SUMMARY
Polyunsaturated fatty acids (PUFAs) exhibit a diverse range of critical functions in biological systems. PUFAs modulate the biophysical properties of membranes and, along with their derivatives, the eicosanoids and endocannabinoids, form a wide array potent lipid signaling molecules. Much of our early understanding of PUFAs and PUFA-derived signaling stems from work in mammals; however, technological advances have made comprehensive lipid analysis possible in small genetic models such as Caenorhabditis elegans and Drosophila melanogaster. These models have a number of advantages, such as simple anatomy and genome-wide genetic screening techniques, which can broaden our understanding of fatty-acid-derived signaling in biological systems. Here we review what is known about PUFAs, eicosanoids, and endocannabinoids in the development and reproduction of C. elegans and D. melanogaster. Fatty acid signaling appears to be fundamental for multicellular organisms, and simple invertebrates often employ functionally similar pathways. In particular, studies in C. elegans and Drosophila are providing insight into the roles of PUFAs and PUFA-derived signaling in early developmental processes, such as meiosis, fertilization, and early embryonic cleavage.
INTRODUCTION
Lipids are fundamental to all biological systems: they define the boundaries of cells and organelles, serve as an important fuel source, and participate in a variety of signaling pathways. Lipidomic analyses, which seek to define all lipid species present in a system, indicate that the lipid composition of eukaryotic systems is enormously complex. An individual cell may have over 1,000 different lipid species, and an organism or tissue between 10,000 and 100,000 different lipid species (Wenk, 2010). The functional relevance of this immense lipid diversity is unclear. Within each class of lipids, a wide variety of different fatty acid chains can be incorporated, contributing to a significant portion of the observed lipid diversity (Shevchenko and Simons, 2010). Fatty acids are hydrocarbon chains that typically have an even number of carbons ranging from 10 to 24 in biological systems. They can be saturated (fully reduced) or unsaturated (containing double bonds), with unsaturated fatty acids containing from one to six double bonds. Fatty acids in biological systems are most often found esterified to another molecule. Triacylglycerol is the primary lipid form for energy storage, and it is comprised of three fatty acid chains esterified to a glycerol backbone. Phospholipids are the major components of membranes, and can be made from either a glycerol or sphingoid base with two acyl chains bonded at positions 1 and 2, and a phosphate at position 3 that can bond to a head group.
Nematodes and fruit flies are emerging as key systems to study lipid biology. The development of highly sensitive chromatographic and spectroscopic methods has enabled comprehensive lipid analysis in these systems. The simple anatomy and wide array of forward and reverse genetic tools available in Caenorhabditis elegans and Drosophila melanogaster make these models ideal for discovering new roles and regulation for lipid metabolism. Additionally, studies in these models can probe lipid metabolism in the context of the whole organism, allowing for the evaluation of the roles of specific lipids in development, lifespan, and other aspects of physiology. Much of the work on lipids in these models has focused on adiposity and metabolic syndromes, revealing conservation of critical regulators such as nuclear receptors and the insulin-like growth factor pathway. Several reviews have recently been published summarizing the studies on the regulation of fat storage in C. elegans (Watts, 2009; Zheng and Greenway, 2012) and D. melanogaster (Baker and Thummel, 2007; Hong and Park, 2010).
Recent work in invertebrate models is revealing that specific fatty acids have functions in development (Vrablik and Watts, 2012). The fatty acid composition of phospholipids can greatly influence membrane properties, such as thickness, fluidity, tension, curvature, and the formation of membrane microdomains. These properties can affect the activity of membrane-associated proteins as well as cellular processes that alter membrane structure such as cytokinesis (Kremmyda et al., 2011; Vrablik and Watts, 2012). Additionally, lipase enzymes can cleave triacylglycerols and phospholipids, releasing free fatty acids that can function independently or be enzymatically processed into signaling molecules. Polyunsaturated fatty acids (PUFAs) and their signaling derivatives, the eicosanoids and endocannabinoids, are involved in a variety of biological processes, including inflammation and immune response, neuroreception, development, and almost all aspects of reproduction (Funk, 2001; Battista et al., 2012; Glass and Olefsky, 2012; Hirata et al., 2012; Katona and Freund, 2012).
While fatty acid-derived signaling is known to play critical roles in mammalian reproduction and development, little is known about how these signals exert their effects. Studies on lipid signaling in simple model systems will promote understanding these signaling pathways with molecular and cellular resolution. This review focuses specifically on the roles of unsaturated fatty acids and their signaling derivatives in the development and reproduction of invertebrate models. The first section of this review provides a broad overview of the synthesis and regulation of PUFAs, eicosanoids, and endocannabinoids. Next, we explore the conserved roles for each of these pathways in the development and reproduction of the invertebrate models C. elegans and D. melanogaster, respectively. We conclude with how studies in invertebrate systems may expand our understanding of fatty acid-derived signaling in mammals, and highlight promising areas for future research.
PATHWAYS FOR THE SYNTHESIS OF PUFA SIGNALING MOLECULES
Polyunsaturated Fatty Acids
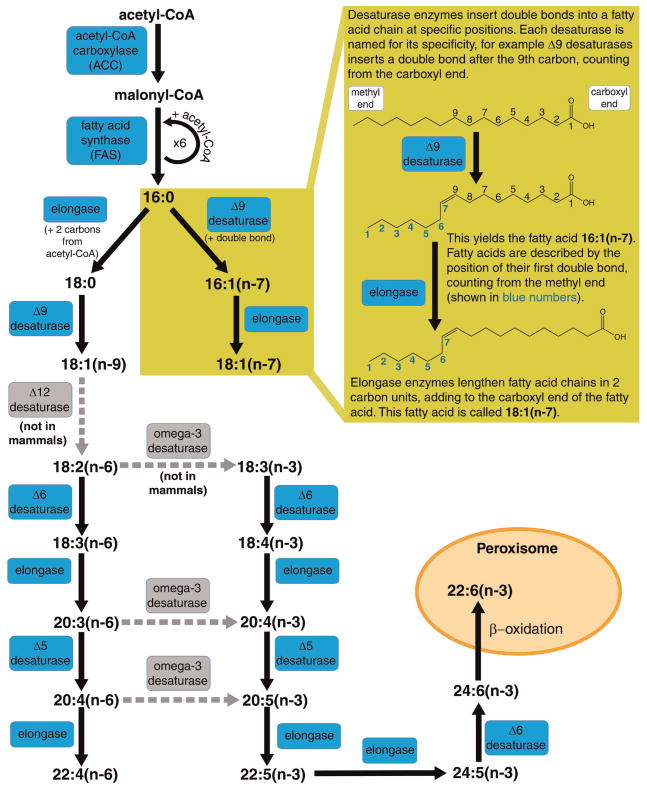
PUFAs are fatty acid chains containing two or more double bonds. The importance of PUFAs has been appreciated since the discovery that omega-3 and omega-6 fatty acids are required in the mammalian diet for viability, and thus termed essential fatty acids. Most eukaryotes can synthesize fatty acids de novo through the activities of acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS), yielding the 16-carbon saturated fatty acid, palmitic acid (16:0). Palmitic acid can then be elongated in two-carbon units by elongase enzymes and double bonds are inserted by fatty acid desaturase enzymes (Fig. 1). Each desaturase enzyme introduces a double bond at a specific carbon in the fatty acid chain, and the desaturase enzyme is usually named after the position in the carbon chain where the double bond is inserted. For example, a Δ9 desaturase inserts a double bond at the 9th carbon of palmitic or stearic acid, creating a monounsaturated fatty acid. The repertoire of fatty acid desaturase enzymes that an organism possesses varies greatly. Mammals lack the Δ12 and omega-3 fatty acid desaturase activities that allow them to convert oleic acid (18:1n-9) to linoleic acid (18:2n-6), or to convert omega-6(n-6) fatty acids to omega-3(n-3) fatty acids. Thus, they must obtain the omega-6 and omega-3 fatty acids, linoleic acid (18:2n-6), and linolenic acid (18:3n-3) from their diet. Mammals can elongate and further desaturate these fatty acids to produce a range of long chain PUFAs, such as arachidonic acid (20:4n-6) and docosahexaenoic acid (22:6n-3) (Fig. 1).
Figure 1.
Pathway for the synthesis of polyunsaturated fatty acids. A diagram of the general synthesis pathway for PUFAs. Mammalian enzymes are drawn in blue accompanied by solid black reaction arrows. The Δ12 and omega-3 desaturase enzymes (gray with dashed gray arrows) are included, as C. elegans possess these desaturase activities; however, C. elegans do not synthesize the 22+ carbon PUFAs produced in mammals.
What are the functional roles of PUFAs that make them essential in mammals? As mentioned early, the PUFA content of membrane phospholipids is critical for optimal membrane properties. Membrane composition can affect the activity of membrane-associated proteins and the structurally dynamic functions of membranes. Free PUFAs are also important regulators of gene expression. In mammals, PUFAs have been found to be ligands for several nuclear hormone receptors (NHRs), including peroxisome proliferator-activated receptors (PPARs) and liver X receptors (LXRs). PUFAs also modulate the activity of the transcription factor sterol regulatory element binding protein (SREBP) (Benatti et al., 2004; Kremmyda et al., 2011), and a series of G-protein coupled receptors (GPCRs) have recently been identified in mammals that are activated by specific PUFAs. GPR120 is activated by long chain omega-3 PUFAs, and is an important regulator of inflammation, insulin resistance, and obesity in mammals (Oh and Olefsky, 2012). Importantly, PUFAs serve as precursors for powerful, locally acting lipid hormone-like signaling molecules called eicosanoids and endocannabinoids, which will be described in the following sections.
Eicosanoids
Eicosanoids is the general name for a broad class of oxygenated derivatives of PUFAs, which includes prostaglandins, thromboxanes, leukotrienes, and lipoxins. They derive the name from the Greek “eikosi,” which means twenty, because the most well characterized of these signaling molecules are formed from 20-carbon PUFAs (Funk, 2001; Kremmyda et al., 2011). Eicosanoids are transient, locally synthesized lipid signaling molecules that mediate a variety of physiological responses. They are most well known for their role in inflammation, but also affect blood pressure and clotting as well as reproductive functions. Prostaglandin signaling is critical for many aspects of reproduction in mammals, including oocyte maturation, ovulation, fertilization, implantation, and parturition (Dey et al., 2004; Wang and Dey, 2005; Takahashi et al., 2006; Dorniak et al., 2012).
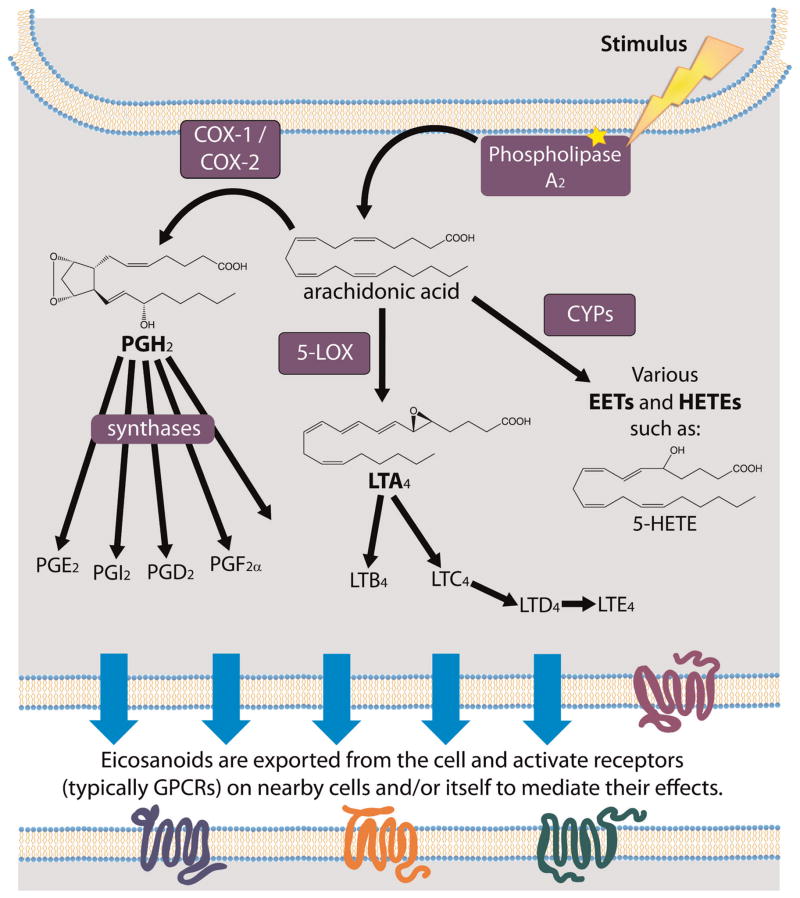
Eicosanoids are synthesized in response to a stimulus, which induces cytoplasmic phospholipase A2 (PLA2) to translocate to the endoplasmic reticulum, Golgi apparatus, or nuclear membrane where it catalyzes the release of a PUFA from a phospholipid membrane (Soberman and Christmas, 2003). Here the pathway diverges, and free PUFA can be processed either by the “cyclic pathway” to form prostaglandins/thromboxanes or the “linear pathway” to form the lipoxin/leukotriene family of eicosanoids (Funk, 2001). In addition, a third pathway uses cytochrome P450 (CYP) enzymes to convert PUFAs into oxygenated signaling molecules that mediate inflammation, cardiac function, and tumor growth (Fleming, 2007; Panigrahy et al., 2010). The cyclic pathway for prostaglandin and thromboxane synthesis is so named because these eicosanoids contain a 5-carbon pentane ring or a 6-member ether-containing ring, respectively. In the first step of the cyclic pathway, cyclooxygenase enzymes (COX-1/2) in the endoplasmic reticulum or nuclear envelope process arachindonic acid (AA, 20:4n-6) to form prostaglandin H2 (PGH2). This is the first committed step for prostaglandin synthesis and is the target for non-steroidal anti-inflammatory drugs (NSAIDs), such as aspirin and ibuprofen (Funk, 2001). Prostaglandin/thromboxane synthases can then process PGH2 into a variety of prostaglandins or thromboxanes (Fig. 2). Prostaglandins are classified as series A through I, while thromboxanes belong to 2 classes, A and B (Funk, 2001; Kremmyda et al., 2011). In the linear pathways, free PUFAs are metabolized by lipoxygenase enzymes (LOX) to form leukotrienes or by CYPs to form molecules such as hydro-peroxyeicosatetraenoic acids (HPETEs), hydroxyeicosate-traenoic acids (HETEs), and epoxyeicosatrienoic acids (EETs). The LOX enzymes (5-, 12-, 15-LOX) are position-and stereospecific, as are the CYPs. In the LOX pathway, the variety of resulting hydroxylated molecules can be further metabolized to produce lipoxins and/or leukotrienes (Kremmyda et al., 2011) (Fig. 2).
Figure 2.
Pathways for the synthesis of eicosanoids. Stimulus induces phospholipase A2 (PLA2) to localize to intracellular membranes (localization is illustrated at a general membrane for simplicity). PLA2 cleaves and releases free PUFA, here shown for arachidonic acid (AA). For prostaglandin synthesis, COX enzymes create the cyclic intermediate PGH2 (prostaglandin H2), which can then be processed into a series of prostaglandins and thromboxanes through their respective synthase enzymes. Alternately, arachidonic acid (shown) or other PUFAs can be processed by 5-LOX for synthesis of leukotriene or by P450 (CYP) enzymes for other linear oxygenated fatty acids. Eicosanoids are then transported out of the cell and engage in autocrine or paracrine signaling through G-protein coupled receptors (GPCRs).
Newly synthesized eicosanoids are transported out of the cell by passive diffusion (Schuster, 2002) and/or through the activity of specific transporters such as multi-drug resistance protein 4 (Russel et al., 2008). Studies supporting efflux through specific transporters were conducted in vitro, so the physiologically relevant mechanism for eicosanoid export is still an area of debate (Reid et al., 2003; Lacroix-Pepin et al., 2011). Eicosanoids are transient signals, lasting on the order of seconds to minutes, that activate GPCRs on neighboring cells to mediate their diverse physiological effects (Funk, 2001). Eicosanoids are taken up by cells, likely via carrier-mediated transport, and rapidly oxidized in the cytosol (Samuelsson et al., 1975; Schuster, 2002; Nomura et al., 2004; Shiraya et al., 2010).
The synthesis of eicosanoids from AA (20:4n-6) is illustrated in Figure 2, but fatty acid-derived oxygenated signaling molecules can also be synthesized from dihomogamma-linolenic acid (DGLA, 20:3n-6), eicosapentaenoic acid (EPA, 20:5n-3), as well as from 18-carbon and 22-carbon PUFA precursors. Interestingly, even though omega-6 and omega-3 PUFAs are substrates for eicosanoid formation, the two substrates are not equal. The COX-1 enzyme oxygenates the omega-3 PUFA EPA at ≤10% of the efficiency observed with the omega-6 PUFA substrate AA (Wada et al., 2007). Also, the 3-series prostaglandins produced from omega-3 fatty acids are, in general, thought to possess anti-inflammatory activity, in contrast with pro-inflammatory activity of 2-series prostaglandins produced from AA, an omega-6 fatty acid. Human consumption of omega-6 fatty acids has increased dramatically in the past decade, and this, coupled with the difference in substrate specificity of COX-1 and in eicosanoid function, may account for observations that diets rich in fish oils containing omega-3 fatty acids are anti-inflammatory and cardioprotective (Marszalek and Lodish, 2005; Smith, 2008).
Endocannabinoids
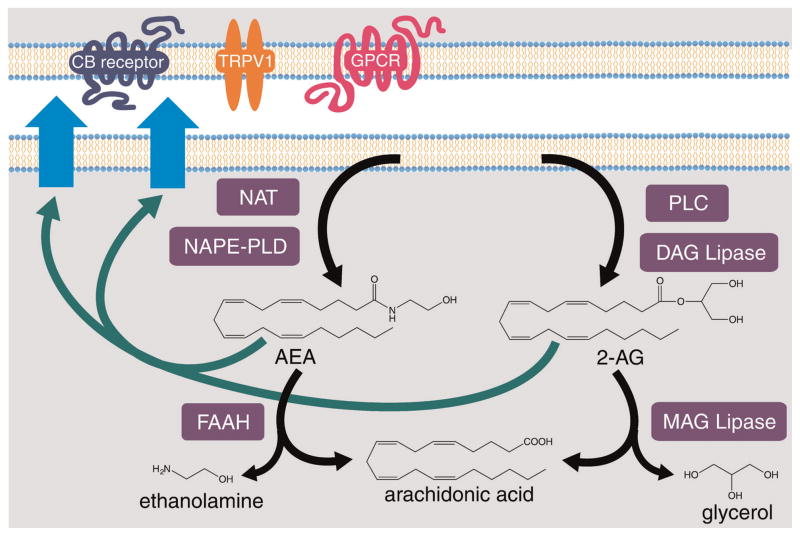
The founding member of the cannabinoid signaling family is Δ9-tetrahydrocannabinol (THC), the active compound in marijuana. Studies investigating the psychoactive mechanism of THC found that it activates GPCRs in the brain, thereby named CB1 and CB2 (Matsuda et al., 1990; Munro et al., 1993). The range of physiological effects of marijuana, from pain alleviation to appetite stimulation, led to the search for the endogenously synthesized ligands for these receptors, the endocannabinoids. Two classes of endocannabinoids have been identified based on their ability to activate CB1 and CB2 receptors: n-acylethanolamines (NAEs), such as arachidonoyl ethanolamide (AEA; anandamide), and 2-arachidonoylglycerol (2-AG).
Endocannabinoids are synthesized from two different pathways, as illustrated in Figure 3. Synthesis of NAEs begins with the transfer of AA or another PUFA from a donor phospholipid to phosphatidylethanolamine (PE) by N-acyltransferase (NAT). This is then hydrolyzed by N-acylphosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD) to yield a fatty acyl ethanolamide. NAEs are exported where they can activate receptors such as CB1 and CB2. This signaling is terminated when NAEs are degraded by fatty acid amide hydrolase (FAAH) enzymes (Elphick and Egertova, 2005; Rouzer and Marnett, 2011). Synthesis of the endocannabinoid 2-AG is initiated by phospholipase C (PLC), which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2), producing diacylglycerol (DAG) and inositol trisphosphate (IP3). DAG lipases then hydrolase DAG to yield 2-AG. Presumably DAG produced from other sources, such as the breakdown of triacylglycerol, can also be used in the synthesis of 2-AG, but the functional relevance of the alternate sources of DAG is unclear. 2-AG is also subject to regulation by both synthesis and degradation. 2-AG is primarily degraded through the activity of monoacylglycerol (MAG) lipases, releasing AA, which can be rapidly incorporated into phospholipids, but can also be hydrolyzed by FAAH enzymes (Elphick and Egertova, 2005; Rouzer and Marnett, 2011).
Figure 3.
Pathway for the synthesis of the endocannabinoids AEA and 2-AG. Arachidonoyl ethanolamide (AEA) is synthesized through the sequential activity of N-acyltransferase (NAT) and N-acylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD). 2-Arachidonoyl-glycerol (2-AG) is synthesized through the sequential activity of phospholipase C (PLC) and diacylglycerol (DAG) lipase. Both endocannabinoids can then be exported from the cell, where they signal through GPCRs (such as CB1/2 receptors or others) or TRPV1. The signaling is terminated by cleavage of the endocannabinoid to generate arachidonic acid. 2-AG is cleaved by monoacylglycerol (MAG) lipases and AEA is cleaved by fatty acid amid hydrolase (FAAH), additionally generating glycerol and ethanolamine, respectively.
In addition to activating the CB1 and CB2 receptors, these ligands can also activate other receptors such as GPR55 and the transient receptor potential vanilloid 1 (TRPV1), a Ca2+ permeable, non-selective cation channel (Pedersen et al., 2005; Nevalainen and Irving, 2010). Therefore, endocannabinoids can activate a variety of receptors to mediate their physiological effects. While endocannabinoids were initially identified for psychoactive/neuronal functions, they also function in other physiological processes and, in particular, are critical regulators of fertility and reproduction. In mice, endocannabinoid signaling regulates essentially all major stages of reproduction. In female mice, both overactive and impaired endocannabinoid signaling disrupts embryo development, implantation, placentation, and parturition (Battista et al., 2012; Sun and Dey, 2012). In males, proper endocannabinoid signaling is important for sperm development and the TRPV1 receptor drives sperm–egg fusion (Battista et al., 2012).
There appears to be significant cross-talk between the eicosanoid and endocannabinoid pathways in mammals, and some eicosanoid synthesis enzymes are able to use endocannabinoids as substrates (Rouzer and Marnett, 2011). This may greatly increase the number of potential signaling molecules derived from PUFAs.
FATTY ACID SIGNALING IN C. elegans
C. elegans Mutants Reveal Roles for PUFAs in Development and Reproduction
Recent work in the roundworm C. elegans has identified many regulatory proteins and downstream effector genes responsible for lipid homeostasis (Watts, 2009; Zheng and Greenway, 2012). While C. elegans stores lipids in intestinal and hypodermal cells rather than dedicated adipose tissue, many aspects of the regulation of fat metabolism closely parallel humans. C. elegans is capable of synthesizing a full complement of 18- and 20-carbon PUFAs from dietary or de novo-synthesized 16:0, and therefore no fatty acids are termed essential (Wallis et al., 2002). C. elegans have seven fatty acid desaturases, including the plant-like Δ12 and omega-3 desaturases (FAT-1 through FAT-7) (Spychalla et al., 1997; Watts and Browse, 2002; Brock et al., 2006), one 3-ketoacyl-CoA reductase (LET-767) (Entchev et al., 2008), and several fatty acid elongases (Watts and Browse, 2002; Kniazeva et al., 2003) that were shown to be necessary for the biosynthesis of PUFAs. Thus, capacity for PUFA synthesis makes C. elegans an excellent system to genetically dissect the role of various fatty acids with resolution not possible in mammals.
Studies of C. elegans mutants defective in fatty acid desaturation and PUFA elongation reveal roles for PUFAs in growth and development. PUFA synthesis is severely disrupted in the fat-2 Δ12 desaturase mutants and in the fat-6;fat-7 Δ9 desaturase double mutants, which are unable to synthesize normal 18- and 20-carbon PUFAs. These mutants grow very slowly, have low fat stores, and display severe reproductive defects, producing only 10% of the number of live progeny compared to wild-type (Watts and Browse, 2002; Brock et al., 2007). Similarly, depletion of elo-1 and elo-2, which cooperate to elongate palmitic acid (16:0) to stearic acid (18:0) and 18- to 20-carbon PUFAs, result in increased saturated fatty acid, decreased PUFA, slow growth, and disrupted reproduction (Kniazeva et al., 2003). The fat-3 Δ6 desaturase mutants produce 18-carbon PUFAs but very little 20-carbon PUFAs. They also display defects in growth rate and reproduction, although not as severe as the fat-2 or fat-6;fat-7 mutants (Watts et al., 2003). The fat-3 mutants were instrumental in revealing roles for specific fatty acid species in neurotransmission and synaptic vesicle recycling (Lesa et al., 2003; Marza et al., 2008), sensory signaling (Kahn-Kirby et al., 2004), and innate immune response (Nandakumar and Tan, 2008). The neurological and reproductive defects in the fat mutants can be rescued by dietary supplementation of various 20-carbon PUFAs (Watts et al., 2003; Kahn-Kirby et al., 2004).
Interestingly, the fat-1 mutants, which lack omega-3 PUFAs and contain an greater proportion of omega-6 PUFAs, show no developmental or reproductive defects when grown in laboratory conditions (Watts and Browse, 2002). Together with the fact that individual omega-6 fatty acids, such as AA, or omega-3 fatty acids, such as EPA, are equally capable of rescuing the developmental and neurological defects in PUFA-deficient fat-2 and fat-3 mutants, indicate a redundant function for 20-carbon omega-3 and omega-6 PUFAs in C. elegans physiology.
Supplementation of C. elegans With Dietary DGLA Destroys Germ Cells
Dietary supplementation of most PUFAs typically leads to improved growth and reproduction in desaturase-deficient worms, but has no adverse effects on wild-type worms; supplementation of a specific omega-6 PUFA, however, leads to reproductive defects in C. elegans. Worms cultured in the presence of DGLA (20:3n-6) became sterile, with DGLA levels reaching approximately 12% of total worm fatty acids (Watts and Browse, 2006). Other PUFAs, including EPA (20:5n-3) and linoleic acid (18:2n-6), did not cause sterility, even when supplemented at higher doses, leading to greater than 35% accumulation in worm fatty acids. Taken together, these experiments demonstrate that exposure to DGLA specifically induces sterility in C. elegans.
The DGLA-induced sterility results from the destruction of germ cells. Exposing developing larvae to DGLA during the critical period of mitotic expansion of germ cells (L2–L3 larval stages) leads to complete sterility in adults, even when they are removed from DGLA exposure during the last larval stage (Watts and Browse, 2006). Adult exposure to DGLA leads to destruction of germ cells in the mitotic and meiotic transition region (Webster et al., 2013). These studies were carried out using hermaphrodite C. elegans, which are anatomically female, but produce sperm for a short period before oogenesis commences so that they can undergo self-fertilization. Male worms that are exposed to DGLA also display a lack of germ cells, indicating that this effect is not sex specific (Watts and Browse, 2006).
The effect of DGLA on germ cells is modulated by homeostatic regulators. Strains carrying mutations in the nuclear hormone receptor genes nhr-80 and nhr-49, which regulate the expression of fatty acid desaturases and other lipid synthesis and degradation enzymes, are extremely sensitive to DGLA and become sterile when exposed to DGLA at levels that have no effect in wild-type (Webster et al., 2013). Conversely, long-lived mutants defective in insulin signaling, such as daf-2, which are resistant to many environmental stressors, show impressive resistance to DGLA (Webster et al., 2013). Thus, the process of DGLA-induced sterility is very sensitive to genetic manipulation. Further genetic analysis and biochemical characterization will be necessary to determine if these effects are mediated by an oxidized metabolite of DGLA. Future characterization of this phenomenon promises to provide insights into the cellular responses to dietary omega-6 fatty acids.
C. elegans Synthesize Prostaglandins That Aid Sperm Migration to Oocytes
A specific role for 20-carbon PUFAs has been identified for C. elegans sperm to locate oocytes (Kubagawa et al., 2006). When wild-type males are mated to fat-2 mutants, which produce oocytes lacking PUFAs, the sperm do not migrate efficiently in the direction of the spermatheca, the region of the uterus where fertilization occurs. Sperm migration improves when the fat-2 mutants are provided dietary PUFAs, however (Kubagawa et al., 2006). These studies demonstrate that for sperm to migrate efficiently to reach the spermatheca, oocytes must contain PUFAs. Depletion of several genes encoding putative prostaglandin synthesis enzymes also showed defective sperm attraction, leading Miller and coworkers to hypothesize that oocytes synthesize prostaglandins (Edmonds et al., 2010). In a direct assay, microinjection of various human prostaglandins was able to rapidly stimulate sperm motility in PUFA-deficient mutants. Furthermore, LC-MS/MS (liquid chromatography–tandem mass spectrometry) detected F-series prostaglandins in C. elegans. Microinjection of nanomolar concentrations of F-series prostaglandins, but not other eicosanoids, was able to promote robust sperm velocity, indicating that the F-series prostaglandins are the signal that guides sperm to the site of fertilization (Edmonds et al., 2010).
Mutants with defective insulin signaling, such as daf-2 (insulin receptor) and age-1 (PI3 kinase), display defects in sperm migration similar to those seen in PUFA-deficient mutants. Mutations in the daf-16 (FOXO) transcription factor partially repress the sperm migration defects in daf-2 mutants. Yet, the same study showed that daf-16 mutants alone have slightly lower brood sizes and minor sperm migration defects, indicating that wild-type levels of DAF-16 finely tune prostaglandin synthesis to optimize fertilization and reproduction (Edmonds et al., 2010). Interestingly, both PUFA and prostaglandin synthesis are regulated by insulin and FOXO signaling (Murphy et al., 2003; Edmonds et al., 2010). Thus, nutritional and endocrine signals regulate the production of PUFAs and prostaglandins to finely control reproductive output.
In a recent study, a range of F-series prostaglandins derived from 20:3n-6 (series 1), 20:4n-6 (series 2) and 20:5n-3 (series 3) were detected in C. elegans. These prostaglandins, which include PGF1α and PGF2α stereo-isomers, are synthesized in the germline and have largely redundant functions (Hoang et al., 2013). In the fat-1 omega-3 fatty acid desaturase mutant strain, omega-3 PUFAs are not synthesized, nor are the series-3 prostaglandins derived from them. Instead, compensatory up-regulation of omega-6 prostaglandins occurs (Hoang et al., 2013). Interestingly, although a wide range of prostaglandin structures have been identified, a clear homolog of the mammalian cyclooxygenase is not present in the C. elegans genome. C. elegans do contain homologs to myeloperoxidase enzymes, which function in the synthesis of prostaglandin-like signaling in D. melanogaster (described further below) (McPartland et al., 2006a). Future studies with C. elegans should illuminate the non-COX mediated mechanisms of prostaglandin formation.
C. elegans Synthesizes CYP-Derived Eicosanoids From PUFAs
While it is difficult to identify direct homologues of COX and LOX enzymes in C. elegans, the CYP family is represented by over 60 genes (Gotoh, 1998). Epoxy and hydroxyl-derivatives of AA and EPA have been identified in C. elegans (Kulas et al., 2008). In vitro, C. elegans microsomal extracts possess NADPH-dependent epoxygenase and hydroxylase activities, as well as cytochrome P450 reductase activity capable of producing EETs from PUFAs (Kulas et al., 2008). One CYP isoform, CYP33E2, when co-expressed with the human NADPH-CYP reductase in insect cells, induced the formation of EET and HETE molecules (Kosel et al., 2011). This gene is expressed in the pharynx of C. elegans, the organ that contracts rhythmically to grind E. coli for digestion, and depletion of the gene encoding CYP33E2 significantly reduced pharyngeal contractions. These studies indicate that eicosanoid signaling may act in pharyngeal cells to regulate muscle contractions necessary for digestion (Kosel et al., 2011).
A requirement for CYP-derived eicosanoids in C. elegans early development has been suggested by studies demonstrating that that depletion of two CYPs, CYP-31A2 or CYP-31A3, lead to polarity defects and early embryo arrest (Benenati et al., 2009). The embryos produced by mothers lacking either CYP isoform are osmotically sensitive and permeable to dyes, similar to embryos produced by mutants lacking the cytochrome P450 reductase EMB-8 (Rappleye et al., 2003). Additionally, the mutant embryos fail to properly finish meiosis and lack the ability to correctly establish their anterior–posterior axis, suggesting a specific lipid requirement for the proper execution of early embryonic events (Rappleye et al., 2003; Benenati et al., 2009). A recent, elegant study used light and immunoelectron microscopy to demonstrate that newly fertilized embryos form a permeability barrier, which is a distinct envelope that underlies the eggshell. The authors showed that the trilaminar eggshell forms after fertilization at anaphase of meiosis I, while the permeability barrier forms later during anaphase of meiosis II (Olson et al., 2012). The establishment of the permeability barrier requires fatty acid synthesis and CYP-31A2/CYP-31A3 activity (Olson et al., 2012). These studies demonstrate that the synthesis of modified fatty acids are specifically required for the formation of a permeability barrier, and that this barrier appears to play an important role in mediating crucial events in early embryogenesis, such as the completion of meiosis and the establishment of anterior–posterior polarity.
C. elegans Synthesizes Endocannabinoids That Are Important for the Decision to Undergo Reproductive Growth
Biochemical analysis detected a diverse range of NAEs in C. elegans, including AEA and eicosapentanoyl ethanolamide (EPEA) (Lehtonen et al., 2008; Lucanic et al., 2011). The levels of NAEs in C. elegans were examined throughout development, and it was found that NAE levels peaked at the L2 larval stage (Lucanic et al., 2011), which is the stage that nematodes commit to reproductive growth. If nutrients are inadequate at the L2 larval stage, C. elegans embarks on an alternative developmental stage called dauer (Fielenbach and Antebi, 2008). Dauer larvae are in a diapause, similar to a hibernation state, until conditions improve and worms can then resume reproductive development. The authors employed a genetic approach to alter NAE levels. Because endocannabinoid levels in mammals are regulated by both synthesis and degradation, they performed over-expression and RNAi knockdown of the fatty acid amide hydrolase (FAAH-1), the enzyme that breaks down NAEs. The worms treated with faah-1 RNAi had increased levels of NAEs, while the worms over-expressing FAAH-1 had reduced levels of NAEs and exhibited delayed growth during larval development (Lucanic et al., 2011). Furthermore, strains carrying a mutation in the insulin receptor gene daf-2 form dauer larvae even in the presence of adequate food. These strains were shown to contain low levels of NAEs. Dietary supplementation of daf-2 mutants with EPEA rescued the constitutive dauer development, inducing them to undergo reproductive growth. These studies suggest that NAEs provide a signal of nutrient availability, and that adequate NAE levels promote reproductive development.
Interestingly, NAE levels are reduced in adult C. elegans raised under dietary restriction, a regime that leads to a long lifespan in nematodes and other animals. Over-expression of FAAH-1 similarly led to lifespan extension and increased stress resistance. Dietary supplementation of EPEA shortens lifespan in wild-type and long-lived daf-2 and target of rapamycin (TOR) pathway mutants (Lucanic et al., 2011). These studies support a role for NAE signaling in the coordination of nutrient status, reproductive development and aging in C. elegans.
FATTY ACID SIGNALING IN DROSOPHILA
D. melanogaster Has a Limited Capacity for PUFA Synthesis
D. melanogaster is a highly developed invertebrate with adipose-like tissues and a lipid transport system. Sophisticated genetic tools have been used in recent years to exploit D. melanogaster in studies of the mechanisms underlying energy balance, fat storage regulation, and lipid droplet physiology (Meyerhof et al., 2010). Yet, D. melanogaster lacks homologs to the Δ5 and Δ6 desaturases, and therefore are unable to synthesize the 20–22 carbon PUFAs from essential fatty acid precursors (Shen et al., 2010). Consequently, flies must obtain PUFAs from their vegetarian diet, which is, for the most part, limited to the essential 18-carbon fatty acids linoleic acid (18:2n-6) and α-linolenic acid (18:3n-3). Physiologically, there appears to be no requirement for 20-carbon PUFAs in D. melanogaster, as flies raised in the absence of 20-carbon PUFAs are healthy and fertile (Shen et al., 2010; Hammad et al., 2011). Interestingly, D. melanogaster larvae have a preference for food enriched in unsaturated fatty acids whereas adults display a preference for saturated fatty acids (Fougeron et al., 2011). Although the reason underlying the stage-specific preferences for fatty acids is yet unknown, this differential preference may reflect the varying nutritional requirements during development (Shen et al., 2010; Hammad et al., 2011).
Because of the lack of 20-carbon PUFAs, fly phospholipids are shorter than those found in mammals; however, the fraction of unsaturated fatty acid chains is approximately 60% in both mammalian and D. melanogaster phospholipids, allowing membranes to remain fluid at relatively low temperatures. Similarly, sphingolipids are also shorter, with a 14-carbon backbone and most often with a 20-carbon arachidic acid linked at the amide position, compared with an 18-carbon backbone and a 24-carbon amide-linked fatty acid in mammals (Rietveld et al., 1999). Even so, D. melanogaster membranes maintain structural properties similar to mammals, such as the ability to form lipid raft domains (Rietveld et al., 1999).
Drosophila Δ9 Desaturase Activity Is Critical for Sex Pheromone Synthesis
While flies have limited fatty acid desaturase capabilities, there has been a unique proliferation and diversification of Δ9 desaturases in insects. The genomes of flies and moths were both found to have six Δ9 desaturase genes (Knipple et al., 2002). This proliferation of Δ9 desaturases is suggested to be integral for the production of gender and species-specific sex pheromones in Drosophila. Sex pheromones in Drosophila are very long hydrocarbon chains that are secreted onto the cuticle and signal whether or not to engage in courtship behaviors. They are typically between 23 and 27 carbons and have 1 or 2 double bonds (Savarit et al., 1999). Sex pheromones are synthesized from saturated fatty acids, which are desaturated and then elongated, and in D. melanogaster females, they are desaturated a second time and further elongated before being decarboxylated and secreted. The position of the double bonds in the hydrocarbon chain can vary because the different Δ9 desaturases have divergent substrate affinity. The Δ9 desaturase desat1 has a preference for 16- and 18-carbon saturated fatty acids, while desat2 has a preference for 14-carbon saturated fatty acids (Dallerac et al., 2000). In D. melanogaster, desatF is a Δ9 desaturase expressed only in females, and it is able to desaturate monounsaturated fatty acids (Chertemps et al., 2006). These different Δ9 desaturase substrate specificities have enabled flies to produce a wide range of mono- and di-unsaturated hydrocarbons chains. Variable expression patterns have evolved for the six Δ9 desaturases in different Drosophila species, as well as gender-specific expression of elongase enzymes, which increases the specificity of pheromone signaling (Chertemps et al., 2007; Fang et al., 2009; Shirangi et al., 2009).
While a significant amount is known about the evolution of Δ9 desaturases in the context of sex pheromone synthesis, relatively little is known about how these enzymes affect the lipid composition, development, or physiology of the fly. Of the six Δ9 desaturases, only desat1 has been reported to have functions beyond courtship behavior. Both the deletion of desat1 and strong over-expression are larval lethal, indicating proper regulation of this activity is critical for development (Kohler et al., 2009). Interestingly, desat1 activity is important for regulating total fatty acid levels, similar to what is observed in C. elegans and mice (Ntambi et al., 2002; Brock et al., 2007). Reduced desat1 activity lowers the total fatty acids levels by 62% in male and 45% in female flies (Ueyama et al., 2005). Reduced desat1 activity, however, only modestly lowers the relative abundance of desaturated fatty acids, though this was significant in the PE and PI phospholipid fractions (Ueyama et al., 2005; Kohler et al., 2009). Further characterization will be necessary to determine the role for desaturase activity in D. melanogaster development and reproduction.
Prostaglandin Signaling Is Critical for the Maturation of D. melanogaster a Egg Follicles
Eicosanoid signaling is integral for D. melanogaster oogenesis and in the immune response to infection (Tootle and Spradling, 2008; Hyrsl et al., 2011). Yet, flies lack 20-carbon PUFAs, so endogenous eicosanoids must be synthesized from 18-carbon PUFA precursors. Interestingly, D. melanogaster do not have clearly identifiable homologs to COX or LOX enzymes (McPartland et al., 2006b), although biochemical analysis demonstrated that flies possess these enzymatic activities (Pages et al., 1986). Tootle and Spradling (2008) found that the D. melanogaster Pxt protein possesses Cox-like activity in vivo. Pxt is a myeloperoxidase, which is the enzyme class from which mammalian COX enzymes evolved. Egg chamber development is impaired in pxt mutants, and this defect is phenocopied by treatment with Cox-1 inhibitors. The follicle maturation defect of pxt mutants is rescued by transgenic expression of mammalian Cox-1. Furthermore, exogenous supplementation of stabilized PGH2 or PGF2α rescued egg follicle defects in both pxt mutants and flies treated with Cox-1 inhibitors (Tootle and Spradling, 2008). Altogether, this evidence strongly argues that Pxt functions as a Cox-like enzyme in D. melanogaster oogenesis. Pxt production of prostaglandin-like signals is critical for several aspects of egg chamber development. The pxt mutants have defects in eggshell structure, altered gene expression, and disrupted actin cytoskeleton dynamics (Tootle et al., 2011; Groen et al., 2012). It is worth noting that F-series prostaglandins restore both D. melanogaster egg follicle maturation and sperm migration in C. elegans, suggesting this may be a conserved reproductive signal.
Prostaglandin signaling is an important regulator of cytoskeletal dynamics in mammals, although the mechanism of this regulation is largely unknown (Peppelenbosch et al., 1993; Birukova et al., 2007). Taking advantage of this powerful genetic model system, Tootle and coworkers performed a pharmacogenetic screen to identify genes that modulate the effects of prostaglandins on the cytoskeleton. Through this screen, they identified Fascin, an actin bundling or cross-linking protein, as a novel downstream target of prostaglandin signaling (Groen et al., 2012). It will be interesting to see if Fascin mediates the prostaglandin-induced actin cytoskeleton rearrangements in mammals.
Limited Evidence for Endocannabinoid Signaling in D. melanogaster
D. melanogaster has been largely dismissed as a system to study endocannabinoid signaling. Flies lack the 20-carbon PUFAs used as precursors for endocannabinoid synthesis, and initial BLAST analysis suggests that flies also lack homologs to the key enzymes for the synthesis, degradation, and reception of cannabinoid signals (Elphick and Egertova, 2005). The synthetic cannabinoid ligands CP 55,940 and SR141716A are not able to specifically bind in fly extracts, and pharmacological analysis concluded that THC did not induce GPCR activity (McPartland et al., 2001). Curiously, treating D. melanogaster with the pharmacological cannabinoid activator CP 55,940 protects flies against paraquat toxicity, conferring both increased survival and improved mobility of paraquat-treated flies. Because flies lack clear cannabinoid receptors, however, the authors suggest that CP 55,940 rescue is mediated by the antioxidant properties of this compound and show a similar rescue with the antioxidant vitamin E (Jimenez-Del-Rio et al., 2008).
On the other hand, one study detected low levels of endogenous 2-AG and palmitoyl ethanolamide (PEA) in fly extracts (McPartland et al., 2001). More rigorous in silico analysis of D. melanogaster sequences suggest that they may possess genes for endocannabinoid signaling, and these distantly related sequences often grouped with the C. elegans orthologs (McPartland et al., 2006b). It is possible that flies utilize cannabinoid-like signals from 18-carbon PUFA derivatives, as is suggested for the eicosanoid pathway.
APPLICATIONS USING INVERTEBRATE PUFA SYNTHESIS GENES
Research on PUFA signaling in invertebrate model systems has great potential to enhance studies in mammals. The discovery of the FAT-1 omega-3 desaturase in C. elegans prompted researchers to express the C. elegans gene in a mouse model, which enabled mice to convert omega-6 to omega-3 fatty acids (Spychalla et al., 1997; Kang et al., 2004). The FAT-1 transgenic mouse has been used to study the roles of endogenously produced omega-3 fatty acids in many processes, including inflammation, cancer, diseases of the nervous system, and metabolism (Kang, 2005, 2007). Most studies show positive outcomes for the fat-1 transgenic mice, such as inhibition of inflammatory pathways (Bellenger et al., 2011), improved learning (He et al., 2009), improved glucose tolerance (Smith et al., 2010), increased bone strength (Lau et al., 2009), and reduced colon cancer and melanoma growth (Xia et al., 2006; Jia et al., 2008). While many physiological improvements can be attributed to altered membrane fatty acid composition in specific tissues, the reduction of specific eicosanoids, such as PGE2 in the fat-1 transgenic mice, likely plays a role in decreased inflammatory responses (Gravaghi et al., 2011).
Reproductive processes appear to be very sensitive to changes in omega-3 and omega-6 fatty acids. In the early experiments demonstrating the essentiality of linoleic acid (18:2n-6), researchers noticed that linolenic acid (18:3n-3) could rescue many fatty acid-deficiency phenotypes, such as growth and skin defects, but omega-3 fatty acids could not rescue parturition deficiencies in fatty acid-deprived rats. Similar parturition deficiencies were later found in COX-1 knockout mice. The phenotypic similarities can be explained by preference for omega-6 fatty acids as a substrate for COX-1, and the importance eicosanoids such as PGF2 in reproductive processes (Wada et al., 2007; Smith, 2008). Recently, it was found that mice expressing FAT-1 primarily in their mammary glands showed unexpected reproductive abnormalities, including an increased rate of post-implantation resorption (Pohlmeier et al., 2011). Taken together, these studies demonstrate that reproductive processes have complex but precise requirements for omega-6 and omega-3 fatty acids to achieve optimal outcomes.
It is well recognized that omega-3 fatty acids are an important dietary nutrient, and some disease states in humans show correlations with low omega-3 fatty acids (Simopoulos, 2010). Farm animals that are common food sources for humans have been engineered to express the C. elegans FAT-1 protein in order to produce meat with higher omega-3 fatty acid content. Researchers have successfully created transgenic swine and cows that have reduced omega-6 to omega-3 fatty acid ratios (Lai et al., 2006; Wu et al., 2012; Zhang et al., 2012). These animals might be used to produce meat and milk enriched in omega-3 fatty acids in order to economically meet an increasing demand for omega-3 PUFAs.
CONCLUSIONS AND FUTURE DIRECTIONS
While our vast knowledge of lipid signaling comes from studies in mammals, genetic model systems are being used to further our understanding of PUFA signaling in development and reproduction. There is presently little known about the roles of specific fatty acids in early developmental processes such as meiosis, fertilization, and early cleavages. One emerging theme in this review is that fatty acid signaling appears to be fundamental for multicellular organisms, and often there are functionally relevant pathways in simple invertebrates. The early developmental events in C. elegans and D. melanogaster are amenable to experimental manipulation, allowing for genetic and biochemical approaches to determine roles for specific lipids in these processes.
C. elegans have a highly simplified anatomy, rapid development, and an impressive conservation of fatty acid synthesis pathways. Studies with C. elegans are beginning to reveal roles for PUFAs, eicosanoids, and endocannabinoids in development and reproduction (see Table 1 for a summary). While omega-3 and omega-6 PUFAs appear to function redundantly in many processes, the discovery that dietary DGLA, an omega-6 fatty acid, destroys germ cells revealed a previously undescribed consequence of a specific dietary fat.
TABLE 1.
Physiological Roles of PUFA, Eicosanoid, and Endocannabinoid Signaling in C. elegans and Drosophila Development and Reproduction

|

|
|---|---|
|
| |
| Polyunsaturated fatty acids | |
|
| |
| Worms are capable of synthesizing a full repetoire of PUFAs (Watts & Browse, 2002). | Flies lack Δ5 and Δ6 desaturases and so are unable to endogenously synthesize PUFAs (Shen et al. 2010). |
| Strains with mutations in fatty acid synthesis genes have defects in growth, development & reproduction (Watts & Browse 2002; Kniazeva et al. 2003; Watts et al. 2003; Brock et al. 2007; Entchev et al. 2008). | Flies have six Δ9 desaturases and their expression is regulated to produce a wide array of gender and species-specific sex pheromones (Saravit et al. 1999; Chertemps et al. 2006; Shirangi et al. 2009). |
| Exogenous DGLA, an omega-6 PUFA, leads to destruction of germ cells (Watts & Browse 2006; Webster 2013). | Loss or overexpression of the Δ9 desaturase desat1 is larval lethal (Kohler et al. 2009). |
|
| |
| Eicosanoids | |
|
| |
| Lack obvious COX homologs, but do have myeloperoxidases (McPartland et al. 2006b). | Flies lack COX enzymes but have myeloperoxidase enzymes (McPartland et al. 2006b) and biochemical analysis indicates they possess Cox-like enzymatic activity (Pages et al. 1986). |
| Worms synthesize more than ten distinct F-series prostaglandins from both omega-3 and omega-6 PUFAs (Hoang et al. 2013). Prostaglandins secreted by oocytes serve as a sperm guidance cue. Impaired PUFA or prostaglandin synthesis causes sperm migration defects, which can be rescued by direct injection of F-series prostaglandins (Kubagawa et al. 2006; Edmonds et al. 2010). | The myeloperoxidase Pxt is critical for several aspects of egg follicle maturation, including eggshell structure, gene expression and actin cytoskeletal dynamics. The egg follicle maturation defect in Pxt mutants is rescued by transgenic expression of Cox1 or supplementation with stabilized F-series prostaglandins (Tootle & Spradling 2008; Tootle et al. 2011; Groen et al. 2012). |
| C. elegans can synthesize EETs from PUFAs (Kulas et al. 2008). Knock down of CYP-31A2 or CYP-31A3 leads to embryo arrest, polarity defects and defective formation of the egg permeability barrier (Benenati et al. 2009; Olson et al. 2012). | The actin bundling/cross-linking protein Fascin is a novel downstream effector of prostaglandin-induced actin remodeling in egg follicle development (Groen et al. 2012). |
|
| |
| Endocannabinoids | |
|
| |
| NAEs serve as a metabolic signal that couples nutrient availablility with reproductive development. Overexpression of FAAH-1 reduces levels of NAEs and results in delayed larval growth, while exogenous EPEA promotes reproductive development in daf-2 mutants (Lucanic et al. 2011). | ? |
Worms are an excellent model to elucidate both the non-COX-dependent synthesis of prostaglandins and the non-Cb1/Cb2-dependent signaling through the endocannabinoid system. C. elegans lack traditional Cb1/Cb2 receptors, but do encode a wide number of other GPCRs as well as the TRPV1 receptor, which could be mediating the observed phenotypes for endocannabinoids (McPartland et al., 2006a). Genetic screens offer an unbiased way to identify the functional fatty acid, prostaglandin and endocannabinoid receptors involved in growth, development, aging, and sperm guidance. Improved metabolomic tools will aid in the identification of additional PUFA-derived signaling molecules in C. elegans. Future studies promise to reveal fundamental developmental processes that depend on fatty acid signaling.
D. melanogaster is proving to be an interesting model to understand the flexible nature of unsaturated fatty acid signaling pathways (see summary in Table 1). Despite having many conserved aspects of lipid storage and metabolic syndrome (Baker and Thummel, 2007; Kuhnlein, 2012), fruit flies do not synthesize long chain PUFAs (Shen et al., 2010). An important task will be to identify the lipid signaling molecules that are synthesized in D. melanogaster from 18-carbon fatty acids. D. melanogaster gametogenesis and early embryonic divisions are sensitive to perturbations in fatty acid synthesis (Jung et al., 2007; Szafer-Glusman et al., 2008; Steinhauer et al., 2009), although the specific roles of PUFAs in these processes are unclear. Studies on Pxt-mediated prostaglandin-like signaling in D. melanogaster egg follicle maturation are already elucidating novel mediators of prostaglandin signaling in vivo (Groen et al., 2012). As in C. elegans, powerful genetic screens in D. melanogaster will uncover receptors and other pathway components that function in reproductive and developmental processes relying on PUFA derived signals.
Lipid signaling is highly complex and fine-tuned in mammals. The vast diversity of lipids and their multiple roles in various organ systems complicates our understanding of fatty acid signaling during development. Simple invertebrate models such as C. elegans and D. melanogaster provide genetic, molecular, and whole organism tools to begin to understand the importance of fatty acids and their derivatives in development and reproduction.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant number: R01-DK074114
The authors thank Dr. Tom Spencer, Dr. Katie Estes, two anonymous reviewers and members of the Watts lab for helpful comments on the manuscript. Research in the Watts lab is funded by the National Institutes of Health (R01-DK074114).
Abbreviations
- 2-AG
2-arachidonoylglycerol
- AA
arachidonic acid
- AEA
arachidonoyl ethanolamide (anandamide)
- COX
cyclooxygenase
- CYP
cytochrome P450DGLA
- DGLA
dihomogamma-linolenic acid
- EET
epoxyeicosatrienoic acid
- EPA
eicosapentaenoic acid
- EPEA
eicosapentanoyl ethanolamide
- FAAH
fatty acid amide hydrolase
- GPCR
G-protein coupled receptor
- HPETE
hydroperoxyeicosatetraenoic acid
- HETE
hydroxyeicosate-traenoic acid
- LOX
lipoxygenase
- NAE
n-acylethanolamine
- PE
phosphatidyl-ethanolamine
- PEA
palmitoyl ethanolamide
- PG
prostaglandin
- PI[P2]
phosphatidylinositol [4,5-bisphosphate]
- PUFA
polyunsaturated fatty acid
- THC
Δ9-tetrahydrocannabinol
- TRPV1
transient receptor potential vanilloid 1
References
- Baker KD, Thummel CS. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 2007;6:257–266. doi: 10.1016/j.cmet.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista N, Meccariello R, Cobellis G, Fasano S, Di Tommaso M, Pirazzi V, Konje JC, Pierantoni R, Maccarrone M. The role of endocannabinoids in gonadal function and fertility along the evolutionary axis. Mol Cell Endocrinol. 2012;355:1–14. doi: 10.1016/j.mce.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Bellenger J, Bellenger S, Bataille A, Massey KA, Nicolaou A, Rialland M, Tessier C, Kang JX, Narce M. High pancreatic n-3 fatty acids prevent STZ-induced diabetes in fat-1 mice: Inflammatory pathway inhibition. Diabetes. 2011;60:1090–1099. doi: 10.2337/db10-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benatti P, Peluso G, Nicolai R, Calvani M. Polyunsaturated fatty acids: Biochemical, nutritional and epigenetic properties. J Am Coll Nutr. 2004;23:281–302. doi: 10.1080/07315724.2004.10719371. [DOI] [PubMed] [Google Scholar]
- Benenati G, Penkov S, Muller-Reichert T, Entchev EV, Kurzchalia TV. Two cytochrome P450s in Caenorhabditis elegans are essential for the organization of eggshell, correct execution of meiosis and the polarization of embryo. Mech Dev. 2009;126:382–393. doi: 10.1016/j.mod.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE2 and PGI2 promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res. 2007;313:2504–2520. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock TJ, Browse J, Watts JL. Genetic regulation of unsaturated fatty acid composition in C. elegans. PLoS Genet. 2006;2:e108. doi: 10.1371/journal.pgen.0020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock TJ, Browse J, Watts JL. Fatty acid desaturation and the regulation of adiposity in Caenorhabditis elegans. Genetics. 2007;176:865–875. doi: 10.1534/genetics.107.071860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertemps T, Duportets L, Labeur C, Ueyama M, Wicker-Thomas C. A female-specific desaturase gene responsible for diene hydrocarbon biosynthesis and courtship behaviour in Drosophila melanogaster. Insect Mol Biol. 2006;15:465–473. doi: 10.1111/j.1365-2583.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- Chertemps T, Duportets L, Labeur C, Ueda R, Takahashi K, Saigo K, Wicker-Thomas C. A female-biased expressed elongase involved in long-chain hydrocarbon biosynthesis and courtship behavior in Drosophila melanogaster. Proc Natl Acad Sci. 2007;104:4273–4278. doi: 10.1073/pnas.0608142104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallerac R, Labeur C, Jallon J-M, Knipple DC, Roelofs WL, Wicker-Thomas C. A delta9 desaturase gene with a different substrate specificity is responsible for the cuticular diene hydrocarbon polymorphism in Drosophila melanogaster. Proc Natl Acad Sci. 2000;97:9449–9454. doi: 10.1073/pnas.150243997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- Dorniak P, Bazer FW, Spencer TE. Biological role of interferon tau in endometrial function and conceptus elongation. J Anim Sci. 2012 doi: 10.2527/jas.2012-5845. [DOI] [PubMed] [Google Scholar]
- Edmonds JW, Prasain JK, Dorand D, Yang Y, Hoang HD, Vibbert J, Kubagawa HM, Miller MA. Insulin/FOXO signaling regulates ovarian prostaglandins critical for reproduction. Dev Cell. 2010;19:858–871. doi: 10.1016/j.devcel.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elphick MR, Egertova M. The phylogenetic distribution and evolutionary origins of endocannabinoid signalling. Handb Exp Pharmacol. 2005;168:283–297. doi: 10.1007/3-540-26573-2_9. [DOI] [PubMed] [Google Scholar]
- Entchev EV, Schwudke D, Zagoriy V, Matyash V, Bogdanova A, Habermann B, Zhu L, Shevchenko A, Kurzchalia TV. LET-767 is required for the production of branched chain and long chain fatty acids in Caenorhabditis elegans. J Biol Chem. 2008;283:17550–17560. doi: 10.1074/jbc.M800965200. [DOI] [PubMed] [Google Scholar]
- Fang S, Ting CT, Lee CR, Chu KH, Wang CC, Tsaur SC. Molecular evolution and functional diversification of fatty acid desaturases after recurrent gene duplication in Drosophila. Mol Biol Evol. 2009;26:1447–1456. doi: 10.1093/molbev/msp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielenbach N, Antebi A. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 2008;22:2149–2165. doi: 10.1101/gad.1701508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I. Epoxyeicosatrienoic acids, cell signaling and angiogenesis. Prostaglandins Other Lipid Mediat. 2007;82:60–67. doi: 10.1016/j.prostaglandins.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Fougeron A-S, Farine J-P, Flaven-Pouchon J, Everaerts C, Ferveur J-F. Fatty-acid preference changes during development in Drosophila melanogaster. PLoS ONE. 2011;6:e26899. doi: 10.1371/journal.pone.0026899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15:635–645. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh O. Divergent structures of Caenorhabditis elegans cytochrome P450 genes suggest the frequent loss and gain of introns during the evolution of nematodes. Mol Biol Evol. 1998;15:1447–1459. doi: 10.1093/oxfordjournals.molbev.a025872. [DOI] [PubMed] [Google Scholar]
- Gravaghi C, La Perle KM, Ogrodwski P, Kang JX, Quimby F, Lipkin M, Lamprecht SA. Cox-2 expression, PGE(2) and cytokines production are inhibited by endogenously synthesized n-3 PUFAs in inflamed colon of fat-1 mice. J Nutr Biochem. 2011;22:360–365. doi: 10.1016/j.jnutbio.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Groen CM, Spracklen AJ, Fagan TN, Tootle TL. Drosophila Fascin is a novel downstream target of prostaglandin signaling during actin remodeling. Mol Biol Cell. 2012;23:4567–4578. doi: 10.1091/mbc.E12-05-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad LA, Cooper BS, Fisher NP, Montooth KL, Karty JA. Profiling and quantification of Drosophila melanogaster lipids using liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2011;25:2959–2968. doi: 10.1002/rcm.5187. [DOI] [PubMed] [Google Scholar]
- He C, Qu X, Cui L, Wang J, Kang JX. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc Natl Acad Sci. 2009;106:11370–11375. doi: 10.1073/pnas.0904835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata T, Narumiya S, Frederick WA. Prostanoids as regulators of innate and adaptive immunity. In: Frederick WA, editor. Advances in immunology. Boston, MA: Academic Press; 2012. pp. 143–174. [DOI] [PubMed] [Google Scholar]
- Hoang HD, Prasain JK, Dorand D, Miller MA. A heterogeneous mixture of F-series prostaglandins promotes sperm guidance in the Caenorhabditis elegans reproductive tract. PLoS Genet. 2013;9:e1003271. doi: 10.1371/journal.pgen.1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J-W, Park KW. Further understanding of fat biology: Lessons from a fat fly. Exp Mol Med. 2010;42:12–20. doi: 10.3858/emm.2010.42.1.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyrsl P, Dobes P, Wang Z, Hauling T, Wilhelmsson C, Theopold U. Clotting factors and eicosanoids protect against nematode infections. J Innate Immun. 2011;3:65–70. doi: 10.1159/000320634. [DOI] [PubMed] [Google Scholar]
- Jia Q, Lupton JR, Smith R, Weeks BR, Callaway E, Davidson LA, Kim W, Fan Y-Y, Yang P, Newman RA, Kang JX, McMurray DN, Chapkin RS. Reduced colitis-associated colon cancer in fat-1 (n-3 fatty acid desaturase) transgenic mice. Cancer Res. 2008;68:3985–3991. doi: 10.1158/0008-5472.CAN-07-6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Del-Rio M, Daza-Restrepo A, Velez-Pardo C. The cannabinoid CP55,940 prolongs survival and improves locomotor activity in Drosophila melanogaster against paraquat: Implications in Parkinson’s disease. Neurosci Res. 2008;61:404–411. doi: 10.1016/j.neures.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Jung A, Hollmann M, Schäfer MA. The fatty acid elongase NOA is necessary for viability and has a somatic role in Drosophila sperm development. J Cell Sci. 2007;120:2924–2934. doi: 10.1242/jcs.006551. [DOI] [PubMed] [Google Scholar]
- Kahn-Kirby AH, Dantzker JLM, Apicella AJ, Schafer WR, Browse J, Bargmann CI, Watts JL. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell. 2004;119:889–900. doi: 10.1016/j.cell.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Kang JX. From fat to fat-1: A tale of omega-3 fatty acids. J Membr Biol. 2005;206:165–172. doi: 10.1007/s00232-005-0790-3. [DOI] [PubMed] [Google Scholar]
- Kang JX. Fat-1 transgenic mice: A new model for omega-3 research. Prostaglandins Leukot Essent Fatty Acids. 2007;77:263–267. doi: 10.1016/j.plefa.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: Fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504–504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- Katona I, Freund TF. Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci. 2012;35:529–558. doi: 10.1146/annurev-neuro-062111-150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeva M, Sieber M, McCauley S, Zhang K, Watts JL, Han M. Suppression of the ELO-2 FA elongation activity results in alterations of the fatty acid composition and multiple physiological defects, including abnormal ultradian rhythms, in Caenorhabditis elegans. Genetics. 2003;163:159–169. doi: 10.1093/genetics/163.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipple DC, Rosenfield C-L, Nielsen R, You KM, Jeong SE. Evolution of the integral membrane desaturase gene family in moths and flies. Genetics. 2002;162:1737–1752. doi: 10.1093/genetics/162.4.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler K, Brunner E, Guan XL, Boucke K, Greber UF, Mohanty S, Barth JMI, Wenk MR, Hafen E. A combined proteomic and genetic analysis identifies a role for the lipid desaturase Desat1 in starvation-induced autophagy in Drosophila. Autophagy. 2009;5:980–990. doi: 10.4161/auto.5.7.9325. [DOI] [PubMed] [Google Scholar]
- Kosel M, Wild W, Bell A, Rothe M, Lindschau C, Steinberg CEW, Schunck WÄ, Menzel R. Eicosanoid formation by a cytochrome P450 isoform expressed in the pharynx of Caenorhabditis elegans. Biochem J. 2011;435:689–700. doi: 10.1042/BJ20101942. [DOI] [PubMed] [Google Scholar]
- Kremmyda L-S, Tvrzicka E, Stankova B, Zak A. Fatty acids as biocompounds: Their role in human metabolism, health and disease—A review. Part 2: Fatty acid physiological roles and applications in human health and disease. Biomed Pap Med Fac Univ Palacky Olomouc. 2011;155:195–218. doi: 10.5507/bp.2011.052. [DOI] [PubMed] [Google Scholar]
- Kubagawa HM, Watts JL, Corrigan C, Edmonds JW, Sztul E, Browse J, Miller MA. Oocyte signals derived from poly-unsaturated fatty acids control sperm recruitment in vivo. Nat Cell Biol. 2006;8:1143–1148. doi: 10.1038/ncb1476. [DOI] [PubMed] [Google Scholar]
- Kuhnlein RP. Lipid droplet-based storage fat metabolism in Drosophila: Thematic review series: Lipid droplet synthesis and metabolism: from yeast to man. J Lipid Res. 2012;53:1430–1436. doi: 10.1194/jlr.R024299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulas J, Schmidt C, Rothe M, Schunck W-H, Menzel R. Cytochrome P450-dependent metabolism of eicosapentaenoic acid in the nematode Caenorhabditis elegans. Arch Biochem Biophys. 2008;472:65–75. doi: 10.1016/j.abb.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Lacroix-Pepin N, Danyod G, Krishnaswamy N, Mondal S, Rong P-M, Chapdelaine P, Fortier MA. The multidrug resistance-associated protein 4 (MRP4) appears as a functional carrier of prostaglandins regulated by oxytocin in the bovine endometrium. Endocrinology. 2011;152:4993–5004. doi: 10.1210/en.2011-1406. [DOI] [PubMed] [Google Scholar]
- Lai L, Kang JX, Li R, Wang J, Witt WT, Yong HY, Hao Y, Wax DM, Murphy CN, Rieke A, Samuel M, Linville ML, Korte SW, Evans RW, Starzl TE, Prather RS, Dai Y. Generation of cloned transgenic pigs rich in omega-3 fatty acids. Nat Biotech. 2006;24:435–436. doi: 10.1038/nbt1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau BYY, Ward WE, Kang JX, Ma DWL. Vertebrae of developing fat-1 mice have greater strength and lower N-6/N-3 fatty acid ratio. Exp Biol Med. 2009;234:632–638. doi: 10.3181/0808-RM-247. [DOI] [PubMed] [Google Scholar]
- Lehtonen M, Reisner K, Auriola S, Wong G, Callaway JC. Mass-spectrometric identification of anandamide and 2-arachidonoylglycerol in nematodes. Chem Biodiversty. 2008;5:2431–2441. doi: 10.1002/cbdv.200890208. [DOI] [PubMed] [Google Scholar]
- Lesa GM, Palfreyman M, Hall DH, Clandinin MT, Rudolph C, Jorgensen EM, Schiavo G. Long chain polyunsaturated fatty acids are required for efficient neurotransmission in C. elegans. J Cell Sci. 2003;116:4965–4975. doi: 10.1242/jcs.00918. [DOI] [PubMed] [Google Scholar]
- Lucanic M, Held JM, Vantipalli MC, Klang IM, Graham JB, Gibson BW, Lithgow GJ, Gill MS. N-acylethanolamine signalling mediates the effect of diet on lifespan in Caenorhabditis elegans. Nature. 2011;473:226–229. doi: 10.1038/nature10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek JR, Lodish HF. Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function: Breastmilk and fish are good for you. Annu Rev Cell Dev Biol. 2005;21:633–657. doi: 10.1146/annurev.cellbio.21.122303.120624. [DOI] [PubMed] [Google Scholar]
- Marza E, Long T, Saiardi A, Sumakovic M, Eimer S, Hall DH, Lesa GM. Polyunsaturated fatty acids influence synaptojanin localization to regulate synaptic vesicle recycling. Mol Biol Cell. 2008;19:833–842. doi: 10.1091/mbc.E07-07-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McPartland J, Di Marzo V, De Petrocellis L, Mercer A, Glass M. Cannabinoid receptors are absent in insects. J Comp Neurol. 2001;436:423–429. doi: 10.1002/cne.1078. [DOI] [PubMed] [Google Scholar]
- McPartland JM, Agraval J, Gleeson D, Heasman K, Glass M. Cannabinoid receptors in invertebrates. J Evol Biol. 2006a;19:366–373. doi: 10.1111/j.1420-9101.2005.01028.x. [DOI] [PubMed] [Google Scholar]
- McPartland JM, Matias I, Di Marzo V, Glass M. Evolutionary origins of the endocannabinoid system. Gene. 2006b;370:64–74. doi: 10.1016/j.gene.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Meyerhof W, Beisiegel U, Joost H-G, Kuhnlein R. Sensory and metabolic control of energy balance. Berlin Heidelberg: Springer; 2010. Energy homeostasis regulation in Drosophila: A lipocentric perspective; pp. 159–173. [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Nandakumar M, Tan M-W. Gamma-linolenic and stearidonic acids are required for basal immunity in Caenorhabditis elegans through their effects on p38 MAP kinase activity. PLoS Genet. 2008;4:e1000273. doi: 10.1371/journal.pgen.1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen T, Irving AJ. GPR55, a lysophosphatidylinositol receptor with cannabinoid sensitivity? Curr Top Med Chem. 2010;10:799–813. doi: 10.2174/156802610791164229. [DOI] [PubMed] [Google Scholar]
- Nomura T, Lu R, Pucci ML, Schuster VL. The two-step model of prostaglandin signal termination: In vitro reconstitution with the prostaglandin transporter and prostaglandin 15 dehydrogenase. Mol Pharmacol. 2004;65:973–978. doi: 10.1124/mol.65.4.973. [DOI] [PubMed] [Google Scholar]
- Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh D-Y, Olefsky JM. Omega 3 fatty acids and GPR120. Cell Metab. 2012;15:564–565. doi: 10.1016/j.cmet.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SK, Greenan G, Desai A, Muller-Reichert T, Oegema K. Hierarchical assembly of the eggshell and permeability barrier in C. elegans. J Cell Biol. 2012;198:731–748. doi: 10.1083/jcb.201206008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages M, Rosello J, Casa J, Gelpi E, Gualde N, Rigaud M. Cyclooxygenase and lipoxygenase-like activity in drosophila melanogaster. Prostaglandins. 1986;32:729–740. doi: 10.1016/0090-6980(86)90195-4. [DOI] [PubMed] [Google Scholar]
- Panigrahy D, Kaipainen A, Greene E, Huang S. Cytochrome P450-derived eicosanoids: The neglected pathway in cancer. Cancer Metastasis Rev. 2010;29:723–735. doi: 10.1007/s10555-010-9264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SF, Owsianik G, Nilius B. TRP channels: An overview. Cell Calcium. 2005;38:233–252. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Peppelenbosch MP, Tertoolen LGJ, Hage WJ, de Laat SW. Epidermal growth factor induced actin remodeling is regulated by 5-lipoxygenase and cyclooxygenase products. Cell. 1993;74:565–575. doi: 10.1016/0092-8674(93)80057-l. [DOI] [PubMed] [Google Scholar]
- Pohlmeier W, Hovey R, Eenennaam A. Reproductive abnormalities in mice expressing omega-3 fatty acid desaturase in their mammary glands. Transgenic Res. 2011;20:283–292. doi: 10.1007/s11248-010-9407-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappleye C, Tagawa A, Le Bot N, Ahringer J, Aroian R. Involvement of fatty acid pathways and cortical interaction of the pronuclear complex in Caenorhabditis elegans embryonic polarity. BMC Dev Biol. 2003;3:8. doi: 10.1186/1471-213X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Wielinga P, Zelcer N, van der Heijden I, Kuil A, de Haas M, Wijnholds J, Borst P. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci. 2003;100:9244–9249. doi: 10.1073/pnas.1033060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld A, Neutz S, Simons K, Eaton S. Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophila raft lipid microdomains. J Biol Chem. 1999;274:12049–12054. doi: 10.1074/jbc.274.17.12049. [DOI] [PubMed] [Google Scholar]
- Rouzer CA, Marnett LJ. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: Cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem Rev. 2011;111:5899–5921. doi: 10.1021/cr2002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel FGM, Koenderink JB, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): A versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol Sci. 2008;29:200–207. doi: 10.1016/j.tips.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Samuelsson B, Granstrom E, Green K, Hamberg M, Hammarstrom S. Prostaglandins. Annu Rev Biochem. 1975;44:669–695. doi: 10.1146/annurev.bi.44.070175.003321. [DOI] [PubMed] [Google Scholar]
- Savarit F, Sureau G, Cobb M, Ferveur J-F. Genetic elimination of known pheromones reveals the fundamental chemical bases of mating and isolation in Drosophila. Proc Natl Acad Sci. 1999;96:9015–9020. doi: 10.1073/pnas.96.16.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster VL. Prostaglandin transport. Prostaglandins Other Lipid Mediat. 2002;68:633–647. doi: 10.1016/s0090-6980(02)00061-8. [DOI] [PubMed] [Google Scholar]
- Shen LR, Lai CQ, Feng X, Parnell LD, Wan JB, Wang JD, Li D, Ordovas JM, Kang JX. Drosophila lacks C20 and C22 PUFAs. J Lipid Res. 2010;51:2985–2992. doi: 10.1194/jlr.M008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Simons K. Lipidomics: Coming to grips with lipid diversity. Nat Rev Mol Cell Biol. 2010;11:593–598. doi: 10.1038/nrm2934. [DOI] [PubMed] [Google Scholar]
- Shirangi TR, Dufour HD, Williams TM, Carroll SB. Rapid evolution of sex pheromone-producing enzyme expression in Drosophila. PLoS Biol. 2009;7:e1000168. doi: 10.1371/journal.pbio.1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraya K, Hirata T, Hatano R, Nagamori S, Wiriyasermkul P, Jutabha P, Matsubara M, Muto S, Tanaka H, Asano S, Anzai N, Endou H, Yamada A, Sakurai H, Kanai Y. A novel transporter of SLC22 family specifically transports prostaglandins and co-localizes with 15-hydroxyprostaglandin dehydrogenase in renal proximal tubules. J Biol Chem. 2010;285:22141–22151. doi: 10.1074/jbc.M109.084426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: Their role in the determination of nutritional requirements and chronic disease risk. Exp Biol Med. 2010;235:785–795. doi: 10.1258/ebm.2010.009298. [DOI] [PubMed] [Google Scholar]
- Smith WL. Nutritionally essential fatty acids and biologically indispensable cyclooxygenases. Trends Biochem Sci. 2008;33:27–37. doi: 10.1016/j.tibs.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Smith BK, Holloway GP, Reza-Lopez S, Jeram SM, Kang JX, Ma DWL. A decreased n-6/n-3 ratio in the fat-1 mouse is associated with improved glucose tolerance. Appl Physiol Nutr Metab. 2010;35:699–706. doi: 10.1139/H10-066. [DOI] [PubMed] [Google Scholar]
- Soberman RJ, Christmas P. The organization and consequences of eicosanoid signaling. J Clin Invest. 2003;111:1107–1113. doi: 10.1172/JCI18338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spychalla JA, Kinney AA, Browse J. Identification of an animal omega-3 fatty acid desaturase by heterologous expression in Arabidopsis. Proc Natl Acad Sci. 1997;94:1142–1147. doi: 10.1073/pnas.94.4.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer J, Gijon MA, Riekhof WR, Voelker DR, Murphy RC, Treisman JE. Drosophila lysophospholipid acyltransferases are specifically required for germ cell development. Mol Biol Cell. 2009;20:5224–5235. doi: 10.1091/mbc.E09-05-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Dey SK. Endocannabinoid signaling in female reproduction. ACS Chem Neurosci. 2012;3:349–355. doi: 10.1021/cn300014e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szafer-Glusman E, Giansanti MG, Nishihama R, Bolival B, Pringle J, Gatti M, Fuller MT. A role for very-long-chain fatty acids in furrow ingression during cytokinesis in Drosophila spermatocytes. Curr Biol. 2008;18:1426–1431. doi: 10.1016/j.cub.2008.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Morrow JD, Wang H, Dey SK. Cyclooxygen-ase-2-derived prostaglandin E(2) directs oocyte maturation by differentially influencing multiple signaling pathways. J Biol Chem. 2006;281:37117–37129. doi: 10.1074/jbc.M608202200. [DOI] [PubMed] [Google Scholar]
- Tootle TL, Spradling AC. Drosophila Pxt: A cyclooxygenase-like facilitator of follicle maturation. Development. 2008;135:839–847. doi: 10.1242/dev.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootle TL, Williams D, Hubb A, Frederick R, Spradling A. Drosophila eggshell production: Identification of new genes and coordination by Pxt. PLoS ONE. 2011;6:e19943. doi: 10.1371/journal.pone.0019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama M, Chertemps T, Labeur C, Wicker-Thomas C. Mutations in the desat1 gene reduces the production of courtship stimulatory pheromones through a marked effect on fatty acids in Drosophila melanogaster. Insect Biochem Mol Biol. 2005;35:911–920. doi: 10.1016/j.ibmb.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Vrablik TL, Watts JL. Emerging roles for specific fatty acids in developmental processes. Genes Dev. 2012;26:631–637. doi: 10.1101/gad.190777.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M, DeLong CJ, Hong YH, Rieke CJ, Song I, Sidhu RS, Yuan C, Warnock M, Schmaier AH, Yokoyama C, Smyth EM, Wilson SJ, FitzGerald GA, Garavito RM, de Sui X, Regan JW, Smith WL. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem. 2007;282:22254–22266. doi: 10.1074/jbc.M703169200. [DOI] [PubMed] [Google Scholar]
- Wallis JG, Watts JL, Browse J. Polyunsaturated fatty acid synthesis: What will they think of next? Trends Biochem Sci. 2002;27:467. doi: 10.1016/s0968-0004(02)02168-0. [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK. Lipid signaling in embryo implantation. Prostaglandins Other Lipid Mediat. 2005;77:84–102. doi: 10.1016/j.prostaglandins.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Watts JL. Fat synthesis and adiposity regulation in Caenorhabditis elegans. Trends Endocrinol Metab. 2009;20:58–65. doi: 10.1016/j.tem.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JL, Browse J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2002;99:5854–5859. doi: 10.1073/pnas.092064799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JL, Browse J. Dietary manipulation implicates lipid signaling in the regulation of germ cell maintenance in C. elegans. Dev Biol. 2006;292:381–392. doi: 10.1016/j.ydbio.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JL, Phillips E, Griffing KR, Browse J. Deficiencies in C20 polyunsaturated fatty acids cause behavioral and developmental defects in Caenorhabditis elegans fat-3 mutants. Genetics. 2003;163:581–589. doi: 10.1093/genetics/163.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster CM, Deline ML, Watts JL. Stress response pathways protect germ cells from omega-6 polyunsaturated fatty acid-mediated toxicity in Caenorhabditis elegans. Dev Biol. 2013;373:14–25. doi: 10.1016/j.ydbio.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk MR. Lipidomics: New tools and applications. Cell. 2010;143:888–895. doi: 10.1016/j.cell.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Wu X, Ouyang H, Duan B, Pang D, Zhang L, Yuan T, Xue L, Ni D, Cheng L, Dong S, Wei Z, Li L, Yu M, Sun Q-Y, Chen D-Y, Lai L, Dai Y, Li G-P. Production of cloned transgenic cow expressing omega-3 fatty acids. Transgenic Res. 2012;21:537–543. doi: 10.1007/s11248-011-9554-2. [DOI] [PubMed] [Google Scholar]
- Xia S, Lu Y, Wang J, He C, Hong S, Serhan CN, Kang JX. Melanoma growth is reduced in fat-1 transgenic mice: Impact of omega-6/omega-3 essential fatty acids. Proc Natl Acad Sci. 2006;103:12499–12504. doi: 10.1073/pnas.0605394103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Zhang Y, Dou H, Yin J, Chen Y, Pang X, Vajta G, Bolund L, Du Y, Ma RZ. Handmade cloned transgenic piglets expressing the nematode fat-1. Cell Reprogram. 2012;14:258–266. doi: 10.1089/cell.2011.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Greenway FL. Caenorhabditis elegans as a model for obesity research. Int J Obes. 2012;36:186–194. doi: 10.1038/ijo.2011.93. [DOI] [PubMed] [Google Scholar]