Abstract
Background
T1ρ MRI is an imaging technique sensitive to proteoglycan (PG) content of hyaline cartilage. However, normative T1ρ values have not been established for the weightbearing cartilage of the hip, and it is not known whether it is uniform or whether there is topographic variation. Knowledge of the T1ρ profile of hyaline cartilage in the normal hip is important for establishing a baseline against which comparisons can be made to experimental and clinical arthritic subjects.
Questions/purposes
In this diagnostic study, we determined (1) the T1ρ MRI values of hyaline cartilage of the normal hip; and (2) whether the T1ρ MRI profile of the normal hip hyaline cartilage is uniform.
Methods
Fourteen asymptomatic volunteers (11 men, three women; mean age, 35 years) prospectively underwent 1.5-T T1ρ MRI of a single hip. The weightbearing hyaline cartilage bilayer of the acetabulum and femoral head was evaluated on sagittal images and segmented into four zones: (1) anterior; (2) anterosuperior; (3) posterosuperior; and (4) and posterior. For the full region of interest and within each zone and each sagittal slice, we calculated the mean T1ρ relaxation value, a parameter that indirectly quantifies PG content, where T1ρ is inversely related to PG concentration.
Results
There was variation in the T1ρ relaxation values depending on zone (anterior to posterior) and slice (medial to lateral). When combining the most anterior quadrants (Zones 1 and 2), the T1ρ relaxation values were lower than those in the combined posterior quadrants (Zones 3 and 4) (30.4 msec versus 32.2 msec, respectively; p = 0.002), reflecting higher PG concentration. There was a difference between the T1ρ relaxation values of the sagittal slices (p = 0.038), most pronounced anteriorly in Zone 1 (26.6 msec, p = 0.001). With a selective combination of zones and slices, there were lower mean T1ρ values in the anterolateral-most region compared with the remainder of the weightbearing portion of the hip (28.6 msec versus 32.2 msec, respectively; p = 0.001).
Conclusions
The T1ρ profile of normal hyaline cartilage of the hip is not uniform with the topographic differences identified suggesting regional variations in PG concentration. This study, through determination of lower T1ρ relaxation values, suggests inherently greater PG concentrations in the more anterolateral region of the normal hip hyaline cartilage. Furthermore, it demonstrates that T1ρ MRI has the ability to detect even subtle, microscopic local differences in hyaline cartilage composition. This technique has the potential to facilitate basic science and clinical research by serving as a noninvasive surrogate or biomarker of cartilage health and thus may be added to the growing repertoire of advanced, biochemical MRI techniques for evaluating hyaline cartilage.
Level of Evidence
Level III, diagnostic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
MRI is a powerful, noninvasive tool for evaluating joint hyaline cartilage [7, 15, 30]. Traditional MRI sequences have been effective in identifying qualitative, macroscopic changes of cartilage such as thickness and volume. Specifically, both routine MRI [17, 24] and MR arthrography [3, 18, 33, 37, 41] have been used to detect chondral abnormality in the hip. However, these gross structural alterations often manifest late in the osteoarthritis pathway at a point where treatment options may be limited to invasive, surgical reconstructive procedures [10]. One of the earliest biochemical changes within cartilage in the pathway of osteoarthritis is proteoglycan (PG) depletion from the extracellular matrix. This leads to weakening of the extracellular matrix collagen framework resulting in microscopic and eventually gross macroscopic structural changes to the cartilage [11, 21, 28]. Thus, the ability to detect early hyaline cartilage changes at the molecular level may facilitate earlier intervention and optimization of the nature and timing of treatment, potentially delaying and preventing onset or progression of arthritic disease. Consequently, advanced MRI techniques are being explored in hope of detecting biochemical changes in the extracellular matrix of cartilage before gross, irreversible morphologic damage occurs [22].
T1ρ MRI is a technique for evaluating hyaline cartilage through its sensitivity to changes in the extracellular matrix, including PG depletion. The T1ρ MRI protocol uses a special technique that is sensitive to the interactions between water and PG molecules of the extracellular matrix; changes in PG concentration will lead to detectable alterations in these interactions, which can be reflected quantitatively with a continuous parameter called the T1ρ relaxation time [1, 12]. Correlation between PG depletion and T1ρ relaxation time changes has been previously established with lower PG levels associated with higher T1ρ values [1, 13, 14, 31, 32, 38].
Two recent studies have demonstrated the feasibility of T1ρ MRI for evaluating hyaline cartilage of the hip, identifying differences between control and symptomatic hips with cam-type femoroacetabular impingement reflecting focal cartilage degeneration [29, 34]. In the normal hip, regional differences in T1ρ values have been identified from an analysis limited to a central strip of the weightbearing portion of the joint from anterior to posterior [34]; this suggests there is topographical variation in PG concentrations in the normal hip, being greater most anteriorly. However, in the normal hip, the entire weightbearing cartilage has not been evaluated to determine the normative T1ρ profile, specifically whether it is uniform throughout or if there is topographic variation. Knowledge of the T1ρ profile of hyaline cartilage of the normal hip is important to serve as a baseline against which future studies with experimental and arthritic subjects can be compared.
The specific aims of this study were to (1) establish the normal T1ρ profile of the weightbearing hyaline cartilage of the normal hip; and (2) determine whether the normal T1ρ profile is uniform or whether any topographic variations exist.
Subjects and Methods
Subjects
This prospective study was approved by our institutional research ethics board with informed consent obtained from all participants. From August 2010 to November 2012, 16 volunteer subjects were enrolled at a tertiary hospital with an orthopaedic service specializing in hip arthritis and surgery. The MR imaging of two subjects was discarded: one as a result of improper positioning of the hip MRI coil that resulted in field and thus image signal inhomogeneity and the other resulting from patient motion artifact. This resulted in a final number of 14 subjects (11 men, three women; mean age, 35 years; range, 24–48 years; all were white). All subjects were asymptomatic and had no clinical history of prior hip surgery or hip pathology. As part of another ongoing study, they had undergone CT of their hips confirming normal osseous morphology and excluding any arthritic changes. For all subjects, the alpha angle was < 51° on oblique axial plane image through the midfemoral neck (mean 42°, SD 5°), the acetabulum was anteverted (mean 17°, SD 7°) with no cases of retroversion, and the lateral center edge angle > 25° (mean 34°, SD 3°). The presence of normal, intact hyaline cartilage along the weightbearing portion of the hip was confirmed by MRI by a musculoskeletal radiologist (KSR) as part of the experimental imaging protocol.
T1ρ MRI Protocol
All subjects underwent T1ρ MRI of a unilateral asymptomatic hip performed on a 1.5-T MRI scanner (Magnetom® Symphony; Siemens Medical Solutions, Erlangen, Germany) with a large, flexible, receive-only, four-channel surface coil wrapped around the hip. Subjects were positioned supine with the leg fixed in neutral rotation. The T1ρ protocol was a turbo spin echo-based sequence acquired in an oblique sagittal plane parallel to the acetabular fossa opening using the following parameters: field of view = 180 mm × 180 mm2, 22 slices, slice thickness = 3 mm, 384 × 384 matrix yielding an in-plane resolution of 0.46 × 0.46 mm2, TR/TE = 274/13 msec, bandwidth = 130 Hz/pixel, sample average (number of excitations [NEX]) = 1, spin-lock amplitude (B1) = 400 Hz, variable times of spin locking = 12, 18, 25, 35, and 45 msec, and total scan time = 21 minutes. A fast-spin echo intermediate-weighted sequence for anatomic depiction of the hip with matched plane, field of view, slice thickness, matrix, and resolution was also performed with TR/TE = 3090/24 msec, NEX = 2, bandwidth = 100 Hz/pixel, and echo train length = 7.
Image Analysis
Images were transferred to a workstation for offline, manual segmentation of articular cartilage and quantification of T1ρ using custom routines written in MATLAB® (The MathWorks Inc, Natick, MA, USA). Segmentation analysis was conducted by an experienced imaging scientist (AC-B). An oblique coronal localizer sequence was used to determine the transverse coverage of the hip that included almost the entire sourcil, the superior weightbearing aspect of the joint. In the majority of subjects, seven sagittal slices were mapped, starting from the lateral rim of acetabular roof, extending inward. The entire sourcil was not included in analysis of some subjects as a result of inherent curvature of the hip, the degree of which varied between subjects. This occurred most medially where the increasing curvature of the articular surface introduced too much volume averaging artifact with indistinctness of the margins of the cartilage. In smaller-habitus patients, the seventh slice that entered the superomedial joint was not included in the analysis for similar volume averaging reason. The region of interest for mapping included the entire visualized hyaline cartilage of both the acetabular roof and femoral head, as a single bilayer, segmented on contiguous slices. The matched, anatomic intermediate-weighted image was used to facilitate delineation of the hyaline cartilage margins for the purpose of segmentation, ensuring that the labrum and subchondral compact bone were excluded from the region of interest. A T1ρ map was calculated by fitting the images, on a pixel-by-pixel basis, to a monoexponential function using a Levenberg-Marquart fitting algorithm [23, 38].
After segmentation, four zones were defined using the center of the femoral head with lines 45° to each other: (1) anterior; (2), anterosuperior; (3) posterosuperior, and (4) posterior. The center of the femoral head was estimated on the sagittal slice through the midfemoral head as follows: (1) a pair of noncontiguous pixels, 1 cm apart, was selected in the anterior and posterior femoral cortical bone; and (2) perpendiculars to the lines connecting each pair of pixels were automatically found and the intersection of the lines deemed the center of the femoral head. A vertical line, passing through the center and the superior apex of the femoral head, was used as a reference to subdivide the four zones (Fig. 1).
Fig. 1.
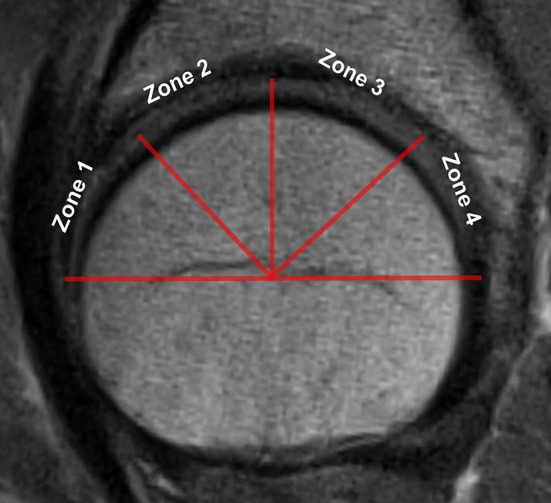
A vertical line, parallel to B0 passing through the center and the superior apex of the femoral head, is used as a reference to subdivide the four zones: (1) anterior; (2) anterosuperior; (3) posterosuperior; and (4) posterior.
Statistical Analysis
A mixed model for statistical analysis was used to compare the various zones and slices to determine whether there were any regional differences in the T1ρ values. Both slice and zone were considered as fixed effects and patients as a random effect in the mixed model. Tukey adjustment was used for multiple comparisons with unequal sample sizes. The mean T1ρ relaxation value for the cartilage bilayer was calculated in each subject for the full region of interest and within each zone and each sagittal slice. The initial analysis in the transverse direction was by individual slice because it was unknown whether any potential nonuniformity in the T1ρ profile would be in the form of focal, abrupt transition in the T1ρ values at a critical or specific location (ie, on a specific slice) or gradual over several slices with a gradient. This would avoid inadvertent masking of any nonuniformity occurring in small focal areas. Select individual zones and slices were also combined to generate larger regions within the region of interest that were also similarly analyzed and compared. All statistical analyses were performed with the SAS® software package (Version 9.2; SAS Institute Inc, Cary, NC, USA) with statistical significance defined as p values < 0.05.
Results
The T1ρ relaxation values of the four zones for all slices combined were different (p = 0.002). Moving from Zones 1 to 4, anterior to posterior, the T1ρ relaxation values increased, suggesting that a gradient exists with the PG concentration greatest most anteriorly (Table 1). For the analysis by individual slices (Table 2), there was a zonal difference only laterally on Slice 1 (p < 0.001). The mean T1ρ relaxation time value for the entire region of interest, combining all slices and all zones, was 31.1 msec (95% confidence interval, 29.9–33.3 msec).
Table 1.
T1ρ relaxation time by zone averaged over all slices and subjects
| T1ρ relaxation time (msec) | |||
|---|---|---|---|
| Anterior | Posterior | ||
| Zone 1 | Zone 2 | Zone 3 | Zone 4 |
| 30.0 (27.6–32.4) | 30.8 (28.4–33.2) | 31.4 (29.0–33.8) | 33.3 (30.8–35.7) |
Values are expressed as mean with 95% confidence interval in parentheses. The T1ρ relaxation times increase from anterior to posterior, from Zone 1 to 4 (p = 0.002).
Table 2.
T1ρ relaxation time averaged over all subjects by individual zone and slice
| Zone | T1ρ relaxation time (msec) | ||||||
|---|---|---|---|---|---|---|---|
| Slice 1* | Slice 2 | Slice 3 | Slice 4 | Slice 5 | Slice 6 | Slice 7 | |
| Zone 1† | 26.6 (23.4–29.9) | 27.5 (24.2–30.7) | 27.9 (24.6–31.2 | 29.2 (25.9–32.4) | 32.3 (29.1–35.6) | 32.9 (29.7–36.2) | 33.8 (27.9–39.7) |
| Zone 2 | 29.9 (26.6–33.2) | 29.4 (26.1–32.7) | 30.5 (27.2–33.7) | 31.8 (28.5–35.0) | 32.0 (28.7–35.3) | 32.8 (29.5–36.1) | 28.9 (23.0–34.8) |
| Zone 3 | 31.7 (28.4–35.0) | 31.2 (28.0–34.5) | 31.5 (28.2–34.8) | 32.3 (29.0–35.5) | 31.3 (28.0–34.5) | 30.8 (27.5–34.1) | 31.3 (25.4–37.2) |
| Zone 4 | 37.8 (33.4–42.3) | 31.5 (27.9–35.0) | 30.0 (26.7–33.4) | 32.9 (29.6–36.3) | 33.3 (30.0–36.6) | 32.3 (29.1–35.6) | 35.2 (29.3–41.1) |
Values are expressed as mean with 95% confidence interval in parentheses; *differences among the four zones, p < 0.001; †differences among the seven slices, p = 0.001.
The T1ρ relaxation values were also different among the seven sagittal slices (p = 0.038) (Table 3) when averaged over all zones. For the analysis by individual zones (Table 2), the Zone 1 T1ρ value (26.6 msec) was lower (p = 0.001) than the other three zones, suggesting higher PG concentration more anteriorly in the joint.
Table 3.
T1ρ relaxation time for all subjects by slice
| T1ρ relaxation time (msec) | ||||||
|---|---|---|---|---|---|---|
| Lateral | Medial | |||||
| Slice 1 | Slice 2 | Slice 3 | Slice 4 | Slice 5 | Slice 6 | Slice 7 |
| 31.5 (29.0–34.1) | 29.9 (27.4–32.4) | 30.0 (27.5–32.4) | 31.5 (29.1–34.0) | 32.2 (29.8–34.7) | 32.2 (29.8–34.7) | 32.3 (28.8–35.9) |
Values are expressed as mean with 95% confidence interval in parentheses; differences among the seven slices, p = 0.038.
Combining slices to generate lateral (Slices 1–3) and medial (Slices 4–7) zones resulted in the splitting of the overall region of interest into four regions: anterolateral (Zones 1, 2; Slices 1–3), anteromedial (Zones 1, 2; Slices 4–7), posterolateral (Zones 3, 4; Slices 1–3), and posteromedial (Zones 3, 4; Slices 4–7). The anterolateral zone had a lower mean T1ρ relaxation value (28.6 msec, p < 0.001) than the other three zones (Table 4).
Table 4.
T1ρ relaxation time for the four regions*
| Region | T1ρ relaxation time (msec) |
|---|---|
| Anterolateral | 28.6 (26.3–31.0) |
| Anteromedial | 31.8 (29.5–34.1) |
| Posterolateral | 31.7 (29.3–34.1) |
| Posteromedial | 32.2 (29.9–34.5) |
| p value (anterolateral versus others) | < 0.001 |
* Anterolateral = Zones 1, 2; Slices 1–3; anteromedial = Zones 1, 2; Slices 4–7; posterolateral = Zones 3, 4; Slices 1–3; posteromedial = Zones 3, 4; Slices 4–7; values are expressed as mean with 95% confidence interval in parentheses.
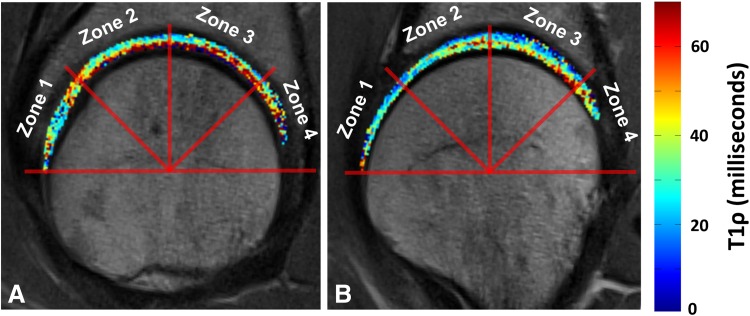
Images from a subject through the lateral and medial portions of the region of interest demonstrated lower T1ρ relaxation values more anteriorly and laterally (Fig. 2).
Fig. 2A–B.
Images from a subject through the (A) lateral and (B) medial portions of the region of interest demonstrate lower T1ρ relaxation values more anteriorly and laterally.
Discussion
Advanced MRI techniques have shown great promise for the noninvasive evaluation of hyaline cartilage at the molecular level [16, 26]. T1ρ MRI is a quantitative cartilage mapping technique sensitive to PG content. Changes in T1ρ relaxation values have been shown to correlate with biochemical measures of PG depletion and alteration of physical properties of cartilage with T1ρ relaxation times being inversely related to PG concentration [13]. The early detection of hip hyaline cartilage biochemical damage, before gross macroscopic and structural changes, may allow for earlier detection and thus intervention to prevent or minimize the impact of arthritis [2]. However, establishing the normative T1ρ profile of hip hyaline cartilage is important to determine the natural distribution of PG, identify any topographic variation, and serve as a baseline for future studies in symptomatic and arthritic hips. In this study, we found differences in the T1ρ relaxation values among the four zones with the lower relaxation values (ie, greater PG content) anterior compared with posterior. These differences were also present in the transverse direction with lower T1ρ relaxation values laterally, but only anteriorly in Zone 1.
There are recognized limitations of this study. The sample size was small, with 14 subjects, although to our knowledge, this is the largest number of control subjects ever evaluated with T1ρ MRI of the hip. A larger group may have resulted in more significant differences in the T1ρ values, strengthening the conclusion of nonuniformity in the cartilage. The sample lacked diversity in terms of sex (three females, 11 males) and race (all white). However, the age range of study subjects matches that of patients with femoroacetabular impingement. Future studies with a larger sample size as well as greater balance and diversity in subject demographics are required to confirm this nonuniformity and establish more definitive normative values. With regard to the MRI protocol, a slice thickness of 3 mm was used; thinner slices could have reduced volume averaging of hyaline cartilage with synovial fluid and subchondral compact bone related to the curvature of the joint space and articular surfaces. Although the in-plane resolution of 0.46 × 0.46 mm2 did reveal statistically significant findings, a higher matrix with smaller pixels would have resulted in greater resolution, reducing any partial volume averaging effects and potentially allowing for accurate separation of the acetabular and femoral cartilages. The current study protocol did not allow for differentiation between acetabular and femoral cartilage. Thus, it is unknown what the contribution of each surface cartilage was to the T1ρ relaxation values and the significant differences identified. The nonweightbearing, more medial articular cartilage of the hip was not evaluated given only sagittal images were acquired. Future studies using higher field strengths and subsequently greater signal-to-noise ratio and higher resolution three-dimensional sequences would allow for global evaluation of joint cartilage with a more exhaustive assessment of the different regions of femoral and acetabular articular surfaces individually. Although the current study used traditional sagittal parallel plane images, radial images have also been successfully used to perform cartilage mapping [4]. Radial images may have allowed for reduced volume averaging artifact, because every slice would be perpendicular to the cartilage surface.
The mean T1ρ relaxation time value for hip hyaline cartilage of 31.1 msec for the region of interest in the current study is lower than that found in the other limited studies on normal control subjects, which reported mean T1ρ values of 38.7 msec [8] and 35.5 msec [34]. However, in both of those studies, the region of interest excluded the far lateral slices, sampling a different and smaller region of hyaline cartilage than in the current project. Furthermore, the spin-lock frequency used in the MRI protocol in the current study (400 Hz) was lower than in the referenced studies (500 Hz). It is known that the T1ρ relaxation value is positively related to the spin-lock frequency, and thus the lower frequency used in our study may be a technical explanation for the lower value obtained [32]. In addition to T1ρ, other MRI techniques for evaluating the biochemical composition and structure of hyaline cartilage have been studied. In the normal, asymptomatic hip, delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) [27, 35], T2 [36], and T2* [4] cartilage mapping have been conducted. dGEMRIC is a PG-sensitive, proton MRI-based technique that requires the intravenous injection of a gadolinium contrast-based agent followed by a regimented exercise protocol before imaging [6, 39]. Comparing normal and diseased hips, dGEMRIC has the ability to prospectively differentiate between normal and abnormal cartilage, respectively, with diseased hips demonstrating lower dGEMRIC indices reflecting lower PG concentration [42]. T2 and T2* relaxation time mapping have been investigated as noncontrast techniques. However, they are predominantly sensitive to changes in collagen content and organization [25]. Recent studies have recognized that T1ρ and T2 may provide complementary information, predominantly but not necessarily exclusively about PG and collagen, respectively [5, 20]. Although the current study used traditional sagittal parallel plane images, radial images have also been successfully used to perform cartilage mapping [4].
Our study found the T1ρ profile to be nonuniform, identifying several regional differences. The variations were in both the anterior-to-posterior and medial-to-lateral directions based on the zonal and slice analyses, respectively. The more anterior and more lateral cartilage demonstrated lower T1ρ relaxation values, suggestive of inherently higher PG concentration. The pattern of increasing T1ρ values from anterior to posterior was also demonstrated in a prior study evaluating normal hip hyaline cartilage [34]. The variation in the transverse direction from lateral to medial has not been previously published with T1ρ, although variations have been observed with T2 mapping, a technique believed to be more specific for collagen content and structure [36]. The difference between the T1ρ relaxation value of the anterolateral region (28.6 msec) and of the other three regions (mean 31.9 msec), an increase of 11.5%, is considered to be at least moderate. As a comparative reference, in a prior study in hips, the T1ρ value of normal control hips was 35.5 msec compared with symptomatic femoroacetabular impingement hips in which the value was 38.8 msec, an increase of 9% [34]. Another study using bovine cartilage explants found that a 6% increase in the T1ρ value was associated with a 12% reduction in PG content [38].
Topographic variation in biochemical composition of hip hyaline cartilage has also been demonstrated with dGEMRIC [4], although the differences were not significant, possibly related to the small sample size. A possible explanation for these zonal differences in PG concentration measured by quantitative cartilage MRI mapping is the known variability in hyaline cartilage thickness within the hip [19, 40]. CT arthrography has demonstrated greater cartilage thickness going from medial to lateral on the acetabular side and from the periphery to the center on the femoral head side with values ranging from 1.4 to 4.8 mm on the acetabular side and 0.95 to 1.36 mm on the femoral side [41]. It has been proposed that cartilage thickness is dependent on the degree of functional stressing [9, 19] whereby greater stresses lead to the development of a thicker articular cartilage [9].
In conclusion, our study demonstrated that the hyaline cartilage of the normal hip did not demonstrate a uniform T1ρ profile. Rather, topographic differences existed with lower T1ρ values found in the anterior- and lateral-most region of the joint, suggestive of higher PG concentrations. The inherently greater PG concentration in particular regions of the joint may be evolutionary or adaptive, reflecting local differences in biomechanical load and stress. Thus, cartilage mapping has the potential to become a tool in compositional analysis of hyaline cartilage, which in turn may facilitate the study and understanding of joint physiology and biomechanics. Furthermore, the study demonstrates that T1ρ MRI has the ability to detect even subtle, microscopic local differences in hyaline cartilage composition. This technique has the potential to facilitate basic science and clinical research by serving as a noninvasive surrogate or biomarker of cartilage health and thus may be added to the growing repertoire of advanced biochemical MRI techniques for evaluating hyaline cartilage.
Acknowledgments
We thank Gillian Parker BSc, for patient recruitment and coordination, Jae-Jin Ryu PhD, for study management, Francine McCullagh for MRI scanning, and Tinghua Zhang MSc, from the Ottawa Hospital Research Institute Method Centres (Ottawa, Ontario, Canada) for statistical analysis.
Footnotes
The institution of one or more of the authors (KSR, GM, PEB) has received, during the study period, funding from an operating grant from the Canadian Institutes of Health Research.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at The Ottawa Hospital, Ottawa, Ontario, Canada.
References
- 1.Akella SV, Regatte RR, Gougoutas AJ, Borthakur A, Shapiro EM, Kneeland JB, Leigh JS, Reddy R. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med. 2001;46:419–423. doi: 10.1002/mrm.1208. [DOI] [PubMed] [Google Scholar]
- 2.Beaule PE, Kim YJ, Rakhra KS, Stelzeneder D, Brown TD. New frontiers in cartilage imaging of the hip. Instr Course Lect. 2012;61:253–262. [PubMed] [Google Scholar]
- 3.Beaule PE, Zaragoza E, Copelan N. Magnetic resonance imaging with gadolinium arthrography to assess acetabular cartilage delamination. A report of four cases. J Bone Joint Surg Am. 2004;86:2294–2298. doi: 10.2106/00004623-200410000-00025. [DOI] [PubMed] [Google Scholar]
- 4.Bittersohl B, Hosalkar HS, Hughes T, Kim YJ, Werlen S, Siebenrock KA, Mamisch TC. Feasibility of T2* mapping for the evaluation of hip joint cartilage at 1.5T using a three-dimensional (3D), gradient-echo (GRE) sequence: a prospective study. Magn Reson Med. 2009;62:896–901. doi: 10.1002/mrm.22096. [DOI] [PubMed] [Google Scholar]
- 5.Blumenkrantz G, Majumdar S. Quantitative magnetic resonance imaging of articular cartilage in osteoarthritis. Eur Cells Mater. 2007;13:76–86. doi: 10.22203/ecm.v013a08. [DOI] [PubMed] [Google Scholar]
- 6.Burstein D, Bashir A, Gray ML. MRI techniques in early stages of cartilage disease. Invest Radiol. 2000;35:622–638. doi: 10.1097/00004424-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Burstein D, Gray ML. Is MRI fulfilling its promise for molecular imaging of cartilage in arthritis? Osteoarthritis Cartilage. 2006;14:1087–1090. doi: 10.1016/j.joca.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Carballido-Gamio J, Link TM, Li X, Han ET, Krug R, Ries MD, Majumdar S. Feasibility and reproducibility of relaxometry, morphometric, and geometrical measurements of the hip joint with magnetic resonance imaging at 3T. J Magn Reson Imaging. 2008;28:227–235. doi: 10.1002/jmri.21411. [DOI] [PubMed] [Google Scholar]
- 9.Carter DR, Beaupre GS, Wong M, Smith RL, Andriacchi TP, Schurman DJ. The mechanobiology of articular cartilage development and degeneration. Clin Orthop Relat Res. 2004;427(Suppl):S69–S77. doi: 10.1097/01.blo.0000144970.05107.7e. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham T, Jessel R, Zurakowski D, Millis MB, Kim YJ. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage to predict early failure of Bernese periacetabular osteotomy for hip dysplasia. J Bone Joint Surg Am. 2006;88:1540–1548. doi: 10.2106/JBJS.E.00572. [DOI] [PubMed] [Google Scholar]
- 11.Dijkgraaf LC, de Bont LG, Boering G, Liem RS. The structure, biochemistry, and metabolism of osteoarthritic cartilage: a review of the literature. J Oral Maxillofac Surg. 1995;53:1182–1192. doi: 10.1016/0278-2391(95)90632-0. [DOI] [PubMed] [Google Scholar]
- 12.Duvvuri U, Goldberg AD, Kranz JK, Hoang L, Reddy R, Wehrli FW, Wand AJ, Englander SW, Leigh JS. Water magnetic relaxation dispersion in biological systems: the contribution of proton exchange and implications for the noninvasive detection of cartilage degradation. Proc Natl Acad Sci U S A. 2001;98:12479–12484. doi: 10.1073/pnas.221471898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duvvuri U, Kudchodkar S, Reddy R, Leigh JS. T(1rho) relaxation can assess longitudinal proteoglycan loss from articular cartilage in vitro. Osteoarthritis Cartilage. 2002;10:838–844. doi: 10.1053/joca.2002.0826. [DOI] [PubMed] [Google Scholar]
- 14.Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38:863–867. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- 15.Gold GE, Burstein D, Dardzinski B, Lang P, Boada F, Mosher T. MRI of articular cartilage in OA: novel pulse sequences and compositional/functional markers. Osteoarthritis Cartilage. 2006;14(Suppl A):A76–A86. doi: 10.1016/j.joca.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Gray ML, Burstein D, Kim YJ, Maroudas A. 2007 Elizabeth Winston Lanier Award Winner. Magnetic resonance imaging of cartilage glycosaminoglycan: basic principles, imaging technique, and clinical applications. J Orthop Res. 2008;26:281–291. doi: 10.1002/jor.20482. [DOI] [PubMed] [Google Scholar]
- 17.James SL, Ali K, Malara F, Young D, O’Donnell J, Connell DA. MRI findings of femoroacetabular impingement. AJR Am J Roentgenol. 2006;187:1412–1419. doi: 10.2214/AJR.05.1415. [DOI] [PubMed] [Google Scholar]
- 18.Kassarjian A, Yoon LS, Belzile E, Connolly SA, Millis MB, Palmer WE. Triad of MR arthrographic findings in patients with cam-type femoroacetabular impingement. Radiology. 2005;236:588–592. doi: 10.1148/radiol.2362041987. [DOI] [PubMed] [Google Scholar]
- 19.Kurrat HJ, Oberlander W. The thickness of the cartilage in the hip joint. J Anat. 1978;126:145–155. [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Pai A, Blumenkrantz G, Carballido-Gamio J, Link T, Ma B, Ries M, Majumdar S. Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med. 2009;61:1310–1318. doi: 10.1002/mrm.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohmander LS. Articular cartilage and osteoarthrosis. The role of molecular markers to monitor breakdown, repair and disease. J Anat. 1994;184:477–492. [PMC free article] [PubMed] [Google Scholar]
- 22.Mamisch TC, Bittersohl B, Hughes T, Kim YJ, Welsch GH, Dudda M, Siebenrock KA, Werlen S, Trattnig S. Magnetic resonance imaging of the hip at 3 Tesla: clinical value in femoroacetabular impingement of the hip and current concepts. Semin Musculoskelet Radiol. 2008;12:212–222. doi: 10.1055/s-0028-1083105. [DOI] [PubMed] [Google Scholar]
- 23.Marquart D. An algorithm for least squares estimation of non-linear parameters. Journal of Society of Industrial and Applied Mathematics. 1963;11:431–441. doi: 10.1137/0111030. [DOI] [Google Scholar]
- 24.Mintz DN, Hooper T, Connell D, Buly R, Padgett DE, Potter HG. Magnetic resonance imaging of the hip: detection of labral and chondral abnormalities using noncontrast imaging. Arthroscopy. 2005;21:385–393. doi: 10.1016/j.arthro.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8:355–368. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 26.Mosher TJ, Walker EA, Petscavage-Thomas J, Guermazi A. Osteoarthritis year 2013 in review: imaging. Osteoarthritis Cartilage. 2013;21:1425–1435. doi: 10.1016/j.joca.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Pollard TC, McNally EG, Wilson DC, Wilson DR, Madler B, Watson M, Gill HS, Carr AJ. Localized cartilage assessment with three-dimensional dGEMRIC in asymptomatic hips with normal morphology and cam deformity. J Bone Joint Surg Am. 2010;92:2557–2569. doi: 10.2106/JBJS.I.01200. [DOI] [PubMed] [Google Scholar]
- 28.Poole AR. An introduction to the pathophysiology of osteoarthritis. Front Biosci. 1999;4:D662–D670. doi: 10.2741/Poole. [DOI] [PubMed] [Google Scholar]
- 29.Rakhra KS, Lattanzio PJ, Cardenas-Blanco A, Cameron IG, Beaule PE. Can T1-rho MRI detect acetabular cartilage degeneration in femoroacetabular impingement? A pilot study. J Bone Joint Surg Br. 2012;94:1187–1192. doi: 10.1302/0301-620X.94B9.29981. [DOI] [PubMed] [Google Scholar]
- 30.Recht MP, Goodwin DW, Winalski CS, White LM. MRI of articular cartilage: revisiting current status and future directions. AJR Am J Roentgenol. 2005;185:899–914. doi: 10.2214/AJR.05.0099. [DOI] [PubMed] [Google Scholar]
- 31.Regatte RR, Akella SV, Borthakur A, Kneeland JB, Reddy R. Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Acad Radiol. 2002;9:1388–1394. doi: 10.1016/S1076-6332(03)80666-9. [DOI] [PubMed] [Google Scholar]
- 32.Regatte RR, Akella SV, Borthakur A, Reddy R. Proton spin-lock ratio imaging for quantitation of glycosaminoglycans in articular cartilage. J Magn Reson Imaging. 2003;17:114–121. doi: 10.1002/jmri.10228. [DOI] [PubMed] [Google Scholar]
- 33.Schmid MR, Notzli HP, Zanetti M, Wyss TF, Hodler J. Cartilage lesions in the hip: diagnostic effectiveness of MR arthrography. Radiology. 2003;226:382–386. doi: 10.1148/radiol.2262020019. [DOI] [PubMed] [Google Scholar]
- 34.Subburaj K, Valentinitsch A, Dillon AB, Joseph GB, Li X, Link TM, Vail TP, Majumdar S. Regional variations in MR relaxation of hip joint cartilage in subjects with and without femoralacetabular impingement. Magn Reson Imaging. 2013;31:1129–1136. doi: 10.1016/j.mri.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tiderius CJ, Jessel R, Kim YJ, Burstein D. Hip dGEMRIC in asymptomatic volunteers and patients with early osteoarthritis: the influence of timing after contrast injection. Magn Reson Med. 2007;57:803–805. doi: 10.1002/mrm.21190. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe A, Boesch C, Siebenrock K, Obata T, Anderson SE. T2 mapping of hip articular cartilage in healthy volunteers at 3T: a study of topographic variation. J Magn Reson Imaging. 2007;26:165–171. doi: 10.1002/jmri.21014. [DOI] [PubMed] [Google Scholar]
- 37.Werlen S. Magnetic resonance arthrography of the hip in femoroacetabular impingement. Oper Tech Orthop. 2005;15:191–203. doi: 10.1053/j.oto.2005.07.007. [DOI] [Google Scholar]
- 38.Wheaton AJ, Dodge GR, Elliott DM, Nicoll SB, Reddy R. Quantification of cartilage biomechanical and biochemical properties via T1rho magnetic resonance imaging. Magn Reson Med. 2005;54:1087–1093. doi: 10.1002/mrm.20678. [DOI] [PubMed] [Google Scholar]
- 39.Williams A, Gillis A, McKenzie C, Po B, Sharma L, Micheli L, McKeon B, Burstein D. Glycosaminoglycan distribution in cartilage as determined by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC): potential clinical applications. AJR Am J Roentgenol. 2004;182:167–172. doi: 10.2214/ajr.182.1.1820167. [DOI] [PubMed] [Google Scholar]
- 40.Wyler A, Bousson V, Bergot C, Polivka M, Leveque E, Vicaut E, Laredo JD. Hyaline cartilage thickness in radiographically normal cadaveric hips: comparison of spiral CT arthrographic and macroscopic measurements. Radiology. 2007;242:441–449. doi: 10.1148/radiol.2422051393. [DOI] [PubMed] [Google Scholar]
- 41.Wyler A, Bousson V, Bergot C, Polivka M, Leveque E, Vicaut E, Laredo JD. Comparison of MR-arthrography and CT-arthrography in hyaline cartilage-thickness measurement in radiographically normal cadaver hips with anatomy as gold standard. Osteoarthritis Cartilage. 2009;17:19–25. doi: 10.1016/j.joca.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 42.Zilkens C, Miese F, Kim YJ, Hosalkar H, Antoch G, Krauspe R, Bittersohl B. Three-dimensional delayed gadolinium-enhanced magnetic resonance imaging of hip joint cartilage at 3T: a prospective controlled study. Eur J Radiol. 2012;81:3420–3425. doi: 10.1016/j.ejrad.2012.04.008. [DOI] [PubMed] [Google Scholar]