Abstract
This is a selective review that provides the context for the study of perinatal affective disorder mechanisms and outlines directions for future research. We integrate existing literature along neural networks of interest for affective disorders and maternal caregiving: (i) the salience/fear network; (ii) the executive network; (iii) the reward/social attachment network; and (iv) the default mode network. Extant salience/fear network research reveals disparate responses and corticolimbic coupling to various stimuli based upon a predominantly depressive versus anxious (post-traumatic stress disorder) clinical phenotype. Executive network and default mode connectivity abnormalities have been described in postpartum depression (PPD), although studies are very limited in these domains. Reward/social attachment studies confirm a robust ventral striatal response to infant stimuli, including cry and happy infant faces, which is diminished in depressed, insecurely attached and substance-using mothers. The adverse parenting experiences received and the attachment insecurity of current mothers are factors that are associated with a diminution in infant stimulus-related neural activity similar to that in PPD, and raise the need for additional studies that integrate mood and attachment concepts in larger study samples. Several studies examining functional connectivity in resting state and emotional activation functional magnetic resonance imaging paradigms have revealed attenuated corticolimbic connectivity, which remains an important outcome that requires dissection with increasing precision to better define neural treatment targets. Methodological progress is expected in the coming years in terms of refining clinical phenotypes of interest and experimental paradigms, as well as enlarging samples to facilitate the examination of multiple constructs. Functional imaging promises to determine neural mechanisms underlying maternal psychopathology and impaired caregiving, such that earlier and more precise detection of abnormalities will be possible. Ultimately, the discovery of such mechanisms will promote the refinement of treatment approaches toward maternal affective disturbance, parenting behaviours and the augmentation of parenting resiliency.
Keywords: postpartum depression, brain imaging, attachment, caregiving
Introduction
Because of the seriousness of the adverse negative impact of maternal psychopathology on child development and challenges to effective maternal treatment, there are growing efforts to understand the neurobiology of maternal psychopathology and caregiving deficits, with the objective of establishing biomarkers for illness severity and treatment response. There has been a false dichotomy in the early affective neuroscience literature in mothers, whereby one set of studies focused on standard emotion processing deficits in samples of women with major depression and another set of studies focused on maternal responses to infant cues to inform the neural circuitry of maternal caregiving. A growing number of recent studies have valiantly begun to integrate the complex concepts of impaired emotion processing and deficient maternal caregiving. For these latter studies, the design typically consists of populations included for presence or absence of a psychiatric disorder, defined using criteria from the Diagnostic and Statistical Manual (e.g. DSM-IV) or related symptom measures, and functional magnetic resonance imaging (fMRI) paradigms typically use ecologically relevant infant and child stimuli, with clinical relevance often demonstrated by correlation with impaired mother–infant interactions. Here, we attempt to summarise and integrate this rich literature along four key neural networks that are consistently described both in the general affective neuroscience literature, as well as the maternal caregiving literature (1): (i) the salience/fear network; (ii) the executive network; (iii) the reward/social attachment network; and (iv) the default mode network. Below, we provide a selective review of each network independently, describing the functional role of each network, as well as what we know about how these networks function in depression and anxiety disorders in nonpostpartum and postpartum populations (Table 1).
Table 1.
Functional magnetic resonance imaging (fMRI) Studies of Mothers Highlighting Contrasting Findings in Healthy Mothers Versus Those With Affective Symptoms, Substance Use, or Characteristics Relating to Impaired Attachment Processes.
| Author (year) (reference) | N; Group | Age of infants/ children |
fMRI paradigm | Findings |
|---|---|---|---|---|
| Salience/fear network | ||||
| Healthy mothers | ||||
| Seifritz et al. (2003) (40) | 20, mothers versus women without children |
< 3 years | Infant cries and laughter versus control sound |
↑ Amygdala, middle cingulate cortex and insula activity in mothers versus women without children |
| Bartels and Zeki (2004) (45) | 19, mothers | 9 months to 6 years |
Face pictures of own versus unknown/familiar child; comparison of maternal versus romantic love |
↑ Insula and dACC activity to own child, which overlapped regions active to romantic others |
| Ranote et al. (2004) (101) | 10, mothers | 4–8 months | Video of own versus other infant |
↑ Amygdala activation to own infant |
| Swain et al. (2008) (42) | 12, vaginal versus caesarean section deliveries |
2–4 weeks | infantcry (own versus other) | ↑ Amygdala activity to infant cry in women with vaginal versus caesarean section deliveries |
| Lenzi et al. (2009) (114) | 16, primiparous mothers |
6–12 months | Viewing and imitating faces of own versus other child |
↑ Insula, amygdala and inferior frontal gyrus activity associated with increased maternal empathy |
| Strathearn et al. (2013) | 39, primiparous mothers |
~11 months | Child faces (own versus other) | ↑ Amygdala activity to own versus other infant faces |
| Ho et al. (2014) (216) | 14, mothers | ~4 years | Parenting decision-making task |
↑ vACC to +ve versus −ve infant feedback. Hypothalamus-septa coupling during −ve feedback inversely related to cortiso reactivity |
| Mothers with affective symptoms, substance use or impaired infant interaction | ||||
| Silverman et al. (2007) (49) | 8, postpartum mothers with EPDS > 12 or <6 |
7–8 weeks | Negative emotional words | ↓ Amygdala but ↑ insula activity to negative words in women with EPDS > 12 |
| Moses-Kolko et al. (2010) (50) | 30, PPD versus HC | 8–10 weeks | Fear/threat adult faces | ↓ DMPFC activity and amygdala- DMPFC connectivity in PPD |
| ↓ Amygdala activity associated with postpartum depression severity |
||||
| Laurent & Ablow (2012) (43) | 22, PDD versus HC | 18 months | infantcry (own versus other) | ↓ dACC and insula activity in PPD |
| Barrett et al. (2012) (39) | 22, mothers, varied affective symptoms |
~3 months | Infant faces (own versus other), with affect rating task |
↓ Amygdala activation to positive own infant faces associated with more depression (EPDS) and anxiety symptoms (STAI-trait) |
| Schechter et al. (2012) (61) | 20, IPV-PTSD versus control |
12–42 months | Videos of child stress during separation (own versus other child) |
↑ Insula and ↓ mPFC activity to child separation videos that correlated with self-reported stress |
| Moser et al. (2013) (63) | 20, IPV-PTSD | 12–42 months | Videos of child stress with separation (own versus other child) |
↓ Cingulate, hippocampus and ↑ DLPFC activity to child separation videos associated with greater dissociative symptoms |
| Landi et al. (2011) (128) | 54, substance using versus non-using postpartum mothers |
1–3 months | Infant sad faces, low distress cries (all other infants) |
↓ Amygdala, parahippocampus, insula, lateral PFC, activity in substance users |
| Musser et al. (2012) (157) | 22, primiparous, varied maternal sensitivity |
18 months | infantcry (own versus other versus control sounds) |
↓ IFG and ↑ insula activity associated with reduced maternal sensitivity and increased maternal intrusiveness |
| Laurent et al. (2012) (156) | 22, primiparous mothers |
15–18 months | infantcry (own versus control sound) |
↑ Parahippocampus/amygdala and insula activity to cry in mothers of insecurely-attached infants |
| Author (year) (reference) | N; Group | Age of infants/ children |
Paradigm, variable | Regional findings |
| Executive network | ||||
| Healthy postpartum versus menstruating nonpostpartum women | ||||
| Bannbers et al. (2013) (76) | 26; postpartum versus menstruating women |
48 h; 4–7 weeks | Go/NoGo task | ↓ VLPFC, precentral gyrus, and dACC at later versus early postpartum timepoints and relative to nonpostpartum women |
| Reward/social attachment network | ||||
| Healthy mothers | ||||
| Lorberbaum et al. (2002) (41) | 10, mothers | 1–2 months | infantcry (other) versus white noise versus baseline rest |
↑ Medial thalamus, medial PFC, right OFC to infant cry |
| Bartels and Zeki (2004) (45) | 19, mothers | 9 months–6 years | Face pictures of own versus unknown/familiar child |
↑ Striatum, thalamus, PAG to own child |
| Leibenluft et al. (2004) (44) | 7, mothers | 5–12 years | Child faces (own versus familiar/unfamiliar other) |
↑ Mentalising circuitry (DMPFC, MPFC, PCC, TPJ, pSTS) to own child > familiar/unknown child |
| Noriuchi et al. (2008) (37) | 13, mothers | 15–20 months | Video clips own versus other infant |
↑ OFC, periaqueductal gray, putamen to own infant |
| Strathearn et at. (2008) (38) | 26, primiparous mothers |
7–10 months | Child happy faces (own versus other) |
↑ Putamen, SN, thalamus, amygdala to own versus other faces |
| Swain et al. (2008) (42) | 12, vaginal versus caesarean section deliveries (VD) |
2–4 weeks | infantcry (own versus other) | ↑ SFG, caudate and thalamus to infant cry in women with vaginal versus caesarean section deliveries. Correlation of parental preoccupation with lenticular nucleus activation |
| Kim et al. (2011) (48) | 17, breast- versus formula-feeding mothers |
2–4 weeks and 3–4 months |
infantcry (own versus other) | ↑ SFG, insula, precuneus, striatum, and amygdala activity to own infant cry in breast- versus formula-feeding mothers |
| ↑ Maternal sensitivity associated with increased SFG and amygdala activity |
||||
| Mothers with affective symptom, impaired infant interaction, or low maternal care received, insecure attachment | ||||
| Silverman et al. (2007) (49) | 8, postpartum mothers with EPDS > 12 or <6 |
7–8 weeks | Positive emotional words | ↓ Striatum to positive words in women with EPDS > 12 |
| Strathearn et al. (2009) (46) | 30, primiparous mothers |
~11 months | Child happy faces (own versus other) |
↓ VST, OFC, mPFC activity to faces in mothers without secure attachment |
| Kim et al. (2010) (53) | 26, mothers | 2–4 weeks | infantcry (other versus control sound) |
↓ MFG, STS and fusiform gyrus to infant cry for women with a history of lower maternal care received |
| Moses-Kolko et al. (2011) (105) |
24, PPD versus HC | 8–10 weeks | Monetary reward task | ↓ Ventral striatal duration of reward-related response in PPD |
| Laurent & Ablow (2011) (43) | 22, PDD versus HC | 18 months | infantcry (own versus control sound) |
↓ Thalamus, caudate, OFC, SFG in PPD, and in association with higher CESD scores |
| Atzil et al. (2011) (125) | 23, mothers | 4–6 months | Mother–infant interaction videos (self versus other mother–infant dyad) |
↑ Striatal activity associated with greater mother–infant behavioral synchrony |
| Default mode network | ||||
| Depressed versus healthy mothers | ||||
| Xiao-juan et al. (2011) (147) | 21, PPD versus HC | Within 16 weeks | Resting state | ↑ PCC index of nearest neighbor connectivity (ReHo) in PPD |
| Chase et al. (2013) (144) | 37, PPD versus HC | 10 weeks | Resting state | ↓ PCC-amygdala connectivity in PPD |
| Deligiannidis et al. (2013) (146) |
17, PPD versus HC | 9 weeks | Resting state | ↓ Connectivity within corticolimbic circuits including the default mode network |
CESD, Center for Epidemiologic Studies Depression Scale; dACC, dorsal anterior cingulate cortex; DMPFC, dorsomedial prefrontal cortex; EPDS, Edinburgh Postnatal Scale for Depression; HC, healthy controls; IFG, inferior frontal gyrus; IPV-PTSD, interpersonal violence-related post-traumatic stress disorder; MPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; OT, oxytocin; PAG, periaqueductal gray; PCC, posterior cingulate cortex; PPD, postpartum depression; pSTS, posterior superior temporal sulcus; ReHo, regional homogeneity or synchronisation between time series of a voxel with that of its nearest neighbors; SFG, superior frontal gyrus; SN, substantia nigra; STAI, Speilberger State Trait Anxiety Inventory; TPJ, temporal–parietal junction; VE, valence. References are listed chronologically. In cases where references have findings that relate to multiple networks, such references have been categorised according to the most relevant network and, in some cases, are listed in two categories if more than one network is adequately assessed.
Primer on fMRI activation studies
Because this review centers on fMRI studies, we include a brief primer on this technology. The fMRI signal is based upon blood-oxygenation-level-dependent (BOLD) measurements that reflect changes in neural activity over a time resolution of seconds. Typically, BOLD signals during different events are measured throughout the brain. Subsequent analyses identify brain regions that show greater activity in response to a condition of interest (e.g. own baby-cry versus other baby-cry) and allow correlation of such brain activity with measures of psychopathology and parental behaviours. Several caveats of current brain imaging approaches must be considered. First, the BOLD signals of the fMRI data are indirect measures of neural activity, such that the data are susceptible to non-neural changes in the participant’s body and environment. These problems are partially controlled with choice of population and averaging. Second, imaging data provide information on the association, rather than causality, between brain and behaviour. Confidence in associations may be strengthened with multiple lines of inquiry, replication and possible analyses under development that consider connectivity and timing in responses in different circuits. Third, because of the limitations of space and movement in the scanner, fMRI studies must use simple tasks and stimuli to simulate the performance of parenting behaviours. This is addressed by regressing brain activity to real-life parenting and by using increasingly sophisticated and realistic in and out of scanner tasks. Finally, differences in parental brain function are subject to many factors that are not typically controlled, including early-life experiences, postpartum timing, social environment, sleep and hormonal concentrations. Investigators are gradually coming to understand and attempt to control for these variables.
Salience/fear network
The role of the salience network is to focus the brain’s resources on the most important environmental stimuli in the service of guiding future actions that will overcome or circumvent threats to organism homeostasis. As initially described by Seeley et al. (2), the dorsal anterior cingulate cortex (dACC), orbital fronto-insular cortex and anterior insula are the key components of the salience network, which are well-poised to integrate sensory, visceral, autonomic, interoceptive and hedonic data through connections with subcortical (amygdala, striatum, dorsomedial thalamus, hypothalamus) and brainstem structures (periaqueductal gray, substantia nigra and ventral tegmental area). The neural salience network (2) responds in proportion to the degree of personal relevance of a given cue and therefore activates strongly to the social threat of angry/fearful faces (3) and threat of shock (4,5). Moreover, the role of the anterior portion of the insula in interoceptive awareness of emotional stimuli (i.e. sensitivity to internal bodily sensations) and its association with autonomic arousal (6) highlights the putative role of this region in reflecting individual differences in anxious arousal during the processing of threat (7).
Neural systems for processing threat overlap with those in the salience network and encompass, most notably, the amygdala, insula and bed nucleus of the stria terminalis (BNST). The amygdala and BNST show greater activity to negative emotional faces and shock with increasing levels of anxious arousal. It has been demonstrated in rodent models that neural responses to acute versus sustained threat may be anatomically segregated, with amygdala activity increasing to acute threat and BNST activity increasing to sustained threat cues and hypervigilance (8). Heightened BNST responses to the threat of electric shock in proportion to levels of trait anxiety (5) support this notion in humans, although the functional segregation may not be as clear as in rodents given that amygdala also activates strongly in threat-of-shock paradigms. Given the neuroimaging challenges of small size and irregular shape of the BNST, the amygdala has been the subcortical structure most frequently studied in response to stimuli of environmental threat. The amygdala serves a critical gating function and sends efferent neurones to the higher-order sensory cortex to evaluate and respond to threat (9).
In unselected samples, activity within the salience network to negative emotional stimuli correlates with state anxiety (e.g. to a particular situation versus characteristic trait-level anxiety) (2) and is considered to be involved mechanistically in the emotional hyper-reactivity that can evolve into a major depressive episode. Across numerous studies, nonpostpartum individuals with depression, anxiety disorders, trait anxiety and neuroticism have shown increased amygdala activity in response to negative emotional stimuli (10). Specifically, amygdala activation to negative faces has been replicated in major depressive disorder (MDD) (11–18) and is associated with the ruminative quality (19) and negative bias to emotional information in MDD (20) that represents exaggerated responses to negative emotional stimuli. Heightened amygdala activity to negative emotional stimuli (11–15,17–21) may also serve as a marker of persistent effects of past MDD, which was shown in some (21–23) but not other studies (14,17,24,25) of individuals who recovered from depression versus controls.
In human research, important regulatory regions, including dACC, ventral ACC and the dorsomedial prefrontal cortex (DMPFC), are active during the perception of emotionally salient and threatening stimuli, such as electric shock. Indeed, a deficit in the regulatory ability of amygdala by medial PFC structures is a widely described mechanism for increased attention to threat, with different patterns of medial PFC-amygdala coupling associated with different anxiety constructs. Generalised anxiety disorder, for example, is associated with decreased functional connectivity between the ventral ACC and amygdala during implicit emotion regulation (26,27), which is considered to represent insufficient regulation of threat. Higher trait anxiety may be associated with greater functional connectivity between DMPFC/dACC and amygdala, denoting increased attention to threat, a circuit of DMPFC–amygdala positive connectivity. Robinson et al. (28) describe this circuit as an ‘aversive amplification’ mechanism whereby threat-responding is increased in the service of protecting the individual from harm.
In rodent studies of early motherhood, the fear and threat circuitry is attenuated in the service of facilitating maternal interaction with, and care/protection of, her offspring. Reduced fear and stress responses in puerperal rodents are associated with decreased amygdala c-fos synthesis (compared to nonpuerperal rodents) (29) and are attributed to increased puerperal amygdalar prolactin and oxytocin inputs (30–32). A novel paradigm that used functional neuroimaging in awake postpartum rodents to examine regional brain BOLD in response to threatening cues revealed that intracerebral oxytocin infusion was associated with an increased percentage BOLD change (threatening cue versus rest) in BNST (33), which may relate to oxytocin-mediated inhibitory neural activity in fear response centers. In a similar paradigm, arginine vasopressin circuits were also shown to influence the degree of neural responses to threat in amygdala (34). A reduction of fear responses in newly maternal rodents is inversely related to an increase of maternal aggression (see also section on Reward/social-attachment network, below) in the service of protection of the offspring, which are functions mediated via brain interactions with prolactin, oxytocin, arginine vasopressin and the hypothalamic-pituitary-adrenal (HPA) axis (35). For example, blockade of arginine vasopressin receptors was found to increase maternal aggression by suppressing amygdala and increasing olfactory and infralimbic activity (34,36).
Functional MRI studies of human mothers have rarely been acquired in the immediate postpartum period. At 2-week or more distal postpartum timeframes, a number of studies have examined healthy mothers responding to negative emotional infant stimuli, including video clips of infants in distress (37), pictures of sad infant faces (38,39) and infant cries (40–43). These studies have revealed that negative infant stimuli activated the amygdala (40,42) (in addition to broader maternal circuitry; see below) which has often been interpreted as a sign of emotional salience (40) or positive emotion associated with attachment (44). One notable exception was a report of amygdala deactivation to own infant pictures (45), which was interpreted as an ‘evolutionarily protective mechanism to invest more energy into reward-oriented rather than fear-oriented emotions and behaviours in motherhood’. Mothers with insecure adult attachment (46), recurrent MDD since adolescence (43), higher levels of depression and anxiety symptoms (39) and psychopathological risk factors (47), as well as mothers with fewer oxytocin/prolactin-stimulating functions [cesarean section deliveries (42) and bottle-feeding (48)], had less robust amygdala activity to infantcry stimuli.
Two additional studies (49,50) of mothers attending to noninfant negative emotional stimuli found that PPD versus healthy status and greater PPD severity was associated with reduced amygdala activity to fearful and threatening faces or negatively valenced words. Taken together, these studies suggest a relatively consistent picture of blunted amygdala activity to negative emotional stimuli and infant distress stimuli among mothers with more depressive and anxious affect, less secure attachment styles, and less prosocial hormone availability.
Little is known about cortical brain regions that directly modulate limbic reactivity to infant and other emotional cues in postpartum women because few studies of within-circuit connectivity have been published. In a single example of such a study, postpartum depressed women demonstrated an absence or disengagement of DMPFC- amygdala connectivity when viewing fearful and threatening faces, whereas healthy mothers had intact DMPFC-amygdala connectivity to fearful and threatening faces (50). The activation of DMPFC to infantcry (41,43), to distressed infant videos (37) and to own child greater than to familiar/unknown faces (44) advances a role for areas of covert appraisal and social cognition in healthy maternal behaviour. A single, small study (n = 8) revealed reduced posterior orbitofrontal cortex (OFC) activity to negative words in women with PPD compared to healthy postpartum women (49). Furthermore, Laurent and Ablow (43) reported that higher depressive symptoms (Center for Epidemiologic Studies Depression Scale) and a diagnosis of MDD were associated with lower left OFC, dACC and medial superior frontal gyrus (SFG) activity to infantcry. The available data therefore suggest that women with PPD disengage critical corticolimbic neurocircuitry that is involved in emotional salience and threat processing during exposure to infant and negative affective stimuli. This requires further examination with techniques for analysing within-circuit regional connectivity.
An important exception, however, is provided by the findings of Swain et al. (42), in which heightened lenticular nucleus and left medial PFC activity to own baby-cry was associated with more depressive symptoms and more anxious intrusive thoughts in a group of 12 mothers scanned 3 weeks postpartum on average. Swain et al. (42) argue for a potentially adaptive function subserved by these apparent anxious/depressive symptoms in nondepressed mothers in the early postpartum mother that is not directly comparable to findings in mothers who are three or more months postpartum. In other studies, Swain et al. (51,52) suggest that maternal neural responses to infant stimuli change with the time since delivery, with a diminution of anxiety-related subcortical responses and an increase in regulatory cortical responses to infantcry among healthy mothers. Indeed, both human (53) and rodent (54) maternal brain research reveal postpartum structural brain growth in orbitofrontal and temporal cortices that may reflect social learning in parents in preparation for changing baby signals over time in addition to the increasingly complex array of possible interactional scenarios. This also opens windows into domains of dysfunction when these adaptations fail to occur, leaving new parents underprepared to respond to increasingly complex situations. Therefore, postpartum duration-dependent analysis of fear/salience network activity and PFC modulatory networks is an important area in need of future investigation.
Summary point
These studies indicate that mothers with syndromal level depression, less secure attachment styles and less pro-social hormone availability have blunted amygdala and insula activity to negative emotional stimuli and infant distress stimuli, which is the opposite of that described in nonpostpartum depressed samples. Heightened intrusive/anxious responses that accompany subsyndromal affective symptoms in very early (3 weeks) postpartum mothers, associated with medial PFC and lenticular nucleus activity to infantcry, may also serve an adaptive role in attaching to the infant that awaits replication.
Post-traumatic stress disorder (PTSD) is an affective disorder with a unique neurobiology for which there are reports, in non-postpartum samples, of increased insula and amygdala activity when listening to traumatic audio clips in the scanner (55). Ventral and dorsal regions of the ACC activity are noted to variably deactivate or activate in association with threat appraisal (56), depending upon the presence of dissociative symptoms (57). Dissociative-type PTSD or individuals with high scores on the Dissociative Experiences Scale demonstrate increased inhibitory modulation of amygdala and insula by the ventromedial prefrontal cortex (VMPFC) and ventral ACC, which suppresses re-experiencing and hyperarousal symptoms and thus constitutes an effective strategy for diminishing attention to threat by keeping threat segregated from other cognitive processes. These and other mechanisms that distinguish PTSD from MDD may also relate to respective differences in HPA axis function (58). In general, individuals with MDD display HPA axis hyper-reactivity, reported as increased baseline cortisol, exaggerated cortisol response to ACTH and non-suppression of the dexamethasone suppression test (DST) (59). By contrast, individuals with PTSD have evidence of HPA axis hyporeactivity, highlighted by a recent meta-analysis showing lower daily cortisol output and lower post-DST cortisol in PTSD compared to healthy controls (60).
Similar to non-postpartum populations, but in contrast to anxious and depressed postpartum mothers, 12–48-month postpartum mothers with PTSD secondary to adult interpersonal violence-related PTSD (IPV-PTSD) had heightened subcortical responses in the fear circuitry. In their elegant study of mothers viewing their own and other child separation versus free play videos, Schechter et al. (61) found that IPV-PTSD mothers activated an ‘unrestrained’ fear-circuitry that was associated with higher subjective ratings of stress. The circuitry described included heightened activity in bilateral anterior entorhinal cortex, left caudate, left insula and reduced frontocortical activity [SFG, Brodmann area (BA) 8, BA10; bilateral superior parietal lobes, BA7] in women with IPV-PTSD compared to healthy mothers. Stress, as measured by the Clinically Administered PTSD Scale and the PTSD Symptom Checklist-short version, was inversely correlated with SFG (BA10) activity and positively associated with caudate activity. Based upon behavioural data in this same population (62), it was suggested that hyperactivity within the fear circuitry associated with IPV-PTSD might mediate reduced maternal emotional availability for coordinated joint attention during play. A sub-analysis within this dataset (63) demonstrated that dissociative symptoms, when present with or without PTSD, were associated with increased cortical (right dorsolateral PFC, ‘DLPFC’) and decreased limbic activity (perirhinal cortex, hippocampus, insula) to separation versus play videos, as has been found previously in other PTSD-dissociative-subtype populations viewing traumatic and nonspecific negative emotional stimuli (64). These findings highlight the importance of clinical characterisation along lines of intersecting dimensions, such as dissociation, PTSD symptoms and other domains of cognitive function that affect maternal sensitivity and child outcome.
Summary point
Mothers, more than 1 year postpartum, who have IPV-PTSD, have a neurobiology that resembles PTSD in nonpostpartum populations, as characterised by hyperactivity within the fear circuitry (amygdala and anterior insula) and alternatively decreased or increased (dissociative-type) voluntary VMPFC/ventral ACC regulation of subcortical activity.
Executive network
As described above, prefrontal cortical regions are involved in the threat processing circuitry with a primary function of regulating activity in subcortical structures. A number of these regulatory regions comprise the executive network, which comes online both during emotional processing tasks and also during performance of attention, working memory and response inhibition tasks. This network primarily includes the DLPFC, the lateral/inferior parietal cortex, and the dACC/DMPFC region, with projections to hippocampus. Activity within the executive network is associated with task performance (2).
The executive dysfunction described in MDD has been associated with altered prefrontal executive network activity on common cognitive neuroscience performance tasks, such as the ‘Go/No-Go Association Task’, testing behavioural inhibition, and the ‘n-BACK task’, measuring cognitive control (65,66). Convergent studies have described decreased activity in emotion regulatory regions including DLPFC, DMPFC and VMPFC (19,67–70), as well as alterations in corticolimbic connectivity during processing of negative faces in unipolar and bipolar depression (71,72). Reduced prefrontal neural activity may normalise following effective depression treatment, such as with transcranial magnetic stimulation (73), and also predict the antidepressant treatment response.
Based upon the evidence of reduced cognitive function in healthy postpartum women (74,75), Bannbers et al. (76) conducted a recent study of response inhibition using the Go/No-Go task to explore a hormone-linked neural basis for this clinical phenomenon. They found that healthy postpartum women showed reduced ventrolateral PFC (VLPFC) activity relative to nonpostpartum women (without concomitant difference in behavioural performance), as well as a lessening over the course postpartum (on average 28 h postpartum and repeated at 34 days postpartum) of task-related VLPFC and dACC activity. This suggests that healthy postpartum women might be operating at a disadvantage for cognitive and emotion-regulatory functions. It is possible that hormone-related blood flow effects could influence these conclusions given similar findings of lower regional activity in follicular (low hormone state) versus luteal (high hormone state) phase (77) and the role of fluctuating neurohormone concentrations in parental response to baby-cry, suckling and baby-smile (78,79). In addition, the early postpartum timeframe has a number of confounding physiological effects that may contribute to altered signal. These intriguing preliminary findings merit continued study, as well as extension to additional executive function cortical regions. In the salience/fear network section above, we describe deficient prefrontal cortical activity in depressed relative to healthy mothers when processing emotional and infant stimuli. Additional studies, both in healthy and depressed postpartum women, are needed to clarify the relationships between duration postpartum, executive function, emotion regulation and affective symptoms.
Summary point
Although little research on executive networks has been conducted in postpartum women to date, this is an area ripe for investigation given the critical role for planning, organisation and attention in parenting and known deficits in affective disorders more broadly.
Reward/social-attachment network
The cortico-striatal-thalamic-cortical loop is a neural circuit that integrates the emotional, behavioural and endocrine aspects of motivated behaviour (80). Together with extrastriatal afferents, dopaminergic output from the ventral tegmental area to the extended ventral striatum (encompassing nucleus accumbens, ventral caudate and putamen) modulates the reinforcing effects of stimuli (81,82). In healthy subjects, fMRI tasks that include anticipation and receipt of monetary rewards have been associated with strong striatal activation (83), whereas medial prefrontal cortex (mPFC) regions, such as VMPFC, are activated during learning of reward-based contingencies (84).
The reward or hedonic neural circuitry has been of great interest to depression researchers because reduced positive affect, in addition to increased negative affect, is an increasingly recognised feature of depressive disorders. Convergent behavioural studies have revealed reduced pursuit and savouring of reward in MDD (85) and molecular imaging and preclinical studies provide evidence that this is mediated by aberrant dopamine signalling in the mesostriatal system (86). The results suggest that behavioural reductions in reward-seeking and pleasure are mediated by hypoactivation of ventral striatum to different aspects of reward (85,87,88) and pleasant stimuli (89–91). In several studies of happy faces processing in nonpostpartum populations, it appears that increased VMPFC activity to happy faces is associated with risk for (92) or the presence of (93) mood and anxiety disturbance and might over-regulate striatal responses to positive cues. Therefore, happy faces might be an important social reward cue that can tap into this neural circuitry.
Increased overall dopaminergic tone in parous rodents and post-partum women has been repeatedly described (94) and is considered to be amplified via oxytocinergic inputs to the mesostriatal reward system. The dopamine reward system plays an important role in maternal care insofar as it motivates mother–infant interaction and facilitates the reinforcing aspects of caregiving. Maternal– pup caregiving is both temporally and quantitatively correlated with increased extracellular dopamine in the nucleus accumbens (95) and suckling of pups is a more powerful reinforcer for mothers than cocaine administration (96). Interactions between central oxytocin and dopamine systems have also been associated with individual differences in maternal behaviour in rodents. For example, stable, individual differences in rat maternal licking/grooming of pups were abolished by oxytocin receptor blockade and this effect was mediated by the direct effect of oxytocin on dopamine release within the mesocorticolimbic dopamine system (97). Other lines of research have explored the neural basis of maternal aggression, a behaviour that promotes offspring protectiveness and is modulated by oxytocin, arginine vasopression and HPA axis activity. Functional MRI paradigms in awake postpartum rodents subjected to intruder stresses to detect maternal aggression have revealed an important role for periaqueductal gray and nucleus accumbens in maternal aggression (36,98–100).
Functional MRI studies of healthy mothers confirm activation of the mesostriatocortical reward circuitry when mothers listen to cries or view pictures of infant stimuli. In healthy, primiparous, breastfeeding, 4–8-week postpartum mothers listening to a standard infantcry, fMRI BOLD activations were present in the midbrain, thalamus, striatum and medial PFC (41). Mothers, 2–4 weeks postpartum, who delivered vaginally rather than surgically (and thus experienced oxytocin release during labour and delivery), had greater caudate and thalamic activation to own baby cries (42).
In a study of maternal response to infant face stimuli, maternal responses to own versus other child pictures similarly activated the reward circuit, including the putamen, thalamus and substantia nigra. Robustness of brain activation to infant face stimuli was highest for happy infant faces and progressively lower for neutral and sad infant faces (38). Similar reward system activation was noted in other studies of mothers viewing their children (37,44,45) but not all studies of maternal responses to child faces (101,102). Variations in fMRI stimuli, age of children and maternal characteristics (delivery mode, breastfeeding, parity, support system, etc.) and generally small sample sizes likely account for inconsistencies among studies.
Dopamine-reward circuit dysregulation in PPD has been hypothesised based upon heightened neuroendocrine response to the dopamine receptor agonist apomorphine in women who later experienced postpartum mood disorders (103) and altered ventral striatal dopamine-2 receptor binding in healthy and depressed mothers (104). Functional MRI studies to date in PPD samples demonstrate reduced ventral striatal responses to pleasant words (49) and monetary stimuli (105), which may signal impaired maternal pleasure from, and attunement to, infants in the context of PPD. In addition, maternal ventral striatal responses to own versus other baby-cry at 18 months postpartum were inversely associated with postpartum depression severity (43).
Summary point
Similar to basic research showing that oxytocinergic inputs to the mesostriatal dopamine reward system enhance maternal motivation and behaviour, healthy mothers have increased ventral striatal and medial PFC activity in response to infant stimuli. Reward-related neural responses to positive words and monetary gain are noted to be reduced in depressed mothers.
Social attachment/social cognition
Social affiliation, social cognition and reward circuits are closely linked systems that facilitate maternal–infant caregiving. A fundamental component of human mothering is being able to mentalise, that is, to understand the thoughts, beliefs and intentions of others in relation to oneself (106,107). Parental social cognition/mentalising/empathy involves appropriate perception, experience and response to another’s emotion and is particularly relevant to maternal caregiving for pre-verbal infants who cannot articulate their needs. Autistic disorders have shed light on the neural systems supporting empathy (108–110), which overlap substantially with brain responses of parents to infant stimuli, and include cingulate and insular cortices (111). Experiments that compare pain perception in oneself or in a loved one reveal that parallel representations of empathy in insula and ACC are necessary for our ability to mentalise. Humans may utilise separate circuitry to ‘decouple’ representations of external versus internal information to understand physical properties and assess personal emotional values. In support of this, a brain network consistently activated during tasks that require mentalising has emerged, including DMPFC, mPFC, precuneus/posterior cingulate cortex (PCC), temporoparietal junction (TPJ) and posterior superior temporal sulcus (pSTS) (112,113).
This social cognition circuitry was activated in healthy mothers when viewing photographs of their own children versus familiar children (44). A novel study (114) tapped into these constructs through an fMRI paradigm in which mothers observed and imitated faces of their own and other children. The study specifically examined the mirror neurone system, localised in the ventral premotor cortex, inferior frontal gyrus (IFG) and posterior parietal cortex, which activates not only when an individual performs an action, but also when observing someone else perform that action. Neural activation in the mirror neurone system was preferentially engaged in response to own child and was correlated with maternal reflective function. Such a mentalising framework promises to facilitate behavioural planning for parent–infant dyads and likely has far-reaching impacts upon maternal and child physical and mental health (115,116).
The development of empathy in parents depends in great part on the care that they themselves received in childhood (117), (see section on Integrative concepts which transcend a neural network approach, below) which is a concept that has been well-elaborated (118,119). Using this framework of analysis, one study examined the effects of early-life experience among mothers in the first post-partum month on brain structure and functional responses to salient infant stimuli (120). Mothers who reported higher perceived maternal care in childhood showed larger grey matter volumes in the superior and middle frontal gyri, orbital gyrus, superior temporal gyrus and fusiform gyrus. In response to infant cries, these mothers also exhibited higher activations in the middle frontal gyrus, superior temporal gyrus and fusiform gyrus, which are all regions involved in processing emotional information and mentalising functions. These findings suggest that an attenuation of maternal care in childhood may be associated with deficits in brain physiology implicated in an appropriate responsivity to infant stimuli in human mothers.
Additionally, the development of secure attachment at a young age in parents promotes healthy dyadic relationships between parent and their social supports and between parent and child. The modulation of threat by secure attachment is well-articulated in social baseline theory (121), which describes how humans evolved to ‘outsource’ neural demands of threat to supportive social resources. By contrast, isolation demands more individual neural resources to regulate threat. This theory is supported by findings that proximity of a significant other or romantic attachment reduces threat-related neural activity in right anterior insula, SFG and hypothalamus (122) and that perceived social support moderates the relationship between threat-related amygdala activity and trait anxiety (123). Oxytocinergic mechanisms have been proposed to mediate the attenuation of threat-related neural activity by secure attachment (122). The significant spatial overlap of affiliation and emotion-processing neural circuitries in humans (124) gives further support for the functional integration of systems of attachment and threat modulation.
Although animal models of maternal behaviour are compelling and have identified key components of putative mothering circuits, paradigms in animal studies are typically approximations of complex human behaviours and ethological relevance is limited. Nevertheless, rodents and nonhuman primate studies that incorporate social stressors have increasing relevance for informing human psychopathology. For example, recent advances allow for fMRI in awake rats, permitting real-time imaging of social paradigms such as introducing a male intruder to a postpartum rodent with and without pups present to study neural correlates of maternal aggression (36).
Although social cognition and mentalising functions have not been tested directly in postpartum affective disorders, mothers with secure, as opposed to insecure, attachment style had increased activity in dopaminergic-rich ventral striatum, as well as reward-processing OFC and mPFC regions, to happy infant faces (46). Insecurely attached mothers showed greater DLPFC activity to infant faces, which concurs with the behavioural phenomenon of avoidance of negative affect, and suggests over-regulation, as predicted by the social baseline theory. Similarly, in a study of ‘synchronous’ and ‘intrusive’ mothers (based upon microcoding of videotaped mother–infant interaction), synchronous mothers, who consistently coordinate positive social processes with their infant, had increased striatal responses to videos of mother–infant interactions (125). Video clips of mother–infant videos have also been used as stimuli to identify relationships for mothers and fathers between amygdala activity and serum oxytocin and vasopressin, respectively (126). However, in this experiment, it is unclear whether this pattern of brain response was a function of synchronous mothers or the viewing of a synchronous mother–infant interaction. More studies will need to characterise the stimuli, as well as the mothers, in increasing detail to disentangle possible confounds.
Substance-using mothers are a unique sample in which to examine the neural basis for documented disruptions in mother– child relationships (127) and empathic failures. Landi et al. (128) investigated neural correlates to infant cries of varying levels of distress and to infant faces of varying emotional expressions (happy, sad, neutral) among substance-using and nonsubstance-using mothers at 1–3 months postpartum. With one minor exception, no brain regions showed greater activation in substance-using mothers compared to non-using mothers in any of the conditions displaying happy, sad or neutral faces. By contrast, nonsubstance-using mothers showed greater activation in a range of prefrontal regions [e.g. DLPFC/medial frontal gyrus (MFG), VMPFC, DMPFC, IFG], limbic system regions (e.g. amygdala, parahippocampal gyrus) and visual/sensorimotor regions for happy, sad and neutral faces compared to substance-using mothers. For cries of both low and high distress, regions involved in emotional processing (amygdala, insula, parahippocampal gyrus), prefrontal regions (MFG) and regions implicated in auditory/sensorimotor processing showed greater activation among non-using mothers than among substance-using mothers. Because these regions comprise networks supporting emotion reactivity and regulation, and are identified as more active in healthy mothers when viewing infant stimuli, substance-using mothers appear to be characterised by a hyporeactivity to visual and auditory infant cues. Thus, blunted neural activity may reduce the salience of cues and contribute to the maladaptive parental responses observed among substance-using mothers. Prenatal foetal exposure to substance use, maternal comorbidities of depression and other psychiatric disorders, and substance use-related high-risk behaviour further compound disrupted maternal care, likely resulting in adverse outcomes for offspring. Further research is needed to explore the extent to which substance use treatments, such as opioid maintenance treatment, compared to high-risk drug-seeking behaviours, confer psychosocial and, potentially, neural benefits through modulation of reward-related behaviours (129).
Summary point
Empathy and mentalisation maternal functions are supported by neural systems including insula, ventral ACC, DMPFC, precuneus/PCC, TPJ and pSTS. The mirror neurone system, localised in the ventral premotor cortex, IFG and posterior parietal cortex, is also involved in maternal reflective function. These regions, in addition to superior and middle frontal gyri, orbital gyrus and fusiform gyrus, also relate in structure and function to the degree of perceived parental care that mothers themselves experienced as children. Regions of this wide neural network support social cognition/mentalisation/empathy in healthy mothers but are hyporesponsive to infant stimuli in mothers with insecure attachment and substance use disorders. This broad domain shows potential as a marker of impaired maternal caregiving that might benefit from greater specificity and standardisation in the future. Detecting neural endophenotypes of disrupted maternal care may allow for earlier inventions and improve the mental and physical health of mother and offspring.
Default mode network
An increasingly popular approach in neuroscience has been to examine the intrinsic functional organisation of brain through examination of spontaneous low frequency BOLD activity when individuals are at rest with eyes closed or attending to a fixation cross. Reviews of this technology (73,130) emphasise that neural processes that occur over minutes (rather than seconds) and utilise significant body energetics (20% of body energy used for ongoing neural activity compared to 5% for task-specific activity) might have greater validity for the study of behaviour. The default mode network (DMN) has emerged as a key network of interest that is more active during passive than active cognitive tasks in meta-analytic resting state positron emission tomography (131,132) and fMRI studies (133,134). Key regions of the DMN include the PCC, ACC, inferior parietal cortex and precuneus.
Functional MRI studies comparing spontaneous/internal cognition to external attention and monitoring may be important for a range of psychopathology (135), including parent–child relational problems and fMRI studies designed to specifically elicit self-referent ideation (136,137). In depressive and anxiety disorders, the DMN is considered to subserve non-adaptive, ruminative, self-referential ideation based upon varied reports of altered PCC connectivity to limbic regions in MDD (138,139), greater cross-network (salience and executive networks) associations with the DMN via the dorsal nexus (140) and increased hippocampal-driven ventral ACC activity (141), amongst others. In several studies, the degree of network connectivity within the DMN was associated with the duration of current depressive episode (138) or rumination severity (142). The study by Johnson et al. (136) on subtypes of self-relevant thought noted the anteroinferior PCC had a more outward, preventative focus on duties and responsibilities and the ventromedial ACC had a more inward, promotional focus on self-related hopes and aspirations. A subsequent study (143) of functional DMN regions during specified tasks of referential ideation found that individuals with MDD had less anterior mPFC activity to the self-evaluative condition and less disengagement/deactivation during a distraction task.
Three published resting BOLD papers have begun to examine DMN activity in PPD. In a study of 14 women with MDD versus 23 healthy women approximately 10 weeks postpartum, Chase et al. (144) selected the PCC as a seed region from which to examine DMN connectivity with whole brain activity during rest because the PCC is a core DMN region involved in self-reflection and social cognition (145), as well as being associated with thinking about duties and responsibilities to others (136). They found that the PCC and right amygdala were negatively coupled in depressed compared to healthy mothers, suggesting that PPD might involve the disruption of outward, preventative aspects of self-relevant thought and perhaps empathy. A second study by Deligiannidis et al. (146) of eight PPD and nine healthy mothers studied at 9 weeks postpartum similarly showed attenuated functional connectivity in numerous corticolimbic circuits, including coupling within the DMN of ACC, inferior parietal cortex and precuneus, as well as between DMN nodes and amygdala. A contradictory report by Xiao-juan et al. (147) revealed heightened PCC connectivity in PPD, using nearest neighbour, regional homogeneity methodology. Additional research related to the content of maternal thought during the resting phase, as well as paradigms that constrain types of self-relevant thought, alongside mother–infant interactional data, will be important to clarify the relationship between rumination, self-reflection, maternal depression and caregiving.
Summary point
More work remains to be carried out in the field of intrinsic neural activity in new mothers, although there are preliminary findings of circuitry disconnectivity that might relate to impaired empathic and self-other-relational processing in PPD. Additional correlative studies are needed.
Integrative concepts which transcend a neural network approach
Although it is convenient to divide neural networks into the four domains above for the purpose of a selective literature review, it is evident that there is significant overlap and interactive effects among these neural networks, just as there are interactive/overlapping themes in the approach to maternal psychopathology and maternal caregiving. Indeed, a developmental perspective of maternal socioemotional neuroscience would suggest that early experience and presence of adversity interacts with current life circumstances to create neural vulnerability or resilience during motherhood. In this vein, what we define as maternal depression or poor caregiving might similarly derive from the neural imprint of adverse early experience. Adverse early-life experiences that impact upon an individual’s attachment to a primary caregiver are of particular interest because convergent research has demonstrated that an individual’s attachment style is a central dimension contributing to the onset of and recovery from affective symptoms (148–150), as well as maternal caregiving (151,152). In addition, current healthy social attachments, which are more likely if the attachment capacity is established at a young age, modulate emotional response to threat and loss, as originally articulated by Bowlby (117), who described that the prototypical response to separation from an attachment figure involves ‘protest’ or anxiety followed by ‘despair’ or depression. However, he noted that individuals with enduring attachments to others may experience anxiety without depression. Because the prevailing context for postpartum affective disorders is a new affiliative relationship between mother and infant accompanied by dramatic changes in pre-existing relationships between the mother and her social network, new motherhood is an ideal ‘laboratory’ in which to test the role of attachment in modulating multiple interacting neural system responses.
Indeed, parental brain findings have been interpreted with respect to maternal attachment behaviours, which themselves affect the development of such circuits in the infant (153,154) and may also be important to more generalised forms of caregiving (155). From the infant attachment perspective, Laurent and Ablow (156) demonstrated higher activity in the amygdala, parahippocampus, and right posterior insula for mothers responding to their own baby-cry versus non-cry control sounds according to decreasing attachment security of their infants. This is in keeping with findings indicating that intrusive and forceful maternal behaviour, as assessed in infant–mother interaction, was related to greater maternal activation in the amygdala when viewing their infant in solitary play and when viewing videos of themselves interacting with their infants (125). Intrusive maternal behaviour was also related to increased insula and temporal pole activation (auditory processing) when listening to cry sounds of their own versus unfamiliar infants (157). This work on parents neatly reinforces earlier work raising the amygdala as a key node in mediating the effects of attachment insecurity (158). Conversely, in a mixed group of breastfeeding and formula-feeding mothers, Kim et al. (48) found that greater cry-related right amygdala and SFG activity at 2– 4 weeks postpartum was related to greater maternal sensitivity to their infants at 3–4 months postpartum. This area of investigation will benefit from increased sample sizes and fMRI paradigm refinement, with the ultimate goal of establishing neural risk markers for maternal caregiving. Inroads into such improvements are seen in the recent work of Wan et al. (159), in which brain responses to visual baby stimuli in a group of 20 mothers was correlated with the quality of her concurrent parental behaviour and with her perceptions of infant warmth.
The neurobiology of maternal affective health and empathy should continue to be informed by the broader literature on mood disorders, in which developmental effects of environment are programmed from infancy (160) through adulthood, most notably through the HPA axis with the important involvement of amygdala, hippocampus and PFC (161). The negative impact of adversity, poor attachment, exposure to stress hormones on neural systems for emotion regulation and self-other relational processing is well-known. Numerous retrospective studies report correlations between own experience of negative or rejecting parenting and adult depression (162), including PPD (163). Although brain imaging research is underway with low socioeconomic status parents, we can begin considering brain-based deficits in emotion response and regulation from the chronic childhood experience of poverty as possibly injurious to parenting behaviours (164). We thus expect future studies to confirm differences already reported from adverse early-life experiences (120), and elaborate the importance of specific forms of adversity on parenting dysfunctions toward better targeted and effective treatments.
It is important to also consider the potential for neuroplasticity inherent in the perinatal period both via the surge of hormone-related brain changes, particularly in the hippocampus and amygdala (165,166) and a richness of environmental support and maternal motivation for change that can accompany the perinatal period. In theory, and perhaps for some women, with the proper environmental support, the neural trajectory can be optimised leading to healthy mood and good social relationships in the puerperium. As described previously and also reported by Lonstein et al. (167), studies of postpartum rodents have demonstrated that pulsatile secretion of oxytocin and prolactin during delivery and lactation are associated with diminished fear responses (30) and increases in mesiostriatal dopaminergic reward system function (168), which may help set the stage for resiliency. Animal studies suggest that structural changes occur in the maternal brain during the early postpartum period in regions such as the hypothalamus, amygdala, parietal lobe and PFC, and such changes are related to the expression of maternal behaviours. In an attempt to explore structural plasticity in new parent brains, Kim et al. (53) conducted a voxel-based morphometry study of mothers’ brains at 2–4 weeks postpartum which was repeated at 3–4 months postpartum. Findings revealed that the gray matter volume increases in the PFC, parietal lobes and midbrain areas over time. Increased gray matter volume in the midbrain, including the hypothalamus, substantia nigra and amygdala, was associated with maternal positive perception of her baby. These results suggest that the first months of motherhood in humans are accompanied by structural changes in brain regions implicated in maternal motivation and behaviours.
Genetics
Advances in molecular biology have highlighted the role of epigenetic mechanisms in regulating gene activity, neurobiology and behaviour, as well as the potential role of environmentally-induced epigenetic variation in linking early-life exposures to long-term bio-behavioural outcomes, including parenting behaviours. Maternal perinatal distress may be associated with both foetal and infant developmental trajectories through epigenetic mechanisms. Later, postpartum experiences may have a critical moderating influence on antenatal effects, the antenatal–postpartum interplay, and the developmental and epigenetic consequences of postpartum mother–infant interactions. The in utero environment is regulated by placental function and there is emerging evidence that the placenta is highly susceptible to maternal distress and a target of epigenetic dysregulation. Of note, recent studies also demonstrate epigenetic paternal (father) effects (169), including the transmission of stress-induced pathologies such as depression (170,171). Integrating studies of antenatal exposures, placental function and postpartum maternal care with the exploration of epigenetic mechanisms may provide novel insights into the pathophysiology induced by maternal distress.
In human and other mammalian mothers, research suggests that early quality of care in the family of origin may affect subsequent parenting (172–175), with clear associations between the maternal history of abuse and child maltreatment. Approximately 30% of mothers who were abused as children go on to abuse their own children, compared to 5% in mothers not reporting abuse (176). Although the biological mechanisms underlying a posited ‘cycle of abuse’ are not well understood, there is support for the intergenerational transmission of parenting styles, bonding, attachment and maternal rejection (177–182). Furthermore, epidemiological studies indicate that children exposed to early adverse experiences, including those related to bonding and attachment, are at increased risk for the development of depression, anxiety and other stress-related disorders in adolescence (183–185) and adulthood (186–188). Protective factors for prospective mothers from the riskiness of their early experiences include social supports in the environment of the developing child (189–193), as well a partner relationship with a supportive spouse in adulthood (194). Thus, the effects of early-experience on the developing brain promise to provide mechanisms for understanding and improving next generations’ capacity for sensitive parental behaviours (118,120,195) with resulting implications for public policy (196).
Although epigenetics may shed light on environmental contributions to PPD, genetic factors under gene mapping research may also help identify differential vulnerability to the changes in gonadal steroid levels that occur at delivery. A genetic study compared nine women with PPD and ten postpartum nondepressed women (197). Gene expression profiles correctly classified 84% of patients as depressed or nondepressed, and gene expression was correlated with the severity of depressive symptoms and clinical course of illness. Moreover, the women with PPD showed a global reduction of gene transcription after delivery and differential immune activation, as well as decreased transcriptional activation, cell proliferation, nucleotide binding, and DNA replication and repair. One large-scale genome-wide association study (n = 1210) examined genetic influences on postpartum depressive symptoms and showed modest evidence of an association between postpartum mood symptoms and a single-nucleotide polymorphism on chromosome 1 in the hemicentin gene HMCN1 (198) that encodes a member of the immuno-globulin superfamily. HMCN1 contains four oestrogen-binding sites and may contribute a phenotype characterised by mood destabilisation in the context of normal changes in gonadal steroid levels at delivery. If such genes have biologically relevant products, they may ultimately guide earlier diagnostic and more specific treatment approaches.
Nosological issues in psychopathology occurring in the puerperium
Because of the profound physiological changes that characterise pregnancy and childbirth (notably the dramatic changes in steroid hormone levels and the perturbations in mood, appetite, energy and sleep associated with childbirth and infant care), it has been suggested that PPD may be distinct from depression that occurs at other times (199). In one study, PPD increased the risk of future PPD but not for depressive episodes that occurred outside of the postpartum period (200). In addition, women whose PPD represented a recurrent depression were at increased risk for depressive episodes occurring outside of the postpartum period but not in the postpartum period. Also, Altemus et al. (201) distinguished between antenatal versus postpartum onset MDD, with the latter being more commonly associated with obsessions and compulsions and the former being come commonly associated with psychosocial stress and nonperinatal MDD.
Notably, a few studies link caesarean section with an increased risk for PPD (202) and PPD is associated with breastfeeding difficulties (203); thus, the role of neuroendocrine perturbations underlying the onset of PPD remain to be examined (204). Additional insights may be gained by considering postpartum psychopathology in the context of birth-related factors, including birthing method (e.g. vaginal, caesarian section), birthing complications, feeding difficulties, sleep patterns, libido, partner intimacy and ongoing medical conditions, such as urinary incontinence and hyperglycaemia.
Hormone withdrawal theories suggest that the withdrawal of oestradiol and progesterone may be a causative link in the chain of events before postpartum blues and depression in some vulnerable women (205,206). In addition, cortisol may be particularly dysregulated in PPD (207). These findings are consistent with depression models that implicate dysregulation of stress hormones, particularly cortisol (208). Indeed, several recent reviews converge on a significant role for dysregulation of the HPA axis in the development of depression in postpartum women (206,209). More work is required to confirm the involvement of gonadal steroids in PPD (207,210–212). Perhaps neuroimaging can help integrate hormone and brain systems in the aetiology and possible treatments of depression by modulating the HPA axis or oestradiol (213–215). As a first step, Ho et al. (216) reported on the connection between cortisol regulation and maternal ventral ACC activity in the discrimination between positive and negative social signals from own-infants in a social decision-making task. Such neurohormonal integration may be disrupted in psychopathology and interfere with normal maternal decision-making in the care for their infant.
Summary
These emerging findings point to the potential capacity of fMRI paradigms that activate the salience/fear circuitry, the maternal reward/attachment network, and systems supporting self-relevant thought to yield critical information for understanding PPD and maternal caregiving. Convergence of findings of hypoactivity and corticolimbic disengagement to emotional, infant and reward cues in mothers with PPD provides a foundation for, and also offers promise for, defining neural biomarkers to estimate PPD risk, as well as guide treatment selection. Personalisation of treatment is needed to increase treatment remission above the currently disappointing rates of 30–50% (217), as well as to decrease the expense of ineffective treatment and decrease the family burden of postpartum mental illness. Neuroimaging is increasingly becoming widespread and feasible to implement. For example, a robust fMRI paradigm of 10 min in duration could cost in the vicinity of $500 for acquisition and analysis. Therefore, it is within the realm of possibility that risk assessment or treatment predicting fMRI could have a place in clinical practice once a set of neuroimaging paradigms is honed based on further study in larger samples. Further decreases in costs are anticipated as a result of advancements in technology.
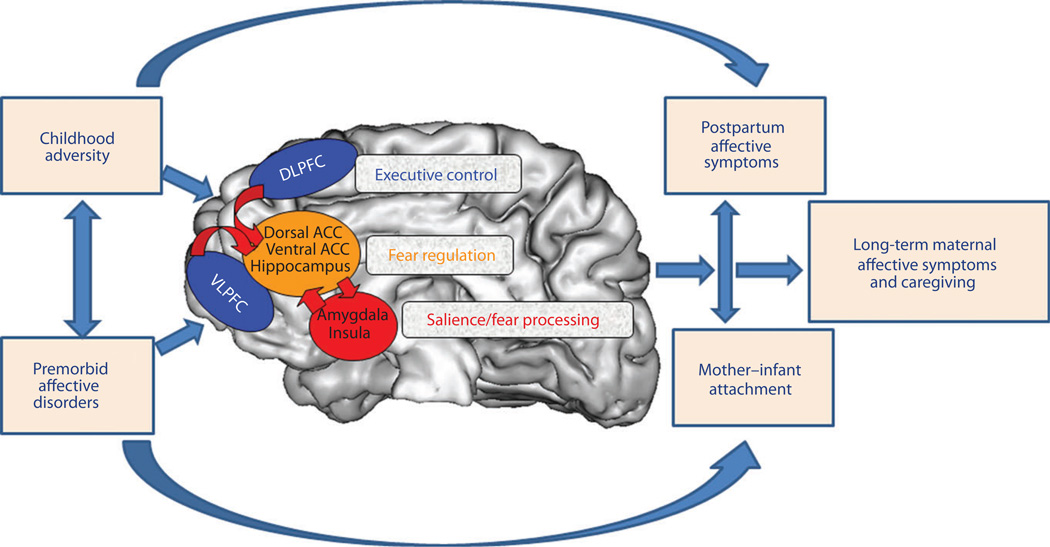
In Fig. 1, we depict a putative model of relationships between multiple early-life factors, representative brain circuits of interest, and maternal affective and caregiving outcomes. One aim of ongoing research in maternal affective neuroscience is to examine complex models of maternal psychopathology and parenting in large maternal samples to identify neural, behavioural and psychosocial predictors of postpartum affective disorders and maternal caregiving behaviours. The application of such predictors to mothers prior to pregnancy or perinatally also holds promise with respect to designing improved treatments and applying early interventions that improve outcomes for mothers and children.
Fig. 1.
Putative model of relationships between multiple early-life factors, representative brain circuits of interest, and maternal affective and caregiving outcomes (note the following network-brain region groupings: executive control [dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC)], fear regulation [dorsal anterior cingulate cortex (ACC), ventral anterior cingulate cortex, hippocampus], and salience/fear processing [amygdala, insula]). Note also that pertinent circuitries are not shown (i.e. reward and attachment) and that other biological mechanisms, specifically genetic, are also left out for the sake of simplicity. An aim of ongoing research in maternal affective neuroscience is to examine complex models of maternal psychopathology and parenting in large maternal samples to identify neural, behavioural, and psychosocial predictors of postpartum affective disorders and maternal caregiving behaviours. Application of such predictors to mothers prior to pregnancy or perinatally shows promise with respect to the design of improved treatments and for applying early interventions to improve outcomes for mothers and children. Adapted from Phillips et al. (222).
Future directions
We conclude by identifying several areas that are ripe for further investigation and stand to greatly enhance our understanding of postpartum affective disturbance.
An under-studied area is how early postpartum hormonal fluctuations modulate neural circuits to result in clinical symptoms. For example, Sacher et al. (218) have demonstrated the value of such work by their findings of greater monoamine catabolism in the early puerperium compared to nonchildbearing time points. Knowing which neural changes are normative versus pathological in the early puerperium could help to define more effective treatments, as well as select the group of women in greatest need of preventive care. Designs using hormonal manipulation, abortion or adoption may be useful in this endeavour.
Ongoing neuroimaging studies would benefit from the implementation of functional and structural connectivity, as well as machine learning analyses, to foster greater understanding of aberrant neural circuits (92,219). Functional MRI paradigms that can tap into standard concepts in the emotion literature can yield useful information about maternal psychopathology, whereas ecologically relevant infant stimuli are necessary to parse the circuitry of maternal caregiving. An important additional goal will be to examine how the different neural circuitries are integrated and whether there is a temporal evolution after childbirth in the predominance of one circuitry over another. For example, a hyperactive salience and fear network may temporally precede a hypoactive reward circuitry, although functional relationships between these two circuitries may not be linear. These questions will benefit greatly from future research.
Subject samples must clearly grow in size and should be selected in a way that hypotheses regarding mood, attachment, parenting quality and sociodemographic context (i.e. paternal, sibling, violence factors) can be studied in concert. For example, a sample size of 120 women and 10 covariates provides > 80% power to detect an R2 of 0.15 (small-medium effect size) with P = 0.05 (220). In addition, dimensions of psychopathology are expected to have greater relevance to the neural mechanisms captured with fMRI paradigms compared to dichotomous diagnoses. As such, efforts to define basic functioning dimensions underlying traditionally defined disorders have high value for establishing brain–behaviour relationships, as suggested by the Research Domain Criteria (RDoC) project set forth by the National Institutes of Mental Health (NIMH) (221).
A recurrent question asked of researchers in this field is the comparability or distinction between PPD and nonpostpartum depression. A well-constructed approach with respect to defining areas of similarity and divergence is needed to clarify the extent to which postpartum affective neuroscience is generalisable to the greater population and vice versa. Furthermore, the effects of parity, multiparity, duration post-delivery and other factors must be closely examined.
As we learn more about maternal psychopathology and parenting, developing standardised fMRI protocols (65) that can isolate the robustness of deficits across overlapping networks will represent an important approach. It might be that futurejne studies will explore graded stimuli, decision-making tasks or responses to more sophisticated and personally tailored aspects of parenting, rather than simply own versus other types of contrast to reveal the important nuances of parent–infant relationships
Integrated studies of neuroimaging and treatment are critically needed to identify which neural circuitry activation and connectivity patterns are linked with the treatment response overall, as well as unique behavioural and pharmacologic treatment modalities.
Acknowledgements
The work was supported by grants HD067185 (EMK, MLP, AEH) and the National Alliance for Research on Schizophrenia and Depression/Brain and Behavior Foundation, Klingenstein Third Generation Foundation, NIMHD/NICHD RC2MD004767-01, the Michigan Institute for Clinical Health Research and the National Center for Advancing Translational Sciences, UL1TR000433, Robert Wood Johnson Health and Society Scholar Award, the University of Michigan Injury Center (JES), and AACAP-NIDA K12 DA000357 (MSH, Physician Scientist Program).
Footnotes
The authors declare that there are no conflicts of interest.
References
- 1.Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 7.Paulus MP, Stein MB. An Insular View of Anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 8.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LeDoux JE. Clues from the brain. Ann Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- 10.Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 11.Anand A, Li Y, Wang Y, Gardner K, Lowe MJ. Reciprocal effects of anti-depressant treatment on activity and connectivity of the mood regulating circuit. J Neuropsychiatry Clin Neurosci. 2007;19:274–282. doi: 10.1176/appi.neuropsych.19.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W, Kersting A, Baune BT, Suslow T. Amygdala reactivity to masked negative faces is associated with automatic judgmental bias in major depression: a 3 T fMRI study. J Psychiatry Neurosci. 2007;32:423–429. [PMC free article] [PubMed] [Google Scholar]
- 13.Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, Mathews J, Sheline YI. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ. Attenuation of the neural response to sad faces in major depressionby antidepressant treatment: a prospective, event-related functional magnetic resonance imagingstudy. Arch Gen Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 15.Fu CH, Williams SC, Brammer MJ, Suckling J, Kim J, Cleare AJ, Walsh ND, Mitterschiffthaler MT, Andrew CM, Pich EM, Bullmore ET. Neural responses to happy facial expressions in major depression following antidepressant treatment.[see comment] Am J Psychiatry. 2007;164:599–607. doi: 10.1176/ajp.2007.164.4.599. [DOI] [PubMed] [Google Scholar]
- 16.Neumeister A, Drevets WC, Belfer I, Luckenbaugh DA, Henry S, Bonne O, Herscovitch P, Goldman D, Charney DS. Effects of a alpha 2C–adrenoreceptor gene polymorphism on neural responses to facial expressions in depression. Neuropsychopharmacology. 2006;31:1550–1556. doi: 10.1038/sj.npp.1301010. [DOI] [PubMed] [Google Scholar]
- 17.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 18.Surguladze SA, Brammer MJ, Young AW, Keedwell P, Travis MJ, Williams S, Phillips ML. A differential pattern of neural response towards sad versus happy facial facial expressions in Major Depressive Disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 19.Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- 20.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 21.Neumeister A, Drevets WC, Belfer I, Luckenbaugh DA, Henry S, Bonne O, Herscovitch P, Goldman D, Charney DS. Effects of a alpha 2C–adrenoreceptor gene polymorphism on neural responses to facial expressions in depression. Neuropsychopharmacology. 2006;31:1750–1756. doi: 10.1038/sj.npp.1301010. [DOI] [PubMed] [Google Scholar]
- 22.Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry. 2010;67:1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulcu E, Lythe K, Elliott R, Green S, Moll J, Deakin JF, Zahn R. Increased amygdala response to shame in remitted major depressive disorder. PLoS ONE. 2014;9:e86900. doi: 10.1371/journal.pone.0086900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnone D, McKie S, Elliott R, Thomas EJ, Downey D, Juhasz G, Williams SR, Deakin JFW, Anderson IM. Increased amygdala responses to sad but not fearful faces in major depression: relation to mood state and pharmacological treatment. Am J Psychiatry. 2012;169:841–850. doi: 10.1176/appi.ajp.2012.11121774. [DOI] [PubMed] [Google Scholar]
- 25.Norbury R, Selvaraj S, Taylor M, Harmer C, Cowen P. Increased neural response to fear in patients recovered from depression: a 3T functional magnetic resonance imaging study. Psychol Med. 2010;40:425. doi: 10.1017/S0033291709990596. [DOI] [PubMed] [Google Scholar]
- 26.Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry. 2011;168:968–978. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- 28.Robinson OJ, Charney DR, Overstreet C, Vytal K, Grillon C. The adaptive threat bias in anxiety: amygdala-dorsomedial prefrontal cortex coupling and aversive amplification. NeuroImage. 2012;60:523–529. doi: 10.1016/j.neuroimage.2011.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wartella J, Amory E, Lomas LM, Macbeth A, McNamara I, Stevens L, Lambert KG, Kinsley CH. Single or multiple reproductive experiences attenuate neurobehavioral stress and fear responses in the female rat. Physiol Behav. 2003;79:373–381. doi: 10.1016/s0031-9384(03)00150-1. [DOI] [PubMed] [Google Scholar]
- 30.Neumann ID. Brain mechanisms underlying emotionalalterations in the peripartum period in rats. Depress Anxiety. 2003;17:111–121. doi: 10.1002/da.10070. [DOI] [PubMed] [Google Scholar]
- 31.Torner L, Neumann ID. The brain prolactin system: involvement in stress response adaptations in lactation. Stress. 2002;5:249–257. doi: 10.1080/1025389021000048638. [DOI] [PubMed] [Google Scholar]
- 32.Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci. 2005;25:6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Febo M, Shields J, Ferris CF, King JA. Oxytocin modulates unconditioned fear response in lactating dams: an fMRI study. Brain Res. 2009;1302:183–193. doi: 10.1016/j.brainres.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caffrey MK, Nephew BC, Febo M. Central vasopressin V1a receptors modulate neural processing in mothers facing intruder threat to pups. Neuropharmacology. 2010;58:107–116. doi: 10.1016/j.neuropharm.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hillerer KM, Neumann ID, Slattery DA. From stress to postpartum mood and anxiety disorders: how chronic peripartum stress can impair maternal adaptations. Neuroendocrinology. 2011;95:22–38. doi: 10.1159/000330445. [DOI] [PubMed] [Google Scholar]
- 36.Ferris CF, Stolberg T, Kulkarni P, Murugavel M, Blanchard R, Blanchard DC, Febo M, Brevard M, Simon NG. Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neurosci. 2008;9:111. doi: 10.1186/1471-2202-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: mother’s response to infant’s attachment behaviors. Biol Psychiatry. 2008;63:415–423. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 38.Strathearn L, Li J, Fonagy P, Montague PR. What’s in a smile? maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrett J, Wonch KE, Gonzalez A, Ali N, Steiner M, Hall GB, Fleming AS. Maternal affect and quality of parenting experiences are related to amygdala response to infant faces. Soc Neurosci. 2011:252–268. doi: 10.1080/17470919.2011.609907. [DOI] [PubMed] [Google Scholar]
- 40.Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, von Bardeleben U, Radue EW, Cirillo S, Tedeschi G, Di Salle F. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiatry. 2003;54:1367–1375. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- 41.Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hammer MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- 42.Swain JE, Tasgin E, Mayes LC, Feldman R, Constable RT, Leckman JF. Maternal brain response to own baby-cry is affected by cesarean section delivery. J Child Psychol Psychiatry. 2008;49:1042–1052. doi: 10.1111/j.1469-7610.2008.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laurent HK, Ablow JC. A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Soc Cogn Affect Neurosci. 2012;7:125–134. doi: 10.1093/scan/nsq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers’ neural activation in reponse to pictures of their children and other children. Biol Psychiatry. 2004;56:225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 45.Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swain J, Ho S, Rosenblum K, Finegood E, Richardson P, Dayton C, Akce L, Marcus S, Phan K, Muzik M. Maternal brain responses to baby stimuli are modulated by mood disorders. Paper Presented at: Biological Psychiatry.2012. [Google Scholar]
- 48.Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, Swain JE. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. J Child Psychol Psychiatry. 2011;52:907–915. doi: 10.1111/j.1469-7610.2011.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silverman ME, Loudon H, Safier M, Protopopescu X, Leiter G, Liu X, Goldstein M. Neural dysfunction in postpartum depression: an fMRI pilot study. CNS Spectr. 2007;12:853–862. doi: 10.1017/s1092852900015595. [DOI] [PubMed] [Google Scholar]
- 50.Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am J Psychiatry. 2010;167:1373–1380. doi: 10.1176/appi.ajp.2010.09081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swain JE, Lorberbaum JP. Imaging the human parental brain. Neuro-biology of the parental brain. 2008:83–100. [Google Scholar]
- 52.Swain JE, Leckman JF, Mayes LC, Feldman R, Hoyt E, Kang H, Kim P, David D, Nguyen S, Constable RT, Schultz RT. Functional brain activations of parents listening to their own baby-cry change over the early postpartum. Dev Psychobiol. 2014 under review. [Google Scholar]
- 53.Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE. The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behav Neurosci. 2010;124:695–700. doi: 10.1037/a0020884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kinsley CH, Meyer EA, et al. The construction of the maternal brain: theoretical comment on Kim. Behav Neurosci. 2010;124:710–714. doi: 10.1037/a0021057. [DOI] [PubMed] [Google Scholar]
- 55.Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: Symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress. 2007;20:713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- 56.Herringa RJ, Phillips ML, Fournier JC, Kronhaus DM, Germain A. Childhood and adult trauma both correlate with dorsal anterior cingulate activation to threat in combat veterans. Psychol Med. 2013;43:1533–1542. doi: 10.1017/S0033291712002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, Spiegel D. Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry. 2010;167:640–647. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- 59.Shea A, Walsh C, Macmillan H, Steiner M. Child maltreatment and HPA axis dysregulation: relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology. 2005;30:162–178. doi: 10.1016/j.psyneuen.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 60.Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32:301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schechter DS, Moser DA, Wang Z, Marsh R, Hao X, Duan Y, Yu S, Gunter B, Murphy D, McCaw J, Kangarlu A, Willheim E, Myers MM, Hofer MA, Peterson BS. An fMRI study of the brain responses of traumatized mothers to viewing their toddlers during separation and play. Soc Cogn Affect Neurosci. 2012;7:969–979. doi: 10.1093/scan/nsr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schechter DS, Willheim E, Hinojosa C, Scholfield-Kleinman K, Turner JB, McCaw J, Zeanah CH, Myers MM. Subjective and objective measures of parent-child relationship dysfunction, child separation distress, and joint attention. Psychiatry. 2010;73:130–144. doi: 10.1521/psyc.2010.73.2.130. [DOI] [PubMed] [Google Scholar]
- 63.Moser DA, Aue T, Wang Z, Rusconi SS, Favez N, Peterson BS, Schechter DS. Limbic brain responses in mothers with post-traumatic stress disorder and comorbid dissociation to video clips of their children. Stress. 2013;16:493–502. doi: 10.3109/10253890.2013.816280. [DOI] [PubMed] [Google Scholar]
- 64.Lanius RA, Frewen PA, Girotti M, Neufeld RWJ, Stevens TK, Densmore M. Neural correlates of trauma script-imagery in posttraumatic stress disorder with and without comorbid major depression: a functional MRI investigation. Psychiatry Res. 2007;155:45–56. doi: 10.1016/j.pscychresns.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 65.Korgaonkar MS, Grieve SM, Etkin A, Koslow SH, Williams LM. Using standardized fMRI protocols to identify patterns of prefrontal circuit dysregulation that are common and specific to cognitive and emotional tasks in major depressive disorder: first wave results from the iSPOT-D study. Neuropsychopharmacology. 2013;38:863–871. doi: 10.1038/npp.2012.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harvey P-O, Fossati P, Pochon J-B, Levy R, LeBastard G, Lehéricy S, Allilaire J-F, Dubois B. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. NeuroImage. 2005;26:860–869. doi: 10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 67.Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P. Unmasking disease -specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychatry. 2002;159:1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- 68.Elliott R, Baker SC, Rogers RD, O’Leary DA, Paykel ES, Frith CD, Dolan RJ, Sahakian BJ. Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychol Med. 1997;27:931–942. doi: 10.1017/s0033291797005187. [DOI] [PubMed] [Google Scholar]
- 69.Goethals I, Audenaert K, Jacobs F, Van de Wiele C, Ham H, Pyck H, Vandierendonck A, Van Heeringen C, Dierckx R. Blunted prefrontal per-fusion in depressed patients performing the Tower of London task. Psychiatry Res. 2005;139:31–40. doi: 10.1016/j.pscychresns.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 70.Okada G, Okamoto Y, Morinobu S, Yamawaki S, Yokota N. Attenuated left prefrontal activation during a verbal fluency task in patients with depression. Neuropsychopharmacology. 2003;47:21–26. doi: 10.1159/000068871. [DOI] [PubMed] [Google Scholar]
- 71.Versace A, Thompson WK, Zhou D, Almeida JR, Hassel S, Klein CR, Kupfer DJ, Phillips ML. Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry. 2010;67:422–431. doi: 10.1016/j.biopsych.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Almeida JR, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, Phillips ML. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hamilton JP, Chen MC, Gotlib IH. Neural systems approaches to understanding major depressive disorder: an intrinsic functional organization perspective. Neurobiol Dis. 2013;52:4–11. doi: 10.1016/j.nbd.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Groot RH, Vuurman EF, Hornstra G, Jolles J. Differences in cognitive performance during pregnancy and early motherhood. Psychol Med. 2006;36:1023–1032. doi: 10.1017/S0033291706007380. [DOI] [PubMed] [Google Scholar]
- 75.Buckwalter JG, Stanczyk FZ, McCleary CA, Bluestein BW, Buckwalter DK, Rankin KP, Chang L, Goodwin TM. Pregnancy, the postpartum, and steroid hormones: effects on cognition and mood. Psychoneuroendo-crinology. 1999;24:69–84. doi: 10.1016/s0306-4530(98)00044-4. [DOI] [PubMed] [Google Scholar]
- 76.Bannbers E, Gingnell M, Engman J, Morell A, Sylven S, Skalkidou A, Kask K, Backstrom T, Wikstrom J, Poromaa IS. Prefrontal activity during response inhibition decreases over time in the postpartum period. Behav Brain Res. 2013;241:132–138. doi: 10.1016/j.bbr.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 77.Amin Z, Epperson CN, Constable RT, Canli T. Effects of estrogen variation on neural correlates of emotional response inhibition. NeuroImage. 2006;32:457–464. doi: 10.1016/j.neuroimage.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 78.Swain JE, Kim P, Ho SS. Neuroendocrinology of parental response to baby-cry. J Neuroendocrinol. 2011;23:1036–1041. doi: 10.1111/j.1365-2826.2011.02212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Swain JE, Mayes LC, Leckman JF. The development of parent-infant attachment through dynamic and interactive signaling loops of care and cry. Behav Brain Sci. 2004;27:472–473. [Google Scholar]
- 80.Robbins TW, Everitt BJ. Motivation and Reward. In: Squire LR, Bloom FE, McConnell SK, Roberts JL, Spitzer NC, Zigmond MJ, editors. Fundamental Neuroscience. San Diego: Elsevier Science; 2003. pp. 1109–1126. [Google Scholar]
- 81.Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7:191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- 82.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 83.Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cogn Affect Behav Neurosci. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- 84.O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 85.Forbes EE, Dahl RE. Neural systems of positive affect: relevance to understanding child and adolescent depression? Dev Psychopathol. 2005;17:827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psych. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 87.Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 90.Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML. Subcortical and ventral pre-frontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 91.Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- 92.Mourao-Miranda J, Oliveira L, Ladouceur CD, Marquand A, Brammer M, Birmaher B, Axelson D, Phillips ML. Pattern recognition and functional neuroimaging help to discriminate healthy adolescents at risk for mood disorders from low risk adolescents. PLoS ONE. 2012;7:e29482. doi: 10.1371/journal.pone.0029482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol Psychiatry. 2005;58:495–503. doi: 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 94.Moses-Kolko EL, Meltzer CC, Berga SL, Wisner KL. Postpartum depression: the clinical disorder and application of PET imaging research methods. In: Bridges RS, editor. Neurobiology of the Parental Brain. Burlington: Academic Press; 2008. pp. 175–199. [Google Scholar]
- 95.Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ferris CF, Kulkarni P, Sullivan JM, Jr, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci. 2005;25:149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pavesi E, Enck MC, De Toledo CAB, Terenzi MG. Disruption of maternal behaviour by acute conspecific interaction induces selective activation of the lateral periaqueductal grey. Eur J Neurosci. 2007;26:2055–2065. doi: 10.1111/j.1460-9568.2007.05806.x. [DOI] [PubMed] [Google Scholar]
- 99.Sukikara MH, Mota-Ortiz SR, Baldo MV, Felicio LF, Canteras NS. The periaqueductal gray and its potential role in maternal behavior inhibition in response to predatory threats. Behav Brain Res. 2010;209:226–233. doi: 10.1016/j.bbr.2010.01.048. [DOI] [PubMed] [Google Scholar]
- 100.Nephew BC, Caffrey MK, Felix-Ortiz AC, Ferris CF, Febo M. Blood oxygen level-dependent signal responses in corticolimbic ‘emotions’ circuitry of lactating rats facing intruder threat to pups. Eur J Neurosci. 2009;30:934–945. doi: 10.1111/j.1460-9568.2009.06875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ranote S, Elliott R, Abel KM, Mitchell R, Deakin JFW, Appleby L. The neural basis of maternal responsiveness to infants: an fMRI study. NeuroReport. 2004;15:1825–1829. doi: 10.1097/01.wnr.0000137078.64128.6a. [DOI] [PubMed] [Google Scholar]
- 102.Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage. 2004;21:583–592. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 103.Wieck A, Kumar R, Hirst AD, Marks MN, Campbell IC, Checkley SA. Increased sensitivity of dopamine receptors and recurrence of affective psychosis after childbirth. Br Med J. 1991;303:613–616. doi: 10.1136/bmj.303.6803.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moses-Kolko EL, Price JC, Wisner KL, Hanusa BH, Meltzer CC, Berga SL, Grace AA, di Scalea TL, Kaye WH, Becker C, Drevets WC. Postpartum and depression status are associated with lower [(11)C]raclopride BP (ND) in reproductive-age women. Neuropsychopharmacology. 2012;37:1422–1432. doi: 10.1038/npp.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moses-Kolko EL, Fraser D, Wisner KL, James JA, Saul AT, Fiez JA, Phillips ML. Rapid habituation of ventral striatal response to reward receipt in postpartum depression. Biol Psychiatry. 2011;70:395–399. doi: 10.1016/j.biopsych.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hein G, Singer T. I feel how you feel but not always: the empathic brain and its modulation. Curr Opin Neurobiol. 2008;18:153–158. doi: 10.1016/j.conb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 108.Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends Cogn Sci. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 109.Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn Sci. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 110.Iacoboni M. Neurobiology of imitation. Curr Opin Neurobiol. 2009;19:661–665. doi: 10.1016/j.conb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 111.Bernhardt BC, Singer T. The neural basis of empathy. Annu Rev Neurosci. 2012;35:1–23. doi: 10.1146/annurev-neuro-062111-150536. [DOI] [PubMed] [Google Scholar]
- 112.Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–534. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 113.Mitchell JP. Inferences about mental states. Philos Trans R Soc Lond B Biol Sci. 2009;364:1309–1316. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lenzi D, Trentini C, Pantano P, Macaluso E, Iacoboni M, Lenzi GL, Ammaniti M. Neural basis of maternal communication and emotional expression processing during infant preverbal stage. Cereb Cortex. 2009;19:1124–1133. doi: 10.1093/cercor/bhn153. [DOI] [PubMed] [Google Scholar]
- 115.Eisenberger NI, Cole SW. Social neuroscience and health: neurophysio-logical mechanisms linking social ties with physical health. Nat Neurosci. 2012;15:669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- 116.Poulin MJ, Brown SL, Dillard AJ, Smith DM. Giving to others and the association between stress and mortality. Am J Public Health. 2013;103:1649–1655. doi: 10.2105/AJPH.2012.300876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bowlby J. Loss: Sadness and Depression. Vol. 3. New York: Basic Books; 1980. [Google Scholar]
- 118.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 119.Buss C, Lord C, Wadiwalla M, Hellhammer DH, Lupien SJ, Meaney MJ, Pruessner JC. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J Neurosci. 2007;27:2592–2595. doi: 10.1523/JNEUROSCI.3252-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim P, Leckman JF, Mayes LC, Newman MA, Feldman R, Swain JE. Perceived quality of maternal care in childhood and structure and function of mothers’ brain. Dev Sci. 2010;13:662–673. doi: 10.1111/j.1467-7687.2009.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Beckes L, Coan JA. Social baseline theory: the role of social proximity in emotion and economy of action. Soc Pers Psychol Compass. 2011;5:976–988. [Google Scholar]
- 122.Coan JA, Schaefer HS, Davidson RJ. Lending a hand: social regulation of the neural response to threat. Psychol Sci. 2006;17:1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- 123.Hyde LW, Gorka A, Manuck SB, Hariri AR. Perceived social support moderates the link between threat-related amygdala reactivity and trait anxiety. Neuropsychologia. 2011;49:651–656. doi: 10.1016/j.neuropsychologia.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Insel TR. A neurobiological basis of social attachment. Am J Psychiatry. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- 125.Atzil S, Hendler T, Feldman R. Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology. 2011;36:2603–2615. doi: 10.1038/npp.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Atzil S, Hendler T, Zagoory-Sharon O, Winetraub Y, Feldman R. Synchrony and specificity in the maternal and the paternal brain: relations to oxytocin and vasopressin. J Am Acad Child Adolesc Psychiatry. 2012;51:798–811. doi: 10.1016/j.jaac.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 127.Molitor A, Mayes LC. Problematic dyadic interaction among toddlers and their polydrug-cocaine-using mothers. Infant Ment Health J. 2010;31:121–140. doi: 10.1002/imhj.20248. [DOI] [PubMed] [Google Scholar]
- 128.Landi N, Montoya J, Kober H, Rutherford HJ, Mencl WE, Worhunsky PD, Potenza MN, Mayes LC. Maternal neural responses to infant cries and faces: relationships with substance use. Front Psychiatry. 2011;2:32. doi: 10.3389/fpsyt.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Swain J, Ho S, Rosenblum K, Marcus S, Muzik M. Maternal Brain Physiology in Health, Mood Disorders and Opiate Exposure. Paper presented at: 60th American Academy of Child and Adolescent Psychiatry; Orlando, FL. 2013. [Google Scholar]
- 130.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 131.Shulman GL, Corbetta M, Fiez JA, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Searching for activations that generalize over tasks. Hum Brain Mapp. 1997;5:317–322. doi: 10.1002/(SICI)1097-0193(1997)5:4<317::AID-HBM19>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 132.Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 133.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 135.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 136.Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self-reflection. Soc Cogn Affect Neurosci. 2006;1:56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Pank-sepp J. Self-referential processing in our brain-a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 138.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6:548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hamilton JP, Glover GH, Hsu JJ, Johnson RF, Gotlib IH. Modulation of subgenual anterior cingulate cortex activity with real-time neurofeedback. Hum Brain Mapp. 2011;32:22–31. doi: 10.1002/hbm.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Johnson MK, Nolen-Hoeksema S, Mitchell KJ, Levin Y. Medial cortex activity, self-reflection and depression. Soc Cogn Affect Neurosci. 2009;4:313–327. doi: 10.1093/scan/nsp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chase HW, Moses-Kolko EL, Zevallos C, Wisner KL, Phillips ML. Disrupted posterior cingulate-amygdala connectivity in postpartum depressed women as measured with resting BOLD fMRI. Soc Cogn Affect Neurosci. 2013;9:1069–1075. doi: 10.1093/scan/nst083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mars RB, Neubert FX, Noonan MP, Sallet J, Toni I, Rushworth MF. On the relationship between the “default mode network” and the “social brain”. Front Hum Neurosci. 2012;6:189. doi: 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Deligiannidis KM, Sikoglu EM, Shaffer SA, Frederick B, Svenson AE, Kopoyan A, Kosma CA, Rothschild AJ, Moore CM. GABAergic neuroactive steroids and resting-state functional connectivity in postpartum depression: a preliminary study. J Psychiatr Res. 2013;47:816–828. doi: 10.1016/j.jpsychires.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xiao-juan W, Jian W, Zhi-hong L, Yan M, Shi-wei Z. Increased posterior cingulate, medial frontal and decreased temporal regional homogeneity in depressed mothers. Procedia Environ Sci. 2011;8:737–743. [Google Scholar]
- 148.Huprich SK, Pouliot GS, Bruner R. Self-other representations mediate the relationship between Five-Factor Model depression and depressive states. Psychiatry. 2012;75:176–189. doi: 10.1521/psyc.2012.75.2.176. [DOI] [PubMed] [Google Scholar]
- 149.Bifulco A, Kwon J, Jacobs C, Moran PM, Bunn A, Beer N. Adult attachment style as mediator between childhood neglect/abuse and adult depression and anxiety. Soc Psychiatry Psychiatr Epidemiol. 2006;41:796–805. doi: 10.1007/s00127-006-0101-z. [DOI] [PubMed] [Google Scholar]
- 150.Fonagy P, Leigh T, Steele M, Steele H, Kennedy R, Mattoon G, Target M, Gerber A. The relation of attachment status, psychiatric classification, and response to psychotherapy. J Consult Clin Psychol. 1996;64:22–31. doi: 10.1037//0022-006x.64.1.22. [DOI] [PubMed] [Google Scholar]
- 151.Fonagy P, Steele H, Steele M. Maternal representations of attachment during pregnancy predict the organization of infant-mother attachment at one year of age. Child Dev. 1991;62:891–905. doi: 10.1111/j.1467-8624.1991.tb01578.x. [DOI] [PubMed] [Google Scholar]
- 152.Van IJzendoorn MH. Adult attachment representations, parental responsiveness, and infant attachment: a meta-analysis on the predictive validity of the Adult Attachment Interview. Psychol Bull. 1995;117:387–403. doi: 10.1037/0033-2909.117.3.387. [DOI] [PubMed] [Google Scholar]
- 153.Mayes LC, Swain JE, Leckman JF. Parental attachment systems: neural circuits, genes, and experiential contributions to parental engagement. Clin Neurosci Res. 2005;4:301–313. [Google Scholar]
- 154.Swain JE. The human parental brain: in vivo neuroimaging. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1242–1254. doi: 10.1016/j.pnpbp.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Swain JE, Konrath S, Brown SL, Finegood ED, Akce LB, Dayton CJ, Ho SS. Parenting and beyond: common neurocircuits underlying parental and altruistic caregiving. Parent Sci Pract. 2012;12:115–123. doi: 10.1080/15295192.2012.680409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Laurent HK, Ablow JC. The missing link: mothers’ neural response to infant cry related to infant attachment behaviors. Infant Behav Dev. 2012;35:761–772. doi: 10.1016/j.infbeh.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Musser ED, Kaiser-Laurent H, Ablow JC. The neural correlates of maternal sensitivity: an fMRI study. Dev Cogn Neurosci. 2012;2:428–436. doi: 10.1016/j.dcn.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lemche E, Giampietro VP, Surguladze SA, Amaro EJ, Andrew CM, Williams SC, Brammer MJ, Lawrence N, Maier MA, Russell TA, Simmons A, Ecker C, Joraschky P, Phillips ML. Human attachment security is mediated by the amygdala: evidence from combined fMRI and psycho-physiological measures. Hum Brain Mapp. 2006;27:623–635. doi: 10.1002/hbm.20206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wan MW, Downey D, Strachan H, Elliott R, Williams SR, Abel KM. The neural basis of maternal bonding. PLoS ONE. 2014;9:e88436. doi: 10.1371/journal.pone.0088436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Maccari S, Morley-Fletcher S, Szyf M. The consequences of early life adversity: neurobiological, behavioural and epigenetic adaptations. J Neuroendocrinol. 2014;26:707–723. doi: 10.1111/jne.12175. [DOI] [PubMed] [Google Scholar]
- 161.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 162.Clark DA, Beck AT, Stewart B. Cognitive specificity and positive-negative affectivity: complementary or contradictory views on anxiety and depression? J Abnorm Psychol. 1990;99:148–155. doi: 10.1037//0021-843x.99.2.148. [DOI] [PubMed] [Google Scholar]
- 163.Gotlib IH, Whiffen VE, Wallace PM, Mount JH. Prospective investigation of postpartum depression: factors involved in onset and recovery. J Abnorm Psychol. 1991;100:122–132. doi: 10.1037//0021-843x.100.2.122. [DOI] [PubMed] [Google Scholar]
- 164.Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, Liberzon I, Phan KL. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc Natl Acad Sci USA. 2013;110:18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kinsley CH, Lambert KG. Reproduction-induced neuroplasticity: natural behavioural and neuronal alterations associated with the production and care of offspring. J Neuroendocrinol. 2008;20:515–525. doi: 10.1111/j.1365-2826.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- 166.Galea LA, Leuner B, Slattery D. Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. J Neuroendocrinol. 2014;26:641–648. doi: 10.1111/jne.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lonstein JS, Maguire J, Meinlschmidt G, Neumann I. Emotion and mood adaptations in the peripartum female: complementary contributions of gamma-aminobutyric acid and oxytocin. J Neuroendocrinol. 2014;26:649–664. doi: 10.1111/jne.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Numan M, Insel TR. Neurochemistry and molecular biology of maternal behavior. In: Numan M, Insel TR, editors. The Neurobiology of Parental Behavior. Vol. 1. New York: Springer-Verlag; 2003. pp. 190–245. [Google Scholar]
- 169.Chong S, Vickaryous N, Ashe A, Zamudio N, Youngson N, Hemley S, Stopka T, Skoultchi A, Matthews J, Scott HS, de Kretser D, O’Bryan M, Blewitt M, Whitelaw E. Modifiers of epigenetic reprogramming show paternal effects in the mouse. Nat Genet. 2007;39:614–622. doi: 10.1038/ng2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dietz DM, Laplant Q, Watts EL, Hodes GE, Russo SJ, Feng J, Oosting RS, Vialou V, Nestler EJ. Paternal transmission of stress-induced pathologies. Biol Psychiatry. 2011;70:408–414. doi: 10.1016/j.biopsych.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dietz DM, Nestler EJ. From father to offspring: paternal transmission of depressive-like behaviors. Neuropsychopharmacology. 2012;37:311–312. doi: 10.1038/npp.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Belsky JJSR, Sligo J, Woodward L, Silva PA. Intergenerational transmission of warm-sensitive-stimulating parenting: a prospective study of mothers and fathers of 3-year-olds. Child Dev. 2005;76:384–396. doi: 10.1111/j.1467-8624.2005.00852.x. [DOI] [PubMed] [Google Scholar]
- 173.Fleming AS, Kraemer GW, Gonzalez A, Lovic V, Rees S, Melo A. Mothering begets mothering: the transmission of behavior and its neurobiology across generations. Pharmacol Biochem Behav. 2002;73:61–75. doi: 10.1016/s0091-3057(02)00793-1. [DOI] [PubMed] [Google Scholar]
- 174.Maestripieri D. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proc Natl Acad Sci USA. 2005;102:9726–9729. doi: 10.1073/pnas.0504122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Champagne F, Meaney MJ. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- 176.Knutson JF. Psychological characteristics of maltreated children: putative risk factors and consequences. Annu Rev Psychol. 1995;46:401–431. doi: 10.1146/annurev.ps.46.020195.002153. [DOI] [PubMed] [Google Scholar]
- 177.Benoit D, Parker KC. Stability and transmission of attachment across three generations. Child Dev. 1994;65:1444–1456. doi: 10.1111/j.1467-8624.1994.tb00828.x. [DOI] [PubMed] [Google Scholar]
- 178.Benoit D, Parker KC, Zeanah CH. Mothers’ representations of their infants assessed prenatally: stability and association with infants’ attachment classifications. J Child Psychol Psychiatry. 1997;38:307–313. doi: 10.1111/j.1469-7610.1997.tb01515.x. [DOI] [PubMed] [Google Scholar]
- 179.Miller L, Kramer R, Warner V, Wickramaratne P, Weissman M. Intergenerational transmission of parental bonding among women. J Am Acad Child Adolesc Psychiatry. 1997;36:1134–1139. doi: 10.1097/00004583-199708000-00022. [DOI] [PubMed] [Google Scholar]
- 180.Serbin LA, Karp J. The intergenerational transfer of psychosocial risk: mediators of vulnerability and resilience. Annu Rev Psychol. 2004;55:333–363. doi: 10.1146/annurev.psych.54.101601.145228. [DOI] [PubMed] [Google Scholar]
- 181.Van Ijzendoorn MH. Intergenerational transmission of attachment. Int J Psychol. 1992;27:208–208. [Google Scholar]
- 182.Moehler E, Biringen Z, Poustka L. Emotional availability in a sample of mothers with a history of abuse. Am J Orthopsychiatry. 2007;77:624–628. doi: 10.1037/0002-9432.77.4.624. [DOI] [PubMed] [Google Scholar]
- 183.Herrenkohl TI, Kosterman R, Mason WA, Hawkins JD, McCarty CA, McCauley E. Effects of childhood conduct problems and family adversity on health, health behaviors, and service use in early adulthood: tests of developmental pathways involving adolescent risk taking and depression. Dev Psychopathol. 2010;22:655–665. doi: 10.1017/S0954579410000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Martin G, Bergen HA, Roeger L, Allison S. Depression in young adolescents - Investigations using 2 and 3 factor versions of the parental bonding instrument. J Nerv Ment Dis. 2004;192:650–657. doi: 10.1097/01.nmd.0000142028.10056.c6. [DOI] [PubMed] [Google Scholar]
- 185.Rey JM. Perceptions of poor maternal-care are associated with adolescent depression. J Affect Disord. 1995;34:95–100. doi: 10.1016/0165-0327(95)00005-8. [DOI] [PubMed] [Google Scholar]
- 186.Enns MW, Cox BJ, Clara I. Parental bonding and adult psychopathology: results from the US National Comorbidity Survey. Psychol Med. 2002;32:997–1008. doi: 10.1017/s0033291702005937. [DOI] [PubMed] [Google Scholar]
- 187.Putnam FW. Ten-year research update review: child sexual abuse. J Am Acad Child Adolesc Psychiatry. 2003;42:269–278. doi: 10.1097/00004583-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 188.Spertus IL, Yehuda R, Wong CM, Halligan S, Seremetis SV. Childhood emotional abuse and neglect as predictors of psychological and physical symptoms in women presenting to a primary care practice. Child Abuse Negl. 2003;27:1247–1258. doi: 10.1016/j.chiabu.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 189.Jaffee SR, Caspi A, Moffitt TE, Polo-Tomas M, Taylor A. Individual, family, and neighborhood factors distinguish resilient from non-resilient maltreated children: a cumulative stressors model. Child Abuse Negl. 2007;31:231–253. doi: 10.1016/j.chiabu.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hankin BL, Nederhof E, Oppenheimer CW, Jenness J, Young JF, Abela JRZ, Smolen A, Ormel J, Oldehinkel AJ. Differential susceptibility in youth: evidence that 5-HTTLPR × positive parenting is associated with positive affect ‘for better and worse’. Transl Psychiat. 2011;1:e44. doi: 10.1038/tp.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Oppenheimer CW, Hankin BL, Jenness JL, Young JF, Smolen A. Observed positive parenting behaviors and youth genotype: evidence for gene-environment correlations and moderation by parent personality traits. Dev Psychopathol. 2013;25:175–191. doi: 10.1017/S0954579412000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci USA. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wind TW, Silvern L. Parenting and family stress as mediators of the long-term effects of child-abuse. Child Abuse Negl. 1994;18:439–453. doi: 10.1016/0145-2134(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 194.Seeman TE, Singer BH, Ryff CD, Love GD, Levy-Storms L. Social relationships, gender, and allostatic load across two age cohorts. Psychosom Med. 2002;64:395–406. doi: 10.1097/00006842-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 195.Francis DD, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 196.Dawson G, Ashman SB, Carver LJ. The role of early experience in shaping behavioral and brain development and its implications for social policy. Dev Psychopathol. 2000;12:695–712. doi: 10.1017/s0954579400004089. [DOI] [PubMed] [Google Scholar]
- 197.Segman RH, Goltser-Dubner T, Weiner I, Canetti L, Galili-Weisstub E, Milwidsky A, Pablov V, Friedman N, Hochner-Celnikier D. Blood mono-nuclear cell gene expression signature of postpartum depression. Mol Psychiatry. 2010;15:102. doi: 10.1038/mp.2009.65. [DOI] [PubMed] [Google Scholar]
- 198.Mahon PB, Payne JL, MacKinnon DF, Mondimore FM, Goes FS, Schweizer B, Jancic D, Consortium NGIBD, Bi GSC, Coryell WH, Holmans PA, Shi J, Knowles JA, Scheftner WA, Weissman MM, Levinson DF, DePaulo JR, Jr, Zandi PP, Potash JB. Genome-wide linkage and follow-up association study of postpartum mood symptoms. Am J Psychiatry. 2009;166:1229–1237. doi: 10.1176/appi.ajp.2009.09030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.O’Hara MW, McCabe JE. Postpartum depression: current status and future directions. Annu Rev Clin Psychol. 2013;9:379–407. doi: 10.1146/annurev-clinpsy-050212-185612. [DOI] [PubMed] [Google Scholar]
- 200.Cooper PJ, Murray L. Course and recurrence of postnatal depression evidence for the specificity of the diagnostic concept. Br J Psychiatry. 1995;166:191–195. doi: 10.1192/bjp.166.2.191. [DOI] [PubMed] [Google Scholar]
- 201.Altemus M, Neeb CC, Davis A, Occhiogrosso M, Nguyen T, Bleiberg KL. Phenotypic differences between pregnancy-onset and postpartum-onset major depressive disorder. J Clin Psychiatry. 2012;73:e1485–e1491. doi: 10.4088/JCP.12m07693. [DOI] [PubMed] [Google Scholar]
- 202.Carter FA, Frampton CM, Mulder RT. Cesarean section and postpartum depression: a review of the evidence examining the link. Psychosom Med. 2006;68:321–330. doi: 10.1097/01.psy.0000204787.83768.0c. [DOI] [PubMed] [Google Scholar]
- 203.Dennis CL, McQueen K. The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics. 2009;123:e736–e751. doi: 10.1542/peds.2008-1629. [DOI] [PubMed] [Google Scholar]
- 204.Stuebe AM, Grewen K, Pedersen CA, Propper C, Meltzer-Brody S. Failed lactation and perinatal depression: common problems with shared neuroendocrine mechanisms? J Women’s Health. 2012;21:264–272. doi: 10.1089/jwh.2011.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.O’Hara MW, Schlechte JA, Lewis DA, Wright EJ. Prospective study of postpartum blues Biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48:801–806. doi: 10.1001/archpsyc.1991.01810330025004. [DOI] [PubMed] [Google Scholar]
- 206.Workman JL, Barha CK, Galea LAM. Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav Neurosci. 2012;126:54–72. doi: 10.1037/a0025538. [DOI] [PubMed] [Google Scholar]
- 207.Bloch M, Rubinow DR, Schmidt PJ, Lotsikas A, Chrousos GP, Cizza G. Cortisol response to ovine corticotropin-releasing hormone in a model of pregnancy and parturition in euthymic women with and without a history of postpartum depression. J Clin Endocr Metab. 2005;90:695–699. doi: 10.1210/jc.2004-1388. [DOI] [PubMed] [Google Scholar]
- 208.Brummelte S, Galea LA. Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:766–776. doi: 10.1016/j.pnpbp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 209.Meltzer-Brody S, Stuebe A, Dole N, Savitz D, Rubinow D, Thorp J. Elevated corticotropin releasing hormone (CRH) during pregnancy and risk of postpartum depression (PPD) J Clin Endocrinol Metab. 2011;96:E40–E47. doi: 10.1210/jc.2010-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- 211.Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry. 2003;44:234–246. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- 212.Daly RC, Kim MY, Bloch M, Schmidt PJ, Purdy RM, Rubinow DR. Neurosteroids in reproductive endocrine-related mood disorders. Biol Psychiatry. 2000;47:5S–5S. [Google Scholar]
- 213.Schiller CE, O’Hara MW, Johnson AK, Rubinow DR. Estradiol withdrawal triggers postpartum depression across species. Biol Psychiatry. 2012;71:279s–279s. [Google Scholar]
- 214.Schiller CE, O’Hara MW, Rubinow DR, Johnson AK. Estradiol modulates anhedonia and behavioral despair in rats and negative affect in a subgroup of women at high risk for postpartum depression. Physiol Behav. 2013;119:137–144. doi: 10.1016/j.physbeh.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Moses-Kolko EL, Berga SL, Kalro B, Sit DKY, Wisner KL. Transdermal estradiol for postpartum depression: a promising treatment option. Clin Obstet Gynecol. 2009;52:516–529. doi: 10.1097/GRF.0b013e3181b5a395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ho SS, Konrath S, Brown S, Swain JE. Empathy and stress related neural responses in maternal decision making. Front Neurosci. 2014;8:152. doi: 10.3389/fnins.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wisner KL, Hanusa BH, Perel JM, Peindl KS, Piontek CM, Sit DKY, Findling RL, Moses-Kolko EL. Postpartum depression: a randomized trial of sertraline vs nortriptyline. J Clin Psychopharmacol. 2006;26:353–360. doi: 10.1097/01.jcp.0000227706.56870.dd. [DOI] [PubMed] [Google Scholar]
- 218.Sacher J, Wilson AA, Houle S, Rusjan P, Hassan S, Bloomfield PM, Stewart DE, Meyer JH. Elevated brain monoamine oxidase a binding in the early postpartum period. Arch Gen Psychiatry. 2010;67:468–474. doi: 10.1001/archgenpsychiatry.2010.32. [DOI] [PubMed] [Google Scholar]
- 219.Van Essen DC, Ugurbil K. The future of the human connectome. Neuro-Image. 2012;62:1299–1310. doi: 10.1016/j.neuroimage.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Soper D. The free statistics calculators website. 2010 http://www.danielsoper.com/statcalc.
- 221.Miller GA, Rockstroh B. Endophenotypes in psychopathology research: where do we stand? Ann Rev Clin Psychol. 2013;9:177–213. doi: 10.1146/annurev-clinpsy-050212-185540. [DOI] [PubMed] [Google Scholar]
- 222.Phillips ML, Ladoucer CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]