Abstract
Low level laser (light) therapy has been used before exercise to increase muscle performance in both experimental animals and in humans. However uncertainty exists concerning the optimum time to apply the light before exercise. The mechanism of action is thought to be stimulation of mitochondrial respiration in muscles, and to increase adenosine triphosphate (ATP) needed to perform exercise. The goal of this study was to investigate the time course of the increases in mitochondrial membrane potential (MMP) and ATP in myotubes formed from C2C12 mouse muscle cells and exposed to light-emitting diode therapy (LEDT). LEDT employed a cluster of LEDs with 20 red (630 ± 10 nm, 25 mW) and 20 near-infrared (850 ± 10 nm, 50 mW) delivering 28 mW/cm2 for 90 sec (2.5 J/cm2) with analysis at 5 min, 3 h, 6 h and 24 h post-LEDT. LEDT-6h had the highest MMP, followed by LEDT-3h, LEDT-24h, LEDT-5min and Control with significant differences. The same order (6h>3h>24h>5min>Control) was found for ATP with significant differences. A good correlation was found (r=0.89) between MMP and ATP. These data suggest an optimum time window of 3-6 h for LEDT stimulate muscle cells.
INTRODUCTION
Mitochondria are the organelles responsible for energy production in cells and for this reason have a very important role in cellular function and maintenance of homeostasis. This organelle has an intriguing and well designed architecture to generate adenosine triphosphate (ATP) that is the basic energy supply for all cellular activity (1, 2).
Mitochondria contain a respiratory electron transport chain (ETC) able to transfer electrons through complexes I, II, III and IV by carrying out various redox reactions in conjunction with pumping hydrogen ions (H+) from the mitochondrial matrix to the intermembrane space. These processes generate water as the metabolic end-product, as oxygen is the final acceptor of electrons from the ETC, that is coupled with synthesis of ATP when H+ ions return back into mitochondrial matrix through complex V (ATP synthase), thus completing the ETC. Changes in the flow of electrons through the ETC and consequently in H+ pumping produce significant modulations in the total proton motive force and ATP synthesis. These changes can be measured by mitochondrial membrane potential (MMP) and content of ATP (1).
Since the earliest evidence that low-level laser (light) therapy (LLLT) can increase ATP synthesis (3, 4), several mechanisms of action have been proposed to explain LLLT effects on mitochondria. One of the first studies reported increased MMP and ATP synthesis measured at an interval of 3 minutes after LLLT (3). Years later, other authors extended the measurement of this “extra” ATP-induced by LLLT in HeLa (human cervical cancer) cells (4). With intervals of 5 to 45 minutes, these authors found no change in ATP synthesis during the first 15 minutes after LLLT, but after 20-25 minutes ATP levels increased sharply and then came back to control levels at 45 minutes (4).
More recent studies have reported LLLT effects on mitochondria in different types of cells (5-9). In neural cells LLLT seems to also increase MMP, protect against oxidative stress (5) and increase ATP synthesis in intact cells (without stressor agents)(6). In mitochondria from fibroblast cells without stressor agents, LLLT also increased ATP synthesis and mitochondrial complex IV activity in a dose-dependent manner (7). In myotubes from C2C12 cells, LLLT could modulate the production of reactive oxygen species (ROS) and mitochondrial function in a dose-dependent manner in intact cells or in cells stressed by electrical stimulation (9).
Increases in mitochondrial metabolism and ATP synthesis have been proposed by several authors as a hypothesis to explain LLLT effects on muscle performance when used for muscular pre-conditioning or muscle recovery post-exercise (10-12). However, there is a lack in the literature to identify immediate and long-term effects of LLLT on mitochondrial metabolism and ATP synthesis in skeletal muscle cells that in turn could confirm these hypotheses.
This current study aimed to identify the time-response for LLLT by light-emitting diode therapy (LEDT) in modulation of MMP and ATP content in myotubes from C2C12 intact cells (mouse muscle cells) only under the stress of the culture. Moreover, the second objective was to correlate MMP with ATP content within a time range of 5 minutes to 24 hours after LLLT. Our goal was to find the best time-response for LLLT which could be useful in future experimental and clinical studies investigating muscular pre-conditioning, muscle recovery post-exercise or any other photobiomodulation in muscle tissue.
MATERIALS AND METHODS
Cell culture
C2C12 cells were kindly provided by the Cardiovascular Division of the Beth Israel Deaconess Medical Center, Harvard Medical School, USA. Cells were grown in culture medium (DMEM, Dulbecco's Modified Eagle's Medium - Sigma-Aldrich) with fetal bovine serum (20% FBS - Sigma-Aldrich) and 1% antibiotic (penicillin and streptomycin) in humidified incubator at 37 °C and 5% CO2.
C2C12 cells were cultured and a total of 1.71 × 105 cells approximately were counted in a Neubauer chamber. Next, these cells were distributed equally into 30 wells (approximately 5.7 × 103 cells per well) into two different plates:
15 wells in black plate (Costar® 96-Well Black Clear-Bottom Plates) for analysis of MMP.
15 wells in white plate (Costar® 96-Well White Clear-Bottom Plates) for analysis of ATP synthesis.
Moreover, both plates were subdivided into five columns with 3 wells per column (triplicate):
LEDT-Control: no LEDT applied to the cells.
LEDT-5min: LEDT applied to the cells and assessments of ATP and MMP after 5 minutes.
LEDT-3h: LEDT applied to the cells and assessments of ATP and MMP after 3 hours.
LEDT-6h: LEDT applied to the cells and assessments of ATP and MMP after 6 hours.
LEDT-24h: LEDT applied to the cells and assessments of ATP and MMP after 24 hours.
After plating C2C12 cells were cultured for nine days in culture medium (DMEM) containing 2% heat-inactivated horse serum (Sigma-Aldrich) in a humidified incubator at 37 °C and 5% CO2 to induce cell differentiation into myotubes, as described in a previous study (9). At the 10th day, LEDT-24h group received LEDT. At 11th day all remaining groups received LEDT and were assessed for ATP and MMP at specific times in accordance with each group.
Light-emitting diode therapy (LEDT)
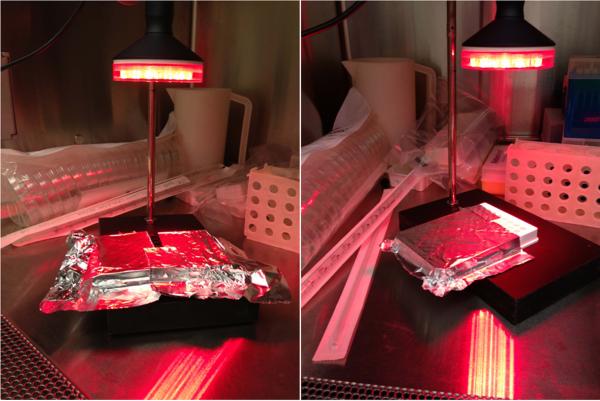
A cluster of 40 LEDs (20 red – 630 ± 10 nm; 20 infrared – 850 ± 20 nm) with a diameter of 76 mm was used in this study. The cluster was positioned at a distance of 156 mm from the top of each plate and irradiation lasted 90 s with fixed parameters as described in table 1. Each group of wells received LEDT individually, and all others wells of each plate (groups) were covered with aluminum foil to avoid light irradiation [Figure 1]. LEDT parameters were measured and calibrated using an optical energy meter PM100D Thorlabs® and sensor S142C (area of 1.13 cm2). In addition, we chose use red and near-infrared light therapy at the same time in order to promote a double band of absorption by cytochrome c oxidase (COX) based on specific bands of absorption reported previously (2, 13-16). The room temperature was controlled (22-23 °C) during LEDT irradiation, which did not increase temperature on the top of plates more than 0.5° C. This increase of 0.5 °C was dissipated to room within 2 minutes after LEDT.
Table 1.
All parameters of light-emitting diode therapy (LEDT). Control did not receive LEDT.
| Number of LEDs (cluster): 40 (20 infrared-IR and 20 red-RED) |
| Wavelength: 850 ± 20 nm (IR) and 630 ± 10 nm (RED) |
| LED spot size: 0.2 cm2 |
| Pulse frequency: continuous |
| Optical output of each LED: 50 mW (IR) and 25 mW (RED) |
| Optical output (cluster): 1,000 mW (IR) and 500 mW (RED) |
| LED cluster size: 45 cm2 |
| Power density (at the top of plate): 28 mW/cm2 |
| Treatment time: 90 s |
| Cluster energy density applied on the top plate: 2.5 J/cm2 |
| Application mode: without contact |
| Distance from plate or power meter: 156 mm |
Figure 1. Myotubes from C2C12 cells.
Experimental setup for irradiation of the white and black plates containing myotubes from C2C12 cells using light-emitting diode therapy (LEDT) without contact.
Mitochondrial membrane potential (TMRM) assay
This analysis was performed using cells placed into black plate. MMP was assessed using tetramethyl rhodamine methyl ester (TMRM – Invitrogen/ Molecular Probes) at a final concentration of 25 nM. Nuclei of myotubes from C2C12 cells were labeled using Hoechst (Sigma-Aldrich) at a concentration of 1mg/ ml. Each well was incubated for 30 min, 37 °C and 5% CO2 with 100 μl of solution containing TMRM and Hoechst. Next, this solution was carefully removed from each well and added 100 μl of buffer solution containing HBSS (Hank's Balanced Salt Solution – Life Technologies Corporation) and 15 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid – Life Technologies Corporation). The myotubes were imaged in a confocal microscope (Olympus America Inc. Center Valley, PA, USA) using an excitation at 559 nm and emission at 610 nm. Three random fields per well were imaged with a magnification of 40x water immersion lens. Images were exported and TMRM fluorescence incorporation into mitochondrial matrix was measured using software Image J (NIH, Bethesda, MD).
Adenosine triphosphate (ATP) assay
This analysis was performed using cells placed into white plate. Firstly, the medium was carefully removed from each well followed by addition of 50 μl/well of CellTiter Glo Luminescent Cell Viability Assay reagent (Promega). After 10 min of incubation at room temperature (25 °C), luminescence signals were measured in a SpectraMax M5 Multi-Mode Microplate Reader (Molecular Devices, Sunnyvale, CA) with integration time of 5 s to increase low signals (17). A standard curve was prepared using ATP standard (Sigma) according to manufacturer's guideline and then ATP concentration was calculated in nanomol (nmol) per well.
Pearson product-moment correlation coefficient (Pearson's r)
The correlation between TMRM and ATP content in myotubes from C2C12 cells was calculated using Pearson's r. The r values were interpreted as recommended previously (18): 0.00–0.19 = none to slight; 0.20–0.39 = low; 0.40–0.69 = modest; 0.70–0.89 = high; and 0.90–1.00 = very high.
Sample size calculation
The sample size was calculated based on that necessary to obtain significant differences among all groups with ATP content. The statistical power of 80% and the effect size (greater than 0.75) were found to be satisfactory.
Statistical analysis
Shapiro-Wilk's W test verified the normality of the data distribution. ATP and TMRM were compared among all groups using one-way analysis of variance (ANOVA) with Tukey HSD post hoc test. Pearson product-moment correlation coefficient (Pearson's r) was conducted between TMRM and ATP. Significance was set at P < 0.05.
RESULTS
Mitochondrial membrane potential (TMRM)
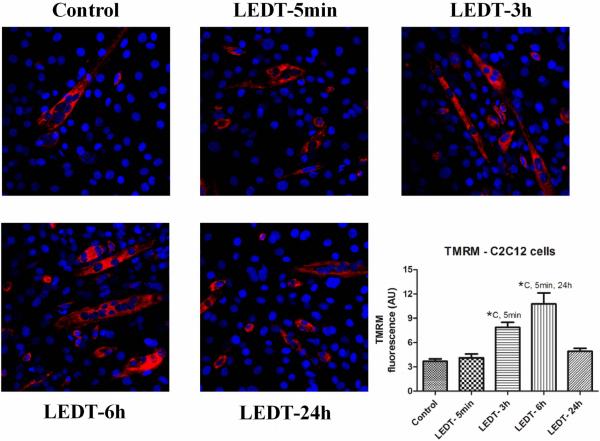
LEDT-6h group increased MMP (10.77 AU, SEM 0.88) compared to: Control (3.79 AU, SEM 0.46): P < 0.001; LEDT-5min (4.11 AU, SEM 0.52): P < 0.001; LEDT-24h (4.91 AU, SEM 0.47): P = 0.001. LEDT-3h (7.87 AU, SEM 0.59) increased MMP compared to Control (P = 0.019) and LEDT-5min (P = 0.031). These results are graphically presented in figure 2. All non-significant results were Control versus LEDT-5min (P = 0.997) and versus LEDT-24h (P = 0.816); LEDT-5min versus LEDT-24h (P = 0.935); LEDT-3h versus LEDT-6h (P = 0.113) and versus LEDT-24h (P = 0.103).
Figure 2. TMRM.
Analysis of mitochondrial membrane potential using tetramethyl rhodamine methyl ester (TMRM) stained in red. Images with a magnification of 40x. Abbreviations: LEDT= light-emitting diode therapy; AU= arbitrary units; C= control group; 5min= LEDT-5min group; 24h= LEDT-24h group; *= statistical significance (P < 0.05) using one-way analysis of variance (ANOVA).
ATP assay
LEDT-6h increased ATP contents (4.53 nmol/ well, SEM 0.19) compared to: Control (1.28 nmol/ well, SEM 0.05): P < 0.001; LEDT-5min (2.01 nmol/ well, SEM 0.16): P < 0.001; LEDT-24h (2.77 nmol/ well, SEM 0.16): P = 0.007. LEDT-3h increased ATP contents (3.73 nmol/ well, SEM 0.17) compared to Control (P < 0.001) and LEDT-5min (P = 0.008). LEDT-24h increased ATP contents compared to Control (P = 0.020). These results are graphically presented in figure 3A. All non-significant results were Control versus LEDT-5min (P = 0.385); LEDT-3h versus LEDT-6h (P = 0.299) and versus LEDT-24h (P = 0.169); LEDT-24h versus LEDT-5min (P = 0.338).
Figure 3. ATP and Pearson's r.
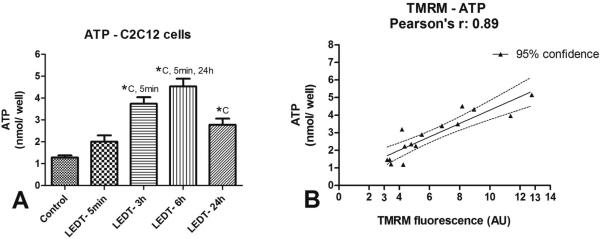
A) Analysis of adenosine triphosphate (ATP) content between groups. B) Pearson product-moment correlation coefficient (Pearson's r)between ATP and mitochondrial membrane potential using TMRM. Abbreviations: LEDT= light-emitting diode therapy; TMRM= tetramethyl rhodamine methyl ester; nmol= nanomol; AU= arbitrary units; C= control group; 5min= LEDT-5min group; 24h= LEDT-24h group; *= statistical significance (P < 0.05) using one-way analysis of variance (ANOVA).
Sample size
The statistical power and the effect size regarding ATP content in all groups were calculated in order to ensure the minimal power of 80% and large effect size (> 0.75). We used the mean ATP content of each group and the highest value of standard deviation among all groups, which was observed in LEDT-6h. Our results demonstrate a difference between groups with a statistical power of 99%, effect size of 3.42 (very large effect) and total sample size of 10, i.e., 2 wells per group (5 groups). These calculations demonstrate that our sample size was small, but adequate (3 wells per group).
Pearson product-moment correlation coefficient (Pearson's r)
TMRM incorporation into mitochondrial matrix of myotubes from C2C12 cells showed a high correlation (r=0.89) with ATP content (P < 0.001). This result is presented in figure 3B.
DISCUSSION
This current study identified a well-defined time-response for the LEDT-mediated increase in MMP and ATP synthesis in myotubes from C2C12 cells under the stress of the cell culture. The light dose used was based on previous study that already reported benefits of LLLT on mitochondria of myotubes (9). In addition, we found a strong correlation between MMP and ATP content measured during a wide range from 5 minutes (immediate effect) to 24h (prolonged effect). To our knowledge this is the first study investigating the time-response for light therapy modulation of mitochondrial metabolism in conjunction with ATP synthesis in muscle cells.
C2C12 is a cell line originally isolated from dystrophic muscles of C3H mice by Yaffe and Saxel (19). In culture it rapidly differentiates into contractile myotubes (muscle fibers) especially when treated with horse serum instead of fetal bovine serum. These myotubes contain multi-nucleated cells that express proteins characteristic of skeletal muscle such as myosin heavy chain and creatine kinase (20).
One of first effects of LLLT reported in literature was a modulation on MMP and ATP synthesis in mitochondria isolated from rat liver (3) and in HeLa cells (4). Our results are in accordance with these previous studies, showing an increased MMP and ATP synthesis in myotubes from C2C12 cells. However, light therapy seems to produce a different time-response of MMP and ATP synthesis among different cell types. While HeLa cells showed a peak of ATP synthesis around 20 minutes after light therapy (4), mitochondria from liver showed an immediate increase in MMP and ATP synthesis (3). In the present study we found that muscle cells need a longer time in the range of 3h to 6h to show the maximum effect of light therapy and convert it into a significant increase in MMP and ATP synthesis, comprising an increase around 200% to 350% over the control values. In addition, we found that 24h after irradiation, myotubes could still produce significantly more ATP compared to LEDT-Control while LEDT-5min showed no significant difference.
Cytochrome c oxidase (COX) has been reported to be the main chromophore in cells exposed to red and near infrared light (2, 21, 16, 15). However, although COX activity is important in the immediate effects of photon absorption, the measurement of its activity may be insufficient to confirm whether light therapy can induce “extra” ATP synthesis. For this reason the measurement of MMP in conjunction with ATP synthesis can provide information on how fast changes occur in the electron transport chain (ETC), and H+ pumping from the mitochondrial matrix to the intermembrane space, as well as how much H+ ions are returning to the mitochondrial matrix (1). In this perspective, our results are consistent with Xu et al. (9) who reported no immediate effects of light therapy on MMP. Moreover, although Xu et al. (9) did not assess ATP content, our results showed no significant responses for ATP increment immediately after light therapy compared to control group.
Our results for MMP in conjunction with ATP content had a high correlation (Pearson's r = 0.89) during the time range of 5min to 24h, suggesting a linear and positive dependence of ATP synthesis on the value of MMP (ETC and H+ pumping) in muscle cells, suggesting a new and more efficient time-response or time-window for LEDT stimulate muscle cells (see figure 4A and 4B). These results are very important for muscle recovery post-exercise (10, 11) because they suggest a prolonged effect of light therapy on ATP synthesis necessary to repair muscle damage. In addition, muscular preconditioning using light therapy for improvement of performance before a bout of exercise (12) could possibly be optimized by application at the appropriate time. However more studies in vivo and clinical trials are needed to confirm our hypotheses.
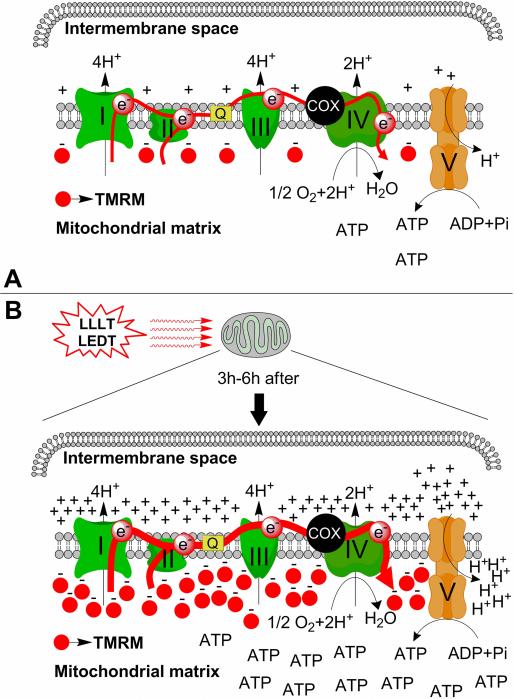
Figure 4. Mechanism of action of LEDT on mitochondria.
A) Mitochondria of myotubes from C2C12 cells without low-level laser therapy (LLLT) or light-emitting diode therapy (LEDT). There is a normal flux of electrons (red arrow) through all complexes of electron transport chain, normal pumping of H+, normal synthesis of ATP and modest take up of TMRM by the mitochondrial matrix. B) Mitochondria of myotubes from C2C12 cells 3 to 6 hours after LEDT. There is an increased flux of electrons (ticker red arrow), increased pumping of H+, increased synthesis of ATP and increased take up of TMRM by the mitochondrial matrix. Abbreviations: I, II, III, IV and V= complexes of the mitochondrial electron transport chain; H+= proton of hydrogen; - = electron of hydrogen; O2= oxygen; H2O= metabolic water; Q= quinone; COX= cytochrome c oxidase; ATP= adenosine triphosphate;TMRM= tetramethyl rhodamine methyl ester.
Muscular pre-conditioning using LLLT or LEDT have been reported as therapeutic approaches to improve muscle performance in both experimental models (22-24) and in clinical trials (12). However, although this improvement reported in the literature has been significant, some studies have not found positive results (25). Furthermore, differences between groups treated with light therapy or placebo seem to be not so large. These differences reported in experimental models varied between 80% to 150% of the values found for control groups for fatigue test induced by electrical stimulation (22-24). In clinical trials these differences varied between 5% to 57% increases in number of repetitions and maximal voluntary contraction (12). Possibly these relatively modest increases could be due to allowing insufficient time necessary for the muscle cells to convert light therapy into biological responses as identified in our study for MMP and ATP synthesis. Consequently, protocols for muscular pre-conditioning that have been done up to now (22-24, 12), i.e., generally applying light 5 minutes before the exercise, may not possibly achieve the best result. Based on our results, we suggest to wait 3h to 6h after light therapy irradiation to obtain the best increase in muscle performance in muscular pre-conditioning regimen, since MMP and ATP availability are important for muscle performance (26, 27). Once more time, we would like to remark the needed of more studies in vivo and clinical trials to confirm our hypotheses. At this point, it is valuable to reference two previous studies that had a similar initiative (28, 29). Hayworth et al. (28) found increments in COX activity 24h after apply LEDT over rats muscles; Albuquerque-Pontes et al. (29) found a time-window, wavelength-dependence and dose-response for COX activity increase also after LLLT in rats muscles. Both studies used animals without any kind of stress, such as this present study used cells only under the stress of the cell culture. We believe that these previous studies combined with our results are extremely valuable for the discovery and understanding of mechanisms of action of LLLT on muscle tissue, and may offer guidance on the future use of LLLT in clinical practice.
Our study was designed to test one dose of light during a time-response to show that there is time-dependency for LLLT to produce secondary responses in muscle cells. For this reason, the current study used a constant dose (fluence) of light as reported in a previous study (9) as well as a constant power density. Since there is a possible biphasic dose response (30, 31), use of different parameters such as fluence, wavelengths or irradiance could produce different responses. In addition, red and near-infrared light therapy was delivered at the same time in order to take advantage of the double bands in COX to absorb the light (2, 13-16).
CONCLUSION
This is the first study reporting the benefits of mixed red and near-infrared light therapy on MMP in conjunction with ATP synthesis in myotubes from C2C12 cells (muscle cells from mice). Moreover, a well defined time-response was found for the increase in ATP synthesis mediated by MMP increased by light therapy in myotubes.
Our data suggest that 3h to 6h could be the best time-response for light therapy to improve muscle metabolism. In addition, our results lead us to think there may be possible cumulative effects if light therapy is applied at intervals less than 24h that may have clinical relevance when LLLT is used for muscle post-exercise recovery. Finally, we believe that use of light therapy for muscular pre-conditioning could be optimized in future studies whether the time-response for increases in ATP and MMP found in this study are taken account.
ACKNOWLEDGMENTS
We would like to thank Professor Zoltan Pierre Arany and his instructor Glenn C. Rowe for the C2C12 cells and Andrea Brissette for assistance with multiple roles including purchase of reagents. Cleber Ferraresi would like to thank FAPESP for his PhD scholarships (numbers 2010/07194-7 and 2012/05919-0). MR Hamblin was supported by US NIH grant R01AI050875.
REFERENCES
- 1.Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50:98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B. 1999;49:1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 3.Passarella S, Casamassima E, Molinari S, Pastore D, Quagliariello E, Catalano IM, Cingolani A. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett. 1984;175:95–99. doi: 10.1016/0014-5793(84)80577-3. [DOI] [PubMed] [Google Scholar]
- 4.Karu T, Pyatibrat L, Kalendo G. Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol B. 1995;27:219–223. doi: 10.1016/1011-1344(94)07078-3. [DOI] [PubMed] [Google Scholar]
- 5.Giuliani A, Lorenzini L, Gallamini M, Massella A, Giardino L, Calza L. Low infra red laser light irradiation on cultured neural cells: effects on mitochondria and cell viability after oxidative stress. BMC Complement Altern Med. 2009;9:8. doi: 10.1186/1472-6882-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oron U, Ilic S, De Taboada L, Streeter J. Ga-As (808 nm) laser irradiation enhances ATP production in human neuronal cells in culture. Photomed Laser Surg. 2007;25:180–182. doi: 10.1089/pho.2007.2064. [DOI] [PubMed] [Google Scholar]
- 7.Houreld NN, Masha RT, Abrahamse H. Low-intensity laser irradiation at 660 nm stimulates cytochrome c oxidase in stressed fibroblast cells. Lasers Surg Med. 2012 doi: 10.1002/lsm.22027. [DOI] [PubMed] [Google Scholar]
- 8.Masha RT, Houreld NN, Abrahamse H. Low-intensity laser irradiation at 660 nm stimulates transcription of genes involved in the electron transport chain. Photomedicine and laser surgery. 2013;31:47–53. doi: 10.1089/pho.2012.3369. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, Zhao X, Liu TC, Pan H. Low-intensity laser irradiation improves the mitochondrial dysfunction of C2C12 induced by electrical stimulation. Photomed Laser Surg. 2008;26:197–202. doi: 10.1089/pho.2007.2125. [DOI] [PubMed] [Google Scholar]
- 10.Ferraresi C, Hamblin MR, Parizotto NA. Low-level laser (light) therapy (LLLT) on muscle tissue: performance, fatigue and repair benefited by the power of light. Photonics Lasers Med. 2012;1:267–286. doi: 10.1515/plm-2012-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borsa PA, Larkin KA, True JM. Does phototherapy enhance skeletal muscle contractile function and postexercise recovery? A systematic review. J Athl Train. 2013;48:57–67. doi: 10.4085/1062-6050-48.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leal-Junior EC, Vanin AA, Miranda EF, de Carvalho PD, Dal Corso S, Bjordal JM. Effect of phototherapy (low-level laser therapy and light-emitting diode therapy) on exercise performance and markers of exercise recovery: a systematic review with meta-analysis. Lasers Med Sci. 2013 doi: 10.1007/s10103-013-1465-4. [DOI] [PubMed] [Google Scholar]
- 13.Karu TI, Pyatibrat LV, Afanasyeva NI. A novel mitochondrial signaling pathway activated by visible-to-near infrared radiation. Photochem Photobiol. 2004;80:366–372. doi: 10.1562/2004-03-25-RA-123. [DOI] [PubMed] [Google Scholar]
- 14.Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomedicine and laser surgery. 2005;23:355–361. doi: 10.1089/pho.2005.23.355. [DOI] [PubMed] [Google Scholar]
- 15.Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Absorption measurements of cell monolayers relevant to mechanisms of laser phototherapy: reduction or oxidation of cytochrome c oxidase under laser radiation at 632.8 nm. Photomedicine and laser surgery. 2008;26:593–599. doi: 10.1089/pho.2008.2246. [DOI] [PubMed] [Google Scholar]
- 16.Karu TI. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life. 2010;62:607–610. doi: 10.1002/iub.359. [DOI] [PubMed] [Google Scholar]
- 17.Khan HA. Bioluminometric assay of ATP in mouse brain: Determinant factors for enhanced test sensitivity. Journal of biosciences. 2003;28:379–382. doi: 10.1007/BF02705114. [DOI] [PubMed] [Google Scholar]
- 18.Weber J, Lamb D. Statistics and research in physical education. C. V. Mosby Co.; Saint Louis: 1970. [Google Scholar]
- 19.Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- 20.Tannu NS, Rao VK, Chaudhary RM, Giorgianni F, Saeed AE, Gao Y, Raghow R. Comparative proteomes of the proliferating C(2)C(12) myoblasts and fully differentiated myotubes reveal the complexity of the skeletal muscle differentiation program. Molecular & cellular proteomics : MCP. 2004;3:1065–1082. doi: 10.1074/mcp.M400020-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.Karu TI. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem Photobiol. 2008;84:1091–1099. doi: 10.1111/j.1751-1097.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 22.Lopes-Martins RA, Marcos RL, Leonardo PS, Prianti AC, Jr., Muscara MN, Aimbire F, Frigo L, Iversen VV, Bjordal JM. Effect of low-level laser (Ga-Al-As 655 nm) on skeletal muscle fatigue induced by electrical stimulation in rats. J Appl Physiol (1985) 2006;101:283–288. doi: 10.1152/japplphysiol.01318.2005. [DOI] [PubMed] [Google Scholar]
- 23.Leal Junior EC, Lopes-Martins RA, de Almeida P, Ramos L, Iversen VV, Bjordal JM. Effect of low-level laser therapy (GaAs 904 nm) in skeletal muscle fatigue and biochemical markers of muscle damage in rats. European journal of applied physiology. 2010;108:1083–1088. doi: 10.1007/s00421-009-1321-1. [DOI] [PubMed] [Google Scholar]
- 24.Santos LA, Marcos RL, Tomazoni SS, Vanin AA, Antonialli FC, Grandinetti VD, Albuquerque-Pontes GM, de Paiva PR, Lopes-Martins RA, de Carvalho PD, Bjordal JM, Leal-Junior EC. Effects of pre-irradiation of low-level laser therapy with different doses and wavelengths in skeletal muscle performance, fatigue, and skeletal muscle damage induced by tetanic contractions in rats. Lasers Med Sci. 2014 doi: 10.1007/s10103-014-1560-1. [DOI] [PubMed] [Google Scholar]
- 25.Higashi RH, Toma RL, Tucci HT, Pedroni CR, Ferreira PD, Baldini G, Aveiro MC, Borghi-Silva A, de Oliveira AS, Renno AC. Effects of low-level laser therapy on biceps braquialis muscle fatigue in young women. Photomed Laser Surg. 2013;31:586–594. doi: 10.1089/pho.2012.3388. [DOI] [PubMed] [Google Scholar]
- 26.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 27.Ferraresi C, de Brito Oliveira T, de Oliveira Zafalon L, de Menezes Reiff RB, Baldissera V, de Andrade Perez SE, Matheucci Junior E, Parizotto NA. Effects of low level laser therapy (808 nm) on physical strength training in humans. Lasers Med Sci. 2011;26:349–358. doi: 10.1007/s10103-010-0855-0. [DOI] [PubMed] [Google Scholar]
- 28.Hayworth CR, Rojas JC, Padilla E, Holmes GM, Sheridan EC, Gonzalez-Lima F. In vivo low-level light therapy increases cytochrome oxidase in skeletal muscle. Photochem Photobiol. 2010;86:673–680. doi: 10.1111/j.1751-1097.2010.00732.x. [DOI] [PubMed] [Google Scholar]
- 29.Albuquerque-Pontes GM, Vieira RD, Tomazoni SS, Caires CO, Nemeth V, Vanin AA, Santos LA, Pinto HD, Marcos RL, Bjordal JM, de Carvalho PD, Leal-Junior EC. Effect of pre-irradiation with different doses, wavelengths, and application intervals of low-level laser therapy on cytochrome c oxidase activity in intact skeletal muscle of rats. Lasers Med Sci. 2014 doi: 10.1007/s10103-014-1616-2. [DOI] [PubMed] [Google Scholar]
- 30.Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7:358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang YY, Sharma SK, Carroll J, Hamblin MR. Biphasic dose response in low level light therapy - an update. Dose Response. 2011;9:602–618. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]