Abstract
Objective
To determine the effect of virally-suppressive antiretroviral therapy on cortical neurodegeneration and associated neurocognitive impairment.
Design
Retrospective, postmortem observational study.
Methods
Clinical neuropsychological and postmortem neuropathology data were analyzed in 90 human immunodeficiency virus-infected volunteers from the general community who had never undergone antiretroviral therapy (n=7, “naïve”) or who had undergone antiretroviral therapy and whose plasma viral load was detectable (n = 64 “unsuppressed”) or undetectable (n = 19, “suppressed”) at the last clinical visit prior to death. Subjects were predominately male (74/90, 82%) with a mean age of 44.7 years (SD 9.8). Cortical neurodegeneration was quantified by measuring microtubule-associated protein (MAP2) and synaptophysin (SYP) density in midfrontal cortex tissue sections.
Results
The suppressed group had higher SYP density than the naïve group (p = 0.007) and higher MAP2 density than the unsuppressed group (p = 0.04). The suppressed group had lower odds of human immunodeficiency virus-associated neurocognitive disorders than naïve (OR 0.07, p = 0.03). Higher SYP was associated with lower likelihood of human immunodeficiency virus-associated neurocognitive disorders in univariable (OR 0.8, p=0.03) and multivariable models after controlling for antiretroviral treatment and brain human immunodeficiency virus p24 protein levels (OR 0.72, p=0.01).
Conclusions
We conclude that virally suppressive antiretroviral treatment protects against cortical neurodegeneration. Further, we find evidence supporting the causal chain from treatment-mediated peripheral and central nervous system viral load suppression to reduced neurodegeneration and improved neurocognitive outcomes.
Keywords: HIV, neurodegenerative disorders, mild cognitive impairment, antiretroviral therapy, acquired immunodeficiency syndrome, brain
Introduction
The widespread use and efficacy of antiretroviral treatment (ART) has fundamentally altered the clinical course of HIV disease and the epidemiology of HIV-associated neurocognitive disorders (HAND). Before the introduction of effective ART, severe HAND was more commonly observed with advanced HIV disease; the prevalence of severe HAND has since decreased from 10-15% in the pre-ART era to around 2%, although milder forms persist [1].
HIV infection may damage neurons through toxic viral factors or through activation of the host immune system that may then amplify neuronal damage [2]. ART likely improves HAND by suppressing systemic and CNS HIV replication and by returning CD4+ T-cells toward normal levels [3-7]. Virally suppressive ART regimens have substantially reduced the prevalence of severe HIV-associated dementia, and antiretroviral regimens that distribute well into the CNS may more effectively prevent neurocognitive decline [5, 7, 8].
Despite these observations, causal pathways relating HIV infection to neurocognitive impairment are incompletely understood and the potential influence of ART on these pathways is unclear. One commonly held model suggests that viral replication drives neurodegeneration, which then leads to HAND, and ART might ameliorate both neurodegeneration and HAND by reducing viral replication. In the present study we evaluated whether ART exerted a protective effect against HAND by reducing brain viral load and neurodegeneration. We hypothesized that suppressive ART, as defined by undetectable plasma viral load, would decrease cortical neurodegeneration relative to non-suppressive ART or no ART. We also sought to determine whether a higher degree of neurodegeneration was predictive of HAND in our cohort. Finally, we hypothesized that suppressive ART would reduce the odds of HAND.
Methods
Participants
We assessed 90 HIV-infected participants enrolled in the California NeuroAids Tissue Network (CNTN). Participants recruited into the larger CNTN parent study were recruited because of suspected risk for death within two years of enrollment based on progression of HIV disease (e.g., CD4 count less than 50 cells/mm3). The participants enrolled in the present study were those with postmortem midfrontal cortex tissue specimens available for characterization and available data on antiretroviral therapy use at their final study visit prior to death. All participants meeting these criteria in the CNTN study were enrolled in this study. The CNTN study was reviewed and approved by the local institutional review board and all participants provided written informed consent.
Neuromedical and Antiretroviral Therapy Assessments
Participants completed in-life neuromedical evaluations that included general physical and neurological examinations, medical and medication history, and laboratory studies including CD4+ lymphocyte counts, plasma and CSF HIV RNA levels, and routine hematology. Information on the use of antiretrovirals was collected at each neuromedical evaluation and through postmortem review of medical chart history. Participants were placed into one of three ART categories. “ART Naïve” individuals had never been exposed to antiretrovirals in the course of their disease. Among those who had taken at least one course of antiretroviral therapy, non-suppressive (NS-ART) and suppressive (S-ART) groups were defined by detectable or undetectable plasma HIV RNA levels, respectively, at the last antemortem clinical evaluation. The detection limit was defined as the lower limit of quantitation (LLQ), which varied from 40 to 400 copies/mL based on the assay available at the time of assessment. Among the 89 individuals with plasma HIV RNA data, the LLQ was 400 copies/mL for 59 (78%), 50 copies/mL for 25 (28%), 40 copies/mL for 3 (3%) and 75 copies/mL for 2 (2%).
Neuropsychological Testing
Clinical neuropsychological (NP) evaluations were scheduled at 6-12 month intervals throughout the course of the study and the last NP assessment prior to death determined the final NP status. As previously described, raw NP scores were corrected for age, education, sex, and ethnicity when possible [8]. Clinical ratings, using a 9-point clinical rating scale for each of seven NP domains, were assigned and applied toward HAND diagnoses [9, 10]. A consensus HAND diagnosis was determined via a multidisciplinary case conference. In order of increasing severity of impairment, HAND was classified as asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HIV-associated dementia (HAD), consistent with the current research guidelines [11]. Among the 69 participants with sufficient data to determine a neurocognitive diagnosis, 20 (29%) were neurocognitively normal, 26 (38%) were diagnosed with HAND, and 23 (33%) were diagnosed with neurocognitive impairment due to a cause other than HIV (NPI-O). Among those diagnosed with HAND, eight were diagnosed with ANI, 11 were diagnosed with MND, and seven were diagnosed with HAD. For all analyses predicting HAND, we excluded participants with a diagnosis of NPI-O.
Quantification of Cortical Neurodegeneration and HIV Levels
As soon as possible after death, tissue blocks measuring 4 cm3 were extracted from the right dorsolateral midfrontal cortex. Tissue blocks were fixed overnight in 4% paraformaldehyde and sectioned at 40 μm with a Leica vibratome (Vienna, Austria). Sections were stored at -30°C in 30% glycerin, 30% ethylene glycol, and 40% phosphate-buffered saline. Fluorescence immunohistochemistry was carried out for synaptophysin (SYP), a marker of presynaptic terminals, and microtubule-associated protein-2 (MAP2), a marker of neuronal cell bodies and dendrites, as described previously [8].
MAP2 and SYP-immunostained sections of the midfrontal cortex were imaged at 63× with a laser scanning confocal microscope (MRC1024 BioRad Scanner, BioRad, Hercules, California, USA) using the LaserSharp Acquisition 3.2 software (Hercules, California, USA). Images were analyzed using ImageJ (v. 1.27, National Institute of Health, Bethesda, Maryland, USA; 2002). The extent of neurodegeneration was measured by quantifying the percentage area of the neuropil occupied by SYP+ presynaptic terminals or MAP2+ neuronal processes, averaged across four images over an area of 1 mm3 [8, 12, 13]. All investigators were blinded to treatment group during data acquisition and analysis.
To obtain an overall measure of neurodegeneration, for each case we combined SYP and MAP2 percentages into a composite measure of neurodegeneration (“ND score”) such that higher ND scores represented greater neurodegeneration, per our previous work [8]. Distributions for SYP and MAP2 were divided into lower 25%, middle 50%, and upper 25% percentiles, which were categorically transformed to scores of 2 (severe ND), 1 (mild/moderate ND), and 0 (no ND). The ND score was calculated as sum of the two scores plus 1.
We measured brain viral load by HIV RNA and HIV p24 protein levels in frontal cortex tissue. Frontal cortex HIV RNA was quantified by qPCR using HIV-1 pol standard. HIV-1 p24 was quantified by ELISA (PerkinElmer, Waltham, MA) on homogenate aliquots containing 20 μg of total protein. HIV encephalitis was defined as the presence of multiple foci of microgliosis, multinucleated giant cells, and astrocytosis [14].
Statistical Analysis
In univariable analyses, measures of MAP2, SYP, and ND were compared between ART groups using ANOVA or Kruskal-Wallis test, if assumptions of normality were not met. Post hoc pairwise contrasts were adjusted for multiple comparisons using Tukey's HSD or false discovery rate methods. Effect sizes for mean differences were estimated by Cohen's d. Jonckheere test for ordered alternatives was used to test for monotonic trend in medians of MAP2, SYP, and ND between the three ART categories. Separate multivariable linear models were used to regress MAP2, SYP, and ND on ART groups and significant covariates. All covariates were initially included in the models and each was subsequently removed if their effect size was not significant at the p < 0.05 level. The full list of covariates, selected a priori based on possible association with HAND, included age, sex, diabetes status, hypertension, hyperlipidemia, tobacco smoking history (ever vs. never), time from last clinical visit to death, hepatitis C virus (HCV) coinfection, CNS penetration effectiveness score, estimated HIV infection duration, and HIV p24 protein. Associations with HAND were analyzed using univariable and multivariable logistic regressions, using Firth's penalized-likelihood methods for data that were too sparse [15]. Effect sizes for predicting HAND were measured using odds ratio (OR). For numeric predictors, OR is calculated for one unit increase. Univariable correlation analyses were performed using Pearson or Spearman method, as appropriate. Values of brain HIV p24 and HIV RNA in plasma, CSF, and frontal cortex were logarithmically transformed to reduce skewness. All tests were two-sided and deemed significant if p < 0.05, unless stated otherwise. All statistical analyses were performed using R 3.0.1 statistical software (R Core Team, 2013).
Results
Participants and Correlations
Participants in this study were predominately men (74/90, 82%) with a mean age of 44.7 years (SD 9.8, range 26 to 70 y/o, median = 44) (Table 1). Evidence of opportunistic infections in the brain (CMV encephalitis, cryptococcosis, progressive multifocal leukencephalopathy, or toxoplasmosis) was found in 14 (15.5%), Alzheimer type II gliosis in 10 (11%), and lymphoma in 4 (4.4%) of 90 subjects. The S-ART group was older and had a higher rate of smoking, diabetes, hypertension, and hyperlipidemia than both the NS-ART and ART Naïve groups. The overall rate of viral suppression among treated individuals was 22.9%. Correlations between plasma, CSF, and frontal cortex HIV RNA, frontal cortex HIV p24 protein, and our measures of neurodegeneration were in general highly significant (Table 2).
Table 1.
Demographic and biologic characteristics of the sample.
| ART Naïve (N=7, 8%) |
Non-Suppressive ART (N=64, 71%) |
Suppressive ART (N=19, 21%) |
All subjects (N=90) |
p-value | |
|---|---|---|---|---|---|
| Age (years) mean (SD, N) | 42.0 (11.0, 7) | 42.5 (8.0, 64) | 53.2 (10.9, 19) | 44.7 (9.8, 90) | < 0.001 |
| Male | 6/7 (85.7%) | 51/64 (79.7%) | 17/19 (89.5%) | 74/90 (82.2%) | 0.8 |
| Smoking ever | 1/5 (20.0%) | 24/62 (38.7%) | 8/19 (42.1%) | 33/86 (38.4%) | 0.7 |
| Months from last clinical visit to autopsy mean (SD, N) | 1.4 (0.84, 2) | 6.3 (9.8, 36) | 5.0 (7.5, 8) | 5.9 (9.2, 46) | 0.7 |
| Estimated duration of HIV infection (years) (SD, N) | 6.1 (5.9, 4) | 12.7 (5.2, 42) | 13.6 (7.2, 15) | 12.5 (5.9, 61) | 0.07 |
| Diabetes | 0 /6 (0%) | 3/63 (4.8%) | 4/19 (21.1%) | 7/88 (8.0%) | 0.08 |
| Hypertension | 1/6 (16.7%) | 19/63 (30.2%) | 9/19 (47.4%) | 29/88 (33.0%) | 0.3 |
| Hyperlipidemia | 0/6 (0%) | 7/63 (11.1%) | 6/19 (31.6%) | 13/88 (14.8%) | 0.09 |
| Hepatitis C coinfection | 4/7 (57.1%) | 17/63 (27.0%) | 10/19 (52.6%) | 31/89 (34.8%) | 0.05 |
| CNS penetration effectiveness score mean (SD, N) | N/Aa | 7.8 (3.1, 16) | 7.7 (2.2, 6) | 7.8 (2.9, 22) | 0.9 |
| HIVE | 4/7 (57.1%) | 13/64 (20.3%) | 1/19 (5.3%) | 18/90 (20.0%) | 0.02 |
| HAND: Impaired | 2/2 (100%) | 21/36 (58.3%) | 3/8 (37.5%) | 26/46 (56.5%) | 0.3 |
| HIV plasma RNA (log10) mean (SD, N) | 5.02 (0.6, 6) | 4.7 (1.3, 64) | N/Ab | 4.71 (1.28, 70) | 0.8 |
| HIV CSF RNA (log10) mean (SD, N) | 3.3 (0, 1) | 3.6 (1.5, 29) | 2.2 (1.3, 6) | 3.34 (1.5, 36) | 0.12 |
| Frontal cortex HIV RNA (log10) mean (SD, N) | 7.7 (0.3, 2) | 4.9 (1.8, 20) | 3.3 (1.0, 3) | 4.9 (1.9, 25) | 0.03 |
| Frontal cortex HIV p24 (log10) mean (SD, N) | 1.76 (0.93, 5) | 1.05 (0.65, 36) | 0.83 (0.23, 10) | 1.08 (0.66, 51) | 0.03 |
HIVE: HIV encephalitis. HAND: HIV associated neurocognitive disorders.
No data available.
S-ART group was defined based on undetectable plasma viral load.
Table 2.
Correlations among measures of viral burden and neurodegeneration.
| CSF RNA | Frontal CTX RNA | Plasma RNA | HIV p24 | % MAP2 | % SYP | ND | |
|---|---|---|---|---|---|---|---|
| CSF RNA | 1 | ||||||
| Frontal cortex RNA | 0.53** (N=24) | 1 | |||||
| Plasma RNA | 0.42*** (N=76) | 0.67*** (N=28) | 1 | ||||
| HIV p24 | 0.42** (N=42) | 0.65** (N=21) | 0.03 (N=45) | 1 | |||
| % MAP2 | -0.39*** (N=76) | -0.54** (N=28) | -0.29** (N=89) | -0.37** (N=52) | 1 | ||
| % SYP | -0.46*** (N=76) | -0.61*** (N=28) | -0.27* (N=89) | -0.38** (N=52) | 0.79*** (N=90) | 1 | |
| ND | 0.41*** (N=76) | 0.58** (N=28) | 0.27* (N=89) | 0.38** (N=52) | N/Aa | N/Aa | 1 |
SYP: Synaptophysin. MAP2: Microtubule-associated protein 2. ND: neurodegeneration score.
Reported values are Spearman coefficients.
p < 0.05;
p < 0.01;
p < 0.001
Since ND score was derived from MAP2 and SYP percentages, correlations among these measures were not performed.
ART and Neurodegeneration: Univariable Analyses
We next examined the effect of ART on measures of neurodegeneration. Representative immunostaining for SYP and MAP2 is shown in Fig. 1. Mean MAP2 percentages were 18.4% for ART Naïve, 19.7% for NS-ART, and 22.5% for S-ART (Fig. 2A). Mean SYP percentages were 16.6% for ART naïve, 19.3% for NS-ART, and 21.1% for S-ART (Fig. 2B). Differences between groups in SYP percentage were statistically significant (p=0.008) and pairwise comparisons identified that the S-ART group differed from the ART Naïve group (Cohen's d = 1.37, p = 0.007). Between-group differences in MAP2 percentage were also statistically significant (p = 0.03) and pairwise comparison identified that the S-ART differed only from the NS-ART group (Cohen's d = 0.64, p = 0.044). Jonckheere test for ordered medians was significant for both SYP (z = 3.11, p < 0.001) and MAP2 (z = 2.67, p = 0.003).
Figure 1. Immunostaining for SYP and MAP2.
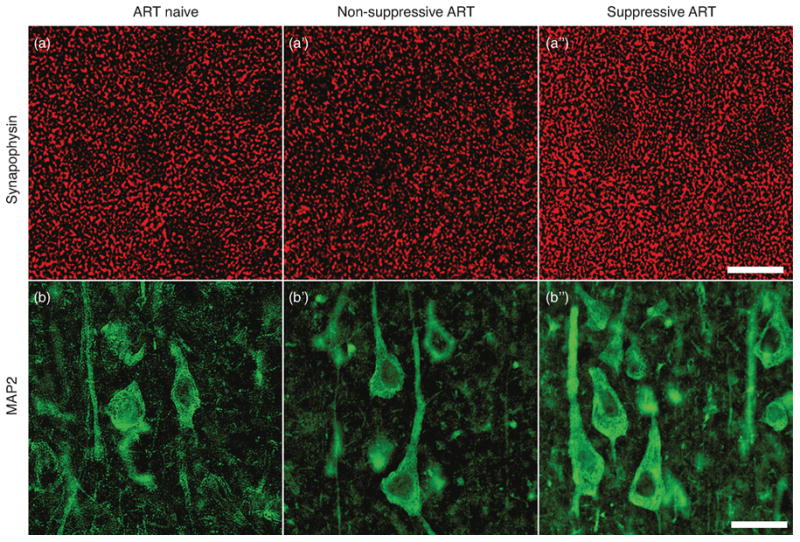
Representative confocal microscopy of dorsolateral midfrontal cortex immunostained for synaptophysin (red, A to A″), present in presynaptic terminals, and MAP2 (green, B to B″), found in neuronal dendrites and cell bodies. See quantitation (Fig. 2A, 2B). Scale bar: 20 μm.
Figure 2. Virally suppressive ART exerts a protective effect against measures of synaptodendritic neurodegeneration.
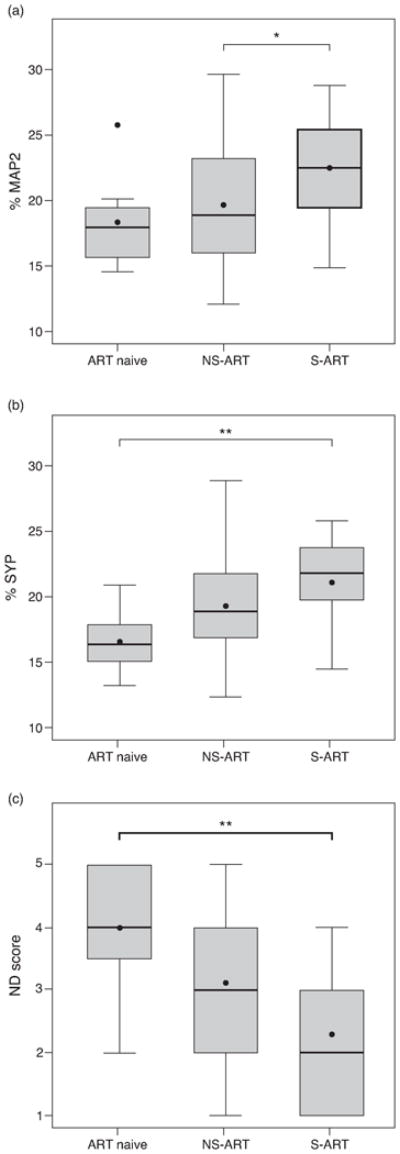
(a) Comparison of MAP2 percentage across ART groups. The S-ART group shows higher MAP2 percentages than the NS-ART group (p = 0.044, one-way ANOVA followed by Tukey's post hoc test). (b) Comparison of SYP percentage across ART groups. The S-ART group shows higher SYP percentage when compared to ART Naïve (p = 0.007, one-way ANOVA followed by Tukey's post hoc test). (c) Comparison of ND score across ART groups. The S-ART group shows lower ND score, representing less neurodegeneration, when compared to ART naïve (p = 0.01, one-way ANOVA followed by Tukey's post hoc test). * p < 0.05; ** p < 0.01.
The composite neurodegeneration (ND) score was constructed using each patient's SYP and MAP2 percentages [8]. Both S- and NS-ART groups had lower mean ND scores (representing greater synaptodendritic abundance) than the ART Naïve group (ART Naïve: 4.0, NS-ART: 3.1, S-ART: 2.3) (Fig. 2C). Differences in ND scores were statistically significant (p = 0.009) and pairwise comparison identified that the ART Naïve and S-ART groups differed (Cohen's d = -0.75, p = 0.01). Comparison between S- and NS-ART groups approached significance (Cohen's d = -0.59, p = 0.068). Jonckheere test for ordered medians was significant (z = 2.84, p = 0.002).
ART and Neurodegeneration: Multivariable Analyses
We then tested the effect of ART category and brain HIV p24 on measures of neurodegeneration in separate multivariable models for MAP2, SYP, and ND score. All covariates were initially included in each model and were subsequently removed if their effect was not significant at the p = 0.05 level. The model predicting MAP2 was statistically significant (p = 0.001, adjusted R2 = 0.29, n = 48) but only higher brain HIV p24 (Cohen's d = -0.93, 95% CI -1.45 to -0.40, p = 0.001) and smoking (Cohen's d = -0.99, 95% CI -1.64 to -0.34, p = 0.004) were associated with lower MAP2 levels. After controlling for smoking and brain HIV p24 the effect of ART category on MAP2 was not significant (p = 0.13). Similar results were found in the model for SYP (overall p < 0.001, adjusted R2 = 0.32, n = 48) in which HIV p24 (Cohen's d = -0.96, 95% CI -1.48 to -0.43, p = 0.001) and smoking (Cohen's d = -0.98, 95% CI -1.63 to -0.33, p = 0.004) were strongly associated with the outcome but ART category reached only a trend level (p = 0.052). In the model predicting ND (overall p < 0.001, adjusted R2 = 0.32, n = 48), HIV p24 (Cohen's d = 0.93, 95% CI 0.40 to 1.45, p = 0.001) and smoking (Cohen's d = 0.93, 95% CI 0.28 to 1.58, p = 0.006) were associated with the outcome, and the overall effect of ART category also reached significance (p = 0.04).
HAND: Univariable and Multivariable Analyses
We next examined the effect of neurodegeneration and ART category on the probability of HAND diagnosis. In a univariable analysis, higher SYP percentage was associated with decreased odds of HAND (OR 0.8, 95% CI 0.65–0.97, p = 0.028). Higher MAP2 percentages trended toward lower odds of HAND (OR 0.87, 95% CI 0.75–1, p = 0.068). Higher ND score was marginally associated with increased odds of HAND (OR 1.57, 95% CI 0.99–2.5, p = 0.056). ART category was not a significant overall predictor of HAND in a univariable analysis (p = 0.094). However, when compared to ART Naïve individuals, the S-ART group was associated with significantly lower odds of HAND (OR 0.07, 95% CI 0.0005–0.78, p = 0.030) and the NS-ART group trended toward significance (p = 0.091).
Separate multivariable models were constructed to further assess the association of ART category with HAND in the presence of potential mediating variables. Measures of neurodegeneration and brain HIV p24 were included as covariates in each model. In their respective models, MAP2, SYP, and ND score were the only variables that remained significant predictors of HAND after controlling for all other variables (MAP2: OR 0.79, 95% CI 0.61–0.97, p = 0.024; SYP: OR 0.72, 95% CI 0.50–0.94, p = 0.014; ND score: OR 2.08, 95% CI 1.06–4.97, p = 0.033).
Discussion
In this postmortem study, virally suppressive ART conferred a significant protective effect against neurodegeneration as measured by markers of synaptic abundance, dendritic complexity, and a composite neurodegeneration score. We further showed strong correlations among measures of neurodegeneration and plasma, CSF, and brain viral load. Neurodegeneration was highly predictive of a diagnosis of HAND, and virally suppressive ART reduced the odds of HAND. These findings are consistent with a model in which viral replication drives neurodegeneration and in which ART ameliorates HAND by reducing neurodegeneration. These findings are also broadly consistent with a number of studies that have examined the relationships among ART, neurodegeneration, and neurocognitive impairment [3-6, 8].
The statistical power of our three measures of neurodegeneration in predicting HAND varied. In univariable analysis, SYP was the strongest predictor of HAND, followed by the composite ND score and then MAP2; in the multivariable models SYP remained the strongest predictor. The relative strength of SYP in predicting neurocognitive impairment is consistent with a previous report which showed that SYP was better correlated with global NP impairment score than MAP2 in midfrontal cortex [8]. We also found that virally suppressive ART treatment was significantly associated with higher SYP, but not MAP2, relative to ART naïve individuals.
We also provide evidence supporting the causative chain from virally suppressive ART to suppression of CNS viral load, decreased neurodegeneration, and reduced odds of HAND. In multivariable models, ART category was not associated with either MAP2 or SYP after controlling for HIV p24; HIV p24 meanwhile remained strongly associated with both measures after controlling for ART category. This suggests that brain viral load may mediate the effect of ART on neurodegeneration. Similarly, in multivariable models predicting HAND, the measures of neurodegeneration were strongly associated with HAND after controlling for ART category and HIV p24, while neither ART category nor HIV p24 was associated with HAND after controlling for neurodegeneration and other covariates. This in turn suggests that neurodegeneration mediates the relationship between ART/brain viral load and HAND.
It is possible that factors other than viral replication may contribute to neurodegeneration in HAND. For example, ART does not eliminate latent provirus in the brain, and viral latency may affect host gene expression in ways that promote neurodegeneration or reduce neuroprotection. Indeed, recent work has demonstrated that viral latency in the brain is associated with dysregulation of inflammatory gene expression [16]. Our findings do not preclude the possibility that nonviral factors such as immune dysregulation and inflammation may contribute to HIV neuropathogenesis, and additional studies will be needed to fully elucidate this pathway.
Our study is subject to several limitations. First, our conclusions on the neuroprotective effect of ART are limited by the small sample size of ART naïve individuals in the cohort. Though our finding that virally suppressive ART therapy improves multiple measures of neurodegeneration is statistically robust, it is possible that a larger cohort of ART naïve individuals would reveal a more complex relationship between ART and neurodegeneration. Second, patients in our study generally had complex ART histories, including multiple stop-start treatment cycles and different lengths of treatment with varying types and number of antiretroviral drugs. Though this complexity presented difficulties in retrospectively defining ART groups, our classification method successfully captures differences in neuropathological and neurocognitive outcomes in a clinically relevant way. However, it is possible that a more tightly controlled, prospective ART trial would reveal different effects of treatment on neurodegeneration and HAND. Third, the plasma viral load assays used to sort participants into ART groups varied, with the lower limit of quantitation ranging from 40 to 400 copies/mL depending on the assay. Finally, our cohort also exhibited an unusually low rate of viral suppression (23% of those who underwent ART). This likely reflects the selection bias inherent in a postmortem study in which the sample is enriched for individuals for whom ART was ineffective.
The findings from the present study point toward several lines of future investigation. We found postmortem MAP2 and SYP to be sensitive markers of HAND. Identifying in vivo correlates of these markers is an essential next step. It is also important to examine factors that may increase susceptibility to (e.g., late ART initiation) or protect against (e.g., physical activity) neurodegeneration, as this may allow those at risk for neurocognitive decline to be identified early and treated appropriately. With the increasing abundance of effective and well-tolerated treatment, viral load suppression early in HIV disease is increasingly observed. The question remains whether the development of HAND can be thwarted or reduced based on earlier ART initiation. In addition, some have argued that ART itself may cause neurotoxicity [16]. If true, the pendulum of ART as neuroprotection may eventually swing toward inducing long-term neural toxicity and this may outweigh the benefits of preventing neurodegeneration. Clearly, adjunct neurological therapies are needed as well as an expanded understanding of the pathophysiology of HAND.
Acknowledgments
The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Reena Deutsch, Ph.D., Anya Umlauf, M.S.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government. CLA, DJM, EM, IG, RJE, and SLL designed the study; BG, CLA, DJM, EM, RJE, SLL, and VS performed data collection; AKB and AU performed the statistical analysis; AKB wrote the manuscript; and all authors provided critical revisions to the manuscript.
This work was supported by NIH U01 MH83506 (PI: DJ Moore, CL Achim). The HIV Neurobehavioral Research Center (HNRC) is supported by Center award P30MH062512 from NIMH.
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
References
- 1.Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- 3.Munoz-Morena JA, Fumaz CR, Ferrer MJ, Prats A, Negredo E, Garolera M, et al. Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res Hum Retroviruses. 2008;24:1301–1307. doi: 10.1089/aid.2007.0310. [DOI] [PubMed] [Google Scholar]
- 4.Cysique LA, Vaida F, Letendre S, Gibson S, Cherner M, Woods SP, et al. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 2009;73:342–348. doi: 10.1212/WNL.0b013e3181ab2b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23:1359–1366. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joska JA, Gouse H, Paul RH, Stein DJ, Flisher AJ. Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. J Neurovirol. 2010;16:101–114. doi: 10.3109/13550281003682513. [DOI] [PubMed] [Google Scholar]
- 7.Vassallo M, Durant J, Biscay V, Lebrun-Frenay C, Dunais B, Laffon M, et al. Can high central nervous system penetrating antiretroviral regimens protect against the onset of HIV-associated neurocognitive disorders? AIDS. 2014;28:493–501. doi: 10.1097/QAD.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 8.Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, et al. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 2006;20:879–887. doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- 9.Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- 10.Blackstone K, Moore DJ, Franklin DR, Clifford DB, Collier AC, Marra CM, et al. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol. 2012;26:894–908. doi: 10.1080/13854046.2012.694479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, et al. Dendritic injury is a pathologic substrate for human immunodeficiency virus-related cognitive disorders. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- 13.Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, et al. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group HIV Neurobehavioral Research Center Brain Pathol. 1999;9:209–217. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budka H, Wiley CA, Kleihues P, Artigas J, Asbury AK, Cho ES, et al. HIV-associated disease of the nervous system: review of nomenclature and proposal for neuropathology-based terminology. Brain Pathol. 1991;1:143–152. doi: 10.1111/j.1750-3639.1991.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 15.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 16.Akay C, Cooper M, Odeleye A, Jensen BK, White MG, Vassoler F, et al. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J Neurovirol. 2014;20:39–53. doi: 10.1007/s13365-013-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


