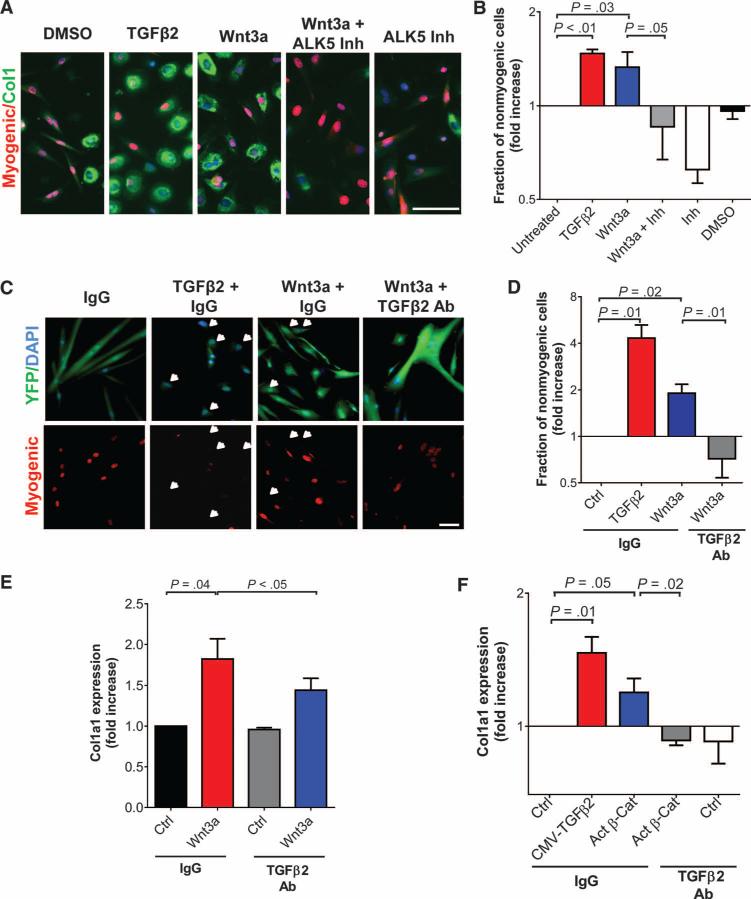
We observed that both C2C12 myoblasts and YFP+ve cells from Pax7CreER;R26RYFP;mdx5Cv mice expressed all three TGFβ receptors capable of transducing signals in response to TGFβ2 (fig. S5, A to C). Moreover, similar to Wnt3a, TGFβ2 repressed the myogenic fate and induced the expression of fibrotic genes such as Collagen1 in cultures of murine myoblasts (Fig. 4, A and B). Myoblasts and fibroblasts exhibit different adhesion properties, the latter adhering more rapidly to cell culture dishes (30). In keeping with a shift from the myogenic to the fibrogenic lineage, cells treated with either Wnt3a or TGFβ2 exhibited enhanced adhesion (fig. S6A). Moreover, immunofluorescence analysis of single cells revealed that the induction of Collagen1 directly correlated with the impairment of the myogenic program (fig. S6B). As evidence that TGFβ acts downstream of Wnt signaling in this context, blocking TGFβ signaling by the addition of an TGFβr1/activin receptor-like kinase 5 (ALK5) inhibitor counteracted the effects of Wnt3a on cell fate (Fig. 4, A and B). Similar results were obtained by adding TGFβ2-specific inhibitors to satellite cell progeny purified from mdx5Cv mice treated with Wnt3a (Fig. 4, C and D). The treatment with an antibody specifically blocking the TGFβ2 isoform partially counteracted the increase in Col1a1 expression induced by Wnt3a or TGFβ2 in C2C12 cultures, without affecting the expression of the Wnt target gene Axin2 (Fig. 4E and fig. S6, C and D). These loss-of-function approaches indicate a functional hierarchical relationship between canonical Wnt and TGFβ2-mediated signaling. These observations suggest that canonical Wnt signaling can induce the expression and secretion of TGFβ2, which promotes the acquisition of fibrogenic features by cells previously in the myogenic lineage. To further corroborate this model, we overexpressed stabilized β-catenin in myoblasts, collected the medium after 24 hours, and treated cultures of myogenic cells with this conditioned medium. We consistently observed an increase in Col1a1 expression in cultures treated with the conditioned medium from cells transfected with a β-catenin expression vector compared to cells transfected with a control vector (Fig. 4F). The addition of a TGFβ2-specific blocking antibody counteracted the effect of the conditioned medium (Fig. 4F). A similar increase in Col1a1 expression was also observed upon transfection with a plasmid expressing TGFβ2 or by addition of recombinant TGFβ2 (Fig. 4F and fig. S6D). These in vitro observations indicate that activation of the Wnt-TGFβ2 axis can alter satellite cell state.
Fig. 4. The Wnt-TGFβ2 axis influences muscle stem cell fate.
(A and B) C2C12 myoblasts were cultured with or without TGFβ2 (10 ng/ml), Wnt3a (100 ng/ml), Wnt3a with addition of ALK5 inhibitor II (5 μM), or ALK5 inhibitor II (Inh) alone. To exclude myotubes, cultures of C2C12 cells were processed as described in Materials and Methods. (A) Cells were stained for myogenic markers (Pax7, MyoD, or myogenin) and Collagen1. (B) The measure of nonmyogenic cells was quantified and expressed as a fraction of untreated cells (logarithmic scale, N = 3). Scale bar, 25 μm. DMSO, dimethyl sulfoxide. (C and D) YFP+ve cells were isolated by FACS from Pax7CreER;R26RYFP;mdx5Cv mice, treated with Wnt3a or TGFβ2 in the presence of a TGFβ2-blocking antibody or control immunoglobulin G (IgG) (each at 12.5 μg/ml), and stained with a cocktail of muscle-specific antibodies (Pax7, MyoD, and myogenin). (C) Examples of nonmyogenic cells are indicated by white arrows. Scale bar, 50 μm. (D) Nonmyogenic cells were quantified and expressed as a fraction of IgG-treated cells (Ctrl) (logarithmic scale, N = 3). Ab, antibody. (E) RT-PCR analysis of the expression of Col1a1 and Axin2 in C2C12 myoblasts cultured with or without Wnt3a (100 ng/ml), with addition of a TGFβ2-blocking antibody or rabbit IgG (both at 12.5 μg/ml). Cells were processed as in (A). Data are expressed as fold increase of the expression in cells treated with IgG (N = 3 for each condition). (F) Primary myoblasts were cultured in conditioned medium from C2C12 myoblasts transfected with plasmids expressing mouse TGFβ2, active β-catenin/mCherry (Act β-Cat) or mCherry (Ctrl) and treated with either a TGFβ2-blocking antibody or a control antibody (each at 12.5 μg/ml) as described in Materials and Methods. The expression of Col1a1 was evaluated by RT-PCR. Data are expressed as fold increase in the expression in cells treated with IgG (logarithmic scale, N = 3). P values are as indicated (unpaired one-tailed t test). CMV, cytomegalovirus promoter.
TGFβ2 expression is elevated in mdx muscles
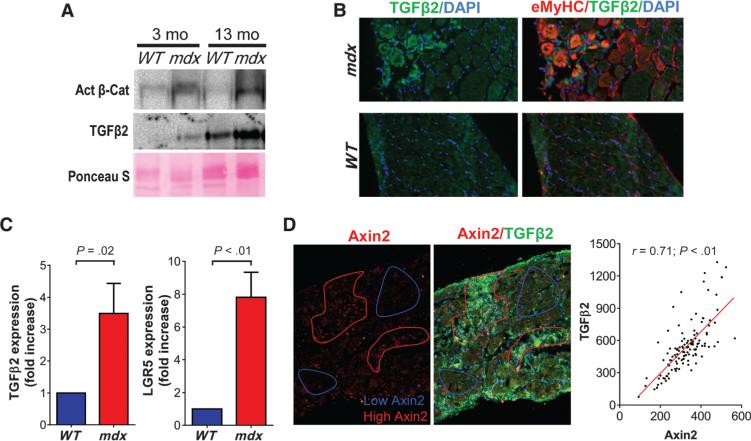
Although several studies have evaluated the expression of TGFβ1 in dystrophic muscle (31, 32), there is little evidence of TGFβ2 expression in relation to the pathogenesis of muscular dystrophies (33). We therefore characterized the expression of TGFβ2 and its relation to canonical Wnt signaling in muscles of mdx5Cv mice. The amounts of both dephosphorylated (stabilized) β-catenin and TGFβ2 were increased in diaphragm muscles from mdx5Cv mice when compared to age-matched controls (Fig. 5A). In particular, the regenerative foci in mdx5Cv muscles, which are characterized by the expression of embryonic myosin heavy chain (eMyHC), stained strongly for TGFβ2 (Fig. 5B and fig. S7A), consistent with an observation in muscles of DMD patients (34). The expression of TGFβ2 and of well-known canonical Wnt signaling targets, such as LGR5, were increased in YFP+ve cells from Pax7CreER; R26RYFP;mdx5Cv mice in comparison to age-matched WT control mice (Fig. 5C and fig. S7B). With the aim of comparing the pattern of Wnt signaling with the pattern of TGFβ2 expression, we costained muscles from mdx5Cv mice with antibodies recognizing TGFβ2 and Axin2, bcatenin, or LGR5 (Fig. 5D and fig. S7, C and D). The regions of muscles that were characterized by strong expression of Axin2, β-catenin, or LGR5 also exhibited strong staining for TGFβ2. These data demonstrate a correlation between elevated Wnt signaling and elevated TGFβ2 signaling, consistent with the data demonstrating the induction of TGFβ2 by canonical Wnt signaling.
Fig. 5. Enhanced TGFβ signaling in dystrophic muscles correlates with increased Wnt activity.
(A) Representative Western blots of active β-catenin and TGFβ2 in diaphragms of WT and mdx5Cv mice at the indicated ages. Ponceau S stain was used as a loading control. (B) Diaphragms of 6-month-old mdx5Cv and WT mice were stained with antibodies recognizing TGFβ2 and eMyHC. (C) RT-PCR analysis of transcript amounts of the Wnt target genes LGR5 and TGFβ2 in YFP+ve cells purified from hindlimb muscles of 8-to 12-month-old Pax7CreER;R26RYFP;mdx5Cv mice (N = 4). Data are expressed as fold increase in expression compared to that for age-matched Pax7CreER;R26RYFP;WT mice (N = 4). (D) Diaphragms of 6-month-old mdx5Cv mice were stained for TGFβ2 and Axin2, and the average intensity of each signal was evaluated in 120 randomly selected regions of the muscles (see Materials and Methods). The correlation between the values obtained for each staining in each region is shown. Linear regression is indicated. Pearson r, 0.71 (P < 0.01; two-tailed t test).
In vivo inhibition of the TGFβ pathway rescues the satellite cell phenotype
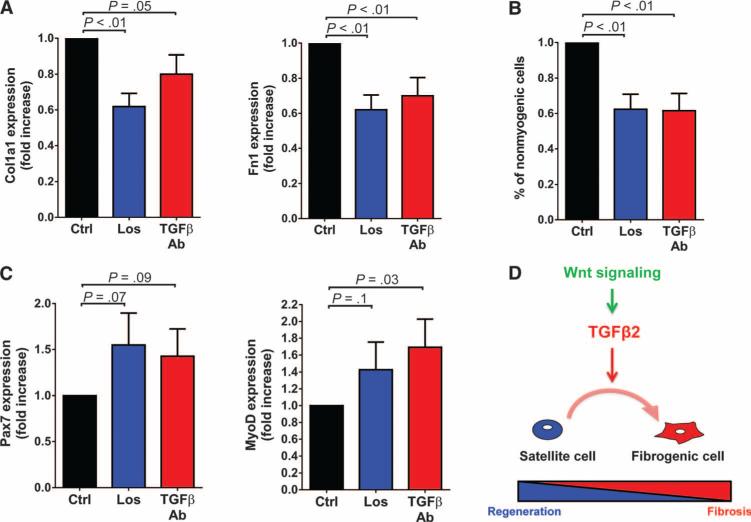
These observations, together with the role of TGFβ2 as a downstream mediator of the effects of Wnt signaling in the control of satellite cell fate, suggest that inhibiting TGFβ signaling might be a way to counteract the aberrant behavior of satellite cells in muscles of mdx mice. To test this possibility, we first treated Pax7CreER;R26RYFP;mdx5Cv mice for 7 months with losartan, a drug that not only is approved for use in humans but also has been shown to efficiently antagonize TGFβ signaling in mdx mice (35). We observed a general reduction in the expression of the fibrotic gene HSP47 and in the abundance of phosphorylated SMAD2/3 (pSMAD2/3), a readout of TGFβ signaling, in response to losartan treatment (fig. S8A). When we focused on the YFP+ve cells (satellite cells and their progeny), we also observed a reduction in the percentage of both pSMAD2/3+ve and HSP47+ve cells (fig. S8B). The link between TGFβ signaling and HSP47 expression was confirmed by the observation that most (~75%) of the YFP+ve cells in mdx5Cv muscles expressing HSP47 also expressed pSMAD2/3. Moreover, gene expression analysis of FACS-sorted cells revealed a reduction in the expression of Col1a1 and Fn1 in cells isolated from mdx5Cv mice treated with losartan (Fig. 6A). In addition to reducing the induction of fibrotic markers in satellite cells, the administration of losartan partially preserved the myogenic program in satellite cells in mdx5Cv mice (Fig. 6B). In keeping with this observation, we noted an increase (not statistically significant) in the expression of the myogenic markers Pax7 and MyoD after treatment with losartan in vivo (Fig. 6C). Similar results to those obtained with losartan were obtained after treating dystrophic mice for 6 months with an anti-TGFβ antibody (Fig. 6, A to C). In agreement with previous reports (35–37), blocking the TGFβ signaling pathway in vivo partially ameliorated the dystrophic phenotype (fig. S9, A and B). These data support the notion that enhanced TGFβ signaling induces both an aberrant behavior in satellite cells and the accumulation of fibrotic tissue in dystrophic muscles.
Fig. 6. Inhibiting satellite cell fate change in mdx5Cv mice by blocking TGFβ signaling.
(A) RT-PCR analysis of transcript amounts of Col1a1 and Fn1 in YFP+ve cells from hindlimb muscles of Pax7CreER; R26RYFP;mdx5Cv mice treated with losartan (Los) (N = 5) or with an antibody that blocked the activity of the three TGFβ isoforms, including TGFβ2 (N = 6). Data are expressed as fold increase in expression over respective controls. (B) YFP+ve cells were isolated from diaphragms of Pax7CreER;R26RYFP;mdx5Cv mice treated with losartan (N = 4) or a TGFβ-blocking antibody (N = 7) and stained for Pax7, MyoD, and myogenin. The percentage of nonmyogenic cells was quantified and expressed as fold increase over the percentage of nonmyogenic cells in control animals. (C) RT-PCR analysis of transcript amounts of Pax7 and MyoD as in (A). Data are expressed as fold increase in expression relative to controls. (D) Model of the mechanisms through which the Wnt-TGFβ2 axis may be affecting satellite cell fate.
DISCUSSION
Here, we present direct evidence that satellite cells have a compromised myogenic potential and enhanced expression of fibrogenic genes in the dystrophic muscles of mdx mice. Our data link enhanced Wnt signaling with a local increase in the expression of TGFβ2 in dystrophic muscles and suggest that the resulting increase in TGFβ activity is responsible for the induction of fibrogenic features in satellite cells induced by the canonical Wnt signaling pathway (Fig. 6D).
These observations support the notion that aberrant behavior of cells in the satellite cell lineage is actively contributing to the dystrophic phenotype, both indirectly by giving rise to a population that less effectively promotes regeneration and directly by producing components of the ECM. In keeping with this view, the pharmacological inhibition of TGFβ signaling both counteracted the fibrogenic activity of satellite cells and reduced the accumulation of fibrotic tissue within the dystrophic muscles. These data do not exclude the possibility that the improved characteristics of the satellite cells in response to inhibition of TGFβ signaling may also be a consequence of the amelioration of the dystrophic phenotype. Nevertheless, the concept that the severity of the dystrophic condition is dependent on the behavior of satellite cells is supported by irradiation studies and by the observation that the severity of the muscular dystrophy in mdx mice is worsened when crossed with a mouse strain in which satellite cell self-renewal is impaired (38, 39). Moreover, several studies in which genes important for the function of the satellite cells have been genetically ablated in mdx mice also documented a worsening of the dystrophic phenotype (40–42). Satellite cells isolated from mdx mice or DMD patients have been reported to exhibit abnormalities in vitro, including decreased clonogenic ability, defective expansion, prolonged doubling times, abnormal differentiation, delayed fusion, altered morphology, presence of crystalline perinuclear inclusions, and reduction in angiogenesis-promoting activity (14, 15, 43–50). Although these studies demonstrate that aberrant function of satellite cells from dystrophic muscle persists during culture in vitro, our study explores the consequences of aberrant satellite cell characteristics in vivo using lineage tracing to track the fate of the satellite cells in dystrophic muscle.
The idea that the progeny of the satellite cells could directly contribute to the accumulation of fibrotic tissue in dystrophic muscles is supported by the observation that a fraction of the mdx satellite cell progeny showed an increased expression of components of the ECM. We observed the presence of satellite cells or their progeny in the interstitium of dystrophic muscle, and we found that these cells expressed generally higher amounts of the profibrotic, collagen-specific processing enzyme HSP47. These observations extend previous electron microscopic observations of satellite cells displaced from myofibers in muscles of DMD patients (51). It is not clear if the aberrant localization of a fraction of the satellite cells depends on the alterations in the architecture of the diseased muscle or reflects intrinsic changes in the satellite cell progeny themselves. Although correlative, the observation of an enhanced expression of TGFβ2 in the regenerative foci of the mdx muscles is suggestive that satellite cells could acquire a fibrogenic potential during the process of regeneration, when a remodeling of the basal lamina is occurring (52).
Although the body of evidence presented here supports an active role of satellite cells in the production of ECM in dystrophic muscles, it does not suggest that satellite cell progeny are the primary source of ECM proteins. Several cell types, including fibroblasts, pericytes, and Sca+ve resident mesenchymal cells, have been proposed as sources of ECM proteins in the context of muscle diseases and regeneration (21, 53–57). The possibility that different cell types could be involved in the abnormal deposition of components of the ECM in the dystrophic setting parallels observations of fibrotic diseases affecting other tissues, such as liver, lung, and kidney, in which different cellular components have been shown to contribute to the etiology of fibrosis (58–64). Recently, an elegant study used newly developed genetic tools to evaluate the relative contribution of distinct lineages to the fibrotic process in the context of kidney fibrosis (65).
Data presented here highlight previously unappreciated similarities in the behavior of satellite cells in the context of muscular dystrophy and aging, two conditions characterized by defective regenerative potential, chronic inflammation, and fibrosis (11). We show that both aging and dystrophic muscles are characterized by increased Wnt signaling and alterations in stem cell fate (12). It is therefore possible that the molecular events we observed in dystrophic muscles could be relevant to abnormalities of aging muscles as well. We are currently exploring the possibility that TGFβ2 could play a detrimental role in the context of muscle aging and other conditions characterized by increased Wnt signaling and stem cell dysfunction (23).
Our data support a key role of a Wnt-TGFβ2 axis in controlling the behavior of satellite cells in dystrophic muscles. These observations place the canonical Wnt signaling pathway and the TGFβ2 pathway in central positions in the complex network of signals that are altered in dystrophic muscles and that have been implicated in the etiology of fibrosis (11). An interaction between Wnt and TGFβ signaling pathways has been described in several physiological and pathophysiological conditions. During cardiac and somite development, Wnt11 can induce TGFβ2 through a noncanonical ATF (activating transcription factor)/ CREB (adenosine 3′,5′-monophosphate response element–binding protein)–dependent mechanism, and recent work investigating kidney development and the molecular changes associated with renal dysplasia supports a link between TGFβ2 expression and canonical Wnt signaling (66, 67). Our data suggest that a canonical Wnt-TGFβ2 axis could be operative in a distinct pathophysiological process in muscle. An increasing body of evidence not only supports the idea that Wnt signaling can modulate TGFβ signaling at different levels (68–70) but also suggests that TGFβ may positively regulate canonical Wnt signaling (71–74). In particular, the recent observation that Wnt signaling is required for TGFβ-mediated fibrosis in an animal model of skin fibrosis (75), together with our identification of a Wnt-TGFβ2 axis in dystrophic muscles, suggests the possibility that an autoreinforcing loop could result from the crosstalk between the Wnt and TGFβ2 signaling pathways. Such a loopwould amplify the events downstream of Wnt and TGFβ and could play an important role in the etiology of fibrosis in muscle and in other tissues.
Several members of the TGFβ superfamily have been previously implicated in DMD pathogenesis (31, 35, 36, 76, 77), but the specific role exerted by each member in determining the enhanced TGFβ signaling observed in dystrophic muscle remains uncertain. We propose that TGFβ2 may be a key player in this context. Intriguingly, it has been described that active TGFβ signaling can induce an autocrine expression of TGFβ1 in myogenic cells, and the overexpression of this cytokine may exert a profibrotic action on muscle cells (78). Because the approaches used in this study to interfere with TGFβ signaling in vivo do not discriminate between TGFβ1 and TGFβ2, it will be of particular interest to define to what extent TGFβ1 is cooperating with TGFβ2 in activating TGFβ signaling in general and altering the state of the satellite cells in the dystrophic muscles. Our observations also raise additional questions that relate to fibrosis in dystrophic muscle and perhaps even more generally. An increased presence of Wnt ligands and receptors and a decreased expression of inhibitors of the Wnt pathway have been proposed to be responsible for the enhanced Wnt signaling reported in aging tissues (79–82). In the case of dystrophic muscle, the cause of the enhanced Wnt signaling is unknown. Also unknown is whether the increase in TGFβ2 expression observed in dystrophic muscles depends exclusively on the enhanced Wnt signaling or whether other molecular pathways are also playing a role in this process. Another important question that derives from our findings is whether the Wnt-TGFβ2 axis is affecting the behavior of other cell types besides satellite cells and to what extent these effects play a role in the etiology of fibrosis. Moreover, the molecular events that are operating downstream of TGFβ2 signaling and are mediating its profibrotic effects also remain largely unknown. Future studies will be important to provide a comprehensive view of the events leading to defective regeneration and excessive fibrosis that ultimately result in compromised functionality of dystrophic muscles.
In conclusion, the observations reported here increase our understanding of local and systemic changes that may affect stem cells in the setting of chronic, degenerative conditions and suggest that aberrant stem cell function could contribute to the development of fibrosis in the muscular dystrophies. Fibrosis is prominent, either as a primary process or as a secondary process, in a variety of degenerative and inflammatory diseases (83). Fibrosis itself both contributes to the symptomatology of such diseases and is also a barrier to effective therapies (84). Therefore, this study, by increasing our understanding of the cellular and molecular mechanisms leading to fibrosis, represents an important step toward the development of effective therapeutic approaches for the treatment of fibrosis in the setting of degenerative disorders of muscle and other tissues (26).
MATERIALS AND METHODS
Study design
The overall objective of this study was to evaluate the fate of satellite cells during the progression of muscular dystrophy by analyzing mdx5Cv mice and comparing them with age-matched WT controls or by analyzing mdx5Cv mice after inhibition of the TGFβ signaling pathway. An additional aim of the study was to investigate the link between Wnt signaling and the expression of TGFβ2 and to evaluate the Wnt-TGFβ2 axis as a regulator of satellite cell behavior. Preliminary data and similar studies previously performed with cells and tissues collected from aging mice indicated that three to seven biological replicates per animal were required in each group to detect a 20% difference in the amounts of gene expression in satellite cells with power of 0.8 and significance level of 0.05. Animals were randomly assigned to experimental and treatment groups based on availability and genotype. No animals were excluded from analysis. With the exception of the microarray analysis and the experiments reported in Figs. 1 (A and D), 2C, and 4B, and figs. S8B and S9 (A and B), the analyses were not blinded. Repeated measures were in experiments with animals and cells as indicated. Both biological and technical replicates were performed in PCR assays.
Mice
Pax7-CreERtm males (85) (herein referred to as Pax7CreER) were crossed to R26RYFP/YFP females (The Jackson Laboratory, no. 006148) to obtain Pax7CreER/WT; R26RYFP/WT males. These breeders were crossed with mdx5Cv/5Cv or WT female (both in the C57BL6 background, The Jackson Laboratory) to obtain the male experimental animals. TMX (Sigma) was dissolved at 50 mg/ml in 92.5% corn oil/7.5% ethanol, and 5 mg was administrated intraperitoneally to 5-week-old experimental mice twice a week for 2 weeks. For specific experiments, R26RLacZ mice were used (The Jackson Laboratory, no. 003474). BAT-GAL mice (The Jackson Laboratory, no. 005317) are engineered with copies of the Wnt-inducible TCF/LEF–responsive element driving the expression of β-Gal. Geno-typing was performed with primers listed in table S2.
In chronic studies, mdx5Cv mice were injected with 300 ng of anti-TGFβ (1D11) or anti-Shigella toxin (control) antibodies on alternate days for 6 months (36). Purified antibodies were provided by Genzyme (37). Losartan was administrated for 7 months (0.6 g/liter in drinking water) (35). Treatments were started at 5 weeks of age. Experiments were done in accordance with protocols approved by the Institutional Animal Care and Use Committee at the Veterinary Medical Unit at the VA Palo Alto Health Care System.
Real-time PCR
RNA was extracted using TRIzol (Invitrogen) and was reverse-transcribed using High Capacity cDNA Reverse Transcription Kit (Applied Bio-systems). Quantitative RT-PCR was carried out on a LightCycler 480II thermocycler (Roche) with LightC480 Probe master or SYBR Green I Master kits (Roche). For SYBR Green assays, primers annealing to different exons were used (table S3). Unless otherwise stated, relative quantification was normalized to mouse HPRT (hypoxanthine-guanine phosphoribosyltransferase). For TaqMan assays, Pax7, MyoD, and GAPDH (glyceraldehyde phosphate dehydrogenase) (normalizer) probes were used (Applied Biosystems).
Immunofluorescence
Cells were processed for immunofluorescence, as previously described (86). For immunofluorescence studies, muscles were fixed for 4 hours using 0.5% electron microscopy–grade paraformaldehyde and transferred to 20% sucrose overnight. Muscles were then frozen in optimum cutting temperature compound (OCT) (Tissue-Tek), cryosectioned at 6 μm, and stained using indirect immunofluorescence or the Zenon labeling kit (Invitrogen). Alexa Fluor 488/594/647 donkey secondary antibodies (Invitrogen) were used. SMAD2/3 immunostaining required additional treatment with 4 N HCl (87). A list of primary antibodies is enclosed in table S4.
Pixel intensity was calculated with Volocity Analysis Software (PerkinElmer). For HSP47 and Collagen1, the maximal pixel intensity was evaluated for each cell. Background intensity of areas devoid of cells was subtracted. For the evaluation of β-catenin, TGFβ2, and Collagen1/3 in WT and mdx5Cv muscle sections, the average pixel intensity of multiple (>8) selected areas was quantified. The background pixel intensity from serial sections stained with only the secondary antibodies was subtracted. Experiments were done in at least three biological replicates. For the correlation between the expression of TGFβ2 and the Wnt-related genes Axin2, LGR5, or β-catenin in mdx5Cv muscles, 120 areas (40 for each mouse; three mice) of 25 μm2 were randomly selected in at least nine different sections, and the corresponding average pixel intensities were quantified. The background pixel intensity from sections stained with only the secondary antibodies was subtracted. Linear regression and correlation (Pearson r) were determined with GraphPad Prism 6.04 software.
X-gal staining
For X-gal staining, sections (10 μM) were incubated for 1 day in X-gal solution. For whole-mount X-gal staining, muscles were fixed for 5 hours in 0.5% paraformaldehyde and incubated for 3 days at 37°C in X-gal solution, as previously described (86).
Cell isolation and culture
To obtain mononucleated cells, adult hindlimb or diaphragm muscles were processed, as previously described (86). For immunostaining with anti-YFP, anti-Pax7, anti-MyoD, anti-myogenin, and anti-HSP47 antibodies, cells isolated from muscles were resuspended in Ham's F-10 nutrient mixture supplemented with 10% horse serum (HS) (Invitrogen). Unless otherwise stated, cells were allowed to adhere overnight on ECM-coated slides (BioCoat, BD) before fixation. To test the effects of Wnt3a and TGFβ2, we added these factors to fresh medium the day after plating and renewed every day.
For RT-PCR, YFP+ve cells from Pax7CreER;R26RYFP mice (WT or mdx5Cv) were further purified using a FACSAria III (BD). Mice without the R26RYFP allele were used to set up the autofluorescence. DAPI dilactate (250 μg/ml, Invitrogen) was used to exclude dead cells. When indicated, satellite cells were purified by positive selection with an anti– vascular cell adhesion molecule antibody (BD Biosciences) and negative selection (Lin−ve) with antibodies recognizing CD31, CD45 (BD Biosciences), and Sca1 (BioLegend), as previously described (86). Cells were processed for RNA extraction after sorting or after 4 days in vitro. When indicated, Wnt3a (100 ng/ml) was added for the last 30 hours.
Primary myoblasts were isolated from 129 (Charles River) or C57 (The Jackson Laboratory) mice, as previously described (88). Cells were cultured on laminin/collagen-coated wells in Ham's F-10 with 20% fetal bovine serum (FBS) and basic fibroblast growth factor (2.5 ng/ml) (Promega) or Dulbecco's modified Eagle's medium (DMEM) with 2% HS to maintain them in a proliferative state or to induce differentiation, respectively.
C2C12 myoblasts [American Type Culture Collection (ATCC)] were expanded in DMEM with 10% FBS. In fate conversion assays, C2C12 myoblasts were maintained in DMEM with 2% HS for 2 days in the presence of indicated factors. Cells were trypsinized [trypsin-EDTA (0.05%)] until complete detachment, filtered through 40-μm strainers to eliminated myotubes, and replated for 2 hours. The kinetics of detachment and adhesion was monitored by time lapse microscopy. In experiments with conditioned medium, C2C12 were cultured in six-well dishes in 800 μl of Ham's F-10 with 10% HS for 24 hours. The medium was filtered through 0.22-μm filters and added to primary myoblasts for 24 hours.
In specific experiments, LSL cells were used to evaluate Wnt activity (89). They were cultured in DMEM with 10% FBS for 24 hours in the presence of XAV-939. Wnt3a was added for 8 hours, and cells were processed to assay luciferase activity (Promega).
Reagents
Reagents used are as follows: XAV-939 (Selleck), BIO (Cayman Chemical), TGFβ1 (Sigma or StemRD), mouse TGFβ2 (Sigma), mouse Wnt3a (R&D or StemRD), mouse Wnt5a (R&D), DMSO (Sigma), ALK5 inhibitor II (Calbiochem), TGFβ2-specific blocking antibody (AB-12-NA, R&D), and rabbit IgG (R&D).
Microarrays
Primary myoblasts isolated from newborn mice were treated for 4 hours in DMEM with 2% HS in the presence or in the absence of Wnt3a (100 ng/ml). RNA was assayed by Affymetrix Mouse 430 2.0 Genome Array.
Transfection
Primary and C2C12 myoblasts were transfected with Lipofectamine 2000 (Invitrogen). Vectors used were mouse TGFβ2 (ATCC, MGC-18603), E[beta]C (28) (Addgene), pLVX-EF1alpha-IRES-mCherry (Clontech), nGFP (90), B9L, and B9LDCter (29). Two days after transfection, cells were trypsinized and purified according to mCherry expression by FACS. DAPI dilactate (250 μg/ml) was used to exclude dead cells.
Western blots
Muscle homogenates were centrifuged, and the protein content of the supernatant was quantified using BCA Protein Assay Kit (Pierce). Equal amounts of protein (50 μg) were separated on 8% SDS–polyacrylamide gel electrophoresis gels and transferred to nitrocellulose membranes (GE). The membranes were stained with Ponceau S to confirm the protein amount and incubated overnight at 4°C with mouse primary antibodies recognizing active β-catenin (Millipore) and TGFβ2 (Abcam). Membranes were incubated with secondary antibodies for 60 min at room temperature, and signals were detected with a ChemiDoc XRS+ Molecular Imager (Bio-Rad) using ECL detection reagents (Amersham).
Statistical analysis
Data are presented as means ± SE. Unless otherwise stated, unpaired one-tailed t tests were used to calculate the statistical significance of observed differences.
Supplementary Material
Acknowledgments
We thank A. Brack, T. Wyss-Coray, K. Wagner, I. Conboy, G. Cossu, and members of the Rando Laboratory for stimulating discussions; J. Brett and Q. Gan for help with the bioinformatics; M. T. Silva, L. Rott, and S. Fernandez-Illescas for technical help; and C. Keller and T. Akiyama for providing reagents. We are also thankful to B. M. Wentworth and Genzyme, a Sanofi company, for supplying us with anti-TGFβ and anti-Shigella toxin antibodies. Funding: This work was supported by grants from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (grant BEX 6650/10-4) to E.H.M. and by grants from the Muscular Dystrophy Association, the NIH (grants P01 AG036695, R37 Method to Extend Research in Time Award AG023806, R01 AR062185, and R01 AR056849), and the Department of Veterans Affairs to T.A.R.
Footnotes
Author contributions: S.B. designed and performed in vitro and in vivo experiments, analyzed data, and wrote the paper. E.H.M. analyzed muscle tissues and performed Western blots. S.D.G. performed ChIP experiments and analyzed data. P.M.M.C. provided technical assistance with the cell culture experiments. T.A.R. designed experiments, analyzed data, and wrote the paper.
Citation: S. Biressi, E. H. Miyabara, S. D. Gopinath, P. M. M. Carlig, T. A. Rando, A Wnt-TGFβ2 axis induces a fibrogenic program in muscle stem cells from dystrophic mice. Sci. Transl. Med. 6, 267ra176 (2014).
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: The microarray data discussed in this publication have been deposited in National Center for Biotechnology Information's Gene Expression Omnibus (GEO) and are accessible through GEO Series accession no. GSE50238. Anti-TGFβ (hybridoma 1D11) and anti-Shigella toxin antibodies were supplied by Genzyme, a Sanofi company.
REFERENCES AND NOTES
- 1.Chargé SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 2.Dhawan J, Rando TA. Stem cells in postnatal myogenesis: Molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15:666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: The stem cell that came in from the cold. J. Histochem. Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- 4.Mauro A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lepper C, Partridge TA, Fan CM. Development; An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration; 2011. pp. 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 7.Mercuri E, Muntoni F. Muscular dystrophies. Lancet. 2013;381:845–860. doi: 10.1016/S0140-6736(12)61897-2. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman EP, Brown Jr RH, Kunkel LM. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 9.Wallace GQ, McNally EM. Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu. Rev. Physiol. 2009;71:37–57. doi: 10.1146/annurev.physiol.010908.163216. [DOI] [PubMed] [Google Scholar]
- 10.Morgan JE, Zammit PS. Direct effects of the pathogenic mutation on satellite cell function in muscular dystrophy. Exp. Cell Res. 2010;316:3100–3108. doi: 10.1016/j.yexcr.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Serrano AL, Mann CJ, Vidal B, Ardite E, Perdiguero E, Muñoz-Cánoves P. Cellular and molecular mechanisms regulating fibrosis in skeletal muscle repair and disease. Curr. Top. Dev. Biol. 2011;96:167–201. doi: 10.1016/B978-0-12-385940-2.00007-3. [DOI] [PubMed] [Google Scholar]
- 12.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 13.Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: A point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 14.Ionasescu V, Ionasescu R. Increased collagen synthesis by Duchenne myogenic clones. J. Neurol. Sci. 1982;54:79–87. doi: 10.1016/0022-510x(82)90220-9. [DOI] [PubMed] [Google Scholar]
- 15.Alexakis C, Partridge T, Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: A self-perpetuating mechanism of collagen overproduction. Am. J. Physiol. Cell Physiol. 2007;293:C661–C669. doi: 10.1152/ajpcell.00061.2007. [DOI] [PubMed] [Google Scholar]
- 16.Danko I, Chapman V, Wolff JA. The frequency of revertants in mdx mouse genetic models for Duchenne muscular dystrophy. Pediatr. Res. 1992;32:128–131. doi: 10.1203/00006450-199207000-00025. [DOI] [PubMed] [Google Scholar]
- 17.Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT, Kelly AM. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- 18.Taguchi T, Nazneen A, Al-Shihri AA, Turkistani KA, Razzaque MS. Heat shock protein 47: A novel biomarker of phenotypically altered collagen-producing cells. Acta Histochem. Cytochem. 2011;44:35–41. doi: 10.1267/ahc.11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishida Y, Nagata K. Hsp47 as a collagen-specific molecular chaperone. Methods Enzymol. 2011;499:167–182. doi: 10.1016/B978-0-12-386471-0.00009-2. [DOI] [PubMed] [Google Scholar]
- 20.Hidestrand M, Richards-Malcolm S, Gurley CM, Nolen G, Grimes B, Waterstrat A, Zant GV, Peterson CA. Sca-1-expressing nonmyogenic cells contribute to fibrosis in aged skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:566–579. doi: 10.1093/gerona/63.6.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trensz F, Haroun S, Cloutier A, Richter MV, Grenier G. A muscle resident cell population promotes fibrosis in hindlimb skeletal muscles of mdx mice through the Wnt canonical pathway. Am. J. Physiol. Cell Physiol. 2010;299:C939–C947. doi: 10.1152/ajpcell.00253.2010. [DOI] [PubMed] [Google Scholar]
- 22.Torres LF, Duchen LW. The mutant mdx: Inherited myopathy in the mouse. Morphological studies of nerves, muscles and end-plates. Brain. 1987;110(Pt. 2):269–299. doi: 10.1093/brain/110.2.269. [DOI] [PubMed] [Google Scholar]
- 23.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 25.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat. Rev. Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauviel A. Transforming growth factor-β: A key mediator of fibrosis. Methods Mol. Med. 2005;117:69–80. doi: 10.1385/1-59259-940-0:069. [DOI] [PubMed] [Google Scholar]
- 28.Fuerer C, Nusse R. Lentiviral vectors to probe and manipulate the Wnt signaling pathway. PLOS One. 2010;5:e9370. doi: 10.1371/journal.pone.0009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adachi S, Jigami T, Yasui T, Nakano T, Ohwada S, Omori Y, Sugano S, Ohkawara B, Shibuya H, Nakamura T, Akiyama T. Role of a BCL9-related β-catenin-binding protein, B9L, in tumorigenesis induced by aberrant activation of Wnt signaling. Cancer Res. 2004;64:8496–8501. doi: 10.1158/0008-5472.CAN-04-2254. [DOI] [PubMed] [Google Scholar]
- 30.Richler C, Yaffe D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev. Biol. 1970;23:1–22. doi: 10.1016/s0012-1606(70)80004-5. [DOI] [PubMed] [Google Scholar]
- 31.Bernasconi P, Di Blasi C, Mora M, Morandi L, Galbiati S, Confalonieri P, Cornelio F, Mantegazza R. Transforming growth factor-β1 and fibrosis in congenital muscular dystrophies. Neuromuscul. Disord. 1999;9:28–33. doi: 10.1016/s0960-8966(98)00093-5. [DOI] [PubMed] [Google Scholar]
- 32.Yamazaki M, Minota S, Sakurai H, Miyazono K, Yamada A, Kanazawa I, Kawai M. Expression of transforming growth factor-β 1 and its relation to endomysial fibrosis in progressive muscular dystrophy. Am. J. Pathol. 1994;144:221–226. [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou L, Porter JD, Cheng G, Gong B, Hatala DA, Merriam AP, Zhou X, Rafael JA, Kaminski HJ. Temporal and spatial mRNA expression patterns of TGF-β1, 2, 3 and TbRI, II, III in skeletal muscles of mdx mice. Neuromuscul. Disord. 2006;16:32–38. doi: 10.1016/j.nmd.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Murakami N, McLennan IS, Nonaka I, Koishi K, Baker C, Hammond-Tooke G. Transforming growth factor-β2 is elevated in skeletal muscle disorders. Muscle Nerve. 1999;22:889–898. doi: 10.1002/(sici)1097-4598(199907)22:7<889::aid-mus12>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 35.Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, Ramirez F, Judge DP, Ward CW, Dietz HC. Angiotensin II type 1 receptor blockade attenuates TGF-β–induced failure of muscle regeneration in multiple myopathic states. Nat. Med. 2007;13:204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andreetta F, Bernasconi P, Baggi F, Ferro P, Oliva L, Arnoldi E, Cornelio F, Mantegazza R, Confalonieri P. Immunomodulation of TGF-beta 1 in mdx mouse inhibits connective tissue proliferation in diaphragm but increases inflammatory response: Implications for antifibrotic therapy. J. Neuroimmunol. 2006;175:77–86. doi: 10.1016/j.jneuroim.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Nelson CA, Hunter RB, Quigley LA, Girgenrath S, Weber WD, McCullough JA, Dinardo CJ, Keefe KA, Ceci L, Clayton NP, McVie-Wylie A, Cheng SH, Leonard JP, Wentworth BM. Inhibiting TGF-β activity improves respiratory function in mdx mice. Am. J. Pathol. 2011;178:2611–2621. doi: 10.1016/j.ajpath.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukada S, Morikawa D, Yamamoto Y, Yoshida T, Sumie N, Yamaguchi M, Ito T, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Yamamoto H. Genetic background affects properties of satellite cells and mdx phenotypes. Am. J. Pathol. 2010;176:2414–2424. doi: 10.2353/ajpath.2010.090887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wakeford S, Watt DJ, Partridge TA. X-irradiation improves mdx mouse muscle as a model of myofiber loss in DMD. Muscle Nerve. 1991;14:42–50. doi: 10.1002/mus.880140108. [DOI] [PubMed] [Google Scholar]
- 40.Garry DJ, Meeson A, Elterman J, Zhao Y, Yang P, Bassel-Duby R, Williams RS. Myogenic stem cell function is impaired in mice lacking the forkhead/winged helix protein MNF. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5416–5421. doi: 10.1073/pnas.100501197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996;10:1173–1183. doi: 10.1101/gad.10.10.1173. [DOI] [PubMed] [Google Scholar]
- 42.Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, Shkreli M, Delp S, Pomerantz JH, Artandi SE, Blau HM. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell. 2010;143:1059–1071. doi: 10.1016/j.cell.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yablonka-Reuveni Z, Anderson JE. Satellite cells from dystrophic (mdx) mice display accelerated differentiation in primary cultures and in isolated myofibers. Dev. Dyn. 2006;235:203–212. doi: 10.1002/dvdy.20602. [DOI] [PubMed] [Google Scholar]
- 44.Rhoads RP, Flann KL, Cardinal TR, Rathbone CR, Liu X, Allen RE. Satellite cells isolated from aged or dystrophic muscle exhibit a reduced capacity to promote angiogenesis in vitro. Biochem. Biophys. Res. Commun. 2013;440:399–404. doi: 10.1016/j.bbrc.2013.09.085. [DOI] [PubMed] [Google Scholar]
- 45.Morgan J, Cohen L. Letter: Crystalline perinuclear inclusions in myoblasts from human dystrophic muscle. N. Engl. J. Med. 1974;290:863. doi: 10.1056/NEJM197404112901525. [DOI] [PubMed] [Google Scholar]
- 46.Melone MA, Peluso G, Petillo O, Galderisi U, Cotrufo R. Defective growth in vitro of Duchenne muscular dystrophy myoblasts: The molecular and biochemical basis. J. Cell. Biochem. 1999;76:118–132. doi: 10.1002/(sici)1097-4644(20000101)76:1<118::aid-jcb12>3.3.co;2-6. [DOI] [PubMed] [Google Scholar]
- 47.Jasmin G, Tautu C, Vanasse M, Brochu P, Simoneau R. Impaired muscle differentiation in explant cultures of Duchenne muscular dystrophy. Lab. Invest. 1984;50:197–207. [PubMed] [Google Scholar]
- 48.Delaporte C, Dehaupas M, Fardeau M. Comparison between the growth pattern of cell cultures from normal and Duchenne dystrophy muscle. J. Neurol. Sci. 1984;64:149–160. doi: 10.1016/0022-510x(84)90033-9. [DOI] [PubMed] [Google Scholar]
- 49.Delaporte C, Dautreaux B, Rouche A, Fardeau M. Changes in surface morphology and basal lamina of cultured muscle cells from Duchenne muscular dystrophy patients. J. Neurol. Sci. 1990;95:77–88. doi: 10.1016/0022-510x(90)90118-7. [DOI] [PubMed] [Google Scholar]
- 50.Blau HM, Webster C, Pavlath GK. Defective myoblasts identified in Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. U.S.A. 1983;80:4856–4860. doi: 10.1073/pnas.80.15.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wakayama Y. Electron microscopic study on the satellite cell in the muscle of Duchenne muscular dystrophy. J. Neuropathol. Exp. Neurol. 1976;35:532–540. doi: 10.1097/00005072-197609000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Vracko R, Benditt EP. Basal lamina: The scaffold for orderly cell replacement. Observations on regeneration of injured skeletal muscle fibers and capillaries. J. Cell Biol. 1972;55:406–419. doi: 10.1083/jcb.55.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vidal B, Serrano AL, Tjwa M, Suelves M, Ardite E, De Mori R, Baeza-Raja B, Martínez de Lagrán M, Lafuste P, Ruiz-Bonilla V, Jardí M, Gherardi R, Christov C, Dierssen M, Carmeliet P, Degen JL, Dewerchin M, Muñoz-Cánoves P. Fibrinogen drives dystrophic muscle fibrosis via a TGFβ/alternative macrophage activation pathway. Genes Dev. 2008;22:1747–1752. doi: 10.1101/gad.465908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Tsuchida K, Yamamoto H, Fukada S. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 2011;124:3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 55.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12+ perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat. Med. 2012;18:1262–1270. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- 58.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am. J. Pathol. 2009;175:1380–1388. doi: 10.2353/ajpath.2009.090096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Broekema M, Harmsen MC, van Luyn MJ, Koerts JA, Petersen AH, van Kooten TG, van Goor H, Navis G, Popa ER. Bone marrow–derived myofibroblasts contribute to the renal interstitial myofibroblast population and produce procollagen I after ischemia/reperfusion in rats. J. Am. Soc. Nephrol. 2007;18:165–175. doi: 10.1681/ASN.2005070730. [DOI] [PubMed] [Google Scholar]
- 63.Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc. Am. Thorac. Soc. 2006;3:377–382. doi: 10.1513/pats.200601-004TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brenner DA, Kisseleva T, Scholten D, Paik YH, Iwaisako K, Inokuchi S, Schnabl B, Seki E, De Minicis S, Oesterreicher C, Taura K. Origin of myofibroblasts in liver fibrosis. Fibrogenesis Tissue Repair. 2012;5S17(Suppl. 1) doi: 10.1186/1755-1536-5-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 2013;19:1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou W, Lin L, Majumdar A, Li X, Zhang X, Liu W, Etheridge L, Shi Y, Martin J, Van de Ven W, Kaartinen V, Wynshaw-Boris A, McMahon AP, Rosenfeld MG, Evans SM. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family–mediated transcriptional activation of TGFβ2. Nat. Genet. 2007;39:1225–1234. doi: 10.1038/ng2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bridgewater D, Di Giovanni V, Cain JE, Cox B, Jakobson M, Sainio K, Rosenblum ND. β-Catenin causes renal dysplasia via upregulation of Tgfβ2 and Dkk1. J. Am. Soc. Nephrol. 2011;22:718–731. doi: 10.1681/ASN.2010050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carthy JM, Garmaroudi FS, Luo Z, McManus BM. Wnt3a induces myofibroblast differentiation by upregulating TGF-β signaling through SMAD2 in a β-catenin-dependent manner. PLOS One. 2011;6:e19809. doi: 10.1371/journal.pone.0019809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo X, Ramirez A, Waddell DS, Li Z, Liu X, Wang XF. Axin and GSK3-β control Smad3 protein stability and modulate TGF-β signaling. Genes Dev. 2008;22:106–120. doi: 10.1101/gad.1590908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei J, Fang F, Lam AP, Sargent JL, Hamburg E, Hinchcliff ME, Gottardi CJ, Atit R, Whitfield ML, Varga J. Wnt/β-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum. 2012;64:2734–2745. doi: 10.1002/art.34424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheon SS, Wei Q, Gurung A, Youn A, Bright T, Poon R, Whetstone H, Guha A, Alman BA. Beta-catenin regulates wound size and mediates the effect of TGF-beta in cutaneous healing. FASEB J. 2006;20:692–701. doi: 10.1096/fj.05-4759com. [DOI] [PubMed] [Google Scholar]
- 72.Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H, Cho KW. Interaction between Wnt and TGF-β signalling pathways during formation of Spemann’s organizer. Nature. 2000;403:781–785. doi: 10.1038/35001602. [DOI] [PubMed] [Google Scholar]
- 73.Satterwhite DJ, Neufeld KL. TGF-β targets the Wnt pathway components, APC and β-catenin, as Mv1Lu cells undergo cell cycle arrest. Cell Cycle. 2004;3:1069–1073. [PubMed] [Google Scholar]
- 74.Zhou S. TGF-β regulates β-catenin signaling and osteoblast differentiation in human mesenchymal stem cells. J. Cell. Biochem. 2011;112:1651–1660. doi: 10.1002/jcb.23079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, Schneider H, Sadowski A, Riener MO, MacDougald OA, Distler O, Schett G, Distler JH. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat. Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wagner KR, McPherron AC, Winik N, Lee SJ. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann. Neurol. 2002;52:832–836. doi: 10.1002/ana.10385. [DOI] [PubMed] [Google Scholar]
- 77.Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–421. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- 78.Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J. Transforming growth factor-β1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: A key event in muscle fibrogenesis. Am. J. Pathol. 2004;164:1007–1019. doi: 10.1016/s0002-9440(10)63188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Naito AT, Sumida T, Nomura S, Liu ML, Higo T, Nakagawa A, Okada K, Sakai T, Hashimoto A, Hara Y, Shimizu I, Zhu W, Toko H, Katada A, Akazawa H, Oka T, Lee JK, Minamino T, Nagai T, Walsh K, Kikuchi A, Matsumoto M, Botto M, Shiojima I, Komuro I. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell. 2012;149:1298–1313. doi: 10.1016/j.cell.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 81.Du J, Klein JD, Hassounah F, Zhang J, Zhang C, Wang XH. Aging increases CCN1 expression leading to muscle senescence. Am. J. Physiol. Cell Physiol. 2014;306:C28–C36. doi: 10.1152/ajpcell.00066.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Doi R, Endo M, Yamakoshi K, Yamanashi Y, Nishita M, Fukada S, Minami Y. Critical role of Frizzled1 in age-related alterations of Wnt/β-catenin signal in myogenic cells during differentiation. Genes Cells. 2014;19:287–296. doi: 10.1111/gtc.12132. [DOI] [PubMed] [Google Scholar]
- 83.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gargioli C, Coletta M, De Grandis F, Cannata SM, Cossu G. PlGF–MMP-9–expressing cells restore microcirculation and efficacy of cell therapy in aged dystrophic muscle. Nat. Med. 2008;14:973–978. doi: 10.1038/nm.1852. [DOI] [PubMed] [Google Scholar]
- 85.Nishijo K, Hosoyama T, Bjornson CR, Schaffer BS, Prajapati SI, Bahadur AN, Hansen MS, Blandford MC, McCleish AT, Rubin BP, Epstein JA, Rando TA, Capecchi MR, Keller C. Biomarker system for studying muscle, stem cells, and cancer in vivo. FASEB J. 2009;23:2681–2690. doi: 10.1096/fj.08-128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Biressi S, Bjornson CR, Carlig PM, Nishijo K, Keller C, Rando TA. Myf5 expression during fetal myogenesis defines the developmental progenitors of adult satellite cells. Dev. Biol. 2013;379:195–207. doi: 10.1016/j.ydbio.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carlson ME, Conboy MJ, Hsu M, Barchas L, Jeong J, Agrawal A, Mikels AJ, Agrawal S, Schaffer DV, Conboy IM. Relative roles of TGF-β1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell. 2009;8:676–689. doi: 10.1111/j.1474-9726.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Quach NL, Biressi S, Reichardt LF, Keller C, Rando TA. Focal adhesion kinase signaling regulates the expression of caveolin 3 and b1 integrin, genes essential for normal myoblast fusion. Mol. Biol. Cell. 2009;20:3422–3435. doi: 10.1091/mbc.E09-02-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blitzer JT, Nusse R. A critical role for endocytosis in Wnt signaling. BMC Cell Biol. 2006;7:28. doi: 10.1186/1471-2121-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berghella L, De Angelis L, De Buysscher T, Mortazavi A, Biressi S, Forcales SV, Sirabella D, Cossu G, Wold BJ. A highly conserved molecular switch binds MSY-3 to regulate myogenin repression in postnatal muscle. Genes Dev. 2008;22:2125–2138. doi: 10.1101/gad.468508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mora-Blanco EL, Mishina Y, Tillman EJ, Cho YJ, Thom CS, Pomeroy SL, Shao W, Roberts CW. Activation of β-catenin/TCF targets following loss of the tumor suppressor SNF5. Oncogene. 2014;33:933–938. doi: 10.1038/onc.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu CI, Hoffman JA, Shy BR, Ford EM, Fuchs E, Nguyen H, Merrill BJ. Function of Wnt/β-catenin in counteracting Tcf3 repression through the Tcf3–β-catenin interaction. Development. 2012;139:2118–2129. doi: 10.1242/dev.076067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ovcharenko I, Loots GG, Giardine BM, Hou M, Ma J, Hardison RC, Stubbs L, Miller W. Mulan: Multiple-sequence local alignment and visualization for studying function and evolution. Genome Res. 2005;15:184–194. doi: 10.1101/gr.3007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Davydov EV, Goode DL, Sirota M, Cooper GM, Sidow A, Batzoglou S. Identifying a high fraction of the human genome to be under selective constraint using GERP++. PLOS Comput. Biol. 2010;6:e1001025. doi: 10.1371/journal.pcbi.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.