Abstract
Endocannabinoids, such as N-arachidonylethanolamine (AEA, also called anandamide), exert potent analgesic and anti-inflammatory effects. Fatty acid amide hydrolase (FAAH) is primarily responsible for degradation of AEA, and deletion of FAAH increases AEA content in various tissues. Since FAAH has been shown to be present in the bladder of various species, we compared bladder function, severity of experimental cystitis, and cystitis-associated referred hyperalgesia in male wild type (WT) and FAAH knock-out (KO) mice. Basal concentrations of AEA were greater, and the severity of cyclophosphamide (CYP)-induced cystitis was reduced in bladders from FAAH KO compared to WT mice. Cystitis-associated increased peripheral sensitivity to mechanical stimuli and enhanced bladder activity (as reflected by increased voiding frequency) were attenuated in FAAH KO compared to WT mice. Further, abundances of mRNA for several pro-inflammatory compounds were increased in bladder mucosa after CYP treatment of WT mice, and this increase was inhibited in FAAH KO mice. These data indicate that endogenous substrates of FAAH, including the cannabinoid AEA, play an inhibitory role in bladder inflammation and subsequent changes in pain perception. Therefore, FAAH could be a therapeutic target to treat clinical symptoms of painful inflammatory bladder diseases.
Keywords: Fatty acid amide hydrolase, N-arachidonylethanolamine, Cystitis, Pain, Mice
Introduction
Visceral pain is the most debilitating symptom of painful bladder syndrome (PBS), which affects 7–8 million patients/year in the U.S. alone (Selo-Ojeme and Onwude, 2004; Stanford et al., 2007; van de Merwe et al., 2008; Schrepf et al., 2014). Little is known about pathophysiological mechanisms underlying this disorder, and no treatment or combination of treatments is consistently effective in alleviating symptoms in PBS patients (Selo-Ojeme and Onwude, 2004; Stanford et al., 2007; van de Merwe et al., 2008; Schrepf et al., 2014). Endocannabinoids, such as N-arachidonylethanolamine (AEA, also called anandamide), exert potent analgesic and anti-inflammatory effects (Alvarez-Jaimes and Palmer, 2011; Maione et al., 2013). Fatty acid amide hydrolase (FAAH) is primarily responsible for degradation of AEA, and the therapeutic potential of enhancing the analgesic effects of endogenous cannabinoids by decreasing their degradation has been recognized (Cravatt and Lichtman, 2003; Jhaveri et al., 2007; Schlosburg et al., 2009; Bisogno and Maccarrone, 2013; Blankman and Cravatt, 2013). Pharmacological inhibition and genetic deletion of FAAH increases AEA content in various tissues, and FAAH knock-out (KO) mice have significantly reduced inflammation and hyperalgesia in response to injection of cargageenin or complete Freund’s adjuvant into the paw, as well as significantly increased analgesia in response to exogenous AEA compared to wild-type (WT) mice (Lichtman et al., 2004; Wise et al., 2007; Schlosburg et al., 2009; Anderson et al., 2014). These observations suggest that endocannabinoids could be useful to suppress clinical symptoms of painful inflammatory bladder diseases. However, the effects of experimentally-induced cystitis have not been investigated in FAAH KO mice.
FAAH mRNA and protein have been detected in human, mouse, and rat urinary bladders (Merriam et al., 2011; Strittmatter et al., 2012; Bakali et al., 2013; Hedlund, 2014). Treatment with a FAAH inhibitor increased voiding intervals, voiding volume and bladder capacity in normal rats via activation of cannabinoid 2 (CB2) receptors (Strittmatter et al., 2012) and rats with bladder overactivity (Gandaglia et al., 2013). Furthermore, FAAH inhibition attenuated afferent activity induced by bladder distension via activation of both cannabinoid 1 (CB1) and CB2 (Aizawa et al., 2014) and suppressed referred hyperalgesia associated with bladder inflammation (Merriam et al., 2011). Collectively, these findings suggest a functional role of FAAH in regulating bladder function and pain sensation during physiological as well as pathophysiological processes through its ability to regulate cannabinoid receptor ligand concentration (Hedlund, 2014; Vizzard, 2014).
Cyclophosphamide (CYP) is an antineoplastic alkylating agent commonly used to treat cancer patients, and an undesirable clinical side effect of CYP is hemorrhagic cystitis. CYP is metabolized by the liver to acrolein that is accumulated in urine, and acrolein is primarily responsible for CYP-induced cystitis (Cox, 1979; Conklin et al., 2009). CYP-induced cystitis in rodents is commonly used as an experimental model to study mechanisms underlying cystitis and associated visceral pain (Bon et al., 1998; Cervero and Laird, 2004; Bjorling et al., 2011; Girard et al., 2012). In the present study, we compared severity of experimental cystitis, cystitisassociated altered bladder function, and referred hyperalgesia in male FAAH KO and congenic WT mice.
Materials and Methods
Induction of cystitis
Breeding pairs of FAAH KO mice were generously provided by Dr. Aron Lichtman (Virginia Commonwealth University). FAAH KO mice were viable, fertile, and largely indistinguishable from WT mice in general appearance, body weight, locomotion, or overt behavior (Cravatt et al., 2001). C57BL/6J WT mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Male mice were used at 3–6 months of age. Experiments were conducted in accordance with National Institutes of Health Guidelines, and all protocols were reviewed and approved by the Animal Care and Use Committee of the University of Wisconsin.
Cystitis was induced by intraperitoneal injection of CYP (150 mg/kg). This dose of CYP was chosen based on results of preliminary experiments in WT mice to induce cystitis of moderate severity. Control mice received an equivalent volume of intraperitoneal saline instead of CYP (Wang et al., 2008).
Mice were euthanized at the end of experiments with pentobarbital (100 mg/kg, ip) and perfused with saline through a cannula inserted into the left ventricle followed by 2% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). Bladders were removed, weighed, and bladder weight (mg) was normalized to body weight (g). Tissues were post-fixed in the same fixative for 4 hours at 4° C and cryoprotected with 30% sucrose in PBS at 4° C. Tissue sections were made with a cryostat at a thickness of 10 µm. Every 4th section was stained with hematoxylin and eosin (H & E) for morphological analysis, and a total of 4–6 sections from each bladder were examined microscopically. Since the increased bladder weight correlated closely with severity of edema and changes in bladder weight reliably reflect the severity of cystitis in this model, bladder weights were used for semi-quantitative analysis of the severity of cystitis (Bjorling et al., 2007; Wang et al., 2008).
Plasma extravasation in bladder
Plasma extravasation was determined using Evans blue dye (Bjorling et al., 1999; Merrill et al., 2013). Mice were sacrificed 24 hours after treatment with CYP or saline, and 15 minutes before sacrifice, mice were injected intravenously with Evans blue dye (300 µl/ 30 g body weight, 3 mg/ml prepared in saline). Mice were then euthanized with pentobarbital (100 mg/kg, ip) and perfused with saline through a cannula inserted into the left ventricle. Bladders were removed, blotted dry, weighed, placed in 0.2 ml formamide (Sigma, St. Louis, MO), and incubated for 24 hours at 55° C to extract Evans blue dye. The concentration of dye in formamide was determined by spectrophotometry at 630 nm using a standard curve generated by increasing concentrations of Evans blue dye (0 to 150 µg/ml). Plasma extravasation was expressed as µg dye/mg wet bladder weight.
Measurement of bladder endocannabinoids content
Animals were sacrificed 3, 24, and 48 hours after administration of CYP, and these time points were chosen based on a previous study in rats (Merriam et al., 2011). Mice were euthanized with pentobarbital (100 mg/kg, ip), and bladders were emptied by application of light abdominal compression. Four bladders were pooled and frozen-pulverized as one sample to obtain sufficient tissue for analysis of AEA and 2-arachidonylglycerol (2-AG; another primary endocannabinoid), and four samples (from 16 mice) were included in each group. The samples were stored at −80° C for subsequent analysis. The frozen-pulverized bladders were mixed with acetonitrile containing [2H8]AEA and [2H8]2-AG and were sonicated in a cooled bath for 30 minutes and incubated overnight at −20° C to precipitate proteins. Particulates were removed from acetonitrile by centrifugation, the solvent was dried, and extracted lipids were resuspended in methanol. AEA and 2-AG were quantified using isotope dilution, atmospheric pressure, chemical ionization liquid chromatography/mass spectrometry (LC-APCI-MS) as previously described (Patel et al., 2003; Merriam et al., 2011).
Measurement of short-term voluntary urination
Mice were treated with saline or CYP (150 mg/kg, ip), and urinary frequency was determined 24 hours later. Freely moving male mice were placed on filter papers for 30 minutes to examine short-term voluntary urinary frequency. Filter papers were then examined under UV light at 302 nm, and photoimages were taken (UVP Imaging System, Upland, CA). The number of small-diameter urine spots (<0.2 cm2) was counted to determine frequency of urination as described previously (Birder et al., 2002; Cornelissen et al., 2008; Merrill et al., 2013; Wang et al., 2013). An increase in number of small diameter urine spots has been considered to be suggestive of irritative voiding and to reflect increased urinary frequency (Birder et al., 2002; Cornelissen et al., 2008; Merrill et al., 2013; Wang et al., 2013).
Peripheral nociception testing
The effect of cystitis on response to peripheral application of mechanical stimuli to hind paws was evaluated. Mechanical sensitivity of the hind paws was assessed using von Frey monofilaments and the up-down method (May and Vizzard, 2010; Wang et al., 2013). Mice were placed in individual Plexiglas chambers with a wire mesh floor. Sensitivity of the hind paws was assessed with a series of six Von Frey filaments of increasing stiffness. Stimulus-related withdrawal of the tested paw was considered a withdrawal response, and the 50% paw withdrawal threshold was determined by the non-parametric method of Dixon (May and Vizzard, 2010; Wang et al., 2013). The individual performing nociceptive testing was unaware of the genotype and treatment of mice.
Semi-quantitative analysis of mRNA
CYP treatment-induced increase in mRNA expression of pro-inflammatory compounds is a relatively early event (Malley and Vizzard, 2002). Mice were sacrificed 3 hours after treatment with CYP or saline. Bladder mucosa was separated from detrusor (Klinger et al., 2008; Wang et al., 2009) and prepared for RNA isolation. Total RNA was extracted with Trizol reagent (Invitrogen, Carlsbad, CA) and treated with DNAse I (Ambion, Austin, TX) to remove genomic DNA. First strand cDNA was generated using a cDNA synthesis kit according to the manufacturer's instructions (Invitrogen, Carlsbad, CA).
Real-time PCR was performed using an ABI 7300 (Foster City, CA) thermocycler. Samples were amplified in duplicate using the following thermal cycling conditions: 94° C for 10 minutes, followed by 40–45 cycles of amplification at 94 °C for 30 seconds and then 60° C for 1 minute to allow denaturing and annealing-extension. Abundance of PCR product was determined semi-quantitatively using a standard curve for each gene. Relative abundance of mRNA in the mucosa was assessed for substances that have been reported to play significant roles in pain and inflammation, including nerve growth factor (NGF), cyclooxygenase-2 (COX-2), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS) (Malley and Vizzard, 2002; Wang et al., 2008; Daly et al., 2011; Birder and Andersson, 2013; Keay et al., 2014; Gonzalez et al., 2014). Expression of each gene was normalized to abundance of mRNA for L19, a constitutively-expressed ribosomal protein in the same sample. Fold changes of expression of each gene in CYP-treated WT and saline- and CYP-treated FAAH KO mice were compared to the corresponding gene expression in saline-treated WT animals (set as 1) (Wang et al., 2008; 2013).
Statistical analysis
Data are presented as arithmetic means ± SEM. The data from multiple groups were analyzed using two-way ANOVA followed by Bonferroni post hoc comparisons (GraphPad Prism, San Diego, CA). Unpaired Student’s t-tests were used as appropriate. p values < 0.05 were considered significant.
Results
Cystitis in WT and FAAH KO mice
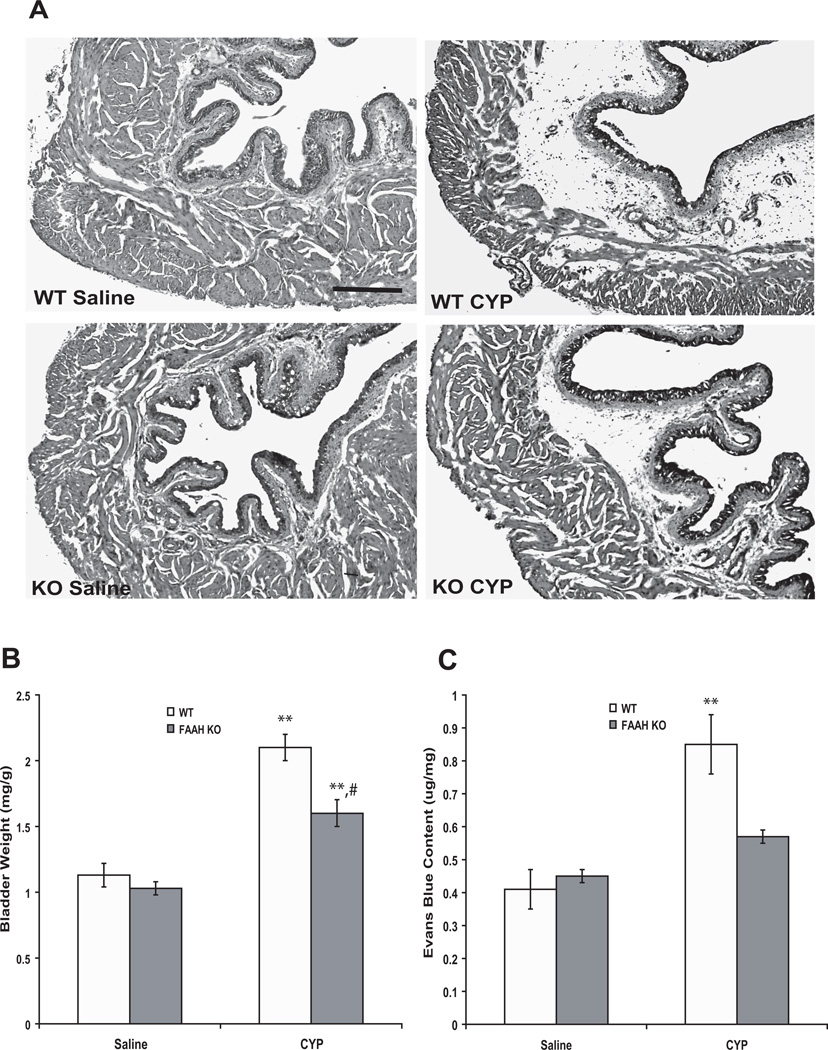
Morphologically, bladders from FAAH KO mice appeared normal and not different from WT mice in saline-treated (control) groups (Fig. 1A). No signs of cystitis were observed in saline-treated animals of either genotype (Fig. 1A). Twenty-four hours after CYP, mean wet bladder weight (mg/g body weight) was increased by 86 % and 55 % relative to saline-treated controls in WT and KO mice, respectively (n = 8, p < 0.01), and the increase was greater in WT than FAAH KO mice (n = 8, p < 0.05) (Fig. 1B). Histological examination of the bladders indicated the presence of cystitis, characterized primarily by edema in the submucosal region (Fig. 1A), and edema appeared to be more severe in WT than in FAAH KO mice. Occasionally, areas of hemorrhage and mild infiltration of inflammatory cells were also observed (Fig. 1A). Treatment with CYP increased plasma extravasation in bladders by 107 % in WT mice (n = 6, p < 0.01 vs saline-treated). Although the plasma extravasation was numerically greater in CYP treated KO mice, this difference did not rise to a significant level (n = 5–6, p > 0.05 vs saline-treated) (Fig. 1C).
Figure 1.
Representative images of bladders from mice of both genotypes treated with saline or CYP (A). CYP (24 hours) induced cystitis characterized primarily by edema in the submucosal region (A). Scale bar indicates 100 µm. Also, CYP increased bladder weight (B, two way ANOVA results: Finteraction = 4.3, p < 0.05; Fgenotype = 63.3, p < 0.0001; Ftreatment = 9.6, p < 0.01) and plasma extravasation (C, two way ANOVA results: Finteraction = 14.2, p < 0.05; Fgenotype = 43.3, p < 0.0001; Ftreatment = 8, p < 0.05). Mean ± S.E.M. n = 5–8 in each group. **: p < 0.01 vs saline-treated group. #: p < 0.05 vs CYP-treated WT mice.
Endocannabinoid contents in bladders
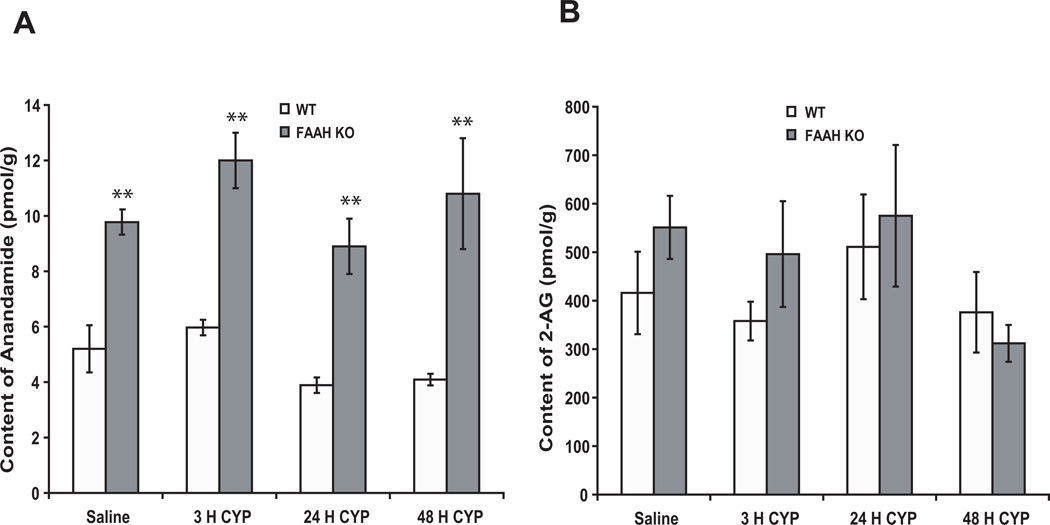
The AEA content was significantly greater in bladders of FAAH KO than WT mice (n = 4, p < 0.01), and treatment with CYP did not significantly alter AEA content in bladders of either WT or FAAH KO mice (Fig. 2A). Bladder content of 2-AG (which is primarily degraded by monoacylglycerol lipase, not FAAH) was similar in both WT and FAAH KO mice, and treatment with CYP did not alter 2-AG content in bladders (Fig. 2B).
Figure 2.
The concentrations of AEA (A) were significantly greater in bladders of KO as compared to WT mice (two way ANOVA results: Finteraction = 1.4, p = 0.7; Fgenotype = 67.6, p < 0.0001; Ftreatment = 7.3, p = 0.09), and treatment with CYP did not significantly alter AEA content in bladders of either WT or KO mice. Bladder 2-AG content was similar in both WT and FAAH KO mice, and treatment with CYP did not alter 2-AG content in bladders (B). Mean ± S.E.M. n = 4 in each group. **: p < 0.01 vs WT mice.
Alterations of bladder function
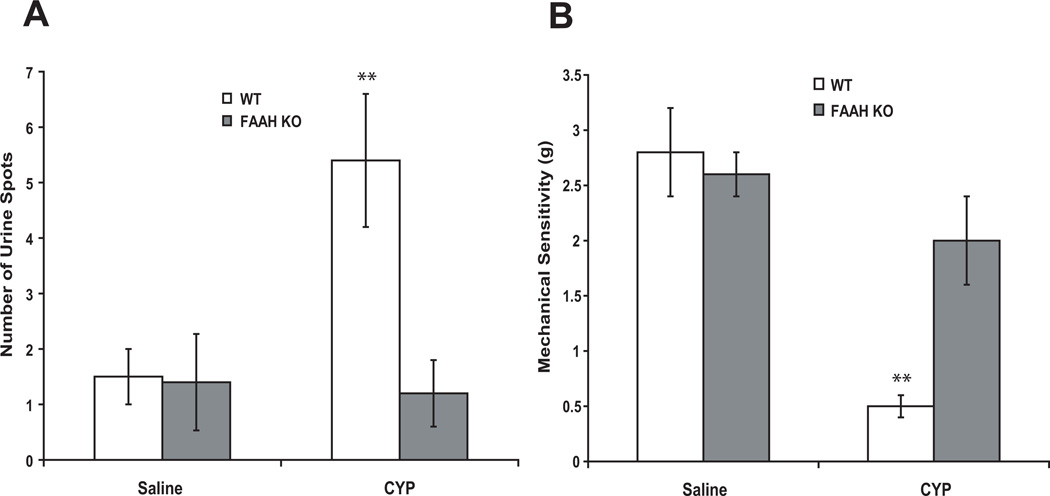
The number of small-diameter urine spots (<0.2 cm2) on filter paper placed under freely moving mice for 30 minutes was similar in saline-treated FAAH KO and WT mice. Twenty-four hours after CYP, the number of urine spots was significantly increased by 241 % in WT (Fig. 3A, n = 6–8, p < 0.01 vs saline-treated), but not in FAAH KO mice (n = 6–8, p > 0.05).
Figure 3.
The number of small diameter urine spot was increased 24 hours after CYP in WT, but not in FAAH KO mice (A, two way ANOVA results: Finteraction = 12.9, p < 0.05; Fgenotype = 14.3, p < 0.05; Ftreatment = 10.3, p < 0.05). Treatment with saline did not affect peripheral mechanical sensitivity (B). However, the mechanical sensitivity threshold was reduced 24 hours after CYP in WT, but not in FAAH KO, mice (B, two way ANOVA results: Finteraction = 12.4, p < 0.01; Fgenotype = 36.2, p < 0.0001; Ftreatment = 7.2, p < 0.05). Mean ± S.E.M. n = 6–8. **: p < 0.01 vs saline treated.
Increase in sensitivity to peripheral mechanical stimuli after CYP treatment
Basal mechanical sensitivity in FAAH KO mice was similar to WT mice (not shown). Treatment with saline did not significantly affect peripheral mechanical sensitivity in either genotype. Peripheral mechanical sensitivity threshold was reduced in WT mice compared to the saline-treated animals 24 hours after treatment with CYP (n =8, p<0.01; Fig. 3B). In FAAH KO mice, treatment with CYP failed to affect peripheral mechanical sensitivity (n =8, p>0.05; Fig. 3B).
Changes in gene expression in bladder mucosa after CYP treatment
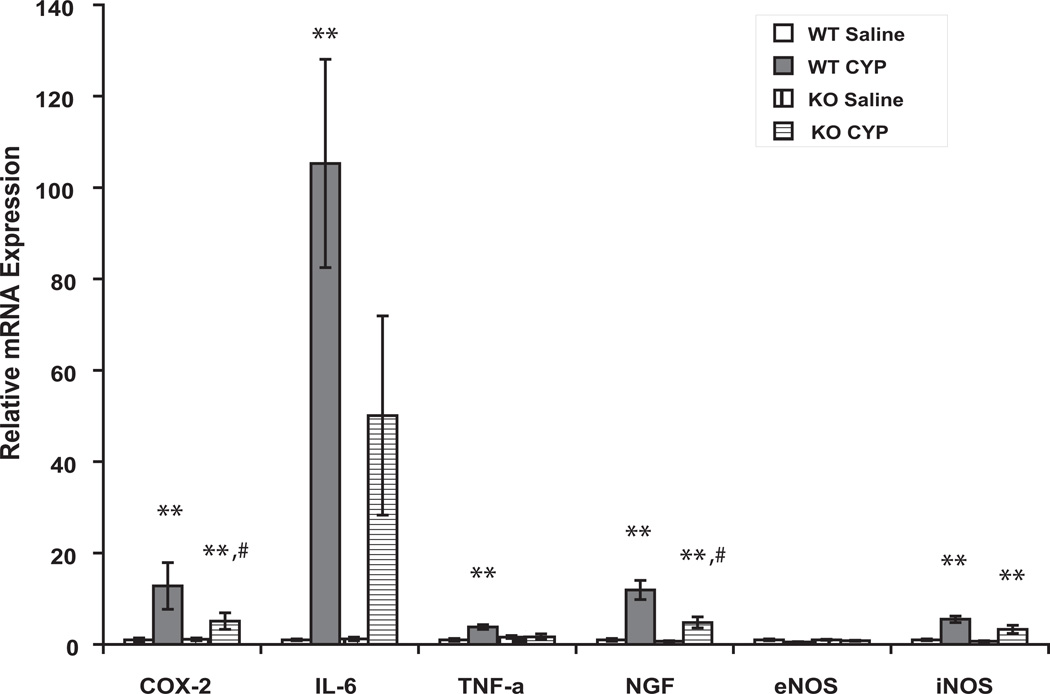
In saline-treated animals, expression of mRNA for NGF, COX-2, eNOS, iNOS, TNF-α and IL-6 was similar in WT and FAAH KO mice. Treatment with CYP for 3 hours augmented expression of NGF, COX-2, iNOS and TNF-α in the mucosa in mice of both genotypes (Fig. 4, n = 6- 8, p < 0.05 or 0.01 vs saline-treated in each genotype). However the increase in abundance of message for NGF and COX-2 was attenuated in FAAH KO mice as compared to WT mice (Fig. 4, n =6–8, p < 0.05 vs CYP-treated WT). Expression of IL-6 mRNA was also significantly increased in CYP treated WT mice (n =6–8, p < 0.01 vs saline-treated). Although IL-6 mRNA was numerically greater in CYP treated KO mice, this difference did not rise to a significant level (n = 5–6, p > 0.05 vs saline- treated).
Figure 4.
Expression of mRNAs of pro-inflammatory compounds 3 hours after CYP. Expression of each gene is normalized to abundance of mRNA for L19, a constitutively-expressed ribosomal protein (house-keeping gene) in the same sample. Fold changes of expression of each gene in CYP-treated WT and saline- and CYP-treated FAAH KO mice were compared to the corresponding gene expression in saline-treated WT animals (set as 1). n = 6–8 for each treatment. *, ** indicate p <0.05 and 0.01, respectively, CYP vs saline for each genotype. # indicates p < 0.05 vs CYP-treated WT mice.
Discussion
In the present study, we found: 1) the concentrations of AEA, but not 2-AG, were greater in bladders from FAAH KO mice than those from WT mice; 2) the severity of CYP-induced cystitis was reduced in FAAH KO mice; 3) cystitis-associated increased peripheral sensitivity to mechanical stimuli was inhibited in FAAH KO mice; 4) inflammation-induced enhanced bladder activity (as reflected by increased voiding frequency) was attenuated in FAAH KO mice; and 5) increased abundances of mRNA for several inflammation-associated compounds in mucosa after CYP treatment were inhibited in FAAH KO mice.
FAAH KO mice have increased AEA concentrations in CNS (brain and spinal cord) and in peripheral tissues (liver) (Cravatt et al., 2001). We provide additional evidence that concentrations of AEA are increased in bladders of FAAH KO mice, although the increase in AEA concentration in bladders was relatively small compared to that observed in brain tissue from FAAH KO mice (Cravatt et al., 2001). Previous studies reported that bladder inflammation increased AEA content in bladders of rats (Dinis et al., 2004; Merriam et al., 2011). In the present study, CYP-induced bladder inflammation did not significantly alter abundance of AEA in the bladders of either WT or FAAH KO mice at the time points examined. This discrepancy in findings may be attributable to differences in experimental design, including variables such as species (mice vs. rats), severity of bladder inflammation, and time lapse after initiation of bladder inflammation. Additionally, we found that the concentrations of 2-AG were similar in both WT and FAAH mice and were not affected by bladder inflammation.
Paw edema induced by injection of lipopolysaccharide or carrageenan was reduced in FAAH KO mice compared to WT mice (Lichtman et al., 2004; Naidu et al., 2010). FAAH KO mice also displayed decreased severity of experimental arthritis and colitis (Storr et al., 2008; Kinsey et al., 2011). Similarly, treatment with the FAAH inhibitors (D’Argenio et al., 2006; Storr et al., 2008; Sałaga et al., 2014) attenuated the severity of experimental colitis (Massa et al., 2004; D'Argenio et al., 2006; Storr et al., 2008; 2009; Salaga et al., 2014a, b). The anti-inflammatory effects observed in FAAH KO mice were primarily mediated by CB2 (Lichtman et al., 2004; Storr et al., 2008; Naidu et al., 2010; Kinsey et al., 2011). Consistent with anti-inflammatory observations associated with FAAH deletion/inhibition in other organs, we observed that the severity of bladder inflammation was reduced in FAAH KO mice. CB2 is present in bladders of various species (Tyagi et al., 2008; Gratzke et al., 2009; 2010; Strittmatter et al., 2012; Hedlund, 2014), and treatment with selective CB2 agonists attenuated bladder inflammation (Wang et al., 2012; 2013; Tambaro et al., 2014). Together, these findings suggest that the elevated concentration of AEA may activate CB2 in bladders, resulting in inhibition of bladder inflammation in FAAH KO mice, although some studies suggest that AEA may be a weak agonist to CB2 (Hillard et al., 1999). Future studies will be needed to clarify the mechanisms underlying activities of AEA in bladders.
Visceral pain is difficult to assess directly (Cervero and Laird, 2004). One hallmark of visceral pain is perception of pain arising from somatic sites distant from the area of visceral injury (referred hyperalgesia) (Bon et al., 1998; Laird et al., 2002; Cervero and Laird, 2004), and this is a relatively common finding in patients with IC/PBS (Tripp et al., 2012). Sensitization of primary afferents by inflammatory mediators at the site of injury plays an important role in development of referred hyperalgesia (Cervero and Laird, 2004). Bladder inflammation increases urination frequency and bladder activity that are also considered as surrogate metrics for assessment of visceral pain related to inflammatory bladder disorders (Cornelissen et al., 2008). FAAH KO mice have been shown to exhibit attenuated inflammatory pain responses that were mediated by CB1, CB2, or both receptors (Cravatt et al., 2001; 2004; Lichtman et al., 2004; Chang et al., 2006; Naidu et al., 2010), and these findings were supported by studies using FAAH inhibitors (Lichtman et al., 2004; Jayamanne et al., 2006; Ahn et al., 2009; Okine et al., 2012; Guindon et al., 2013). In the present study, we demonstrated that bladder inflammation-associated increased urination frequency and enhanced mechanical sensitivity were attenuated in FAAH KO mice. Collectively, our findings provide additional evidence that inhibition/deletion of FAAH suppresses inflammatory visceral pain. It should be noted that urine spot assay is one of many methods used to determine bladder functions and other techniques, such as cytometry, are needed to validate these findings in future studies.
FAAH has been shown to be present in bladders, especially in urothelium, of various species, including human beings (Merriam et al., 2011; Strittmatter et al., 2012; Hedlund, 2014). While urothelium has historically been viewed as a simple barrier separating the bladder wall from urine, increasing evidence also suggests that the urothelial cells play a critical role in physiological and pathophysiological processes in the bladder (Daly et al., 2011; Birder and Andersson, 2013; Hill, 2014; Keay et al., 2014; Gonzalez et al., 2014). Specifically, urothelial cells have the capacity to secrete a variety of signaling molecules such as PGE2, NGF, nitric oxide, and cytokines in response to various stimuli (Malley and Vizzard, 2002; Wang et al., 2008; Birder and Andersson, 2013; Hill, 2014; Keay et al., 2014; Gonzalez et al., 2014). Conceivably, chemical mediators derived from urothelial cells could significantly influence bladder function and pain sensation during bladder inflammation. AEA has been shown to inhibit production and release of pro-inflammatory mediators in various cell types (Cencioni et al., 2010; Clapper et al., 2010; Krishnan and Chatterjee, 2012). Similarly, we found that abundances of mRNA for several pro-inflammatory compounds, such as NGF and COX-2, were increased in bladder mucosa after CYP treatment of WT mice, and this increase was inhibited in FAAH KO mice. It is possible that increased concentrations of AEA due to FAAH deletion inhibit production and release of inflammatory mediators from urothelial cells, reducing severity of cystitis and associated enhanced pain sensation. Further, AEA may also exert a direct inhibitory action on afferent nerves in bladders (Walczak and Cervero, 2011; Aizawa et al., 2014). Therefore, suppression of bladder inflammation and associated increased bladder activity and referred mechanical hyperalgesia in FAAH KO mice may result from the combined effects of reduced inflammation and inhibition of afferent nerve activity. It should be noted that concentrations of AEA are also increased in spinal cord and brain of FAAH KO mice, and these structures are obviously involved in moderating bladder function and pain sensation. It was reported that intrathecal injection of a FAAH inhibitor decreased micturition frequency in normal rats and decreased frequency and bladder pressures in rats with bladder overactivity (Füllhase et al., 2013). Additional studies are needed to examine the regulation of expression of pro-inflammatory compounds at protein level and to further clarify the mechanisms underlying the effects of FAAH deletion or inhibition on enhanced pain sensation associated with bladder inflammation.
In conclusion, the severity of CYP-induced cystitis was reduced, and cystitis-associated increased peripheral sensitivity to mechanical stimuli was inhibited in FAAH KO mice. Similarly, inflammation-induced enhanced bladder activity (as reflected by increased voiding frequency) was reduced in FAAH KO mice. Our data indicate that endogenous AEA inhibits bladder inflammation and associated changes in pain perception and bladder function. Importantly, FAAH KO mice exhibit normal motility and thermoregulation (Cravatt et al., 2001), and treatment of animals with FAAH inhibitors do not cause symptoms such as catalepsy (rigid immobility), hypothermia and hyperphagia (increased food intake), that are associated with exogenously administered cannabinoids (Lichtman et al., 2004). Therefore, selective FAAH inhibitors may possess therapeutic potential to treat painful inflammatory bladder disorders without undesirable side effects generally associated with exogenously administered cannabinoids.
Acknowledgements
This study was supported by NIH R01 DK 066349 (DEB).
Funding: NIH DK R01 088806 (DEB)
Footnotes
The authors declare no conflict of interest.
References
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, Lazerwith S, Stiff C, Kamtekar S, Bhattacharya K, Zhang Y, Swaney S, Van Becelaere K, Stevens RC, Cravatt BF. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009;16:411–420. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa N, Hedlund P, Füllhase C, Ito H, Homma Y, Igawa Y. Inhibition of Peripheral FAAH Depresses Activities of Bladder Mechanosensitive Nerve Fibers of the Rat. J Urol. 2014 doi: 10.1016/j.juro.2014.04.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Alvarez-Jaimes LJ, Palmer JA. The role of endocannabinoids in pain modulation and the therapeutic potential of inhibiting their enzymatic degradation. Curr Pharm Biotechnol. 2011;12:1644–1659. doi: 10.2174/138920111798357357. [DOI] [PubMed] [Google Scholar]
- Anderson WB, Gould MJ, Torres RD, Mitchell VA, Vaughan CW. Actions of the dual FAAH/MAGL inhibitor JZL195 in a murine inflammatory pain model. Neuropharmacology. 2014;81:224–230. doi: 10.1016/j.neuropharm.2013.12.018. [DOI] [PubMed] [Google Scholar]
- Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- Birder L, Andersson KE. Urothelial signaling. Physiol Rev. 2013;93:653–680. doi: 10.1152/physrev.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Maccarrone M. Latest advances in the discovery of fatty acid amide hydrolase inhibitors. Expert Opin Drug Discov. 2013;8:509–522. doi: 10.1517/17460441.2013.780021. [DOI] [PubMed] [Google Scholar]
- Bjorling DE, Wang ZY, Bushman W. Models of inflammation of the lower urinary tract. Neurourol Urodyn. 2011;30:673–682. doi: 10.1002/nau.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Cravatt BF. Chemical probes of endocannabinoid metabolism. Pharmacol. 2013;65:849–871. doi: 10.1124/pr.112.006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cencioni MT, Chiurchiù V, Catanzaro G, Borsellino G, Bernardi G, Battistini L, Maccarrone M. Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors. PLoS One. 2010;5:e8688. doi: 10.1371/journal.pone.0008688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F, Laird JM. Understanding the signaling and transmission of visceral nociceptive events. J Neurobiol. 2004;61:45–54. doi: 10.1002/neu.20084. [DOI] [PubMed] [Google Scholar]
- Chang L, Luo L, Palmer JA, Sutton S, Wilson SJ, Barbier AJ, Breitenbucher JG, Chaplan SR, Webb M. Inhibition of fatty acid amide hydrolase produces analgesia by multiple mechanisms. Br J Pharmacol. 2006;148:102–113. doi: 10.1038/sj.bjp.0706699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, Tontini A, Sanchini S, Sciolino NR, Spradley JM, Hohmann AG, Calignano A, Mor M, Tarzia G, Piomelli D. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 2010;13:1265–1270. doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen LL, Misajet B, Brooks DP, Hicks A. Influence of genetic background and gender on bladder function in the mouse. Auton Neurosci. 2008;140:53–58. doi: 10.1016/j.autneu.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Lichtman AH. Fatty acid amide hydrolase: an emerging therapeutic target in the endocannabinoid system. Curr Opin Chem Biol. 2003;7:469–475. doi: 10.1016/s1367-5931(03)00079-6. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly DM, Collins VM, Chapple CR, Grundy D. The afferent system and its role in lower urinary tract dysfunction. Curr Opin Urol. 2011;21:268–274. doi: 10.1097/MOU.0b013e3283476ea2. [DOI] [PubMed] [Google Scholar]
- D'Argenio G, Valenti M, Scaglione G, Cosenza V, Sorrentini I, Di Marzo V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006;20:568–570. doi: 10.1096/fj.05-4943fje. [DOI] [PubMed] [Google Scholar]
- Dinis P, Charrua A, Avelino A, Yaqoob M, Bevan S, Nagy I, Cruz F. Anandamide-evoked activation of vanilloid receptor 1 contributes to the development of bladder hyperreflexia and nociceptive transmission to spinal dorsal horn neurons in cystitis. J Neurosci. 2004;24:11253–11263. doi: 10.1523/JNEUROSCI.2657-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füllhase C, Russo A, Castiglione F, Benigni F, Campeau L, Montorsi F, Gratzke C, Bettiga A, Stief C, Andersson KE, Hedlund P. Spinal cord FAAH in normal micturition control and bladder overactivity in awake rats. J Urol. 2013;189:2364–2370. doi: 10.1016/j.juro.2012.11.165. [DOI] [PubMed] [Google Scholar]
- Gandaglia G, Strittmatter F, La Croce G, Benigni F, Bettiga A, Castiglione F, Moschini M, Mistretta F, Gratzke C, Montorsi F, Stief C, Hedlund P. The fatty acid amide hydrolase inhibitor oleoyl ethyl amide counteracts bladder overactivity in female rats. Neurourol Urodyn. 2013 doi: 10.1002/nau.22482. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Gonzalez EJ, Merrill L, Vizzard MA. Bladder sensory physiology: neuroactive compounds and receptors, sensory transducers, and target-derived growth factors as targets to improve function. Am J Physiol Regul Integr Comp Physiol. 2014;306:R869–R878. doi: 10.1152/ajpregu.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzke C, Streng T, Park A, Christ G, Stief CG, Hedlund P, Andersson KE. Distribution and function of cannabinoid receptors 1 and 2 in the rat, monkey and human bladder. J Urol. 2009;181:1939–1948. doi: 10.1016/j.juro.2008.11.079. [DOI] [PubMed] [Google Scholar]
- Gratzke C, Streng T, Stief CG, Downs TR, Alroy I, Rosenbaum JS, Andersson KE, Hedlund P. Effects of cannabinor, a novel selective cannabinoid 2 receptor agonist, on bladder function in normal rats. Eur Urol. 2010;57:1093–1100. doi: 10.1016/j.eururo.2010.02.027. [DOI] [PubMed] [Google Scholar]
- Hedlund P. Cannabinoids and the endocannabinoid system in lower urinary tract function and dysfunction. Neurourol Urodyn. 2014;33:46–53. doi: 10.1002/nau.22442. [DOI] [PubMed] [Google Scholar]
- Hill WG. Control of Urinary Drainage and Voiding. Clin J Am Soc Nephrol. 2014 doi: 10.2215/CJN.04520413. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, Murphy V, Pertwee RG, Campbell WB. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J Pharmacol Exp Ther. 1999;289:1427–1433. [PubMed] [Google Scholar]
- Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D, Vaughan CW. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol. 2006;147:281–288. doi: 10.1038/sj.bjp.0706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay SK, Birder LA, Chai TC. Evidence for bladder urothelial pathophysiology in functional bladder disorders. Biomed Res Int. 2014 doi: 10.1155/2014/865463. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Naidu PS, Cravatt BF, Dudley DT, Lichtman AH. Fatty acid amide hydrolase blockade attenuates the development of collagen-induced arthritis and related thermal hyperalgesia in mice. Pharmacol Biochem Behav. 2011;99:718–725. doi: 10.1016/j.pbb.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger MB, Dattilio A, Vizzard MA. Expression of cyclooxygenase-2 in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol. 2007;293:R677–R685. doi: 10.1152/ajpregu.00305.2007. [DOI] [PubMed] [Google Scholar]
- Krishnan G, Chatterjee N. Endocannabinoids alleviate proinflammatory conditions by modulating innate immune response in muller glia during inflammation. Glia. 2012;60:1629–1645. doi: 10.1002/glia.22380. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Maione S, Costa B, Di Marzo V. Endocannabinoids: a unique opportunity to develop multitarget analgesics. Pain. 2013;154:S87–S93. doi: 10.1016/j.pain.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Malley SE, Vizzard MA. Changes in urinary bladder cytokine mRNA and protein after cyclophosphamide-induced cystitis. Physiol Genomics. 2002;9:5–13. doi: 10.1152/physiolgenomics.00117.2001. [DOI] [PubMed] [Google Scholar]
- Massa F, Marsicano G, Hermann H, Cannich A, Monory K, Cravatt BF, Ferri GL, Sibaev A, Storr M, Lutz B. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004;113:1202–1209. doi: 10.1172/JCI19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Rademacher DJ, Hillard CJ. Differential regulation of the endocannabinoids anandamide and 2-arachidonylglycerol within the limbic forebrain by dopamine receptor activity. J Pharmacol Exp Ther. 2003;306:880–888. doi: 10.1124/jpet.103.054270. [DOI] [PubMed] [Google Scholar]
- Salaga M, Mokrowiecka A, Zakrzewski PK, Cygankiewicz A, Leishman E, Sobczak M, Zatorski H, Małecka-Panas E, Kordek R, Storr M, Krajewska WM, Bradshaw HB, Fichna J. a Experimental colitis in mice is attenuated by changes in the levels of endocannabinoid metabolites induced by selective inhibition of fatty acid amide hydrolase (FAAH) J Crohns Colitis. 2014 doi: 10.1016/j.crohns.2014.01.025. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaga M, Sobczak M, Fichna J. b Inhibition of fatty acid amide hydrolase (FAAH) as a novel therapeutic strategy in the treatment of pain and inflammatory diseases in the gastrointestinal tract. Eur J Pharm Sci. 2014 doi: 10.1016/j.ejps.2013.11.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Kinsey SG, Lichtman AH. Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation. AAPS J. 2009;11:39–44. doi: 10.1208/s12248-008-9075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrepf A, O'Donnell M, Luo Y, Bradley CS, Kreder K, Lutgendorf S. Inflammation and inflammatory control in interstitial cystitis/bladder pain syndrome: Associations with painful symptoms. Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network. Pain. 2014 doi: 10.1016/j.pain.2014.05.029. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selo-Ojeme DO, Onwude JL. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal.Interstitial cystitis. J Obstet Gynaecol. 2004;24:216–225. doi: 10.1080/01443610410001660652. [DOI] [PubMed] [Google Scholar]
- Stanford EJ, Dell JR, Parsons CL. The emerging presence of interstitial cystitis in gynecologic patients with chronic pelvic pain. Urology. 2007;69:53–59. doi: 10.1016/j.urology.2006.05.049. [DOI] [PubMed] [Google Scholar]
- Storr MA, Keenan CM, Emmerdinger D, Zhang H, Yüce B, Sibaev A, Massa F, Buckley NE, Lutz B, Göke B, Brand S, Patel KD, Sharkey KA. Targeting endocannabinoid degradation protects against experimental colitis in mice: involvement of CB1 and CB2 receptors. J Mol Med (Berl) 2008;86:925–936. doi: 10.1007/s00109-008-0359-6. [DOI] [PubMed] [Google Scholar]
- Storr MA, Keenan CM, Zhang H, Patel KD, Makriyannis A, Sharkey KA. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm Bowel Dis. 2009;15:1678–1685. doi: 10.1002/ibd.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter F, Gandaglia G, Benigni F, Bettiga A, Rigatti P, Montorsi F, Gratzke C, Stief C, Colciago G, Hedlund P. Expression of fatty acid amide hydrolase (FAAH) in human, mouse, and rat urinary bladder and effects of FAAH inhibition on bladder function in awake rats. Eur Urol. 2012;61:98–106. doi: 10.1016/j.eururo.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Tambaro S, Casu MA, Mastinu A, Lazzari P. Evaluation of selective cannabinoid CB(1) and CB(2) receptor agonists in a mouse model of lipopolysaccharide-induced interstitial cystitis. Eur J Pharmacol. 2014 doi: 10.1016/j.ejphar.2014.02.013. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Tyagi V, Philips BJ, Su R, Smaldone MC, Erickson VL, Chancellor MB, Yoshimura N, Tyagi P. Differential expression of functional cannabinoid receptors in human bladder detrusor and urothelium. J Urol. 2009;181:1932–1938. doi: 10.1016/j.juro.2008.11.078. [DOI] [PubMed] [Google Scholar]
- van de Merwe JP, Nordling J, Bouchelouche P, Bouchelouche K, Cervigni M, Daha LK, Elneil S, Fall M, Hohlbrugger G, Irwin P, Mortensen S, van Ophoven A, Osborne JL, Peeker R, Richter B, Riedl C, Sairanen J, Tinzl M, Wyndaele JJ. Visceral pain is the most debilitating symptom of painful bladder syndrome. Eur Urol. 2008;53:60–67. doi: 10.1016/j.eururo.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Walczak JS, Cervero F. Local activation of cannabinoid CB1 receptors in the urinary bladder reduces the inflammation-induced sensitization of bladder afferents. Mol Pain. 2011;7:31. doi: 10.1186/1744-8069-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Wang P, Merriam FV, Bjorling DE. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain. 2008;139:158–167. doi: 10.1016/j.pain.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Wang P, Bjorling DE. Role of mast cells and protease-activated receptor-2 in cyclooxygenase-2 expression in urothelial cells. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1127–R1135. doi: 10.1152/ajpregu.00310.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Wang P, Bjorling DE. Activation of cannabinoid receptor 2 inhibits experimental cystitis. Am J Physiol Regul Integr Comp Physiol. 2013;304:R846–R853. doi: 10.1152/ajpregu.00585.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Wang P, Bjorling DE. Treatment with a cannabinoid receptor 2 agonist decreases severity of established cystitis. J Urol. 2014;191:1153–1158. doi: 10.1016/j.juro.2013.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LE, Cannavacciulo R, Cravatt BF, Martin BF, Lichtman AH. Evaluation of fatty acid amides in the carrageenan-induced paw edema model. Neuropharmacology. 2007;54:181–188. doi: 10.1016/j.neuropharm.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]