Abstract
Background:
Transmission of Mycobacterium tuberculosis (M. tuberculosis) can occur in different ways. Furthermore, drug resistant in M. tuberculosis family is a major problem that creates obstacles in treatment and control of tuberculosis (TB) in the world. One of the most prevalent families of M. tuberculosis is Haarlem, and it is associated with drug resistant. Our objectives of this study were to determine the prevalence and occurrence rate of M. tuberculosis Haarlem family multi-drug resistant (MDR) in the worldwide using meta-analysis based on a systematic review that performed on published articles.
Materials and Methods:
Data sources of this study were 78 original articles (2002-2012) that were published in the literatures in several databases including PubMed, Science Direct, Google Scholar, Biological abstracts, ISI web of knowledge and IranMedex. The articles were systematically reviewed for prevalence and rate of MDR. Data were analyzed using meta-analysis and random effects models with the software package Meta R, Version 2.13 (P < 0.10).
Results:
Final analysis included 28601 persons in 78 articles. The highest and lowest occurrence rate of Haarlem family in M. tuberculosis was in Hungary in 2006 (66.20%) with negative MDR-TB and in China in 2010 (0.8%), respectively. From 2002 to 2012, the lowest rate of prevalence was in 2010, and the highest prevalence rate was in 2012. Also 1.076% were positive for MDR and 9.22% were negative (confidence interval: 95%).0020.
Conclusion:
Many articles and studies are performed in this field globally, and we only chose some of them. Further studies are needed to be done in this field. Our study showed that M. tuberculosis Haarlem family is prevalent in European countries. According to the presence of MDR that was seen in our results, effective control programs are needed to control the spread of drug-resistant strains, especially Haarlem family.
Keywords: Haarlem family, multi-drug resistant, Mycobacterium tuberculosis, prevalence
INTRODUCTION
Mycobacterium tuberculosis (M. tuberculosis) is a primarily human pathogen that causes tuberculosis (TB) disease.[1] This disease damages the lungs, central nervous system, lymphatic system and circulatory system.[2] Rate of infection with M. tuberculosis is associated with host's inherited susceptibility, environmental risk factors and genetic variations.[3] There are approximately 8 million new TB cases each year and over 2 million deaths because of this disease.[4] Different studies showed that the one-third of the worldwide population is infected with M. tuberculosis and new cases occur at a rate of 1/s.[5] Transmission of TB can occur through different ways such as: Human-to-human and human-to-animal, poor hygienic conditions, blood and fecal contamination (transmission) and inhalation of contaminated droplets that are released from the lungs of an infected individual (coughing and inhalation). Prevention and control of TB infection can be achieved through the prompt detection and treatment of infectious patients, air related precautions, and treatment of people who are suspected or confirmed to have TB disease and vaccination with the TB vaccine (Bacillus Calmette — Guerin [BCG]). There are several kinds of tests that are used to detect TB bacteria: The tuberculin skin test and TB blood tests (Centers for Disease Control or test). The typing method is based on DNA presence of polymorphism at one particular chromosomal locus, the “Direct Repeat” (DR) region, which is in M. tuberculosis complex bacteria.[6] This locus was first described in Mycobacterium bovis BCG. The DR region in M. bovis BCG consists of directly repeated sequences of 36 base pairs, which are interspersed by nonrepetitive DNA spacers, each 35-41 base pairs in length.[7] The main genotype families of M. tuberculosis include Beijing, Haarlem, Africa, East-African-Indian, Latin American and Mediterranean strains. The Haarlem genotype is associated with widespread epidemics and drug resistance.[4,8] Several meta-analyses are conducted on different aspects of M. tuberculosis around the world: Trunz et al. showed by a meta-analysis that BCG vaccination was effective against childhood TB, meningitis and TB in the military and the number of cases that were prevented was highest in South East Asia (46%), sub-Saharan Africa (27%) and in the western Pacific region (15%).[9] Ling et al. (2008) in Canada by a meta-analysis investigated on the multi-drug resistant (MDR) TB based on genotype MTBDR assays and they showed that the sensitivity and specificity were 98.1% and 98.7% for rifampicin resistance, respectively.[10] Yang et al. (2011) in China using a systematic review and Meta-Analysis showed that the prevalence of drug-resistant TB in new cases was 27.9% and in previously treated cases was 60.3%.[11] Nowadays, this MDR quality is a major problem in TB treatment and control. Also, drug resistance in TB patients will increase in cases of improper use of antibiotics. Drug resistance is raising in areas with weak TB control programs. Also, transmission of TB to other individuals by drug-resistant patients is easily possible. Determining the M. tuberculosis prevalence and occurrence rate of MDR-TB can help to control and plan therapies. So it is important to know specific families that are over represented among drug-resistant cases, and in particular if these resistant strains are successfully transmitted within the community. Haarlem family is associated with drug resistance and rapid clonal expansion; this genotype has epidemic potential.[12] The aim of this study was to determine the prevalence and distribution rate of the Haarlem family MDR in worldwide using a meta-analysis based on a systematic review that performed on published articles.
MATERIALS AND METHODS
Search strategy
The different literature databases and original articles that were published in the time span of 2002-2012 in English language for determination of M. tuberculosis Haarlem family prevalence and occurrence rate of MDR in this family in worldwide population were obtained from valid and credible web sites: PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Science Direct (http://www.sciencedirect.com), Google Scholar (http://www.scholar.google.co.uk), Biological Abstracts (http://www.science.thomsonreuters.com) and ISI web of knowledge (http://www.pcs.webofknowledge.com) in Thomsonreuters, the years of coverage of these web sites are 1955, 1977, 2006, 2009 and 1958, respectively. Also, some article was found in IranMedex (http://www.iranmedex.com). We contacted several study authors in the cellular and molecular research center and microbiology department of Kurdistan University of medical sciences, located in Sanandaj, Iran to identify some additional studies. The last search was performed in the winter of 2012. For the means of searching in different websites, several key words were entered. For example TB Haarlem, molecular epidemiology, MDR and spoligotyping were entered in PubMed (Advanced search part). In some cases, search process got limited because in order to reach to some of the papers’ full text, we should have had a specific username and password and hence we only used their abstracts which were available or free.
Data extraction and assessment of studies quality
Process for selecting the studies
All the information in different articles and papers used in this study were surveyed by authors. Two reviewers did the searching independently. When there was any disagreement between them for selecting specific articles, they discoursed about selecting it. Also, eligibility for each paper was determined in Excel data sheets (CEB603, Chino-Excel Technology). The following data were extracted from original publications: Number of cases, websites, Author, Study place (country), Year of the research, Sample size, and prevalence of Haarlem and MDR association.
Inclusion and exclusion criteria
Inclusion criteria
Research articles with full text,
Articles with abstract in English.
Excluded studies
Review articles,
Congress abstracts,
Studies that reported in languages other than English and
Studies that were not available for us in abstract or full text,
Studies that their sampling location was uncertain,
Studies that locations of sampling was performed at the same time,
Studies that their data were not clear.
Data synthesis and meta-analysis
Variables of our study were prevalence of TB infection and Haarlem family MDR in different countries (2002-2012). According to different studies performed in various countries, it was concluded that high levels of prevalence of TB infection and Haarlem family MDR can exist in different countries. Hence, the prevalence of Haarlem family of TB in 78 studies was the main outcome. The variance of the prevalence was computed using the binomial distribution (confidence interval [CI]: 95%). Meta-analysis with the random effect model was applied to combine the prevalence among studies. There was sensitivity (how the uncertainty in the output of a mathematical model or system can be apportioned to different sources of uncertainty in its inputs) and heterogeneity among studies. I2 and Q (P < 0.10) statistical tests were used to check out this heterogeneity (I2 static is percentage of observed total variation across studies that are due to heterogeneity rather than chance. It is calculated as I2 = 100% × (Q − df)/Q, where Q is Cochran's heterogeneity statistic and df degrees of freedom. Negative values of I2 are put equal to zero so that I2 lies between 0% and 100%. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity. Q is the weighted of squares on a standardized scale. It is reported with a P value with low P values indicating the presence of heterogeneity. This test however is known to have low power to detect heterogeneity, and it is suggested to use a value of 0.01 as a cut of for significance. Conversely, Q has too much power as a test of heterogeneity if the number of studies is large. Subgroup analyses were assessed using Chi-square tests, and it was done for continents). Stratified analyses were subsequently performed with respect to geographical locations and MDR-TB among the Haarlem family isolates.[13] Meta-analysis was carried out using the software package Meta R (Version 2.13.2, Copyright 2011, The R Foundation for Statistical Computing).
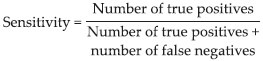
Publication bias
There was some evidence of publication bias in the subsample of studies. Some of these studies did not find a positive association between antibiotic consumption and antibiotic resistance. Also, excluded studies that were introduced above are publication Bias.
RESULTS
Description of included and excluded studies
According to our investigation in PubMed, Science Direct, Google Scholar, Biological Abstracts, ISI web of knowledge and IranMedex we had 78 inclusions (research articles with full text and abstracts in English) and in end 26 exclusions (Review articles, congress abstracts, studies that reported in languages other than English, studies that were not available for us in abstract or full text, studies that their sampling location was uncertain, studies that locations of sampling was performed at the same time, studies that their data were not clear) for this meta-analysis [Figure 1].
Figure 1.
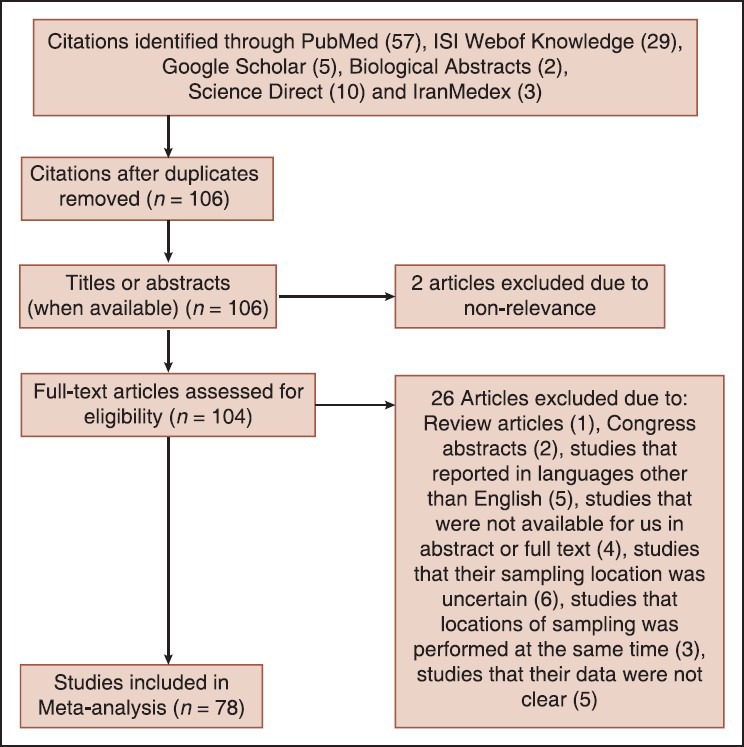
Flow diagram for study selection
Samples information
In our study, 78 articles were reviewed from 33 different countries. The names of these countries are mentioned in Table 1.
Table 1.
Data that were extracted from published documents about country, year, sample size, prevalence and M. tuberculosis Haarlem family MDR
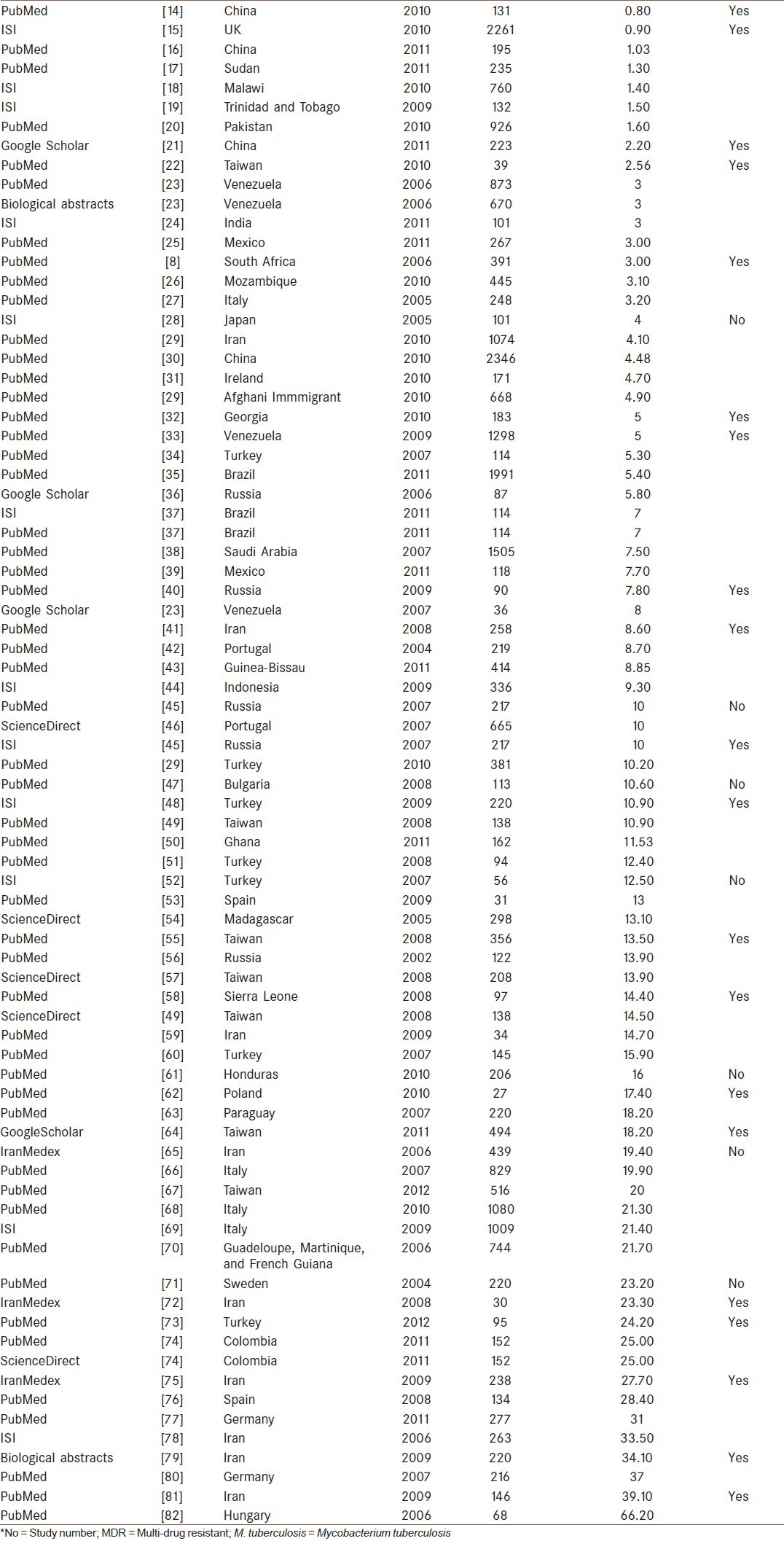
The total population for prevalence of Haarlem family of M. tuberculosis that was included in this meta-analysis was 28,601. The major risk factors for M. tuberculosis infection were geographical areas and MDR-TB among Haarlem family. The typing method described in these studies was based on DNA polymorphism that exists at one particular chromosomal locus, the DR region, which is uniquely present in M. tuberculosis complex bacteria.
Prevalence of Mycobacterium tuberculosis Haarlem family in the worldwide population
In this study, 78 different studies with a total number of 28,601 persons from 2002 to 2012 were subjected. For this meta-Analysis, prevalence of M. tuberculosis Haarlem Family in worldwide population was analyzed according to the year of occurrence and MDR-TB. The highest rate of prevalence was in Hungary in 2006 (66.20%) with negative MDR-TB and the lowest rate was in China in 2010 (0.8%), also MDR-TB was observed in these studies (CI: 95%) [Table 1].
Year
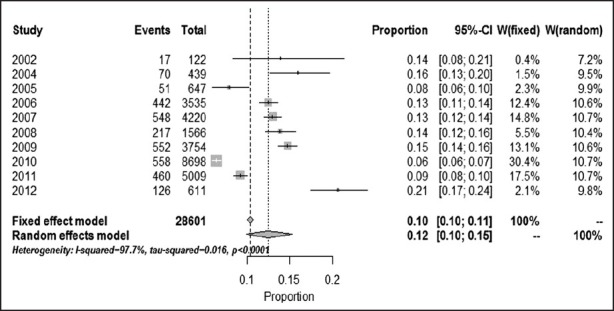
The data for 11 groups were analyzed for the period of 2002-2012. The lowest rate of prevalence was in 2010, and stood at 6.42% (95% CI: 5.91-6.95%). But the highest prevalence was attained in 2012, and stood at 20.62% (95% CI: 17.48-24.05%). The heterogeneity, I2 = 97.7, heterogeneity Chi-squared = 383. 86 (d.f = 9) P < 0.00, estimate of between-study variance tau-squared = 0.01 and P = 0.00. Also results of publication bias presented in Figures 2-4 and Table 2.
Figure 2.
Pooled sensitivity (confidence interval: 95%) and heterogeneity for Mycobacterium tuberculosis Haarlem family in worldwide population based on year (Method: Inverse variance method [Freeman Turkey double arcsine transformation])
Figure 4.
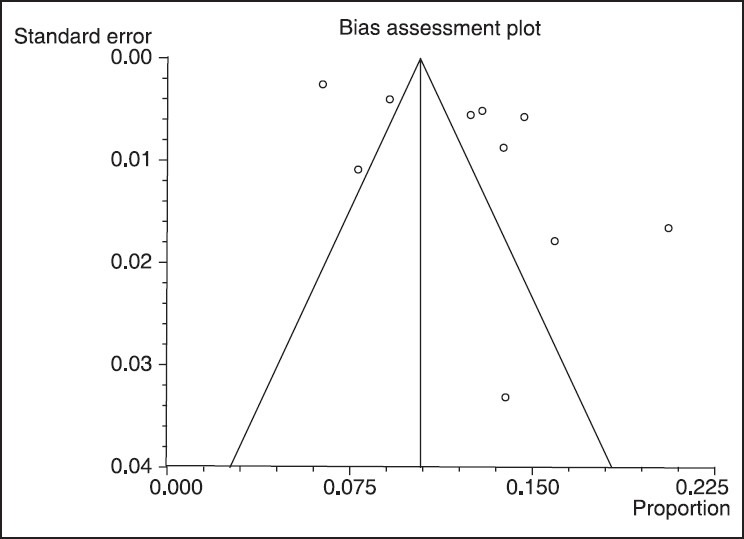
Results of publication bias in for Mycobacterium tuberculosis Haarlem family in worldwide population based on year (confidence interval: 95%)
Table 2.
Pooled sensitivity (CI: 95%) and heterogeneity for M. tuberculosis Haarlem family in worldwide population based on year
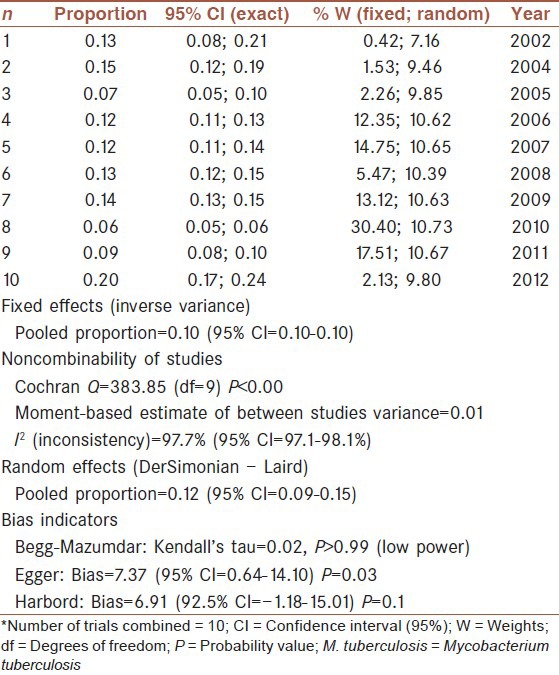
Figure 3.
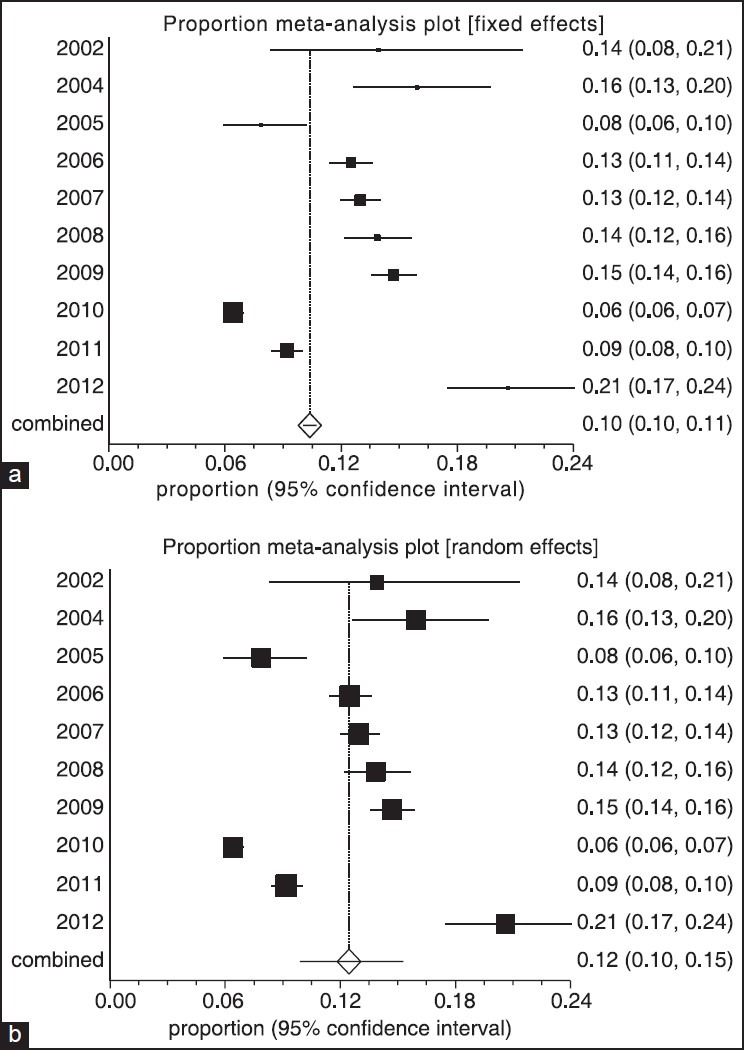
Forest plot of fixed (a) and random (b) effect estimates for pooled sensitivity and heterogeneity estimates for prevalence of Haarlem family of Mycobacterium tuberculosis in worldwide population based on year (confidence interval: 95%)
Multi-drug resistant tuberculosis
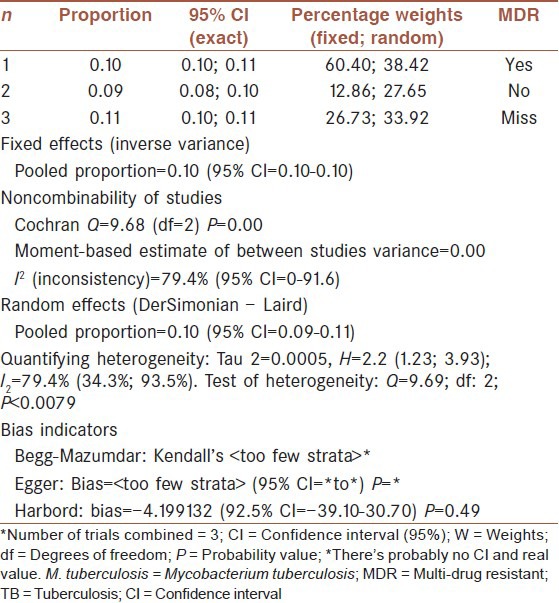
Multi-drug resistant tuberculosis prevalence analysis in our 78 previously mentioned studies, showed that 1.076% (95% CI: 10.30-11.23%) were positive MDR. But, prevalence for negative MDR was 9.22% (95% CI: 8.3-10.2%). Heterogeneity, I-squared = 79.4, heterogeneity Chi-squared = 9.69 (d.f = 2) P < 0.001, Estimate of between-study variance Tau-squared = 0.00 and P = 0.00. Also there is no publication bias [Figures 5 and 6, Table 3].
Figure 5.
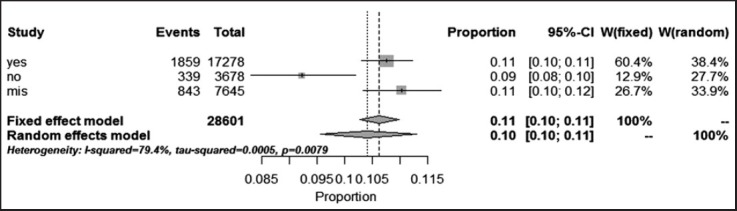
Pooled sensitivity (confidence interval: 95%) and heterogeneity estimates for prevalence of Haarlem family of Mycobacterium tuberculosis in worldwide population based on multi-drug resistant tuberculosis (Method: Inverse variance method [Freeman Turkey double arcsine transformation])
Figure 6.
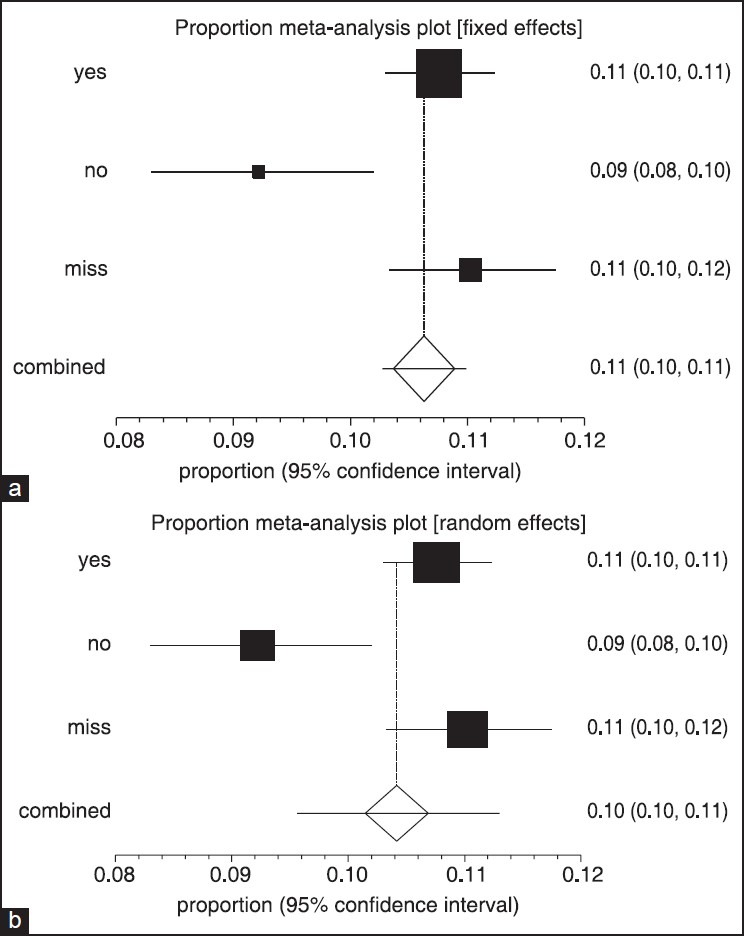
Forest plot of fixed (a) and random (b) effect estimates for pooled sensitivity (confidence interval [CI]) and heterogeneity estimates for prevalence of Haarlem family of Mycobacterium tuberculosis in worldwide population based on multi-drug resistant tuberculosis (CI: 95%)
Table 3.
Pooled sensitivity (CI: 95%) and heterogeneity estimates for prevalence of Haarlem family of M. tuberculosis in worldwide population based on MDR-TB
DISCUSSION
Mycobacterium tuberculosis causes TB disease and possibly death.[3] The aim of our study was to determine the prevalence and rate of occurrence of MDR Haarlem family of M. tuberculosis in the worldwide using meta-analysis and a systematic review based on published articles in the period of 2002-2012. Molecular epidemiology fulfills to establish a dynamic transmission of disease. Results in our study showed that the lowest and highest rates of prevalence were in 2010 and 2013, respectively. 1.076% of samples were positive for MDR and 9.22% were negative for MDR. TB cases have decreased since 2005 and new cases since 2002. For example China has a % 80 decline in its TB mortality rate. 80% of the population in Asian and African countries has positive tuberculin test, but 5-10% of the USA population has positive tuberculin test. In 2007, Swaziland with 1200 cases per 100,000 people had the highest incidence rate of TB. India with 2.0 million new cases had the largest total incidence.[5,83,84] In our study the lowest rate of Haarlem family prevalence was 6.42% in 2010 and the highest prevalence rate was 20.62% in 2012. TB morbidity has decreased worldwide but an increase in the incidence and prevalence of MDR-TB can be observed.[85] Also in our results the highest and lowest rates of occurrence of Haarlem family of M. tuberculosis in worldwide population were in Hungary in 2006 (66.20%) and in China in 2010 (0.8%), respectively. Risk factors that are important for considering when we talk about M. tuberculosis occurrence rate are poor living conditions, unhealthy work environments, crowded places, poverty, and lack of access to proper medical care, and factors that impair the host's defense against TB infection such as HIV infection, malnutrition, smoking, diabetes, alcohol abuse, and indoor air pollution. These factors can be effective on prevalence of M. tuberculosis family in different regions according to our study as compared to other studies.[86,87] These data suggest that different factors such as spread of strains of MDR-TB (primary drug resistance) or the development of drug resistance in the drug-susceptible M. tuberculosis strains due to treatment default or failure (acquired resistance) in patients are important factors for MDR-TB existence. Durmaz et al. in Turkey showed that genetic diversity and major spoligotype families of drug-resistant M. tuberculosis from different regions of Turkey in clinical isolates was for T super-family (29%), Latin-American and Mediterranean (33.5%), Haarlem (14%), and the S lineage (3%).[88] Thus, genetic variation in TB plays an important role in the prevalence of the disease.[3] Based on these results genotyping of M. tuberculosis is an important factor for TB control. Several molecular techniques exist to identity M. tuberculosis such as variable-number tandem repeats of different families of genetic elements that are called mycobacterial interspersed repetitive units and polymerase chain reaction-based spoligotyping.[89,90] In another study Marais et al. in South Africa by spoligotyping that they performed for drug susceptibility testing on 391 isolates; 12.5% were resistant to isoniazid, and 20% were resistant to both isoniazid and rifampin. In Marais study, Haarlem genotype family with 53.1% was significantly associated with drug resistance, so association between antibiotic resistance and prevalence of Haarlem family can be confirmed by spoligotyping.[8] The most common method that is used in clinical molecular epidemiology of TB is spoligotyping that is used extensively.[91,92] This technique showed various profiles of Haarlem family of M. tuberculosis that were obtained from different populations.[93] Bazira et al. in Uganda by spoligotyping on 125 isolates revealed that 79 spoligotype patterns, with an overall diversity of 63.2%. Resistance mutations to either rifampicin or isoniazid were detected in 6.4% of the isolates. Multidrug resistance was seen in 1.6% of the cases.[94] Mutation in bacterium and diversion of the genotypes are largely in M. tuberculosis families and their resistance to drugs may be different among various families.[95] Research articles of our study were gathered for meta-analysis from different websites, including newest researches in PubMed, Science Direct, Google Scholar, Biological Abstracts and ISI web of knowledge in Thomsonreuters in regard to prevalence of TB in world health population. The difference in percentages in databases is logical based on published medical journals. Also we used the Endnote software for our search and deleted overlap studies. In this review, 78 studies were selected for analysis; therefore power of statistical analysis is great and association with drug resistance document in total published reports. Since Haarlem strains are largely transmitted in cities of different countries, causing health problems and outbreak of MDR-TB in populations. This family isolated from patients in different locations in Europe, Asia and USA, so its detection and prevention is necessary.[3,96] We hope results of this study can be effective on control and prevention of Haarlem Family of M. tuberculosis in world populations.
CONCLUSION
This study's results indicate the dynamics of transmission of Haarlem family and its association in drug resistance and they can improve our vision about TB in worldwide populations. Tracking and designing programs for controlling TB infection and MDR is necessary for public health because M. tuberculosis families can be transmitted between different populations in the world. TB infection has been decreasing in recent years, but our results showed that the prevalence rate in 2010 was more than other years that we surveyed about. Therefore, control of its transmission and drug-resistant patients are required. This meta-analysis provides comprehensive and reliable data on the prevalence and occurrence rate of M. tuberculosis Haarlem family MDR among different countries and may be helpful in prevention of its occurrence.
AUTHOR'S CONTRIBUTION
RR contributed in original idea and protocol, conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. DR was statistical consulting. PS contributed in wrote and editing of this manuscript. SR contributed in wrote and editing of this manuscript conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
ACKNOWLEDGMENTS
This is part of our project. The authors wish to extend their gratitude to the Research Deputy of Kurdistan University of Medical Sciences for financial support. The authors declare that there is no conflict of interest.
Footnotes
Source of Support: Authors wish to extend their gratitude to the Research Deputy of Kurdistan University of Medical Sciences for financial support.
Conflict of Interest: None declared.
REFERENCES
- 1.Moravkova M, Slany M, Trcka I, Havelkova M, Svobodova J, Skoric M, et al. Human-to-human and human-to-dog Mycobacterium tuberculosis transmission studied by IS6110 RFLP analysis: A case report. Vet Med. 2011;56:314–7. [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). Trends in tuberculosis-United States, 2004. MMWR Morb Mortal Wkly Rep. 2005;18:245–9. [PubMed] [Google Scholar]
- 3.Cubillos-Ruiz A, Sandoval A, Ritacco V, López B, Robledo J, Correa N, et al. Genomic signatures of the haarlem lineage of Mycobacterium tuberculosis: Implications of strain genetic variation in drug and vaccine development. J Clin Microbiol. 2010;48:3614–23. doi: 10.1128/JCM.00157-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alix E, Godreuil S, Blanc-Potard AB. Identification of a Haarlem genotype-specific single nucleotide polymorphism in the mgtC virulence gene of Mycobacterium tuberculosis. J Clin Microbiol. 2006;44:2093–8. doi: 10.1128/JCM.00278-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geneva, Switzerland: WHO; 2010. World Health Organization (WHO). Tuberculosis Fact sheet N 104. www.who.int . [Google Scholar]
- 6.Trung P, Bjune G, Dahle UR, Trao VT, Duong BD. University of Oslo Faculty of Medicine Institute of General Practice and Community Medicine Section for International Health; 2007. Distribution of Mycobacterium tuberculosis lineages overview in the North of Vietnam, “Thesis submitted as a part of the Master of Philosophy Degree in International Community Health”. [Google Scholar]
- 7.Abubakar A. A Thesis Submitted to the University of Plymouth in Partial Fulfilment for the Degree of Doctor of Philosophy School of Biological Sciences Faculty of Sciences, September 2007 2; 2007. Epidemiology of human and bovine tuberculosis in the federal capital territory and kaduna state of Nigeria; pp. 1–184. [Google Scholar]
- 8.Marais BJ, Victor TC, Hesseling AC, Barnard M, Jordaan A, Brittle W, et al. Beijing and Haarlem genotypes are overrepresented among children with drug-resistant tuberculosis in the Western Cape Province of South Africa. J Clin Microbiol. 2006;44:3539–43. doi: 10.1128/JCM.01291-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: A meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–80. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 10.Ling DI, Zwerling II, Pai M. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. Eur Respir J. 2008;32:1165–74. doi: 10.1183/09031936.00061808. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Li X, Zhou F, Jin Q, Gao L. Prevalence of drug-resistant tuberculosis in mainland China: Systematic review and meta-analysis. PLoS One. 2011;6:e20343. doi: 10.1371/journal.pone.0020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parwati I, van Crevel R, van Soolingen D. Possible underlying mechanisms for successful emergence of the Mycobacterium tuberculosis Beijing genotype strains. Lancet Infect Dis. 2010;10:103–11. doi: 10.1016/S1473-3099(09)70330-5. [DOI] [PubMed] [Google Scholar]
- 13.Marais BJ, Victor TC, Hesseling AC, Barnard M, Jordaan A, Brittle W, et al. Beijing and Haarlem genotypes are overrepresented among children with drug-resistant tuberculosis in the western cape province of south Africa. J Clin Microbiol. 2006;44:3539–43. doi: 10.1128/JCM.01291-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Y, Hoffner S, Jiang W, Wang W, Xu B. Extensive transmission of isoniazid resistant M. tuberculosis and its association with increased multidrug-resistant TB in two rural counties of eastern China: A molecular epidemiological study. BMC Infect Dis. 2010;10:43. doi: 10.1186/1471-2334-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown T, Nikolayevskyy V, Velji P, Drobniewski F. Associations between Mycobacterium tuberculosis strains and phenotypes. Emerg Infect Dis. 2010;16:272–80. doi: 10.3201/eid1602.091032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou A, Nawaz M, Duan Y, Moore JE, Millar BC, Xu J, et al. Molecular characterization of isoniazid-resistant Mycobacterium tuberculosis isolates from Xi’an, China. Microb Drug Resist. 2011;17:275–81. doi: 10.1089/mdr.2010.0135. [DOI] [PubMed] [Google Scholar]
- 17.Sharaf Eldin GS, Fadl-Elmula I, Ali MS, Ali AB, Salih AL, Mallard K, et al. Tuberculosis in Sudan: A study of Mycobacterium tuberculosis strain genotype and susceptibility to anti-tuberculosis drugs. BMC Infect Dis. 2011;11:219. doi: 10.1186/1471-2334-11-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dou HY, Huang SC, Su IJ. Prevalence of Mycobacterium tuberculosis in Taiwan: A model for strain evolution linked to population migration. Int J Evol Biol 2011. 2011 doi: 10.4061/2011/937434. 937434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baboolal S, Millet J, Akpaka PE, Ramoutar D, Rastogi N. First insight into Mycobacterium tuberculosis epidemiology and genetic diversity in Trinidad and Tobago. J Clin Microbiol. 2009;47:1911–4. doi: 10.1128/JCM.00535-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merza MA, Farnia P, Salih AM, Masjedi MR, Velayati AA. First insight into the drug resistance pattern of Mycobacterium tuberculosis in Dohuk, Iraq: Using spoligotyping and MIRU-VNTR to characterize multidrug resistant strains. J Infect Public Health. 2011;4:41–7. doi: 10.1016/j.jiph.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Hu Y, Mathema B, Jiang W, Kreiswirth B, Wang W, Xu B. Transmission pattern of drug-resistant tuberculosis and its implication for tuberculosis control in eastern rural China. PLoS One. 2011;6:e19548. doi: 10.1371/journal.pone.0019548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai CC, Tan CK, Lin SH, Liao CH, Huang YT, Chou CH, et al. Clinical and genotypic characteristics of extensively drug-resistant and multidrug-resistant tuberculosis. Eur J Clin Microbiol Infect Dis. 2010;29:597–600. doi: 10.1007/s10096-010-0874-6. [DOI] [PubMed] [Google Scholar]
- 23.Aristimuño L, Armengol R, Cebollada A, España M, Guilarte A, Lafoz C, et al. Molecular characterisation of Mycobacterium tuberculosis isolates in the first national survey of anti-tuberculosis drug resistance from Venezuela. BMC Microbiol. 2006;6:90. doi: 10.1186/1471-2180-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas SK, Iravatham CC, Moni BH, Kumar A, Archana BV, Majid M, et al. Modern and ancestral genotypes of Mycobacterium tuberculosis from Andhra Pradesh, India. PLoS One. 2011;6:e27584. doi: 10.1371/journal.pone.0027584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nava-Aguilera E, López-Vidal Y, Harris E, Morales-Pérez A, Mitchell S, Flores-Moreno M, et al. Clustering of Mycobacterium tuberculosis cases in Acapulco: Spoligotyping and risk factors. Clin Dev Immunol 2011. 2011 doi: 10.1155/2011/408375. 408375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viegas SO, Machado A, Groenheit R, Ghebremichael S, Pennhag A, Gudo PS, et al. Molecular diversity of Mycobacterium tuberculosis isolates from patients with pulmonary tuberculosis in Mozambique. BMC Microbiol. 2010;10:195. doi: 10.1186/1471-2180-10-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lari N, Rindi L, Sola C, Bonanni D, Rastogi N, Tortoli E, et al. Genetic diversity, determined on the basis of katG463 and gyrA95 polymorphisms, Spoligotyping, and IS6110 typing, of Mycobacterium tuberculosis complex isolates from Italy. J Clin Microbiol. 2005;43:1617–24. doi: 10.1128/JCM.43.4.1617-1624.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millet J, Miyagi-Shiohira C, Yamane N, Sola C, Rastogi N. Assessment of mycobacterial interspersed repetitive unit-QUB markers to further discriminate the Beijing genotype in a population-based study of the genetic diversity of Mycobacterium tuberculosis clinical isolates from Okinawa, Ryukyu Islands, Japan. J Clin Microbiol. 2007;45:3606–15. doi: 10.1128/JCM.00348-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merza MA, Farnia P, Salih AM, Masjedi MR, Velayati AA. The most predominant spoligopatterns of Mycobacterium tuberculosis isolates among Iranian, Afghan-immigrant, Pakistani and Turkish tuberculosis patients: A comparative analysis. Chemotherapy. 2010;56:248–57. doi: 10.1159/000316846. [DOI] [PubMed] [Google Scholar]
- 30.Dong H, Liu Z, Lv B, Zhang Y, Liu J, Zhao X, et al. Spoligotypes of Mycobacterium tuberculosis from different Provinces of China. J Clin Microbiol. 2010;48:4102–6. doi: 10.1128/JCM.00549-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ojo OO, Sheehan S, Corcoran DG, Nikolayevsky V, Brown T, O’Sullivan M, et al. Molecular epidemiology of Mycobacterium tuberculosis clinical isolates in Southwest Ireland. Infect Genet Evol. 2010;10:1110–6. doi: 10.1016/j.meegid.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Niemann S, Diel R, Khechinashvili G, Gegia M, Mdivani N, Tang YW. Mycobacterium tuberculosis Beijing lineage favors the spread of multidrug-resistant tuberculosis in the Republic of Georgia. J Clin Microbiol. 2010;48:3544–50. doi: 10.1128/JCM.00715-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abadia E, Zhang J, dos Vultos T, Ritacco V, Kremer K, Aktas E, et al. Resolving lineage assignation on Mycobacterium tuberculosis clinical isolates classified by spoligotyping with a new high-throughput 3R SNPs based method. Infect Genet Evol. 2010;10:1066–74. doi: 10.1016/j.meegid.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Kisa O, Albay A, Baylan O, Tozkoparan E, Acikel CH, Doganci L. Genetic diversity of Mycobacterium tuberculosis isolates at the Military medical Academy in Ankara, Turkey. Res Microbiol. 2007;158:318–23. doi: 10.1016/j.resmic.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Gomes HM, Elias AR, Oelemann MA, Pereira MA, Montes FF, Marsico AG, et al. Spoligotypes of Mycobacterium tuberculosis complex isolates from patients residents of 11 states of Brazil. Infect Genet Evol. 2012;12:649–56. doi: 10.1016/j.meegid.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 36.Ignatova A, Dubiley S, Stepanshina V, Shemyakin I. Predominance of multi-drug-resistant LAM and Beijing family strains among Mycobacterium tuberculosis isolates recovered from prison inmates in Tula Region, Russia. J Med Microbiol. 2006;55:1413–8. doi: 10.1099/jmm.0.46575-0. [DOI] [PubMed] [Google Scholar]
- 37.Miranda SS, Carvalho Wda S, Suffys PN, Kritski AL, Oliveira M, Zarate N, et al. Spoligotyping of clinical Mycobacterium tuberculosis isolates from the state of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 2011;106:267–73. doi: 10.1590/s0074-02762011000300003. [DOI] [PubMed] [Google Scholar]
- 38.Al Hajoj S, Rastogi N. The emergence of Beijing genotype of Mycobacterium tuberculosis in the Kingdom of Saudi Arabia. Ann Thorac Med. 2010;5:149–52. doi: 10.4103/1817-1737.65045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molina-Torres CA, Moreno-Torres E, Ocampo-Candiani J, Rendon A, Blackwood K, Kremer K, et al. Mycobacterium tuberculosis spoligotypes in Monterrey, Mexico. J Clin Microbiol. 2010;48:448–55. doi: 10.1128/JCM.01894-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mokrousov I, Otten T, Zozio T, Turkin E, Nazemtseva V, Sheremet A, et al. At Baltic crossroads: A molecular snapshot of Mycobacterium tuberculosis population diversity in Kaliningrad, Russia. FEMS Immunol Med Microbiol. 2009;55:13–22. doi: 10.1111/j.1574-695X.2008.00470.x. [DOI] [PubMed] [Google Scholar]
- 41.Farnia P, Masjedi MR, Varahram M, Mirsaeidi M, Ahmadi M, Khazampour M, et al. The recent-transmission of Mycobacterium tuberculosis strains among Iranian and Afghan relapse cases: A DNA-fingerprinting using RFLP and spoligotyping. BMC Infect Dis. 2008;8:109. doi: 10.1186/1471-2334-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.David S, Portugal C, Antunes A, Cardoso A, Calado A, Barros V, et al. Molecular identification using Spoligotyping of strains from the Mycobacterium tuberculosis complex isolated from the hospital Fernando Fonseca. Rev Port Pneumol. 2004;10:195–204. doi: 10.1016/s0873-2159(15)30581-x. [DOI] [PubMed] [Google Scholar]
- 43.Groenheit R, Ghebremichael S, Svensson J, Rabna P, Colombatti R, Riccardi F, et al. The Guinea-Bissau family of Mycobacterium tuberculosis complex revisited. PLoS One. 2011;6:e18601. doi: 10.1371/journal.pone.0018601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Crevel R, Parwati I, Sahiratmadja E, Marzuki S, Ottenhoff TH, Netea MG, et al. Infection with Mycobacterium tuberculosis Beijing genotype strains is associated with polymorphisms in SLC11A1/NRAMP1 in Indonesian patients with tuberculosis. J Infect Dis. 2009;200:1671–4. doi: 10.1086/648477. [DOI] [PubMed] [Google Scholar]
- 45.Lipin MY, Stepanshina VN, Shemyakin IG, Shinnick TM. Association of specific mutations in katG, rpoB, rpsL and rrs genes with spoligotypes of multidrug-resistant Mycobacterium tuberculosis isolates in Russia. Clin Microbiol Infect. 2007;13:620–6. doi: 10.1111/j.1469-0691.2007.01711.x. [DOI] [PubMed] [Google Scholar]
- 46.David S, Ribeiro DR, Antunes A, Portugal C, Sancho L, de Sousa JG. Contribution of spoligotyping to the characterization of the population structure of Mycobacterium tuberculosis isolates in Portugal. Infect Genet Evol. 2007;7:609–17. doi: 10.1016/j.meegid.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Valcheva V, Mokrousov I, Narvskaya O, Rastogi N, Markova N. Utility of new 24-locus variable-number tandem-repeat typing for discriminating Mycobacterium tuberculosis clinical isolates collected in Bulgaria. J Clin Microbiol. 2008;46:3005–11. doi: 10.1128/JCM.00437-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Otlu B, Durmaz R, Gunal S, Sola C, Zozio T, Rastogi N. Beijing/W and major spoligotype families of Mycobacterium tuberculosis strains isolated from tuberculosis patients in Eastern Turkey. New Microbiol. 2009;32:255–63. [PubMed] [Google Scholar]
- 49.Chuang PC, Liu H, Sola C, Chen YM, Jou R. Spoligotypes of Mycobacterium tuberculosis isolates of a high tuberculosis burden aboriginal township in Taiwan. Infect Genet Evol. 2008;8:553–7. doi: 10.1016/j.meegid.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Yeboah-Manu D, Asante-Poku A, Bodmer T, Stucki D, Koram K, Bonsu F, et al. Genotypic diversity and drug susceptibility patterns among M. tuberculosis complex isolates from South-Western Ghana. PLoS One. 2011;6:e21906. doi: 10.1371/journal.pone.0021906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aktas E, Zozio T, Cömert FB, Külah C, Aydin O, Rastogi N, et al. A first insight into the genetic diversity and population structure of Mycobacterium tuberculosis in Zonguldak, Turkey. Clin Microbiol Infect. 2008;14:55–9. doi: 10.1111/j.1469-0691.2007.01882.x. [DOI] [PubMed] [Google Scholar]
- 52.Bicmen C, Esen N, Graviss EA, Williams-Bouyer N, Ramaswamy SV, Yulug N. Molecular characterization of Mycobacterium tuberculosis isolates from Izmir, Turkey. New Microbiol. 2007;30:229–40. [PubMed] [Google Scholar]
- 53.López-Calleja AI, Gavín P, Lezcano MA, Vitoria MA, Iglesias MJ, Guimbao J, et al. Unsuspected and extensive transmission of a drug-susceptible Mycobacterium tuberculosis strain. BMC Pulm Med. 2009;9:3. doi: 10.1186/1471-2466-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferdinand S, Sola C, Chanteau S, Ramarokoto H, Rasolonavalona T, Rasolofo-Razanamparany V, et al. A study of spoligotyping-defined Mycobacterium tuberculosis clades in relation to the origin of peopling and the demographic history in Madagascar. Infect Genet Evol. 2005;5:340–8. doi: 10.1016/j.meegid.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Dou HY, Tseng FC, Lin CW, Chang JR, Sun JR, Tsai WS, et al. Molecular epidemiology and evolutionary genetics of Mycobacterium tuberculosis in Taipei. BMC Infect Dis. 2008;8:170. doi: 10.1186/1471-2334-8-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shemiakin IG, Stepanshina VN, Korobova OV, Anisimova VA, Ivanov IIu, Lipin MIu, et al. Genetic typing of Mycobacterium tuberculosis strains by spoligotyping and genome fingerprinting techniques. Zh Mikrobiol Epidemiol Immunobiol. 2002:30–5. [PubMed] [Google Scholar]
- 57.Dou HY, Tseng FC, Lu JJ, Jou R, Tsai SF, Chang JR, et al. Associations of Mycobacterium tuberculosis genotypes with different ethnic and migratory populations in Taiwan. Infect Genet Evol. 2008;8:323–30. doi: 10.1016/j.meegid.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 58.Homolka S, Köser C, Archer J, Rüsch-Gerdes S, Niemann S. Single-nucleotide polymorphisms in Rv2629 are specific for Mycobacterium tuberculosis genotypes Beijing and Ghana but not associated with rifampin resistance. J Clin Microbiol. 2009;47:223–6. doi: 10.1128/JCM.01237-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doustdar F, Khosravi AD, Farnia P. Mycobacterium tuberculosis genotypic diversity in pyrazinamide-resistant isolates of Iran. Microb Drug Resist. 2009;15:251–6. doi: 10.1089/mdr.2009.0066. [DOI] [PubMed] [Google Scholar]
- 60.Durmaz R, Zozio T, Gunal S, Allix C, Fauville-Dufaux M, Rastogi N. Population-based molecular epidemiological study of tuberculosis in Malatya, Turkey. J Clin Microbiol. 2007;45:4027–35. doi: 10.1128/JCM.01308-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosales S, Pineda-García L, Ghebremichael S, Rastogi N, Hoffner SE. Molecular diversity of Mycobacterium tuberculosis isolates from patients with tuberculosis in Honduras. BMC Microbiol. 2010;10:208. doi: 10.1186/1471-2180-10-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jagielski T, Augustynowicz-Kopec E, Zozio T, Rastogi N, Zwolska Z. Spoligotype-based comparative population structure analysis of multidrug-resistant and isoniazid-monoresistant Mycobacterium tuberculosis complex clinical isolates in Poland. J Clin Microbiol. 2010;48:3899–909. doi: 10.1128/JCM.00572-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Candia N, Lopez B, Zozio T, Carrivale M, Diaz C, Russomando G, et al. First insight into Mycobacterium tuberculosis genetic diversity in Paraguay. BMC Microbiol. 2007;7:75. doi: 10.1186/1471-2180-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang CW, Wu MH, Chuang PC, Jou R. Characteristics of multidrug-resistant Mycobacterium tuberculosis in Taiwan: A population-based study. Infect Genet Evol. 2011;11:633–9. doi: 10.1016/j.meegid.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 65.Ramazanzadeh R, Farnia P, Amirmozafari N, Ghazi F, Ghadertotonchi Z, Kamran J, et al. Comparison between molecular epidemiology, geographical regions and drug resistance in Mycobacterium tuberculosis strains isolated from Iranian and Afghan patients. Chemotherapy. 2006;52:316–20. doi: 10.1159/000095971. [DOI] [PubMed] [Google Scholar]
- 66.Lari N, Rindi L, Bonanni D, Rastogi N, Sola C, Tortoli E, et al. Three-year longitudinal study of genotypes of Mycobacterium tuberculosis isolates in Tuscany, Italy. J Clin Microbiol. 2007;45:1851–7. doi: 10.1128/JCM.00170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang SF, Su WJ, Dou HY, Feng JY, Lee YC, Huang RM, et al. Association of Mycobacterium tuberculosis genotypes and clinical and epidemiological features – a multi-center study in Taiwan. Infect Genet Evol. 2012;12:28–37. doi: 10.1016/j.meegid.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Garzelli C, Lari N, Cuccu B, Tortoli E, Rindi L. Impact of immigration on tuberculosis in a low-incidence area of Italy: A molecular epidemiological approach. Clin Microbiol Infect. 2010;16:1691–7. doi: 10.1111/j.1469-0691.2009.03149.x. [DOI] [PubMed] [Google Scholar]
- 69.Lari N, Rindi L, Cristofani R, Rastogi N, Tortoli E, Garzelli C. Association of Mycobacterium tuberculosis complex isolates of BOVIS and Central Asian (CAS) genotypic lineages with extrapulmonary disease. Clin Microbiol Infect. 2009;15:538–43. doi: 10.1111/j.1469-0691.2009.02712.x. [DOI] [PubMed] [Google Scholar]
- 70.Brudey K, Driscoll JR, Rigouts L, Prodinger WM, Gori A, Al-Hajoj SA, et al. Mycobacterium tuberculosis complex genetic diversity: Mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 2006;6:23. doi: 10.1186/1471-2180-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brudey K, Gordon M, Moström P, Svensson L, Jonsson B, Sola C, et al. Molecular epidemiology of Mycobacterium tuberculosis in western Sweden. J Clin Microbiol. 2004;42:3046–51. doi: 10.1128/JCM.42.7.3046-3051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Doustdar F, Khosravi AD, Farnia P, Masjedi MR, Velayati AA. Molecular analysis of isoniazid resistance in different genotypes of Mycobacterium tuberculosis isolates from Iran. Microb Drug Resist. 2008;14:273–9. doi: 10.1089/mdr.2008.0842. [DOI] [PubMed] [Google Scholar]
- 73.Vasankari T, Soini H, Liippo K, Ruutu P. MDR-TB in Finland – still rare despite the situation in our neighbouring countries. Clin Respir J. 2012;6:35–9. doi: 10.1111/j.1752-699X.2011.00242.x. [DOI] [PubMed] [Google Scholar]
- 74.Cerezo I, Jiménez Y, Hernandez J, Zozio T, Murcia MI, Rastogi N. A first insight on the population structure of Mycobacterium tuberculosis complex as studied by spoligotyping and MIRU-VNTRs in Bogotá, Colombia. Infect Genet Evol. 2012;12:657–63. doi: 10.1016/j.meegid.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 75.Tajeddin E, Farnia P, Kargar M, Noroozi J, Ahmadi M, Kazempour M, et al. Identification of Mycobacterium tuberculosis beijing genotype using three different molecular methods Koomesh. J Sem Univ Med Sci. 2009;11:7–14. [Google Scholar]
- 76.Alonso Rodríguez N, Chaves F, Iñigo J, Bouza E, García de Viedma D, et al. TB Molecular Epidemiology Study Group of Madrid. Transmission permeability of tuberculosis involving immigrants, revealed by a multicentre analysis of clusters. Clin Microbiol Infect. 2009;15:435–42. doi: 10.1111/j.1469-0691.2008.02670.x. [DOI] [PubMed] [Google Scholar]
- 77.Roetzer A, Schuback S, Diel R, Gasau F, Ubben T, di Nauta A, et al. Evaluation of Mycobacterium tuberculosis typing methods in a 4-year study in Schleswig-Holstein, Northern Germany. J Clin Microbiol. 2011;49:4173–8. doi: 10.1128/JCM.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Farnia P, Masjedi MR, Mirsaeidi M, Mohammadi F, Jallaledin-Ghanavi, Vincent V, et al. Prevalence of Haarlem I and Beijing types of Mycobacterium tuberculosis strains in Iranian and Afghan MDR-TB patients. J Infect. 2006;53:331–6. doi: 10.1016/j.jinf.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 79.Ramazanzadeh R, Farnia P, Amirmozafari N. Characterization of Mycobacterium tuberculosis complex isolated from iranian and afghani patients by spoligotyping method. Braz J Microbiol. 2009;40:314–20. doi: 10.1590/S1517-838220090002000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oelemann MC, Diel R, Vatin V, Haas W, Rüsch-Gerdes S, Locht C, et al. Assessment of an optimized mycobacterial interspersed repetitive- unit-variable-number tandem-repeat typing system combined with spoligotyping for population-based molecular epidemiology studies of tuberculosis. J Clin Microbiol. 2007;45:691–7. doi: 10.1128/JCM.01393-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Velayati AA, Masjedi MR, Farnia P, Tabarsi P, Ghanavi J, Ziazarifi AH, et al. Emergence of new forms of totally drug-resistant tuberculosis bacilli: Super extensively drug-resistant tuberculosis or totally drug-resistant strains in iran. Chest. 2009;136:420–5. doi: 10.1378/chest.08-2427. [DOI] [PubMed] [Google Scholar]
- 82.Ködmön C, Niemann S, Lukács J, Sör E, Dávid S, Somoskövi A. Molecular epidemiology of drug-resistant tuberculosis in Hungary. J Clin Microbiol. 2006;44:4258–61. doi: 10.1128/JCM.01254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geneva, Switzerland: WHO; 2011. World Health Organization (WHO). Global Tuberculosis Control: WHO report 2011; p. 246. [Google Scholar]
- 84.Kumar V, Abbas AK, Fausto N, Mitchell R. 8th ed. United States: Saunders/Elsevier; 2007. Robbins Basic Pathology; pp. 516–22. [Google Scholar]
- 85.Afanas’ev MV, Ikryannikova LN, Il’ina EN, Kuz’min AV, Larionova EE, Smirnova TG, et al. Molecular typing of Mycobacterium tuberculosis circulated in Moscow, Russian Federation. Eur J Clin Microbiol Infect Dis. 2011;30:181–91. doi: 10.1007/s10096-010-1067-z. [DOI] [PubMed] [Google Scholar]
- 86.Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: The role of risk factors and social determinants. Soc Sci Med. 2009;68:2240–6. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 87.Albanna AS, Reed MB, Kotar KV, Fallow A, McIntosh FA, Behr MA, et al. Reduced transmissibility of East African Indian strains of Mycobacterium tuberculosis. PLoS One. 2011;6:e25075. doi: 10.1371/journal.pone.0025075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Durmaz R, Zozio T, Gunal S, Yaman A, Cavusoglu C, Guney C, et al. Genetic diversity and major spoligotype families of drug-resistant Mycobacterium tuberculosis clinical isolates from different regions of Turkey. Infect Genet Evol. 2007;7:513–9. doi: 10.1016/j.meegid.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 89.Diab AM, Al-Turk IM. ERIC and RAPD PCR-based DNA fingerprinting techniques application for microbial source tracking (MST) at Al-Madinah Al-Munwwarah, KSA. J Taibah Univ Sci. 2011;5:31–8. [Google Scholar]
- 90.Allix C, Walravens K, Saegerman C, Godfroid J, Supply P, Fauville-Dufaux M. Evaluation of the epidemiological relevance of variable-number tandem-repeat genotyping of Mycobacterium bovis and comparison of the method with IS6110 restriction fragment length polymorphism analysis and spoligotyping. J Clin Microbiol. 2006;44:1951–62. doi: 10.1128/JCM.01775-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shabbeer A, Ozcaglar C, Yener B, Bennett KP. Web tools for molecular epidemiology of tuberculosis. Infect Genet Evol. 2012;12:767–81. doi: 10.1016/j.meegid.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 92.Amirmozafari N, Ramezanzadeh R, Farnia P, Ghazi F. The frequency of beijing genotype of Mycobacterium tuberculosis isolated from tuberculosis patients. Razi J Med Sci. 2006;13:7–17. [Google Scholar]
- 93.Brudey K, Filliol I, Sola C, Bébéar C, Elia-Pasquet S, Texier-Maugein J, et al. Molecular characterization and biodiversity of Mycobacterium tuberculosis in the Antilles-Guiana region and comparative analysis in a metropolitan region, Aquitaine. Pathol Biol (Paris) 2003;51:282–9. doi: 10.1016/s0369-8114(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 94.Bazira J, Asiimwe BB, Joloba ML, Bwanga F, Matee MI. Mycobacterium tuberculosis spoligotypes and drug susceptibility pattern of isolates from tuberculosis patients in South-Western Uganda. BMC Infect Dis. 2011;11:81. doi: 10.1186/1471-2334-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Augustynowicz-Kopec E, Jagielski T, Zwolska Z. Genetic diversity of isoniazid-resistant Mycobacterium tuberculosis isolates collected in Poland and assessed by spoligotyping. J Clin Microbiol. 2008;46:4041–4. doi: 10.1128/JCM.01315-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bifani PJ, Mathema B, Kurepina NE, Kreiswirth BN. Global dissemination of the Mycobacterium tuberculosis W-Beijing family strains. Trends Microbiol. 2002;10:45–52. doi: 10.1016/s0966-842x(01)02277-6. [DOI] [PubMed] [Google Scholar]


