Abstract
The small ubiquitin-related modifier (SUMO) participates in various cellular processes, including maintenance of genome integrity, nuclear transport, transcription and signal transduction. However, the biological function of sumoylation in hematopoiesis has not been fully explored. We show here that definitive hematopoietic stem/progenitor cells (HSPCs) are depleted in SUMO-deficient zebrafish embryos. Impairment of sumoylation attenuates HSPC generation and proliferation. The hyposumoylation triggered HSPC defects are CCAAT/enhancer-binding protein α (C/ebpα) dependent. Critically, a SUMO-C/ebpα fusion rescues the defective hematopoiesis in SUMO-deficient embryos, at least in part through restored runx1 expression. While C/ebpα-dependent transcription is involved in myeloid differentiation, our studies here reveal that C/ebpα sumoylation is essential for HSPC development during definitive hematopoiesis.
In vertebrates, hematopoiesis is a dynamic process involving primitive and definitive waves. Primitive hematopoiesis is transitory and mainly produces erythrocytes and macrophages. Definitive hematopoiesis generates self-renewing pluripotent HSPCs capable of giving rise to all blood cell lineages1. Over the last decade, the zebrafish (Danio rerio) has emerged as an excellent model organism in hematopoiesis research2,3,4. In zebrafish, the primitive wave of hematopoiesis gives rise to myeloid cells in the anterior lateral mesoderm (ALM) and to mostly erythrocytes and some myeloid cells in the posterior lateral mesoderm (PLM), which later becomes the intermediate cell mass (ICM) at 18 hours post fertilization (hpf). The definitive wave of hematopoiesis that contains HSPCs originates in the aorta-gonad-mesonephros (AGM) region at 30 hpf. Subsequently, the HSPCs migrate to the caudal hematopoietic tissue (CHT) in the posterior region of the tail at 48 hpf, and finally colonize the kidney where adult hematopoiesis occurs at 4 days post fertilization (dpf)5. Hematopoiesis is evolutionarily conserved from zebrafish to mammals. The AGM, the CHT and the kidney of zebrafish are functional analogs to the AGM, the fetal liver and the bone marrow of mammals, respectively6.
The SUMO proteins belong to the growing family of ubiquitin-like proteins (UBLs) involved in posttranslational modification. Sumoylation is carried out by a multistep enzymatic cascade reaction consisting of a SUMO-activating enzyme (E1), a heterodimer of Sae1 and Sae2, a unique SUMO-conjugating enzyme (E2), Ubc9, and SUMO ligases (E3), which facilitate attachment of SUMO to the substrates7. In vertebrates, at least three SUMO paralogues have been identified, designated SUMO1-3. Sumoylation regulates a wide variety of cellular processes such as transcription, DNA repair, trafficking and signal transduction8. Our previous work showed that sumoylation played an important role in the myelo-erythroid progenitor cell (MPC) fate decision during primitive hematopoiesis of zebrafish9. However, the functional role of sumoylation during definitive hematopoiesis is still unknown.
Here we show that loss of SUMO conjugation results in decreased numbers of HSPC. The impairment of HSPC in SUMO-deficient embryos is due to reduced HSPC generation and proliferation. Mechanistically, hyposumoylation-triggered HSPC depletion is C/ebpα dependent and a SUMO-C/ebpα fusion protein can restore defective definitive hematopoiesis. Thus, the fine-tuning of C/ebpα sumoylation is critical for definitive HSPCs homeostasis.
Results
Loss of SUMO leads to definitive hematopoiesis defects
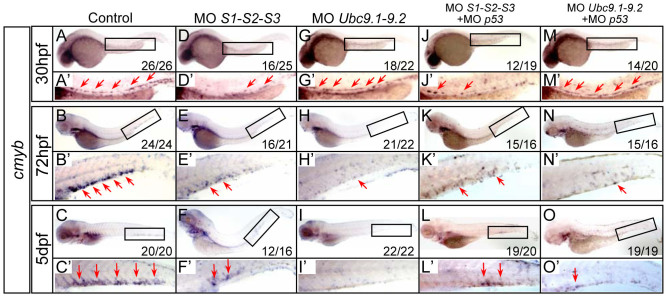
As our previous studies demonstrated that lack of sumoylation biased primitive hematopoiesis9, here we questioned its role in definitive hematopoiesis. To evaluate the effects of sumoylation on definitive hematopoietic development, the temporal and spatial expression patterns of a panel of HSPC and lineage-specific markers were examined by whole-mount mRNA in situ hybridization (WISH) after antisense MO-mediated knock-down. The MOs efficacy has been successfully validated previously10,11. Compared with control embryos (Fig. 1 A–C), the expression of cmyb, a marker of HSPCs, was strikingly diminished in the AGM from 30 hpf onward in SUMO-deficient embryos (Fig. 1D), and almost no cmyb signals were detected in the CHT at 5 dpf (Fig. 1F), consistent with the phenotypes observed in the sae1 mutant, a SUMO E1 mutant line12. Similarly, the expression of another HSPCs marker, runx1, was also markedly decreased in the AGM in SUMOs morphants (Supplementary Fig. 1A). Two Ubc9 genes (Ubc9.1 and Ubc9.2), encoding a unique SUMO-conjugating enzyme of sumoylation pathway, have been identified in zebrafish11,13. As expected, knockdown of Ubc9 also led to depleted HSPCs in the CHT, as indicated by reduced cmyb expression at 72 hpf and 5 dpf (Fig. 1 H–I). The cmyb expression level was relatively unchanged in Ubc9 morphants up to 30 hpf (Fig. 1G), possibly due to maternal Ubc9 protein11. Consistent with WISH data, loss of SUMOs or Ubc9 resulted in considerably reduced cmyb-EGFP positive cells in the CHT of a Tg (cmyb-EGFP) transgenic line (Supplementary Fig. 1 B–C). To rule out the MOs off-target effects mediated through p53 activation14, SUMOs or Ubc9 MO was injected with p53 MO or into p53 null mutants, respectively. Similar phenotypes were observed in the co-injected embryos or p53 null mutants (Fig. 1 J–O and Supplementary Fig. 1D). Collectively, these data indicate that impairment of sumoylation leads to depletion of HSPCs during definitive hematopoiesis.
Figure 1. HSPCs are depleted in SUMO-deficient embryos.
(A–O) WISH assay of cmyb at 30 hpf (A, D, G, J, M), 72 hpf (B, E, H, K, N) and 5 dpf (C, F, I, L, O). Boxed regions indicate the AGM or CHT, respectively. (A′–O′) Magnified images of corresponding boxed regions from A to O, respectively. Red arrows identify cmyb-positive cells in the AGM or CHT, respectively. MO, morpholino oligonucleotides.
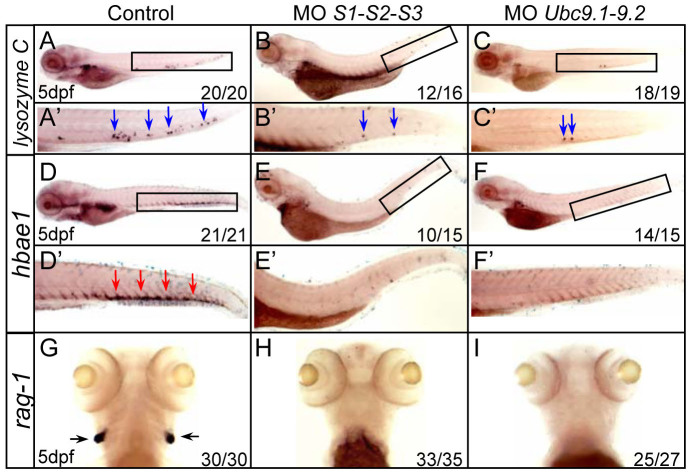
To further confirm that HSPCs were depleted in SUMO-deficient embryos, lineage-specific markers related to definitive hematopoiesis were examined. Expression of the myeloid lineage marker lysozyme C and erythroid lineage marker hbae1 were drastically decreased in both SUMOs and Ubc9 morphants (Fig. 2 A–F). Expression of the lymphoid lineage marker rag1 was absent in the developing thymus compared with control siblings (Fig. 2 G–I). Thus, these results demonstrate that the three major definitive hematopoitic lineages are all compromised in SUMO-deficient embryos.
Figure 2. Multiple blood cell lineages are impaired in SUMO-deficient embryos.
(A–I) WISH assays of lysozyme C (A–C), hbae1 (D–F) and rag1 (G–I) at 5 dpf. Boxed regions indicate the CHT (A–F). (A′–F′) Magnified images of corresponding boxed regions from A to F, respectively. Blue arrows identify lysozyme C-positive cells in the CHT (A′–C′). Red arrows identify hbae1-positive cells in the CHT (D′–F′). Black arrows identify rag1-positive cells in the thymus (G–I).
The defects of definitive hematopoiesis are due to reduced HSPC generation and proliferation
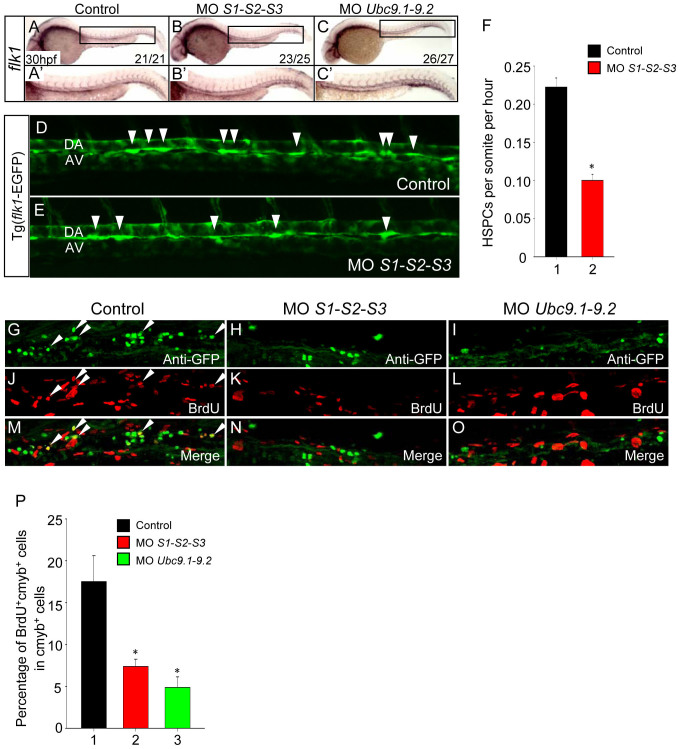
HSPCs arise directly from the aortic endothelium during embryogenesis15,16,17. To assess whether the depletion of HSPCs in SUMO-deficient embryos was caused by improper vascular morphogenesis, the expression of flk1, a marker of endothelial cells, was examined. No overt differences were observed between control siblings and SUMOs or Ubc9 morphants (Fig. 3 A–C), suggesting that the vascular system remains intact. Accordingly, a similar phenotype was also detected in the Tg (flk1-EGFP) transgenic line (data not shown). Next, we investigated whether the HSPC budding process was affected in SUMO-deficient embryos by confocal time-lapse imaging experiments. The results showed that the frequency of HSPC generation was profoundly decreased in SUMO-deficient embryos (0.1 cells per somite per hour, n = 3) compared to that of the control siblings (0.22 cells per somite per hour, n = 3) (Fig. 3 D–F; Supplementary video S1 and S2). To further explore whether cell apoptosis or proliferation was affected following HSPC generation, TUNEL and BrdU incorporation assays were performed in the Tg (cmyb-EGFP) transgenic line. Double immunostaining revealed no significant differences in apoptotic HSPC numbers between control siblings and SUMO-deficient embryos (Supplementary Fig. 2). In contrast, the percentage of cmyb-EGFP/BrdU double positive cells very significantly decreased in the CHT region of both SUMOs and Ubc9 morphants compared with control siblings (Fig. 3 G–P). Taken together, these data suggest that the impairment of definitive hematopoiesis is attributed, at least in part, to reduced HSPC generation and subsequent hypoproliferation.
Figure 3. Loss of SUMO reduces HSPC generation and proliferation.
(A–C) The vasculature is intact in SUMOs or Ubc9 morphants. WISH assay of flk1 at 30 hpf. (A′–C′) Magnified images of corresponding boxed regions from A to C, respectively. (D–E) The frequency of HSPC generation was reduced in SUMO-deficient embryos. Time-lapse confocal imaging analyses of HSPC generation from Tg (flk1-EGFP) line from 30 to 50 hpf. Representative pictures were captured from Video S1 and Video S2, respectively. Arrowheads indicate the budding cells. DA, dorsal aorta; AV, axial vein. (F) Statistical analyses of the frequency of HSPC generation. Data shown are the mean ± SEM, n = 3, *P < 0.01 by student's t-test. (G–O) The proliferation of HSPC is reduced in SUMOs or Ubc9 morphants. Double immunostaining of cmyb-EGFP (G–I) and BrdU (J–L) in the CHT of Tg (cmyb-EGFP) line at 72 hpf. The bottom panel shows merged images (M–O). (P) Quantification of BrdU and cmyb-EGFP double positive cells in the CHT of Tg (cmyb-EGFP) line at 72 hpf. Data shown are the mean ± SD, n ≥ 3, *P < 0.01 by student's t-test.
Sumoylation of C/ebpα is implicated in HSPC development
C/ebpα is a member of the basic leucine zipper protein family of transcription factors, which contain a basic region (BR) and a leucine zipper domain at the C-terminus18,19. In the mouse haematopoietic system, C/ebpα not only plays a pivotal role in granulopoiesis20, but also regulates the self-renewal and proliferation of HSPC at a much earlier stage21,22,23,24. Both C/ebpα-deficient fetal and adult HSPCs exhibit enhanced proliferation and competitive repopulation activities21,22,24, whilst activation of C/ebpα in HSPCs leads to their reduced self-renewal and proliferation23. We have previously shown that zebrafish C/ebpα can be sumoylated, triggering transcriptional repression9. Thus, herein we investigated the potential role of C/ebpα sumoylation in the regulation of HSPC homeostasis.
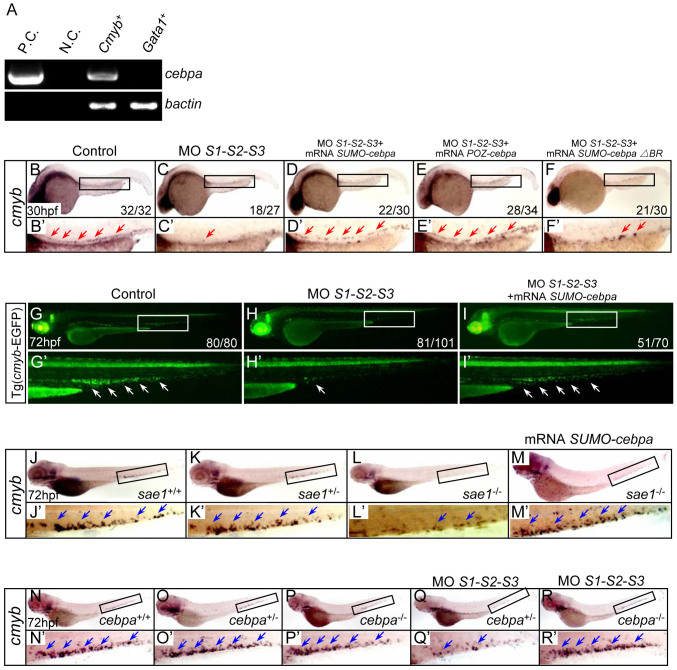
Firstly, to examine whether cebpa is expressed in HSPCs, homogenous EGFP positive cells were sorted from the Tg (cmyb-EGFP) transgenic line. In parallel, EGFP positive cells sorted from the Tg (gata1-EGFP) transgenic line served as a control. RT-PCR analysis showed that cebpa was expressed in the cmyb-EGFP positive cells, whilst no signal was detected in the gata1-EGFP positive cells (Fig. 4A), suggesting that cebpa may be expressed in the HSPCs of zebrafish, consistent with previous findings observed in mice25.
Figure 4. Sumoylation of C/ebpα is involved in HSPC development.
(A) The expression of cebpa was examined by RT-PCR analysis. EGFP positive cells were sorted from the Tg (cmyb-EGFP) and Tg (gata1-EGFP) transgenic lines at 72 hpf, respectively, and subjected to RT-PCR. As a control, transcripts of the bactin gene were amplified. P.C., positive control (plasmid). N.C., negative control (H2O). (B–F) WISH assay of cmyb at 30 hpf. Boxed regions indicate the AGM. (B′–F′) Magnified images of corresponding boxed regions from B to F, respectively. Red arrows identify cmyb-positive cells in the AGM. (G–I) Fluorescent images of the Tg (cmyb-EGFP) line at 72 hpf. (G′–I′) Magnified images of corresponding boxed regions from G to I, respectively. White arrows identify cmyb-positive cells in the CHT. (J–M) WISH assay of cmyb in the sae1 mutant embryos and siblings at 72 hpf. (N–R) WISH assay of cmyb in the cebpa mutant embryos and siblings at 72 hpf. (J′–R′) Magnified images of corresponding boxed regions from J to R, respectively. Blue arrows identify cmyb-positive cells in the CHT.
Secondly, to determine whether sumoylation of C/ebpα was involved in the defects of HSPC mediated by SUMO-deficiency, a series of rescue assays were carried out. Our previous work showed that, a SUMO-C/ebpα fusion protein, mimicking the constitutively sumoylated form of C/ebpα, and a POZ-C/ebpα fusion protein, mimicking the SUMO-mediated repressive form of C/ebpα, could significantly inhibit the transcriptional activity of wide type (WT) C/ebpα9. This allowed us to assess the rescue effects of the fusion proteins in the SUMO-deficient embryos. Critically, in vivo rescue assays showed that SUMO-C/ebpα or POZ-C/ebpα could effectively restore the SUMO-deficiency-mediated phenotypes (Fig. 4 B–E and Supplementary Fig. 3A), whereas SUMO-C/ebpα ΔBR (which lacks the basic region of C/ebpα and has no transcriptional activity and no repressive effect on the WT C/ebpα) (Supplementary Fig. 3B), was ineffective (Fig. 4F). Consistently, similar rescue effects using the fusion proteins were also observed in the Tg (cmyb-EGFP) transgenic line (Fig. 4 G–I). Overexpression of SUMO-C/ebpα, POZ-C/ebpα or SUMO-C/ebpα ΔBR alone had no obvious effect on HSPC development (Supplementary Fig. 3C). Collectively, these data suggest that sumoylation of C/ebpα is implicated in the phenotypes triggered by SUMO-deficiency.
Similarly, in the sae1 mutation zebrafish line (encoding a key subunit of the E1 sumoylation pathway enzyme), systemic sumoylation was reduced and led to depletion of HSPCs in the CHT12. Again, SUMO-C/ebpα was also able to rescue the defects of HSPC in this mutant (Fig. 4 J–M), further confirming that sumoylation of C/ebpα is essential for the maintenance of HSPCs during definitive hematopoiesis.
To further confirm that sumoylation of C/ebpα is required for HSPC development, a cebpa mutant zebrafish line was generated using TALEN technology (Supplementary Fig. 4). There was no significant difference in the expression of cmyb between cebpa mutants and control siblings (Fig. 4 N–P). However, the expression of cmyb was only markedly reduced in SUMO-deficient cebpa heterozygous embryos (Fig. 4Q), and not changed in SUMO-deficient cebpa null homozygous embryos (Fig. 4R). These data demonstrate that C/ebpα is the major effector responsible for the defects triggered by SUMO-deficiency during definitive hematopoiesis in zebrafish.
Runx1 is involved in C/ebpα sumoylation-dependent HSPC development
It has been reported that C/ebpα inhibits cell proliferation through regulating p21 protein stability26,27. To examine whether distinct C/ebpα sumoylation patterns could affect p21 protein stability, a p21 pulse-chase experiment was performed in the presence of either WT C/ebpα, unsumoylatable C/ebpα K125R or SUMO-C/ebpα. No significant differences in the p21 protein half-lives were observed (data not shown), suggesting that the defects of HSPC in SUMO-deficient embryos may not be due to the stabilization of p21.
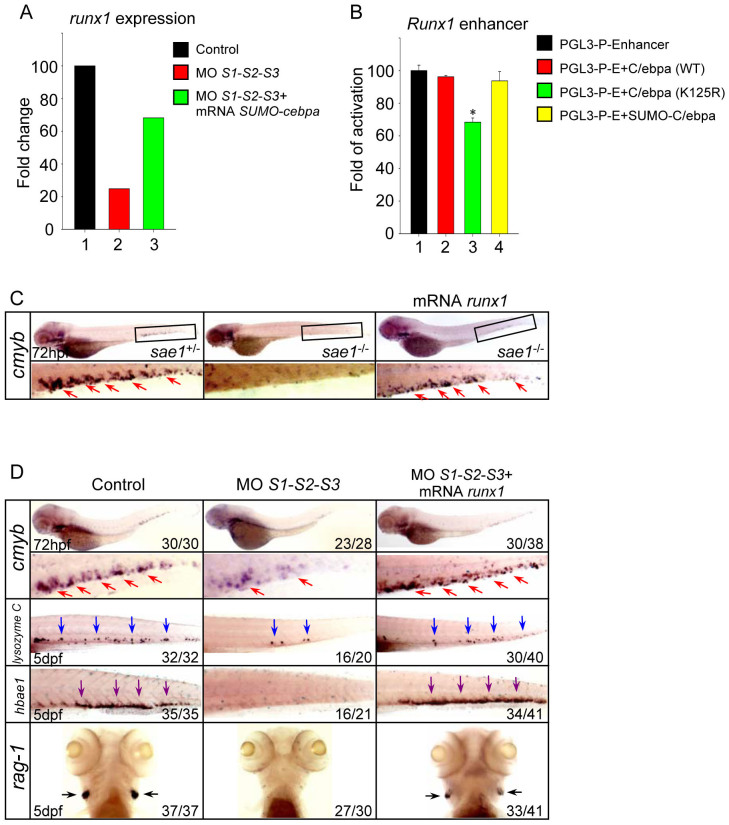
The basic region of C/ebpα plays a key role in the binding of DNA, which is essential for transcriptional activation (Supplementary Fig. 3A). The SUMO-C/ebpα ΔBR fusion protein, which lacks the basic region, was unable to rescue SUMO-deficient embryos, indicating that the transcriptional activity of C/ebpα is essential. To determine the potential downstream genes of C/ebpα, Affymetrix-based global gene expression analysis was performed on cmyb-positive HSPCs sorted from the Tg (cmyb-EGFP) line injected with either SUMO MOs or SUMO MOs plus SUMO-cebpa mRNA. Microarray analysis showed that the expression of runx1, a key regulator of definitive hematopoiesis28, was down-regulated in SUMOs morphants, while SUMO-C/ebpα could restore its expression (Fig. 5A), implying that runx1 might be regulated by the distinct sumoylation status of C/ebpα. In order to assess whether the sumoylation status of C/ebpα had a distinct effect on runx1 expression, the mouse Runx1 enhancer was cloned into a pGL3-Promoter vector29 and luciferase assays performed. The data revealed that C/ebpα K125R, the unsumoylatable form, significantly inhibited Runx1 enhancer activity (Fig. 5B), demonstrating that desumoylated C/ebpα could negatively regulate runx1 expression. To further investigate whether Runx1 was implicated in the defects of HSPC mediated by SUMO-deficiency in vivo, runx1 mRNA was injected at the 1-cell stage in the sae1 mutant embryos or SUMOs morphants. The results showed that Runx1 could efficiently rescue the reduced HSPCs both in the sae1 mutants and SUMOs morphants (Fig. 5 C–D). Furthermore, the expression of lineage-specific markers was also restored (Fig. 5D). Thus, our data suggests that Runx1, negatively regulated by desumoylated C/ebpα, is implicated in the phenotypes triggered by SUMO-deficiency.
Figure 5. Runx1 is implicated in the hematopoietic defects triggered by SUMO-deficiency.
(A) The relative expression level of runx1 revealed by microarray analysis. The result shown is expressed as fold difference compared with the level (set to 100) detected in control embryos. (B) Luciferase activity assays were performed in 293T cells using the various C/ebpα constructs indicated. The Renilla plasmid was used as an internal control. Data shown are the mean ± SD of three independent experiments. *P < 0.05 by student's t-test. (C) WISH assay of cmyb in the sae1 mutant embryos and siblings at 72 hpf. (D) WISH assay of cmyb at 72 hpf and lysozyme C, hbae1 and rag1 at 5 dpf. Note that runx1 overexpression rescued the hematopoietic defects in the SUMO-deficient embryos. Red, blue, purple and black arrows identify cmyb, lysozyme C, hbae1 and rag1-positive cells, respectively.
Discussion
The biological function of sumoylation in hematopoiesis during early embryonic development has not been extensively studied. Ubc9-deficient mice die at the early postimplantation stage before hematopoiesis occurs30, precluding exploration of the functional role of sumoylation in hematopoiesis. Recently, a zebrafish mutant with defects in definitive hematopoiesis has been characterized. This phenotype is caused by a nonsense mutation of sae1 gene, a subunit of the sumoylation pathway E1 enzyme12. However, the molecular mechanism involved is still poorly understood. In this study, we show that depletion of SUMO or Ubc9 leads to a decreased number of HSPCs during embryogenesis. The defects of definitive hematopoiesis are attributed primarily to reduced HSPC generation and proliferation. C/ebpα, as a key sumoylated target, is essential for HSPC development during definitive hematopoiesis in zebrafish. These defective HSPCs triggered by hyposumoylation are probably caused by a cell-autonomous effect, as demonstrated in published chimera generation experiments12.
The transcription factor C/ebpα is a critical factor for granulopoiesis20. C/ebpα regulates the expression of a number of myeloid-specific genes31,32,33,34. Our previous work showed that C/ebpα desumoylation increased its transcriptional activity and promoted myelopoiesis of myelo-erythroid progenitor cells during primitive hematopoiesis9. Recently, an increasing number of reports have implicated C/ebpα as an important modulator of HSPCs function21,22,23,24. In mice, C/ebpα-deficient HSPCs possess enhanced proliferation and repopulation activity21,22,24, while C/ebpα activation in HSPCs results in their reduced self-renewal and proliferation23, suggesting a role for C/ebpα in limiting HSPC proliferation. Here we demonstrate for the first time that, under physiological conditions, desumoylation-activated C/ebpα leads to reduced proliferation of HSPC during zebrafish definitive hematopoiesis, thus highlighting the conserved role of C/ebpα in HSPC regulation across vertebrate species.
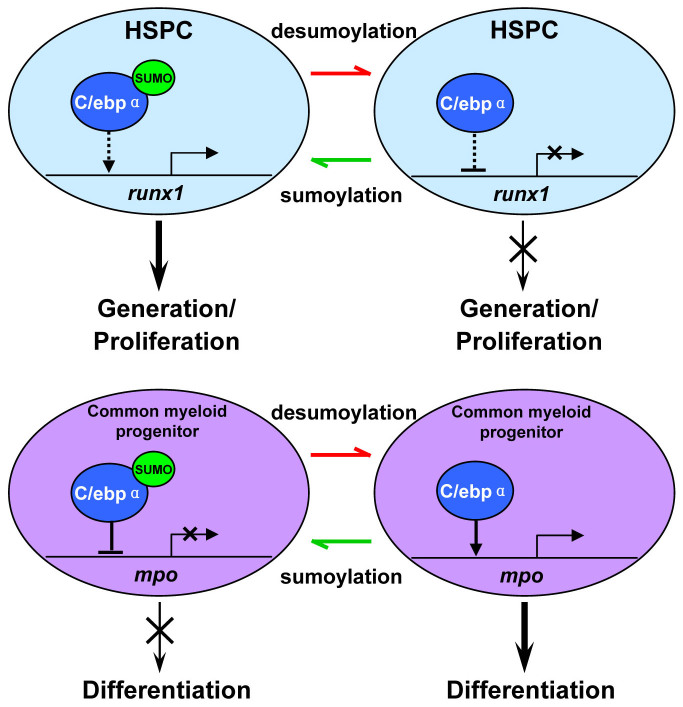
Mechanistically, our microarray analysis identified that upon the loss of SUMOs, runx1 (a critical transcription factor capable of regulating HSPC induction and proliferation35) was strongly down-regulated. Yet, SUMO-C/ebpα could largely restore its expression and rescue the definitive HSPC defects. Furthermore, runx1 mRNA was also able to rescue the hematopoietic defects in the SUMO-deficient embryos, implying that runx1 might be a downstream gene of C/ebpα. Based on our studies and those of others, we hypothesized that C/ebpα may exert distinct functions depending on its sumoylation status and specific given cell compartment (Fig. 6). The role of C/ebpα sumoylation in HSPCs appears to be quite distinct from its function in myeloid progenitor cells development. Within HSPCs, desumoylated C/ebpα acts as a transcriptional repressor and down-regulates runx1 directly or indirectly, which results in the attenuated HSPC generation and proliferation. Under physiological conditions, sumoylated C/ebpα, through an as yet unknown mechanism, likely promotes protein structural changes, de-represses runx1 transcription, and ultimately participates in controlling normal HSPC development (Fig. 6, top panel). In contrast, during myeloid development, the desumoylation-activated C/ebpα is required to promote myeloid progenitor cells differentiation through activation of myeloid-specific gene expression (Fig. 6, bottom panel).
Figure 6. Schematic depiction of the distinct functions of C/ebpα sumoylation in the regulation of HSPC and myeloid progenitor development during hematopoiesis.
HSPC homeostasis is maintained by the fine-tuning of C/ebpα sumoylation. Sumoylated C/ebpα de-represses runx1 transcription, which in turn participates in regulating normal HSPC development (top panel, left). In SUMO-deficient embryos, C/ebpα is desumoylated, which subsequently inhibits Runx1 activity, likely through direct transcriptional repression. As a consequence, HSPC generation and proliferation is reduced (top panel, right). In contrast, during myeloid cell development, inactivation of C/ebpα triggered by sumoylation blocks myeloid progenitor differentiation (bottom panel, left), while the activated form triggered by desumoylation promotes myelopoiesis (bottom panel, right).
ChIP assays aimed to verify whether runx1 expression was directly regulated by C/ebpα. Unfortunately however, due to both a lack of an endogenous zebrafish C/ebpα specific antibody, and cebpa mRNA overexpression causing embryonic death during gastrulation prior to the onset of hematopoiesis, further studies were not feasible. Nevertheless, it has been demonstrated by other groups that C/ebpα can bind to the runx1 promoter in both U937 cells and K562 cells36. Further work to investigate the ability of C/ebpα to regulate runx1 expression in zebrafish HSPCs is envisioned.
In summary, we provide novel evidence that sumoylation of C/ebpα is essential for HSPC development and demonstrate that protein posttranslational modification participates in the maintenance of HSPC properties during definitive hematopoiesis in zebrafish. Our studies shed new light on the role of protein sumoylation in HSPC regulation and may provide rationale for targeting of SUMO pathway in hematologic disorders.
Methods
Zebrafish
Zebrafish maintenance and staging were performed as described previously37. The transgenic lines, Tg (cmyb:EGFP)38, Tg (mpo:EGFP)9, Tg (gata1:EGFP)39 and Tg (flk1:EGFP), and sae1 mutants12, p53 mutants40 were used. The zebrafish facility and study were approved by the Institutional Review Board of the Institute of Health Sciences, Shanghai Institutes of Biological Sciences, Chinese Academy of Sciences (Shanghai, China) and the methods were carried out in accordance with the approved guidelines.
Generation of constructs
The mouse Runx1 enhancer29 and zebrafish runx1 were amplified by RT-PCR with indicated primers (Supplementary table S1), then cloned into pGL3-Promoter vector and pCS2+ vector, respectively. C/ebpα ΔBR and SUMO-C/ebpα ΔBR was generated with indicated primers, respectively (Supplementary table S1).
Morpholinos and mRNAs microinjection
MOs were purchased from Gene Tools. SUMO1 MO GTCTCCGTGTCTGACATGATATTCC; SUMO2 MO CATGGTTATTGTATTTGCGCTTCTC; SUMO3 MO TAGGCTTGTCTTCGGACATTTTTGC; Ubc9.1 MO TCAGAGCAATGCCAGACATGACCAC; Ubc9.2 MO GACGACTCAATGCTATACCAGACAT; p53 MO TCTTGGCTGTCGTTTTGCGCCATTG. The doses of injection per embryo were: SUMO1-SUMO2-SUMO3 MOs combination, 4.15 ng each; Ubc9.1-Ubc9.2 MOs combination, 4.15 ng each; p53 MO, 4.15 ng. Capped mRNAs were transcribed from linearized pCS2+ plasmid (mMessage Machine, Ambion), purified and diluted to 50 ng/μl (SUMO-cebpα, SUMO-cebpα ΔBR, POZ-cebpα and runx1 mRNA) for microinjection.
Whole-mount mRNA in situ hybridization
Digoxigenin-labeled antisense RNA probes were transcribed from linearized constructs using T3 or T7 polymerase (Roche). Whole-mount mRNA in situ hybridization was performed as described previously9. The probes were detected using alkaline phosphatase (AP)-coupled anti-digoxigenin Fab fragment antibody (Roche) with BCIP/NBT staining (Vector Laboratories).
Time-lapse confocal fluorescence imaging
Olympus FV 1000 confocal microscopy was used for the four-dimensional time-lapse fluorescence imaging15,16. Z stacks were taken every 2 min from 30 to 50 hpf. Videos were created after processing with the FV10-ASW version3 software.
Bromodeoxyuridine (BrdU) labeling assay, TUNEL assay and double immunostaining
Briefly, Tg(cmyb:EGFP) embryos were incubated with 10 mM BrdU (Sigma) for 30 minutes and incubated with egg water for another 2 hours, then fixed in 4% paraformaldehyde (PFA) at 3 dpf. After dehydration and rehydration, the embryos were treated with Proteinase K (10 mg/ml, Finnzyme) for 30 minutes and re-fixed in 4% PFA for 30 minutes. After blocking with blocking buffer (2 mg/ml BSA, 10% FBS, 0.3% Triton-X100 and 1% DMSO in PBST), the embryos were stained with rabbit anti-GFP (Invitrogen) primary antibody and Alexa Fluor 488-conjugated anti-rabbit (Invitrogen) secondary antibodies. The embryos were fixed again, treated with Proteinase K for the second time, and re-fixed in 4% PFA. The embryos were then incubated with 2 N HCl for 1 hour and stained with mouse anti-BrdU (Roche) and rabbit anti-GFP (Invitrogen) antibodies. Finally, Alexa Fluor 594-conjugated anti-mouse and Alexa Fluor 488-conjugated anti-rabbit (Invitrogen) secondary antibodies were used. Images were taken using Olympus FV 1000 confocal microscopy equipped with the FV10-ASW version3 software.
Terminal transferase UTP nick end labeling (TUNEL) was performed using the In Situ Cell Death Detection Kit, TMR red (Roche) according to the manufacturer's recommendations.
Luciferase reporter assay
293 T cells were transfected with indicated plasmids using Effectene Transfection Reagent (QIAGEN). Cells were harvested 36 hours after transfection and luciferase activities were analyzed using the Dual Luciferase Reporter Assay Kit (Promega) according to the manufacturer's protocols. Luciferase activity was normalized to Renilla activity.
Generation of TALEN-mediated cebpa mutant zebrafish
TAL Effector Nucleotide Targeter software was used to design candidate TALEN target site for cebpa gene41. TALEN expression vectors were constructed as previously described42. The corresponding mRNAs were prepared using SP6 mMESSAGE mMACHINE Kit (Ambion) and were injected in pairs into one-cell stage zebrafish embryos at the dosage of 150 pg (for each TALEN, 300 pg in total). To examine the targeting effect of cebpa TALENs, genomic DNA of 10 normally developing embryos was extracted as a pool at 30 hpf. A 867 bp DNA fragment containing the TALEN target site was amplified by PCR (forward primer: 5′-ATGGAGCAAGCAAACCTCTACGAGG-3′, reverse primer: 5′-TTAAGCGCAGTTGCCCATGGCTTTG-3′). 10 μl of the PCR product was digested by Sal I at 37°C overnight and fractionated through 2.5% agarose gel. The uncleaved band was recovered after gel electrophoresis and cloned for sequencing analysis. To screen the heritable mutants, the injected F0 founder embryos were raised to adulthood and outcrossed with wild type zebrafish. From each cross, genomic DNA of 10 F1 embryos was extracted as a pool and analyzed via PCR amplification and sequencing as described above. Siblings of F1 embryos carrying potential indel mutations were raised to adulthood. Genomic DNA was isolated from tail clips of these F1 fish and the status of the target site was analyzed via PCR amplification and sequencing to identify mutants. F1 zebrafish carrying 2 bp deletion in target site and their offspring were used for further study.
Sudan black staining
Sudan black staining was performed as described previously43.
Cell sorting and microarray
About 400 cmyb-EGFP transgenic embryos at 72 hpf were collected and digested with 0.5% trypsin (GIBCO) for 15 minutes at 37°C. Single-cell suspension was obtained by centrifugation at 400 g for 5 minutes, washing twice with PBS, and passing through a 40 μm nylon mesh filter. Fluorescence-activated cell sorting was performed with MoFlo FACS (DakoCytomation) to obtain homogenous EGFP positive cells. Then, RNA was extracted using Trizol (Invitrogen), which were subsequently subject to RT-PCR or microarray (Shanghai Biochip Co. Ltd).
Author Contributions
H.Y. designed the research, performed experiments, analyzed data and wrote the manuscript; T.Z., X.H.L. and M.D. performed experiments; W.Q.Z., Z.L.W., S.J.C., Z.C. and H.d.T. provided suggestions on experimental design and analyzed data; J.Z. and J.Z. designed experiments, analyzed data and wrote the manuscript.
Supplementary Material
Supplementary Files
Supplementary Video S1
Supplementary Video S2
Acknowledgments
We appreciate Dr. Gavine for proofreading this manuscript. The authors would also like to thank Dr. Bo Zhang and Dr. Marella F. T. R. de Bruijn for kindly providing TALEN plasmids and Runx1 enhancer plasmids, respectively. We are grateful for Mei Dong, Juan Chen, Yi Chen and Yi Jin for their excellent technical support. This work was supported by grants from the National Basic Research Program of China (973 Program) (2012CB910300), the National Natural Science Foundation of China (31371479), the Natural Science Foundation of Shanghai City (13ZR1425800) and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning.
References
- Orkin S. H. & Zon L. I. Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132, 631–644 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen J. S. Zebrafish make a big splash. Cell 87, 969–977 (1996). [DOI] [PubMed] [Google Scholar]
- Carradice D. & Lieschke G. J. Zebrafish in hematology: sushi or science? Blood 111, 3331–3342 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatman S. et al. Assaying hematopoiesis using zebrafish. Blood Cells Mol Dis 51, 271–276 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik E. J. & Zon L. I. Hematopoietic development in the zebrafish. Int J Dev Biol 54, 1127–1137 (2010). [DOI] [PubMed] [Google Scholar]
- Jagannathan-Bogdan M. & Zon L. I. Hematopoiesis. Development 140, 2463–2467 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. S. Protein modification by SUMO. Annu Rev Biochem 73, 355–382 (2004). [DOI] [PubMed] [Google Scholar]
- Flotho A. & Melchior F. Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem 82, 357–385 (2013). [DOI] [PubMed] [Google Scholar]
- Yuan H. et al. Sumoylation of CCAAT/enhancer-binding protein alpha promotes the biased primitive hematopoiesis of zebrafish. Blood 117, 7014–7020 (2011). [DOI] [PubMed] [Google Scholar]
- Yuan H. et al. Small ubiquitin-related modifier paralogs are indispensable but functionally redundant during early development of zebrafish. Cell Res 20, 185–196 (2010). [DOI] [PubMed] [Google Scholar]
- Nowak M. & Hammerschmidt M. Ubc9 regulates mitosis and cell survival during zebrafish development. Mol Biol Cell 17, 5324–5336 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Lan Y., Xu J., Zhang W. & Wen Z. SUMO1-activating enzyme subunit 1 is essential for the survival of hematopoietic stem/progenitor cells in zebrafish. Development 139, 4321–4329 (2012). [DOI] [PubMed] [Google Scholar]
- Kurtzman A. L. & Schechter N. Ubc9 interacts with a nuclear localization signal and mediates nuclear localization of the paired-like homeobox protein Vsx-1 independent of SUMO-1 modification. Proc Natl Acad Sci U S A 98, 5602–5607 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robu M. E. et al. p53 activation by knockdown technologies. PLoS Genet 3, 0787–0801 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J. Y. et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464, 108–111 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissa K. & Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464, 112–115 (2010). [DOI] [PubMed] [Google Scholar]
- Boisset J. C. et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464, 116–120 (2010). [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., Adashi E. Y., Graves B. J. & McKnight S. L. Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev 2, 786–800 (1988). [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F. & McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science 240, 1759–1764 (1988). [DOI] [PubMed] [Google Scholar]
- Zhang D. E. et al. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc Natl Acad Sci U S A 94, 569–574 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P. et al. Enhancement of hematopoietic stem cell repopulating capacity and self-renewal in the absence of the transcription factor C/EBP alpha. Immunity 21, 853–863 (2004). [DOI] [PubMed] [Google Scholar]
- Heath V. et al. C/EBPalpha deficiency results in hyperproliferation of hematopoietic progenitor cells and disrupts macrophage development in vitro and in vivo. Blood 104, 1639–1647 (2004). [DOI] [PubMed] [Google Scholar]
- Fukuchi Y., Ito M., Shibata F., Kitamura T. & Nakajima H. Activation of CCAAT/enhancer-binding protein alpha or PU.1 in hematopoietic stem cells leads to their reduced self-renewal and proliferation. Stem cells 26, 3172–3181 (2008). [DOI] [PubMed] [Google Scholar]
- Ye M. et al. C/EBPa controls acquisition and maintenance of adult haematopoietic stem cell quiescence. Nat Cell Biol 15, 385–394 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T. et al. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. Dev Cell 3, 137–147 (2002). [DOI] [PubMed] [Google Scholar]
- Timchenko N. A., Wilde M., Nakanishi M., Smith J. R. & Darlington G. J. CCAAT/enhancer-binding protein alpha (C/EBP alpha) inhibits cell proliferation through the p21 (WAF-1/CIP-1/SDI-1) protein. Genes Dev 10, 804–815 (1996). [DOI] [PubMed] [Google Scholar]
- Timchenko N. A. et al. CCAAT/enhancer binding protein alpha regulates p21 protein and hepatocyte proliferation in newborn mice. Mol Cell Biol 17, 7353–7361 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T., van Deursen J., Hiebert S. W., Grosveld G. & Downing J. R. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84, 321–330 (1996). [DOI] [PubMed] [Google Scholar]
- Nottingham W. T. et al. Runx1-mediated hematopoietic stem-cell emergence is controlled by a Gata/Ets/SCL-regulated enhancer. Blood 110, 4188–4197 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacerddine K. et al. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev Cell 9, 769–779 (2005). [DOI] [PubMed] [Google Scholar]
- Smith L. T., Hohaus S., Gonzalez D. A., Dziennis S. E. & Tenen D. G. PU.1 (Spi-1) and C/EBP alpha regulate the granulocyte colony-stimulating factor receptor promoter in myeloid cells. Blood 88, 1234–1247 (1996). [PubMed] [Google Scholar]
- Oelgeschlager M., Nuchprayoon I., Luscher B. & Friedman A. D. C/EBP, c-Myb, and PU.1 cooperate to regulate the neutrophil elastase promoter. Mol Cell Biol 16, 4717–4725 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford A. M. et al. Regulation of the myeloperoxidase enhancer binding proteins Pu1, C-EBP alpha, -beta, and -delta during granulocyte-lineage specification. Proc Natl Acad Sci U S A 93, 10838–10843 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwama A. et al. Use of RDA analysis of knockout mice to identify myeloid genes regulated in vivo by PU.1 and C/EBPalpha. Nucleic Acids Res 26, 3034–3043 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. D. Cell cycle and developmental control of hematopoiesis by Runx1. J Cell Physiol 219, 520–524 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompouki E. et al. Lineage regulators direct BMP and Wnt pathways to cell-specific programs during differentiation and regeneration. Cell 147, 577–589 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B. & Schilling T. F. Stages of embryonic development of the zebrafish. Dev Dyn 203, 253–310 (1995). [DOI] [PubMed] [Google Scholar]
- North T. E. et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447, 1007–1011 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y. F. et al. Mir-144 selectively regulates embryonic alpha-hemoglobin synthesis during primitive erythropoiesis. Blood 113, 1340–1349 (2009). [DOI] [PubMed] [Google Scholar]
- Berghmans S. et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci U S A 102, 407–412 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak T. et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res 39, 1–11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P. et al. Heritable gene targeting in zebrafish using customized TALENs. Nat Biotechnol 29, 699–700 (2011). [DOI] [PubMed] [Google Scholar]
- Le Guyader D. et al. Origins and unconventional behavior of neutrophils in developing zebrafish. Blood 111, 132–141 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Files
Supplementary Video S1
Supplementary Video S2