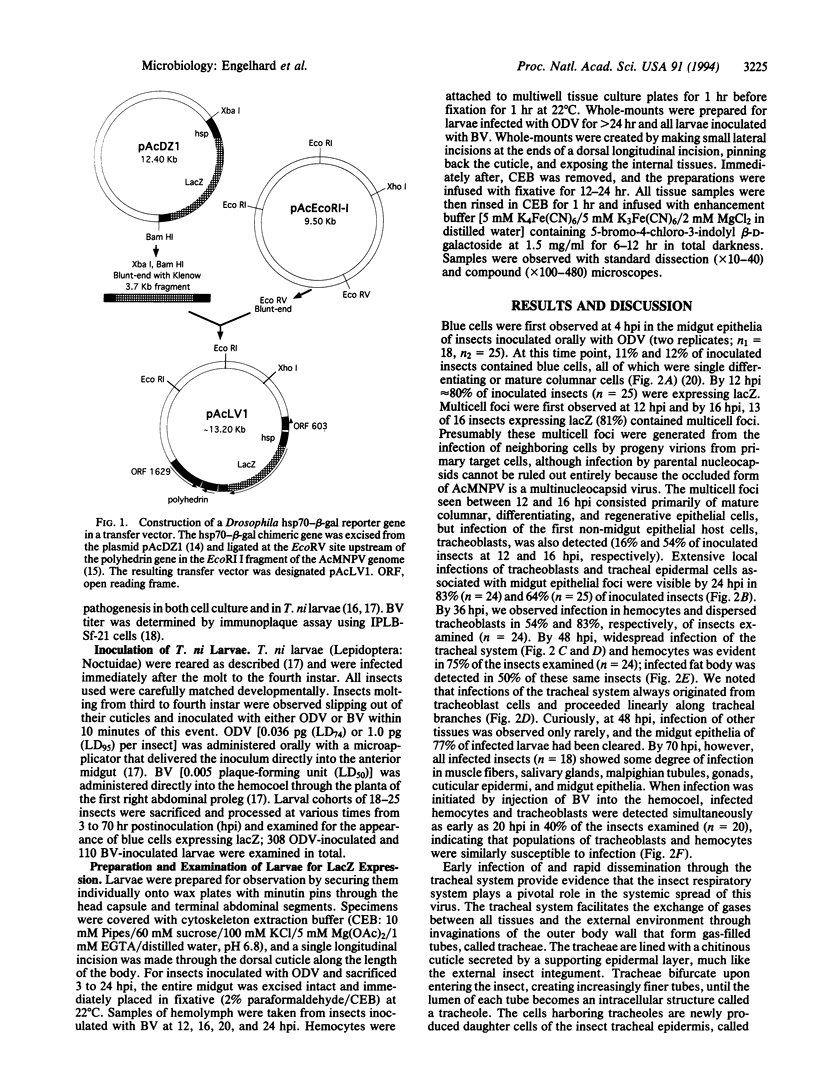
Abstract
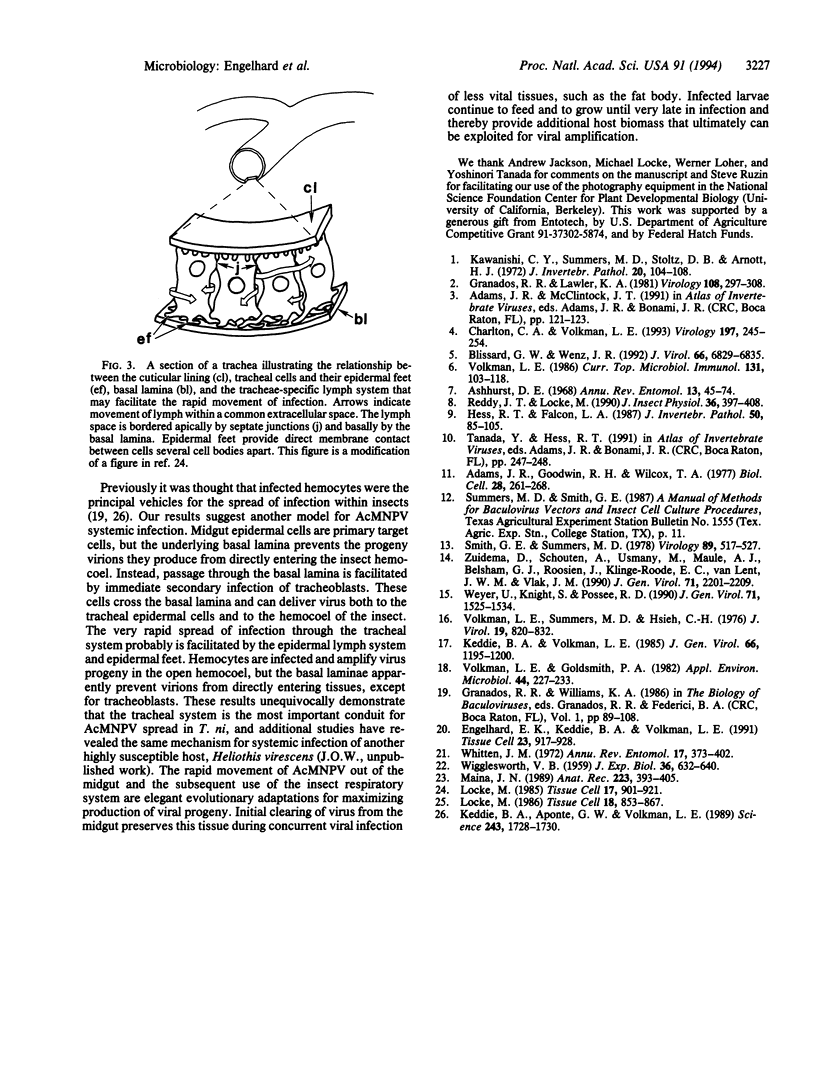
Baculoviruses establish systemic infections within susceptible insect hosts, even though host tissues are surrounded by basal laminae, extracellular matrices that exclude particles smaller than these viruses. Using a recombinant Autographa californica M nuclear polyhedrosis virus containing a lacZ reporter gene under the control of a constitutive promoter, we followed the progression of infection in Trichoplusia ni larvae. We discovered that infection of the larval insect tracheal system (and not hemocytes, as thought previously) provides the major conduit for this virus to pass through basal laminae and to spread throughout the host. Tracheal epidermal cells, the only known cellular components of the tracheal system, share a common lymph system. Locally these cells contact one another by interdigitating cytoplasmic extensions called epidermal feet. These two features of the tracheal system are likely to facilitate the rapid systemic spread of the virus. The findings reported here have major implications for the fields of insect pathology and biological control and usher in an important consideration regarding host-range factors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blissard G. W., Wenz J. R. Baculovirus gp64 envelope glycoprotein is sufficient to mediate pH-dependent membrane fusion. J Virol. 1992 Nov;66(11):6829–6835. doi: 10.1128/jvi.66.11.6829-6835.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton C. A., Volkman L. E. Penetration of Autographa californica nuclear polyhedrosis virus nucleocapsids into IPLB Sf 21 cells induces actin cable formation. Virology. 1993 Nov;197(1):245–254. doi: 10.1006/viro.1993.1585. [DOI] [PubMed] [Google Scholar]
- Kawanishi C. Y., Summers M. D., Stoltz D. B., Arnott H. J. Entry of an insect virus in vivo by fusion of viral envelope and microvillus membrane. J Invertebr Pathol. 1972 Jul;20(1):104–108. doi: 10.1016/0022-2011(72)90088-2. [DOI] [PubMed] [Google Scholar]
- Keddie B. A., Aponte G. W., Volkman L. E. The pathway of infection of Autographa californica nuclear polyhedrosis virus in an insect host. Science. 1989 Mar 31;243(4899):1728–1730. doi: 10.1126/science.2648574. [DOI] [PubMed] [Google Scholar]
- Maina J. N. Scanning and transmission electron microscopic study of the tracheal air sac system in a grasshopper Chrotogonus senegalensis (Kraus)--Orthoptera: Acrididae: Pyrgomorphinae. Anat Rec. 1989 Apr;223(4):393–405. doi: 10.1002/ar.1092230408. [DOI] [PubMed] [Google Scholar]
- Volkman L. E., Goldsmith P. A. Generalized Immunoassay for Autographa californica Nuclear Polyhedrosis Virus Infectivity In Vitro. Appl Environ Microbiol. 1982 Jul;44(1):227–233. doi: 10.1128/aem.44.1.227-233.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman L. E., Summers M. D., Hsieh C. H. Occluded and nonoccluded nuclear polyhedrosis virus grown in Trichoplusia ni: comparative neutralization comparative infectivity, and in vitro growth studies. J Virol. 1976 Sep;19(3):820–832. doi: 10.1128/jvi.19.3.820-832.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman L. E. The 64K envelope protein of budded Autographa californica nuclear polyhedrosis virus. Curr Top Microbiol Immunol. 1986;131:103–118. doi: 10.1007/978-3-642-71589-1_6. [DOI] [PubMed] [Google Scholar]
- Weyer U., Knight S., Possee R. D. Analysis of very late gene expression by Autographa californica nuclear polyhedrosis virus and the further development of multiple expression vectors. J Gen Virol. 1990 Jul;71(Pt 7):1525–1534. doi: 10.1099/0022-1317-71-7-1525. [DOI] [PubMed] [Google Scholar]
- Zuidema D., Schouten A., Usmany M., Maule A. J., Belsham G. J., Roosien J., Klinge-Roode E. C., van Lent J. W., Vlak J. M. Expression of cauliflower mosaic virus gene I in insect cells using a novel polyhedrin-based baculovirus expression vector. J Gen Virol. 1990 Oct;71(Pt 10):2201–2209. doi: 10.1099/0022-1317-71-10-2201. [DOI] [PubMed] [Google Scholar]



