Abstract
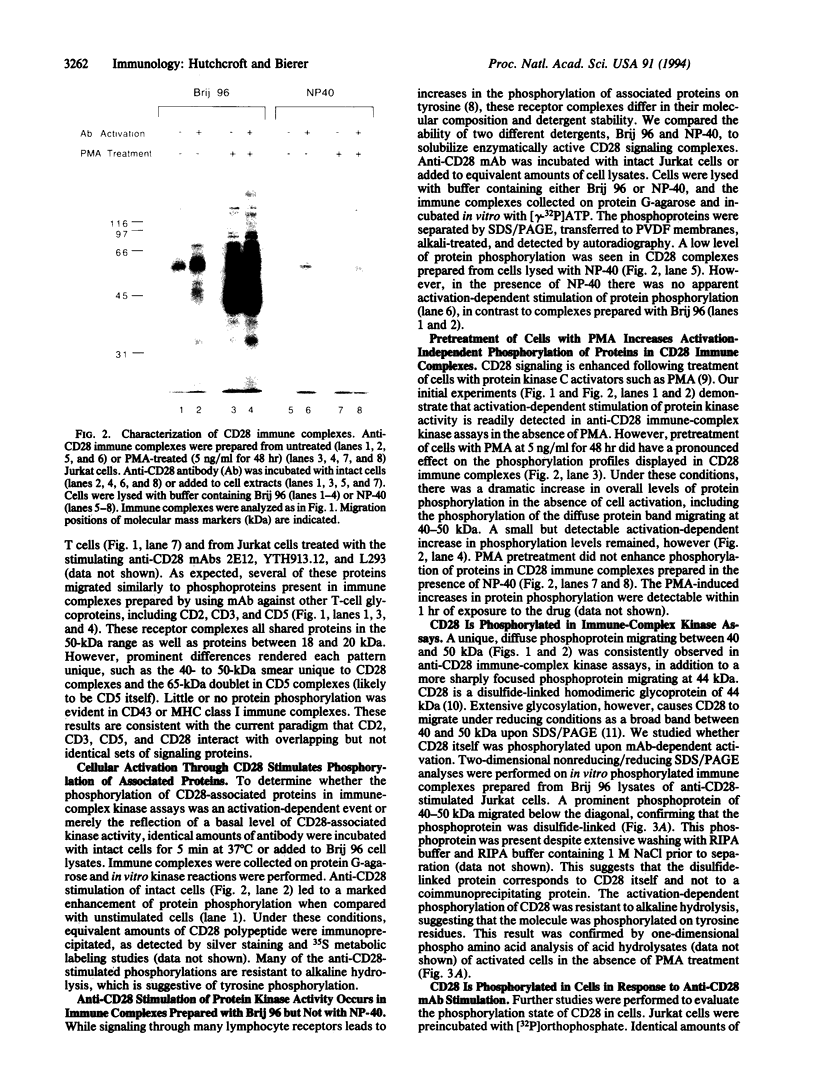
CD28 is a costimulatory receptor that can provide the second signal necessary for T-cell activation and function in response to stimulation through the T-cell antigen receptor/CD3 complex. We found that a distinct array of proteins was phosphorylated on tyrosine following stimulation with anti-CD28 monoclonal antibody, as detected by immune-complex kinase assays. Anti-CD28 stimulation of in vitro kinase activity was detergent-dependent, occurring in immune complexes prepared with Brij 96 but not Nonidet P-40. Pretreatment of cells with low concentrations of phorbol ester increased the activation-independent phosphorylation of proteins in CD28 immune complexes. Reimmunoprecipitation studies indicated that the cytoplasmic protein-tyrosine kinases Lck and Fyn were associated with CD28. CD28 itself was phosphorylated both in vitro and in vivo in an activation-dependent manner, as detected by nonreducing/reducing SDS/PAGE analyses. The activation-stimulated phosphorylation of CD28 may play a key role in signaling through this receptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma M., Ito D., Yagita H., Okumura K., Phillips J. H., Lanier L. L., Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993 Nov 4;366(6450):76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Freeman G. J., Gribben J. G., Boussiotis V. A., Ng J. W., Restivo V. A., Jr, Lombard L. A., Gray G. S., Nadler L. M. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993 Nov 5;262(5135):909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- Gmünder H., Lesslauer W. A 45-kDa human T-cell membrane glycoprotein functions in the regulation of cell proliferative responses. Eur J Biochem. 1984 Jul 2;142(1):153–160. doi: 10.1111/j.1432-1033.1984.tb08263.x. [DOI] [PubMed] [Google Scholar]
- Hara T., Fu S. M., Hansen J. A. Human T cell activation. II. A new activation pathway used by a major T cell population via a disulfide-bonded dimer of a 44 kilodalton polypeptide (9.3 antigen). J Exp Med. 1985 Jun 1;161(6):1513–1524. doi: 10.1084/jem.161.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T., Fu S. M. Human T cell activation. I. Monocyte-independent activation and proliferation induced by anti-T3 monoclonal antibodies in the presence of tumor promoter 12-o-tetradecanoyl phorbol-13 acetate. J Exp Med. 1985 Apr 1;161(4):641–656. doi: 10.1084/jem.161.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Samelson L. E. T cell antigen receptor activation pathways: the tyrosine kinase connection. Cell. 1991 Mar 8;64(5):875–878. doi: 10.1016/0092-8674(91)90310-u. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Imboden J. B., Schieven G. L., Grosmaire L. S., Rabinovitch P. S., Lindsten T., Thompson C. B., June C. H. CD28 ligation in T-cell activation: evidence for two signal transduction pathways. Blood. 1990 Apr 1;75(7):1531–1539. [PubMed] [Google Scholar]
- Lesslauer W., Gmünder H. Biochemical characterization of the 9.3 antigens of human T-cells: simultaneous expression of disulfide-bonded 90-kilodalton dimers and free subunits at the cell surface. Mol Immunol. 1986 Mar;23(3):271–278. doi: 10.1016/0161-5890(86)90053-2. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Ledbetter J. A. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- Lu Y., Granelli-Piperno A., Bjorndahl J. M., Phillips C. A., Trevillyan J. M. CD28-induced T cell activation. Evidence for a protein-tyrosine kinase signal transduction pathway. J Immunol. 1992 Jul 1;149(1):24–29. [PubMed] [Google Scholar]
- Perlmutter R. M., Levin S. D., Appleby M. W., Anderson S. J., Alberola-Ila J. Regulation of lymphocyte function by protein phosphorylation. Annu Rev Immunol. 1993;11:451–499. doi: 10.1146/annurev.iy.11.040193.002315. [DOI] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Kenney D. M., Parkman R., Cairns L., Savage B., Rosen F. S. Characterization of a human lymphocyte surface sialoglycoprotein that is defective in Wiskott-Aldrich syndrome. J Exp Med. 1984 Jun 1;159(6):1705–1723. doi: 10.1084/jem.159.6.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992 Dec 24;71(7):1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- Turka L. A., Ledbetter J. A., Lee K., June C. H., Thompson C. B. CD28 is an inducible T cell surface antigen that transduces a proliferative signal in CD3+ mature thymocytes. J Immunol. 1990 Mar 1;144(5):1646–1653. [PubMed] [Google Scholar]
- Vandenberghe P., Freeman G. J., Nadler L. M., Fletcher M. C., Kamoun M., Turka L. A., Ledbetter J. A., Thompson C. B., June C. H. Antibody and B7/BB1-mediated ligation of the CD28 receptor induces tyrosine phosphorylation in human T cells. J Exp Med. 1992 Apr 1;175(4):951–960. doi: 10.1084/jem.175.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A., Horak I. D., Bolen J. B. Post-translational alterations of the tyrosine kinase p56lck in response to activators of protein kinase C. Oncogene Res. 1988 May;2(4):385–401. [PubMed] [Google Scholar]