Abstract
OBJECTIVE
To test the hypothesis that greater chocolate-candy intake is associated with more weight gain in postmenopausal women.
DESIGN AND METHODS
Prospective cohort study involving 107,243 post-menopausal American women aged 50–79 years (mean=60.7) at enrolment in the Women’s Health Initiative (WHI), with three-year follow up. Chocolate-candy consumption was assessed by food frequency questionnaire and body weight was measured. Linear mixed models, adjusted for demographic, socio-economic, anthropomorphic and behavioral variables, were used to test our main hypotheses.
RESULTS
Compared to women who ate a 1 oz (~28 g) serving of chocolate candy <1 per month, those who ate this amount 1 per month to <1 per week, 1 per week to < 3 per week and ≥3 per week showed greater three-year prospective weight gains (kg) of 0.76 (95% CI: 0.66, 0.85), 0.95 (0.84, 1.06) and 1.40 (1.27, 1.53), respectively, (p for linear trend<0.0001). Each additional 1 oz/day was associated with a greater three-year weight gain (kg) of 0.92 (0.80, 1.05). The weight gain in each chocolate-candy intake level increased as BMI increased above the normal range (18.5–25 kg/m2), and as age decreased.
CONCLUSIONS
Greater chocolate-candy intake was associated with greater prospective weight gain in this cohort of post-menopausal women.
Keywords: Nutrition, chocolate, weight gain, body weight, weight management, women's Health
INTRODUCTION
Obesity remains a major health problem, with women being particularly vulnerable to weight gain during the early postmenopausal years (1). Numerous factors may contribute to weight gain including consumption of energy dense foods such as chocolate (2). While an emerging body of scientific evidence suggests that dark chocolate may have the ability to decrease cardiovascular disease risk over the short-term (3), much is still unknown about the relationship between long-term chocolate-candy consumption and body weight.
In a recent prospective analysis with a six-year follow up in the Atherosclerosis Risk in Communities cohort (ARIC), adult male and female participants who ate 1 oz of chocolate candy at least weekly experienced a mean increase in Body Mass Index (BMI in kg/m2) of 0.39 (95% confidence interval: 0.23, 0.55) compared to those who ate this amount less often than monthly (2). Conversely, four cross-sectional studies (2, 4–6), which are less rigorous than prospective studies for determining temporality (7), found that chocolate-candy intake was higher among lighter participants, found that chocolate-candy intake was higher among lighter participants. One other cross-sectional study (8) observed a significantly lower body mass index (BMI) and waist circumference among elderly males who preferred chocolate candy than among those who preferred non-chocolate candy.
Given these inconsistent findings, the objective of the current study was to investigate the association between chocolate-candy intake and prospective 3-year weight change in the Women’s Health Initiative (WHI), a large sample of racial/ethnically diverse American postmenopausal women. Our primary a-priori hypothesis, based primarily on the findings in the ARIC cohort (2), was that chocolate-candy intake would be positively associated with weight gain.
METHODS AND PROCEDURES
Subjects
The design and methods of the WHI have been described in detail elsewhere (9). In brief, the WHI enrolled 161,808 postmenopausal women 50–79 years of age between 1993–1998 into the OS or four overlapping clinical trials.
Our study included participants from the WHI Observational Study (OS) and Clinical Trial control arms (CT-controls).
Data collection
At baseline (year 0) and year 3 data related to medical history, and health behaviors such as diet, smoking and physical activity were collected. Weight and height were measured during in-clinic visits (see Outcome Variables). Data on physical functioning and psychosocial factors were collected using standardized questionnaires. Information on the standard operating procedures and validity of the baseline measures have been described previously (9–11). A robust set of variables for evaluation as regression-model confounders, which are described below, were collected at year 0 and year 3.
Assessment of Chocolate-candy Intake
Data on chocolate-candy intake were collected at year 0 and 3. This information was collected in the form of responses to a semi-quantitative food-frequency questionnaire (FFQ) designed for the WHI (12). The FFQ contained a single line item asking for the frequency and portion size of “chocolate candy and candy bar” intake over the prior 3 months. Participants were asked to specify their usual serving size as small (1/2 oz), medium (1 oz) or large (1 1/2 oz), and to indicate the frequency of intake as one of nine response options: from never or <1 per month to ≥2 per day. In order to provide adequate statistical power we converted the original nine categories of chocolate-candy intake frequency into four levels of a 1 oz serving: <1 per month; ≥1 per month to <1 per 3 weeks; ≥1 per 3 weeks to <3 per week; and ≥3 per week. This allowed us to assess and compare temporal changes in body weight during the 3-year period between year 0 and 3 across these four categories. Total chocolate-candy intake, calculated from portion size and frequency of consumption, was used to assess the association between a 1 oz increment in chocolate-candy intake and weight gain during the 3-year period.
Outcome Variable
Body weight was measured at year 0 and 3 by trained and certified personnel using standardized procedures and calibrated beam scales in all WHI participants (9). Our outcome variable, weight change, was calculated as year 3 weight minus year 0 weight.
Statistical Methods
We built two regression models to examine the association between chocolate-candy intake and body weight change. Both models were linear mixed models with random coefficients that contained random effects for intercept and time, with a banded main diagonal covariance matrix. This matrix structure is relatively parsimonious in that it only has two parameters. Further it accounted for the observed heterogeneous variances and small covariance between the random factors, intercept and time, in our analysis. We used likelihood ratio tests to assess results of different random effects and covariance matrix structures on model fit (13). Chocolate intake was our exposure independent variable, body weight was the dependent variable, and we included the cross product of chocolate-candy intake with time to allow for estimation of the change in body weight during the follow up period. We selected height squared as the baseline covariate in our linear mixed model. Height squared was weakly correlated with the dependent variable in our model (body weight), and yielded good model fit and a stable model.
The variables tested as potential confounders were: age (years); time (binary, year 0, 3); race/ethnicity (non-Hispanic white, other); smoking status (never, past, current <15 cigarettes/day, current >=15 cigarettes/day); physical activity, total activity at work, sports and leisure (in MET-hrs/wk); educational level (<high school; some high school -<college; some college-<postgraduate study; >=postgraduate study or degree); non-chocolate-candy daily caloric intake (kcal/day (14)); WHI study arm (OS or CT-control); self-reported prior diagnosis of a major chronic disease at year 0 (heart attack, stroke, cancer or diabetes - yes/no); self-reported prior diagnosis of hypertension at year 0 (yes/no); family income (8 levels); employment status (3 levels); marital status (5 levels); caffeine consumption (mg/day); modified alternative healthy eating index (15); limitations due to emotional problems (16); emotional well being (16); depression (17); sleep disturbances (18); social functioning (16); illness symptoms (18); Activities of Daily Living (18); and physical functioning (16). Our criterion for elimination/inclusion of confounders was at least a 10% change in the regression coefficient for our exposure variable, chocolate-candy consumption. The confounders in the two regression models are in table footnotes.
Paired t-tests were used to test the a-priori hypothesis, based on prior evidence (2), that obese (BMI≥30.0 kg/m2 ) women would decrease their chocolate-candy intake and body weight after a diagnosis of a serious chronic disease. The a-priori hypothesis that the prospective association between chocolate-candy intake and body weight would be different among those with and without a serious chronic disease was tested by inserting a cross-product interaction term of chocolate-candy intake and the prevalence of serious chronic disease in the regression model. We used similar cross-product terms to explored other subgroup analyses for age and BMI without prior hypotheses Significant interactions were elucidated by means of stratified analyses. All linear mixed models analyses were conducted with SAS (v. 9.3, SAS Institute Inc., Cary, North Carolina). Other analyses were performed using IBM SPSS Statistics (v. 20, IBM Corp., Armonk, NY).
Ethics
Our procedures were in accordance with the Helsinki Declaration of 1975 as revised in 1983. This manuscript follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations (19,20). The WHI study protocol was approved by institutional review boards at each participating institution, and all participants provided written informed consent. The ClinicalTrials.gov Identifier is NCT00000611.
RESULTS
Of the original 161,808 women, 93,676 were in the OS and 68,132 were in the clinical trials, of whom 26,515 were in the clinical trial control groups (CT-control participants). We included all OS and CT-control participants, with 120,191 at year 0 and 100,215 at year 3, representing a follow-up rate of 83.8%. We excluded participants with: 1) implausible FFQ energy intakes (21) defined as mean intakes <600 kcal/d or >5,000 kcal/d (n=4,103); 2) extreme BMI values, defined as <15 kg/m2 or >50 kg/m2 (n=1,878); or 3) height <122 cm - 4ft (n=975). We excluded women with extreme values of BMI and low height because these values could have resulted from coding errors, and these two exclusions substantially reduced the extreme values of our outcome variable, weight change during the three year study period, from 42.7 to 25.6 kg/yr. After these exclusions there were 114,281 women at year 0 and 75,489 at year 3. None of these criteria was responsible for excluding more than 3.4% of participants for implausible FFQ energy values at year 0. From these women we then excluded those with missing values on any exposure, dependent or confounder variables, leaving an analytic sample of 107,243 women at year 0 and 70,624 at year 3 who provided data for our multivariable analysis (Table 2). None of these variables had missing rates higher than 3.6% at year 0 or 3. For instance, at year 3 the missing rates for body weight, chocolate-candy intake, physical activity and smoking were 0%, 0%, 2.2% and 3.6%, respectively.
Table 2.
Chocolate Candy Intake1 and Three-year Change in Body Weight among Post-menopausal Women in the Women’s Health Initiative (WHI) Cohort.
| Chocolate Candy Intake (1 oz Serving) | Adjusted for Age, Race/ethnicity & WHI Study Arm | Adjusted for all Confounders 4 | ||||
|---|---|---|---|---|---|---|
| N 2 at Year 0 | N at Year 3 | Body Weight Change (kg) 3 | N at Year 0 | N at Year 3 | Body Weight Change (kg) | |
| <1/month | 35205 | 22602 | 0 (REFERENT) | 33636 | 21594 | 0 (REFERENT) |
| ≥1/month – <1/week | 39208 | 24920 | 0.85 (0.76, 0.95) | 37279 | 23761 | 0.76 (0.66, 0.85) |
| ≥1/week – <3/ week | 21983 | 15743 | 1.16 (1.05, 1.27) | 20938 | 15029 | 0.95 (0.84, 1.06) |
| ≥3/ week | 16296 | 10695 | 1.78 (1.65, 1.90) | 15390 | 10240 | 1.40 (1.27, 1.53) |
| An Additional 1 oz per Day | 112692 | 73960 | 1.29 (1.16, 1.41) | 107243 | 70624 | 0.92 (0.80, 1.05) |
| P for Linear Trend5 | <.0001 | <.0001 | ||||
| P for Quadratic Trend5 | <.0001 | <.0001 | ||||
Data are presented as mean (95% confidence interval) estimated by means of a linear mixed effects model, in which the exposure and outcome variable and confounders were updated at year 3 (see text for details).
Frequency of chocolate-candy intake was assessed by means of a semi-quantitative food frequency question.
N is the number of participants who provided data values in each of the categories of chocolate-candy intake at year 0 & 3.
Body weight change is the change in kg during the three year period between year 0 and 3:1) among participants in a particular chocolate-candy intake category, compared to participants who reported eating chocolate candy <1/ month (referent category); and 2) among all participants. The change is associated with the consumption of an additional 1 oz of chocolate candy per day.
Adjusted for age (year); time (year 0 and 3); chocolate-candy intake (1 oz servings/day)*time; baseline height squared; ethnicity (white, other); WHI study arm (2 groups); smoking status (never, past, current <15/day, current >=15/day); physical activity (total activity at work, sports and leisure, in MET-hrs/wk); educational level (<high school; Some high school -<college; some college-<postgraduate study; >=postgraduate study or degree); non-chocolate daily caloric intake (kcal/day); modified alternative health eating index. All confounders were continuous or binary variables.
Tests for linear trend were performed by putting the linear version of chocolate-candy intake in the model. Tests for quadratic trend were performed by putting the linear and quadratic version of chocolate-candy intake in the model.
Participant Characteristics
Women who reported more frequent chocolate-candy intake reported lower physical activity, dietary quality and prevalence of serious chronic disease. They exhibited greater likelihood of Non-Hispanic White race/ethnicity. They also reported greater dietary energy intake, level of illness symptoms and level of social strain (Table 1).
Table 1.
Characteristics 1 of post-menopausal women in relation to Chocolate Candy Intake in the Women’s Health Initiative, Mean (SD) or %.
| Characteristics- | Frequency of Consumption of a 1oz Serving of Chocolate Candy | |||
|---|---|---|---|---|
| <1 per month (N=27,960) | ≥1 per month to <1 week (N=31,877) | >1 week to <3 per week (N=17,843) | ≥3 per week (N=13,191) | |
| Chocolate Candy (1 oz servings/day) | 0.00 (0.01) | 0.06 (0.03) | 0.21 (0.07) | 0.79 (.53) |
| Body Weight (kg) 2 | 70.1 (14.9) | 72.3 (14.9) | 71.9 (14.8) | 74.9 (15.9) |
| Age (years) | 63.9 (7.2) | 63.2 (7.1) | 63.6 (7.3) | 63.3 (7.3) |
| Race/ethnicity (%) | ||||
| non-Hispanic white | 81.9 | 86.8 | 90.2 | 90.2 |
| non-Hispanic black | 9.6 | 7.2 | 4.7 | 5.3 |
| Other | 8.5 | 5.9 | 5.1 | 4.5 |
| Education (%) 3 | ||||
| <high school | 20.9 | 20.1 | 19.6 | 20.7 |
| high school -<college | 36.9 | 36.6 | 37.2 | 37.9 |
| college-<postgrad. study | 23.1 | 24.1 | 23.9 | 23.5 |
| >=postgrad. study/degree | 19.1 | 19.2 | 19.3 | 17.8 |
| Physical Activity (MET-hrs/wk) 3 | 14.8 (15.1) | 13.2 (13.7) | 12.34 (13.1) | 11.1 (12.9) |
| Total dietary calories (kcal/day) | 1423.3 (531.8) | 1556.8 (560.7) | 1689.2 (598.9) | 2013.1 (720.3) |
| Non-chocolate calories(kcal/day) | 1422.9 (531.8) | 1548.9 (560.3) | 1662.0 (598.3) | 1909.8 (707.8) |
| Smoking (%) | ||||
| Never | 50.3 | 50.0 | 52.2 | 50.1 |
| Former | 43.8 | 43.7 | 41.0 | 42.1 |
| current <15/day | 3.1 | 3.4 | 3.5 | 3.4 |
| current >=15/day | 2.7 | 2.9 | 3.3 | 4.4 |
| Healthy Eating Index 4 | 51.5 (10.0) | 49.5 (9.7) | 48.3 (9.6) | 46.3 (9.9) |
| Family Income ‡ | 4.29 (1.88) | 4.37 (1.80) | 4.38 (1.80) | 4.33 (1.80) |
| Serious Chronic Disease (%) 5 | 19.6 | 16.0 | 15.4 | 15.2 |
| Illness Symptoms 6 | 0.41 (0.27) | 0.43 (0.26) | 0.43 (0.26) | 0.46 (0.27) |
| Physical Functioning 6 | 81.9 (20.1) | 82.3 (19.2) | 81.8 (19.0) | 80.2 (19.9) |
| Social Strain 6 | 6.4 (2.5) | 6.5 (2.5) | 6.5 (2.5) | 6.7 (2.5) |
| Postmenopausal Hormones (%) | ||||
| Never | 32.9 | 29.8 | 29.6 | 30.5 |
| >3mo ago | 19.6 | 19.3 | 19.6 | 20.4 |
| <=3mo ago | 47.5 | 51.0 | 50.8 | 49.2 |
There were significant differences across levels of chocolate candy intake (p<.05), based on the analysis of variance, Kruskal-Wallis test, or Chi-square test.
Data are given as mean (SD) for continuous variables and as percentages for categorical variables. Data are for participants with no missing values for any of the characteristics in this table.
Body weight, measured weight in lb.
Educational level, Physical activity and Family Income were quantified by WHI researchers. Physical activity was total energy expended in recreational physical activity. Family Income ranged from 1–8).
the Modified Alternative Healthy Eating Index (15).
Serious chronic illness was self-reported preexisting diagnosis of heart attack, stroke, diabetes or cancer.
Association between Chocolate-candy Consumption and Body Weight Change
The mean three-year weight gain in the entire cohort was 0.88 kg (SD=7.84). After adjusting for confounders, and with women who ate a 1 oz (~28 g) serving of chocolate candy <1 per month as referent, those who ate this amount more frequently gained significantly more weight over the three year follow-up, and the weight gain increased steadily as the frequency of consumption increased (Table 2), suggesting a dose-response relationship. There was no significant interaction between chocolate intake and diagnosis of serious chronic disease regardless of the time of diagnosis, either prior to the baseline (p=.09) or after baseline but prior to the year 3 follow-up (p=.19). When we added serious chronic disease diagnosed prior to baseline as a confounder to our full model, an extra 1 oz of chocolate-candy per day was associated with a three-year weight gain of 0.93 (0.80, 1.06), which is essentially the same as that from the model without serious chronic disease as a confounder (Table 2).
Change in Chocolate-candy Intake After Diagnosis of a Serious Chronic Disease
Compared to obese, disease-free women, obese women diagnosed with serious chronic disease during the follow-up period between year 0 and 3 showed significant decreases in the consumption of chocolate candy, energy and fat, and body weight, across the three years. As a percentage of mean year 0 values, the mean decreases were 20.8%, 4.3%, 5.3% and 1.8%, respectively.
Secondary Analyses
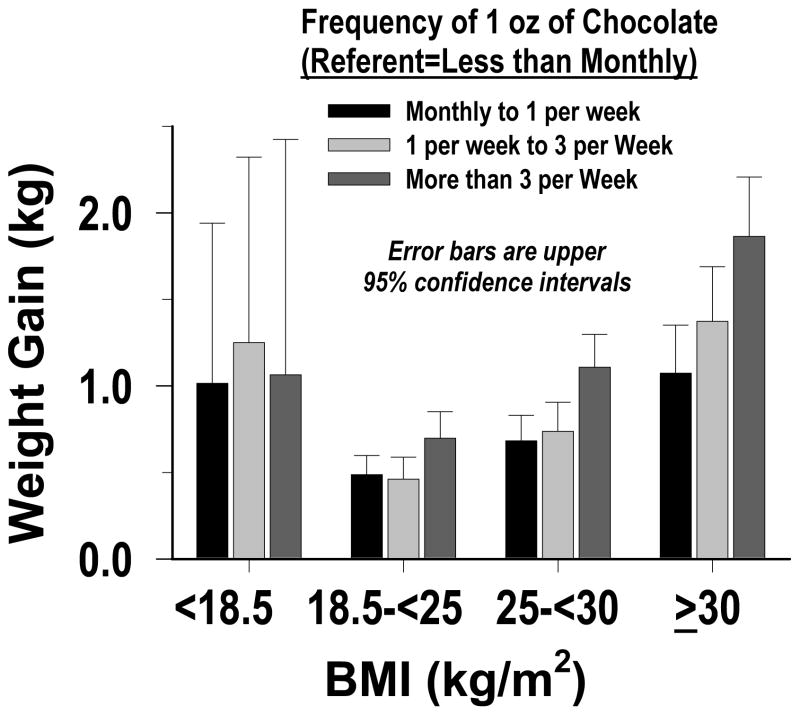
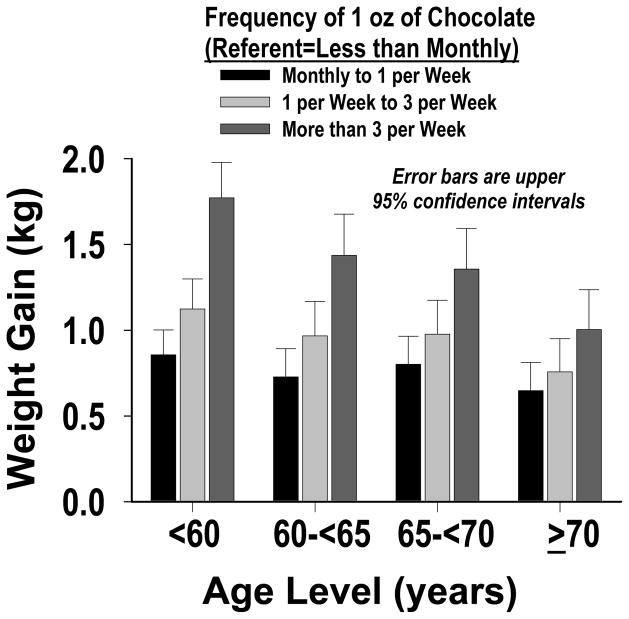
We performed a separate analysis to assess the effect of adjusting for total energy intake, rather than non-chocolate energy intake, as a confounder in our main full-model. The results were essentially the same as the main results in Table 2. While more frequent chocolate-candy consumption was associated with greater weight gain in all age groups, more frequent intake was associated with greater weight gain across each of the following three BMI groups: normal weight (BMI 18.5–<25 kg/m2), overweight (25–<30) and obese (≥30); with the weight gain being greatest for obese women (p-value<0.0001 for interaction by BMI, and for linear trend - Figure 1). No clear pattern was observed in the relatively small subgroup of women who were underweight (BMI <18.5 kg/m2). We also found that the weight gain was greater in younger than older women (p-value for interaction by age <0.0001 - Figure 2). We conducted a sensitivity analysis that repeated our main full-model analysis four times - with no baseline covariate, and with three other variables as the baseline covariate: waist-to-hip ratio, waist circumference and body weight. For no baseline covariate and waist-to-hip ratio the results were essentially the same as those in Table 2. For body weight, compared to women who ate a 1 oz (~28 g) serving of chocolate candy <1 per month, those who ate this amount 1 per month to <1 per week, 1 per week to < 3 per week and ≥3 per week showed three-year greater prospective weight gains (kg) of 0.55 (95% CI: 0.49, 0.61), 0.64 (0.57, 0.71) and 0.94 (0.86, 1.02), respectively. For waist circumference, the estimates were intermediate between those for height squared and body weight.
Figure 1.
Three-year Weight Gain Associated With Chocolate Candy, in Different BMI Levels (p-value for interaction by BMI <0.0001).
Figure 2.
Three-year Weight Gain Associated With Chocolate Candy, in Different Age Levels (p-value for interaction by age <0.0001).
We repeated our main full-model analysis in Table 2 without energy intake as a covariate. Compared to women who ate a 1 oz (~28 g) serving of chocolate candy <1 per month, those who ate this amount 1 per month to <1 per week, 1 per week to < 3 per week and ≥3 per week showed greater three-year prospective relative weight gains (kg) of 0.86 (95% CI: 0.77, 0.96), 1.17 (1.06, 1.28) and 1.79 (1.66, 1.92), respectively. The differences between these results and those in Table 2 could be because energy intake is in the causal pathway between chocolate candy intake and body-weight change. It seems more likely that energy intake is a regular confounder as chocolate intake constituted only 1.4% (SD=2.3%) of total energy intake in our sample.
DISCUSSION
Our main finding was that more chocolate-candy consumption was associated with greater weight gain during our three-year study period in the WHI cohort. This finding was robust in that it did not change in sensitivity analyses which tested different confounders and baseline covariates. Each additional 1 oz daily chocolate-candy serving was associated with a mean extra three-year weight gain of 0.92 kg (95% confidence interval: 0.80, 1.05). While our estimates of weight change are subject to biases, as described below, this estimate agrees reasonably well with results from the ARIC cohort, the only previous epidemiological study on chocolate-candy consumption and prospective weight change (2). In that cohort there was a 1.09 (0.64, 1.34) kg greater increase in weight over a six-year period among ARIC participants of average height (1.68 m) who ate a 1 oz serving ≥1 per week compared to those who ate it <1 per month (2).
Our main finding, that long-term consumption of chocolate candy was associated with weight gain, most likely applies primarily to milk-chocolate candy, as milk chocolate was more popular than dark chocolate (22) when the WHI FFQ was administered. Flavanols are the compounds in cocoa that are thought to be responsible for the observed decreases in cardiovascular risk (3). Evidence from three human trials suggests that the minimum dose of flavanols needed to significantly increase vascular dilation and blood flow - the basis of the cardiovascular benefit - is 200 mg (22). If this dose is consumed daily solely through solid milk chocolate, about 10 oz/day would be needed (23), an amount likely to result in a substantial weight gain in the absence of a compensatory reduction in caloric intake from other foods. About 2 oz of solid dark chocolate are needed to provide 200 mg of flavanols (23). Consequently a dark chocolate habit seems more likely than a milk chocolate habit to be able to yield long-term cardiovascular benefits with lower risk of weight gain. In support of this possibility there is evidence suggesting that compounds in cocoa, which tend to be more concentrated in dark than milk chocolate, may help counteract weight gain (31). These compounds are postulated to decrease expression of genes involved in fatty acid synthesis, and decrease digestion and absorption of fats and carbohydrates (24). Also, dark chocolate may be able to induce stronger feelings of satiety and lead to lower energy intake than milk chocolate (25). Unfortunately, we were not able to investigate the effects of dark chocolate on body weight because the WHI data do not distinguish between different types of chocolate (e.g. white, milk, dark).
While our study, and the one other prior prospective analysis (2), found a direct association between chocolate-candy consumption and weight gain, the four prior less-rigorous cross-sectional studies (7) found an inverse association (2,4–6). The divergent findings from these two types of studies could be due to confounding effects of serious chronic disease. In obese participants in the WHI, we found a three-year decrease in chocolate-candy consumption and body weight of 20.8% and 1.8%, respectively, after a first diagnosis of a serious chronic disease. The ARIC analysis yielded a similar pattern with decreases in chocolate-candy consumption of ~33% and body weight of ~3% over a six year period (2). This pattern could explain an inverse association between chocolate-candy consumption and body weight in a cross-sectional analysis. Notably, there was a significant interaction effect for serious chronic disease in the cross-sectional ARIC analysis; but the interaction was not significant in the prospective ARIC analysis (2), nor in our prospective analysis.
We found that higher chocolate-candy intake was associated with weight gain among women in all age groups, but the weight gain was greater in younger than older women (p-value for interaction by age <0.0001). This trend was most apparent for women in our highest intake-frequency level - a 1 oz serving more than 3 times a week: the three-year weight gain for women younger than 60 years (1.77 kg) was 77% higher than for women older than 70 years (1.00 kg). This observation could be explained by the fact that aging tends to be accompanied by decreases in body, bone, muscle and organ mass (26).
Similarly, among women who were either normal-weight, overweight or obese at baseline the weight gain associated with chocolate consumption increased as BMI increased (p-values for interaction and linear trend <.0001), and was greatest for obese women. This pattern could be due to the fact that a particular increase in caloric intake is likely to sustain a larger body weight increase in persons with higher adiposity (27). It could also be due to disruptions in gut peptide signaling leading to attenuated satiety in persons with diet-induced obesity (28, 29).
Our study has several strengths. First, the WHI cohort is large and provides adequate statistical power to detect small effects in the subgroups we examined. There were 107,243 women at year 0 and 70,624 at year 3 who provided data for our multivariable analysis. Second, the WHI dataset contains standardized body weight measurements made at two sequential time points, which allowed us to apply linear mixed model techniques to optimally use all available data to obtain precise estimates of changes in body weight over time. Third, the WHI data have been collected and validated using extensive modern empirically-proven quality-control techniques (9–11). Finally, the WHI dataset provides a wide range of possible confounding variables which allowed us to account for many factors known to affect our exposure and outcome variables. Our study also has several limitations. First, we did not have data on the type of chocolate consumed by WHI participants, as discussed above. Second, our exposure variable, chocolate-candy intake, was self reported (12). Although FFQ data are considered to be reliable for ranking participants by level of dietary intake, these data are subject to intra-individual variation, including measurement error (30). However, intra-individual variation often moves estimates closer to the null (31), so that our estimates of body weight change associated with different levels of chocolate-candy intake are likely to be underestimates. Third, our baseline covariate may not be optimal (32, 33), only two sequential measurements were available for our analysis (32), and we may not have fully accounted for confounding. Hence our estimates of weight change should be interpreted with caution.
In conclusion, we found that greater chocolate-candy consumption was associated with greater prospective three-year weight gain in a large cohort of postmenopausal women. The weight gain increased monotonically with increasing frequency of chocolate-candy consumption.
Supplementary Material
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The effect of chocolate-candy consumption on body weight has not been clarified;
One prior prospective study in the Atherosclerosis Risk in Communities (ARIC) cohort found a positive association between chocolate-candy consumption and weight gain;
five prior cross-sectional studies found inverse associations between chocolate-candy consumption and body weight.
WHAT THIS STUDY ADDS
Higher chocolate-candy consumption was associated with a subsequent greater weight gain over a three-year period in the WHI cohort;
Our findings suggest that long-term regular chocolate-candy consumption may be associated with cumulative weight gain among postmenopausal women.
Our estimates of body weight change should be interpreted with caution as they are based on only two sequential waves of data, and are subject to residual confounding and other sources of bias.
Acknowledgments
The Women's Health Initiative Study is registered at Clinical-Trials.gov (Identifier NCT00959270).
WHI Investigators
We wish to acknowledge and thank the WHI Investigators for their efforts in the collection of the data that we used in this study (see Supporting Information).
Funding Agencies
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. JEM had partial support from HHSN268201100001C from NIH / NHLBI, the Women’s Health Initiative program. LW had partial support from grant HL095649 from the National Heart, Lung, and Blood Institutes. MLN and LT had partial support provided by NIH grant HHSN268201100046C. MEW had partial support provided by NIH grants KL2TR000160 and 1U01HL105268. Funding agencies had no direct role in these analyses.
ABBREVIATIONS
- WHI
Women's Health Initiative
- BMI
Body Mass Index
- FFQ
food-frequency questionnaire
- CT-controls
WHI clinical trial control arms
- OS
WHI observational study
SHORT LIST OF WOMENS' HEALTH INITIATIVE INVESTIGATORS
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
For a list of all the investigators who have contributed to WHI science, please visit:https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf
Footnotes
Author Contributions
JEM, MAW and CAT collected data. JAG reviewed the literature, provided the initial design of the study, analyzed the data, interpreted results, and drafted and edited the manuscript. JEM, BB, LW and LT helped improve the design of the study. JEM, BB, MLN, LW and MEW helped interpret results. All authors were involved in critical review of the paper and had final approval of the submitted and published versions.
CONFLICTS OF INTEREST
The only author with a disclosure is Dr. JoAnn Manson. She and colleagues at Brigham and Women’s Hospital, Harvard Medical School are recipients of funding from Mars Symbioscience for an investigator-initiated randomized trial of cocoa flavanols and cardiovascular disease.
References
- 1.Howard BV, Manson JE, Stefanick ML, Beresford SA, Frank G, Jones B, et al. Low-fat dietary pattern and weight change over 7 years: the Women's Health Initiative Dietary Modification Trial. JAMA. 2006 Jan 4;295(1):39–49. doi: 10.1001/jama.295.1.39. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg J, Buijsse B. Habitual Chocolate Consumption May Increase Body Weight in a Dose-Response Manner. Plos One. 2013;8:e70271. doi: 10.1371/journal.pone.0070271. http://dx.plos.org/10.1371/journal.pone.0070271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corti R, Flammer AJ, Hollenberg NK, Lüscher TF. Cocoa and cardiovascular health. Circulation. 2009;119(10):1433–41. doi: 10.1161/CIRCULATIONAHA.108.827022. [DOI] [PubMed] [Google Scholar]
- 4.Golomb BA, Koperski S, White HL. Association between more frequent chocolate consumption and lower body mass index. Arch Intern Med. 2012;172(6):519–21. doi: 10.1001/archinternmed.2011.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Neil CE, Fulgoni VL, 3rd, Nicklas TA. Candy consumption was not associated with body weight measures, risk factors for cardiovascular disease, or metabolic syndrome in US adults: NHANES 1999–2004. Nutr Res. 2011;31(2):122–30. doi: 10.1016/j.nutres.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Cuenca-García M, Ruiz JR, Ortega FB, Castillo MJ HELENA study group. Association between chocolate consumption and fatness in European adolescents. Nutrition. 2014 Feb;30(2):236–9. doi: 10.1016/j.nut.2013.07.011. Epub 2013 Oct 17. [DOI] [PubMed] [Google Scholar]
- 7.Green MD, Freedman DM, Gordis L. Reference Guide on Epidemiology. 2. United States: Federal Judicial Center. Matthew Bender Publishing Co; 2000. [Web. last accessed on May 22, 2014]. Reference Manual on Scientific Evidence; pp. 339–354. < http://www.fjc.gov/public/pdf.nsf/lookup/sciman06.pdf/$file/sciman06.pdf>. [Google Scholar]
- 8.Strandberg TE, Strandberg AY, Pitkälä K, Salomaa VV, Tilvis RS, Miettinen TA. Chocolate, well-being and health among elderly men. Eur J Clin Nutr. 2008;62(2):247–53. doi: 10.1038/sj.ejcn.1602707. [DOI] [PubMed] [Google Scholar]
- 9.WHI Study Group. Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/S0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 10.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(S9):S107–S121. doi: 10.1016/S1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 11.Margolis KL, Wei F, de Boer IH, Howard BV, Liu S, Manson JE, et al. Women’s Health Initiative Investigators. A diet high in low-fat dairy products lowers diabetes risk in postmenopausal women. J Nutr. 2011 Nov;141(11):1969–74. doi: 10.3945/jn.111.143339. Epub 2011 Sep 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999 Apr;9(3):178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 13.West BT, Welch KB, Galecki AT. Linear Mixed Models: a practical guide using statistical software. Chapman & Hall/CRC; New York: 2006. p. 39. [Google Scholar]
- 14.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999 Mar 15;149(6):531–40. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 15.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012 Jun;142(6):1009–18. doi: 10.3945/jn.111.157222. Epub 2012 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1. 0. Health Econ. 1993;2:217–27. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 17.Tuunainen A, Langer RD, Klauber MR, Kripke DF. Short version of the CES-D (Burnam screen) for depression in reference to the structured psychiatric interview. Psychiatry Res. 2001;103:261–70. doi: 10.1016/s0165-1781(01)00278-5. [DOI] [PubMed] [Google Scholar]
- 18.Women's Health Initiative. Women's Health Initiative [WWW document] 2013 URL http://www.whi.org.
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 20.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. STROBE initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007 Oct 16;147(8):W163–94. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]
- 21.Thomson CA, Wertheim BC, Hingle M, Wang L, Neuhouser ML, Gong Z, Garcia L, Stefanick ML, Manson JE. Alcohol consumption and body weight change in postmenopausal women: results from the Women's Health Initiative. Int J Obes (Lond) 2012 Sep;36(9):1158–64. doi: 10.1038/ijo.2012.84. Epub 2012 Jun 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NPD. NPD Data Insights: Chocolate Candy. Port Washington (NY): The NPD Group, Inc; 2011. Mar, [Google Scholar]
- 23.Miller KB, Hurst WJ, Flannigan N, Ou B, Lee CY, Smith N, et al. Survey of commercially available chocolate- and cocoa-containing products in the United States. 2. Comparison of flavan-3-ol content with nonfat cocoa solids, total polyphenols, and percent cacao. J Agric Food Chem. 2009 Oct 14;57(19):9169–80. doi: 10.1021/jf901821x. [DOI] [PubMed] [Google Scholar]
- 24.Farhat G, Drummond S, Fyfe L, Al-Dujaili EA. Dark Chocolate: An Obesity Paradox or a Culprit for Weight Gain? Phytother Res. 2013 Sep 2; doi: 10.1002/ptr.5062. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Sørensen LB, Astrup A. Eating dark and milk chocolate: a randomized crossover study of effects on appetite and energy intake. Nutr Diabetes. 2011 Dec 5;1:e21. doi: 10.1038/nutd.2011.17. doi:10.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morley JE. Pathophysiology of the anorexia of aging. Curr Opin Clin Nutr Metab Care. 2013 Jan;16(1):27–32. doi: 10.1097/MCO.0b013e328359efd7. doi:10.1097. [DOI] [PubMed] [Google Scholar]
- 27.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378(9793):826–37. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maljaars J. Overeating makes the gut grow fonder; new insights in gastrointestinal satiety signaling in obesity. Curr Opin Gastroenterol. 2013 Mar;29(2):177–83. doi: 10.1097/MOG.0b013e32835d9fe0. [DOI] [PubMed] [Google Scholar]
- 29.Hellström PM. Satiety signals and obesity. Curr Opin Gastroenterol. 2013 Mar;29(2):222–7. doi: 10.1097/MOG.0b013e32835d9ff8. [DOI] [PubMed] [Google Scholar]
- 30.Knuiman MW, Divitini ML, Buzas JS, Fitzgerald PE. Adjustments for regression dilution in epidemiological regression analysis. Ann Epidemiol. 1998;8:56–63. doi: 10.1016/s1047-2797(97)00107-5. [DOI] [PubMed] [Google Scholar]
- 31.Spearman C. The proof and measurement of association between two things. Am J Psychol. 1904;15:72–101. [PubMed] [Google Scholar]
- 32.Chiolero A, Paradis G, Rich B, Hanley JA. Assessing the Relationship between the Baseline Value of a Continuous Variable and Subsequent Change Over Time. Front Public Health. 2013;1:29. doi: 10.3389/fpubh.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005 Aug 1;162(3):267–278. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.