Abstract
Background
Cancer survivors’ disclosure of complementary health approaches (CHA) to their follow-up care physicians is necessary to ensure safe and optimal use of such approaches. Rates of disclosure of CHA are variable and may be facilitated by patient-centered communication.
Methods
This cross-sectional study conducted in 2003–2004 examined a population-based sample (N=623) of leukemia, colorectal, and bladder cancer survivors who were 2–5 years post-diagnosis. A subset of participants who reported using CHA (N=196) was analyzed using multivariable logistic regression analyses to examine the association between patients’ perception of their physician’s patient-centered communication (i.e., information exchange, affective behavior, knowledge of patient-as-person) and patients’ disclosure of CHA use to their physician adjusting for physician, patient and patient-physician relationship factors.
Results
Thirty-one percent of the full sample used CHA and 47.6% of CHA users disclosed their use to their physicians. Disclosure was significantly associated with patient-centered communication even when adjusting for hypothesized covariates (OR=1.37; 95% Confidence Interval [CI; 1.09, 1.71]). Perceived physician knowledge of the patient-as-person (OR=1.28; CI [1.10, 1.48]) and information exchange (OR=1.27; CI [1.02, 1.60]) were the aspects of patient-centered communication that contributed to this association. The main reason for nondisclosure assessed in the survey was that survivors did not think it was important to discuss CHA (67.0%). A majority of physicians encouraged continued use of CHA, when disclosed (64.8%).
Conclusion
Results support that improving the overall patient-centeredness of cancer follow-up care and improving disclosure of CHA use are potentially synergistic clinical goals.
Keywords: Neoplasms, Survivors, Complementary Therapies, Complementary Medicine, Communication, Patient-centered Care
Background
There are currently over 13 million cancer survivors in the United States and this number continues to increase.1 After completion of cancer treatment, survivors need follow-up care to monitor for cancer recurrence, treatment-related toxicities, late effects, and psychosocial challenges.2 In many instances, survivors use complementary health approaches (CHA) that have developed outside of mainstream medicine (i.e., natural products, mind and body practices) to feel more in control of their health and address unmet needs experienced in multiple life domains (e.g., emotional, physical).3,4 Accordingly, the prevalence of CHA use is higher in cancer survivors than the general population5 with approximately 40% of cancer survivors at various stages of treatment using CHA including cancer survivors more than five years post-diagnosis.6,7
It is important for cancer survivors to discuss CHA use with their physicians to ensure safe and optimal use.8,9 Some therapies may interact with cancer treatment (e.g., natural products such as herbs),10 or the quality of products ingested may present concerns for patient safety.11–13 In addition, there is growing evidence of positive effects of CHA such as yoga or acupuncture to reduce cancer-related stress and treatment side-effects.14 Thus, increased communication about CHA may facilitate optimal use among cancer survivors by allowing physicians to both monitor possible contraindications and educate patients regarding the evidence supporting the potential benefits of CHA.
Reported rates of non-disclosure of CHA use to medical providers are variable, ranging from 20%–70%.8,15 The association of the general quality of patient-physician communication with patient disclosure of CHA has not been well-investigated.16 One qualitative study of breast cancer patients highlighted the potential importance of patient-centered care (e.g., having a respectful discussion regardless of agreement) to a patient’s decision to disclose CHA use.17 Patient-centered communication can conceptually be influenced by patient factors (e.g., sociodemographic characteristics, health status), physician factors (e.g., specialty), patient-physician relationship factors (e.g., duration of the relationship, number of recent visits), and health system factors (e.g., access to care).18 Patient-perceived components of physician communication that influence overall quality of care include: affective behavior, information exchange, and physician knowledge of the patient-as-person.19
The purpose of the present analysis was to examine CHA disclosure to follow-up care physicians in a diagnostically diverse sample of cancer survivors by describing rates, reasons, and predictors of CHA disclosure with a focus on patient-centered communication and responses from physicians. Results add to the limited literature on predictors of CHA disclosure8,16,20 and understanding of disclosure of CHA within the broader context of patient-centered communication in cancer care.21,22
Methods
Study Population
We analyzed survey data collected as part of the Assessment of Patient Experiences of Cancer Care study (APECC; http://appliedresearch.cancer.gov/surveys/apecc/). APECC is a population-based study that aimed to assess cancer survivors’ experiences of their follow-up cancer care. This included a comprehensive assessment of physicians’ communication behavior that is previously presented in detail along with the other methods of this study.19,23 Briefly, respondents were sampled from the Cancer Prevention Institute of California (CPIC) Surveillance Epidemiology and End Results (SEER) registry. To be eligible, survivors had to read English; be diagnosed with leukemia, bladder or colorectal cancer 2–5 years before enrolling (i.e., diagnosed in 1998–2001); be at least 20 years old at diagnosis; have received cancer treatment; have the cancer of interest as their first cancer diagnosis; not have any other cancer between their initial diagnosis and the start of the study; and have no objections from their physician to their participation. These cancer types were selected because they encompassed both genders and a broad age range. Data collection took place in April 2003–November 2004. Study procedures were approved by CPIC’s Institutional Review Board.
Measures
Use of CHA
We asked participants to report their use of 24 examples of CHA modalities during the 12 months prior to interview. We categorized these modalities to be consistent with the definition used by the National Center for Complementary and Alternative Medicine: (1) Natural products (high-dose or mega vitamins [not including 1-a-day multivitamins], nutritional supplements, or herbal remedies); (2) Mind and body practices (movement or physical therapies such as yoga, tai chi, massage, chiropractic, or electromagnetic therapy; mind-body therapies such as guided imagery/visualization, biofeedback, meditation, relaxation techniques, hypnosis/hypnotherapy; energy healing, therapeutic touch, or music therapy; oriental therapies such as acupuncture, acupressure, Qigong, or Shiatsu; faith healing, laying on of hands, or any other spiritual or religious group participation); and (3) Other CHA (homeopathy).24 Approaches such as special diets, support groups; psychological therapy or counseling from a psychologist, psychiatrist, social worker, or any other mental health professional5 were not included in our definition of CHA use because these practices have emerged within conventional medicine.24 Prevalence rates for these practices were reported to facilitate comparisons to studies published using prior definitions of CHA.
CHA Disclosure
Cancer survivors who reported use of CHA were asked, “Did you discuss your use of any of these complementary and alternative therapies with your follow-up care doctor in the last 12 months?” (yes/no).
Reasons for Not Disclosing
Cancer survivors who reported not discussing CHA to their physician were asked to select any of the following reasons that applied for not doing so: “Your doctor never asked”; “You thought that your doctor wouldn’t approve”; “It wasn’t important for you to tell your doctor”; “You felt your doctor might refuse to continue to be your doctor”; or “Other.”
Physician Response to Disclosure
Survivors who disclosed CHA were asked which best characterized their physician’s response: “Encouraged you to use it”; “Didn’t care whether you used it or not”; “Told you about the risks in using it”; “Encouraged you to stop using it”; “Made no comment”; or “Other.”
Physician Communication
Patient response to a 17-item measure of perceived patient-centeredness was assessed with three scales confirmed in a factor analysis and described in detail in a prior publication of these data19 and then summarized into one combined index score. The three scales addressed: information exchange: 10 items (e.g., “How often did your follow-up care doctor answer your cancer-related questions to your satisfaction?”; α=0.92), physician’s affective behavior: 4 items (e.g., “How often was your follow-up care doctor caring and kind?”; α=0.92), and physician’s knowledge of the patient-as-person including: 3 items (e.g., “How would you rate your follow-up care doctor’s knowledge of how cancer and the medical treatments you received for cancer have affected the quality of your life?”; α=0.86).19 All scale scores were linearly transformed to a 0 to 100 range, with a higher score representing more positive quality assessments. The combined index created for this study was a mean of these three transformed and highly correlated (r’s=0.64–0.79) scores.
Patient Factors
Sociodemographic characteristics included age, gender, race/ethnicity (white/non-Hispanic, other), married/living as married (or other), and education completed (high school graduate/GED or less, some college/technical/vocational school or more). Clinical characteristics included type of cancer and perceived health status (fair/poor, good, very good/excellent).
Physician Factors
Physician specialty was assessed (primary care or hematology/oncology specialty, other specialty including gastroenterologist, radiation oncologist, surgeon, or urologist) to account for physician factors.
Patient-Physician Relationship Factors
Relationship factors assessed were the duration of relationship with physician (<2 years, ≥2 years) and number of visits to the physician in the last 12 months (≤3 visits, >3 visits).
Data Analysis
The analytic sample was comprised of 623 survivors who had seen a physician for follow-up care in the last 12 months (Figure 1). To describe the sample, chi-square tests were used for categorical patient variables and t-tests for continuous patient variables. Analyses of CHA disclosure with follow-up care physicians was limited to those survivors who had used at least one of the 24 CHA modalities assessed (N=196). A logistic regression analysis was conducted to evaluate the unadjusted association between patient-centeredness of communication and disclosure. Subsequently, patient, physician, and patient-physician relationship factors found to be significantly related to disclosure of CHA and ratings of physician communication in previous studies8,19,20,25,26 or that were conceptually important18 were selected for inclusion in four adjusted logistic regression analyses that assessed the influence of overall patient-centered communication and three specific aspects of communication on disclosure of CHA. Each measure of patient-centered communication was transformed from a scale from 0–100 to a scale from 0–10 to assist in the interpretation of odds ratios. Alpha values were two-sided at 0.05. In addition, descriptive analysis of survivors’ reasons for not disclosing CHA and follow-up care physician’s responses to disclosure of CHA were performed. Participants with missing responses (< 3%) were not included in analyses of relevant variables. All analyses were conducted using SPSS software (IBM SPSS Statistics: Version 21).
Figure 1.
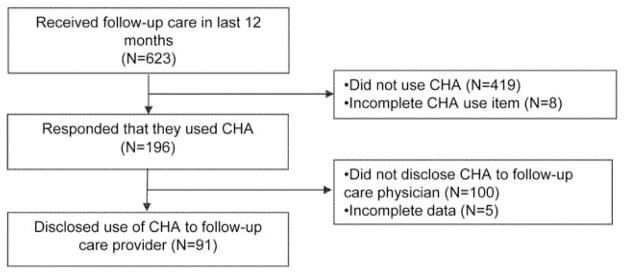
Survey response and data analysis. CHA indicates complementary health approach.
Results
Sample Description
The sample included 623 survivors, diagnosed 2–5 years before study interview and between the ages of 23 and 95 (Table 1). Overall, 31.5% of the sample reported having used CHA (44.3% of the sample including special diets and psychological support). Of those who used CHA, 42.3% used natural products, 78.1% used mind and body practices, and 7.7% other CHA. CHA use was more prevalent among survivors who were younger (p<0. 01), female (p<0.001), not married or living as married (p<0.05), and more highly educated (p<0.01).
Table 1.
Description of patient characteristics for total sample, users of Complementary Health Approaches and non CHA users
| Total N = 623 |
Used CHA† N = 196 |
Did Not Use CHA N = 419 |
|
|---|---|---|---|
|
| |||
| Age M(SD) | 62.6 (12.9) | 60.0 (12.6)** | 63.8 (12.89) |
|
| |||
| N (%) | N (%) | N (%) | |
| Gender | *** | ||
| Male | 353 (56.7) | 89 (45.4) | 259 (61.8) |
| Female | 270 (43.3) | 107 (54.6) | 160 (38.2) |
|
| |||
| Race/ethnicity | NS | ||
| White, Non-Hispanic | 460 (73.8) | 141 (71.9) | 316 (75.4) |
| Other | 163 (26.2) | 55 (28.1) | 103 (24.6) |
|
| |||
| Marital status | * | ||
| Other | 177 (28.5) | 67 (34.1) | 104 (24.9) |
| Married/living as married | 445 (71.4) | 129 (65.8) | 314 (75.1) |
|
| |||
| Education completed | ** | ||
| High school or less | 130 (20.9) | 25 (12.8) | 100 (23.9) |
| Some college or more | 492 (79.0) | 171 (87.2) | 318 (76.1) |
|
| |||
| Type of cancer | NS | ||
| Colorectal | 371 (59.6) | 122 (62.2) | 244 (58.2) |
| Bladder | 163 (26.2) | 41 (20.9) | 121 (28.9) |
| Leukemia | 89 (14.3) | 33 (16.8) | 54 (12.9) |
|
| |||
| Perceived health status | NS | ||
| Poor/Fair | 101 (16.2) | 29 (15.0) | 72 (17.2) |
| Good | 220 (35.3) | 65 (33.7) | 155 (37.0) |
| Very good/Excellent | 291 (46.7) | 99 (51.3) | 192 (45.8) |
p<.05,
p<.01,
p<.001
Significance tests compared those who endorsed the reported value to those who did not.
Note. Total scores that do not add up to 100% are due to missing responses.
Disclosure of CHA to Physician
Of those who reported using CHA in the past 12 months, less than half (47.6%) discussed CHA with their follow-up care physician. Unadjusted analyses characterizing survivors who disclosed are presented in Table 2. The unadjusted odds ratio for overall patient-centeredness of the interaction was 1.31 (95% CI [1.08, 1.59]) with a mean difference of 0.72 points, calculated by subtracting the mean scores presented in Table 2. That is, a one unit increase in the overall patient-centeredness (range 0–10) increased the odds of disclosure by 31%.
Table 2.
Unadjusted factors associated with cancer survivors’ disclosure of use of complementary health approaches (CHA) to follow-up care physicians (N=191)
| Total N (%) |
Disclosed N (%) |
Did not Disclose N (%) |
|
|---|---|---|---|
|
Patient factors
| |||
| Gender | |||
| Male | 85 (44.5) | 43 (50.6) | 42 (49.4) |
| Female | 106 (55.5) | 48 (45.3) | 58 (54.7) |
|
| |||
| Race* | |||
| White, Non-Hispanic | 138 (72.3) | 59 (42.7) | 79 (57.3) |
| Other | 53 (27.7) | 32 (60.4) | 21 (39.6) |
|
| |||
| Marital status | |||
| Other | 66 (34.6) | 32 (48.5) | 34 (51.5) |
| Married/living as married | 125 (65.4) | 59 (47.2) | 66 (52.8) |
|
| |||
| Education completed | |||
| High school or less | 23 (12.0) | 9 (39.1) | 14 (60.9) |
| Some college or more | 168 (88.0) | 82 (48.8) | 86 (51.2) |
|
| |||
| Perceived health status | |||
| Poor/Fair | 27 (14.1) | 15 (55.6) | 12 (44.4) |
| Good | 63 (33.0) | 23 (36.5) | 40 (63.5) |
| Very good/Excellent | 98 (51.3) | 51 (52.0) | 47 (48.0) |
|
| |||
|
Physician factors
| |||
| Specialty** | |||
| Primary care or hematologist/oncologist | 129 (67.5) | 70 (54.3) | 59 (45.7) |
| Other specialist | 60 (31.4)) | 20 (33.3) | 40 (66.7) |
|
| |||
|
Patient-physician relationship factors
| |||
| Duration of relationship | |||
| < 2 years | 40 (20.9) | 17 (42.5) | 23 (57.5) |
| ≥ 2 years | 151 (79.1) | 74 (49.0) | 77 (51.0) |
|
| |||
| Number visits in past year** | |||
| ≤ 3 | 112 (58.6) | 44 (39.3) | 68 (60.7) |
| > 3 | 79 (41.4) | 47 (59.5) | 32 (40.5) |
| M (SD) | M (SD) | M (SD) | |
|---|---|---|---|
| Patient factors | |||
| Age | 60.06 (12.58) | 59.38 (12.7) | 60.67 (12.5) |
| Physician communication – Total** (R: 0–10) | 8.37 (1.72) | 8.75 (1.41) | 8.03 (1.90) |
| Information exchange (R: 0–10)† | 8.91 (1.57) | 9.14 (1.22) | 8.70 (1.81) |
| Affective behavior (R: 0–10) | 9.13 (1.69) | 9.35 (1.26) | 8.93 (1.99) |
| Knowledge of survivor (R:0–10)*** | 7.08 (2.50) | 7.75 (2.31) | 6.48 (2.53) |
p = .05;
p<0.05,
p<.01,
p<.001
Note. R= Range; Total scores that do not add up to 100% are due to missing responses; 5 participants had incomplete data.
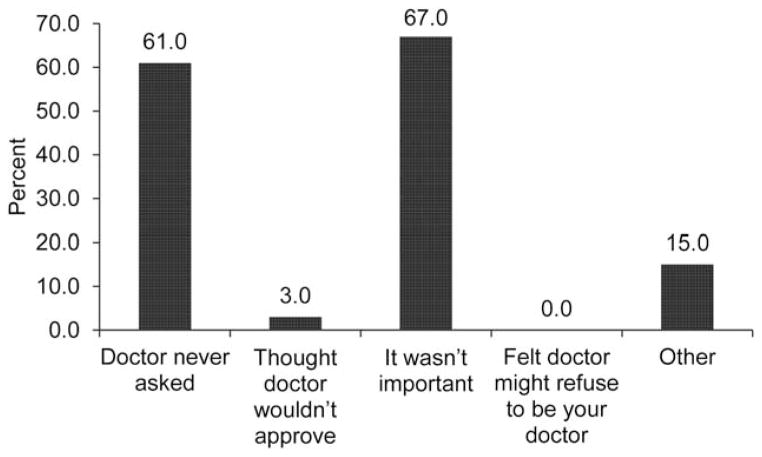
In adjusted analysis, disclosure of CHA was significantly associated with the overall patient-centeredness of physician communication with an odds ratio of 1.37 (Table 3). Further analyses revealed that patients’ perception of the physician’s knowledge of the patient-as-person and information exchange were significantly associated with disclosure, whereas affective behavior was not (Table 3). Several patient (male gender, minority race/ethnicity), physician (primary care or oncology/hematology as compared to other specialties) and patient-physician relationship (number of visits in the past year) factors were also significantly related to CHA disclosure in the overall patient-centered communication model (p’s< 0.05; Table 3). In addition, patients with better perceived health were more likely to disclose use of CHA (very good/excellent vs. good). The two most common reasons that survivors did not discuss CHA were that it wasn’t perceived as important for them to tell their doctor (67.0%; Figure 2) and their doctor never asked (61.0%). Of those who discussed CHA, most (64.8%) reported that their doctors encouraged CHA (Figure 3).
Table 3.
Logistic regression models predicting cancer survivors’ disclosure of complementary health approaches (CHA) to follow-up care physicians (N=191)
| AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | |
|---|---|---|---|---|
| Physician communicationa | 1.37 (1.09, 1.71)** | |||
|
| ||||
| Information exchangea | 1.27 (1.02, 1.60)* | |||
|
| ||||
| Affective behaviora | 1.21 (0.97, 1.51) | |||
|
| ||||
| Knowledge of survivora | 1.28 (1.10, 1.48)** | |||
|
| ||||
| Patient factors | ||||
|
| ||||
| Age | 1.00 (0.97, 1.02) | 1.00 (0.97, 1.02) | 1.00 (0.97, 1.02) | 1.00 (0.97, 1.02) |
|
| ||||
| Gender | * | * | ||
| Male | 2.04 (1.00, 4.15) | 1.88 (0.94, 3.76) | 1.90 (0.94, 3.80) | 2.06 (1.01, 4.23) |
| Female | Reference | Reference | Reference | Reference |
|
| ||||
| Race | ** | ** | ** | ** |
| White, Non-Hispanic | Reference | Reference | Reference | Reference |
| Other | 3.50 (1.59, 7.71) | 3.24 (1.50. 7.00) | 3.26 (1.51, 7.02) | 3.65 (1.64, 8.11) |
|
| ||||
| Marital status | ||||
| Other | 1.03 (0.50, 2.12) | 1.03 (0.50, 2.10) | 1.01 (0.50, 2.05) | 1.00 (0.48, 2.06) |
| Married/living as married | Reference | Reference | Reference | Reference |
|
| ||||
| Education completed | ||||
| High school or less | Reference | Reference | Reference | Reference |
| Some college or more | 1.37 (0.47, 3.98) | 1.45 (0.51, 4.17) | 1.31 (0.45, 3.81) | 1.44 (0.50, 4.20) |
|
| ||||
| Perceived health status | * | |||
| Poor/Fair | 0.67 (0.25, 1.80) | 0.65 (0.25, 1.73) | 0.63 (0.24, 1.65) | 0.69 (0.26, 1.87) |
| Good | 0.40 (0.18, 0.86)* | 0.41 (0.19, 0.87)* | 0.38 (0.18, 0.80)* | 0.40 (0.18, 0.86)* |
| Very good/Excellent | Reference | Reference | Reference | Reference |
|
| ||||
| Physician factors | ||||
|
| ||||
| Specialty | * | * | * | * |
| Primary care or hematologist/oncologist | Reference | Reference | Reference | Reference |
| Other specialist | 0.46 (0.22, 0.95) | 0.46 (0.22, 0.94) | 0.49 (0.24, 1.00) | 0.45 (0.21, 0.94) |
|
| ||||
| Patient-physician relationship factors | ||||
|
| ||||
| Duration of relationship | ||||
| < 2 years | 0.84 (0.36, 1.96) | 0.78 (0.34, 1.79) | 0.80 (0.35, 1.84) | 0.86 (0.37, 2.01) |
| ≥ 2 years | Reference | Reference | Reference | Reference |
|
| ||||
| Number visits in past year | ** | ** | ** | ** |
| ≤ 3 | 0.36 (0.18, 0.73) | 0.34 (0.17, 0.70) | 0.34 (0.17, 0.69) | 0.38 (0.19, 0.77) |
| > 3 | Reference | Reference | Reference | Reference |
p<0.05,
p<0.01
Odds ratios displayed are per 10-point change
Note. AOR = adjusted odds ratio, adjusted for the other variables in the table; CI = confidence interval.
Figure 2.
Reasons that patients did not disclose complementary health approaches to physicians (n=100). Patients could endorse more than 1 response, so the total is >100%.
Figure 3.
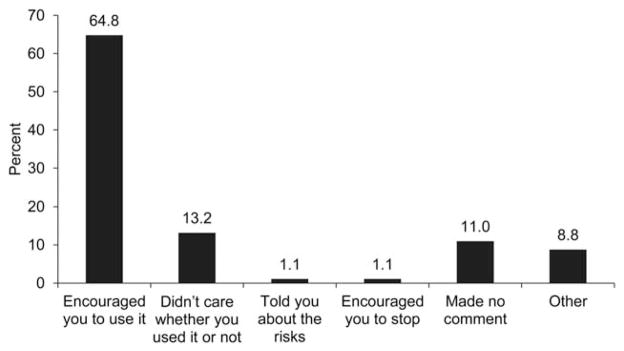
Descriptions of patient reports of physicians’ responses to the disclosed use of complementary health approaches (n=91).
Discussion
In this population-based study of leukemia, colorectal, and bladder cancer survivors 2–5 years post-diagnosis, we found that almost a third (31.5%) reported using CHA, and overall patient-centeredness of communication with physicians was associated with the likelihood of disclosing CHA. The size of this effect was approximately half a standard deviation, which is considered clinically meaningful for quality of life, although not specifically for this construct.27 Unique components of communication associated with disclosure were the physician’s (1) contextual knowledge of cancer survivors and (2) quality of information exchange. Factors related to the physician (specialty: primary care or hematologist/oncologist), patient (male gender, minority race/ethnicity), and patient-physician relationship (longer duration of relationship) were also significantly associated with increased disclosure.
Estimates of CHA used in this sample are comparable to other studies of cancer survivors.5–7 Less than half of participants reported disclosing CHA to their follow-up care physicians, a disclosure rate also similar to those found in other studies (generally 40–50%).8 Further, an important finding was that whereas CHA use did not differ by perceived health status, patients with very good/excellent self-reported health were more likely to disclose CHA to their physicians than those who rated their health as good. These results suggest that physicians need to more actively solicit information about CHA from patients who perceive their health as worse.
Consistent with other studies, survivors in this study indicated that they did not disclose CHA to their physician because they thought it was not important to discuss or because their physician did not ask them.8,15 Cancer survivors and physicians need to be educated about the importance of discussing CHA use given safety concerns as well as possible health benefits.10–12,14 This study further supports that a majority of physicians have a positive response to disclosure of CHA.8,28 Results from other studies add that discussion of CHA has the potential to improve physician-patient relationships and satisfaction with care.29,30 Expertise in CHA is not necessary to initiate a respectful discussion,31 and investigators are beginning to explore how to enhance such discussions 32–34 by providing clinicians and patients with supplemental education on CHA and referring patients to support services.32 Efforts to train “integrative health coaches,” who emphasize patient-centered care35 and use of CHA36 are also growing.
Results from the current study demonstrate that physician knowledge of patient-as-person and information exchange quality are associated with increased disclosure of CHA and add to the literature describing the role of patient-centered communication in promoting positive health outcomes.37 This is clinically relevant because implementing robust patient-centered communication may have the secondary benefit of prompting discussions of unmet needs that cancer survivors are attempting to address with CHA.3 Furthermore, patient-centered discussions can encourage patients’ involvement in their care and increase sense of control,37 both of which are reasons frequently given for CHA use.4 Therefore, enhancing overall patient-centered communication used by physicians, absent a specific CHA-focused intervention, may begin to decrease patients’ need to seek unconventional care without first discussing potential risks and benefits with their physician.
This study has some limitations. The data are cross-sectional, so it is not possible to determine the direction of the relationship between CHA disclosure and patient-centeredness of communication. Another interpretation could be that disclosing CHA increases patient’s perception of the patient-centeredness of communication.30 In addition, participants were leukemia, bladder, and colorectal cancer survivors recruited from Northern California. Although results on disclosure rates and reasons were consistent with those found in breast cancer survivors, they may not be generalizable to survivors of all cancer types or in other regions. Furthermore, these data were collected approximately ten years ago, and disclosure rates may have since changed due to an increase in information provided on CHA related to cancer care;38 however, rates of disclosure in more recent studies are similarly variable to prior studies (e.g., 20–71%).5, 39 Relationships among variables were the primary focus of this study and are even less likely to change over time. Lastly, future studies may choose to further explore if disclosure of specific modalities varied within the broad categories of CHA used in these analyses.
Strengths of this study include the comprehensive assessment of patient-centered communication not included in other studies of CHA disclosure. Thus a unique contribution of our study includes the finding that patient-centered communication and CHA disclosure are positively associated, which suggests that improving patient-centered communication and disclosure of CHA are potentially synergistic clinical goals. In addition, results regarding the patient, physician, and patient-physician relationship factors associated with disclosure of CHA uniquely add to broader understanding of how improving patient-centered care can result in a positive impact on follow-up care for cancer survivors. Future research is needed to determine the direction of the relationship between patient-centered communication and CHA disclosure and whether specifically educating physicians and patients on the benefits of CHA disclosure and potential harms of non-disclosure will further increase discussions of CHA use.
Acknowledgments
This manuscript was supported by the National Institutes of Health (NIH) contract N01-PC-35136/PC/NCI NIH HHS/United States and grant Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Scholar Program (2K12HD043483-11).
Footnotes
There are no financial disclosures from any authors.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Howlader N, Noone AN, Krapcho M, Garshell J, Neyman N, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2010 [Internet] Bethesda, MD: National Cancer Institute; [accessed Jan 7, 2014]. Available from URL: http://seer.cancer.gov/csr/1975_2010/ [Google Scholar]
- 2.Institute of Medicine (IOM) Cancer care for the whole patient: Meeting psychosocial health needs. Washington DC: National Academies Press; 2008. [PubMed] [Google Scholar]
- 3.Mao JJ, Palmer SC, Straton JB, Cronholm PF, Keddem S, Knott K, et al. Cancer survivors with unmet needs were more likely to use complementary and alternative medicine. J Cancer Surviv. 2008;2:116–124. doi: 10.1007/s11764-008-0052-3. [DOI] [PubMed] [Google Scholar]
- 4.Verhoef MJ, Balneaves LG, Boon HS, Vroegindewey A. Reasons for and characteristics associated with complementary and alternative medicine use among adult cancer patients: A systematic review. Integr Cancer Ther. 2005;4:274–286. doi: 10.1177/1534735405282361. [DOI] [PubMed] [Google Scholar]
- 5.Mao J, Palmer C, Healy K, Desai K, Amsterdam J. Complementary and alternative medicine use among cancer survivors: a population-based study. J Cancer Surviv. 2011;5:8–17. doi: 10.1007/s11764-010-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horneber M, Bueschel G, Dennert G, Less D, Ritter E, Zwahlen M. How many cancer patients use complementary and alternative medicine: a systematic review and meta-analysis. Integr Cancer Ther. 2012;11:187–203. doi: 10.1177/1534735411423920. [DOI] [PubMed] [Google Scholar]
- 7.Sohl SJ, Weaver KE, Birdee G, Kent EE, Danhauer SC, Hamilton AS. Characteristics associated with the use of complementary health approaches among long-term cancer survivors. Support Care Cancer. 2014;22:927–36. doi: 10.1007/s00520-013-2040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis EL, Oh B, Butow PN, Mullan BA, Clarke S. Cancer patient disclosure and patient-doctor communication of complementary and alternative medicine use: a systematic review. Oncologist. 2012;17:1475–1481. doi: 10.1634/theoncologist.2012-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briggs JP, Killen J. Perspectives on complementary and alternative medicine research. JAMA. 2013;310:691–692. doi: 10.1001/jama.2013.6540. [DOI] [PubMed] [Google Scholar]
- 10.Chen J-L, Wang J-Y, Tsai Y-F, Lin Y-H, Tseng L-M, Chang W-C, et al. In vivo and in vitro demonstration of herb-drug interference in human breast cancer cells treated with tamoxifen and trastuzumab. Menopause. 2013;20:646–54. doi: 10.1097/gme.0b013e31827b2240. [DOI] [PubMed] [Google Scholar]
- 11.Fong HHS. Integration of herbal medicine into modern medical practices: issues and prospects. Integr Cancer Ther. 2002;1:287–293. doi: 10.1177/153473540200100313. [DOI] [PubMed] [Google Scholar]
- 12.Perlman A, Lontok O, Huhmann M, Parrott JS, Simmons LA, Patrick-Miller L. Prevalence and correlates of postdiagnosis initiation of complementary and alternative medicine among patients at a comprehensive cancer center. J Oncol Pract. 2013;9:34–41. doi: 10.1200/JOP.2012.000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newmaster SG, Grguric M, Shanmughanandhan D, Ramalingam S, Ragupathy S. DNA barcoding detects contamination and substitution in North American herbal products. BMC Med. 2013;11:222. doi: 10.1186/1741-7015-11-222. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Chandwani KD, Ryan JL, Peppone LJ, Janelsins MM, Sprod LK, Devine K, et al. Cancer-related stress and complementary and alternative medicine: a review. Evid-Based Complement Altern Med. 2012;2012:979213. doi: 10.1155/2012/979213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson A, McGrail MR. Disclosure of CAM use to medical practitioners: a review of qualitative and quantitative studies. Complement Ther Med. 2004;12:90–98. doi: 10.1016/j.ctim.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Chao MT, Wade C, Kronenberg F. Disclosure of complementary and alternative medicine to conventional medical providers: variation by race/ethnicity and type of CAM. J Natl Med Assoc. 2008;100:1341–1349. doi: 10.1016/s0027-9684(15)31514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adler SR, Fosket JR. Disclosing complementary and alternative medicine use in the medical encounter: a qualitative study in women with breast cancer. J Fam Pract. 1999;48:453–458. [PubMed] [Google Scholar]
- 18.Epstein RM, Franks P, Fiscella K, Shields CG, Meldrum SC, Kravitz RL, et al. Measuring patient-centered communication in patient–physician consultations: Theoretical and practical issues. Soc Sci Med. 2005;61:1516–1528. doi: 10.1016/j.socscimed.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Arora NK, Reeve BB, Hays RD, Clauser SB, Oakley-Girvan I. Assessment of quality of cancer-related follow-up care from the cancer survivor’s perspective. J Clin Oncol. 2011;29:1280–1289. doi: 10.1200/JCO.2010.32.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge J, Fishman J, Vapiwala N, Li SQ, Desai K, Xie SX, et al. Patient-physician communication about complementary and alternative medicine in a radiation oncology setting. Int J Radiat Oncol. 2013;85:e1–e6. doi: 10.1016/j.ijrobp.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epstein RM, Street RL. Patient-Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. Bethesda, MD: National Cancer Institute; 2007. NIH Publication No. 07-6225. [Google Scholar]
- 22.McCormack LA, Treiman K, Rupert D, Williams-Piehota P, Nadler E, Arora NK, et al. Measuring patient-centered communication in cancer care: a literature review and the development of a systematic approach. Soc Sci Med. 2011;72:1085–1095. doi: 10.1016/j.socscimed.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Kent EE, Mitchell SA, Oakley-Girvan I, Arora NK. The importance of symptom surveillance during follow-up care of leukemia, bladder, and colorectal cancer survivors. Support Care Cancer. 2014;22:163–72. doi: 10.1007/s00520-013-1961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. [accessed August 14, 2013];Complementary, Alternative, or Integrative Health: What’s In a Name? [Internet]; Available from URL: http://nccam.nih.gov/health/whatiscam.
- 25.Saxe GA, Madlensky L, Kealey S, Wu DPH, Freeman KL, Pierce JP. Disclosure to physicians of CAM use by breast cancer patients: findings from the Women’s Healthy Eating and Living Study. Integr Cancer Ther. 2008;7:122–129. doi: 10.1177/1534735408323081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashikaga T, Bosompra K, O’Brien P, Nelson L. Use of complementary and alternative medicine by breast cancer patients: prevalence, patterns and communication with physicians. Support Care Cancer. 2002;10:542–548. doi: 10.1007/s00520-002-0356-1. [DOI] [PubMed] [Google Scholar]
- 27.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 28.Tasaki K, Maskarinec G, Shumay DM, Tatsumura Y, Kakai H. Communication between physicians and cancer patients about complementary and alternative medicine: exploring patients’ perspectives. Psychooncology. 2002;11:212–220. doi: 10.1002/pon.552. [DOI] [PubMed] [Google Scholar]
- 29.Roberts CS, Baker F, Hann D, Runfola J, Witt C, McDonald J, et al. Patient-physician communication regarding use of complementary therapies during cancer treatment. J Psychosoc Oncol. 2005;23:35–60. doi: 10.1300/j077v23n04_03. [DOI] [PubMed] [Google Scholar]
- 30.Oh B, Butow P, Mullan B, Clarke S, Tattersall M, Boyer M, et al. Patient-doctor communication: use of complementary and alternative medicine by adult patients with cancer. J Soc Integr Oncol. 2010;8:56–64. [PubMed] [Google Scholar]
- 31.Schofield P, Diggens J, Charleson C, Marigliani R, Jefford M. Effectively discussing complementary and alternative medicine in a conventional oncology setting: communication recommendations for clinicians. Patient Educ Couns. 2010;79:143–151. doi: 10.1016/j.pec.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 32.Balneaves LG, Truant TLO, Verhoef MJ, Ross B, Porcino AJ, Wong M, et al. The Complementary Medicine Education and Outcomes (CAMEO) program: A foundation for patient and health professional education and decision support programs. Patient Educ Couns. 2012;89:461–466. doi: 10.1016/j.pec.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Parker PA, Urbauer D, Fisch MJ, Fellman B, Hough H, Miller J, et al. A multisite, community oncology-based randomized trial of a brief educational intervention to increase communication regarding complementary and alternative medicine. Cancer. 2013;119:3514–22. doi: 10.1002/cncr.28240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frenkel M, Cohen L. Effective communication about the use of complementary and integrative medicine in cancer care. J Altern Complement Med. 2014;20:12–8. doi: 10.1089/acm.2012.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolever RQ, Simmons LA, Sforzo GA, Dill D, Kaye M, Bechard EM, et al. A systematic review of the literature on health and wellness coaching: Defining a key behavioral intervention in healthcare. Glob Adv Health Med. 2013;2:34–53. doi: 10.7453/gahmj.2013.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolever RQ, Caldwell KL, Wakefield JP, Little KJ, Gresko J, Shaw A, et al. Integrative health coaching: an organizational case study. Explore. 2011;7:30–36. doi: 10.1016/j.explore.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Street RL, Jr, Makoul G, Arora NK, Epstein RM. How does communication heal? Pathways linking clinician-patient communication to health outcomes. Patient Educ Couns. 2009;74:295–301. doi: 10.1016/j.pec.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 38.Brauer JA, El Sehamy A, Metz JM, Mao JJ. Complementary and alternative medicine and supportive care at leading cancer centers: a systematic analysis of websites. J Altern Complement Med. 2010;16:183–6. doi: 10.1089/acm.2009.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King N, Balneaves L, Card C, Nation J, Nguyen T, Carlson L. Surveys of cancer patients and cancer care providers regarding complementary therapy use, communication and information needs. J Altern Complement Med. 2014;20:A98. doi: 10.1177/1534735415589984. [DOI] [PubMed] [Google Scholar]