Abstract
Objective
To investigate the post-prostatectomy and long-term outcomes of men presenting with an elevated pretreatment prostate-specific antigen (PSA) level (>10 ng/mL), but otherwise low-risk features (biopsy Gleason score ≤6 and clinical stage ≤T2a).
Patients and Methods
PSA-incongruent intermediate-risk (PII) cases were defined as those patients with preoperative PSA >10 and ≤20 ng/mL but otherwise low-risk features, and PSA-incongruent high-risk (PIH) cases were defined as men with PSA >20 ng/mL but otherwise low-risk features. Our institutional radical prostatectomy database (1992–2012) was queried and the results were stratified into D’Amico low-, intermediate- and high risk, PSA-incongruent intermediate-risk and PSA-incongruent high-risk cases. Prostate cancer (PCa) features and outcomes were evaluated using appropriate comparative tests. Multivariable analyses were adjusted for age, race and year of surgery.
Results
Of the total cohort of 17 608 men, 1132 (6.4%) had PII-risk disease and 183 (1.0%) had PIH-risk disease. Compared with the low-risk group, the odds of upgrading at radical prostatectomy (RP) were 2.20 (95% CI 1.93–2.52; P < 0.001) for the PII group and 3.58 (95% CI 2.64–4.85; P < 0.001) for the PIH group, the odds of extraprostatic disease at RP were 2.35 (95% CI 2.05–2.68; P < 0.001) for the PII group and 6.68 (95% CI 4.89–9.15; P < 0.001) for the PIH group, and the odds of positive surgical margins were 1.97 (95% CI 1.67–2.33; P < 0.001) for the PII group and 3.54 (95% CI 2.50–4.95, P < 0.001) for the PIH group. Compared with low-risk disease, PII-risk disease was associated with a 2.85-, 2.99- and 3.32-fold greater risk of biochemical recurrence (BCR), metastasis and PCa-specific mortality, respectively, and PIH-risk disease was associated with a 5.32-, 6.14- and 7.07-fold greater risk of BCR, metastasis and PCa-specific mortality, respectively (P ≤ 0.001 for all comparisons). For the PII group, the higher risks of positive surgical margins, upgrading, upstaging and BCR were dependent on PSA density (PSAD): men in the PII group who had a PSAD <0.15 ng/mL/g were not at higher risk compared with those in the low-risk group. Men in the PII group with a PSAD ≥0.15 ng/mL/g and men in the PIH group were more likely to have an anterior component of the dominant tumour (59 and 64%, respectively) compared with those in the low- (35%) and intermediate-risk group (39%) and those in the PII-risk group with PSAD <0.15 ng/mL/g (29%).
Conclusions
Men with PSA >20 ng/mL or men with PSA >10 and ≤20 ng/mL with a PSAD ≥0.15 ng/mL/g, but otherwise low-risk PCa, are at greater risk of adverse pathological and oncological outcomes and may be inappropriate candidates for active surveillance. These men are at greater risk of having anterior tumours that are undersampled at biopsy, so if treatment is deferred, ancillary testing such as anterior zone sampling or magnetic resonance imaging should be strongly encouraged. Men with elevated PSA levels >10 and ≤20 ng/mL but low PSAD have outcomes similar to those in the low-risk group, and consideration of surveillance is appropriate in these cases.
Keywords: prostate-specific antigen, prostate cancer, risk, outcome, prostatectomy
Introduction
Physicians sometimes encounter men who present with clinically localised Gleason score (GS) 6 prostate cancer (PCa) on DRE and biopsy but with serum PSA levels in the intermediate or high range. Particularly when only low-volume disease is detected on extended core biopsy, there may be a temptation to counsel these men who present with elevated pretreatment PSA in the intermediate- or high-risk range (>10 ng/mL) but otherwise D’Amico low-risk PCa (biopsy GS ≤6 or clinical stage ≤T2a) as if they have less aggressive disease.
The D’Amico criteria, developed in 1998 by D’Amico et al. [1], are part of a prediction model that stratifies patients into low-, intermediate- or high-risk PCa groups. The model uses clinical stage, serum PSA and biopsy GS for risk stratification and is widely used to predict oncological outcomes after radical prostatectomy (RP) or radiotherapy, as well as to select appropriate candidates for surveillance. Patients are deemed to be at low risk if they present with clinical stage ≤T2a, PSA ≤10 ng/mL and biopsy GS ≤6. Patients deemed to be at intermediate risk have clinical stage T2b disease or PSA >10 and ≤20 ng/mL or biopsy GS 7. Those presenting with clinical stage ≥T2c or serum PSA >20 ng/mL or biopsy GS 8–10 are considered to be at high risk. In surgical series, the prevalence of elevated PSA (>10 ng/mL) as the single risk factor for intermediate- or high-risk disease varies from 4 to 18% [2–5].
To further delineate outcomes between risk groups, numerous studies have attempted to explain which risk factors are the strongest predictors of recurrence and outcome. In several surgical cohorts, Gleason grade was the primary predictor of biochemical recurrence (BCR) and PCa-specific mortality [2,3,6,7], whereas serum PSA level has been shown to be a weaker variable for risk stratification [4,8]. As a result of downward stage migration and increased prostate sampling at biopsy in the contemporary PSA screening era, pretreatment PSA levels may have less impact in decision-making and patient counselling [2,9].
Currently, the long-term outcomes of the subset of men presenting with ‘PSA-incongruence’ (PSA >10 ng/mL) but otherwise low-risk disease have not been thoroughly explored in contemporary series. In the present study, we describe the outcomes of men with PSA-incongruent PCa at RP from a single, high-volume tertiary referral centre and examine the BCR-free survival, metastasis-free survival and PCa-specific survival of these men.
Patients and Methods
The Johns Hopkins institutional review board-approved radical prostatectomy database was queried to obtain data from 1992 to 2012 for men with clinically localised disease (n = 19 468). The time period was chosen such that all patients had undergone treatment in the PSA era but also had enough follow-up for oncological outcomes to be determined. We excluded men who received neoadjuvant therapy (n = 876) and those with incomplete preoperative risk stratification data (n = 984). The final study cohort of 17 608 men was stratified into D’Amico low-risk, intermediate- and high-risk groups, a PSA-incongruent intermediate-risk and a PSA-incongruent high-risk group. We defined PSA-incongruent intermediate-risk (PII) cases as those men with preoperative PSA >10 and ≤20 ng/mL but otherwise low-risk features (clinical stage ≤T2a and biopsy GS ≤6). PSA-incongruent high-risk (PIH) cases were men with PSA>20 ng/mL but otherwise low-risk features (clinical stage ≤T2a and biopsy GS ≤6). In comparisons among risk groups, PSA-incongruent cases were excluded from the traditional D’Amico classifications to prevent double-counting (intermediate risk except PII and high risk except PIH).
Biopsy and RP specimens were reviewed at Johns Hopkins by genitourinary pathologists, as previously described [10]. Follow-up included serial PSA measurements and in the setting of PSA recurrence, DRE, radionuclide bone scan and axial imaging.
Preoperative features, pathological findings and oncological outcomes were evaluated according to risk group. PSA density (PSAD) was calculated using pathological prostate weights with adjustment (preoperative serum PSA/[pathological prostate weight in g–7 g for seminal vesicles]) as a proxy for volume measured by TRUS (Epstein JI, Pers. Commun.) [11]. Oncological outcomes evaluated with follow-up were BCR-free survival, metastasis-free survival and PCa-specific survival. BCR was defined as a single postoperative PSA of ≥0.2 ng/mL.
Preoperative features and pathological outcomes were compared among risk groups using one-way ANOVA for continuous variables and Pearson’s chi-squared test for categorical variables. Odds ratios for pathological outcomes were computed using logistic regression analyses. BCR-free survival, metastasis-free survival and PCa-specific survival (time 0 was defined as the date of surgery) were compared among risk groups using the Kaplan–Meier method and log-rank tests. Associations between risk group and BCR, metastasis and PCa-specific mortality were assessed with univariate and multivariable Cox proportional hazards models. All statistical analyses were performed using STATA 11.0 (StataCorp, College Station, TX, USA). Two-tailed P values < 0.05, adjusted for multiple comparisons when applicable, were considered to indicate statistical significance.
Results
Of 17 608 men treated with RP, 1315 (7.4%) were identified as having PSA-incongruent disease: 1132 (6.4%) with PII disease and 183 (1.0%) with PIH disease (Table 1). Men in the PII risk group had a median PSA level of 12.5 ng/mL, and men in the PIH risk group had a median PSA level of 24.8 ng/mL. The median PSA values for D’Amico low-, intermediate- and high-risk groups were 4.9, 5.6 and 7.4 ng/mL, respectively. The median PSAD was 0.22 ng/mL/g for men in the PII group and 0.46 ng/mL/mg for men in the PIH group; for D’Amico low-, intermediate- and high-risk groups, the median PSADs were 0.09, 0.12, and 0.16 ng/mL/g, respectively. By definition, all men in the PII and PIH groups presented with biopsy GS ≤6 and clinical stage T1c–T2a disease. In comparison, only 13.0% of men in the intermediate-risk group and 15.1% of men in the high-risk group presented with biopsy GS ≤6, and 76.6% of the intermediate-risk group and 57.4% of the high-risk group presented with T1c–T2a disease.
Table 1.
Preoperative characteristics and pathological findings from the study cohort stratified by risk category.
| Risk category | P | |||||
|---|---|---|---|---|---|---|
| Low | PII | Intermediate except PII | PIH | High except PIH | ||
| No. men (%) | 10 388 (59.) | 1132 (6.4) | 4670 (27.0) | 183 (1.0) | 1235 (7.0) | – |
| Mean (range) age, years | 57.3 (33–73) | 58.4 (39–73) | 59.1 (34–76) | 58.3 (39–73) | 59.4 (37–76) | <0.001 |
| Race, n (%) | ||||||
| Black | 798 (7.7) | 111 (9.8) | 471 (10.1) | 24 (13.1) | 133 (10.8) | <0.001 |
| White/Other | 9 590 (92.7) | 1021 (90.2) | 4199 (89.9) | 159 (86.9) | 1102 (89.2) | |
| Median (IQR) year of surgery, | 2 003 (2000, 2007) | 2000 (1996, 2004) | 2005 (2000, 2009) | 1999 (1996, 2003) | 2004 (1996, 2008) | <0.001 |
| Follow-up, years | ||||||
| Mean | 6.0 | 7.5 | 5.7 | 6.8 | 6.8 | <0.001 |
| Median | 2.0 | 2.0 | 2.0 | 3.0 | 2.0 | |
| Median (IQR) PSA, ng/mL | 4.9 (3.7, 6.4) | 12.5 (10.9, 14.8) | 5.6 (4.3, 8.0) | 24.8 (22.3, 31.9) | 7.4 (5.0, 15.0) | <0.001 |
| Median (IQR) PSAD, ng/mL/g | 0.09 (0.07, 0.13) | 0.22 (0.17, 0.28) | 0.12 (0.08, 0.17) | 0.46 (0.32, 0.60) | 0.16 (0.10, 0.29) | <0.001 |
| Median (IQR) prostate weight, g | 50.9 (42.0, 64.0) | 58.0 (46.7, 77.9) | 48.0 (40.4, 59.4) | 58.0 (45.0, 81.0) | 51.0 (42.0, 62.3) | <0.001 |
| Biopsy GS, n (%) | ||||||
| ≤6 | 10 388 (100.0) | 1132 (100.0) | 607/4666 (13.0) | 183 (100.0) | 187 (15.1) | <0.001 |
| 7 (3 + 4) | 0 (0.0) | 0 (0.0) | 2934 (62.9) | 0 (0.0) | 179 (14.5) | |
| 7 (4 + 3) | 0 (0.0) | 0 (0.0) | 1125 (24.1) | 0 (0.0) | 100 (8.1) | |
| 8–10 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 769 (62.3) | |
| Clinical stage, n (%) | ||||||
| T1c | 8 558/10 382 (82.4) | 922/1132 (81.4) | 2558/4670 (54.8) | 154/183 (84.2) | 462/1215 (38.0) | <0.001 |
| T2a | 1 824 (17.6) | 210 (18.6) | 1018 (21.8) | 29 (15.8) | 236 (19.4) | |
| T2b | 0 (0.0) | 0 (0.0) | 1 094 (23.4) | 0 (0.0) | 191 (15.7) | |
| T2c | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 261 (21.5) | |
| T3a | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 63 (5.2) | |
| T3b | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.2) | |
| Pathological GS, n (%) | ||||||
| ≤6 | 8 058/10 385 (77.6) | 691/1130 (61.2) | 1182/4669 (25.3) | 90/183 (49.2) | 139/1233 (11.3) | <0.001 |
| 7 (3 + 4) | 1 903 (18.3) | 320 (28.3) | 2111 (45.2) | 60 (32.8) | 295 (24.0) | |
| 7 (4 + 3) | 295 (2.8) | 78 (6.9) | 1014 (21.7) | 23 (12.6) | 254 (20.6) | |
| 8–10 | 129 (1.2) | 41 (3.6) | 362 (7.8) | 10 (5.5) | 545 (44.2) | |
| Pathological stage | ||||||
| T2 | 8 432/10 361 (81.4) | 736/1131(65.1) | 2496/4653 (53.6) | 72/182 (39.6) | 412/1234 (33.4) | <0.001 |
| T3a | 1 771 (17.1) | 334 (29.5) | 1699 (36.5) | 88 (48.4) | 527 (42.7) | |
| T3b | 119 (1.1) | 41 (3.6) | 309 (6.6) | 11 (6.0) | 163 (13.2) | |
| N1 | 39 (0.4) | 20 (1.9) | 149 (3.2) | 11 (6.0) | 132 (10.7) | |
| Positive surgical margins | 1 052/10 363 (10.2) | 206/1130 (18.2) | 742/4663 (15.9) | 52/182 (28.6) | 328/1232 (26.6) | <0.001 |
| Upgrade (pathological GS ≥ 7) | 2 327/10 385 (22.4) | 439/1130 (38.8) | 576/4669 (12.3) | 93/183 (50.8) | 269/1233 (21.8) | <0.001 |
| Upstage (pathological stage ≥pT3a) | 1 929/10 361 (18.6) | 395/1131 (34.9) | 2157/4653 (46.4) | 110/182 (60.4%) | 764/1234 (61.9) | <0.001 |
PII, PSA-incongruent intermediate risk; PIH, PSA-incongruent high risk; IQR, interquartile range; PSAD, PSA density; GS, Gleason score.
Examining pathological upgrading and upstaging at surgery, 22.4% of the low-risk group were upgraded to GS ≥7 at RP and 18.6% were upstaged to pathological stage ≥ pT3a. By comparison, 38.8% of the PII group were upgraded and 34.9% were upstaged and, of the PIH group, 50.8% were upgraded and 60.4% were upstaged (Table 1). The odds of upgrading at RP were 2.20 (95% CI 1.93–2.52; P < 0.001) for the PII group and 3.58 (95% CI 2.64–4.85; P < 0.001) for the PIH group compared with the low-risk group (Table 2A). Similarly, men in the PII and PIH groups had odds ratios (ORs) of extraprostatic disease at RP of 2.35 (95% CI 2.05–2.68; P < 0.001) and 6.68 (95% CI 4.89–9.15; P < 0.001), respectively, compared with those in the D’Amico low-risk group. The risks of positive surgical margins among men in the PII and PIH groups were also higher (OR 1.97, 95% CI 1.67–2.33, P < 0.001 and OR 3.54, 95% CI 2.50–4.95; P < 0.001, respectively) compared with men in the low-risk group, and the rates of positive surgical margins (18.2 and 28.6%, respectively) were more similar to those in the respective D’Amico intermediate- or high-risk groups (15.9 and 26.6%, respectively; Tables 1,2A).
Table 2.
| A Risk of positive surgical margins, upgrading (pathological Gleason sum ≥7), and upstaging (pathological stage ≥pT3a) at radical prostatectomy among the risk categories compared with those classified as D’Amico low risk. | |||
|---|---|---|---|
| OR | 95% CI | P | |
| Positive surgical margins | |||
| Low | 1 | – | – |
| PII | 1.97 | 1.67–2.33 | <0.001 |
| Intermediate except PII | 1.67 | 1.51–1.86 | <0.001 |
| PIH | 3.54 | 2.50–4.95 | <0.001 |
| High except PIH | 3.21 | 2.78–3.71 | <0.001 |
| Upgrading | |||
| Low | 1 | – | – |
| PII | 2.20 | 1.93–2.51 | <0.001 |
| Intermediate except PII | 0.49 | 0.44–0.54 | <0.001 |
| PIH | 3.58 | 2.64–4.85 | <0.001 |
| High except PIH | 0.97 | 0.84–1.11 | 0.6379 |
| Upstaging | |||
| Low | 1 | – | – |
| PII | 2.35 | 2.05–2.68 | <0.001 |
| Intermediate except PII | 3.78 | 3.50–4.08 | <0.001 |
| PIH | 6.68 | 4.89–9.15 | <0.001 |
| High except PIH | 7.11 | 6.28–8.07 | <0.001 |
| B Risk of positive surgical margins, upgrading (pathological Gleason sum ≥7), and upstaging (pathologic stage ≥pT3a) at radical prostatectomy among the cohort of men with PSA-incongruent intermediate-risk or PSA-incongruent high-risk prostate cancer, stratified by PSA density <0.15 ng/mL/g or PSA ≥0.15 ng/mL/g, compared with those classified as having D’Amico low-risk prostate cancer. | |||
|---|---|---|---|
| OR | 95% CI | P | |
| Positive surgical margins | |||
| Low | 1 | – | – |
| PII, PSAD <0.15 ng/mL/g | 0.59 | 0.30–1.07 | 0.079 |
| PII, PSAD ≥0.15 ng/mL/g | 2.32 | 1.94–2.78 | <0.001 |
| PIH, PSAD <0.15 ng/mL/g | 0.00 | 0.00–6.81 | 0.452 |
| PIH, PSAD ≥0.15 ng/mL/g | 3.55 | 2.48–5.02 | <0.001 |
| Upgrading | |||
| Low | 1 | – | – |
| PII, PSAD <0.15 ng/mL/g | 1.01 | 0.70–1.43 | 0.972 |
| PII, PSAD ≥0.15 ng/mL/g | 2.60 | 2.25–3.00 | <0.001 |
| PIH, PSAD <0.15 ng/mL/g | 0.87 | 0.02–8.75 | 0.897 |
| PIH, PSAD ≥0.15 ng/mL/g | 3.71 | 2.71–5.09 | <0.001 |
| Upstaging | |||
| Low | 1 | – | – |
| PII, PSAD <0.15 ng/mL/g | 0.51 | 0.30–0.82 | 0.004 |
| PII, PSAD ≥0.15 ng/mL/g | 2.99 | 2.58–3.46 | <0.001 |
| PIH, PSAD <0.15 ng/mL/g | 2.91 | 0.24–25.45 | 0.220 |
| PIH, PSAD ≥0.15 ng/mL/g | 7.13 | 5.16–9.90 | <0.001 |
PII, PSA-incongruent intermediate risk; PIH, PSA-incongruent high risk; OR, odds ratio.
PII, PSA-incongruent intermediate risk; PIH, PSA-incongruent high risk; PSAD, PSA density; OR, odds ratio.
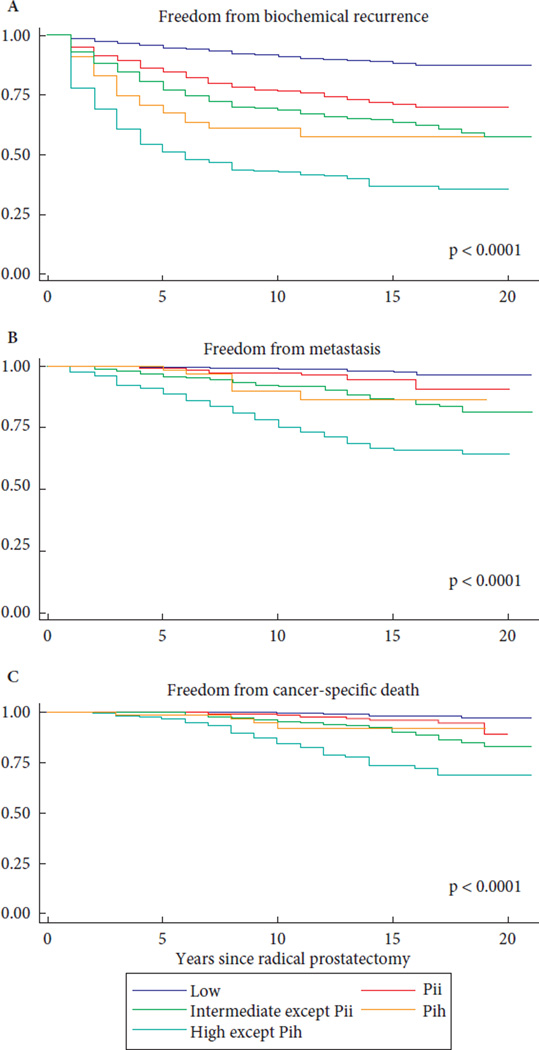
When compared with the low-risk group, BCR-free survival, metastasis-free survival and PCa-specific survival were significantly worse for the PII and PIH groups, although, in general, men in the PII and PIH groups had more favourable outcomes than men in the intermediate- and high-risk groups, respectively (Fig. 1). Similar results were additionally found in multivariable regression analyses adjusting for age, race and year of surgery (Table 3A). The improved 10-year survival rates for the PII and PIH groups in comparison with their respective D’Amico intermediate- and high-risk groups may be explained by the presence of multiple risk factors in the latter groups. We performed additional Kaplan–Meier analyses examining the outcomes of men in the PII group/men in the PIH group in comparison with men with biopsy GS or clinical stage as their only intermediate- or high-risk feature. We found that the presence of elevated Gleason grade as the only intermediate- or high-risk factor was associated with slightly but significantly worse outcomes when compared with the PII or the PIH groups (Figs S1–S4). By contrast, men with higher clinical stage as their only intermediate- or high-risk feature had similar metastasis-free survival and PCa-specific survival to those of men in the PII and PIH groups.
Fig. 1.
Cancer-specific outcomes stratified by risk category. (A) Biochemical recurrence free survival. Proportion of patients free from biochemical recurrence at 10 years was 77% and 61%, for Pii and Pih patients respectively. In comparison, low, intermediate, and high risk patients had 10 year BFS of 91%, 68%, and 43%, respectively. P < 0.0001 for all comparisons. (B) Metastasis free survival. Proportion of patients free from metastasis at 10 years was 97% and 90%, for Pii and Pih patients respectively. In comparison, low, intermediate, and high risk patients had 10 year MFS of 99%, 92%, and 75%, respectively. P < 0.0001 for all comparisons. (C) Cancer-specific survival. Proportion of patients free from cancer-specific death at 10 years was 99% and 92%, for Pii and Pih patients respectively. In comparison, low, intermediate, and high risk patients had 10 year CSS of 99.5%, 96%, and 85%, respectively. P < 0.0001 for all comparisons.
Table 3.
| A Univariate and multivariable hazard ratios predicting biochemical recurrence, metastasis and prostate cancer-specific mortality. | ||||||
|---|---|---|---|---|---|---|
| Univariate | Multivariable | |||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Biochemical recurrence | ||||||
| Risk category | ||||||
| Low (referent) | 1 | – | – | 1 | – | – |
| PII | 3.03 | 2.49, 3.69 | <0.001 | 2.85 | 2.33, 3.47 | <0.001 |
| Intermediate except PII | 4.26 | 3.74, 4.85 | <0.001 | 4.20 | 3.86, 4.78 | <0.001 |
| PIH | 5.95 | 4.21, 8.42 | <0.001 | 5.32 | 3.75, 7.54 | <0.001 |
| High except PIH | 10.61 | 9.18, 12.26 | <0.001 | 10.05 | 8.68, 11.63 | <0.001 |
| Age | 1.02 | 1.01, 1.03 | <0.001 | 1.00 | 0.99, 1.01 | 0.402 |
| Black race | 1.70 | 1.43, 2.01 | <0.001 | 1.56 | 1.32, 1.85 | <0.001 |
| Year of surgery | 0.96 | 0.95, 0.97 | <0.001 | 0.98 | 0.97, 0.99 | <0.001 |
| Metastasis | ||||||
| Risk category | ||||||
| Low (referent) | 1 | – | – | 1 | – | – |
| PII | 3.17 | 1.87, 5.38 | <0.001 | 2.99 | 1.76, 5.08 | <0.001 |
| Intermediate except PII | 7.66 | 5.45, 10.78 | <0.001 | 7.47 | 5.30, 10.53 | <0.001 |
| PIH | 6.59 | 2.81, 15.47 | <0.001 | 6.14 | 2.61, 14.46 | <0.001 |
| High except PIH | 22.75 | 16.09, 32.18 | <0.001 | 21.33 | 15.01, 30.31 | <0.001 |
| Age | 1.03 | 1.01, 1.05 | 0.003 | 1.01 | 0.99, 1.02 | 0.526 |
| Black race | 0.96 | 0.58, 1.59 | 0.883 | 0.86 | 0.52, 1.42 | 0.544 |
| Year of surgery | 0.93 | 0.90, 0.96 | <0.001 | 0.97 | 0.94, 0.99 | 0.016 |
| Prostate cancer-specific mortality | ||||||
| Risk category | ||||||
| Low (referent) | 1 | – | – | 1 | – | – |
| PII | 3.51 | 1.77, 6.98 | <0.001 | 3.32 | 1.67, 6.61 | 0.001 |
| Intermediate except PII | 8.28 | 5.15, 13.30 | <0.001 | 7.98 | 4.95, 12.86 | <0.001 |
| PIH | 7.64 | 2.63, 22.19 | <0.001 | 7.07 | 2.43, 20.59 | <0.001 |
| High except PIH | 26.00 | 16.28, 41.53 | <0.001 | 23.92 | 14.84, 38.56 | <0.001 |
| Age | 1.02 | 1.00, 1.05 | 0.040 | 1.00 | 0.98, 1.02 | 0.916 |
| Black race | 1.23 | 0.67, 2.26 | 0.504 | 1.04 | 0.57, 1.91 | 0.900 |
| Year of surgery | 0.89 | 0.85, 0.93 | <0.001 | 0.96 | 0.92, 1.00 | 0.058 |
| B Univariate and multivariable hazard ratios predicting biochemical recurrence and metastasis among the cohort of men with PSA-incongruent intermediate risk prostate cancer. | ||||||
|---|---|---|---|---|---|---|
| Univariate | Multivariable | |||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Biochemical recurrence | ||||||
| PII, PSAD ≥0.15 ng/mL/g | 3.79 | 1.85, 7.76 | <0.001 | 4.04 | 1.96,8.31 | <0.001 |
| Age | 1.02 | 0.99, 1.05 | 0.108 | 1.04 | 1.01,1.07 | 0.009 |
| Black race | 2.23 | 1.35, 3.69 | 0.002 | 2.35 | 1.42,3.91 | 0.001 |
| Year of surgery | 0.96 | 0.92, 1.00 | 0.066 | 0.96 | 0.92,1.01 | 0.106 |
| Metastasis | ||||||
| PII, PSAD ≥0.15 ng/mL/g | 4.33 | 0.58, 32.44 | 0.154 | 4.32 | 0.56,33.23 | 0.159 |
| Age | 1.02 | 0.95, 1.10 | 0.561 | 1.04 | 0.96,1.12 | 0.326 |
| Black race | 2.86 | 0.83, 9.87 | 0.096 | 2.82 | 0.81,9.78 | 0.102 |
| Year of surgery | 0.92 | 0.79, 1.07 | 0.305 | 0.94 | 0.81,1.10 | 0.423 |
PII, PSA-incongruent intermediate risk; PIH, PSA-incongruent high risk; HR, hazard ratio.
PII, PSA-incongruent intermediate risk; PIH, PSA-incongruent high risk; PSAD, PSA density; HR, hazard ratio.
Further analysis was undertaken to assess the volume of cancer on biopsy among different risk categories. Among the men who also underwent biopsy at our institution (such that complete pathological review of all positive and benign cores was available), the number of positive cores at diagnosis and the maximum percent core involvement at diagnosis were examined (Tables 4,5). Of the men in the low-risk group (n = 6135), 78.1% had PCa involving ≤3 cores. Similarly, 79.9% of men in the PII group and 74.6% of men in the PIH group had ≤3 positive cores at biopsy. Men in the PII group had significantly fewer positive cores (P < 0.001) and significantly less core involvement (P < 0.001) than men in the intermediate-risk group. Similarly, men in the PIH group had significantly fewer positive cores (P < 0.001) and significantly less core involvement (P < 0.001) than men in the high-risk group. Of the men in the low-risk group, 55.5% had ≤3 positive cores and <50% core involvement. This proportion was similar among men in the PII group (56.9%) and slightly reduced among men in the PIH group (41.4%). By comparison, only 25.4 and 19.5% of other men with intermediate- or high-risk disease had these features. On subset analysis, including only those men with PII- or PIH-risk disease, we found that rates of upgrading, upstaging and positive surgical margins were higher for men in the PII group with >3 positive cores on biopsy compared with those with ≤3 positive cores (P = 0.031, P = 0.003, P < 0.001, respectively) but this was not true for men in the PIH group (P = 0.597, 0.539 and 0.455, respectively).
Table 4.
Number of positive cores at diagnosis stratified by risk category (among patients with both biopsies and radical prostatectomy performed at our institution).
| Risk category | Number of positive cores | P | ||
|---|---|---|---|---|
| ≤3 positive cores, n (%) | 4–6 positive cores, n (%) | ≥7 positive cores, n (%) | ||
| Low, n = 6135 | 4792 (78.1) | 1068 (17.4) | 275 (4.5) | – |
| PII, n = 508 | 406 (79.9) | 78 (15.4) | 24 (4.7) | 0.3414 |
| Intermediate except PII, n = 2725 | 1406 (51.6) | 912 (33.5) | 407 (14.9) | <0.001 |
| PIH, n = 71 | 53 (74.6) | 12 (16.9) | 6 (8.5) | 0.4834 |
| High except PIH, n = 588 | 251 (42.7) | 203 (34.5) | 134 (22.8) | <0.001 |
PII, PSA-incongruent intermediate risk; PIH, PSA-incongruent high risk. P values are for comparisons with low-risk disease.
Table 5.
Maximum percent of core involvement at diagnosis stratified by risk category (among patients with both biopsies and radical prostatectomy performed at our institution).
| <50% core involvement, n (%) |
≥50% core involvement, n (%) |
P | |
|---|---|---|---|
| Low, n = 6004 | 3845 (64.0) | 2159 (36.0) | – |
| PII, n = 483 | 312 (64.6) | 171 (35.4) | 0.8066 |
| Intermediate except PII, n = 3170 | 1089 (34.4) | 2081 (65.6) | <0.001 |
| PIH, n = 71 | 41 (57.7) | 30 (42.3) | 0.2721 |
| High except PIH, n = 703 | 183 (26.0) | 520 (74.0) | <0.001 |
PII, PSA-incongruent intermediate risk; PIH, PSA-incongruent high risk. P values are for comparisons with low-risk disease.
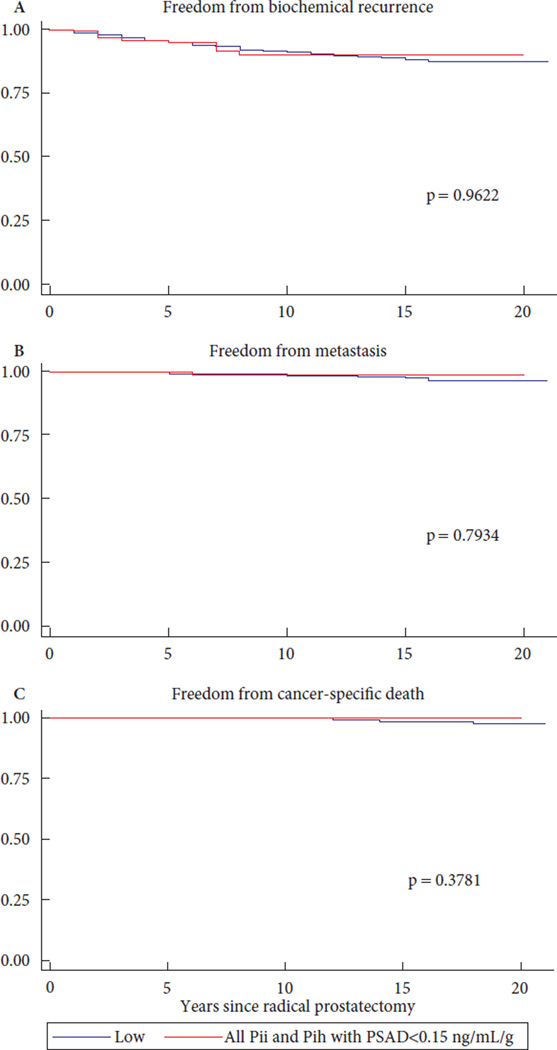
Previously, PSAD, particularly values <0.15 ng/mL/g, has been used as a predictor of very-low-risk PCa in surveillance populations [12–14], so we sought to determine whether PSAD could stratify risk among men with a PSA level >10ng/mL as their only risk factor for intermediate- and high-risk disease. Among men in the PSA-incongruent groups with available PSAD information, 191 in the PII group (17.9%) had PSAD <0.15 ng/mL/g and 878 (82.1%) had PSAD ≥0.15 ng/mL/g. Only five men in the PIH group (2.8%) had PSAD <0.15 ng/mL/g, while 172 men (97.2%) had PSAD ≥0.15 ng/mL/g. Because of the small number of men in the PIH group with low PSAD values, further PSAD analyses within the PIH cohort were not meaningful. When considering men with PII-risk disease, the higher risks of positive surgical margins, upgrading and upstaging were highly dependent on PSAD. For these men, a PSAD of <0.15 ng/mL/g conferred risks of positive surgical margins, upgrading and upstaging at RP that were similar to those for men in the D’Amico low-risk group (Table 2B). In addition, for all men in the PSA-incongruent groups with low PSAD (<0.15 ng/mL/g), BCR-free survival, metastasis-free survival and PCa-specific survival rates were similar to those for men with low-risk disease (Fig. 2). Correlating to this, among men in the PII group, a PSAD ≥0.15 ng/mL/g was associated with a hazard ratio for BCR of 4.04 (95% CI 1.96–8.31; P < 0.001 [Table 3B]). When compared with the low-risk group, men in the PII group with PSAD <0.15 ng/mL/g were not at higher risk of BCR or metastasis, and there was no PCa-specific mortality after RP among men with these features (Table S1).
Fig. 2.
Kaplan Meier curves comparing all PSA-incongruent men (both Pii and Pih risk) having PSA density <0.15 ng/mL/g to D’Amico low risk men. (A) Biochemical recurrence free survival, P = 0.9622. (B) Metastasis free survival, P = 0.7934. (C) Cancer-specific survival, P = 0.3781.
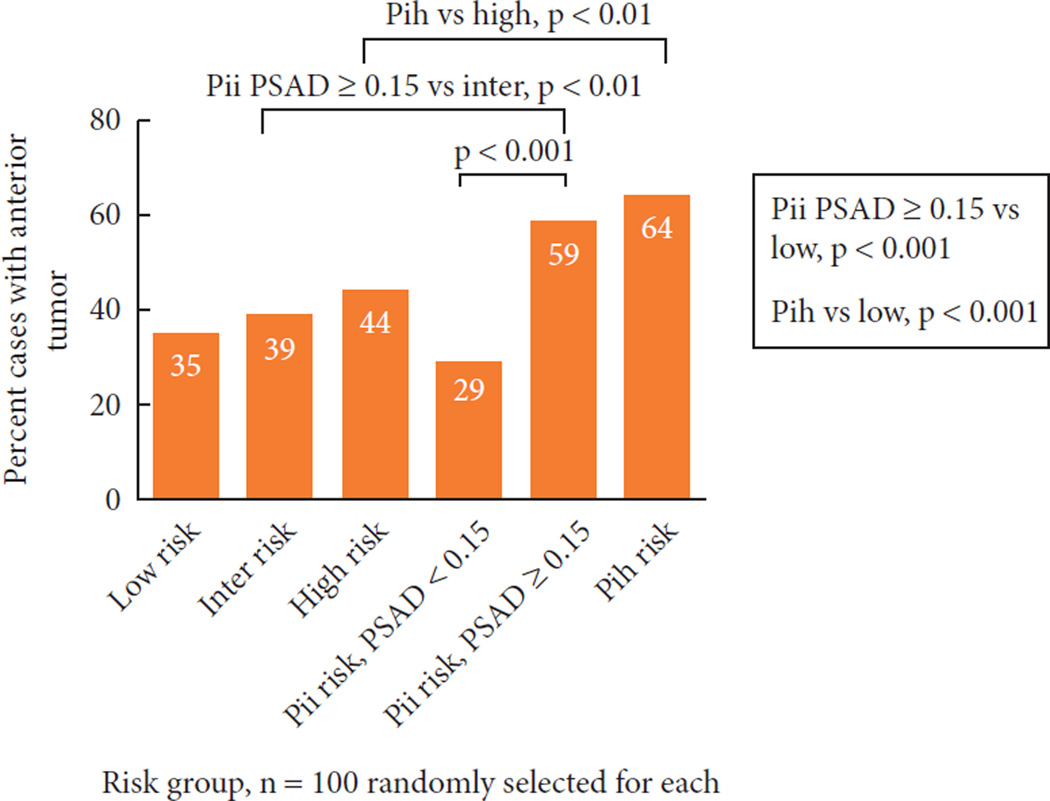
The high frequency of upgrading and upstaging among men in the PSA-incongruent groups, along with the low numbers of cores involved, suggested that tumour locations in these men might have been difficult to sample by standard extended biopsy templates. To examine this, we assessed tumour location in men in the low-, intermediate- and high-risk groups, men in the PII group with PSAD < 0.15 ng/mL/g, men in the PII group with PSAD ≥0.15 ng/mL/g, and men in the PIH group (Fig. 3): 35% of men in the low-risk group, 29% of men in the PII group with PSAD < 0.15 ng/mL/g, 39% of men in the intermediate-risk group, and 44% of men in the high-risk group had dominant nodules with an anterior location. By contrast, 59% of men in the PII group with PSAD ≥0.15 ng/mL/g and 64% of men in the PIH group had dominant tumour nodules with anterior prostate involvement at RP.
Fig. 3.
Percentage of tumors with an anterior component found at RP, stratified by random samples of 100 patients per risk group.
Discussion
Since 1998, the D’Amico classification system has used PSA level, clinical stage and biopsy GS as risk factors to predict biochemical progression after definitive local therapy, but the original risk stratification used by D’Amico et al. [1] was derived from six-core biopsy data. Because sampling of the prostate has since increased, clinicians may view PSA as a less relevant risk factor. Indeed, several studies have argued that among the three risk factors, PSA level is the least relevant in predicting outcomes [4,8]; therefore, patients who present with elevated PSA levels but with biopsy GS≤6 and clinical stage ≤T2a may be counselled to undergo less aggressive treatment because their more ‘relevant’ risk stratifiers are consistent with low-risk disease, particularly if the core involvement with cancer is low.
In the present study, we explored the post-RP findings of men classified as having D’Amico intermediate- or high-risk disease based on PSA only. We find that an elevated PSA level in the setting of other low-risk features does significantly increase the risk of adverse pathological and oncological outcomes. Importantly, this increased risk is highly dependent on PSAD: men with a PSA between 10 and 20 ng/mL but a PSAD <0.15ng/mL/g (~18% of the study population) have risks that are similar to men in the low-risk group. Very few men with PSA >20 ng/mL had a low PSAD, and so it remains unclear as to whether PSAD can act as a similar risk stratifier in this subgroup.
Consistent with previous studies, we found that elevated preoperative PSA levels in men with low-risk biopsy GS and low-risk clinical stage increases the risk of upgrading, extraprostatic disease and positive surgical margins at RP [15–18]. This finding, coupled with a lower number and percent core involvement for men in the PII and PIH groups compared with men in the other intermediate- or high-risk groups, suggested systematic under-sampling of the prostate at TRUS biopsy, possibly caused by anterior tumour locations [19–21]. Indeed, in pathological analysis, men in the PII and PIH groups had the highest percentages of anterior dominant tumour nodules. Consistent with the effect of PSAD on outcomes, when the PII group was stratified by PSAD, men with low PSAD had similar proportions of tumours with anterior components to men in the low-risk group and almost half as many as men in the PII group with PSA ≥0.15 ng/mL/g. For this reason, we suggest that if men with PSA >10 ng/mL and PSAD ≥0.15 ng/mL/g or with PSA >20 ng/mL are to be considered for surveillance, they should undergo additional anterior sampling at biopsy or multiparametric MRI [21–23].
The present study is limited by its retrospective nature and reflects the experiences of a single tertiary centre. Although a large number of men were studied, the data reflect an 18-year period in which changes in biopsy scheme, Gleason grading and surgical technique may have influenced outcomes among D’Amico risk groups. To account for the 2005 changes in Gleason grading, we reviewed a contemporary cohort (2005–2012) of 7268 men: 56.3% with D’Amico low-risk, 3.5% with PII-risk, 32.3% with intermediate-risk, 0.4% with PIH-risk and 7.5% with high-risk PCa (Tables S2–S4, Figs S5,S6). The pathological outcome risks were similar between the original cohort and the contemporary cohort, and again these risks were only higher for men in the PII group who had PSAD ≥0.15 ng/mL/g; however, we were unable to assess the long-term oncological outcome risks for the contemporary cohort using multivariable analysis because of the short follow-up. Another limitation is the fact that our study cohort was from a largely referral-based centre which introduces the potential of selection bias and limits generalisability. Comorbidity and socio-economic data were not available for analysis and are potential confounders.
In conclusion, men with an elevated PSA level (>10 ng/mL), low biopsy GS and low clinical stage are at higher risk of adverse pathological and oncological outcomes when compared with men with only low-risk features. A subpopulation of these men (those with PSA between 10 and 20 ng/mL and with PSAD <0.15 ng/mL/g), however, are not at higher risk. For these men with lower PSAD, active surveillance may be an ideal option. Men with elevated PSA levels and high PSA density as their only risk factors at diagnosis are at higher oncological risk and often harbour anterior tumours that may be under-sampled at biopsy. For these men, caution should be used if recommending surveillance, and ancillary testing (i.e. MRI, additional anterior sampling at biopsy) should be strongly encouraged if surveillance is chosen as an option.
Supplementary Material
Acknowledgement
A.E.R was supported by the Johns Hopkins Clinician Scientist Award.
Abbreviations
- PCa
prostate cancer
- RP
radical prostatectomy
- BCR
biochemical recurrence
- PSAD
PSA density
- GS
Gleason score
- PII
PSA-incongruent intermediate risk
- PIH
PSA-incongruent high risk
- OR
odds ratio
Footnotes
Conflict of Interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Fig. S1 Kaplan–Meier curves comparing outcomes of the PII risk group with men in the D’Amico intermediate-risk group with biopsy Gleason score 7 as their only intermediate-risk feature. (A) Biochemical recurrence-free survival, P = 0.102. (B) Metastasis-free survival, P = 0.002. (C) Cancer-specific survival, P = 0.039.
Fig. S2 Kaplan–Meier curves comparing outcomes of the PII risk group with men in the D’Amico intermediate-risk group with clinical stage T2b as their only intermediate-risk feature. (A) Biochemical recurrence-free survival, P = 0.003. (B) Metastasis-free survival, P = 0.685. (C) Prostate cancer-specific survival, P = 0.33.
Fig. S3 Kaplan–Meier curves comparing outcomes of men in the PIH-risk group with men in the D’Amico high-risk group with biopsy Gleason score 8–10 as their only high-risk feature. (A) Biochemical recurrence-free survival, P = 0.025. (B) Metastasis-free survival, P = 0.013. (C) Prostate cancer-specific survival, P = 0.0234.
Fig. S4 Kaplan–Meier curves comparing outcomes of the PIH-risk group with men in the D’Amico high-risk group with clinical stage ≥T2c as their only high-risk feature. (A) Biochemical recurrence-free survival, P = 0.006. (B) Metastasis-free survival, P = 0.489. (C) Prostate cancer-specific survival, P = 0.714.
Fig. S5 Cancer-specific outcomes stratified by risk category for the 2005–2012 cohort. (A) Biochemical recurrence-free survival, P < 0.001 for all comparisons. (B) Metastasis-free survival, P < 0.001 for all comparisons. (C) Prostate cancer-specific survival, P = 0.008 for all comparisons.
Fig. S6 Kaplan–Meier curves comparing men with PSA-incongruent risk from the 2005–2012 cohort having PSA density <0.15 ng/mL/g (PII: n = 46, PIH: n = 0) with the D’Amico low-risk group. (A) Biochemical recurrence-free survival, P = 0.405. (B) Metastasis-free survival, P = 0.828. (C) Prostate cancer-specific survival, P = 0.899.
Table S1 Among the PII and PIH cohorts, stratified by PSAD <0.15 ng/mL/g or PSAD ≥0.15 ng/mL/g, univariate and multivariable hazard ratios predicting biochemical recurrence, metastasis and prostate cancer-specific mortality.
Table S2 Preoperative characteristics and pathological findings of a 2005–2012 cohort stratified by risk category.
Table S3 (A) Risk of positive surgical margins, upgrading (pathological Gleason sum ≥7), and upstaging (pathological stage ≥pT3a) at radical prostatectomy compared with those classified as having D’Amico low-risk disease for the 2005–2012 cohort. (B) Among the cohort of men with PIIor PIH-risk disease from 2005–2012, stratified by PSAD <0.15 ng/mL/g or PSA ≥0.15 ng/mL/g, risk of positive surgical margins, upgrading (pathologic Gleason sum ≥7), and upstaging (pathological stage ≥pT3a) at radical prostatectomy compared with those classified as having D’Amico low-risk disease.
Table S4 Univariate and multivariable hazard ratios predicting biochemical recurrence, metastasis and prostate cancer-specific mortality for the 2005–2012 cohort.
References
- 1.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 2.Pierorazio PM, Ross AE, Han M, et al. Evolution of the clinical presentation of men undergoing radical prostatectomy for high-risk prostate cancer. BJU Int. 2011;109:988–993. doi: 10.1111/j.1464-410X.2011.10514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Amico AV, Cote K, Loffredo M, et al. Pretreatment predictors of time to cancer specific death after prostate specific antigen failure. J Urol. 2003;169:1320–1324. doi: 10.1097/01.ju.0000049200.30192.d1. [DOI] [PubMed] [Google Scholar]
- 4.Walz J, Joniau S, Chun FK, et al. Pathological results and rates of treatment failure in high-risk prostate cancer patients after radical prostatectomy. BJU Int. 2010;107:765–770. doi: 10.1111/j.1464-410X.2010.09594.x. [DOI] [PubMed] [Google Scholar]
- 5.D’Amico AV, Chen MH, Catalona WJ, et al. Prostate cancer-specific mortality after radical prostatectomy or external beam radiation therapy in men with 1 or more high-risk factors. Cancer. 2007;110:56–61. doi: 10.1002/cncr.22737. [DOI] [PubMed] [Google Scholar]
- 6.Stamey TA, Yemoto CM, McNeal JE, et al. Prostate cancer is highly predictable: a prognostic equation based on all morphological variables in radical prostatectomy specimens. J Urol. 2000;163:1155–1160. doi: 10.1016/s0022-5347(05)67713-0. [DOI] [PubMed] [Google Scholar]
- 7.Stamey TA, McNeal JE, Yemoto CM, et al. Biological determinants of cancer progression in men with prostate cancer. JAMA. 1999;281:1395–1400. doi: 10.1001/jama.281.15.1395. [DOI] [PubMed] [Google Scholar]
- 8.Olumi AF, Richie JP, Schultz DJ, et al. Calculated volume of prostate cancer identifies patients with clinical stage T1c disease at high risk of biochemical recurrence after radical prostatectomy: a preliminary study. Urology. 2000;56:273–277. doi: 10.1016/s0090-4295(00)00644-0. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez DJ, Nielsen ME, Han M, et al. Contemporary evaluation of the D’Amico risk classification of prostate cancer. Urology. 2007;70:931–935. doi: 10.1016/j.urology.2007.08.055. [DOI] [PubMed] [Google Scholar]
- 10.Epstein JI, Pizov G, Walsch PC. Correlation of pathologic findings with progression after radical retropubic prostatectomy. Cancer. 1993;71:3582–3593. doi: 10.1002/1097-0142(19930601)71:11<3582::aid-cncr2820711120>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Epstein JI, Walsh PC, Carmichael M, et al. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–374. [PubMed] [Google Scholar]
- 12.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185–2190. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 13.Thomsen FB, Brasso K, Klotz LH, et al. Active surveillance for clinically localized prostate cancer: a systematic review. J Surg Oncol. 2014;109:830–835. doi: 10.1002/jso.23584. [DOI] [PubMed] [Google Scholar]
- 14.Reese AC, Landis P, Han M, et al. Expanded criteria to identify men eligible for active surveillance of low risk prostate cancer at Johns Hopkins: a preliminary analysis. J Urol. 2013;190:2033–2038. doi: 10.1016/j.juro.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 15.D’Amico AV, Renshaw AA, Arsenault L, et al. Clinical predictors of upgrading to Gleason grade 4 or 5 disease at radical prostatectomy: potential implications for patient selection for radiation and androgen suppression therapy. Int J Radiat Oncol Biol Phys. 1999;45:841–846. doi: 10.1016/s0360-3016(99)00260-6. [DOI] [PubMed] [Google Scholar]
- 16.Mehta V, Rycyna K, Baesens BM, et al. Predictors of Gleason score upgrading on subsequent prostatectomy: a single institution study in a cohort of patients with GS 6. Int J Clin Exp Pathol. 2012;5:496–502. [PMC free article] [PubMed] [Google Scholar]
- 17.Kulkarni GS, Lockwood G, Evans A, et al. Clinical predictors of Gleason score upgrading: implications for patients considering watchful waiting, active surveillance, or brachytherapy. Cancer. 2007;109:2432–2438. doi: 10.1002/cncr.22712. [DOI] [PubMed] [Google Scholar]
- 18.Vora A, Large T, Aronica J, et al. Predictors of Gleason score upgrading in a large African-American population. Int Urol Nephrol. 2013;45:1257–1262. doi: 10.1007/s11255-013-0495-y. [DOI] [PubMed] [Google Scholar]
- 19.Sundi D, Kryvenko ON, Carter HB, et al. Pathological examination of radical prostatectomies in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in black American men. J Urol. 2014;191:60–67. doi: 10.1016/j.juro.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koppie TM, Bianco FJ, Jr, Kuroiwa K, et al. The clinical features of anterior prostate cancers. BJU Int. 2006;98:1167–1171. doi: 10.1111/j.1464-410X.2006.06578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawrentschuk N, Haider MA, Daljeet N, et al. Prostatic evasive anterior tumors: the role of magnetic resonance imaging. BJU Int. 2009;105:1231–1236. doi: 10.1111/j.1464-410X.2009.08938.x. [DOI] [PubMed] [Google Scholar]
- 22.Komai Y, Numao N, Yoshida S, et al. High diagnostic ability of multiparametric magnetic resonance imaging to detect anterior prostate cancer missed by transrectal 12-core biopsy. J Urol. 2013;190:867–873. doi: 10.1016/j.juro.2013.03.078. [DOI] [PubMed] [Google Scholar]
- 23.Cirillo S, Petracchini M, Della Monia P, et al. Value of endorectal MRI and MRS in patients with elevated prostate-specific antigen levels and previous negative biopsies to localize peripheral zone tumours. Clin Radiol. 2008;63:871–879. doi: 10.1016/j.crad.2007.10.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.