Abstract
Hypothalamic neuropeptides play essential roles in regulating energy and body weight balance. Energy imbalance and obesity have been linked to hypothalamic signaling defects in regulating neuropeptide genes; however, it is unknown whether dysregulation of neuropeptide exocytosis could be critically involved. This study discovered that synaptotagmin-4, an atypical modulator of synaptic exocytosis, is expressed most abundantly in oxytocin neurons of the hypothalamus. Synaptotagmin-4 negatively regulates oxytocin exocytosis, and dietary obesity is associated with increased vesicle binding of synaptotagmin-4 and thus enhanced negative regulation of oxytocin release. Overexpressing synaptotagmin-4 in hypothalamic oxytocin neurons and centrally antagonizing oxytocin in mice are similarly obesogenic. Synaptotagmin-4 inhibition prevents against dietary obesity by normalizing oxytocin release and energy balance under chronic nutritional excess. In conclusion, the negative regulation of synaptotagmin-4 on oxytocin release represents a hypothalamic basis of neuropeptide exocytosis in controlling obesity and related diseases.
INTRODUCTION
The hypothalamus in the central nervous system (CNS) is known as the central regulator of feeding, energy and body weight homeostasis (Coll et al., 2007; Flier and Maratos-Flier, 1998; Mobbs, 2007; Park and Bloom, 2005; Schwartz et al., 2000; Ukropec et al., 2006). All these hypothalamic functions are critically mediated by various hypothalamic neuropeptides. Several well-appreciated examples of such neuropeptides include α-MSH (α-melanocyte stimulating hormone), CART (cocaine and amphetamine regulated transcript), NPY (neuropeptide Y) and AGRP (agouti-related peptide). These neuropeptides have been shown to be controlled at the gene transcriptional levels (Bates et al., 2003; Kim et al., 2006; Kitamura et al., 2006; Xu et al., 2005) by nuclear transcription factors which sense nutrient and metabolic cues of the body (Ahima et al., 1996; Air et al., 2002; Friedman and Halaas, 1998). Interestingly, recent research has begun to recognize the importance of neuropeptide post-transcriptional modulation (Plum et al., 2009), indicating that the control of neuropeptide gene expression represents only an initial step in the whole cascade of neuropeptide regulation. Logically, this process ultimately involves regulation of neuropeptide release to precisely control the biological functions of neuropeptides. However, how hypothalamic neuropeptide exocytosis is regulated and whether it is critical for metabolic physiology and disease have not been explored.
Recent research in basic science has obtained significant knowledge regarding the general principles of neuropeptide/neurotransmitter vesicular exocytosis (Stojilkovic, 2005). Studies based on synaptic neurotransmitter release have identified vesicular exocytosis as a process that is mediated by SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) complex (Jahn and Scheller, 2006; Sudhof and Rothman, 2009) under the regulation of synaptotagmins (Syts) (Chapman, 2008; Sudhof, 2002). Syts are a group of Ca2+-binding proteins that catalyze the formation of SNARE complex to provide the force and energy required for exocytosis. The mammalian Syt family is comprised of 17 members. While most of them are predominantly present in the CNS, some of them are involved in the vesicular functions of endocrine cells such as pancreatic α- and β-cells (Fukuda and Mikoshiba, 1999; Gao et al., 2000; Gauthier et al., 2008; Iezzi et al., 2005) and glucose-transport metabolic cells (Hudson and Birnbaum, 1995; Li et al., 2007). These interesting studies, which were mainly based on peripheral endocrine systems, have raised the recent alert on the potential implication of Syts in diabetes (Gauthier and Wollheim, 2008). However, research to date addressing Syts in hypothalamic neuroendocrine neurons is still missing.
Syt4 is an inducible Syt isoform detectable only in the brain and the neuroendocrine system (Vician et al., 1995), hinting at a possible role in neuroendocrine physiology. Notably, compared to other Syt family members, the puzzling aspect of Syt4 is its lack of a critical Ca2+-binding amino acid (von et al., 1997) and related inability to induce Ca2+-dependent exocytosis in biophysical models (Chapman et al., 1998; Thomas et al., 1999). Recent biophysical research reported that Syt4 inhibits exocytotic activities in the posterior pituitary (Zhang et al., 2009) and in cultured PC12 cells (Machado et al., 2004), suggesting a potential neuroendocrine role of Syt4. In this research, through targeting the regulators of vesicle exocytosis including SNARE complex proteins and Syt family members, we identified a hypothalamic program of oxytocin (OXT) release regulated by Syt4 and established the physiological significance of this regulation in the hypothalamic control of energy balance and related diseases.
RESULTS
Abundant distribution of Syt4 in the hypothalamic PVN
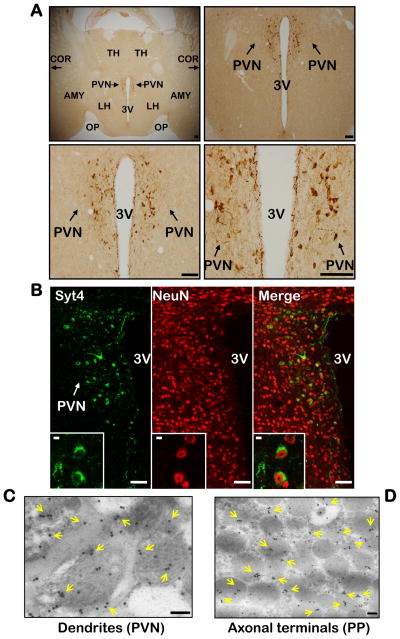
The hypothalamus regulates energy balance essentially through neuropeptide release. Therefore, we explored whether the hypothalamus has a regulatory program for neuropeptide release that is critical for metabolic physiology, and whether alteration of this program causes metabolic disease. We adopted a candidate screening strategy by targeting neuropeptide release regulators, including SNARE complex proteins and Syt family proteins in the hypothalamus. Western blots initially confirmed that Syt4 was detectable in the brain and the posterior pituitary, but not in the peripheral tissues. Immunostaining further revealed that Syt4 was expressed abundantly in a subpopulation of cells in the paraventricular nucleus (PVN) (Fig. 1A) and the supra-optic nucleus of the hypothalamus, but only modestly in a few other brain regions such as hippocampus and the cortex (data not shown). The specificity of Syt4 antibody (Zhang et al., 2009) was verified by Western blot and immunostaining analyses of hypothalamic samples from Syt4 knockout (Syt4−/−) mice (Ferguson et al., 2000a) (suppl. Fig. 1A–C). In addition, both mRNA in situ hybridization (suppl. Fig. 1D) and real-time RT-PCR (suppl. Fig. 1E) confirmed that Syt4 expression was uniquely enriched in the hypothalamus. High-magnification images of Syt4 immunostaining (Fig. 1A) revealed that Syt4 was present in the cell bodies and projections of a subpopulation of cells with neuronal morphology in the PVN. Subsequent co-immunostaining of Syt4 with NeuN (a neuronal marker) confirmed that Syt4 was exclusively expressed in neurons but not other cell types (Fig. 1B). High-resolution images of co-immunostaining revealed that Syt4 co-localized with vesicle-like structures in the cytoplasm and projections of neurons (Fig. 1B inserts). In support of this observation, Syt4 immunogold labeling and electron microscopy analysis showed that Syt4 was abundantly localized in vesicles of a subpopulation of PVN neurons (Fig. 1C) and in axonal terminals of the PVN neurons that projected into the posterior pituitary (Fig. 1D). Thus, in line with previous report that Syt4 is present only in the brain and neuroendocrine system (Vician et al., 1995), our data revealed that Syt4 is abundantly expressed in a specific subtype of PVN neurons, which may indicate a previously unappreciated role of Syt4 in hypothalamic control of physiology.
Figure 1. Syt4 distribution in hypothalamic PVN.
A. Distribution of Syt4 in the hypothalamic PVN. Immunohistochemical staining of Syt4 in hypothalamic sections across the PVN was examined under a light microscope. Scale bar=100 μm.
B. Neuronal expression of Syt4 in the PVN. PVN sections were co-immunostained for Syt4 (green) and neuronal marker NeuN (red). Co-localization of two fluorescent signals within the same cells indicates Syt4 expression in neurons. Scale bar=50 μm. Inserts: Intracellular distribution of Syt4 in neurons by co-immunostaining at high magnification (insert scale bar=5 μm).
C&D. Syt4 immunogold labeling in hypothalamic PVN (C) and posterior pituitary (D) sections were examined by electron microscopy. Yellow arrows indicate Syt4 immunogold labeling. Scale bar=100 nm.
A–D: All experimental mice were adult males, chow-fed, and in C57BL/6 background. AMY: amygdala, COR: cortex, LH: lateral hypothalamus, OP: optic tract, PVN: paraventricular nucleus, PP: posterior pituitary, TH: thalamus, 3V: third ventricle.
Nutritional excess promotes Syt4 expression and vesicular localization
Since the PVN is one of the hypothalamic regions that critically regulate energy and metabolic balance, we subsequently explored whether nutritional excess could affect the expression and vesicular profiles of Syt4 in the hypothalamus-pituitary axis. First, quantitative real-time RT-PCR was used to measure Syt4 mRNA levels in the hypothalamus of C57BL/6 mice that were chronically maintained on a normal chow vs. high-fat diet (HFD). Results showed that HFD feeding increased Syt4 mRNA levels in the hypothalamus but not in other brain regions (suppl. Fig. 1E), suggesting that Syt4-directed vesicular exocytosis could be involved in the hypothalamic control of HFD-induced metabolic disorders. To further evaluate this possibility, Syt4 immunogold labeling and electron microscopy were employed to examine whether HFD feeding could affect vesicular localization of Syt4 in PVN neurons. Results showed that HFD feeding nearly doubled the number of vesicle-bound Syt4 particles in PVN dendrites and axonal terminals (suppl. Fig. 1F&G). Thus, obesity development under nutritional excess is associated with hypothalamic changes of Syt4 expression and in particular Syt4 vesicular distribution.
Syt4 inhibition prevents obesity by normalizing energy balance
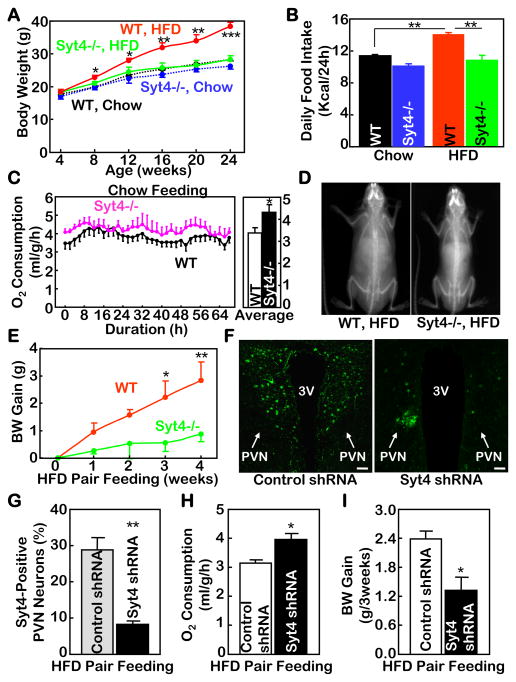
Syt4−/− mice were then employed to test if Syt4 ablation could affect obesity and related metabolic diseases. Based on our observation as well as the literature (Vician et al., 1995), Syt4 is expressed in the brain but not peripheral tissues; therefore, Syt4 knockout functionally targets the brain. We first confirmed that Syt4−/− mice have normal growth, appearance, viabilities and physical activities, and thus represent a suitable model for metabolic research without involving confounding developmental changes. Syt4−/− mice and wildtype (WT) littermate controls were maintained on a normal chow upon weaning. At young ages, Syt4−/− mice displayed similar body weight compared to WT controls (Fig. 2A) despite slightly reduced food intake (Fig. 2B) and evidently increased energy expenditure (Fig. 2C). On the other hand, a long-term follow-up revealed that Syt4−/− mice were completely protected from the development of post-adult weight gain (suppl. Fig. 2A&B). Therefore, the catabolic effects of Syt4 ablation on body weight were accumulative and required an adequate age to manifest. Taken together, Syt4 inhibition is catabolic; while its impact on body weight is minor at young ages, it can provide accumulative benefits against age-related fat expansion and weight gain at post-adult ages.
Figure 2. Metabolic profiles of mice with Syt4 ablation.
A&B. Syt4−/− mice (blue and green curves/bars) and WT littermate controls (black and red curves/bars) were maintained on either a normal chow (black and blue curves/bars) or a HFD (red and green curves/bars) since weaning (3 weeks old) and longitudinally monitored for body weight (A) and food intake (B). Data in Panel B represent the average daily food intake over a 6-month follow-up period.
C. Normal chow-fed Syt4−/− mice (pink curves) and WT littermate controls (black curves) at 12 weeks of age were measured for O2 consumption using metabolic chambers. Data represent real-time (left panel) and daytime average (right panel) O2 consumption over a 64-hour period. The levels of O2 consumption were normalized by lean body mass of individual mice.
D. Representative DEXA scanning images of Syt4−/− mice vs. WT littermate controls that received 4 months of HFD feeding since weaning.
E. Normal chow-fed Syt4−/− mice (green curve) and WT littermate controls (red curve) at 6 weeks of age were switched to HFD pair feeding (food supply based on the average ad libitum HFD intake of Syt4−/− mice), and body weight (BW) gain of these mice were followed up for 4 weeks.
F–I. HFD-fed C57BL/6 mice received bilateral PVN injections of Syt4 shRNA lentiviruses or matched control shRNA lentiviruses and were subsequently subjected to HFD pair feeding post injection. Lentiviral shRNA-mediated Syt4 ablation was verified by Syt4 immunostaining (green fluorescent) in the PVN sections (F) and by counting Syt4-positive neurons across serial PVN sections (G). Mice were measured for O2 consumption at Week 1 post injection (H) and followed up for body weight (BW) gain over a 3-week period (I). Scale bar=50 μm.
A–I: Data in these experiments were based on male mice. Data in A–D also represented similar observations in female mice. *P<0.05, **P<0.01, ***P<10−3, compared to genotype/treatment-matched controls; n=6–10 per group (A&B), and n=4–6 per group (C, E, G–I).
To better elucidate the potential anti-obesity effect of Syt4 ablation, the paradigm of HFD feeding was applied to Syt4−/− mice and WT littermate controls after weaning. Data revealed that while WT controls developed profound obesity over a 6-month period, Syt4−/− mice maintained completely normal body weight (Fig. 2A&D). A prolonged follow-up of 12-month HFD feeding showed that the body weight of WT mice increased to a morbid level, but the matched Syt4−/− mice remained intact (suppl. Fig. 2C&D). DEXA scanning and histological analyses (suppl. Fig. 2E–H) confirmed that the anti-obesity phenotype of Syt4−/− mice was due to the resistance to fat mass expansion without loss of lean body mass. Daily HFD intake of these mice was longitudinally monitored, showing that Syt4−/− mice consumed reduced amount of HFD compared to WT mice (Fig. 2B). Indirect calorimetry was applied to Syt4−/− and WT mice during the initial stage (1~2 weeks) of HFD feeding when mice of both groups still had comparable body weight. Syt4−/− mice upon switching to a HFD had higher energy expenditure levels than matched WT mice (data not shown), which was similar to the energy expenditure pattern under chow feeding condition (Fig. 2C). To further assess whether increased energy expenditure contributed to the anti-obesity phenotype of Syt4−/− mice, HFD pair feeding was provided to adult Syt4−/− and WT mice. Although the total calories provided through HFD pair feeding were reduced compared to ad libitum feeding, adult WT mice still gained weight over a 4-week period – an outcome of impaired energy expenditure resulting from HFD-associated malnutrition. In contrast, a significant less magnitude of weight gain was observed in Syt4−/− mice under the same HFD feeding regime (Fig. 2E), and such weight gain reduction can be attributed to elevated energy expenditure induced by Syt4 ablation.
Finally, we explored whether obesity development could be acutely attenuated by Syt4 ablation through the control of energy expenditure in addition to food intake. HFD-fed C57BL/6 mice received bilateral intra-PVN injections of Syt4 shRNA lentiviruses or control lentiviruses (Fig. 2F&G). HFD pair feeding was then provided to both groups post injection (suppl. Fig. 2I). Compared to the control mice, Syt4 shRNA-injected mice had higher energy expenditure levels at Week 1 post injection (Fig. 2H), a time point when body weights of both groups were still comparable. Further follow-up revealed that Syt4 shRNA-injected mice gained less body weight than control mice (Fig. 2I, suppl. Fig. 2J). Such anti-weight gain effect of Syt4 ablation can be specifically related to increased energy expenditure, since HFD intake was controlled at the same level via pair feeding. Therefore, in conjunction with the food intake data in Fig. 2B, it can be concluded that the anti-obesity effect of Syt4 inhibition under nutritional excess is mediated by both energy expenditure increase and energy (food) intake restriction.
Syt4 inhibition prevents against obesity co-morbidities
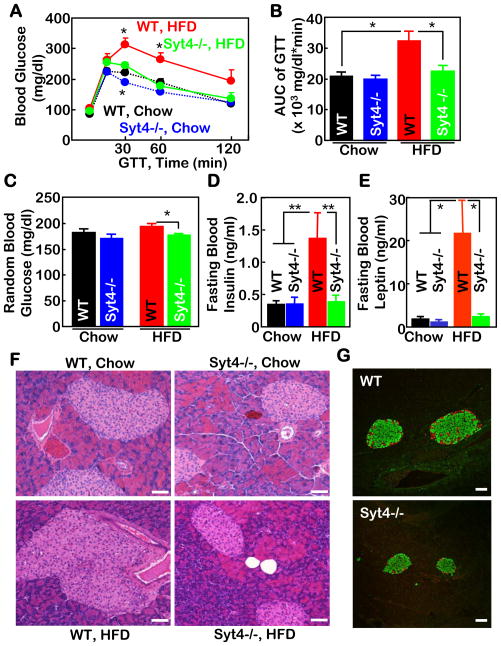
The frequent disease consequences of obesity include glucose intolerance, insulin resistance, and tissue lipid infiltrations. In context of Syt4’s relevance to obesity, we additionally assessed whether Syt4 inhibition was sufficient to prevent against obesity-associated diseases. Under HFD feeding, WT mice developed severe glucose intolerance (Fig. 3A&B) and slight hyperglycemia (Fig. 3C); in contrast, Syt4−/− mice were completely normal. Consistent with these metabolic profiles, HFD-treated WT mice, but not Syt4−/− mice, developed profound insulin resistance and leptin resistance, presented in the forms of hyperinsulinemia (Fig. 3D), hyperleptinemia (Fig. 3E), and pancreatic islet hypertrophy (Fig. 3F). Double immunostaining of insulin and glucagon in pancreatic sections confirmed that pancreatic islets of HFD-treated WT mice displayed centralization of glucagon-producing cells, a morphological indicator of islet damage, but such pathologic changes were not seen in HFD-treated Syt4−/− mice (Fig. 3G). Finally, the mice were assessed for lipid infiltration profiles in the liver and skeletal muscles. HFD-treated WT mice showed severe hepatic steatosis (suppl. Fig. 3A) and muscular lipid deposits (suppl. Fig. 3B); in contrast, HFD-treated Syt4−/− mice were completely or significantly protected from developing these lipid abnormalities. All these metabolic benefits of Syt4−/− mice were predicted to be mainly secondary to obesity prevention, but it is also possible that Syt4 ablation may directly improve obesity-related glucose and lipid homeostasis.
Figure 3. Counteraction against insulin and leptin resistance in Syt4−/− mice.
A–E. Syt4−/− mice (blue and green curves/bars) and WT littermate controls (black and red curves /bars) were maintained on a chow (black and blue curves/bars) vs. a HFD (red and green curves /bars) since weaning. At 4 months of age, mice were analyzed for glucose tolerance test (GTT) (A&B), random blood glucose levels (C), and fasting blood insulin (D) and leptin (E) levels. AUC: area under curve of GTT. *P<0.05, **P<0.01, n=5–7 per group.
F&G. Syt4−/− mice and WT littermate controls were maintained on a HFD for 4 months since weaning. Pancreases were collected and sectioned for H&E staining (F) and co-immunostaining of insulin (green) and glucagon (red) (G). Scale bar=100 μm.
A–G: Data presented were based on male mice but representatives of both sexes.
Co-localization of Syt4 and OXT in the PVN neurons
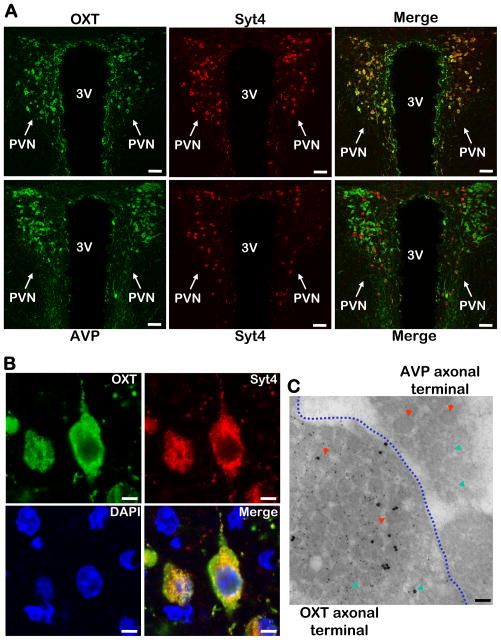
Next our study aimed to understand which neuropeptide(s) were directed by hypothalamic Syt4 to account for the physiological effects of Syt4 knockout. Since Syt4 is abundantly expressed in the PVN neurons (Fig. 1), and PVN importantly integrates many other hypothalamic nuclei to control energy balance, we focused our study on this hypothalamic site. Co-immunostaining of Syt4 with various PVN neuropeptides revealed that Syt4 was enriched in oxytocin (OXT)-expressing neurons in the PVN (Fig. 4A upper panels). For comparison, PVN sections were co-immunostained for Syt4 and arginine vasopressin (AVP), a neuropeptide structurally closely related to OXT. Surprisingly, Syt4 was undetectable in the majority of AVP neurons except for a few that co-expressed OXT and AVP (Fig. 4A lower panels). These results indicate that OXT and AVP probably employ different Syt-directed regulatory systems to control their exocytosis, and such difference may form a basis of their different physiological functions.
Figure 4. Co-localization of Syt4 with OXT in hypothalamic PVN.
A. OXT (upper panels, green) or AVP (lower panels, green) in the PVN was co-immunostained with Syt4 (all panels, red) and merged to display their co-localization (indicated by yellow color). Scale bar=50 μm.
B. High-magnification images of Syt4 (red) and OXT (green) co-immunostaining. Yellow color in merged images indicates intracellular co-localization of Syt4 and OXT. DAPI staining (blue) revealed nuclei of all cells in the sections. Scale bar=5 μm.
C. OXT and Syt4 co-immunogold labeling in OXT vs. AVP axonal terminals. The posterior pituitary from normal C57BL/6 mice were sectioned and co-immunogold labeled with OXT (small particles) and Syt4 (large particles). The image represents a junction region that contains both OXT axonal terminals and AVP axonal terminals (separated by a blue dotted line). Red arrows indicate dense-core vesicles, and green arrows indicate micro-vesicles. Scale bar=100 nm.
A–C: All experimental mice were adult males, chow-fed, and in C57BL/6 background.
Subsequently, high-magnification imaging of Syt4 and OXT co-immunostaining was used to examine the morphology of Syt4-containing subcellular structures in detail. As shown in Fig. 4B, the subcellular organelles recognized by Syt4 immunostaining displayed the appearance of aggregated vesicles that were also encompassed by OXT, suggesting that Syt4 is physically present in OXT vesicles. To prove this hypothesis, immunogold co-labeling of Syt4 with OXT and electron microscopy analysis were performed, and data confirmed that Syt4 was selectively present in the dendritic and axonal vesicles of OXT neurons (Fig. 4C). In comparison, Syt4-positive vesicles were not detected in adjacent AVP neurons (Fig. 4C). In addition to the dense-core vesicles, Syt4 particles appeared to be modestly present in micro-vesicles, suggesting that regulation of neurotransmitter release by Syt4 in these neurons is also possible. Overall, Syt4 is expressed specifically in OXT neurons and might provide a critical regulation on OXT release to modulate the biological functions of hypothalamic OXT.
Syt4 in OXT neurons mediates metabolic actions
The connection between Syt4 and OXT prompted further investigation on whether Syt4 acts in OXT neurons to mediate the anti-obesity benefit of Syt4−/− mice. To do so, we examined whether an exogenous induction of Syt4 in OXT neurons within the PVN could recapitulate the effects of HFD feeding to cause weight gain. Oxytocin gene promoter (Zhang et al., 2002) was used to generate OXT neuron-specific Syt4 or control (GFP) lentiviruses. The cell specificity of these lentiviruses was verified by examining the PVN of virus-injected mice (Fig. 5A&B). As expected, delivery of Syt4 into OXT neurons within the PVN mimicked HFD feeding to promote food intake (Fig. 5C) and weight gain (Fig. 5D&E) in C57BL/6 mice despite normal chow feeding condition. Importantly, both effects were abolished by daily OXT injections via the third ventricle (Fig. 5C&D), indicating that OXT in the brain can antagonize the metabolic action of Syt4. In parallel, we examined whether the anti-obesity phenotype of Syt4−/− mice could be reversed by restoring Syt4 in OXT neurons. OXT neuron-specific Syt4 or control GFP lentiviruses were injected into the PVN of HFD-fed Syt4−/− mice and matched WT controls. All mice were maintained on a HFD post injection. Quantitative real-time RT-PCR analysis revealed a 42% restoration of Syt4 mRNA in the PVN of Syt4−/− mice (Fig. 5F). Longitudinal food intake and body weight monitoring indicated that the delivery of Syt4 significantly abrogated the anti-obesity phenotype of Syt4−/− mice (Fig. 5G), and these mice regained hyperphagia (Fig. 5H). The reversal of metabolic changes was partial, which might be related to the technical limitation of viral injection approach, or may point to the possibility that other neurons in the brain also contribute to the metabolic phenotype of Syt4−/− mice. Altogether, based on the gain-of-function study using conventional C57BL/6 mice and the rescue experiment using Syt4−/− mice, it can be concluded that OXT neurons are important for the metabolic effects of Syt4.
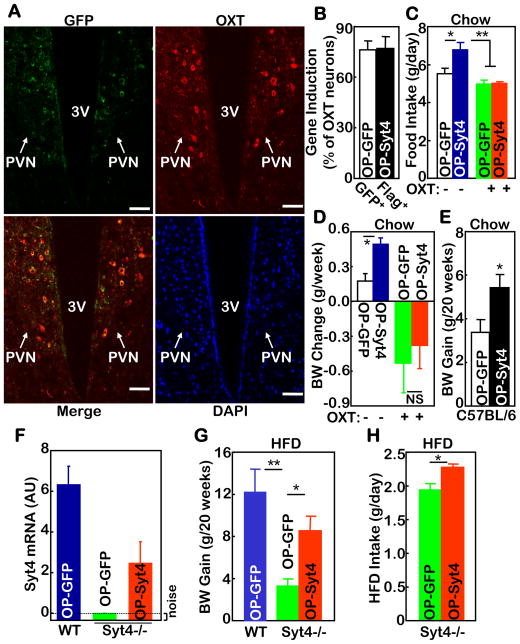
Figure 5. Metabolic effects of Syt4 in hypothalamic OXT neurons.
A–E. Chow-fed C57BL/6 mice received bilateral intra-PVN injections of oxytocin promoter-driven Flag-tagged Syt4 lentiviruses (OP-Syt4) vs. oxytocin promoter-driven GFP lentiviruses (OP-GFP). A. Lentivirus-induced GFP expression (green) was co-immunostained with endogenous OXT expression (red) in the PVN. Merged image indicates GFP expression in OXT neurons. DAPI staining (blue) revealed the nuclei of all cells in the section. Scale bar=50 μm. B. The percentage of OXT neurons immunoreactive for GFP vs. Flag in serial PVN sections that were co-immunostained for GFP or Flag with OXT. C&D. Mice received daily injections of OXT (+) vs. control vehicle (−) via pre-implanted third ventricle cannula for one week, and daily food intake and weekly body weight (BW) gain were measured. E. Lentivirus-injected mice were longitudinally monitored for BW gain over a 20-week period.
F–H. Chow-fed Syt4−/− mice and WT littermate mice were bilaterally injected with OP-Syt4 vs. OP-GFP lentiviruses in the PVN. F. Syt4 mRNA in the hypothalamus was measured at Week 1 post injection. G&H. Lentivirus-injected mice were placed on a HFD, and monitored for BW gain (G) and HFD intake (H) over a 20-week period. AU: arbitrary unit.
A–H: Mice in these experiments were males. *P<0.05, **P<0.01, NS, non-significant, n=5–7 per group (A–D), and n= 6–8 per group (E–H).
Syt4 negatively regulates OXT release in the PVN
Following the above physiological studies, we aimed to investigate if and how Syt4 can regulate OXT exocytosis. Experiments were designed to test the effects of Syt4 loss-of-function on OXT release via OXT ex vivo release assay, an established method to study OXT release in the PVN (Jin et al., 2007; Sladek and Somponpun, 2008). Live PVN slices from the hypothalamus of chow-fed Syt4−/− mice or WT controls were used for OXT release measurement. Results revealed that Syt4 ablation significantly enhanced OXT release in the PVN slices under both basal and KCl-depolarized conditions (Fig. 6A). This finding was confirmed by an independent assay which analyzed the 60-min dynamics of OXT release in the PVN tissues from Syt4−/− vs. WT mice (suppl. Fig. 4A&B). Hypothalamic OXT mRNA levels were comparable between chow-fed Syt4−/− and WT mice (suppl. Fig. 5A), which excluded the involvement of OXT gene expression. These data indicate that Syt4 negatively regulates OXT exocytosis, which is supported by the inhibitory structural (Chapman et al., 1998; Thomas et al., 1999) and biophysical (Zhang et al., 2009) characteristics of Syt4. We further inferred that the blood OXT levels should be higher in Syt4−/− mice than in WT controls regardless of dietary conditions. Measurements of circulating OXT levels in Syt4−/− and WT mice on either a normal chow or a HFD confirmed this prediction (Fig. 6B). We also measured hypothalamic OXT mRNA levels of HFD-fed Syt4−/− and WT mice. Compared to chow feeding, HFD enhanced OXT mRNA levels in WT mice (suppl. Fig. 5A), which was likely a compensatory response to suppressed OXT release under HFD. In contrast, hypothalamic OXT mRNA levels were comparable between HFD-fed and chow-fed Syt4−/− mice (suppl. Fig. 5A), indirectly indicating that HFD-fed Syt4−/− mice did not suffer OXT release impairment. To summarize, exocytosis but not gene expression of OXT is negatively regulated by Syt4.
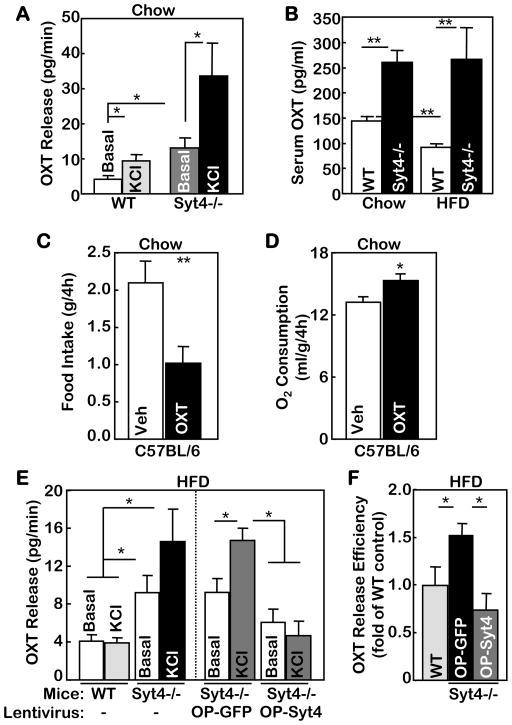
Figure 6. Regulation of OXT release by Syt4 and OXT’s metabolic action.
A. PVN slices were prepared from chow-fed Syt4−/− mice and WT littermate controls at 3 months of age. Tissues were incubated in Locke’s solution, washed every 5 min for 10 times, and incubated for 5 min in Locke’s solution in the presence or absence of 70 mM KCl. Aliquots of the final solution were used to measure OXT release. *P<0.05, n=6–8 per group.
B. Syt4−/− mice and WT littermate controls were maintained on a normal chow vs. a HFD for 4 months since weaning, and serum samples were collected to measure serum OXT concentrations. **P<0.01, n= 8–10 per group.
C&D. Adult C57BL/6 mice received third ventricle injection of OXT (4 μg) vs. vehicle (Veh) and were monitored for food intake (C) and O2 consumption (D) over a 4-hour period post injection. The levels of O2 consumption were normalized by lean body mass of individual mice. *P<0.05, **P<0.01, n=11 per group (C), and n=4 per group (D).
E&F. 3-month-old Syt4−/− mice and WT littermate controls received HFD feeding for 3 months. A subgroup of mice received bilateral injections of OP-Syt4 or OP-GFP lentiviruses in the PVN prior to the 3-month HFD treatment (E right panel). The PVN slices from these mice were prepared and incubated in Locke’s solution, and measured for OXT release under basal and KCl-stimulated conditions. F. OXT release efficiency of PVN slices from the virus-injected Syt4−/− mice was calculated as the ratio of KCl-stimulated OXT release to the basal OXT release, and data are presented as fold changes compared to WT control. *P<0.05, n= 4–8 per group.
A–F: Mice in all these experiments were males.
Brain OXT restricts food intake and promotes energy expenditure
OXT is a hypothalamic neuropeptide that is synthesized by OXT neurons and released from both axon terminals and somato-dendritic regions. In addition to its classical role in reproductive physiology, OXT also regulates various social behaviors such as care, love, emotion and trust (Ferguson et al., 2000b; Keverne and Curley, 2004; Kosfeld et al., 2005). Of note, many of these classical and non-classical actions of OXT were associated with feeding changes (Douglas et al., 2007; Leng et al., 2008). More recently, genetic studies reported that hyperphagia and obesity developed in mice that were genetically deficient of OXT (Amico et al., 2005; Kublaoui et al., 2008) or OXT receptor (Takayanagi et al., 2008). While these genetic approaches targeted the whole body, the brain-specific role of OXT in metabolic physiology has not been defined. To study the central action of OXT, we injected OXT into the brain of normal C57BL/6 mice via third ventricle cannula. Central administration of OXT readily suppressed food intake (Fig. 6C) and elevated energy expenditure (Fig. 6D). Importantly, brain injection of OXT did not yield side effects on the general health, as confirmed by multiple behavioral tests including kaolin intake test (suppl. Fig. 5B), conditioned taste aversion test (suppl. Fig. 5C), elevated plus maze (suppl. Fig. 5D–F), and open field test (suppl. Fig. 5G). In conclusion, OXT in the brain can exert a catabolic regulation on energy balance by restricting food intake and promoting energy expenditure.
Obesity is associated with impaired OXT release
Following the observations that HFD-induced obesity is associated with increased hypothalamic Syt4 mRNA levels and Syt4 vesicular distribution (suppl. Fig. 2B&C), we predicted that dietary obesity might be causally related to altered OXT release. This hypothesis was also suggested by the data in Fig. 6B showing that circulating levels of OXT in HFD-fed mice were reduced by ~40% compared to chow-fed mice. To test this hypothesis, we employed ex vivo OXT release assay to determine if HFD feeding could affect OXT release of the hypothalamic PVN. KCl-induced depolarization elicited OXT release response in the PVN slices of chow-fed WT mice (Fig. 6A), but this effect was blunted by HFD feeding (Fig. 6E left panel). In contrast, HFD did not blunt depolarization-induced OXT release in the PVN slices of Syt4−/− mice (Fig. 6E left panel). Moreover, when Syt4 expression was restored into OXT neurons by lentivirus-mediated gene delivery, depolarization failed to elicit OXT release in the PVN slices of HFD-fed Syt4−/− mice (Fig. 6E right panel & 6F). In sum, while obesity causes impaired OXT release of the PVN, the anti-obesity phenotype of Syt4−/− mice is associated with retained sensitivity of OXT release.
Obesogenic effects of OXT antagonists in WT and Syt4−/− mice
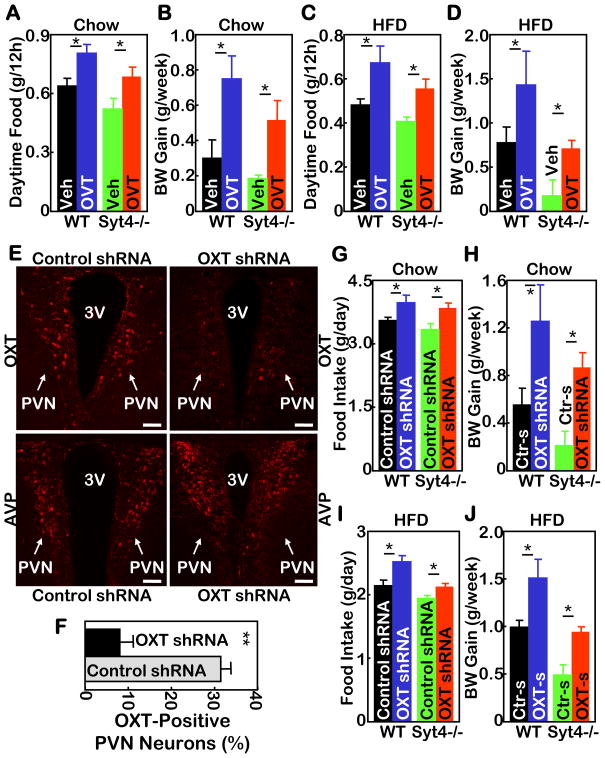
To further analyze the significance of hypothalamic Syt4-OXT pathway in obesity development, we examined whether OXT antagonists could be obesogenic. First, a pharmacological approach was employed by daily injections of OVT (an OXT antagonist) into the third ventricle of WT and Syt4−/− mice under chow or HFD feeding condition. Compared to vehicle injections, OVT increased food intake in chow-fed WT and Syt4−/− mice (Fig. 7A), resulting in increased weight gain over a 2-week follow-up period (Fig. 7B). Similarly, OVT increased food intake in HFD-fed WT and Syt4−/− mice (Fig. 7C). As a result, the effect of HFD in promoting weight gain was exacerbated in WT mice, and the anti-obesity phenotype in HFD-fed Syt4−/− mice was significantly abrogated (Fig. 7D). Next, OXT shRNA lentivirus was used to evaluate the Syt4-OXT connection in the brain control of feeding and body weight. Syt4−/− mice and WT controls, maintained on either chow or HFD feeding, received bilateral intra-PVN injections of OXT shRNA lentiviruses or control lentiviruses. Immunostaining confirmed that OXT shRNA delivery sufficiently ablated the expression of OXT but not a control neuropeptide AVP in the PVN (Fig. 7E&F). As shown in Fig. 7G–J, shRNA-mediated OXT ablation increased food intake and weight gain similarly in WT mice and Syt4−/− mice under either chow or HFD condition. Compared to OVT injection, the obesogenic effects of OXT shRNA were stronger, consistent with the observation that OXT shRNA affected 24-hour food intake while the effect of OVT injection lasted only ~12 hours post injection. In summary, results from both pharmacological and shRNA lentiviral injection experiments indicate that OXT critically mediates the role of Syt4 in hypothalamic control of weight gain and obesity.
Figure 7. Hypothalamic Syt4-OXT links HFD feeding to disease.
A–D. Syt4−/− mice and WT littermate controls were maintained on a normal chow (A&B) vs. a HFD (C&D) for 3 months since weaning, and subsequently implanted with cannula into the third ventricle. After 2 weeks of post-operative recovery, mice received daily third ventricle injections of OVT (4 μg) vs. vehicle (Veh) for 2 weeks, and were monitored for daily 12-hour daytime food intake post injection (A&C) and weekly body weight (BW) gain (B&D) during the 2-week treatment period. *P<0.05, n= 5–6 per group. Mice in all these experiments were males.
E–J. Syt4−/− mice and WT littermate controls were maintained on a normal chow (E–H) vs. a HFD (I&J) for 5 months since weaning, and subsequently received bilateral intra-PVN injection of OXT shRNA (OXT-s) lentiviruses or control shRNA (Ctr-s) lentiviruses. E&F: Lentivirus-mediated OXT ablation was assessed by OXT immunostaining (E upper panels) and quantitated by counting OXT-expressing neurons in the serial PVN sections (F). AVP immunostaining of matched PVN sections (E lower panels) was used as a control. 3V: third ventricle; scale bar=50 μm. G–J: Mice were monitored for daily food intake (G&I) and weekly BW gain (H&J) during a 3-week follow-up. *P<0.05, n= 4–7 per group. Mice in all these experiments were males.
Therapeutic potential of OXT in preventing obesity
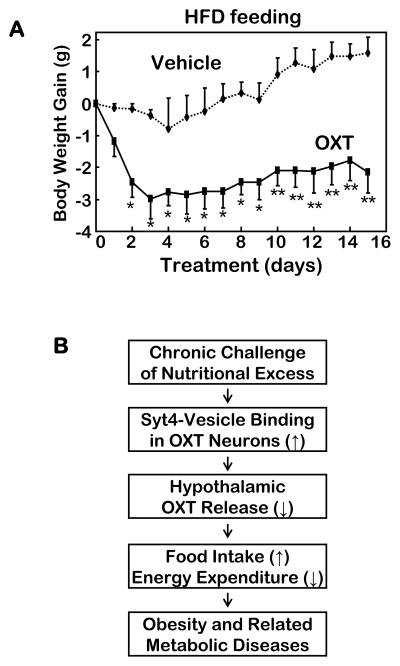
The overall results in Figs. 1–7 demonstrated that chronic nutritional excess can promote hypothalamic Syt4 to suppress OXT release, leading to energy imbalance and obesity. Following these findings, we explored the therapeutic relevance of this conceptual model by testing if central administration of OXT could treat dietary obesity in mice. C57BL/6 mice were first allowed to develop obesity via 5-month HFD feeding, and subsequently implanted with third ventricle cannula. Following post-surgical recovery, mice received daily ICV injections of OXT or vehicle control for a period of 2 weeks. Injections were given every night before the light was off so that the duration (~4 hours) of OXT action overlapped the peak food-consuming period (~6 hours) of mice. Data revealed that OXT treatment rapidly reduced the magnitude of obesity during the first 3 days of the treatment and subsequently prevented HFD from causing weight gain (Fig. 7A). Comparatively, we assessed whether OXT treatment might affect body weight of chow-fed mice – which is mainly comprised of lean body mass. 3 days of OXT injections were found to only slightly reduce the body weight of chow-fed mice, while the same treatment had more evident anti-obesity effect in age- and sex-matched HFD-fed mice (suppl. Fig. 5H). Thus, OXT has strong anti-obesity effect, but its impact on lean body mass was minimal, highlighting the potential value of OXT analogues in obesity treatment. To summarize this study, Syt4-directed OXT release in the hypothalamus is critical for obesity development (Fig. 8B), and Syt4 inhibitors or OXT analogues bear clinical potentials for treating obesity and related health problems.
Figure 8. Anti-obesity therapy by OXT and the Syt4-OXT model in disease.
A. Male C57BL/6 mice with dietary obesity (via 4 months of HFD feeding) received daily third ventricle injections of OXT (1 μg/day) vs. vehicle for 15 days, and were monitored for body weight gain during the treatment period. *P<0.05, **P<0.01, n=5–10 per group.
B. Model of hypothalamic Syt4-OXT in the neural mechanism of obesity and related diseases. Chronic nutritional excess sensitively upregulates Syt4 in the hypothalamus to suppress OXT release, leading to energy imbalance and the development of obesity and co-morbidities. Syt4 and OXT represent two molecular targets for the intervention of obesity and related diseases.
DISCUSSION
Neuropeptide exocytosis in hypothalamic control of energy balance
The CNS regulates whole-body energy balance primarily by the mediobasal region and the PVN of the hypothalamus (Elmquist and Flier, 2004; Schwartz and Porte, Jr., 2005). Recent research advances have significantly elucidated the neuronal subtypes and molecular pathways in these hypothalamic regions that direct the central control of feeding and energy expenditure (Balthasar et al., 2005; Bouret et al., 2004; Cone, 2005; Cowley et al., 2001; Elmquist and Flier, 2004). The underlying molecular basis involves transcriptional regulation of neuropeptide genes in response to dynamic changes of the body’s energy status (Bruning et al., 2000; Coll et al., 2007; Cota et al., 2006; Flier and Maratos-Flier, 1998; Friedman and Halaas, 1998; Minokoshi et al., 2004; Mobbs, 2007; Munzberg and Myers, Jr., 2005; Park and Bloom, 2005; Schwartz et al., 2000). However, compared to the appreciated neuropeptide gene regulation, the role of neuropeptide exocytotic regulation in metabolic actions remains unexplored, despite recent basic research advances showing that neuropeptide release from secretory granules is a cellular event with sophisticated regulation (Stojilkovic, 2005). The present work discovered that exocytosis regulator Syt4 is expressed predominantly in a neuronal subtype of hypothalamic PVN, OXT neurons. Moreover, Syt4 is revealed to negatively regulate OXT release from OXT neurons. The physiological role of this negative regulation by hypothalamic Syt4 is anabolic, and under the environment of chronic nutritional excess, this Syt4 program is further enhanced by unidentified mechanism(s) to become obesogenic. While forcefully suppressing this Syt4 program does not evidently impact normal body weight homeostasis – which might be due to re-balance by other anabolic/catabolic systems, it provides a remarkable and nearly complete protection against the development of obesity and various other metabolic diseases under nutritional excess. These findings suggest that the significance of hypothalamic neuropeptide exocytotic regulation to obesity development and control is substantial. Along this line, further research is needed to explore the exocytotic regulations of other hypothalamic neuropeptides/neurotransmitters and the implications of these potential regulations in hypothalamic control of physiology and disease.
Inhibitory regulation of Syt4 on OXT exocytosis
The identification of hypothalamic Syt4-OXT pathway by this work can provide an answer to the question regarding the physiological function of Syt4. Unlike other Syt members, Syt4 contains an atypical C2A domain in amid acid sequence (von et al., 1997) and fails to catalyze the Ca2+-dependent exocytosis (Chapman et al., 1998; Thomas et al., 1999). The physiological relevance of Syt4 became more puzzling with the relatively normal phenotype of Syt4−/− mice under normal feeding condition (Ferguson et al., 2000a), calling into question whether Syt4 can really regulate vesicle exocytosis in vivo. A recent electrophysiological study pointed to the neuroendocrine relevance of Syt4 (Zhang et al., 2009); however, it offered only a biophysical characterization rather than a physiological study of Syt4 function in neuroendocrine and related diseases. In the current work, we obtained results establishing Syt4 as an important negative regulator of OXT exocytosis in the hypothalamic neuroendocrine system. Considering that Syt4 is present not only in dense-core vesicles but also in micro-vesicles, we speculate that Syt4 may also contribute to the regulation of neurotransmitter release. While such a possibility was not examined in the current study, it represents an interesting subject for the next-step investigation. Other immediate questions stemming the current research include whether and how other Syt members might be involved in the hypothalamic control of neuropeptide/neurotransmitter release to direct metabolic physiology and disease. Studies towards understanding these questions could form an interesting research area which addresses the role of vesicular exocytosis in the hypothalamic control of endocrine and metabolic physiology.
Central actions of OXT in controlling energy balance
OXT, a hypothalamic neuropeptide known for its role in mediating reproductive activities, is synthesized by neurons that are localized predominantly in the PVN of the hypothalamus. OXT is released on demand to the blood stream from neuronal axon terminals that innervate the posterior pituitary. Recent attention has been drawn to OXT’s role in regulating social behaviors, including care, love, emotion and trust (Ferguson et al., 2000b; Keverne and Curley, 2004; Kosfeld et al., 2005), and local release of OXT in the brain seems to underlie these regulations (Ludwig et al., 2002; Ludwig and Leng, 2006). In addition, OXT has catabolic effects through suppressing food intake and promoting physical activities (Douglas et al., 2007; Leng et al., 2008). More recently, several genetic studies reported the development of overeating and obesity in mice that were deficient of OXT (Amico et al., 2005; Kublaoui et al., 2008) or OXT receptor (Takayanagi et al., 2008). The current research focused on OXT exocytosis regulation and provided evidence supporting the notion that regulation of OXT’s local release is critical for hypothalamic control of energy and body weight balance. This concept is in line with recent literature that linked OXT to the hypothalamic actions of leptin (Kutlu et al., 2010) and nesfatin (Maejima et al., 2009). Moreover, our research revealed a reverse relationship between OXT and feeding, i.e., excessive nutrient intake through HFD feeding suppresses OXT release. This observation is supported by a recent study showing that chronic sugar intake dampened the feeding-related c-Fos expression (an indicator of neuronal activities) in OXT neurons (Mitra et al., 2010). Therefore, compromised action of OXT represents a significant hypothalamic mechanism for the development of dietary obesity and co-morbidities. In addition to the mechanistic understanding, our experiments further confirmed that brain injection of OXT was effective in treating mouse obesity without causing appreciable non-specific or side effects. However, given that OXT has a short half-life, the use of OXT for human obesity treatment remains challenging until appropriate OXT analogues and practical delivery methods could be developed.
Syt4: a potential anti-obesity target
The hypothalamus has been known as a pathogenic culprit for overeating, obesity and related diseases. The underlying molecular mechanisms have been related to hypothalamic leptin and insulin resistance which involve SOCS3, PTP1B, IKK/NF- B, MyD88 and ER stress (Bence et al., 2006; Howard and Flier, 2006; Kievit et al., 2006; Kleinridders et al., 2009; Zhang et al., 2008). The current research demonstrated that chronic nutritional excess suppresses OXT release to promote obesity development, and more importantly, Syt4 is identified as the mediator for this dysregulation and hence a molecular target to prevent/reverse the pathogenesis of obesity. Such potentials of Syt4 were verified in animals by the current research showing that Syt4 inhibition was sufficient to prevent or reverse obesity. Notably, the catabolic effect of Syt4 inhibition on body weight in lean animals was modest, reflecting only a “miniature” level of negative energy balance; such small quantity of negative energy balance is insufficient to cause deleterious effects on health, but accumulatively is sufficient to prevent or treat obesity. Thus, in addition to OXT analogues, developing Syt4 modulators could be another avenue for targeting Syt4-OXT pathway to combat obesity and related diseases.
Experimental Procedures
Animals and Phenotyping
C57BL/6 mice were purchased from Jackson Laboratory. Syt4−/− mice were previously described (Ferguson et al., 2000a) and backcrossed into C57BL/6 for more than 5 generations. All mice were housed in standard conditions. HFD was from Research Diets, Inc. The Institutional Animal Care and Use Committee approved all the procedures. Mouse body weight was regularly measured and food intake was determined on a daily basis by individual housing. Pair feeding: individually housed mice were daily provided a defined amount of food, which was based on mouse group with lower ad libitum food intake prior to pair feeding. Mice that did not consume the whole amount of supplied food were excluded from the final analysis. Energy expenditure was determined using metabolic chambers (Columbus Instrument, Inc.) at DRTC core facility of Albert Einstein College of Medicine. GTT: overnight-fasted mice were IP injected with glucose (2 g/kg body weight). Blood glucose was measured using Glucometer Elite (Bayer). Blood insulin and leptin were measured using ELISA kits (Crystal Chem.). Serum OXT levels were measured using Oxytocin EIA kit (Assay Design).
Third Ventricle Cannulation and Animal Treatment
As previously described (Zhang et al., 2008), an ultra-precise small animal stereotactic apparatus (Kopf Instruments) was employed to implant a guide cannula into the third ventricle of anesthetized mice at the midline coordinates of 1.8 mm posterior to the bregma and 5.0 mm below the skull surface. Mice were allowed 1~2 weeks for post-surgical recovery. Individually housed mice received OXT injection (Bachem California, Inc.) via pre-implanted cannula.
Lentiviruses and Intra-PVN Injection
Lentiviral vectors using mouse oxytocin gene cassette (Zhang et al., 2002) to direct oxytocin neuron-specific expression of Flag-tagged Syt4 or GFP were created as previously described (Zhang et al., 2008). Briefly, DNA of mouse oxytocin gene cassette (AI-03) was provided by Dr. H. Gainer (Zhang et al., 2002), and the translation start of exon I was mutated and inserted with Flag-tagged Syt4 or GFP cDNA in the truncated exon III. Lentiviral shRNA against mouse OXT or Syt4 and the matched control lentiviral vector were purchased from Sigma. Lentiviruses were produced from HEK293T cells through co-transfection of target plasmids with their packaging plasmids using Ca3(PO4)2. Lentiviruses were purified by ultracentrifugation. As previously described (Zhang et al., 2008), an ultra-precise stereotax was employed to bilaterally inject lentiviruses into the PVN at the coordinates of 0.85 mm posterior to the bregma, 0.15 mm lateral to the midline, and 4.8 mm below the skull surface.
Ex vivo OXT Release Test
The protocol has been described previously (Jin et al., 2007). Briefly, the PVN slices were dissected from the hypothalamus. In some experiments, the PVN slices were cut into small pieces at the size of ~0.5 mm. The PVN slices were immediately incubated in Locke’s solution constantly supplied with 95% O2 and 5% CO2 at 37°C. The solution was changed every 5 to 10 min during the 60-min experimental period. An aliquot of Locke’s solution was collected to measure the basal levels of OXT release. Depolarization was induced during the final 5 to 10 min by adding KCl to Locke’s solution at a final concentration of 70 mM, and an aliquot of Locke’s solution was collected to measure KCl-stimulated release of OXT. PVN slices were also collected in some experiments, washed and lysated for the measurement of tissue OXT content. Oxytocin EIA kit (Assay Design) was used to determine the OXT levels in the solution and tissues.
Heart Perfusion and Brain Immunostaining
Mice under anesthesia were trans-heart perfused with 4% PFA, and brains were removed, post-fixed in 4% PFA for 4 hours, and infiltrated in 20%–30% sucrose. Brain sections (20-μm thickness) were made using a cryostat at −20°C. Fixed brain sections were blocked with serum of the appropriate species, penetrated with 0.2% Triton-X 100, treated with primary antibodies including rabbit anti-Syt4 (Synaptic Systems), mouse anti-NeuN (Chemicon), guinea pig anti-OXT, and anti-AVP (Peninsula Lab) IgGs, and followed by a reaction with Alexa Fluor® 488 or 555 secondary antibody (Invitrogen) or a reaction using an ABC kit (Vector Lab). Control IgGs of the appropriate species were used as negative controls. A light microscope was used to detect color staining and a con-focal microscope to detect fluorescence.
Immunogold Labeling and Electron Microscopy
Mice under anesthesia were trans-heart perfused with 1% PFA and 1% glutaraldehyde, and tissues were isolated, post-fixed, dehydrated, embedded in London Resin White, and sectioned at 100 nm. For immunogold labeling, tissue sections on nickel grids were quenched, blocked, and incubated with rabbit anti-Syt4 antibody, guinea pig anti-OXT antibody overnight at 4°C, then with gold particle (size: 10 nm or 20 nm)-conjugated secondary antibodies (Ted Pella) for 2 h at room temperature. Sections were post-stained with uranyl acetate and lead citrate, examined on an electron microscope (Hitachi 7500), and imaged using AMT digital imaging equipment.
Quantitative RT-PCR
Total RNA from the homogenized tissue samples was extracted using TRIzol (Invitrogen). Complementary DNA was synthesized using the Advantage RT-for PCR kit (Clontech). PCR amplification of Syt4 mRNA was quantified using SYBR® Green PCR Master Mix (Applied Biosystems). Results were normalized against the expression of house-keeping genes including TATA box-binding protein (TBP) or β-actin.
Statistical Analyses
Two-tailed Student’s t-tests were used for 2-group comparisons. ANOVA and appropriate post hoc analyses were used for comparisons of >2 groups. Data were presented as mean ± SEM. P<0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank H. Herschman for providing Syt4−/− mice, H. Gainer for mouse oxytocin promoter, and G.Y. Wen for assistance in electron microscopy, M. Hendrickson for assistance in con-focal microscopy, E. Chapman and M. Jackson for suggestions, and Cai lab members for general assistance. This study was supported by NIH R01 DK078750 and R01 AG031774, and American Diabetes Association Junior Faculty Award 1-07-JF-09 (all to D. Cai).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- Air EL, Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin combine additively to reduce food intake and body weight in rats. Endocrinology. 2002;143:2449–2452. doi: 10.1210/endo.143.6.8948. [DOI] [PubMed] [Google Scholar]
- Amico JA, Vollmer RR, Cai HM, Miedlar JA, Rinaman L. Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1798–R1806. doi: 10.1152/ajpregu.00558.2005. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Chapman ER. How does synaptotagmin trigger neurotransmitter release? Annu Rev Biochem. 2008;77:615–641. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- Chapman ER, Desai RC, Davis AF, Tornehl CK. Delineation of the oligomerization, AP-2 binding, and synprint binding region of the C2B domain of synaptotagmin. J Biol Chem. 1998;273:32966–32972. doi: 10.1074/jbc.273.49.32966. [DOI] [PubMed] [Google Scholar]
- Coll AP, Farooqi IS, O’Rahilly S. The hormonal control of food intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Johnstone LE, Leng G. Neuroendocrine mechanisms of change in food intake during pregnancy: a potential role for brain oxytocin. Physiol Behav. 2007;91:352–365. doi: 10.1016/j.physbeh.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Flier JS. Neuroscience. The fat-brain axis enters a new dimension. Science. 2004;304:63–64. doi: 10.1126/science.1096746. [DOI] [PubMed] [Google Scholar]
- Ferguson GD, Anagnostaras SG, Silva AJ, Herschman HR. Deficits in memory and motor performance in synaptotagmin IV mutant mice. Proc Natl Acad Sci U S A. 2000a;97:5598–5603. doi: 10.1073/pnas.100104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000b;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Flier JS, Maratos-Flier E. Obesity and the hypothalamus: novel peptides for new pathways. Cell. 1998;92:437–440. doi: 10.1016/s0092-8674(00)80937-x. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Mikoshiba K. A novel alternatively spliced variant of synaptotagmin VI lacking a transmembrane domain. Implications for distinct functions of the two isoforms. J Biol Chem. 1999;274:31428–31434. doi: 10.1074/jbc.274.44.31428. [DOI] [PubMed] [Google Scholar]
- Gao Z, Reavey-Cantwell J, Young RA, Jegier P, Wolf BA. Synaptotagmin III/VII isoforms mediate Ca2+-induced insulin secretion in pancreatic islet beta-cells. J Biol Chem. 2000;275:36079–36085. doi: 10.1074/jbc.M004284200. [DOI] [PubMed] [Google Scholar]
- Gauthier BR, Duhamel DL, Iezzi M, Theander S, Saltel F, Fukuda M, Wehrle-Haller B, Wollheim CB. Synaptotagmin VII splice variants alpha, beta, and delta are expressed in pancreatic beta-cells and regulate insulin exocytosis. FASEB J. 2008;22:194–206. doi: 10.1096/fj.07-8333com. [DOI] [PubMed] [Google Scholar]
- Gauthier BR, Wollheim CB. Synaptotagmins bind calcium to release insulin. Am J Physiol Endocrinol Metab. 2008;295:E1279–E1286. doi: 10.1152/ajpendo.90568.2008. [DOI] [PubMed] [Google Scholar]
- Howard JK, Flier JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab. 2006;17:365–371. doi: 10.1016/j.tem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Hudson AW, Birnbaum MJ. Identification of a nonneuronal isoform of synaptotagmin. Proc Natl Acad Sci U S A. 1995;92:5895–5899. doi: 10.1073/pnas.92.13.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iezzi M, Eliasson L, Fukuda M, Wollheim CB. Adenovirus-mediated silencing of synaptotagmin 9 inhibits Ca2+-dependent insulin secretion in islets. FEBS Lett. 2005;579:5241–5246. doi: 10.1016/j.febslet.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, Fujita K, Takasawa S, Yokoyama S, Koizumi K, Shiraishi Y, Tanaka S, Hashii M, Yoshihara T, Higashida K, Islam MS, Yamada N, Hayashi K, Noguchi N, Kato I, Okamoto H, Matsushima A, Salmina A, Munesue T, Shimizu N, Mochida S, Asano M, Higashida H. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Curley JP. Vasopressin, oxytocin and social behaviour. Curr Opin Neurobiol. 2004;14:777–783. doi: 10.1016/j.conb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab. 2006;4:123–132. doi: 10.1016/j.cmet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, Kim KS, Kim SW, Kim HS, Park JY, Kim YB, Lee KU. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9:901–906. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- Kleinridders A, Schenten D, Konner AC, Belgardt BF, Mauer J, Okamura T, Wunderlich FT, Medzhitov R, Bruning JC. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 2009;10:249–259. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol. 2008;22:1723–1734. doi: 10.1210/me.2008-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu S, Aydin M, Alcin E, Ozcan M, Bakos J, Jezova D, Yilmaz B. Leptin modulates noradrenaline release in the paraventricular nucleus and plasma oxytocin levels in female rats: a microdialysis study. Brain Res. 2010;1317:87–91. doi: 10.1016/j.brainres.2009.12.044. [DOI] [PubMed] [Google Scholar]
- Leng G, Onaka T, Caquineau C, Sabatier N, Tobin VA, Takayanagi Y. Oxytocin and appetite. Prog Brain Res. 2008;170:137–151. doi: 10.1016/S0079-6123(08)00413-5. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang P, Xu J, Gorelick F, Yamazaki H, Andrews N, Desir GV. Regulation of insulin secretion and GLUT4 trafficking by the calcium sensor synaptotagmin VII. Biochem Biophys Res Commun. 2007;362:658–664. doi: 10.1016/j.bbrc.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- Machado HB, Liu W, Vician LJ, Herschman HR. Synaptotagmin IV overexpression inhibits depolarization-induced exocytosis in PC12 cells. J Neurosci Res. 2004;76:334–341. doi: 10.1002/jnr.20072. [DOI] [PubMed] [Google Scholar]
- Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, Takano E, Yoshida N, Koike M, Uchiyama Y, Fujiwara K, Yashiro T, Horvath TL, Dietrich MO, Tanaka S, Dezaki K, Oh I, Hashimoto K, Shimizu H, Nakata M, Mori M, Yada T. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab. 2009;10:355–365. doi: 10.1016/j.cmet.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Mitra A, Gosnell BA, Schioth HB, Grace MK, Klockars A, Olszewski PK, Levine AS. Chronic sugar intake dampens feeding-related activity of neurons synthesizing a satiety mediator, oxytocin. Peptides. 2010;31:1346–1352. doi: 10.1016/j.peptides.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs CV. The “domino theory” of hunger: the hypothalamus is hot. Cell Metab. 2007;5:1–2. doi: 10.1016/j.cmet.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Myers MG., Jr Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;8:566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- Park AJ, Bloom SR. Neuroendocrine control of food intake. Curr Opin Gastroenterol. 2005;21:228–233. doi: 10.1097/01.mog.0000153358.05901.3f. [DOI] [PubMed] [Google Scholar]
- Plum L, Lin HV, Dutia R, Tanaka J, Aizawa KS, Matsumoto M, Kim AJ, Cawley NX, Paik JH, Loh YP, DePinho RA, Wardlaw SL, Accili D. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med. 2009;15:1195–1201. doi: 10.1038/nm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Sladek CD, Somponpun SJ. Estrogen receptors: their roles in regulation of vasopressin release for maintenance of fluid and electrolyte homeostasis. Front Neuroendocrinol. 2008;29:114–127. doi: 10.1016/j.yfrne.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojilkovic SS. Ca2+-regulated exocytosis and SNARE function. Trends Endocrinol Metab. 2005;16:81–83. doi: 10.1016/j.tem.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. Synaptotagmins: why so many? J Biol Chem. 2002;277:7629–7632. doi: 10.1074/jbc.R100052200. [DOI] [PubMed] [Google Scholar]
- Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport. 2008;19:951–955. doi: 10.1097/WNR.0b013e3283021ca9. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Ferguson GD, Herschman HR, Elferink LA. Functional and biochemical analysis of the C2 domains of synaptotagmin IV. Mol Biol Cell. 1999;10:2285–2295. doi: 10.1091/mbc.10.7.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukropec J, Anunciado RV, Ravussin Y, Kozak LP. Leptin is required for uncoupling protein-1-independent thermogenesis during cold stress. Endocrinology. 2006;147:2468–2480. doi: 10.1210/en.2005-1216. [DOI] [PubMed] [Google Scholar]
- Vician L, Lim IK, Ferguson G, Tocco G, Baudry M, Herschman HR. Synaptotagmin IV is an immediate early gene induced by depolarization in PC12 cells and in brain. Proc Natl Acad Sci U S A. 1995;92:2164–2168. doi: 10.1073/pnas.92.6.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von PC, Ichtchenko K, Shao X, Rizo J, Sudhof TC. The evolutionary pressure to inactivate. A subclass of synaptotagmins with an amino acid substitution that abolishes Ca2+ binding. J Biol Chem. 1997;272:14314–14319. doi: 10.1074/jbc.272.22.14314. [DOI] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest. 2005;115:951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang BJ, Kusano K, Zerfas P, Iacangelo A, Young WS, III, Gainer H. Targeting of green fluorescent protein to secretory granules in oxytocin magnocellular neurons and its secretion from neurohypophysial nerve terminals in transgenic mice. Endocrinology. 2002;143:1036–1046. doi: 10.1210/endo.143.3.8700. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Bhalla A, Dean C, Chapman ER, Jackson MB. Synaptotagmin IV: a multifunctional regulator of peptidergic nerve terminals. Nat Neurosci. 2009;12:163–171. doi: 10.1038/nn.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.