Abstract
The incidence of type 2 diabetes and obesity in children and adolescents has risen at staggering rates. Studies have shown that treating type 2 diabetes with oral medications in children may be more difficult than treating in adults. Compounding this problem is the fact that most of the medications available for treating type 2 diabetes have not been studied in children. Recently, the American Diabetes Association and the Pediatric Endocrine Society have collaborated to create a guideline for the treatment of type 2 diabetes in children. Similar to the treatment of adults with type 2 diabetes, metformin remains the mainstay of therapy along with diet and exercise. Adjunctive therapy should be based on the limited clinical evidence available as well as on patient preference. In order to avoid detrimental microvascular and macrovascular complications, patients, clinicians, and family members should work together to ensure adequate treatment of type 2 diabetes in children.
INDEX TERMS: adolescents, children, guidelines, treatment, type 2 diabetes
INTRODUCTION
The incidence of childhood obesity is increasing at a staggering rate, correlating with the incidence of type 2 diabetes mellitus (T2DM). Recently, the American Academy of Pediatrics (AAP) released a clinical practice guideline to aid providers in treating T2DM in children between 10 and 18 years of age. Many organizations, including the American Diabetes Association (ADA) and the Pediatric Endocrine Society, collaborated to develop this guideline, which emphasizes treatments focused on improving clinical outcomes in pediatric patients.1 This article provides an overview of T2DM and reviews clinical practice guidelines in conjunction with clinical trial data regarding the use of available pharmacological treatments.1,2
EPIDEMIOLOGY
The incidence of diabetes among youth is on the rise. The 2008 National Health and Nutrition Examination Survey (NHANES) found the prevalence of T2DM among youth 12 to 19 years of age increased from 9% in 1999 to 2000 to 23% in 2007 to 2008.3 Incidence rates of T2DM are known to vary by ethnicity. Research findings have demonstrated a global increase in the incidence of T2DM over the last 2 decades, with the greatest incidence observed in non-white ethnic groups.4
With limited population-based data, the SEARCH study aimed to estimate the incidence of both T2DM and type 1 diabetes mellitus (T1DM).5 This multiethnic observational study was conducted in subjects younger than 20 years of age according to race, ethnicity, and type of diabetes. Results of the SEARCH study found the incidence of T2DM among non-Hispanic whites 10 to 19 years of age was 3.0/100,000 person-years compared to 15.7/100,000 person-years in African American youth.5 Comparing age groups, T2DM was most prevalent in youth 15 to 19 years of age, with the greatest incidence noted in the American Indian subgroup at 49.4/100,000 person-years. A higher incidence was also observed in females than in males.5
PATHOPHYSIOLOGY
Under normal physiological conditions, insulin is secreted from pancreatic β-cells in response to elevation in blood glucose.6 In fasting states, glucagon is secreted from pancreatic α-cells and prevents hypoglycemia by activating hepatic glu-coneogenesis.7 Development of T2DM involves a progressive imbalance in glucose homeostasis. In both adults and children, impaired insulin secretion from pancreatic β-cells and reduced insulin sensitivity of the peripheral tissues represent the defining physiologic defects.
Insulin resistance within the peripheral tissues leads to hypersecretion of insulin from pancreatic β-cells. This compensatory secretion is thought to lead to overtaxing of the β-cells, which contributes to further decline in overall β-cell function, paving the way for glucose intolerance.7 In the liver, insulin resistance leads to less regulation of glucagon concentrations. Poor peripheral uptake of plasma glucose in the fed state coupled with ongoing hepatic glucose production leads to a worsening glycemic state.6,8
In children, the role of obesity in the development of insulin resistance and, ultimately T2DM, is well established.9,10 Excessive visceral fat has been independently associated with the development of diabetes due to an increase in insulin resistance.11 The NHANES compiled data from 3281 children and adolescents (2–19 years of age) and 719 infants and toddlers (birth-2 years of age) to evaluate the prevalence of high body mass index (BMI). Results from the 2007 to 2008 NHANES found that among children 6 to 11 years of age, obesity rates increased from 6.5% in 1976 to 1980 to 16.9% in 2007 to 2008.12,13 Overall, Hispanic males had a significantly higher incidence of elevated BMIs. NHANES data from 2009 to 2010 indicate the percentage of children and adolescents 2 to 19 years of age defined as obese or overweight was approximately 33%.14 Children who are obese or overweight are at increased risk of developing T2DM. In addition, their health may be further effected by an increased risk of comorbid conditions such as cardiovascular disease and sleep apnea.15–19 Furthermore, T2DM has been associated with development of a number of microvascular and macrovascular complications. Due to the multiple health risks associated with obesity in children and adolescents, prevention of T2DM in overweight or obese youth is imperative.
DIAGNOSIS
The increase in childhood obesity obscures the diagnosis between T2DM and T1DM. Up to 25% of children who have T1DM are obese.2 Diagnosis of T2DM in children and adolescents focuses on blood glucose concentrations and the presence of key symptoms. These characteristic symptoms include polyuria, polydipsia, blurred vision, weight loss in association with glucosuria, and potentially ketonuria. Diagnosis of T2DM must be accompanied by these symptoms as well as a random plasma glucose concentration >200 mg/dL. Diabetes is also diagnosed by a fasting plasma glucose (FPG) concentration >126 mg/dL or a postchallenge (i.e., 75 mg of anhydrous glucose in water) glucose concentration of >200 mg/dL or hemoglobin A1c (A1c) ≥6.5%.20 Unfortunately, diagnosis of diabetes is often delayed until complications are present.1,2 Because of this, children who have BMI in the 85th to 95th percentile with a family history, signs of insulin resistance (e.g., acanthosis nigricans), or comorbidities of insulin resistance (e.g., hypertension, dyslipidemia, polycystic ovarian syndrome, and others) are considered high risk and should be screened for T2DM.2,20 Children with a BMI >95th percentile should be screened, regardless.2,20
TREATMENT
Treatment of T2DM should be focused on decreasing complications in children and adolescents. Few studies exist in children with T2DM; however, data from studies in children with T1DM and adults with T2DM suggest that tight glycemic control reduces the risk of microvascular complications.1 A1c and fasting blood sugar (BS) goals (<7% and 70–130 mg/dL, respectively) are the same for children and adults with T2DM.1 Most clinical evidence surrounding T2DM and pediatric patients involves the use of metformin and insulin. However, many patients will remain uncontrolled with these medications. The following section reviews lifestyle modifications. In addition, each of the drug classes and their potential role in this specific patient population will be addressed.
Lifestyle Modifications
Treatment of every child with T2DM should begin with lifestyle modifications, including physical activity and nutrition. Lifestyle modifications are the cornerstone of treatment for T2DM, yet they are frequently met with limited long-term success. Only 10% of pediatric patients with T2DM achieve their BS goals with lifestyle modifications alone. This is likely secondary to loss of follow-up; a high rate of depression in teenagers, which affects adherence; and peer pressure steering toward unhealthy eating habits.1 It is also possible that patients do not understand the importance of diet, as it is not a medication being prescribed. In fact, observational studies show treatment of diabetes with lifestyle modifications alone is associated with a higher rate of loss to follow-up compared to those prescribed medications.21 Consequently, most of these patients will require addition of drug therapy.1
Patients should be encouraged to engage in moderate to vigorous physical activity for at least 60 minutes per day.1 Moderate physical activity is characterized by increased respiration and perspiration. During such exercise, the patient should be able to talk but not sing; during vigorous physical exercise, the patient should be unable to talk without pausing to take a breath. When planning physical exercise, it is important to consider the family's circumstances. Not all children can engage in physical exercise in a structured environment due to financial or logistical constraints. Individualizing the exercise program will allow the patient to incorporate physical activity into their daily routine while taking into account their limitations and preferences. Also, non-academic screen time (e.g., computer, television) should be limited to less than 2 hours per day.1
The AAP guideline recommends clinicians incorporate Academy of Nutrition and Dietetics' Pediatric Weight Management Evidence-Based Nutrition Practice Guidelines into their patient education and counseling.1 Ideally, a registered dietician should provide initial comprehensive detailed nutritional education. The patient's primary care provider should deliver ongoing nutritional counseling to maximize adherence and outcomes. These recommendations should be culturally sensitive and financially feasible and provided to all caregivers.2 Common dietary recommendations should include eating regular meals and healthy snacks, reducing portion sizes, choosing calorie-free beverages (other than milk), limiting juice to 1 cup per day, increasing dietary intake of fruits and vegetables, consuming 3 to 4 servings of low-fat dairy products per day, limiting dietary intake of high-fat foods, limiting frequency and size of snacks, and reducing calories consumed in fast food meals.1 Currently, many restaurants include calorie counts on their menu. For those who do not, information available online can assist in choosing meals. In addition, smart phone applications can assist in helping patients to make decisions about fast food, track calories and exercise, and achieve weight loss goals.
Insulin
The use of exogenous insulin helps regulate serum glucose by increasing its uptake into muscle and adipose tissue and decreasing hepatic glucose production. Weight gain, hypoglycemia, and peripheral hyperinsulinemia are the most common adverse events associated with insulin.22 The following insulin types are indicated for use in children: aspart, glulisine, lispro, regular, neutral protamine Hagedorn (NPH), detemir, and glargine. Regimens that have shown success in this patient population include a single dose of long-acting insulin at bedtime with or without an oral agent as well as basal/bolus insulin regimens. The latter regimens require patients to be willing to count carbohydrates and administer the bolus insulin at meals based on a specific calculation.1
Practitioners often delay the use of insulin in children with T2DM due to regimen complexity and adverse events. However, the AAP clinical practice guideline states that insulin should be used as a first-line treatment in children with T2DM who are ketotic or are in ketoacidosis and in whom a distinction between T1DM and T2DM is unclear (Figure). In addition, insulin should be used, at least in the short term, in those patients with random blood sugar concentrations =250 mg/dL or A1c >9%.1 The use of insulin early in the disease process allows glucose concentrations to normalize while β- cells are provided a chance to “rest and recover.” After this initial time period, many patients can be titrated from insulin and maintained with oral therapy.1
Figure.
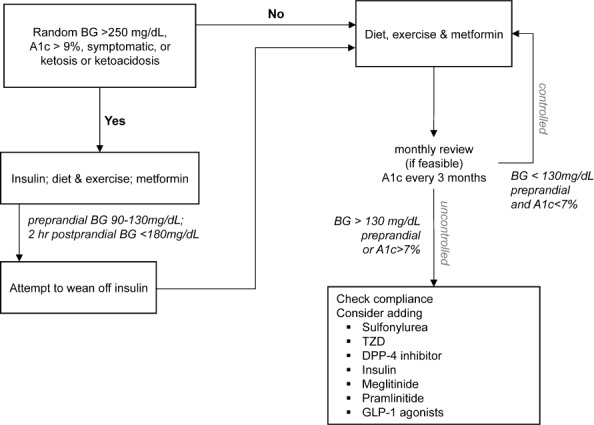
Treatment algorithm for type 2 diabetes mellitus in children and adolescents.
BG, blood glucose; DPP-4, dipeptidyl-peptidase 4; GLP-1, glucagon like peptide-1; TZD, thiazolidinediones Adapted from Rosenbloom and Copeland1,2
Limited evidence in children is available to support the use of other medications used in the treatment of T2DM. In fact, much of the evidence has been extrapolated from adult data. The Table provides a summary of these medications, including their mechanisms of action, doses studied in children (if applicable), and monitoring parameters.
Table.
Current Treatments Available for T2DM
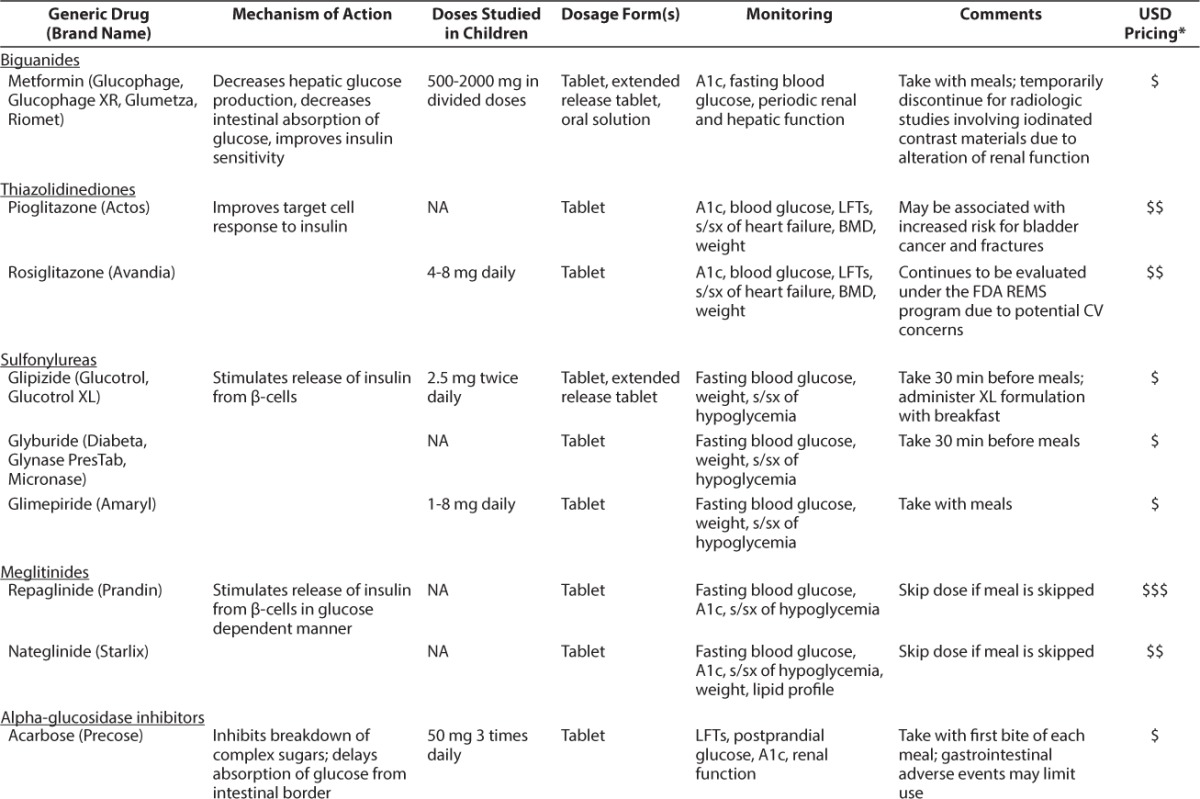
Table.
Current Treatments Available for T2DM (cont.)
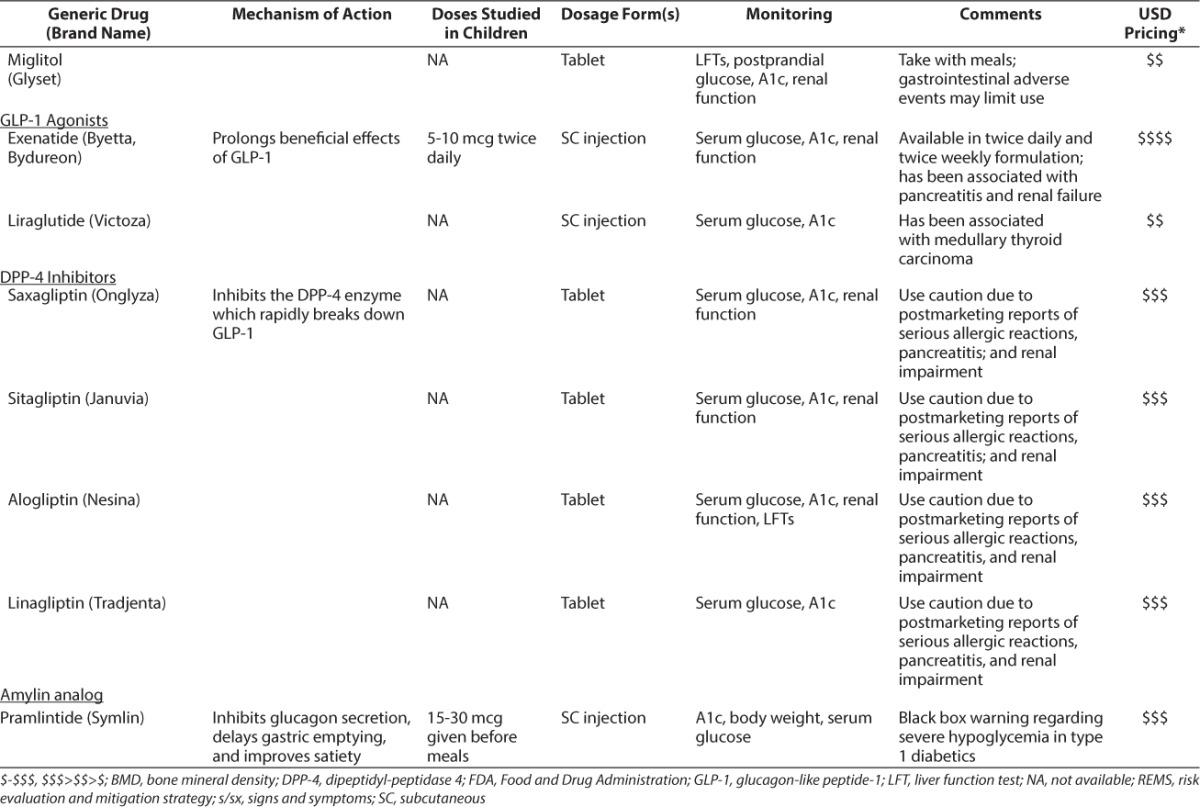
Biguanides
Unless there are contraindications, all children with T2DM should be started on metformin. Metformin works by decreasing hepatic glucose production and stimulating glucose uptake into peripheral tissues.23 Metformin should be initiated in children 10 to 16 years of age at a dose of 500 mg daily with food and titrated up by increments of 500 mg every 1 to 2 weeks until a target dose of 2000 mg daily is reached.1 The most common adverse events associated with metformin are gastrointestinal upset, including abdominal pain and diarrhea.24 Although rare, lactic acidosis may occur in patients with renal and/or hepatic impairment or cardiac/respiratory insufficiency or in those undergoing studies involving iodinated contrast dye.25 Contraindications to metformin therapy include renal dysfunction (defined as serum creatinine ≥1.5 mg/dL in adult males and ≥1.4 mg/dL in adult females) and acute or chronic metabolic acidosis.26 Specific cutoffs for serum creatinine in children are unknown; however, pediatric-specific monitoring of renal function is recommended. When used appropriately, metformin lowers A1c 1 to 2% and aids in weight loss.27 Gastrointestinal adverse events may limit achieving the target dose in some patients.
Clinical evidence supporting the use of metformin in pediatric patients is limited. In a clinical trial of 82 children and adolescents 10 to 16 years of age with T2DM, patients were randomized to receive metformin or placebo.28 The dose of metformin was titrated to the largest tolerable dose but did not exceed 2000 mg daily. The mean FPG significantly decreased in the metformin group while it increased in the placebo group (−42.9 mg/dL vs. +21.4 mg/dL, p<0.001). Mean A1c concentrations fell from 8.2% to 7.5% in the metformin group and from 8.9% to 8.6% in the placebo group. The between-group difference was found to be statistically significant (p<0.001). Other metabolic effects were observed in the study. Mean serum cholesterol decreased from baseline in the metformin group (−9.7 mg/dL), reflecting the reduction noted in low-density lipoprotein (−4.2 mg/dL vs. +4 mg/dL in placebo); p=0.053). Consequently, the placebo group experienced a slight increase in total cholesterol (0.7 mg/dL; p=0.043). A greater weight loss and BMI reduction were noted in the metformin group. Mean weight change from baseline was −1.5 kg in the metformin group versus −0.9 kg in the placebo group, and BMI reduction was slightly more in the metformin group (p values not reported). Although adverse events (e.g., abdominal pain, diarrhea, nausea/vomiting, and headache) occurred more frequently in the metformin group, no patient discontinued treatment due to an adverse event. The authors concluded that metformin in doses ≤2000 mg/day is safe and effective for the treatment of T2DM in pediatric patients.
A more recent trial (TODAY Study), examined the durability of metformin in 699 patients 10 to 17 years of age with T2DM.29 After a 2- to 6-month run-in period of metformin monotherapy (target dose 2000 mg daily), patients were randomized to metformin alone, to metformin plus rosiglitazone 4 mg twice daily, or to metformin plus lifestyle interventions. The primary outcome, treatment failure, was defined as a persistently elevated A1c concentration (≥8%) over a period of 6 months. Rates of treatment failure were 51.7% in the metformin group, 38.6% in the metformin plus rosiglitazone group, and 46.6% in the metformin plus lifestyle intervention group (p values not reported). Metformin plus rosiglitazone produced a 25.3% decrease in treatment failure compared to metformin alone (p=0.006). Of note, the group taking metformin plus rosiglitazone experienced the greatest increase in BMI, but this was not a determinant of treatment failure. Serious adverse events, including diabetic ketoacidosis (n=11), hyperglycemia (n=10), and hypoglycemia (n=4) and 1 case of non-fatal lactic acidosis, were reported in 19.2% of study participants. The TODAY authors concluded that the glycemic durability of metformin plus rosiglitazone was superior to therapy with metformin alone in children with T2DM, despite a small increase in BMI.29
Thiazolidinediones
Thiazolidinediones (TZDs) work to decrease blood glucose by increasing insulin sensitivity in liver, muscle, and adipose tissue and to decrease hepatic glucose synthesis output. This class of drugs is not approved for use in children; however, rosiglitazone in doses of 4 to 8 mg daily have been studied.30 Pioglitazone has not been studied in children; therefore, dosing recommendations are not available. Adverse events commonly seen in adults taking TZDs include edema, weight gain, anemia, and potential for elevation of liver enzymes. Typically, long-term use of TZDs in adults will decrease A1c an average of 1%.31 TZDs have been associated with a potential increased risk of bladder cancer and fracture rates.32,33 Interim results from a long-term study examining the association between pioglitazone use and bladder cancer revealed patients with the longest exposure to and highest cumulative dose of pioglitazone experienced an increased risk of bladder cancer.33 Use of rosiglitazone was previously restricted due to concerns of increased cardiovascular ischemic risk observed in a meta-analysis.34 At the time of this publication, the US Food and Drug Administration requires removal of the prescribing and dispensing restrictions for rosiglitazone. Rosiglitazone continues to be monitored under the Risk Evaluation and Mitigation Strategy (REMS); however, the REMS has been modified to reflect the aforementioned changes.35
Rosiglitazone use was evaluated in a pilot study of obese adolescents with impaired glucose tolerance (IGT).30 In that study, 21 obese adolescents 13 to 18 years of age with IGT or IGT combined with impaired FPG were randomized to receive rosiglitazone 4 mg daily (titrated to 8 mg daily) or placebo. The investigators found 58% of patients treated with rosiglitazone returned to normal glucose tolerance compared with 44% of patients treated with placebo (p=0.528). Although few adverse events surfaced during this short-term treatment, the authors concluded that, given the cardiovascular concerns with rosiglitazone in adults and the small amount of data regarding its use in children, it would be premature to recommend rosiglitazone in children.
Sulfonylureas
Sulfonylureas work by stimulating pancreatic β-cells to secrete insulin. Although not labeled for use in pediatrics, sulfonylureas have been used safely in this population.36–39 Doses used in studies include glimepiride, 1 to 8 mg once daily,36,39 and glipizide, 2.5 mg twice daily.38 The most common adverse events associated with sulfonylureas include weight gain and hypoglycemia.40 The use of sulfonylureas in adults provides lowering of A1c approximately 1.25%.41
Data regarding the use of sulfonylureas in children are limited. A recent study compared the safety and efficacy of glimepiride to that of metformin in 285 pediatric patients with T2DM.36 Patients were randomized to receive metformin 500 to 1000 mg twice daily or glimepiride 1 to 8 mg once daily for 26 weeks. Final mean doses were 3.8 mg daily for glimepiride and 1408 mg daily for metformin. Hemoglobin A1c significantly decreased from baseline in both the glimepiride group (−0.54%; p=0.001) and the metformin group (−0.71%; p=0.0002). In addition, 42.4% of patients in the glimepiride group and 48.1% of patients in the metformin group achieved an A1c concentration of <7%. No significant differences between groups were noted for blood glucose, serum lipids, or hypoglycemic events; however, patients in the glimepiride group experienced more weight gain. The authors concluded glimepiride was as effective as metformin in reducing A1c, with more weight gain, in pediatric patients with T2DM.
Although not specific to children with T2DM, several additional studies have evaluated the use of sulfonylureas in children. A prospective trial of 12 patients 9 to 29 years of age evaluated the effect of sulfonylureas (mainly chlorpropamide) on glucose-induced insulin secretion in patients with maturity-onset diabetes of the young.37 The authors found that long-term administration of sulfonylureas significantly enhanced glucose-induced insulin concentrations by approximately 68%. Another small study of 6 patients with cystic fibrosis who had developed impaired glucose tolerance examined the effect of glipizide 2.5 mg twice daily.38 The investigators found that glipizide improved glucose tolerance, and only mild, symptomatic hypoglycemia was noted. Finally, a small study involving 40 children with T1DM randomized patients to receive glimepiride 4 mg daily or placebo.39 The investigators found no differences between groups in weight, blood pressure, insulin dosage, fasting serum glucose, rate of hypoglycemia, A1c concentration, or serum lipids; however, it is important to note that these were not the intended primary outcomes of the study.
Meglitinides
Repaglinide and nateglinide work by stimulating insulin release from the pancreas in a glucose-dependent manner. Neither agent is approved for use in children. Data regarding use in children are limited to a few case reports; therefore, dosage information is not available.42 The most common adverse events associated with this class of agents include hypoglycemia, upper respiratory tract infection, diarrhea, and headache.43,44 The use of meglitinides in adults affords patients approximately 0.75% in A1c lowering.41
Alpha-Glucosidase Inhibitors
Acarbose and miglitol slow the absorption of carbohydrates in the distal small intestine, thereby decreasing postprandial serum glucose.45 The decrease in A1c achieved with acarbose and miglitol is approximately 0.5% to 1%.40 Common adverse events such as flatulence, diarrhea, and abdominal cramps may limit the use of these medications in children and adolescents.46
The alpha-glucosidase inhibitors have not been well studied in this population. Kentrup et al47 evaluated acarbose in patients with IGT related to cystic fibrosis.47 In this double-blind randomized crossover trial, the efficacy and safety of acarbose was evaluated in 12 glucose-intolerant patients over a 5-day period. The researchers observed that acarbose 50 mg 3 times daily caused a significant decrease in serum glucose concentrations compared to placebo. Of note, 67% of subjects in the acarbose group reported gastrointestinal disturbance. The authors concluded acarbose attenuated increases in postprandial glucose and decreased insulin secretion response in patients with cystic fibrosis but at the expense of gastrointestinal side effects.
GLP-1 Agonists
Exenatide and liraglutide work as a glucagon-like peptide-1 (GLP-1) agonists. GLP-1 is an incretin hormone released from the gut in response to meals. Its beneficial effects include slowed gastric emptying, enhanced insulin biosynthesis, improved β-cell function, and decreased appetite.48 Neither agent is approved for pediatric patients. Exenatide doses of 5 to 10 mcg twice daily have been studied in pediatric patients. Liraglutide has not been studied in children, therefore, dosage recommendations are not available. The most common adverse events associated with GLP-1 agonists include nausea, hypoglycemia, vomiting, headache, and diarrhea.49,50 Exenatide should be avoided in patients with a history of pancreatitis and severe renal impairment or end-stage renal disease.49 Liraglutide should be avoided in patients with a history of pancreatitis and a personal or family history of medullary thyroid carcinoma.50 Data from adults indicate GLP-1 agonists are very effective, lowering the A1c by approximately 1% to 1.5%.51
Clinical evidence surrounding the use of exenatide in children is limited to the treatment of obesity and T1DM. In a study of exenatide in 12 extremely obese children 9 to 16 years of age, patients were randomized to receive placebo plus lifestyle modifications or exenatide plus lifestyle modifications for 3 months.52 Patients were then crossed over to receive the opposite treatment for the next 3 months. Exenatide was given at 5 mcg twice daily and then increased (if tolerated) to 10 mcg twice daily. The investigators found that exenatide significantly reduced BMI, body weight, and fasting insulin. In addition, exenatide was well tolerated, with nausea being the most common adverse event reported. From this study, the authors concluded exenatide deserves further investigation for weight loss in obese youth. A follow-up study examined the use of exenatide 5 mcg twice daily (increased to 10 mcg twice daily after 1 month), in severely obese adolescents 12 to 19 years of age.53 Patients were randomized to receive exenatide or placebo for 3 months, followed by an open-label extension period of 3 months in which all patients were offered active treatment. The investigators found that exenatide afforded patients a greater percentage of reduction in BMI than placebo (p=0.03). Adverse events were mild to moderate and included nausea, abdominal pain, diarrhea, headache, and vomiting; no patient experienced hypoglycemia or pancreatitis. The authors concluded that exenatide is safe and effective for the treatment of obesity in adolescents. Raman et al54 investigated the role of exenatide as adjunctive treatment to insulin in children with T1DM. In that study, 8 adolescents 13 to 22 years of age were randomized to receive 2 doses of exenatide (1.25 mcg and 2.5 mcg) or insulin monotherapy. The investigators found exenatide decreased postprandial hyperglycemia but not glucagon suppression. Two patients experienced nausea requiring antiemetic therapy; 1 patient experienced hypoglycemia. The authors concluded exenatide may be an effective adjunctive treatment to insulin in T1DM and deserves further investigation.
DPP-4 Inhibitors
GLP-1 is rapidly degraded by dipeptidyl-peptidase 4 (DPP-4) in vivo. By inhibiting DPP-4, the beneficial effects of GLP-1 remain active. Agents currently marketed in the United States include sitagliptin, saxagliptin, linagliptin, and alogliptin. DPP-4 inhibitors have not been studied in children; therefore, dosage recommendations are not available. The most common adverse events associated with DPP-4 inhibitors include upper respiratory tract infection, urinary tract infection, headache, and nasopharyngitis.55–58 Due to postmarketing reports of pancreatitis, renal impairment, and serious allergic reactions, these agents should be used with extreme caution or avoided in certain populations.55–58 A1c lowering with use of DPP-4 inhibitors in adults is approximately 0.75 %.41
Amylin Analog
Pramlintide is a synthetic analog of amylin, a hormone produced in vivo by β-cells and cosecreted with insulin. Amylin inhibits glucagon secretion, delays gastric emptying, and improves satiety.59 The doses of pramlintide studied in pediatric patients are 15 to 30 mcg given prior to meals.60–64 The most common adverse events associated with pramlintide include hypoglycemia, nausea, headache, anorexia, and abdominal pain.65 The package insert contains a black box warning regarding severe hypoglycemia in T1DM; suggestions to avoid this risk include careful patient selection, patient education, and appropriate insulin dose adjustments.65 Data for use in adults with T2DM indicate an A1c lowering of approximately 0.5%.66
Clinical evidence regarding the use of pramlintide in children is limited to T1DM.60–64 Many of the studies were very small, enrolling only 8 to 13 patients.60–63 Results were similar among studies; pramlintide was shown to decrease post-prandial glucose with minimal adverse events. One study investigated the effects of pramlintide on A1c, body weight, and postprandial glucose in children with T1DM.64 In that study, 10 adolescents 13 to 17 years of age were randomized to active treatment with 15 mcg of pramlintide before meals (titrated to 30 mcg before meals if tolerated) or to control for 28 days. The investigators found pramlintide decreased A1c, body weight, and total insulin dose compared to the effects in the control group (p≤0.02 for each). The authors concluded that use of pramlintide in this preliminary study led to improvements in A1c, body weight, and insulin dose in adolescents with T1DM and that pramlintide therapy warrants further investigation.
TREATMENT ALGORITHM
In T2DM, the rate of treatment failure with monotherapy may be higher in the pediatric population.29 Evidence from the TODAY study indicates treatment failure with metformin monotherapy was higher in the pediatric population than in the adult population. This treatment failure could not be explained by differences in baseline characteristics, BMI, insulin sensitivity, body composition, or adherence rates. In contrast, a study assessing time to failure with oral therapy reported patients for whom monotherapy failed were non-compliant with metformin treatment.67 In addition, characteristics associated with a higher incidence of failure included initial insulin therapy, presenting A1c level, and black race or Hispanic ethnicity. It is possible that the decrease in durability of glycemic control in the pediatric population reflects a biological or pathophysiological difference.29
Guidelines from the AAP and the International Society for Pediatric and Adolescent Diabetes Consensus Guidelines are clear about when to use insulin or metformin.1,2 How to treat patients who present with moderate concentrations of hyperglycemia (random BS concentration of 200–249 mg/dL) or those patients who remain uncontrolled on monotherapy with metformin is less clear. In patients with moderate hyperglycemia, metformin alone, metformin plus insulin, or insulin alone would all be reasonable choices.1 The question of what to do next remains unclear. As previously discussed, many of the medications used to treat T2DM have not been studied in children, and data are often extrapolated from data in adults. If a patient's A1c remains above the goal of <7%, the following options exist: increase frequency of office visits, increase BS monitoring, add 1 or more medications to the current regimen, refer patient to a registered dietician and/or diabetes educator, or intensify the diet and exercise plan.1 A variety of medications are available for combination therapy; however, one should keep in mind the limited clinical evidence for each agent. Additional agents that may be considered include sulfonylureas, thiazolidinediones, DPP-4 inhibitors, meglitinides, GLP-1 agonists, or pramlintide (Figure). Adverse events, dosages, administration, and monitoring should all be taken into account when making this decision.
Recommendations for monitoring BS in children with T2DM should follow the ADA recommendations for adults. For patients using multiple insulin injections or an insulin pump, BS should be monitored at least 3 times daily. For patients using non-insulin therapies, less intense insulin regimens, or lifestyle interventions alone, BS can be monitored less frequently, and results should be used to help guide therapy. The goal for fasting BS should be 70 to 130 mg/dL for most patients. Two-hour postprandial BS, with a goal of <180 mg/dL, should be monitored in patients whose FPG is controlled but A1c remains uncontrolled.1
CONCLUSIONS
T2DM has emerged as a serious epidemic in the pediatric population. The lack of clinical evidence supporting use of various medications makes treating this group of patients difficult. Lifestyle interventions including exercise and nutrition should be the cornerstone of therapy. Patients and family members should be involved in educational efforts involving diet and exercise. The AAP guideline recommends metformin or insulin as first-line therapy in children with a diagnosis of T2DM, depending upon clinical presentation. Insulin is preferred if the need to reverse glucose toxicity is present, such as ketoacidosis or significant hyperglycemia. Lifestyle interventions alone are met with limited long-term success, and metformin and insulin are the only antidiabetic agents approved for children. With limited pediatric data, the choice of an additional agent presents a unique challenge to the health care practitioner. Choosing among these agents should take into account patient preference and available data for safety and efficacy in children and adults. Because providers are diagnosing T2DM in children more frequently, future research should explore whether these oral agents could potentially preserve β-cell function and become effective tools in combating this aggressive disease.
ABBREVIATIONS
- AAP
American Academy of Pediatrics
- ADA
American Diabetes Association
- BS
blood sugar
- BMI
body mass index
- FPG
fasting plasma glucose
- A1c
hemoglobin A1c
- IGT
impaired glucose tolerance
- NHANES
National Health and Nutrition Examination Survey
- REMS
risk evaluation and mitigation strategy
- TZDs
thiazolidinedione's
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Copeland KC, Silverstein J, Moore KR et al. Management of newly diagnosed type 2 diabetes mellitus (T2DM) in children and adolescents. Pediatrics. 2013;131(2):364–382. doi: 10.1542/peds.2012-3494. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbloom AL, Silverstein JH, Amemiya S et al. Type 2 diabetes in children and adolescents (ISPAD Clinical Practice Consensus Guidelines 2009 Compendium) Pediatr Diabetes. 2009;10(suppl 12):17–32. [Google Scholar]
- 3.May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999–2008. Pediatrics. 2012;129(6):1035–1041. doi: 10.1542/peds.2011-1082. [DOI] [PubMed] [Google Scholar]
- 4.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146(5):693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 5.Writing Group for the SEARCH for Diabetes in Youth Study Group. Incidence of diabetes in youth in the United States. JAMA. 2007;297(24):2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 6.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378(9786):169–181. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- 7.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50(11):2444–2450. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- 9.Caprio S. Insulin resistance in childhood obesity. J Pediatr Endocrinol Metab. 2002;15(suppl 1):487–492. [PubMed] [Google Scholar]
- 10.Molnár D. The prevalence of the metabolic syndrome and type 2 diabetes mellitus in children and adolescents. Int J Obesity. 2004;28(suppl 3):S70–S74. doi: 10.1038/sj.ijo.0802811. [DOI] [PubMed] [Google Scholar]
- 11.Neeland IJ, Turer AT, Ayers CR et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308(11):1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogden CL, Carroll MD, Curtin LR et al. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 13.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288(14):1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 14.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goran MI, Ball DC, Cruz ML. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J Clin Endocrinol Metab. 2003;88(4):1417–1427. doi: 10.1210/jc.2002-021442. [DOI] [PubMed] [Google Scholar]
- 16.Dietz WH. Overweight in childhood and adolescence. N Engl J Med. 2004;350(9):855–857. doi: 10.1056/NEJMp048008. [DOI] [PubMed] [Google Scholar]
- 17.Franks PW, Hanson RL, Knowler WC et al. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362(6):485–493. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Ford ES, Zhao G, Mokdad AH. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among US adolescents: NHANES 2005–2006. Diabetes Care. 2009;32(2):342–347. doi: 10.2337/dc08-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. http://www.cdc.gov/diabetes/pubs/fact-sheet11.htm. Accessed October 31, 2014.
- 20.Dileepan K, Feldt M. Type 2 diabetes mellitus in children and adolescents. Pediatr Rev. 2013;34(12):541–547. doi: 10.1542/pir.34-12-541. [DOI] [PubMed] [Google Scholar]
- 21.Reinehr T, Schober E, Roth CL et al. Type 2 diabetes in children and adolescents in a 2-year follow-up: insufficient adherence to diabetes centers. Horm Res. 2008;69(2):107–113. doi: 10.1159/000111814. [DOI] [PubMed] [Google Scholar]
- 22.Ludvigsson J. Prevention of adverse events in juvenile diabetes. Minerva Pediatr. 2004;56(3):277–290. [PubMed] [Google Scholar]
- 23.DeFronzo RA, Barzilai N, Simonson DC. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J Clin Endocrinol Metab. 1991;73(6):1294–1301. doi: 10.1210/jcem-73-6-1294. [DOI] [PubMed] [Google Scholar]
- 24.DeFronzo RA, Goodman AM, the Multicenter Metformin Study Group Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333(9):541–549. doi: 10.1056/NEJM199508313330902. [DOI] [PubMed] [Google Scholar]
- 25.Stang MR, Wysowski DK, Butler-Jones D. Incidence of lactic acidosis in metformin users. Diabetes Care. 1999;22(6):925–927. doi: 10.2337/diacare.22.6.925. [DOI] [PubMed] [Google Scholar]
- 26.Glucophage [package insert] New York, NY: Bristol-Myers Squibb Co.; 2014. [Google Scholar]
- 27.Garber AJ, Duncan TG, Goodman AM et al. Efficacy of metformin in type II diabetes: results of a double blind, placebo-controlled, dose-response trial. Am J Med. 1997;103(6):491–497. doi: 10.1016/s0002-9343(97)00254-4. [DOI] [PubMed] [Google Scholar]
- 28.Jones KL, Park JS, Arslanian S et al. Effect of metformin in pediatric patient with type 2 diabetes. Diabetes Care. 2002;25(1):89–94. doi: 10.2337/diacare.25.1.89. [DOI] [PubMed] [Google Scholar]
- 29.TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247–2256. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cali AMG, Pierpont BM, Taksali SE et al. Rosiglitazone improves glucose metabolism in obese adolescents with impaired glucose tolerance: a pilot study. Obesity. 2011;19(1):94–99. doi: 10.1038/oby.2010.109. [DOI] [PubMed] [Google Scholar]
- 31.Aronoff S, Rosenblatt S, Braithwaite S et al. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes. Diabetes Care. 2000;23(11):1605–1611. doi: 10.2337/diacare.23.11.1605. [DOI] [PubMed] [Google Scholar]
- 32.Loke YK, Singh S, Furberg C. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ. 2009;180(1):32–39. doi: 10.1503/cmaj.080486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Food and Drug Administration. FDA drug safety communication: update to ongoing safety review of Actos (pioglitazone) and increased risk of bladder cancer. http://www.fda.gov/drugs/drugsafety/ucm259150.htm. Accessed October 24, 2014.
- 34.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 35.US Food and Drug Administration. FDA drug safety communication: FDA requires removal of some prescribing and dispensing restrictions for rosiglitazone-containing diabetes medicines. http://www.fda.gov/Drugs/DrugSafety/ucm376389.htm, accessed October 24, 2014.
- 36.Gottschalk M, Danne T, Vlajnic A, Cara J. Glimepiride versus metformin as monotherapy in pediatric patients with type 2 diabetes. Diabetes Care. 2007;30(4):790–794. doi: 10.2337/dc06-1554. [DOI] [PubMed] [Google Scholar]
- 37.Fajans SS, Brown MB. Administration of sulfonylureas can increase glucose-induced insulin secretion for decades in patient with maturity-onset diabetes of the young. Diabetes Care. 1993;16(9):1254–1261. doi: 10.2337/diacare.16.9.1254. [DOI] [PubMed] [Google Scholar]
- 38.Culler FL, McKean LP, Buchanan CN et al. Glipizide treatment of patients with cystic fibrosis and impaired glucose tolerance. J Pediatr Gastroenterol Nutr. 1994;18(3):375–378. doi: 10.1097/00005176-199404000-00021. [DOI] [PubMed] [Google Scholar]
- 39.Wudy SA, Hogel J, Dollinger B et al. Glimepiride treatment and IGF-I in adolescents with type 1 diabetes. Diabetes Care. 2003;26(4):1312. doi: 10.2337/diacare.26.4.1312. [DOI] [PubMed] [Google Scholar]
- 40.Rosenbloom A. Increasing incidence of type 2 diabetes in children and adolescents: treatment considerations. Pediatr Drugs. 2002;4(4):209–221. doi: 10.2165/00128072-200204040-00001. [DOI] [PubMed] [Google Scholar]
- 41.Sherifali D, Cheng JE, Nerenberg K et al. The effect of oral antidiabetic agents on A1c levels. Diabetes Care. 2010;33(8):1859–1864. doi: 10.2337/dc09-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker M, Galler A, Raile K. Meglitinide analogues in adolescent patients with HNF1A-MODY (MODY 3) Pediatrics. 2014;133(3):e775–779. doi: 10.1542/peds.2012-2537. [DOI] [PubMed] [Google Scholar]
- 43.Repaglinide [package insert] Clayton, NC: Novo Nordisk Inc.; 2014. [Google Scholar]
- 44.Nateglinide [package insert] Parsippany, NJ: Watson Laboratories, Inc.; 2014. [Google Scholar]
- 45.Coniff RF, Shapiro JA, Robbins D et al. Reduction of glycosylated hemoglobin and postprandial hyperglycemia by acarbose in patient with NIDDM. Diabetes Care. 1995;18(6):817–824. doi: 10.2337/diacare.18.6.817. [DOI] [PubMed] [Google Scholar]
- 46.Acarbose [package insert] Wayne, NJ: Bayer Health Care Pharmaceuticals; 2014. [Google Scholar]
- 47.Kentrup H, Bongers H, Spengler M et al. Efficacy and safety of acarbose in patients with cystic fibrosis and impaired glucose tolerance. Eur J Pediatr. 1999;158(6):455–459. doi: 10.1007/s004310051119. [DOI] [PubMed] [Google Scholar]
- 48.Miller SA, St Onge EL. Sitagliptin: a dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Ann Pharmacother. 2006;40(7–8):1336–1343. doi: 10.1345/aph.1G665. [DOI] [PubMed] [Google Scholar]
- 49.Exenatide [package insert] New York, NY: Bristol-Myers Squibb Co.; 2014. [Google Scholar]
- 50.Liraglutide [package insert] Clayton, NC: Novo Nordisk Inc.; 2014. [Google Scholar]
- 51.Peters A. Incretin-based therapies: review of current clinical trial data. Am J Med. 2010;123(suppl 3):S28–S37. doi: 10.1016/j.amjmed.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 52.Kelly AS, Metzig AM, Rudser KD et al. Exenatide as a weight-loss therapy in extreme pediatric obesity: a randomized, controlled pilot study. Obesity. 2012;20(2):364–370. doi: 10.1038/oby.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly AS, Rudser KD, Nathan BM et al. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity. JAMA Pediatr. 2013;167(4):355–360. doi: 10.1001/jamapediatrics.2013.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raman VS, Yu X, Mason KJ et al. The role of adjunctive exenatide therapy in pediatric type 1 diabetes. Diabetes Care. 2010;33(6):1294–96. doi: 10.2337/dc09-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sitagliptin [package insert] Kenilworth, NJ: Merck & Company, Inc.; 2014. [Google Scholar]
- 56.Saxagliptin [package insert] New York, NY: Bristol-Myers Squibb Co.; 2014. [Google Scholar]
- 57.Alogliptin [package insert] Deerfield, IL: Takeda Pharmaceuticals America, Inc.; 2014. [Google Scholar]
- 58.Linagliptin [package insert] Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2014. [Google Scholar]
- 59.Schmitz O, Brock B, Rungby J. Amylin agonists: a novel approach in the treatment of diabetes. Diabetes. 2004;53(suppl 3):S233–238. doi: 10.2337/diabetes.53.suppl_3.s233. [DOI] [PubMed] [Google Scholar]
- 60.Weinzimer SA, Carria L, Sherr JL et al. Effect of pramlintide on prandial glycemic excursions during closed-loop control in adolescents and young adults with type 1 diabetes. Diabetes Care. 2012;35(10):1994–1999. doi: 10.2337/dc12-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chase HP, Lutz K, Pencek R et al. Pramlintide lowered glucose excursions and was well-tolerated in adolescents with type 1 diabetes: results from a randomized, single-blind, placebo-controlled, crossover study. J Pediatr. 2009;155(3):369–373. doi: 10.1016/j.jpeds.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 62.Heptulla RA, Rodriguez LM, Mason KJ, Haymond MW. Twenty-four-hour simultaneous subcutaneous basal-bolus administration of insulin and amylin in adolescents with type 1 diabetes decreases postprandial hyperglycemia. J Clin Endocrinol Metab. 2009;94(5):1608–1611. doi: 10.1210/jc.2008-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hassan K, Heptulla RA. Reducing post-prandial hyperglycemia with adjuvant premeal pramlintide and postmeal insulin in children with type 1 diabetes mellitus. Pediatric Diabetes. 2009;10(4):264–268. doi: 10.1111/j.1399-5448.2008.00490.x. [DOI] [PubMed] [Google Scholar]
- 64.Kishiyama C, Burdick PL, Cobry EC et al. A pilot trial of pramlintide home usage in adolescents with type 1 diabetes. Pediatrics. 2009;124(5):1344–1347. doi: 10.1542/peds.2008-3750. [DOI] [PubMed] [Google Scholar]
- 65.Pramlintide [package insert] San Diego, CA: Amylin Pharmaceuticals, Inc.; 2014. [Google Scholar]
- 66.Hoogwerf BJ, Doshi KB, Diab D. Pramlintide, the synthetic analogue of amylin: physiology, pathophysiology, and effects on glycemic control, body weight, and selected biomarkers of vascular risk. Vasc Health Risk Manag. 2008;4(2):355–362. doi: 10.2147/vhrm.s1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barnes NS, White PC, Hutchison MR. Time to failure of oral therapy in children with type 2 diabetes: a single center retrospective chart review. Pediatr Diabetes. 2012;13(7):578–582. doi: 10.1111/j.1399-5448.2012.00873.x. [DOI] [PubMed] [Google Scholar]


