Summary
The ability of caffeine, enprofylline (3-propylxanthine), 8-phenyltheophylline, 8-p-sulphophenyltheophylline, 8-(4′-carboxymethyloxyphenyl)-1,3-dipropylxanthine, and 8-(4′-carboxymethyloxyphenyl)-1,3-dipropylxanthine-2-aminoethylamide (XAC) to antagonize the effects of an adenosine analogue, N-5′-ethylcarboxamidoadenosine, on heart rate and blood pressure in anesthetized rats was examined. The first five xanthine derivatives were equally active in antagonizing the two responses. By contrast, XAC was ~20 times more potent in antagonizing the heart rate response than the blood pressure response. Measurements of the concentration of XAC in plasma and brain indicate that it penetrates the central nervous system poorly. It is concluded that XAC is a cardioselective adenosine antagonist, and since adenosine is supposed to reduce heart rate via an effect on A1-receptors, and the blood pressure via A2-receptors, XAC may be a selective A1-adenosine receptor antagonist in vivo.
Keywords: Xanthines, Rat, Blood pressure, Heart rate, Adenosine, Adenosine receptor
Adenosine exerts various biological effects via an action on cell-surface adenosine receptors (1). These adenosine receptors are of two types: A1 and A2. The A1-receptors are operationally defined as those receptors at which several N6-substituted adenosine analogs such as R-phenylisopropyladenosine (R-PIA) and cyclohexyladenosine (CHA) are more potent than 2-chloroadenosine and N-5′-ethylcarboxamidoadenosine (NECA). At A2-receptors the order of potency is instead NECA > 2-chloroadenosine > R-PIA > CHA (1–3). At both types of adenosine receptors, alkylxanthines—theophylline and caffeine being the prototypes—are competitive antagonists. These classical xanthines show affinities in the 10−5–10−4 range and show little or no selectivity for either receptor type (4). Substitution of the xanthine moiety at the 1- and 3-positions with ethyl or propyl groups or at the 8-position with a phenyl group results in considerably more active antagonists. Furthermore, some analogs of 8-phenyltheophylline have shown evidence of selectivity with regard to the A1-adenosine receptor (5,6). Unfortunately, the combined effects of multiple hydrophobic substituents in analogs such as 8-(2-amino-4-chlorophenyl)-1,3-dipropylxanthine (400-fold A1-selective in vitro; ref. 5) often lead to low aqueous solubility, thus precluding meaningful in vivo testing. The presence of a permanently charged group, such as sulphonate, directly on the ring increases water solubility but does not result in selective analogs.
We have recently described a “functionalized congener” approach to xanthines (6), which has produced highly potent and water-soluble xanthine analogs. This approach has also been applied to adenosine analogs (7). By this approach a drug analog is synthesized with a chain terminating in a chemically reactive group such as a carboxylic acid or an amine. To the analog may be attached, via the reactive group, well-defined “carrier” molecules. The resultant conjugate can still retain the ability to bind to the receptor site (e.g., high molecular weight receptor probes based on the biotin–avidin complex; see ref. 8) and have biological activity. The carrier may be a synthetic peptide, a protein, or other biopolymer. Drug conjugates may be step-wise modified by adding further groups to modify potency, specificity, and duration of action, as well as physicochemical properties such as water solubility. In vitro screening of xanthine functionalized congeners indicated that both potency and adenosine receptor subtype selectivity may be modulated through distal structural changes on the attached chain. In particular, a high degree of selectivity toward A1-receptors was associated with analogs having a free amino group on the chain, whereas other congeners showed little or no selectivity. These preliminary studies on the receptor selectivity were carried out only under in vitro conditions and it was necessary to investigate the important question as to whether or not such selectivity can also be observed under in vivo conditions.
It is well known that adenosine produces a marked decrease in blood pressure (9). This blood pressure reduction is probably dependent upon an A2-receptor–mediated decrease in peripheral resistance (10–13). Adenosine analogs are also able to decrease heart rate (9, 14). This effect is probably mediated via adenosine receptors of the A1-subtype (13, 15). In the present series of experiments we therefore examined the ability of several xanthine analogs, including two recently synthesized functionalized congeners of 1,3-dipropyl-8-phenylxanthine, to antagonize the hypotensive and negative chronotropic effects of a stable adenosine analog (NECA) under in vivo conditions. A preliminary account of some of the results has been given (16).
MATERIAL AND METHODS
Experimental procedure
The experiments were conducted on male Sprague–Dawley rats (Alab strain) weighing 250–300 g. Anesthesia was induced with sodium pentobarbital (60 mg/kg i.p.). The rats were tracheotomized and polyethylene catheters were inserted into the common artery for continuous blood pressure and heart rate recording. Another polyethylene catheter was inserted into the external jugular vein for drug administration. The xanthine derivatives were administered intraperitoneally in the doses given in the text. Thirty minutes after the administration of the xanthine derivative, increasing doses of NECA were given intravenously and the changes in blood pressure and heart rate were recorded. Immediately after injection there was a very marked drop in blood pressure and there was also a transient fall in heart rate. A short-lasting decrease in blood pressure was often encountered, even after injection of pure saline, whereas NECA produced a more long-lasting blood pressure reduction, which has been used in this study. The effect of vehicles on this blood pressure reduction is negligible or nonexistent. After a period of 10–20 min a steady level of heart rate and blood pressure is obtained (13). This steady level was used to quantitate the NECA effect. Full dose–response curves were constructed in at least three animals at each dose of the xanthine. The EC50 values were estimated by Hill plots of transformed data. Based on the shift in the NECA dose–response curves, pA2 values were estimated by Schild plot (when several doses of the xanthine were given) or by the formula, [log (concentration of antagonist) − log(DR − 1)] = −pA2; where DR is the ratio of concentration agonist in the presence of antagonist to agonist concentration in the absence of antagonist producing the same effect. It should be emphasized that the pA2 value, although calculated as described by Schild, does not necessarily represent the receptor antagonist affinity, since the in vivo situation is more complex than, for instance, membrane receptor binding in vitro.
The plasma concentrations of the two novel xanthine derivatives were determined by high-performance liquid chromatography (HPLC). Blood samples were taken at various times, usually 30, 60, 90, and 120 min after administration. The rats were thereafter killed by decapitation. The brains were rapidly taken out and homogenized in 5 vol buffer containing 0.1 M triethylammonium acetate and 70% methanol at pH 5.2. After centrifugation at 1,800 g for 15 min, the supernatant of the brain homogenate and the plasma were used to determine the drug concentration using HPLC. Little or no xanthine remained in the pelleted material. The stationary phase was a micro Bondapac C18 column, 30 cm long. The mobile phase consisted of 0.1 M triethylammonium acetate at pH 4.2 containing 60–70% methanol. Absorbance was measured by Waters model 440 Absorbance Monitor at 280 nm. Aqueous standard solutions were used to quantitate the xanthines.
Chemicals
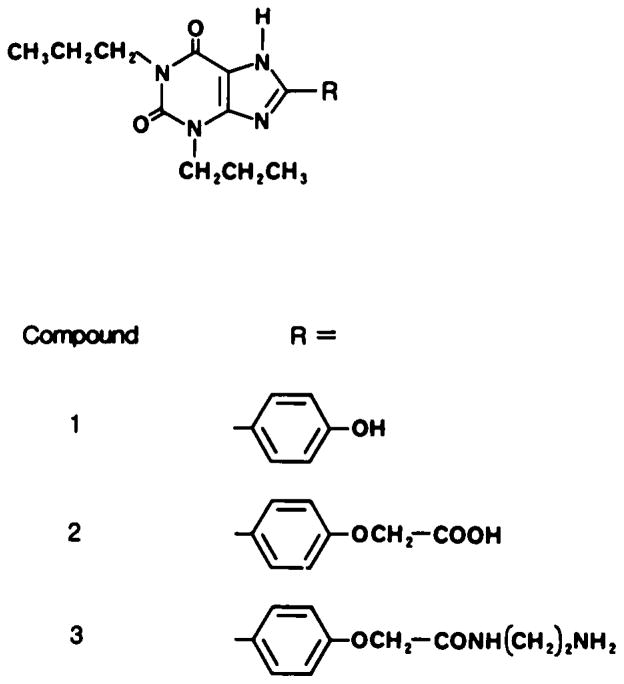
NECA was a gift from Byk-Gulden AG, Constanz, BRD. 8-Phenyltheophylline was obtained from Calbiochem Behring, and 8-p-sulphophenyltheophylline was a gift from Dr. Lars Gustafsson, Stockholm, Sweden. Enprofyllin (3-propylxanthine) was obtained from AB Draco, Lund, Sweden. The two functionalized congeners of 1,3-dipropyl-8-phenylxanthine, XAC and XCC, were synthetized as described elsewhere (6). The structures of these compounds are given in Fig. 1. The drugs were dissolved in a minimal amount of sodium hydroxide (0.1 M) and diluted with isotonic sodium chloride before injection.
FIG. 1.
Structures of two novel xanthine derivatives, compound 2 (XCC) and compound 3 (XAC), which are functionalized congeners related to 1,3-dipropyl-8-(p-hydroxyphenyl) xanthine (compound 1; not used in this study).
RESULTS
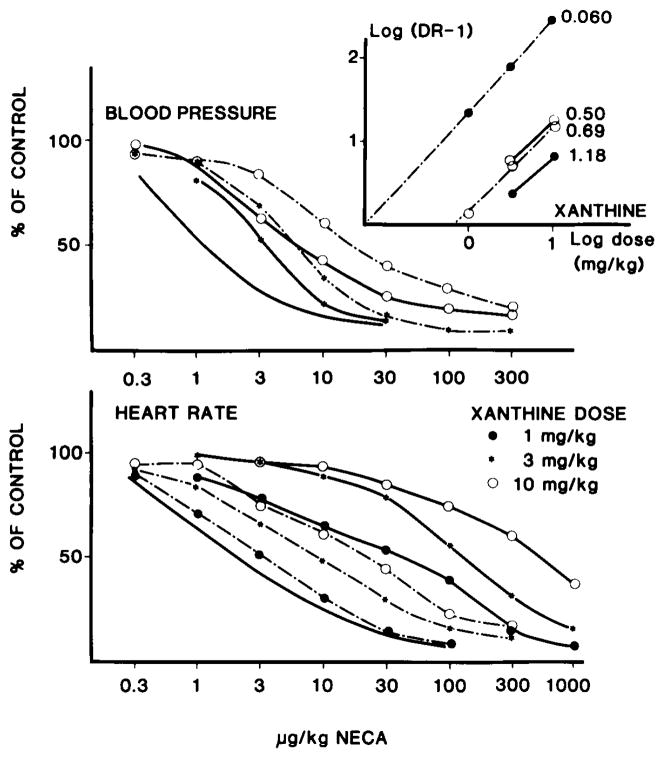
As seen in Fig. 2 NECA caused a dose-dependent decrease in heart rate and in blood pressure in the anesthetized rats. The xanthine derivatives did not significantly affect blood pressure. The acute changes were as follows: caffeine—+10 ± 13.9 mm Hg (20 mg/kg); enprofylline—−29 ± 24 mm Hg (10 mg/kg); XCC—1.6 ± 10.3 mm Hg (1 mg/kg), +5 ± 6 mm Hg (3 mg/kg), +2.5 ± 0 mm Hg (10 mg/kg); XAC—8.8 ± 6.5 mm Hg(1 mg/kg), −12.5 ± 12.5 mm Hg (3 mg/kg), −15 ± 29 mm Hg (10 mg/kg); 8-phenyltheophylline—+13 ± 12 mm Hg (2 mg/kg); and 8-p-sulphophenyltheophylline— 8 ± 6 mm Hg (2 mg/kg). Similarly, the heart rate effects were minor (<5% change for all the xanthines). The amino congener of 1,3-dipropyl-8-phenylxanthine (XAC) produced a much larger shift of the NECA dose–response curve for heart rate than for blood pressure at the same dose (Fig. 2). By contrast, the carboxylic acid congener (XCC) produced equivalent shifts to the right of the two dose–response curves (Fig. 2). The results are summarized in Table 1, which also gives the results for 8-phenyltheophylline, 8-p-sulphophenyltheophylline, enprofylline, and caffeine. It may be seen that, with the exception of the amino congener XAC, all the xanthine derivatives were approximately equipotent on heart rate and blood pressure.
FIG. 2.
Effect of increasing doses of N-5′-ethylcarboxamidoadenosine (NECA) on blood pressure and heart rate in anesthetized rats in the presence of increasing doses of two novel derivatives (XAC and XCC) (compounds 2 and 3 in Fig. 1). Upper graph: effect of the two compounds on the blood pressure response to NECA. The solid line without symbols shows the effect of NECA. The solid lines with symbols show the effect of XAC and the hatched lines the effect of XCC. Lower graph: corresponding effects on heart rate. Insert: Schild plots for the compounds and the estimated potentials (in mg/kg), calculated from the pA2 values. Solid lines represent effects on blood pressure, hatched lines effects on heart rate. Dots show the effect of XAC, open circles the effect of XCC. The initial blood pressure/heart rate and SD and number of experiments were as follows: control, 138 ± 28/411 ± 36 (n = 6); XAC (1–10 μg/kg), 136 ± 21/458 ± 42 (n = 10); and XCC 113 ± 23/410 ± 58 (n = 10).
TABLE 1.
Potency of xanthine derivatives as adenosine receptor antagonists in vivo in rats
| Drug | Heart rate
|
Blood pressure
|
Ratio | ||
|---|---|---|---|---|---|
| mg/kg | μmol/kg | mg/kg | μmol/kg | ||
| Caffeinea | 3.39 | 17.5 | 3.63 | 18.7 | 1.07 |
| Enprofyllinea | 14.5 | 63.6 | 17.0 | 74.6 | 1.17 |
| 8-Phenyltheophyllinea | 0.062 | 0.22 | 0.041 | 0.14 | 0.66 |
| 8-p-Sulphophenyltheophyllinea | 0.85 | 2.3 | 1.71 | 4.70 | 2.01 |
| XCCb | 0.50 | 1.1 | 0.69 | 1.5 | 1.38 |
| XACb | 0.060 | 0.14 | 1.18 | 2.8 | 20 |
Results are expressed as the doses of the xanthine derivatives that produce a dose ratio of 2 for N-5′-ethylcarboxamidoadenosine (NECA). The control EC50 for NECA ranged between 1.18 and 3.1 μg/kg (blood pressure) and 1.8 and 6.1 μg/kg (heart rate). Caffeine was tested in a single dose of 20 mg/kg. enprofylline in a dose of 10 mg/kg. and 8-phenyltheophylline and 8-p-sulphophenyltheophylline in a dose of 2 mg/kg. The Hill coefficients for the NECA dose–effect curves were as follows (blood pressure/heart rate; mean and 95% confidence interval): control—0.78 ± 0.16/1.03 ± 0.15; caffeine—1.09 ± 0.13/1.19 ± 0.12; enprofylline—0.73 ± 0.13/1.06 ± 0.07; 8-phenyltheophylline—0.69 ± 0.15/0.62 ± 0.26; and 8-p-sulphophenyltheophylline—0.55 ± 0.06/0.79 ± 0.14. The fact that the Hill coefficients were sometimes different from unity probably indicates that the slope of the dose–response curve was significantly affected by factors other than the drug–receptor interaction. XCC, 8-(4′-carboxymethyloxyphenyl)-1,3-dipropylxanthine; XAC. 8-(4′-carboxymethyloxyphenyl)-1,3-dipropylxanthine-2-aminoethylamide.
Calculated from: antilog [log (xanthine) − log (DR − 1)] (see Methods).
Calculated from Schild plot (Fig. 2).
The data shown in Table 2 illustrate that at a dose of 3 mg/kg the two functionalized congeners of 1,3-dipropyl-8-phenylxanthine produced plasma concentrations between 10 and 20 μM after 30 min. After 90 min the levels remained close to 70% of the value observed after 30 min. By contrast, it was impossible to measure the levels of these derivatives in brain tissue. Thus, the level in brain is at most 1/20 of that in liver (not shown) and ⅕ of that in plasma after 3 mg/kg XAC. It is probable that XAC penetrates the central nervous system (CNS) poorly, since the concentration is much higher in the periphery than in the CNS.
TABLE 2.
Plasma concentrations of two novel xanthine derivatives after intraperitoneal administration at a dose of 3 mg/kg
| Dose given i.p. | XCC (n = 3) (6.4 μmol/kg) | XAC (n = 5) (7.0 μmol/kg) |
|---|---|---|
| 30 min | 14.1 ± 0.8 μM | 12.4 ± 0.9 μM |
| 60 min | ND | 12.4 ± 0.5 μM |
| 90 min | 10.7 ± 0.2 μM | 9.8 ± 0.7 μM |
| 120 min | ND | 9.4 ± 0.7 μM |
Abbreviations as in Table 1. ND, not determined.
DISCUSSION
The para position of the 8-phenyl ring has been identified as a site on the drug that may accommodate a functional chain without the loss of biological activity (6). In in vitro studies XCC was nonselective, with a Ki at the A1-receptors in brain membranes of 58 ± 3 nM and a Ki at the A2-receptors of the brain slice assay of 34 ± 13 nM (6). XAC, on the other hand, had a 40-fold higher affinity for A1-adenosine receptors, with a Ki of 1.2 ± 0.5 nM, as compared with a Ki at the A2-receptors of the brain slice assay of 49 ± 17 nM (6). The compounds were relatively water soluble. In 0.1 M sodium phosphate buffer (pH 7.2), the maximum solubility of XCC and XAC was 1,200 and 90 μM, respectively (6). Simple 8-phenylxanthines show very low water solubility, which limits their usefulness for in vivo studies. For example, the maximum solubility of 1,3-dipropyl-8-(p-hydroxyphenyl)xanthine (compound 1 in Fig. 1) in the same buffer is 3.2 μM.
The major finding of the present investigation is that the amino congener of 8-phenyl-1,3-dipropyl-xanthine, XAC, is much more potent in antagonizing the effects of adenosine analogs on heart rate than on blood pressure. Since the adenosine analog–induced effects on the heart seem to be mediated via A1-receptors (13, 15), and those on blood pressure via A2-receptors (10–13), these results suggest that this novel xanthine derivative is more active as an antagonist at A1-receptors than at A2-receptors under in vivo conditions. By contrast, another (carboxylic acid) congener (XCC) of the same basic structure did not show such selectivity, nor was it exhibited by caffeine, enprofyllin, 8-phenyltheophylline, or 8-p-sulphophenyltheophylline. These in vivo results thus agree with the selectivity data obtained in vitro.
It could be estimated that the dose–response curve for NECA was shifted by a factor of 2 to the right after administration of 0.06 mg/kg of the novel derivative. A dose of 3 mg/kg was associated with a plasma concentration of ~12 μM. Assuming that the compound does not exhibit dose-dependent pharmacokinetics, it may be calculated that the affinity of the antagonist at A1-receptors in vivo is in the 10−7 M range. By contrast, under in vitro conditions in a binding assay, an affinity in the 10−9 range was observed (6).
We found no evidence that XCC or XAC entered the brain to any substantial degree. This is also compatible with the finding that the plasma concentration of the drug would correspond to ~6 mg/L at a dose of 3 mg/kg, indicating a preferential distribution in the water space. Such a distribution would also provide a partial explanation for the relatively low activity of the compound under in vivo conditions, since it could suggest that the penetration of the compound through tissues is incomplete. The pharmacokinetic information presented here does not allow conclusions about the half-life of the compounds, but suggests that the compounds are not extensively metabolized in vivo.
Thus, the present data shows that a novel xanthine derivative is a cardioselective adenosine antagonist and that this selectivity may be explained by A1-adenosine receptor selectivity. Furthermore, little, if any, of the drug is distributed to the CNS. Such a compound could be a valuable research tool, for example, in elucidating which adenosine actions are mediated via peripheral and which via central adenosine receptors. There is also the intriguing possibility that a compound of this type could have clinical potential. For example, adenosine analogs have potent actions on blood vessels and on blood pressure in humans and animals, but the clinical use is limited by negative inotropic and chronotropic effects on the heart (see ref. 17). A cardioselective xanthine derivative may allow utilization of the full potential of the adenosine vasodilatation.
Acknowledgments
These studies were supported by grants from the Swedish Medical Research Council (proj. no. 2553), by NIH, by Ostermans foundation, and by Karolinska Institutet.
References
- 1.Daly JW. Adenosine receptors: characterization with radioactive ligands. In: Daly JW, Kuroda Y, Phillis JW, Schimizu H, Ui M, editors. Physiology and pharmacology of adenosine derivatives. New York: Raven Press; 1983. pp. 59–70. [Google Scholar]
- 2.Fredholm BB. Adenosine receptors. Med Biol. 1982;60:289–93. [PubMed] [Google Scholar]
- 3.Burnstock G, Buckley N. The classification of receptors for adenosine and adenine nucleotides. In: Paton DM, editor. Methods in pharmacology. Vol. 6. New York: Plenum; 1985. pp. 193–212. [Google Scholar]
- 4.Fredholm BB, Persson CG. Xanthine derivatives as adenosine receptor antagonists. Eur J Pharmacol. 1982;81:673–6. doi: 10.1016/0014-2999(82)90359-4. [DOI] [PubMed] [Google Scholar]
- 5.Daly JW, Padgett W, Shamin MT, Butts-Lamb P, Waters J. 1,3-Dialkyl-8-p-sulfophenylxanthines: potent water-soluble antagonists for A1- and A2-adenosine receptors. J Med Chem. 1985;28:487–92. doi: 10.1021/jm00382a018. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson KA, Kirk KL, Padgett WL, Daly JW. Functionalized congeners of 1,3-dialkylxanthines: preparation of analogs with high affinity for adenosine receptors. J Med Chem. 1985;28:1334–40. doi: 10.1021/jm00147a038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson KA, Kirk KL, Padgett WL, Daly JW. Functionalized congeners of adenosine: preparation of analogs with high affinity for A1-adenosine receptors. J Med Chem. 1985;28:1341–6. doi: 10.1021/jm00147a039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson KA, Kirk KL, Padgett W, Daly JW. Probing the adenosine receptor with adenosine and xanthine biotin conjugates. FEBS Lett. 1985;184:30–5. doi: 10.1016/0014-5793(85)80646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drury AN, Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol (Lond) 1929;28:213–37. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kusachi S, Thompson RD, Olsson RA. Ligand selectivity of dog coronary adenosine receptor resembles that of adenylate cyclase stimulatory (Ra) receptors. J Pharmacol Exp Ther. 1983;227:316–21. [PubMed] [Google Scholar]
- 11.Edvinsson L, Fredholm BB. Characterization of adenosine receptors in isolated cerebral arteries of cat. Br J Pharmacol. 1983;80:631–8. doi: 10.1111/j.1476-5381.1983.tb10052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sollevi A, Lagerkranser M, Andreen M, Irestedt L. Relationship between arterial and venous adenosine levels and vasodilatation during ATP- and adenosine-infusion in dogs. Acta Physiol Scand. 1984;120:171–6. doi: 10.1111/j.1748-1716.1984.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 13.Jonzon B, Bergqvist A, Li YO, Fredholm BB. Effects of adenosine and two stable adenosine analogues on blood pressure, heart rate and colonic temperature in the rat. Acta Physiol Scand. 1986;126:491–8. doi: 10.1111/j.1748-1716.1986.tb07846.x. [DOI] [PubMed] [Google Scholar]
- 14.Belardinelli L, West A, Crampton R, Berne RM. Chronotropic and dromotropic effects of adenosine. In: Berne RM, Rall TV, Rubio R, editors. Regulatory function of adenosine. The Hague/Boston: Martinus Nijhoff Publishers; 1983. pp. 377–98. [Google Scholar]
- 15.Collis MG. Evidence for an A1-adenosine receptor in the guinea-pig atrium. Br J Pharmacol. 1983;78:207–12. doi: 10.1111/j.1476-5381.1983.tb09381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson KA, Kirk KL, Daly JW, Jonzon B, Li YO, Fredholm BB. A novel 8-phenyl-substituted xanthine derivative is a selective antagonist at adenosine A1-receptors in vivo. Acta Physiol Scand. 1985;125:341–2. doi: 10.1111/j.1748-1716.1985.tb07725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fredholm BB, Sollevi A. Cardiovascular effects of adenosine. Clin Physiol. 1986;6:1–21. doi: 10.1111/j.1475-097x.1986.tb00139.x. [DOI] [PubMed] [Google Scholar]