Abstract
Background
Balanced activation and inhibition of the immune system ensures pathogen clearance while avoiding hyperinflammation. Siglecs, sialic acid binding proteins found on subsets of immune cells, often inhibit inflammation: Siglec-8 on eosinophils and Siglec-9 on neutrophils engage sialoglycan ligands on airways to diminish ongoing inflammation. The identities of human siglec ligands and their expression during inflammation are largely unknown.
Objective
The histological distribution, expression and molecular characteristics of siglec ligands were explored in healthy and inflamed human upper airways and in a cellular model of airway inflammation.
Methods
Normal and chronically inflamed upper airway tissues were stained for siglec ligands. The ligands were extracted from normal and inflamed tissues and from human Calu-3 cells for quantitative analysis by siglec blotting and isolation by siglec capture.
Results
Siglec-8 ligands were expressed on a subpopulation of submucosal gland cells of human inferior turbinate, whereas Siglec-9 ligands were expressed more broadly (submucosal glands, epithelium, connective tissue); both were significantly upregulated in chronic rhinosinusitis patients. Human airway (Calu-3) cells expressed Siglec-9 ligands on mucin 5B under inflammatory control via the NF-κB pathway, and mucin 5B carried sialoglycan ligands of Siglec-9 on human upper airway tissue.
Conclusion
Inflammation results in upregulation of immune inhibitory Siglec-8 and Siglec-9 sialoglycan ligands on human airways. Siglec-9 ligands were upregulated via the NF-κB pathway resulting in their enhanced expression on mucin 5B. Siglec sialoglycan ligand expression in inflamed cells and tissues may contribute to the control of airway inflammation.
Keywords: chronic rhinosinusitis, inflammation, submucosal gland cells, siglecs, glycobiology, MUC5B, NF-κB
INTRODUCTION
An appropriate balance between positive and negative inflammatory signals is essential to the effective clearance of pathogens while avoiding autoimmune and hyperinflammatory syndromes.1 Among the molecules that regulate the level of inflammation are members of the siglec family, cell surface molecules on leukocytes that bind to sialylated glycans and translate glycan binding into changes in immune cell function.2 There are fourteen human siglecs, most of which are expressed on specific subsets of cells in the innate and adaptive immune systems. Siglecs are single-pass membrane proteins containing multiple extracellular immunoglobulin-like domains that mediate glycan binding and short cytoplasmic tails, most of which express immunoreceptor tyrosine based inhibitor motifs (ITIMs) that negatively regulate the immune cells on which they are expressed. Human Siglec-8 is expressed on eosinophils, mast cells and basophils where ligation results in eosinophil apoptosis and inhibition of mast cell mediator release.3–5 In models of allergic inflammation, mice lacking the paralog of Siglec-8 (Siglec-F) display exacerbated eosinophilic infiltration.6,7 Siglec-9 is expressed on neutrophils, monocytes, dendritic cells and natural killer cells. Its ligation on neutrophils results in their apoptotic death.8 Mice lacking the homolog of Siglec-9 (Siglec-E) display exaggerated neutrophil recruitment to the lung in an acute inflammation model (aerosolized lipopolysaccharide).9 The effects of Siglec-8 on eosinophils and Siglec-9 on neutrophils are more pronounced when the cells are activated,8,10 implicating siglec function in the down-regulation of ongoing inflammatory responses.
In the airways, Siglec-8 and Siglec-9 may be involved in the regulation of allergic and non-allergic inflammation in diseases such as asthma and chronic obstructive pulmonary disease (COPD), which involve activated eosinophils and neutrophils. During an ongoing immune response the activated inflammatory cells come into contact with the appropriate siglec ligands on airways, resulting in the inflammatory response subsiding. Knowing the molecular characteristics of human siglec ligands and control of their expression in human airways during inflammation may provide additional insight into this pathway of immune system regulation in health and disease.2 We report here the tissue distribution and molecular characteristics of Siglec-8 and Siglec-9 ligands in normal and inflamed human upper airways.
Chronic rhinosinusitis (CRS) is a common disorder defined by inflammation of the upper airways and sinuses with symptoms persisting longer than 12 weeks even with medical intervention.11 Its etiology is heterogeneous, may be related to allergies or infections, and is believed to result in hyperinflammation of different types.12 Histological data from its two major clinical sub-classifications, with or without nasal polyps, reveals both eosinophilic and neutrophilic infiltration that depends on the stage, etiology and geography of the affected populations.12,13 As a clinically accessible source of human inflamed airway tissue, we focused the current study on the expression of Siglec-8 and Siglec-9 ligands in CRS.
METHODS
Patients and biopsy specimens
Patients with CRS (Table E1) were recruited from the Allergy-Immunology and Otolaryngology Clinics of the Northwestern Medical Faculty Foundation and the Northwestern Sinus Center at the Northwestern Medical Faculty Foundation. Sinonasal tissues were obtained from routine functional endoscopic sinus surgery in patients with CRS. All patients met the criteria for CRS, as defined by the American Academy of Otolaryngology-Head and Neck Surgery Chronic Rhinosinusitis Task Force. Unless otherwise indicated, CRS in this manuscript refers to patients without nasal polyps (CRSsNP). Patients with an established immunodeficiency, pregnancy, coagulation disorder, diagnosis of classic allergic fungal sinusitis, Samter’s triad, Churg-Strauss syndrome, or cystic fibrosis did not participate in the study. All subjects signed informed consent, and the protocol and consent forms governing procedures for the study were approved by the Institutional Review Board of Northwestern University Feinberg School of Medicine.
Siglec-8 and Siglec-9 ligand histochemistry
Siglec ligands were detected by overlaying upper airway histological sections with Siglec-8 or Siglec-9 chimeras consisting of the complete extracellular domain of each siglec fused to human Fc. Expression plasmids for the chimeras were cloned behind an EF1α promoter by replacing the GFP sequence in pEF-GFP (Addgene, Cambridge, MA) with the extracellular sequence of each siglec followed in frame with human IgG Fc. Plasmids were transiently transfected into HEK293T cells and soluble chimeras purified from the supernatant by affinity chromatography on Protein G Sepharose (HiTrap, GE Healthcare, Pittsburgh, PA). Eluted purified protein was dialyzed against Dulbecco’s phosphate buffered saline (PBS) and quantified using a bicinchoninic acid assay (BCA, Thermo Fisher Scientific, Rockford, IL).
Nasal tissue was dehydrated, paraffin-embedded, and sectioned at 3 μm onto glass slides using a Leica RM2245 Cryostat (Leica Microsystems, Buffalo Grove, IL). After xylene deparaffinization and rehydration, slides were immersed in 10 mM sodium citrate pH 6.0 at 95–100°C for 15 min and then blocked with Dako enzyme block (Dako, Carpinteria, CA) and Fc receptor blocker (Innovex, Richmond, CA). Siglec-8-Fc (20 μg/mL) or Siglec-9-Fc (15 μg/mL) were pre-complexed by incubation with 2 μg/mL alkaline phosphatase conjugated goat anti-human Fcγ (Jackson Immunoresearch, West Grove, PA) in ice-cold PBSTr (PBS supplemented with 0.1% Triton X-100) for 30 min. Blocked slides were washed in PBSTr and overlaid with 200 μL of the pre-complexed siglec for 2 h at ambient temperature. After washing three times with PBSTr, bound siglec-Fc chimeras were detected using Vector Red Substrate (SK-5100, Vector Laboratories, Burlingame, CA). For detection of Mucin 5B (MUC5B), slides were blocked and washed as above and then were overlaid with 1:200 rabbit anti-MUC5B primary antibody (Santa Cruz Biotechnology, Dallas, TX) in PBSTr for 2 h at ambient temperature, followed by alkaline-phosphatase-conjugated goat anti-rabbit IgG (1:1000 in PBSTr) for 1 h. Binding was detected as above. As indicated, control slides were treated with 80 mU/mL of V. cholerae sialidase14 for 2.5 h at 37°C and then washed with PBSTr prior to probing with siglec-Fc chimeras.
Images of stained sections were captured using a Nikon Eclipse 90i automated research microscope (Nikon Instruments, Melville, NY). Siglec ligand and MUC5B expression were quantified in randomly chosen well-demarcated submucosal glands containing at least 50 gland cells. The positively stained (red pixel) area was determined relative to the total area of the submucosal glands using NIS-Elements image analysis software (Nikon).
Inferior turbinate protein extracts
Freshly obtained tissue specimens were weighed and 1 mL of PBS supplemented with 0.05% Tween 20 and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) was added for every 100 mg of tissue. The tissue was then homogenized with a Bullet Blender Blue (Next Advance, Averil Park, NY) at setting 7 for 8 min at 4°C. After homogenization, the suspension was centrifuged at 2000 x g for 20 min at 4°C, and supernatants were stored at −80°C until analyzed.
Human tracheobronchial gland cell and Calu-3 cell culture, inflammatory mediators and glycan inhibitors
Human tracheobronchial submucosal gland cells were prepared as described with modifications.15,16 Trachea and main bronchi from organ donors were opened and digested with 0.1% protease in Ham’s F12 medium at 4°C overnight to remove epithelial cells. Submucosal tissue was then dissected and incubated for 24 h in 0.01% dispase/collagenase (Roche, Indianapolis, IN) in Dulbecco’s modified Eagle medium supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 2.5 μg/mL amphotericin and 50 μg/mL gentamicin. Gland cells were recovered mechanically, washed in the same medium, and then resuspended in 0.25% trypsin-EDTA (LifeTechnologies, Grand Island, NY) and triturated to dissociate the clumps of gland cells. Digestion was stopped by addition of fetal bovine serum and cells collected and resuspended in gland cell medium comprised of a 1:1 mixture of Dulbecco’s modified Eagle medium and Ham’s F12 medium supplemented with 0.5 μg/mL hydrocortisone, 5 μg/mL insulin, 10 μl/mL transferrin, 0.5 μg/mL epinephrine, 6.5 ng/mL triiodothyronine, and 25 ng/mL human epidermal growth factor. Cells were plated on collagen-coated dishes and cultured at 37°C in a 5% CO2 atmosphere. As indicated, cells were cultured for 12 h in gland cell medium containing one of the following inflammatory mediators: 100 ng/mL E. coli lipopolysaccharides (LPS, L2637, Sigma-Aldrich), 10 ng/mL TNF-α (Life Technologies) or 10 ng/mL IL-13 (Life Technologies).
Calu-3 human lung adenocarcinoma cells (HTB-55, ATCC, Manassas, VA) were maintained on plastic tissue culture dishes in gland cell medium. For experiments, cells were grown to 80% confluence and then were washed with PBS and their medium switched to Eagle’s minimal essential medium containing 10% fetal bovine serum with or without 10 mU/mL V. cholerae sialidase14 for 12 h. Cells were then washed with PBS and cultured for an additional 12 h in gland cell medium containing inflammatory mediators as above. After incubation, cells were extracted with 300 μL/well of extraction buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM CaCl2, 2 mM MgCl2, 0.3% CHAPS, 1% NP-40 and protease inhibitor cocktail (Sigma-Aldrich)) for 2 h at 4°C with gentle shaking. The extract was centrifuged at 13,500 x g for 5 min and the supernatant stored at −20°C prior to analysis. Protein concentration in each sample was determined using the BCA assay. As indicated, Calu-3 cultures were treated for 12 h prior to extraction with swainsonine, benzyl-2-acetamido-2-deoxy-α-D-galactopyranoside (benzyl-α-GalNAc), N-acetyl-2,3-dehydro-2-deoxyneuraminic acid (Neu5Ac2en), or BAY 11-7082 all from Sigma-Aldrich, or with 3Fax-peracetyl Neu5Ac from EMD-Millipore, Darmstadt.
Siglec-9 ligand precipitation
Calu-3 or inferior turbinate extracts containing 100 μg protein were incubated at 4°C for 16 h in 100 μL extraction buffer containing Siglec-9-Fc (10 μg) with end-over-end mixing. To capture the Siglec-9-Fc with bound Siglec-9 ligands, magnetic Protein-G Sepharose beads (25 μL, GE Healthcare) were added and the reaction incubated for an additional 3–4 h at 4°C with gentle shaking. The magnetic beads with bound Siglec-9-Fc and Siglec-9 ligands were then collected and washed three times with Tris-buffered saline (50 mM Tris-HCl, 150 mM NaCl, pH 7.5). Bound proteins were eluted in 100 μL NuPAGE LDS Sample Buffer (Life Technologies) and subjected to gel electrophoresis and blotting. Control precipitations were performed identically but without addition of Siglec-9-Fc.
Immunoblotting
Detergent-extracted proteins from Calu-3 cells were resolved by SDS gel electrophoresis on composite agarose-acrylamide gels (2% agarose, 1.5% acrylamide) for 2 h at 100 V, conditions suitable for resolution of large glycoprotein molecules (>500 kDa). Resolved proteins were electro-blotted onto PVDF membranes (iBlot, Life Technologies). Membranes were blocked with 5% nonfat milk in PBS containing 0.1% Tween 20 (PBSTw) for 30 min. Siglec-8-Fc (0.5 μg/mL) or Siglec-9-Fc (0.25 μg/mL) were pre-incubated for 30 min on ice with 1:10,000 horseradish peroxidase (HRP)-conjugated anti-human Fc (Sigma-Aldrich). Blots were incubated with precomplexed siglecs for 16 h at 4°C. Alternatively, blots were incubated with rabbit polyclonal anti-MUC5B (1:500 in PBSTw) with subsequent detection using 1:1000 HRP-conjugated goat anti-rabbit IgG (Jackson Immunoresearch). Blots were washed three times in PBSTw and then Siglec and antibody binding were detected using enhanced chemiluminescence (ECL, GE Healthcare).
For detection of nuclear NF-κB p65, cells were collected by scraping, washed in PBS, and nuclear extracts selectively isolated using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific) using the manufacturer’s protocol. Nuclear proteins were resolved by SDS gel electrophoresis on 4–12% NuPAGE Bis-Tris polyacrylamide gels. After blotting to PVDF membranes as above, NF-κB p65 was detected using 0.4 μg/mL of rabbit polyclonal antibody (sc-372, Santa Cruz) followed by secondary antibody and detection as above.
As a loading control, extracts were resolved on 4–12% NuPAGE gels, blotted to PVDF, and GAPDH detected using mouse monoclonal anti-GAPDH antibody (G8795, Sigma-Aldrich) as primary and 1:10,000 HRP-conjugated anti-mouse IgG (Cell Signaling, Danvers, MA) as secondary antibody, with ECL detection as above. ECL images were collected using a CareStream Gel Logic 4000 analyzer, bands quantified using CareStream software, normalized to GAPDH expression, and expressed relative to control.
MUC5B siRNA
To investigate the relationship of Siglec-9 ligand and MUC5B expression in Calu-3 cells, MUC5B siRNA (Santa Cruz, sc-106263) was used to knockdown MUC5B expression. MUC5B siRNA or non-targeting (control) siRNA-A (sc-37007), each at 1 μg/mL, was transfected into Calu-3 cells with Lipofectamine RNAiMAX Transfection Reagent (Life Technologies). Transfected cells were cultured for 72 h prior to further treatments.
Statistics
Data are expressed as mean ± SEM. Statistical significance was tested using paired or unpaired Student’s t-test or ANOVA with Tukey post hoc analysis as indicated.
RESULTS
Siglec-8 and Siglec-9 ligand distribution in human upper airway
Siglecs on the surfaces of inflammatory cells encounter their sialoglycan ligands on target tissues to regulate ongoing inflammation.2 To determine the histological distribution of ligands for the major siglecs of eosinophils and neutrophils, sections from normal human airways were stained with expressed and tagged forms of Siglec-8 and Siglec-9 (Fig. 1, Fig. E1).
FIG. 1.
Siglec-8 and Siglec-9 ligands on human inferior turbinate tissues. Serial tissue sections of normal human inferior turbinate were stained with hematoxylin and eosin (A), Siglec-8-Fc (B,C) or Siglec-9-Fc (D,E). Control sections (C,E) were pretreated with sialidase. Bars = 100 μm. Images are representative of results using tissue sections from six subjects.
Siglec-8 ligands were expressed very selectively on a subpopulation of sub-mucosal gland cells (Fig. 1, B). Intense Siglec-8-Fc binding was detected on cells of the inferior turbinate with the morphological characteristics of serous cells, whereas no binding was detected to cells with mucous cell morphology, to connective tissue or to the airway epithelium. In contrast, Siglec-9 ligands were more broadly distributed on normal inferior turbinate tissue (Fig. 1, D). In addition to strong staining of serous cells of the submucosal glands and the airway epithelium, less intense staining was detected on mucous cells and in connective tissue. Binding of both siglecs was completely absent after the tissue was treated with sialidase, indicating that all binding of the tagged siglecs was to sialoglycan ligands (Fig. 1, C & E). The cellular and tissue distributions of Siglec-8 and Siglec-9 ligands were similar when comparing inferior turbinate with uncinate tissues (Fig. E1).
Siglec-8 and Siglec-9 ligands are upregulated on inflamed upper airways
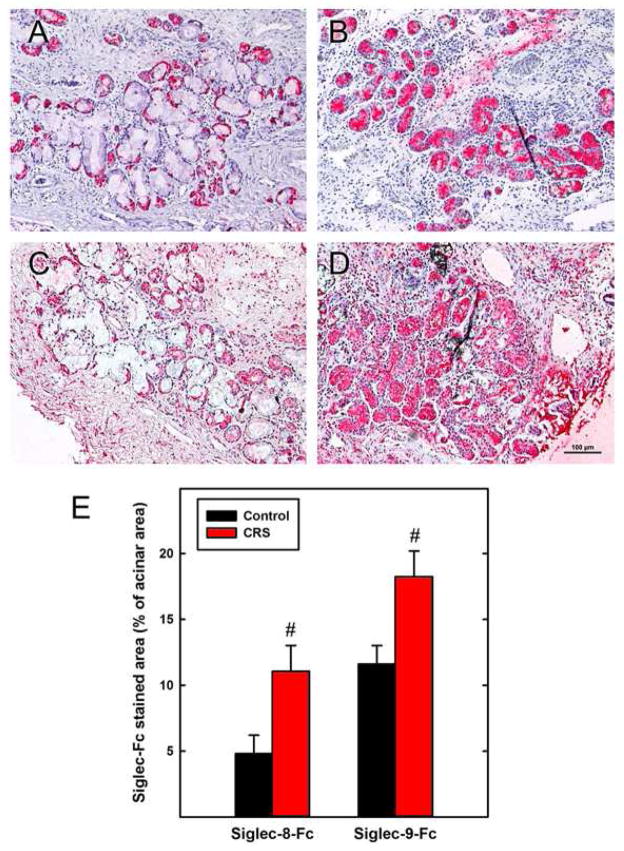
We hypothesized that an ongoing eosinophilic and/or neutrophilic inflammatory response may be counteracted by increased expression of Siglec-8 and Siglec-9 ligands respectively. Consistent with this hypothesis, a comparison of CRS and normal inferior turbinate tissue sections revealed markedly increased binding of both Siglec-8 and Siglec-9 chimeras to chronically inflamed tissues (Fig. 2). Siglec-8 ligand expression extended from serous cells (mostly demilunes on the border of acini) in non-inflamed tissue to stain acini more broadly and intensely in CRS tissues (Fig. 2, A & B). Staining by Siglec-9-Fc also intensified in acini, in connective tissue and on the airway epithelium of CRS compared to non-inflamed tissues (Fig. 2, C & D). Quantitative image analyses revealed that siglec ligands in CRS inferior turbinate covered significantly greater acinar areas compared to normal (Fig. 2, E). These data are consistent with an inflammatory response in human tissues that includes upregulation of siglec ligands.
FIG. 2.
Expression of Siglec-8 and Siglec-9 ligands is increased in CRS. Human inferior turbinate from control subjects (A,C) and CRS patients (B,D) were stained with Siglec-8-Fc (A,B) or Siglec-9-Fc (C,D). Quantification of stained areas within acini (E) demonstrated significant increases in Siglec-8 and Siglec-9 ligand expression in CRS compared to controls (#, p < 0.03, t-test, n=6). Bar = 100 μm.
Comparison of differential Siglec-8 and Siglec-9 ligand expression in inferior turbinate and uncinate tissues from the two major sub-classifications of CRS, those with nasal polyps (CRSwNP) and those without (CRSsNP), revealed quantitatively similar changes (Fig. E1).
Human airway tracheal gland cells and Calu-3 cells in culture express Siglec-9 ligands under inflammatory mediator control
Since Siglec-8 and Siglec-9 ligands are expressed on inferior turbinate submucosal glands, gland cells were cultured from fresh human airways and tested for siglec ligand expression. Although a proportion of the freshly cultured airway gland cells expressed ligands for Siglec-8 and Siglec-9, expression of ligands for Siglec-8 quickly diminished as cells were cultured in vitro (data not shown). Ligands for Siglec-9 were retained on cultured airway gland cells sufficiently long to allow in vitro testing of inflammatory mediators for their effects on Siglec-9 ligand expression (Fig. E2). When cells were treated with bacterial lipopolysaccharide (LPS), tumor necrosis factor alpha (TNF-α) or IL-13, Siglec-9-Fc binding increased.
To provide a ready source of Siglec ligand-expressing cells, the stable human airway cell line Calu-3, a human lung adenocarcinoma cell line with serous cell-like properties,17,18 was tested for siglec expression. Siglec-Fc overlay binding studies revealed that these cells, when grown as submerged cultures, express Siglec-9 ligands (Fig. 3, A) but not Siglec-8 ligands (data not shown). To test whether Calu-3 cells are a model for inflammatory mediator control of Siglec-9 ligand expression, cells were treated with LPS, TNF-α or IL-13 (Fig. 3, B–D). In each case, Siglec-9-Fc binding to treated Calu-3 cells was significantly increased compared to untreated cells (Fig. 3, E). These data were confirmed by protein extraction, gel electrophoresis, and Siglec-9-Fc blotting of ligands from Calu-3 cells (Fig. 4). Since initial studies (not shown) revealed that prominent Siglec-9 ligand bands were all >500 kDa, composite agarose-acrylamide gels were used to resolve very high molecular weight glycoproteins.19 Siglec-9-Fc overlay detection of blotted ligands from Calu-3 cells revealed 3–4 molecular weight species of varied binding intensities ranging in size from ~500 to >4,000 kDa based on their migration relative to ultra-high molecular weight proteins extracted from rat soleus muscle.20 The ligand electrophoretic migration pattern was reproducible, with some experimental variation in the relative binding intensity of each of the molecular weight species. Treatment with inflammatory mediators significantly increased Siglec-9 ligand expression (Fig. 4). Sialidase treatment of intact cells neutralized all Siglec-9 ligands as measured by Siglec-9-Fc blotting of electrophoretic blots (Fig. E3). After sialidase treatment, Calu-3 cells fully recovered their Siglec-9 ligands over a 12 h-period.
FIG. 3.
Inflammatory mediators increase Siglec-9 ligands on Calu-3 cells. Cells were fixed and stained with Siglec-9-Fc. Control cultures (A) are compared to cultures treated with LPS (B), TNF-α (C) or IL-13 (D). Quantification of stained areas (E) demonstrated that each inflammatory mediator increased Siglec-9 ligand expression (†, p < 0.01, ANOVA, n=3). Bar = 100 μm.
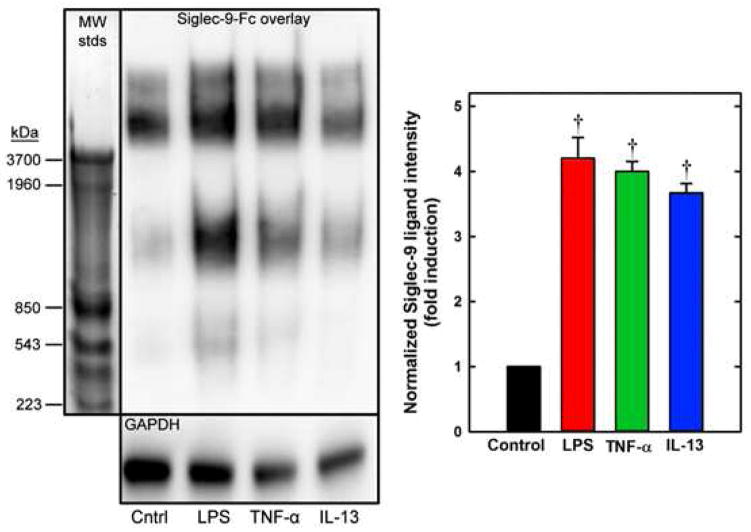
FIG. 4.
Siglec-9 ligands from Calu-3 cells. Left: Calu-3 cell proteins were resolved by gel electrophoresis and stained with Siglec-9-Fc or anti-GAPDH (loading control). Molecular weight standards (MW stds) from rat muscle20 were stained with Coomassie Brilliant Blue. Calu-3 cultures were treated 12 h with LPS, TNF-α, or IL-13 as indicated. Right: Total Siglec-9-Fc band densities relative to GAPDH (†, p < 0.01, paired t-test vs control, n=3).
Mucin 5B is a sialoglycan carrier protein for Siglec-9 ligands
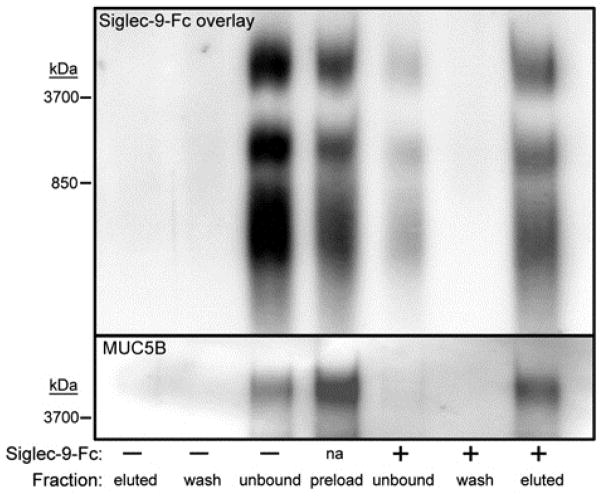
The finding that major ligands for Siglec-9 in Calu-3 cells are very high molecular weight sialoglycoproteins (Fig. 4) suggested that the sialoglycan carrier protein may be a mucin. Mucins range in size from several hundred thousand to several million daltons and carry a large number and variety of sialylated glycans primarily on serine and threonine residues (O-linked glycans).21 The major mucins of airways include MUC1, MUC4, MUC5AC, MUC5B, and MUC16. To determine whether any of these mucins carry Siglec-9 ligand, proteins were extracted from Calu-3 cells and subjected to Siglec-9-Fc lectin affinity capture. Complete capture of Siglec-9 ligands resulted in complete capture of MUC5B (Fig. E4). The other major airway mucins were not detected (data not shown). To determine whether MUC5B carries Siglec-9 ligand sialoglycans in human upper airway tissue, mild detergent extracts of normal human inferior turbinate were subjected to Siglec-9-Fc lectin capture. As with Calu-3 cell extracts, when Siglec-9 ligands were captured from inferior turbinate extracts so was MUC5B (Fig. 5). As will be addressed below, MUC5B sialoglycans represent a substantial subpopulation of Siglec-9 ligands that migrate at very high molecular weight (>4,000 kDa) by composite agarose-acrylamide gel electrophoresis.
FIG. 5.
MUC5B carries Siglec-9 ligands in human inferior turbinate. Detergent extracts of human inferior turbinate were incubated with or without Siglec-9-Fc, then the siglec with bound ligands was captured using Protein G beads. Extract prior to Siglec-9-Fc capture (preload), unbound, wash, and eluted proteins were resolved by gel electrophoresis and stained with Siglec-9-Fc or anti-MUC5B. Data are representative of duplicate experiments.
Inferior turbinate tissues from CRS patients expressed significantly greater amounts of MUC5B than normal subjects, as determined by immunohistochemistry (Fig. 6). MUC5B immunostaining was mostly in submucosal glands and collecting ducts, consistent with the conclusion that it represents a subpopulation of the total Siglec-9 ligands (compare Fig. 6 and Fig. 2, C & D). Consistent with these findings, treatment of Calu-3 cells with inflammatory mediators (LPS, TNF-α or IL-13) resulted in significant increases in multiple Siglec-9 ligand species as well as MUC5B expression migrating at the highest molecular weight of these (Fig. E5).
FIG. 6.
Expression of MUC5B is increased in CRS submucosal gland acini. Human inferior turbinate from a control subject (A) and CRS patient (B) were stained with anti-MUC5B. Quantification of stained areas within acini (C) demonstrated a significant increase in MUC5B expression in CRS tissues (†, p < 0.01, t-test, n=6). Bar = 100 μm.
Further evidence that MUC5B is a carrier of Siglec-9 sialoglycan ligands in Calu-3 cells was obtained using RNA interference. Transfection of Calu-3 cells with MUC5B siRNA resulted in a 72% decrease in MUC5B expression, and a marked decrease in Siglec-9-Fc binding to the largest (> 4,000 kDa) molecular weight ligand (Fig. 7, A). Notably, total Siglec-9-Fc binding to extracted proteins was not reduced by MUC5B siRNA under control conditions (without LPS), since loss of binding to the highest molecular weight species (~4,000 kDa) was matched by coordinately increased binding to a lower molecular weight sialoglycan (~1,000 kDa).
FIG. 7.
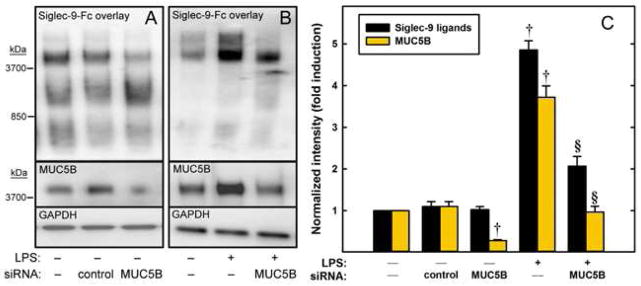
Inhibiting MUC5B expression alters Siglec-9 ligand expression in Calu-3 cells. (A) Cells were transfected with control or MUC5B siRNA or were not transfected. After 72 h, proteins were extracted. (B) Transfected or non-transfected cells were cultured 72 h and then treated for 12 h with sialidase to remove endogenous Siglec-9 ligands. After sialidase washout, cells were treated with or without LPS for an additional 12 h prior to protein extraction. Proteins were resolved by gel electrophoresis, blotted, and stained with Siglec-9-Fc, anti-MUC5B, or anti-GAPDH. (C) Total band densities relative to GAPDH. †, p < 0.01 compared to matched treatment control (n=3, paired t-test). §, p < 0.001 compared to LPS-treated non-transfected cells (n=3, t-test).
These results are consistent with other glycosylation recognition systems. The ligands for siglecs, like other lectins, are the glycans which are added to proteins post-translationally by a suite of Golgi-resident glycosyltransferases. In some cells, when the major protein carrier for a functional glycan is no longer expressed, the target glycan is still constructed, but on an alternate carrier protein. This has been well documented previously in the study of selectin ligands on cancer cells.22
In contrast to untreated Calu-3 cells, re-expression of Siglec-9 ligands on LPS-treated Calu-3 cells was markedly reduced by MUC5B siRNA. Most of the Siglec-9-Fc binding re-expressed by Calu-3 cells in the presence of LPS for 12 h was to the highest molecular weight band(s), and transfection with MUC5B siRNA significantly reduced Siglec-9 ligand levels (Fig. 7, B). We interpret these results as indicating that much of the LPS-induced increase in Siglec-9 ligands is due to sialoglycans on MUC5B, and that the capacity for biosynthesis of increased Siglec-9 ligands on other carrier proteins is quantitatively limited.
LPS-induced increases in Siglec-9 ligand and MUC5B expression occur via NF-κB activation
LPS binds to toll-like receptor 4 (TLR4) to activate NF-κB.23 To evaluate the potential role of this pathway in the increased expression of Siglec-9 ligands, an inhibitor of NF-κB activation, BAY 11-7082,24 was used. As the concentration of the inhibitor was increased there was a coordinate decrease in the LPS-mediated enhanced expression of Siglec-9 ligands and MUC5B until both were equivalent to control levels (no LPS treatment, Fig. 8). The concentration required for half-maximal inhibition (3–6 μM) is consistent with the effects of BAY 11-7082 on other NF-κB-mediated cellular responses.24
FIG. 8.
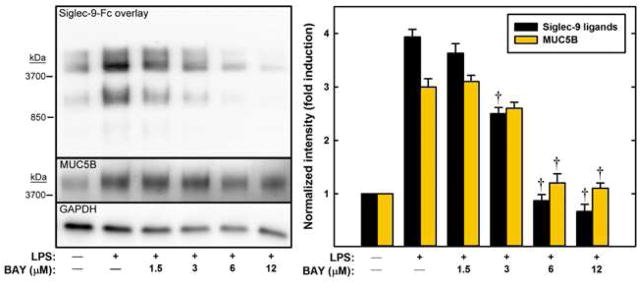
Inhibition of NF-κB translocation blocks LPS-induced increases in the expression of Siglec-9 ligands and MUC5B. Calu-3 cells were treated with LPS (as indicated) for 12 h in the presence of BAY 11-7082. Extracted proteins were resolved by gel electrophoresis, blotted, and stained with Siglec-9-Fc, anti-MUC5B, and anti-GAPDH. Band densities relative to GAPDH are shown. †, p < 0.01 compared to LPS-treated cells in the absence of inhibitor (n=3, ANOVA).
To evaluate LPS enhancement of Siglec-9 ligand glycan biosynthesis, Calu-3 cells were treated with four different glycosylation pathway inhibitors: swainsonine to block N-linked glycan maturation; α-benzyl N-acetylgalactosamine (benzyl-α-GalNAc) to block O-linked glycan maturation; 3-fluoro(axial)sialic acid (3Fax-Neu5Ac) to block sialyltransferases; and Neu5Ac2en to block sialidases. None of the inhibitors significantly reduced total MUC5B protein expression, as measured by immunoblotting. In contrast, benzyl-α-GalNAc and 3Fax-Neu5Ac significantly reduced LPS enhancement of Siglec-9 ligand expression, whereas swainsonine and Neu5Ac2en had no effect (Fig. E6). For comparison, BAY 11-7082 completely blocked LPS-enhanced expression of Siglec-9 ligands and MUC5B in the same experiment. These data indicate that the Siglec-9 ligands of LPS-treated Calu-3 cells are primarily O-linked sialoglycans that are carried on MUC5B.
DISCUSSION
Glycans and glycan binding proteins play important roles in immune system regulation from pathogen recognition to inflammatory cell trafficking to the control of ongoing immune responses.2,25–29 Siglecs expressed on more or less restricted sets of immune cells are often involved in immune inhibition, with many carrying immunoreceptor tyrosine-based inhibitory motifs (ITIMs) on their short cytoplasmic tails.2 Exemplary are Siglec-8 on allergic inflammatory cells (eosinophils, mast cells and basophils) and Siglec-9 on neutrophils, monocytes, dendritic cells and natural killer cells. Each has three extracellular Ig-like domains (the outermost carrying the primary sialic acid binding domain) and ITIM sequences on their cytoplasmic tails.30 Ligation of each siglec on the surfaces of immune cells on which they are carried results in immune inhibition, including apoptosis. Siglec-8 and Siglec-9 likely encounter multivalent target sialoglycan ligands on tissues, leading to their ligation and initiation of their inhibitory program. Activated eosinophils and neutrophils are more susceptible to siglec-mediated inhibition than their naïve counterparts, suggesting that siglecs are well positioned to moderate ongoing inflammatory responses.8,10 Inflammatory target tissues synthesize and express specific multivalent sialoglycan ligands that engage one or more siglecs, leading to directed effects on the subpopulations of immune cells on which the complementary siglecs are expressed.2 A complete understanding of this immune regulatory system will require knowledge of the expression and binding specificities of the different siglecs, as well as the expression of their natural ligands on target tissues. The value of this knowledge is that variations in glycan expression and recognition may contribute to immune function and dysfunction. In addition, synthetic molecules that mimic siglec targets may therapeutically inhibit inflammation.31
The major findings reported here are that Siglec-8 and Siglec-9 ligands are expressed at markedly higher levels in inflamed compared to normal human airways. The sharp increase in both Siglec-8 and Siglec-9 ligand expression in human upper airways from CRS patients is consistent with the hypothesis that these sialoglycans are part of a tissue response to inflammation. Since the same general increase in both Siglec-8 and Siglec-9 ligands was detected in tissues from CRS patients with or without polyps, and in both inferior turbinate and uncinate tissue sections (Fig. E1), we hypothesize that increased Siglec-8 and Siglec-9 ligand expression may be a broadly distributed response to inflammatory states in which there is eosinophilic or neutrophilic infiltration respectively. In the case of CRS, enhanced Siglec-8 and Siglec-9 ligand expression apparently did not prevent disease, although the extent (if any) to which it moderated disease is unknown. Siglec engagement is well documented to lessen inflammation in animal models. Expression of ligands for mouse Siglec-F, a functional paralog of Siglec-8, is increased in mice after allergic challenge or parasitic infection.7,32 Mice engineered to lack Siglec-F or its sialoglycan ligands display exacerbated eosinophilic responses in an allergic disease model,6,7,33 indicating that siglec engagement mitigates inflammation.
The restricted distribution of Siglec-8 ligands compared to those for Siglec-9 (Fig. 2) implies that their increase is not due to a non-specific increase in cross-reacting sialoglycans throughout inflamed tissues, but is a directed response of cells expressing siglec-specific ligands. The restricted expression of Siglec-8 ligands to a subset of submucosal gland cells in upper airway tissue suggests that the target is a secreted molecule that has yet to be identified. This is especially apparent in CRS tissue, where Siglec-8 ligands are upregulated and appear in collecting ducts as well as in the cells where they are produced. Identification of the Siglec-8 ligands will require other methods than those used here, since mild detergent extraction was inefficient at extracting Siglec-8 targets from inferior turbinate tissues, and Calu-3 cells failed to express Siglec-8 ligands under the growth conditions used (data not shown). In contrast, Siglec-9 ligands were amenable to analysis both in mild detergent extracts of inferior turbinate and Calu-3 cells.
Calu-3 is a human bronchial adenocarcinoma cell line with properties in common with human airway submucosal serous cells.17,18 Since Siglec-9 ligands are more broadly distributed in airways than are those of Siglec-8 (Fig. 1), it was not surprising that an airway carcinoma cell line retained the capacity to express Siglec-9 ligands but not Siglec-8 ligands. The ability of multiple inflammatory mediators to acutely induce an increase in Siglec-9 ligands in Calu-3 cells is consistent with the hypothesis that siglec ligand expression is a direct response of airway cells to inflammatory mediators. The availability of a cell line as a model for inflammation allowed a more thorough analysis of both the molecular nature of the Siglec-9 ligand and the signaling pathway responsible for its enhanced expression. The results indicate that MUC5B is a significant (but not the only) protein carrier of the Siglec-9 binding sialoglycan. It is notable that under inflammatory conditions, the proportion of Siglec-9 binding to MUC5B increases, suggesting a directed response that favors biosynthesis of this particular anti-inflammatory sialoglycan on this particular mucin carrier. The data using Calu-3 cells are supported by findings in inferior turbinate, where CRS tissue expressed increased amounts of MUC5B protein along with increased Siglec-9 ligands compared to normal tissue. It is also notable that when Siglec-9-Fc was used to capture ligands extracted from human inferior turbinate most of the MUC5B appeared in the Siglec-9 bound fraction (Fig. 5). Since the expression of the carrier protein occurs independently of the sialoglycans that decorate it,34 this finding indicates highly directed addition of Siglec-9 sialoglycan ligands to MUC5B in inflamed tissue. It is also apparent that MUC5B is one of multiple protein carriers of Siglec-9 sialoglycan ligands, and that other carriers increase their Siglec-9 binding capacity when MUC5B expression is blocked (Fig. 7, A). Since MUC5B is heavily glycosylated, it (or other sialoglycoproteins) can coordinately be a carrier for different siglec ligand glycans under different conditions. Consistent with this concept, mouse Siglec-F, a Siglec-8 paralog, binds to glycans on mouse MUC5B.35
LPS was particularly effective in enhancing Siglec-9 ligand expression in Calu-3 cells. The ability to block this enhancement using an NF-κB inhibitor suggests that biosynthesis of this sialoglycan ligand is under control of TLR4 activation via downstream translocation of NF-κB to the nucleus.36 The coordinate inhibition of Siglec-9 ligands and MUC5B by BAY 11-7082 is consistent with the conclusion that in response to inflammation the sialoglycan ligand for Siglec-9 is biosynthesized largely on MUC5B.
Increased secretions are characteristic of airway inflammation. Consistent with mucus biochemistry,37 we found increased levels of MUC5B by immunohistochemistry in submucosal glands within inferior turbinates of patients compared to controls. Although we did not comprehensively analyze gland density, this finding suggests that the mechanism of increased mucus secretion in inflamed airways may involve both hyperplasia of glands and activation of secretory glandular cells. Another finding of potential interest clinically is that the siglec ligands tended to be localized within the glandular cells and in the ductal spaces rather than parenchymal tissue. If siglec ligand sialoglycans expressed on MUC5B are regulating the survival or activation of neutrophils in airways inflammatory diseases, it may be doing so primarily within the airway lumen. A recent study consistent with this reported that MUC5B was significantly increased in mucus secretion in pediatric CRS.38 In asthma, abundant eosinophils and neutrophils are found within the airway lumen. Thus, it is worthwhile to investigate further whether the apoptosis of inflammatory cells within airways is facilitated by secreted mucins bearing siglec ligands.
Much remains unknown about siglec ligands and their expression in human tissues. Detailed structural information about the Siglec-9 binding site on MUC5B (and other protein carriers) will be informative. Cell surfaces throughout the body are rich in sialic acids carried as diverse sialoglycans on glycoproteins and on glycolipids.39 A challenge for siglecs is to distinguish and selectively bind to a restricted subset of sialoglycans in a metaphorical sea of cell surface sialic acids. This is achieved, in part, by binding specificities that depend on the linkage of the sialic acid to the other glycans in an oligosaccharide and to other binding substituents on the glycan. Using synthetic glycan arrays, we found that Siglec-8 binds with high specificity to an unusual sulfated sialylated trisaccharide (NeuAc α2,3 6-SO3-Gal β1,4 GlcNAc).40 Siglec-9 binds preferentially to a distinct but closely related structure (NeuAc α2,3 Gal β1,4 6-SO3-GlcNAc).2 The extent to which these particular sialoglycan structures are found on human airways has not been well studied. By analogy with other glycan binding proteins, it is likely that the extended glycan structures, additional substituents (such as sulfate), the display of glycans in multivalent arrays, and/or additional interacting groups on the amino acids of the protein carrier itself (distinct from the sialoglycan) cooperate to ensure siglec binding avidity and specificity.41
From a clinical perspective, there are additional factors that may impact siglec ligand expression and function that were not addressed in this study. Most of the patients studied here were non-smokers, and a minority reported steroid use of any type (Table E1). Since both smoking and steroid use would be expected to alter inflammatory responses,42 it would be worthwhile to include these factors in the study of siglec ligand expression and function.
Knowledge of the structures, specificities and expression of siglec ligands on normal and inflamed human tissues promises to reveal the details of this layer of control of immune responses. This information may contribute to enhanced glycomimetic anti-inflammatory drug development.31,43
Supplementary Material
Key Messages.
Sialoglycan ligands for the immune inhibitory sialic acid binding proteins Siglec-8 on eosinophils and Siglec-9 on neutrophils are upregulated in chronically inflamed airways.
Inflammatory mediators enhance the expression of Siglec-9 ligands, including mucin 5B, on human Calu-3 cells via the NF-κB pathway.
Enhanced biosynthesis of inhibitory siglec ligands may be a tissue-level response to dampen ongoing inflammation.
Acknowledgments
Supported by the Lung Inflammatory Disease Program of Excellence in Glycoscience from the National Institutes of Health/National Heart, Lung, and Blood Institute (P01 HL107151). R Schleimer was supported by R37 HL068546 and P01 AI106683 from the NIH, and by the Ernest S. Bazley Foundation. A Gonzalez was supported in part by the NIH-funded Chemistry-Biology Interface Program at Johns Hopkins University (T32 GM080189).
We thank the members of the Northwestern Sinus Center who provided tissues or laboratory support: David Conley, MD, Bruce Tan, MD, Kathleen Harris, MA, James Norton, MS, Lydia Suh, MS and Derek Carter, BA.
Abbreviations used
- BCA
bicinchoninic acid protein assay
- benzyl-α-GalNAc
benzyl-2-acetamido-2-deoxy-α-D-galactopyranoside
- ECL
enhanced chemiluminescence
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HRP
Horseradish peroxidase
- LPS
bacterial lipopolysaccharide
- MUC5B
Mucin 5B
- Neu5Ac2en
N-acetyl-2,3-dehydro-2-deoxyneuraminic acid
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PBS
Dulbecco’s phosphate-buffered saline
- PBSTr
PBS supplemented with 0.1% Triton X-100
- PBSTw
PBS supplemented with 0.1% Tween 20
- PVDF
polyvinylidene fluoride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kane BA, Bryant KJ, McNeil HP, Tedla NT. Termination of Immune Activation: An Essential Component of Healthy Host Immune Responses. J Innate Immun. 2014;6:727–38. doi: 10.1159/000363449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14:653–66. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yokoi H, Choi OH, Hubbard W, Lee HS, Canning BJ, Lee HH, et al. Inhibition of FcepsilonRI-dependent mediator release and calcium flux from human mast cells by sialic acid-binding immunoglobulin-like lectin 8 engagement. J Allergy Clin Immunol. 2008;121:499–505. doi: 10.1016/j.jaci.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Kiwamoto T, Katoh T, Tiemeyer M, Bochner BS. The role of lung epithelial ligands for Siglec-8 and Siglec-F in eosinophilic inflammation. Curr Opin Allergy Clin Immunol. 2013;13:106–11. doi: 10.1097/ACI.0b013e32835b594a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol Ther. 2012;135:327–36. doi: 10.1016/j.pharmthera.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho JY, Song DJ, Pham A, Rosenthal P, Miller M, Dayan S, et al. Chronic OVA allergen challenged Siglec-F deficient mice have increased mucus, remodeling, and epithelial Siglec-F ligands which are up-regulated by IL-4 and IL-13. Respir Res. 2010;11:154. doi: 10.1186/1465-9921-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 2007;109:4280–7. doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Gunten S, Yousefi S, Seitz M, Jakob SM, Schaffner T, Seger R, et al. Siglec-9 transduces apoptotic and nonapoptotic death signals into neutrophils depending on the proinflammatory cytokine environment. Blood. 2005;106:1423–31. doi: 10.1182/blood-2004-10-4112. [DOI] [PubMed] [Google Scholar]
- 9.McMillan SJ, Sharma RS, McKenzie EJ, Richards HE, Zhang J, Prescott A, et al. Siglec-E is a negative regulator of acute pulmonary neutrophil inflammation and suppresses CD11b beta2-integrin-dependent signaling. Blood. 2013;121:2084–94. doi: 10.1182/blood-2012-08-449983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters Siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008;38:121–4. doi: 10.1165/rcmb.2007-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan BK, Schleimer RP, Kern RC. Perspectives on the etiology of chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2010;18:21–6. doi: 10.1097/MOO.0b013e3283350053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114:155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–8. doi: 10.1016/j.jaci.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Mountney A, Zahner MR, Lorenzini I, Oudega M, Schramm LP, Schnaar RL. Sialidase enhances recovery from spinal cord contusion injury. Proc Natl Acad Sci U S A. 2010;107:11561–6. doi: 10.1073/pnas.1006683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sommerhoff CP, Finkbeiner WE. Human tracheobronchial submucosal gland cells in culture. Am J Respir Cell Mol Biol. 1990;2:41–50. doi: 10.1165/ajrcmb/2.1.41. [DOI] [PubMed] [Google Scholar]
- 16.Casalino-Matsuda SM, Monzon ME, Conner GE, Salathe M, Forteza RM. Role of hyaluronan and reactive oxygen species in tissue kallikrein-mediated epidermal growth factor receptor activation in human airways. J Biol Chem. 2004;279:21606–16. doi: 10.1074/jbc.M309950200. [DOI] [PubMed] [Google Scholar]
- 17.Banga A, Flaig S, Lewis S, Winfree S, Blazer-Yost BL. Epinephrine stimulation of anion secretion in the Calu-3 serous cell model. Am J Physiol Lung Cell Mol Physiol. 2014;306:L937–L946. doi: 10.1152/ajplung.00190.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bridges RJ. Mechanisms of bicarbonate secretion: lessons from the airways. Cold Spring Harb Perspect Med. 2012;2:a015016. doi: 10.1101/cshperspect.a015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Issa SM, Schulz BL, Packer NH, Karlsson NG. Analysis of mucosal mucins separated by SDS-urea agarose polyacrylamide composite gel electrophoresis. Electrophoresis. 2011;32:3554–63. doi: 10.1002/elps.201100374. [DOI] [PubMed] [Google Scholar]
- 20.Greaser ML, Warren CM. Protein electrophoresis in agarose gels for separating high molecular weight proteins. Methods Mol Biol. 2012;869:111–8. doi: 10.1007/978-1-61779-821-4_10. [DOI] [PubMed] [Google Scholar]
- 21.Lillehoj EP, Kato K, Lu W, Kim KC. Cellular and molecular biology of airway mucins. Int Rev Cell Mol Biol. 2013;303:139–202. doi: 10.1016/B978-0-12-407697-6.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas SN, Schnaar RL, Konstantopoulos K. Podocalyxin-like protein is an E-/L-selectin ligand on colon carcinoma cells: comparative biochemical properties of selectin ligands in host and tumor cells. Am J Physiol Cell Physiol. 2009;296:C505–C513. doi: 10.1152/ajpcell.00472.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Neill LA, Bryant CE, Doyle SL. Therapeutic targeting of Toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacol Rev. 2009;61:177–97. doi: 10.1124/pr.109.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, et al. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- 25.Johnson JL, Jones MB, Ryan SO, Cobb BA. The regulatory power of glycans and their binding partners in immunity. Trends Immunol. 2013;34:290–8. doi: 10.1016/j.it.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabinovich GA, van Kooyk Y, Cobb BA. Glycobiology of immune responses. Ann N Y Acad Sci. 2012;1253:1–15. doi: 10.1111/j.1749-6632.2012.06492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008;9:593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 29.Schnaar RL. Glycans and glycan binding proteins in immune regulation: A concise introduction to glycobiology for the allergist. J Allergy Clin Immunol. 2014:135. doi: 10.1016/j.jaci.2014.10.057. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bochner BS, Zimmermann N. Role of siglecs and related glycan-binding proteins in immune responses and immunoregulation. J Allergy Clin Immunol. 2014:135. doi: 10.1016/j.jaci.2014.11.031. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ernst B, Magnani JL. From carbohydrate leads to glycomimetic drugs. Nat Rev Drug Discov. 2009;8:661–77. doi: 10.1038/nrd2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patnode ML, Cheng CW, Chou CC, Singer MS, Elin MS, Uchimura K, et al. Galactose 6-O-sulfotransferases are not required for the generation of Siglec-F ligands in leukocytes or lung tissue. J Biol Chem. 2013;288:26533–45. doi: 10.1074/jbc.M113.485409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiwamoto T, Brummet ME, Wu F, Motari MG, Smith DF, Schnaar RL, et al. Mice deficient in the St3gal3 gene product alpha2,3 sialyltransferase (ST3Gal-III) exhibit enhanced allergic eosinophilic airway inflammation. J Allergy Clin Immunol. 2014;133:240–7. doi: 10.1016/j.jaci.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An HJ, Froehlich JW, Lebrilla CB. Determination of glycosylation sites and site-specific heterogeneity in glycoproteins. Curr Opin Chem Biol. 2009;13:421–6. doi: 10.1016/j.cbpa.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiwamoto T, Katoh T, Evans CM, Janssen WJ, Brummet ME, Hudson SA, et al. Endogenous airway mucins carry glycans that bind Siglec-F and induce eosinophil apoptosis. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.10.027. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 37.Viswanathan H, Brownlee IA, Pearson JP, Carrie S. MUC5B secretion is up-regulated in sinusitis compared with controls. Am J Rhinol. 2006;20:554–7. doi: 10.2500/ajr.2006.20.2935. [DOI] [PubMed] [Google Scholar]
- 38.Saieg A, Brown KJ, Pena MT, Rose MC, Preciado D. Proteomic analysis of pediatric sinonasal secretions shows increased MUC5B mucin in CRS. Pediatr Res. 2014 doi: 10.1038/pr.2014.187. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.Cohen M, Varki A. The sialome--far more than the sum of its parts. OMICS. 2010;14:455–64. doi: 10.1089/omi.2009.0148. [DOI] [PubMed] [Google Scholar]
- 40.Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, et al. Glycan array screening reveals a candidate ligand for siglec-8. J Biol Chem. 2005;280:4307–12. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- 41.Taylor ME, Drickamer K. Convergent and divergent mechanisms of sugar recognition across kingdoms. Curr Opin Struct Biol. 2014;28C:14–22. doi: 10.1016/j.sbi.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamilos DL. Chronic rhinosinusitis: epidemiology and medical management. J Allergy Clin Immunol. 2011;128:693–707. doi: 10.1016/j.jaci.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Chang J, Patton JT, Sarkar A, Ernst B, Magnani JL, Frenette PS. GMI-1070, a novel pan-selectin antagonist, reverses acute vascular occlusions in sickle cell mice. Blood. 2010;116:1779–86. doi: 10.1182/blood-2009-12-260513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.