Summary
Interventions that delay ageing mobilize mechanisms that protect and repair cellular components1–3, but it is unknown how these interventions might slow the functional decline of extracellular matrices4,5, which are also damaged during ageing6,7. Reduced Insulin/IGF-1 signalling (rIIS) extends lifespan across the evolutionary spectrum, and in juvenile C. elegans also allows the transcription factor DAF-16/FOXO to induce development into dauer, a diapause that withstands harsh conditions (Supplementary Discussion)1,2. It has been suggested that rIIS delays C. elegans ageing through activation of dauer-related processes during adulthood2,8,9, but some rIIS conditions confer robust lifespan extension unaccompanied by any dauer-like traits1,10,11. Here we show that rIIS can promote C. elegans longevity through an program that is genetically distinct from the dauer pathway, and requires the Nrf (NF-E2-related factor) ortholog SKN-1 acting in parallel to DAF-16. SKN-1 is inhibited by IIS and has been broadly implicated in longevity12–14, but is rendered dispensable for rIIS lifespan extension by even mild activity of dauer-related processes. When IIS is decreased under conditions that do not induce dauer traits, SKN-1 most prominently increases expression of collagens and other extracellular matrix (ECM) genes. Diverse genetic, nutritional, and pharmacological pro-longevity interventions delay an age-related decline in collagen expression. These collagens mediate adulthood ECM remodelling, and are needed for ageing to be delayed by interventions that do not involve dauer traits. By genetically delineating a dauer-independent rIIS ageing pathway, our results show that IIS controls a broad set of protective mechanisms during C. elegans adulthood, and may facilitate elucidation of processes of general importance for longevity. The importance of collagen production in diverse anti-ageing interventions implies that ECM remodelling is a generally essential signature of longevity assurance, and that agents promoting ECM youthfulness may have systemic benefit.
Results and Discussion
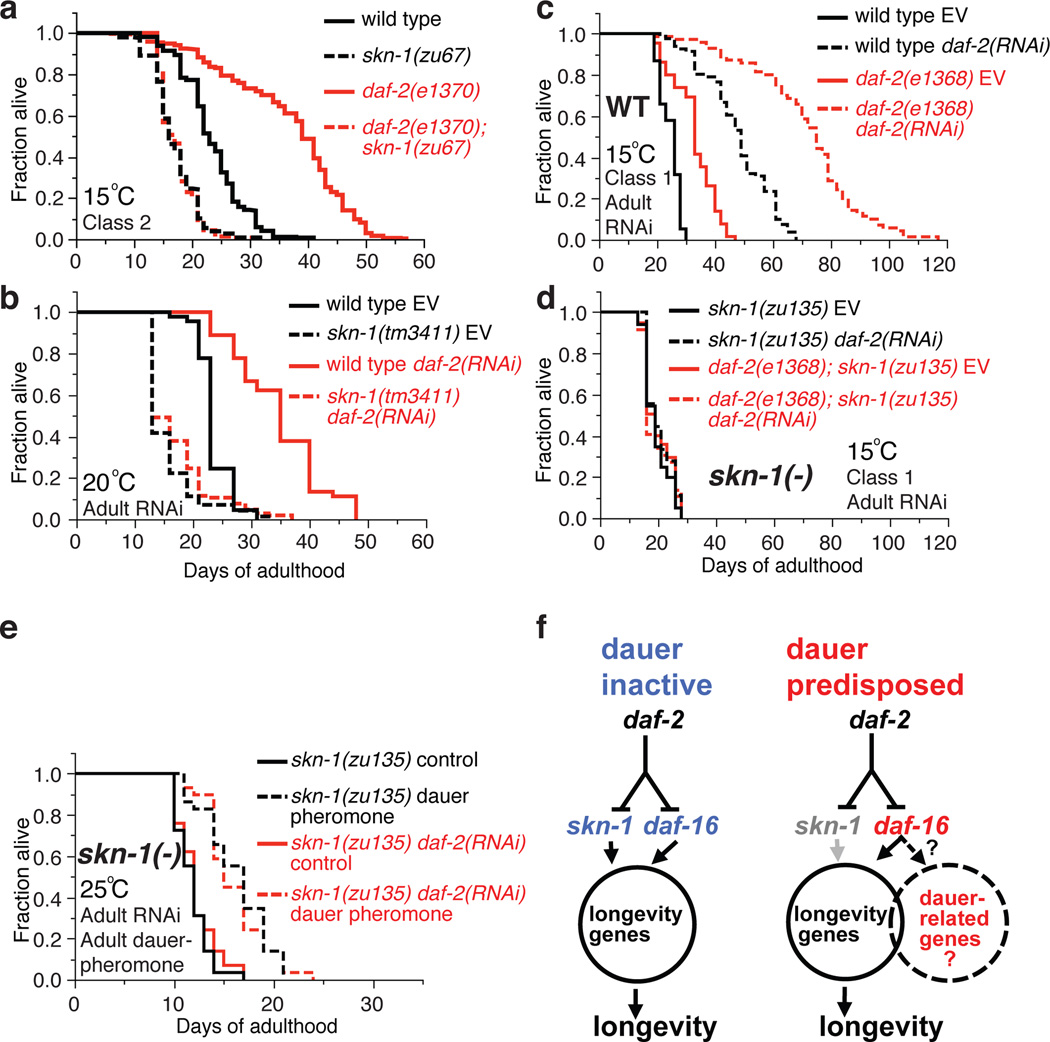
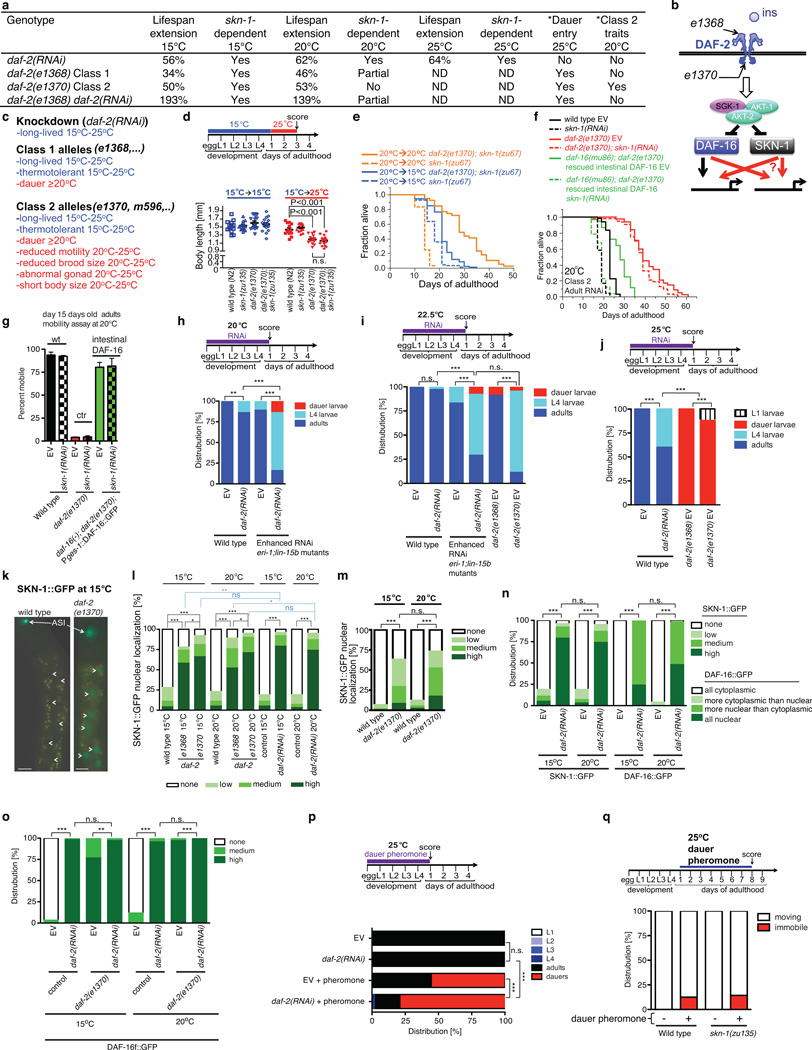
We hypothesized that SKN-1 would be required for rIIS lifespan extension under conditions in which dauer-associated processes are inactive. Class 2 mutations in the insulin/IGF-1 receptor DAF-2 induce adulthood dauer-related traits that are mild at 20°C, and severe at 22.5°C or above, but Class 1 mutations do not (Video 1, 2; Supplementary Discussion)10. SKN-1 is inhibited by IIS phosphorylation but is dispensable for dauer development13, adulthood dauer-related traits (Extended Data Fig. 1a–d; Supplementary Table 1), or lifespan extension by Class 2 daf-2 mutations at 20°C (Extended Data Fig. 1a and Supplementary Table 2)13. By contrast, at 15°C SKN-1 was completely required for longevity in the same Class 2 daf-2 mutants (Fig. 1a; Extended Data Fig. 1a, 1e, Extended Data Table 1, and Supplementary Table 2), which do not show dauer traits at 15°C10 because low temperature inhibits dauer entry (Supplementary Discussion). skn-1 was also essential at 20°C in Class 2 daf-2; daf-16 double mutants that expressed DAF-16 specifically in the intestine, a condition that rescues longevity but not dauer development1,15 or traits (Extended Data Fig. 1f, 1g and Table 1). Finally, skn-1 was required at 15°C, 20°C, or 25°C for lifespan extension from daf-2 RNA interference (RNAi) (Fig. 1b, Extended Data Fig. 1a and Table 1, and Supplementary Table 2), which promotes dauer entry only at extreme temperature and does not induce dauer traits in adults (Extended Data Fig. 1h–j). In these last two scenarios, the absence of dauer traits may reflect DAF-16 insufficiency in neurons, which are central to dauer regulation15,16 and resistant to RNAi (Extended Data Fig. 1h, 1i, and Table 1). Lifespan extension is extremely robust when daf-2 RNAi is performed in the Class 1 mutant daf-2(e1368)11, which lacks adulthood dauer traits but predisposes to dauer entry10. skn-1 was largely required for this lifespan extension at 20°C, and was essential for the even greater healthy lifespan extension seen at 15°C (117 days maximum; Fig.1c, 1d; Extended Data Fig. 1a and Table 1).
Figure 1. Dauer-independent rIIS longevity requires SKN-1.
a, b, skn-1-dependent rIIS lifespan extension in the absence of dauer traits. c, d, skn-1-dependent extreme rIIS longevity. EV: empty RNAi vector. e, skn-1-independent longevity from adulthood dauer pheromone treatment but not daf-2(RNAi). f, Longevity assurance programs regulated by IIS. Under conditions that predispose to dauer traits (right panel) some SKN-1 functions may be assumed by DAF-16, possibly including ECM remodelling. Statistics and additional lifespan data are in Extended Data Table 1 and Supplementary Table 2.
The skn-1-dependence of rIIS longevity tracked inversely with predisposition to dauer entry or adulthood dauer traits, and was not determined by temperature (Extended Data Fig. 1a). skn-1-dependence also did not correlate with the magnitude of rIIS lifespan extension, suggesting that it was not determined by the extent of IIS reduction (Extended Data Fig. 1a). Accordingly, DAF-16 and SKN-1 nuclear localization was increased as robustly by daf-2 RNAi as by Class 1 or Class 2 daf-2 mutations, and was similar in daf-2 mutants at 15°C and 20°C (Extended Data Fig. 1k–o). Activation of dauer processes in adults by a mechanism other than genetic IIS reduction should extend lifespan without skn-1. Accordingly, skn-1 was dispensable for lifespan extension from adulthood dauer pheromone exposure (Fig. 1e, Extended Data Fig. 1p, 1q and Table 1).
We conclude that skn-1 is needed for rIIS longevity specifically when dauer-associated mechanisms are inactive (Extended Data Fig. 1a). This genetic requirement for skn-1 reveals that rIIS extends lifespan through two downstream pathways that may overlap (Fig. 1f). During the reproductive life cycle, IIS inhibits a protective program that requires both DAF-16 and SKN-1, and does not involve dauer-specific processes. This program may be controlled mainly by IIS acting outside the nervous system. The requirement for SKN-1 for lifespan extension is relieved under conditions that activate vestiges of the dauer developmental pathway in adults.
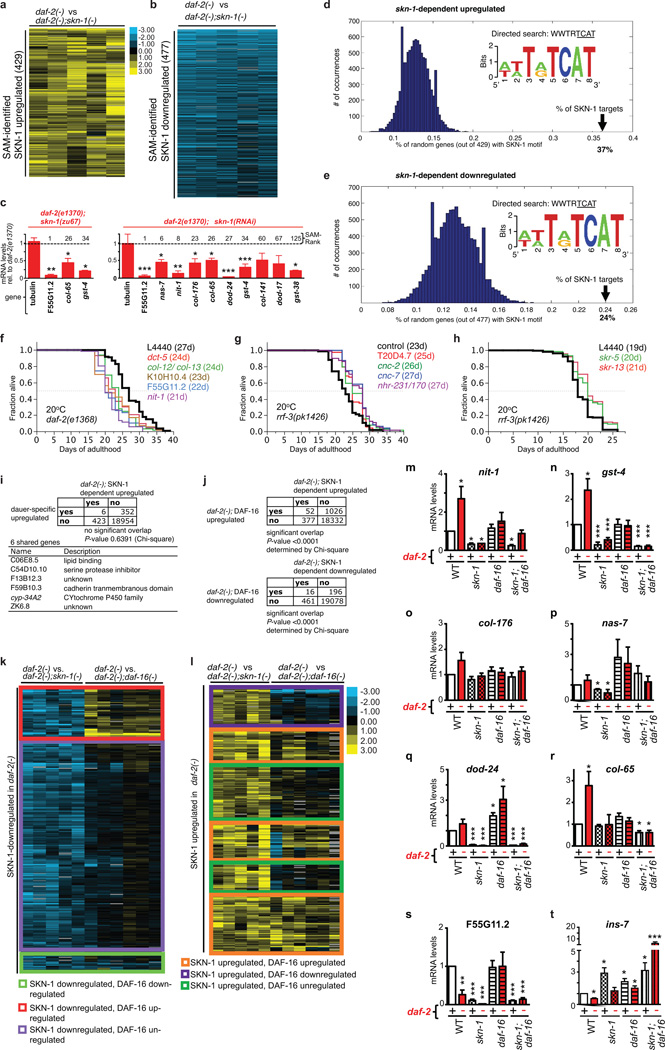
Analyses of how rIIS affects ageing have typically involved conditions that predispose to mild or even severe dauer-related traits (Supplementary Discussion), and would therefore allow skn-1-independent lifespan extension. We investigated the basis for dauer-independent rIIS longevity by identifying genes that are regulated by SKN-1 in daf-2 mutants at 15°C. At a false discovery rate of <3%, microarrays identified 429 genes with higher expression in daf-2(−) than daf-2(−); skn-1(−) animals (SKN-1-upregulated daf-2(−) genes), and 477 SKN-1-downregulated daf-2(−) genes, including direct and indirect SKN-1 targets (Extended Data Fig. 2a–e; Supplementary Table 3). Many of these genes affected lifespan as would be predicted by these expression patterns (Extended Data Fig. 2f–h; Supplementary Table 4, 5). Overlap with a dauer-expressed gene set was insignificant, as was overlap between SKN-1- and DAF-16-downregulated daf-2(−) genes (Extended Data Fig. 2i–k). However, many SKN-1-upregulated daf-2(−) genes were activated by DAF-16 (Extended Data Fig. 2j, 2l-t), which is also required for daf-2 lifespan extension at 15°C17, indicating that SKN-1 responds to rIIS by functioning in parallel to and independently of DAF-16.
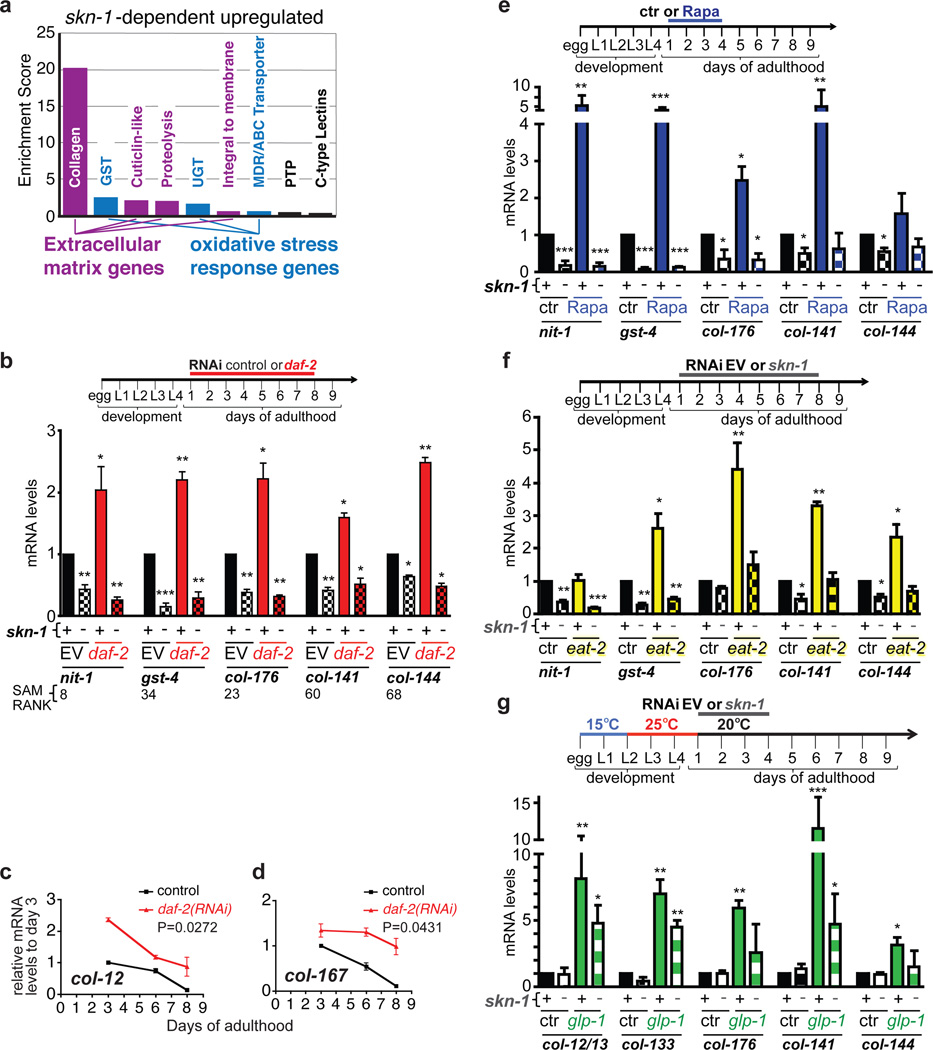
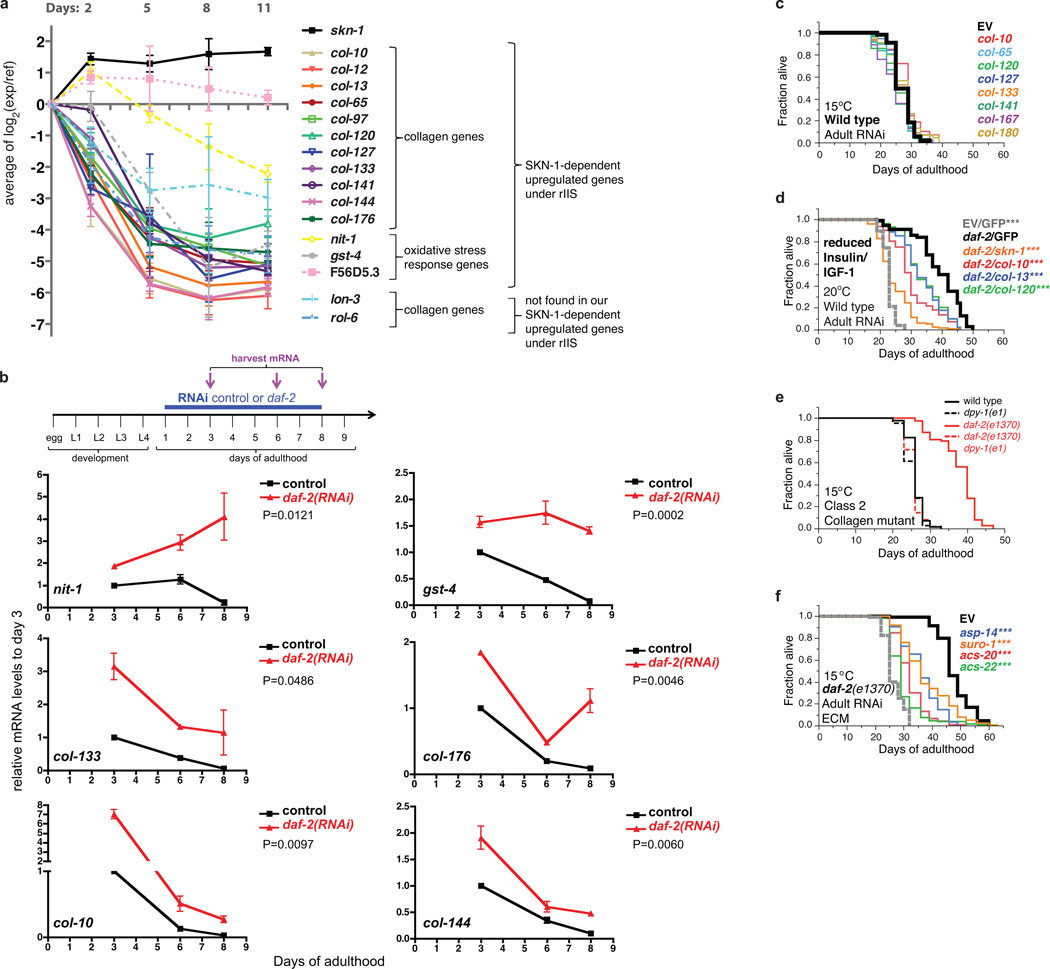
SKN-1 has conserved functions in stress defence, protein homeostasis, and metabolism12,18,19 and was required for daf-2 oxidative stress resistance (Supplementary Table 6)13, but only 40/429 SKN-1-upregulated daf-2(−) genes had been identified under normal or stress conditions (Extended Data Fig. 3a–g; Supplementary Table 7)18. Unexpectedly, by far the most overrepresented functional group within the SKN-1-upregulated daf-2(−) gene set consisted of collagen genes, which seemed to be regulated by SKN-1 indirectly (Fig. 2a, Supplementary Table 3, 8, and 9). In humans, collagens constitute about 1/3 of all protein and accumulate damage during ageing, leading to functional decline in tissues throughout the body6,7. C. elegans collagens form basement membranes as well as the cuticle, a complex structure that covers the animal, lines the buccal cavity, pharynx, and rectum, and becomes thickened and wrinkled with age20. The SKN-1-upregulated daf-2(−) collagens are of the type that forms the cuticle, but are expressed in multiple tissues (Extended Data Fig. 3h; Supplementary Table 9). Collagen production decreases in human skin during ageing21, and 27 SKN-1-upregulated daf-2(−) collagens are among a set of genes that decline in expression as C. elegans ages22 (Supplementary Table 10). These and other collagens were prominently upregulated in each of 20 C. elegans longevity-associated gene sets we examined (Extended Data Table 2; Supplementary Table 10). Moreover, in mice extracellular matrix genes were overrepresented in some longevity or Nrf2-dependent sets (Supplementary tables 11, 12), and in silico analysis of longevity-associated genes identified a predicted ECM network23. The possible significance of these expression signatures has not been explored.
Figure 2. Longevity-promoting interventions increase skn-1 dependent collagen expression in adults.
a, Functional categories enriched in SKN-1-upregulated daf-2(−) gene sets, identified by DAVID. Enrichment scores ≥ 1.3 are shown. b-d, Collagen upregulation by adulthood daf-2 RNAi. mRNA expression in wild type (+) or skn-1(zu135) (−) animals, assayed by qRT-PCR. nit-1 and gst-4 are canonical SKN-1 targets18. SAM score ranks are in Supplementary Table 3. e, Rapamycin-treated (100 µM) wild type and skn-1(zu67) animals are compared. f. Expression in the DR model eat-2. RNAi-sensitized control (rrf-3(pk1426)) (ctr) or eat-2(ad1116); rrf-3(pk1426) (eat-2) adults were exposed to EV or skn-1 RNAi. g. Upregulation after germline stem cell proliferation block induced by glp-1(bn18) temperature shift. 3 replicates of 200 worms were analysed at the indicated days (c, d) or at the end of treatment. Data are represented as mean ± s.e.m. P value *<0.05, **<0.001, ***<0.0001 relative to wild type or control, by one sample t-test, two-tailed, hypothetical mean of 1.
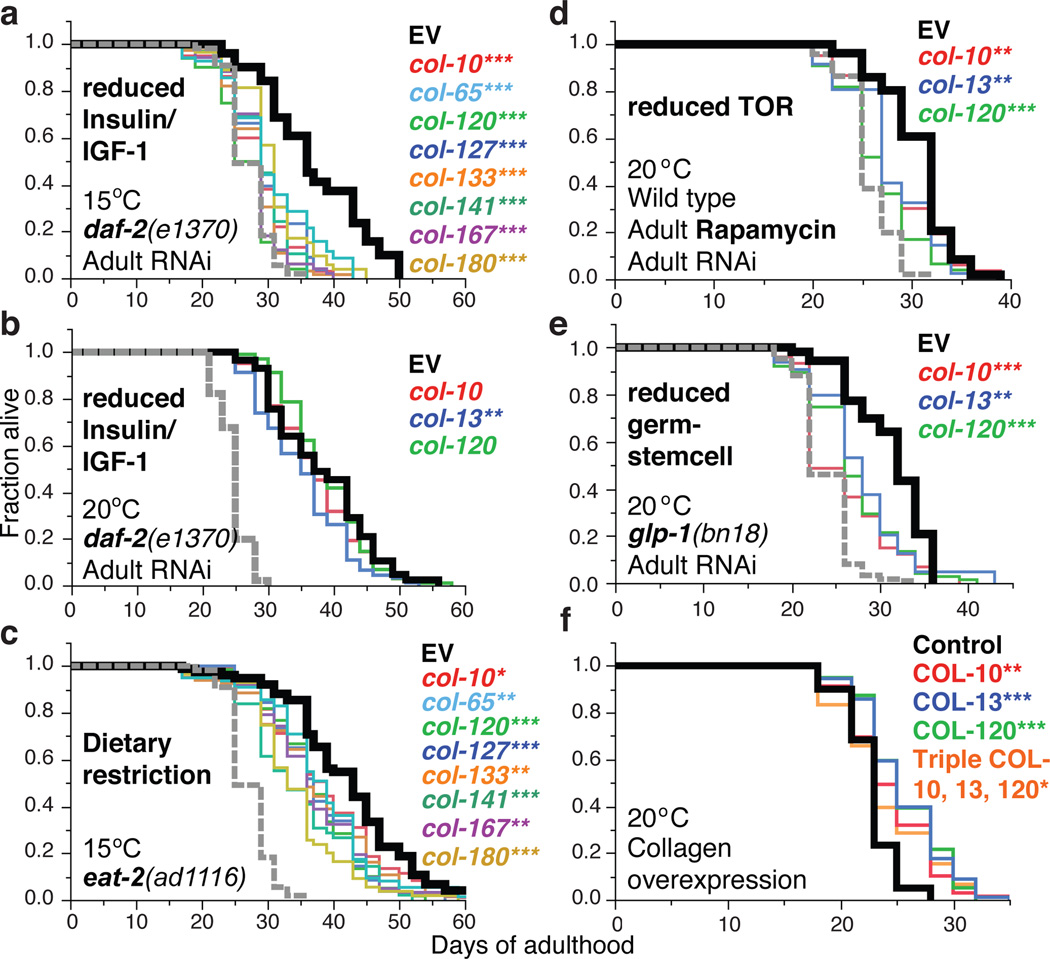
We investigated the functional importance of specific SKN-1-upregulated daf-2(−) collagen genes that decline during aging, and are upregulated in other longevity-associated gene sets (Extended Data Table 2). SKN-1 increased expression of these genes during adulthood, and delayed their age-related decline in expression in response to multiple interventions that promote longevity: daf-2 RNAi, rapamycin (mTOR kinase inhibitor24), the dietary restriction (DR) model eat-2, and inhibition of germ cell proliferation (glp-1(−))1 (Fig. 2b–g; Extended Data Fig. 3i–k, 4a, 4b). Adulthood knockdown of these collagen genes did not affect wild type lifespan, but dramatically reduced longevity of the canonical daf-2 Class 2 mutant e1370 at 15°C but not 20°C (Fig. 3a, 3b; Extended Data Fig. 4c and Table 3; Supplementary Table 13), at which skn-1 is dispensable for longevity (see above). Additionally, knockdown of these collagens significantly reduced lifespan extension from daf-2 RNAi at 20°C, and from other skn-1-dependent14,24,25 longevity interventions (Fig. 3c–e; Extended Data Fig. 4d and Table 3; Supplementary Table 13). Most of these genes include regions related to other collagens, but col-120 is unique (Supplementary Table 14), and at 15°C, daf-2(e1370) but not wild-type lifespan was reduced by the collagen mutation dpy-1(e1) (Extended Data Fig. 4e, Supplementary Table 13). Lack of a single critical collagen can therefore impair lifespan extension. At 15°C, daf-2(e1370) lifespan was also decreased by adulthood knockdown of certain extracellular protease genes from the SKN-1-upregulated daf-2(−) set, or other genes important for cuticle formation (Extended Data Fig. 4f, 13, 15). Remarkably, transgenic overexpression of key collagens from the SKN-1-upregulated daf-2(−) gene set but not other collagens modestly but consistently increased lifespan (Fig. 3f, Supplementary Table 13). Adulthood SKN-1-dependent expression of particular collagen and ECM genes therefore promotes lifespan extension in diverse pathways that slow C. elegans ageing.
Figure 3. Adulthood collagen expression promotes longevity.
a, b, SKN-1-upregulated collagens are needed for daf-2(e1370) longevity at 15°C but not 20°C. c, Adulthood collagen knockdown reduced eat-2(ad1116) lifespan at 15°C. Trial run in parallel with (a, and Extended Data Fig. 4c). d, Adulthood collagen expression is required for rapamycin lifespan extension. Rapamycin treatment and RNAi were initiated at adulthood day one. e, Longevity from reduced germline stem cell number requires adult collagen expression. glp-1(bn18) was exposed to RNAi or EV control after downshift from the non-permissive temperature to 20°C. In (a-e), the grey dashed line shows the wild-type or control lifespan. f, Overexpression of collagens COL-10, COL-13, and COL-120 individually or in combination increased lifespan. P value *<0.05, **<0.001, ***<0.0001 by Log-Rank. Statistics and additional lifespan data are in Extended Data Table 3 and Supplementary Table 13.
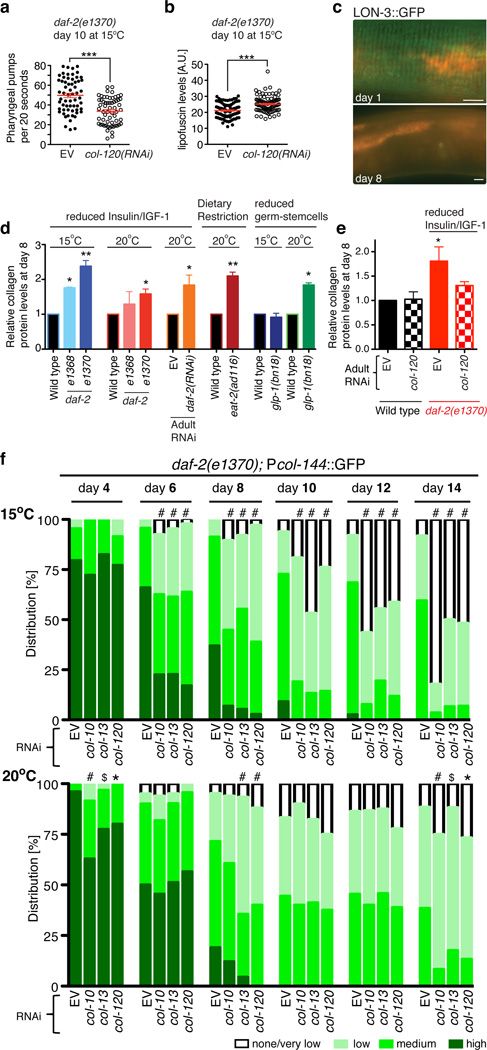
Adulthood collagen RNAi did not affect body size, detectably impair cuticle function, or increase markers of various stresses (Extended Data Fig. 5a–v, 6a–i). Collagen RNAi sensitized to exogenous oxidative stress, however, and increased the prominence of ageing markers in daf-2 mutants at 15°C, and in rapamycin-treated animals (Fig. 4a, 4b, Extended Data Fig. 6j–m; Supplementary Table 16). Apparently, knockdown of these collagens interfered with the capacity of these interventions to delay ageing.
Figure 4. Importance of ECM remodelling for longevity assurance.
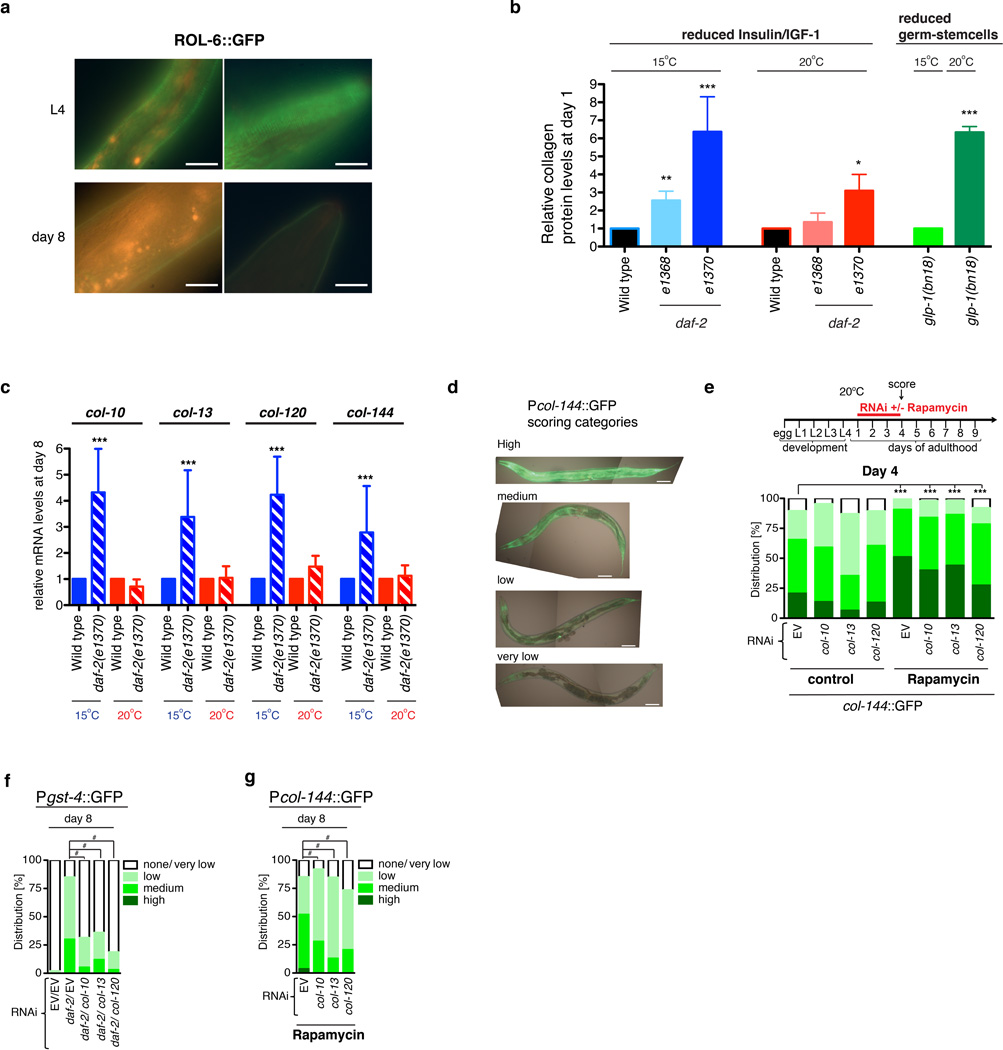
a, b, Adulthood collagen expression is required for rIIS to delay appearance of ageing markers. The same animals were scored in each panel, N>60. Each dot represents an animal; 2 merged trials; P value ***<0.0001 determined with unpaired t-test, two-tailed. c, Disappearance of the LON-3 collagen from the cuticle during ageing. Typical animals at the indicated days of adulthood are shown. Midsections from representative LON-3::GFP (green fluorescent protein) adults are shown, ventral side down, anterior to the left, scale bar = 10 µm. d, Interventions that increase longevity induce adulthood ECM deposition. Total collagen in day-8 adults is indicated by hydroxyproline content. daf-2 RNAi was initiated at day-one. glp-1(bn18) mutants were kept at the permissive temperature (15°C) or shifted to 25°C until day-one of adulthood then kept at 20°C. e, Loss of a single collagen interferes with rIIS-induced collagen deposition. In d, e, N>3000 per sample. Data are represented as mean ± s.e.m. P -value *<0.05 relative to control, by one sample t-test, two-tailed, hypothetical mean of 1. f, Dependence of a collagen promoter (col-144) on adulthood expression of other SKN-1-upregulated collagens in daf-2(e1370) under dauer-independent conditions. Scoring is described in Extended Data Fig. 7d RNAi initiated at day 1 of adulthood had a much more severe effect at 15°C (upper panel) than 20°C (lower panel), starting at day 6. N>60 for each condition, 1 representative trial is shown, with P value by chi2 (*=P<0.05; $=P<0.001; #=P<0.0001).
ECM gene upregulation might allow ECM remodelling to occur in adults. During ageing the collagens LON-3 and ROL-6 decline in expression22 and largely disappear from the cuticle (Fig. 4c; Extended Data Fig. 4a and 7a), indicating that C. elegans ECM proteins turn over. Adulthood daf-2 RNAi and other anti-ageing interventions increased total collagen in older C. elegans (Fig. 4d), indicating deposition of new ECM. This also occurred in daf-2(e1370) (Class 2) at 20°C, even though by adulthood day 8 expression of SKN-1 upregulated daf-2(−) collagens was not generally maintained in older daf-2(e1370) adults under these conditions (Fig. 4d; Extended Data Fig. 7b, 7c). Perhaps different genes might promote ECM remodelling under dauer-predisposed conditions, consistent with dauers having a distinct cuticle structure (Supplementary Discussion).
Longevity interventions delay ageing by acting through non cell-autonomous signalling pathways1. Adulthood col-120 knockdown reduced total daf-2 collagen levels (Fig. 4e), implying that individual collagens and the ECM influence these pathways. Adulthood collagen RNAi also inhibited SKN-1-responsive gene expression in adults that would otherwise be long-lived (Fig. 4f, Extended Data Fig. 7d–g), possibly explaining the importance of these collagens for oxidative stress resistance. These longevity interventions therefore require adulthood expression of particular ECM genes in order to maintain their beneficial regulatory program. Why would diverse longevity interventions induce and depend upon ECM remodelling? Under conditions of low nutrient availability, it might be advantageous to allocate resources towards ECM maintenance. The ECM also may directly affect signalling that orchestrates these longevity pathways, consistent with studies in other systems that identified signalling functions of collagens, and critical effects of the ECM on signalling pathways26–28.
We determined that in adult animals rIIS can activate a longevity program that is distinguished from the dauer developmental pathway by its lack of dauer-like traits, and its dependence upon skn-1 and SKN-1-dependent collagens (Fig. 1f). Further analyses will determine which rIIS longevity mechanisms are linked to the dauer program, and which are dauer-independent and possibly more broadly involved in pathways that promote longevity. Considerable effort has been devoted to enhancing collagen function in order to maintain youthful human skin during ageing29. By demonstrating that increased collagen expression is a shared feature of multiple conserved longevity pathways, our results suggest strategies for promoting ECM function that may be widely applicable. The long-lived naked mole rat is remarkably cancer resistant at least in part because it produces a uniquely dense hyaluronan, an ECM component30. Our results suggest that functional enhancement of the ECM may be generally important for longevity assurance per se. We speculate that interventions that promote collagen and ECM function systemically are likely to be beneficial in human chronic disease and ageing.
Methods
Strains
Caenorhabditis elegans strains were maintained on NGM plates and OP50 Escherichia coli bacteria at 20°C as described (Brenner, 1974)31, except that daf-2 mutants (and corresponding controls for a given assay) were maintained at 15°C unless otherwise noted. The wild-type strain was N2 Bristol31. Mutant strains used are described in Wormbase (www.wormbase.org): LGI: daf-16(mgDf47, mu86); LGII: eat-2(ad1116); LGIII: daf-2(e1368, e1370, and m596), rrf-3(pk1462), glp-1(bn18); and LGIV: eri-1(mg366), skn-1(tm3411, zu67, zu129, and zu135). LGX: lin-15B(n744). Transgenic lines used were: jgIs5 [ROL- 6::GFP;TTX-3::GFP]32, BC12533 dpy-5(e907); sEx12533 [Pcol-89::GFP; dpy-5(+)]33, CF1660 daf-16(mu86); daf-2(e1370); muIs84 [Psod-3::GFP; pRF4 rol-6(su1006gf)]; muEx211 [Pges-1::DAF-16::GFP; pRF4 rol-6(su1006gf)]15, CL2166 dvIs19 [Pgst-4::GFP; pRF4 rol-6(su1006gf)]34, EE86 mup-4(mg36); upIs1 [MUP-4::GFP; pRF4 rol-6(su1006gf)]35, HT1883 daf-16(mgDf50); daf-2(e1370) unc-119(ed3); lpIs14 [Pdaf-16::DAF-16f::GFP + unc-119(+)]36, IG274 frIs7 [Pcol-12::DsRed; Pnlp-29::GFP]37, LD001 ldIs007 [Pskn-1::SKN-1b/c::GFP; pRF4 rol-6(su1006gf)]38, MH2051 kuIs55 [LON-3::GFP; unc-119(+)]39, SJ4005 zcIs4 [Phsp-4::GFP; lin-15(+)]40, SJ4103 zcIs14 [myo-3::GFP(mit)]41, TB1682 chEx1682 [QUA-1::GFP; pRF4 rol-6(su1006gf)]42, TJ356 zIs356 [Pdaf-16::DAF-16a/b::GFP; pRF4 rol-6(su1006gf)]43, TP12 kaIs12 [COL-19::GFP]44.
Construction of transgenic lines
To construct the collagen overexpression transgenes, the genomic region of each gene, including approximately 3 kb of promoter, the coding region, and 3’UTR sequences that encompass at least 2 predicted cleavage/polyadenylation sites, were amplified by PCR. These PCR products were injected at 50 ng/µl together with 100 ng/µl of pRF4 rol-6(su1006gf) into wild-type (N2) animals. For the triple collagen gene transgenic line (ldEx111), 50 ng/µl each of PCR products for col-10, col-13, col-120 were injected together with 50 ng/µl of pRF4 rol-6(su1006gf). For the control line (ldEx102), pBluescript KS(+) 50 ng/µl was injected along with 100 ng/µl of pRF4 rol-6(su1006gf)). Lines were isolated from at least 2 independent transgenic P0 animals. For col-10, a 4.4 kb genomic region was amplified using the primers 5’-CCACCAACAACTCCATCCACC-3’ and 5’- GTAAAGTGGGCAGGCCGTAG-3’. The resulting transgenic lines were ldEx103 and ldEx104. For col-13, a 4.3 kb genomic region was amplified using the primers 5’-TAGCCCAAGTCTGACCGAAG-3’ and 5’- CGGATCTTCCCAACCAGGAG-3’. The resulting transgenic lines were ldEx105, ldEx106, ldEx107, and ldEx108. For col-120, a 4.4 kb genomic region was amplified using the primers 5’-CAATATGACCCGAGGCGCTG-3’ and 5’-CGCCAGAATCGTAAGGCTCC-3’. The resulting transgenic lines were: ldEx109 and ldEx110. Transgene overexpression levels were determined by qRT-PCR of one-day old adults.
Scoring of phenotypic experiments
No statistical methods were used in choosing sample sizes. In analyses of fluorescent reporters, either all or representative trials were scored blindly. All other phenotypic assays were not scored blindly.
Body length measurements
Animals were maintained at 15°C and either kept at 15°C, or shifted to 25°C at the first day of adulthood. At day 3 of adulthood, animals were mounted on 2% agar pads, immobilized with 0.06% tetramisole and images were taken at 10x magnification with a Zeiss Axioskop 2 microscope and with a Zeiss AxioCam HRc digital camera. Body lengths were measured by placing a line through the middle of the body starting from head to tail using Zeiss AxioVision V 4.8.2.0 program (Extended Data Fig. 1d).
Lifespan assays
Strains were age-synchronized by picking L4 animals onto fresh OP50 plates, then day-one adults were placed on either OP50 or RNAi plates containing 50 µM 5-Fluoro-2’deoxyuridine (FUdR), unless otherwise indicated, and assayed either at 15°C, 20°C, or 25°C as described in14. All lifespans were plotted with L4 as time-point=0. For glp-1(bn18) lifespans, wild type (N2) and glp-1(bn18) were maintained at 15°C, then shifted to 25°C at the mid-L1 stage as described in45. At the first day of adulthood they were placed on plates containing FUdR and RNAi bacteria for lifespan assay at 20°C (Fig. 4g; Extended Data Table 3). For rapamycin lifespans, one-day old animals were placed on plates containing FUdR, RNAi bacteria, and either rapamycin (100µM) dissolved in 0.2% DMSO or 0.2% DMSO control as described in14. Lifespan was determined at 20°C (Fig. 3d; Extended Data Table 3; Supplementary Table 13). For dauer pheromone experiments, day-one adults were placed on plates containing FUdR, RNAi bacteria, and either crude dauer pheromone (a gift from Piali Sengupta) dissolved in 6% ethanol, or 6% ethanol control as described in46. Those lifespans were determined at 25°C (Fig. 1e; Extended Data Table 1; Supplementary Table 2). Animals were classified as dead animals if they failed to respond to prodding. Exploded or bagged animals were excluded from the statistics. The estimates of survival functions were calculated using the product-limit (Kaplan-Meier) method. The log-rank (Mantel-Cox) method was used to test the null hypothesis and calculate P values (JMP software v.9.0.2.).
Scoring of transgenic protein nuclear accumulation or expression
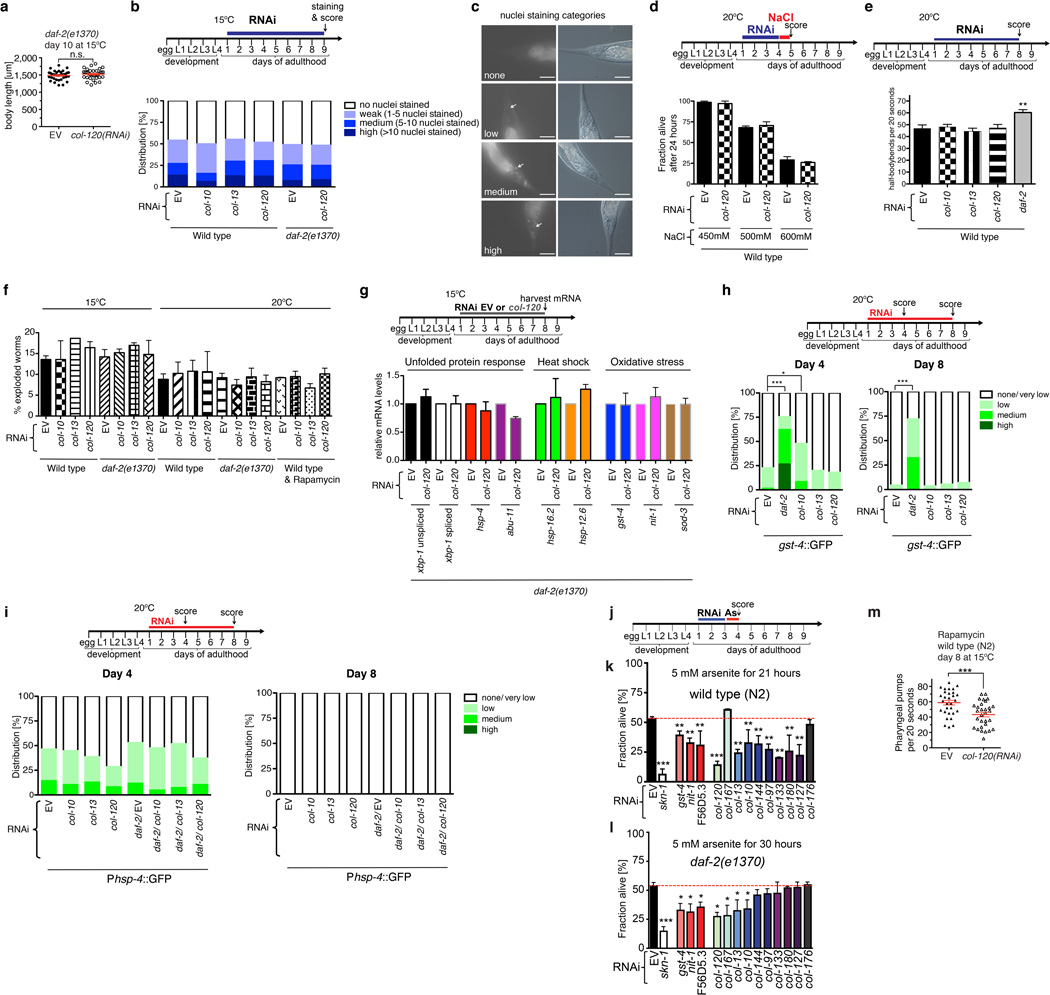
Nuclear accumulation of SKN-1 that was expressed from the SKN-1bc::GFP transgene (LD001 strain), which encodes two of the three SKN-1 isoforms, was scored blindly after mounting on slides essentially as in14 (Extended Data Fig. 1k–n). none = no GFP observed in nuclei; low = some nuclei show GFP; medium = more than half of the nuclei show GFP; high = all intestinal nuclei show GFP. Nuclear accumulation of DAF-16a/b::GFP (zIs356) was scored as described in47 (Extended Data Fig. 1n). Nuclear accumulation of DAF-16f::GFP (lpIs14) was scored as none = no GFP observed in nuclei; medium = more than half of the nuclei show GFP; high = all intestinal nuclei show GFP (Extended Data Fig. 1o). For Pcol-12::dsRED, Pcol-144::GFP, Pgst-4::GFP, and Phsp-4::GFP: one-day adult animals were placed on RNAi and three and/or seven days later the green or red fluorescence intensity was scored by using a Zeiss AxioSKOP2 microscope. Green or red fluorescence was categorized in none/very low, low, medium, or high intensity and was scored blindly (Fig. 4f,Extended Data Fig. 3j–k, 6h–j, 7d–g).
RNA interference
RNAi clones were picked from the Ahringer48 or Vidal49 libraries. Cultures were grown overnight in LB with 12.5 µg/ml tetracycline and 100 µg/ml ampicillin, diluted to an OD600 of 1, and induced with 1 mM IPTG. This culture was seeded onto NGM agar plates containing tetracycline, ampicillin, and additional IPTG. Empty vector (EV) plasmid pL4440 was used as control. For double RNAi, clones were grown separately in parallel and after spin-down equal amounts of two clones were mixed and spread on plates.
RNA isolation for microarray analysis
After a timed egg-lay on HT115 E. coli, daf-2(e1368) and daf-2(e1368);skn-1(zu67) or daf-2(e1370) and daf-2(e1370);skn-1(zu67) worms were grown at 15°C until the late L4 stage. Approximately 200 worms were collected and washed three times in M9 buffer31 to remove bacteria. TriReagent (Sigma) was added, and samples were snap frozen in liquid nitrogen. Total RNA was isolated using TriReagent and an RNA purification column (RNAeasy, Qiagen). RNA quality was determined by visualization of 28S and 18S rRNA bands on a denaturing formaldehyde gel, or an RNAse-free 1.5–2% agarose TBE gel.
RNA preparation, hybridization and data collection for microarray experiments
RNA (325 ng) was linearly amplified and labeled using the Agilent Low RNA Input Linear Amplification Kit, with Cy3- or Cy5-CTP (Perkin Elmer), and cRNA was hybridized on Agilent 4×44k C. elegans arrays. A dye swap replicate was performed for each set of biological replicate samples as previously described18. Data were extracted with Agilent Feature Extraction software and submitted to the Princeton University Microarray database for storage and filtering (http://puma.princeton.edu).
Microarray analysis
Data were filtered to remove spots that were not above background intensity in both channels, and replicate spots within each array were averaged. Genes for which more than 20% of data were missing across replicates were removed from further analysis. One-class SAM analysis was used to identify genes that were significantly up- or downregulated across all replicates in a set50. Expression profiles were clustered using Cluster 3.051 and visualized using Java TreeView52. Up- and downregulated genes identified by SAM analysis were submitted to DAVID53 to identify overrepresented functional annotations. Annotations used were: Gene ontology (GO) Biological Process FAT (GO BP, filtered by DAVID to remove the broadest GO terms), GO Molecular Function, Kegg Pathway, and Interpro Protein Domains. The Benjamini test for multiple hypothesis testing was applied to P values. Up- and downregulated genes were also submitted to GOToolBox to perform a hypergeometric test using the Benjamini-Hochberg correction. Enriched GO Terms were submitted to ReviGO to remove redundant terms. Co-occurrence between our datasets and previously published datasets was visualized with GeneVenn54 and BioInfoRx Area-Proportional Venn Diagram.
Motif analysis
We used two distinct algorithms, Weeder55 and FIRE56 to perform an unbiased search for overrepresented sequences in the promoters of SKN-1-regulated genes that were identified by SAM. We submitted upstream sequences (1000 bp) to Weeder and performed a scan for motifs of length 6 and 8 (“Normal” scan mode). FIRE was run using default parameters, with all genes partitioned into three groups to identify motifs that are informative about each group: SKN-1-upregulated, SKN-1-downregulated, and background. To search in a directed manner for occurrence of the consensus SKN-1 binding motif, we used RSATools57 to search the 600 bp upstream of up- and downregulated targets for the SKN-1 binding motif (WWTRTCAT). For comparison with the percentage of promoters in a random sample of genes that would be expected to contain the SKN-1 motif, we searched for the motif in 10,000 random samplings of gene promoter sets of equal size to the number of up- or downregulated genes, to empirically determine a distribution. To calculate a P value, we z-transformed the percentage of SKN-1 target promoters (z = (%skn-1 - mu)/sigma), where mu and sigma are the mean and standard deviation of the distribution.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Assays
For validation of the microarray data with skn-1 and daf-16 mutants, C. elegans were allowed to lay eggs for 3–4 hours on RNAi plates. After 2–4 days (depending upon the temperature and strain), 200 L4 worms were harvested (15°C for Extended Data Fig. 2c; 20°C Extended Data Fig. 2m–t). For adult RNAi, one-day adults were placed on RNAi plates and 3–8 days later 100–200 worms were harvested (Fig. 2a–c and Extended Data Fig. 4b). For rapamycin treatment, one-day old animals were placed on plates containing rapamycin (100µM) dissolved in 0.2% DMSO or in 0.2% DMSO control as described in14 and three days later mRNA was harvested for qRT-PCR (Extended Data Fig. 3l–m). For the glp-1 experiment, glp-1(bn18) or wild type (N2) animals were maintained at 15°C and L2 worms were upshifted to 25°C. One-day adults were placed on L4440 (empty vector RNAi) plates at 20°C and 3 days later 200 worms were harvested (Extended Data Fig. 3p–q). RNA was isolated with Trizol (TRI REAGENT Sigma), DNAse-treated, and cleaned over a column (RNA Clean & Concentrator™ ZYMO Research). First-strand cDNA was synthesized in duplicate from each sample (Invitogen SuperScript III). SYBR green was used to perform qRT-PCR (ABI 7900). For each primer set, a standard curve from genomic DNA accompanied the duplicate cDNA samples58. mRNA levels relative to N2 control were determined by normalizing to the number of worms and the geometric mean of three reference genes (cdc-42, pmp-3, and Y45F10D.459). Primer sequences are listed in Supplementary Table 17. Except for col-12/13, primers bind uniquely to the corresponding gene transcript (Supplementary Table 14 and 17). At least two biological replicates were examined for each sample. For statistical analysis, one sample t-test, two-tailed, hypothetical mean of 1 was used for comparison using Prism 4.0a software (GraphPad).
Oxidative stress assays
In oxidative stress assays, day-one daf-2 or skn-1 adults were placed in 5 mM sodium arsenite (in 1mL H2O) at 20°C and scored for survival hourly (Supplementary Table 7). For RNAi oxidative stress assays, wild type (N2) or daf-2(e1370) day-one adults were placed on RNAi plates at 15°C, and three days later animals were placed either on plates containing 15.4 mM t-BOOH and scored hourly at 20°C, or in 5 mM sodium arsenite (in 1mL M9 buffer) and scored after 21 hours (N2) or 30 hours (daf-2) at 20°C (Extended Data Fig. 6j–l and Supplementary Table 16).
Age-related phenotypic marker and body-size assays
Age-related phenotypes were described in60. One-day old animals were placed on RNAi food until day 10 of adulthood and the following phenotypes were scored: (1) Pharyngeal pumping was determined by counting grinder movements in 20 second intervals when the animals were placed on food (Fig. 4a and Extended Data Fig. 6m); (2) Lipofuscin levels were determined by mounting animals onto slides and taking bright field and DAPI channel pictures with a Zeiss Imager M2 microscope. Blue fluorescence from the DAPI channel pictures were analysed in Image J (imagej.nih.gov/ij/) by selecting the intestine and measuring the mean grey value minus the background (Fig. 4b); (3) The body size was determined from bright field images by drawing a line through the middle of the worm from anterior to posterior by using Zeiss Zen 2012 software (Extended Data Fig. 6a).
Collagen assays
Synchronized L1 larvae were placed on 10 cm NGM plates containing OP50 bacteria at 15°C, 20°C, or 25°C and monitored for development to the L4 stage. After an additional day, day-one adults were either harvested for the assay (Extended Data Fig. 7b), or placed on either 10 cm OP50 or RNAi plates containing 50 µM 5-Fluoro-2’deoxyuridine (FUdR) and maintained at the corresponding temperature. At day 8 of adulthood, the remaining animals were harvested (Fig. 4d, 4e). In each case, the animals were washed 3 times with M9, the number of worms was determined, and at least 3000 worms per strain and condition were used for the assay. Collagen levels were determined using the QuickZyme Biosciences Total Collagen Kit (QZBTOTCOL1), which detects Hydroxyproline 61, according to the manufacturer’s instructions.
Barrier function assay
One-day old adults were placed on RNAi food and at day 9 were harvested, washed 3 times with M9 and incubated in 1µg/ml Hoechst (Hoechst 33342, which is cuticle-impermeable but membrane-permeable) for 15 minutes in darkness at room temperature. The animals were then washed 3 times in M9, allowed to recover for 10 minutes on plates with food, and mounted for microscopy (Extended Data Fig. 6b, 6c; method adapted from 62).
Extended Data
Extended Data Figure 1. Analyses of rIIS under dauer-independent and dauer-predisposed conditions.
a, Data from this study illustrating that rIIS longevity dependence upon skn-1 correlates with low dauer pathway activity, not temperature or percent increase in mean lifespan extension. b, Partial schematic of the IIS pathway in C. elegans. Insulin-like peptides (ins) bind to DAF-2, leading to activation of the AKT-1/2 and possibly SGK-1 kinases1,13,80, which phosphorylate DAF-16 and SKN-1. Class 1 daf-2 mutations are typically located on the extracellular portion of DAF-2, whereas most Class 2 mutations affect its intracellular domains81. c, daf-2 mutant phenotypes. Red indicates penetrance specifically at higher temperatures (Supplementary Discussion). d, The Class 2 (dauer-related) daf-2 trait of reduced body length is skn-1-independent. Each dot represents an animal, with P values determined by one-way ANOVA with post hoc Tukey. e, Dependence of dauer-independent daf-2 longevity on adulthood skn-1. daf-2(e1370) lifespan extension requires skn-1 when the temperature is downshifted to 15°C specifically during adulthood (blue). For additional information see Supplementary Table 2. f, skn-1-dependence of daf-2(e1370) longevity at 20°C when DAF-16 is expressed specifically in the intestine (strain description in Extended Data Table 1). g, Intestine-specific DAF-16 expression fails to rescue a Class 2 dauer-like trait (immobility) in daf-2(e1370). h-j, Condition-specific induction of dauer by daf-2 RNAi. daf-2 RNAi fails to induce dauer entry even at 25°C (j), although some dauers are seen under more extreme conditions (27°C)82. The activity of IIS and DAF-16 in neurons is critical for dauer regulation15,16,83, and in the wild-type RNAi is comparatively ineffective in neurons84, suggesting that the extremely weak dauer propensity of daf-2 RNAi might derive from a failure to reduce IIS sufficiently in neurons. Supporting this idea, daf-2 RNAi induced dauer entry even at 20°C in eri-1(mg366); lin-15B(n744) mutants, in which neuronal RNAi is robust85 (h). N>100 for each condition, 2 merged trials. k-n, Robust SKN-1 and DAF-16 nuclear localization under conditions of dauer inactivity. SKN-1 nuclear accumulation is inhibited comparably by IIS at 15°C and 20°C. SKN-1 is constitutively localized to ASI neuron nuclei in wild type animals, and accumulates in intestinal nuclei in daf-2(e1370)13. k, Extent of IIS reduction from daf-2(e1370) at 15°C, indicated by nuclear SKN-1::GFP. Chevrons indicate intestinal nuclei, Scale bar = 20 µm. SKN-1::GFP (LD001) in intestinal nuclei is quantified in (l, m). N> 60 for each condition and trial, 3 merged trials with P values determined by chi2 test. Nuclear accumulation was scored as in Methods. n, daf-2 RNAi comparably induces SKN-1::GFP (LD001) and DAF-16::GFP (TJ356) intestinal nuclear localization at 15°C and 20°C. (N>60 for each condition, 1 trial with all experimental conditions done in parallel). o, Comparable nuclear accumulation of DAF-16f::GFP (lpIs14) induced by daf-2 RNAi and daf-2(e1370) at 15°C and 20°C (N>60 for each condition, 1 trial performed in parallel). p, q, Induction of dauer development (p) and dauer-like traits (skn-1-independent) (q) by the crude dauer pheromone preparation used in lifespan assays (Fig. 1e; Extended Data Table 1, Supplementary Table 2). In p; N>100 for each condition, 1 trial. In q; N=30 for each condition, 3 merged trials. For h-j, l-o: P values were determined by chi2 test. n.s.= not significant, *<0.05, **<0.001, ***<0.0001.
Extended Data Figure 2. Identification of SKN-1-regulated daf-2(−) genes.
a, Heatmap of 429 genes identified by SAM as significantly upregulated by SKN-1 in daf-2 mutants. b, 477 genes identified by SAM as significantly downregulated by SKN-1 in daf-2 mutants (Supplementary Table 3). The SKN-1-downregulated daf-2(−) set was enriched for genes involved in ubiquitin-mediated proteolysis (E3 ligase/SCF, F-box; Supplementary Table 8). Columns represent biological samples. blue = down; black = unregulated. c, Confirmation of microarray data for SKN-1-upregulated daf-2(−) genes by qRT-PCR at 15°C. One and three biological replicates were analysed in the left and right panels, respectively. SAM scores are in Supplementary Table 3. Data are represented as mean ± s.e.m. P values *<0.05, **<0.001, ***<0.0001 relative to daf-2 were determined by one sample t-test, two-tailed, hypothetical mean of 1. d-e, Enrichment of SKN-1 binding sites upstream of SKN-1-regulated daf-2(−) genes. An unbiased search using the Weeder and FIRE algorithms did not detect any overrepresented form of the consensus SKN-1 binding motif (WWTRTCAT) (W=A/T, R=G/A)86. Given the degeneracy of this motif, we used RSA Tools to perform a directed search of 600 bp upstream of SKN-1 upregulated (d) and downregulated (e) genes. This search window was based upon the location of SKN-1 binding sites identified by genome-wide ChIP-seq using transgenically-expressed SKN-187. A SKN-1 motif was detected at only 13% of a random sample of 10,000 genes, but at 37% and 24% of the SKN-1-upregulated (out of 429 genes) and downregulated genes (out of 477 genes), respectively. f, Importance of SKN-1-upregulated daf-2(−) genes for daf-2(e1368) lifespan. The Class 1 daf-2 allele e1368 is partially dependent upon skn-1 for lifespan extension at 20°C (Extended Data Table 1)13. Adult RNAi against 5 of 12 genes tested reduced daf-2(e1368) lifespan at 20°C. g-h, Several SKN-1-downregulated daf-2(−) genes decrease lifespan. Knockdown was performed in the RNAi-sensitive strain rrf-3(pk1426)88. (g) Genes for which RNAi knockdown increased lifespan, from 12 that were analysed without regard to their function. (h) Analysis of 6 Skp1-related genes, an overrepresented category among SKN-1-downregulated daf-2(−) genes (Supplementary Table 8). Only genes that affected lifespan are shown. Other data and all statistics are in Supplementary Table 6. For 15 other SKN-1-downregulated daf-2(−) genes, it has been shown previously that RNAi increases lifespan (Supplementary Table 5). (f) and (g) each show a single trial, and a composite of 3 trials is shown in (h). In (g) the negative RNAi control is elpc-4(RNAi) instead of L4440. Mean lifespan (days) is indicated for each gene. i, Overlap between the daf-2(−); SKN-1-dependent upregulated gene set (429 genes, this study) and a set of genes preferentially upregulated in dauers (358 genes)89. The overlap of 6 genes was not significant (P-value 0.6391 by two-sided Chi-square). The number of genes that were present in neither set (no/no) was determined by subtracting the total number in both gene sets from the total number of genes encoded in C. elegans 19,73590. j, Overlaps between SKN-1-regulated daf-2(−) and DAF-16-regulated daf-2(−) gene sets72. For both up- and down-regulated daf-2(−) genes, overlaps between the SKN-1- and DAF-16-regulated sets were significant (P-value <0.0001 determined by two-sided Chi-square). Moreover, hierarchical clustering identified additional SKN-1-upregulated daf-2(−) genes that were also upregulated by DAF-16 even though they were not present in this list of highest-confidence DAF-16-regulated genes (l). The number of genes that were in neither set (no/no) was determined as (i). k, Hierarchical clustering of SKN-1-downregulated daf-2(−) gene sets with DAF-16-regulated genes. SKN-1-regulated genes identified here were queried as to how they were influenced by DAF-16 in a comparison of daf-2(e1370) vs daf-2(e1370);daf-16(mu86) animals raised at 20°C72. 393 SKN-1-upregulated daf-2(−) genes that were present in this DAF-16-regulated dataset are shown. Most SKN-1-downregulated daf-2(−) genes did not appear to be regulated by DAF-16. l, Hierarchical clustering of SKN-1-upregulated daf-2(−) genes with DAF-16-regulated genes that were identified by comparing daf-2(e1370) vs daf-2(e1370);daf-16(mu86) at 20°C72. 272 SKN-1-upregulated daf-2(−) genes that were present in this DAF-16-regulated dataset are shown, 46% of which were upregulated by both SKN-1 and DAF-16. yellow = up; blue = down; black = unregulated. m-t, Effects of SKN-1 and DAF-16 on individual genes in response to daf-2 RNAi at 20°C. A qRT-PCR analysis of skn-1(zu67) daf-16(mgDf47), and skn-1;daf-16 skn-1(zu67); daf-16(mgDf47) double mutants indicated that many genes are upregulated by daf-2(RNAi) (red) in a skn-1-dependent manner, but also that these genes vary in how they are affected by DAF-16. DAF-16 and SKN-1 increased activity of gst-4 col-65, and col-176, but DAF-16 seemed to downregulate dod-24 nas-7, and F55G11.2. All of these genes except ins-7 were identified in our daf-2;skn-1 data sets. For each condition, 3 biological samples of 200 worms each were analysed by qRT-PCR. All data are represented as mean ± s.e.m. P values of *<0.05, **<0.001, ***<0.0001 relative to wild type RNAi control were determined by one sample t-test, two-tailed, hypothetical mean of 1.
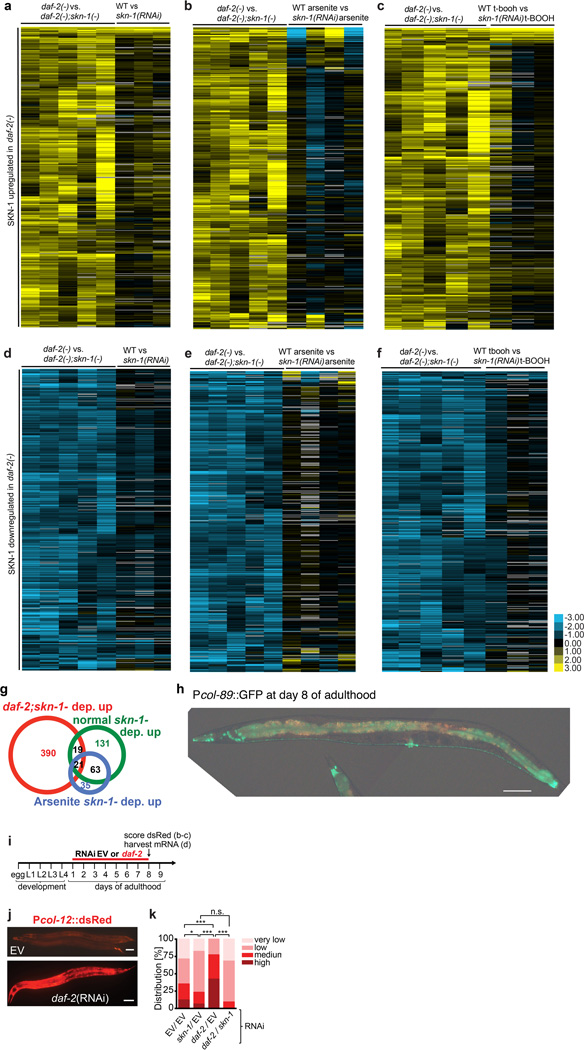
Extended Data Figure 3. Analyses of SKN-1-regulated daf-2(−) genes.
a–f, SKN-1-upregulated (a–c) and downregulated (d–f) daf-2(−) gene sets were examined by hierarchical clustering to determine how they were previously found to be affected by SKN-1 under unstressed or oxidative stress conditions18. t-BOOH= tert-butyl hydroperoxide. g, Proportional Venn diagrams show comparisons of SKN-1-upregulated genes identified under daf-2(−), normal, or arsenite treatment conditions18 (Supplementary Tabel 7). In each case, L4 larvae were analysed to avoid embryogenesis effects. Heatmaps are shown in (a-f). h, The SKN-1-upregulated daf-2(−) collagen col-89 is expressed in neurons and the intestine, but not in hypodermis. Transgenic Pcol-89::GFP (BC12533) at day 8 of adulthood is shown. Anterior to the left, ventral side down, scale bar = 100 µm. i-k, SKN-1-mediated collagen gene activation in day 8 daf-2(RNAi) adults. Adulthood daf-2 knockdown (i) activated a Pcol-12::dsRED reporter (j; Scale bar = 100 µm). (k). skn-1-dependence of Pcol-12::dsRED expression. EV: empty RNAi vector. N> 60 for each condition, 3 merged trials, with P value by chi2 (*=P<0.05; ***=P<0.0001; n.s.= not significant).
Extended Data Figure 4. rIIS delays age-associated decline in collagen expression.
a, Age-associated decline in expression of selected collagen and SKN-1-dependent detoxification genes. 88 collagens are among many genes that decline in expression as C. elegans ages22. Fifty of these age-downregulated genes were in our SKN-1 upregulated daf-2(−) gene set, including 27 collagen genes (Supplementary Table 10). These daf-2(−); SKN-1-dependent collagens were neither flanked by SKN-1 binding sites nor bound by SKN-1 in a genome-wide survey (Supplementary Table 9)87, suggesting that they are regulated by SKN-1 indirectly. The average Cy5-labeled cDNA values of day 2–11 adults (indicated as “exp”) are plotted in binary logarithm (log2) relative to cy3-labeled reference cDNA from mixed stage hermaphrodites (indicated as “ref”). Data are from22. nit-1 gst-4, and F56D5.3 are predicted to encode a nitrilase, glutathione S-transferase, and NADPH oxidoreductase, respectively (WormBase). b, Expression of SKN-1-regulated collagen and oxidative stress response genes (nit-1 and gst-4) are maintained during ageing in daf-2(RNAi) animals. One-day old adult wild type (N2) animals were placed on either empty vector control (L4440)(black) or daf-2 RNAi (red) at 20°C. mRNA was harvested at days 3, 6 and 8. mRNA levels are shown relative to wild type (N2) day 3 adults on empty vector control (L4440) RNAi and are represented as mean ± s.e.m. For each condition, 2 biological samples of >100 worms each were analysed by qRT-PCR. For each gene, statistical difference of relative mRNA expression levels between L4440 and daf-2(RNAi) treatment over the time-course (day 3,6,8) is shown by two-way ANOVA (repeated measures). c, Adulthood knockdown of SKN-1-upregulated collagens did not affect wild-type lifespan (for statistics and additional trials: Extended Data Table 3 and Supplementary Table 13). d, Importance of SKN-1-upregulated collagens for daf-2(RNAi) longevity. Adulthood RNAi knockdown of daf-2 with combined with collagens or skn-1 at 20°C is shown. GFP was the RNAi control. (for statistics and additional data: Supplementary Table 13). e, Suppression of daf-2(e1370) but not wild-type longevity at 15°C by the collagen mutation dpy-1(e1), which affects the cuticle31,91, but was not present in our SKN-1-regulated gene set. For details and statistics see Supplementary Table 13. f, daf-2(e1370) longevity at 15°C requires the SKN-1-upregulated extracellular proteases asp-14 and suro-1, along with cuticle integrity genes acs-20 and acs-2262, suggesting a general importance of ECM gene expression. For details and statistics see Supplementary Table 13.
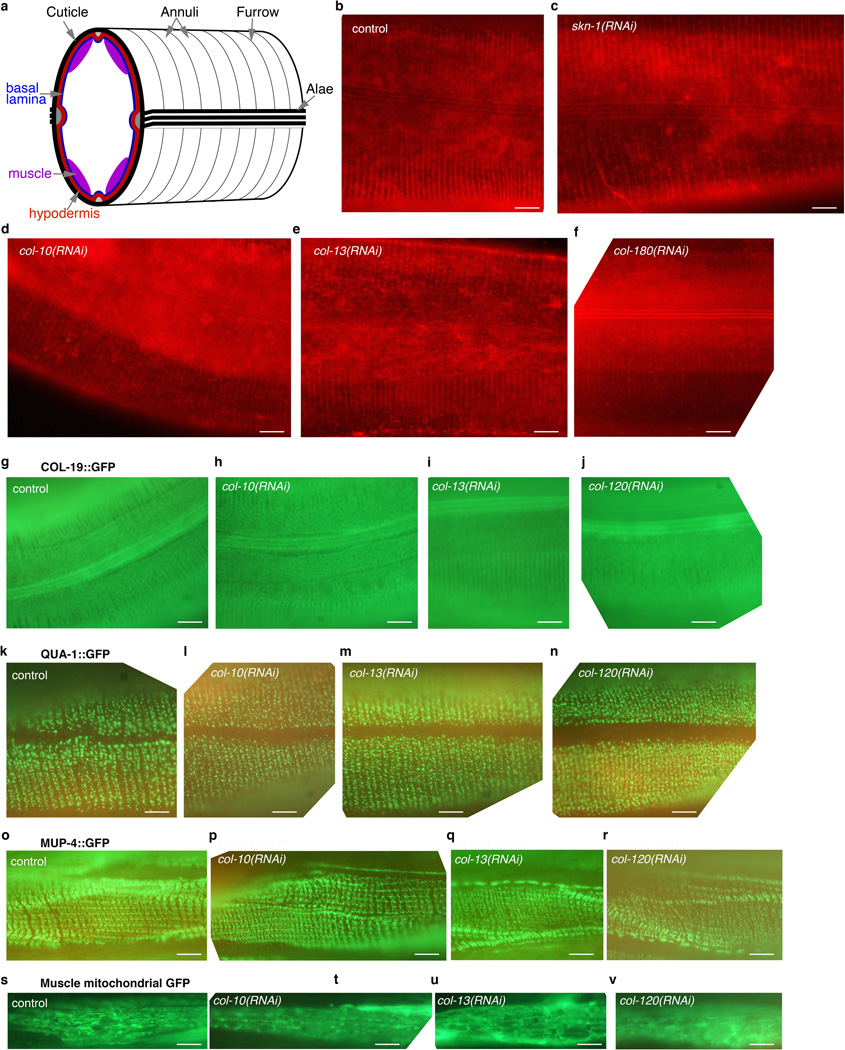
Extended Data Figure 5. Adulthood knockdown of collagens important for longevity does not affect morphology of cuticle-associated structures.
a, Schematic cross-section of C. elegans illustrating the proximity of the cuticle (black), hypodermis (red), basal lamina (blue), and bodywall muscles (purple). Annuli, Furrow, and Alae are characteristic cuticle structures. b-j, Adulthood RNAi against SKN-1-upregulated daf-2(−) collagens does not affect cuticle morphology. b-f, One-day old wild-type animals were exposed to either empty vector (control) or the indicated RNAi clone by feeding. 10 days later animals were incubated in DiI for 16 hours, the cuticle was imaged as described in92. N>30 animals per condition scored, with typical images shown. Scale bar = 10 µm. g-j, Cuticle morphology revealed by the collagen COL-19, detected by a translational fusion protein (kaIs12 [COL-19::GFP]). We did not identify col-19 as being regulated by daf-2 and skn-1, and daf-2(RNAi) did not detectably alter COL-19::GFP levels (not shown). k-n, Adulthood knockdown of SKN-1-upregulated daf-2(−) collagens does not affect the pattern of chEx1682 QUA-1::GFP, a marker of cuticle adhesion. QUA-1 encodes a hedgehog related protein required for molting, cuticle adhesion, and alae formation42. o-r, Adulthood RNAi against SKN-1-upregulated daf-2(−) collagens does not affect the pattern of muscle-hypodermis-cuticle adhesion, as indicated by upIs1 MUP-4::GFP. MUP-4 is a transmembrane protein that is part of a complex that attaches hypodermis and muscles to the cuticle35. s-v, Adulthood collagen knockdown does not affect mitochondrial morphology in muscle. For g-v, animals where placed on RNAi at the first day of adulthood and scored and imaged at day 8 of adulthood. N>30 animals per condition scored, with typical images shown. Scale bar = 10 µm.
Extended Data Figure 6. Phenotypic analyses of collagens important for longevity.
a, Adulthood col-120 knockdown does not affect daf-2(e1370) body size at 15°C. daf-2(e1370) animals were placed on RNAi food as day-one adults, and at day 10 body size, pharyngeal pumping and lipofuscin levels were scored in parallel in the same animals (N>30; 1 trial; see Fig. 4a, 4b). b, c, Adulthood knockdown of SKN-1-upregulated collagens does not alter barrier function. b, Upper panel: animals were placed on RNAi food on adulthood day one, and at day 9 were incubated in 1µg/ml Hoechst 33342, which is membrane-permeable but cuticle-impermeable. For details see full methods, adapted from62. b, Lower panel: Barrier permeability was not affected by daf-2 mutation or collagen knockdown. Permeability was assessed by nuclear Hoechst staining in the tail62 (N>50 per condition; 1 trial). Approximately half of the animals in each group showed nuclear staining in the tail that is likely to have arisen through uptake in the intestine, as suggested by the high levels of intestinal Hoechst staining (c). Uptake through the cuticle would have resulted in a much wider distribution of stained nuclei. c, representative pictures of quantification categories. Arrow indicates Hoechst-stained tail nuclei. Scale bar = 50 µm. d, Adulthood knock-down of col-120 did not sensitize to hypertonic stress. Day-one adult wild-type animals were placed RNAi food for 3 days, and then on plates containing food and high concentrations of salt for 24 hours prior to assay (NaCl: 450 mM, 500 mM, 600 mM; N>60 per condition; 2 trials). e, Adulthood knockdown of SKN-1-upregulated collagens did not impair body movement. Neither the frequency nor morphology (not shown) of body movement was affected. In parallel, the daf-2 RNAi control increased movement frequency because these animals were chronologically younger. (**P-value < 0.001, One-way ANOVA post hoc Tukey compared to EV). f, Adulthood collagen RNAi did not increase vulval rupturing during ageing. The bar graph shows the mean ± s.e.m percentage of exploded worms that were censored during lifespan assays (Extended Data Table 3 and Supplementary Table 13). g-i, Adulthood col-120 knockdown did not induce unfolded protein, heat-shock stress, or oxidative stress responses. In (g), daf-2(e1370) mutants were placed on RNAi food as day-one adults, and assayed at day 8 (upper panel). Relative levels of these stress response gene mRNAs were determined by qRT-PCR (2 independent trials with each 200 worms per condition). h, Adulthood collagen RNAi does not activate the oxidative stress response marker Pgst-4::GFP34, assayed after 4 and 8 days of RNAi. As a control, daf-2 RNAi induced SKN-1 to increase gst-4 expression (Fig. 2a). i, Adulthood collagen RNAi does not activate the unfolded protein response marker Phsp-4::GFP40. j-l, Importance of collagens for oxidative stress resistance. Day-one adults were exposed to empty vector (EV) or RNAi food at 15°C, then at day three were placed in 5 mM arsenite (As) and scored for survival. Knockdown of collagens and other SKN-1 upregulated daf-2(−) genes sensitized to oxidative stress from arsenite. nit-1 (nitrilase), gst-4 (glutathione S-transferase), F56D5.3 (NADPH oxidoreductase). P value *<0.05, **<0.01, ***<0.001 relative to control (EV), determined by one-way ANOVA with post hoc Tukey. t-BOOH experiments are described in Supplementary Table 16. m, Adulthood collagen expression required for rapamycin to delay appearance of an ageing marker (pharyngeal pumping). N>30; each dot represents an animal; P value ***<0.0001 determined with unpaired t-test, two-tailed.
Extended Data Figure 7. Cuticle remodelling in adults.
a, The collagen ROL-6 is present in the cuticle during development and early adulthood93, then largely disappears during aging. The upper panels show the mid-body (left) and head (right) regions in an L4 animal. Day-one adults exhibited similar levels and patterns of jgIs5 ROL-6::GFP fluorescence (not shown). Lower panels show the corresponding regions in a day-8 adult, in which jgIs5 ROL-6::GFP levels are reduced. The orange signal corresponds to gut autofluorescence. Representative images are shown, with scale bar = 20 µm. N=30 for each sample set (L4, day 1, and day 8). b, Total collagen levels are elevated in long-lived animals at the first day of adulthood. Note that these long-lived animals also maintain higher collagen levels in later life despite an age-related decline (Fig. 4d). Relative collagen levels were estimated by a hydroxyproline assay61. In daf-2 mutants total collagen levels were elevated at both temperatures but the increase was greater at 15°C, at which skn-1 and SKN-1-dependent collagens are required for lifespan extension (Fig. 3, Supplementary Table 13). Temperature-sensitive glp-1(bn18) mutants were maintained at 15°C (permissive temperature), or upshifted to 25°C (restrictive temperature) as L1 larvae until the L4 stage, then placed at 20°C. c, SKN-1-dependent collagen genes from the daf-2(−) set are not upregulated in 8 day-old daf-2(e1370) adults at 20°C. Expression of these collagens remains increased at this age in daf-2(e1370) at 15°C or after daf-2 RNAi at 20°C (Fig. 2a, Extended Data Fig. 2c, 4b), conditions in which the dauer pathway is inactive and lifespan extension is skn-1-dependent (see text). 200 day-8 adults were assayed in each sample, with 3 merged independent trials shown. d, Scoring categories for the Pcol-144::GFP reporter are shown in (e, g; Fig. 4f; scale bar = 100 µm). e, Adulthood rapamycin treatment increases col-144 promoter activity. Knockdown of col-10, col-13, or col-120 did not reduce Pcol-144::GFP levels at day 4, but significantly decreased Pcol-144::GFP levels by day 8 (g). N>60 for each condition, 2 merged trials, with P value by chi2 (#=P<0.0001 against untreated EV control animals). f, Dependence of the SKN-1 target gene gst-4 on adulthood SKN-1-upregulated collagen expression in daf-2(RNAi) animals. Collagen or empty vector (EV) control RNAi was initiated at day 1 of adulthood at 20°C, together with daf-2 knockdown. g, Adulthood collagen RNAi decreases col-144 promoter activity in rapamycin-treated animals. As is seen in daf-2 mutants at 15°C (Fig. 4f), activity of this rapamycin-activated promoter is unaffected by adulthood collagen RNAi at day 4 (e), but reduced at day 8. For f and g, N>60 for each condition, 2 merged trials, with P value by chi2 (#=P<0.0001).
Extended Data Table 1. skn-1 dependence of daf-2 lifespan extension in the absence of dauer-related mechanisms.
Lifespans were measured from the L4 stage, and animals that left the plates, buried into the agar, bagged, or exploded were censored. Analyses performed in parallel are grouped. L4440 empty vector was used as the RNAi control. Each skn 1 mutant analysed is a strong loss-of-function and possible null. The Class 2 alleles daf-2(e1370) and daf-2(m596) have comparably extended lifespans at 20°C and 15°C (Supplementary Table 2). daf-2(e1370);skn-1 double mutants lived 55% longer at 20°C than at 15°C (Supplementary Table 2), because skn-1–independent dauer-related processes increase their lifespan at the higher temperature (see text). This finding is striking given that C. elegans generally lives longer at lower temperatures1 (Supplementary Table 2). Previous analyses of these transgenically-rescued daf-16 strains showed that DAF-16 expression specifically in neurons rescues the dauer but not longevity phenotypes of daf-2(e1370), whereas intestine-specific DAF-16 rescue allows lifespan extension but not dauer entry15.
| Strain | Temp [°C] |
Mean lifespan ± S.E.M. [Days] |
75th percentile [Days] |
N assayed / Initial N |
% mean lifespan change to N2 or control |
% mean lifespan change to skn-1 |
P-value (log- rank) vs. N2 |
P-value (log- rank) vs. skn-1 |
P-value (log- rank) vs. daf-2 |
Figure |
|---|---|---|---|---|---|---|---|---|---|---|
| 3 merged trials at 15°C * | ||||||||||
| wild type (N2) | 15 | 23.4 ± 0.3 | 27 | 279/332 | 1a | |||||
| skn-1(zu67) | 15 | 16.9 ±0.2 | 19 | 278/315 | −28 | <0.0001 | 1a | |||
| daf-2(e1370) | 15 | 36.7 ±0.5 | 44 | 372/396 | +57 | +117 | <0.0001 | <0.0001 | 1a | |
| daf-2(e1370); skn-1(zu67) | 15 | 17.1 ±0.2 | 19 | 308/327 | +1 | <0.0001 | 0.7993 | <0.0001 | 1a | |
| Trial at 15°C and 20°C | ||||||||||
| wild type (N2) L4440(RNAi) | 15 | 25.4 ± 0.5 | 27 | 74/83 | ||||||
| wild type (N2) daf-2(RNAi) | 15 | 39.1 ±1.2 | 42 | 64/73 | +54 | +146 | <0.0001 | <0.0001 | ||
| skn-1(tm3411) L4440(RNAi) | 15 | 15.9 ±0.4 | 16 | 116/136 | −37 | <0.0001 | <0.0001 | |||
| skn-1(tm3411) daf-2(RNAi) | 15 | 16.6 ±0.7 | 16 | 84/103 | +6 | <0.0001 | 0.6424 | <0.0001 | ||
| wild type (N2) L4440(RNAi) | 20 | 23.6 ± 0.5 | 26 | 45/51 | 1b | |||||
| wild type (N2) daf-2(RNAi) | 20 | 34.8 ±1.1 | 40 | 45/50 | +47 | +120 | <0.0001 | <0.0001 | 1b | |
| skn-1(tm3411) L4440(RNAi) | 20 | 15.8 ±0.5 | 16 | 93/108 | −33 | <0.0001 | <0.0001 | 1b | ||
| skn-1(tm3411) daf-2(RNAi) | 20 | 17.1 ±0.5 | 16 | 115/124 | +8 | <0.0001 | 0.0491 | <0.0001 | 1b | |
| Trial at 20°C | ||||||||||
| wild type (N2) L4440(RNAi) | 20 | 23.3 ± 0.2 | 23 | 67/74 | ||||||
| wild type (N2)skn-1(RNAi) | 20 | 18.6 ±0.2 | 19 | 96/101 | −20 | <0.0001 | ED 1f | |||
| daf-16(mu86);daf-2(e1370) L4440(RNAi) | 20 | 17.0 ±0.2 | 19 | 73/83 | −27 | −9 | <0.0001 | <0.0001 | <0.0001 | ED 1f |
| daf-16(mu86);daf-2(e1370)skn-1(RNAi) | 20 | 16.5 ±0.2 | 19 | 78/89 | −29 | −11 | <0.0001 | <0.0001 | <0.0001 | |
| daf-2(e1370) L4440(RNAi) | 20 | 41.7 ±0.9 | 47 | 82/87 | +79 | +124 | <0.0001 | <0.0001 | ED 1f | |
| daf-2(e1370) skn-1 (RNAi) | 20 | 38.9 ±0.8 | 42 | 96/102 | +67 | +109 | <0.0001 | <0.0001 | 0.0153 | ED 1f |
| DAF-16 rescued in all tissues:daf-16(mgDf50);daf-2(e1370); IplsU [Pdaf-16::DAF-16f::GFP] L4440(RNAi) | 20 | 53.5 ±1.0 | 61 | 77/83 | +130 | +188 | <0.0001 | <0.0001 | <0.0001 | |
| DAF-16 rescued in all tissues:daf-16(mgDf50);daf-2(e1370); Ipls14 [Pdaf-16::DAF-16f::GFP] skn-1(RNAi) | 20 | 50.8 ±0.9 | 58 | 86/93 | +118 | +173 | <0.0001 | <0.0001 | <0.0001 | |
| DAF-16 rescued in neurons:daf-16(mu86);daf-2(e1370); muEx169 [Punc119::GFP::DAF-16]L4440(RNAi) | 20 | 16.4 ±0.2 | 17 | 71/77 | −30 | −12 | <0.0001 | <0.0001 | <0.0001 | |
| DAF-16 rescued in neurons:daf-16(mu86);daf-2(e1370); muEx169 [Punc119::GFP::DAF-16]skn-1(RNAi) | 20 | 16.5 ±0.2 | 17 | 92/97 | −29 | −11 | <0.0001 | <0.0001 | <0.0001 | |
| DAF-16 rescued in intestine:daf-16(mu86);daf-2(e1370); muEx211 [Pges-1::GFP::DAF-16] L4440(RNAi) | 20 | 27.5 ±0.6 | 30 | 89/98 | +18 | +48 | <0.0001 | <0.0001 | <0.0001 | ED 1f |
| DAF-16 rescued in intestine:daf-16(mu86);daf-2(e1370); muEx211 [Pges-1::GFP::DAF-16]skn 1(RNAi) | 20 | 18.5 ±0.3 | 19 | 86/93 | −21 | −0.5 | <0.0001 | 0.2671 | <0.0001 | ED 1f |
| Trial at 15°C and 20°C | ||||||||||
| wild type (N2) L4440(RNAi) | 15 | 24.5 ± 0.6 | 28 | 68/76 | 1c | |||||
| wild type (N2) daf-2(RNAi) | 15 | 47.8 ±1.3 | 57 | 81/94 | +95 | +148 | <0.0001 | <0.0001 | <0.0001$ | 1c |
| skn-1(zu135) L4440(RNAi) | 15 | 19.3 ±0.9 | 21 | 61/73 | −21 | <0.0001 | <0.0001$ | 1d | ||
| skn-1(zu135) daf-2(RNAi) | 15 | 20.3 ±1.1 | 26 | 68/77 | −17 | +5 | 0.0026 | 0.3805 | <0.0001$ | 1d |
| daf-2(e1368) L4440(RNAi) | 15 | 32.9 ±0.9 | 40 | 65/72 | +34 | +70 | <0.0001 | <0.0001 | 1c | |
| daf-2(e1368) daf-2(RNAi) | 15 | 71.7 ±2.3 | 82 | 70/78 | +193 | +272 | <0.0001 | <0.0001 | <0.0001$ | 1c |
| daf-2(e1368); skn-1(zu135) L4440(RNAi) | 15 | 19.9 ±0.7 | 26 | 48/57 | −19 | +3 | 0.0002 | 0.4624 | <0.0001$ | 1d |
| daf-2(e1368); skn-1(zu135) daf-2(RNAi) | 15 | 19.3 ±0.9 | 26 | 60/65 | −21 | 0 | 0.0004 | 0.7268 | <0.0001$ | 1d |
| wild type (N2) L4440(RNAi) | 20 | 22.9 ± 0.3 | 24 | 64/75 | ||||||
| wild type (N2) daf-2(RNAi) | 20 | 38.9 ±0.7 | 45 | 82/91 | +69 | +133 | <0.0001 | <0.0001 | <0.0001$ | |
| skn-1(zu135) L4440(RNAi) | 20 | 16.7 ±0.2 | 17 | 93/104 | −27 | <0.0001 | <0.0001$ | |||
| skn-1(zu135) daf-2(RNAi) | 20 | 16.6 ±0.2 | 17 | 101/112 | −28 | −0.6 | <0.0001 | 0.6062 | <0.0001$ | |
| daf-2(e1368) L4440(RNAi) | 20 | 33.4 ± 0.9 | 38 | 51/63 | +46 | +100 | <0.0001 | <0.0001 | ||
| daf-2(e1368) daf-2(RNAi) | 20 | 54.8 ± 0.9 | 64 | 62/73 | +139 | +228 | <0.0001 | <0.0001 | <0.0001$ | |
| daf-2(e1368); skn-1(zu135) L4440(RNAi) | 20 | 21.6 ±0.6 | 26 | 112/124 | −6 | +29 | 0.7408 | <0.0001 | <0.0001$ | |
| daf-2(e1368); skn-1(zu135) daf-2(RNAi) | 20 | 25.9 ±1.2 | 36 | 81/94 | +13 | +55 | 0.0678 | <0.0001 | <0.0001$ | |
| Trial of crude dauerpheromone at 25°C | ||||||||||
| wild type (N2) L4440(RNAi) control | 25 | 12.9 ±0.4 | 14 | 30/30 | ||||||
| wild type (N2) L4440(RNAi) crude dauerpheromone | 25 | 17.7 ±0.8 | 21 | 30/30 | +37 | +50 | <0.0001 | <0.0001 | ||
| wild type (N2) daf-2(RNAi) control | 25 | 21.1 ±0.7 | 24 | 30/30 | +64 | +79 | <0.0001 | <0.0001 | ||
| wild type (N2) daf-2(RNAi) crude dauerpheromone | 25 | 25.6 ±0.9 | 28 | 29/30 | +98 | +117 | <0.0001 | <0.0001 | <0.0001 | |
| skn-1(zu135) L4440(RNAi) control | 25 | 11.8 ±0.3 | 13 | 29/30 | −9 | 0.0235 | <0.0001 | 1e | ||
| skn-1(zu135) L4440(RNAi) crude dauerpheromone | 25 | 16.2 ±0.6 | 19 | 29/30 | +26 | +37 | <0.0001 | <0.0001 | <0.0001 | 1e |
| skn-1(zu135) daf-2(RNAi) control | 25 | 12.2 ±0.4 | 13 | 29/30 | −5 | +3 | 0.2311 | 0.4030 | <0.0001 | 1e |
| skn-1 (zu135) daf-2(RNAi) crude dauerpheromone | 25 | 16.1 ±0.6 | 21 | 29/30 | +25 | +36 | <0.0001 | <0.0001 | <0.0001 | 1e |
(N) = number of animals observed.
a merger of three trials shown in Supplementary Table 2. ED indicates data shown in an Extended Data Figure. P values were determined by Log-Rank. Additional experiments are shown in Supplementary Table 2.
Extended Data Table 2. Collagen genes are upregulated by diverse interventions that increase lifespan.
Collagens were overrepresented in each C. elegans longevity-associated gene set we examined. Gene Ontology (GO) enrichment clusters were identified by DAVID, using high stringency classification. Enrichment scores were ranked from highest (1) to lowest (>10). Additional information is provided in Supplementary Table 10, including P-values that were determined by DAVID using the Fisher Exact test53,78,79.
| Experimental condition | Total # of genes upregulated |
Reference | Enrichment score rank of collagens |
# of collagens upregulated |
# of collagens shared with daf-2; skn-1 upregulated collagens |
shared collagens tested in lifespan assays (in this study) |
|---|---|---|---|---|---|---|
| COLLAGENS UPREGULATED BY DRUG TREATMENTS THAT INCREASE C. ELEGANS LIFESPAN | ||||||
| Resveratrol treatment in young wild-type adults | 116 | 63 | 2 | 8 | 0 | |
| Resveratrol treatment in young daf-16(−) adults | 1027 | 63 | 1 | 85 | 28 | col-12, col-13, col-65, col-97, col-120, col-127, col-133, |
| Humic acid treatment in 11 days old wild-type adults | 740 | 64 | 1 | 27 | 5 | col-13,col-167,col-133 |
| Tannic acid treatment in young wild-type adults | 2842 | 65 | 1 | 74 | 33 | col-10, col-12, col-13, col-65, col-97, col-133, col-141, col-144, col-167, col-180 |
| Quercetin treatment in young wild-type adults | 1562 | 65 | 1 | 67 | 18 | col-12, col-13, col-97, col-133 |
| MAHMA (nitric oxide donor) in wild-type L4 worms | 65 | 66 | 1 | 8 | 1 | col-97 |
| MAHMA (nitric oxide donor) in hsf-1(sy441) L4 worms | 99 | 66 | 1 | 21 | 1 | col-97 |
| Rotenone treatment in young wild-type adults | 2380 | 67 | 1 | 64 | 27 | col-10, col-65, col-97, col-133, col-141 |
| COLLAGENS UPREGULATED IN GENETIC BACKGROUNDS THAT INCREASE C. ELEGANS LIFESPAN | ||||||
| Mixed-stage wdr-23(tm1817) mutants compared to wild type | 2285 | 68 | 7 | 41 | 15 | col-10, col-144, col-167 |
| Young age-1(mg44) adults compared to wild type | 791 | 69 | 1 | 54 | 9 | col-141 |
| daf-2(e1370) at day 5 of adulthood vs wild type* | 869 | 70 | 1 | 57 | 19 | col-10, col-12, col-65, col-89, col-97, col-144, col-167 |
| daf-2(m41) at day 10 vs. wild type at day 6 of adulthood at 25.5°C | 48 | 71 | 2 | 17 | 1 | col-141 |
| DAF-16-dependent genes expressed in daf- 2(e1370) in day 1 adults at 20°C** | 1078 | 72 | 1 | 43 | 16 | col-141 |
| TGFβ-dependent in day 1 adults | 2181 | 72 | 1 | 90 | 30 | col-13, col-65, col-127, col-141, col-144, col-167, col-180 |
| AMPK and downstream signaling (shared transcriptional output of loss of crh-1 (CREB) / loss of tax-6 (calrectulin) / AAK-2 (AMPK) overexpression) in L4 larvae | 549 | 73 | 1 | 31 | 17 | col-12, col-13, col-127, col-133, col-141, col-167, col-180 |
| ash-2 RNAi in animals that lacked a germline in day 8 adults | 592 | 74 | 1 | 21 | 4 | col-12, col-133 |
| Young isp-1 mutant adults compared to wild type | 709 | 75 | 3 | 15 | 2 | |
| cyc-1 RNAi in young adults | 2459 | 75 | 1 | 51 | 18 | col-12, col-13, col-97, col-120, col-141, col-144 |
| 2 day old rsks-1(ok1255) adults | 155 | 76 | 1 | 13 | 3 | col-133 |
| Young ctbp-1(ok498) adults | 213 | 77 | 1 | 30 | 16 | col-65, col-97, col-120, col-144, col-180 |
Temperature not specified.
daf-2(e1370) vs daf-2(e1370);daf-16(mu86).
Extended Data Table 3. Suppression of lifespan extension by adulthood collagen gene knockdown.
Lifespans were measured and presented as in Extended Data Table 1, and one-day old animals were placed on RNAi plates. Additional related experiments are shown in Supplementary Table 13. col-10 and col-12 share >99% protein sequence identity with col-144 col-13, respectively. In analysis of glp-1(bn18), both N2 and glp-1 animals were upshifted from 15°C to 25°C from the mid-L1 stage until the first day of adulthood, then lifespan analysis was performed at 20°C.
| Strain / RNAi | Mean lifespan ± S.E.M. [Days] |
75th percentile [Days] |
N dead/ Initial N |
% mean lifespan change to control |
P-value (log-rank) vs. control |
Figure |
|---|---|---|---|---|---|---|
| Trial of collagen genes from SKN-1-upregulated dat −2(−)set at 15°C | ||||||
| rrf-3(pk1426) RNAi L4440 (control) | 27.2 ±0.4 | 29 | 55/64 | ED Fig. 4c | ||
| rrf-3(pk1426) RNAi col-10 | 28.2 ±0.6 | 29 | 70/77 | +4 | 0.0253 | ED Fig. 4c |
| rrf-3(pk1426) RNAi col-65 | 26.4 ±0.5 | 29 | 57/70 | −3 | 0.4555 | ED Fig. 4c |
| rrf-3(pk1426) RNAi col-120 | 25.7 ±0.7 | 29 | 46/56 | −6 | 0.2542 | ED Fig. 4c |
| rrf-3(pk1426) RNAi col-127 | 26.7 ±0.5 | 29 | 48/61 | −2 | 0.4797 | ED Fig. 4c |
| rrf-3(pk1426) RNAi col-133 | 27.2 ±0.7 | 31 | 58/69 | 0 | 0.4623 | ED Fig. 4c |
| rrf-3(pk1426) RNAi col-141 | 25.3 ± 0.8 | 29 | 29/43 | −7 | 0.0668 | ED Fig. 4c |
| rrf-3(pk1426) RNAi col-167 | 24.8 ±0.7 | 29 | 47/64 | −9 | 0.1128 | ED Fig. 4c |
| rrf-3(pk1426) RNAi col-180 | 27.3 ±0.7 | 29 | 47/61 | 0 | 0.4668 | ED Fig. 4c |
| P-value and % mean lifespan change are relative to rrf-3(pk1426) RNAi L4440 | ||||||
| daf-2(e1370); rrf-3(pk1426) RNAi L4440 | 37.3 ±1.1 | 43 | 51/62 | Fig. 3a | ||
| daf-2(e1370);rrf-3(pk1426) RNAi col-10 | 28.6 ± 0.6 | 31 | 69/80 | −23 | <0.0001 | Fig. 3a |
| daf-2(e1370); rrf-3(pk1426) RNAi col-65 | 30.4 ±0.8 | 36 | 71/86 | −18 | <0.0001 | Fig. 3a |
| daf-2(e1370);rrf-3(pk1426) RNAi col-120 | 26.6 ±0.6 | 29 | 53/66 | −29 | <0.0001 | Fig. 3a |
| daf-2(e1370);rrf-3(pk1426) RNAi col-127 | 29.5 ±0.7 | 33 | 56/62 | −21 | <0.0001 | Fig. 3a |
| daf-2(e1370); rrf-3(pk1426) RNAi col-133 | 28.2 ±0.5 | 31 | 66/75 | −24 | <0.0001 | Fig. 3a |
| daf-2(e1370);rrf-3(pk1426) RNAi col-141 | 29.1 ±0.6 | 31 | 58/71 | −22 | <0.0001 | Fig. 3a |
| daf-2(e1370);rrf-3(pk1426) RNAi col-167 | 28.3 ±0.6 | 29 | 50/59 | −24 | <0.0001 | Fig. 3a |
| daf-2(e1370);rrf-3(pk1426) RNAi col-180 | 30.7 ±0.7 | 33 | 53/65 | −18 | <0.0001 | Fig. 3a |
| P-value and % mean lifespan change are relative to daf-2(e1370); rrf-3(pk1426) RNAi L4440 | ||||||
| eat-2(ad1116); rrf-3(pk1426) RNAi L4440 | 42.1 ±1.0 | 47 | 75/81 | Fig. 3c | ||
| eat-2(ad1116); rrf-3(pk1426) RNAi col-10 | 38.9 ±0.9 | 45 | 97/102 | −7 | 0.0356 | Fig. 3c |
| eat-2(ad1116); rrf-3(pk1426) RNAi col-65 | 38.0 ±1.0 | 43 | 75/79 | −10 | 0.0038 | Fig. 3c |
| eat-2(ad1116); rrf-3(pk1426) RNAi col-120 | 37.1 ±0.9 | 43 | 60/64 | −12 | <0.0001 | Fig. 3c |
| eat-2(ad1116); rrf-3(pk1426) RNAi col-127 | 37.4 ±0.8 | 43 | 83/85 | −11 | <0.0001 | Fig. 3c |
| eat-2(ad1116); rrf-3(pk1426) RNAi col-133 | 37.3 ±1.1 | 45 | 75/83 | −11 | 0.0022 | Fig. 3c |
| eat-2(ad1116); rrf-3(pk1426) RNAi col-141 | 34.7 ±1.2 | 43 | 49/54 | −18 | <0.0001 | Fig. 3c |
| eat-2(ad1116); rrf-3(pk1426) RNAi col-167 | 36.9 ±1.0 | 43 | 62/69 | −12 | 0.0002 | Fig. 3c |
| eat-2(ad1116); rrf-3(pk1426) RNAi col-180 | 34.5 ±0.9 | 37 | 55/58 | −18 | <0.0001 | Fig. 3c |
| P-value and %mean lifespan change are relative to eat-2(ad1116), rrf-3(pk1426) RNAi L4440 | ||||||
| Trial of collagen genes from SKN-1-upregulated daf-2(−) set at 20°C | ||||||
| wild type (N2) RNAi L4440 (control) | 24.6 ±0.2 | 25 | 98/107 | Fig. 3b | ||
| daf-2(e1370); rrf-3(pk1426) RNAi L4440 | 38.2 ±0.8 | 44 | 86/93 | Fig. 3b | ||
| daf-2(e1370);rrf-3(pk1426) RNAi col-10 | 37.4 ±0.7 | 42 | 104/110 | −2 | 0.4014 | Fig. 3b |
| daf-2(e1370);rrf-3(pk1426) RNAi col-13 | 35.2 ±0.7 | 42 | 92/99 | −7 | 0.0043 | Fig. 3b |
| daf-2(e1370);rrf-3(pk1426) RNAi col-120 | 38.8 ±0.6 | 44 | 103/110 | +2 | 0.9386 | Fig. 3b |
| P-value and % mean lifespan change are relative to daf-2(e1370); rrf-3(pk1426) RNAi L4440 | ||||||
| Trial of collagen genes from the SKN-1-upregulated daf-2(−) set at 20°C | ||||||
| wild type (N2) RNAi L4440 (control) 0.2% DMSO | 25.7 ±0.3 | 29 | 96/103 | Fig. 3d | ||
| wild type (N2) RNAi col-10 0.2% DMSO | 25.7 ±0.4 | 28 | 92/100 | 0 | 0.1545 | |
| wild type (N2) RNAi col-13 0.2% DMSO | 26.4 ±0.3 | 27 | 102/111 | +3 | 0.0811 | |
| wild type (N2) RNAi col-120 0.2% DMSO | 26.6 ±0.2 | 29 | 98/104 | +4 | 0.0147 | |
| P-value and % mean lifespan change are relative to wild type (N2) RNAi L4440 (control) 0.2% DMSO | ||||||
| wild type (N2) RNAi L4440 (control) 0.2% DMSO 100µM Rapamycin | 30.7 ±0.3 | 32 | 107/118 | Fig. 3d | ||
| wild type (N2) RNAi col-10 0.2% DMSO 100µM Rapamycin | 28.6 ±0.5 | 32 | 83/93 | −7 | 0.0083 | Fig. 3d |
| wild type (N2) RNAi col-13 0.2% DMSO 100µM Rapamycin | 28.0 ±0.5 | 32 | 83/90 | −8 | 0.0003 | Fig. 3d |
| wild type (N2) RNAi col-120 0.2% DMSO 100µM Rapamycin | 26.8 ±0.5 | 29 | 77/87 | −13 | <0.0001 | Fig. 3d |
| P-value and % mean lifespan change are relative to wild type (N2) RNAi L4440 (control) 0.2% DMSO 100µM Rapamycin | ||||||
| Trial of collagen genes from SKN-1-upregulated daf-2(−) set at 20°C | ||||||
| wild type (N2) RNAi L4440 (control) | 23.8 ±0.3 | 26 | 126/142 | Fig. 3e | ||
| wild type (N2) RNAi col-10 | 23.7 ±0.3 | 26 | 94/108 | 0 | 0.9994 | |
| wild type (N2) RNAi col-13 | 23.3 ±0.3 | 26 | 97/112 | −2 | 0.2122 | |
| wild type (N2) RNAi col-120 | 23.1 ±0.3 | 26 | 71/84 | −3 | 0.1610 | |
| P-value and % mean lifespan change are relative to wild type (N2) RNAi L4440 (control) | ||||||
| glp-1(bn 18) RNAi L4440 (control) | 31.2 ±0.6 | 34 | 53/64 | Fig. 3e | ||
| glp-1(bn 18) RNAi col-10 | 25.8 ±0.6 | 30 | 74/90 | −17 | <0.0001 | Fig. 3e |
| glp-1(bn 18) RNAi col-13 | 27.8 ±0.7 | 30 | 64/84 | −11 | 0.0001 | Fig. 3e |
| glp-1(bn18) RNAi col-120 | 27.0 ±0.6 | 30 | 75/98 | −13 | <0.0001 | Fig. 3e |
| P-value and % mean lifespan change are relative to glp-1(bn 18) RNAi L4440 (control) | ||||||
Supplementary Material
“When maintained at 15°C, daf-2(e1370) mutants (Class 2) are mobile throughout their lifespan as shown here at day 14 of adulthood (10 min; 8x speed).”
“When upshifted from 15°C to 20°C at the first day of adulthood, daf-2(e1370) is typical of other Class 2 mutants in that it becomes relatively immobile during much of its adult lifespan, as shown here at day 14 (10 min; 8x speed).”
Acknowledgements
We thank Cynthia Kenyon, Shohei Mitani, and Jaegal Shim for strains, Piali Sengupta for dauer pheromone, Carolin Obieglo, Lorenza Moronetti, Monet Bland, and Krina Patel for assistance, and Javier Apfeld, Eric Greer, Cynthia Kenyon, William Mair, and Blackwell lab members for discussions or comments on the manuscript. Some strains were provided by the CGC, which is funded by the NIH office of Research Infrastructure Programs (P40 OD010440). Supported by funding from the NIH to TKB (GM062891), CTM (New Innovator), and JPA (5T32DK007260), a DRC award to the Joslin Diabetes Center (P30DK036836), and fellowships from the NSF to JNL, and the Swiss National Science Foundation (PBSKP3_140135) to CYE.
Footnotes
Author Contributions
All authors participated in designing the experiments, and analysing and interpreting the data. JNL and JPA obtained samples for microarray analysis, performed the microarray experiments, analysed the expression profiling data, and performed the lifespan studies in Extended Data Fig. 2f–h and Supplementary Table 4 CYE performed all other experiments. CYE and TKB wrote the manuscript in consultation with the other authors.
The authors have no competing interests to declare.
References
- 1.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. doi:nature08980 [pii] 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 2.Shore DE, Ruvkun GA. cytoprotective perspective on longevity regulation. Trends in cell biology. 2013;23:409–420. doi: 10.1016/j.tcb.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkinson JE, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. doi:S0168952503003196 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Toyama BH, Hetzer MW. Protein homeostasis: live long, won’t prosper. Nat Rev Mol Cell Biol. 2013;14:55–61. doi: 10.1038/nrm3496. doi:nrm3496 [pii] 10.1038/nrm3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Partridge L, Harvey PH. Gerontology Methuselah among nematodes. Nature. 1993;366:404–405. doi: 10.1038/366404a0. [DOI] [PubMed] [Google Scholar]
- 9.McElwee JJ, Schuster E, Blanc E, Thomas JH, Gems D. Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J Biol Chem. 2004;279:44533–44543. doi: 10.1074/jbc.M406207200. doi:10.1074/jbc.M406207200 M406207200 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Gems D, et al. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans . Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arantes-Oliveira N, Berman JR, Kenyon C. Healthy animals with extreme longevity. Science. 2003;302:611. doi: 10.1126/science.1089169. doi:10.1126/science.1089169 302/5645/611 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Sykiotis GP, Bohmann D. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci Signal. 2010;3:re3. doi: 10.1126/scisignal.3112re3. doi:scisignal.3112re3 [pii] 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tullet JM, et al. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C elegans . Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. doi:S0092-8674(08)00130-X [pii] 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robida-Stubbs S, et al. TOR Signaling and Rapamycin Influence Longevity by Regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012;15:713–724. doi: 10.1016/j.cmet.2012.04.007. doi:S1550-4131(12)00147-7 [pii] 10.1016/j.cmet.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Libina N, Berman JR, Kenyon C. Tissue-specific activities of C elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. doi:S0092867403008894 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of Celegans life-span by insulinlike signaling in the nervous system. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147. doi:8880 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Narasimhan SD, et al. PDP-1 links the TGF-beta and IIS pathways to regulate longevity, development, and metabolism. PLoS Genet. 2011;7:e1001377. doi: 10.1371/journal.pgen.1001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliveira RP, Porter Abate J, Dilks K, et al. Condition-adapted stress and longevity gene regulation by Caenorhabditis elegans SKN-1/Nrf. Aging Cell. 2009;8:524–541. doi: 10.1111/j.1474-9726.2009.00501.x. doi:ACE501 [pii] 10.1111/j.1474-9726.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang S, Lynn DA, Lo JY, Paek J, Curran SP. SKN-1 and Nrf2 couples proline catabolism with lipid metabolism during nutrient deprivation. Nature communications. 2014;5:5048. doi: 10.1038/ncomms6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herndon LA, et al. Stochastic genetic factors influence tissue-specific decline in ageing C elegans . Nature. 2002;419:808–814. doi: 10.1038/nature01135. doi:10.1038/nature01135 nature01135 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Varani J, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. The American journal of pathology. 2006;168:1861–1868. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budovskaya YV, et al. An elt-3/elt-5/elt-6 GATA transcription circuit guides aging in C elegans . Cell. 2008;134:291–303. doi: 10.1016/j.cell.2008.05.044. doi:S0092-8674(08)00707-1 [pii] 10.1016/j.cell.2008.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Argmann C, et al. Ppargamma2 is a key driver of longevity in the mouse. PLoS Genet. 2009;5:e1000752. doi: 10.1371/journal.pgen.1000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. doi:nature11861 [pii] 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilchez D, et al. RPN-6 determines C elegans longevity under proteotoxic stress conditions. Nature. 2012;489:263–268. doi: 10.1038/nature11315. [DOI] [PubMed] [Google Scholar]
- 26.Seeger-Nukpezah T, Golemis EA. The extracellular matrix and ciliary signaling. Current opinion in cell biology. 2012;24:652–661. doi: 10.1016/j.ceb.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munger JS, Sheppard D. Cross talk among TGF-beta signaling pathways, integrins, and the extracellular matrix. Cold Spring Harbor perspectives in biology. 2011;3:a005017. doi: 10.1101/cshperspect.a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu HL, et al. Discoidin domain receptors: unique receptor tyrosine kinases in collagen-mediated signaling. J Biol Chem. 2013;288:7430–7437. doi: 10.1074/jbc.R112.444158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumann L. Skin ageing and its treatment. The Journal of pathology. 2007;211:241–251. doi: 10.1002/path.2098. [DOI] [PubMed] [Google Scholar]
- 30.Tian X, et al. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature. 2013;499:346–349. doi: 10.1038/nature12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brenner S. The genetics of Caenorhabditis elegans . Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim TH, et al. Tyrosylprotein sulfotransferase regulates collagen secretion in Caenorhabditis elegans . Molecules and cells. 2010;29:413–418. doi: 10.1007/s10059-010-0049-4. [DOI] [PubMed] [Google Scholar]
- 33.McKay SJ, et al. Gene expression profiling of cell, tissues, developmental stages of the nematode C elegans . Cold Spring Harb Symp Quant Biol. 2003;68:159–169. doi: 10.1101/sqb.2003.68.159. [DOI] [PubMed] [Google Scholar]
- 34.Link CD, Johnson CJ. Reporter transgenes for study of oxidant stress in Caenorhabditis elegans . Methods in enzymology. 2002;353:497–505. doi: 10.1016/s0076-6879(02)53072-x. [DOI] [PubMed] [Google Scholar]
- 35.Hong L, et al. MUP-4 is a novel transmembrane protein with functions in epithelial cell adhesion in Caenorhabditis elegans . J Cell Biol. 2001;154:403–414. doi: 10.1083/jcb.200007075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon ES, Narasimhan SD, Yen K, Tissenbaum HA. A new DAF-16 isoform regulates longevity. Nature. 2010;466:498–502. doi: 10.1038/nature09184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pujol N, et al. Distinct innate immune responses to infection and wounding in the C elegans epidermis. Curr Biol. 2008;18:481–489. doi: 10.1016/j.cub.2008.02.079. doi:S0960-9822(08)00361-8 [pii] 10.1016/j.cub.2008.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.An JH, Blackwell TK. SKN-1 links C elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17:1882–1893. doi: 10.1101/gad.1107803. doi:10.1101/gad.1107803 1107803 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki Y, Morris GA, Han M, Wood WB. A cuticle collagen encoded by the lon-3 gene may be a target of TGF-beta signaling in determining Caenorhabditis elegans body shape. Genetics. 2002;162:1631–1639. doi: 10.1093/genetics/162.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calfon M, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. doi:10.1038/415092a 415092a [pii] [DOI] [PubMed] [Google Scholar]
- 41.Benedetti C, Haynes CM, Yang Y, Harding HP, Ron D. Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics. 2006;174:229–239. doi: 10.1534/genetics.106.061580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hao L, et al. The hedgehog-related gene qua-1 is required for molting in Caenorhabditis elegans . Developmental dynamics : an official publication of the American Association of Anatomists. 2006;235:1469–1481. doi: 10.1002/dvdy.20721. [DOI] [PubMed] [Google Scholar]
- 43.Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11:1975–1980. doi: 10.1016/s0960-9822(01)00594-2. doi:S0960-9822(01)00594-2 [pii] [DOI] [PubMed] [Google Scholar]
- 44.Thein MC, et al. Caenorhabditis elegans exoskeleton collagen COL-19: an adult-specific marker for collagen modification and assembly, and the analysis of organismal morphology. Developmental dynamics : an official publication of the American Association of Anatomists. 2003;226:523–539. doi: 10.1002/dvdy.10259. [DOI] [PubMed] [Google Scholar]
- 45.Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans . Science. 2002;295:502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- 46.Neal SJ, Kim K, Sengupta P. Quantitative assessment of pheromone-induced Dauer formation in Caenorhabditis elegans . Methods in molecular biology. 2013;1068:273–283. doi: 10.1007/978-1-62703-619-1_20. [DOI] [PubMed] [Google Scholar]
- 47.Curran SP, Ruvkun G. Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet. 2007;3:e56. doi: 10.1371/journal.pgen.0030056. doi:07-PLGE-RA-0112R1 [pii] 10.1371/journal.pgen.0030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamath RS, Martinez-Campos M, Zipperlen P, Fraser AG, Ahringer J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans . Genome Biol. 2001;2 doi: 10.1186/gb-2000-2-1-research0002. RESEARCH0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rual JF, et al. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 2004;14:2162–2168. doi: 10.1101/gr.2505604. doi:14/10b/2162 [pii] 10.1101/gr.2505604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. doi:10.1073/pnas.091062498 091062498 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saldanha AJ. Java Treeview--extensible visualization of microarray data. Bioinformatics. 2004;20:3246–3248. doi: 10.1093/bioinformatics/bth349. doi:10.1093/bioinformatics/bth349 bth349 [pii] [DOI] [PubMed] [Google Scholar]
- 53.Dennis G, Jr, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:3. [PubMed] [Google Scholar]
- 54.Pirooznia M, Nagarajan V, Deng Y. GeneVenn - A web application for comparing gene lists using Venn diagrams. Bioinformation. 2007;1:420–422. doi: 10.6026/97320630001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pavesi G, Mereghetti P, Mauri G, Pesole G. Weeder Web: discovery of transcription factor binding sites in a set of sequences from co-regulated genes. Nucleic Acids Res. 2004;32:W199–W203. doi: 10.1093/nar/gkh465. doi:10.1093/nar/gkh465 32/suppl_2/W199 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elemento O, Slonim N, Tavazoie S. A universal framework for regulatory element discovery across all genomes and data types. Mol Cell. 2007;28:337–350. doi: 10.1016/j.molcel.2007.09.027. doi:S1097-2765(07)00666-1 [pii] 10.1016/j.molcel.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas-Chollier M, et al. RSAT: regulatory sequence analysis tools. Nucleic Acids Res. 2008;36:W119–W127. doi: 10.1093/nar/gkn304. doi:gkn304 [pii] 10.1093/nar/gkn304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glover-Cutter K, Kim S, Espinosa J, Bentley DL. RNA polymerase II pauses and associates with pre-mRNA processing factors at both ends of genes. Nat Struct Mol Biol. 2008;15:71–78. doi: 10.1038/nsmb1352. doi:nsmb1352 [pii] 10.1038/nsmb1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoogewijs D, Houthoofd K, Matthijssens F, Vandesompele J, Vanfleteren JR. Selection validation of a set of reliable reference genes for quantitative sod gene expression analysis in C elegans . BMC Mol Biol. 2008;9:9. doi: 10.1186/1471-2199-9-9. doi:1471-2199-9-9 [pii] 10.1186/1471-2199-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans . Proc Natl Acad Sci U S A. 2004;101:8084–8089. doi: 10.1073/pnas.0400848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zito E, Hansen HG, Yeo GS, Fujii J, Ron D. Endoplasmic reticulum thiol oxidase deficiency leads to ascorbic acid depletion and noncanonical scurvy in mice. Mol Cell. 2012;48:39–51. doi: 10.1016/j.molcel.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kage-Nakadai E, et al. Two very long chain fatty acid acyl-CoA synthetase genes, acs-20 and acs-22, have roles in the cuticle surface barrier in Caenorhabditis elegans . PLoS One. 2010;5:e8857. doi: 10.1371/journal.pone.0008857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C elegans life span. Dev Cell. 2005;9:605–615. doi: 10.1016/j.devcel.2005.09.017. doi:S1534-5807(05)00379-5 [pii] 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 64.Menzel R, et al. The Nematode Caenorhabditis elegans, Stress and Aging: Identifying the Complex Interplay of Genetic Pathways Following the Treatment with Humic Substances. Front Genet. 2012;3:50. doi: 10.3389/fgene.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pietsch K, et al. Meta-Analysis of Global Transcriptomics Suggests that Conserved Genetic Pathways are Responsible for Quercetin and Tannic Acid Mediated Longevity in C elegans . Front Genet. 2012;3:48. doi: 10.3389/fgene.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gusarov I, et al. Bacterial nitric oxide extends the lifespan of C elegans . Cell. 2013;152:818–830. doi: 10.1016/j.cell.2012.12.043. doi:S0092-8674(13)00014-7 [pii] 10.1016/j.cell.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 67.Schmeisser S, et al. Neuronal ROS signaling rather than AMPK/sirtuin-mediated energy sensing links dietary restriction to lifespan extension. Molecular Metabolism. 2013;2:1–11. doi: 10.1016/j.molmet.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Staab TA, et al. The Conserved SKN-1/Nrf2 Stress Response Pathway Regulates Synaptic Function in Caenorhabditis elegans . PLoS Genet. 2013;9:e1003354. doi: 10.1371/journal.pgen.1003354. doi:10.1371/journal.pgen.1003354 PGENETICS-D-12-02463 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iser WB, Wilson MA, Wood WH, Becker K, 3rd, Wolkow CA. Co-regulation of the DAF-16 target gene cyp-35B1/dod-13, by HSF-1 in C elegans dauer larvae and daf-2 insulin pathway mutants. PLoS One. 2011;6:e17369. doi: 10.1371/journal.pone.0017369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zarse K, et al. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab. 2012;15:451–465. doi: 10.1016/j.cmet.2012.02.013. doi:S1550-4131(12)00094-0 [pii] 10.1016/j.cmet.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Halaschek-Wiener J, et al. Analysis of long-lived C elegans daf-2 mutants using serial analysis of gene expression. Genome Res. 2005;15:603–615. doi: 10.1101/gr.3274805. doi:gr.3274805 [pii] 10.1101/gr.3274805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaw WM, Luo S, Landis J, Ashraf J, Murphy CT. The C elegans TGF-beta Dauer pathway regulates longevity via insulin signaling. Curr Biol. 2007;17:1635–1645. doi: 10.1016/j.cub.2007.08.058. doi:S0960-9822(07)01914-8 [pii] 10.1016/j.cub.2007.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mair W, et al. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature. 2011;470:404–408. doi: 10.1038/nature09706. doi:nature09706 [pii] 10.1038/nature09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greer EL, et al. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C elegans . Nature. 2010;466:383–387. doi: 10.1038/nature09195. doi:nature09195 [pii] 10.1038/nature09195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cristina D, Cary M, Lunceford A, Clarke C, Kenyon C. A regulated response to impaired respiration slows behavioral rates and increases lifespan in Caenorhabditis elegans . PLoS Genet. 2009;5:e1000450. doi: 10.1371/journal.pgen.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen D, et al. Germline signaling mediates the synergistically prolonged longevity produced by double mutations in daf-2 rsks-1 in C elegans . Cell reports. 2013;5:1600–1610. doi: 10.1016/j.celrep.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen S, et al. The conserved NAD(H)-dependent corepressor CTBP-1 regulates Caenorhabditis elegans life span. Proc Natl Acad Sci U S A. 2009;106:1496–1501. doi: 10.1073/pnas.0802674106. doi:0802674106 [pii] 10.1073/pnas.0802674106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. doi:gkn923 [pii] 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nature Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 80.Hertweck M, Gobel C, Baumeister R. C elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev Cell. 2004;6:577–588. doi: 10.1016/s1534-5807(04)00095-4. doi:S1534580704000954 [pii] [DOI] [PubMed] [Google Scholar]
- 81.Patel DS, et al. Clustering of genetically defined allele classes in the Caenorhabditis elegans DAF-2 insulin/IGF-1 receptor. Genetics. 2008;178:931–946. doi: 10.1534/genetics.107.070813. doi:genetics.107.070813 [pii] 10.1534/genetics.107.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/IGF-1 signaling in C elegans . Science. 2002;298:830–834. doi: 10.1126/science.1074240. doi:10.1126/science.1074240 298/5594/830 [pii] [DOI] [PubMed] [Google Scholar]
- 83.Apfeld J, Kenyon C. Cell nonautonomy of C elegans daf-2 function in the regulation of diapause and life span. Cell. 1998;95:199–210. doi: 10.1016/s0092-8674(00)81751-1. [DOI] [PubMed] [Google Scholar]
- 84.Timmons L, Tabara H, Mello CC, Fire AZ. Inducible systemic RNA silencing in Caenorhabditis elegans . Mol Biol Cell. 2003;14:2972–2983. doi: 10.1091/mbc.E03-01-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kennedy S, Wang D, Ruvkun G. A conserved siRNA-degrading RNase negatively regulates RNA interference in C elegans . Nature. 2004;427:645–649. doi: 10.1038/nature02302. [DOI] [PubMed] [Google Scholar]
- 86.Blackwell TK, Bowerman B, Priess JR, Weintraub H. Formation of a monomeric DNA binding domain by Skn-1 bZIP and homeodomain elements. Science. 1994;266:621–628. doi: 10.1126/science.7939715. [DOI] [PubMed] [Google Scholar]
- 87.Niu W, et al. Diverse transcription factor binding features revealed by genome-wide ChIP-seq in C elegans . Genome Res. 2011;21:245–254. doi: 10.1101/gr.114587.110. doi:gr.114587.110 [pii] 10.1101/gr.114587.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Simmer F, et al. Loss of the putative RNA-directed RNA polymerase RRF-3 makes C elegans hypersensitive to RNAi. Curr Biol. 2002;12:1317–1319. doi: 10.1016/s0960-9822(02)01041-2. doi:S0960982202010412 [pii] [DOI] [PubMed] [Google Scholar]
- 89.Jones SJ, et al. Changes in gene expression associated with developmental arrest and longevity in Caenorhabditis elegans . Genome Res. 2001;11:1346–1352. doi: 10.1101/gr.184401. [DOI] [PubMed] [Google Scholar]
- 90.Hillier LW, et al. Genomics in C elegans: so many genes, such a little worm. Genome Res. 2005;15:1651–1660. doi: 10.1101/gr.3729105. [DOI] [PubMed] [Google Scholar]
- 91.Hedgecock EM, Herman RK. The ncl-1 gene and genetic mosaics of Caenorhabditis elegans . Genetics. 1995;141:989–1006. doi: 10.1093/genetics/141.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schultz RD, Gumienny TL. Visualization of Caenorhabditis elegans cuticular structures using the lipophilic vital dye DiI. J Vis Exp. 2012:e3362. doi: 10.3791/3362. doi:3362 [pii] 10.3791/3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim TH, Kim YJ, Cho JW, Shim J. A novel zinc-carboxypeptidase SURO-1 regulates cuticle formation and body morphogenesis in Caenorhabditis elegans . FEBS letters. 2011;585:121–127. doi: 10.1016/j.febslet.2010.11.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
“When maintained at 15°C, daf-2(e1370) mutants (Class 2) are mobile throughout their lifespan as shown here at day 14 of adulthood (10 min; 8x speed).”
“When upshifted from 15°C to 20°C at the first day of adulthood, daf-2(e1370) is typical of other Class 2 mutants in that it becomes relatively immobile during much of its adult lifespan, as shown here at day 14 (10 min; 8x speed).”