Abstract
Background. Human ehrlichioses are emerging life-threatening diseases transmitted by ticks. Animal models have been developed to study disease development; however, there is no valid small animal model that uses a human ehrlichial pathogen. The objective of this study was to develop a mouse model for ehrlichiosis with the newly discovered human pathogen, Ehrlichia muris–like agent (EMLA).
Methods. Three strains of mice were inoculated with different doses of EMLA by the intravenous, intraperitoneal, or intradermal route and evaluated for clinical and pathologic changes during the course of infection.
Results. EMLA infected C57Bl/6, BALB/c, and C3H/HeN mice and induced lethal or persistent infection in a route- and dose-dependent manner. The clinical chemistry and hematologic changes were similar to those of human infection by Ehrlichia chaffeensis or EMLA. Bacterial distribution in tissues differed after intradermal infection, compared with the distribution after intravenous or intraperitoneal injection. Lethal infection did not cause remarkable pathologic changes, but it caused fluid imbalance. EMLA infection of endothelium and mononuclear cells likely plays a role in the severe outcome.
Conclusions. The EMLA mouse model mimics human infection and can be used to study pathogenesis and immunity and for development of a vector transmission model of ehrlichiosis.
Keywords: tick-borne disease, ehrlichiosis, Ehrlichia muris-like, human pathogen, emerging infectious disease
Ehrlichioses are emerging tick-borne diseases that affect several mammals, including humans. The genus Ehrlichia comprises Ehrlichia chaffeensis, Ehrlichia canis, Ehrlichia ewingii, Ehrlichia ruminantium, Panola Mountain Ehrlichia, and Ehrlichia muris; however, a new species closely related to E. muris, designated E. muris–like agent (EMLA), has been identified in human patients and ticks [1, 2]. All ehrlichial species except E. muris have been reported to cause infection in humans, which can lead to life-threatening disease, such as human monocytotropic ehrlichiosis (HME) caused by E. chaffeensis [2–6]. E. chaffeensis infection does not provide a relevant animal model, since it does not induce progressive infection in laboratory animals. E. chaffeensis infection in immunocompromised mice with severe combined immunodeficiency does not provide an ideal model for studying pathogenesis or immunity. Other murine models have been developed to better understand the mechanisms of ehrlichial infection and disease mechanisms: E. muris, a nonlethal agent inducing persistent infection, and Ixodes ovatus Ehrlichia (IOE), a lethal model [7, 8]. IOE has been detected only in I. ovatus ticks in Japan [9].
EMLA has been detected in patients from the upper Midwestern United States since 2009 [2]. The new bacterium has been identified in different stages of Ixodes scapularis ticks collected from the same region as the human patients. The disease caused by EMLA is similar to E. chaffeensis infection, with fever, malaise, fatigue, headache, nausea, and vomiting. Clinical laboratory findings include elevated hepatic aminotransferase levels, thrombocytopenia, and lymphopenia [2, 10].
An ideal animal model to study monocytotropic ehrlichiosis infection should use a human pathogen and induce dose-dependent sublethal and lethal infection [11–13]. The objective of this research was to develop and characterize a better mouse model of human ehrlichiosis, using EMLA, which will be used for future studies of the vector-host-pathogen interaction, ehrlichial pathogenesis, and immunity.
MATERIALS AND METHODS
Ehrlichia
The newly isolated Ehrlichia species from Wisconsin (EMLA), generously provided by Dr Ulrike Munderloh (University of Minnesota), was cultivated in RF/6A monkey endothelial cells. After infection was achieved in 80%–90% of cells, the monolayer was harvested and stored in liquid nitrogen. An aliquot was quantified by real-time polymerase chain reaction (PCR), as described below. The stock was prepared as 5 × 106 infected cells/mL, with approximately 100 bacteria per cell.
Animals
Female C57BL/6 mice aged 6–8 weeks (Jackson Laboratories, Bar Harbor, ME) were inoculated by different routes with EMLA-infected cultured cells or spleen homogenate from infected C57BL/6 mice. BALB/c and C3H/HeN mice were also evaluated for infection by EMLA, using cell culture inocula; however, EMLA infection in C57BL/6 mice was characterized in greater detail. All experiments were performed with groups of 4 mice for each time point. Euthanasia was performed by overdose of isoflurane followed by cervical dislocation. All experiments were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee.
Inoculum
The preliminary studies were performed using EMLA-infected cell culture. Cell culture inoculum was prepared on the basis of a previously determined amount of bacteria per cell by real time-PCR. In subsequent studies, we used splenocyte inoculum, which was prepared from homogenate of spleens of infected mice inoculated with a lethal dose of EMLA-infected cell culture stock. When animals showed signs of illness, they were euthanized, and spleens were collected. Tissues were homogenized with a Dounce homogenizer and sonicated for tissue disruption and cell lysis. The inoculum dose was determined by titration of lethality of intravenous infection in C57BL/6 mice with serial 10-fold dilutions of homogenized splenocytes, starting with 103 bacteria to a maximum of 108 bacteria.
Routes of Inoculation
Animals were inoculated by the intradermal, intraperitoneal, or intravenous route with EMLA to evaluate the disease course. Intradermal inoculations were performed on shaved skin over the sternum. The tail vein was used for intravenous inoculations. Control mice were inoculated with similarly prepared uninfected splenic tissue by the same routes.
Collection of Samples
Whole blood, spleen, liver, lung, lymph nodes (brachial and inguinal), kidney, brain, and bone marrow specimens were collected from the animals for determination of bacterial burdens. All tissue samples including heart and intestine were fixed in 10% neutral buffered formalin for histopathologic analysis. Aliquots of whole blood collected in ethylenediaminetetraacetic acid were used for determining blood cell counts (by use of the species-specific Hemavet analyzer, Drew Scientific, Dallas, TX) and evaluation of circulating CD4+ and CD8+ T cells by flow cytometry. In addition, blood samples were obtained for serum separation to measure antibody and alanine transaminase (ALT) levels (Clinical Chemistry Laboratory, University of Texas Medical Branch, Galveston).
Determination of Bacterial Loads
Samples of whole blood and organs were processed for DNA extraction using DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA) with a few modifications. The tissue-lyser disruption system (Qiagen, Valencia, CA) was used to optimize extraction of nucleic acids. The final concentration of DNA was determined by NanoDrop Spectrophotometer (Thermo Scientific, Waltham, MA).
Tissue and blood samples were evaluated for levels of ehrlichial DNA by targeting the disulfide bond formation (dsb) gene (GenBank number AY236484). Real time PCR reaction was performed as described by Stevenson et al [14]. Bacterial numbers in the organs were normalized to the total DNA in the sample. The limit of detection was 6 copies of plasmid carrying Ehrlichia dsb gene.
Histopathologic and Immunohistochemical (IHC) Analyses
Tissue samples were processed and stained with hematoxylin and eosin. Sections of each tissue sample were also prepared for immunohistochemical detection of EMLA. Sectioned tissues were deparaffinized, hydrated, and treated with proteinase K (Dako, Carpinteria, CA), followed by incubation with a polyclonal rabbit anti-E. muris antibody at a dilution of 1 in 200 and with a biotinylated secondary goat anti-rabbit IgG antibody (Vector Laboratories, Burlingame, CA) at a dilution of 1 in 200. For detection, sections were incubated with streptavidin AP (Thermo Scientific, Waltham, MA) and then Fast Red (Dako, Carpinteria, CA). Counterstaining with hematoxylin was performed. Naive rabbit serum was used as primary antibody negative control.
Transmission Electron Microscopy
Samples from lung, spleen, liver, lymph nodes, heart, and thymus were collected during the late stage (days 9 and 10 after infection) of the EMLA lethal infection. The tissues were fixed and processed as described by Sotomayor et al [7].
Determination of Antibody Titer by Enzyme-Linked Immunosorbent Assay (ELISA)
The serum samples were assayed for immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies, using the Protein Detector ELISA Kit (KPL, Gaithersburg, MD). Lysates of EMLA cultivated in RF6A cells and of uninfected RF6A cells were coated on ELISA plates. Serum samples were diluted at 1:100 and added to the plates, according to the manufacturer's instructions. Antibody levels were calculated based on the absorbance of an equivalent dilution in a standard curve of IgM antibody– or IgG antibody–containing reference serum [15].
Flow Cytometric Analysis of Peripheral Blood T Lymphocytes
Percentages of T cells and their subsets in the blood of EMLA-infected mice were determined by flow cytometry. Briefly, a 100-µL blood sample was centrifuged, washed with fluorescence-activated cell sorter (FACS) buffer, and then incubated with anti-Fc II/III receptor monoclonal antibodies (eBioscience, San Diego, CA) at 4°C for 15 minutes. Subsequently, cells were labeled with fluorochrome-conjugated monoclonal antibodies (eBioscience, San Diego, CA) specific for mouse CD3, CD4, or CD8. Cells were fixed, and flow cytometric data were collected using the LSRII FACS (BD Immunocytometry Systems, San Jose, CA). Lymphocytes were gated on the based of forward and side scattering, and at least 20 000 lymphocytes were analyzed. Data were analyzed using FCS Express, version 3 (De Novo Software, Los Angeles, CA).
Statistical Analysis
Experimental data were analyzed using GraphPad Prism software, version 5.01 for Windows (GraphPad Software, San Diego, CA). All data were analyzed by the 2-tailed unpaired Student t test for comparison of infected and naive or control groups. Statistical significance was determined at 95% (P < .05). Data presented are expressed as means plus standard deviations.
RESULTS
The new ehrlichial pathogen, EMLA, infected all strains of mice tested, with very similar clinical and pathologic outcomes. We characterized EMLA infection in C57BL/6 mice because of the availability of specific gene knockout animals on the C57BL/6 background, which will allow future mechanistic studies of pathogenesis and immunity.
Outcome of EMLA Infection in C57BL/6 Mice Following Different Routes of Inoculation
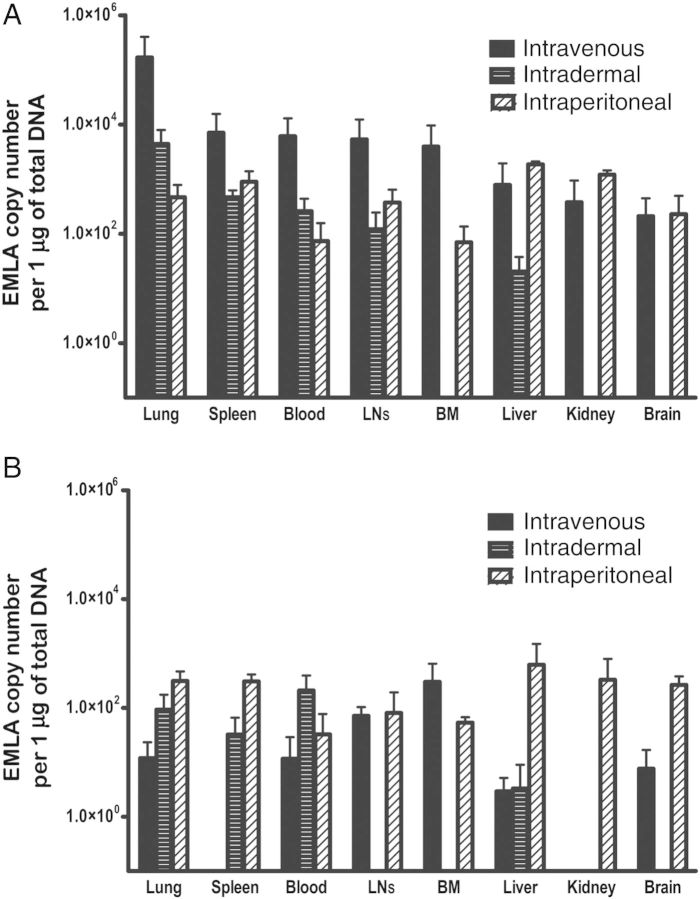
Inoculation of EMLA by intraperitoneal and intravenous routes induced disease in C57BL/6 mice, which progressed to a lethal or persistent infection in a dose-dependent manner. The lethal dose of infection in both intraperitoneal and intravenous models induced lethargy, dehydration, hunched posture, and roughened fur. Mice inoculated with low-dose or high-dose EMLA by the intradermal route and mice infected with a sublethal dose by the intraperitoneal or intravenous route did not show signs of disease but developed persistent infection. Macroscopic evaluation of the organs revealed peritoneal and/or pleural effusions in mice inoculated by the intraperitoneal route but not in intravenously or intradermally infected mice. Mild-to-moderate splenomegaly and lymphadenopathy were more evident in lethal infections, although slight increases in spleen and lymph node sizes were observed in sublethal infection. Comparison of the bacterial burden after different routes of infection indicated that intravenous and intraperitoneal inoculation led to disseminated infection in all organs tested. Intradermal infection resulted in bacterial distribution to all organs except the kidney, brain, and bone marrow (Figure 1A). Also, the levels of bacteria in tissues differed depending on the route of infection. After intraperitoneal inoculation, higher levels of bacteria were observed in the liver, spleen, and kidney (organs associated with the site of inoculation), followed by the lung, lymph node, brain, and bone marrow. Lethal intravenous and sublethal intradermal infection resulted in the highest bacterial concentrations in the lung, followed by the spleen and lymph nodes, with lower levels in the liver. The bacterial distribution varied depending on the route of infection on day 30 after infection (Figure 1B).
Figure 1.
Bacterial loads in organs of mice infected with Ehrlichia muris–like agent (EMLA) by different routes. A, EMLA distribution in organs at day 9 after infection, in intravenously, intradermally, and intraperitoneally inoculated mice. B, EMLA distribution in organs at day 30 after infection in mice infected by intravenous, intradermal, and intraperitoneal routes. Abbreviations: BM, bone marrow; LN, lymph node.
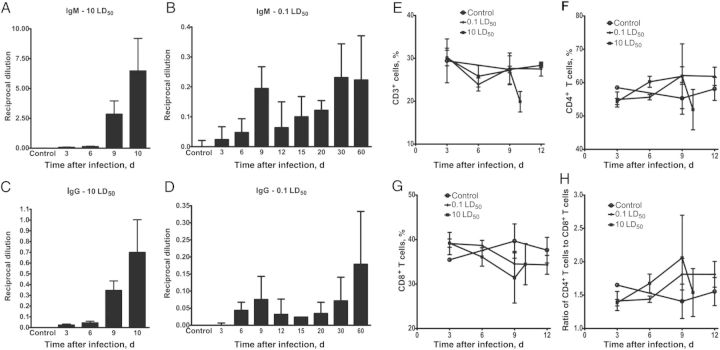
Mice infected with EMLA by different routes of inoculation developed both IgM and IgG antibody responses by day 9 after infection (Supplementary Figure 1A and 1B). IgG antibody responses in mice infected by different routes were very similar on day 30 after infection (Supplementary Figure 1C and 1D).
Mice infected with EMLA by the intradermal route and mice infected with a sublethal dose by the intravenous route that survived through day 60 after infection were challenged with an ordinarily lethal dose of EMLA by the intravenous route. Mice primarily infected by the intravenous route and challenged intravenously died around day 9 after infection, and all mice primarily inoculated intradermally survived the intravenous challenge (data not shown).
Determination of the Median Lethal Dose (LD50) by the Intravenous Route of Inoculation
All animals that received at least 1 × 105 bacteria died or became moribund and were euthanized around day 10–12 after infection. Mice receiving 1 × 103 bacteria survived for >45 days after infection. Of 5 animals inoculated with 1 × 104 bacteria, 3 became moribund and were euthanized, and 2 survived >45 days after infection (Supplementary Figure 2). We determined by using the Reed-Muench equation that the LD50 of EMLA by intravenous inoculation is approximately 1 × 104 bacteria.
We tested doses as high as 1 × 108 EMLA by intradermal inoculation, but no animals developed clinical signs of disease or died (data not shown).
EMLA Infection Course in Mice Following Inoculation of Lethal and Sublethal Doses by the Intravenous Route
We used the intravenous model to study the lethal disease by inoculation of 10 LD50 and to study the sublethal infection by inoculation of 0.1 LD50.
Clinical Signs
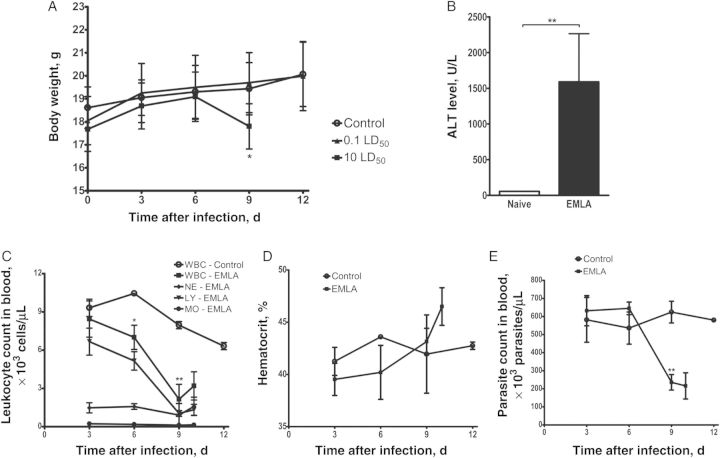
Lethal infection induced clinical signs approximately 24 hours before death. The animals developed hunched posture, lethargy, changes in breathing pattern, and roughened fur; became moribund; and died or were euthanized on day 10–12. No signs of disease were observed after inoculation of the sublethal dose. Body weights revealed remarkable weight loss during lethal infection around the time animals became moribund; however, no significant change was identified during sublethal infection (Figure 2A).
Figure 2.
Clinical and hematologic findings during the acute phase of infection in mice infected with Ehrlichia muris–like agent (EMLA) by the intravenous route. A, Change in the body weight of mice infected with lethal and sublethal doses of EMLA. B, Serum concentration of alanine aminotransferase (ALT) in mice lethally infected with EMLA on day 10 after infection. C, Total white blood cell (WBC) and differential cell counts in mice lethally infected with EMLA. D, Hematocrit values in lethally infected animals. E, Platelet counts in lethally infected mice. Bars represent means ± SD. Control and EMLA-infected groups were compared by an unpaired t test. *P < .05 and **P < .01. Abbreviations: LD50, median lethal dose; LY, lymphocyte; MO, monocyte; NE, neutrophil.
Serum Liver Enzyme
Evaluation of the serum ALT level demonstrated a significant increase in concentrations during the late stage (day 10 after infection) of lethal infection by EMLA, compared with control mice, with levels as high as 2400 U/L (Figure 2B).
Complete Blood Counts
Infection with a lethal dose of EMLA induced significant changes in blood cell counts. The total leukocyte count showed a progressive decrease from days 6 to 9 after infection, with severe leukopenia mainly due to lymphopenia, and mild-to-moderate neutropenia and monocytopenia (Figure 2C). On day 10 after infection, the animals developed a moderate increase in leukocyte counts. The concentration of red blood cells increased during lethal infection, particularly just preceding death, on day 10 after infection (Figure 2D). Platelet counts decreased precipitously on days 9 and 10 after infection in lethally infected mice, compared with control mice (Figure 2E).
Animals inoculated with a sublethal dose of EMLA did not show significant changes in blood cell counts during the course of disease through day 60 after infection.
Tissue Distribution of Bacteria
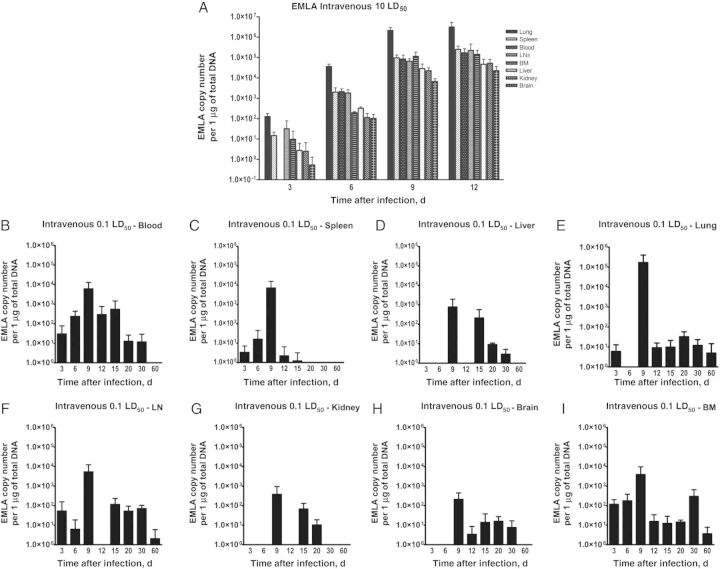
During lethal infection with EMLA, bacterial levels in spleen, liver, lung, lymph nodes, kidney, brain, and bone marrow increased progressively, rising from day 3 until death (Figure 3A). Animals infected with a sublethal dose had low bacterial loads in the organs, which varied during the course of infection and peaked on day 9 after infection. EMLA persisted in lung, lymph nodes, and bone marrow until day 60 after infection (Figure 3B–I).
Figure 3.
Bacterial distribution in the organs throughout the course of Ehrlichia muris–like agent (EMLA) infection. A, Bacterial load in organs during the lethal intravenous infection. B–I, Bacterial loads in different organs during the course of sublethal intravenous infection. Abbreviations: BM, bone marrow; LD50, median lethal dose; LN, lymph node.
Histopathologic and Immunohistochemical Findings
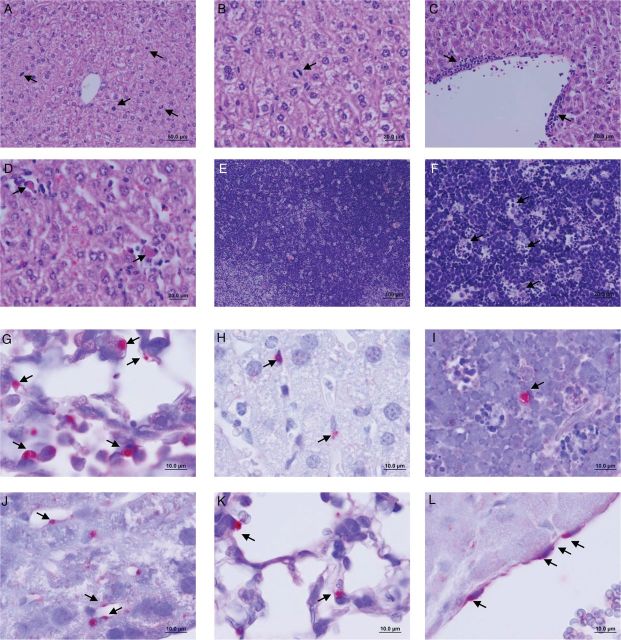
Histopathologic analysis of the organs infected with EMLA revealed progressive mild changes during the course of infection. Lethal infection initially induced proliferation of hepatocytes, observed as numerous mitotic figures (Figure 4A and 4B). With progression of the disease, a few more changes were observed in the tissues. In the liver, mitotic figures continued to be present but were less numerous; however, increased frequency of small foci of mononuclear cellular infiltration and adjacent apoptotic hepatocytes were identified diffusely throughout the liver (Figure 4C and 4D). Very mild mononuclear cell infiltration was observed in the lungs. Thymus, spleen, and lymph nodes presented similar changes characterized by individual cell fragments consumed by phagocytes, which gave the appearance of clear spaces filled with cellular debris suggestive of ingested apoptotic bodies (so-called starry sky; Figure 4E and 4F). The same lesions were observed in animals less than 24 hours before death. These lesions underwent rapid progression when the animals were moribund, with more foci of apoptotic cells in liver, increased mononuclear cell infiltration in liver and lungs, and more prominent apoptotic bodies in the thymus, spleen, and lymph nodes. Vascular congestion was observed in the majority of the organs at the late stage of infection.
Figure 4.
Histopathologic changes and bacterial localization in the organs of mice infected with Ehrlichia muris–like agent (EMLA). A and B, Mitotic figures (arrows) on day 5 after infection in liver of mice infected with a lethal dose of EMLA (20× and 40× original magnification, respectively). C, Perivascular cellular infiltration on day 9 after infection in the liver of a lethally infected mouse (40× original magnification). D, Apoptotic cells (arrows) on day 9 after infection in the liver of a lethally infected mouse (40× original magnification). E and F, Apoptotic bodies (arrows), so-called starry sky lesion, on day 10 after infection in thymus of lethally infected mice (10× and 40× original magnification, respectively). G, EMLA morulae (arrows) in the lung of a lethally infected mouse (100× original magnification). H, EMLA morulae (arrows) in the hepatic sinusoidal lining cells in a lethally infected mouse (100× original magnification). I, Thymus cell infected with EMLA (arrows) in lethal disease (100× original magnification). J–L, EMLA morulae (arrows) in the endothelial cells in liver, lung, and cardiac atrium (100× original magnification), respectively, during lethal infection.
Sublethal infection by the intravenous route did not result in significant pathologic changes. An increase of Kupffer cells was observed in the liver on days 12–13 after infection.
IHC staining revealed the presence of EMLA in all evaluated tissues, especially at the late stage of lethal infection. Staining of morulae of EMLA was identified in mononuclear cells in the lungs, liver, lymph nodes, spleen, and thymus (Figure 4G–I) and in pulmonary endothelial cells. Other organs that contained infected cells, apparently endothelial cells, were the heart (especially the atria), intestine, kidney, and bone marrow (Figure 4J–L).
Ultrastructure of EMLA in Infected Tissue
The 2 developmental forms of Ehrlichia (dense core; reticulate cells) were observed within morulae in infected lung cells, some of which were endothelial cells (Figure 5).
Figure 5.
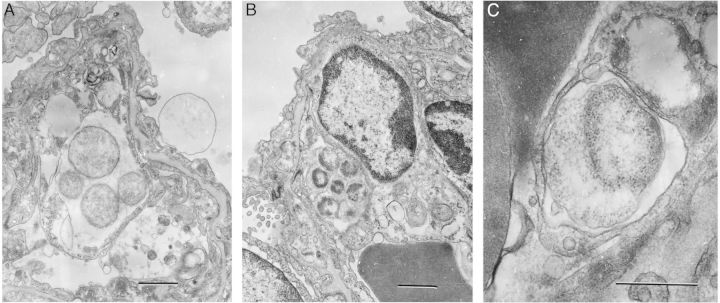
Ultrastructure of Ehrlichia muris–like agent in infected lung during lethal infection. A, A morula-containing reticulate cells (RC) in the cytoplasm of a lung cell. The bar denotes 1 µm. B, A morula with dense-core cells and intramorular fibrillar material in the cytoplasm of an endothelial cell. The bar denotes 1 µm. C, A small morula with an RC with expanded periplasmic space in the cytoplasm of an endothelial cell. Dark masses on the left represent parts of erythrocytes in a capillary lumen. The bar denotes 0.5 µm.
Antibody Titers
The highest levels of anti-EMLA IgM and IgG antibodies were induced by lethal infection 9–10 days after infection (Figure 6A and 6C). In sublethal infection, lower titers of both IgM and IgG antibodies were detected in the serum during the course of the disease. IgM antibodies to EMLA were present on day 3 after infection and were maintained through the course of infection, with increased levels on days 30 and 60 after infection (Figure 6B). Anti-EMLA IgG antibodies appeared on day 6 and persisted until day 60 after infection (Figure 6D).
Figure 6.
Antibody concentrations and T-cell responses during the course of lethal and sublethal Ehrlichia muris–like agent (EMLA) infection. A, Serum anti-EMLA immunoglobulin M (IgM) antibody concentrations during lethal infection. B, Serum anti-EMLA IgM antibody concentrations during sublethal infection. C, Serum anti-EMLA immunoglobulin G (IgG) antibody concentrations during lethal infection. D, Serum anti-EMLA IgG antibody concentrations during sublethal infection. E, Percentage of T cells in mice infected with lethal and sublethal doses of EMLA early during infection. F, Percentage of CD4+ T cells early during infection in lethally and sublethally infected mice. G, Percentage of CD8+ T cells early during infection in lethally and sublethally infected mice. H, Ratio of CD4+ to CD8+ T cells in peripheral blood of lethally and sublethally infected mice during early infection. Bars represent means ± SD. Abbreviation: LD50, median lethal dose.
CD4+ and CD8+ T Cells in the Peripheral Blood
Quantification of T cells in circulating blood was determined by flow cytometry. Lethal infection induced a decrease in the percentage of CD3+ T cells at the time the animals become moribund. However, at early time points, the percentage of total T cells was unchanged, but the ratio of CD4+ T cells to CD8+ T cells increased on day 6 after infection, with a greater increase on day 9 after infection, and then decreased before death (Figure 6E–H). However, the changes observed in the ratio of CD4+ T cells to CD8+ T cells did not achieve statistical significance. Levels of CD3+ T cells and their subtypes did not change significantly in mice with sublethal infection.
DISCUSSION
This study demonstrated that the recently identified species, EMLA, caused infection in mice that mimicked the abnormalities observed in cases of human disease induced by EMLA and E. chaffeensis. Human cases present nonspecific clinical signs, with pancytopenia and elevated serum hepatic transaminases [2, 12, 16]. Mice infected with EMLA also developed nonspecific signs of disease but only in severe cases, and the clinical signs appeared only around 24 hours before death. Changes in blood counts were similar to those in other ehrlichial infections (thrombocytopenia, leukopenia, and lymphopenia), and elevated hepatic transaminase concentrations during acute infection mimicked those during human infection.
EMLA induced lethal or persistent infection in mice in a dose- and route-dependent manner, which differed from findings for the uniformly sublethal E. muris infection (persistent model) [7, 8]. Lethal cases of HME have been reported, as well as sublethal infections; however, few cases of occurrence of prolonged infection caused by E. chaffeensis have been reported [17]. No lethal cases of EMLA in humans have been reported [2, 16, 18]. However, the EMLA mouse model provides a tool to study the disease caused by a human ehrlichial pathogen, since our results show productive infection by intravenous, intraperitoneal, and intradermal routes. Furthermore, preliminary studies have indicated that larval and nymphal I. scapularis ticks readily acquire EMLA from infected mice and transmit the bacterium to naive uninfected mice, indicating the feasibility of developing a tick transmission model of ehrlichiosis (data not shown). Interesting differences in disease manifestations were observed in mice inoculated with EMLA by different routes. In contrast with other routes, intraperitoneal infection induced pleural and peritoneal effusions, especially the accumulation of peritoneal fluid, which have not been described in typical human cases of HME or EMLA. Intradermal infection did not induce severe disease but caused persistent infection, and mice inoculated by this route were protected from subsequent challenge with an ordinarily lethal dose. Conversely, intravenous infection caused severe disease or persistent infection depending on the dose; however, it did not induce protection from subsequent lethal challenge. These observations did not correlate with antibody levels because no significant differences were observed in antibody levels in mice inoculated by different routes.
Lethal infection resulted in a progressive increase in bacterial loads in all the organs and in antibody titers, a decrease in blood cell counts, and an increase in serum ALT concentration until the animals died from the infection. However, no clinical signs were observed until the last 24 hours before death. Also, no dramatic histopathologic lesions were observed in this severe infection. The final stage of lethal infection was characterized by a rapid decrease in body weight, hemoconcentration, and increased urine specific gravity (data not shown), which suggested severe dehydration or vascular fluid leak as a possible cause of death. However, no clear source of fluid loss was identified in kidney or intestines. As bacteria were observed in endothelial cells, we hypothesize that vascular leakage played a role in hypotension and death [19, 20]. Also, it is consistent with toxic shock–like syndrome, which induces a fluid and electrolyte imbalance as a cause of death in the absence of dramatic pathologic lesions, as observed in this study. Liver was the tissue demonstrated to have more morphologic changes during lethal infection by EMLA than other organs. Interestingly, EMLA initially induced hepatocellular mitoses followed by apoptotic cell death in liver and also in lymphoid tissues at the time when animals manifested clinical signs 24 hours before death. The previously described lethal IOE model of infection also induces liver damage and increased levels of cytokines, such as tumor necrosis factor α, which was suggested to be related to toxic shock–like syndrome [21]. Other cytokines and T-cell types have also been implicated in the development of toxic shock–like syndrome [22, 23]; however, we have yet to evaluate the host immune response in detail. Other pathologic changes observed in the EMLA model were cell infiltrations in lungs and apoptosis in lymphoid tissues. HME cases are reported to develop multifocal necrosis in liver and lymphoid tissues, interstitial pneumonia, and perivascular lymphohistocytic infiltration [11, 24–26]. Similarly, the lethal IOE model demonstrates pathologic involvement of the liver, lymphoid tissues, and lungs [7]. Tissues involved are similar in other ehrlichial infections.
In conclusion, the development of a new animal model of ehrlichiosis, using a recently discovered human pathogen, provided an opportunity to investigate pathogenesis and host immune responses of ehrlichiosis. This new ehrlichial species induced a range of severity of illness, from subclinical to lethal disease in mice, in a dose- and route-dependent manner, which can be used to discover principles that apply to the monocytotropic ehrlichioses. Furthermore, our preliminary studies suggest the feasibility of developing a highly relevant natural vector transmission model for monocytotropic ehrlichiosis, using this new animal model.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Kenneth Escobar and Kerry Graves from the histopathology core, and ARC personnel, mainly Vallerie Meador, for research support during the performance of this project.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grants AI102304 and AI089973).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dumler JS, Barbet AF, Bekker CP, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–65. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- 2.Pritt BS, Sloan LM, Johnson DK, et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med. 2011;365:422–9. doi: 10.1056/NEJMoa1010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez M, Bodor M, Zhang C, Xiong Q, Rikihisa Y. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann N Y Acad Sci. 2006;1078:110–7. doi: 10.1196/annals.1374.016. [DOI] [PubMed] [Google Scholar]
- 4.Allsopp MT, Louw M, Meyer EC. Ehrlichia ruminantium—an emerging human pathogen. S Afr Med J. 2005;95:541. [PubMed] [Google Scholar]
- 5.Buller RS, Arens M, Hmiel SP, et al. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148–55. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 6.Dumler JS, Bakken JS. Ehrlichial diseases of humans: emerging tick-borne infections. Clin Infect Dis. 1995;20:1102–10. doi: 10.1093/clinids/20.5.1102. [DOI] [PubMed] [Google Scholar]
- 7.Sotomayor EA, Popov VL, Feng HM, Walker DH, Olano JP. Animal model of fatal human monocytotropic ehrlichiosis. Am J Pathol. 2001;158:757–69. doi: 10.1016/S0002-9440(10)64018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olano JP, Wen G, Feng HM, McBride JW, Walker DH. Histologic, serologic, and molecular analysis of persistent ehrlichiosis in a murine model. Am J Pathol. 2004;165:997–1006. doi: 10.1016/S0002-9440(10)63361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibata S, Kawahara M, Rikihisa Y, et al. New Ehrlichia species closely related to Ehrlichia chaffeensis isolated from Ixodes ovatus ticks in Japan. J Clin Microbiol. 2000;38:1331–8. doi: 10.1128/jcm.38.4.1331-1338.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson BE, Sims KG, Olson JG, et al. Amblyomma americanum: a potential vector of human ehrlichiosis. Am J Trop Med Hyg. 1993;49:239–44. doi: 10.4269/ajtmh.1993.49.239. [DOI] [PubMed] [Google Scholar]
- 11.Paddock CD, Sumner JW, Shore GM, et al. Isolation and characterization of Ehrlichia chaffeensis strains from patients with fatal ehrlichiosis. J Clin Microbiol. 1997;35:2496–502. doi: 10.1128/jcm.35.10.2496-2502.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olano JP, Hogrefe W, Seaton B, Walker DH. Clinical manifestations, epidemiology, and laboratory diagnosis of human monocytotropic ehrlichiosis in a commercial laboratory setting. Clin Diagn Lab Immunol. 2003;10:891–6. doi: 10.1128/CDLI.10.5.891-896.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fishbein DB, Dawson JE, Robinson LE. Human ehrlichiosis in the United States, 1985 to 1990. Ann Intern Med. 1994;120:736–43. doi: 10.7326/0003-4819-120-9-199405010-00003. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson HL, Jordan JM, Peerwani Z, Wang HQ, Walker DH, Ismail N. An intradermal environment promotes a protective type-1 response against lethal systemic monocytotropic ehrlichial infection. Infect Immun. 2006;74:4856–64. doi: 10.1128/IAI.00246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008;26:193–200. doi: 10.1016/j.vaccine.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson DKH, Nietzel DF, Miller TK, et al. Five years' experience with the novel human Ehrlichia sp. in the upper Midwestern United States: 2009–2013 [LB-2399]. Presented at: 62nd Annual Meeting of the American Society of Tropical Medicine and Hygiene,; 13–17 November 2013; Washington, D. C. [Google Scholar]
- 17.Dumler JS, Sutker WL, Walker DH. Persistent infection with Ehrlichia chaffeensis. Clin Infect Dis. 1993;17:903–5. doi: 10.1093/clinids/17.5.903. [DOI] [PubMed] [Google Scholar]
- 18.Johnson DKH, Neitzel DF, McFadden JD, et al. Novel Ehrlichia sp. pathogenic for humans in the midwestern United States: human cases 2009–2011 and results of tick, rodent, and deer studies [LB-2074]. Presented at: 60th Annual Meeting of the American Society of Tropical Medicine and Hygiene; 4–8 December 2011; Philadelphia, Pennsylvania. [Google Scholar]
- 19.Druey KM, Greipp PR. Narrative review: the systemic capillary leak syndrome. Ann Intern Med. 2010;153:90–8. doi: 10.1059/0003-4819-153-2-201007200-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapoor P, Greipp PT, Schaefer EW, et al. Idiopathic systemic capillary leak syndrome (Clarkson's disease): the Mayo Clinic experience. Mayo Clin Proc. 2010;85:905–12. doi: 10.4065/mcp.2010.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ismail N, Soong L, McBride JW, et al. Overproduction of TNF-alpha by CD8+ type 1 cells and down-regulation of IFN-gamma production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J Immunol. 2004;172:1786–800. doi: 10.4049/jimmunol.172.3.1786. [DOI] [PubMed] [Google Scholar]
- 22.Ismail N, Stevenson HL, Walker DH. Role of tumor necrosis factor alpha (TNF-alpha) and interleukin-10 in the pathogenesis of severe murine monocytotropic ehrlichiosis: increased resistance of TNF receptor p55- and p75-deficient mice to fatal ehrlichial infection. Infect Immun. 2006;74:1846–56. doi: 10.1128/IAI.74.3.1846-1856.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevenson HL, Crossley EC, Thirumalapura N, Walker DH, Ismail N. Regulatory roles of CD1d-restricted NKT cells in the induction of toxic shock-like syndrome in an animal model of fatal ehrlichiosis. Infect Immun. 2008;76:1434–44. doi: 10.1128/IAI.01242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sehdev AE, Dumler JS. Hepatic pathology in human monocytic ehrlichiosis. Ehrlichia chaffeensis infection. Am J Clin Pathol. 2003;119:859–65. doi: 10.1309/F7EA-B5P7-3217-16LJ. [DOI] [PubMed] [Google Scholar]
- 25.Dumler JS, Brouqui P, Aronson J, Taylor JP, Walker DH. Identification of Ehrlichia in human tissue. N Engl J Med. 1991;325:1109–10. doi: 10.1056/NEJM199110103251517. [DOI] [PubMed] [Google Scholar]
- 26.Fordham LA, Chung CJ, Specter BB, Merten DF, Ingram DL. Ehrlichiosis: findings on chest radiographs in three pediatric patients. AJR Am J Roentgenol. 1998;171:1421–4. doi: 10.2214/ajr.171.5.9798890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.