Abstract
Coproporphyrinogen oxidase (EC 1.3.3.3) catalyzes the sixth step in the heme biosynthetic pathway, the oxidation of coproporphyrinogen III to protoporphyrinogen IX. The activity of this enzyme is deficient in the disease hereditary coproporphyria. The sequence of the cDNA and predicted amino acid sequence of the human coproporphyrinogen oxidase are presented. The human protein sequence contains a region completely homologous to that we obtained by sequencing an 11-amino acid peptide fragment from purified murine liver coproporphyrinogen oxidase. Results of Southern blotting were consistent with the presence of a single human coproporphyrinogen oxidase gene, and Northern blotting demonstrated one transcript of similar size in erythroid and nonerythroid cell lines. Expression of the cDNA coding for the putative mature human coproporphyrinogen oxidase in Escherichia coli resulted in a 17-fold increase in coproporphyrinogen activity over endogenous activity.
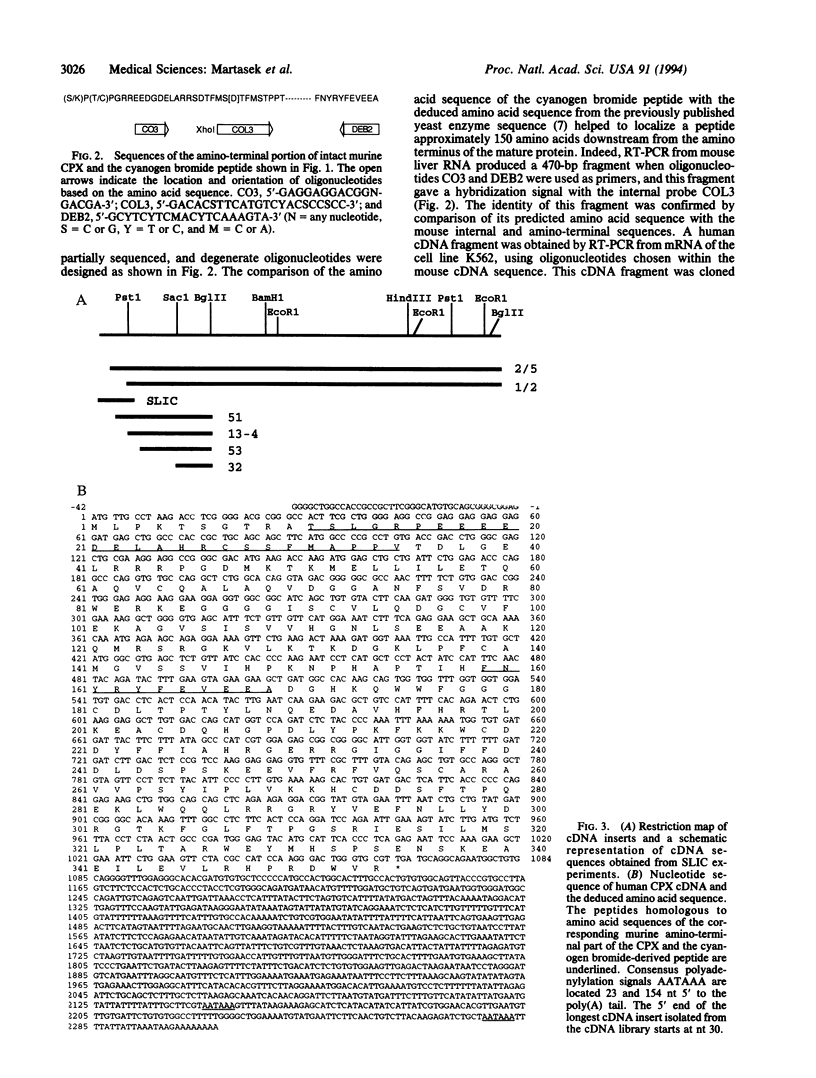
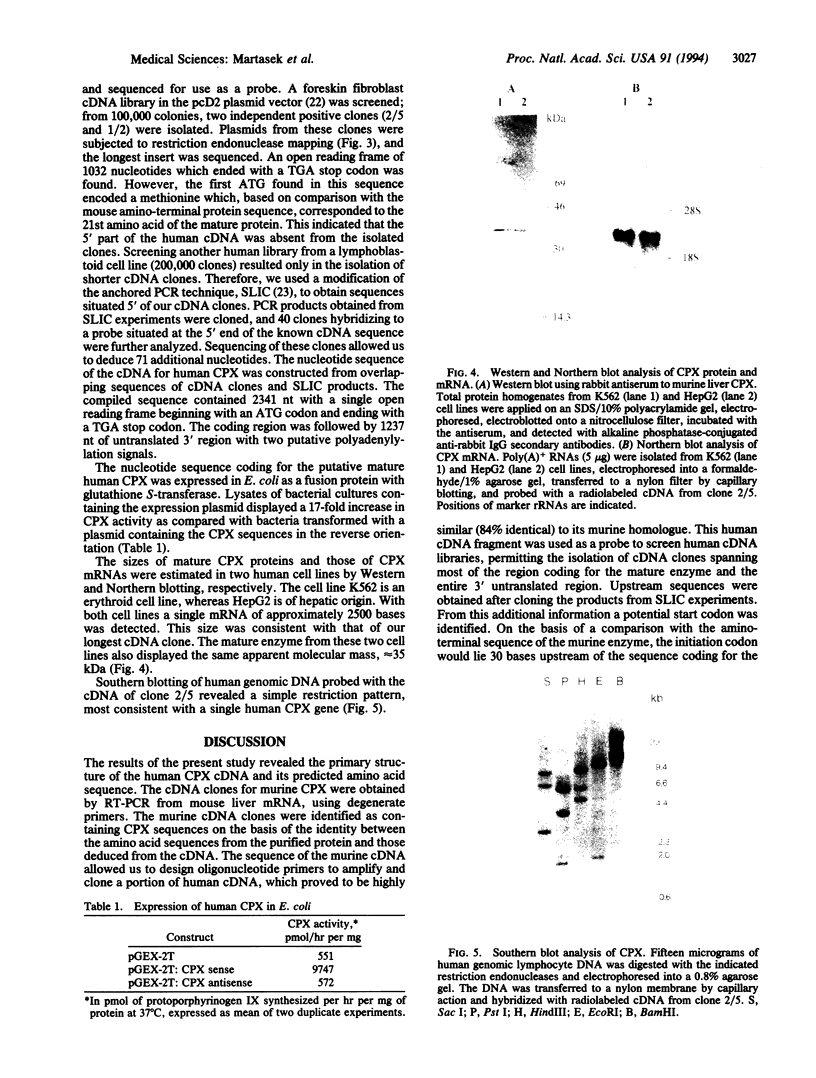
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansorge W., Sproat B. S., Stegemann J., Schwager C. A non-radioactive automated method for DNA sequence determination. J Biochem Biophys Methods. 1986 Dec;13(6):315–323. doi: 10.1016/0165-022x(86)90038-2. [DOI] [PubMed] [Google Scholar]
- Bogard M., Camadro J. M., Nordmann Y., Labbe P. Purification and properties of mouse liver coproporphyrinogen oxidase. Eur J Biochem. 1989 May 1;181(2):417–421. doi: 10.1111/j.1432-1033.1989.tb14741.x. [DOI] [PubMed] [Google Scholar]
- Bottomley S. S., Muller-Eberhard U. Pathophysiology of heme synthesis. Semin Hematol. 1988 Oct;25(4):282–302. [PubMed] [Google Scholar]
- Camadro J. M., Chambon H., Jolles J., Labbe P. Purification and properties of coproporphyrinogen oxidase from the yeast Saccharomyces cerevisiae. Eur J Biochem. 1986 May 2;156(3):579–587. doi: 10.1111/j.1432-1033.1986.tb09617.x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Conder L. H., Woodard S. I., Dailey H. A. Multiple mechanisms for the regulation of haem synthesis during erythroid cell differentiation. Possible role for coproporphyrinogen oxidase. Biochem J. 1991 Apr 15;275(Pt 2):321–326. doi: 10.1042/bj2750321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmetic talc powder. Lancet. 1977 Jun 25;1(8026):1348–1349. [PubMed] [Google Scholar]
- Elder G. H., Evans J. O. Evidence that the coproporphyrinogen oxidase activity of rat liver is situated in the intermembrane space of mitochondria. Biochem J. 1978 May 15;172(2):345–347. doi: 10.1042/bj1720345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B. S., Beasley E. M., Schatz G. Protein sorting in mitochondria. Trends Biochem Sci. 1992 Nov;17(11):453–459. doi: 10.1016/0968-0004(92)90487-t. [DOI] [PubMed] [Google Scholar]
- Grandchamp B., Phung N., Nordmann Y. The mitochondrial localization of coproporphyrinogen III oxidase. Biochem J. 1978 Oct 15;176(1):97–102. doi: 10.1042/bj1760097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M., Nagata A., Jinno S., Suto K., Okayama H. Wee1(+)-like gene in human cells. Nature. 1991 Sep 5;353(6339):80–83. doi: 10.1038/353080a0. [DOI] [PubMed] [Google Scholar]
- Kohno H., Furukawa T., Yoshinaga T., Tokunaga R., Taketani S. Coproporphyrinogen oxidase. Purification, molecular cloning, and induction of mRNA during erythroid differentiation. J Biol Chem. 1993 Oct 5;268(28):21359–21363. [PubMed] [Google Scholar]
- Labbe P., Camadro J. M., Chambon H. Fluorometric assays for coproporphyrinogen oxidase and protoporphyrinogen oxidase. Anal Biochem. 1985 Aug 15;149(1):248–260. doi: 10.1016/0003-2697(85)90502-0. [DOI] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Madsen O., Sandal L., Sandal N. N., Marcker K. A. A soybean coproporphyrinogen oxidase gene is highly expressed in root nodules. Plant Mol Biol. 1993 Oct;23(1):35–43. doi: 10.1007/BF00021417. [DOI] [PubMed] [Google Scholar]
- Nordmann Y., Grandchamp B., de Verneuil H., Phung L., Cartigny B., Fontaine G. Harderoporphyria: a variant hereditary coproporphyria. J Clin Invest. 1983 Sep;72(3):1139–1149. doi: 10.1172/JCI111039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romana M., Grandchamp B., Dubart A., Amselem S., Chabret C., Nordmann Y., Goossens M., Romeo P. H. Identification of a new mutation responsible for hepatoerythropoietic porphyria. Eur J Clin Invest. 1991 Apr;21(2):225–229. doi: 10.1111/j.1365-2362.1991.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Sassa S. Heme stimulation of cellular growth and differentiation. Semin Hematol. 1988 Oct;25(4):312–320. [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Xu K., Elliott T. An oxygen-dependent coproporphyrinogen oxidase encoded by the hemF gene of Salmonella typhimurium. J Bacteriol. 1993 Aug;175(16):4990–4999. doi: 10.1128/jb.175.16.4990-4999.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga T., Sano S. Coproporphyrinogen oxidase. I. Purification, properties, and activation by phospholipids. J Biol Chem. 1980 May 25;255(10):4722–4726. [PubMed] [Google Scholar]
- Zagorec M., Buhler J. M., Treich I., Keng T., Guarente L., Labbe-Bois R. Isolation, sequence, and regulation by oxygen of the yeast HEM13 gene coding for coproporphyrinogen oxidase. J Biol Chem. 1988 Jul 15;263(20):9718–9724. [PubMed] [Google Scholar]