Abstract
A murine model was developed that recapitulates key features of clinical hypersensitivity to Escherichia coli asparaginase. Sensitized mice developed high levels of anti-asparaginase IgG antibodies and had immediate hypersensitivity reactions to asparaginase upon challenge. Sensitized mice had complete inhibition of plasma asparaginase activity (P = 4.2 × 10−13) and elevated levels of mouse mast cell protease 1 (P = 6.1 × 10−3) compared with nonsensitized mice. We investigated the influence of pretreatment with triprolidine, cimetidine, the platelet activating factor (PAF) receptor antagonist CV-6209 [2-(2-acetyl-6-methoxy-3,9-dioxo-4,8-dioxa-2,10-diazaoctacos-1-yl)-1-ethyl-pyridinium chloride], or dexamethasone on the severity of asparaginase-induced allergies. Combining triprolidine and CV-6209 was best for mitigating asparaginase-induced hypersensitivity compared with nonpretreated, sensitized mice (P = 1.2 × 10−5). However, pretreatment with oral dexamethasone was the only agent capable of mitigating the severity of the hypersensitivity (P = 0.03) and partially restoring asparaginase activity (P = 8.3 × 10−4). To rescue asparaginase activity in sensitized mice without requiring dexamethasone, a 5-fold greater dose of asparaginase was needed to restore enzyme activity to a similar concentration as in nonsensitized mice. Our results suggest a role of histamine and PAF in asparaginase-induced allergies and indicate that mast cell–derived proteases released during asparaginase allergy may be a useful marker of clinical hypersensitivity.
Introduction
Asparaginase is one of the integral components of combination chemotherapy regimens used in the treatment of acute lymphoblastic leukemia (ALL) and lymphoma. The mechanism of action of asparaginase is not completely understood, but the active enzyme depletes asparagine and possibly glutamine systemically (Wu et al., 1978; Asselin et al., 1989; Chan et al., 2014). Common adverse events to asparaginase include allergic reactions, often accompanied by the development of anti-asparaginase IgG antibodies (Pieters et al., 2011). Serum anti-asparaginase IgG levels have been found to increase before the onset of allergic reactions (Liu et al., 2012), suggesting that high antibody titers are required to induce clinical hypersensitivity. Furthermore, lower serum asparaginase activity was found in patients with anti-asparaginase antibodies compared with patients without detectable antibodies, and the enzyme activities were inversely correlated with the antibody levels (Liu et al., 2012). Anti-asparaginase IgG antibodies have been proposed to directly neutralize asparaginase activity (Albertsen et al., 2002; Pieters et al., 2011), and lower systemic exposure to asparaginase is associated with lower exposure to concomitantly administered dexamethasone and an increased risk of central nervous system relapse (Yang et al., 2008; Kawedia et al., 2012), although some studies showed no associations between anti-asparaginase IgG antibodies and ALL outcomes (Cheung et al., 1986; Larson et al., 1998; Asselin, 1999; Woo et al., 2000; Panosyan et al., 2004).
Classic allergies involve cell-associated antigen-specific IgE antibodies and require low doses of antigen, low titers of circulating antibody, and are mediated by the release of histamine (Finkelman et al., 2005). An alternative pathway for allergy, which appears to play a role in asparaginase-induced reactions (Liu et al., 2012), requires repeated exposure to the antigen, high antigen-specific IgG antibody levels, a large antigen dose, and the release of platelet activating factor (PAF) (Finkelman et al., 2005; Finkelman, 2007). Immunologic studies have shown that antihistamines or PAF receptor antagonist can block the symptoms of an allergic reaction depending on the mechanism of allergy induced by the antigen (Strait et al., 2002). Understanding the pathway of asparaginase allergy will inform strategies for ameliorating the severity of hypersensitivity reactions and can help identify possible markers for detecting sensitized patients before receiving the offending drug.
To investigate therapeutic strategies for mitigating allergies and maintaining plasma concentrations of asparaginase, we created a murine model of asparaginase allergy. The model recapitulates several features of clinical hypersensitivity reactions developed to Escherichia coli asparaginase. Our results indicate the involvement of both histamine and PAF in asparaginase-induced allergies and support the importance of monitoring asparaginase activity if pretreatment of patients with antihistamines or glucocorticoids is used to prevent allergy.
Materials and Methods
Asparaginase Sensitization Protocol.
Eight-week-old female BALB/c mice received 10 μg i.p. doses of E. coli asparaginase (BioVendor Laboratory Medicine Inc., Candler, NC; >96.0% purity as determined by reverse phase high-performance liquid chromatography and SDS-PAGE) formulated with aluminum hydroxide adjuvant (Imject Alum; Thermo Scientific, Rockford, IL) on days 0 and 14 of treatment (Fig. 1A) to be sensitized to asparaginase. Control (nonsensitized) mice received intraperitoneal doses of adjuvant with vehicle alone (normal saline). Asparaginase allergies were induced in sensitized mice by challenging with a 100 μg i.v. dose of E. coli asparaginase on day 24 of treatment. The onset of hypersensitivity was detected by monitoring decreases in rectal temperature using a digital thermometer (model BAT-12; Physitemp Instruments, Clifton, NJ) for 2 hours after the asparaginase challenge. Prechallenge plasma samples for determining anti-asparaginase antibody levels were collected on day 23 of treatment by retro-orbital puncture (Fig. 1A), and postchallenge samples were collected by cardiac puncture at the end of the experiment for measuring antibody levels, asparaginase activity, and mouse mast cell protease 1 (mMCP-1) levels. The area under the temperature versus time curve was calculated using the trapezoidal rule. Lower area under the curve (AUC) values indicate more severe reaction (drop in rectal temperature), and differences in the severity of asparaginase-induced allergies between treatment groups was determined by comparing the AUC values of different groups. Mice were housed in an American Association of Laboratory Animal Care–accredited facility and treated using Institutional Animal Care and Use Committee–approved protocols in accordance with National Institutes of Health guidelines
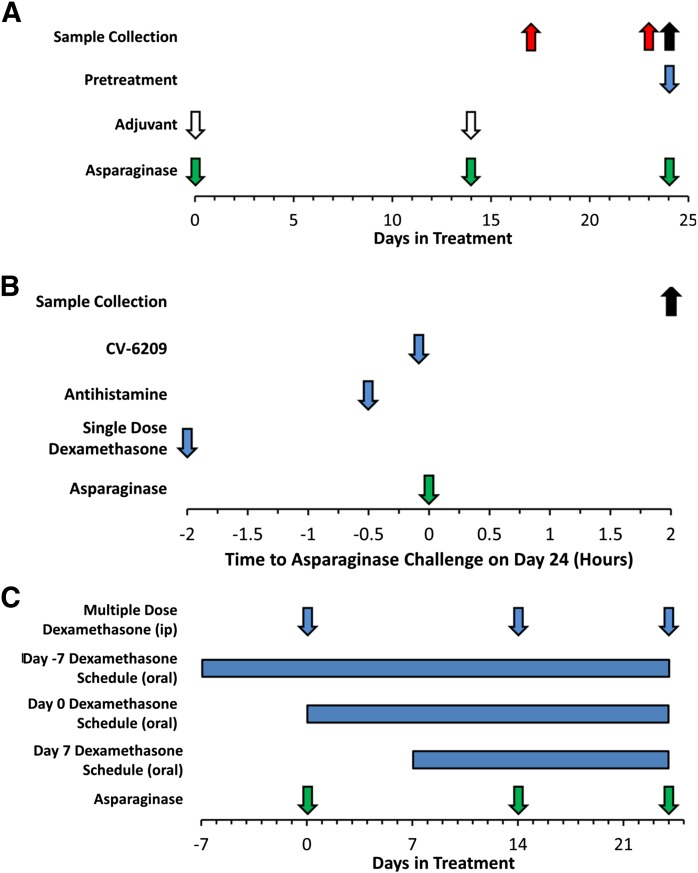
Fig. 1.
Schedule for sensitization to E. coli asparaginase and sample collection. (A) Mice received intraperitoneal injections of E. coli asparaginase (10 μg or 2.25 IU per mouse, green arrows) formulated with aluminum hydroxide adjuvant (1 mg, white arrows) on day 0 and day 14 of the immunization schedule to sensitize mice to asparaginase. On day 24, mice were challenged with an intravenous dose of E. coli asparaginase (100 μg or 22.5 IU per mouse, green arrow) to induce hypersensitivity reactions. Samples collected on day 23 (red arrow) were used to assess anti-asparaginase antibody titers before the asparaginase challenge on day 24. For a subset of mice, including those receiving oral dexamethasone, prechallenge samples were available only on day 17 of treatment (red arrow, n = 39). Mice received pretreatment on day 24 (blue arrow) with CV-6209, antihistamine (triprolidine, cimetidine), or a single dose of dexamethasone (i.p.) in an attempt to mitigate the severity of the allergic reaction. (B) These pretreatment agents were administered 5 minutes, 30 minutes, or 2 hours before the asparaginase challenge, respectively. (C) Additional mitigation strategies included pretreatment with multiple doses of dexamethasone (i.p.) on days 0, 14, and 24 of treatment, and pretreatment with continuous oral dexamethasone starting 7 days before, same day, or 7 days after the initial asparaginase immunization dose. Samples collected 2 hours after the challenge on day 24 (black arrow) were used to measure asparaginase activity, antibody titers, and mMCP-1. Data in subsequent figures are color-matched accordingly to indicate the sample used for the laboratory value [black corresponding to day 24 samples (postchallenge dose of asparaginase) and red to day 23 or day 17 samples (prechallenge)].
Pretreatment Protocol.
Four different agents were investigated for their ability to mitigate asparaginase-induced allergic reactions: the antihistamines cimetidine (H2 receptor antagonist; Sigma-Aldrich, St. Louis, MO) and triprolidine (H1 receptor antagonist; Sigma-Aldrich), the PAF receptor antagonist CV-6209 [2-(2-acetyl-6-methoxy-3,9-dioxo-4,8-dioxa-2,10-diazaoctacos-1-yl)-1-ethyl-pyridinium chloride; Santa Cruz Biotechnology, Santa Cruz, CA], and dexamethasone (American Pharmaceutical Partners, Inc., Schaumburg, IL). As described in Fig. 1B, antihistamine (cimetidine and triprolidine, 200 μg i.p.) and CV-6209 (66 μg i.v.) were given 30 and 5 minutes before the asparaginase challenge on day 24, respectively. Five different regimens of dexamethasone were investigated as described in Fig. 1, B and C. Single-dose dexamethasone was given 2 hours before the asparaginase challenge on day 24 (2 mg/kg i.p.), and multiple-dose dexamethasone was given 2 hours before each asparaginase dose on days 0, 14, and 24 (2 mg/kg i.p.). Pretreatment with oral continuous dexamethasone (4 mg/l in drinking water with a consumption rate of 5 ml/day yields an approximate dose of 0.8–1.0 mg/kg per day for the mice) starting 7 days before, same day (day 0), or 7 days after the initial asparaginase immunization dose was also evaluated for its influence on the severity of asparaginase-induced allergies. This regimen of dexamethasone achieves similar plasma dexamethasone concentrations as those achieved clinically (10–200 nM) in pediatric ALL patients given doses of 8 mg/m2 per day (Yang et al., 2008, 2009; Kawedia et al., 2011).
Asparaginase Activity Determination.
The asparaginase activity in plasma samples was determined by monitoring the enzymatically coupled oxidation of NADH to NAD+ in a 96-well format, as described previously (Fernandez et al., 2013). Briefly, 10 μl of plasma from each sample was added to a 96-well microplate (Corning, Lowell, MA), and an enzyme reaction mixture containing asparagine, α-ketoglutaric acid, glutamic oxalacetic transaminase, β-NADH, and malic dehydrogenase was added to each sample. A BioTek EL x 808 IU microplate reader (Winooski, VT) was used to determine the asparaginase activity. All asparaginase standards used for calibration purposes were prepared using mouse plasma, and samples with values over the standard curve were diluted with mouse plasma to get their enzyme activities within the linear range of the assay.
Determining the Ex Vivo Asparaginase Activity Inhibition of Samples from Sensitized Mice.
The neutralization of asparaginase activity by postimmunization plasma was determined by adding 0.2 IU/ml of asparaginase to plasma samples collected after the asparaginase challenge (day 24). Plasma from sensitized mice, nonsensitized mice, or nonsensitized mice after addition of 5 μg/μl of rabbit polyclonal anti-asparaginase IgG antibodies (Abcam, Cambridge, MA) were tested for residual asparaginase activity as described above.
Detection of Anti-Asparaginase IgG Antibodies.
An enzyme-linked immunosorbent assay–based method was used to detect anti–E.coli asparaginase IgG antibodies, as described previously (Wang et al., 2000; Liu et al., 2012). Plasma samples from nonsensitized mice were used as negative controls, and rabbit polyclonal anti-asparaginase IgG antibodies were used as positive controls. Anti-mouse IgG (Sigma-Aldrich), anti-mouse IgE (Thermo Scientific), and anti-rabbit IgG secondary antibodies (Sigma-Aldrich) were used for detection of antibodies in samples or controls.
Determining mMCP-1 Concentrations.
Concentrations of mMCP-1 were determined by enzyme-linked immunosorbent assay using the Ready-SET-Go commercial kit from eBioscience (San Diego, CA).
Statistical Analysis.
Differences between rectal temperature AUC, asparaginase activity, anti-asparaginase IgG levels, and mMCP-1 concentrations were determined using a pairwise Wilcoxon rank sum test. All statistical analysis was performed with the R statistical software (version 2.13.2).
Results
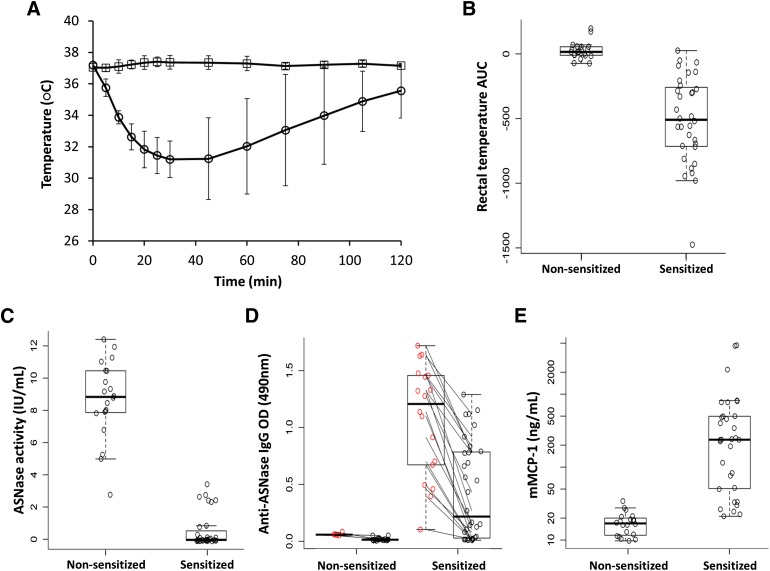
Mice were sensitized to E. coli asparaginase using the adjuvant aluminum hydroxide to create a murine immune response similar to clinical hypersensitivity reactions to asparaginase (Figs. 1A and 2, A–E). Sensitized mice challenged with 100 μg of asparaginase (22.5 IU or ∼1125 IU/kg per mouse) had immediate hypersensitivity reactions characterized by a sudden drop in rectal temperature (Fig. 2A) and a decrease in physical activity, whereas nonsensitized mice did not experience changes in rectal temperature (Fig. 2B; P = 3.3 × 10−10) or activity upon challenge. Compared with nonsensitized controls, sensitized mice experienced a peak drop in rectal temperature >6°C within 30 minutes of the asparaginase challenge and had negligible asparaginase activity in samples collected 2 hours after the challenge (Fig. 2C; P = 4.2 × 10−13). The loss of enzyme activity is likely due to high levels of anti-asparaginase IgG antibodies detected before the challenge on day 23 relative to nonsensitized mice (Fig. 2D, red open circles; P = 1.1 × 10−7). The antibodies bind to asparaginase upon challenge and result in a drop in the measurable antibody titer (Fig. 2D; P = 6.7 × 10−5, comparing black to red open circles of sensitized mice), presumably due to the formation of immune complexes that lead to a decrease in asparaginase activity. Using an ex vivo activity inhibition assay, we found that plasma samples from sensitized mice neutralize >30% of the added asparaginase activity (0.2 IU/ml), with the degree of activity inhibition correlated with anti-asparaginase IgG levels (Supplemental Fig. 1, A and B; R2 = 0.161, P = 0.04). Consistent with the reports of elevated levels of mMCP-1 in various pathways of anaphylaxis (Strait et al., 2002), the mMCP-1 concentrations measured in sensitized mice were elevated nearly 35-fold compared with nonsensitized mice (Fig. 2E; P = 6.1 × 10−3).
Fig. 2.
Mice sensitized to E. coli asparaginase developed asparaginase-induced hypersensitivity reactions when challenged. (A) The rectal temperature of sensitized (○) and nonsensitized (□) mice was monitored for 2 hours after intravenous administration of E. coli asparaginase (100 μg) at day 24 (as per Fig. 1A). (B) Compared with nonsensitized controls, sensitized mice with asparaginase-induced allergies experienced a drop in rectal temperature that was quantified by the AUC of the rectal temperature versus time curve (P = 3.3 × 10−10). (C) Sensitized mice had low to no detectable asparaginase activity (P = 4.2 × 10−13) and (D) developed high anti-asparaginase IgG antibody titers compared with nonsensitized mice (red open circles, P = 1.1 × 10−7). Sensitized mice showed a drop in antibody levels when comparing the prechallenge and postchallenge samples at day 23 versus day 24 (red versus black open circles, P = 6.7 × 10−5), suggesting the formation of asparaginase–anti-asparaginase IgG immune complexes. (E) Sensitized mice with asparaginase-induced allergies had elevated levels of mMCP-1 compared with nonsensitized mice in samples collected 2 hours after the challenge (P = 6.1 × 10−3).
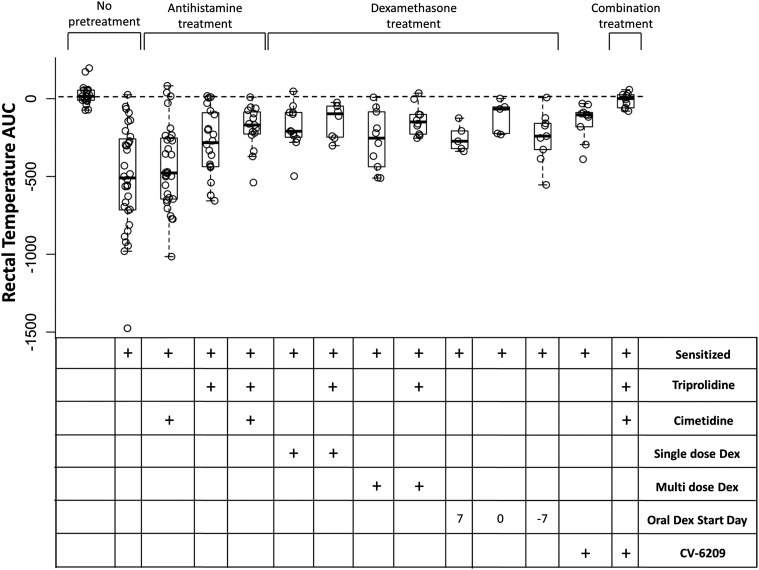
Having established a model of asparaginase-induced allergy, we next tried to develop pretreatment strategies to mitigate the severity of the reactions. The initial pretreatment agents we selected were based on receptor antagonists of known chemical meditators of allergy (Fig. 1, B and C). We found that cimetidine, triprolidine, CV-6209, or the three agents used in combination all mitigated the severity of the reaction compared with no pretreatment (Fig. 3; P < 1.3 × 10−3). Antihistamines and CV-6209 mitigated the drop in rectal temperature, but pretreatment with the combination of antihistamines and CV-6209 reduced the severity of the allergic reaction compared with no pretreatment (Fig. 3; P = 1.2 × 10−5), antihistamine alone (Fig. 3; P = 1.6 × 10−4), and single agent CV-6209 (Fig. 3; P = 3.2 × 10−4). It was unclear whether triprolidine and/or cimetidine were responsible for reducing the severity of asparaginase-induced allergy; therefore, we tested each antihistamine as a single agent in sensitized mice. Cimetidine showed little effect compared with nonpretreated sensitized mice, and this group had more severe reactions compared with mice pretreated with a combination of cimetidine and triprolidine (Fig. 3; P = 1.1 × 10−3). In contrast, mice pretreated with single agent triprolidine showed a decrease in the severity of allergies compared with nonpretreated, sensitized mice (Fig. 3; P = 0.02), and their rectal temperature AUC was similar to that of mice pretreated with cimetidine and triprolidine in combination (Fig. 3; P > 0.05). These results suggest that triprolidine but not cimetidine is required for mitigating asparaginase-induced allergies in our murine model.
Fig. 3.
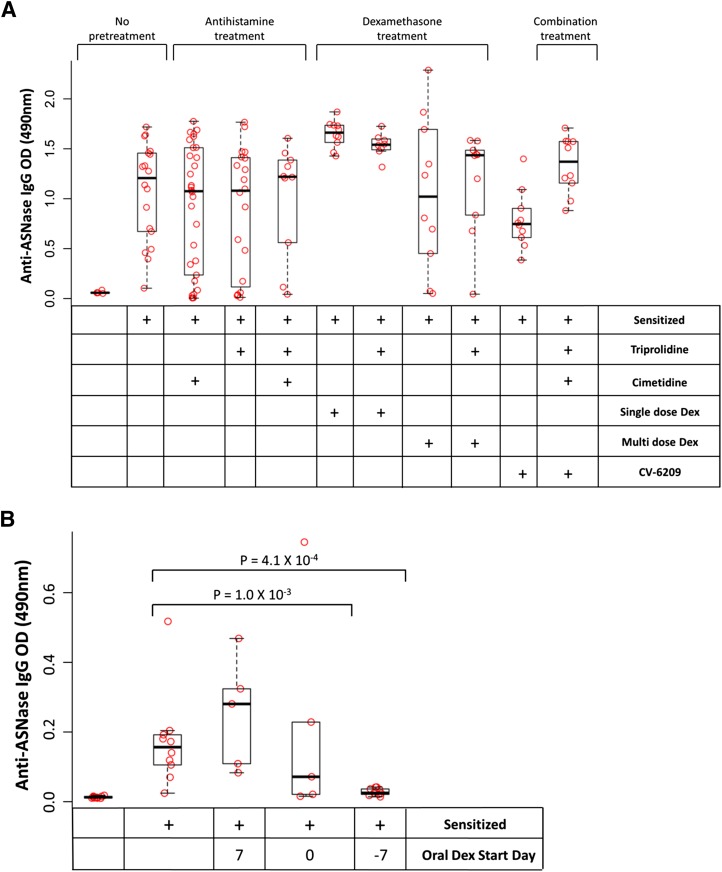
Pretreatment with triprolidine, CV-6209, or dexamethasone (Dex) mitigates the severity of asparaginase-induced allergies. Mice sensitized to E. coli asparaginase were pretreated with triprolidine, cimetidine, the PAF receptor antagonist CV-6209, single-dose dexamethasone, or a combination of the agents as per Fig. 1B; multiple-dose dexamethasone or oral dexamethasone was given as per Fig. 1C. All pretreatment agents except for cimetidine and oral dexamethasone given 7 days after the initial asparaginase immunization dose (at day 0) were able to mitigate the severity of the reaction compared with nonpretreated, sensitized mice (P < 0.03); however, the combination of antihistamine and CV-6209 showed the best ability to reduce the severity of the reaction compared with nonpretreated, sensitized mice (P = 1.2 × 10−5).
PAF receptor antagonists, such as CV-6209, are not approved for clinical use in most countries. Therefore, we wanted to test whether dexamethasone could achieve similar effects as CV-6209 on asparaginase-induced allergies. Five dexamethasone pretreatment strategies were investigated: single-dose dexamethasone (Fig. 1B; 2 mg/kg i.p.) given 2 hours before the asparaginase challenge, multiple doses of dexamethasone (Fig. 1C; 2 mg/kg i.p.) given 2 hours before each immunization dose (Fig. 1C; days 0 and 14) and before the challenge (day 24), and oral dexamethasone (Fig. 1C; 4 mg/l in drinking water) starting at 7 or 0 days before or 7 days after the initial asparaginase immunization dose. Pretreatment with single or multiple doses of dexamethasone was also evaluated with concomitant triprolidine. We found that mice pretreated with any of the dexamethasone regimens showed a decrease in the severity of the reaction compared with nonpretreated, sensitized mice (Fig. 3; P ≤ 0.03), except for mice receiving oral dexamethasone 7 days after the initial asparaginase immunization. We found no difference in rectal temperature AUC between single-dose dexamethasone pretreatment (with or without concomitant triprolidine), multiple-dose dexamethasone pretreatment (with or without concomitant triprolidine), and mice receiving oral dexamethasone (Fig. 3; P > 0.05). Furthermore, all mice pretreated with any regimen containing dexamethasone had more severe reactions compared with mice pretreated with antihistamines and CV-6209 in combination (Fig. 3; P ≤ 0.06).
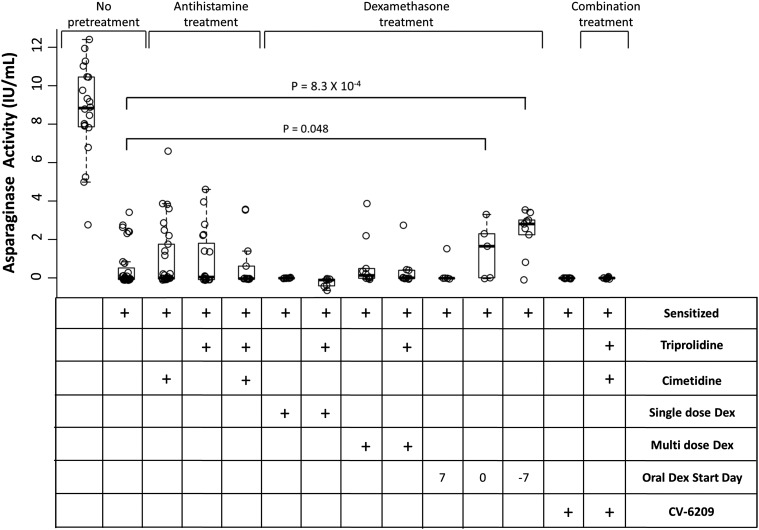
Asparaginase activity was determined in samples collected after the asparaginase-induced hypersensitivity to determine if pretreatment was able to restore activity (Fig. 4). Only oral dexamethasone given 0 or 7 days before the initial asparaginase was able to partially rescue activity compared with nonpretreated, sensitized mice (Fig. 4; P = 4.8 × 10−2 and 8.3 × 10−4, respectively). All other pretreatment strategies resulted in similar activity levels as nonpretreated, sensitized mice (Fig. 4; P > 0.05). Nevertheless, mice receiving oral dexamethasone 0 or 7 days before the initial asparaginase had lower asparaginase activity than nonsensitized mice (Fig. 4; P = 3.8 × 10−5 and 1.9 × 10−6, respectively), suggesting that other methods of lowering anti-asparaginase IgG antibody levels may be required to completely rescue asparaginase activity. Similar pretreatment experiments with antihistamine, CV-6209, and dexamethasone were also tested in mice that were not sensitized to asparaginase to confirm that there was no direct influence of premedication on asparaginase activity (Supplemental Fig. 2). These results indicate that H1 and PAF receptor antagonists mitigate allergic reactions but do not influence the asparaginase activity.
Fig. 4.
Oral dexamethasone given 7 days before asparaginase sensitization can partially restore asparaginase activity. Nonpretreated mice sensitized to asparaginase showed low to no detectable asparaginase activity compared with nonsensitized mice (P = 3.2 × 10−14). Pretreatment with antihistamine, CV-6209, or intraperitoneal dexamethasone (Dex) was not able to rescue asparaginase activity relative to nonpretreated, sensitized mice (P > 0.05). However, mice receiving continuous oral dexamethasone starting 0 or 7 days before asparaginase sensitization had partially restored asparaginase activity compared with nonpretreated, sensitized mice (P = 4.8 × 10−2 and 8.3 × 10−4, respectively).
The inability of pretreatment strategies to fully rescue asparaginase activity is likely due to high anti-asparaginase IgG levels that are unaffected by the pretreatment strategies we used. Mice immunized with asparaginase all developed high levels of anti-asparaginase IgG antibodies in prechallenge samples (day 23) compared with nonsensitized mice (Fig. 5A; P < 0.04), including mice that received multiple doses of dexamethasone. However, mice pretreated with oral dexamethasone on the day of or 7 days before the initial asparaginase immunization showed diminished antibody levels compared with sensitized, nonpretreated mice (Fig. 5B; P = 1.0 × 10−3 and 4.1 × 10−4, respectively) in samples available on day 17 of treatment (Fig. 1A). These results indicate that oral dexamethasone was able to partially restore asparaginase activity due to its effect on anti-asparaginase IgG antibody levels.
Fig. 5.
Dexamethasone effects the development of anti-asparaginase IgG antibodies. (A) On day 23 [before pretreatment with intraperitoneal single-dose dexamethasone (Dex), triprolidine, cimetidine, or CV-6209], all sensitized mice had elevated anti-asparaginase IgG levels compared with nonsensitized controls (P < 0.04). Mice that received pretreatment with multiple doses of parenteral dexamethasone (with or without triprolidine) on days 0 and 14 before the day 23 blood collection had similar antibody levels as sensitized, nonpretreated mice (P > 0.05). (B) Mice receiving continuous oral dexamethasone starting 0 or 7 days before the asparaginase sensitization had lower measured anti-asparaginase IgG levels compared with sensitized, nonpretreated mice (P = 1.0 × 10−3 and 4.1 × 10−4, respectively) in samples available on day 17 of treatment (before the asparaginase challenge).
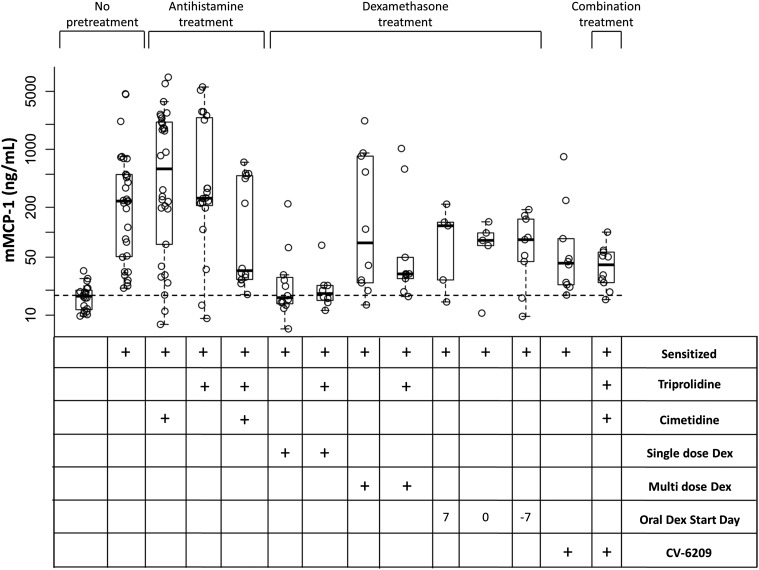
We hypothesized that many of the pretreatment strategies were masking the severity of the hypersensitivity reactions rather than blocking the release of the chemical mediators of allergy. To confirm this, we measured concentrations of mMCP-1 in samples that were collected after the asparaginase-induced hypersensitivity. All sensitized mice showed elevated levels of mMCP-1 compared with nonsensitized mice (Fig. 6; P ≤ 0.02), except for mice receiving a single dose of dexamethasone (with or without concomitant triprolidine, P > 0.05). Interestingly, this observation was not observed with any other dexamethasone pretreatment regimen. It is possible that the pretreatment with a single dose of dexamethasone mitigated the reactions by inhibiting the release of histamine during the allergic reaction, whereas other dexamethasone regimens masked the hypersensitivity through other mechanisms that likely affect antibody production. Similar data were previously reported, suggesting that dexamethasone can inhibit the release of histamine from mast cells and basophils (Schleimer et al., 1982, 1987; Berenstein et al., 1987).
Fig. 6.
Elevated levels of mMCP-1 were detected in sensitized mice after asparaginase-induced allergies. Elevated levels of mMCP-1 were detected in all sensitized mice after asparaginase-induced allergies (P < 0.02), except for mice pretreated with a single dose of intraperitoneal dexamethasone (Dex) or a single dose of intraperitoneal dexamethasone in combination with triprolidine.
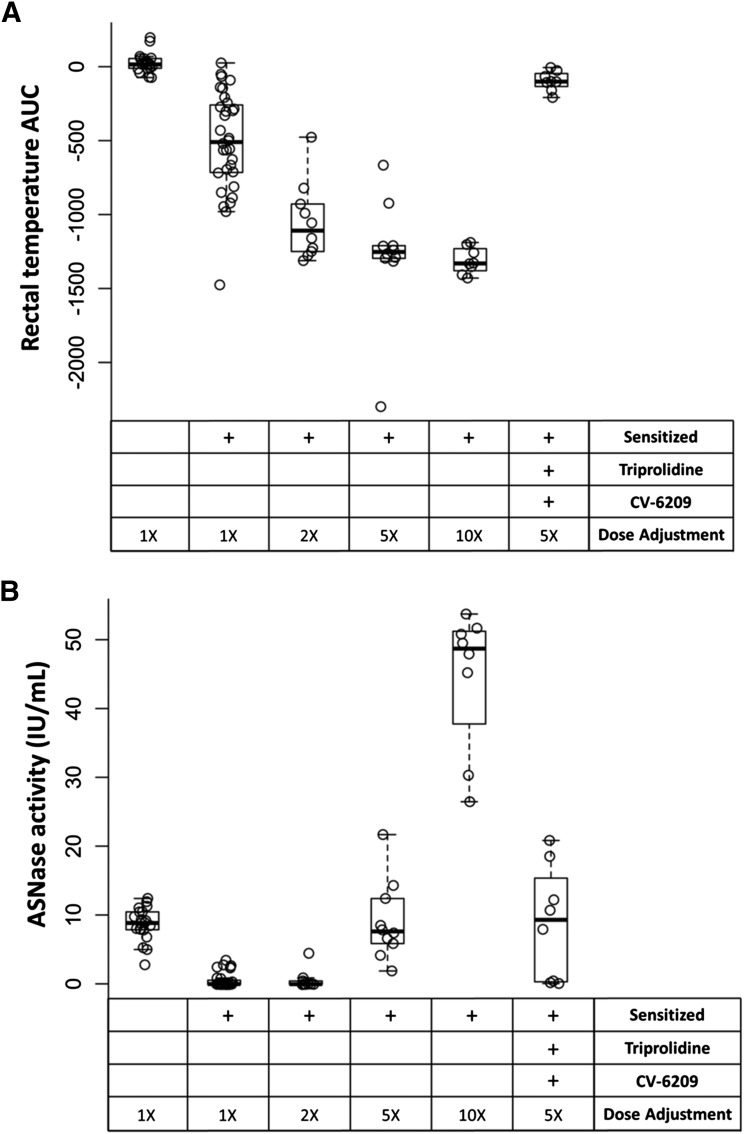
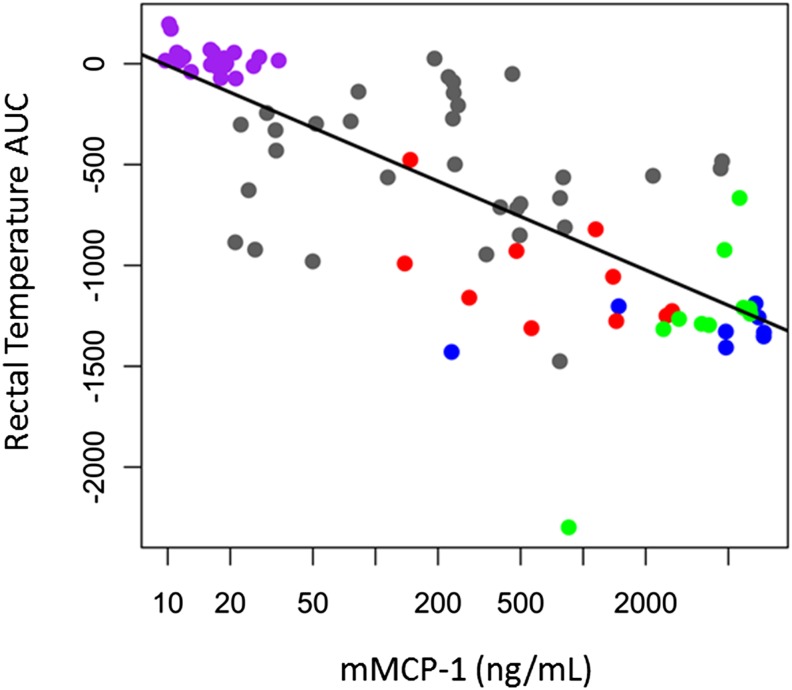
We next investigated if increasing the dose of asparaginase used during the challenge could overcome the high antibody titers and restore asparaginase activity without requiring dexamethasone. In the absence of pretreatment, the severity of asparaginase-induced reactions increased in a dose-dependent manner, and all groups challenged with higher doses of asparaginase had more severe reactions compared with sensitized mice challenged with the standard 100-μg dose (Fig. 7A; P ≤ 2.1 × 10−4). Furthermore, dose increases ≥5-fold (5× and 10×) were able to restore enzyme activity to concentrations similar to or higher than that of nonsensitized mice (Fig. 7B). Although increasing the dose of asparaginase used during the challenge from 1- to 10-fold showed a trend toward lower antibody titers in samples collected after the challenge (data not shown), the trend failed to reach statistical significance. mMCP-1 levels were elevated in a dose-dependent manner and correlated with the severity of the reactions (Fig. 8; R2 = 0.577, P = 3.0 × 10−16).
Fig. 7.
Asparaginase dose adjustments restored activity levels in sensitized mice. Sensitized mice were challenged with 2-, 5-, or 10-fold higher asparaginase doses. (A) The severity of the reaction (drop in rectal temperature) showed a dose-dependent relationship (P < 1.0 × 10−3 compared with 1×, sensitized), and mice pretreated with triprolidine and CV-6209 receiving a 5-fold higher dose were able to mitigate the reaction relative to nonpretreated mice (P = 9.8 × 10−4). (B) Furthermore, dose adjustments ≥5-fold were able to restore asparaginase activity in samples collected 2 hours after the challenge compared with nonsensitized mice (P > 0.05).
Fig. 8.
The severity of asparaginase-induced allergies was correlated with mMCP-1 concentrations. The lower the rectal temperature AUC, the more severe the allergic reaction, and the higher the mMCP-1 concentrations from samples collected after the challenge on day 24 for nonsensitized mice (purple circle) and for sensitized mice receiving 100 μg (gray circle), 200 μg (red circle), 500 μg (green circle), or 1 mg (blue circle) of asparaginase per mouse during the challenge (R2 = 0.577, P = 3.0 × 10−16).
Our pretreatment studies showed that triprolidine and CV-6209 were the best for mitigating the severity of asparaginase-induced allergies, and our dose adjustment experiments indicated that a 500-μg dose of asparaginase per mouse was required to rescue asparaginase activity in sensitized mice. Therefore, we combined both strategies to determine if we could restore activity while simultaneously mitigating the severity of the allergy. We found that the pretreatment, even at the higher dose of asparaginase, was capable of blocking the allergic reaction compared with sensitized, nonpretreated mice (Fig. 7A; P = 9.8 × 10−4), and the dose adjustment restored asparaginase activity to similar concentrations as nonsensitized mice (Fig. 7B; P > 0.05). The anti-asparaginase antibody levels and mMCP-1 concentrations measured for pretreated and nonpretreated mice given a 5-fold increased dose were similar (P > 0.05, data not shown), suggesting that mice in both groups had similar potential for developing hypersensitivity reactions of comparable severities.
Discussion
Clinical immune responses to asparaginase during the treatment of leukemia almost exclusively occur upon re-exposure to asparaginase after an initial exposure (Albertsen et al., 2002; Pieters et al., 2011; Fernandez et al., 2014a), implying that these patients were sensitized to asparaginase administered initially. Reactions in sensitized patients are usually characterized by an immediate allergic response after administration of asparaginase, by the development of high levels of anti-asparaginase IgG antibodies, and by low serum asparaginase activity concentrations (Peterson et al., 1971; Spiegel et al., 1980; Muller et al., 2001; Panosyan et al., 2004; Pieters et al., 2011; Liu et al., 2012). The sensitization protocol we used in mice (Fig. 1, A and B) resulted in the development of an asparaginase-mediated immune response with a similar phenotype as clinical hypersensitivity to E. coli asparaginase.
Our results have several implications for improving use of asparaginase. Our data support that an H1 histamine receptor antagonist (triprolidine) used in combination with a PAF receptor antagonist (CV-6209) is best for mitigating asparaginase-induced reactions (Fig. 3). Although we did not measure IgE antibodies, these response data are consistent with a role for both anti-asparaginase IgG (through FcγR-dependent release of PAF) and IgE antibodies (through FcεR1-dependent release of histamine) being involved in eliciting asparaginase hypersensitivity reactions. However, pretreatment with these agents did not rescue plasma asparaginase activity (Fig. 4). These experiments clearly demonstrate that the symptoms and signs of asparaginase allergies can be completely masked by pretreatment and yet result in subtherapeutic drug concentrations, thus emphasizing the importance of monitoring asparaginase activity after pretreatment with antihistamines, glucocorticoids, or any other medication used for mitigating the severity of allergic reactions. In addition, the low asparaginase activity measured in sensitized mice is likely due to an accelerated clearance and antibody neutralization, because the maximum ex vivo inhibition we were able to induce using rabbit anti-asparaginase IgG antibodies (5 μg/μl) was only 50% (Supplemental Fig. 1). Similar neutralization results were previously reported (Peterson et al., 1971; Baechtel and Prager, 1973), suggesting that anti-asparaginase IgG antibodies cannot completely neutralize asparaginase activity and that other mechanisms are involved in the rapid in vivo disappearance of enzyme activity. This is further supported by the >50% loss of asparaginase activity (Fig. 4) seen in mice pretreated with dexamethasone 7 days before the initial asparaginase immunization dose, although they had negligible antibody levels (Fig. 5B) compared with other pretreatment groups. This is similar to the accelerated asparaginase clearance seen in pediatric patients with relapsed ALL receiving E. coli asparaginase and negative for anti-asparaginase IgG antibodies (Panetta et al., 2009).
We found that dexamethasone could be used to mitigate the reaction, although at the dosage schedules we studied it was not as effective in masking the allergic reaction as pretreatment with triprolidine and CV-6209 in combination (Fig. 3; P ≤ 0.06). However, pretreating mice with oral dexamethasone before exposure to asparaginase lowered anti-asparaginase IgG levels (Fig. 5B) and partially maintained asparaginase activity (Fig. 4). These results are similar to recent clinical findings that showed a lower incidence of asparaginase hypersensitivity in patients that received dexamethasone 4 days before asparaginase administration during remission induction and support the use of glucocorticoids before beginning asparaginase (Pui, 2013; Vora et al., 2013).
Our dose adjustment studies indicate that strategies to lower anti-asparaginase IgG antibody titers (by overcoming their inhibitory effects by presenting a higher concentration of antigen) can restore enzyme activity. However, the effect of large asparaginase doses on treatment-related toxicities is unknown. The dose-adjustment experiments also show that the severity of asparaginase-induced reactions increases in a dose-dependent manner (Fig. 7A). Thus, when challenging patients after a suspected reaction, a lower dose of asparaginase might be expected to result in a less serious reaction but may not overcome the antibody levels and result in lower serum asparaginase activity levels.
Histamine and PAF are considered primary chemical mediators of allergic shock by acting on smooth muscle endothelial cells (Finkelman, 2007). However, histamine or PAF are unlikely to be useful biomarkers of asparaginase-induced allergies due to their short serum half-life. Histamine concentrations reach peak levels within 5 minutes of the onset of symptoms and have a serum half-life of a few minutes (Ferrer et al., 2010), whereas PAF has a serum half-life <15 minutes (Vadas et al., 2008). Several studies have shown that elevated levels of tryptase can be used as a biomarker of clinical hypersensitivity (Ordoqui et al., 1997; He et al., 2004; Payne and Kam, 2004; Fernandez et al., 2014b), and in accord with those studies, we found that mMCP-1 was elevated in sensitized mice from samples collected 2 hours after the asparaginase challenge (mMCP-1 is a mucosal mast cell granule-specific β-chymase that is released during basophil or mast cell degranulation in mice similar to tryptase in humans; Caughey, 2007). Furthermore, mMCP-1 concentrations were correlated with the severity of allergic reactions (Fig. 8), and previous studies showed that increases in plasma mMCP-1 levels may be induced by either IgG or IgE-mediated allergies (Strait et al., 2002). Unlike histamine and PAF, tryptase reaches peak concentrations ∼30–60 minutes after allergy onset, has a serum half-life of 90 minutes, and exhibits low serum concentrations in normal, nonallergic individuals (Laroche et al., 1991). Although other studies have suggested that histamine levels may be a more useful immediate (e.g., 10 minutes) indicator of the onset of allergies compared with the release of mast cell proteases in mice (Nabe et al., 2013), a biomarker of allergy that is elevated and stable for several hours after an allergic reaction may be more useful as an eventual clinical biomarker of asparaginase-induced allergies. Taken together, our results suggest that tryptase may be a possible clinical biomarker for confirming episodes of hypersensitivity to asparaginase.
Aluminum hydroxide adjuvant was required for mice to develop immune responses to asparaginase, as mice immunized with asparaginase without adjuvant did not develop allergic reactions when challenged (data not shown). Selection of an adjuvant can play a large role in determining the characteristics of the immune response generated (e.g., antibody isotypes, subclass, affinity, and titer) (Hadjipetrou-Kourounakis and Moller, 1984; Kenney et al., 1989; Muller and Boos, 1998). Anti-asparaginase antibodies in ALL patients have been reported to be predominantly IgG1 and IgG4 (Cheung et al., 1986; Korholz et al., 1987), which is consistent with a TH2 response (Visciano et al., 2012). Immunization with aluminum hydroxide generally elicits a TH2 response with the development of antigen-specific IgG1 and IgE antibodies (Kenney et al., 1989). In accordance with these reports, asparaginase-sensitized mice developed anti-asparaginase IgG1 antibodies, but no IgE antibodies were detected (data not shown). Therefore, clinical hypersensitivity reactions to asparaginase that are associated with the development of anti-asparaginase IgG antibodies and the asparaginase-induced allergic reaction in our murine model both appear to be TH2 driven. Nevertheless, there are several similarities and differences between the murine and human immune system that may influence the translation of our results to human allergies. Both species produce IgE antibodies that bind with high affinity to the FcεRI receptor, and cross-linking the receptor results in the release of chemical mediators of anaphylaxis, including histamine and PAF (Finkelman, 2007). Both species also produce IgG antibodies that induce the release of PAF by binding to the FcγRIII receptor, and both can mediate allergic reactions by the activation of complement through the production of anaphylatoxins. In contrast, the human and murine immune systems differ in the ability to activate complement through their respective IgG isotypes (Snapper and Finkelman, 1999), in the cells that express FcεR1 (Bieber et al., 1992; Maurer et al., 1994, 1996; Kinet, 1999), and in the quantity of granules within basophils, which influences the role of chemical mediators of allergy during hypersensitivity reactions (Lee and McGarry, 2007; Tsujimura et al., 2008). It is also possible that other chemical mediators of allergies in addition to PAF and histamine, such as cysteinyl leukotrienes and prostaglandin D2, play a role during human allergic reactions to asparaginase (Ono et al., 2009).
In conclusion, we created the first murine model of immediate hypersensitivity reaction to asparaginase. Several previous studies have sensitized mice to asparaginase and demonstrated the development of anti-asparaginase antibodies (Roberts et al., 1966; Baechtel and Prager, 1973; Goldberg et al., 1973; Uren and Ragin, 1979; Kamisaki et al., 1981; Yagura et al., 1981; Fernandes and Gregoriadis, 2001; Cantor et al., 2011) and demonstrated delayed hypersensitivity reactions to asparaginase (Kawamura et al., 1985; Gaspar et al., 1996). Our model is the first to show the development of acute symptomatic allergies in sensitized mice and to identify methods of reducing the severity of the symptoms and restoring asparaginase activity. We demonstrate that pretreatment with H1 receptor antagonists, PAF receptor antagonist, or dexamethasone can all partially mitigate the severity of asparaginase-induced allergic reactions to a similar extent; however, only dexamethasone was able to partially maintain plasma asparaginase activity. This may be particularly important for selecting a pretreatment agent in patients that are at a high risk of developing glucocorticoid-induced osteonecrosis (Vora, 2011). Our results also support the need for a clinically available PAF receptor antagonist for mitigating immune responses (Roberts et al., 1988; Hozawa et al., 1995; Kingsnorth et al., 1995) and emphasize that it may be important to monitor asparaginase activity if pretreatment medications are used. Furthermore, our results indicate that strategies that can lower anti-asparaginase antibodies might be useful to restore enzyme activity in a similar manner as our dose escalation approach.
Supplementary Material
Abbreviations
- ALL
acute lymphoblastic leukemia
- AUC
area under the curve
- CV-6209
2-(2-acetyl-6-methoxy-3,9-dioxo-4,8-dioxa-2,10-diazaoctacos-1-yl)-1-ethyl-pyridinium chloride
- mMCP-1
mouse mast cell protease 1
- PAF
platelet activating factor
Authorship Contributions
Participated in research design: Fernandez, Smith, Finkelman, Relling.
Conducted experiments: Fernandez.
Performed data analysis: Fernandez, Smith.
Wrote or contributed to the writing of the manuscript: Fernandez, Smith, Karol, Ramsey, Liu, Pui, Jeha, Evans, Finkelman, Relling.
Footnotes
The study was supported by the National Institutes of Health National Cancer Institute [Grants P30-CA21765, R01-CA142665, R37-CA36401]; and by the American Lebanese Syrian Associated Charities.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Albertsen BK, Schrøder H, Jakobsen P, Avramis VI, Müller HJ, Schmiegelow K, Carlsen NT. (2002) Antibody formation during intravenous and intramuscular therapy with Erwinia asparaginase. Med Pediatr Oncol 38:310–316. [DOI] [PubMed] [Google Scholar]
- Asselin BL. (1999) The three asparaginases. Comparative pharmacology and optimal use in childhood leukemia. Adv Exp Med Biol 457:621–629. [PubMed] [Google Scholar]
- Asselin BL, Ryan D, Frantz CN, Bernal SD, Leavitt P, Sallan SE, Cohen HJ. (1989) In vitro and in vivo killing of acute lymphoblastic leukemia cells by L-asparaginase. Cancer Res 49:4363–4368. [PubMed] [Google Scholar]
- Baechtel S, Prager MD. (1973) Basis for loss of therapeutic effectiveness of L-asparaginase in sensitized mice. Cancer Res 33:1966–1969. [PubMed] [Google Scholar]
- Berenstein EH, Garcia-Gil M, Siraganian RP. (1987) Dexamethasone inhibits receptor-activated phosphoinositide breakdown in rat basophilic leukemia (RBL-2H3) cells. J Immunol 138:1914–1918. [PubMed] [Google Scholar]
- Bieber T, de la Salle H, Wollenberg A, Hakimi J, Chizzonite R, Ring J, Hanau D, de la Salle C. (1992) Human epidermal Langerhans cells express the high affinity receptor for immunoglobulin E (Fc epsilon RI). J Exp Med 175:1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor JR, Yoo TH, Dixit A, Iverson BL, Forsthuber TG, Georgiou G. (2011) Therapeutic enzyme deimmunization by combinatorial T-cell epitope removal using neutral drift. Proc Natl Acad Sci USA 108:1272–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey GH. (2007) Mast cell tryptases and chymases in inflammation and host defense. Immunol Rev 217:141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WK, Lorenzi PL, Anishkin A, Purwaha P, Rogers DM, Sukharev S, Rempe SB, Weinstein JN. (2014) The glutaminase activity of L-asparaginase is not required for anticancer activity against ASNS-negative cells. Blood 123:3596–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung NK, Chau IY, Coccia PF. (1986) Antibody response to Escherichia coli L-asparaginase. Prognostic significance and clinical utility of antibody measurement. Am J Pediatr Hematol Oncol 8:99–104. [PubMed] [Google Scholar]
- Fernandes AI, Gregoriadis G. (2001) The effect of polysialylation on the immunogenicity and antigenicity of asparaginase: implication in its pharmacokinetics. Int J Pharm 217:215–224. [DOI] [PubMed] [Google Scholar]
- Fernandez CA, Cai X, Elozory A, Liu C, Panetta JC, Jeha S, Molinelli AR, Relling MV. (2013) High-throughput asparaginase activity assay in serum of children with leukemia. Int J Clin Exp Med 6:478–487. [PMC free article] [PubMed] [Google Scholar]
- Fernandez CA, Smith C, Yang W, Daté M, Bashford D, Larsen E, Bowman WP, Liu C, Ramsey LB, Chang T, et al. (2014a) HLA-DRB1*07:01 is associated with a higher risk of asparaginase allergies. Blood 124:1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez CA, Stewart E, Panetta JC, Wilkinson MR, Morrison AR, Finkelman FD, Sandlund JT, Pui CH, Jeha S, Relling MV, et al. (2014b) Successful challenges using native E. coli asparaginase after hypersensitivity reactions to PEGylated E. coli asparaginase. Cancer Chemother Pharmacol 73:1307–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer M, Nunez-Cordoba JM, Luquin E, Grattan CE, De la Borbolla JM, Sanz ML, Schwartz LB. (2010) Serum total tryptase levels are increased in patients with active chronic urticaria. Clin Exp Allergy 40:1760–1766. [DOI] [PubMed] [Google Scholar]
- Finkelman FD. (2007) Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol 120:506–515; quiz 516–507. [DOI] [PubMed] [Google Scholar]
- Finkelman FD, Rothenberg ME, Brandt EB, Morris SC, Strait RT. (2005) Molecular mechanisms of anaphylaxis: lessons from studies with murine models. J Allergy Clin Immunol 115:449–457, quiz 458. [DOI] [PubMed] [Google Scholar]
- Gaspar MM, Perez-Soler R, Cruz ME. (1996) Biological characterization of L-asparaginase liposomal formulations. Cancer Chemother Pharmacol 38:373–377. [DOI] [PubMed] [Google Scholar]
- Goldberg AI, Cooney DA, Glynn JP, Homan ER, Gaston MR, Milman HA. (1973) The effects of immunization to L-asparaginase on antitumor and enzymatic activity. Cancer Res 33:256–261. [PubMed] [Google Scholar]
- Hadjipetrou-Kourounakis L, Möller E. (1984) Adjuvants influence the immunoglobin subclass distribution of immune responses in vivo. Scand J Immunol 19:219–225. [DOI] [PubMed] [Google Scholar]
- He SH, Xie H, He YS. (2004) Induction of tryptase and histamine release from human colon mast cells by IgE dependent or independent mechanisms. World J Gastroenterol 10:319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozawa S, Haruta Y, Ishioka S, Yamakido M. (1995) Effects of a PAF antagonist, Y-24180, on bronchial hyperresponsiveness in patients with asthma. Am J Respir Crit Care Med 152:1198–1202. [DOI] [PubMed] [Google Scholar]
- Kamisaki Y, Wada H, Yagura T, Matsushima A, Inada Y. (1981) Reduction in immunogenicity and clearance rate of Escherichia coli L-asparaginase by modification with monomethoxypolyethylene glycol. J Pharmacol Exp Ther 216:410–414. [PubMed] [Google Scholar]
- Kawamura K, Igarashi T, Fujii T, Kamisaki Y, Wada H, Kishimoto S. (1985) Immune responses to polyethylene glycol modified L-asparaginase in mice. Int Arch Allergy Appl Immunol 76:324–330. [DOI] [PubMed] [Google Scholar]
- Kawedia JD, Kaste SC, Pei D, Panetta JC, Cai X, Cheng C, Neale G, Howard SC, Evans WE, Pui CH, et al. (2011) Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood 117:2340–2347, quiz 2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawedia JD, Liu C, Pei D, Cheng C, Fernandez CA, Howard SC, Campana D, Panetta JC, Bowman WP, Evans WE, et al. (2012) Dexamethasone exposure and asparaginase antibodies affect relapse risk in acute lymphoblastic leukemia. Blood 119:1658–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JS, Hughes BW, Masada MP, Allison AC. (1989) Influence of adjuvants on the quantity, affinity, isotype and epitope specificity of murine antibodies. J Immunol Methods 121:157–166. [DOI] [PubMed] [Google Scholar]
- Kinet JP. (1999) The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol 17:931–972. [DOI] [PubMed] [Google Scholar]
- Kingsnorth AN, Galloway SW, Formela LJ. (1995) Randomized, double-blind phase II trial of Lexipafant, a platelet-activating factor antagonist, in human acute pancreatitis. Br J Surg 82:1414–1420. [DOI] [PubMed] [Google Scholar]
- Korholz D, Urbanek R, Nurnberger W, Jobke A, Gobel U, Wahn V. (1987) [Formation of specific IgG antibodies in l-asparaginase treatment. Distribution of IgG subclasses]. Monatsschrift Kinderheilkunde. Organ der Deutschen Gesellschaft fur Kinderheilkunde 135:325–328. [PubMed] [Google Scholar]
- Laroche D, Vergnaud MC, Sillard B, Soufarapis H, Bricard H. (1991) Biochemical markers of anaphylactoid reactions to drugs. Comparison of plasma histamine and tryptase. Anesthesiology 75:945–949. [DOI] [PubMed] [Google Scholar]
- Larson RA, Fretzin MH, Dodge RK, Schiffer CA. (1998) Hypersensitivity reactions to L-asparaginase do not impact on the remission duration of adults with acute lymphoblastic leukemia. Leukemia 12:660–665. [DOI] [PubMed] [Google Scholar]
- Lee JJ, McGarry MP. (2007) When is a mouse basophil not a basophil? Blood 109:859–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Kawedia JD, Cheng C, Pei D, Fernandez CA, Cai X, Crews KR, Kaste SC, Panetta JC, Bowman WP, et al. (2012) Clinical utility and implications of asparaginase antibodies in acute lymphoblastic leukemia. Leukemia 26:2303–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D, Fiebiger E, Reininger B, Wolff-Winiski B, Jouvin MH, Kilgus O, Kinet JP, Stingl G. (1994) Expression of functional high affinity immunoglobulin E receptors (Fc epsilon RI) on monocytes of atopic individuals. J Exp Med 179:745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D, Fiebiger S, Ebner C, Reininger B, Fischer GF, Wichlas S, Jouvin MH, Schmitt-Egenolf M, Kraft D, Kinet JP, et al. (1996) Peripheral blood dendritic cells express Fc epsilon RI as a complex composed of Fc epsilon RI alpha- and Fc epsilon RI gamma-chains and can use this receptor for IgE-mediated allergen presentation. J Immunol 157:607–616. [PubMed] [Google Scholar]
- Müller HJ, Beier R, Löning L, Blütters-Sawatzki R, Dörffel W, Maass E, Müller-Weihrich S, Scheel-Walter HG, Scherer F, Stahnke K, et al. (2001) Pharmacokinetics of native Escherichia coli asparaginase (Asparaginase medac) and hypersensitivity reactions in ALL-BFM 95 reinduction treatment. Br J Haematol 114:794–799. [DOI] [PubMed] [Google Scholar]
- Müller HJ, Boos J. (1998) Use of L-asparaginase in childhood ALL. Crit Rev Oncol Hematol 28:97–113. [DOI] [PubMed] [Google Scholar]
- Nabe T, Matsuya K, Akamizu K, Fujita M, Nakagawa T, Shioe M, Kida H, Takiguchi A, Wakamori H, Fujii M, et al. (2013) Roles of basophils and mast cells infiltrating the lung by multiple antigen challenges in asthmatic responses of mice. Br J Pharmacol 169:462–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono E, Taniguchi M, Mita H, Fukutomi Y, Higashi N, Miyazaki E, Kumamoto T, Akiyama K. (2009) Increased production of cysteinyl leukotrienes and prostaglandin D2 during human anaphylaxis. Clin Exp Allergy 39:72–80. [DOI] [PubMed] [Google Scholar]
- Ordoqui E, Zubeldia JM, Aranzábal A, Rubio M, Herrero T, Tornero P, Rodríguez VM, Prieto A, Baeza ML. (1997) Serum tryptase levels in adverse drug reactions. Allergy 52:1102–1105. [DOI] [PubMed] [Google Scholar]
- Panetta JC, Gajjar A, Hijiya N, Hak LJ, Cheng C, Liu W, Pui CH, Relling MV. (2009) Comparison of native E. coli and PEG asparaginase pharmacokinetics and pharmacodynamics in pediatric acute lymphoblastic leukemia. Clin Pharmacol Ther 86:651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panosyan EH, Seibel NL, Martin-Aragon S, Gaynon PS, Avramis IA, Sather H, Franklin J, Nachman J, Ettinger LJ, La M, et al. Children’s Cancer Group Study CCG-1961 (2004) Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children’s Cancer Group Study CCG-1961. J Pediatr Hematol Oncol 26:217–226. [DOI] [PubMed] [Google Scholar]
- Payne V, Kam PC. (2004) Mast cell tryptase: a review of its physiology and clinical significance. Anaesthesia 59:695–703. [DOI] [PubMed] [Google Scholar]
- Peterson RG, Handschumacher RE, Mitchell MS. (1971) Immunological responses to L-asparaginase. J Clin Invest 50:1080–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters R, Hunger SP, Boos J, Rizzari C, Silverman L, Baruchel A, Goekbuget N, Schrappe M, Pui CH. (2011) L-asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer 117:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pui CH. (2013) Reducing delayed intensification therapy in childhood ALL. Lancet Oncol 14:178–179. [DOI] [PubMed] [Google Scholar]
- Roberts J, Prager MD, Bachynsky N. (1966) The antitumor activity of Escherichia coli L-asparaginase. Cancer Res 26:2213–2217. [PubMed] [Google Scholar]
- Roberts NM, Page CP, Chung KF, Barnes PJ. (1988) Effect of a PAF antagonist, BN52063, on antigen-induced, acute, and late-onset cutaneous responses in atopic subjects. J Allergy Clin Immunol 82:236–241. [DOI] [PubMed] [Google Scholar]
- Schleimer RP, MacGlashan DW, Jr, Gillespie E, Lichtenstein LM. (1982) Inhibition of basophil histamine release by anti-inflammatory steroids. II. Studies on the mechanism of action. J Immunol 129:1632–1636. [PubMed] [Google Scholar]
- Schleimer RP, Undem BJ, Meeker S, Bollinger ME, Adkinson NF, Jr, Lichtenstein LM, Adams GK., 3rd (1987) Dexamethasone inhibits the antigen-induced contractile activity and release of inflammatory mediators in isolated guinea pig lung tissue. Am Rev Respir Dis 135:562–566. [DOI] [PubMed] [Google Scholar]
- Snapper C, Finkelman F. (1999) Immunoglobulin Class Switching, in Fundamental Immunology (Paul W. ed) Lippincott-Raven, Philadelphia. [Google Scholar]
- Spiegel RJ, Echelberger CK, Poplack DG. (1980) Delayed allergic reactions following intramuscular L-asparaginase. Med Pediatr Oncol 8:123–125. [DOI] [PubMed] [Google Scholar]
- Strait RT, Morris SC, Yang M, Qu XW, Finkelman FD. (2002) Pathways of anaphylaxis in the mouse. J Allergy Clin Immunol 109:658–668. [DOI] [PubMed] [Google Scholar]
- Tsujimura Y, Obata K, Mukai K, Shindou H, Yoshida M, Nishikado H, Kawano Y, Minegishi Y, Shimizu T, Karasuyama H. (2008) Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity 28:581–589. [DOI] [PubMed] [Google Scholar]
- Uren JR, Ragin RC. (1979) Improvement in the therapeutic, immunological, and clearance properties of Escherichia coli and Erwinia carotovora L-asparaginases by attachment of poly-DL-alanyl peptides. Cancer Res 39:1927–1933. [PubMed] [Google Scholar]
- Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, Simons FE, Simons KJ, Cass D, Yeung J. (2008) Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med 358:28–35. [DOI] [PubMed] [Google Scholar]
- Visciano ML, Tagliamonte M, Tornesello ML, Buonaguro FM, Buonaguro L. (2012) Effects of adjuvants on IgG subclasses elicited by virus-like particles. J Transl Med 10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora A. (2011) Management of osteonecrosis in children and young adults with acute lymphoblastic leukaemia. Br J Haematol 155:549–560. [DOI] [PubMed] [Google Scholar]
- Vora A, Goulden N, Wade R, Mitchell C, Hancock J, Hough R, Rowntree C, Richards S. (2013) Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol 14:199–209. [DOI] [PubMed] [Google Scholar]
- Wang B, Hak LJ, Relling MV, Pui CH, Woo MH, Storm MC. (2000) ELISA to evaluate plasma anti-asparaginase IgG concentrations in patients with acute lymphoblastic leukemia. J Immunol Methods 239:75–83. [DOI] [PubMed] [Google Scholar]
- Woo MH, Hak LJ, Storm MC, Sandlund JT, Ribeiro RC, Rivera GK, Rubnitz JE, Harrison PL, Wang B, Evans WE, et al. (2000) Hypersensitivity or development of antibodies to asparaginase does not impact treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol 18:1525–1532. [DOI] [PubMed] [Google Scholar]
- Wu MC, Arimura GK, Yunis AA. (1978) Mechanism of sensitivity of cultured pancreatic carcinoma to asparaginase. Int J Cancer 22:728–733. [DOI] [PubMed] [Google Scholar]
- Yagura T, Kamisaki Y, Wada H, Yamamura Y. (1981) Immunological studies on modified enzymes. I. soluble L-asparaginase/mouse albumin copolymer with enzyme activity and substantial loss of immunogenicity. Int Arch Allergy Appl Immunol 64:11–18. [PubMed] [Google Scholar]
- Yang L, Boyd K, Kaste SC, Kamdem Kamdem L, Rahija RJ, Relling MV. (2009) A mouse model for glucocorticoid-induced osteonecrosis: effect of a steroid holiday. J Orthop Res 27:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Panetta JC, Cai X, Yang W, Pei D, Cheng C, Kornegay N, Pui CH, Relling MV. (2008) Asparaginase may influence dexamethasone pharmacokinetics in acute lymphoblastic leukemia. J Clin Oncol 26:1932–1939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.