Abstract
Decades of work have identified the signaling pathways that regulate the differentiation of chondrocytes during bone formation, from their initial induction from mesenchymal progenitor cells to their terminal maturation into hypertrophic chondrocytes. Here, we review how multiple signaling molecules, mechanical signals and morphological cell features are integrated to activate a set of key transcription factors that determine and regulate the genetic program that induces chondrogenesis and chondrocyte differentiation. Moreover, we describe recent findings regarding the roles of several signaling pathways in modulating the proliferation and maturation of chondrocytes in the growth plate, which is the ‘engine’ of bone elongation.
Keywords: Chondrogenesis, Chondrocyte hypertrophy, Growth plate, Sox9, Ihh, PTHrP, Fgfr3
Summary: This Review article discusses how signaling molecules, mechanical signals and morphological cell features are integrated to regulate chondrogenesis and bone development.
Introduction
The commitment of mesenchymal cells to the chondrogenic lineage is a key event in the formation of bones. With the exception of the bones in the cranial vault, parts of the jaw and the medial part of the clavicle [which all form via intramembranous ossification (Hall and Miyake, 1992; Ornitz and Marie, 2002)], the vertebrate skeleton is formed by the process of endochondral bone formation. During this process, each skeletal element is first established from mesesenchymal progenitors and is then patterned as cartilage, which is later replaced by bone (Fig. 1). Mesenchymal progenitors that originate from the cranial neural crest, somites and lateral plate mesoderm contribute to the craniofacial, axial and limb skeleton, respectively. Condensations of such mesenchymal cells express the transcription factor Sox9, which is a key regulator of chondrogenesis, and give rise to cartilage primordia consisting of round immature chondrocytes that continue to express Sox9 (Bi et al., 1999; Akiyama et al., 2002). Cells lying within the central regions of the cartilage primordia then undergo maturation (Fig. 1). During this process, chondrocytes withdraw from the cell cycle and increase ∼20-fold in volume (Cooper et al., 2013), giving rise to cells that are termed hypertrophic chondrocytes. As the cartilage continues to grow longitudinally, it continually deposits hypertrophic chondrocytes in its wake. These are subsequently replaced by bone in a region known as the primary ossification center. The production and maturation of chondrocytes is eventually restricted to the end of the bone (the epiphysis) in a structure termed the growth plate. A secondary ossification center then arises within the epiphysis, separating the growth plate from the distal ends of the long bones.
Fig. 1.
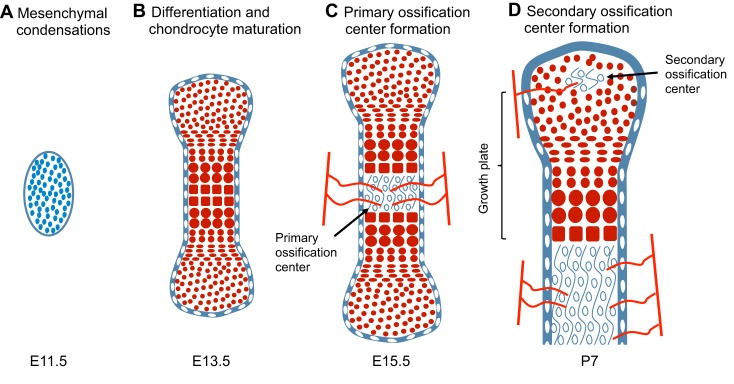
The stages of endochondral bone formation in the developing mouse hindlimb. (A) Schematic representation of mesenchymal cells (blue) that begin to form condensations at E11.5 in the hindlimb buds. (B) By E13.5, mesenchymal cells differentiate into chondrocytes (red cells), and the process of chondrocyte maturation and hypertrophy initiates. The cartilage anlage is surrounded by a layer of perichondrium (white cells). (C) By ∼E15.5, vascularization (represented by red lines) takes place in the center of the cartilage anlage, resulting in replacement of chondrocytes with endochondral bone (open circles) in the primary ossification center. (D) The secondary ossification center (SOC) forms postnatally, at ∼P7, and also becomes vascularized. The timing of SOC formation varies slightly between different bones. The schematics of the developing bones are approximately drawn to scale.
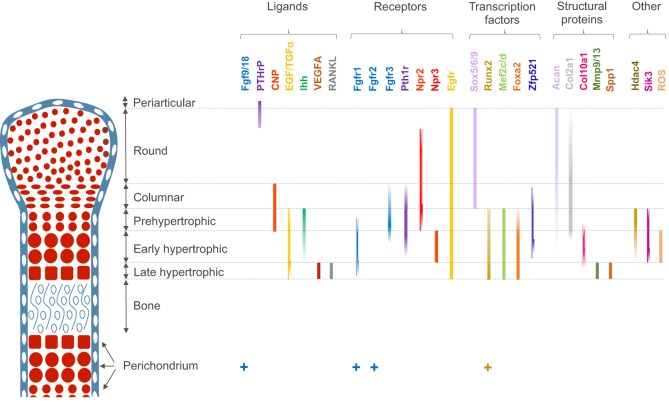
During the process of endochondral bone formation, the cellular features and expression profiles of chondrocytes progressively change (Fig. 2). Within the growth plate, small round distally located chondrocytes initially give rise to flattened chondrocytes that are more centrally located in the cartilage primordia, and which proliferate and stack into longitudinal columns. These immature chondrocytes express the transcription factors Sox5, Sox6 and Sox9, and the structural proteins collagen, type II, α1 (Col2a1) and aggrecan (Acan). The next stage of maturation into prehypertrophic chondrocytes is marked by the expression of both parathyroid hormone 1 receptor (Pth1r) and Indian hedgehog (Ihh). This is followed by maturation into early hypertrophic chondrocytes that express collagen, type X, α1 (Col10a1). Notably the induction of Pth1r, Ihh and, subsequently, Col10a1 correlates with the loss of Sox5, Sox6, Sox9, Col2a1 and Acan expression. Finally, Col10a1-expressing cells lose expression of this collagen and progress to become late hypertrophic chondrocytes, which express vascular endothelial growth factor A (VEGFA), matrix metalloproteinase 13 (Mmp13) and secreted phosphoprotein 1 (also known as, osteopontin/bone sialoprotein 1; Spp1). VEGFA and Mmp13 expression herald the invasion of the growth plate by endothelial cells, osteoclasts and osteoblast precursors. Osteblast precursors that arise from both the perichondrium (Maes et al., 2010) and the hypertrophic chondrocytes (Yang et al., 2014) work together with osteoclasts to remodel the growth plate matrix to form trabecular bone.
Fig. 2.
Summary of regionalized gene expression in the growth plate. Schematic representation of the various zones of chondrocyte maturation are displayed for a E15.5-E16.5 mouse long-bone growth plate. The expression of various classes of genes and/or proteins is displayed. Ligands: Fgf9/18 (Liu et al., 2002; Hung et al., 2007), PTHrP (St-Jacques et al., 1999), CNP (Chusho et al., 2001), EGF/TGFα (Ren et al., 1997), Ihh (Vortkamp et al., 1996), VEGFA (Zelzer et al., 2001) and RANKL (Zhang et al., 2011). Receptors: Fgfr1 (Jacob et al., 2006), Fgfr2 (Yu et al., 2003), Fgfr3 (de Frutos et al., 2007), Pth1r (Lee et al., 1996; de Frutos et al., 2007), Npr2 (Yamashita et al., 2000), Npr3 (Yamashita et al., 2000) and Egfr (Zhang et al., 2013). Transcription factors: Sox5/6/9 (Lefebvre et al., 1998), Runx2 (Inada et al., 1999; Kim et al., 1999), Mef2c and Mef2d (Mef2c/d) (Arnold et al., 2007), Foxa2 (Ionescu et al., 2012), and Zfp521 (Correa et al., 2010). Structural proteins: aggrecan (Acan) (Inada et al., 1999), Col2a1 (de Frutos et al., 2007), Col10a1 (de Frutos et al., 2007), Mmp9 and Mmp13 (Mmp9/13) (Inada et al., 1999), and Spp1 (Inada et al., 1999). Others: Hdac4 (Vega et al., 2004), Sik3 (Sasagawa et al., 2012) and reactive oxygen species (ROS) (Morita et al., 2007). For a comprehensive review of the expression pattern of BMP and TGFβ ligands and receptors, see Minina et al. (2005).
Work over the past few decades, using both in vitro and in vivo systems, has identified a multitude of signaling and transcription factors, as well as changes in cell shape (see Box 1), that regulate these progressive changes in chondrocytes, from their initial induction to their terminal maturation. These findings have implications both for understanding the basic biology of cartilage and bone, and for understanding how disruption of this finely tuned process of chondrocyte maturation results in various skeletal pathologies. In this Review, we first describe the signaling pathways and transcription factors that regulate the specification of mesenchymal cells as chondroprogenitors. We then concentrate on the establishment of the growth plate and the factors that regulate the balance between chondrocyte proliferation and differentiation.
Box 1. Cell shape and cytoskeletal changes regulate chondrogenesis
Limb bud mesenchymal cells can undergo chondrogenesis in culture when plated at extremely high density (termed micromass culture) (Ahrens et al., 1977; Osdoby and Caplan, 1979) or at low density but only when these cells are plated in either suspension culture (Levitt and Dorfman, 1972; Solursh and Reiter, 1975) or collagen gels (Solursh et al., 1982). This leads to speculation that a spherical cell shape might promote chondrogenesis (Zanetti and Solursh, 1984). Consistent with these findings, recent work has established that the culture of ‘dedifferentiated’ primary chondrocytes in either agarose (Benya and Shaffer, 1982) or alginate (Bonaventure et al., 1994; Reginato et al., 1994; Kumar and Lassar, 2009) can induce re-expression of the chondrocyte phenotype and simultaneously induce depolymerization of the actin cytoskeleton. Other studies have established that RhoA activation can block both Sox9 expression and chondrogenic differentiation, and that inhibition of RhoA kinase signaling or pharmacological disruption of the actin cytoskeleton can promote both Sox9 expression and chondrogenic differentiation in some but not all cellular contexts (Woods et al., 2005; Woods and Beier, 2006). In addition to controlling Sox9 expression, RhoA signaling and modulation of actin polymerization can directly control the transcriptional activity of Sox9 (Kumar and Lassar, 2009).
The initiation of chondrogenesis
The molecular events that regulate the differentiation of mesenchymal cells into chondrocytes are still largely unknown. Although some of the signaling molecules that are necessary for the induction of this process have been identified, our understanding of the downstream molecular pathways that promote chondrogenesis is still a work in progress. Below, we summarize current knowledge of the molecular players that participate in initiating chondrogenesis.
Shh induces chondrogenic competence in sclerotomal cells by inducing Sox9 expression
During development, the somite undergoes a stereotypical process of differentiation. The ventral region becomes mesenchymal and forms the sclerotome, precursor to the vertebrae and to the medial part of the ribs (Kato and Aoyama, 1998). A signaling molecule known to be crucial for sclerotome induction is sonic hedgehog (Shh) (Chiang et al., 1996), a member of the hedgehog family of proteins that is expressed by both the notochord and the floorplate of the neural tube. Interactions between Shh and the patched transmembrane receptors Ptch1 and Ptch2 cause another transmembrane protein, smoothened (Smo), to translocate to the base of the primary cilium. This leads to activation of Gli transcription factors (Gli1, Gli2 and Gli3), which in turn regulate the expression of hedgehog-responsive genes (reviewed by Wilson and Chuang, 2010). Shh signaling has been found to play multiple roles in sclerotome formation: promoting the survival of somitic cells (Teillet et al., 1998), an epithelial-mesenchymal transition of the sclerotome via induction of Snail (Li et al., 2006) and, finally, the induction of sclerotome-specific markers such as Pax1, Sox9 and Nkx3-2 (reviewed by Monsoro-Burq, 2005). In addition to Shh, noggin (Nog) (which is expressed in the notochord) and gremlin 1 (Grem1) (which is expressed in the dorsal neural tube and somites) cooperate to maintain a BMP signaling-free zone that is crucial for Shh-mediated sclerotome induction (Stafford et al., 2011). Mice engineered to lack Shh fail to form vertebrae (Chiang et al., 1996), indicating that Shh signaling is crucial for the formation of vertebral cartilage. Nevertheless, the subsequent differentiation of sclerotome into cartilage does not depend on maintained Shh signaling (Murtaugh et al., 1999). Instead, Shh signals induce the expression of Sox9 and the transcription factor Nkx3-2, which indirectly maintains Sox9 expression in somitic cells (Zeng et al., 2002). In addition, bone morphogenetic protein (BMP) signals maintain the expression of these genes after their initial induction by Shh, although they cannot induce the expression of either Sox9 or Nkx3-2 in presomitic paraxial mesodermal cells that have not yet been exposed to Shh signaling (Murtaugh et al., 2001; Zeng et al., 2002). Provided that BMP signals are present, Sox9 can both regulate its own expression (Kumar and Lassar, 2009; Mead et al., 2013) and induce expression of Nkx3-2 (Zeng et al., 2002). Nkx3-2 is a BMP-dependent transcriptional repressor (Kim and Lassar, 2003) that blocks the expression of inhibitor(s) of Sox9 transcription (Zeng et al., 2002). It was recently found that Nkx3-2 blocks BMP-dependent expression of several GATA transcription factors (Gata4, Gata5 and Gata6) in explants of paraxial mesoderm, and that these GATA factors can in turn block Shh-dependent induction of Sox9 gene expression (Daoud et al., 2014) (Fig. 3).
Fig. 3.
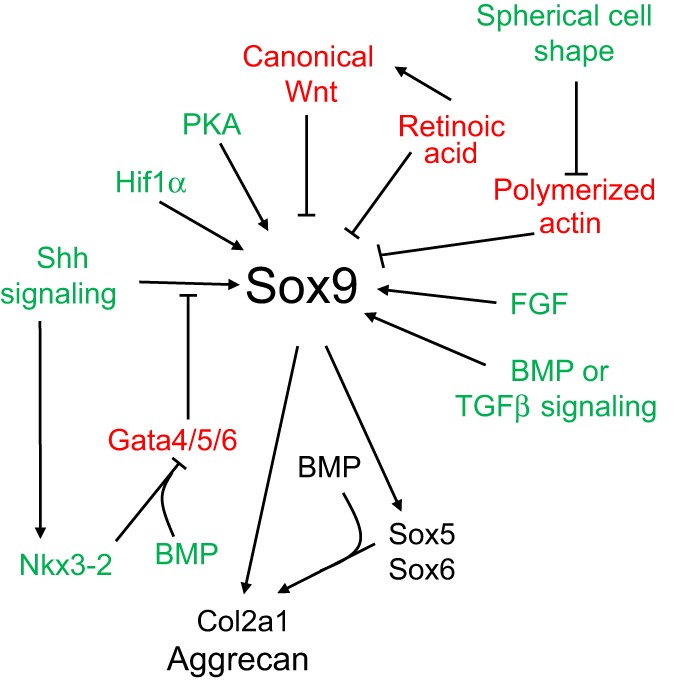
Multiple signaling pathways regulate the expression and activity of Sox9 during chondrogenesis. Signals that are known to activate Sox9 expression or activity are shown in green The signals that inhibit Sox9 expression are shown in red. Sox9 and some of its downstream targets are indicated in black. See text for details. BMP, bone morphogenetic protein; Col2a1, collagen, type II, α1; FGF, fibroblast growth factor; PKA, protein kinase A; Shh, sonic hedgehog; TGFβ, transforming growth factor β.
Chondrogenic competence in the limb bud is differentially modulated by Wnt and FGF signaling
In the limb bud, the formation of cartilage is restricted to the core of the limb bud mesenchyme by signals from the ectoderm that block cartilage formation in the periphery of this tissue (Solursh, 1984). The ectopic expression of Wnts that signal via β-catenin/Lef1/Tcf block cartilage formation in the limb bud (Rudnicki and Brown, 1997; Hartmann and Tabin, 2000). Indeed, conditional loss of β-catenin expression in either limb or head mesenchymal progenitors both increases the expression of Sox9 in these progenitor cells and induces chondrocyte formation at the expense of osteoblasts (Day et al., 2005; Hill et al., 2005). In addition to Wnts secreted by the limb bud ectoderm, FGFs secreted by the apical ectodermal ridge (AER) are necessary to maintain: (1) limb bud outgrowth (Niswander et al., 1993; Fallon et al., 1994; Sun et al., 2002); (2) the viability of a chondrogenic precursor pool that gives rise to the cartilage templates of the limb (Dudley et al., 2002; Sun et al., 2002); and (3) the competence for limb bud mesenchymal cells to undergo chondrogenesis once the Wnt signals are removed (ten Berge et al., 2008). In addition, FGF signals have been demonstrated to boost the expression of Sox9 in primary chondrocytes via a mitogen-associated protein kinase (MAPK)-dependent pathway (Murakami et al., 2000).
Recently, it was demonstrated that Wnt signals induce both a repressive chromatin mark (H3K27me3) and DNA methylation over the Sox9 promoter, and that Wnt-induced irreversible silencing of the Sox9 gene requires DNA methylation of this locus that is specifically countered by FGF signals (Kumar and Lassar, 2014). FGF blocks the recruitment of the de novo DNA methyltransferase DNMT3A to the Sox9 promoter by inducing the interaction and phosphorylation of DNMT3A by extracellular-regulated kinase (ERK) 1 and ERK2, and thereby controls whether the expression of Sox9 is irreversibly or reversibly silenced by Wnt signals in limb bud mesenchymal cells (Kumar and Lassar, 2014). Taken together, these findings suggest that Wnts secreted by the ectoderm act via a β-catenin-dependent pathway to block Sox9 expression and cartilage formation in limb bud mesenchymal cells, and that FGF signaling maintains chondrogenic competence of these cells by blocking DNA methylation of the Sox9 promoter. Although the signaling centers and the signals they produce to regulate limb and vertebra patterning and growth have been identified, we still lack a complete understanding of how these signals are interpreted and integrated into the genetic program that drives the induction of chondrogenesis.
TGFβ signaling in chondrogenesis
The role of transforming growth factor β (TGFβ) signaling in chondrogenesis has long been controversial. Gain-of-function studies in cell culture models suggest that TGFβ triggers chondrogenesis (Carrington et al., 1991; Leonard et al., 1991; Chimal-Monroy and Diaz de Leon, 1997; Merino et al., 1998; Karamboulas et al., 2010). However, blocking TGFβ signaling in the limb mesenchyme by targeting the expression of TGFβ receptor 2 (Tgfbr2) has some effect on chondrocyte differentiation and on joint formation in the digits (Seo and Serra, 2007; Spagnoli et al., 2007) but appears not to affect the initial stages of chondrogenesis. Recent findings have demonstrated that the cartilaginous anlagen of long bones are formed modularly, from two distinct pools of progenitor cells (Blitz et al., 2013). The first pool, which contains Sox9-positive progenitors, forms the primary structure of the cartilaginous anlage. The second pool differs from the first in that it contains progenitors that express both Sox9 and the transcription factor scleraxis (Scx). These Sox9+Scx+ progenitors give rise to bone eminences (Sugimoto et al., 2013): the superstructures that serve for articulation and for muscle insertion via tendons. Although TGFβ signaling inhibition has limited effects on the formation of Sox9+Scx− progenitors, TGFβ signaling is absolutely necessary for both formation of the Sox9+Scx+ progenitor population and the subsequent development of bone eminences (Blitz et al., 2013). It may be relevant in this regard that Smad2 and Smad3, which transduce TGFβ signaling, have been documented to interact with Sox9, recruit the transcriptional co-activators CBP/p300 to this transcription factor and increase Sox9 transcriptional activity (Furumatsu et al., 2005).
BMP and PKA signaling positively regulate the initiation of chondrogenesis, whereas RA signaling blocks this process
BMPs also play a crucial role in the formation and differentiation of cartilage. The mis-expression of BMPs or of activated BMP receptors in the limb bud results in ectopic chondrogenesis (Duprez et al., 1996; Zou et al., 1997). Conversely, inhibiting BMP signaling with dominant-negative BMP receptors or with the soluble BMP antagonist Nog inhibits the formation of cartilage in vivo and in vitro (Kawakami et al., 1996; Zou et al., 1997; Capdevila and Johnson, 1998; Murtaugh et al., 1999). In micromass cultures, BMP signaling initially regulates the compaction of mesenchymal cells that is required to acquire a cohesive cell behavior (Barna and Niswander, 2007) and subsequently is required to support chondrocyte differentiation (Roark and Greer, 1994). The BMP receptors Bmpr1a and Bmpr1b have overlapping functions; mice lacking both of these receptors display a lack of Sox5, Sox6 and Sox9 expression in precartilaginous condensations and a marked absence of chondrocyte formation (Yoon et al., 2005). In addition, conditional knockout of BMP-regulated Smad proteins (i.e. Smad1, Smad5 and Smad8) in chondrocytes leads to severe chondrodysplasia (Retting et al., 2009), indicating that BMP signaling is necessary not only for the initiation of chondrogenesis but also for the maintenance of this differentiation program. Recently, BMP-regulated Smads were demonstrated to regulate gene expression in early Xenopus embryos via interaction with Sox5 (Nordin and Labonne, 2014). This study demonstrated that both Sox5 and Sox6 could bind to Smad1 (Nordin and Labonne, 2014), so it seems plausible that these transcription factors may also transduce BMP signals to activate chondrogenic differentiation. Lyons and colleagues noted that the chondrocyte-specific knockout of Bmpr1a and Bmpr1b resulted in both defective maturation of chondrocytes, from a resting to a columnar proliferating state, and in an inability of hypertrophic chondrocytes to complete terminal differentiation (Yoon et al., 2006). The requirement for BMP signaling to promote chondrocyte maturation is consistent with the finding that C-terminal phosphorylated Smad1/5 is most evident in columnar proliferating chondrocytes in the growth plate (Yoon et al., 2006; Retting et al., 2009).
Another factor that plays an important role in chondrocyte differentiation is protein kinase A (PKA). Pharmacological inhibition of PKA with the PKA antagonist H89 efficiently blocks the chondrogenic differentiation of limb bud micromass cultures (Lee and Chuong, 1997; Yoon et al., 2000). PKA-mediated phosphorylation of Sox9 increases the transcriptional activity of this crucial chondrogenic transcription factor (Huang et al., 2000), which may explain the necessity for this signaling pathway in the initiation of chondrogenesis. In contrast to PKA signaling, which promotes chondrogenesis, retinoic acid (RA) signaling represses the induction of this differentiation program. The inhibition of RA receptor-mediated signaling in primary cultures of mouse limb bud mesenchyme increases both Sox9 expression and activation of Sox9 transcriptional targets (Weston et al., 2002). Administration of RA to murine epiphyseal chondrocytes induces the expression of both Wnt ligands and their receptors, and thereby increases the expression of Lef/Tcf reporters (Yasuhara et al., 2010), suggesting that the RA signaling pathway may block Sox9 expression in part by promoting canonical Wnt signaling (Fig. 3).
Hif1α is a positive regulator of chondrogenesis
During the initial stages of chondrogenesis, the limb vasculature undergoes a remodeling process that renders the condensing mesenchyme and the developing cartilaginous template avascularized. The regression of blood vessels from the developing cartilage induces a localized reduction in oxygen tension, thus forming hypoxic niches. Several studies have identified hypoxia-inducible factor 1 α (Hif1α), a basic helix-loop-helix transcription factor that is expressed in such hypoxic conditions, as a positive regulator of chondrogenesis. Hif1α was shown to promote the expression of Sox9 and to elevate the expression of glycolytic enzymes and glucose transporters to allow chondrocyte differentiation and adaptation to hypoxic conditions (Pfander et al., 2003; Amarilio et al., 2007). Hif1α was also shown to upregulate VEGFA expression in the growth plate (Schipani et al., 2001; Zelzer et al., 2004) as well as promote collagen hydroxylation to enable collagen secretion by hypoxic chondrocytes (Bentovim et al., 2012).
In summary, although we know some of the signaling pathways that drive the differentiation of mesenchymal cells into chondrocytes, the transcriptional regulatory elements that lie downstream of these pro-chondrogenic signaling pathways have yet to be completely elucidated. We speculate that the identification of the regulatory elements that control Sox9 expression downstream of these signaling pathways may reveal additional molecular players that transduce patterning information to coordinate skeletal development.
The transcriptional regulation of chondrocyte maturation
In contrast to our limited knowledge about the mechanisms that regulate the initial steps of chondrogenesis, we currently have a good understanding of the transcriptional regulation of chondrocyte differentiation and maturation, once these cells have committed to the chondrogenic lineage. Below, we describe both the positive and negative regulators of the chondrogenic differentiation program, specifically focusing on molecular interplay between different transcriptional regulators (summarized in Fig. 4).
Fig. 4.
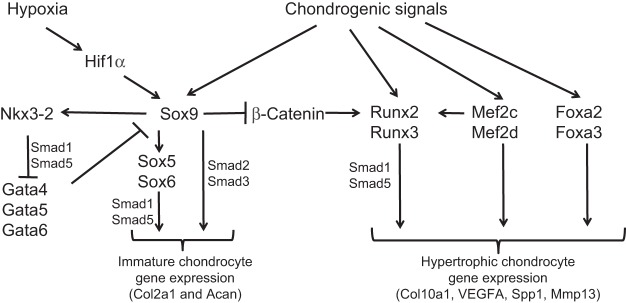
Different combinations of transcription factors drive tissue-specific gene expression in immature versus hypertrophic chondrocytes. The early steps of chondrogenesis, including mesenchymal condensation and the expression of chondrocyte-specific extracellular matrix proteins, are crucially dependent upon Sox-family transcription factors, including Sox9, Sox5 and Sox6 (de Crombrugghe et al., 2001; Lefebvre, 2002). By contrast, the process of chondrocyte hypertrophy is regulated by Runx2 and Runx3 (Inada et al., 1999; Kim et al., 1999; Yoshida et al., 2004), Mef2c and Mef2d (Arnold et al., 2007), and Foxa2/3 (Ionescu et al., 2012). Both direct interactions and/or regulatory relationships between these and other transcriptional regulators are displayed. See text for details. Acan, aggrecan; Col2a1, collagen, type II, α1; Col10a1, collagen, type X, α1; Mmp13, matrix metalloprotein 13; Spp1, osteopontin/bone sialoprotein 1; VEGFA, vascular endothelial growth factor A.
Multiple roles for Sox9 in chondrocyte differentiation and maturation
The transcription factor Sox9 is necessary for mesenchymal cells to commit to and to execute the chondrogenic differentiation program; in its absence, chondrogenesis is blocked (Bi et al., 1999; Akiyama et al., 2002). Sox9 both directly activates chondrocyte differentiation markers (Bell et al., 1997; Lefebvre et al., 1997, 1998; Ng et al., 1997; Zhou et al., 1998) and induces the expression of Sox5 and Sox6 (Akiyama et al., 2002), which work together with Sox9 to activate the chondrocyte differentiation program (Lefebvre et al., 1998; Smits et al., 2001). Sox9 expression is also necessary to sustain chondrocyte survival through a PI3K-AKT pathway (Ikegami et al., 2011; Dy et al., 2012) and has been noted to block chondrocyte maturation into hypertrophic cells. Sox9 loss of function leads to premature maturation of immature chondrocytes into hypertrophic cells (Bi et al., 2001; Akiyama et al., 2002). Conversely, overexpression of Sox9 in either immature or hypertrophic chondrocytes of the growth plate slows the process of chondrocyte hypertrophy (Akiyama et al., 2004).
There are several possible mechanisms by which Sox9 might repress chondrocyte hypertrophy. First, Sox9 was found to interact with β-catenin, induce its degradation and thereby block canonical Wnt signaling (Akiyama et al., 2004; Topol et al., 2009). As this signaling pathway has been demonstrated to promote chondrocyte hypertrophy (Hartmann and Tabin, 2000; Enomoto-Iwamoto et al., 2002; Tamamura et al., 2005; Später et al., 2006), Sox9-mediated destruction of β-catenin may thereby disrupt this maturation process. Second, Sox9 has been demonstrated both to interact directly with and to block the activity of the transcription factor Runx2 (Runt-related transcription factor 2) (Zhou et al., 2006), which plays a crucial role in directing chondrocyte maturation (discussed below). Third, Sox9 can directly repress genes that are normally expressed by hypertrophic chondrocytes, such as Col10a1 and Vegfa (Hattori et al., 2010; Leung et al., 2011). Last, it has been shown that Sox9 is necessary to maintain proliferation of columnar chondrocytes, to delay expression of markers of prehypertrophic chondrocyte differentiation and, strikingly, to prevent the differentiation of chondrocytes into osteoblasts by lowering both β-catenin signaling and Runx2 expression (Dy et al., 2012). In addition, it has been shown that Sox9 plays an additional role to activate the expression of Col10a1 specifically in early hypertrophic chondrocytes by directly binding to the promoter of this gene (Dy et al., 2012). Taken together, these findings suggest that Sox9 blocks the maturation of immature columnar chondrocytes into prehypertrophic ones, while promoting the initial induction of Col10a1 in early hypertrophic chondrocytes.
Positive transcriptional regulators of chondrocyte hypertrophy in the growth plate
Runx family transcription factors
The Runt family transcription factors Runx2 and Runx3 (also known as Cbfa1 and Cbfa3, respectively) are vital for promoting chondrocyte hypertrophy. Runx2 is expressed in prehypertrophic and hypertrophic chondrocytes of the growth plate and in the perichondrium (Inada et al., 1999; Kim et al., 1999). The activation of canonical Wnt signaling in chicken chondrocytes increases Runx2 expression (Dong et al., 2006), which may in part explain how this signaling pathway promotes chondrocyte hypertrophy. Either genetic ablation of Runx2 or the expression of dominant-negative Runx2 in mice results in delayed and greatly diminished chondrocyte hypertrophy (Inada et al., 1999; Kim et al., 1999; Ueta et al., 2001). Conversely, the ectopic expression of Runx2 in immature chondrocytes drives premature cellular maturation and induces the expression of Col10a1 and other hypertrophic markers, both in vivo (Takeda et al., 2001; Ueta et al., 2001; Stricker et al., 2002) and in vitro (Enomoto et al., 2000). Several studies demonstrate that Runx2 can directly bind to the regulatory sequences that drive the expression of Ihh (Yoshida et al., 2004), Vegfa (Zelzer et al., 2001), Col10a1 (Drissi et al., 2003; Zheng et al., 2003) and Mmp13 (Selvamurugan et al., 2000). Runx2 has also been found to interact with BMP-regulated Smads (Hanai et al., 1999; Zhang et al., 2000; Javed et al., 2008) and is thus a likely candidate to transduce BMP signals to activate hypertrophic chondrocyte gene expression. There is redundancy between Runx-family members in the control of chondrocyte hypertrophy. The genetic ablation of Runx3 in mice results in a slight delay in both chondrocyte hypertrophy and vascular invasion into the cartilage; neonatal skeletal development was normal in these animals (Yoshida et al., 2004). By contrast, Runx2;Runx3 double knockout animals display a complete absence of chondrocyte maturation, a phenotype that is considerably more severe than that seen following Runx2 inactivation alone (Yoshida et al., 2004).
Mef2 family transcription factors
Mef2c and Mef2d, which are members of the myocyte enhancer factor 2 (Mef2) family of transcription factors, are also expressed in prehypertrophic and hypertrophic chondrocytes. Mef2c loss of function in chondrocytes results in shortening of the bones, a delay in chondrocyte hypertrophy and downregulation of Runx2 expression (Arnold et al., 2007). Conversely, gain-of-function experiments in transgenic mice that express an activated Mef2c-VP16 fusion protein in chondrocytes results in the opposite phenotype of premature and excessive endochondral ossification (Arnold et al., 2007). These results indicate that Mef2c functions upstream of Runx2, and is necessary to either induce or maintain Runx2 expression in hypertrophic chondrocytes.
FoxA family transcription factors
When overexpressed, Runx2 and Mef2c can readily activate the expression of hypertrophic chondrocyte-specific genes, such as Col10a1, in chondrocytes (Zheng et al., 2003; Arnold et al., 2007); however, they fail to activate Col10a1 expression in fibroblasts (Kempf et al., 2007; Ionescu et al., 2012), suggesting that they require another chondrocyte-specific co-factor for this activity. Recently, members of the forkhead box A (FoxA) family of transcription factors were identified as part of the transcriptional network that regulates chondrocyte differentiation. Foxa3 is expressed in both immature and hypertrophic chondrocytes, although Foxa3-null animals do not display any obvious skeletal phenotype (Kaestner et al., 1998; Shen et al., 2001). Foxa2 expression, by contrast, is restricted to hypertrophic cells (Ionescu et al., 2012). Loss of function of Foxa2 in the chondrocytes of Foxa3 knockout mice leads to decreased expression of hypertrophic markers such as Col10a1 and Mmp13 (Ionescu et al., 2012). Furthermore, the skeletal defects resulting from chondrocyte-specific loss of Foxa2 are exacerbated in a Foxa3-null background (Ionescu et al., 2012), suggesting that Foxa2 and Foxa3 share overlapping roles in the growth plate to promote chondrocyte hypertrophy.
Negative regulators of chondrocyte hypertrophy in the growth plate
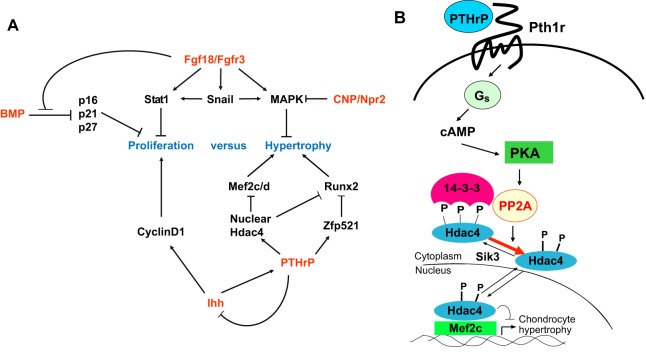
The transcriptional network that modulates chondrocyte differentiation also includes negative regulators. One of the best characterized of these is histone deacetylase 4 (Hdac4), a member of the class II HDACs that was found to repress both Runx2 and Mef2 activity (Vega et al., 2004; Kozhemyakina et al., 2009; Correa et al., 2010) (Fig. 5A). Hdac4 is expressed in prehypertrophic chondrocytes, consistent with the finding that Hdac4 loss of function causes premature chondrocyte hypertrophy (Vega et al., 2004). Hdac4 can either directly interact with Runx2 and inhibit its activity (Vega et al., 2004) or indirectly repress Runx2 transcriptional activity by binding to the zinc-finger transcriptional co-regulator Zfp521, which in turn binds to Runx2 and blocks its transcriptional activity (Correa et al., 2010). The activity of class II HDACs is, in turn, regulated by signaling pathways (Fig. 5B) that either promote or block HDAC phosphorylation (on S246 in Hdac4) and thereby modulate its interaction with 14-3-3 proteins (Grozinger and Schreiber, 2000; McKinsey et al., 2000b). Many 14-3-3 family members are located in the cytoplasm, where they interact with other proteins containing a phosphorylated serine located in an RXX-SXP consensus binding motif. As S246 in Hdac4 is located in one such 14-3-3-binding motif, phosphorylation of S246 results in the interaction of Hdac4 with 14-3-3 proteins in the cytoplasm (McKinsey et al., 2000a; Wang et al., 2000). This interaction shields the Hdac4 nuclear localization signal and simultaneously blocks entry of Hdac4 into the nucleus and stimulates its nuclear export (Wang and Yang, 2001). As a consequence, nuclear-localized Runx and Mef2 family members do not interact with Hdac4, and thus are free to activate their transcriptional targets efficiently.
Fig. 5.
Regulatory pathways downstream of BMP, Fgf18, CNP, Ihh or PTHrP signaling control chondrocyte proliferation and hypertrophy. (A) Summary of how various transcriptional regulators and signaling pathways regulate chondrocyte proliferation and hypertrophy. (B) PTHrP signals repress chondrocyte hypertrophy via the PKA-induced dephosphorylation of phospho-S246 on Hdac4 by PP2A. This dephosphorylation event enhances the nuclear localization of Hdac4 and thereby inhibits Mef2 function. See text for details. BMP, bone morphogenetic protein; CNP, C-type natriuretic peptide; Fgf18, fibroblast growth factor 18; Hdac4, histone deacetylase 4; MAPK, mitogen-activated protein kinase; Npr2, natriuretic peptide receptor 2; PKA, protein kinase A; PP2A, protein phosphatase 2A; Pth1r, parathyroid hormone 1 receptor; PTHrP, parathyroid hormone-related protein; Sik1, salt-inducible kinase 1.
In immature chondrocytes, which are found in both the resting and columnar zones of the growth plate, the paracrine hormone parathyroid hormone-related protein (PTHrP) induces PKA activity to promote dephosphorylation of S246 on Hdac4 by protein phosphatase 2A (PP2A). This allows Hdac4 to translocate into the nucleus and to inhibit the activity of both Mef2 and Runx family members (Kozhemyakina et al., 2009; Correa et al., 2010). As chondrocytes mature, salt-inducible kinase 3 (Sik3), which is specifically expressed in both prehypertrophic and hypertrophic chondrocytes of the growth plate (Sasagawa et al., 2012), phosphorylates Hdac4 [presumably on S246; as does Sik1 (Berdeaux et al., 2007; Kozhemyakina et al., 2009)] and thus promotes Hdac4 phosphorylation and interaction with 14-3-3 proteins in the cytoplasm, with consequent activation of Mef2 transcriptional activity (Fig. 5B). Indeed, loss of Sik3 causes severe inhibition of chondrocyte hypertrophy in mice (Sasagawa et al., 2012). Taken together, these observations suggest that Sik3-induced phosphorylation of Hdac4 liberates both Mef2 and Runx family members from interaction with Hdac4, and thus permits the induction of chondrocyte hypertrophy target genes by these key transcriptional regulators. Other transcription factors, such as Nkx3-2 (also known as Bapx1) also play a significant role in attenuating maturation of columnar chondrocytes in the growth plate (Provot et al., 2006).
Multiple signaling pathways control chondrocyte maturation in the growth plate
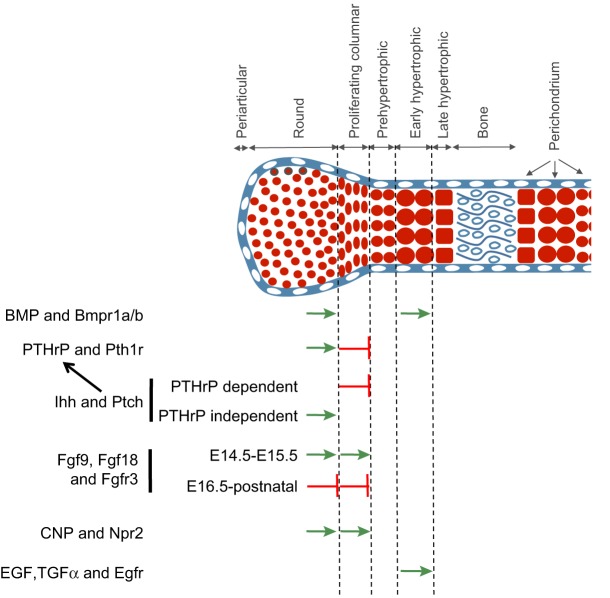
The signals that control the induction of cartilage primordia and/or chondrocyte maturation in the growth plate do so by controlling the expression and/or the activity of the above discussed transcription factors. In the following sections, we discuss how the subsequent initiation and progression of chondrocyte hypertrophy is regulated by a number of key ligands, including PTHrP, Ihh, Fgf9, Fgf18, C-type natriuretic peptide (CNP), insulin-like growth factor (IGF), epidermal growth factor (EGF) and transforming growth factor α (TGFα) (summarized in Fig. 6).
Fig. 6.
Summary of the signaling pathways that regulate crucial transitions during chondrocyte maturation. Signals known to promote either proliferation and/or hypertrophic differentiation are indicated by green arrows; signals that block these steps are indicated by a red bar-headed arrow. The transitions regulated by these signals are indicated by the dotted line to the right of each arrow. See text for details. BMP, bone morphogenetic protein; Bmpr, bone morphogenetic protein receptor; CNP, C-type natriuretic peptide; EGF, epidermal growth factor; Egfr, epidermal growth factor receptor; Fgf, fibroblast growth factor; Fgfr3, fibroblast growth factor receptor 3; Ihh, Indian hedgehog; Npr2, natriuretic peptide receptor; Ptch, patched; Pth1r, parathyroid hormone 1 receptor; PTHrP, parathyroid hormone-related protein; TGFα, transforming growth factor α.
PTHrP-mediated regulation of chondrocyte hypertrophy
The paracrine hormone PTHrP and its receptor Pth1r are crucial regulators of chondrocyte proliferation and hypertrophy. PTHrP is expressed at high levels in periarticular resting cells of the growth plate and at lower levels in early proliferating chondrocytes (Lee et al., 1996; St-Jacques et al., 1999). Knockout of PTHrP (Pthlh – Mouse Genome Informatics) gene results in diminished chondrocyte proliferation in the growth plate, premature chondrocyte maturation and accelerated bone formation (Karaplis et al., 1994; Lee et al., 1996). Conversely, PTHrP overexpression in mouse chondrocytes results in delayed chondrocyte differentiation during early development (Weir et al., 1996). The PTHrP receptor Pth1r is produced at low levels by proliferating growth plate chondrocytes and at a higher level in prehypertrophic cells (Vortkamp et al., 1996; St-Jacques et al., 1999). Knockout of Pth1r in mice results in a phenotype that is similar to that of PTHrP-null mice, marked by a reduction of proliferation and premature mineralization of chondrocytes in the growth plate (Lanske et al., 1996).
How does Pth1r signaling slow the progression of chondrocyte hypertrophy? Pth1r is a G-protein-coupled receptor and its activation by PTHrP results in stimulation of both Gs(α) and Gq(α) signaling pathways, which surprisingly have opposing actions on chondrocyte hypertrophy (Guo et al., 2002). Stimulation of the Gs(α) pathway results in activation of adenylate cyclase (AC) and the production of cAMP, which in turn activates PKA (Guo et al., 2002). As discussed above, PTHrP-induced PKA activation promotes the translocation of Hdac4 into the nucleus, where it binds to and inhibits the transcriptional activity of Mef2 family members (Kozhemyakina et al., 2009). In addition, PTHrP signaling increases the expression of Zfp521 that, as mentioned above, blocks Runx2 transcriptional activity via recruitment of Hdac4 (Correa et al., 2010). Thus, by both inducing nuclear translocation of Hdac4 and increasing expression of Zfp521, Pth1r signaling blocks the activity of two key families of transcription factors, Mef2 and Runx, that regulate chondrocyte hypertrophy (Fig. 5A).
A PTHrP-Ihh signaling loop maintains a pool of immature chondrocyte progenitors
PTHrP synthesis in the growth plate is controlled by Ihh (Vortkamp et al., 1996), a member of the Hedgehog family of proteins. In the growth plate, Ihh is expressed and secreted by prehypertrophic and early hypertrophic cells (Vortkamp et al., 1996). The deletion of Ihh in mice causes a reduction in chondrocyte proliferation, premature chondrocyte hypertrophy and a failure of osteoblast development in endochondral bones (St-Jacques et al., 1999). Likewise, the conditional deletion of Smo in a cartilage-specific manner decreases chondrocyte proliferation (Long et al., 2001). How does Ihh control chondrocyte proliferation and maturation in the growth plate? The misexpression of Ihh in chick embryos was found to induce PTHrP expression in the periarticular perichondrium of the growth plate (Vortkamp et al., 1996). Conversely, PTHrP expression is absent from the growth plate in Ihh-deficient mice, which display a phenotype that is similar to that of PTHrP-null animals (St-Jacques et al., 1999). Although increased levels of exogenous Ihh inhibit chondrocyte hypertrophy in the growth plate, the deletion of either PTHrP or Pth1r in mice abolishes this effect (Lanske et al., 1996; Vortkamp et al., 1996). Notably, the expression of constitutively active Pth1r in the growth plate of Ihh−/− mice reverses premature chondrocyte hypertrophy but fails to rescue decreased chondrocyte proliferation (Karp et al., 2000). These findings suggest that Ihh controls proliferation and maturation of chondrocytes by both a PTHrP-dependent pathway that negatively regulates chondrocyte hypertrophy and a PTHrP-independent pathway that positively regulates chondrocyte proliferation (Karp et al., 2000).
Ihh promotes chondrocyte proliferation, at least in part by increasing the expression of cyclin D1, which regulates cell cycle progression (Long et al., 2001). In addition, Ihh promotes the transition of small round chondrocytes into proliferating chondrocytes, independently of PTHrP expression (Kobayashi et al., 2005), by inhibiting the repressor activity of Gli3 (i.e. Gli3R) (Koziel et al., 2005). The analysis of Ihh−/−;Gli3−/− double knockout embryos revealed that Gli3 is the key effector of Ihh signaling in chondrocytes. Deletion of Gli3 bypasses the requirement for Ihh to promote proliferation and maturation of chondrocytes, demonstrating that Ihh normally regulates PTHrP expression by antagonizing Gli3R repressor activity (Hilton et al., 2005; Koziel et al., 2005). Coupling the maintained expression of PTHrP to Ihh expression in prehypertrophic chondrocytes [which is itself repressed by PTHrP signaling (Vortkamp et al., 1996)] ensures that not all immature chondrocytes will simultaneously initiate the prehypertrophic/hypertrophic differentiation program and thus deplete the chondrogenic progenitor pool. Thus, Ihh-dependent activation of PTHrP expression maintains a population of immature chondrocytes in the resting and columnar zones of the growth plate. At the time of puberty in humans, the growth plate closes in a manner that is dependent upon estrogen receptor signaling (reviewed by Borjesson et al., 2012), suggesting that estrogen somehow disrupts maintenance of the PTHrP/Ihh loop.
FGF signaling regulates chondrocyte proliferation and the initiation of chondrocyte hypertrophy
Several FGFs and their cognate receptors exhibit distinct domains of expression in the growth plate and the surrounding perichondrium/periosteum. Fgfr1 is specifically expressed in both the perichondrium/periosteum and in hypertrophic chondrocytes of the growth plate, while being excluded from proliferating chondrocytes (Jacob et al., 2006). The conditional deletion of Fgfr1 in chondrocytes results in a transient increase in the hypertrophic zone, with no impact on chondrocyte proliferation (Jacob et al., 2006). Fgfr2 is initially expressed at high levels in the condensed mesenchyme that will give rise to cartilage and bone, and is subsequently expressed in both the perichondrium and periosteum (reviewed by Ornitz and Marie, 2002). The deletion of Fgfr2 in mesenchymal cells results in marked postnatal dwarfism with reduced thickness of the hypertrophic zone, a failure in osteoprogenitor maturation and abnormal function of mature osteoblasts (Yu et al., 2003). Fgfr3, which is expressed in the proliferating zone of the growth plate but downregulated in the hypertrophic zone, exhibits a pattern of expression that is complementary to that of Fgfr1 and Fgfr2 (Peters et al., 1993; Deng et al., 1996; Koziel et al., 2005; de Frutos et al., 2007). Fgfr3-null mice display postnatal elongation of long bones, correlating with an increase in chondrocyte proliferation and elongation of the hypertrophic zone (Colvin et al., 1996; Deng et al., 1996). Conversely, the overexpression of activated Fgfr3 in the mouse growth plate gives rise to a decrease in chondrocyte proliferation and a small hypertrophic zone, leading to dwarfism (Naski et al., 1998; Chen et al., 1999; Wang et al., 1999b; Segev et al., 2000; Iwata et al., 2001). Accordingly, several identified human gain-of-function mutations in Fgfr3 result in a spectrum of developmental disorders, including achondroplasia, hypochondroplasia and thanatophoric dysplasia, all of which are marked by rhizomelic shortening of the limbs (see review by Foldynova-Trantirkova et al., 2012). Therefore, Fgfr3 plays a significant role in growth plate development, acting to inhibit both the rate of chondrocyte proliferation and the initiation of chondrocyte hypertrophy. In addition, it was observed that FGF application accelerates late hypertrophic differentiation in cartilage explant cultures (Minina et al., 2002), as opposed to inhibiting it, raising the interesting possibility that this signaling pathway may block the initiation of chondrocyte hypertrophy (in prehypertrophic chondrocytes) but subsequently promote the progression of early hypertrophic chondrocytes (i.e. those that express Col10a1) into late hypertrophic chondrocytes (i.e. those that express Mmp13).
Although multiple FGF ligands have been detected during endochondral ossification (for a review, see Degnin et al., 2010), so far only Fgf9 and Fgf18 have been shown to be relevant for chondrogenesis in vivo (Hung et al., 2007; Liu et al., 2007). Fgf9 is expressed in the perichondrium/periosteum, and Fgf9-deficient mice manifest disproportional shortening of proximal skeletal elements, decreased proliferation and delayed chondrocyte hypertrophy during early stages of chondrogenesis (Hung et al., 2007), a phenotype resembling an early phenotype of mice with conditional inactivation of Fgfr1 (Jacob et al., 2006). In addition to regulating chondrocyte proliferation and hypertrophy directly, Fgf9 also regulates chondrocyte maturation indirectly via the Ihh/PTHrP pathway; Ihh, Pth1r and Ptc1 levels are all decreased in Fgf9-null mice (Hung et al., 2007). Fgf18 is another ligand expressed in the perichondrium, with low levels of expression also detected in chondrocytes (Liu et al., 2002, 2007). Fgf18−/− mice exhibit decreased chondrocyte proliferation and delayed initiation of chondrocyte hypertrophy during their early stages of development (E14.5-E15.5) (Liu et al., 2007) and thus phenocopy Fgf9-null mice at this stage of development (Hung et al., 2007). By contrast, at later embryonic stages (E16.5-E18.5), Fgf18−/− mice display an increase in proliferating and hypertrophic zone thickness and an enlarged growth plate, indicating that, at this stage of development, Fgf18 signaling negatively regulates proliferation and maturation of growth plate chondrocytes (Liu et al., 2002). Consistent with these findings, an activating mutation in Fgfr3 increases chondrocyte proliferation specifically in E14-E15 mice but not in older E18 embryos (Iwata et al., 2000). At this later stage of development, the phenotype of Fgf18−/− mice closely resembles that of Fgfr3−/− mice, which similarly display an increase in chondrocyte proliferation and elongation of the hypertrophic zone (Colvin et al., 1996; Deng et al., 1996), suggesting that Fgf18 is likely to signal through Fgfr3. Together, these findings imply that the response of chondrocytes to Fgf18 signaling in mice is biphasic: during early stages of development (E14-E15) this signaling pathway promotes both proliferation and the initiation of chondrocyte hypertrophy, while in older embryos (and postnatal mice) this signaling pathway acts to block chondrocyte proliferation and delays the initiation of chondrocyte hypertrophy (Liu et al., 2007). It is not clear what controls these strikingly different responses to Fgf18 signaling at differing stages of growth plate development.
How does Fgfr3 signaling negatively regulate both chondrocyte proliferation and the initiation of chondrocyte hypertrophic differentiation? Two principal signaling pathways – the Stat1 and MAPK pathways – have been reported to mediate Fgfr3 signaling in chondrocytes. The role of the transcription factor Stat1 in FGF-induced inhibition of chondrocyte proliferation was initially proposed by Sahni et al. (1999). Although the issue of whether Stat1 is downstream of FGF signaling is still debated (Krejci et al., 2008), deletion of Stat1 was found to reverse both the reduced proliferation and the chondrodysplasic phenotype in the growth plates of mice overexpressing human FGF2 (Sahni et al., 2001). Notably, however, although Fgfr3-null mice show dramatic postnatal expansion of both the proliferative and hypertrophic chondrocyte zones (Colvin et al., 1996; Deng et al., 1996), the growth plate of Stat1-knockout mice displays expansion of the proliferative zone only during early postnatal development, which is attenuated at adult stages (Sahni et al., 2001). Moreover, the ablation of Stat1 in transgenic mice expressing an activated form of Fgfr3 restored proliferation of growth plate chondrocytes but failed to reverse the deficit in chondrocyte hypertrophy induced by activated Fgfr3 signaling (Murakami et al., 2004). Taken together, these studies suggest that Fgfr3 regulates chondrocyte proliferation via a Stat1-dependent pathway but represses the initiation of chondrocyte hypertrophic differentiation via a distinct signaling pathway. Indeed, Murakami et al. demonstrated that transgenic mice expressing a constitutively active form of MEK1 (Map2k1, mitogen-activated protein kinase kinase 1), which is the activating kinase in the MAPK pathway, in the growth plate displayed dwarfism accompanied by incomplete chondrocyte hypertrophy and a delay in endochondral ossification, while chondrocyte proliferation was unaffected (Murakami et al., 2004). In addition, the same group found that overexpression of constitutively active MEK1 in the growth plate of Fgfr3-null mice inhibited skeletal overgrowth, which usually occurs in Fgfr3-null mice. Based on their findings, Murakami et al. proposed that Fgfr3 signaling inhibits the initiation of chondrocyte hypertrophy through the MAPK pathway, yet attenuates chondrocyte proliferation through Stat1 (Murakami et al., 2004). In addition, application of Fgf18 to cultured mouse limb buds attenuates the accumulation of phosphorylated-Smad1/5 (Retting et al., 2009) and thereby represses BMP signaling in the growth plate. Because BMP signaling in turn represses expression of the cyclin-dependent kinase (CDK) inhibitors p16, p21 and p27 in the growth plate (Yoon et al., 2006), it is possible that Fgfr3/MAPK signaling also blocks chondrocyte proliferation in part by inhibiting BMP-mediated repression of these CDK inhibitors. Finally, recent work showed that overexpression of the transcriptional repressor Snail1 in the growth plate leads to achondroplasia in mice, and demonstrated that Snail1 acts downstream of Fgfr3 signaling in chondrocytes (Fig. 5A) to positively promote both the Stat1 and MAPK pathways (de Frutos et al., 2007).
CNP-mediated antagonism of Fgfr3 signaling modulates the onset of chondrocyte hypertrophy
Natriuretic peptides comprise a family of three structurally related peptides: atrial natriuretic peptide, brain natriuretic peptide and C-type natriuretic peptide (CNP). Natriuretic peptides transmit their signals by activating natriuretic peptide receptors: Npr1 (also known as NPR-A or GC-A) and Npr2 (also known as NPR-B or GC-B), both of which have a guanylate cyclase domain, and Npr3 (also known as NPR-C), which lacks guanylate cyclase activity and is thought to play the role of a clearance receptor that acts to internalize and destroy the ligand (reviewed by Kishimoto et al., 2011). CNP has the highest affinity for Npr2 and can also bind Npr3, both of which are expressed in chondrocytes (Kishimoto et al., 2011). In the growth plate, Npr2 is predominantly expressed in proliferating and prehypertrophic chondrocytes (Yamashita et al., 2000; Tamura et al., 2004), the same regions that express the ligand CNP (Chusho et al., 2001). Npr3 is specifically expressed in hypertrophic chondrocytes (Yamashita et al., 2000). Targeted deletion of the gene encoding CNP (Nppc) in mice results in dwarfism, with a decreased height of both the proliferating and hypertrophic zones of the growth plate, and a reduced rate of differentiation into hypertrophic chondrocytes (Chusho et al., 2001), a phenotype resembling that seen following the activation of Fgfr3 signaling (Naski et al., 1998; Chen et al., 1999; Wang et al., 1999b; Segev et al., 2000; Iwata et al., 2001). Notably, this dwarf phenotype can be rescued by cartilage-specific overexpression of CNP (Chusho et al., 2001), indicating that CNP acts locally within the growth plate. Consistent with these results, the cartilage-specific overexpression of CNP results in bone overgrowth, with increased thickness of both the proliferating and hypertrophic zones within the growth plate (Yasoda et al., 2004). Moreover, CNP overexpression in cartilage can prevent the dwarfism that results from the expression of activated Fgfr3 in growth plate chondrocytes, indicating that CNP signaling works antagonistically to Fgfr3 signaling (Yasoda et al., 2004). Remarkably, the systemic administration of either CNP or a CNP pharmacological analog significantly reverses the dwarf phenotype and growth-plate defects in mouse models of achondroplasia (Yasoda et al., 2009; Lorget et al., 2012).
By binding to its receptor Npr2, CNP promotes the accumulation of intracellular cyclic GMP (cGMP) in chondrocytes and thereby regulates cellular function (reviewed by Nakao et al., 1992). In the ATDC5 murine chondrocyte cell line, the administration of either CNP or 8-bromo-cGMP strongly and dose-dependently inhibits the induction of ERK/MAPK phosphorylation by either Fgf2 or Fgf18 while having no effect on phosphorylation of Stat1 (Ozasa et al., 2005). It was also found that the administration of either Fgf2 or Fgf18 markedly reduced CNP-dependent intracellular cGMP production (Ozasa et al., 2005). Thus, CNP and FGF activate mutually antagonistic signaling pathways. Furthermore, in rat chondrosarcoma (RCS) chondrocytes, CNP signaling blocks Fgf2-mediated activation of both MEK1 and Raf1 but not that of the Ras oncogene (Krejci et al., 2005), demonstrating that CNP blocks the MAPK pathway at the level of Raf1. These and other observations prompted Krejci et al. to propose that CNP-mediated elevation in cGMP activates protein kinase G, which in turn phosphorylates Raf1 at Ser43, resulting in the uncoupling of the Ras/Raf1 interaction and inactivation of the MAPK pathway (Krejci et al., 2005). Consistent with the notion that CNP/Npr2 signaling counters that of Fgfr3 in the growth plate, a loss-of-function mutation in Npr2 results in dwarfism in mice (Tamura et al., 2004; Tsuji and Kunieda, 2005) and thus phenocopies gain-of-function mutations in Fgfr3. Taken together, these studies have established that antagonistic signaling pathways cued by either CNP/Npr2 or Fgf18/Fgfr3 are very finely balanced in the growth plate to maintain the appropriate rate of chondrocyte hypertrophy.
A nexus of signaling pathways regulates initiation of the hypertrophic chondrocyte differentiation program
As discussed above, four key ligands modulate the initiation of chondrocyte hypertrophy: PTHrP, Ihh, Fgf18 and CNP. The highest expression levels for both Fgfr3 (which transduces the Fgf18 signal from the perichondrium) and Pth1r (which transduces the PTHrP signal from periarticular chondrocytes) in the growth plate are found in prehypertrophic chondrocytes (de Frutos et al., 2007). For both these receptors, engagement with their cognate ligand blocks the progression of chondrocyte hypertrophy. However, what triggers the onset of chondrocyte hypertrophy? The relative level of PTHrP signaling in columnar chondrocytes is controlled by both the distance these cells lie from the source of PTHrP and the level of Ihh secreted by prehypertrophic chondrocytes, which induces expression of PTHrP in chondrocytes adjacent to the joint. Thus, only cells that lie at a sufficient distance from the source of PTHrP, and therefore display both decreased expression of Zfp521 and nuclear-localized Hdac4, can initiate Mef2/Runx-driven hypertrophic chondrocyte gene expression. As discussed above, CNP and Fgf18 activate antagonistic signaling pathways via Npr2 and Fgfr3, respectively, which differentially regulate the MAPK pathway that blocks the initiation of chondrocyte hypertrophy (possibly by maintaining the expression of Snail1 and/or Sox9). Thus, the level of activated MAPK in prehypertrophic chondrocytes is determined by the relative level of Npr2 signaling (which represses this pathway) and Fgfr3 signaling (which activates this pathway) in these cells. Although Fgf18 is secreted by cells in the perichondrium, CNP is secreted by both proliferating and prehypertrophic chondrocytes. Thus, as more proliferating and prehypertrophic chondrocytes are generated, these immature chondrocytes will secrete greater amounts of CNP. Eventually, when CNP/Npr2 signaling is sufficient to negate Fgfr3-driven activation of MAPK in prehypertrophic chondrocytes, hypertrophic differentiation of these cells will commence. In this sense, the mutual antagonism between the CNP and Fgf18 signaling pathways can be viewed as a sensing mechanism to ensure that a sufficient number of proliferating and prehypertrophic chondrocytes are present to initiate the hypertrophic chondrocyte differentiation program in that subset of these cells that lies distal to the source of PTHrP signals.
IGF1 signaling controls the last phase of growth during chondrocyte hypertrophy
Loss of IGF1 signaling is known to severely decrease long bone growth in both mice (Liu et al., 1993; Powell-Braxton et al., 1993) and human (Woods et al., 1996). In mice engineered to lack IGF1, chondrocyte numbers and proliferation are normal in the growth plates, but the size of the hypertophic chondrocytes is ∼30% smaller in the direction of elongation than in wild-type mice (Wang et al., 1999a). Recently, Cooper and colleagues have extended these findings to document that chondrocyte hypertrophy proceeds via three phases of volume increase, with the final phase involving a proportional increase in dry mass with a relatively low density of cellular contents (Cooper et al., 2013). This last phase of chondrocyte enlargement is dependent upon IGF1 signaling (Cooper et al., 2013).
The terminal phases of chondrocyte hypertrophy: regulation by EGFR and ROS
Initiation of the terminal phase of chondrocyte hypertrophy is marked by a loss of Col10a1 expression, coupled with the induction of VEGFA and the metalloproteinases, Mmp9 and Mmp13. VEGFA signaling is necessary to promote vascular invasion and consequent remodeling of the cartilage tissue by osteoblasts and osteoclasts (Zelzer et al., 2002, 2004). Mmp9 and Mmp13 similarly play a crucial role in promoting vascular invasion of the hypertrophic matrix; mice that lack both these metalloproteinases display a considerable increase in the zone of the growth plate containing Col10a1-expressing hypertrophic chondrocytes (Inada et al., 2004; Stickens et al., 2004). What signals trigger this terminal phase of chondrocyte hypertrophy? Recent studies have demonstrated that signaling via the EGF receptor (Egfr) plays a crucial role in orchestrating this transition. Mice that have been engineered to express herstatin (a soluble Erbb2 receptor that acts in a dominant-negative manner to inhibit Egfr signaling) in developing limb buds display an expanded hypertrophic zone in their limbs (Fisher et al., 2007). Furthermore, Qin and colleagues have demonstrated that the administration of an Egfr-specific small-molecule inhibitor, gefitinib, to 1-month-old rats results in significant epiphyseal growth plate thickening and massive accumulation of early hypertrophic chondrocytes that express Col10a1 (Zhang et al., 2011). The growth plates in gefitinib-treated animals display both increased expression of Col10a1 and markedly decreased expression of Mmp9, Mmp13 and RANK ligand (RANKL; Tnfsf11 – Mouse Genome Informatics), which supports osteoclast development (Zhang et al., 2011). Consistent with these findings, the same workers demonstrated that the chondrocyte-specific knockout of Egfr significantly enlarges the length of the hypertrophic zone in postnatal mice (Zhang et al., 2011). Two ligands for the EGF receptor, EGF and TGFα, are expressed in both the prehypertrophic and hypertrophic zones, but not in the proliferative zone of chick growth plates during early postnatal growth (Ren et al., 1997). Importantly, Tgfa-null mice display transient expansion of the early hypertrophic zone of the growth plate, coupled with decreased expression of Runx2, RANKL and Mmp13 in this structure (Usmani et al., 2012). Taken together, these findings strongly support the notion that Egfr signaling (cued by both EGF and TGFα) plays a crucial role in orchestrating the transition of Col10a1-expressing early hypertrophic chondrocytes into late hypertrophic cells that express Mmp9, Mmp13 and RANKL.
How does EGFR signaling act to block the expression of Col10a1 while promoting that of Mmp9, Mmp13 and RANKL? Qin and colleagues have noted that, although upregulation of Mmp9 and RANKL by Egfr signaling is partially mediated by the canonical Wnt/β-catenin pathway, Egfr-induced expression of Mmp13 is mediated via a distinct pathway (Zhang et al., 2013). Conditional knockout of both Erk1 and Erk2 in the mesenchymal precursors of the appendicular skeleton resulted in a remarkable expansion of the Col10a1 expression domain within the growth plate, coupled with a lack of expression of late hypertrophic markers such as VEGFA and RANKL in this tissue (Matsushita et al., 2009). This is similar to the phenotype observed following loss of Egfr signaling in the growth plate (Zhang et al., 2011). In addition to activating the ERK and p38 pathways (outlined in Zhang et al., 2013), growth factors such as EGF are known to stimulate the generation of hydrogen peroxide via activation of the NADPH oxidase Nox1, by a mechanism involving PI3K and Rac1 (Bae et al., 1997; Fan et al., 2005). Notably, Morita et al. found that reactive oxygen species (ROS) are specifically generated in the hypertrophic zone of the growth plate (Morita et al., 2007). Moreover, this same group demonstrated that the treatment of newborn mice with an antioxidant (N-acetylcysteine) decreases the length of the hypertrophic zone in their growth plates, and that application of hydrogen peroxide to a chondrocyte cell line robustly induces the expression of Mmp13 (Morita et al., 2007). Taken together, these findings suggest that Egfr signaling may promote the terminal stages of chondrocyte hypertrophy in a manner that is dependent upon both ERK signaling and ROS production.
The ultimate fate of growth plate chondrocytes
For many years it has been appreciated that apoptosis is detectable at the chondro-osseous front in the growth plate (i.e. the border between late hypertrophic chondrocytes and bone matrix) (Gibson, 1998; Shapiro et al., 2005), suggesting that initiation of an apoptotic program of cell death is the ultimate fate of late hypertrophic chondrocytes. However, the morphology of some late hypertrophic chondrocytes also suggests that some of these cells may undergo transformation into osteoblasts (reviewed by Roach et al., 1995). By employing mice in which Cre recombinase has been knocked into one allele of the Col10a1 locus, or those in which the expression of a Cre-transgene is driven by Col10a1 regulatory elements, Cheah and colleagues recently demonstrated that a considerable number of hypertrophic chondrocytes that previously expressed Col10a1 do indeed go onto become osteocytes located in the trabeculae of bones (Yang et al., 2014). The parameters that dictate whether a hypertrophic chondrocyte will either undergo apoptosis or initiate the osteogenic differentation program and survive in the bone matrix are not yet understood.
Conclusions
During the past decade, excellent progress has been made in elucidating both the signaling pathways and the transcriptional regulatory networks that control the induction of chondrogenesis and the maturation of immature chondrocytes into hypertrophic chondrocytes in the growth plate. Mechanical signals (see Box 2) have also been shown to be important regulators of both chondrocyte proliferation and bone morphology. However, the details regarding how these various signals modulate either the expression and/or activity of the relevant transcription factors is only beginning to be explored. Such a detailed molecular understanding will be necessary to fully comprehend how either the initiation of chondrogenesis or growth plate expansion is regulated with such precision during vertebrate development. In addition, we anticipate that this type of knowledge could be harnessed to develop novel therapeutics that regulate the process of endochondral ossification to reverse various skeletal pathologies, including chondrodysplasias.
Box 2. The effect of mechanical signals during chondrogenesis
Studies of paralyzed chick and mouse embryos have revealed that various cartilaginous skeletal elements were shorter and bone eminences were significantly smaller (Hamburger and Waugh, 1940; Hosseini and Hogg, 1991; Rot-Nikcevic et al., 2006; Blitz et al., 2009; Nowlan et al., 2010), and that the size of the proliferative zone (and number of proliferating chondrocytes) in the growth plates of long bones is reduced relative to control embryos (Germiller and Goldstein, 1997; Roddy et al., 2011). Further support for the involvement of mechanical stimulation in chondrocyte proliferation is provided by a study in which cyclic mechanical stimulation of rabbit premaxillae accelerated the rate of chondrocyte proliferation (Wang and Mao, 2002). The mechanical regulation of chondrocyte proliferation affects not only the length of the bone but also its morphology. For example, both the ‘mini’ growth plate of bone eminences and the endochondral ossification-mediated repair of bone fractures are controlled by muscle loading (Blitz et al., 2009; Rot et al., 2014). Another way to affect growth plate activity is by regulating chondrocyte intercalation into columns. This process highly resembles convergent extension, a well-studied morphogenetic process whereby changes in cell organization affect tissue/organ shape. This process facilitates skeletal elongation and contributes to morphogenesis. Mechanical force is a key regulator of chondrocyte intercalation in zebrafish craniofacial development, whereas in mice it contributes to elongation of chondrocyte columns (Shwartz et al., 2012). The planar cell polarity pathway is also known to play an important role in directing appropriate morphogenesis of the growth plate (reviewed by Gao and Yang, 2013), and it will be interesting to elucidate how mechanical motion is integrated with this pathway.
Acknowledgements
We apologize to our colleagues whose work we could not cite due to space limitations.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
Work in the Lassar lab is supported by grants from the National Institutes of Health. Work in the Zelzer lab is supported by grants from European Research Council (ERC) and the Minerva Foundation. Deposited in PMC for release after 12 months.
References
- Ahrens P. B., Solursh M. and Reiter R. S. (1977). Stage-related capacity for limb chondrogenesis in cell culture. Dev. Biol. 60, 69-82 10.1016/0012-1606(77)90110-5 [DOI] [PubMed] [Google Scholar]
- Akiyama H., Chaboissier M.-C., Martin J. F., Schedl A. and De Crombrugghe B. (2002). The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 16, 2813-2828 10.1101/gad.1017802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H., Lyons J. P., Mori-Akiyama Y., Yang X., Zhang R., Zhang Z., Deng J. M., Taketo M. M., Nakamura T., Behringer R. R. et al. (2004). Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 18, 1072-1087 10.1101/gad.1171104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarilio R., Viukov S. V., Sharir A., Eshkar-Oren I., Johnson R. S. and Zelzer E. (2007). HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development 134, 3917-3928 10.1242/dev.008441 [DOI] [PubMed] [Google Scholar]
- Arnold M. A., Kim Y., Czubryt M. P., Phan D., McAnally J., Qi X., Shelton J. M., Richardson J. A., Bassel-Duby R. and Olson E. N. (2007). MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev. Cell 12, 377-389 10.1016/j.devcel.2007.02.004 [DOI] [PubMed] [Google Scholar]
- Bae Y. S., Kang S. W., Seo M. S., Baines I. C., Tekle E., Chock P. B. and Rhee S. G. (1997). Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 272, 217-221 10.1074/jbc.272.1.217 [DOI] [PubMed] [Google Scholar]
- Barna M. and Niswander L. (2007). Visualization of cartilage formation: insight into cellular properties of skeletal progenitors and chondrodysplasia syndromes. Dev. Cell 12, 931-941 10.1016/j.devcel.2007.04.016 [DOI] [PubMed] [Google Scholar]
- Bell D. M., Leung K. K. H., Wheatley S. C., Ng L. J., Zhou S., Ling K. W., Sham M. H., Koopman P., Tam P. P. L. and Cheah K. S. E. (1997). SOX9 directly regulates the type-II collagen gene. Nat. Genet. 16, 174-178 10.1038/ng0697-174 [DOI] [PubMed] [Google Scholar]
- Bentovim L., Amarilio R. and Zelzer E. (2012). HIF1alpha is a central regulator of collagen hydroxylation and secretion under hypoxia during bone development. Development 139, 4473-4483 10.1242/dev.083881 [DOI] [PubMed] [Google Scholar]
- Benya P. D. and Shaffer J. D. (1982). Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30, 215-224 10.1016/0092-8674(82)90027-7 [DOI] [PubMed] [Google Scholar]
- Berdeaux R., Goebel N., Banaszynski L., Takemori H., Wandless T., Shelton G. D. and Montminy M. (2007). SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nat. Med. 13, 597-603 10.1038/nm1573 [DOI] [PubMed] [Google Scholar]
- Bi W., Deng J. M., Zhang Z., Behringer R. R. and de Crombrugghe B. (1999). Sox9 is required for cartilage formation. Nat. Genet. 22, 85-89 10.1038/8792 [DOI] [PubMed] [Google Scholar]
- Bi W., Huang W., Whitworth D. J., Deng J. M., Zhang Z., Behringer R. R. and de Crombrugghe B. (2001). Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc. Natl. Acad. Sci. USA 98, 6698-6703 10.1073/pnas.111092198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz E., Viukov S., Sharir A., Shwartz Y., Galloway J. L., Price B. A., Johnson R. L., Tabin C. J., Schweitzer R. and Zelzer E. (2009). Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev. Cell 17, 861-873 10.1016/j.devcel.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz E., Sharir A., Akiyama H. and Zelzer E. (2013). Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development 140, 2680-2690 10.1242/dev.093906 [DOI] [PubMed] [Google Scholar]
- Bonaventure J., Kadhom N., Cohen-Solal L., Ng K. H., Bourguignon J., Lasselin C. and Freisinger P. (1994). Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp. Cell Res. 212, 97-104 10.1006/excr.1994.1123 [DOI] [PubMed] [Google Scholar]
- Borjesson A. E., Windahl S. H., Karimian E., Eriksson E. E., Lagerquist M. K., Engdahl C., Antal M. C., Krust A., Chambon P., Savendahl L. et al. (2012). The role of estrogen receptor-alpha and its activation function-1 for growth plate closure in female mice. Am. J. Physiol. Endocrinol. Metab. 302, E1381-E1389 10.1152/ajpendo.00646.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J. and Johnson R. L. (1998). Endogenous and ectopic expression of noggin suggests a conserved mechanism for regulation of BMP function during limb and somite patterning. Dev. Biol. 197, 205-217 10.1006/dbio.1997.8824 [DOI] [PubMed] [Google Scholar]
- Carrington J. L., Chen P., Yanagishita M. and Reddi A. H. (1991). Osteogenin (bone morphogenetic protein-3) stimulates cartilage formation by chick limb bud cells in vitro. Dev. Biol. 146, 406-415 10.1016/0012-1606(91)90242-U [DOI] [PubMed] [Google Scholar]
- Chen L., Adar R., Yang X., Monsonego E. O., Li C., Hauschka P. V., Yayon A. and Deng C.-X. (1999). Gly369Cys mutation in mouse FGFR3 causes achondroplasia by affecting both chondrogenesis and osteogenesis. J. Clin. Invest. 104, 1517-1525 10.1172/JCI6690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C., Litingtung Y., Lee E., Young K. E., Corden J. L., Westphal H. and Beachy P. A. (1996). Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, 407-413 10.1038/383407a0 [DOI] [PubMed] [Google Scholar]
- Chimal-Monroy J. and Diaz de Leon L. (1997). Differential effects of transforming growth factors beta 1, beta 2, beta 3 and beta 5 on chondrogenesis in mouse limb bud mesenchymal cells. Int. J. Dev. Biol. 41, 91-102. [PubMed] [Google Scholar]
- Chusho H., Tamura N., Ogawa Y., Yasoda A., Suda M., Miyazawa T., Nakamura K., Nakao K., Kurihara T., Komatsu Y. et al. (2001). Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc. Natl. Acad. Sci. USA 98, 4016-4021 10.1073/pnas.071389098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin J. S., Bohne B. A., Harding G. W., McEwen D. G. and Ornitz D. M. (1996). Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat. Genet. 12, 390-397 10.1038/ng0496-390 [DOI] [PubMed] [Google Scholar]
- Cooper K. L., Oh S., Sung Y., Dasari R. R., Kirschner M. W. and Tabin C. J. (2013). Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature 495, 375-378 10.1038/nature11940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa D., Hesse E., Seriwatanachai D., Kiviranta R., Saito H., Yamana K., Neff L., Atfi A., Coillard L., Sitara D. et al. (2010). Zfp521 is a target gene and key effector of parathyroid hormone-related peptide signaling in growth plate chondrocytes. Dev. Cell 19, 533-546 10.1016/j.devcel.2010.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoud G., Kempf H., Kumar D., Kozhemyakina E., Holowacz T., Kim D.-W., Ionescu A. and Lassar A. B. (2014). BMP-mediated induction of GATA4/5/6 blocks somitic responsiveness to SHH. Development 141, 3978-3987 10.1242/dev.111906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T. F., Guo X., Garrett-Beal L. and Yang Y. (2005). Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell 8, 739-750 10.1016/j.devcel.2005.03.016 [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B., Lefebvre V. and Nakashima K. (2001). Regulatory mechanisms in the pathways of cartilage and bone formation. Curr. Opin. Cell Biol. 13, 721-728 10.1016/S0955-0674(00)00276-3 [DOI] [PubMed] [Google Scholar]
- de Frutos C. A., Vega S., Manzanares M., Flores J. M., Huertas H., Martínez-Frías M. L. and Nieto M. A. (2007). Snail1 is a transcriptional effector of FGFR3 signaling during chondrogenesis and achondroplasias. Dev. Cell 13, 872-883 10.1016/j.devcel.2007.09.016 [DOI] [PubMed] [Google Scholar]
- Degnin C. R., Laederich M. B. and Horton W. A. (2010). FGFs in endochondral skeletal development. J. Cell Biochem. 110, 1046-1057 10.1002/jcb.22629 [DOI] [PubMed] [Google Scholar]
- Deng C., Wynshaw-Boris A., Zhou F., Kuo A. and Leder P. (1996). Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 84, 911-921 10.1016/S0092-8674(00)81069-7 [DOI] [PubMed] [Google Scholar]
- Dong Y.-F., Soung D. Y., Schwarz E. M., O'Keefe R. J. and Drissi H. (2006). Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J. Cell Physiol. 208, 77-86 10.1002/jcp.20656 [DOI] [PubMed] [Google Scholar]
- Drissi M. H., Li X., Sheu T. J., Zuscik M. J., Schwarz E. M., Puzas J. E., Rosier R. N. and O'Keefe R. J. (2003). Runx2/Cbfa1 stimulation by retinoic acid is potentiated by BMP2 signaling through interaction with Smad1 on the collagen X promoter in chondrocytes. J. Cell Biochem. 90, 1287-1298 10.1002/jcb.10677 [DOI] [PubMed] [Google Scholar]
- Dudley A. T., Ros M. A. and Tabin C. J. (2002). A re-examination of proximodistal patterning during vertebrate limb development. Nature 418, 539-544 10.1038/nature00945 [DOI] [PubMed] [Google Scholar]
- Duprez D., Bell E. J. d. H., Richardson M. K., Archer C. W., Wolpert L., Brickell P. M. and Francis-West P. H. (1996). Overexpression of BMP-2 and BMP-4 alters the size and shape of developing skeletal elements in the chick limb. Mech. Dev. 57, 145-157 10.1016/0925-4773(96)00540-0 [DOI] [PubMed] [Google Scholar]
- Dy P., Wang W., Bhattaram P., Wang Q., Wang L., Ballock R. T. and Lefebvre V. (2012). Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev. Cell 22, 597-609 10.1016/j.devcel.2011.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto H., Enomoto-Iwamoto M., Iwamoto M., Nomura S., Himeno M., Kitamura Y., Kishimoto T. and Komori T. (2000). Cbfa1 is a positive regulatory factor in chondrocyte maturation. J. Biol. Chem. 275, 8695-8702 10.1074/jbc.275.12.8695 [DOI] [PubMed] [Google Scholar]
- Enomoto-Iwamoto M., Kitagaki J., Koyama E., Tamamura Y., Wu C., Kanatani N., Koike T., Okada H., Komori T., Yoneda T. et al. (2002). The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev. Biol. 251, 142-156 10.1006/dbio.2002.0802 [DOI] [PubMed] [Google Scholar]
- Fallon J. F., Lopez A., Ros M. A., Savage M. P., Olwin B. B. and Simandl B. K. (1994). FGF-2: apical ectodermal ridge growth signal for chick limb development. Science 264, 104-107 10.1126/science.7908145 [DOI] [PubMed] [Google Scholar]
- Fan C., Katsuyama M., Nishinaka T. and Yabe-Nishimura C. (2005). Transactivation of the EGF receptor and a PI3 kinase-ATF-1 pathway is involved in the upregulation of NOX1, a catalytic subunit of NADPH oxidase. FEBS Lett. 579, 1301-1305 10.1016/j.febslet.2005.01.021 [DOI] [PubMed] [Google Scholar]
- Fisher M. C., Clinton G. M., Maihle N. J. and Dealy C. N. (2007). Requirement for ErbB2/ErbB signaling in developing cartilage and bone. Dev. Growth Differ. 49, 503-513 10.1111/j.1440-169X.2007.00941.x [DOI] [PubMed] [Google Scholar]
- Foldynova-Trantirkova S., Wilcox W. R. and Krejci P. (2012). Sixteen years and counting: the current understanding of fibroblast growth factor receptor 3 (FGFR3) signaling in skeletal dysplasias. Hum. Mutat. 33, 29-41 10.1002/humu.21636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumatsu T., Tsuda M., Taniguchi N., Tajima Y. and Asahara H. (2005). Smad3 induces chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment. J. Biol. Chem. 280, 8343-8350 10.1074/jbc.M413913200 [DOI] [PubMed] [Google Scholar]
- Gao B. and Yang Y. (2013). Planar cell polarity in vertebrate limb morphogenesis. Curr. Opin. Genet. Dev. 23, 438-444 10.1016/j.gde.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germiller J. A. and Goldstein S. A. (1997). Structure and function of embryonic growth plate in the absence of functioning skeletal muscle. J. Orthop. Res. 15, 362-370 10.1002/jor.1100150308 [DOI] [PubMed] [Google Scholar]
- Gibson G. (1998). Active role of chondrocyte apoptosis in endochondral ossification. Microsc. Res. Tech. 43, 191-204 [DOI] [PubMed] [Google Scholar]
- Grozinger C. M. and Schreiber S. L. (2000). Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc. Natl. Acad. Sci. USA 97, 7835-7840 10.1073/pnas.140199597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Chung U.-I., Kondo H., Bringhurst F. R. and Kronenberg H. M. (2002). The PTH/PTHrP receptor can delay chondrocyte hypertrophy in vivo without activating phospholipase C. Dev. Cell 3, 183-194 10.1016/S1534-5807(02)00218-6 [DOI] [PubMed] [Google Scholar]
- Hall B. K. and Miyake T. (1992). The membranous skeleton: the role of cell condensations in vertebrate skeletogenesis. Anat. Embryol. (Berl.) 186, 107-124 10.1007/BF00174948 [DOI] [PubMed] [Google Scholar]
- Hamburger V. and Waugh M. (1940). The primary development of the skeleton in nerveless and poorly innervated limb transplants of chick embryos. Phsiol. Zool. 13, 367-384. [Google Scholar]
- Hanai J.-i., Chen L. F., Kanno T., Ohtani-Fujita N., Kim W. Y., Guo W.-H., Imamura T., Ishidou Y., Fukuchi M., Shi M.-J. et al. (1999). Interaction and functional cooperation of PEBP2/CBF with Smads: synergistic induction of the immunoglobulin germline Calpha promoter. J. Biol. Chem. 274, 31577-31582 10.1074/jbc.274.44.31577 [DOI] [PubMed] [Google Scholar]
- Hartmann C. and Tabin C. J. (2000). Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development 127, 3141-3159. [DOI] [PubMed] [Google Scholar]
- Hattori T., Muller C., Gebhard S., Bauer E., Pausch F., Schlund B., Bosl M. R., Hess A., Surmann-Schmitt C., von der Mark H. et al. (2010). SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development 137, 901-911 10.1242/dev.045203 [DOI] [PubMed] [Google Scholar]
- Hill T. P., Spater D., Taketo M. M., Birchmeier W. and Hartmann C. (2005). Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev. Cell 8, 727-738 10.1016/j.devcel.2005.02.013 [DOI] [PubMed] [Google Scholar]
- Hilton M. J., Tu X., Cook J., Hu H. and Long F. (2005). Ihh controls cartilage development by antagonizing Gli3, but requires additional effectors to regulate osteoblast and vascular development. Development 132, 4339-4351 10.1242/dev.02025 [DOI] [PubMed] [Google Scholar]
- Hosseini A. and Hogg D. A. (1991). The effects of paralysis on skeletal development in the chick embryo. II. Effects on histogenesis of the tibia. J. Anat. 177, 169-178. [PMC free article] [PubMed] [Google Scholar]
- Huang W., Zhou X., Lefebvre V. and de Crombrugghe B. (2000). Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase A enhances SOX9's ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol. Cell. Biol. 20, 4149-4158 10.1128/MCB.20.11.4149-4158.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I. H., Yu K., Lavine K. J. and Ornitz D. M. (2007). FGF9 regulates early hypertrophic chondrocyte differentiation and skeletal vascularization in the developing stylopod. Dev. Biol. 307, 300-313 10.1016/j.ydbio.2007.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami D., Akiyama H., Suzuki A., Nakamura T., Nakano T., Yoshikawa H. and Tsumaki N. (2011). Sox9 sustains chondrocyte survival and hypertrophy in part through Pik3ca-Akt pathways. Development 138, 1507-1519 10.1242/dev.057802 [DOI] [PubMed] [Google Scholar]
- Inada M., Yasui T., Nomura S., Miyake S., Deguchi K., Himeno M., Sato M., Yamagiwa H., Kimura T., Yasui N. et al. (1999). Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev. Dyn. 214, 279-290 [DOI] [PubMed] [Google Scholar]
- Inada M., Wang Y., Byrne M. H., Rahman M. U., Miyaura C., Lopez-Otin C. and Krane S. M. (2004). Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc. Natl. Acad. Sci. USA 101, 17192-17197 10.1073/pnas.0407788101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu A., Kozhemyakina E., Nicolae C., Kaestner K. H., Olsen B. R. and Lassar A. B. (2012). FoxA family members are crucial regulators of the hypertrophic chondrocyte differentiation program. Dev. Cell 22, 927-939 10.1016/j.devcel.2012.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata T., Chen L., Li C.-L., Ovchinnikov D. A., Behringer R. R., Francomano C. A. and Deng C.-X. (2000). A neonatal lethal mutation in FGFR3 uncouples proliferation and differentiation of growth plate chondrocytes in embryos. Hum. Mol. Genet. 9, 1603-1613 10.1093/hmg/9.11.1603 [DOI] [PubMed] [Google Scholar]
- Iwata T., Li C.-L., Deng C.-X. and Francomano C. A. (2001). Highly activated Fgfr3 with the K644M mutation causes prolonged survival in severe dwarf mice. Hum. Mol. Genet. 10, 1255-1264 10.1093/hmg/10.12.1255 [DOI] [PubMed] [Google Scholar]
- Jacob A. L., Smith C., Partanen J. and Ornitz D. M. (2006). Fibroblast growth factor receptor 1 signaling in the osteo-chondrogenic cell lineage regulates sequential steps of osteoblast maturation. Dev. Biol. 296, 315-328 10.1016/j.ydbio.2006.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed A., Bae J.-S., Afzal F., Gutierrez S., Pratap J., Zaidi S. K., Lou Y., van Wijnen A. J., Stein J. L., Stein G. S. et al. (2008). Structural coupling of Smad and Runx2 for execution of the BMP2 osteogenic signal. J. Biol. Chem. 283, 8412-8422 10.1074/jbc.M705578200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner K. H., Hiemisch H. and Schutz G. (1998). Targeted disruption of the gene encoding hepatocyte nuclear factor 3gamma results in reduced transcription of hepatocyte-specific genes. Mol. Cell. Biol. 18, 4245-4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamboulas K., Dranse H. J. and Underhill T. M. (2010). Regulation of BMP-dependent chondrogenesis in early limb mesenchyme by TGFbeta signals. J. Cell Sci. 123, 2068-2076 10.1242/jcs.062901 [DOI] [PubMed] [Google Scholar]
- Karaplis A. C., Luz A., Glowacki J., Bronson R. T., Tybulewicz V. L., Kronenberg H. M. and Mulligan R. C. (1994). Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 8, 277-289 10.1101/gad.8.3.277 [DOI] [PubMed] [Google Scholar]
- Karp S. J., Schipani E., St-Jacques B., Hunzelman J., Kronenberg H. and McMahon A. P. (2000). Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Development 127, 543-548. [DOI] [PubMed] [Google Scholar]
- Kato N. and Aoyama H. (1998). Dermomyotomal origin of the ribs as revealed by extirpation and transplantation experiments in chick and quail embryos. Development 125, 3437-3443. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Ishikawa T., Shimabara M., Tanda N., Enomoto-Iwamoto M., Iwamoto M., Kuwana T., Ueki A., Noji S. and Nohno T. (1996). BMP signaling during bone pattern determination in the developing limb. Development 122, 3557-3566. [DOI] [PubMed] [Google Scholar]
- Kempf H., Ionescu A., Udager A. M. and Lassar A. B. (2007). Prochondrogenic signals induce a competence for Runx2 to activate hypertrophic chondrocyte gene expression. Dev. Dyn. 236, 1954-1962 10.1002/dvdy.21205 [DOI] [PubMed] [Google Scholar]
- Kim D.-W. and Lassar A. B. (2003). Smad-dependent recruitment of a histone deacetylase/Sin3A complex modulates the bone morphogenetic protein-dependent transcriptional repressor activity of Nkx3.2. Mol. Cell. Biol. 23, 8704-8717 10.1128/MCB.23.23.8704-8717.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I. S., Otto F., Zabel B. and Mundlos S. (1999). Regulation of chondrocyte differentiation by Cbfa1. Mech. Dev. 80, 159-170 10.1016/S0925-4773(98)00210-X [DOI] [PubMed] [Google Scholar]
- Kishimoto I., Tokudome T., Nakao K. and Kangawa K. (2011). Natriuretic peptide system: an overview of studies using genetically engineered animal models. FEBS J. 278, 1830-1841 10.1111/j.1742-4658.2011.08116.x [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Soegiarto D. W., Yang Y., Lanske B., Schipani E., McMahon A. P. and Kronenberg H. M. (2005). Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J. Clin. Invest. 115, 1734-1742 10.1172/JCI24397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhemyakina E., Cohen T., Yao T.-P. and Lassar A. B. (2009). Parathyroid hormone-related peptide represses chondrocyte hypertrophy through a protein phosphatase 2A/histone deacetylase 4/MEF2 pathway. Mol. Cell. Biol. 29, 5751-5762 10.1128/MCB.00415-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziel L., Wuelling M., Schneider S. and Vortkamp A. (2005). Gli3 acts as a repressor downstream of Ihh in regulating two distinct steps of chondrocyte differentiation. Development 132, 5249-5260 10.1242/dev.02097 [DOI] [PubMed] [Google Scholar]
- Krejci P., Masri B., Fontaine V., Mekikian P. B., Weis M., Prats H. and Wilcox W. R. (2005). Interaction of fibroblast growth factor and C-natriuretic peptide signaling in regulation of chondrocyte proliferation and extracellular matrix homeostasis. J. Cell Sci. 118, 5089-5100 10.1242/jcs.02618 [DOI] [PubMed] [Google Scholar]
- Krejci P., Salazar L., Goodridge H. S., Kashiwada T. A., Schibler M. J., Jelinkova P., Thompson L. M. and Wilcox W. R. (2008). STAT1 and STAT3 do not participate in FGF-mediated growth arrest in chondrocytes. J. Cell Sci. 121, 272-281 10.1242/jcs.017160 [DOI] [PubMed] [Google Scholar]
- Kumar D. and Lassar A. B. (2009). The transcriptional activity of Sox9 in chondrocytes is regulated by RhoA signaling and actin polymerization. Mol. Cell. Biol. 29, 4262-4273 10.1128/MCB.01779-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D. and Lassar A. B. (2014). Fibroblast growth factor maintains chondrogenic potential of limb bud mesenchymal cells by modulating DNMT3A recruitment. Cell Rep. 8, 1419-1431 10.1016/j.celrep.2014.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanske B., Karaplis A. C., Lee K., Luz A., Vortkamp A., Pirro A., Karperien M., Defize L. H. K., Ho C., Mulligan R. C. et al. (1996). PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science 273, 663-666 10.1126/science.273.5275.663 [DOI] [PubMed] [Google Scholar]
- Lee Y.-S. and Chuong C.-M. (1997). Activation of protein kinase A is a pivotal step involved in both BMP-2- and cyclic AMP-induced chondrogenesis. J. Cell Physiol. 170, 153-165 [DOI] [PubMed] [Google Scholar]
- Lee K., Lanske B., Karaplis A. C., Deeds J. D., Kohno H., Nissenson R. A., Kronenberg H. M. and Segre G. V. (1996). Parathyroid hormone-related peptide delays terminal differentiation of chondrocytes during endochondral bone development. Endocrinology 137, 5109-5118. [DOI] [PubMed] [Google Scholar]
- Lefebvre V. (2002). Toward understanding the functions of the two highly related Sox5 and Sox6 genes. J. Bone Miner. Metab. 20, 121-130 10.1007/s007740200017 [DOI] [PubMed] [Google Scholar]
- Lefebvre V., Huang W., Harley V. R., Goodfellow P. N. and de Crombrugghe B. (1997). SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol. Cell. Biol. 17, 2336-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V., Li P. and de Crombrugghe B. (1998). A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 17, 5718-5733 10.1093/emboj/17.19.5718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard C. M., Fuld H. M., Frenz D. A., Downie S. A., Massague J. and Newman S. A. (1991). Role of transforming growth factor-beta in chondrogenic pattern formation in the embryonic limb: stimulation of mesenchymal condensation and fibronectin gene expression by exogenenous TGF-beta and evidence for endogenous TGF-beta-like activity. Dev. Biol. 145, 99-109 10.1016/0012-1606(91)90216-P [DOI] [PubMed] [Google Scholar]
- Leung V. Y. L., Gao B., Leung K. K. H., Melhado I. G., Wynn S. L., Au T. Y. K., Dung N. W. F., Lau J. Y. B., Mak A. C. Y., Chan D. et al. (2011). SOX9 governs differentiation stage-specific gene expression in growth plate chondrocytes via direct concomitant transactivation and repression. PLoS Genet. 7, e1002356 10.1371/journal.pgen.1002356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D. and Dorfman A. (1972). The irreversible inhibition of differentiation of limb-bud mesenchyme by bromodeoxyuridine. Proc. Natl. Acad. Sci. USA 69, 1253-1257 10.1073/pnas.69.5.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Deng W., Nail C. D., Bailey S. K., Kraus M. H., Ruppert J. M. and Lobo-Ruppert S. M. (2006). Snail induction is an early response to Gli1 that determines the efficiency of epithelial transformation. Oncogene 25, 609-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. P., Baker J., Perkins A. S., Robertson E. J. and Efstratiadis A. (1993). Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75, 59-72. [PubMed] [Google Scholar]
- Liu Z., Xu J., Colvin J. S. and Ornitz D. M. (2002). Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 16, 859-869 10.1101/gad.965602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Lavine K. J., Hung I. H. and Ornitz D. M. (2007). FGF18 is required for early chondrocyte proliferation, hypertrophy and vascular invasion of the growth plate. Dev. Biol. 302, 80-91 10.1016/j.ydbio.2006.08.071 [DOI] [PubMed] [Google Scholar]
- Long F., Zhang X. M., Karp S., Yang Y. and McMahon A. P. (2001). Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development 128, 5099-5108. [DOI] [PubMed] [Google Scholar]
- Lorget F., Kaci N., Peng J., Benoist-Lasselin C., Mugniery E., Oppeneer T., Wendt D. J., Bell S. M., Bullens S., Bunting S. et al. (2012). Evaluation of the therapeutic potential of a CNP analog in a Fgfr3 mouse model recapitulating achondroplasia. Am. J. Hum. Genet. 91, 1108-1114 10.1016/j.ajhg.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C., Kobayashi T., Selig M. K., Torrekens S., Roth S. I., Mackem S., Carmeliet G. and Kronenberg H. M. (2010). Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 19, 329-344 10.1016/j.devcel.2010.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T., Chan Y. Y., Kawanami A., Balmes G., Landreth G. E. and Murakami S. (2009). Extracellular signal-regulated kinase 1 (ERK1) and ERK2 play essential roles in osteoblast differentiation and in supporting osteoclastogenesis. Mol. Cell. Biol. 29, 5843-5857 10.1128/MCB.01549-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey T. A., Zhang C. L. and Olson E. N. (2000a). Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc. Natl. Acad. Sci. USA 97, 14400-14405 10.1073/pnas.260501497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey T. A., Zhang C.-L., Lu J. and Olson E. N. (2000b). Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408, 106-111 10.1038/35040593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead T. J., Wang Q., Bhattaram P., Dy P., Afelik S., Jensen J. and Lefebvre V. (2013). A far-upstream (-70 kb) enhancer mediates Sox9 auto-regulation in somatic tissues during development and adult regeneration. Nucleic Acids Res. 41, 4459-4469 10.1093/nar/gkt140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino R., Gañan Y., Macias D., Economides A. N., Sampath K. T. and Hurle J. M. (1998). Morphogenesis of digits in the avian limb is controlled by FGFs, TGFbetas, and noggin through BMP signaling. Dev. Biol. (Orlando) 200, 35-45 10.1006/dbio.1998.8946 [DOI] [PubMed] [Google Scholar]
- Minina E., Kreschel C., Naski M. C., Ornitz D. M. and Vortkamp A. (2002). Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev. Cell 3, 439-449 10.1016/S1534-5807(02)00261-7 [DOI] [PubMed] [Google Scholar]
- Minina E., Schneider S., Rosowski M., Lauster R. and Vortkamp A. (2005). Expression of Fgf and Tgfbeta signaling related genes during embryonic endochondral ossification. Gene Expr. Patterns 6, 102-109 10.1016/j.modgep.2005.04.012 [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq A.-H. (2005). Sclerotome development and morphogenesis: when experimental embryology meets genetics. Int. J. Dev. Biol. 49, 301-308 10.1387/ijdb.041953am [DOI] [PubMed] [Google Scholar]
- Morita K., Miyamoto T., Fujita N., Kubota Y., Ito K., Takubo K., Miyamoto K., Ninomiya K., Suzuki T., Iwasaki R. et al. (2007). Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J. Exp. Med. 204, 1613-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S., Kan M., McKeehan W. L. and de Crombrugghe B. (2000). Up-regulation of the chondrogenic Sox9 gene by fibroblast growth factors is mediated by the mitogen-activated protein kinase pathway. Proc. Natl. Acad. Sci. USA 97, 1113-1118 10.1073/pnas.97.3.1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S., Balmes G., McKinney S., Zhang Z., Givol D. and de Crombrugghe B. (2004). Constitutive activation of MEK1 in chondrocytes causes Stat1-independent achondroplasia-like dwarfism and rescues the Fgfr3-deficient mouse phenotype. Genes Dev. 18, 290-305 10.1101/gad.1179104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh L. C., Chyung J. H. and Lassar A. B. (1999). Sonic hedgehog promotes somitic chondrogenesis by altering the cellular response to BMP signaling. Genes Dev. 13, 225-237 10.1101/gad.13.2.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh L. C., Zeng L., Chyung J. H. and Lassar A. B. (2001). The chick transcriptional repressor Nkx3.2 acts downstream of Shh to promote BMP-dependent axial chondrogenesis. Dev. Cell 1, 411-422 10.1016/S1534-5807(01)00039-9 [DOI] [PubMed] [Google Scholar]
- Nakao K., Ogawa Y., Suga S.-i. and Imura H. (1992). Molecular biology and biochemistry of the natriuretic peptide system. II: natriuretic peptide receptors. J. Hypertens. 10, 1111-1114 10.1097/00004872-199210000-00002 [DOI] [PubMed] [Google Scholar]
- Naski M. C., Colvin J. S., Coffin J. D. and Ornitz D. M. (1998). Repression of hedgehog signaling and BMP4 expression in growth plate cartilage by fibroblast growth factor receptor 3. Development 125, 4977-4988. [DOI] [PubMed] [Google Scholar]
- Ng L.-J., Wheatley S., Muscat G. E. O., Conway-Campbell J., Bowles J., Wright E., Bell D. M., Tam P. P. L., Cheah K. S. E. and Koopman P. (1997). SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev. Biol. 183, 108-121 10.1006/dbio.1996.8487 [DOI] [PubMed] [Google Scholar]
- Niswander L., Tickle C., Vogel A., Booth I. and Martin G. R. (1993). FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. Cell 75, 579-587 10.1016/0092-8674(93)90391-3 [DOI] [PubMed] [Google Scholar]
- Nordin K. and LaBonne C. (2014). Sox5 is a DNA-binding cofactor for BMP R-smads that directs target specificity during patterning of the early ectoderm. Dev. Cell 31, 374-382 10.1016/j.devcel.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowlan N. C., Bourdon C., Dumas G., Tajbakhsh S., Prendergast P. J. and Murphy P. (2010). Developing bones are differentially affected by compromised skeletal muscle formation. Bone 46, 1275-1285 10.1016/j.bone.2009.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D. M. and Marie P. J. (2002). FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 16, 1446-1465 10.1101/gad.990702 [DOI] [PubMed] [Google Scholar]
- Osdoby P. and Caplan A. I. (1979). Osteogenesis in cultures of limb mesenchymal cells. Dev. Biol. 73, 84-102 10.1016/0012-1606(79)90140-4 [DOI] [PubMed] [Google Scholar]
- Ozasa A., Komatsu Y., Yasoda A., Miura M., Sakuma Y., Nakatsuru Y., Arai H., Itoh N. and Nakao K. (2005). Complementary antagonistic actions between C-type natriuretic peptide and the MAPK pathway through FGFR-3 in ATDC5 cells. Bone 36, 1056-1064 10.1016/j.bone.2005.03.006 [DOI] [PubMed] [Google Scholar]
- Peters K., Ornitz D., Werner S. and Williams L. (1993). Unique expression pattern of the FGF receptor 3 gene during mouse organogenesis. Dev. Biol. 155, 423-430 10.1006/dbio.1993.1040 [DOI] [PubMed] [Google Scholar]
- Pfander D., Cramer T., Schipani E. and Johnson R. S. (2003). HIF-1alpha controls extracellular matrix synthesis by epiphyseal chondrocytes. J. Cell Sci. 116, 1819-1826 10.1242/jcs.00385 [DOI] [PubMed] [Google Scholar]
- Powell-Braxton L., Hollingshead P., Warburton C., Dowd M., Pitts-Meek S., Dalton D., Gillett N. and Stewart T. A. (1993). IGF-I is required for normal embryonic growth in mice. Genes Dev. 7, 2609-2617 10.1101/gad.7.12b.2609 [DOI] [PubMed] [Google Scholar]
- Provot S., Kempf H., Murtaugh L. C., Chung U.-i., Kim D.-W., Chyung J., Kronenberg H. M. and Lassar A. B. (2006). Nkx3.2/Bapx1 acts as a negative regulator of chondrocyte maturation. Development 133, 651-662 10.1242/dev.02258 [DOI] [PubMed] [Google Scholar]
- Reginato A. M., Iozzo R. V. and Jimenez S. A. (1994). Formation of nodular structures resembling mature articular cartilage in long-term primary cultures of human fetal epiphyseal chondrocytes on a hydrogel substrate. Arthritis Rheum. 37, 1338-1349 10.1002/art.1780370912 [DOI] [PubMed] [Google Scholar]
- Ren P., Rowland G. N. III and Halper J. (1997). Expression of growth factors in chicken growth plate with special reference to tibial dyschondroplasia. J. Comp. Pathol. 116, 303-320 10.1016/S0021-9975(97)80005-9 [DOI] [PubMed] [Google Scholar]
- Retting K. N., Song B., Yoon B. S. and Lyons K. M. (2009). BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development 136, 1093-1104 10.1242/dev.029926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach H. I., Erenpreisa J. and Aigner T. (1995). Osteogenic differentiation of hypertrophic chondrocytes involves asymmetric cell divisions and apoptosis. J. Cell Biol. 131, 483-494 10.1083/jcb.131.2.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roark E. F. and Greer K. (1994). Transforming growth factor-beta and bone morphogenetic protein-2 act by distinct mechanisms to promote chick limb cartilage differentiation in vitro. Dev. Dyn. 200, 103-116 10.1002/aja.1002000203 [DOI] [PubMed] [Google Scholar]
- Roddy K. A., Prendergast P. J. and Murphy P. (2011). Mechanical influences on morphogenesis of the knee joint revealed through morphological, molecular and computational analysis of immobilised embryos. PLoS ONE 6, e17526 10.1371/journal.pone.0017526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rot C., Stern T., Blecher R., Friesem B. and Zelzer E. (2014). A mechanical jack-like mechanism drives spontaneous fracture healing in neonatal mice. Dev. Cell 31, 159-170 10.1016/j.devcel.2014.08.026 [DOI] [PubMed] [Google Scholar]
- Rot-Nikcevic I., Reddy T., Downing K. J., Belliveau A. C., Hallgrímsson B., Hall B. K. and Kablar B. (2006). Myf5-/- :MyoD-/- amyogenic fetuses reveal the importance of early contraction and static loading by striated muscle in mouse skeletogenesis. Dev. Genes Evol. 216, 1-9 10.1007/s00427-005-0024-9 [DOI] [PubMed] [Google Scholar]
- Rudnicki J. A. and Brown A. M. C. (1997). Inhibition of chondrogenesis by Wnt gene expression in vivo and in vitro. Dev. Biol. 185, 104-118 10.1006/dbio.1997.8536 [DOI] [PubMed] [Google Scholar]
- Sahni M., Ambrosetti D.-C., Mansukhani A., Gertner R., Levy D. and Basilico C. (1999). FGF signaling inhibits chondrocyte proliferation and regulates bone development through the STAT-1 pathway. Genes Dev. 13, 1361-1366 10.1101/gad.13.11.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni M., Raz R., Coffin J. D., Levy D. and Basilico C. (2001). STAT1 mediates the increased apoptosis and reduced chondrocyte proliferation in mice overexpressing FGF2. Development 128, 2119-2129. [DOI] [PubMed] [Google Scholar]
- Sasagawa S., Takemori H., Uebi T., Ikegami D., Hiramatsu K., Ikegawa S., Yoshikawa H. and Tsumaki N. (2012). SIK3 is essential for chondrocyte hypertrophy during skeletal development in mice. Development 139, 1153-1163 10.1242/dev.072652 [DOI] [PubMed] [Google Scholar]
- Schipani E., Ryan H. E., Didrickson S., Kobayashi T., Knight M. and Johnson R. S. (2001). Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 15, 2865-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev O., Chumakov I., Nevo Z., Givol D., Madar-Shapiro L., Sheinin Y., Weinreb M. and Yayon A. (2000). Restrained chondrocyte proliferation and maturation with abnormal growth plate vascularization and ossification in human FGFR-3(G380R) transgenic mice. Hum. Mol. Genet. 9, 249-258 10.1093/hmg/9.2.249 [DOI] [PubMed] [Google Scholar]
- Selvamurugan N., Pulumati M. R., Tyson D. R. and Partridge N. C. (2000). Parathyroid hormone regulation of the rat collagenase-3 promoter by protein kinase A-dependent transactivation of core binding factor alpha1. J. Biol. Chem. 275, 5037-5042 10.1074/jbc.275.7.5037 [DOI] [PubMed] [Google Scholar]
- Seo H.-S. and Serra R. (2007). Deletion of Tgfbr2 in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints. Dev. Biol. 310, 304-316 10.1016/j.ydbio.2007.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro I. M., Adams C. S., Freeman T. and Srinivas V. (2005). Fate of the hypertrophic chondrocyte: microenvironmental perspectives on apoptosis and survival in the epiphyseal growth plate. Birth Defects Res. C Embryo Today 75, 330-339 10.1002/bdrc.20057 [DOI] [PubMed] [Google Scholar]
- Shen W., Scearce L. M., Brestelli J. E., Sund N. J. and Kaestner K. H. (2001). Foxa3 (hepatocyte nuclear factor 3gamma) is required for the regulation of hepatic GLUT2 expression and the maintenance of glucose homeostasis during a prolonged fast. J. Biol. Chem. 276, 42812-42817 10.1074/jbc.M106344200 [DOI] [PubMed] [Google Scholar]
- Shwartz Y., Farkas Z., Stern T., Aszódi A. and Zelzer E. (2012). Muscle contraction controls skeletal morphogenesis through regulation of chondrocyte convergent extension. Dev. Biol. 370, 154-163 10.1016/j.ydbio.2012.07.026 [DOI] [PubMed] [Google Scholar]
- Smits P., Li P., Mandel J., Zhang Z., Deng J. M., Behringer R. R., de Crombrugghe B. and Lefebvre V. (2001). The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev. Cell 1, 277-290 10.1016/S1534-5807(01)00003-X [DOI] [PubMed] [Google Scholar]
- Solursh M. (1984). Ectoderm as a determinant of early tissue pattern in the limb bud. Cell Differ. 15, 17-24 10.1016/0045-6039(84)90025-3 [DOI] [PubMed] [Google Scholar]
- Solursh M. and Reiter R. S. (1975). Determination of limb bud chondrocytes during a transient block of the cell cycle. Cell Differ. 4, 131-137 10.1016/0045-6039(75)90034-2 [DOI] [PubMed] [Google Scholar]
- Solursh M., Linsenmayer T. F. and Jensen K. L. (1982). Chondrogenesis from single limb mesenchyme cells. Dev. Biol. 94, 259-264 10.1016/0012-1606(82)90090-2 [DOI] [PubMed] [Google Scholar]
- Spagnoli A., O'Rear L., Chandler R. L., Granero-Molto F., Mortlock D. P., Gorska A. E., Weis J. A., Longobardi L., Chytil A., Shimer K. et al. (2007). TGF-beta signaling is essential for joint morphogenesis. J. Cell Biol. 177, 1105-1117 10.1083/jcb.200611031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Später D., Hill T. P., O'Sullivan R. J., Gruber M., Conner D. A. and Hartmann C. (2006). Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development 133, 3039-3049 10.1242/dev.02471 [DOI] [PubMed] [Google Scholar]
- St-Jacques B., Hammerschmidt M. and McMahon A. P. (1999). Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 13, 2072-2086 10.1101/gad.13.16.2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D. A., Brunet L. J., Khokha M. K., Economides A. N. and Harland R. M. (2011). Cooperative activity of noggin and gremlin 1 in axial skeleton development. Development 138, 1005-1014 10.1242/dev.051938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickens D., Behonick D. J., Ortega N., Heyer B., Hartenstein B., Yu Y., Fosang A. J., Schorpp-Kistner M., Angel P. and Werb Z. (2004). Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development 131, 5883-5895 10.1242/dev.01461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker S., Fundele R., Vortkamp A. and Mundlos S. (2002). Role of Runx genes in chondrocyte differentiation. Dev. Biol. 245, 95-108 10.1006/dbio.2002.0640 [DOI] [PubMed] [Google Scholar]
- Sugimoto Y., Takimoto A., Akiyama H., Kist R., Scherer G., Nakamura T., Hiraki Y. and Shukunami C. (2013). Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development 140, 2280-2288 10.1242/dev.096354 [DOI] [PubMed] [Google Scholar]
- Sun X., Mariani F. V. and Martin G. R. (2002). Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature 418, 501-508 10.1038/nature00902 [DOI] [PubMed] [Google Scholar]
- Takeda S., Bonnamy J.-P., Owen M. J., Ducy P. and Karsenty G. (2001). Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev. 15, 467-481 10.1101/gad.845101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamura Y., Otani T., Kanatani N., Koyama E., Kitagaki J., Komori T., Yamada Y., Costantini F., Wakisaka S., Pacifici M. et al. (2005). Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J. Biol. Chem. 280, 19185-19195 10.1074/jbc.M414275200 [DOI] [PubMed] [Google Scholar]
- Tamura N., Doolittle L. K., Hammer R. E., Shelton J. M., Richardson J. A. and Garbers D. L. (2004). Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc. Natl. Acad. Sci. USA 101, 17300-17305 10.1073/pnas.0407894101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teillet M., Watanabe Y., Jeffs P., Duprez D., Lapointe F. and Le Douarin N. M. (1998). Sonic hedgehog is required for survival of both myogenic and chondrogenic somitic lineages. Development 125, 2019-2030. [DOI] [PubMed] [Google Scholar]
- ten Berge D., Brugmann S. A., Helms J. A. and Nusse R. (2008). Wnt and FGF signals interact to coordinate growth with cell fate specification during limb development. Development 135, 3247-3257 10.1242/dev.023176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol L., Chen W., Song H., Day T. F. and Yang Y. (2009). Sox9 inhibits Wnt signaling by promoting beta-catenin phosphorylation in the nucleus. J. Biol. Chem. 284, 3323-3333 10.1074/jbc.M808048200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T. and Kunieda T. (2005). A loss-of-function mutation in natriuretic peptide receptor 2 (Npr2) gene is responsible for disproportionate dwarfism in cn/cn mouse. J. Biol. Chem. 280, 14288-14292 10.1074/jbc.C500024200 [DOI] [PubMed] [Google Scholar]
- Ueta C., Iwamoto M., Kanatani N., Yoshida C., Liu Y., Enomoto-Iwamoto M., Ohmori T., Enomoto H., Nakata K., Takada K. et al. (2001). Skeletal malformations caused by overexpression of Cbfa1 or its dominant negative form in chondrocytes. J. Cell Biol. 153, 87-100 10.1083/jcb.153.1.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usmani S. E., Pest M. A., Kim G., Ohora S. N., Qin L. and Beier F. (2012). Transforming growth factor alpha controls the transition from hypertrophic cartilage to bone during endochondral bone growth. Bone 51, 131-141 10.1016/j.bone.2012.04.012 [DOI] [PubMed] [Google Scholar]
- Vega R. B., Matsuda K., Oh J., Barbosa A. C., Yang X., Meadows E., McAnally J., Pomajzl C., Shelton J. M., Richardson J. A. et al. (2004). Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell 119, 555-566 10.1016/j.cell.2004.10.024 [DOI] [PubMed] [Google Scholar]
- Vortkamp A., Lee K., Lanske B., Segre G. V., Kronenberg H. M. and Tabin C. J. (1996). Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science 273, 613-622 10.1126/science.273.5275.613 [DOI] [PubMed] [Google Scholar]
- Wang X. and Mao J. J. (2002). Chondrocyte proliferation of the cranial base cartilage upon in vivo mechanical stresses. J. Dent. Res. 81, 701-705 10.1177/154405910208101009 [DOI] [PubMed] [Google Scholar]
- Wang A. H. and Yang X.-J. (2001). Histone deacetylase 4 possesses intrinsic nuclear import and export signals. Mol. Cell. Biol. 21, 5992-6005 10.1128/MCB.21.17.5992-6005.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhou J. and Bondy C. A. (1999a). Igf1 promotes longitudinal bone growth by insulin-like actions augmenting chondrocyte hypertrophy. FASEB J. 13, 1985-1990. [DOI] [PubMed] [Google Scholar]
- Wang Y., Spatz M. K., Kannan K., Hayk H., Avivi A., Gorivodsky M., Pines M., Yayon A., Lonai P. and Givol D. (1999b). A mouse model for achondroplasia produced by targeting fibroblast growth factor receptor 3. Proc. Natl. Acad. Sci. USA 96, 4455-4460 10.1073/pnas.96.8.4455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Kruhlak M. J., Wu J., Bertos N. R., Vezmar M., Posner B. I., Bazett-Jones D. P. and Yang X.-J. (2000). Regulation of histone deacetylase 4 by binding of 14–3-3 proteins. Mol. Cell. Biol. 20, 6904-6912 10.1128/MCB.20.18.6904-6912.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir E. C., Philbrick W. M., Amling M., Neff L. A., Baron R. and Broadus A. E. (1996). Targeted overexpression of parathyroid hormone-related peptide in chondrocytes causes chondrodysplasia and delayed endochondral bone formation. Proc. Natl. Acad. Sci. USA 93, 10240-10245 10.1073/pnas.93.19.10240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston A. D., Chandraratna R. A. S., Torchia J. and Underhill T. M. (2002). Requirement for RAR-mediated gene repression in skeletal progenitor differentiation. J. Cell Biol. 158, 39-51 10.1083/jcb.200112029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. W. and Chuang P.-T. (2010). Mechanism and evolution of cytosolic Hedgehog signal transduction. Development 137, 2079-2094 10.1242/dev.045021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A. and Beier F. (2006). RhoA/ROCK signaling regulates chondrogenesis in a context-dependent manner. J. Biol. Chem. 281, 13134-13140 10.1074/jbc.M509433200 [DOI] [PubMed] [Google Scholar]
- Woods K. A., Camacho-Hübner C., Savage M. O. and Clark A. J. L. (1996). Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N. Engl. J. Med. 335, 1363-1367 10.1056/NEJM199610313351805 [DOI] [PubMed] [Google Scholar]
- Woods A., Wang G. and Beier F. (2005). RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J. Biol. Chem. 280, 11626-11634 10.1074/jbc.M409158200 [DOI] [PubMed] [Google Scholar]
- Yamashita Y., Takeshige K., Inoue A., Hirose S., Takamori A. and Hagiwara H. (2000). Concentration of mRNA for the natriuretic peptide receptor-C in hypertrophic chondrocytes of the fetal mouse tibia. J. Biochem. 127, 177-179 10.1093/oxfordjournals.jbchem.a022591 [DOI] [PubMed] [Google Scholar]
- Yang L., Tsang K. Y., Tang H. C., Chan D. and Cheah K. S. E. (2014). Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc. Natl. Acad. Sci. USA 111, 12097-12102 10.1073/pnas.1302703111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasoda A., Komatsu Y., Chusho H., Miyazawa T., Ozasa A., Miura M., Kurihara T., Rogi T., Tanaka S., Suda M. et al. (2004). Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat. Med. 10, 80-86 10.1038/nm971 [DOI] [PubMed] [Google Scholar]
- Yasoda A., Kitamura H., Fujii T., Kondo E., Murao N., Miura M., Kanamoto N., Komatsu Y., Arai H. and Nakao K. (2009). Systemic administration of C-type natriuretic peptide as a novel therapeutic strategy for skeletal dysplasias. Endocrinology 150, 3138-3144 10.1210/en.2008-1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara R., Yuasa T., Williams J. A., Byers S. W., Shah S., Pacifici M., Iwamoto M. and Enomoto-Iwamoto M. (2010). Wnt/beta-catenin and retinoic acid receptor signaling pathways interact to regulate chondrocyte function and matrix turnover. J. Biol. Chem. 285, 317-327 10.1074/jbc.M109.053926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y.-M., Oh C.-D., Kang S.-S. and Chun J.-S. (2000). Protein kinase A regulates chondrogenesis of mesenchymal cells at the post-precartilage condensation stage via protein kinase C-alpha signaling. J. Bone Miner. Res. 15, 2197-2205 10.1359/jbmr.2000.15.11.2197 [DOI] [PubMed] [Google Scholar]
- Yoon B. S., Ovchinnikov D. A., Yoshii I., Mishina Y., Behringer R. R. and Lyons K. M. (2005). Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc. Natl. Acad. Sci. USA 102, 5062-5067 10.1073/pnas.0500031102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon B. S., Pogue R., Ovchinnikov D. A., Yoshii I., Mishina Y., Behringer R. R. and Lyons K. M. (2006). BMPs regulate multiple aspects of growth-plate chondrogenesis through opposing actions on FGF pathways. Development 133, 4667-4678 10.1242/dev.02680 [DOI] [PubMed] [Google Scholar]
- Yoshida C. A., Yamamoto H., Fujita T., Furuichi T., Ito K., Inoue K.-i., Yamana K., Zanma A., Takada K., Ito Y. et al. (2004). Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. 18, 952-963 10.1101/gad.1174704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K., Xu J., Liu Z., Sosic D., Shao J., Olson E. N., Towler D. A. and Ornitz D. M. (2003). Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development 130, 3063-3074 10.1242/dev.00491 [DOI] [PubMed] [Google Scholar]
- Zanetti N. C. and Solursh M. (1984). Induction of chondrogenesis in limb mesenchymal cultures by disruption of the actin cytoskeleton. J. Cell Biol. 99, 115-123 10.1083/jcb.99.1.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelzer E., Glotzer D. J., Hartmann C., Thomas D., Fukai N., Soker S. and Olsen B. R. (2001). Tissue specific regulation of VEGF expression during bone development requires Cbfa1/Runx2. Mech. Dev. 106, 97-106 10.1016/S0925-4773(01)00428-2 [DOI] [PubMed] [Google Scholar]
- Zelzer E., McLean W., Ng Y. S., Fukai N., Reginato A. M., Lovejoy S., D'Amore P. A. and Olsen B. R. (2002). Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development 129, 1893-1904. [DOI] [PubMed] [Google Scholar]
- Zelzer E., Mamluk R., Ferrara N., Johnson R. S., Schipani E. and Olsen B. R. (2004). VEGFA is necessary for chondrocyte survival during bone development. Development 131, 2161-2171 10.1242/dev.01053 [DOI] [PubMed] [Google Scholar]
- Zeng L., Kempf H., Murtaugh L. C., Sato M. E. and Lassar A. B. (2002). Shh establishes an Nkx3.2/Sox9 autoregulatory loop that is maintained by BMP signals to induce somitic chondrogenesis. Genes Dev. 16, 1990-2005 10.1101/gad.1008002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-W., Yasui N., Ito K., Huang G., Fujii M., Hanai J.-i., Nogami H., Ochi T., Miyazono K. and Ito Y. (2000). A RUNX2/PEBP2alpha A/CBFA1 mutation displaying impaired transactivation and Smad interaction in cleidocranial dysplasia. Proc. Natl. Acad. Sci. USA 97, 10549-10554 10.1073/pnas.180309597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Siclari V. A., Lan S., Zhu J., Koyama E., Dupuis H. L., Enomoto-Iwamoto M., Beier F. and Qin L. (2011). The critical role of the epidermal growth factor receptor in endochondral ossification. J. Bone Miner. Res. 26, 2622-2633 10.1002/jbmr.502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhu J., Li Y., Lin T., Siclari V. A., Chandra A., Candela E. M., Koyama E., Enomoto-Iwamoto M. and Qin L. (2013). Epidermal growth factor receptor (EGFR) signaling regulates epiphyseal cartilage development through beta-catenin-dependent and -independent pathways. J. Biol. Chem. 288, 32229-32240 10.1074/jbc.M113.463554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q., Zhou G., Morello R., Chen Y., Garcia-Rojas X. and Lee B. (2003). Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocyte-specific expression in vivo. J. Cell Biol. 162, 833-842 10.1083/jcb.200211089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Lefebvre V., Zhang Z., Eberspaecher H. and de Crombrugghe B. (1998). Three high mobility group-like sequences within a 48-base pair enhancer of the Col2a1 gene are required for cartilage-specific expression in vivo. J. Biol. Chem. 273, 14989-14997 10.1074/jbc.273.24.14989 [DOI] [PubMed] [Google Scholar]
- Zhou G., Zheng Q., Engin F., Munivez E., Chen Y., Sebald E., Krakow D. and Lee B. (2006). Dominance of SOX9 function over RUNX2 during skeletogenesis. Proc. Natl. Acad. Sci. USA 103, 19004-19009 10.1073/pnas.0605170103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H., Wieser R., Massague J. and Niswander L. (1997). Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes Dev. 11, 2191-2203 10.1101/gad.11.17.2191 [DOI] [PMC free article] [PubMed] [Google Scholar]