Abstract
Preeclampsia is associated with oxidative stress which is suspected to play a role in hypertension, placental ischemia and fetal demise associated with the disease. Various cellular sources of oxidative stress such as neutrophils, monocytes and CD4+T cells have been suggested as culprits in the pathophysiology of preeclampsia. The objective of this study was to examine a role for circulating and placental CD4+T cells in oxidative stress in response to placental ischemia during pregnancy. CD4+T cells and oxidative stress was measured in preeclamptic and normal pregnant women, placental ischemic and normal pregnant rats and normal pregnant recipient rats of placental ischemic CD4+ T cells. Preeclamptic women had significantly increased circulating (p=0.02) and placental CD4+T cells (p=0.0001); lymphocyte secretion of myeloperoxidase (p=0.004); and placental reactive oxygen species (p=0.0004) compared to normal pregnant women. CD4+T cells from placental ischemic rats cause many facets of preeclampsia when injected into normal pregnant recipient rats on gestational day 13. On gestational day 19 blood pressure increased in normal pregnant recipients of placental ischemic CD4+T cells (p=0.002) compared to normal pregnant rats. Similar to preeclamptic patients, CD4+ T cells from placental ischemic rats secreted significantly more myeloperoxidase (p=0.003) and induced oxidative stress in cultured vascular cells (p=0.003) than normal pregnant rat CD4+Tcells. Apocynin, an NADPH inhibitor, attenuated hypertension, and all oxidative stress markers in placental ischemic and normal pregnant recipient rats of placental ischemic CD4+Tcells (p=0.05). These data demonstrate an important role for CD4+T cells in mediating another factor, oxidative stress, to cause hypertension during preeclampsia.
Keywords: Hypertension, Preeclampsia, Oxidative stress, CD4+ T cells, Placental Ischemia
INTRODUCTION
Preeclampsia, defined as new onset hypertension and proteinuria during pregnancy, is one of the leading causes of maternal and perinatal morbidity1, 2. Abnormal trophoblast invasion into the maternal uterine spiral arteries is postulated to be the initiating event in preeclampsia resulting in reduced uterine perfusion pressure to the placenta3. As preeclampsia develops, dysregulation of various immune cells such as monocytes, macrophages, T lymphocytes and endothelial cells occur thus leading to an alteration in various circulating factors that are thought to increase blood pressure and decrease renal excretory function4, 5. One such factor is oxidative stress. Macrophages and neutrophils and proinflammatory T cells are capable of converting molecular oxygen into reactive oxygen species (ROS) in pathological disease states, such as in preeclampsia4, 6. These immune cells, released upon placental ischemia can generate superoxide within the maternal vascular endothelium7. Indeed increased levels of ROS and 8-isoPGF2α, the free form of isoprostane, are found in placentas and in the circulation of women with preeclampsia8, 9, indicating that preeclampsia is accompanied by an increase in pro-oxidant factors. During hypertensive disorders peroxynitrite concentrations10, 11 and ROS are also released by the maternal vascular endothelium and smooth muscle cells (SMC) stimulated by stress or angiotensin II. In the case of preeclampsia, SMC and endothelial cells (HUVECS) in culture have been shown to release increased ROS when stimulated by autoantibodies to the angiotensin II type I receptor (AT1-AA) or by antiangiogenic factors sFlt-112, 13. Therefore, during preeclampsia there are multiple stimulators and pathways of oxidative stress, which are known to be important mediators of endothelial dysfunction and hypertension. However, not many studies have illustrated a direct role for T cells as producers or mediators of oxidative stress during preeclampsia. Many studies have demonstrated, however, an increase in circulating CD4+ T cells in preeclamptic women compared to normal pregnant (NP) women. In addition, this profile has been further characterized by an increase in Thelper 17(Th17) and decrease Tregulatory (Treg) cells. Additionally, women with preeclampsia were found to have decreased mRNA expression of transcription factors directly associated with Treg cells and increased mRNA expression of transcription factors directly associated with Th17 cells in both the blood and the decidua when compared to normal pregnant women14. It is believed that placental ischemia stimulates factors circulating in preeclampsia that cause much of the disease pathology. However, no studies have identified if this altered circulating T cell ratio is reflective of that occurring within the placenta or if it is associated with elevated ROS.
Our laboratory has recently demonstrated in an animal model of reduced uterine perfusion pressure (RUPP), that markers of oxidative stress and circulating CD4+ T cells, are increased compared to NP rats, similar to what is seen in preeclamptic women15, 16. Previous work has shown that malondialdehyde, superoxide and myeloperoxidase activity is increased in RUPP placentas similar to what is observed in placentas from women with preeclampsia17, 18. We have further shown that adoptive transfer of placental ischemia stimulated CD4+ T cells into NP recipient rats, resulted in hypertension, increases in inflammatory cytokines, AT1-AA and endothelial activation in NP recipient rats15, 16, 19, 20. One mechanism whereby hypertension and oxidative stress may be increased during placental ischemia is via T cells21, 22.
Although, antioxidant therapy has shown not to benefit mother and baby during preeclampsia, these studies only supplemented mothers and did not attempt to target cellular sources to reduce oxidative free radicals from being released23, 24. It is understandable that such studies may be difficult to perform in a clinical cohort, therefore, it is important to ascertain such knowledge from reliable and repeatable animal models of disease. Therefore the objective of this study was 2-fold, first to determine if placental CD4+ T cells have the same profile as circulating T cells from preeclamptic women and whether or not they are important mediators of oxidative stress. Secondly was to determine if placental ischemic stimulated CD4+ T cells are a source of oxidative stress and if specific therapy targeting reactive oxygen species could reduce blood pressure or T cells in animal models of preeclampsia.
MATERIALS AND METHODS
Protocol 1: Human study subjects
Women diagnosed with preeclampsia25 or as having a normal pregnancy at the University of Mississippi Medical Center (UMMC) were consented and enrolled in this study approved by the Institutional Review Board. All participants were scheduled for caesarian section delivery, after which placentas were immediately collected. Plasma was collected for analysis of immune cells.
Isolation of CD4+ placental lymphocytes
Placental white blood cells were isolated from the placenta and used for the next set of studies (Supplemental Methods). CD4+ lymphocytes were isolated from placentas to determine their role in contributing to oxidative stress. Briefly, lymphocytes were resuspended in degassed MACS running buffer (Miltenyi Biotec) according to manufacturer's directions (Supplemental Methods). The resulting CD4+ lymphocytes were cultured overnight at standard atmospheric conditions in lymphocyte media.
Determination of CD4+ Treg and Th17 lymphocytes
Flow cytometry was used to measure CD4+ T cell subpopulations in lymphocytes isolated from placentas and whole blood samples. To determine if Treg and Th17 cells were decreased and increased, respectively, we measured markers for Treg cells (CD4+CD25High+FOXP3+) and Th17 (CD4+CD25−RORγ+) cells (Supplemental Methods; Gating strategies are in S1 & S2).
Determination of oxidative stress
Superoxide production in the placenta was measured using the lucigenin technique21, 26 (Supplemental Methods). Each sample was repeated 5 times and the average used for data transformation. The protein concentration was measured using a protein assay. Data are expressed as RLU/min/mg protein.
Myeloperoxidase (MPO) was measured in plasma and cell culture supernatants according to manufacturer's directions (RnD Systems).
To determine if placentas and placental CD4+ T lymphocytes could cause an increase in vascular ROS production, previously collected tissue and cell culture media was placed over human umbilical vein endothelial cells (HUVECs) and oxidative stress was measured via dihydroethidium (DHE) staining (Supplemental Methods).
Protocol 2: Animal study
All animal studies were performed in 250g timed-pregnant Sprague Dawley rats (Harlan, Indianapolis, IN). Animals were housed in a temperature controlled room with a 12:12 light:dark cycle. All experimental procedures were in accordance with the National Institutes of Health guidelines for use and care of animals and approved by the Institutional Animal Care and Use Committee at UMMC.
Reduced Uterine Perfusion Pressure (RUPP rat model of preeclampsia)
Reduction in uterine perfusion pressure was performed on gestational day (GD)14 as previously described15, 16, 27 (Supplemental Methods). Mean Arterial Pressure (MAP) was measured via the carotid artery (Supplemental Methods), blood, placentas, urine and spleens were isolated and pups collected and weighed on GD19.
Placental explants were cultured as previously described for 24hrs, after which culture media was collected28. To determine if placentas and splenic CD4+ T lymphocytes from RUPP rats could cause an increase in vascular ROS production, we repeated the HUVEC and DHE studies described above.
Adoptive transfer of RUPP CD4+ T cells into NP recipient rats
The current study was designed to determine the effect of RUPP CD4+ T cells to increase oxidative stress as a mechanism of hypertension in NP recipient rats. As such CD4+ T cells were isolated from NP and RUPP spleens as previously described16, 20, 28 (Supplemental methods). The four groups examined were: NP rats (n=7); NP rats injected with NP CD4+ T cells (NP+NPTCells; n=3); RUPP rats (n=8) and NP rats injected with CD4+ RUPP T cells (NP+RPTCells; n=6). As adoptive transfer of NP or RUPP CD4+ T cells into virgin rats has been shown to not increase blood pressure, these groups were not examined16.
Isolation and measurement of rat lymphocytes from whole blood
1mL of whole blood was mixed with 9mL's of RPMI 1640 and placed over a Lymphoprep® gradient and centrifuged at 1200RPM for 25min. Flow cytometry was used to measure CD4+ T cells after cells were stained with fluorescein isothyiocyanate conjugated mouse anti-rat CD4 (BD Pharmingen)16. As we have previously reported that experimental placental ischemia does not significantly increase circulating CD8+ T cells we did not measure them in the current study28.
Determination of oxidative stress
MPO was measured in CD4+ T cell culture supernatants and 15-isoprostane F2t, a marker of in vivo oxidative stress was measured in urine, according to manufacturer's protocol (RnD Systems; Oxford Biomedical Research, Rochester Hills, MI). Superoxide production in the placenta and renal cortex was measured using lucigenin techniques21, 26.
The effect of anti-oxidant therapy on CD4+ T cell induced hypertension
As the generation of oxygen free radicals and NADPH oxidase activity are contributors to hypertension8, 17, 21, 26, 29, we sought to determine if suppression of either of these mechanisms affects hypertension in response to adoptive transfer of RUPP stimulated CD4+ T cells. The superoxide dismutase mimetic Tempol (TEM; Sigma) and the antioxidant, apocynin (APO; Sigma) were utilized. On GD13 Tempol (5mg/kg/day) or apocynin (100mg/kg/day) were administered in the drinking water ad libitum of pregnant rats until GD19. The groups examined were as follows: NP+TEM (n=6); NP+NPTCells+TEM (n=3); RUPP+TEM (n=4); RPTCells+TEM (n=6) and NP+APO (n=6); NP+NPTCells+APO (n=2); RUPP+APO (n=4); NP+RPTCells+APO (n=6). MAP and tissues were measured/collected in all groups of pregnant rats on GD19.
Statistical Analysis
All data are expressed as mean±standard error mean. Differences between control and experimental groups were analyzed via one-way analysis of variance and post-hoc analyses were obtained through Bonferroni post-hoc test. Student's Ttest was used to compare groups treated with Tempol or APO to their untreated groups. For confocal studies, three separate frames per experimental condition were taken per experiment; n=6 per experimental condition. All conditions of image collections, including gain, offset, pinhole and laser power were identical among all samples. Values of p < 0.05 were considered significant.
RESULTS
Protocol 1: Human study
Twenty women undergoing scheduled cesarean section were enrolled in the current study. There was not a significant difference between preeclamptic women (n=10) and NP women (n=10) in maternal age at delivery (p=0.19; Table 1) or in body mass index at admission (p=0.583; Table 1). Women with preeclampsia delivered at a significantly earlier gestational age compared to NP women (p=0.0001) and had significantly smaller babies (p=0.0001, Table 1). MAP in women with preeclampsia was significantly higher compared to NP (p=0.0001; Table 1), as were systolic (p=0.0001) and diastolic (p=0.0001) pressures.
Table 1.
Demographic data for women with normal and preeclamptic pregnancies.
| Normal Pregnant (n=10) | Preeclamptic (n=10) | ||
|---|---|---|---|
| Patient Parameters | Mean ± SEM (Range) | Mean ± SEM Range | P Value |
| Maternal age (years) | 28.90± 1.78 (17-37) | 25.60 ± 1.66 (19-35) | 0.191 |
| BMI at admission | 34.33 ± 3.03 (25.1-49.4) | 37.18 ± 4.10 (21-55.6) | 0.583 |
| Gestational age (weeks) | 38.91 ± 0.15 (38.2-39.4) | 33.93 ± 0.91 (30-38.2)* | 0.0001 |
| Fetal Birth Weight (grams) | 3509 ± 100.8 (3155-4005) | 1697 ± 244.9 (951-3645)* | 0.0001 |
| MAP | 84.20 ± 2.16 (71-93.67) | 114.5 ± 4.86 (93.33-133.3)* | 0.0001 |
| Systolic | 119.4 ± 2.01 (111-131) | 155.1 ± 6.65 (131-200)* | 0.0001 |
| Diastolic | 66.5 ± 2.54 (54-78) | 94.2 ± 4.65 (73-115)* | 0.0001 |
BMI (body mass index), SEM (standard error mean).
p < 0.05 compared to normal pregnant women.
CD4+ T cells are increased in preeclamptic women
In the current study preeclamptic women had significantly increased circulating (23.39±3.04% vs. 10.84±1.6%; p=0.006; Figure 1A) CD4+ T cells. Preeclamptic women had significantly decreased Tregs compared to NP women (0.51±0.29% vs. 4.58±1.2%; p=0.01) and significantly increased Th17s (15.75±1.18% vs. 6.84±1.25%; p=0.0008; Figure 1A). Preeclamptic women had increased CD4+ T cells compared to NP women (15.91±2.9% vs. 2.81±0.86%; p=0.003). Tregs were significantly decreased (0.67±0.23% vs. 2.08±0.45%, p=0.02) and Th17s were increased (14.02±1.7% vs. 0.64±0.34, p=0.0001) compared to NP women (Figure 1B). There was a negative correlation between the number of Tregs and the gestational age at delivery in both circulating (r=0.663, n=10, p=0.037) and placental Tregs (r=0.653, n=10, p=0.041). CD8+ T cells were not significantly increased in the circulation (p=0.55) but were significantly increased in the placenta (p=0.021; Data Supplement).
Figure 1. CD4+ T cells and oxidative stress are increased in women with preeclampsia.
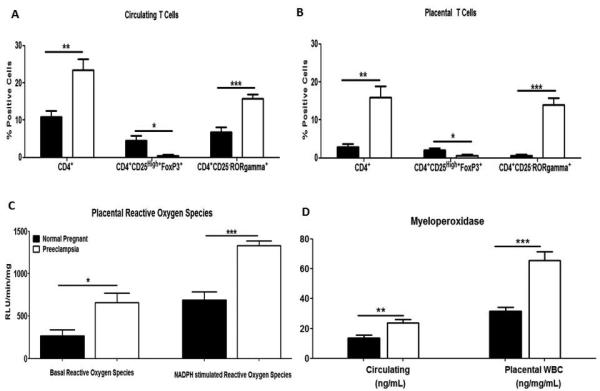
Circulating (A) and placental (C) CD4+ T cells are increased in preeclamptic women compared to normal pregnant women. Additionally Tregs (Tregulatory) were decreased and THelper 17 (Th17) was increased in preeclamptic women compared to normal pregnant women. Placental tissue from preeclamptic women produced significantly more reactive oxygen species compared to normal pregnant women (C). Preeclamptic women had significantly higher circulating and placental levels of myeloperoxidase compared to normal pregnant women (D). Data is expressed as mean±standard error mean. *p <0.05, **p<0.005, ***p<0.0005 compared to normal pregnant women.
Oxidative stress is increased in women with preeclampsia
As demonstrated in Figure 1C placentas from women with preeclampsia (n=5) produce significantly more ROS compared to NP women (n=5; 658.5±112.8 vs. 267.8±72.8RLU/min/mg; p=0.019). The same is true when stimulated with NADPH oxidase (1333±53.7 vs. 688.1±98.9RLU/min/mg; p=0.0004). Preeclamptic women had significantly higher levels of circulating MPO compared to NP women (23.81±2.4 vs. 13.78±1.8ng/mL; p=0.004; Figure 1D). Placental WBCs from preeclamptic women secreted significantly more MPO into cell culture media compared to NP women (65.67±6 vs. 31.53±2.7ng/mg/mL; p=0.0004; Figure 1D).
Conditioned media from preeclamptic placental explants tended to increase ROS production in HUVECs (59.68±4.4% signal intensity; n=4) compared to conditioned media from NP explants (46.98±3.87%, n=4, p=0.07; Figure 2). The mean intensity of HUVECs exposed to media from preeclamptic CD4+ T cells was 78.87±1.86% vs. 67.52±5.7% in HUVECs exposed to media from NP+NPTcells (p=0.107; Figure 2A-E).
Figure 2. Placental ischemia induced CD4+ T cells increase oxidative stress in vascular cells.
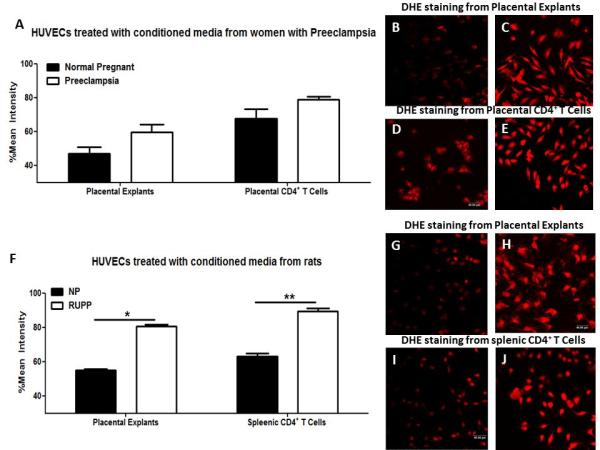
The mean signal intensity of dihydroethidium (DHE) was measured from human umbilical vein endothelial cells (HUVECs) exposed to conditioned media from normal pregnant and preeclamptic women (A) and conditioned media from normal pregnant or reduced uterine perfusion pressure (RUPP) rats (F). Representative photomicrographs of DHE staining in HUVECs; B-E from humans and G-J of rats where pictures on the left correspond with normal pregnant and pictures on the left with preeclamptic or RUPP. Data is expressed as mean±standard error mean. *p<0.05, **p<0.005, compared to normal pregnant rats.
Protocol 2: Animal study
Placental ischemia stimulates oxidative stress
Conditioned media from RUPP placentas (62.98±1.96%) significantly increased ROS in HUVECs compared to placentas from NP rats (54.97±0.96%; n=4 per group; p=0.01; Figure 2F). Conditioned media from CD4+ T cells from RUPP rats (89.43±1.54%) significantly increased ROS in HUVECs compared to media from NP+NPTcells (80.54±1.1%, n=4 per group; p=0.003; Figure 2F-J).
CD4+ T cells isolated from the spleens of RUPP rats secreted significantly more MPO compared NP rats (n=6; 116.9±15.2 vs. 48.5±8.3ng/mg/mL; p=0.003; Figure 3A).
Figure 3. Adoptive transfer of RUPP CD4+ T cells into normal pregnant (NP) rats increases mean arterial pressure (MAP) and circulating CD4+ T cells.
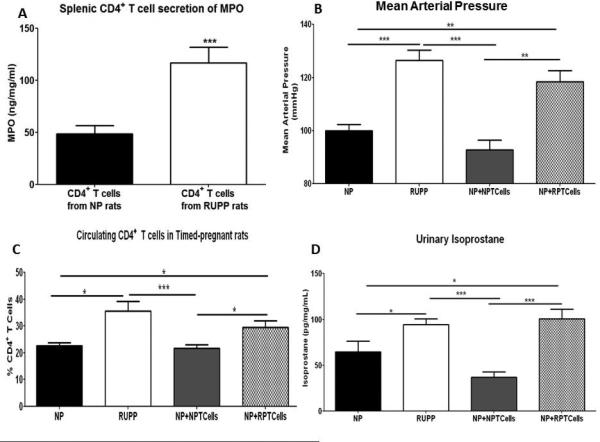
Splenic CD4+ T cells isolated from placental ischemic (RUPP) rats and cultured for 24hrs produced significantly more MPO compared to CD4+ T cells isolated from NP rats (A). MAP was significantly increased in RUPP rats compared to NP rats and to NP+NPCD4+ T cell rats (NP+NPTcells) (B). NP recipients of RUPP CD4+ T cells (NP+RPTcells) had significantly increased circulating CD4+ T cells compared to NP+NP Tcell rats and NP rats. CD4+ T cells are increased in placental ischemic RUPP rats and in NP+RPTcell rats compared to NP rats and NP+NPTcell rats (C). Excretion of urinary isoprostane was significantly increased in RUPP and NP+RPTcell rats compared to NP and NP+NPTcell rats (D). Data is expressed as mean±standard error mean. *p <0.05, **p<0.005, ***p<0.0005 between indicated groups.
Adoptive Transfer of RUPP CD4+ T cells increases MAP and CD4+ T cells in recipient NP rats
In the current study MAP increased from 100±2mmHg in NP rats to 126±4mmHg in RUPP rats (p=0.0001) and to 118±4mmHg in NP+RPTcell rats (p=0.002) which was significantly greater than NP+NPTcells (93±4mmHg; p=0.006;Figure 3B). MAP in RUPP rats was significantly increased compared to NP+NPTcell rats (p=0.0008; Figure 3B). Litter survival and pup weight for all groups treated with and without APO and TEM were unchanged among the groups but is reported in the Data Supplement.
Compared to NP rats (22.6±1.2%) circulating levels of CD4+ T cells were significantly increased in RUPP (35.51±3.8%; p=0.01; Figure 3C) and NP+RPTcell rats (29.55±2.5%; p=0.03). There were no significant differences in circulating levels of CD4+ T cells between NP+NPTcells (21.68±1.4%) and NP rats (p=0.64) which would indicate that RUPPCD4+ T cells stimulate endogenous T cells in the circulation in NP recipient rats.
Oxidative stress is increased in response to adoptive transfer of RUPP CD4+ T cells
Urinary isoprostane excretion was significantly increased in RUPP and NP+RPTcell rats compared to NP and NP+NPTcell rats (Data Supplement; Figure 3D). Basal placental production of ROS between the groups was not statistically significant (p=0.403; data not shown). With the addition of NADPH oxidase, placental ROS production in RUPP rats (830.6±68 RLU/min/mg) and NP+RPTcell rats (726.2±43.73 RLU/min/mg) was significantly increased compared to NP rats (420.7±51.4 RLU/min/mg; p=0.009 and p=0.004) and NP+NPTcell rats (449±36.58 RLU/min/mg; p=0.002 and p=0.001; Figure 4A).
Figure 4. Local reactive oxygen species production is increased in RUPP and NP recipients of RUPP CD4+ T cells.
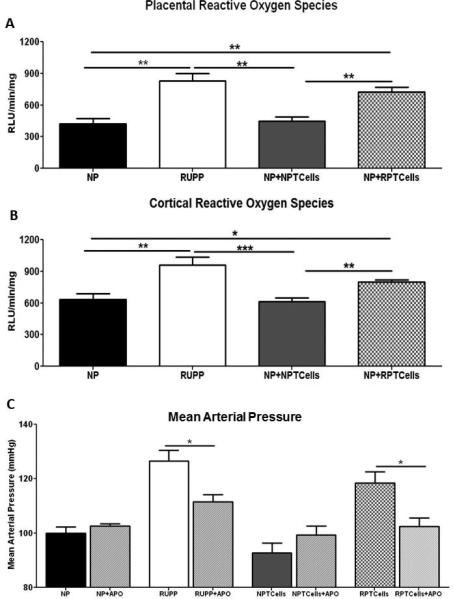
Placental (A) and renal cortical (B) production of reactive oxygen species was significantly increased upon addition of NADPH oxidase. MAP (mean arterial pressure) was significantly decreased in RUPP and NP recipients of RUPP CD4+ T cells (NP+RPTcells) upon apocynin administration (C). Data is expressed as mean±standard error mean. *p <0.05, **p<0.005, ***p<0.0005 between indicated groups.
Basal renal cortical ROS production between the groups was not statistically significant (p=0.808; data not shown). With the addition of NADPH oxidase renal cortical ROS production in RUPP rats (966.9±53.15RLU/min/mg) was significantly increased compared to NP rats (634.6±54.4RLU/min/mg; p=0.007) and NP+NPTcell rats (348.9±71.9RLU/min/mg; p=0.0007; Figure 4B). NP+RPTcell rats (798.9±22RLU/min/mg) produced significantly more cortical ROS compared to NP controls (p=0.025) and NP+NPTcell rats (p=0.004; Figure 4B).
Apocynin decreases MAP and CD4+ T cells in NP recipients of RUPP CD4+ T cells
MAP in RUPP rats treated with apocynin (111±2mmHg) was significantly decreased compared to untreated RUPP rats (p=0.03; Figure 4C). Administration of TEM did not significantly decrease RUPPCD4+ T cell induced MAP (Data supplement), however administration of APO to NP+RPTcell rats significantly decreased MAP (102±3 mmHg; p=0.01) compared to NP+RPTcell rats not receiving APO. There was not a significant effect of APO on NP rats (p=0.330) or NP+NPTcell rats (p=0.877; Figure 4C) compared to untreated NP rats.
Importantly, CD4+ T cells were decreased upon APO administration in both RUPP rats (decreased to 17.28±1.8%; p=0.002) and NP+RPTcell rats (decreased to 15.96±3.1%; p=0.008; Figure 5A).
Figure 5. Apocynin (APO) decreases CD4+ T cells and reactive oxygen species.
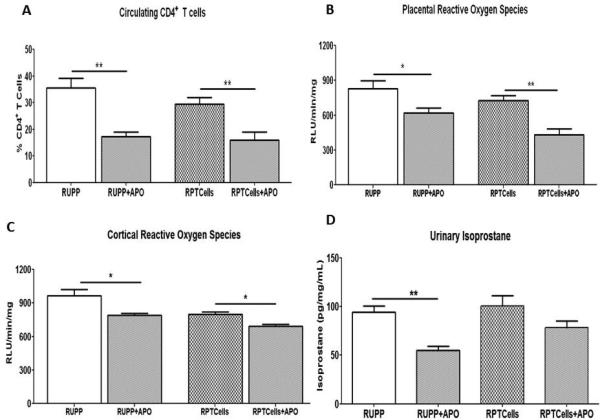
APO administration decreased circulating CD4+ T cells (A), and placental (B) and cortical (C) reactive oxygen species production. Administration of APO to RUPP and NP recipients of RUPP CD4+ T cell rats significantly decreased excretion of urinary isoprostane compared to the untreated groups (D). Data is expressed as mean±standard error mean. *p <0.05, **p<0.005 between indicated groups.
Apocynin (APO) decreases tissue ROS production in NP recipients of RUPP CD4+ T cells
APO administration decreased NADPH induced placental ROS production in RUPP rats (622.5±40.3RLU/min/mg; p=0.05) and in NP+RPTcell rats (431.4±52.5RLU/min/mg; p=0.006; Figure 5B). Cortical ROS production in RUPP rats treated with APO was significantly decreased (789.3±17RLU/min/mg; p=0.04) compared to untreated RUPP rats. APO administration significantly decreased cortical ROS production in NP+RPTcell rats (693.9±15.7RLU/min/mg; p=0.02; Figure 5C).
Administration of APO decreased urinary excretion of isoprostane from RUPP rats (54.83±4.4ng/mg/mL; p=0.003; Figure 5D). Urinary isoprostane excretion showed a trend to decrease in NP+RPTcell rats administered APO (78.57ng/mg/mL; p=0.13; Figure 5D) but did not reach statistical significance.
DISCUSSION
Many characteristics of preeclampsia are associated with those of various immune diseases such as elevated circulating inflammatory cytokines, oxidative stress and an imbalance in T cells. In this study we demonstrate for the first time that when measured by flow cytometry, placental T cell ratio mirrors that of circulating T cell alterations among preeclamptic patients (Fig1). In addition we demonstrate that circulating and placental cells are a source of oxidative stress from preeclamptic patients and not from those with normal pregnancies (Fig1). Furthermore, exposure of endothelial cells from conditioned media from preeclamptic T cells or placental explants stimulated endothelial cell oxidative stress indicating the importance of these cells to cause vascular oxidative stress systemically (Fig2).
Additionally, we demonstrate in our model of adoptive transfer of placental ischemic CD4+ T cells from RUPP rats into NP rats that placental and renal oxidative stress and mean arterial pressure are all increased in recipient rats. Furthermore, like preeclamptic CD4+ T cell media, RUPPCD4+ T cell media directly stimulated oxidative stress in vascular endothelial cells (Fig2). In addition, conditioned media from RUPP rat placental explants stimulated oxidative stress in vascular endothelial cells. Importantly, we demonstrate hypertension and circulating CD4+ T cells both decreased upon treatment with apocynin, an inhibitor of NADPH oxidase, which correlated with lower placental and renal NADPH stimulated oxidative stress (Fig5). Although previous supplementation of preeclamptic women with anti-oxidants vitamin E and C didn't prove beneficial, we demonstrate here that oxidative stress stimulated via CD4+ T cells plays an important role in hypertension during pregnancy. This study highlights the importance of continued drug discovery for safe alternatives to suppress CD4+ T cells as a mechanism to decrease oxidative stress and many other important mediators in the pathology of preeclampsia which would prove beneficial for the health of both moms and babies affected by this disease.
There is increasing evidence demonstrating a role of T cells in the pathogenesis of preeclampsia. In the current study we demonstrate that T cells are increased in the circulation and placentas of preeclamptic women compared to those with normal pregnancies. As Tregs peak during the 2nd trimester and then begin to decrease as the pregnancy continues30, 31, we determined if the changes in the number of Tregs between NP and preeclamptic women was possibly a function of pregnancy. There was a negative correlation between gestational age at delivery and the number of Tregs, indicating that the decrease in Tregs in the preeclamptic group is not due to an earlier gestational delivery date. Furthermore, we show that WBCs from preeclamptic placentas secrete greater MPO, suggesting that these cells, once activated by placental ischemia, may play a role in contributing to placental oxidative stress. Although there are studies that trophoblasts and vascular smooth muscle cells release reactive oxygen species during preeclampsia, to our knowledge there is currently no data on the role of CD4+ T cells in contributing to the development of oxidative stress mediated hypertension in preeclampsia. In the current study media collected from cultured placental CD4+ T cells from women with preeclampsia did not significantly stimulate oxidative stress in HUVECs. It is possible that since our isolated CD4+ cells from the placenta were less than 99% pure, there were potentially other cell types involved, which could contribute to the lack of statistical significance in the HUVEC studies. We have previously demonstrated that by adoptively transferring RUPP stimulated CD4+ T cells into NP recipient rats, we can mimic some of the factors associated with preeclampsia16, 19, 20, thereby suggesting that CD4+ T cells play an important role in the hypertension and pathophysiological features associated with preeclampsia. The present study reveals that the hypertension associated with adoptive transfer of RUPP CD4+ T cells is accompanied by increases in the production of placental and renal ROS. Furthermore we demonstrate that CD4+T cells also secrete greater MPO and stimulate MPO in NP recipient rats, indicating their role to mediate multiple oxidative stress pathways as a pathophysiological mechanism triggered in response to placental ischemia. Interestingly it has recently been reported that MPO-specific CD4+ T cells contribute to renal injury in mice adoptively transferred with T cells, further supporting the idea that CD4+ T cells are indeed inducing oxidative stress32.
As oxidative stress is associated with hypertension in both clinical and experimental models26, 33, 34, we sought to determine if the increase in oxidative stress led to the development of the hypertension. Treatment with TEM has previously been shown to decrease oxidative stress and hypertension in experimental animal models of hypertension during pregnancy17, 21, 26, however administration of Tempol did not significantly decrease the blood pressure in NP recipients of RUPPCD4+ T cells. A study by De Miguel et al, demonstrated that administration of TEM to Dahl SS rats decreased hypertension but not T cell infiltration33. Therefore it is possible that in this model of adoptive transfer of RUPPCD4+ T cells, the action of TEM is inefficient to decrease blood pressure. Therefore, we tried a more specific inhibitor of NADPH oxidase. We found that administration of APO, an NADPH inhibitor did significantly decrease the blood pressure in NP recipients of RUPPCD4+ T cells. In addition, administration of APO also decreased placental and cortical ROS in NP recipients of RUPP CD4+ T cells circulating CD4+ T cells in both RUPP control rats and NP recipient rats of RUPP CD4+ T cells (Fig 5). Importantly, urinary isoprostane excretion was not significantly decreased in NP+RPTcell rats receiving APO, which may indicate that while APO is effective at reducing NADPH induced oxidative stress, other oxidative stress pathways may still be active in response to placental ischemic stimulated CD4+ T cells. In addition, while T cells seemed to have been decreased in NP+RPTcell+APO, other immune cells that could be present in our cell prep used for adoptive transfer such as neutrophils or monocytes could be present releasing ROS molecules in these rats. These cell types however, were not measured and may not have been affected by APO and could be contributing to the elevation in urinary isoprostanes. This study illustrates the importance of cellular communication between reactive oxygen molecules with immune cells and highlights how multifactorial placental ischemia can be in regard to immune activation. Although we acknowledge the importance of monocytes and neutrophils as producers of oxidative stress we demonstrate a causal role for CD4+ T lymphocytes to mediate oxidative stress pathways as a mechanism of hypertension during pregnancy. This study further supports the importance of drug discovery to find novel immunosuppressive therapies applicable during pregnancy.
Perspectives
Preeclampsia has long been suggested to result from immunological origins. Many studies worldwide have demonstrated preeclamptic women have exacerbated immune responses characterized by elevated TNF alpha, IL-6, IL-17, autoantibodies, activated immune cells and oxidative stress. Most recently much focus has turned to characterizing the T cell subsets activating and playing a role in this disease. We have focused our research attentions to deciphering a role for placental ischemia as a stimulus for dysregulation among the T cell subsets Tregs/Th17s. In this study we demonstrated that the placental T cell profile mirrors that in the circulation (increased Th17/decreased Tregs) and that these cells are secretors of oxidative stress molecules and stimulate reactive oxygen species from vascular cells in vitro. Furthermore, we demonstrate for the first time that transferring CD4+ T cells mediates oxidative stress and we have previously shown that placental ischemic stimulated CD4+ T cells mediate other pathologies associated with preeclampsia. The increase in placental and cortical oxidative stress, urinary isoprostane and MPO in recipients of RUPPCD4+ T cells indicates the importance of CD4+ T cells in stimulating oxidative stress during pregnancy. Importantly, NADPH oxidase inhibition attenuated oxidative stress, hypertension, and most importantly T cells from increasing in response to placental ischemia or in response to adoptive transfer of T cells during pregnancy. Collectively the studies indicate the importance of continued research into drug discovery to improve oxidation and clinical symptoms and outcomes in preeclamptic women.
Supplementary Material
Novelty and Significance.
What is new?
Placental T cells mirror the profile previously described in the circulation of preeclamptic women. Those T cells are mediators of oxidative stress. Administration of NADPH oxidase inhibitor versus SOD mimetic or antioxidant supplementation decreased immune cells and oxidative signaling molecules in a rat model of hypertension during pregnancy This decrease of immune cells and oxidative stress molecules resulted in lower blood pressures in pregnant rat models of preeclampsia.
What is relevant?
Blockade of this inflammatory cascade versus antioxidant supplementation could be used to improve pregnancy outcomes in preeclamptic women
Summary
This study highlights the importance of immune mechanisms to cause the increase in blood pressure and oxidative stress that is associated with preeclampsia, both of which can be corrected by administration of specific anti-inflammatories during the later gestational stages.
ACKNOWLEDGEMENTS
We would like to thank Heather Drummond, PhD for the use of the confocal core facilities used to assess DHE staining in the current study.
FUNDING SOURCES
This work was supported by a NIH grant 1F32HL108558-01A1 to KW, NICHD 1R01HD067541-01A1 to BL.
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Fisher S, Martin J. Hypertensive Disorders in Pregnancy. Chelsey Hypertensive Disorders in Pregnancy. 1998:377–394. [Google Scholar]
- 2.Roberts J, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 3.Roberts J, Lain K. Recent Insights into the pathogenesis of preeclampsia. Placenta. 2002;23:359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 4.Lamarca B, Cornelius D, Wallace K. Elucidating Immune Mechanisms Causing Hypertension During Pregnancy. Physiology. 2013;28:225–233. doi: 10.1152/physiol.00006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laresgoiti-Servitje E. A leading role for the immune system in the pathophysiology of preeclampsia. J Leukoc Biol. 2013 doi: 10.1189/jlb.1112603. doi:10.1189/jlb.1112603. [DOI] [PubMed] [Google Scholar]
- 6.Gelderman K, Hultqvist M, Pizzolla A, et al. Macrophages suppress T cell responses and arthritis development in mice by producing reactive oxygen species. Journal of Clinical Investigation. 2007;117:3020–3028. doi: 10.1172/JCI31935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfort R, Stokes K, Granger D. CD4+ T lymphocytes mediate hypercholesterolemia-induced endothelial dysfunction via a NAD(P)H oxidase-dependent mechanism. Am J Physiol Heart Circ Physiol. 2008;294:H2619–H2626. doi: 10.1152/ajpheart.00989.2007. [DOI] [PubMed] [Google Scholar]
- 8.Raijmakers M, Dechend R, Poston L. Oxidative stress and preeclampsia: rationale for antioxidant clinical trials. Hypertension. 2004;44:374–380. doi: 10.1161/01.HYP.0000141085.98320.01. [DOI] [PubMed] [Google Scholar]
- 9.Mihu D, Sabau L, Costin N, Ciortea R, Maulutan A, Mihu C. Implications of maternal systemic oxidative stress in normal pregnancy and in pregnancy complicated by preeclampsia. J Maternal Fetal and Neonatal Medicine. 2012;25:944–951. doi: 10.3109/14767058.2011.600796. [DOI] [PubMed] [Google Scholar]
- 10.Roggensack A, Zhang Y, Davidge S. Evidence for peroxynitrite formation in the vasculature of women with preeclampsia. Hypertension. 1999;33:83–89. doi: 10.1161/01.hyp.33.1.83. [DOI] [PubMed] [Google Scholar]
- 11.Hung T, Burton G. Hypoxia and reoxygenation: a possible mechanism for placental oxidative stress in preeclampsia. Taiwan J Obstet Gynecol. 2006;45:189–200. doi: 10.1016/S1028-4559(09)60224-2. [DOI] [PubMed] [Google Scholar]
- 12.Brewer R, Liu R, Lu Y, et al. Endothelin-1, oxidative stress, and endogenous angiotensin II: mechanisms of angiotensin II type 1 receptor autoantibody-enhanced renal and blood pressure response during pregnancy. Hypertension. 2013;62:886–892. doi: 10.1161/HYPERTENSIONAHA.113.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam Tam K, Lamarca B, Arany M, et al. Role of reactive oxygen species during hypertension in response to chronic antiangiogenic factor (sFlt-1) excess in pregnant rats. American Journal of Hypertension. 2011;24:110–113. doi: 10.1038/ajh.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jianjun Z, Yali H, Zhiqun W, Mingming Z, Xia Z. Imbalance of T-cell transcription factors contributes to the Th1 type immunity predominant in pre-eclampsia. American Journal of Reproductive Immunology. 2010;63:38–45. doi: 10.1111/j.1600-0897.2009.00763.x. [DOI] [PubMed] [Google Scholar]
- 15.Lamarca B, Wallace K, Herse F, et al. Hypertension in response to placental ischemia during pregnancy: role of B lymphocytes. Hypertension. 2011;57:864–871. doi: 10.1161/HYPERTENSIONAHA.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace K, Richards S, Dhillion P, et al. CD4+ T Helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension. 2011;57:949–955. doi: 10.1161/HYPERTENSIONAHA.110.168344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sedeek M, Gilbert J, LaMarca B, et al. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertension. 2008;21:1152–1156. doi: 10.1038/ajh.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sedeek M, Llinas M, Drummond H, et al. Role of reactive oxygen species in endothelin-induced hypertension. Hypertension. 2003;42:806–810. doi: 10.1161/01.HYP.0000084372.91932.BA. [DOI] [PubMed] [Google Scholar]
- 19.Novotny S, Wallace K, Heath J, et al. Activating autoantibodies to the angiotensin II type 1 receptor play an important role in mediating hypertension in response to adoptive transfer of CD4+ T lymphocytes from placental ischemic rats. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2012;301:R1197–1201. doi: 10.1152/ajpregu.00623.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace K, Novotny S, Heath J, et al. Hypertension in response to CD4+ T cells from reduced uterine perfusion pregnant rats is associated with activation of the endothelin-1 system. Am J Physiol Regul Integr Comp Physiol. 2012;303:R144–149. doi: 10.1152/ajpregu.00049.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parrish M, Wallace K, Tam Tam K, et al. Hypertension in Response to AT1-AA: Role of Reactive Oxygen Species in Pregnancy-Induced Hypertension. American Journal of Hypertension. 2011;24:835–840. doi: 10.1038/ajh.2011.62. [DOI] [PubMed] [Google Scholar]
- 22.Dechend R, Viedt C, Muller D, et al. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107:1632–1639. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 23.Parrish M, Martin J, Jr, Lamarca B, et al. Randomized, placebo-controlled, double blind trial evaluating early pregnancy phytonutrient supplementation in the prevention of preeclampsia. J Perinatol. 2013;33:593–599. doi: 10.1038/jp.2013.18. [DOI] [PubMed] [Google Scholar]
- 24.Spinnato J, Freire S, Pinto e Silva J, et al. Antioxidant therapy to prevent preeclampsia: a randomized controlled trial. Obstet Gynecol. 2007;110:1311–1318. doi: 10.1097/01.AOG.0000289576.43441.1f. [DOI] [PubMed] [Google Scholar]
- 25.ACOG Diagnosis and management of preeclampsia and eclampsia. Practice Bulletin. 2002:33. [Google Scholar]
- 26.Dhillion P, Wallace K, Scott J, et al. IL-17 mediated oxidative stress is an important stimulator of AT1-AA and hypertension during pregnancy. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2012;303:R353–R358. doi: 10.1152/ajpregu.00051.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granger J, LaMarca B, Cockrell K, et al. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods in Molecular Medicine. 2006;122:383–392. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 28.Novotny S, Wallace K, Herse F, et al. CD4+ T cells play a critical role in mediating hypertension in response to placental ischemia. Journal of Hypertension. 2013;2:1–6. doi: 10.4172/2167-1095.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrison D, Gongora M. Oxidative Stress and Hypertension. Med Clin N Am. 2009;93:621–635. doi: 10.1016/j.mcna.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Matrougui K, Zakaria A, Kassan M, et al. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am J Pathol. 2011;178:434–441. doi: 10.1016/j.ajpath.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aluvihare V, Kallikourdis M, Betz A. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 32.Gan P, Holdsworth S, Kitching A, Ooi J. Myeloperoxidase (MPO)-specific CD4+ T cells contribute to MPO-anti-neutrophil cytoplasmic antibody (ANCA) associated glomerulonephritis. Cellular Immunology. 2013;282:21–27. doi: 10.1016/j.cellimm.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 33.De Miguel C, Guo C, Lund H, Feng D, Mattson D. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol. 2011;300:F734–742. doi: 10.1152/ajprenal.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redon J, Oliva M, Tormos C, et al. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension. 2003;41:1096–1101. doi: 10.1161/01.HYP.0000068370.21009.38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


