Abstract
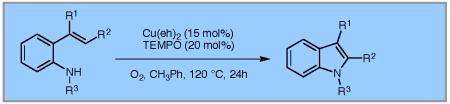
A copper-catalyzed intramolecular alkene oxidative amination that utilizes TEMPO as co-catalyst and O2 as the terminal oxidant has been developed. The method furnishes N-aryl and N-sulfonyl indoles from N-aryl and N-sulfonyl 2-vinylanilines, respectively. Additionally, sequential copper-catalyzed reactions where initial Chan-Lam coupling of 2-vinylanilines with arylboronic acids is followed by oxidative amination of the alkene can generate N-aryl indoles in one pot.
Keywords: copper, oxidation, indoles, oxygen, TEMPO
Methods for the synthesis of indoles have been studied extensively due to their prevalence in natural products and bioactive compounds.1 While classical reactions such as the Fischer indole synthesis and the Bartoli indole synthesis feature prominently in synthetic routes,2 palladium-catalyzed intermolecular coupling methods such as Larock’s indole synthesis using alkynes as well as intramolecular methods such as Heck reactions of 2-halo-N-allylanilines have gained considerable usage in modern indole synthesis.1,2,3 Conversely, Pd-catalyzed routes involving intramolecular oxidative cyclizations of 2-vinylanilines have been used comparatively less, presumably because they have not been as general or efficient.4 Along these lines, Zheng and co-workers recently reported a comparatively more general method for the synthesis of N-(4-methoxyphenyl) indoles using a ruthenium catalyst under photo-redox conditions.5a A new method for the synthesis of a variety of 2,3-unsubstituted indoles from N-acetyl- and N-carbamoyl-2-vinylanilines using hypervalent iodine reagents has also appeared.5b
Synthetic methods for indole synthesis based on comparatively less expensive copper catalysts have also emerged.6 We recently reported a copper-catalyzed alkene C-H amination whose scope includes the synthesis of N-sulfonyl- and N-phenyl indoles.6a The reported method utilized a Cu(OTf)2•bis(oxazoline) complex as catalyst and 300 mol % MnO2 as terminal oxidant (Scheme 1).6a In an effort to reduce reliance on the stoichiometric metal oxidant, we pursued development of a procedure based on terminal aerobic oxidation. The ability of O2 to oxidize Cu(I) to Cu(II) is dependant on the solvent and copper coordination sphere.7a Under the reaction conditions generally effective for copper-catalyzed alkene amination, and with the copper complexes most effective for this purpose [Cu(OTf)2*bis(oxazoline) and Cu(2-ethylhexanoate)2], aerobic oxidation is minimally effective (e.g., Table 1, entry 1). We were aware, however, that when 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) is used as a co-catalyst in conjunction with an O2 atmosphere, turnover can be achieved in a variety of transition metal-catalyzed reactions.7
Scheme 1.
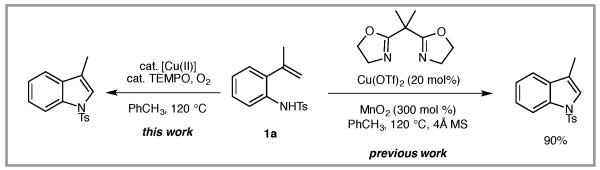
Previous method (uses 300 mol % MnO2) and present study (uses O2, 1 atm) for the copper-catalyzed indole synthesis.
Table 1.
Optimization of Reaction Conditions
| |||
|---|---|---|---|
|
| |||
| entry | [Cu] (mol %) | TEMPO (mol %) | yield 2aa |
| 1 | Cu(eh)2 (15) | - | 28% |
| 2 | Cu(eh)2 (20) | 50 | 41%b |
| 3 | Cu(OTf)2•3 (20) | 50 | 65%c |
| 4 | Cu(OTf)2•4(20) | 50 | 65%c |
| 5 | Cu(eh)2 (20) | 20 | 73% |
| 6 | Cu(eh)2 (15) | 20 | 71% |
| 7 | Cu(eh)2 (15) | 10 | 40%c |
| 8 | - | 20 | NR |
Isolated yield.
20% of 2aa was also isolated.
Conversion % based on crude 1H NMR.
Cu(eh)2 = copper(II) 2-ethylhexanoate, NR = No reaction
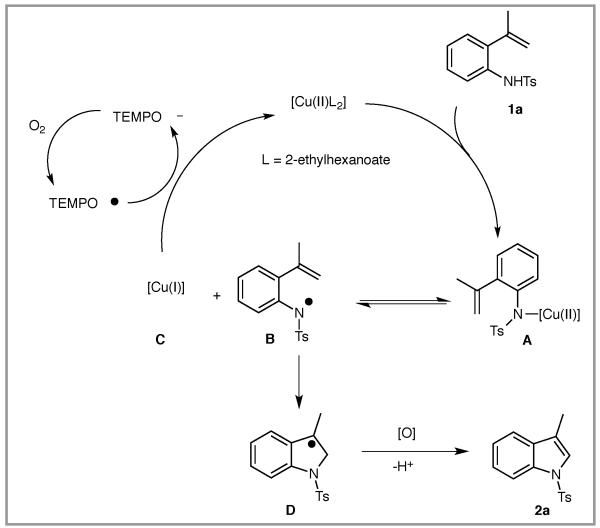
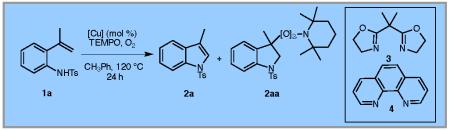
When 50 mol % of TEMPO was used in the presence of O2 (1 atm, via balloon) under Cu(2-ethylhexanoate)2 catalysis (20 mol%), 41% of indole 2a was isolated along with 20% of a TEMPO-peroxide 2aa. The presence of 2aa is consistent with the presence of a carbon radical reaction intermediate (vide infra).6a
Changing to Cu(OTf)2 and bis(oxazoline) ligand 3, 65% conversion was achieved with the remainder being starting 2-vinyl aniline 1a (Table 1, entry 3). Use of 1,10-phenanthroline 4 as ligand with Cu(OTf)2 gave similar results (Table 1, entry 4). We hypothesized that the presence of side product 2aa formed in Table 1, entry 2, could be reduced if the TEMPO loading were reduced. Gratifyingly, use of 20 mol% of both Cu(2-ethylhexanoate)2 and TEMPO under O2 (1 atm) gave 73% yield of indole 2a with only trace 2aa (Table 1, entry 5). Reduction of the copper loading to 15 mol% provided 2a in comparable yield (71%, Table 1, entry 6). Further reduction in TEMPO loading resulted in decreased conversion to 2a (Table 1, entry 7). In the absence of copper there is no reaction (Table 1, entry 8).
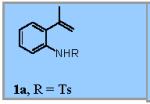
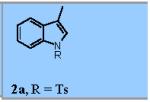
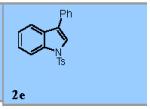
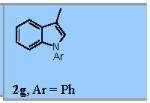
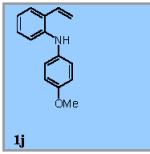
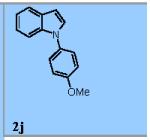
Using the optimized conditions (Table 1, entry 6) the reaction scope was explored (Table 2). Various N-sulfonyl 2-vinylanilines underwent the oxidative cyclization in moderate to good yield (Table 2, entries 1-5). Interestingly, only the (E)-isomer was reactive when a mixture of internal alkenes 1f (E:Z ratio = 2:3) was submitted to the reaction, giving indole 2f in 22% yield along with 22% recovered (Z)-1f (Table 2, entry 6). Exclusive reaction with (E)-1f gave 43% of indole 2f (Table 2, entry 7). N-Aryl-2-vinylanilines 1g, 1h, and 1i were the most reactive substrates, giving 81-85% yield range (Table 2, entries 8-10). Substrates with less alkene substitution, e.g. 1j, gave reduced conversion to the indole (55% isolated 2j, Table 2, entry 10). The N-tosyl variant of 1j was unreactive.
Table 2.
Substrate Scopea
| |||
|---|---|---|---|
|
| |||
| entry | substrate | product | yieldb |
| 1 |
|
|
71% |
| 2 | 1b, R = SES | 2b, R = SES | 55% |
| 3 | 1c, R = Ns | 2c, R = Ns | 51% |
| 4 | 1d, R = Ms | 2d, R = Ms | 50% |
| 5 |
|
|
73% |
| 6 |
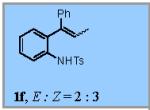
|
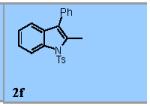
|
22% |
| 7 |
|
|
43% |
| 8 |
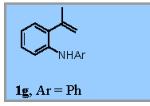
|
|
83% |
| 8 | 1h, Ar = 4-F-Ph | 2h, Ar = 4-F-Ph | 81% |
| 9 | 1i, Ar = 4-MeO-Ph | 2i, Ar = 4-MeO-Ph | 85% |
| 10 |
|
|
55% |
Reaction conditions used: 1a (0.174 mmol, 1 equiv), Cu(eh)2 (0.026 mmol, 15 mol %), TEMPO (0.035 mmol, 20 mol %), toluene (1.74 mL) and O2 (1 atm, balloon).
Isolated yield after column chromatography
22% of the (Z)-isomer of 1f was recovered.
SES = 2-(trimethylsilyl)ethanesulfonyl, Ns = 4-nitrophenylsulfonyl, Ms = methane sulfonyl.
Taken in sum, these results indicate the efficiency of the reaction is dependent on the electronics and sterics of the alkene as well as the electronics of the amine. In general alkenes that accept radicals more readily (e.g. 1,1-disubstituted alkenes), and N-substituents able to stabilize an N-radical are more reactive. Primary anilines and N-alkyl anilines failed to react under the optimized conditions.
This new aerobic Cu-catalyzed indole synthesis is largely complementary in substrate scope to recently reported oxidative amination reactions of 2-vinylanlinines.5 When compared to the Ru-catalyzed photocatalytic oxidative cyclization of N-(4-methoxyphenyl)-2-vinylanilines,5a the N-substituent scope of this aerobic copper-catalyzed reaction is much wider, but the alkene substituent scope is more narrow. When compared to the hypervalent iodine-mediated oxidative cyclization of 2-vinylaniline derivatives where unsubstituted alkenes such as 1j perform best,5b this Cu-catalyzed reaction performs better with higher substituted, 1,1-disubstituted alkenes but is less efficient with less substituted alkenes such as 1j. In comparison to our previously reported method where 300 mol % MnO2 was used as the terminal oxidant,6a this new aerobic method requires less reagents and creates less waste. Isolated yields of the indoles are comparable in some cases and somewhat lower in others, and the substrate scope is more narrow (e.g. intermolecular C-H aminations do not work as well under the aerobic conditions, not shown).
A catalytic cycle consistent with the observations in Tables 1 and 2 is illustrated in Scheme 2.6a Complexation of N-tosyl-2-vinylaniline 1a and Cu(2-ethylhexanoate)2 gives A. Reversible N-Cu(II) homolysis provides A in equilibrium with nitrogen radical B and [Cu(I)] complex C. Addition of the amidyl radical to the pendant alkene generates benzylic carbon radical D, which under oxidizing conditions proceeds to indole 2a. The [Cu(I)] complex C is then oxidized by TEMPO radical, thereby regenerating the catalyst and TEMPO anion.7m The latter is oxidized by O2 and re-enters the cycle. The relatively lower reactivity of the less substituted vinylarene 1j argues against an alternative mechanism involving outer-sphere electrophilic activation of the alkene by [Cu(II)] since such C-[Cu] bond formation should be more favorable on less substituted alkenes (in analogy to hypervalent iodine promoted reactions).5b
Scheme 2.
Proposed Catalytic Cycle
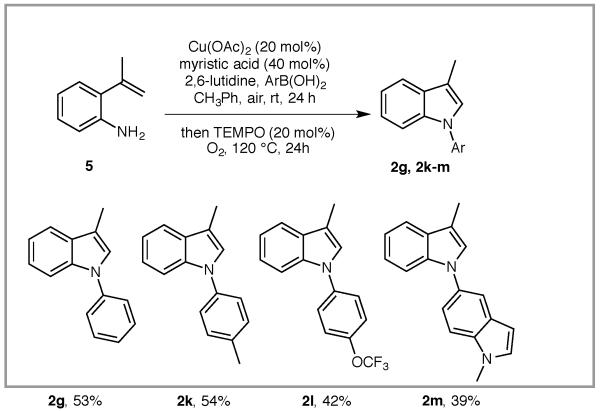
A tandem reaction involving two different copper-catalyzed C-N bond formations was next explored. N-aryl anilines are efficiently synthesized via copper-catalyzed Chan-Lam coupling8 between the corresponding anilines and boronic acids. In the event, commercially available 2-isopropenylaniline 5 was subjected to Cu(OAc)2-catalyzed couplings with various aryl boronic acids at room temperature under air (Scheme 3). After 24 h, TEMPO (20 mol %) and O2 (1 atm, balloon) were introduced and the mixture heated to 120 °C for 24 h. This concise process yielded N-aryl indoles 2g and 2k, 2l and 2m in moderate yields.
Scheme 3.
One-Pot Tandem Chan-Lam Coupling8 / C-H Amination
In conclusion, a new indole synthesis method involving oxidative cyclization of N-sulfonyl and N-aryl-2-vinylanilines catalyzed by copper under aerobic reaction conditions has been developed. A further illustration of the synthetic potential was demonstrated by a one-pot Chan-Lam coupling / oxidative amination sequence. The reaction is complementary to existing indole synthesis protocols and is especially applicable to the synthesis of 3-substituted N-sulfonyl and N-aryl indoles starting from 2-vinyl anilines. Substrate reactivity trends are consistent with amidyl radical reactivity. A catalytic sequence implicating TEMPO as a co-catalyst that functions as intermediary between the copper(II) carboxylate and molecular O2 is proposed.
Supplementary Material
Acknowledgment
We thank the National Institutes of Health (RO1 GM078383) for generous financial support of this research.
Footnotes
Supporting Information for this article is available online at http://www.thieme-connect.com/ejournals/toc/synlett.
Primary Data for this article are available online at http://www.thieme-connect.com/ejournals/toc/synlett and can be cited using the following DOI: (number will be inserted prior to online publication).
Contributor Information
Timothy W. Liwosz, Department of Chemistry, The State University of New York at Buffalo, Buffalo, NY, 14620, United States
Sherry R. Chemler, Department of Chemistry, The State University of New York at Buffalo, Buffalo, NY, 14620, United States.
References
- (1).For selected reviews on synthesis and bioactivity of indoles, see: Cacchi S, Fabrizi G. Chem. Rev. 2011;111:PR215. doi: 10.1021/cr100403z. Humphrey GR, Kuethe JT. Chem. Rev. 2006;106:2875. doi: 10.1021/cr0505270. Kochanowska-Karamyan AJ, Hamann MT. Chem. Rev. 2010;110:4489. doi: 10.1021/cr900211p. Taber DF, Tirunahari PK. Tetrahedron. 2011;67:7195. doi: 10.1016/j.tet.2011.06.040.
- (2).For classical indole syntheses, see: Fischer E, Jourdan F. 1883;16:2241. Robinson B. Chem. Rev. 1963;63:373. Bartoli G, Palmieri G, Bosco M, Dalpozzo R. Tetrahedron Lett. 1989;30:2129. Larock RC, Yum EK. J. Am. Chem. Soc. 1991;113:6689.
- (3).For selected recent indole synthesis methods, see: Taddei M, Mura MG, Rajamäki S, Luca LD, Porcheddu A. Adv. Synth. Catal. 2013;355:3002. Muralirajan K, Cheng C-H. Adv. Synth. Catal. 2014;356:1571. Dawande SG, Kanchupalli V, Kalepu J, Chennamsetti H, Lad BS, Katukojvala S. Angew. Chem. Int. Ed. 2014;53:4076. doi: 10.1002/anie.201400161. Stokes BJ, Liu S, Driver TG. J. Am. Chem. Soc. 2011;133:4702. doi: 10.1021/ja111060q. Ackermann L, Lygin AV. Org. Lett. 2012;14:764. doi: 10.1021/ol203309y. Yamaguchi M, Manabe K. Org. Lett. 2014;16:2386. doi: 10.1021/ol500711z. Hao W, Geng W, Zhang W-X, Xi Z. Chem. Eur. J. 2014;20:2605. doi: 10.1002/chem.201304215. Cacchi S, Fabrizi G, Goggiamani A, Molinaro C, Verdiglione R. J. Org. Chem. 2013;79:401. doi: 10.1021/jo401456x. Wang Q, Huang L, Wu X, Jiang H. Org. Lett. 2013;15:5940. doi: 10.1021/ol4027683. Zheng L, Hua R. Chem. Eur. J. 2014;20:2352. doi: 10.1002/chem.201304302. Miura T, Funakoshi Y, Murakami M. J. Am. Chem. Soc. 2014;136:2272. doi: 10.1021/ja412663a. Liu B, Song C, Sun C, Zhou S, Zhu J. J. Am. Chem. Soc. 2013;135:16625. doi: 10.1021/ja408541c. Kiruthika SE, Perumal PT. Org. Lett. 2013;16:484. doi: 10.1021/ol403365t. Zhu C, Ma S. Org. Lett. 2013;15:2782. doi: 10.1021/ol401115a. Jensen T, Petersen H, Bang-Andersen B, Madsen R, Jorgensen M. Angew. Chem. Int. Ed. 2008;47:888. doi: 10.1002/anie.200703763. Pena-Lopez M, Neumann H, Beller M. Chem. Eur. J. 2014;20:1818. doi: 10.1002/chem.201304432. Zhao D, Shi Z, Glorius F. Angew. Chem. Int. Ed. 2013;52:12426. doi: 10.1002/anie.201306098. Shi Z, Zhang C, Li S, Pan D, Ding S, Cui Y, Jiao N. Angew. Chem. Int. Ed. 2009;48:4572. doi: 10.1002/anie.200901484. Wei Y, Deb I, Yoshikai N. J. Am. Chem. Soc. 2012;51:9098. doi: 10.1021/ja3030824. Wagaw S, Yang BH, Buchwald SL. J. Am. Chem. Soc. 1999;121:10251. Wang C, Huang Y. Org. Lett. 2013;15:5294. doi: 10.1021/ol402523x. Varela-Fernandez A, Varela JA, Saa C. Synthesis. 2012;44:3285.
- (4).(a) Harrington PJ, Hegedus LS. J. Org. Chem. 1984;49:2657. [Google Scholar]; (b) Harrington PJ, Hegedus LS, McDaniel KF. J. Am. Chem. Soc. 1987;109:4335. [Google Scholar]; (c) Larock RC, Hightower TR, Hasvold LA, Peterson KP. J. Org. Chem. 1996;61:3584. doi: 10.1021/jo952088i. [DOI] [PubMed] [Google Scholar]; (d) Izumi T, Nishimoto Y, Kohei K, Kasahara A. J. Heterocycl. Chem. 1990;27:1419. [Google Scholar]; (e) Tsvelikhovsky D, Buchwald SL. J. Am. Chem. Soc. 2010;132:14048. doi: 10.1021/ja107511g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).(a) Maity S, Zheng N. Angew. Chem. Int. Ed. 2012;51:9562. doi: 10.1002/anie.201205137. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fra L, Millan A, Souto JA, Muniz K. Angew. Chem. Int. Ed. 2014;53:7349. doi: 10.1002/anie.201402661. [DOI] [PubMed] [Google Scholar]
- (6).Liwosz TW, Chemler SR. Chem. Eur. J. 2013;19:12771. doi: 10.1002/chem.201301800. For alternative copper-catalyzed indole syntheses, primarily using alkynes, see: Ackermann L. Org. Lett. 2005;7:439. doi: 10.1021/ol047649j. Oda Y, Hirano K, Satoh T, Miura M. Org. Lett. 2012;14:664. doi: 10.1021/ol203392r. Frischmuth A, Knochel P. Angew. Chem. Int. Ed. 2013;52:10084. doi: 10.1002/anie.201304380. Zhu Z, Yuan J, Zhou Y, Qin Y, Xu J, Peng Y. Eur. J. Org. Chem. 2014:511.
- (7).For selected reviews and examples, see: Allen SE, Walvoord RR, Padilla-Salinas R, Kozlowski MC. Chem. Rev. 2013;113:6234. doi: 10.1021/cr300527g. Ortgies DH, Chen F, Forgione P. Eur. J. Org. Chem. 2014:3917. Steves JE, Stahl SS. J. Am. Chem. Soc. 2013;135:15742. doi: 10.1021/ja409241h. Han B, Yang X-L, Wang C, Bai Y-W, Pan T-C, Chen X, Yu W. J. Org. Chem. 2011;77:1136. doi: 10.1021/jo2020399. Liu J, Ma S. Org. Lett. 2013;15:5150. doi: 10.1021/ol402434x. Yin W, Wang C, Huang Y. Org. Lett. 2013;15:1850. doi: 10.1021/ol400459y. Chen C, Liu B, Chen W. Synthesis. 2013;45:3387. Qifa L, Ming L, Fei Y, Wei W, Feng S, Zhenbang Y, Sufeng H. Synth. Comm. 2010;40:1106. Liwosz TW, Chemler SR. Org. Lett. 2013;15:3034. doi: 10.1021/ol401220b. Paderes MC, Keister JB, Chemler SR. J. Org. Chem. 2012;78:506. doi: 10.1021/jo3023632. Fuller PH, Kim J-W, Chemler SR. J. Am. Chem. Soc. 2008;130:17638. doi: 10.1021/ja806585m. Zeng W, Chemler SR. J. Am. Chem. Soc. 2007;129:12948. doi: 10.1021/ja0762240. Sheldon RA, Arends IWCE. J. Mol. Catal. A: Chem. 2006;251:200.
- (8).Recent example and review: Antilla JC, Buchwald S. Org. Lett. 2001;3:2077. doi: 10.1021/ol0160396. Qiao JX, Lam PYS. Synthesis. 2011:829–856. Qiao JX, Lam PYS. Boronic Acids. 2nd Ed. John Wiley & Sons; 2011.
- (9).Representative Procedure for the Copper-Catalyzed Intramolecular Alkene C-H Amination To an oven dried 100 mL round bottom flask was charge with 1a (50 mg, 0.174 mmol, 1 equiv), Cu(2-ethylhexanoate)2 (9 mg, 0.026 mmol, 15 mol %), TEMPO (5 mg, 0.035 mmol, 20 mol %) and 1.74 mL of dry toluene (0.1M). The flask was purged with O2, put under an O2 atmosphere using an O2 balloon and stirred for 24 h at 120 °C. Filtration of the cooled reaction mixture through a pad of silica gel with ethyl acetate (100 mL), and subsequent evaporation of the solvent in vacuo afforded crude mixture. Flash chromatography of the resulting crude mixture on silica gel (0-20% ethyl acetate in hexanes gradient) afforded indole 2a (36 mg, 71% yield) as an off-white solid.6 1H NMR (400 MHz, CDCl3): δ 7.99 (d, J = 8.4 Hz, 1H), 7.74 (d, J = 8.0 Hz, 2H), 7.45 (d, J = 7.2 Hz, 1H), 7.34-7.29 (m, 2H), 7.26-7.22 (m, 1H), 7.19 (d, J = 8.4 Hz, 2H), 2.32 (s, 3H), 2.24 (d, J = 0.8 Hz, 3H).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.