Abstract
The aging of the population in the United States and throughout the developed world has increased morbidity and mortality attributable to lung disease, while the morbidity and mortality from other prevalent diseases has declined or remained stable. Recognizing the importance of aging in the development of lung disease, the American Thoracic Society (ATS) highlighted this topic as a core theme for the 2014 annual meeting. The relationship between aging and lung disease was discussed in several oral symposiums and poster sessions at the annual ATS meeting. In this article, we used the input gathered at the conference to develop a broad framework and perspective to stimulate basic, clinical, and translational research to understand how the aging process contributes to the onset and/or progression of lung diseases. A consistent theme that emerged from the conference was the need to apply novel, systems-based approaches to integrate a growing body of genomic, epigenomic, transcriptomic, and proteomic data and elucidate the relationship between biologic hallmarks of aging, altered lung function, and increased susceptibility to lung diseases in the older population. The challenge remains to causally link the molecular and cellular changes of aging with age-related changes in lung physiology and disease susceptibility. The purpose of this review is to stimulate further research to identify new strategies to prevent or treat age-related lung disease.
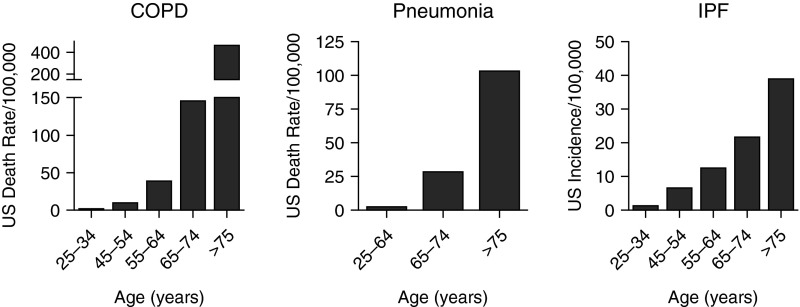
From 2000 to 2010, the proportion of the U.S. population aged 65 years or older increased from 12 to 15% and it is expected to increase to 20% by 2030 (1). Worldwide, life expectancy has dramatically increased from 47 years in 1950–1955, to 69 years in 2005–2010, and is expected to increase to 76 years by 2050 (2). The aging of the population in the United States and throughout the developed world has been associated with increases in morbidity and mortality attributable to lung disease, whereas the morbidity and mortality from other prevalent diseases has declined or remained stable. For example, chronic obstructive pulmonary disease (COPD) has risen to become the fourth leading cause of death worldwide and the third leading cause of death in the United States (3). There is growing recognition that aging contributes to the pathogenesis of a number of chronic lung diseases; indeed, most lung diseases are either largely restricted to the elderly or are more severe in older individuals. For example, in the United States, the prevalence of COPD was estimated at 3.2% among those aged 25–44 years and 10.3% among those 65–74 years (4). Similarly, the mortality attributable to COPD and pneumonia and the incidence of idiopathic pulmonary fibrosis (IPF) all increase with age (Figure 1). Advancing age (i.e., aging) has been associated with increased susceptibility to, and severity of, both viral and bacterial pneumonia (5, 6). Older patients are at increased risk for developing the acute respiratory distress syndrome (ARDS), likely reflecting increased severity of both pneumonia and sepsis, major risk factors for ARDS in elderly patients (7, 8). Advanced age increases the risk for nontuberculous mycobacterial infections and venous thromboembolism, and an expanding body of literature describes a surprisingly high incidence and severity of asthma associated with airway remodeling in elderly patients (9–11).
Figure 1.
Lung disease is more common in the elderly. Estimates of annual U.S. death rates for chronic obstructive pulmonary disease (COPD) and pneumonia and the estimated annual incidence rate for idiopathic pulmonary fibrosis (IPF) are shown. Data from References 4, 107, and 108.
Aging research has attracted the curiosity and imagination of the scientific research community throughout the history of humankind. “Biological aging” is characterized by a progressive loss of physiological integrity, leading to impaired function, increased frailty, and increased vulnerability to death, which is common to most living organisms (12). This disruption is often associated with the slow and gradual buildup of molecular damage from environmental and metabolic stressors, leading to a decrease in fitness and greater susceptibility to disease. Aging researchers debate whether these processes are inevitable; at conception, two cells that are chronologically decades old combine to form a cell with a chronological age of zero, suggesting the presence of biological mechanisms for cellular rejuvenation, a finding confirmed by experimental success in cloning and in the generation of induced pluripotent stem cells (13). Investigators have been further energized by discoveries in model organisms suggesting that the rate of aging is at least partially controlled by genetic pathways and biochemical processes conserved in evolution that can be manipulated to extend the life span (14). More importantly, many of these life span–extending interventions are associated with improvements in physiological function, offering the promise of improving both health span and life span (14, 15). Rapid advances in new technologies including high-throughput tools and systems-based analyses are likely to lead to new discoveries, including the identification of key nodes in aging pathways that can be targeted to slow down or treat age-related lung diseases. We are in an era of resetting the aging clock (13).
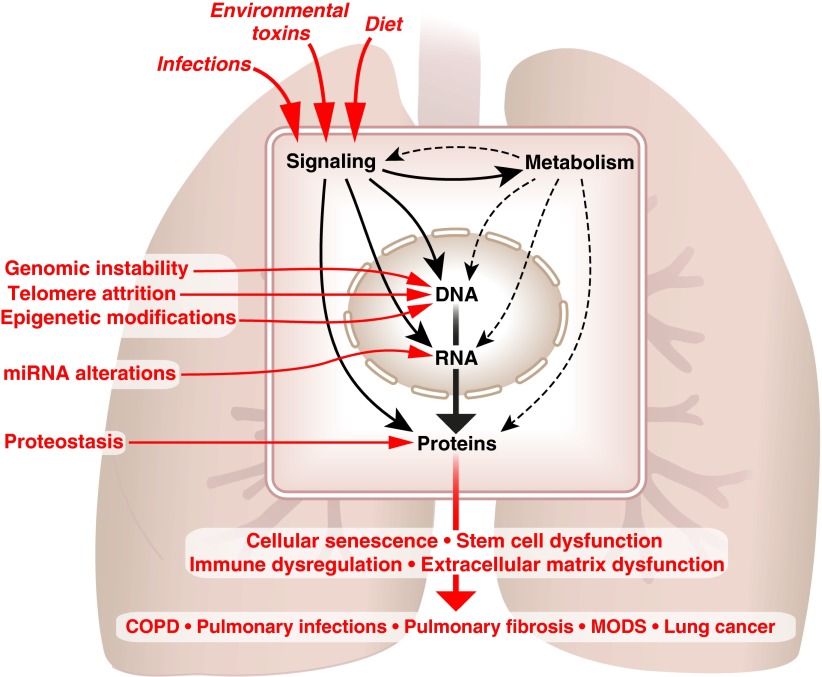
Recognizing the importance of aging in the development of lung disease, the American Thoracic Society (ATS) chose this topic as the basic science core theme for the 2014 annual meeting, held in San Diego, California. The relationship between aging and lung disease, including COPD, emphysema, and others, was discussed in both oral symposiums and poster sessions at the ATS meeting. In this article, we used the input gathered from the participants to develop a broad framework designed to stimulate basic, clinical, and translational research into how the aging process contributes to the genesis and/or progression of lung diseases. A consistent theme that emerged from the ATS meeting was the need to apply novel, systems-based approaches, which can integrate a growing body of genomic, epigenomic, transcriptomic, and proteomic data to explain the relationship between biologic hallmarks of aging, altered lung function, and lung diseases (Figure 2).
Figure 2.
Understanding aging hallmarks at the molecular level. Environmental factors including infections, environmental toxins, and dietary risk factors can act through signaling pathways to directly alter the structure and function of cellular proteins or can affect the proteome indirectly by damaging genomic DNA or modulating gene transcription. In addition, signaling pathways activated in response to environmental stress alter metabolism, which supplies energy and biosynthetic intermediates for genome maintenance, gene transcription, and proteostasis. Some of the hallmarks of aging (genomic instability, telomere attrition, epigenetic alterations, changes in noncoding RNAs including microRNAs and alterations in proteostasis) are known to act primarily at the level of DNA, RNA, or protein; however, their integrated effects on the genome, transcriptome, proteome, and metabolome are not known. A similar approach combining fundamental mechanistic biology with systems-based strategies is needed to understand how aging hallmarks including cellular senescence, stem cell dysregulation, immune dysregulation and extracellular matrix dysfunction contribute to the development of age-related lung disease. COPD = chronic obstructive pulmonary disease; miRNA = micro-RNA; MODS = multiple organ dysfunction syndrome.
Several outstanding reviews in aging biology have been published (16–19). In one of these, the authors identified “tentative hallmarks” characteristic of mammalian aging: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication (16). We have begun to see incorporation of these hallmarks into the studies of lung aging. As shown in Figure 2, genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, and mitochondrial dysfunction directly alter the genome, transcriptome, proteome, or metabolome to drive biological phenotypes including cellular senescence, stem cell dysfunction, and immune dysregulation. We suggest that extracellular matrix (ECM) alterations represent another consequence of the cellular changes of aging with particular relevance to the lung (Figure 2). In this article, we discuss these aging hallmarks to elucidate the biology of aging, with special emphasis on lung pathophysiology. The need to causally link these molecular and cellular hallmarks of aging with the physiological changes seen in age-related lung diseases represents an important challenge in aging research.
Molecular Damage and Subcellular Changes with Aging
The molecular basis for some of the hallmarks of aging has been described; however, the interaction between these molecular events and their contributions to aging phenotypes in the complex milieu of the lung remain obscure. Over the past two decades, we have witnessed the complete descriptions of the human genome, the transcriptome down to the level of an individual cell and, most recently, an early draft of the proteome (20). New and faster bioinformatics allow investigators to develop and organize large datasets to identify molecular signatures and networks that can be examined over the life span at the systems level. Although these studies are in their infancy, current technology allows examination of the interaction between age-related telomere attrition, epigenetic changes (DNA methylation, histone acetylation, and miRNA alterations), and proteostasis at the level of the genome, transcriptome, and proteome, using systems-based approaches (21). A challenge for lung researchers will be understanding how discrete changes at the cellular and molecular levels that underlie these hallmarks of aging affect intracellular, intercellular, and endocrine networks and their dynamic integration into the function of the cell, lung, and the entire organism, respectively (21). Doing so will help us define the complex interrelated events that occur during aging and develop potential new approaches and therapeutics to prevent, diagnose, and treat the many lung diseases that disproportionately affect the elderly.
The Aging Genome
The maintenance of the approximately 3 billion base pairs of DNA in lung cells that constitute the human genome over thousands of cell divisions over a lifetime represents a remarkable achievement. The lung is continuously exposed to environmental stressors and toxins, including air pollution, biomass particles, cigarette smoke, and inhaled occupational toxins, which damage the genome via the generation of oxidative stress and other mechanisms. There is substantial evidence that the efficiency of DNA repair processes declines with age, resulting in the development of genomic instability, a major aging hallmark (16, 22). Nevertheless, our understanding of the consequences of this genomic instability on gene expression and protein function is incomplete.
One of the first molecular mechanisms of aging was discovered by researchers asking how the ends of chromosomal DNA could be reliably replicated, as the mammalian DNA polymerase complex cannot replicate DNA sequences at the termini of chromosomes. In work that was recognized by the Nobel Prize in Physiology in 2009, Elizabeth Blackburn, Carol Greider, and Jack Szostak discovered that to prevent the loss of genetic information during replication, cells are equipped with repetitive DNA sequences that cap the ends of the chromosomes, referred to as telomeres (23). The progressive shortening of telomeres with each cell division limits the replicative life span of the cell (24). Cells in the germline and stem cells express telomerase, which allows the regeneration of telomeres after cell division, extending the replicative life span. Telomere attrition has been implicated in the pathogenesis of chronic lung diseases of aging; in particular, patients with shortened telomeres are at increased risk of developing pulmonary fibrosis (25, 26) and telomere shortening has been noted in patients with emphysema (27), and the syndrome of combined pulmonary fibrosis and emphysema (28). Although telomere shortening has been strongly associated with these disorders, the underlying molecular mechanisms remain obscure. Interestingly, shortening of telomeres does not affect the fibrotic response after an acute lung injury in a commonly used animal model of fibrosis (29, 30). Researchers are challenged to understand the specific lung cell types in which telomere attrition is limiting for renewal, and the impact of telomerase-related cellular phenotypes on the histopathological, morphological, and physiological changes seen in chronic lung diseases.
Modification of the Transcriptome during Aging
All of the cells in the body contain the same complement of DNA, yet the phenotype of each cell differs substantially. These changes are in part attributable to epigenetic alterations, which are mediated by DNA methylation and histone modifications through acetylation (histone acetyltransferase) and deacetylation (histone deacetylase), and noncoding RNAs such as micro-RNAs. Epigenetic changes can be induced by environmental factors, many of which have been associated with the development of lung disease including diet, cigarette smoke, and air pollution exposure. Some epigenetic changes may be long lasting and can be passed on to daughter cells, thereby providing a mechanism by which environmental exposures early in life, even in utero, can influence the pattern of gene expression decades later or in offspring of the exposed individual. Aging is associated with marked alterations in gene expression (31, 32), which is at least partly attributable to epigenetic alterations (13). For example, pioneering work in stem cell biology is providing hints that the accumulation of epigenetic changes in resident tissue stem cells might represent an “aging clock” and, even more importantly, that this clock can be manipulated to produce more youthful phenotypes (13, 33, 34). As lung researchers study epigenetic changes in the aging lung, it will be important to understand whether they represent signatures/biomarkers of disease or whether they influence the dynamics of aging and age-associated diseases such as COPD, emphysema, and fibrosis. For example, changes in histone acetylation have been suggested to increase the risk of pulmonary fibrosis and alter the response to therapy in patients with COPD (35–37). Epigenomic studies of other age-related chronic lung diseases are underway, with more recent studies reporting the results of global DNA methylation in patients with IPF (38–40). Understanding the impact of these epigenetic changes on functional networks in the lung and determining whether reversing them could delay lung aging or age-associated lung diseases is an active area of investigation. Certain epigenetics-modifying drugs (e.g., SIRT1 activators) are in development for COPD and some agents currently used in the treatment of COPD may exert their effects by inhibiting histone deacetylase activity (35–37, 41).
Noncoding micro-RNAs recognize specific sequences in mRNA molecules, reducing the expression of the proteins they encode. Each micro-RNA may modulate the expression of hundreds of genes. Increased or decreased expression of micro-RNAs has been implicated in the pathogenesis of both COPD (42–44) and IPF (45–50). Of particular interest is micro-RNA-29, which is down-regulated in IPF (51), and targets a number of genes encoding extracellular matrix proteins while inducing fibroblast proliferation (52). Large-scale sequencing analyses have revealed the presence of hundreds of other noncoding RNAs; determining their function and role in the development of lung disease is an important area of investigation.
Maintenance of the Aging Proteome through Proteostasis
Before the last decade, gene expression was seldom considered beyond the translation of mRNA into protein; however, the goal of gene transcription and the ultimate determinant of cellular phenotype and function is the generation of a functional proteome. This is a massive undertaking requiring the synthesis of close to 6 amino acids per second from 3 million ribosomes in each cell and requiring on average 3,000 ATP molecules for each protein (53). These nascent proteins must be folded and transported to the proper intracellular location to function, and rigorous quality control mechanisms must be present to remove proteins that are improperly synthesized or folded, aggregated, or damaged. This generation and destruction of proteins is dynamically regulated through a series of interconnected pathways that collectively maintain protein homeostasis, referred to as “proteostasis” (54). Proteostasis begins during nascent protein synthesis on the ribosome, where a coordinated system of molecular chaperones ensures proper folding to achieve the native state. Cytosolic and compartmentalized (exocytic and endocytic) pathways are managed by distinct proteostasis networks reflecting their roles in cell, tissue, and host biology. With aging, some proteins are mistranslated, misfolded, or incorrectly trafficked and their function is compromised either spontaneously or in response to posttranslational modifications induced by environmental or intrinsic metabolic stressors (55). These proteins are sequestered and targeted for degradation through three major protein degradation hubs in the cell: the ubiquitin–proteasome system (UPS), cytosolic autophagy pathways, and the compartmentalized lysosomal pathway, the latter serving as the ultimate end point of autophagy. Failure of these pathways can result in the accumulation of misfolded or damaged proteins, which can compete with nascent proteins for folding chaperones and form toxic aggregates. Environmental factors, including cigarette smoke or air pollution exposure, and diet, either directly or via changes in metabolism, can accelerate protein damage and induce proteostatic stress.
Disordered proteostasis plays a fundamental role in neurodegeneration during aging and is becoming an active area of investigation in the lung (56). The function of the proteasome, which is responsible for the ubiquitin-mediated destruction of proteins, may be impaired by environmental challenges, disrupting proteostasis and contributing to COPD pathogenesis (57). Consistent with these findings, the function of the UPS declines during aging and may contribute to the development of disease (58). The activation of autophagy pathways has been implicated in the life span–extending effects of nutrient deprivation; paradoxically, increased autophagy has been shown to contribute to the development of COPD (59) and acute lung injury (60), whereas decreased autophagy has been reported in IPF (61). Genetic mutations in the cystic fibrosis transmembrane conductance regulator that underlie the development of cystic fibrosis result in improper folding and trafficking of the protein (62). Similarly, mutations in the gene encoding α1-antitrypsin result in the accumulation of toxic intracellular aggregates (63). Mutations in surfactant protein C that result in the accumulation of misfolded protein, and defects in intracellular trafficking pathways in patients with Hermansky–Pudlak syndrome, have been shown to play a critical role in familial fibrotic disorders (64). In each of these examples, chaperone and degradative systems attempt to maintain proteostasis, which may be overwhelmed by additional challenges imposed by environmental stressors such as cigarette smoke, suggesting a possible genetic and environmental age limit for proteostasis management. Consistent with this view, stress responses designed to combat the accumulation of misfolded proteins in the aging cell, collectively referred to as the “heat shock response,” were suggested to paradoxically worsen the trapping of slightly misfolded or nonfunctional proteins, creating a vicious cycle of proteostatic stress and functional decline (65). The publication of the draft human proteome and ongoing technological advances in proteomics will allow investigators to systematically examine changes to the proteome and its response to stress with advancing age (20).
Metabolomics and Aging
The synthesis and maintenance of the genome, transcriptome, and proteome require high-energy intermediates and biosynthetic substrates provided by a group of biochemical pathways collectively referred to as metabolism (66). In the genomic era, metabolism has been considered to play a passive role relative to signaling, gene transcription, and cellular function through the production of substrates and energy. It is increasingly recognized, however, that many environmental factors that drive disease pathogenesis, for example, diet and exercise, act primarily by changing the activity of metabolic pathways. Indeed, many of the interventions that prolong the life span in model organisms activate signaling through metabolic pathways. In mice, manipulating the levels of dietary folate can alter the level of gene methylation (discussed previously) to cause observable phenotypes. These results suggest that alterations in metabolism might play a primary role in the onset and development of age-related lung disease.
Mitochondria serve as metabolic hubs of the cell, providing ATP and metabolic intermediates required for DNA, RNA, and amino acid synthesis, and as signaling organelles important for cellular adaptation to environmental challenges. Changes in mitochondrial function have been implicated in regulating the life span of model organisms (14). Aging is associated with a decline in the number of mitochondria, mitochondrial DNA copy number, and mitochondrial protein levels in rodents and humans (67–71). This may lead to a loss of mitochondrial capacity and place a metabolic limit on cellular function, resulting in increased susceptibility to catastrophic metabolic failure in response to genomic and proteomic stress, thereby contributing to lung frailty during aging. Other investigators have challenged this paradigm, suggesting that reduced mitochondrial capacity is an adaptive strategy undertaken by the cell to create an imbalance between mitochondrial proteins encoded by the nuclear and mitochondrial DNA and/or promote the generation of nanomolar concentrations of reactive oxygen species to induce regenerative or reparative processes (72, 73). A possible resolution comes from the observation that both reduced and increased mitochondrial capacity can activate the mitochondrial unfolded protein response, a coordinated stress response that activates transcription of nuclear DNA–encoded mitochondrial chaperones to preserve proteostasis and resilience (73). A more detailed understanding of the impact of the complex metabolic changes that occur with aging on subcellular, cellular, and organismal genomic and proteomic signaling networks may explain why some therapies, for example, dietary antioxidants, have failed to show direct benefit, or may even be harmful in the prevention and treatment of age-related lung disease.
Cellular Phenotypes of Aging
Environmental factors and changes in gene expression alter the proteome and metabolome over the life span to induce profound changes in cellular phenotypes that are strongly associated with lung aging. These cellular phenotypes include cellular senescence, stem cell dysfunction, immune dysregulation, and ECM alterations among others. The molecular pathways that drive many of these phenotypes have been described in detail; however, we are only beginning to understand how these processes contribute to age-related lung disease in the context of intracellular and intercellular functional and signaling networks.
Cellular Senescence and Aging
Cellular senescence is broadly defined as a state of stable growth arrest in combination with distinctive phenotypic changes that include profound alterations in chromatin and in the secretome (74, 75). A number of stimuli, both intrinsic and extrinsic, can mediate senescence via well-described signaling networks that converge on tumor suppressor pathways to induce stable cell cycle arrest (75). There is an emerging view that cellular senescence may mediate beneficial or detrimental effects depending on its contextual roles in embryogenesis, tissue repair, tumor suppression, aging, and age-related diseases (74, 75). There is evidence for senescence of a variety of cell types in lungs of patients with both emphysema (76, 77) and IPF (78, 79). The differences in tissue remodeling in these clinical syndromes may ultimately be dependent on the specific cell types involved (80), or on the resulting fate of senescent cells, for example, apoptosis (81) versus apoptosis resistance (79).
Stem Cells and Aging
The maintenance of tissues/organs and their function are intricately coupled to innate regenerative processes that maintain not only proper cell numbers (homeostasis), but also repair and replace damaged cells after injury. Evidence suggests that the regenerative potential in most tissues is dictated by functional stem and progenitor cells, which respond to cues provided by the tissue microenvironment to restore tissue integrity and function. The importance of the stem cell niche in the development of aging phenotypes and the susceptibility of tissues and organs to disease is increasingly recognized and has been the subject of several reviews (13, 19). It is hypothesized that during aging, the maintenance and/or restorative function of the stem cell pools progressively declines, perhaps via the accumulation of epigenetic marks (13). This concept of stem cell exhaustion has been suggested to play a role in age-related diseases including emphysema and IPF; however, the evidence is not definitive (80, 82). Several groups of investigators have worked to identify stem cell populations in the lung and determine their role in tissue homeostasis and function during lung aging and disease. Although the low turnover rate of cells within the mouse lung makes these studies challenging, these investigators have identified a hierarchically arranged system of partially differentiated self-renewing cells scattered throughout the airways and the distal lung epithelium (83) including basal cells in the trachea and bronchi (84) and type 2 cells in the alveolar compartment (85, 86). In addition, investigators have identified a population of lung-resident mesenchymal stem cells (MSCs) (87, 88), which may modulate epithelial stem cell behavior via interactions in stem cell niches (89) and serve as stromal cell progenitors for tissue repair. At present, the precise identity and/or location of MSCs and other mesenchymal progenitors are unclear, even in the mouse lung, and little is known about the location, function, and markers of stem cell pools in the human lung. Emerging data suggest that mesenchymal cells/fibroblasts in fibroblastic foci in lungs of patients with IPF display a senescent phenotype, and this change may contribute to the persistent fibrosis observed in the lungs of aged injured mice (79). Identification of factors or signals that modulate stem/progenitor cell proliferation and quiescence in both the epithelial and mesenchymal compartments would be invaluable for the design of stem cell–based therapies to restore lung resilience and ameliorate lung disease.
Immune Dysregulation and Aging
Aging introduces alterations in both innate and adaptive immunity that negatively impact cellular defense mechanisms against pathogens and environmental insults. Innate immune responses such as recognition of bacterial antigens through Toll-like receptors are blunted (90), and the chemotaxis and phagocytic capacity of neutrophils and macrophages is reduced (91). Abnormalities in macrophage polarization (92) and bioenergetics can lead to dysregulated inflammation. Adaptive immune abnormalities are seen in both T and B cells (93). Antigen-presenting cells such as dendritic cells demonstrate poor homing and migration during a viral challenge such as influenza; both the quality and quantity of antibody production to immunizations are inhibited (94, 95).
Immune dysregulation in aging may also be characterized by nonspecific inflammation in the absence of an antigenic challenge; this is often associated with an increase in circulating cytokines such as tumor necrosis factor-α, IL-β, and IL-6 and has been described as inflamm-aging (96). Inflamm-aging may represent part of the spectrum of the senescence-associated secretory phenotype that may exert a profound influence on neighboring cells (97). It has been suggested that the chronic low-level inflammation seen in the elderly might explain the increased burden of disease in elderly patients with asthma (98). Further studies are required to determine the specific roles for inflamm-aging in chronic lung diseases associated with aging, and the role of immunosenescence in the impaired responses to infections in the elderly.
Extracellular Matrix Changes during Aging
Aging is known to be associated with changes in the ECM and the extracellular proteome in almost all organs (99), although the precise alterations and their specific roles in disorders of tissue remodeling in the lung are largely unknown. Most chronic lung diseases, in particular COPD and IPF, are characterized by alterations in the expression, deposition, degradation, and/or turnover of the ECM (100). Initially considered an end consequence of tissue injury, ECM remodeling is now recognized to occur early after lung injury, and is proposed to render the host susceptible to disrepair and drive disease progression (51, 101). This is possible because lung cells express surface receptors (e.g., integrins) capable of binding ligands in the ECM to activate biochemical and biomechanical signals that regulate gene expression and influence biological processes ranging from cellular proliferation and differentiation to inflammation, fibrogenesis, and carcinogenesis.
The aging lung is characterized by higher levels of collagen deposition with decreased elastin expression, among other findings, which promulgated the long-held view of “senile emphysema” (102, 103). Studies in decellularized human lungs and in aging rodent lungs confirm alterations in the expression of collagens, fibronectin, and matrix metalloproteinases (104, 105). Aging-related biochemical changes of the ECM may involve differential rates of collagen fiber biosynthesis, enzymatic or nonenzymatic cross-linking reactions, and turnover of ECM components. Such changes will alter the biomechanical and topographic properties of the ECM, resulting in aberrant cell signaling and phenotypes. Studies designed to evaluate the relative contribution of distinct ECM components to tissue physiology (e.g., tension) and biological processes during aging and age-related disease should be pursued.
Challenges in Studying the Aging Lung
As chronological aging begins at birth, the first challenge that aging investigators encounter is deciding when to begin looking for age-related functional and cellular/molecular changes in complex pulmonary systems. Although studies focused on developmental pathways have provided remarkable insights, they should be complemented by studies conducted in aged organisms. Another challenge is identifying experimentally tractable model systems for the study of lung aging. Invertebrate models, with life spans measured in days or weeks, have proven invaluable for identifying the molecular underpinnings of aging. Although the usefulness of rodent models is limited, the use of murine models in particular offers the opportunity to genetically examine the importance of age-related cellular/molecular pathways relevant to human aging. These and other aged mammalian models are necessary to determine whether the age-related decline in lung function and the increased severity of lung disease in older mammals are driven by stochastic processes that develop dysynchronously in different organs in response to accumulating environmental damage, or by coordinated, genetically programmed largely synchronous process as described in model organisms (106). In recognition of the cost and time needed to age animals for studies in the laboratory, the National Institute on Aging (NIA) maintains several biological and data informatics resources, including aged rodent colonies, an aged rodent tissue bank, and a nonhuman primate tissue bank. These resources are available for NIA- and NIH-supported investigators (http://www.nia.nih.gov/research/scientific-resources). Finally, researchers are faced with the challenge of integrating aging pathways or hallmarks identified in cells, tissues, and model systems into a conceptual framework to identify common key nodes in aging biology that can be targeted for the development of therapeutics to prevent or treat age-related lung disease.
Conclusions
Aging is a major risk factor for the development of virtually every lung disease. Many of the important hallmarks of aging are found in the aging lung, and an increasing body of evidence suggests that these changes contribute to the high incidence of lung diseases in the elderly. In this era of unprecedented technology framed by the perspectives of personalized medicine, we are presented with both the opportunity and challenge of integrating the hallmarks of aging to define common aging and disease-relevant pathways in the lung. Newly evolving technologies and informatics tools allow for studies of the genome, transcriptome, and proteome at the resolution of single cells and their associated ECM over the life span. These tools will also allow us to link aging hallmarks with morphological, metabolic, and cellular changes that impact lung physiology. More importantly, these tools offer the promise of translating findings in isolated cells and mice to the study of the intact lungs of elderly and diseased patients in vivo. It is possible to envision a future in which these advancements will become the framework for devising new strategies to prevent, diagnose, and treat lung diseases that impact the life span and health span of our aging population.
Acknowledgments
Acknowledgment
Serge Adnot, Hôpital Henri Mondor, Créteil, France; Alvar Agustí, Universitat de Barcelona, Barcelona, Spain; Mary Armanios, Johns Hopkins, Baltimore, MD; Hoeke Baarsma, Helmholtz Center Munich, Munich, Germany; Christina Barkauskas, Duke University, Durham, NC; Serpil Erzurum, Cleveland Clinic, Cleveland, OH; Konstantin Birukov, University of Chicago, Chicago, IL; Jorge Boczkowski, Hôpital Henri Mondor, Créteil, France; Marta Bueno, University of Pittsburgh Medical Center, Pittsburgh, PA; Hillary Coller, University of California Los Angeles, Los Angeles, CA; Kristina Crothers, University of Washington, Seattle, WA; Kelvin Davies, Medical University of South Carolina, Charleston, SC; Deepak Deshpande, Helmholtz Center Munich, Munich, Germany; Isis Fernandez, Helmholtz Center Munich, Munich, Germany; Kelsa Gabehart, National Jewish Health, Denver, CO; George Garinis, Institute of Molecular Biology and Biotechnology, Heraklion, Greece; Lindsay Godin, University of Minnesota, Minneapolis, MN; Andrew Halayko, University of Manitoba, Winnipeg, Canada; Kevin High, Wake Forest Baptist Medical Center, Winston-Salem, NC; Claude Jourdan LeSaux, University of Texas Health Science Center at San Antonio, San Antonio, TX; Melanie Königshoff, Helmholtz Center Munich, Munich, Germany; Edward Lakatta, NIH, Bethesda, MD; Joseph Lasky, University of Tulane, New Orleans, LA; Patty J. Lee, Yale University, New Haven, CT; Klaus Ley, La Jolla Institute for Allergy & Immunology, La Jolla, CA; Gustavo Matute-Bello, University of Washington, Seattle, WA; Herman Meurs, University of Groningen, Groningen, The Netherlands; Bethany Moore, University of Michigan, Ann Arbor, MI; Ana Mora, University of Pittsburgh Medical Center, Pittsburgh, PA; Gokhan Mutlu, University of Chicago, Chicago, IL; Enid Neptune, Johns Hopkins, Baltimore, MD; Mitchell Olman, Cleveland Clinic, Cleveland, OH; Lawrence Ostrowski, University of North Carolina, Chapel Hill, NC; Angela Panoskaltsis-Mortari, University of Minnesota, Minneapolis, MN; Sem Phan, University of Michigan, Ann Arbor, MI; Kent Pinkerton, University of California at Davis, Davis, CA; Lisa Postow, NIH, Bethesda, MD; Irfan Rahman, University of Rochester, Rochester, NY; Joe Ramsdell, University of California at San Diego, San Diego, CA; Mauricio Rojas, University of Pittsburgh Medical Center, Pittsburgh, PA; Jesse Roman, University of Louisville, Louisville, KY; Ivan Rosas, Brigham and Women’s Hospital, Harvard, Cambridge, MA; Cecilia G. Sanchez, University of Tulane, New Orleans, LA; Lynn Schnapp, Medical University of South Carolina, Charleston, SC; William Sonntag, University of Oklahoma Health Science Center, Oklahoma City, OK; Anup Srivastava, Yale University, New Haven, CT; Viranuj Sueblinvong, Emory University, Atlanta, GA; Carlos Vaz Fragoso, Yale University, New Haven, CT.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201410-1876PP on January 15, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Vincent GK, Velkoff VA.The next four decades: the older population in the United States: 2010–2050. Population estimates and projections. Washington, DC: U.S. Department of Commerce Census Bureau; 2010
- 2.United Nations, Department of Economic and Social AffairsPopulation Division. World population prospects: the 2012 revision, key findings and advance tables. Working paper no. ESA/P/WP.227. New York: United Nations; 2013
- 3.Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung HC. Deaths: final data for 2009. Natl Vital Stat Rep. 2011;60:1–116. [PubMed] [Google Scholar]
- 4.Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. COPD surveillance—United States, 1999-2011. Chest. 2013;144:284–305. doi: 10.1378/chest.13-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan V, Angus DC, Griffin MF, Clermont G, Scott Watson R, Linde-Zwirble WT. Hospitalized community-acquired pneumonia in the elderly: age- and sex-related patterns of care and outcome in the United States. Am J Respir Crit Care Med. 2002;165:766–772. doi: 10.1164/ajrccm.165.6.2103038. [DOI] [PubMed] [Google Scholar]
- 6.Ortiz JR, Neuzil KM, Rue TC, Zhou H, Shay DK, Cheng P-Y, Cooke CR, Goss CH. Population-based incidence estimates of influenza-associated respiratory failure hospitalizations, 2003 to 2009. Am J Respir Crit Care Med. 2013;188:710–715. doi: 10.1164/rccm.201212-2341OC. [DOI] [PubMed] [Google Scholar]
- 7.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 8.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rota PA, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology. 1990;175:59–68. doi: 10.1016/0042-6822(90)90186-u. [DOI] [PubMed] [Google Scholar]
- 10.Tsai CL, Lee WY, Hanania NA, Camargo CA., Jr Age-related differences in clinical outcomes for acute asthma in the United States, 2006–2008. J Allergy Clin Immunol. 2012;129:1252–1258.e1. doi: 10.1016/j.jaci.2012.01.061. [DOI] [PubMed] [Google Scholar]
- 11.Paul Glezen W, Schmier JK, Kuehn CM, Ryan KJ, Oxford J. The burden of influenza B: a structured literature review. Am J Public Health. 2013;103:e43–e51. doi: 10.2105/AJPH.2012.301137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finch CE, Ruvkun G. The genetics of aging. Annu Rev Genomics Hum Genet. 2001;2:435–462. doi: 10.1146/annurev.genom.2.1.435. [DOI] [PubMed] [Google Scholar]
- 13.Rando TA, Chang HY. Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock. Cell. 2012;148:46–57. doi: 10.1016/j.cell.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morimoto RI, Cuervo AM. Proteostasis and the aging proteome in health and disease. J Gerontol A Biol Sci Med Sci. 2014;69:S33–S38. doi: 10.1093/gerona/glu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowery EM, Brubaker AL, Kuhlmann E, Kovacs EJ. The aging lung. Clin Interv Aging. 2013;8:1489–1496. doi: 10.2147/CIA.S51152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 19.Oh J, Lee YD, Wagers AJ. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 2014;20:870–880. doi: 10.1038/nm.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim M-S, Pinto SM, Getnet D, Nirujogi RS, Manda SS, Chaerkady R, Madugundu AK, Kelkar DS, Isserlin R, Jain S, et al. A draft map of the human proteome. Nature. 2014;509:575–581. doi: 10.1038/nature13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valdes AM, Glass D, Spector TD. Omics technologies and the study of human ageing. Nat Rev Genet. 2013;14:601–607. doi: 10.1038/nrg3553. [DOI] [PubMed] [Google Scholar]
- 22.Vijg J, Suh Y. Genome instability and aging. Annu Rev Physiol. 2013;75:645–668. doi: 10.1146/annurev-physiol-030212-183715. [DOI] [PubMed] [Google Scholar]
- 23.Szostak JW, Blackburn EH. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982;29:245–255. doi: 10.1016/0092-8674(82)90109-x. [DOI] [PubMed] [Google Scholar]
- 24.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88:557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 25.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, Vulto I, Xie M, Qi X, Tuder RM, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA. 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alder JK, Guo N, Kembou F, Parry EM, Anderson CJ, Gorgy AI, Walsh MF, Sussan T, Biswal S, Mitzner W, et al. Telomere length is a determinant of emphysema susceptibility. Am J Respir Crit Care Med. 2011;184:904–912. doi: 10.1164/rccm.201103-0520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunes H, Monnet I, Kannengiesser C, Uzunhan Y, Valeyre D, Kambouchner M, Naccache JM. Is telomeropathy the explanation for combined pulmonary fibrosis and emphysema syndrome?: report of a family with TERT mutation. Am J Respir Crit Care Med. 2014;189:753–754. doi: 10.1164/rccm.201309-1724LE. [DOI] [PubMed] [Google Scholar]
- 29.Liu T, Ullenbruch M, Young Choi Y, Yu H, Ding L, Xaubet A, Pereda J, Feghali-Bostwick CA, Bitterman PB, Henke CA, et al. Telomerase and telomere length in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2013;49:260–268. doi: 10.1165/rcmb.2012-0514OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degryse AL, Xu XC, Newman JL, Mitchell DB, Tanjore H, Polosukhin VV, Jones BR, McMahon FB, Gleaves LA, Phillips JA, III, et al. Telomerase deficiency does not alter bleomycin-induced fibrosis in mice. Exp Lung Res. 2012;38:124–134. doi: 10.3109/01902148.2012.658148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahar R, Hartmann CH, Rodriguez KA, Denny AD, Busuttil RA, Dollé ME, Calder RB, Chisholm GB, Pollock BH, Klein CA, et al. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature. 2006;441:1011–1014. doi: 10.1038/nature04844. [DOI] [PubMed] [Google Scholar]
- 32.Brunet A, Berger SL. Epigenetics of aging and aging-related disease. J Gerontol A Biol Sci Med Sci. 2014;69:S17–S20. doi: 10.1093/gerona/glu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, Kung S, Jiang KP, Conboy IM. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun. 2014;5:4082. doi: 10.1038/ncomms5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes PJ. Role of HDAC2 in the pathophysiology of COPD. Annu Rev Physiol. 2009;71:451–464. doi: 10.1146/annurev.physiol.010908.163257. [DOI] [PubMed] [Google Scholar]
- 36.Mizuno S, Yasuo M, Bogaard HJ, Kraskauskas D, Natarajan R, Voelkel NF. Inhibition of histone deacetylase causes emphysema. Am J Physiol Lung Cell Mol Physiol. 2011;300:L402–L413. doi: 10.1152/ajplung.00207.2010. [DOI] [PubMed] [Google Scholar]
- 37.Sanders YY, Hagood JS, Liu H, Zhang W, Ambalavanan N, Thannickal VJ. Histone deacetylase inhibition promotes fibroblast apoptosis and ameliorates pulmonary fibrosis in mice. Eur Respir J. 2014;43:1448–1458. doi: 10.1183/09031936.00095113. [DOI] [PubMed] [Google Scholar]
- 38.Sanders YY, Ambalavanan N, Halloran B, Zhang X, Liu H, Crossman DK, Bray M, Zhang K, Thannickal VJ, Hagood JS. Altered DNA methylation profile in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:525–535. doi: 10.1164/rccm.201201-0077OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabinovich EI, Kapetanaki MG, Steinfeld I, Gibson KF, Pandit KV, Yu G, Yakhini Z, Kaminski N. Global methylation patterns in idiopathic pulmonary fibrosis. PLoS ONE. 2012;7:e33770. doi: 10.1371/journal.pone.0033770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabinovich EI, Selman M, Kaminski N. Epigenomics of idiopathic pulmonary fibrosis: evaluating the first steps. Am J Respir Crit Care Med. 2012;186:473–475. doi: 10.1164/rccm.201208-1350ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao H, Chung S, Hwang JW, Rajendrasozhan S, Sundar IK, Dean DA, McBurney MW, Guarente L, Gu W, Rönty M, et al. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest. 2012;122:2032–2045. doi: 10.1172/JCI60132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassan T, Carroll TP, Buckley PG, Cummins R, O’Neill SJ, McElvaney NG, Greene CM. miR-199a-5p silencing regulates the unfolded protein response in chronic obstructive pulmonary disease and α1-antitrypsin deficiency. Am J Respir Crit Care Med. 2014;189:263–273. doi: 10.1164/rccm.201306-1151OC. [DOI] [PubMed] [Google Scholar]
- 43.Christenson SA, Brandsma CA, Campbell JD, Knight DA, Pechkovsky DV, Hogg JC, Timens W, Postma DS, Lenburg M, Spira A. miR-638 regulates gene expression networks associated with emphysematous lung destruction. Genome Med. 2013;5:114. doi: 10.1186/gm519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savarimuthu Francis SM, Davidson MR, Tan ME, Wright CM, Clarke BE, Duhig EE, Bowman RV, Hayward NK, Fong KM, Yang IA. MicroRNA-34c is associated with emphysema severity and modulates SERPINE1 expression. BMC Genomics. 2014;15:88. doi: 10.1186/1471-2164-15-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dakhlallah D, Batte K, Wang Y, Cantemir-Stone CZ, Yan P, Nuovo G, Mikhail A, Hitchcock CL, Wright VP, Nana-Sinkam SP, et al. Epigenetic regulation of miR-17∼92 contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2013;187:397–405. doi: 10.1164/rccm.201205-0888OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milosevic J, Pandit K, Magister M, Rabinovich E, Ellwanger DC, Yu G, Vuga LJ, Weksler B, Benos PV, Gibson KF, et al. Profibrotic role of miR-154 in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;47:879–887. doi: 10.1165/rcmb.2011-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardoso WV, Lü J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pandit KV, Corcoran D, Yousef H, Yarlagadda M, Tzouvelekis A, Gibson KF, Konishi K, Yousem SA, Singh M, Handley D, et al. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;182:220–229. doi: 10.1164/rccm.200911-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selman M, Pardo A. Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis. an integral model. Am J Respir Crit Care Med. 2014;189:1161–1172. doi: 10.1164/rccm.201312-2221PP. [DOI] [PubMed] [Google Scholar]
- 51.Parker MW, Rossi D, Peterson M, Smith K, Sikström K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest. 2014;124:1622–1635. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suh EJ, Remillard MY, Legesse-Miller A, Johnson EL, Lemons JM, Chapman TR, Forman JJ, Kojima M, Silberman ES, Coller HA. A microRNA network regulates proliferative timing and extracellular matrix synthesis during cellular quiescence in fibroblasts. Genome Biol. 2012;13:R121. doi: 10.1186/gb-2012-13-12-r121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolff S, Weissman JS, Dillin A. Differential scales of protein quality control. Cell. 2014;157:52–64. doi: 10.1016/j.cell.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 55.Bouchecareilh M, Balch WE. Proteostasis: a new therapeutic paradigm for pulmonary disease. Proc Am Thorac Soc. 2011;8:189–195. doi: 10.1513/pats.201008-055MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balch WE, Sznajder JI, Budinger S, Finley D, Laposky AD, Cuervo AM, Benjamin IJ, Barreiro E, Morimoto RI, Postow L, et al. Malfolded protein structure and proteostasis in lung diseases. Am J Respir Crit Care Med. 2014;189:96–103. doi: 10.1164/rccm.201306-1164WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meiners S, Keller IE, Semren N, Caniard A. Regulation of the proteasome: evaluating the lung proteasome as a new therapeutic target. Antioxid Redox Signal. 2014;21:2364–2382. doi: 10.1089/ars.2013.5798. [DOI] [PubMed] [Google Scholar]
- 58.Kevei É, Hoppe T. Ubiquitin sets the timer: impacts on aging and longevity. Nat Struct Mol Biol. 2014;21:290–292. doi: 10.1038/nsmb.2806. [DOI] [PubMed] [Google Scholar]
- 59.Lam HC, Cloonan SM, Bhashyam AR, Haspel JA, Singh A, Sathirapongsasuti JF, Cervo M, Yao H, Chung AL, Mizumura K, et al. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J Clin Invest. 2013;123:5212–5230. doi: 10.1172/JCI69636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun Y, Li C, Shu Y, Ju X, Zou Z, Wang H, Rao S, Guo F, Liu H, Nan W, et al. Inhibition of autophagy ameliorates acute lung injury caused by avian influenza A H5N1 infection. Sci Signal. 2012;5:ra16. doi: 10.1126/scisignal.2001931. [DOI] [PubMed] [Google Scholar]
- 61.Patel AS, Lin L, Geyer A, Haspel JA, An CH, Cao J, Rosas IO, Morse D. Autophagy in idiopathic pulmonary fibrosis. PLoS ONE. 2012;7:e41394. doi: 10.1371/journal.pone.0041394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 63.Bouchecareilh M, Conkright JJ, Balch WE. Proteostasis strategies for restoring α1-antitrypsin deficiency. Proc Am Thorac Soc. 2010;7:415–422. doi: 10.1513/pats.201001-016AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Young LR, Gulleman PM, Bridges JP, Weaver TE, Deutsch GH, Blackwell TS, McCormack FX. The alveolar epithelium determines susceptibility to lung fibrosis in Hermansky-Pudlak syndrome. Am J Respir Crit Care Med. 2012;186:1014–1024. doi: 10.1164/rccm.201207-1206OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roth DM, Hutt DM, Tong J, Bouchecareilh M, Wang N, Seeley T, Dekkers JF, Beekman JM, Garza D, Drew L, et al. Modulation of the maladaptive stress response to manage diseases of protein folding. PLoS Biol. 2014;12:e1001998. doi: 10.1371/journal.pbio.1001998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu Z, Zhai G, Singmann P, He Y, Xu T, Prehn C, Römisch-Margl W, Lattka E, Gieger C, Soranzo N, et al. Human serum metabolic profiles are age dependent. Aging Cell. 2012;11:960–967. doi: 10.1111/j.1474-9726.2012.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herbener GH. A morphometric study of age-dependent changes in mitochondrial population of mouse liver and heart. J Gerontol. 1976;31:8–12. doi: 10.1093/geronj/31.1.8. [DOI] [PubMed] [Google Scholar]
- 68.Stocco DM, Cascarano J, Wilson MA. Quantitation of mitochondrial DNA, RNA, and protein in starved and starved-refed rat liver. J Cell Physiol. 1977;90:295–306. doi: 10.1002/jcp.1040900215. [DOI] [PubMed] [Google Scholar]
- 69.Stocco DM, Hutson JC. Quantitation of mitochondrial DNA and protein in the liver of Fischer 344 rats during aging. J Gerontol. 1978;33:802–809. doi: 10.1093/geronj/33.6.802. [DOI] [PubMed] [Google Scholar]
- 70.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brierley EJ, Johnson MA, James OF, Turnbull DM. Mitochondrial involvement in the ageing process. Facts and controversies. Mol Cell Biochem. 1997;174:325–328. [PubMed] [Google Scholar]
- 72.Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, Williams RW, Auwerx J. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013;497:451–457. doi: 10.1038/nature12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 76.Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med. 2006;174:886–893. doi: 10.1164/rccm.200509-1374OC. [DOI] [PubMed] [Google Scholar]
- 77.Holz O, Zühlke I, Jaksztat E, Müller KC, Welker L, Nakashima M, Diemel KD, Branscheid D, Magnussen H, Jörres RA. Lung fibroblasts from patients with emphysema show a reduced proliferation rate in culture. Eur Respir J. 2004;24:575–579. doi: 10.1183/09031936.04.00143703. [DOI] [PubMed] [Google Scholar]
- 78.Minagawa S, Araya J, Numata T, Nojiri S, Hara H, Yumino Y, Kawaishi M, Odaka M, Morikawa T, Nishimura SL, et al. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-β-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L391–L401. doi: 10.1152/ajplung.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, Thannickal VJ. Reversal of persistent fibrosis in aging by targeting Nox4–Nrf2 redox imbalance. Sci Transl Med. 2014;6:231ra247. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chilosi M, Carloni A, Rossi A, Poletti V. Premature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema. Transl Res. 2013;162:156–173. doi: 10.1016/j.trsl.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 81.Taraseviciene-Stewart L, Voelkel NF. Molecular pathogenesis of emphysema. J Clin Invest. 2008;118:394–402. doi: 10.1172/JCI31811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chilosi M, Doglioni C, Murer B, Poletti V. Epithelial stem cell exhaustion in the pathogenesis of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27:7–18. [PubMed] [Google Scholar]
- 83.Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC, Niklason L, Calle E, Le A, Randell SH, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lama VN, Smith L, Badri L, Flint A, Andrei AC, Murray S, Wang Z, Liao H, Toews GB, Krebsbach PH, et al. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest. 2007;117:989–996. doi: 10.1172/JCI29713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Summer R, Fitzsimmons K, Dwyer D, Murphy J, Fine A. Isolation of an adult mouse lung mesenchymal progenitor cell population. Am J Respir Cell Mol Biol. 2007;37:152–159. doi: 10.1165/rcmb.2006-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Volckaert T, De Langhe S. Lung epithelial stem cells and their niches: Fgf10 takes center stage. Fibrogenesis Tissue Repair. 2014;7:8. doi: 10.1186/1755-1536-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 91.Mariani E, Pulsatelli L, Meneghetti A, Dolzani P, Mazzetti I, Neri S, Ravaglia G, Forti P, Facchini A. Different IL-8 production by T and NK lymphocytes in elderly subjects. Mech Ageing Dev. 2001;122:1383–1395. doi: 10.1016/s0047-6374(01)00270-6. [DOI] [PubMed] [Google Scholar]
- 92.Dace DS, Apte RS. Effect of senescence on macrophage polarization and angiogenesis. Rejuvenation Res. 2008;11:177–185. doi: 10.1089/rej.2007.0614. [DOI] [PubMed] [Google Scholar]
- 93.Zhao J, Zhao J, Legge K, Perlman S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest. 2011;121:4921–4930. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cummins NW, Weaver EA, May SM, Croatt AJ, Foreman O, Kennedy RB, Poland GA, Barry MA, Nath KA, Badley AD. Heme oxygenase-1 regulates the immune response to influenza virus infection and vaccination in aged mice. FASEB J. 2012;26:2911–2918. doi: 10.1096/fj.11-190017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, et al. Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 96.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 97.Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yanez A, Cho SH, Soriano JB, Rosenwasser LJ, Rodrigo GJ, Rabe KF, Peters S, Niimi A, Ledford DK, Katial R, et al. Asthma in the elderly: what we know and what we have yet to know. World Allergy Org J. 2014;7:8. doi: 10.1186/1939-4551-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Robert L. Mechanisms of aging of the extracellular matrix: role of the elastin-laminin receptor. Gerontology. 1998;44:307–317. doi: 10.1159/000022034. [DOI] [PubMed] [Google Scholar]
- 100.Thannickal VJ, Henke CA, Horowitz JC, Noble PW, Roman J, Sime PJ, Zhou Y, Wells RG, White ES, Tschumperlin DJ. Matrix biology of idiopathic pulmonary fibrosis: a workshop report of the national heart, lung, and blood institute. Am J Pathol. 2014;184:1643–1651. doi: 10.1016/j.ajpath.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013;123:1096–1108. doi: 10.1172/JCI66700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.D’Errico A, Scarani P, Colosimo E, Spina M, Grigioni WF, Mancini AM. Changes in the alveolar connective tissue of the ageing lung. An immunohistochemical study. Virchows Arch A Pathol Anat Histopathol. 1989;415:137–144. doi: 10.1007/BF00784351. [DOI] [PubMed] [Google Scholar]
- 103.Faner R, Rojas M, Macnee W, Agustí A. Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:306–313. doi: 10.1164/rccm.201202-0282PP. [DOI] [PubMed] [Google Scholar]
- 104.Sueblinvong V, Neujahr DC, Mills ST, Roser-Page S, Ritzenthaler JD, Guidot D, Rojas M, Roman J. Predisposition for disrepair in the aged lung. Am J Med Sci. 2012;344:41–51. doi: 10.1097/MAJ.0b013e318234c132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186:866–876. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Labbadia J, Morimoto RI. Proteostasis and longevity: when does aging really begin? F1000Prime Rep. 2014;6:7. doi: 10.12703/P6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61:1–117. [PubMed] [Google Scholar]
- 108.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]